Abstract
The proapoptotic Bcl-2 protein Bax can commit a cell to apoptosis by translocation from the cytosol to the mitochondria and permeabilization of the outer mitochondrial membrane. Prosurvival Bcl-2 family members, such as Bcl-xL, control Bax activity. Bcl-xL recognizes Bax after a conformational change in the N-terminal segment of Bax on the mitochondria and retrotranslocates it back into the cytoplasm, stabilizing the inactive form of Bax. Here we show that Bax retrotranslocation depends on the C-terminal helix of Bcl-xL. Deletion or substitution of this segment reduces Bax retrotranslocation and correlates with the accumulation of GFP-tagged or endogenous Bax on the mitochondria of non-apoptotic cells. Unexpectedly, the substitution of the Bcl-xL membrane anchor by the corresponding Bax segment reverses the Bax retrotranslocation activity of Bcl-xL, but not that of Bcl-xL shuttling. Bax retrotranslocation depends on interaction to the Bcl-xL membrane anchor and interaction between the Bax BH3 domain and the Bcl-xL hydrophobic cleft. Interference with either interaction increases mitochondrial levels of endogenous Bax. In healthy cells, mitochondrial Bax does not permeabilize the outer mitochondrial membrane, but increases cell death after apoptosis induction.
Keywords: apoptosis, Bcl-2 proteins, mitochondrial dysfunction
The balance between cell survival and programmed cell death in multicellular organisms often depends on the activities of Bcl-2 proteins.1 Proapoptotic members of the Bcl-2 family such as Bax can commit cells to apoptosis and are controlled by their prosurvival counterparts of the Bcl-2 family.2 The diverse group of ‘BH3-only' proteins can also participate in apoptosis control by directly or indirectly initiating the proapoptotic activities of Bax-like proteins.3, 4 Bax largely localizes to the cytoplasm of healthy cells, but accumulates on the outer mitochondrial membrane (OMM) upon apoptosis induction.5, 6 The activity of mitochondrial Bax is regulated by major conformational changes.7, 8, 9 Active Bax initiates mitochondrial dysfunction resulting in the release of cytochrome c (cyt c) from the mitochondrial intermembrane space.10, 11
Although Bax primarily resides in the cytoplasm of healthy cells,7 a fraction of Bax is associated with the mitochondria.12 The mitochondrial Bax pool results from constant translocation of cytosolic Bax.9 Accumulation of mitochondrial Bax is prevented by constant Bax retrotranslocation from the OMM into the cytoplasm in the absence of apoptosis signaling.9 Bax retrotranslocation depends on a conformational change of mitochondrial Bax and subsequent recognition of the Bax BH3 domain by prosurvival Bcl-2 proteins, such as Bcl-xL. The retrotranslocation activity of Bcl-xL correlates with its binding to the BH3 domain of Bax13 and co-retrotranslocation of Bcl-xL.9 Bcl-2 protein-mediated shuttling of Bax from the mitochondria back into the cytosol constitutes an equilibrium with constant translocation of Bax and Bcl-xL from the cytosol. Large shifts in the subcellular Bax distribution have also been observed, for instance, during anoikis,14 but are reversible, if Bax does not undergo further activation.15 In non-apoptotic cells, Bax retrotranslocation maintains the predominant cytosolic Bax localization and minimizes the danger of proapoptotic Bax activity.
Mitochondrial Bax and Bcl-xL seem to be involved in the regulation of mitochondrial morphology,16, 17, 18 and Bax has been shown to promote the assembly of the GTPase mitofusin 2 (Mfn2).16, 17 Mfn2 is one of two large GTPases that mediate OMM fusion.19 Bax increases the efficiency of Mfn2 homodimers to promote mitochondrial fusion and thus shifts cells with predominant Bax expression toward an interconnected mitochondrial network.20 Bax-mediated Mfn2 stimulation occurs only when Bax undergoes partial conformational changes on the mitochondria, but active Bax is incompetent to stimulate Mfn2-dependent OMM fusion. Similar partial conformational changes of Bax regulate Bax retrotranslocation.9
The retrotranslocation of Bax requires the interaction of the Bax BH3 motif with the hydrophobic groove in Bcl-xL, because mutations in the BH3 domain of Bax or the hydrophobic groove of Bcl-xL and the BH3 mimetic ABT-737 prevent Bax shuttling9 as effectively as they interfere with the inhibition of the proapoptotic activity of Bax by prosurvival Bcl-2 proteins.13, 21 However, it appears that the interaction between Bax BH3 and hydrophobic groove of Bcl-xL does not stabilize a heterodimeric complex of Bax and Bcl-xL, but participates in the transient process of Bax retrotranslocation. The dynamic character of Bax shuttling suggests that beyond the initial recognition of Bax BH3 by Bcl-xL further interactions could mediate Bax retrotranslocation.
Interestingly, the membrane anchor of Bcl-xL has been shown to interact potentially with the hydrophobic groove of Bax.22 In OMM-associated Bax and Bcl-xL, the hydrophobic grooves of both proteins are not likely to be occupied by the respective membrane anchors, as these segments mediate membrane association.23, 24 In this study, we show the importance of the C-terminal membrane anchor of Bcl-xL for both Bax and Bcl-xL retrotranslocation. Abrogation of retrotranslocation by preventing interactions between Bax and Bcl-xL leads to Bax accumulation on the mitochondria. Partial and complete substitution or deletion of the Bcl-xL membrane anchor shows that Bax retrotranslocation fully depends on interaction to Bcl-xL mediated by the membrane anchor, while Bcl-xL shuttling is independent of interactions with Bax or Bcl-xL.
Results
Bcl-xL ΔC does not shuttle Bax off the mitochondria
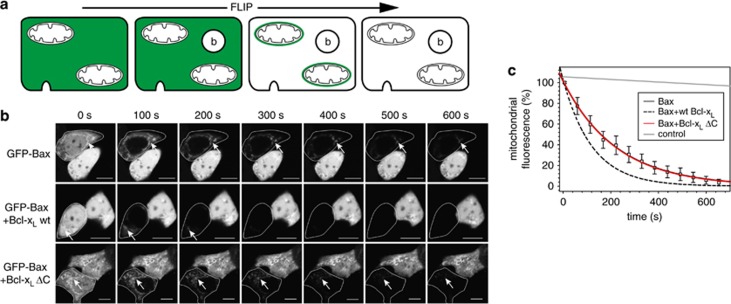
Interactions between Bax and Bcl-xL have been shown to be mediated by either the BH3 domain of Bax and the hydrophobic groove of Bcl-xL25 or by the membrane anchor of Bcl-xL and the hydrophobic groove of Bax.22 The Bax BH3 domain recognition is required for Bax retrotranslocation.9 We thus measured the influence of the Bcl-xL membrane anchor, mediating the second interaction, on Bax retrotranslocation by fluorescence loss in photobleaching (FLIP). In FLIP experiments, HCT116 Bax/Bak DKO cells overexpressing GFP-Bax and different Bcl-xL variants were monitored in repeated cycles of bleaching (Figure 1a). FLIP removes rapidly the cytosolic GFP-Bax fluorescence in an analyzed cell; the mitochondrial GFP-Bax pool becomes readily apparent and the decreasing mitochondrial fluorescence is analyzed (Figures 1a and b). Overexpression of wild-type (wt) Bcl-xL accelerates Bax retrotranslocation, resulting in the reduction of mitochondrial GFP-Bax fluorescence during FLIP measurements (Figures 1b and c). In contrast, overexpression of Bcl-xL ΔC, lacking the C-terminal membrane anchor, did not increase the rate of Bax retrotranslocation (Figures 1b and c). Bax retrotranslocation in the absence of Bcl-xL ΔC (Figure 1c, black solid line) is identical to Bax shuttling in the presence of overexpressed Bcl-xL ΔC (Figure 1c, red solid line). The absence of Bax shuttling activity of overexpressed Bcl-xL ΔC could indicate the requirement of the Bcl-xL membrane anchor for Bax retrotranslocation.
Figure 1.
Bcl-xL ΔC has no Bax retrotranslocation activity. (a) Bax retrotranslocation monitored by fluorescence loss in photobleaching (FLIP) measurements. Before FLIP, a targeted cell has fluorescent green fluorescent protein (GFP)-Bax molecules in the cytosol and on the mitochondria (left). FLIP measurements monitor the target cell during repeated bleaching (b) in the cytosol (second from left). After the first cycles of bleaching, the cytosolic Bax fluorescence is diminished and mitochondrial Bax becomes readily apparent (third from left). During FLIP measurements, the reduction of mitochondrial GFP-Bax fluorescence is monitored until mitochondrial and cytosolic GFP-Bax molecules are bleached (right). (b) Bcl-xL ΔC does not increase Bax retrotranslocation. FLIP of GFP-Bax in the absence (top) and presence of overexpressed wild-type Bcl-xL (center) and Bcl-xL ΔC (bottom) diminishes GFP-Bax fluorescence in the cytoplasm of the targeted cells (circled) completely after 100 s and GFP fluorescence is detected only on the mitochondria (arrows). The mitochondrial GFP-Bax fluorescence in the presence of overexpressed wild-type (wt) Bcl-xL is decreased faster than in the absence of wt Bcl-xL or presence of Bcl-xL ΔC overexpression. Time points in seconds are displayed on top. A scale of 10 μm is shown by the white bar in every image. (c) Bax retrotranslocation is not influenced by Bcl-xL ΔC. FLIP of mitochondrial GFP-Bax in the absence (solid black line) and presence of overexpressed Bcl-xL ΔC (red line, square) reveals identical rates for both conditions resulting in complete overlap of both data fits, while overexpression of wt Bcl-xL (broken black line) accelerates Bax retrotranslocation. Fluorescence of the neighboring cell is shown as control (gray line). Data represent normalized averages±S.E.M. from 20 region of interest (ROI) measurements per condition
The substitution of the Bcl-xL membrane anchor by the C-terminal Bax helix reverses the Bcl-xL activity
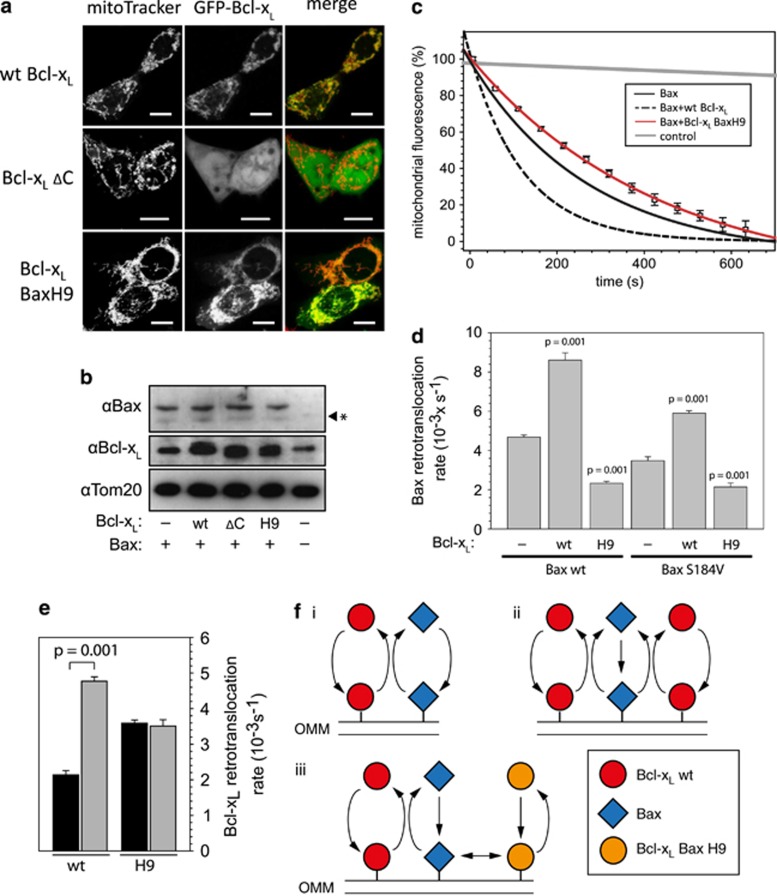
In contrast to the wt, Bcl-xL ΔC resides primarily in the cytoplasm (Figure 2a). The lack of Bcl-xL ΔC retrotranslocation activity could be caused by the absence of an essential interaction site or merely by insufficient mitochondrial Bcl-xL levels to retrotranslocate Bax. Mitochondrial Bcl-xL localization was restored by the substitution of the Bcl-xL membrane anchor with the corresponding helix in Bax (Bcl-xL BaxH9). In parallel to the wt, Bcl-xL BaxH9 localizes primarily to the mitochondria in the presence and absence of Bax overexpression at comparable expression levels, but does not contain the Bcl-xL membrane anchor (Figures 2a and b and Supplementary Figure S1).
Figure 2.
Bax retrotranslocation is inhibited in the presence of Bcl-xL BaxH9. (a) Confocal images of HCT116 Bax/Bak DKO cells transfected with wild-type (wt) green fluorescent protein (GFP)-Bcl-xL (top), GFP-Bcl-xL ΔC (center) or GFP-Bcl-xL BaxH9 (bottom), displaying GFP-Bcl-xL fluorescence in the center panels and in green in the merge on the right. Mitochondria are stained with Mito Tracker-far red (left, red in the merge). Colocalization between Bcl-xL variants and the mitochondria is shown as yellow in the merge (right). The white line in the low right corner of every image is the scale of 10 μm. (b) Western blot analysis of GFP-Bax expression in the presence of different Bcl-xL variants in HCT116 Bax/Bak DKO cells. Equal loading of the samples was controlled using anti-Tom20 antibodies. An unspecific protein band is detected by the anti-Bax antibody (*). (c) Fluorescence loss in photobleaching (FLIP) measurements of mitochondrial GFP-Bax without (solid black line) and with overexpressed wt Bcl-xL (broken black line) or Bcl-xL BaxH9 (red line, square). Fluorescence of the neighboring cell is shown as control (gray line). Data represent averages±S.E.M. from 20 region of interest (ROI) measurements per condition. (d) Retrotranslocation rates measured for wt Bax and Bax S184V in the absence and presence of wt Bcl-xL and Bcl-xL BaxH9. Data represent averages±S.D. P-values according to a Mann–Whitney test between data in the absence and presence of the Bcl-xL variants are displayed. (e) Retrotranslocation rates measured for wt Bcl-xL and Bcl-xL BaxH9 in the absence (black) and presence of Bax (gray). Data represent averages±S.D. (f) Schematic depiction of Bax and Bcl-xL shuttling on and off the outer mitochondrial membrane (OMM) in healthy cells overexpressing wt Bcl-xL and Bcl-xL BaxH9. In the absence of Bcl-xL overexpression, Bax (blue square) and endogenous wt Bcl-xL (red circle) co-retrotranslocate from the mitochondria and translocate independently back to the OMM (i). Overexpressed wt Bcl-xL (red circle), like endogenous Bcl-xL, co-retrotranslocates with Bax increasing the rate of Bax retrotranslocation (ii). In the presence of Bcl-xL BaxH9 (orange circle), the retrotranslocation rate of Bax is reduced. Bcl-xL BaxH9 does not co-retrotranslocate with Bax, although both proteins may interact (iii)
Surprisingly, Bcl-xL BaxH9 reduces Bax retrotranslocation in context of endogenous prosurvival Bcl-2 proteins (Figure 2c) in contrast to the activity of wt Bcl-xL overexpression. This result suggests a function of the Bcl-xL membrane anchor in Bax retrotranslocation that is independent of the requirement of sufficient mitochondrial Bcl-xL. Retrotranslocation of the primarily mitochondrial Bax S184V is accelerated by wt Bcl-xL and diminished by Bcl-xL BaxH9 below the level measured for Bax S184V retrotranslocation by endogenous prosurvival Bcl-2 proteins, corroborating the results with wt Bax and ruling out the influence of shifting protein localization on the measured retrotranslocation rate (Figure 2d). Despite its inhibitory effect on Bax retrotranslocation, Bcl-xL BaxH9 shuttles itself on and off the mitochondria (Figure 2e). Bcl-xL BaxH9 retrotranslocates faster than wt Bcl-xL in the absence of Bax, but this shuttling is not influenced by the presence of Bax. The absence of an inhibitory influence of Bax on Bcl-xL BaxH9 retrotranslocation excludes the possibility of competition between Bax and Bcl-xL BaxH9 for Bcl-xL-mediated shuttling. The adverse effect of Bcl-xL BaxH9 on Bax retrotranslocation compared with wt Bcl-xL is thus not based on competition between Bax and Bcl-xL BaxH9. On the other hand, Bcl-xL BaxH9 is not permanently sequestering Bax on the mitochondria as it shuttles itself. Apparently, Bcl-xL BaxH9 shuttling off the mitochondria does neither depend on wt Bcl-xL co-retrotranslocation nor on the Bcl-xL membrane anchor. Given the inhibitory effect of Bcl-xL BaxH9 on Bax shuttling, transient interactions between both proteins possibly occur on the OMM (Figure 2f). However, such interactions are not rate limiting for Bcl-xL BaxH9 retrotranslocation from the mitochondria.
The Bcl-xL membrane anchor is critical for Bax retrotranslocation
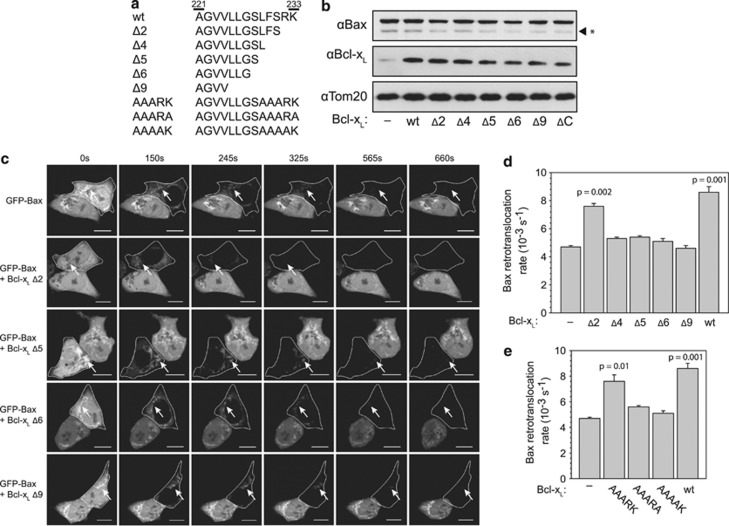
Bcl-xL variants differing in the C-terminal segment were expressed to comparable levels in HCT116 Bax/Bak DKO cells together with GFP-Bax and subjected to FLIP measurements to dissect the influence of the Bcl-xL membrane anchor on Bax retrotranslocation (Figures 3a and b). FLIP revealed that the deletion of four to five amino acids at the Bcl-xL C terminus reduces the acceleration of Bax retrotranslocation by Bcl-xL to about 10% of shuttling in the presence of wt Bcl-xL (Figures 3c and d). The Bax shuttling activity was completely abolished in Bcl-xL Δ9, while the deletion of the two very C-terminal amino acids results in only a minor reduction of Bcl-xL activity (Figures 3c and d). The substitution of the residues L229, F230 and S231 to alanine had only a slight effect on Bax retrotranslocation, although deletion of these residues has a dramatic effect on Bax shuttling (Figures 3c–e). The additional substitution of a charged residue to an alanine at the very C terminus mimicked the dramatic reduction in Bax retrotranslocation activity observed with the variants with C-terminal deletions of four or more residues (Figures 3d and e). These results also differentiate between Bcl-xL membrane anchor-mediated mitochondrial localization of Bcl-xL and Bax retrotranslocation activity, because Bcl-xL Δ2, Δ4, AAARK, AAARA and AAAAK share a similar mitochondrial localization,22 but have fundamentally different Bax retrotranslocation activities (Supplementary Table S1). Of note, Bcl-xL Δ2 and Δ4 do also localize to the ER and ER marker proteins are associated with the mitochondria in the HM fractions of HCT116 Bax/Bak DKO cells (Supplementary Figure S2). Thus, Bcl-xL molecules could also localize to ER membranes and those in close proximity could retrotranslocate mitochondria-associated Bax back into the cytosol. The C terminus of Bcl-xL is important for the Bax retrotranslocation activity, but instead of a single key residue the integrity of this helix seems to be essential for Bax shuttling.
Figure 3.
The Bcl-xL C terminus is required for Bax retrotranslocation. (a) Comparison of the sequence of the C termini of different Bcl-xL variants beginning with residue A221. (b) Western blot analysis of green fluorescent protein (GFP)-Bax expression in the presence of different Bcl-xL variants in HCT116 Bax/Bak DKO cells. Equal loading of the samples was controlled using anti-Tom20 antibodies. An unspecific protein band detected by the anti-Bax antibody is marked on the right (*). (c) Fluorescence loss in photobleaching (FLIP) of GFP-Bax in the absence (top) and presence of overexpressed C-terminal deletion variants of Bcl-xL. GFP-Bax fluorescence is diminished in the cytoplasm of the targeted cells (circled) after 150 s and GFP fluorescence is detected only on the mitochondria (arrows). Time points in seconds are displayed above the images. A scale of 10 μm is displayed by the white bar in every image. (d) Retrotranslocation rates measured for Bax in the absence and presence of wild-type (wt) Bcl-xL and C-terminal deletion variants of Bcl-xL. Data represent averages±S.D. (e) Bax retrotranslocation measured in the absence and presence of wt Bcl-xL and Bcl-xL variants containing alanine substitutions in the C-terminal segment. Data represent averages±S.D.
Decreased Bax retrotranslocation increases mitochondrial Bax levels and apoptotic cell death
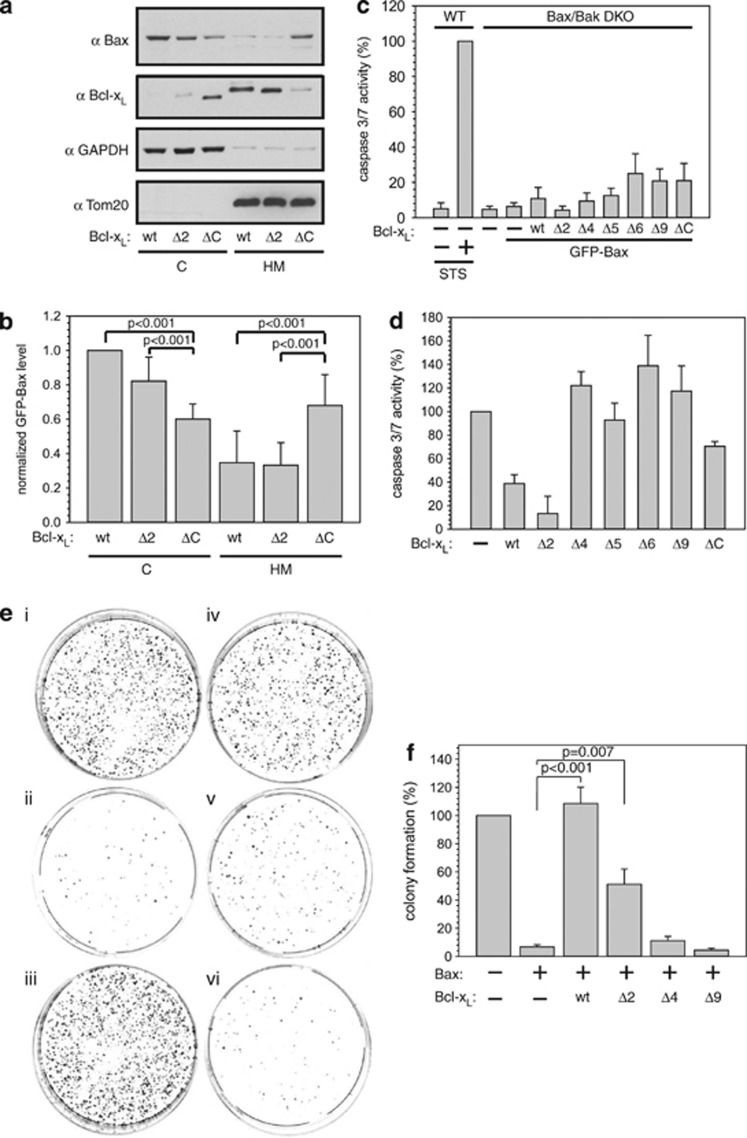
Different Bax retrotranslocation activities of Bcl-xL variants are directly reflected in mitochondrial Bax levels (Figure 4a and Supplementary Figure S3). Low levels of Bax are found in the heavy membrane fractions of cells expressing wt Bcl-xL or Bcl-xL Δ2 that possess Bax retrotranslocation activity (Figures 4a and b). In contrast, mitochondrial Bax accumulation can be observed in cells expressing Bcl-xL ΔC that does not accelerate Bax shuttling. Mitochondrial Bax pools do not induce cyt c release and subsequent caspase activation in the absence of apoptosis signaling (Figure 4c). However, after apoptosis induction different initial levels of mitochondrial Bax alter the induced cell death (Figure 4d). Bax retrotranslocation rates and Bax accumulation on the OMM influence not only the initial caspase-3/7 activity but also the cell fate after apoptosis induction by 1 μM staurosporine (STS) (Figure 4e). Bcl-xL variants with Bax retrotranslocation activity protect cells from Bax-dependent apoptosis (Figure 4f). In contrast, expression of Bcl-xL variants with C-terminal truncations of four or more amino acids does not influence the colony formation of HCT116 Bax/Bak DKO cells expressing Bax after STS treatment. These results establish a direct correlation between Bcl-xL membrane anchor functionality in Bax retrotranslocation and the protection of cells from mitochondrial Bax accumulation, OMM permeabilization and cell death following Bax activation (Supplementary Table S1).
Figure 4.
Bax accumulates on mitochondria at low retrotranslocation rates. (a) Western blot analysis of Bax localization in the presence of different Bcl-xL variants. Cytosol (C) and heavy membrane fraction (HM) of HCT116 cells expressing green fluorescent protein (GFP)-Bax and different variants of Bcl-xL. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Tom20 serve as fractionation controls. (b) Quantification of GFP-Bax levels in the C and in the HM is dependent on the presence of different Bcl-xL variants revealed by western blot. P-values according to a one-way analysis of variance (ANOVA) test are depicted (n=4). (c) Apoptosis signaling based on caspase-3/7 activity measured in HCT116 Bax/Bak DKO cells overexpressing GFP-Bax and either wt Bcl-xL or C-terminal deletion variants of Bcl-xL. Measured caspase activities are displayed as normalized to untransfected cells and relative to the activity detected in HCT116 wt cells after apoptosis induction by staurosporine (STS, 1 μM) for 18 h. Untreated HCT116 cells and mock-transfected HCT116 Bax/Bak DKO cells served as controls. Data represent averages±S.E.M. (n≥3 × 3) wells. (d) STS (1 μM)-induced apoptosis activity of GFP-Bax in the presence of different Bcl-xL variants based on caspase-3/7 activity measured in HCT116 Bax/Bak DKO cells relative to the activity obtained in the absence of Bcl-xL overexpression and normalized to mock-transfected cells. Data represent averages±S.E.M. (n≥3 × 3 wells). (e) HCT116 Bax/Bak DKO cells were transfected with pcDNA (i), GFP-Bax+pcDNA (ii), GFP-Bax+wt Bcl-xL (iii), GFP-Bax+Bcl-xL Δ2 (iv), GFP-Bax+Bcl-xL Δ4 (v) and GFP-Bax+Bcl-xL Δ9 (vi), 1 μM STS was added for 24 h, cells were replated and colonies were stained with methylene blue 14 days after treatment. (f) Quantification of colony formation depicted in (e) relative to colony formation of pcDNA-transfected HCT116 Bax/Bak cells. Data are presented±S.E.M. (n=8). P-values according to Student's t-test are shown
Bcl-xL retrotranslocation depends on the C-terminal membrane anchor
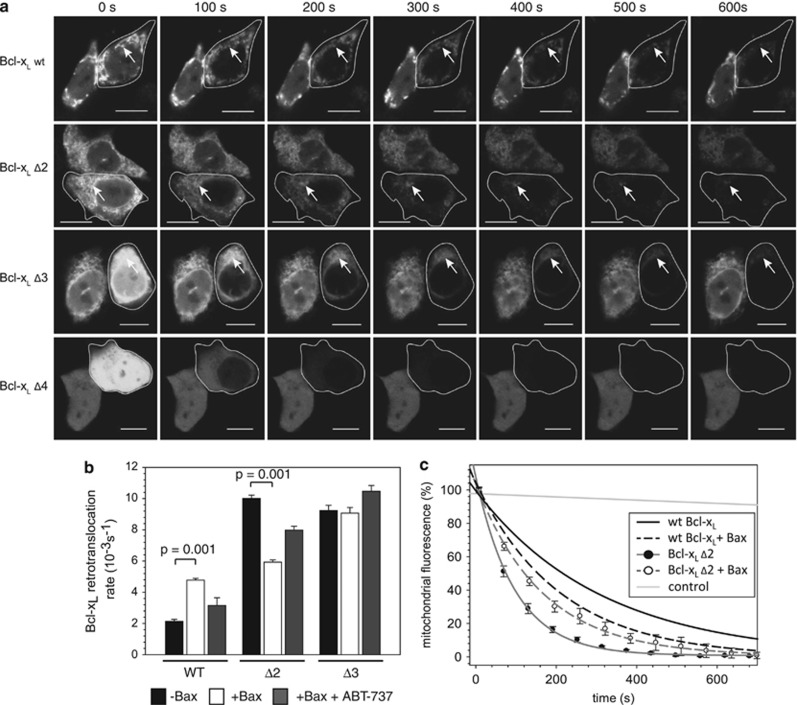
The Bcl-xL C-terminal residues are important for Bax retrotranslocation, raising the question whether Bcl-xL shuttling and localization is also affected in C-terminal deletion Bcl-xL variants. The FLIP analysis of GFP-Bcl-xL wt and variants with C-terminal deletions shows coherence between increasing C-terminal deletions and accelerated disappearance of mitochondrial GFP-Bcl-xL signal (Figure 5a). In parallel to faster Bcl-xL retrotranslocation, the localization of Bcl-xL shifts from the mitochondria to the cytosol with increasing C-terminal deletions, suggesting also the involvement of decreased membrane affinity of Bcl-xL. In cells expressing Bcl-xL variants lacking five or more C-terminal amino acids, mitochondrial Bcl-xL was not detectable. Bcl-xL lacking the two or three most C-terminal amino acids localizes primarily to the mitochondria with increased cytosolic pools compared with the wt (Figure 5a). Their retrotranslocation rates increased about fourfold compared with the rate of wt Bcl-xL (Figure 5b).
Figure 5.
Bcl-xL localization and retrotranslocation is influenced by the C terminus. (a) Fluorescence loss in photobleaching (FLIP) measurements of wild-type (wt) green fluorescent protein (GFP)-Bcl-xL (top) or C-terminal deletion variants of Bcl-xL in the absence of Bax. Cytosolic GFP fluorescence of the targeted cells (circled) is reduced after 100 s and Bcl-xL is detected only on the mitochondria (arrows). Time points in seconds are displayed above the images. A scale of 10 μm is shown by the white bar in every image. (b) Retrotranslocation rates measured for wt Bcl-xL and C-terminal deletion variants of Bcl-xL in the absence (black) and presence of Bax (white) or in the presence of Bax and 1 μM ABT-737 (gray). Data represent averages±S.D. P-values according to a one-way analysis of variance (ANOVA) test are displayed. (c) FLIP measurements of mitochondrial wt GFP-Bcl-xL without (solid black line) and with overexpressed Bax (broken black line) and measurements for mitochondrial GFP-Bcl-xL Δ2 in the absence (solid dark gray line, full circle) and presence of overexpressed Bax (broken dark gray line, open circles) are displayed. Fluorescence of the neighboring cell is shown as control (light gray line). Data represent averages±S.E.M. from 20 region of interest (ROI) measurements per condition
Interestingly, the retrotranslocation rate of Bcl-xL Δ2 changes with the overexpression of Bax as dramatically as observed with the wt; however, while the rate of wt Bcl-xL almost doubles in the presence of Bax, the rate of Bcl-xL Δ2 is reduced in the same experiment (Figure 5c). In spite of different rates in the absence of Bax, wt Bcl-xL and Bcl-xL Δ2 have similar retrotranslocation rates when Bax is present (Figures 5b and c). In contrast, the retrotranslocation rate of Bcl-xL Δ3 remains unaltered in the presence of Bax, although Bcl-xL Δ3 has a similar localization pattern as Bcl-xL Δ2. These results again suggest a role of the Bcl-xL membrane anchor in Bax retrotranslocation that is distinct from localization of Bcl-xL on the mitochondria. The effect of Bax on the shuttling of wt Bcl-xL and Bcl-xL Δ2 can be reversed by ABT-737 (Figure 5b). The similar rates for Bcl-xL Δ2 and wt Bcl-xL retrotranslocation in the presence of Bax suggest that Bax retrotranslocation determines the rate of Bcl-xL shuttling.
Endogenous Bax accumulates on the mitochondria upon inhibition of Bax/Bcl-xL interactions
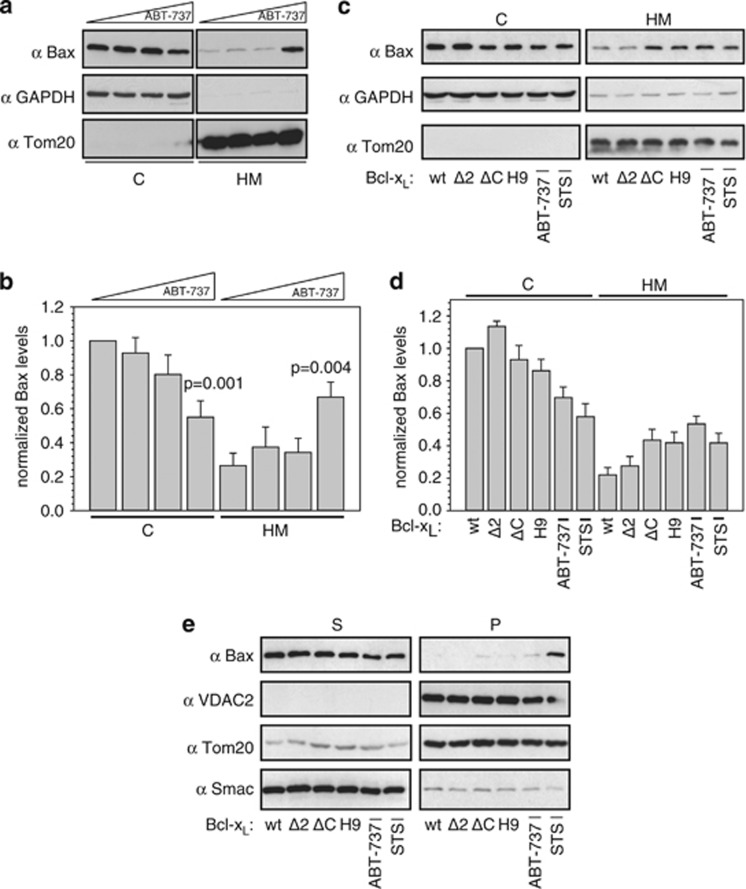
The effect of prevented Bax retrotranslocation on the localization of endogenous Bax was studied by the treatment of Mcl-1 KO MEFs with increasing concentrations of ABT-737. The BH3 mimetic ABT-737 should completely abolish the interaction between Bax BH3 and the hydrophobic cleft of prosurvival Bcl-2 family members in these cells lacking Mcl-1. In the presence of 1 μM ABT-737, endogenous Bax accumulates on the mitochondria and the cytosolic Bax pool of Mcl-1 KO MEFs is markedly reduced (Figures 6a and b). Prevention of interactions between the Bax BH3 domain and prosurvival Bcl-2 proteins results in the accumulation of endogenous Bax on the mitochondria in the absence of additional apoptotic stimuli.
Figure 6.
Endogenous Bax accumulates on the mitochondria in the absence of retrotranslocation. (a) Western blot analysis of endogenous Bax localization in Mcl-1 knockout (KO) mouse embryonic fibroblasts (MEFs) after 6 h treatment with 0, 0.01, 0.1 and 1 μM ABT-737 (from left to right, respectively). Cytosol (C) and heavy membrane fraction (HM) of Mcl-1 KO cells are displayed. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Tom20 serve as controls for the fractionation. (b) Quantification of endogenous Bax levels in the C and in the HM is dependent on the application of different ABT-737 concentrations revealed by western blot. P-values according to a one-way analysis of variance (ANOVA) test are depicted (n=7). (c) Analysis of endogenous Bax localization in HCT116 wt cells either overexpressing different Bcl-xL variants or after 6 h treatment with 1 μM ABT-737 by western blot. Apoptosis induction by 1 μM staurosporine (STS) serves as control. C and HM of HCT116 wt cells are displayed. GAPDH and Tom20 serve as control for equal protein loading. (d) Quantification of endogenous Bax levels in the C and in the HM of HCT116 cells either overexpressing different Bcl-xL variants or after treatment with 1 μM ABT-737 or 1 μM STS as analyzed by western blot (n=3). (e) Western blot analysis of carbonate extraction of membrane-associated endogenous Bax in HCT116 wt cells either overexpressing different Bcl-xL variants or after administration of 1 μM ABT-737 or 1 μM STS. Displayed are supernatant (S) and pellet (P) of the carbonate extraction. VDAC2, Tom20 and Smac served as controls
The subcellular localization of endogenous Bax in HCT116 wt cells can also be shifted by inhibiting either one of the two essential interactions mediated by the Bax BH3 motif or by the Bcl-xL membrane anchor, respectively. In the presence of wt Bcl-xL or Bcl-xL Δ2, endogenous Bax is largely localized to the cytoplasm with a minor pool associated with the heavy membrane fraction (Figures 6c and d and Supplementary Figure S4). Overexpression of the Bax retrotranslocation-inactive Bcl-xL ΔC or the inhibitory Bcl-xL BaxH9 increases the concentration of endogenous Bax in the heavy membrane fraction with decreased levels of Bax in the cytosol compared with the overexpression of wt Bcl-xL. A similar subcellular distribution of endogenous Bax is observed in samples of HCT116 wt cells treated with 1 μM ABT-737 or 1 μM STS. The inhibition of either interaction led to a similar accumulation of endogenous Bax on the mitochondria as is observed after apoptosis induction.
However, the analysis of membrane-associated endogenous Bax under the different conditions by carbonate extraction reveals a clear difference in Bax accumulation following apoptosis induction and inhibition of Bax retrotranslocation. Membrane-associated endogenous Bax is either completely or largely found in the supernatant of carbonate extraction when Bax accumulation is caused by the inhibition of Bax retrotranslocation (Figure 6e). In contrast, a significant fraction of Bax was found in the pellet of the carbonate extraction after apoptosis induction. Thus, apoptosis induction leads to further conformational changes and membrane insertion of Bax, while the inhibition of Bax retrotranslocation only increases the pool of mitochondria-associated but not membrane-integrated Bax. These results corroborate measurements of the apoptotic activity of GFP-Bax pools on the mitochondria following reduced Bax retrotranslocation (Figure 4). Inhibition of Bax retrotranslocation leads to the accumulation of Bax on the mitochondria susceptible for activation but not per se active. Thus, Bax retrotranslocation targets OMM-associated Bax that exposes the BH3 domain after a conformational change9 in the N-terminal segment.
Discussion
The proapoptotic Bcl-2 family member Bax can commit cells to apoptosis when it localizes to the OMM.26 In healthy cells, Bax constantly translocates to the mitochondria, but the inactive, cytosolic form of Bax is stabilized by retrotranslocation in dependence on the activities of the antiapoptotic Bcl-2 proteins.9 Bcl-xL and other prosurvival Bcl-2 proteins recognize mitochondrial Bax after a conformational change exposing the Bax BH3 domain and shuttle it back into the cytoplasm. Interference with Bax retrotranslocation shows an inverse correlation between the Bax retrotranslocation rates and mitochondrial levels of GFP-tagged or endogenous Bax. In fact, inhibition of Bax retrotranslocation separates the two processes of Bax translocation and Bax activation that occur simultaneously following apoptosis induction. Decreased Bax retrotranslocation results in an increased pool of OMM-associated but not integrated Bax that is susceptible for activation but not active. Bax retrotranslocation regulates the pool of Bax that is ready for activation and may depend on the level of cell stress.2 A shift of the Bax population towards the mitochondria can either be caused by a reduction in the retrotranslocation rate or by an increase in Bax translocation to the mitochondria that is independent of prosurvival Bcl-2 activities.9 The equilibrium between both processes allows reversible shifts in the subcellular localization of Bax, as they have been observed during transient detachment of cells from the extracellular matrix.14 The analysis of myc-deficient cells suggests that mitochondrial Bax accumulation and Bax activation are separate processes.27 Inhibition of Bax retrotranslocation allows separation of mitochondrial association from membrane integration in living cells.
Mitochondrial Bax accumulation in non-apoptotic cells in the presence of Bcl-xL variants with C-terminal truncations does not per se lead to OMM permeabilization, but can result in increased cell death after apoptosis induction. Bax can accumulate on the OMM after apoptosis induction despite Bcl-xL overexpression,4 but in a slower process compared with the absence of overexpressed Bcl-xL. The retrotranslocation activity of overexpressed Bcl-xL prevents mitochondrial Bax accumulation after apoptosis induction by shifting Bax towards the cytosolic inactive form and increasing the cellular tolerance for increased mitochondrial Bax levels as Bax is shuttled from the mitochondria into the cytoplasm prior activation. Bcl-xL retrotranslocation activity prevents the commitment to apoptosis and protects cells from Bax-dependent cell death. The inhibition of Bax retrotranslocation produces a mitochondrial Bax pool with increased susceptibility for activation by, for instance, BH3-only proteins already present on the mitochondria.28
Bax retrotranslocation depends on two interactions: recognition of the Bax BH3 domain by the hydrophobic groove of Bcl-xL and binding of the Bcl-xL membrane anchor to Bax. While the binding of the Bax BH3 domain to the hydrophobic groove of Bcl-xL seems to be critical in Bax regulation,13 the Bcl-xL membrane anchor has been shown to mediate potentially interactions to Bax or another Bcl-xL molecule.22 The binding of Bcl-xL to the hydrophobic cleft of Bax is unlikely to occur when this binding site is obstructed by the Bax membrane anchor in cytosolic Bax,28 but mediates the binding of Bax and Bcl-xL when this hydrophobic cleft of Bax is solvent accessible.29, 22 On the mitochondria, the membrane anchor of Bax mediates the association with the lipid bilayer and thus does not likely occupy the hydrophobic groove, leaving the interaction site accessible for the Bcl-xL membrane anchor. The importance of this interaction for Bax shuttling is directly reflected in the Bax retrotranslocation rates in the presence of different Bcl-xL variants, corresponding mitochondrial Bax levels and cell fate after apoptosis induction.
Bcl-xL shuttling off the mitochondria is also influenced by C-terminal truncations. The deletion of amino acids from the C terminus of Bcl-xL likely reduces the affinity of the prosurvival Bcl-2 protein for the OMM.22 However, Bcl-xL BaxH9 retrotranslocation shows that the movement of Bcl-xL from the mitochondria to the cytosol does not require the integrity of the C-terminal Bcl-xL segment. The retrotranslocation rates of different Bcl-xL variants converge in the presence of Bax. Bax shuttling seems to be rate limiting under these conditions, suggesting the formation of a transient complex involved in the co-retrotranslocation of Bax and Bcl-xL. However, Bcl-xL does not require binding to Bax or interactions with the C-terminal helix of another Bcl-xL molecule for the shuttling from the mitochondria, as the retrotranslocation of Bcl-xL BaxH9 was not affected by overexpression of Bax. These insights into the Bax retrotranslocation process suggest that Bax is the freight and Bcl-xL is the carrier for shuttling from the mitochondria into the cytoplasm.
Materials and Methods
Cell culture and transfection
HCT116 cells and HCT116 Bax/Bak DKO cells30 were cultured in McCoy's 5A medium supplemented with 10% heat-inactivated fetal bovine serum and 10 mM HEPES in 5% CO2 at 37 °C. Mcl-1 KO MEFs were cultured in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum and 10 mM HEPES in 5% CO2 at 37 °C. Cells were transfected with Turbofect (Fermentas, St. Leon-Rot, Germany) or Lipofectamine LTX (Invitrogen, Darmstadt, Germany) typically with 100 ng of the GFP-Bax construct according to the manufacturer's instructions and cells were incubated for 6–8 h for confocal imaging.
Confocal microscopy and FLIP
HCT116 Bax/Bak DKO cells were seeded on a chambered cover glass (Thermo Scientific, Bonn, Germany) in McCoy's 5A medium, grown for 20—24 h and transfected for 6–8 h. The cells were then incubated with Mito Tracker-far red (Invitrogen) for 10 min and imaged using a Zeiss LSM 510 META confocal microscope (Zeiss, Oberkochen, Germany) equipped with argon (458/488/514 nm lines) and HeNe (543/633 nm) lasers.
In FLIP experiments, a single spot with a diameter of 1 μm within the nucleus was repeatedly bleached with two iterations of 100% power of a 488 nm laser line (100% output) using a Zeiss LSM510 META confocal microspcope with 63 × PlanFluor lens. The average diameter of a single z-axis plane varied between 2 and 2.5 μm. Two images were collected after each bleach pulse, with 30 s between bleach pulses. After collecting 30 images, two separate measurements on the mitochondria were taken to analyze the fluorescence loss. Unbleached control cells were monitored for photobleaching because of image acquisition. The rate of loss in fluorescence on the mitochondria was calculated from fluorescence intensity measurements using the Zeiss LSM software (Zeiss). The fluorescence intensities were normalized by setting the initial fluorescence to 100% signal. Plots are shown as normalized fluorescence over time.
Apoptosis activity assays
For caspase-3/7 measurements, HCT116 Bax/Bak DKO cells were seeded in 96-well plates and transfected with plasmids containing different Bcl-xL variants in combination with GFP-Bax wt. HCT116 Bax/Bak DKO cells transfected with a control vector or HCT116 wt cells served as positive and negative controls. Then, cells were treated with 1 μM STS or left untreated. After 10 h incubation, Apo-ONE caspase-3/7 Reagent (Promega, Mannheim, Germany) was applied according to the manufacturer's protocol. The samples were incubated for 1 h at 37 °C and then fluorescence signal was measured 30 times at 2 min intervals with an excitation wavelength of 490 nm and an emission wavelength of 520 nm in a plate reader. The increasing fluorescence correlated linearly with the amount of caspase-3 in each sample.
Whole cell lysis and subcellular fractionation
For whole cell lysates, cells were harvested after indicated time points, re-suspended in SDS sample buffer and boiled for 10 min at 95 °C. To obtain mitochondrial and cytosolic fractions, cells were harvested and centrifuged at 1200 × g for 5 min at 4 °C. The cell pellet was re-suspended in SEM buffer (10 mM HEPES, 250 mM sucrose, pH 7.2) supplemented with protease inhibitors and homogenized. Then, samples were centrifuged at 500 × g for 3 min at 4 °C. The supernatant was transferred to a new tube and subsequently subjected to a centrifugation step at 13 000 × g for 30 min at 4 °C. The supernatant of this step, the cytosolic fraction, was ultracentrifuged at 100 000 × g for 1 h at 4 °C, followed by an ultrafiltration step. Finally, samples were separated by SDS-PAGE and subjected to western blot analysis. Sedimented mitochondria were washed two times and then analyzed by SDS-PAGE and subsequent western blotting.
Clonogenic survival assay
For the evaluation of clonogenic survival, HCT 116 Bax/Bak DKO cells were seeded in six-well plates and transfected with plasmids containing different Bcl-xL variants in combination with GFP-Bax wt. Cells transfected only with GFP-Bax or with a control vector (pcDNA 3.1) served as controls. Then, cells were treated with 1 μM STS for 24 h. After 14 days, surviving colonies were fixed and stained with 1% methylene blue.
Acknowledgments
We thank S Liebscher for superb technical assistance. This work is supported by the Emmy Noether program of the German Research Council (Deutsche Forschungsgemeinschaft, DFG) and the NINDS intramural programs.
Glossary
- OMM
outer mitochondrial membrane
- cyt c
cytochrome c
- FLIP
fluorescence loss in photobleaching
- STS
staurosporine
- Mfn2
mitofusin 2
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by C Borner
Supplementary Material
References
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Martinou J-C, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ, et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens TW, Valentijn AJ, Upton JP, Keeble J, Zhang L, Lindsay J, et al. Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK. Cell Death Differ. 2009;16:1551–1562. doi: 10.1038/cdd.2009.102. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong S-Y, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, Goebbels S, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, et al. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S-Y, Gaume B, Lee YJ, Hsu YT, Ryu SW, Yoon SH, et al. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 2004;23:2146–2155. doi: 10.1038/sj.emboj.7600225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, et al. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, et al. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucie EL, Annis MG, Sedivy J, Filmus J, Leber B, Andrews DW, et al. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol Cell Biol. 2001;21:4725–4736. doi: 10.1128/MCB.21.14.4725-4736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ. Predominant requirement of Bax for apoptosis in HCT116 cells is determined by Mcl-1's inhibitory effect on Bak. Oncogene. 2011;31:3177–3189. doi: 10.1038/onc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.