Abstract
Background
Cognitive impairment is common among patients with end-stage renal disease receiving hemodialysis 3x-per-week.
Study Design
Randomized Clinical Trial
Setting & Participants
218 subjects participating in the Frequent Hemodialysis Network (FHN) Daily Trial and 81 subjects participating in the FHN Nocturnal Trial.
Intervention
The Daily Trial tested in-center hemodialysis 6x-per-week versus 3x-per-week. The Nocturnal Trial tested home nocturnal hemodialysis 6x-per-week versus home or in-center hemodialysis 3x-per-week.
Outcomes
Cognitive function was measured at baseline, month four, and month 12. The primary outcome was performance on the Trail-Making Test, Form B, a measure of executive function, and a secondary outcome was performance on the Modified Mini-Mental State Examination, a measure of global cognition. The domains of attention, psychomotor speed, memory and verbal fluency were assessed in 59 participants in the Daily Trial and 19 participants in the Nocturnal Trial.
Results
We found no benefit of frequent hemodialysis in either trial for the primary cognitive outcome (Daily Trial: OR for improvement, 0.99; 95% CI, 0.59–1.66; Nocturnal Trial: OR, 1.19; 95% CI, 0.48–2.96). Similarly, there was no benefit of frequent hemodialysis in either trial on global cognition, the secondary cognitive outcome. Exploratory analyses in the Daily Trial suggested possible benefits of frequent hemodialysis on memory and verbal fluency, but not on attention and psychomotor speed. Exploratory analyses in the Nocturnal Trial suggested no benefit of frequent hemodialysis on attention, psychomotor speed, memory, or verbal fluency.
Limitations
Unblinded intervention, small sample
Conclusions
Frequent hemodialysis did not improve executive function or global cognition.
Keywords: dialysis, end-stage renal disease, cognitive function
Impairments in cognitive function have long been recognized as a debilitating complication of end-stage renal disease (ESRD) 1. Early observational studies reported that impairments in cognitive function and electroencephalographic (EEG) abnormalities improved after the initiation of maintenance dialysis or kidney transplantation, suggesting that retained uremic solutes were responsible for the cognitive manifestations of kidney failure 1. The National Cooperative Dialysis Study provided additional support for this hypothesis by demonstrating that increased fractional removal of urea during the dialysis session reduced EEG abnormalities among patients receiving maintenance hemodialysis 2.
Contemporary studies indicate up to 30% of patients with ESRD receiving thrice weekly maintenance hemodialysis suffer from moderate to severe cognitive impairment despite meeting urea removal standards advocated by clinical practice guidelines 3,4. It is not clear whether these findings reflect residual uremia 5, side effects of the conventional thrice weekly hemodialysis procedure 6,7, or other conditions that may affect cognitive function, such as depression, cerebrovascular disease or use of certain medications 8–10. Compared to conventional 3x-per-week hemodialysis schedules, frequent hemodialysis augments removal of uremic solutes, attenuates wide fluctuations in solute concentrations, and reduces hemodynamic instability during dialysis treatments. In an uncontrolled study of 12 subjects, conversion from 3x-per-week hemodialysis to long nocturnal dialysis was associated with improvements in several domains of cognitive function 11. Conversely, in another study of 13 subjects, conversion from 3x-per-week hemodialysis to short daily hemodialysis did not improve cognitive function 12. Cognitive test scores may improve with repeat administration, a phenomenon known as 'practice effects' 13. In the absence of a control group, it is difficult to interpret the significance of changes in cognitive function over time, especially when retesting occurs over a short period, since practice effects cannot be separated from the intervention's effect.
The Frequent Hemodialysis Network (FHN) Trials consist of two randomized clinical trials conducted in patients with ESRD receiving maintenance hemodialysis: a trial of incenter 3x-per-week hemodialysis versus in-center 6x-per-week hemodialysis ('Daily Trial'), and a trial of home or in-center 3x-per-week hemodialysis versus home 6x-per-week nocturnal hemodialysis ('Nocturnal Trial'). Executive function, as measured by the completion rate of the Trail Making B (Trails B) test, was designated as the main cognitive outcome in the FHN Trials and did not significantly improve with more frequent hemodialysis schedules 14,15. In this report we describe in more detail the effect of the interventions on executive function and additional domains of cognitive function, including global cognition, attention, psychomotor speed, memory and verbal fluency.
Methods
Participants
The design, recruitment, measurements and primary outcome of the FHN Trials have been published 14–16. Briefly, from January 2006 to March 2009 we recruited subjects with ESRD requiring maintenance hemodialysis from clinical sites in the United States and Canada for both trials. Major exclusion criteria included age <13 (Daily) or <18 (Nocturnal) years, inability to achieve a mean equilibrated Kt/V (eKt/V)urea ≥1.0 on two occasions, life expectancy less than six months, medical need for hemodialysis > 3x-per-week, residual urea clearance >3 ml/min (Daily), history of poor adherence to hemodialysis, inability to communicate in English or Spanish and anticipated kidney transplantation or relocation within the next 14 months. The study was reviewed by Institutional Review Boards at each clinical center and all subjects signed informed consent. For these analyses, we included all randomized individuals who completed baseline cognitive function testing and at least one follow-up test after randomization.
Intervention
Subjects enrolled in the Daily Trial were randomized to receive either in-center 3x-per-week hemodialysis targeting a minimum equilibrated Kt/Vurea of 1.1 per session and a session length of 2.5 to 4 hours, or to receive in-center hemodialysis 6x-per-week targeting a minimum equilibrated Kt/Vurea of 0.9 per session and a session length of 1.5 to 2.75 hours. Subjects enrolled in the Nocturnal Trial were randomized to receive either in-center or home 3x-per-week hemodialysis targeting a minimum equilibrated Kt/Vurea of 1.1 per session and a session length of 2.5 to 4 hours, or to receive home nocturnal hemodialysis 6x-per-week targeting a minimum weekly standard Kt/Vurea of 4.0 and a session length of 6 to 8 hours. In the Daily Trial, the total weekly dialysis time was 2.3 hours longer and the weekly standard Kt/Vurea was 40% higher in the intervention arm compared to the control arm 14. In the Nocturnal Trial, the total weekly dialysis time was 18 hours longer and the weekly standard Kt/Vurea was 73% higher in the intervention arm compared to the control arm 15.
Cognitive Assessment
We assessed cognitive function in all subjects with two tests administered by trained study coordinators in English or Spanish. Testing was performed at baseline, four months after randomization and 12 months after randomization. The Trails B test is a timed test of executive function17, and was designated the primary cognitive outcome in both trials. The Modified Mini-Mental State Examination (3MS) is a test of global cognitive function which includes an assessment of orientation, attention, calculation, language and short-term memory 18. The 3MS was designated as the secondary cognitive outcome.
English-speaking subjects enrolled after January 2007 from 5 of the 11 clinical sites in the Daily Trial and subjects enrolled after July 2008 from all clinical sites in the Nocturnal Trial completed a more extensive cognitive assessment battery which assessed domains of attention, psychomotor speed, memory and verbal fluency (see Box 1). The pre-specified main outcomes for the expanded cognitive study were scores on the Digit Symbol Substitution (for the Daily Trial) and Digit Symbol Coding (a subset of the Wechsler Adult Intelligence Scale, third revision; for the Nocturnal Trial) tests, which assess attention. Where available, we used alternate test forms for follow-up visits. For the Trails A and B and the grooved pegboard tests, lower scores indicate better performance, whereas for all other tests, higher scores indicate better performance. In the Daily Trial, the protocol targeted testing prior to a mid-week dialysis session. In the Nocturnal Trial, the protocol targeted testing 24 hours after the last completed dialysis session. Both protocols recommended against testing within six hours after completing a dialysis session.
Box 1.
Neurocognitive Assessment Battery Administered in FHN Trials.
| Global cognitive function |
| Modified Mini-Mental State Examination |
| Executive function |
| Trail-Making Test, Form B |
| Attention |
| Digit Symbol Substitution Test (Daily Trial) * |
| Digit Symbol Coding (Nocturnal Trial) * |
| Trail-Making Test, Form A* |
| Psychomotor speed |
| Grooved pegboard (Daily Trial) * |
| Memory |
| Rey Auditory Verbal Learning Test, immediate and delayed recall * |
| Letter-Number Sequencing * |
| Verbal fluency |
| Controlled Oral Word Association Test* |
indicates tests administered to a subset of FHN participants
FHN, Frequent Hemodialysis Network
Analytical Methods
Analyses were conducted separately for each trial and for each cognitive test. Because Trails B scores were not normally distributed after transformation, we first categorized subjects according to the change in Trails B scores from baseline to 12 months as follows: completed test in less than 300 seconds at baseline but not follow-up, follow-up score ≥30 seconds longer than baseline, follow-up score 1–29 seconds longer than baseline, no change in follow-up score, follow-up score 1–29 seconds shorter than baseline, follow-up score ≥30 seconds shorter than baseline, and completed test in less than 300 seconds at follow-up but not baseline. We then analyzed the resulting categories using ordinal logistic regression, expressing results as odds ratios (OR) with 95% confidence intervals (CI). For the 3MS, the main treatment effects were evaluated using mixed effects models adjusted for baseline score and, in the Daily Trial, for clinical center. Correlations within subjects over time were accounted for using an unstructured covariance matrix. In post-hoc analyses we analyzed the change in Trails B scores using mixed effects models after transforming raw scores into t-scores based on age, sex and education level 19. The ancillary cognitive test scores were analyzed in a similar manner with adjustment for baseline score. To facilitate comparisons across cognitive tests, we derived standardized effect sizes for each test by dividing the mean treatment group difference by the baseline standard deviation (SD).
Pre-specified subgroup analyses were performed for Trails B and 3MS scores according to age (stratified by median), education (post high school education versus not), depressive symptoms (Beck Depression Inventory score <15, ≥15) 20, and baseline 3MS score (stratified by median). We also conducted post-hoc subgroup analyses according to baseline use of central nervous system (CNS) medications - anticonvulsants, antidepressants, antihistamines, benzodiazepines, hypnotics and opioids.
We conducted two separate sensitivity analyses. The first set of sensitivity analyses assessed whether differences in the interdialytic interval preceding cognitive testing may have biased cognitive function scores. For these analyses, we modeled cognitive test scores as a quadratic function of the interdialytic interval after controlling for the number of treatments during the month. The second set of sensitivity analyses assessed whether loss to follow-up affected the findings. For these analyses, we imputed missing Trails B and 3MS scores based on baseline age, sex, race, language, diabetes, vintage and center, and all available measurements of the Medical Outcomes Study Physical Health Composite, Mental Health Composite, Feeling Thermometer, Beck Depression Inventory, weight, serum albumin concentration, and hospitalization rate. The Trails B was converted to an ordinal variable of 10 second intervals and then imputed using the discriminant function method to accommodate the bimodal distribution. We imputed the 3MS using the regression method.
We estimated the Daily Trial had 80% power to detect an effect size of 0.40 on the Trails B using a two-sided significance level of 0.05 and a sample size of 250 subjects with 20% of randomized subjects missing follow-up measurements. We estimated the Nocturnal Trial had 80% power to detect an effect size of 0.67 on the Trails B using a two-sided significance level of 0.05 and a sample size of 90 subjects with 20% of randomized subjects missing follow-up measurements. Two-tailed P-values <0.05 were considered statistically significant unless otherwise indicated. Analyses were conducted using SAS, version 9.2 (SAS Institute Inc).
Results
Daily Trial Cohort Characteristics
Of the 245 subjects randomized in the Daily Trial who completed baseline testing, 215 completed testing at month 4, 175 completed testing at the end of the study, and 218 completed at least one cognitive test during follow-up (Fig S1, provided as online supplementary material). Reasons for test non-completion at the final assessment were similar in the intervention and control groups. Subject characteristics according to trial and treatment arm are shown in Table 1. Mean age in the Daily Trial was 50.4 ± 13.9 years and median ESRD vintage was 3.6 (range, 0.6–17.3) years. At baseline 70% of subjects in the 3x-per-week group and 74% of subjects in the 6x-per-week group had a Trails B score within 300 seconds (median scores, 105 and 96, respectively). There were 9 subjects (4%) who had a 3MS score of 100 (the maximum) at baseline.
Table 1.
Baseline Characteristics of Participants.
| Baseline characteristic | Daily Trial | Nocturnal Trial | ||
|---|---|---|---|---|
|
| ||||
| 3x/wk | 6x/wk | 3x/wk | 6x/wk | |
|
| ||||
| No. of patients | 101 | 117 | 40 | 41 |
|
| ||||
| Age (y) | 51.9 ± 13.8 | 48.9 ± 13.4 | 54.9 ± 12.2 | 51.1 ± 14.5 |
|
| ||||
| Male sex | 61 (60.4) | 72 (61.5%) | 26 (65.0%) | 27 (65.9%) |
|
| ||||
| Race /Ethnicity | ||||
| Black | 53 (44.2%) | 49 (39.2%) | 11 (26.2%) | 12 (26.7%) |
| White | 46 (38.3%) | 43 (34.4%) | 21 (50.0%) | 27 (60.0%) |
| Native American, Aboriginal Canadian, Alaskan Native, First Nation | 4 (3.3%) | 4 (3.2%) | 2 (4.8%) | 1 (2.2%) |
| Asian | 5 (4.2%) | 11 (8.8%) | 7 (16.7%) | 5 (11.1%) |
| Native Hawaiian or other | 3 (2.5%) | 1 (0.8%) | 0 (0%) | 0 (0%) |
| Pacific Islander | ||||
| Other/Mixed/Unknown | 9 (7.5%) | 17 (13.6%) | 1 (2.4%) | 0 (0%) |
| Hispanic/Latino Ethnicity* | 31 (26%) | 38 (30%) | 0 (0%) | 0 (0%) |
|
| ||||
| Primary language English | 85 (84.2%) | 88 (75.2%) | 34 (85.0%) | 37 (90.0%) |
|
| ||||
| ESRD vintage (y) | 3.02 (0.6–12.5) | 3.85 (0.6–17.3) | 0.5 (0.08–6.1) | 1.3 (0.09–12.6) |
|
| ||||
| Education | ||||
|
| ||||
| < High school graduate | 20 (20.0%) | 27 (23.5%) | 5 (12.5%) | 8 (20.0%) |
|
| ||||
| High school graduate | 29 (29.0%) | 25 (21.7%) | 9 (22.5%) | 11 (27.5%) |
|
| ||||
| Post high school | 51 (51.0%) | 63 (54.8%) | 26 (65.0%) | 21 (52.5%) |
|
| ||||
| Diabetes | 42 (41.6%) | 48 (41.0%) | 18 (45.0%) | 16 (39.0%) |
|
| ||||
| Stroke | 6 (5.9%) | 8 (6.8%) | 1 (2.5%) | 1 (2.4%) |
|
| ||||
| Charlson comorbidity index | 1.5 ± 1.8 | 1.4 ± 1.6 | 1.6 ± 1.7 | 1.2 ± 1.3 |
|
| ||||
| Beck depression index | 12.7 ± 9.9 | 12.5 ± 8.6 | 11.5 ± 8.7 | 11.7 ± 8.2 |
|
| ||||
| Predialysis SBP (mm Hg) | 146 ± 18 | 147 ± 19 | 154 ± 22 | 144 ± 13 |
|
| ||||
| Predialysis DBP (mm Hg) | 78 ± 12 | 81 ± 11 | 83 ± 14 | 80 ±11 |
|
| ||||
| Hemoglobin (g/dL) | 12.0 ± 1.3 | 11.8 ± 1.2 | 11.9 ± 1.1 | 11.6 ± 1.1 |
|
| ||||
| Albumin (g/dL) | 4.0 ± 0.5 | 3.9 ± 0.4 | 3.9 ± 0.5 | 3.9 ± 0.5 |
|
| ||||
| Anticonvulsant | 22 (21.8%) | 22 (18.8%) | 8 (20.0%) | 7 (18.4%) |
|
| ||||
| Antidepressant | 13 (12.9%) | 14 (15.8%) | 9 (22.5%) | 9 (23.7%) |
|
| ||||
| Antihistamine | 24 (23.8%) | 21 (18.0%) | 14 (35.0%) | 18 (43.9%) |
|
| ||||
| Benzodiazepine | 10 (9.9%) | 15 (12.8%) | 7 (17.5%) | 7 (17.1%) |
|
| ||||
| Opioid | 15 (14.9%) | 21 (18.0%) | 7 (17.5%) | 10 (24.4%) |
Note: Values are for participants in the Frequent Hemodialysis Network Trials who completed baseline and at least 1 follow-up cognitive test. Values for categorical variables are given as number (percentage); values for continuous variables are given as mean +/− SD or median (range).
Abbreviations: ESRD, end-stage renal disease; SBP - systolic blood pressure, DBP - diastolic blood pressure
Persons in this category may also be counted in other categories.
Of the 60 Daily Trial subjects who completed the expanded battery of cognitive tests at baseline, 51 completed testing at the month 4 time point, 36 completed testing at the end of the study, and 59 completed at least one cognitive test during follow-up (Fig S1). Drop-out resulting from death or transplantation was similar in the two groups. Characteristics of subjects who were included in the expanded cognitive study were similar to those who were not, except that subjects who participated in the expanded study were six years older on average and had completed more years of education (P<0.01 for both) (Table S1).
Daily Trial Primary and Secondary Cognitive Outcomes
Cognitive tests were administered 43 (IQR, 22–44) hours after the last dialysis treatment at baseline. At month 12, cognitive tests were administered 43 (IQR, 25–44) hours after the last dialysis treatment in the 3x-per-week group and 21 (IQR, 20–29) hours after the last dialysis treatment in the 6x-per-week group. Overall, 55% of subjects had an improvement in Trails B performance after 12 months. The odds of improvement did not differ between the intervention and control groups at month four (OR, 1.15; 95% CI, 0.72–1.86) or at month 12 (OR, 0.99; 95% CI, 0.59–1.66; Figure 1). Post-hoc analyses utilizing Trails B t-scores resulted in similar findings. There was no evidence for effect-modification by age, education, baseline 3MS scores, baseline depressive symptoms or baseline CNS medication use on Trails B scores (all P-values for interaction terms >0.05).
Figure 1.
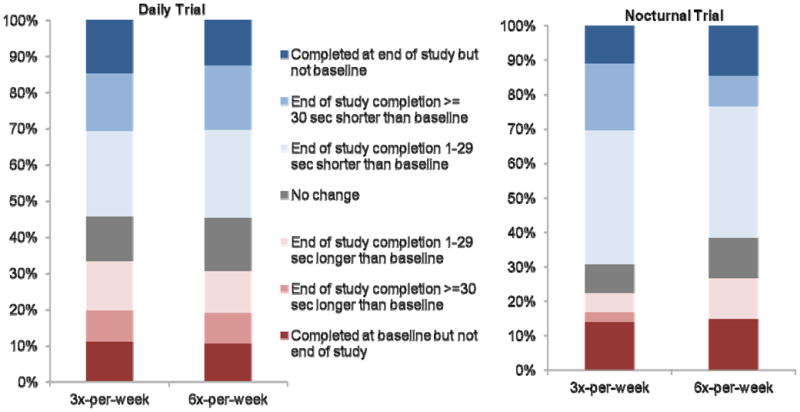
Change in Trail B score from baseline to month 12 in Daily Trial (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.59–1.66) and Nocturnal Trial (OR, 1.19; 95% CI, 0.48–2.96).
Mean scores on the 3MS increased (improved) over time in both groups and did not differ according to treatment group (Table 2). There was no consistent evidence for effect-modification by age, education, baseline 3MS scores, depressive symptoms or CNS medications (P-values >0.05).
Table 2.
3MS scores in the Daily trial and Nocturnal Trial
| Trial | Treatment arm | Observed Data | Adjusted Means and Treatment Effect (± SE or with 95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Mo 0 | Mo 4 | Mo 12 | Mo 4 | Mo 12 | ||||
| Mo 4 vs Mo 0 | 6x/wk vs. 3x/wk | Mo 12 vs Mo 0 | 6x/wk vs. 3x/wk | |||||
| Daily Trial* | 3x/wk | 87 ± 10 | 88 ± 10 | 89 ± 9 | +1.1 ± 0.7 | −0.0 (−1.8 to 1.5) | +1.9 ± 0.8 | +0.2 (−1.8 to 2.2) |
| 6x/wk | 87 ± 9 | 88 ±10 | 89 ± 10 | +1.0 ± 0.6 | +2.1 ± 0.7 | |||
| Nocturnal Trial** | 3x/wk | 90 ± 6 | 94 ± 6 | 95 ± 4 | +3.5 ± 0.8 | −2.1 (−4.3 to 0.0) | +4.4 ± 1.1 | −2.3 (−5.4 to0.8) |
| 6x/wk | 90 ± 8 | 92 ± 10 | 93 ± 12 | +1.3 ± 0.8 | +2.1 ± 1.1 | |||
Note: Values for observed data are given as mean ± SD; other values given as adjusted mean ± SE or treatment effect (95% CI).
Values adjusted for baseline score and clinical center
Values adjusted for baseline score
Abbreviations: 3MS, Modified Mini-Mental State Examination; SD - standard deviation, SE - standard error, CI - confidence interval
The maximum (best) score on the 3MS is 100.
In sensitivity analyses, there was no association between the interdialytic interval and scores on the Trails B or 3MS. When we used multiple imputation to account for loss to follow-up, the magnitude of improvement in test scores was smaller in both groups. Consistent with the primary analyses, there was no significant benefit of frequent hemodialysis on either Trails B or 3MS scores.
Daily Trial Expanded Cognitive Battery
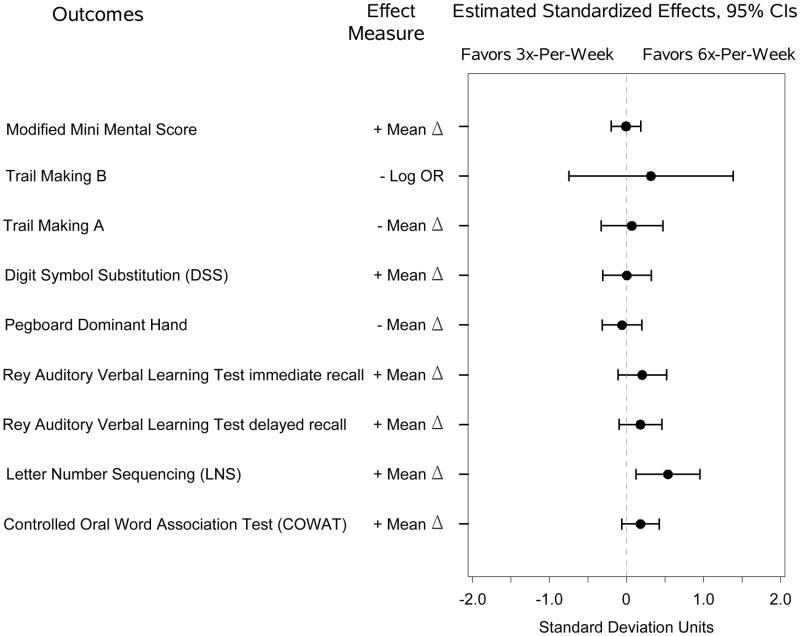
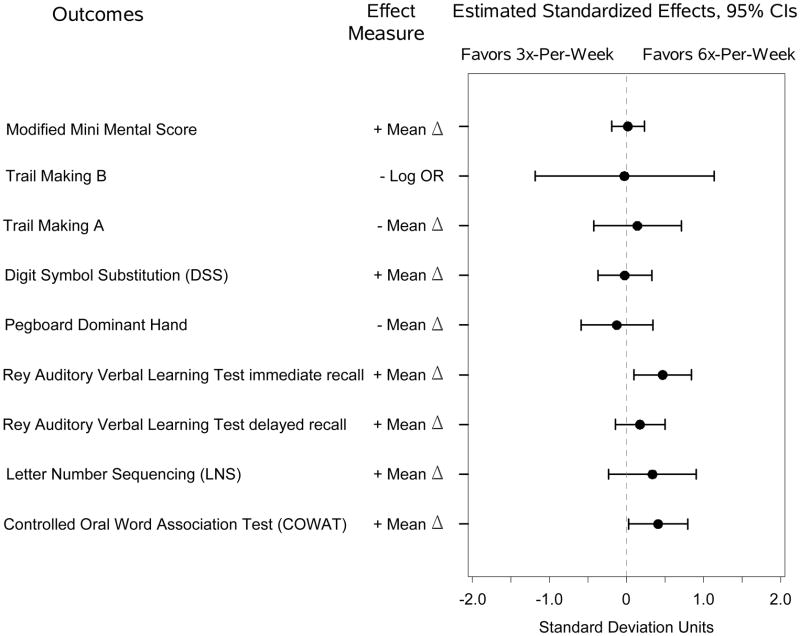
Among the subset of Daily Trial subjects who completed the expanded cognitive battery, there were no significant differences in tests of attention or psychomotor speed between the intervention and control groups (Figure 2A-B and Table S2). At four months there was a significantly larger improvement in the Letter-Number Sequencing subtest of the Wechsler Adult Intelligence Scale for the 6x-per-week group versus the 3x-per-week group, and similar but not significant trends for other tests of memory and verbal fluency. At 12 months, there were significantly larger improvements on the Rey Auditory Verbal Learning Test Immediate Recall and the Controlled Oral Word Association Test for the 6x-per-week group compared to the 3x-per-week group.
Figure 2.
(A) Estimated standardized effects on cognitive outcomes comparing 6-times-per-week versus 3-times-per-week hemodialysis in the Daily Trial at month four. n=218 for the Modified Mini-Mental State Examination; n=209, Trail-Making Test, Form B; and n=59, other tests. (B). Estimated standardized effects on cognitive outcomes comparing 6-times-per-week versus 3-times-per-week hemodialysis in the Daily Trial at month 12. n=176 for the Modified Mini-Mental State Examination; n=176, Trail-Making Test, Form B; and n=36, other tests. CI, confidence interval; OR, odds ratio.
Nocturnal Trial Cohort Characteristics
Of the 87 subjects randomized in the Nocturnal Trial who completed baseline testing, 81 completed testing at month 4 and 71 completed testing at the end of the study. Reasons for test non-completion were similar between the intervention and control groups (Figure S2). Mean age in the Nocturnal Trial was 52.8 ± 13.6 years and median ESRD vintage was 0.9 (range, 0.1–12.6) years (Table 1). At baseline 81% of subjects in the 3x-per-week group and 75% of subjects in the 6x-per-week group had a Trails B score within 300 seconds (median scores, 87 and 77, respectively). There were 2 subjects (2%) who had a 3MS score of 100 at baseline. Of the 21 Nocturnal Trial subjects who completed the expanded cognitive battery at baseline, 19 completed testing at month four while 17 completed testing at the end of follow-up. Characteristics of subjects who completed the expanded cognitive battery were similar to the overall trial cohort (Table S1).
Nocturnal Trial Primary and Secondary Cognitive Outcomes
Cognitive tests were administered 43 (IQR, 27–44) hours after the last dialysis treatment at baseline. At month 12, cognitive tests were administered 25 (IQR, 14–42) hours after the last dialysis treatment in the control group and 31 (IQR, 18–33) hours after the last dialysis treatment in the intervention group; in other words, cognitive testing occurred following a night “off” more often in the 6x-per-week group than in the 3x-per-week group. Overall, 66% of subjects had an improvement in Trails B performance after 12 months. The odds of improvement did not differ between the intervention and control groups at month 4 (OR, 1.01; 95% CI, 0.44–2.30) or month 12 (OR, 1.19; 95% CI, 0.48–2.96; Figure 1). Post-hoc analyses utilizing Trails B t-scores had similar findings. There was no evidence for effect-modification by age, education, depressive symptoms or individual CNS medication use (all P-values for interaction terms >0.05).
Scores on the 3MS increased over time in both groups (Table 2). Improvements were less pronounced in the 6x-per-week group; this difference was of borderline significance at four months, and not statistically significant at 12 months. There was no evidence for effect-modification by age, education, baseline 3MS score, depressive symptoms or individual CNS medication use (all P-values for interaction terms >0.05).
In sensitivity analyses, there was no association between the interdialytic interval and scores on the Trails B or 3MS. Multiple imputation models were consistent with the main analyses.
Nocturnal Trial Expanded Cognitive Battery
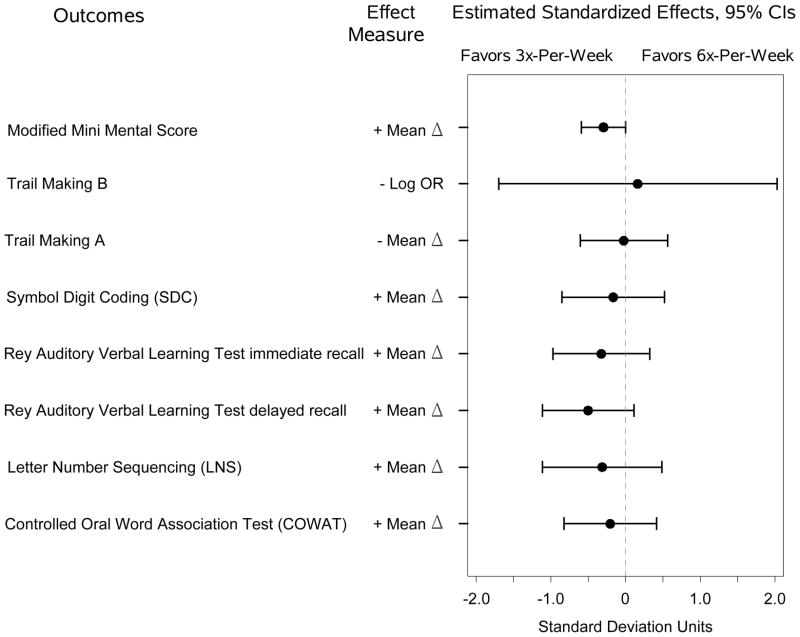
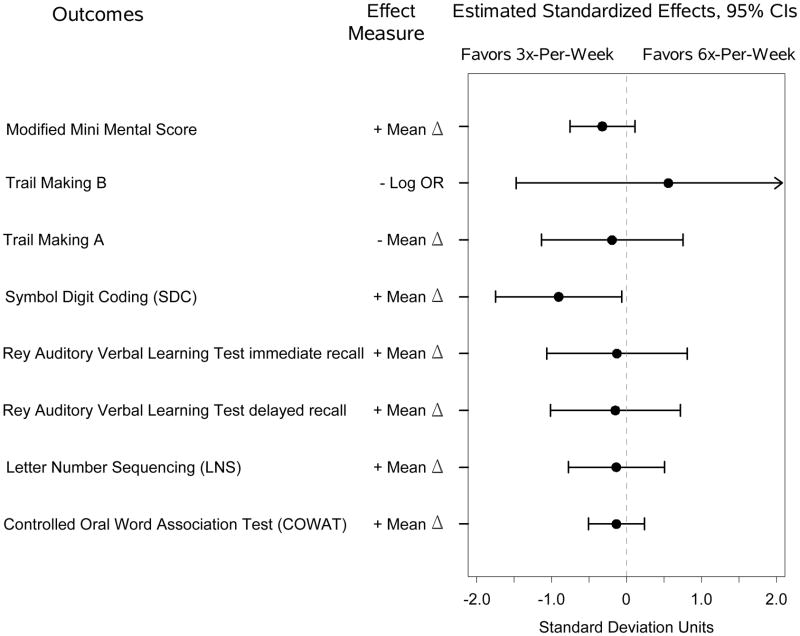
On the expanded cognitive battery, there were no significant effects of frequent hemodialysis at 4 months (Figure 3A) but there was a statistically significant poorer performance on the Digit Symbol Coding for the 6x-per-week group compared to the 3x-per-week group at 12 months (Figure 3B). There were no other significant differences in test scores, though in all cases the trends favored the control group.
Figure 3.
(A) Estimated standardized effects on cognitive outcomes comparing 6-times-per-week versus 3-times-per-week hemodialysis in the Nocturnal Trial at month four. n=80 for the Modified Mini-Mental State Examination; Trail-Making Test, Form B, and n=21 for other tests.
(B) Estimated standardized effects on cognitive outcomes comparing 6-times-per-week versus 3-times-per-week hemodialysis in the Nocturnal Trial at month four. n=70 for the Modified Mini-Mental State Examination and Trail-Making Test, Form B, and n=19 for other tests. CI, confidence interval; OR, odds ratio.
Discussion
We found that frequent hemodialysis did not improve the primary outcome of executive function or the secondary outcome of global cognition. These results were not modified by age, education, depressive symptoms, baseline cognition or individual CNS medication use. In exploratory analyses in the Daily Trial, frequent hemodialysis improved performance on tests of memory and verbal fluency but not attention or psychomotor speed. In exploratory analyses in the Nocturnal Trial, frequent hemodialysis was associated with significantly poorer performance on one test of attention, but there were no significant differences in other cognitive domains.
To our knowledge, the FHN Trials are the only randomized trials of frequent hemodialysis that have evaluated cognitive function. Jassal and colleagues studied cognitive function in 12 patients converted from home hemodialysis 3x-per-week to nocturnal hemodialysis 5 to 7x-per-week 11. They observed significant improvements in the domains of psychomotor efficiency, processing speed, attention and working memory after six months of follow-up; however their study did not include a control group. Vos and colleagues studied cognitive function in 13 patients who converted from home hemodialysis 3x-per-week to 6x-per-week 12. After six months of follow-up, they found no significant differences in several cognitive domains compared to matched controls who had remained on 3x-per-week hemodialysis.
Unique features of the study design and patient cohort may affect how our results are interpreted. First, the FHN Trials were randomized and had longer follow-up. This eliminated confounding by indication or patient selection and allowed us to control for practice effects attributable to repeated assessments of cognitive function. Second, we tested in-center short daily hemodialysis and home long nocturnal hemodialysis in two separate trials. By using the same cognitive battery in both trials, we could qualitatively compare the effects of short daily hemodialysis and long nocturnal hemodialysis. The FHN Trials had an older study population with more comorbidity compared to previous studies of frequent dialysis. This enhances the generalizability of our results, but may have contributed to higher long-term drop-out rates. It might also have obscured a benefit to frequent dialysis evident only in a younger population.
The FHN findings are important for several reasons. First, cognitive impairment is common among patients receiving conventional hemodialysis but until now there have been no randomized trials to measure the effect of more frequent dialysis. Second, although there were no statistically significant differences in the primary or secondary cognitive outcomes in either trial, the magnitude of improvement in memory and verbal fluency in the Daily Trial may be clinically important. For example, frequent hemodialysis was associated with an average improvement in immediate recall by almost six words and an average increased verbal fluency of almost five words. These effects are larger than the reported benefits of physical activity among older adults with mild cognitive impairment 21,22. Third, they suggest that memory and verbal fluency domains may be more sensitive to uremia than other cognitive functions. Impairments in attention and executive function, although common among patients receiving maintenance hemodialysis, may not be primarily related to residual uremia. For example, aging, depressive symptoms, cerebrovascular disease and certain medications have been linked with executive dysfunction 8,9,23. It is also possible these results are attributable to differential drop-out or to chance, since similar findings were not seen in the Nocturnal Trial.
In the Nocturnal Trial, we found no benefit from more frequent hemodialysis across multiple cognitive domains. Furthermore, exploratory analyses found performance on tests of global cognition and attention was poorer in the intervention group. Whether the differing findings on secondary cognitive measures in the Nocturnal Trial versus the Daily Trial are due to differences in baseline patient characteristics, differences in the measurement of cognitive function, chance (due to the large number of cognitive tests performed and the small number of subjects), or due to a true adverse effect of nocturnal hemodialysis is not clear. Poorer cognitive function in the frequent arm of the Nocturnal Trial was not explained by differences in CNS medication use at baseline or over the course of the trial (data not shown). Alternatively, sleep disturbance from nocturnal dialysis may have had a detrimental effect on cognitive function.
Our study has several limitations. Though larger than previous studies, our sample size was small, particularly for the expanded cognitive battery. The subjects and study personnel were not blinded, so it is possible knowledge of the treatment arm influenced cognitive performance. There were differences in the interdialytic interval preceding cognitive testing between treatment arms in addition to higher than expected drop-out at 12 months, reflecting the complexity and intensity of the interventions. In sensitivity analyses, these differences had no significant effect on the primary or secondary outcomes. Finally, practice effects were evident on many cognitive tests. Sensitivity analyses indicated the magnitude of practice effects would be smaller if subjects who were lost to follow-up remained in the study, since these subjects tended to be sicker and have poorer cognitive function.
In conclusion, we found that frequent hemodialysis did not improve executive function or global cognition, suggesting that residual uremia is not primarily responsible for these impairments. Exploratory analyses indicated possible improvements in memory and verbal fluency in the Daily Trial, and a possible detrimental effect on attention and global cognition in the Nocturnal Trial that merit further investigation.
Supplementary Material
Acknowledgments
We dedicate this manuscript to our friend, colleague, and collaborator Dr John Stokes.
Support: This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK03005 and R01DK74715 to JBS), a Paul B. Beeson Career Development Award in Aging (K23AG028952 to MKT) from the National Institute on Aging, a Norman S. Coplon Award from Satellite Research (MKT), and by the Department of Veterans Affairs (JBS).
Footnotes
Trial registration: www.ClinicalTrials.gov; study numbers: NCT00264758 and NCT00271999.
Financial Disclosure:Dr Kurella Tamura has received funding and served on an advisory board for Amgen. Dr Nissenson is Chief Medical Officer of DaVita. The other authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Table S1: Characteristics of FHN Trial patients completing extended cognitive study.
Table S2: Change in cognitive function scores in the Daily Trial extended cognitive study.
Table S3: Change in cognitive function scores in the Nocturnal Trial extended cognitive study.
Figure S1. Flow of participants in the FHN Daily Trial.
Figure S2. Flow of participants in the FHN Nocturnal Trial.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.Teschan PE. Electroencephalographic and other neurophysiological abnormalities in uremia. Kidney Int Suppl. 1975;(2):210–216. [PubMed] [Google Scholar]
- 2.Teschan PE, Bourne JR, Reed RB, Ward JW. Electrophysiological and neurobehavioral responses to therapy: the National Cooperative Dialysis Study. Kidney Int Suppl. 1983;(13):S58–65. [PubMed] [Google Scholar]
- 3.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30(1):41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 4.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006 Jul 25;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 5.Williams MA, Sklar AH, Burright RG, Donovick PJ. Temporal effects of dialysis on cognitive functioning in patients with ESRD. Am J Kidney Dis. 2004 Apr;43(4):705–711. doi: 10.1053/j.ajkd.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Mizumasa T, Hirakata H, Yoshimitsu T, et al. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97(1):c23–30. doi: 10.1159/000077592. [DOI] [PubMed] [Google Scholar]
- 7.Giang LM, Weiner DE, Agganis BT, et al. Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol. 2011;33(1):33–38. doi: 10.1159/000322611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agganis BT, Weiner DE, Giang LM, et al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2010 Oct;56(4):704–712. doi: 10.1053/j.ajkd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010 Aug;5(8):1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner DE, Scott TM, Giang LM, et al. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2011 Nov;58(5):773–781. doi: 10.1053/j.ajkd.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jassal SV, Devins GM, Chan CT, Bozanovic R, Rourke S. Improvements in cognition in patients converting from thrice weekly hemodialysis to nocturnal hemodialysis: A longitudinal pilot study. Kidney Int. 2006;70(5):956–962. doi: 10.1038/sj.ki.5001691. [DOI] [PubMed] [Google Scholar]
- 12.Vos PF, Zilch O, Jennekens-Schinkel A, et al. Effect of short daily home haemodialysis on quality of life, cognitive functioning and the electroencephalogram. Nephrol Dial Transplant. 2006 Sep;21(9):2529–2535. doi: 10.1093/ndt/gfl256. [DOI] [PubMed] [Google Scholar]
- 13.Collie A, Darby DG, Falleti MG, Silbert BS, Maruff P. Determining the extent of cognitive change after coronary surgery: a review of statistical procedures. Ann Thorac Surg. 2002 Jun;73(6):2005–2011. doi: 10.1016/s0003-4975(01)03375-6. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010 Dec 9;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011 Jul 20; doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007 Feb;71(4):349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 17.Yeudall LT, Reddon JR, Gill DM, Stefanyk WO. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol. 1987 May;43(3):346–367. doi: 10.1002/1097-4679(198705)43:3<346::aid-jclp2270430308>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 19.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004 Mar;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 20.Watnick S, Wang PL, Demadura T, Ganzini L. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005 Nov;46(5):919–924. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama. 2008 Sep 3;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 22.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010 Jan;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004 Nov 9;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.