The nature of the immunoglobulin light chain affects peripheral B cell tolerance and autoreactivity.
Abstract
The important subtleties of B cell tolerance are best understood in a diverse immunoglobulin (Ig) repertoire context encoding a full spectrum of autoreactivity. To achieve this, we used mice expressing Igκ transgenes that confer varying degrees of autoreactivity within a diverse heavy chain (HC) repertoire. These transgenes, coupled with a biomarker to identify receptor-edited cells and combined with expression cloning of B cell receptors, allowed us to analyze tolerance throughout B cell development. We found that both the nature of the autoantigen and the Ig HC versus light chain (LC) contribution to autoreactivity dictate the developmental stage and mechanism of tolerance. Furthermore, although selection begins in the bone marrow, over one third of primary tolerance occurs in the periphery at the late transitional developmental stage. Notably, we demonstrate that the LC has profound effects on tolerance and can lead to exacerbated autoantibody production.
Random rearrangement of the heavy chain (HC) and light chain (LC) genes encoding the B cell receptor (BCR) creates a diverse repertoire capable of recognizing a wide array of antigens but at the cost of generating self-reactive specificities that can predispose for widespread autoimmune disease. An important stage for removal of self-reactive B cells from the developing repertoire, termed primary tolerance, occurs when the BCR is first expressed on the cell surface at the immature stage of development in the BM. At this stage, receptor editing, or secondary rearrangement of Ig genes to alter the specificity of an autoreactive BCR (Gay et al., 1993; Radic et al., 1993; Tiegs et al., 1993), is the default mechanism for the removal of autoreactive B cells (Melamed and Nemazee, 1997; Halverson et al., 2004). It is estimated that one quarter of all mature B cells that enter peripheral lymphoid organs such as the spleen have been subjected to receptor editing (Casellas et al., 2001). If receptor editing proves unsuccessful at reducing BCR self-reactivity, immature B cells are removed by clonal deletion (Nossal, 1983; Nemazee and Bürki, 1989) or are rendered anergic, particularly if they react to soluble or low avidity self-antigens (Goodnow et al., 1988).
Censoring of the B cell repertoire also occurs in peripheral B cells. BCR transgenic B cells undergo deletion when they encounter their cognate antigen expressed solely in peripheral tissues (Russell et al., 1991; Lang et al., 1997; Aït-Azzouzene et al., 2006; Duong et al., 2010; Ota et al., 2010). The acute sensitivity of transitional B cells to undergo apoptosis upon BCR engagement and the restriction of the BCR repertoire between immature B cells and the splenic mature naive pool further demonstrate that selection also occurs after B cells exit the BM (Gu et al., 1991; Carsetti et al., 1995; Norvell et al., 1995; Levine et al., 2000; Allman et al., 2001). A study in humans has also demonstrated that the relative number of self-reactive B cells decreases from ∼40% to 20% as newly formed immature B cells transition into the naive mature B cell compartment (Wardemann et al., 2003).
Much of what we know about central and peripheral B cell tolerance mechanisms has been gleaned from studying the development of self-reactive B cells expressing a transgenic HC or HC/LC pair that recognize a well-defined self-antigen (Shlomchik, 2008). These studies have defined many of the fundamental concepts of tolerance, but understanding the role of each tolerance mechanism and the developmental stage where tolerance occurs in a more physiological setting has been challenging. A major goal of this study, therefore, was to quantify the relative contributions of central versus peripheral tolerance mechanisms in honing the mature repertoire in the context of a highly diverse polyclonal B cell repertoire. To do so, we used mice containing HCs generated by endogenous rearrangement of the HC loci with a κ LC knockin transgene, which allows us to identify receptor-edited cells. To characterize editing and selection for a wide spectrum of self-reactivity, we studied two different κ LC knockin transgenic mice. First, we used the prototypical anti-DNA–associated LC Vκ4-Jκ4 (Vκ4; Shlomchik et al., 1987; Prak and Weigert, 1995), in which editing was discovered by the observation that continued VJ recombination efficiently replaced this LC to reduce the anti-DNA reactivity of the 3H9 HC (Gay et al., 1993; Radic et al., 1993; Chen et al., 1997). Second, we used the anti-HEL Vκ5-45/Jκ2 LC (αHelκ) as an “innocuous” LC (Casellas et al., 2001). When paired with randomly rearranged HCs, the Vκ4-containing BCRs are predicted to have a propensity for autoreactivity, and tolerance mechanisms such as receptor editing or deletion will be induced. Conversely, the αHelκ LC is not predicted to contribute to and may indeed lessen the autoreactive nature of a BCR and so should only rarely be edited or counter-selected when paired with random HCs.
Using these two different LC transgenic mice, we determined the frequency of edited cells and prevalence of self-reactive B cells at various B cell developmental stages on both a wild-type (C57BL/6 [B6]) and an autoimmune background (MRL/lpr). This approach also allows the rapid assessment of mAb autoreactivity from random VH-D-JH rearrangements paired with the fixed transgenic LC or endogenous LCs rearranged as a consequence of receptor editing. With this system, we show that the Vκ4 LC–expressing cells are more self-reactive, leading to extensive editing to other κ genes during B cell development in the BM. Conversely, the αHelκ LC is typically not self-reactive when paired with random HCs, resulting in a low frequency of edited cells at the immature B cell stage. Surprisingly, once the B cells developed from the transitional stages to maturity in the periphery, attrition of unedited cells doubled the frequency of cells that had undergone receptor editing in the mature B cell repertoire. This was abrogated in MRL/lpr mice, correlating with accelerated development of IgG double-stranded DNA (dsDNA) antibodies in Vκ4 LC transgenic mice. When coupled with other known mechanisms such as central deletion and anergy, we conclude that the impact of receptor editing on the mature B cell repertoire is greatly amplified by peripheral selection events that occur after the molecular process of receptor editing has ceased. As a whole, the data presented demonstrate that the precise stage and mechanism of self-tolerance induced for any particular self-reactive B cell can be quite variable and is dictated by both the type of autoantigen bound and by the relative contribution of the HC or LC to BCR autoreactivity.
RESULTS
Tracking B cell tolerance mechanisms in the context of a diverse polyclonal BCR repertoire
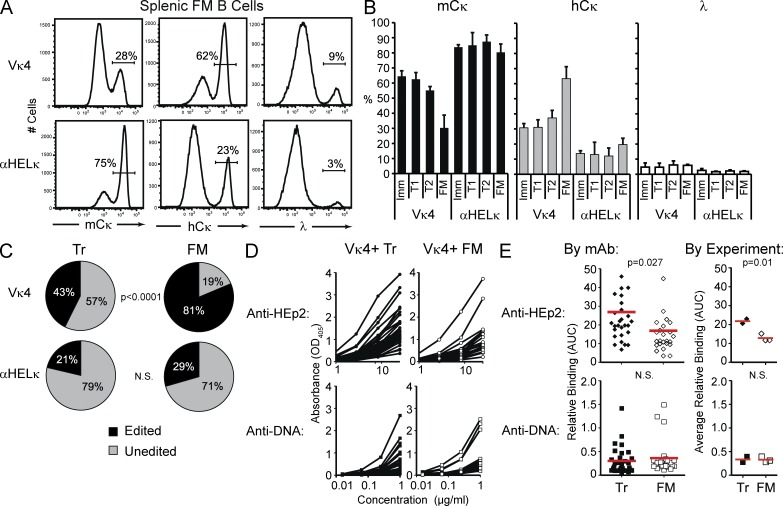
We established a mouse model in which fixed Igκ LC transgenes influence the preselection autoreactivity of the B cell repertoire when paired with a polyclonal repertoire of endogenously rearranged HCs. To do so, we used mice containing a predicted “innocuous” αHelκ LC and “self-reactive prone” Vκ4 LC knocked into the variable region of one κ allele. To monitor receptor editing, these LC transgenic mice were crossed with mice containing the human κ constant (hCκ) region knocked in downstream of unrearranged variable region genes (Igκh) of the endogenous κ locus (Fig. 1 A). In these Igκαhel/h or IgκVκ4/h mice, upon receptor editing, the hCκ allele undergoes VJ rearrangement and pairs with the endogenous HCs (Casellas et al., 2001, 2007). Thus, we can track the frequency of receptor-edited B cells and their subsequent selection by flow cytometry for mouse versus human Igκ and/or by RT-PCR and sequence analysis of the VκJκ region of the expressed LC.
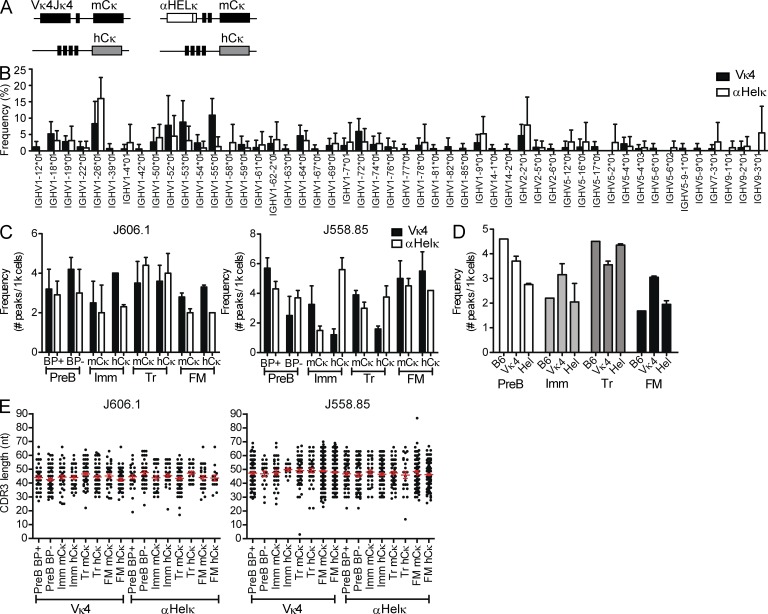
Figure 1.
VH gene diversity in LC transgenic mice. (A) Diagram of κ loci in IgκVκ4/h and Igκαhel/h knockin mice. (B) VH gene usage of edited and unedited AA4− mature cells sorted from IgκVκ4/h and Igκαhel/h mice. Analysis of 141 HC sequences from five IgκVκ4/h mice and 96 HC sequences from five Igκαhel/h mice is shown. ANOVA indicated no significant difference between the VH gene repertoires of Vκ4 and αHELκ mice. Error bars indicate SD of the mean. (C) Frequencies of VH-JH2 rearrangements. B cells from 12-wk-old mice were sorted into different fractions based on developmental stage (as specified in Fig. S1) and LC cell surface phenotype (where applicable). Genomic DNA from each cell fraction was analyzed by CDR3 spectratyping, and the numbers of peaks were counted per 1,000 cells for VH J606.1–JH2 (left) and J558.85–JH2 (right). Mean VH peak numbers with ± SEM are shown for BM B220+CD43−IgM−pre-B (BP+ vs. BP−) and mCκ versus hCκ immature (Imm), splenic transitional (Tr), and FM B cells from IgκVκ4/h (closed bars) and Igκαhel/h (open bars) mice. Shown are data from cells sorted from two individual mice for each genotype in two independent experiments. (D) Similar frequencies of VH J606.1-JH2 rearrangements in B6, IgκVκ4/h, and Igκαhel/h. VH peak number analysis in pre-B, Imm, Tr, and FM of B6, IgκVκ4/h, and Igκαhel/h mice is shown. The same data for IgκVκ4/h and Igκαhel/h mice from panel B are replotted here, but rearrangements per 1,000 sorted B cells were calculated by combining both the sorted mCκ and hCκ populations to facilitate comparison with wild-type B6 mice (which have two mouse κ alleles). Plotted are the frequencies of VH J606.1–JH2 with mean ± SEM in both IgκVκ4/h and Igκαhel/h mice. No SEM is given for B6 because the B6 analysis was performed on two pooled 12-wk-old mice. (E) VH CDR3 length distribution comparison in B subsets of IgκVκ4/h and Igκαhel/h mice. The CDR3 lengths (nt) of VH-JH2 rearrangements in genomic DNA of sorted cell populations were calculated based on CDR3 spectratyping performed in B and C. Each dot represents a single rearrangement. Error bars indicate SEM.
We first verified that the fixed transgenic LC in these mice is not significantly skewing or restricting the HC repertoire. Comparison of the HC VH gene usage in the splenic mature compartment showed similar and diverse usage of VH genes in Igκαhel/h or IgκVκ4/h mice in both edited and unedited B cells (Fig. 1 B and not depicted). Likewise, spectratyping of VH genes J558.85 and J606.1 in sorted B cell subsets showed a varied, polyclonal HC repertoire at all stages of development, comparable with a wild-type B6 mouse for both HCs (Fig. 1, C–E). This was true in cells that expressed the transgenic LC or endogenous LC. J558.85 was specifically chosen because its germline sequence is similar to the 3H9 HC and has been found in other autoantibodies in its unmutated form (Shlomchik et al., 1990). Conversely, J606.1 is normally abundantly rearranged (Meng et al., 2011) and has not been described among autoantibodies to our knowledge. Collectively, these results suggest that alterations in LC rearrangement between Igκαhel/h or IgκVκ4/h mice are not caused by severe limitations in HC/LC pairing or strongly altered HC selection at the pro-B to pre-B transition.
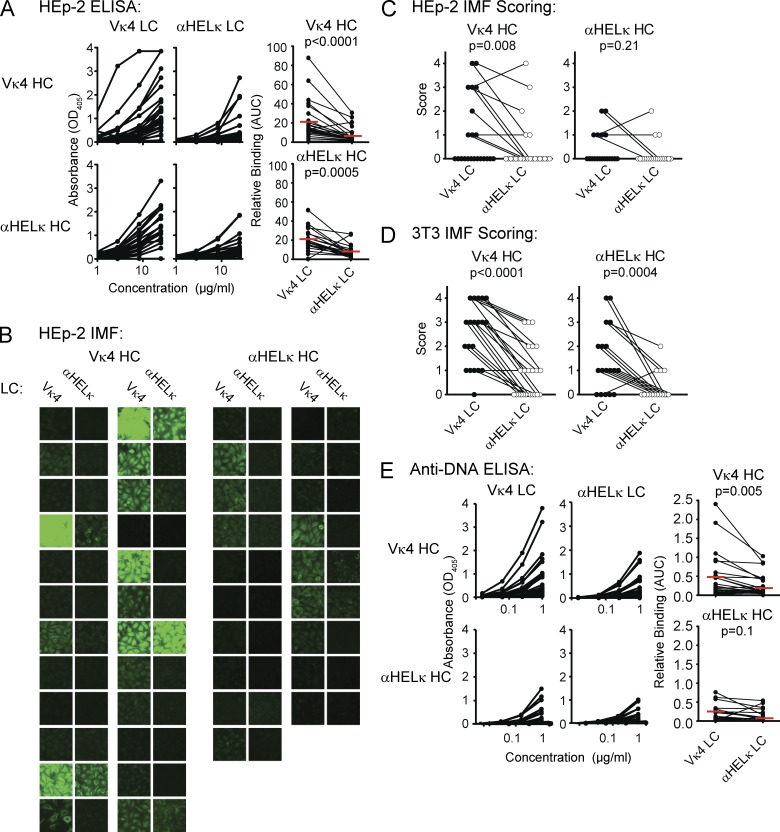
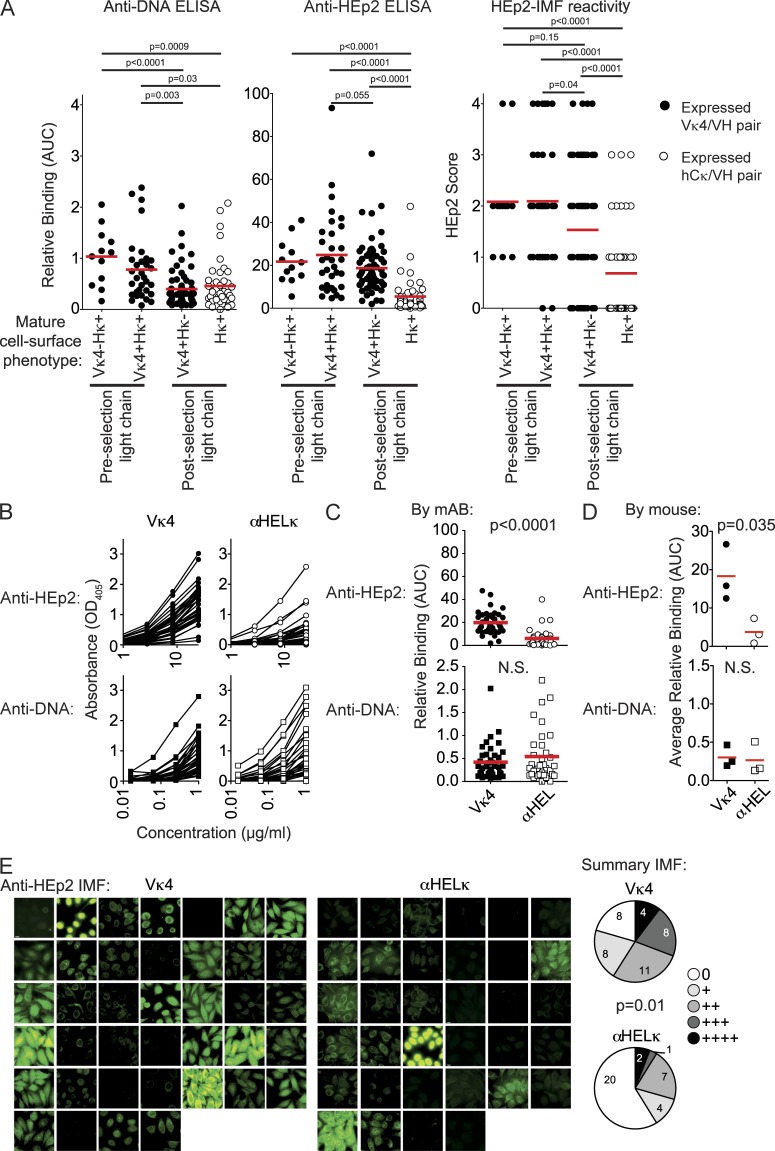
Next, we confirmed that antibodies encoded by randomly rearranged VH genes paired with the Vκ4 LC were indeed more self-reactive than those encoded by the αHelκ LC. For this, we directly compared the self-reactivity of the BCR composed of the Vκ4 versus the αHELκ LC transgene paired with 43 VH genes that were isolated from single-cell sorted mature (AA4−) B cells from the spleens of Igκαhel/h and IgκVκ4/h mice. VH genes from both types of mice were sequenced and cloned into expression vectors and cotransfected with either transgenic LC into 293A cells to express recombinant mAbs. The mAbs were expressed as chimeric antibodies using identical human IgG1/Cκ constant regions to normalize detection in all assays. There was no clonal relation between any two individual B cells (not depicted). The 43 pairs of VH/Vκ4 and VH/αHELκ encoded mAbs were tested side by side for their ability to bind HEp-2 cells either by ELISA or immunofluorescence (IMF). When VH genes isolated from either mouse strain were paired with the Vκ4 LC, in all but two cases, the resulting antibody was more HEp-2 reactive by ELISA or IMF than when paired with the αHelκ LC (Fig. 2, A–C). As we are testing antibodies with mouse-derived variable regions on a human cell line, we further confirmed that we are measuring reactivity to self and not foreign antigen by testing the ability of the antibodies to bind 3T3 mouse fibroblast cells. As with HEp-2 cells, binding of VH/Vκ4 antibodies to 3T3 cells was higher than VH/αHELκ antibodies by IMF (Fig. 2 D). None of the antibodies bound better to HEp-2 cells compared with 3T3 cells, although several of the VH/Vκ4 antibodies had more intensive staining on 3T3 cells than HEp-2 cells. Whether this is caused by differences in the species from which the cell is derived or the cell fixation process is unclear. We went on further to measure by ELISA the reactivity of antibody pairs to the common self-antigen, dsDNA. Antibody binding to dsDNA mirrored reactivity to LPS and insulin (not depicted), and we therefore used this assay as a measure of polyreactivity. Similar to self-reactivity as determined by HEp-2 binding, the affinity of polyreactive antibodies was also reduced when the HC was paired with the αHelκ LC (Fig. 2 E). Thus, the antibodies encoded by the Vκ4 LC are indeed self-reactive prone and more polyreactive when paired with HCs isolated from either LC transgenic mouse. In contrast, the αHELκ LC tends to control or reduce HC-mediated self-reactivity and polyreactivity.
Figure 2.
A mouse model of selection in the context of a polyclonal repertoire. (A) Recombinant mAbs were expressed from pairing different HC genes of the B220+AA4− mature mCκ+hCκ− B cells isolated from IgκVκ4/h mice (a total of 24 VH genes; top) or Igκαhel/h mice (a total of 19 VH genes; bottom) with vectors containing either the Vκ4 or αHELκ LC genes. Each pair of antibodies sharing the same VH gene was tested simultaneously. ELISA binding curves are shown in the left (VH/Vκ4 antibodies) and middle (VH/αHELκ antibodies) panels. Each dot in the right panel represents the area under the binding curve (AUC) of one mAb. The red line indicates the mean value for that group. (B and C) Binding to HEp-2 cells by IMF of mAbs described in A. Shown are representative images of cell binding of each antibody (B) and pairwise comparison of the mode of IMF intensity of each VH gene paired with the Vκ4 or αHELκ LC (C) scored on a scale of 0–4 (whole numbers only). (D) Antibody binding to 3T3 cells detected and scored in the same manner as HEp-2 cells in C. (E) dsDNA binding by ELISA of antibodies described in A and analyzed as in A. Antibodies with shared VH genes were paired both for comparing ELISA binding curves (AUC) and for scoring HEp-2 IMF. Statistical significance was calculated using the paired Student’s t tests. Shown are representative data of at least three independent experiments.
Receptor editing to endogenous LCs reduces the self-reactivity of the BCR
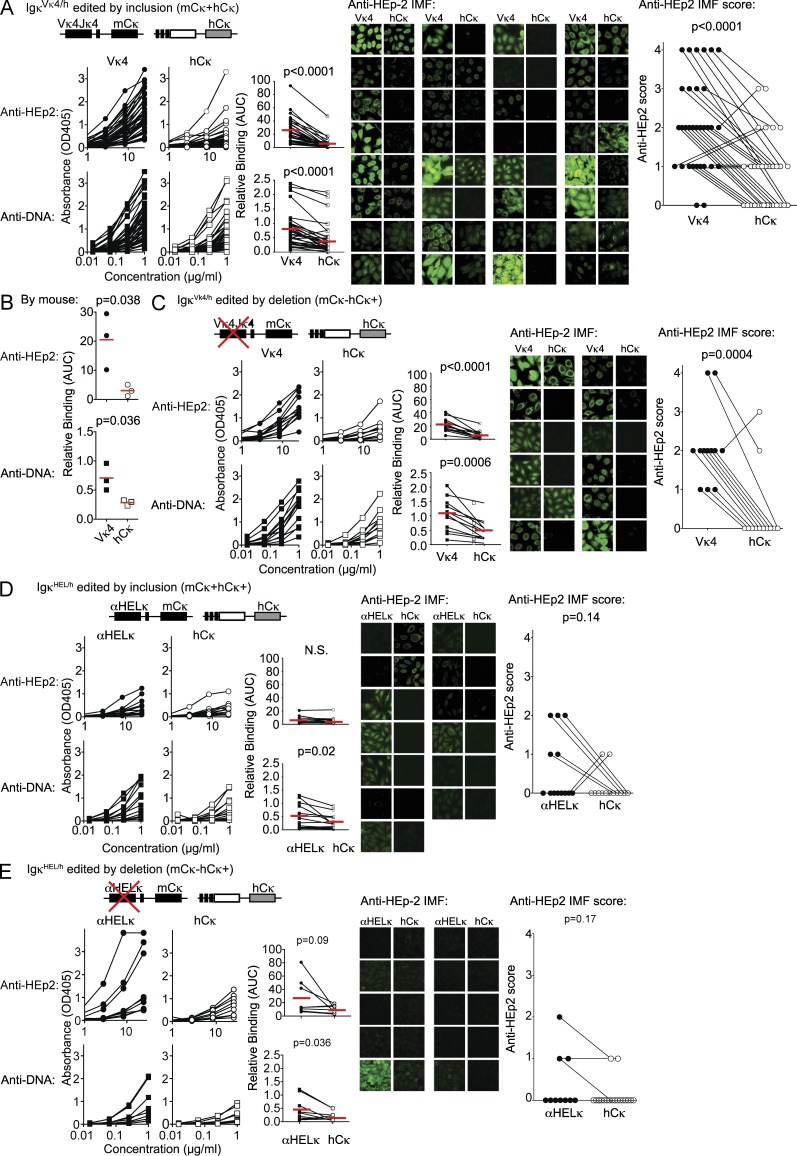
In the fixed LC knockin mice used herein, the endogenous Vκ/Jκ gene segments on the hCκ allele are rearranged almost solely for receptor editing, either with deletion of the original LC (editing by deletion: mCκ−hCκ+ cells) or by coexpression with the transgene (editing by inclusion: mCκ+hCκ+ cells). This dilutes the self-reactivity of the cell to levels where the cell can avoid deletion (Gay et al., 1993; Li et al., 2002; Wardemann et al., 2004; Liu et al., 2005; Huang et al., 2006; Casellas et al., 2007; Velez et al., 2007). In either case, the majority of HCs from hCκ+ B cells are predicted to be less self-reactive when paired to hCκ rather than with the Vκ4 or αHELκ LC transgenes. This provides an easily measurable biomarker identifying B cells with random specificities but reduced self-reactivity. To formally demonstrate this, we isolated B cells from the LC transgenic mice that express hCκ, indicating they had undergone receptor editing. Some HCs may not pair well with the knockin transgenes, so in mCκ−hCκ+ B cells, the use of the hCκ gene may sometimes occur because of structural reasons rather than selection to reduce self-reactivity. Therefore, we first looked at B cells that had undergone receptor editing by allelic inclusion (mCκ+hCκ+ cells) because in these cells we could verify that both the mCκ transgene (the edited LC) and the hCκ endogenous LC (the editor) could efficiently pair with the HC expressed by that particular B cell. Using single-cell RT-PCR, we isolated and sequenced the HC and the hCκ+ VκJκ rearrangements. The HC genes were then cotransfected with either the hCκ+ (editor) gene or the original Vκ4 or αHEL transgenes, and the resulting antibodies were tested for self-reactivity by HEp-2 ELISA and IMF and dsDNA ELISAs. As indicated in Fig. 3 A, most (24/32) of the mAbs cloned from allelically included (mCκ+hCκ+) B cells were significantly more self-reactive when the HC was paired with the Vκ4 transgene rather than with the hCκ. This observation was consistent when we grouped the antibodies based on the individual mouse they were cloned from (Fig. 3 B). Similarly, by all three assays, all but one of the antibodies from allelically excluded mCκ−hCκ+ B cells from an IgκVκ4/h mouse bound better to self-antigen when expressed with Vκ4 compared with hCκ (Fig. 3 C). Thus, although structural incompatibility between the HC and LC may play some role in receptor editing, LC editing primarily occurs to reduce autoreactivity in IgκVκ4/h mice. It is more difficult to detect a difference in self-reactivity between hCκ- and αHelκ-expressing antibodies as very few mature B cells in Igκαhel/h mice, regardless of the LC, bind self-antigen at detectable levels in the assays used here. However, the HCs from both mCκ+hCκ+ and mCκ−hCκ+ B cells cloned from an Igκαhel/h mouse were more often dsDNA reactive when expressed with the αHELκ transgene (Fig. 3, D and E). Furthermore, though not significantly increased on average, there were several clear examples where HEp-2 ELISA and or IMF positivity was increased when the HC was paired with the αHELκ transgene compared with the hCκ editor (Fig. 3, D and E). We can reliably conclude, then, that in this transgenic LC system, receptor editing and expression of the hCκ allele serve as a tolerance mechanism to reduce the autoreactivity of the mature B cell repertoire. Thus, by quantifying the loss of transgenic mCκ LC using B cells (and the concomitant accumulation of edited hCκ+ and λ+ B cells), we have a biomarker to analyze central and peripheral immune tolerance events in mice containing a diverse, polyclonal HC repertoire.
Figure 3.
Receptor editing to endogenous LCs reduces the autoreactivity of the BCR. (A–C) The level of autoreactivity of mAbs cloned from mature B cells in IgVκ4/h mice that were allelically included mCκ+hCκ+ (A) or mCκ−hCκ+ excluded (C). A total of 88 mAbs from 44 allelically included cells (sorted from three IgVκ4/h mice) and 12 mAbs from allelically excluded cells were tested. Analysis by mouse for allelically included cells is provided in B. Shown is total binding to HEp-2 cells and dsDNA by ELISA and to HEp-2 cells by IMF as described for Fig 2. Statistics were calculated by the paired Student’s t tests. (D and E) The level of autoreactivity of mAbs cloned from mature B cells in Igαhel/h mice that were allelically included mCκ+hCκ+ (D) or mCκ−hCκ+ excluded (E). mAbs from a total of 13 allelically included cells and 9 allelically excluded cells were tested. Binding to autoantigens was performed and analyzed as in A–C. (A–E) Red lines indicated the mean AUC.
Vκ4-expressing B cells are first counter-selected in the BM
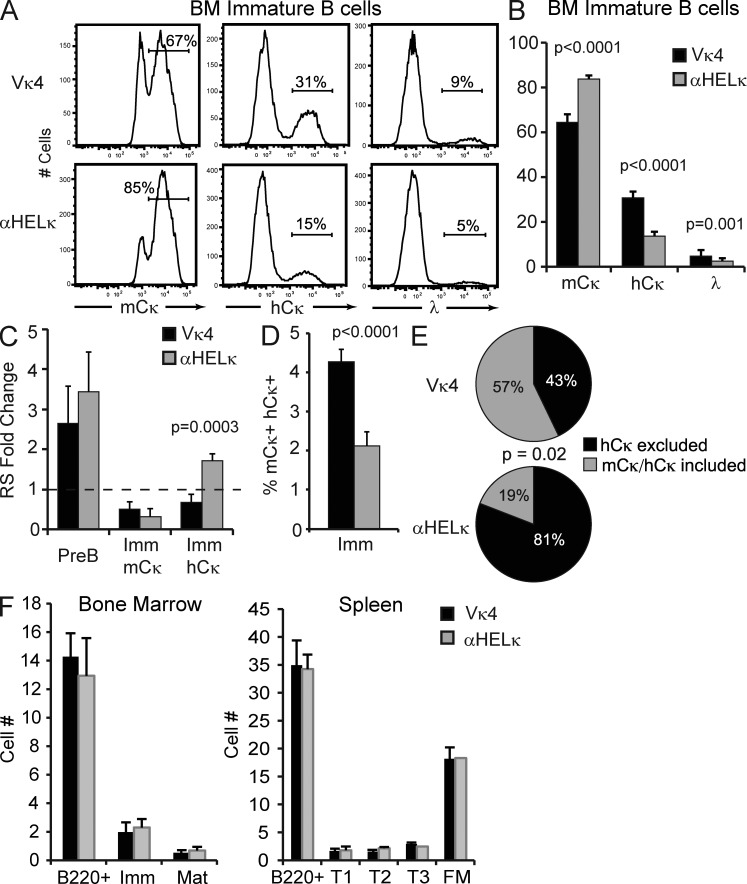
Given their relative self-reactive properties, we would expect substantially more counter-selection of B cells expressing the Vκ4 over the αHELκ transgene beginning at the earliest stage of B cell development when the HC/LC pair is first expressed on the cell surface. Indeed, when we measured by flow cytometry the percentage of immature B cells (B220+IgM+CD23−; Fig. S1 A) in the BM that were mCκ+, hCκ+, or λ+, a substantially higher percentage of immature cells in the IgκVκ4/h mice had undergone receptor editing and expressed an hCκ or λ LC (Fig. 4, A and B), similar to data published previously (Casellas et al., 2001, 2002). On average, 85% of immature B cells from Igκαhel/h mice expressed the mCκ LC, whereas only ∼65% of the immature B cells did so in the IgκVκ4/h mice (Fig. 4 B).
Figure 4.
Central tolerance in IgκVκ4/h and Igκαhel/h mice. (A) Representative example of LC usage of B220+IgM+CD23− immature BM B cells as determined by flow cytometry. (B) Percentage of mCκ+, hCκ+, or λ+ cells within the immature BM B cell fraction in IgκVκ4/h and Igκαhel/h mice. Mean with SD of at least 12 mice from five independent experiments is shown. (C) The levels of RS elements were determined by quantitative PCR from genomic DNA isolated from sorted BM B cell fractions. Shown is the fold change in RS levels relative to splenic FM κ+ B cells sorted from wild-type B6 mice. Mean with SD of at least three experiments is shown. (D) Percentage of immature BM B cells coexpressing on the cell surface mCκ and hCκ as determined by flow cytometry. Mean with SD of nine mice from three independent experiments is shown. (E) mCκ−hCκ+ surface positive transitional B cells were single-cell sorted, and the presence of mCκ and hCκ mRNA was determined by RT-PCR in each sorted cell. Shown is the percentage of sorted cells from each mouse type that expressed only the hCκ allele (hCκ excluded) or both the mCκ+ and hCκ+ allele (mCκ+hCκ+ included) from a total of 87 (IgκVκ4/h) or 46 (Igκαhel/h) cells sorted in two independent experiments. Statistic used was the χ2 test p-value. (F) Absolute number of B cell developmental subsets in the BM and spleen in IgκVκ4/h and Igκαhel/h knockin mice. Mean with SD of six mice is shown.
The different frequencies of edited cells in the two strains could be caused by either increased levels of receptor editing or greater deletion of unedited mCκ+ cells in the IgκVκ4/h mice during development in the BM. In an attempt to differentiate these two possibilities, we compared the relative levels of receptor editing in Igκαhel/h mice versus IgκVκ4/h mice by measuring levels of recombining sequence (RS) rearrangements formed during the primary stage of receptor editing in BM B220+CD43−IgM− pre-B cells (Panigrahi et al., 2008), leading to the inactivation of the original κ LC allele. There was no significant difference in the relative frequency of RS rearrangements between IgκVκ4/h and Igκαhel/h mice at the pre-B cell stage (Fig. 4 C), suggesting overall levels of receptor editing might be the same. However, editing can occur without terminal RS rearrangements (Nemazee, 2006), allowing the possibility of allelic inclusion. When we looked in more detail, we found that as expected, there were lower levels of RS rearrangements in sorted, largely unedited, mCκ+ immature B cells of either strain in comparison with total Igκ+ splenic mature B cells from wild-type B6 mice. Also, predictably, in the Igκαhel/h mice there were more RS rearrangements in the immature cells that had undergone receptor editing (the hCκ+ fraction) compared with the unedited mCκ+ fraction. Interestingly however, RS rearrangement levels were virtually unaltered between the edited and unedited immature B cell fractions sorted from IgκVκ4/h mice (Fig. 4 C). This reduction in Vκ RS terminal rearrangements correlates with a twofold increase in the percentage of allelically included mCκ+hCκ+ surface double-positive immature B cells in IgκVκ4/h mice (Fig. 4 D). More strikingly, when we single-cell sorted splenic transitional edited mCκ−hCκ+ cells and determined by RT-PCR the κ chain or chains expressed, we found that an estimated 57% of cells isolated from IgκVκ4/h mice still expressed at the transcript level both the original fixed LC and the newly rearranged hCκ+ LC, whereas only ∼20% of the transitional hCκ+ cells from Igκαhel/h mice expressed two LCs (Fig. 4 E). This difference in the editing mechanism between the two transgenic mice makes it difficult to determine exactly the relative level of receptor editing. However, significantly higher levels of self-antigen–mediated deletion in the BM were not apparent in IgκVκ4/h mice as total numbers of B cells at all developmental stages were not altered in the BM or spleen (Fig. 4 F). We conclude from these experiments that increased receptor editing and limited levels of deletion are induced to mitigate LC-mediated autoreactivity at the immature stage of development in the BM.
A substantial proportion of primary B cell tolerance occurs in the periphery
When we compared the level of edited cells between immature BM and mature splenic follicular mature (FM; AA4−CD21intCD23+) B cells in IgκVκ4/h mice, we found that the percentage of edited hCκ+ cells had almost doubled from ∼30% in the immature B cell subset to >60% in the splenic FM compartment (Fig. 5, A and B). The percentage of hCκ+ cells also increased in the periphery of Igκαhel/h from a mean of 13% in the BM immature subset to around 20% at the mature stage in the spleen (Fig. 5, A and B). There was little difference in LC usage between mature B cells in the spleen, LNs, and BM in both LC transgenic mice (not depicted). To further delineate at what point of peripheral B cell development the loss of mCκ+ cells is occurring, we measured by flow cytometry LC usage in AA4+IgMhiCD24hiCD21loCD23− early transitional (T1) and AA4+IgMhiCD24hiCD21intCD23+ late transitional (T2) splenic B cell subsets (Fig. S1 B). No difference in LC usage was detected between immature B cells in the BM and T1 B cells in the spleen in either mouse strain, and only a small decrease in mCκ usage was observed between the T1 and T2 compartment in the IgκVκ4/h mice (Fig. 5 B). Thus, the loss of transgenic LC-expressing cells is primarily occurring between the late transitional (T2) and the FM B cell developmental subsets.
Figure 5.
Peripheral tolerance in IgκVκ4/h and Igκαhel/h mice. (A) Representative example of LC usage of splenic FM B cells as determined by flow cytometry. (B) Mean percentage of BM immature, splenic T1 and T2, and FM B cells expressing mCκ, hCκ, or λ on their cell surface. Shown is the mean of at least 16 mice from five independent experiments. Error bars indicate SD of the mean. Student’s t test p-values are as follows: Vκ4-mCκ: T1 versus FM, P < 0.0001; T1 versus T2, P < 0.0001; T2 versus FM, P < 0.0001. Vκ4-hCκ: T1 versus FM, P < 0.0001; T1 versus T2, P = 0 0.006; T2 versus FM, P < 0.0001. αHELκ-mCκ: T1 versus FM, P = 0.07; T2 versus FM, P = 0.0004. αHELκ-hCκ: T1 versus FM, P = 0.007; T2 versus FM, P = 0.0001. (C) Percentage of splenic Tr and FM B cells expressing the LC knockin (unedited) or an alternative LC (edited mCκ, hCκ, or λ) through receptor editing. To differentiate between mCκ+ cells that still expressed the LC knockin or had undergone receptor editing on the mCκ allele, mCκ surface–positive cells were single-cell sorted, and the variable portion of the κ allele was sequenced. Statistic used was the χ2 test p-value. (D and E) The HC variable region cloned from single-cell sorted unedited Tr (closed symbols) and FM (open symbols) B cells isolated from pooled IgκVκ4/h mice in two (Tr) or three (FM) independent experiments was cloned and paired with the Vκ4 LC to express mAbs. These were then tested for autoreactivity by HEp-2 cell and dsDNA ELISAs as described for Fig 2. Shown in D are the binding curves of each antibody and in E the AUC of HEp-2 or dsDNA binding for each antibody or the mean AUC (red lines) for antibodies isolated in each experiment.
Some receptor editing can occur on the mCκ allele of both the Vκ4 and αHelκ transgene as both still contain downstream endogenous Jκ gene segments. Therefore, to determine more accurately the percentage of cells still expressing the knockin LC in the peripheral B cell compartment, we single-cell sorted mCκ+hCκ− cells, amplified by RT-PCR the expressed κ chain from each cell, and sequenced it. Based on the analysis of >80 sequences from each B cell population sorted from six mice, we determined that >90% of AA4+CD24hiIgMhi transitional (inclusive of both T1 and T2; Tr) B cells from both mouse strains still expressed the knockin LC. However, although 87% of the mCκ+hCκ− FM B cells from Igκαhel/h mice expressed the αHelκ LC, only 62% of FM B cells in IgκVκ4/h mice remained Vκ4+. If we combine this with the flow cytometry data, the proportion of cells between the Tr and FM compartment in IgκVκ4/h mice that had a receptor-edited LC almost doubled (43 to 82%; Fig. 5 C). Even in Igκαhel/h mice expressing an innocuous LC, the level of edited cells increased by about a third (21% in Tr to 29% in FM) during peripheral B cell development, presumably because of largely HC-mediated autoreactivity that the αHelκ LC is unable to mask.
To measure the impact of peripheral tolerance on removing self-reactive and dsDNA-binding polyreactive B cells from the naive mature B repertoire, we single-cell sorted mCκ+hCκ− Tr and FM B cells from IgκVκ4/h mice (Fig. S2 A), verified by sequence analysis that they expressed the Vκ4 LC, and produced mAbs with the endogenously rearranged HCs and Vκ4 LC from 30 Tr and 25 FM B cells. When we compared self-antigen binding of antibodies expressed by Tr versus FM B cells, we saw a significant reduction by HEp-2 cell ELISA in the overall level of autoreactivity between Tr and the FM B cell compartment (Fig. 5, D and E). We detected little dsDNA-binding polyreactivity, however, in either peripheral B cell compartment (Fig. 5, D and E).
These experiments show that a substantial proportion of primary tolerance occurs in peripheral locations rather than just in the BM. This is particularly striking in the IgκVκ4/h mice but is also evident in the Igκαhel/h mice. Finally, we show in mice with a polyclonal B cell repertoire that the transition from T2 to FM is an important stage in primary selection during peripheral B cell development.
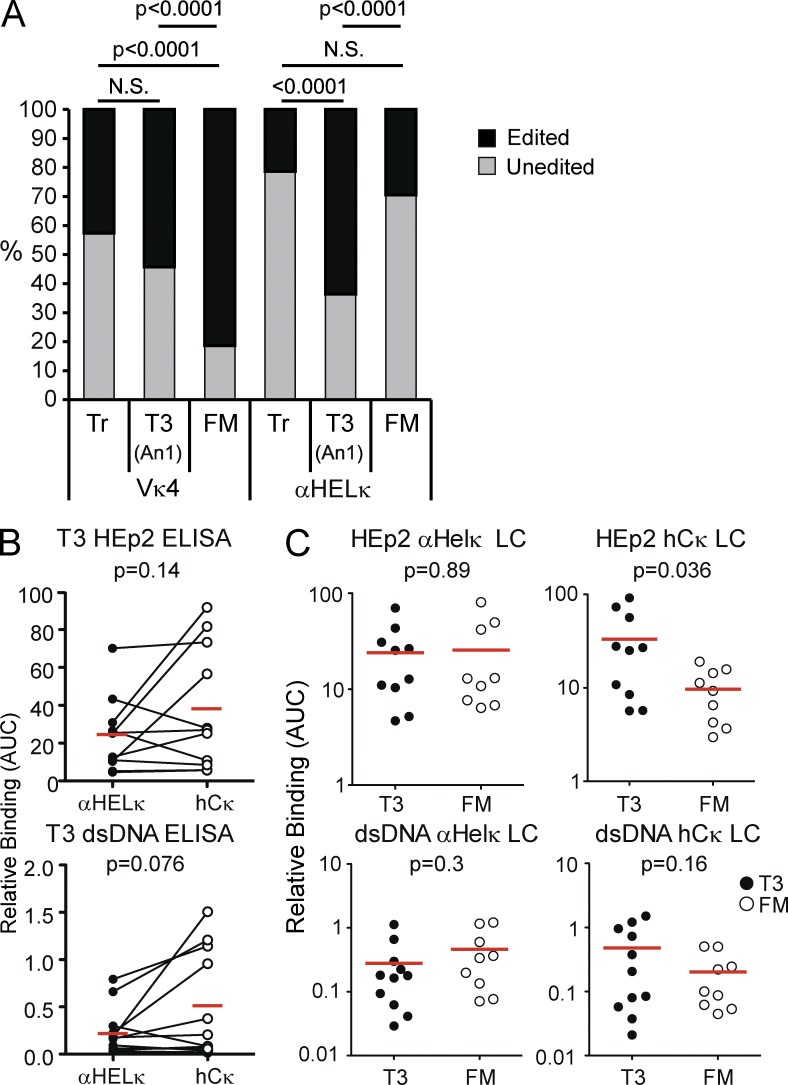
Primary B cell tolerance in the periphery occurs predominantly through deletion and anergy
Deletion of self-reactive B cells is thought to be the default negative selection mechanism operating in the periphery, not receptor editing as in the BM. Indeed, similar to past studies of RAG reporter mice (Yu et al., 1999; Gärtner et al., 2000), we were unable to detect RAG transcript expression by single-cell RT-PCR in transitional cells in the periphery of IgκVκ4/h or Igκαhel/h mice (not depicted), nor was there a significant number of Ig-low cells, which can be indicative of ongoing receptor editing (Fig. S1 B). These results indicate that continuing receptor editing during the transitional phase of development does not account for the substantial change in proportion of edited cells within the mature compartment.
We also looked for evidence of Vκ4+ cells being rendered anergic by encounter with self-antigen, thus preventing development into FM B cells. The absolute number of T3 (An1) AA4+CD23+IgMlo cells previously described as anergic B cells (Merrell et al., 2006) was not different between Igκαhel/h and IgκVκ4/h mice, and surface IgM levels of other subsets were normal (Fig. 4 F and Fig. S1 B). In addition, Vκ4+ cells were not enriched in the T3 B cell compartment, which in fact contained fewer Vκ4+ cells than the IgMhi Tr compartment (Fig. 6 A). Interestingly, however, >60% of the T3 population in Igκαhel/h mice no longer expressed the αHelκ LC compared with ∼30% in the FM compartment (Fig. 6 A). We hypothesized that these AA4+CD23+IgMlo receptor-edited cells likely express an HC with intrinsic self-reactive properties, triggering receptor editing and eventually anergy when LC rearrangement proved unsuccessful at completely correcting self-reactivity. To test whether these hCκ+ T3 cells were cells in which receptor editing failed to reduce self-reactivity, we single-cell sorted T3 cells from Igκαhel/h mice (Fig. S2 B) and cloned the HC and hCκ LC and expressed antibodies containing the HC paired with either the unedited αHELκ or the rearranged hCκ LC. In contrast to FM B cells (Fig. 3 E), in 9/11 T3 antibody pairs tested, expression of a rearranged LC did not lead to reduction in self-reactivity compared with antibodies with the unedited αHELκ LC (Fig. 6 B). Indeed, when we directly compared the self-reactivity of antibody pairs from edited FM and T3 B cells, although there was no difference between the two populations in the ability of αHELκ-containing antibodies to bind HEp-2 cells, T3 hCκ+ antibodies were more self-reactive than their FM counterparts (Fig. 6 C). Though not statistically significant, a similar trend was observed in the ability to bind dsDNA (Fig. 6 C). Together, these data indicate that unedited self-reactive cells primarily undergo deletion in the periphery, whereas cells that have undergone editing in BM but retain some level of HC-mediated autoreactivity are maintained in an anergic state in the periphery.
Figure 6.
Tolerance by anergy predominates in Igκαhel/h mice. (A) Percentage of cells in the splenic Tr and T3 (or An1) and FM B cells fractions that express the LC knockin or receptor-edited LCs as determined by flow cytometry and sequence analysis of LC transcripts. Flow cytometry data are the mean of at least 16 mice from five independent experiments. Sequencing of LC transcripts was performed on at least 40 single cells for each B cell population. Statistic used was the χ2 test p-value. (B) The HC variable region cloned from single-cell sorted edited T3 (n = 11) B cells isolated from Igκαhel/h mice was cloned and paired with the αHELκ LC or cloned rearranged hCκ LC to express mAbs and tested for autoreactivity by HEp-2 cells and dsDNA ELISAs as described for Fig 2. Antibodies with shared VH genes were paired for comparing ELISA binding curves (AUC) of HEp-2 (top) or dsDNA (bottom) reactivity with the edited (hCκ) or original αHELκ LC. Statistical significance was calculated using the paired Student’s t tests. (C) Comparison of binding curves (AUC) with HEp-2 cells or dsDNA binding between antibodies composed of HCs cloned from edited (hCκ+) T3 and FM B cells in Igκαhel/h mice and expressed with the respective αHELκ (left) or hCκ LC (right). (B and C) Red lines indicated the mean AUC.
Many self-reactive B cells are removed from the repertoire at the T2 to mature transition
To better understand the level of BCR self-reactivity that triggers different forms of tolerance, we compared the levels of autoreactivity of cells that had or had not undergone receptor editing in IgVκ4/h mice. To estimate the preselection reactivity of cells induced to undergo receptor editing, we took HC genes from cells that no longer expressed the Vκ4 LC (Vκ4−hCκ+) or were allelically included (Vκ4+hCκ+) and paired them with the original Vκ4 LC. The autoreactivity of these preselection HC/LC pairs was then compared with antibodies containing the HC paired with an edited, rearranged hCκ LC (postselection hCκ+) and to antibodies cloned from cells that had reached maturity without undergoing receptor editing (Vκ4+hCκ−; Fig. 7 A). As expected, the two groups of antibodies representing the preselection condition had the highest levels of autoreactivity as measured by HEp-2 ELISA and IMF (Fig. 7 A). The affinity and number of dsDNA-binding polyreactive antibodies was also increased in preselected antibodies. Notably, as suggested by our previous publication (Casellas et al., 2007), many allelically included B cells escaped primary immune tolerance controls despite continued expression of HC/LC self-reactive pairs. It is also interesting that both Tr B cells, the earliest population to have exited central selection but have not completed peripheral selection, and edited and unedited post-selection antibodies isolated from mature B cells had very low levels of dsDNA-binding compared with preselection antibodies (Figs. 5 E and 7 A). This suggests that B cells expressing polyreactive antibodies capable of binding this autoantigen were strongly counter-selected during central B cell development before these cells reached the periphery. HEp-2 reactivity, however, declined between unedited cells at the Tr developmental stage and full maturation (Figs. 5 E and 7 A). The level of autoreactivity was even lower in mature cells that had undergone receptor editing. Overall, these experiments indicate first that receptor editing results in a significant reduction in the mean degree of autoreactivity to a level that allows them to reach the mature naive B cell stage. Second, whereas polyreactive cells capable of binding dsDNA were removed or edited primarily during the central stage of tolerance, those binding other autoantigens as detected by HEp-2 ELISA and IMF were predominantly removed during the peripheral stage of tolerance.
Figure 7.
Developmental sites and stages of B cell immune tolerance. (A) Comparison of dsDNA and HEp-2 cell binding of antibodies representing different stages of selection in the IgVκ4/h mice. All antibody binding data for HEp-2 cell and dsDNA by ELISA and the blinded scores for anti–HEp-2 IMF were compiled to compare the relative levels of autoreactivity before and after B cell selection. To do so, preselection recombinant mAbs composed of VH genes from allelically included (Vκ4κ+hCκ+) or allelically excluded (Vκ4κ−hCκ+) edited mature cells paired with the Vκ4 LC and postselection recombinant mAbs composed of VH genes from mature unedited cells (Vκ4+hCκ−) paired with the Vκ4 LC or VH genes from mature edited cells (Vκ4−hCκ+) with the hCκ edited LC were compared. Recombinant antibodies were made with VH genes from 12 Vκ4−hCκ+ mature cells, 32 Vκ4+hCκ+ mature cells, 62 postselection mature Vκ4+hCκ− cells, and 44 Vκ4κ−hCκ+ edited mature cells. (B–E) Autoreactivity of mAbs cloned from single-cell sorted mature (AA4−) unedited Vκ4+hCκ− B cells from the spleens of Igκαhel/h and IgκVκ4/h mice. Antibodies were generated from a total of 37 B cells from three IgκVκ4/h mice (n = 10, 10, and 17 mAbs by mouse) and a total of 34 B cells from three Igκαhel/h mice (n = 11, 7, and 16 mAbs by mouse). The frequency of autoreactivity was tested and analyzed as in Figs. 2 and 3 using HEp-2 cells and anti-dsDNA ELISAs (B–D) and by HEp-2 IMF (E). In E, the results of blinded scoring on a scale of 0–4 are shown in pie charts. All assays were repeated at least three times. Statistic used was the χ2 test p-value for E. (A, C, and D) Red lines indicated the mean AUC.
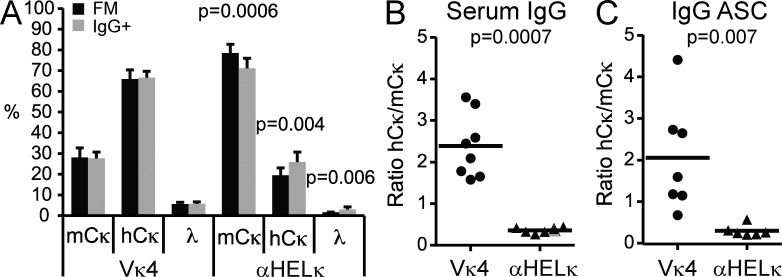
The LC repertoire of activated IgG+ B cells mirrors the naive FM compartment
Despite the extensive peripheral deletion occurring in the IgVκ4/h mice, the remaining Vκ4+ cells in the naive mature compartment were, as a whole, more HEp-2 reactive compared with edited hCκ+ cells in the same mice or mature αHelκ LC–expressing cells (Fig. 7, A–E). We therefore looked for evidence of further selection against Vκ4+ cells during B cell activation, or secondary tolerance. We saw similar LC usage when we compared splenic FM naive B cells and IgG+ B cells in IgκVκ4/h mice but detected a small, but reproducible decrease in mCκ+ cells in isotype-switched IgG+ compartment in the Igκαhel/h mice (Fig. 8 A). This may be because the naive mature cells in the small, but more diverse hCκ+ mature pool are more likely to encounter foreign antigen and enter an immune response. The proportion of serum IgG antibodies expressing mCκ versus hCκ (edited) LC and IgG antibody–secreting cells also correlated with the number of naive mature B cells expressing these LCs (Fig. 8, B and C). Thus, despite the higher level of autoreactivity still present in the unedited fraction of naive FM B cells in IgκVκ4/h compared with Igκαhel/h mice (Fig. 7, B–E), we detected no further selection against cells expressing the self-reactive prone Vκ4 LC during B cell activation and differentiation into antibody-secreting cells and isotype-switched cells on a B6 background.
Figure 8.
Secondary tolerance in IgκVκ4/h and Igκαhel/h mice. (A) Splenic naive FM B cells and IgG1/2+ isotype-switched cells were gated, and the percentage of cells in each population that expressed mCκ, hCκ, or λ was determined by flow cytometry. Mean with SD of nine mice from three independent experiments is shown. (B) mCκ or hCκ serum IgG levels were determined by ELISA. Shown is the ratio of the OD values of hCκ relative to mCκ for each mouse. (C) IgG+ plasmablasts expressing the mCκ or hCκ allele in the spleen were detected by ELISPOT. Shown is the ratio of hCκ+ to mCκ+IgG+ antibody-secreting cells (ASC) for each mouse. (B and C) Horizontal lines indicate the mean.
Systemic autoimmune disease in MRL/lpr mice is exacerbated by expression of the Vκ4 LC
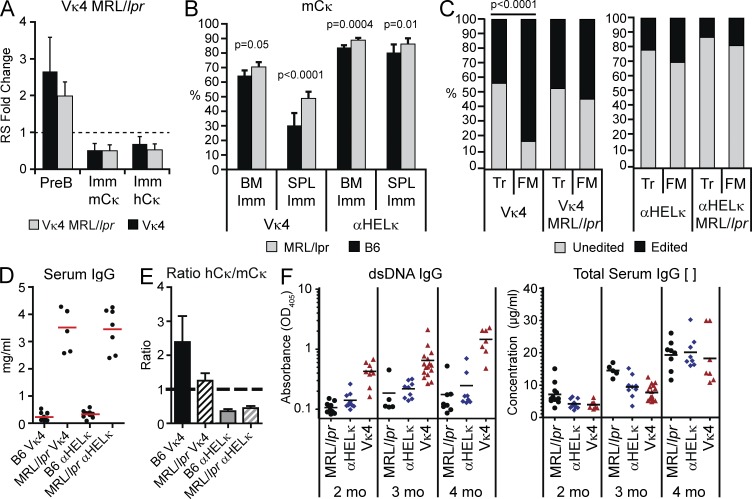
To study the importance of tolerance mechanisms during B cell development, we crossed the IgκVκ4/h and Igκαhel/h mice onto the autoimmune MRL/lpr background where defects in central and peripheral B and T cell tolerance lead to systemic lupus–like disease. On the MRL/lpr background, there was no significant difference in the relative level of RS elements in pre-B or immature B cells in the BM (Fig. 9 A) or in the levels of allelically included cells at the transcript or protein level (not depicted). This suggests that the levels of receptor editing in IgVκ4/h mice were not altered between the two genetic backgrounds. However, as published previously (Panigrahi et al., 2008), we did detect lower levels of RS elements in MRL/lpr mice without a fixed LC compared with B6 mice (not depicted). Although there was only a modest increase in the levels of mCκ+ (self-reactive prone) cells at the BM immature stage in the IgκVκ4/h MRL/lpr mice, there was a substantial increase in these cells at the FM stage of development compared with the B6 background (Fig. 9 B). Indeed, when we determined the percentage of splenic B cells by flow cytometry and single-cell sequence analysis of the κ locus that still expressed the transgenic LC, we saw little to no difference in LC usage at the Tr stage in IgκVκ4/h mice between B6 and MRL/lpr mice (Fig. 9 C). At the FM stage, however, whereas 80% of the cells in the B6 mice were receptor edited, in the MRL/lpr mice, only 65% of the cells had an edited LC (Fig. 9 C). Likewise, fewer receptor-edited FM B cells were detected in MRL/lpr Igκαhel/h mice. Thus, in the MRL/lpr mouse model of lupus, a significant proportion of the defect in primary immune tolerance occurs during the peripheral stages of B cell selection.
Figure 9.
Tolerance in MRL/lpr mice. (A) Quantification of RS elements from sorted BM B cell fractions of IgκVκ4/h mice on the B6 or MRL/lpr background. Shown is the fold change in RS levels relative to splenic FM κ+ B cells sorted from wild-type B6 mice. Mean with SD of at least three experiments is shown. There were no statistical differences by Student’s t tests. (B) Percentage of mCκ+ cells in splenic T1 and FM B cells from IgκVκ4/h and Igκαhel/h mice on the B6 or MRL/lpr background. Mean with SD of nine mice from four independent experiments. (C) Percentage of cells in the splenic Tr and FM fractions that express the LC knockin or receptor-edited LCs as determined by flow cytometry and sequence analysis of LC transcripts. Sequencing of LC transcripts was performed on at least 40 single cells for each B cell population in each mouse strain. Statistic used was the χ2 test p-value. (D) Total serum IgG concentrations were quantified by ELISA in IgκVκ4/h and Igκαhel/h mice on the B6 or MRL/lpr background at 2 mo of age. Red lines indicated the mean mg/ml. (E) Relative serum levels of IgG+mCκ+ or hCκ+ antibodies. Shown is the ratio of hCκ relative to mCκ OD values for each mouse strain at 2 mo of age. Error bars indicate SD of the mean. (F) The level of IgG dsDNA reactive antibodies (left) or total IgG concentration (right) was determined by ELISA in serum of 2-, 3-, and 4-mo-old non-Ig transgenic MRL/lpr mice (black circles) and IgκVκ4/h (blue diamonds) and Igκαhel/h (red triangles) knockin mice on the MRL/lpr background. Horizontal lines indicate the mean. P-values at 2 mo are as follows: Vκ4 versus αHELκ or Mrl/lpr, P < 0.0001. P-values at 3 mo are as follows: Vκ4 versus αHELκ, P = 0.04; Vκ4 versus Mrl/lpr, P = 0.001. P-values at 4 mo are as follows: Vκ4 versus αHELκ, P = 0.003; Vκ4 versus Mrl/lpr, P = 0.001.
We then wanted to determine whether this difference in peripheral tolerance impacted the progression of autoantibody production in MRL/lpr mice. Total serum IgG levels were very high in both Igκαhel/h and IgκVκ4/h mice on the MRL/lpr background (Fig. 9 D). However, in agreement with the decreased levels of hCκ+ FM B cells, the ratio of hCκ to mCκ+ serum IgG levels decreased in the MRL/lpr IgκVκ4/h mice (Fig. 9 E). Thus, altered peripheral selection of Vκ4 LC–expressing cells on the MRL/lpr background resulted in more Vκ4+ serum IgG antibodies. Most importantly, this alteration in peripheral selection corresponded with accelerated development of IgG dsDNA antibodies, which were detectable as early as 2 mo of age in the IgκVκ4/h MRL/lpr mice, in contrast to normal MRL/lpr or Igκαhel/h MRL/lpr mice (Fig. 9 F, left). This increased production of dsDNA antibodies continued as the mice aged, although total serum IgG levels were comparable between the two transgenic mice at all ages tested (Fig. 9 F, right). IgG dsDNA-specific serum antibodies were almost undetectable in both LC knockin mice on the B6 background up to 11 mo of age (not depicted). Thus, expression of an autoimmune-permissive LC can accelerate production of self-reactive antibodies when peripheral tolerance mechanisms are impaired.
DISCUSSION
It has long been appreciated that a portion of primary tolerance must occur as newly formed B cells transition to maturity in the periphery. However, it had been difficult to establish the relative contributions of central versus peripheral tolerance mechanisms in honing the mature repertoire that, as a pool, recognizes a multitude of self and nonself antigens. We therefore established a mouse model to track tolerance mechanisms operating in a diverse polyclonal B cell repertoire in the context of two significantly different starting levels of LC-mediated autoreactivity. The HC CDR3 forms the epicenter of the Ig antigen–binding site and thus more easily trumps LC effects on specificity (Kindt and Capra, 1984) and has been demonstrated to have profound associations with B cell selection and tolerance (Zemlin et al., 2005, 2008; Ippolito et al., 2006; Schelonka et al., 2008). However, as illustrated by the high-affinity anti-DNA 3H9 antibody isolated from MRL/lpr mice, the LC can have a profound influence on binding affinity and to a lesser extent on antigen specificity (Radic et al., 1991; Li et al., 2001). When paired with the 3H9 HC, variant LCs with acidic CDRs are able to reduce anti-DNA binding, whereas LCs with more neutral or basic CDRs such as the Vκ4 LC used in the study fail to do so. Thus, the Vκ4 LC in 3H9/Vκ4 and 3H9R/Vκ4 double transgenic BALB/c mice is almost invariably replaced by endogenously rearranged LCs (Gay et al., 1993; Chen et al., 1997). This, together with studies demonstrating extensive receptor editing in Vκ4 LC knockin mice, suggests that this LC has strong inherent self-binding properties (Prak and Weigert, 1995; Casellas et al., 2001). Here, we formally demonstrate that the Vκ4 LC is self-reactive when paired with a wide selection of HCs isolated from mature B cells. This led to profound selection against Vκ4+ cells both centrally and in the periphery, making this a useful mouse model to decipher the key selection checkpoints. We coupled this transgenic mouse system with cloning and expression of BCRs to track qualitatively and quantitatively the self-reactivity of B cells that have undergone tolerance at different stages of development. Together, these methods have allowed us to gain unprecedented insight into stage-specific B cell tolerance in mice containing a diverse BCR repertoire.
We demonstrate that receptor editing of the original fixed LC by the endogenous VJκ genes as detected by hCκ+ usage reduced autoreactivity. Thus, changes in LC usage by receptor editing that we detected appear to be largely caused by negative selection aimed at reducing autoreactivity, as has been shown in many other mouse tolerance models (Retter and Nemazee, 1998; Wardemann et al., 2004; Ait-Azzouzene et al., 2005). Although the possibility that changes in B cell LC usage over the course of development are caused by positive selection cannot be formally ruled out, the demonstrated autoreactivity of Vκ4-encoded antibodies strongly suggests that changes in the repertoire were mediated by negative selection. Therefore, it was not surprising that in IgκVκ4/h mice, a much larger percentage of immature B cells compared with Igκαhel/h mice no longer expressed the LC knockin as the result of central tolerance. Strikingly, however, although the percentage of edited cells changed little between splenic early (T1) and late transitional (T2) cells, there was an almost 50% reduction in Vκ4+-expressing cells between the T2 developmental stage and FM B cells. Although it is well established that deletion of self-reactive cells occurs in the periphery (Russell et al., 1991; Fulcher et al., 1996; Wardemann et al., 2003; Aït-Azzouzene et al., 2006; Merrell et al., 2006), the specific transitional stage at which this occurs in a polyclonal repertoire has not been well characterized. Factors regulating B cell survival clearly change between the AA4+CD23−CD21lo T1 and AA4+CD23+CD21int T2 transitional subsets, although in vitro they both undergo apoptosis upon BCR stimulation (Allman et al., 2001; Duong et al., 2010). In T2 cells, tonic BCR signaling becomes vital for survival, and up-regulation of BAFFR levels coincides with responsiveness to the survival signal provided by BAFF (Hsu et al., 2002; Chung et al., 2003). Mice lacking BCR signaling components and BAFF expression have a block in development between the T1 and T2 stage, and conversely, mice overexpressing BAFF or lacking the proapoptotic gene Bcl2l11 have selective survival of cells at the T2 stage and beyond (Batten et al., 2000; Schiemann et al., 2001; Craxton et al., 2005). Given these functional differences, we expected that negative selection might occur to a significant degree between the T1 and T2 stage. However, we show that the majority of self-reactive cells that reach the periphery are lost between the T2 and FM compartments. This would suggest that positive selection through appropriate BCR/BAFF signals can occur at the T2 stage of development, but attrition is primarily occurring later for self-reactive B cells. Although maintenance of self-reactive cells this late into maturity is potentially dangerous, it allows for selection of certain polyreactive low-affinity B cells that may be important in innate-like responses (i.e., marginal zone or B1 cells) as previously demonstrated in M167 and 81x transgenic mice (Martin and Kearney, 2001; Meyer-Bahlburg et al., 2008).
In contrast to our results, in mice expressing an Igκ superantigen only in the periphery, negative selection appears to primarily occur earlier, between the T1 and T2 stage (Aït-Azzouzene et al., 2006; Ota et al., 2011). However, in this model, B cells are first encountering a high-affinity autoantigen in the periphery, resulting in deletion at first exposure to self-antigen at the early T1 transitional stage. By using an LC-mediated model of autoreactivity, we are able to study tolerance against a broad range of specificities (evident from the various affinities to HEp-2 cell extracts or dsDNA and the variant staining patterns of HEp-2 cells that were detected from the Vκ4-encoded antibodies) encountered in their physiological settings. Under these conditions, it appears that high-affinity self-reactive cells are removed earlier in the BM. Although the peripheral selection stage is still significant in magnitude, it appears to primarily remove moderate-affinity autoantibodies after the T2 stage.
Importantly, we detected very limited Vκ4-encoded polyreactive dsDNA binding in peripheral B cells, although anti-dsDNA reactivity was commonly encoded by VH genes from edited (hCκ+) cells when expressed with the Vκ4 transgene to resemble the cell’s preselection specificity. It is clear that different factors including the affinity to self-reactivity influences the mechanism of tolerance (Shlomchik, 2008), but simple differences in binding affinity cannot fully explain what dictates central versus peripheral tolerance. Indeed, very low-affinity anti-MHC BCRs have been shown to be efficiently tolerized in the BM (Lang et al., 1996), suggesting that ignorance, such as seen in the AM14 rheumatoid factor transgenic mouse model (Hannum et al., 1996), not just affinity, can explain why some self-reactive B cells reach and persist in the periphery. In any case, we can clearly establish a stepwise progression in tolerance as B cells develop.
Anergic B cells and B cells that have undergone receptor editing by allelic inclusion remain self-reactive in the periphery (Goodnow et al., 1988; Hartley et al., 1991; Kenny et al., 2000; Li et al., 2002; Liu et al., 2005; Huang et al., 2006; Casellas et al., 2007; Velez et al., 2007; Duty et al., 2009; Isnardi et al., 2010), but the prevalence of these two partial forms of tolerance varied between the two LC transgenic models. Given the high level of peripheral attrition of Vκ4+ B cells, it is somewhat surprising that more Vκ4+ cells weren’t found in the T3 IgMlo B cell population. Work with Hel, ArsA1, superantigen-induced tolerance, and other model systems indicates that a major mechanism of tolerance is the presence of anergic cells in the periphery with reduced survival (Goodnow et al., 1988; Erikson et al., 1991; Santulli-Marotto et al., 1998; Benschop et al., 2001; Ota et al., 2011). But it is unclear from the literature whether peripheral deletion is necessarily preceded by low surface Ig levels and induction of an anergic state and that all deleted cells would transit through the T3 stage before undergoing apoptosis. It may be that induction of anergy (whether caused by antigen encounter in the BM or periphery) and peripheral induction of apoptosis through strong BCR signals could be two separate tolerance mechanisms. In contrast to other model systems in which peripheral attrition of cells anergized by antigen encounter is observed, we are characterizing tolerance induced in response to a myriad of different self-antigens present in different anatomical locations and with different affinities and avidities. It is probable that a given antigen that would induce frank apoptosis in the BM may only be encountered in the periphery whereupon deletion occurs.
Although there were no significant increases in the numbers of anergic Vκ4+ cells in IgVκ4/h mice, edited hCκ and Igλ+ cells were enriched in the T3 IgMlo B cell subset in Igκαhel/h mice, and these edited cells retained a high level of self-reactivity compared with edited FM B cells. As autoreactivity is largely mediated by the HC in these mice, it is likely that these T3 B cells represent cells with self-reactive prone HCs in which receptor editing failed to mitigate the self-reactive nature of the BCR, leading to anergy. If the LC is the major contributor of autoreactivity, however, as is more often the case in IgVκ4/h mice, receptor editing of the offending LC is more likely to result in a self-tolerant BCR. Cells that have both a highly self-reactive HC and LC are most likely deleted early during central tolerance. Collectively, it seems that receptor editing and deletion primarily deal with LC-mediated autoreactivity, whereas anergy plays a more significant role in HC-mediated autoreactivity.
Allelic inclusion or coexpression of two different LCs in the same cell has also been described in several different systems as a byproduct of receptor editing that allows self-reactive BCRs to escape detection and reach maturity (Gay et al., 1993; Kenny et al., 2000; Li et al., 2002; Liu et al., 2005; Huang et al., 2006; Casellas et al., 2007; Velez et al., 2007). In support of this model, when we compared the self-reactivity of BCRs with either the original Vκ4 LC or with the hCκ editor cloned from mature cells coexpressing similar levels of both LCs, hCκ+ BCRs were more self-tolerant. The proportion of allelically included cells detected by flow cytometry was doubled in IgVκ4/h mice compared with Igκαhel/h mice at the same stage in the BM. Furthermore, as detected by single-cell PCR, about half of the IgVκ4/h cells isolated by flow cytometry as hCκ+ but mCκ negative at the protein level expressed the Vκ4 transgene at the transcript level, whereas much fewer did so in the Igαhel/h mice. This suggests strong selection for cells in which the rearranged VJκ gene on the hCκ allele outcompeted the preexisting Vκ4 LC for pairing with the HC, which in effect resulted in receptor editing and self-tolerance. The reason for this increase in allelically included cells in IgVκ4/h mice is not entirely clear. It is possible that this is an artifact of the transgenic LC as it’s been shown that the Vκ4 LC, but not the αHel LC, has impaired allelic exclusion in an OcaB−/− knockout (Casellas et al., 2002). This phenotype is associated with a specific need for this transcriptional regulator for Vκ4 but not αHel expression. However, whether allelic exclusion of Vκ4 transgenic cells undergoing editing is reduced on a wild-type background, as in the case here, was not investigated. Alternatively, exacerbated allelic inclusion in the IgκVκ4/h mice could be a physiological mechanism. It may be that the LC-mediated autoreactivity of Vκ4-expressing cells is more easily diluted by the presence of any competing LC. Thus, during receptor editing, any additional LC that functionally rearranges and can more favorably interact with the HC can remove the LC-mediated autoreactivity. Conversely, HC-induced autoreactivity that predominates in the Igκαhel/h mice must be directly controlled at the chemical level dependent on the properties of the editing Vκ gene. For example, the presence of LC aspartate residues adjacent to HC arginines in the CDRs is essential to reduce anti-DNA reactivity in the 3H9 system (Li et al., 2001). Our observation demonstrates that Vκ genes that are poor editors and the autoantibodies they encode are particularly prone to be maintained in mature B cells by allelic inclusion, which may have contributed to the exacerbated autoimmune disease when the Vκ4 transgene was expressed on the MRL/lpr background herein.
The last stage of immune tolerance is to control the activation of self-reactive B cells during immune responses, some of which likely arise as the result of somatic mutations that alter specificity. Despite increased receptor editing and peripheral attrition, the fully mature Vκ4+ B cells from IgVκ4/h mice still bind better on average to HEp-2 antigens than their counterpart in Igκαhelκ/h mice. We hypothesized that the level of self-reactive cells might be further reduced during B cell activation and secondary tolerance. This did not appear to be case, however, as the levels of edited cells did not change between the naive and IgG compartments in IgVκ4/h mice. On the MRL/lpr background, however, we saw accelerated development of IgG dsDNA antibodies in IgVκ4/h mice likely caused by defects in both primary and secondary tolerance. In the classical 3-83 BCR transgenic tolerance model, tolerance against central or peripheral expression of the H-2k self-antigen is intact on the MRL/lpr background (Rubio et al., 1996; Kench et al., 1998). Likewise, tolerance for soluble and membrane-bound Hel is unchanged in lpr/lpr mice (Rathmell and Goodnow, 1994). However, in anti-DNA models of autoimmunity, there are breaks in tolerance at multiple levels, leading to accelerated production of autoantibodies in MRL/lpr mice. For example, in 3H9 HC or 3H9/Vκ8 HC/LC transgenic mice on the MRL/lpr background, central tolerance appears to be largely intact, but peripheral tolerance is compromised. In these mice, DNA-reactive B cells normally limited to the peripheral immature or anergic compartment with a high turnover rate reach maturity and with T cell help become activated and differentiate into anti-DNA–secreting plasmablasts (Brard et al., 1999; Mandik-Nayak et al., 2000; Chen et al., 2006). Allelically included cells found in the 3H9/Vκ8 transgenic mice with greater frequency in the FM compartment further contributed to the loss of tolerance and DNA-reactive B cells in MRL/lpr mice (Brard et al., 1999). Similarly, though we saw little change in central tolerance in these LC transgenic mice, there was a significant increase in unedited Vκ4+ and αHelκ LC–expressing cells in the periphery. Thus, it appears that defects in peripheral B cell tolerance increasing the level of naive precursor self-reactive cells, in combination with the availability of self-reactive T cell help, are instrumental in leading to accelerated autoimmunity in MRL/lpr mice.
This LC-mediated model of self-reactivity highlights the importance and complexity of peripheral tolerance. Furthermore, we demonstrate that LC-mediated autoreactivity alone can lead to accelerated development of autoantibodies. LCs matter.
MATERIALS AND METHODS
Mice.
Igκαhel/h and IgκVκ4/h mice were provided by R. Casellas at the National Institute of Allergy and Infectious Diseases (Bethesda, MD), and the MRL/MPJ and MRL/lpr mice were purchased from the Jackson Laboratory. Igκαhel/h and IgκVκ4/h mice were bred as previously described (Casellas et al., 2001). The Igκαhel/αhel, IgκVκ4/Vκ4, and IgκhCκ/hCκ were backcrossed to MRL/MPJ mice for six generations and then crossed with MRL/lpr mice for two generations. To get Igκαhel/h or IgκVκ4/h on the MRL/lpr background, mice that were MRL/lpr/Igκαhel/αhel or MRL/lpr/IgκVκ4/Vκ4 were crossed with MRL/lpr/IgκhCκ/hCκ mice. B6 and MRL/lpr without a LC transgene were bred separately in the same facility. Mice were kept and bred in the animal facility at the Oklahoma Medical Research Foundation and University of Chicago, and experiments were approved by the Institutional Animal Care and Use Committee. Unless stated otherwise, all mice were 7–10 wk of age and age matched for each experiment.
Flow cytometry.
Single-cell suspensions were derived from mouse BM, LNs, and spleens. Cells were then stained with antibodies recognizing mouse κ (187.1; BD), human κ (goat anti–human κ F(ab′)2 mouse absorbed; SouthernBiotech), λ (JC-5; SouthernBiotech), B220 (RA3-6B2; BD), IgM (1B4B1; SouthernBiotech), CD93 (AA4.1; eBioscience), CD21 (7G6; BD), CD23 (B3B4; eBioscience), CD24 (M1/69; BioLegend), IgG1/2 (A85-1/R2-40; SouthernBiotech), and CD43 (S7; BD). Flow cytometry was performed on an LSR II (BD), and data were analyzed using FlowJo software (Tree Star).
Single-cell sorting and RT-PCR.
B cells from mouse spleens were enriched using a RosetteSep murine B cell isolation kit (STEMCELL Technologies) or MACS CD43 depletion (Miltenyi Biotec) and stained with antibodies described above. Cells were presorted in bulk using a FACSAria cytometer (BD), and single cells were resorted into 96-well plates containing 10 mM Tris-HCl with 40 U/µl RNase inhibitor (Promega). Single-cell RT-PCR for RAG expression was performed as previously described (Yannoutsos et al., 2001). For antibody variable gene amplification, each cell was amplified in a one-step RT-PCR reaction (OneStep RT-PCR kit; QIAGEN) using a cocktail of sense primers specific for the leader region and antisense primers specific for either mCκ or hCκ, or Fcµ/δ for HC genes (primer sequences are available in Table S1). RT-PCR conditions were as follows: 50°C for 30 min, 95°C for 15 min, followed by 39 cycles of 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, and, finally, 72°C for 10 min. 1 µl from each RT-PCR reaction was reamplified using nested PCR primers specific for FW1 regions of the various Vκ genes and antisense primers specific for either mCκ or hCκ, or Fcµ/δ for HC genes. Nested PCR reaction conditions were as follows: 95°C for 15 min, followed by 39 cycles of 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, and finally 72°C for 10 min. To amplify LCs from both human and mouse alleles in allelically included cells, cDNA synthesis from single cells was first performed using random primers, followed by two-step amplification of Ig transcripts using specific primers as described above. Efficiency of LC amplification was typically ∼70%.
mAb expression.
mAbs were produced as previously published (Wardemann et al., 2003; Wrammert et al., 2008; Duty et al., 2009; Smith et al., 2009). In brief, the Ig HC and LC variable genes from single-sorted B cells amplified by RT-PCR were sequenced, amplified, and cloned into expression vectors and expressed with human IgG constant regions in the 293A human cell line. The antibodies produced were then purified from the serum-free tissue culture media using Protein A beads.
dsDNA and HEp-2 ELISA.
To measure the autoreactivity of the mAbs, we tested each antibody for HEp-2 binding activity using a human HEp-2 ANA ELISA kit (Alpha Diagnostic) according to the manufacturer’s instructions. For the anti-dsDNA ELISAs, microtiter plates (Costar; Corning) were coated with 10 µg/ml calf thymus dsDNA (Invitrogen). Goat anti–human IgG (γ chain specific) peroxidase conjugate (Jackson ImmunoResearch Laboratories, Inc.) was used to detect binding of the recombinant antibodies followed by development with AquaBlue ELISA substrate (eBioscience). Paired antibodies sharing the same HC were tested on the same plate at the same time. Absorbencies were measured at OD405 when the control antibody 3H9 included on every plate reached an OD of 3.0. mAbs were diluted to a starting concentration of 25 µg/ml followed by three additional threefold serial dilutions for HEp-2 cell ELISAs and 1 µg/ml followed by three additional fourfold serial dilutions for dsDNA ELISAs. Data were plotted, and area under the curve (AUC) was determined with Prism software (GraphPad Software). To determine Ig concentrations in mouse sera, 96-well plates were coated with anti–mouse IgG and blocked with PBS/0.5% BSA. Sera were diluted to 1:10,000 (B6) or 1:100,000 (MRL/lpr) to fall within the concentration range of the IgG standard control. Detection was performed with HRP-conjugated anti–mouse IgG (SouthernBiotech). To determine the levels of anti-dsDNA antibodies in mouse sera, 1:100 dilutions of serum were added to microtiter plates coated with calf-thymus DNA and blocked with PBS + 5% BSA. Bound Igs were detected with HRP-conjugated anti–mouse IgG. To normalize results across plates, each plate had serum from the same MRL/lpr mouse, and the absorbance was read when this sample reached an OD405 of 3.0.
ELISPOT.
Multiscreen HTS filter plates (EMD Millipore) were coated overnight with mouse or human anti-κ antibody followed by incubation with 3 × 106/well total splenocytes overnight and detection of secreted antibodies with biotin-conjugated anti–mouse IgG (SouthernBiotech) and streptavidin peroxidase.
HEp-2 and 3T3 cell staining.
mAbs at 50 µg/ml were screened on commercial ANA (HEp-2 cell) slides (Bion Enterprises, Ltd.) according to the manufacturer’s recommended protocol. 3T3 cells were grown on chambered slides and fixed with acetone before staining with mAbs according to the same protocol as with the HEp-2 slides. Paired antibodies sharing the same HC were tested on the same slide at the same time. HEp-2 and 3T3 slides were analyzed using a fluorescent microscope (Axioplan II; Carl Zeiss). All HEp-2 and 3T3 reactivity was compared with positive and negative control sera included in the ANA commercial kit. Binding to HEp-2 and 3T3 cells by IMF was independently scored in a blinded manner on a scale of 0–4 by several individuals.
RS element detection.
Detection of RS rearrangements was performed as described previously (Panigrahi et al., 2008). In brief, genomic DNA was isolated from sorted cells using a Puregene kit (QIAGEN). Quantitative PCR was then performed using Taqman technology (Applied Biosystems) with primers and probes specific to RS elements and actin (Meng et al., 2011).
Spectratyping.
Genomic DNA was isolated from sorted cells, purified according to the manufacturer’s directions using Puregene, and amplified using the J606.1 or J558.85 VH primers and a labeled JH2 reverse primer as described previously (Meng et al., 2011). All primers were synthesized by Integrated DNA Technologies. 2 µl PCR product per reaction was resolved by capillary electrophoresis on an ABI 3100 analyzer (Applied Biosciences). Peak scanner version 1.0 was used to generate and analyze the spectratypes (Applied Biosciences) To measure the frequency of individual rearrangements accurately, the input DNA was diluted to very low concentrations (up to 2,000 cells) in each independent PCR reaction, so that each spectratype of a given size corresponds to only one rearrangement.
Statistics.
Statistics were performed using Prism software (analyzing column and grouped data by Student’s t test and by ANOVA) or Microsoft Excel (for χ2 test statistics). Unless stated otherwise in the figure legend, statistical significance was determined using a two-tailed Student’s t test of independent sample means.
Online supplemental material.
Fig. S1 shows B cell developmental subsets in LC transgenic mice. Fig. S2 shows LC usage in mature B cell populations and sort purity. Table S1 lists primers used in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20120525/DC1.
Supplementary Material
Acknowledgments
We thank Marin Weigert for helpful discussions.
This work was supported by National Institutes of Health grants to P.C. Wilson (including RO1AI76585-01 and U19AI082724-02) and grants from the Arthritis Foundation and Lupus Research Institute to E.T.L. Prak.
The authors declare no financial or commercial conflicts of interest.
Footnotes
Abbreviations used:
- AUC
- area under the curve
- BCR
- B cell receptor
- dsDNA
- double-stranded DNA
- FM
- follicular mature
- HC
- heavy chain
- IMF
- immunofluorescence
- LC
- light chain
- RS
- recombining sequence
References
- Ait-Azzouzene D., Verkoczy L., Peters J., Gavin A., Skog P., Vela J.L., Nemazee D. 2005. An immunoglobulin Cκ-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J. Exp. Med. 201:817–828 10.1084/jem.20041854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aït-Azzouzene D., Verkoczy L., Duong B., Skog P., Gavin A.L., Nemazee D. 2006. Split tolerance in peripheral B cell subsets in mice expressing a low level of Igkappa-reactive ligand. J. Immunol. 176:939–948 [DOI] [PubMed] [Google Scholar]
- Allman D., Lindsley R.C., DeMuth W., Rudd K., Shinton S.A., Hardy R.R. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167:6834–6840 [DOI] [PubMed] [Google Scholar]
- Batten M., Groom J., Cachero T.G., Qian F., Schneider P., Tschopp J., Browning J.L., Mackay F. 2000. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 192:1453–1466 10.1084/jem.192.10.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop R.J., Aviszus K., Zhang X., Manser T., Cambier J.C., Wysocki L.J. 2001. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 14:33–43 10.1016/S1074-7613(01)00087-5 [DOI] [PubMed] [Google Scholar]
- Brard F., Shannon M., Prak E.L., Litwin S., Weigert M. 1999. Somatic mutation and light chain rearrangement generate autoimmunity in anti–single-stranded DNA transgenic MRL/lpr mice. J. Exp. Med. 190:691–704 10.1084/jem.190.5.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R., Köhler G., Lamers M.C. 1995. Transitional B cells are the target of negative selection in the B cell compartment. J. Exp. Med. 181:2129–2140 10.1084/jem.181.6.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas R., Shih T.A., Kleinewietfeld M., Rakonjac J., Nemazee D., Rajewsky K., Nussenzweig M.C. 2001. Contribution of receptor editing to the antibody repertoire. Science. 291:1541–1544 10.1126/science.1056600 [DOI] [PubMed] [Google Scholar]
- Casellas R., Jankovic M., Meyer G., Gazumyan A., Luo Y., Roeder R., Nussenzweig M. 2002. OcaB is required for normal transcription and V(D)J recombination of a subset of immunoglobulin kappa genes. Cell. 110:575–585 10.1016/S0092-8674(02)00911-X [DOI] [PubMed] [Google Scholar]
- Casellas R., Zhang Q., Zheng N.Y., Mathias M.D., Smith K., Wilson P.C. 2007. Igκ allelic inclusion is a consequence of receptor editing. J. Exp. Med. 204:153–160 10.1084/jem.20061918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Prak E.L., Weigert M. 1997. Editing disease-associated autoantibodies. Immunity. 6:97–105 10.1016/S1074-7613(00)80673-1 [DOI] [PubMed] [Google Scholar]
- Chen C., Li H., Tian Q., Beardall M., Xu Y., Casanova N., Weigert M. 2006. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J. Immunol. 176:5183–5190 [DOI] [PubMed] [Google Scholar]
- Chung J.B., Silverman M., Monroe J.G. 2003. Transitional B cells: step by step towards immune competence. Trends Immunol. 24:342–349 10.1016/S1471-4906(03)00119-4 [DOI] [PubMed] [Google Scholar]
- Craxton A., Draves K.E., Gruppi A., Clark E.A. 2005. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J. Exp. Med. 202:1363–1374 10.1084/jem.20051283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong B.H., Ota T., Aït-Azzouzene D., Aoki-Ota M., Vela J.L., Huber C., Walsh K., Gavin A.L., Nemazee D. 2010. Peripheral B cell tolerance and function in transgenic mice expressing an IgD superantigen. J. Immunol. 184:4143–4158 10.4049/jimmunol.0903564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty J.A., Szodoray P., Zheng N.Y., Koelsch K.A., Zhang Q., Swiatkowski M., Mathias M., Garman L., Helms C., Nakken B., et al. 2009. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 206:139–151 10.1084/jem.20080611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Radic M.Z., Camper S.A., Hardy R.R., Carmack C., Weigert M. 1991. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 349:331–334 10.1038/349331a0 [DOI] [PubMed] [Google Scholar]
- Fulcher D.A., Lyons A.B., Korn S.L., Cook M.C., Koleda C., Parish C., Fazekas de St Groth B., Basten A. 1996. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J. Exp. Med. 183:2313–2328 10.1084/jem.183.5.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F., Alt F.W., Monroe R.J., Seidl K.J. 2000. Antigen-independent appearance of recombination activating gene (RAG)-positive bone marrow B cells in the spleens of immunized mice. J. Exp. Med. 192:1745–1754 10.1084/jem.192.12.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999–1008 10.1084/jem.177.4.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K., et al. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682 10.1038/334676a0 [DOI] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. 1991. Most peripheral B cells in mice are ligand selected. J. Exp. Med. 173:1357–1371 10.1084/jem.173.6.1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson R., Torres R.M., Pelanda R. 2004. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 5:645–650 10.1038/ni1076 [DOI] [PubMed] [Google Scholar]
- Hannum L.G., Ni D., Haberman A.M., Weigert M.G., Shlomchik M.J. 1996. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J. Exp. Med. 184:1269–1278 10.1084/jem.184.4.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S.B., Crosbie J., Brink R., Kantor A.B., Basten A., Goodnow C.C. 1991. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 353:765–769 10.1038/353765a0 [DOI] [PubMed] [Google Scholar]
- Hsu B.L., Harless S.M., Lindsley R.C., Hilbert D.M., Cancro M.P. 2002. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J. Immunol. 168:5993–5996 [DOI] [PubMed] [Google Scholar]
- Huang H., Kearney J.F., Grusby M.J., Benoist C., Mathis D. 2006. Induction of tolerance in arthritogenic B cells with receptors of differing affinity for self-antigen. Proc. Natl. Acad. Sci. USA. 103:3734–3739 10.1073/pnas.0600214103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito G.C., Schelonka R.L., Zemlin M., Ivanov I.I., Kobayashi R., Zemlin C., Gartland G.L., Nitschke L., Pelkonen J., Fujihashi K., et al. 2006. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J. Exp. Med. 203:1567–1578 10.1084/jem.20052217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnardi I., Ng Y.S., Menard L., Meyers G., Saadoun D., Srdanovic I., Samuels J., Berman J., Buckner J.H., Cunningham-Rundles C., Meffre E. 2010. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 115:5026–5036 10.1182/blood-2009-09-243071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kench J.A., Russell D.M., Nemazee D. 1998. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J. Exp. Med. 188:909–917 10.1084/jem.188.5.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J.J., Lustig A., Longo D.L. 2000. Positive selection of low affinity autoreactive B cells. Curr. Top. Microbiol. Immunol. 252:39–45 10.1007/978-3-642-57284-5_5 [DOI] [PubMed] [Google Scholar]
- Kindt T.J., Capra J.D. 1984. The Antibody Enigma. Plenum Press, New York: 270 pp [Google Scholar]
- Lang J., Jackson M., Teyton L., Brunmark A., Kane K., Nemazee D. 1996. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J. Exp. Med. 184:1685–1697 10.1084/jem.184.5.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J., Arnold B., Hammerling G., Harris A.W., Korsmeyer S., Russell D., Strasser A., Nemazee D. 1997. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J. Exp. Med. 186:1513–1522 10.1084/jem.186.9.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.H., Haberman A.M., Sant’Angelo D.B., Hannum L.G., Cancro M.P., Janeway C.A., Jr, Shlomchik M.J. 2000. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc. Natl. Acad. Sci. USA. 97:2743–2748 10.1073/pnas.050552997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jiang Y., Prak E.L., Radic M., Weigert M. 2001. Editors and editing of anti-DNA receptors. Immunity. 15:947–957 10.1016/S1074-7613(01)00251-5 [DOI] [PubMed] [Google Scholar]
- Li Y., Li H., Weigert M. 2002. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med. 195:181–188 10.1084/jem.20011453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Velez M.G., Humann J., Rowland S., Conrad F.J., Halverson R., Torres R.M., Pelanda R. 2005. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J. Immunol. 175:5067–5076 [DOI] [PubMed] [Google Scholar]
- Mandik-Nayak L., Seo S., Eaton-Bassiri A., Allman D., Hardy R.R., Erikson J. 2000. Functional consequences of the developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Immunol. 164:1161–1168 [DOI] [PubMed] [Google Scholar]
- Martin F., Kearney J.F. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195–201 10.1016/S0952-7915(00)00204-1 [DOI] [PubMed] [Google Scholar]
- Melamed D., Nemazee D. 1997. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 94:9267–9272 10.1073/pnas.94.17.9267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Yunk L., Wang L.S., Maganty A., Xue E., Cohen P.L., Eisenberg R.A., Weigert M.G., Mancini S.J., Prak E.T. 2011. Selection of individual VH genes occurs at the pro-B to pre-B cell transition. J. Immunol. 187:1835–1844 10.4049/jimmunol.1100207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell K.T., Benschop R.J., Gauld S.B., Aviszus K., Decote-Ricardo D., Wysocki L.J., Cambier J.C. 2006. Identification of anergic B cells within a wild-type repertoire. Immunity. 25:953–962 10.1016/j.immuni.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A., Andrews S.F., Yu K.O., Porcelli S.A., Rawlings D.J. 2008. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J. Exp. Med. 205:155–168 10.1084/jem.20071088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. 2006. Receptor editing in lymphocyte development and central tolerance. Nat. Rev. Immunol. 6:728–740 10.1038/nri1939 [DOI] [PubMed] [Google Scholar]
- Nemazee D.A., Bürki K. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 337:562–566 10.1038/337562a0 [DOI] [PubMed] [Google Scholar]
- Norvell A., Mandik L., Monroe J.G. 1995. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J. Immunol. 154:4404–4413 [PubMed] [Google Scholar]
- Nossal G.J. 1983. Cellular mechanisms of immunologic tolerance. Annu. Rev. Immunol. 1:33–62 10.1146/annurev.iy.01.040183.000341 [DOI] [PubMed] [Google Scholar]
- Ota M., Duong B.H., Torkamani A., Doyle C.M., Gavin A.L., Ota T., Nemazee D. 2010. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J. Immunol. 185:4128–4136 10.4049/jimmunol.1002176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T., Ota M., Duong B.H., Gavin A.L., Nemazee D. 2011. Liver-expressed Igκ superantigen induces tolerance of polyclonal B cells by clonal deletion not κ to λ receptor editing. J. Exp. Med. 208:617–629 10.1084/jem.20102265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi A.K., Goodman N.G., Eisenberg R.A., Rickels M.R., Naji A., Luning Prak E.T. 2008. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J. Exp. Med. 205:2985–2994 10.1084/jem.20082053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prak E.L., Weigert M. 1995. Light chain replacement: a new model for antibody gene rearrangement. J. Exp. Med. 182:541–548 10.1084/jem.182.2.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radic M.Z., Mascelli M.A., Erikson J., Shan H., Weigert M. 1991. Ig H and L chain contributions to autoimmune specificities. J. Immunol. 146:176–182 [PubMed] [Google Scholar]
- Radic M.Z., Erikson J., Litwin S., Weigert M. 1993. B lymphocytes may escape tolerance by revising their antigen receptors. J. Exp. Med. 177:1165–1173 10.1084/jem.177.4.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J.C., Goodnow C.C. 1994. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J. Immunol. 153:2831–2842 [PubMed] [Google Scholar]
- Retter M.W., Nemazee D. 1998. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188:1231–1238 10.1084/jem.188.7.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C.F., Kench J., Russell D.M., Yawger R., Nemazee D. 1996. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J. Immunol. 157:65–71 [PubMed] [Google Scholar]
- Russell D.M., Dembić Z., Morahan G., Miller J.F., Bürki K., Nemazee D. 1991. Peripheral deletion of self-reactive B cells. Nature. 354:308–311 10.1038/354308a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli-Marotto S., Retter M.W., Gee R., Mamula M.J., Clarke S.H. 1998. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 8:209–219 10.1016/S1074-7613(00)80473-2 [DOI] [PubMed] [Google Scholar]
- Schelonka R.L., Zemlin M., Kobayashi R., Ippolito G.C., Zhuang Y., Gartland G.L., Szalai A., Fujihashi K., Rajewsky K., Schroeder H.W., Jr 2008. Preferential use of DH reading frame 2 alters B cell development and antigen-specific antibody production. J. Immunol. 181:8409–8415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B., Gommerman J.L., Vora K., Cachero T.G., Shulga-Morskaya S., Dobles M., Frew E., Scott M.L. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 293:2111–2114 10.1126/science.1061964 [DOI] [PubMed] [Google Scholar]
- Shlomchik M.J. 2008. Sites and stages of autoreactive B cell activation and regulation. Immunity. 28:18–28 10.1016/j.immuni.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Shlomchik M.J., Aucoin A.H., Pisetsky D.S., Weigert M.G. 1987. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc. Natl. Acad. Sci. USA. 84:9150–9154 10.1073/pnas.84.24.9150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M.Z., Pisetsky D., Marshak-Rothstein A., Weigert M. 1990. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 171:265–292 10.1084/jem.171.1.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Garman L., Wrammert J., Zheng N.Y., Capra J.D., Ahmed R., Wilson P.C. 2009. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 4:372–384 10.1038/nprot.2009.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs S.L., Russell D.M., Nemazee D. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009–1020 10.1084/jem.177.4.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M.G., Kane M., Liu S., Gauld S.B., Cambier J.C., Torres R.M., Pelanda R. 2007. Ig allotypic inclusion does not prevent B cell development or response. J. Immunol. 179:1049–1057 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377 10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Hammersen J., Nussenzweig M.C. 2004. Human autoantibody silencing by immunoglobulin light chains. J. Exp. Med. 200:191–199 10.1084/jem.20040818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J., Smith K., Miller J., Langley W.A., Kokko K., Larsen C., Zheng N.Y., Mays I., Garman L., Helms C., et al. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 453:667–671 10.1038/nature06890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoutsos N., Wilson P., Yu W., Chen H.T., Nussenzweig A., Petrie H., Nussenzweig M.C. 2001. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J. Exp. Med. 194:471–480 10.1084/jem.194.4.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., Nussenzweig M.C. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687 10.1038/23287 [DOI] [PubMed] [Google Scholar]
- Zemlin M., Ippolito G.C., Zemlin C., Link J., Monestier M., Schroeder H.W., Jr 2005. Adult lupus-prone MRL/MpJ2+ mice express a primary antibody repertoire that differs in CDR-H3 length distribution and hydrophobicity from that expressed in the C3H parental strain. Mol. Immunol. 42:789–798 10.1016/j.molimm.2004.07.049 [DOI] [PubMed] [Google Scholar]
- Zemlin M., Schelonka R.L., Ippolito G.C., Zemlin C., Zhuang Y., Gartland G.L., Nitschke L., Pelkonen J., Rajewsky K., Schroeder H.W., Jr 2008. Regulation of repertoire development through genetic control of DH reading frame preference. J. Immunol. 181:8416–8424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.