Abstract
The Dlx and Msx homeodomain transcription factors play important roles in the control of limb development. The combined disruption of Msx1 and Msx2, as well as that of Dlx5 and Dlx6, lead to limb patterning defects with anomalies in digit number and shape. Msx1;Msx2 double mutants are characterized by the loss of derivatives of the anterior limb mesoderm which is not observed in either of the simple mutants. Dlx5;Dlx6 double mutants exhibit hindlimb ectrodactyly. While the morphogenetic action of Msx genes seems to involve the BMP molecules, the mode of action of Dlx genes still remains elusive. Here, examining the limb phenotypes of combined Dlx and Msx mutants we reveal a new Dlx-Msx regulatory loop directly involving BMPs. In Msx1;Dlx5;Dlx6 triple mutant mice (TKO), beside the expected ectrodactyly, we also observe the hallmark morphological anomalies of Msx1;Msx2 double mutants suggesting an epistatic role of Dlx5 and Dlx6 over Msx2. In Msx2;Dlx5;Dlx6 TKO mice we only observe an aggravation of the ectrodactyly defect without changes in the number of the individual components of the limb. Using a combination of qPCR, ChIP and bioinformatic analyses, we identify two Dlx/Msx regulatory pathways: 1) in the anterior limb mesoderm a non-cell autonomous Msx-Dlx regulatory loop involves BMP molecules through the AER and 2) in AER cells and, at later stages, in the limb mesoderm the regulation of Msx2 by Dlx5 and Dlx6 occurs also cell autonomously. These data bring new elements to decipher the complex AER-mesoderm dialogue that takes place during limb development and provide clues to understanding the etiology of congenital limb malformations.
Introduction
The developing vertebrate limb is widely adopted as a model to study cell-cell signaling, pattern formation and morphogenesis, and has provided a wealth of knowledge of the function and regulation of specific transcription factors and signaling molecules [1], [2], [3]. The phenotype of a large set of mutant mice with limb defects led to the identification of genes and regulatory pathways essential for normal limb development. The spatio-temporal organization of the complex network of signaling and transcriptional regulations has been elucidated only in part. In brief, genes and regulatory modules can be related to the activation/maintenance/regression of three signaling systems: a) Sonic hedgehog (SHH) and the Zone of Polarizing Activity (ZPA), for the control of digit patterning along the antero-posterior axis [4], [5], [6], b) Fibroblast Growth Factors (FGFs) and the Apical Ectodermal Ridge (AER), for the control of proximo-distal growth and for ZPA maintenance [7], [8], [9], [10], [11], and c) Lmx1B, in the mesoderm, and Wnt7a and En-1, in the ectoderm, for dorso-ventral specification [12], [13], [14], [15], [16]. The three signaling systems are organized in precise time- and space-restricted manners, and are integrated in self-regulatory modules that assure the acquisition or the correct digit complements, limb morphogenesis and overall growth [4], [17], [18], [19]. The signaling molecules of the Bone Morphogenetic Protein (BMP) class have been proposed to link these three systems together [20], [21], [22], [23], [24], [25], thus they are regarded as key players in the coordination of limb patterning, morphogenesis and growth along the three axes.
Proximo-distal limb development and digit extension is directly controlled by the signaling activity of the AER via expression of morphogens of the FGF family [7], [8], [10], [26] and their regulation by other signaling molecules such as SHH and BMP antagonists [20], [21], [27], [28]. In this scenario, the Dlx and Msx homeobox transcription factors, expressed in the AER and the mesoderm of the limb buds, play an important morphogenetic role, although their functions and regulations are yet to be defined at the molecular level. Dlx5;Dlx6 double knock-out (DKO) mice are characterized by loss of the central digit(s) and/or fusion with the lateral ones [29], [30]; these mice constitute a model of the human congenital defect ectrodactyly or Split Hand Foot Malformation type I, a condition linked to genomic alterations encompassing the DLX5;DLX6 region, and for which point mutations in the DNA-binding domain of DLX5 have recently been found [31]. The double inactivation of Msx1 and Msx2 genes results in moderate-penetrance polydactyly of the forelimbs (FL) and oligodactyly of the hindlimbs (HL) (loss of the anterior digits and part of the tibia in the zeugopod) [32], [33], [34].
Studies on the cellular and molecular functions of Dlx and Msx proteins during limb development have met with difficulties owing to several reasons. First, none of the single knockout for Dlx5, Dlx6, Msx1 or Msx2 shows evident limb defects [29], [32], [35], [36], [37], [38], [39], [40], [41], suggesting some degree of functional redundancy. Second, members of the Dlx and Msx families are expressed both in the AER and in restricted regions of the limb mesoderm [42], [43]. Third, in vitro the Dlx and Msx proteins compete for the same DNA binding sites, form heterodimers via their homeodomain and reciprocally inhibit their transcriptional activities [44], due to the high degree of homology of their homeodomains [37], [43], [45], [46], [47]. However, current literature suggest that Dlx and Msx proteins have distinct functions: Msx1 and Msx2 are known to control cell proliferation and differentiation in a variety of cell types [48], [49], [50], [51], while Dlx genes are implicated in the differentiation of specific cell lineages, such as forebrain interneurons [52], [53], [54], olfactory receptor neurons [55], osteoblasts [35], [56], and the AER and ectoderm [29], [30], [57], [58]. Notably, Dlx and Msx genes have been shown to cooperate only in specific cases [59], [60], [61], but not at all sites where they are co-expressed. However, Dlx5 has been shown to regulate Msx2 transcription in the AER, via homeodomain binding elements present in the gene’s promoter [62], [63].
We investigate possible interactions between Dlx5;Dlx6 and Msx1 or Msx2 in limb development, by generating double and triple Dlx;Msx compound mutant mice and analyzing their limb phenotypes. The limb phenotype of Msx1;Dlx5;Dlx6 triple knock-out (TKO) mice shows features of the Msx1;Msx2 DKO phenotype [32], leading to the conclusion that Dlx5;Dlx6 control the expression of Msx2, but not of Msx1. In contrast, the limb phenotype of Msx2;Dlx5;Dlx6 TKO mice is consistent with a severe aggravation of the ectrodactyly defect. We also re-examine the spatio-temporal expression of Dlx and Msx genes in FLs and HLs and observe that in the AER these genes are co-expressed whereas in the anterior mesenchyme Msx expression precedes that of Dlx, ruling out a direct regulation of Msx by Dlx in this territory. Combining these findings with qPCR expression analyses on the limb buds of different compound embryos, and with ChIP data, we propose that two modes of regulation coexist during limb development: 1) a direct transcriptional regulation of Msx2 by Dlx proteins in the AER and, later on, in the limb mesoderm, and 2) a Bmp2- and Bmp4-mediated non-cell autonomous regulatory loop between the AER and the anterior limb mesoderm.
Materials and Methods
Mouse Strains and Breeding
All animal procedures were reviewed and approved by the Ethical Committee of the University of Torino and University of Genova, the Italian Ministry of Health and the French Ministère de l’Enseignement supérieur et de la Recherche. No surgery or other manipulation on adult animals was used in this study. All efforts were made to minimize suffering. Generation and genotyping of the Dlx5lacZ/+ (hereafter named Dlx5 +/−); Dlx5;Dlx6neo/+ (hereafter named Dlx5;Dlx6 +/−); Msx1lacZ/+ and Msx2lacZ/+ (hereafter named, respectively, Msx1 +/− and Msx2 +/−) have been previously reported [29], [32], [35], [39]. These mutations were maintained on a C57B6;DBA F1 mixed genetic background, throughout. TKO embryos and newborn were obtained by crossbreeding either the Msx1+/− or the Msx2+/− single heterozygous parents with the Dlx5;Dlx6+/− double heterozygous ones, and then crossing the triple heterozygotes. Following mating, the day of the vaginal plug was considered as embryonic age 0.5 (E0.5).
Skeletal Preparation and β-galactosidase Detection
Cartilage staining (with Alcian Blue) of E14.5 embryos as well as bone and cartilage staining (with Alcian Blue and Alizarine Red) of E18.5 embryos were carried out as previously described [35]. For lacZ expression analysis E10.5 embryos were fixed for 15–30 min in 2% paraformaldehyde in PBS, while E14.5 embryos were fixed for 15–30 min in 4% PFA. X-gal staining was performed as described [35]. For detection of β-gal on limb sections, E10.5 and E11.5 FLs and HLs were fixed with 4% paraformadehyde for 8–12 hrs at 4°C, washed in PBS, cryoprotected with 30% sucrose, frozen at −70°C, sectioned (thickness 11 µm) and stained as described [35].
Whole-mount RNA in situ Hybridization
For RNA:RNA whole-mount in situ hybridization (WMISH), embryos at the desired age were dissected in cold RNAse-free PBS, fixed in cold 4% PFA for 12–16 hrs, rinsed with PBS, permeabilized by treatment with proteinase-K, prehybridized and hybridized as previously published [60]. Digoxygenin (DIG)-UTP (Roche)-labeled antisense RNA probes were used, synthesized by in vitro transcription with conventional methods. WMISH was carried out following described procedures [64], the signal was detected using an alkaline phosphatase-conjugated anti-DIG antibody and developed with the chromogenic mix NBT-BCIP (Roche). With each probe, at least two normal and two mutant specimens were examined. The Dlx5 probe comprised 780 bp and was linearized with EcoRI and transcribed with T7 RNA polymerase [57]. The Dlx6 probe is a 350 bp fragment spanning exons 3–4 [53]. The Msx1 probe was a 550 bp 3′ spanning the homeodomain, containing exon 1; the Msx2 probe corresponded to 378 bp in the first exon of Msx2 cDNA, the Fgf8 probe corresponded to most of the mouse coding sequence, the Bmp4 probe (a kind gift from B. Hogan) contains the 3′ UTR and most of the coding sequence from a mouse cDNA [65], the probe for Gremlin corresponded to the entire murine coding sequence (a kind gift from R. Zeller). After hybridization, the signal was revealed with the NBT-BCIP chromogenic reaction.
RNA Quantification by Real-time PCR
Embryonic FLs and HLs at the age E11 were dissected in cold RNase-free PBS, the anterior and posterior halves were separated and pooled in RNA-later (Ambion). Pools of two half limbs from the same embryos were used to extract total RNA, using the Tissue Lyser II reagent followed by elution through RNA micro-kit plus (Qiagen). cDNA synthesis was done using standard conditions, 3 ng of each cDNA sample were used to carry out qPCRs on a CFX96 equipment (Biorad) using the SybrSafe system (Invitrogen). Samples were analyzed in technical triplicates, and for each genotypes (except for the Msx1+/−;Dlx5−/−;Dlx6 −/−) biological triplicates could be analyzed. Primer sequences were designed with the Primer Express online tool. RNA quality, primer efficiency and correct size were tested by RT-PCR and agarose gel electrophoresis. Standard curve were performed using WT cDNA with four calibration points: 1∶10; 1∶40; 1∶160; 1∶640. Specificity and absence of primer dimers was controlled by denaturation curves. Rps9 mRNA abundance was used for normalization (primer sequences provided in Table S1).
Genome-wide Identification of Dlx Binding Sites and Target Genes
We used the Position-Weight matrix (PWM) provided by JASPAR under accession PH0024.1. The score of a site was computed with standard log-likelihood ratios, using as null model the nucleotide frequencies computed over the whole intergenic fraction of the mouse genome. We considered for further analysis putative sites scoring 50% of the maximum possible score or better. We selected among the sites identified above the ones that are conserved in at least two of 8 vertebrate species (genome sequence version in parenthesis): mouse (mm9), human (hg19), cow (bosTau4), opossum (monDom5), platypus (ornAna1), chicken (galGal3), frog (xenTro2), zebrafish (danRer6) and lamprey (petMar1). A site is defined as conserved with species S if it lies in a region of the mouse genome which is aligned with a region of the S genome and the aligned sequence in/S/is a site according to the same definition used for mouse sites. All genomic sequences and pre-computed “Net” alignments were obtained from UCSC.
A ranked list of putative Dlx target genes was obtained from the sites determined above by associating each site conserved in at least one species to its closest Refseq mRNA, and then selecting the sites located either within 10 kb upstream of the TSS, or within the non-coding portion of the first exon, or in the first intron. We then associated to each putative target a score equal to the sum of the conservation scores (number of species) of its associated sites.
Chromatin Immunoprecipitation
A DLX5-myc expression vectors (OriGene, USA) containing the full-length human DLX5 cDNA with an in-frame insertion of the myc-TAG at the C-terminus, was used as described [66]. The Q178P point mutation [31] was inserted in the DLX5-myc expression vector indicated above, by site directed mutagenesis and sequence verified (Bio-Fab Research, Rome).
The U2Os human osteosarcoma cells were used; these cells express low or undetectable levels of DLX5 mRNA endogenously, but have been shown to respond to Dlx expression with activation of the p63 promoter [57]. Eight µg of the DLX5-myc expression vectors were used for transfections, which yielded an efficiency of 35% (number of myc-positive cells over total counted nuclei). Chromatin was crosslinked, sonicated, immunoprecipitated with either the anti-myc TAG (A-14 sc-789, SantaCruz, USA) or the anti-acetyl-Histone H4 (06-866, ChIP Grade, Upstate Biotechnology USA,) antibodies and de-crosslinked according to instructions (EZ Magna ChipG, Millipore). Fragments of the human BMP2 and BMP4 loci spanning the identified conserved regions were PCR-amplified and analysed by gel electrophoresis (sequences provided in Table S2). Total chromatin was used as positive control (input), chromatin from cells transfected with an empty vector was used as negative control.
Results
Msx and Dlx Coexpression Analyses, in silico
Previous evidences indicate that Dlx5 binds to homeodomain-responsive elements in a proximal region of the Msx2 promoter, and thereby regulates its transcription [62], [63]. To further support this possibility, we have used a human-mouse co-expression network, generated using published profiling datasets [67], [68] and found that DLX5 and MSX genes are connected, i.e. each ranks in the first 1% of the co-expression lists of the other (p<0.01, data not shown).
Next, we screened conserved regions of the vertebrate genome for the presence of consensus Dlx DNA-binding sites, as defined by the Dlx5 PWM [69] present in the Jaspar database (accession N° PH0024.1) [70] and reported in Table S3. As the PWM for Dlx5 reported in Jaspar is not highly informative, it is not surprising that a total 565,995 putative binding sites were initially identified in the mouse genome. However, by introducing evolutionary conservation with at least two (out of 8) species examined as a further criteria, this number is reduced to 11,262 sites, and with conservation in three species is further reduced to 4085 (Table S3, complete lists available upon request). The full annotation on the UCSC genome browser is available upon request. As positive controls, the well defined Dlx sites present in the Dlx5;Dlx6 intergenic region [71] and in the Msx2 promoter [62], [63] were correctly predicted (Fig. S1). The evolutionary conservation of the sites suggests the presence of positive selection pressure to maintain the sites, confirming their functional relevance.
We then generated a ranked list of putative Dlx targets, based on the position of predicted conserved Dlx sites in the genome, as indicated in the Methods sections. The ranked list contains 3,051 Refseq mRNAs associated to 2,412 unique Entrez gene IDs (available upon request). The Msx1 and Msx2 genes were found in the top 10% of the list of putative Dlx targets, strengthening the possibility that Dlx proteins might directly regulate Msx expression.
Expression of Msx and Dlx Genes during Limb Development
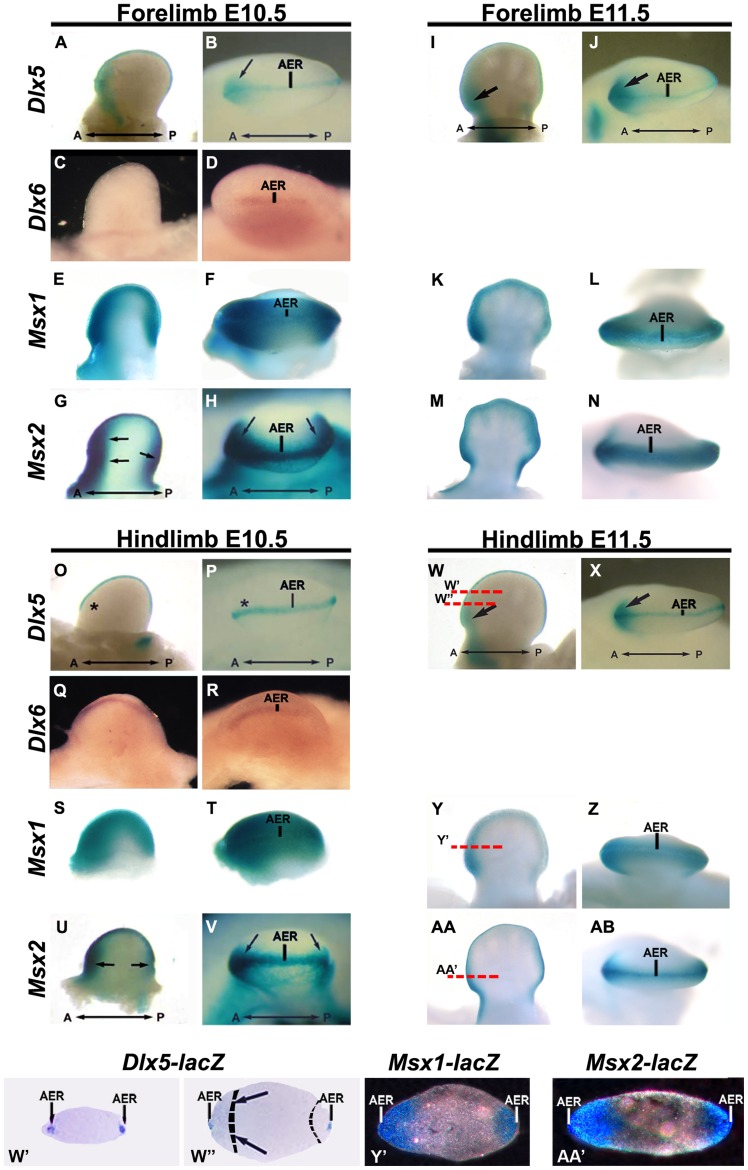
We examined expression of Dlx5, Dlx6, Msx1 and Msx2 in the FL and HL of normal embryos, at E10.5 and E11.5, by X-gal staining of heterozygous embryos carrying an allele with inserted lacZ reporter (Dlx5, Msx1, Msx2), and by WMISH (Dlx6) (Fig. 1). WMISH for Dlx5, Msx1 and Msx2 have been previously reported with comparable results. In the AER, all four genes are co-expressed starting at E9.5/E10. On the contrary, in the limb mesoderm they are expressed with a different time-of-onset. At E10.5 Msx1 and Msx2 are mainly expressed in two mesoderm territories (anterior and posterior) of both the FL and the HL (Fig. 1E–H, S–V), whereas at the same stage the Dlx5 transcript is detected only in the anterior mesoderm of the FL, and not that of the HL (compare Fig. 1A–B with O–P). The Dlx6 transcript is not detected in the mesoderm at this stage (Fig. 1C–D, Q–R). In the HLs, mesodermal expression of Dlx5 starts around E11.5 and is confined to the anterior margin (Fig. 1W–X). At this stage, Msx1 and Msx2 are expressed in the AER and in a larger region underneath the AER (Fig. 1K–N, Y-AB). Histological sections of X-gal stained limb buds from Dlx5 +/−, Msx1 +/− and Msx2 +/− embryos (Fig. 1W’, W’’, Y’ and AA’) reveal that Msx1 and Dlx5 are truly co-expressed in the AER (strong signal in W’ and W’’) and in the anterior HL mesoderm (a weak signal is present in W’’, indicated with black arrows).
Figure 1. Spatio-temporal expression of Dlx-Msx in developing limbs.
A-N, forelimbs. O-AB, hindlimbs, at E10.5 (A-H, O-V) and E11.5 (I-N, W-AB). Whole-mount X-gal staining on FLs and HLs from Dlx5+/−, Msx1+/− and Msx2+/− (lacZ+) heterozygous embryos are shown. Expression of Dlx6 was detected by WMISH on embryonic limbs at the same ages and shown. At E10.5 Msx2 and Msx1 are expressed in the AER and the anterior and posterior mesoderm of HLs and FLs. Dlx5 and Dlx6, at E10.5, are expressed in the AER of HLs and FLs and in the anterior limb mesoderm only of the FLs, but not of the HLs. At later stages (E11.5), Dlx5 and Dlx6 are then expressed in the anterior mesoderm of HLs. Black arrows indicate mesodermal expression. The AER is also indicated. Black asterisks indicate absence of expression. W’,W’’ histologic transversal sections of E11.5 HLs from Dlx5+/− embryos, stained with Xgal. Y’,AA’ histologic transversal sections of HLs from Msx1+/− (Y’) and Msx2+/− (AA’) embryos, to compare AER and mesodermal expression between these genes. Section planes and position are reported with red lines (in W, Y and AA). The extent of the Msx1-positive anterior and posterior mesoderm regions, based on the micrographs in AA’ and AC’, are indicated with dashed lines. A strong Dlx5-lacZ signal is detected in the AER (W’ and W’’), a weak Dlx5-lacZ signal, overlapping with the Msx1-lacZ and the Msx2-lacZ signal, is detected in the anterior mesoderm (W’’, indicated by black arrows).
In summary, Msx expression precedes that of Dlx in the HL anterior mesoderm, consequently in this location Dlx genes are unlikely to regulate Msx gene transcription cell-autonomously and Dlx and Msx proteins are unlikely to interact in the anterior limb mesoderm, at early stages.
Msx2 Expression is Downregulated in Dlx5;Dlx6 DKO Limbs
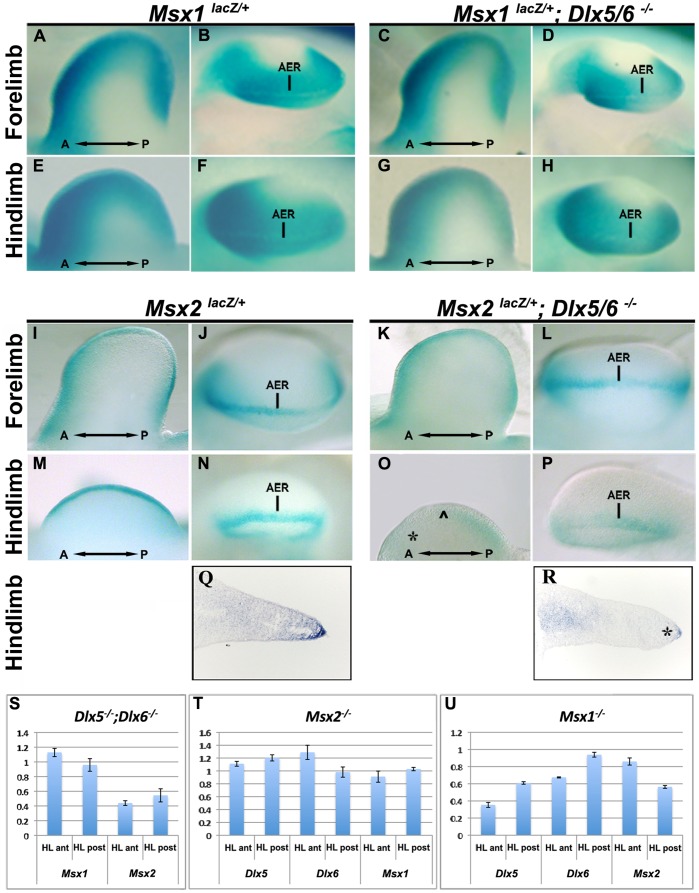
To investigate interactions between the Dlx and the Msx genes during limb development, we used animals DKO for Dlx5;Dlx6 and analyzed the expression of the Msx-lacZ reporter in this genetic background. To do this, we compared the expression patterns of the lacZ reporter between Msx1+/− and Msx1+/−;Dlx5−/−;Dlx6−/− mice (Fig. 2A–H), and between Msx2+/− and Msx2+/−;Dlx5−/−;Dlx6−/− mice (Fig. 2I–P). We observed a clear reduction of Msx2 expression in the anterior mesoderm and AER exclusively in the HLs, whereas neither expression of Msx1 in the HLs nor expression of Msx1 and Msx2 in the FLs were significantly changed (Fig. 2A–P). To further document the reduction of Msx2 expression in the AER of Dlx5;Dlx6 DKO limbs, we carried out WMISH for Msx2 on cryostatic sections of Dlx5;Dlx6 DKO HLs, at E11. Msx2 expression was strongly reduced or absent in the AER and the underlying mesoderm of a central wedge of the mutant limbs, as compared to the same region of normal limbs (Fig. 2Q,R).
Figure 2. Reduction of Msx2 expression in Dlx5/Dlx6 DKO HLs.
A-P. Whole-mount X-gal staining to detect Msx1 and Msx2 expression in Dlx5;Dlx6 DKO. In the FL (A-D,I-L) no changes of expression is observed whereas in the HL (E-H, M-P), Msx2 expression is reduced in the AER and in the anterior limb mesoderm of the Dlx5;Dlx6 DKO HLs. Q,R. Sections of WT (Q) and Dlx5;Dlx6 DKO mutant HLs (R) hybridized in situ to detect Msx2, showing a drastically reduced Msx2 signal in the AER and in the underlying mesoderm, but not in a proximal mesoderm territory. S-U. Quantification of the expression of Dlx5, Dlx6, Msx1 and Msx2 mRNAs by qRT-PCR in HLs from Dlx5;Dlx6 DKO (S), Msx2−/− (T) and Msx1−/− (U), relative to WT. The results show a reduction of 45% of Msx2 expression in the Dlx5;Dlx6 DKO HLs compared to WT, but not of Msx1. Dlx5 and Dlx6 expression is downregulated in Msx1 KO HLs but not in Msx2 KO HLs. Expression of the knocked-out genes was also tested, as control, and always found to be reduced to undetectable levels (not shown).
Since WMISH is not a quantitative method, we used quantitative Real Time PCR (qRT-PCR) to quantify the reduction of Msx2 mRNA on samples extracted from the HLs of normal and Dlx5;Dlx6 DKO embryos, at E11.5. Limb buds were divided in two halves on the proximo-distal axis, to determine gene expression in the anterior and posterior mesoderm separately. In such samples, the AER cells contributed minimally, while most of the RNA derives from the mesenchyme. Msx2 mRNA abundance was reduced by 60% in the anterior half, and by 50% in the posterior half of Dlx5;Dlx6 DKO HLs from the same embryo, as compared to WT. In the same samples, expression of Msx1 was minimally or not changed (Fig. 2S). We then determined the abundance of Msx and Dlx mRNAs in the anterior half of the HLs of Msx1−/− and Msx2−/− (single homozygous) mutant embryos, as compared to WT. In the Msx2−/− mutant the expression level of Dlx5, Dlx6 and Msx1 was unchanged, whereas in the Msx1−/− mutant the expression levels of Dlx5, Dlx6 and Msx2 were reduced by 65%, 40% and 15%, respectively. Moreover, in the posterior half of the HLs from the Msx1−/− mutant we observed a reduction of 40% in the abundance of Dlx5 and Msx2 mRNA, whereas Dlx6 did not change (Fig. 2T,U).
Limb Phenotype of Msx2;Dlx5;Dlx6 Triple Mutants
To reveal possible functional interactions between the Dlx and the Msx gene products during limb development, we generated TKO mice with genotype Msx1−/−;Dlx5−/−;Dlx6−/− or Msx2−/−;Dlx5−/−;Dlx6−/−. TKO newborns were obtained at a frequency lower than expected, and all died shortly after birth, due to severe craniofacial malformations and consequent breathing impairment. Quadruple knock-out embryos were never obtained in spite of several attempts.
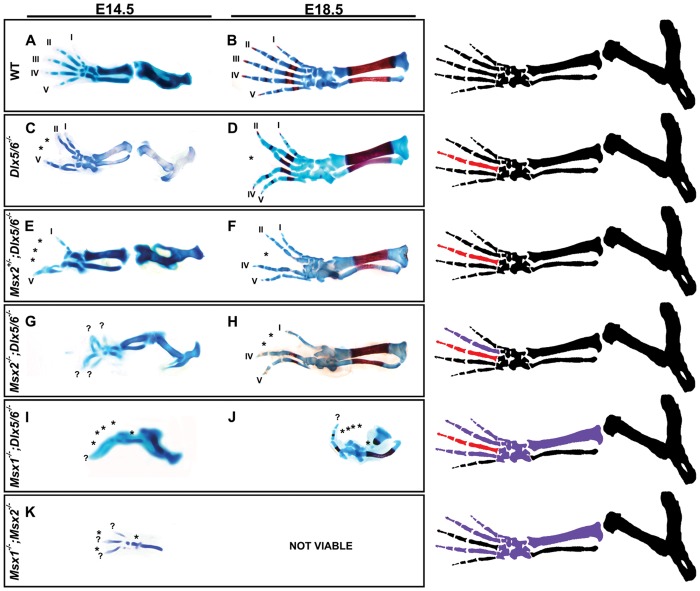
We examined the limb skeleton in the TKO animals at two ages: E14.5 (with Alcian-Blue) and E18.5/birth (with Alcian-Blue and Alizarine-Red, which stain, respectively, cartilages and mineralized bones). In Msx2;Dlx5;Dlx6 TKO animals, the HLs were severely affected (Fig. 3G,H) whereas no evident alteration was observed in the FLs (data not shown). The central digit was always missing, rarely (<10%) the two central digits were missing, while the remaining digits extended pairwise towards the opposite (anterior-posterior) sides. Importantly, no loss of anterior digits or of zeugopod elements was ever observed (Fig. 3G,H). The limb phenotype observed in the Msx2;Dlx5;Dlx6 TKO mutant animals is consistent with a significant aggravation of the ectrodactyly phenotype seen in Dlx5;Dlx6 DKO [29], [30] (compare Fig. 3G with 3C, and 3H with 3D), without appearance of other recognizable phenotypes. Remarkably a very similar phenotype, although less severe, was observed in animals with genotype Msx2+/−;Dlx5−/−;Dlx6−/−, i.e. in the presence of a single wild-type Msx2 allele (Fig. 3E,F).
Figure 3. Skeletal preparations of the HLs of single and combined Msx;Dlx mutant animals.
Chondroskeletal preparation of the HLs of E14.5 embryos (micrographs on the left) and full skeletal preparation on newborn animals (micrographs on the right), representing single and combined Dlx;Msx mutant genotypes (indicated on the left). The HLs of Msx2 +/−;Dlx5;Dlx6 DKO animals (E,F) display an aggravated ectrodactyly phenotype compared to Dlx5;Dlx6 DKO ones (C,D), with fusion of the external digit (1with 2, 4 with 5) and hypoplasia of the central digit. Msx2;Dlx5;Dlx6 TKO HLs (G,H) display a further aggravated ectrodactyly phenotype, with the external digits fused and extended towards the opposite (anterior-posterior) sides, and a complete absence of the central digit. The limbs of Msx1+/−;Dlx5;Dlx6 DKO mice (not shown) show ectrodactyly similar to that observed in Dlx5;Dlx6 DKO mice, whereas Msx1;Dlx5;Dlx6 TKO mice (I,J) show ectrodactyly and loss of skeletal elements deriving from the anterior mesoderm of the autopod and zeugopod, a phenotype seen in Msx1;Msx2 DKO mutant embryos (K). The stylopod shows no evident defects. The anterior-posterior orientation is shown. The numbers 1–5 indicate the digits (1 is the toe). Asterisks indicate hypoplasia or absence of skeletal structures. The drawings on the left schematically illustrate the Dlx-related (red elements) and the Msx-related (purple elements) skeletal defects, corresponding to the genotypes examined.
Limb Phenotype of Msx1;Dlx5;Dlx6 Triple Mutants
In Msx1;Dlx5;Dlx6 TKO mice the FL were usually normal, although in one case (1/6) anterior polydactyly was observed (data not shown). Of note, polydactyly has been reported for the FLs in Msx1;Msx2 DKO mice with a moderate penetrance [32]. On the contrary, the TKO HLs displayed a severe phenotype consisting in the loss of the tibia and of 3–4 preaxial digits (Fig. 3I,J). Since the HLs of Msx1;Msx2 DKO usually exhibit loss of 1–2 anterior digits and of the tibia [32], we interpreted the TKO phenotype as a combination of ectrodactyly, caused by the loss of Dlx5;Dlx6 [29], [30] (Fig 3C,D), and the preaxial adactyly as observed upon the loss of Msx1;Msx2 [32] (Fig. 3K).
The appearance of features of the Msx1;Msx2 DKO phenotype in the Msx1;Dlx5;Dlx6 TKO animals, which retain two functional Msx2 alleles, argues in favor of a severe reduction of Msx2 expression in the TKO limbs, as compared to the Dlx5;Dlx6 DKO.
Indeed, removing a single Msx1 allele in the context of Dlx5;Dlx6 DKO background (i.e.Msx1+/−;Dlx5−/−;Dlx6−/− embryos) results in a 50% reduction of Msx2 mRNA in both the anterior and the posterior half of the embryonic HLs, as compared to WT. Moreover, expression of Msx2 in the anterior and posterior halves of Msx1 homozygus mutant HLs was reduced by 30% compared to Msx1 heterozygous HLs (data not shown). This indicates that reduced Msx2 expression may result from the combination of (1) loss of Dlx5 and Dlx6 (Fig. 2U, Fig. S2), upstream regulators of Msx2, and (2) the loss of Msx1, with the consequent decrease of Msx2 expression (Fig. 2V).
Expression of Bmp4 and Gremlin in Dlx5;Dlx6 Mutant Limbs
The phenotype of the Msx1;Dlx5;Dlx6 TKO HLs shares some similarities with the Msx1;Msx2 DKO phenotype [32]. However, defects in the anterior autopod and zeugopod cannot be simply explained by a direct Dlx5;Dlx6 control on Msx2 expression, since in the presumptive territory of the anterior zeugopod and autopod the expression of Msx1 and Msx2 precedes that of Dlx5 and Dlx6 (Fig. 1). Therefore we hypothesized that a non-cell autonomous regulation should take place, linking a defective AER with Msx2 misexpression in the anterior limb mesoderm. We focused on Bmps as candidate signaling molecules since they are expressed in the AER as well as the anterior and posterior mesoderm [21], and since BMP signaling is known to induce Msx expression in several embryonic territories [20], [72].
We carried out WMISH for Bmp4 on Dlx5;Dlx6 DKO embryos at E11 and found that expression is decreased specifically in the central sector of the AER of the mutant HLs (3 of 4 embryos) but not significantly in the anterior mesoderm (Fig. 4C,D). Bmp4 expression was not significantly changed in the FLs of the same embryos (Fig. 4A,B), as expected considering the lack of any morphological defects in the FLs. As a further control, Bmp4 expression was retained in the pharyngeal arches region (Fig. 4E,F). We also examined the expression of Gremlin in the limbs of E10.5 embryos. Gremlin is a BMP antagonist that participates in a regulatory loop between Shh in the posterior limb mesoderm and FGF4 in the posterior AER, which maintains the AER and ZPA and promotes digit patterning and limb outgrowth [17], [73], [74]. The anterior-posterior patterning and expression level of Gremlin did not significantly change in either the FLs or the HLs of Dlx5;Dlx6 DKO embryos (Fig. 4G–L). Thus, we concluded that the inactivation of Dlx5 and Dlx6 does not lead to significant alterations of limb antero-posterior patterning. Finally, the expression of Bmp7 was not significantly changed (data not shown).
Figure 4. Expression of Bmp4 in Dlx5;Dlx6 DKO limbs.
A-D. Detection of Bmp4 mRNA by WMISH on WT (left) and Dlx5;Dlx6 DKO mutant (right) limbs, at E11. FLs are on the top, HLs are on the bottom. E,F. In situ detection of Bmp4 mRNA in the pharyngeal arches region of WT (left) and Dlx5;Dlx6 DKO mutant (right), at E11, as a control for RNA preservation. G-L. Detection of Gremlin mRNA in FLs (G,H) and HLs (I-L) of WT (left) and Dlx5;Dlx6 DKO mutant embryos (right), at E10.5. While Bmp4 expression in the anterior mesoderm of the FLs (A,B) or the HLs (C,D) is unchanged (green arrowheads), expression in the central wedge of the AER of mutant embryos is diminished in the HLs, but not in the FLs (red arrows in D). Gremlin expression is unchanged both in the FLs (G,H) and in the HLs (I-L) of Dlx mutant embryos (red arrowheads). Genotypes and probes are reported on the top. The Anterior-Posterior (A-P) orientation is indicated. M,N. whole-mount photographs documenting the reduced size of mutant embryos and justifying the slightly reduced size of the mutant limbs, often observed. O. Quantification of Bmp2 and Bmp4 mRNAs by qRT-PCR in the anterior and posterior halves of HLs from embryos with the genotype indicated on the top of each graph, compared to WT.
Bmp2 and Bmp4 Expression Levels are Synergistically Reduced by Msx and Dlx Mutations
The WMISH results reported above suggest that Bmp4 expression is unchanged in the anterior mesoderm of the limbs; however this technique is poorly quantitative and cannot reveal minor changes in gene expression level. To further investigate the mechanism that might induce and/or sustain Msx2 expression in the anterior half of the HLs, we determined the relative abundance of Bmp2 and Bmp4 mRNAs by qRT-PCR in the anterior and posterior halves of the embryonic HLs (E11) with different genotypes. Expression of Bmp2 is mainly decreased in the anterior (about 50%), and minimally in the posterior half (20%) of the HLs from Dlx5;Dlx6 DKO embryos (Fig. 4O). Bmp2 expression is similarly reduced in Msx1−/− HLs. Bmp4 expression is minimally or not affected in the Msx1−/− mutants, while in the Dlx5;Dlx6 DKO Bmp4 expression is reduced of about 30% and 20%, respectively, in the anterior and in the posterior halves (Fig. 4O). Of note, Bmp4 expression is also reduced in Msx2−/− embryonic HLs, less severely in the anterior (30%) than the posterior half (50%) (data not shown). Strikingly we observed a strong synergy between Msx1 and Dlx5;Dlx6 in controlling BMP expression. When combined with the Dlx5;Dlx6 DKO mutation, the loss of a single Msx1 allele was sufficient to reduce Bmp2 and Bmp4 expression to levels lower than 10% of that of control samples, both in the anterior and in the posterior halves of the HLs. Considering that Msx2 is a known target of Bmp2 and Bmp4 in several tissues [72], [75], [76], this may explain how Msx2 expression is reduced in the Msx1;Dlx5;Dlx6 TKO, and how this mutant shows a limb phenotype with similarities to the Msx1;Msx2 DKO.
Finally, we determined the abundance of Fgf8 and Shh mRNAs in HL samples from the relevant genotypes (Fig. S3). The Abundance of Fgf8 mRNA was slightly reduced in the absence of Dlx5 and Dlx6 (DKO samples), and was further drastically reduced when one Msx1 allele was eliminated in the absence of Dlx5;Dlx6. Similarly, the abundance Shh mRNA (in the posterior half of the limb buds) was slightly reduced in the absence of Dlx5 and Dlx6, and was further reduced when one Msx1 allele was simultaneously eliminated. While reduced Fgf8 expression is not surprising [30], reduced Shh expression is novel and may contribute to the altered digit number and morphogenesis seen in the Msx1,Dlx5;Dlx6 TKO embryos.
Dlx5 Binds to Conserved Sequences in the Proximity of the Bmp2 and Bmp4 Loci
To search for possible direct regulations of the Bmp2 and Bmp4 loci by Dlx5, we first exploited the genome-wide predictions of conserved Dlx sites, described above. In the proximity of the Bmp2 locus we identified three Dlx consensus binding sequences, named B2-RE1, located 30 kb upstream of the transcription start site (TSS), B2-RE2 and B2-RE3, located respectively 3.5 kb and 18 kb downstream of the end of the Bmp2 transcript. All these Dlx binding elements are conserved, although not identical, in at least two vertebrate species, and fall within stretches of conserved genomic sequence (Fig. 5A,B). The B2–R3 element was recognized as conserved only between human and mouse, and contains 6 (of 16 bases) mismatches, in non-critical positions. In the proximity of the Bmp4 locus we identified two Dlx consensus sequences, named B4-RE1 and B4-RE2, located respectively 430 bp upstream of the TSS and within the second intron, conserved in at least two mammalian species (Fig. 5A,B). Also these predicted Dlx binding sites fall within stretches of conserved genomic sequence (sequences and genomic locations provided in Tables S4 and S5).
Figure 5. Binding of DLX5 on predicted conserved elements close to BMP2 and BMP4. A.
Location of predicted conserved Dlx binding sites in regions of the mammalian genome around the BMP2 (top) and BMP4 (bottom) loci. Sites are indicated with colour vertical bars, the chromosomal position and coordinates are shown. The mammalian genomic conservation is reported on the bottom. With the exception of B2-RE3, all elements fall within stretches of conserved sequences. B. Sequences and alignment of the predicted Dlx binding elements. The sequence corresponding to the PWM is shown in red. Dashed lines indicate the degenerated part of the binding sequence. C. Western blot analysis to demonstrate expression of DLX5-myc and DLX5-Q184P-myc proteins in U2Os cells. The molecular weight of the detected proteins is indicated on the left. D. ChIP analyses on the predicted Dlx binding sites in the human U2Os cells, transfected with the DLX5-myc and DLX5-Q178P-myc expression vector, or with the control empty vector. The input chromatin (positive control) is shown on the left, ChIP with or without anti-myc are shown in the mid panels. ChIP with and without anti-H4Ac on the same elements are shown on the right.
To demonstrate that the Dlx5 protein physically binds to these putative responsive elements, we transfected a human DLX5-myc-tag expression vector [66] and the same one modified to harbor the disease-causing point mutation Q178P [31] in the U2Os osteoblast cells (Fig. 5C). These cells were chosen since they are of human origin, they express low endogenous level of DLX5, have been shown to respond to Dlx5 expression by activation of the p63 and other promoters [57], are of osteoblastic origin (Dlx5 plays a role in late osteoblast differentiation [35]), and are easily transfected. The crosslinked chromatin was immunoprecipitated with an anti-myc antibody, or with anti-acetylated Histone 4 (H4Ac) and subjected to PCR amplification with primers flanking the predicted DLX sites in the human genome. With the exception of the B2-RE3 element (not shown), all the other elements showed an enrichment of PCR amplification products from chromatin of DLX5-myc transfected cells, as compared to the chromatin from cells transfected with the empty vector (Fig. 5D). The same elements immunoprecipitated with anti-H4Ac, suggesting that in these locations the chromatin is available for transcription regulation. Interestingly, the Q178P mutant DLX5 protein did not show any binding to these sequences, thus this disease-causing mutation is associated with loss of DNA-binding activity on the Bmp elements. As further controls, PCR amplification of two sequences containing no identifiable homeodomain-binding sequences (BMP2 exon 3 and BMP4 exon 5), as well as an irrelevant sequence (IL10) did not show any enrichment (not shown). These results reinforce the notion that DLX proteins physically interact with four (out of five) conserved elements close to the BMP2 and the BMP4 loci, and might exert a direct transcription regulatory activity, relevant for normal limb development.
Discussion
Dlx5, Dlx6, Msx1 and Msx2 genes encode homeodomain transcription factors involved in limb development. The targeted disruption of each of these genes, individually, does not lead to limb defects, however double Msx1/Msx2 [33], [34] and Dlx5/Dlx6 [29], [30] mutant mice present severe limb malformations indicating that these genes participate in the control of digit number and morphogenesis. The Dlx and Msx homeodomains show a high degree of sequence similarity and similar DNA binding sequences in vitro [43]. In spite of these similarities, there is no evidence suggesting that these homeoproteins cooperate or interact, except in two specific developmental processes: elevation and fusion of the palatal shelves [60], [61] and development of the frontal bone [59]. In this work, we shed light on direct and indirect interactions between these two classes of homeodomain genes during limb development.
Direct and Indirect Dlx-Msx Regulations during Limb Bud Development
Although Msx2;Dlx5;Dlx6 TKO mice show an aggravation of the ectrodactyly phenotype, as compared to Dlx5;Dlx6 DKO, they do not present any obvious additional defect. Furthermore, inactivation of either Dlx5;Dlx6 or Msx2 does not modify the level of Msx1 expression, suggesting that the Msx2;Dlx5;Dlx6 TKO phenotype represents the exclusive contribution of the absence of Msx2 in the Dlx5;Dlx6 mutant context. This leads us to conclude that either Msx2 has a rather minor function in limb development, or that its function is largely compensated by Msx1, in agreement with the limited defects observed in Msx1+/−;Msx2−/− compound mutant animals [32] (Y. Lallemand, unpublished). On the contrary, Msx1;Dlx5;Dlx6 TKO mice display a limb defect which can be interpreted as the sum of the limb anomalies found in the Msx1;Msx2 DKO and in the Dlx5;Dlx6 DKO. These results strongly suggest that the expression of Msx2 is suppressed in this genetic context, and that therefore Dlx5;Dlx6 are genetically upstream of Msx2.
We show that in Dlx5;Dlx6 DKO limbs Msx2 expression is diminished in the central sector of the AER. As starting at E10.5 Dlx and Msx genes are co-expressed in the AER, it is possible to hypothesize that in this territory Dlx proteins bind directly on the Msx2 promoter, as previously reported [62], [63]. On the contrary, in the anterior limb mesenchyme, the expression of Msx1 and Msx2 precedes that of Dlx5 and Dlx6. This precludes the possibility of a direct regulation of Msx genes by Dlx proteins. Nevertheless, our qRT-PCR analyses show that Msx2 expression is reduced in the anterior limb mesoderm of Dlx5;Dlx6 DKO limbs, implying the existence of a non cell-autonomous mode of regulation between the AER and the anterior limb mesoderm. It is possible, therefore, that a diffusible protein, expressed by AER cells in a Dlx-dependent fashion, is required to initiate and/or sustain Msx2 expression in the anterior limb mesoderm.
Bmps as Signaling Relays between Dlx and Msx
Msx genes are well documented downstream effectors of BMP signaling in several developing structures [72], [77], [78], [79], [80], [81], [82]. Enhanced BMP signaling in the limb in mice deficient for the BMP antagonist Gremlin, results in upregulation of both Msx1 and Msx2 expression [73]. Conversely, blocking BMP signaling in the limb ectoderm by ectopic expression of Noggin, another BMP antagonist, results in decreased Msx2 expression [23]. In the chick limb buds, expression of a constitutively-active BMP receptor, or misexpression of Msx1, in the dorsal ectoderm, induces the formation of ectopic AERs [20]. In addition, the combined inactivation of Msx1 and Msx2 leads to a phenotype that mimics, in some aspects, the loss of BMP signaling [32].
Noticeably, Msx genes are also upstream of Bmp4 in several developmental systems. During tooth germ development, mesodermal Msx1 is needed for efficient Bmp4 expression [83], [84], [85], [86]. Palatal development, which is impaired in Msx1 −/− mice, can be rescued by a Bmp4-expressing transgene [87]. In the limb itself, Msx genes are required in the mesoderm for maintenance of Bmp4 expression [34]. Thus, BMP signaling is both upstream and downstream of Msx genes, depending on the context and the developing structure. The possibility that Bmp2 and Bmp4 participate in a Dlx-Msx signaling loop between the limb bud AER and mesoderm is clearly in line with previously identified roles of these molecules.
Therefore, Bmps represented likely candidates to mediate a non cell-autonomous regulation between AER-expressed Dlx5;Dlx6 and mesodermal Msx2. Indeed, we find that Bmp2 and Bmp4 expression is significantly reduced in Dlx5;Dlx6 DKO hindlimbs, at E11 when no evident defect is yet visible. Reduction for Bmp2 and Bmp4 in the anterior part of the HL is too high (50 and 30%, respectively) to be accounted for by the ectoderm alone, and rather indicates a downregulation of these Bmps in the mesoderm, too. This could mean that Bmps produced in the ectoderm activate Bmps also in the mesoderm, either directly or via Msx1. Indeed, further inactivation of one Msx1 allele in the context of the loss of Dlx5;Dlx6 nearly abolishes Bmp2 and Bmp4 expression, necessarily implying both the ectoderm and the mesoderm (Fig. 4K). This downregulation would explain a reduction in Msx2 expression in the anterior mesenchyme in the Dlx5;Dlx6 mutant limbs, at the same embryonic ages. The direct action of Dlx over Bmp is further supported by our ChIP data which indicate that DLX5, but not its mutated variant Q178P [31], binds to conserved regions near the BMP2 and BMP4 loci. However formal evidence that DLX5 activates BMP2 and BMP4 transcription is still lacking, as no suitable AER-related cell line is available for such experiments. It would be of interest to further investigate whether MSX1 also binds to the same conserved sequences in the BMP2 and BMP4 promoters.
Such a non cell-autonomous mode of regulation is strikingly similar to that occurring during development of the palatal shelves and the tooth primordia, both of which involve diffusion of Bmp between adjacent epithelial/mesodermal cell layers [60], [87], and in the case of the tooth germ, an induction of Bmp4 expression in the mesoderm by ectodermal Bmp4 via expression of the Bmp target Msx1 [85], [86].
The loss of mesenchymal Bmp2, 4 and 7 expression, on the other side, has been shown to be required for osteogenic differentiation, in a dose-dependent fashion and with Bmp molecules acting in a partially redundant way [88]. In their work, the selective loss of Bmp2 and Bmp4 in the limb mesenchyme affects zeugopod development and skeletogenesis, and less severely the autopod. On a similar note, another work [22] shows that the gradual elimination of Bmp4 from the limb mesenchyme is required to rescue the Grem1−/− phenotype and a normal digit organization, implying a right amount of mesenchyme-derived Bmps is essential for AER function and for autopod morphogenesis. In the Msx1;Dlx5;Dlx6 TKO mutants we observe a quite severe autopod defect (loss of digits) accompanied, however, by less severe zeugopod defect. Thus, it appears that both AER- and mesenchyme-derived Bmps are involved in the Msx;Dlx defects. The possibility that misexpression of AER-Bmps alone directly cause the TKO defects is unlikely. Rather, in light of the well known self-regulatory system of signalling loops comprising FGFs, SHH and BMPs [22], and considering that we observe changes in the expression levels of Fgf8 and Shh upon loss of Msx1, Dlx5 and Dlx6 genes, most likely the overall nature of the limb defects in TKO mutant embryos is a quantitative misregulation of the “slow module” of this loop. Dlx and Msx genes can be regarded as new players in this complex regulation. Since Shh participates in the “slow module”, a more critical role for it could be envisioned, as suggested by resemblance of the phenotype of Msx1;Dlx5;Dlx6 TKO limbs with that exhibited by Shh KO embryos [89].
In conclusion, we propose a model that involves a complex epithelial-mesodermal dialogue between Dlx and Msx (Fig. 6), entailing two distinct modes of regulation in the limb buds: a direct, cell-autonomous, regulation intrinsic to AER cells, and an indirect regulation between the AER cells and the anterior limb mesoderm. We further provide data suggesting that Bmp2 and Bmp4 mediate a non cell-autonomous control of Dlx over Msx, establishing a dialogue between the AER and the anterior mesoderm of the developing limb.
Figure 6. A dynamic model for Dlx-Msx-Bmp functional interactions during HL development.
Schematic drawing to summarize our results and illustrate our model of functional interaction between Dlx5;Dlx6, Msx1, Msx2, Bmp2 and Bmp4. On the top, a scheme of the limb bud, the AER (in light blue color) and the mesoderm (in pink color) is reported. Below, the proposed dynamic model of gene regulations, shown for an Early (E9.5–E10, on the left) and a Late (E10–E10.5, on the right) phases of HL development, using the same color code as above. The anterior mesenchyme is framed with a dotted black box; the Ant-Post and Prox-Dist directions are shown. Bmp2 and Bmp4 are placed at the interface between the AER and the Ant Mes, to indicate that these are diffusible signaling molecules.
Genotypes, Morphotypes, Gene Dosage and Expression Levels
When comparing the limb phenotypes of the genetic series Dlx5;Dlx6 DKO vs. Msx2+/−;Dlx5−/−;Dlx6−/− vs. Msx2;Dlx5;Dlx6 TKO (Fig. 3C–H), we observe a clear increase in severity of the ectrodactyly defect. This interesting observation can be explained by introducing the notion that Msx2 expression depends on allelic dosage, and that a threshold level of Msx2 expression is critical to drive normal morphogenesis.
The importance of allelic dosage, hence quantitative gene expression, is increasingly being recognized, even when phenotypes are not evident (see [88], [90]). As a further example, we detect a reduction of Msx2 mRNA level in the Msx2+/− mice, in which no evident phenotype can be seen. Our explanation, in this case, is that reduced Msx2 expression alone is not sufficient to cause limb malformations due to the presence of two functional Msx1 alleles. Loss of one Msx2 allele in the context of Dlx5;Dlx6 DKO, instead, aggravates the phenotype. We explain this by proposing that Msx2 expression is severely reduced, approaching that of the null condition, due to the combination of a) the genetic inactivation of one allele, and b) the lack of Dlx5;Dlx6 genes.
Likewise, in Msx1;Dlx5;Dlx6 TKO animals, Msx2 expression is further reduced. A residual level is nonetheless observed; however, this is not sufficient to compensate for the loss of Msx1. In conclusion, allelic dosage and quantitative gene expression are crucial factors to be considered in the interpretation of a series of phenotypes, especially when related genes are involved.
Conclusions
In human, the DLX5 and DLX6 genes cause the SHFM-type-1 congenital malformation when lost or mutated, while the MSX1 and MSX2 genes cause cleft palate and tooth agenesis. By crossbreeding mutant mouse strains, we show that the Dlx5;Dlx6 and Msx1;Msx2 genes cooperate for normal limb development and morphogenesis. At least two modes of regulation have emerged, one in which Dlx5;Dlx6 control expression of Msx2 cell-autonomously, the other in which the AER and the anterior mesenchyme interact non cell-autonomously, entailing Bmps as signaling molecules. We further show that the BMP2 and BMP4 loci comprise Dlx5-binding elements, occupied by Dlx5. Thus, the highly related homeodomain genes Dlx and Msx are two key players of a novel set of molecular and histological interactions during limb development.
Supporting Information
Top. Location of predicted conserved Dlx binding sites in the Dlx5-Dlx6 intergenic genomic region. Sites are indicated with colour vertical bars (asterisk) and annotated with the species conservation. The chromosomal position and coordinates are also reported. The mammalian genomic conservation is reported on the bottom. The known i56i element is correctly predicted by the PWM bioinformatic approach we have adopted. Bottom. Same as above, relative to the Msx2 proximal promoter. Two known conserved Dlx binding sites are correctly predicted.
(PDF)
Quantification of the Msx2 mRNAs by qRT-PCR in the anterior and posterior halves of HLs from Msx1+/−;Dlx5−/−;Dlx6−/− embryos, relative to the corresponding WT samples (set = 1).
(PDF)
Quantification of the Fgf8 and Shh mRNAs by qRT-PCR in the HLs from Msx1−/− (top left), Dlx5−/−;Dlx6−/− (top right), Msx1+/−;Dlx5+/−;Dlx6+/− (bottom left) and Msx1+/−;Dlx5−/−;Dlx6−/− (bottom right) embryos, relative to the corresponding WT samples (set = 1).
(PDF)
Sequences of the oligonucleotides used for real-time qPCR on mouse embryonic tissues.
(PDF)
Sequences of the oligonucleotides used for ChIP analysis on the predicted Dlx elements near the human BMP2 and BMP4 loci.
(PDF)
The Dlx5 Position-Weight matrix and results of the prediction of Dlx5 binding sites based on genomic conservation.
(PDF)
Sequences of the mouse and human conserved genomic regions containing predicted Dlx5 binding sites near the BMP2 locus.
(PDF)
Sequences of the mouse and human conserved genomic regions containing predicted Dlx5 binding sites near the BMP4 locus.
(PDF)
Acknowledgments
We thank Dr. Rolf Zeller (Univ. of Basel, Switzerland) for providing probes and reagents. We thank Dr. Ferdinando DiCunto (Univ. of Torino, Italy) for bioinformatic analyses. We also thank Dr. Denis Duboule (Geneva, Switzerland), Drs. Massimo Santoro and Emilio Hirsch (Univ. of Torino, Italy) for helpful comment on the manuscript.
Funding Statement
Giorgio R. Merlo is supported by the Italian Telethon Foundation (GGP11097), the Fondazione per la Ricerca Biomedica (Torino, Italy), and the Compagnia di San Paolo (Torino, Italy). Ottavia Barbieri is supported by the Italian Telethon Foundation (GP0218Y01). Benoit Robert is supported by Institut Pasteur and the Centre National de la Recherche Scientifique (France). Benoit Robert and Giovanni Levi are supported by the Agence Nationale de la Recherche (France) grant “GENDACTYL.” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Allard P, Tabin CJ (2009) Achieving bilateral symmetry during vertebrate limb development. Semin Cell Dev Biol 20: 479–484. [DOI] [PubMed] [Google Scholar]
- 2. Zakany J, Duboule D (2007) The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev 17: 359–366. [DOI] [PubMed] [Google Scholar]
- 3. Zeller R (2010) The temporal dynamics of vertebrate limb development, teratogenesis and evolution. Curr Opin Genet Dev 20: 384–390. [DOI] [PubMed] [Google Scholar]
- 4. Bastida MF, Ros MA (2008) How do we get a perfect complement of digits? Curr Opin Genet Dev 18: 374–380. [DOI] [PubMed] [Google Scholar]
- 5. Riddle RD, Johnson RL, Laufer E, Tabin C (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75: 1401–1416. [DOI] [PubMed] [Google Scholar]
- 6. Zeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM, et al. (2001) A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411: 716–720. [DOI] [PubMed] [Google Scholar]
- 7. Mariani FV, Ahn CP, Martin GR (2008) Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 453: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Mariani FV, Martin GR (2002) Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418: 501–508. [DOI] [PubMed] [Google Scholar]
- 9. Niswander L, Tickle C, Vogel A, Booth I, Martin GR (1993) FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 75: 579–587. [DOI] [PubMed] [Google Scholar]
- 10. Lu P, Yu Y, Perdue Y, Werb Z (2008) The apical ectodermal ridge is a timer for generating distal limb progenitors. Development 135: 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez-Teran M, Ros MA (2008) The Apical Ectodermal Ridge: morphological aspects and signaling pathways. Int J Dev Biol 52: 857–871. [DOI] [PubMed] [Google Scholar]
- 12. Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, et al. (1996) The mouse Engrailed-1 gene and ventral limb patterning. Nature 382: 360–363. [DOI] [PubMed] [Google Scholar]
- 13. Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374: 350–353. [DOI] [PubMed] [Google Scholar]
- 14. Cygan JA, Johnson RL, McMahon AP (1997) Novel regulatory interactions revealed by studies of murine limb pattern in Wnt-7a and En-1 mutants. Development 124: 5021–5032. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Johnson RL (2002) Interactions between dorsal-ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. Int J Dev Biol 46: 937–941. [PubMed] [Google Scholar]
- 16. Qiu Q, Chen H, Johnson RL (2009) Lmx1b-expressing cells in the mouse limb bud define a dorsal mesenchymal lineage compartment. Genesis 47: 224–233. [DOI] [PubMed] [Google Scholar]
- 17. Benazet JD, Zeller R (2009) Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system. Cold Spring Harb Perspect Biol 1: a001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeller R, Lopez-Rios J, Zuniga A (2009) Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet 10: 845–858. [DOI] [PubMed] [Google Scholar]
- 19. Tickle C (2006) Making digit patterns in the vertebrate limb. Nat Rev Mol Cell Biol 7: 45–53. [DOI] [PubMed] [Google Scholar]
- 20. Pizette S, Abate-Shen C, Niswander L (2001) BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development 128: 4463–4474. [DOI] [PubMed] [Google Scholar]
- 21. Robert B (2007) Bone morphogenetic protein signaling in limb outgrowth and patterning. Dev Growth Differ 49: 455–468. [DOI] [PubMed] [Google Scholar]
- 22. Benazet JD, Bischofberger M, Tiecke E, Goncalves A, Martin JF, et al. (2009) A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science 323: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 23. Wang CK, Omi M, Ferrari D, Cheng HC, Lizarraga G, et al. (2004) Function of BMPs in the apical ectoderm of the developing mouse limb. Dev Biol 269: 109–122. [DOI] [PubMed] [Google Scholar]
- 24. Zuniga A, Haramis AP, McMahon AP, Zeller R (1999) Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature 401: 598–602. [DOI] [PubMed] [Google Scholar]
- 25. Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB 3rd (2001) BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development 128: 4449–4461. [DOI] [PubMed] [Google Scholar]
- 26. Tickle C (2003) Patterning systems–from one end of the limb to the other. Dev Cell 4: 449–458. [DOI] [PubMed] [Google Scholar]
- 27. Robert B, Lallemand Y (2006) Anteroposterior patterning in the limb and digit specification: contribution of mouse genetics. Dev Dyn 235: 2337–2352. [DOI] [PubMed] [Google Scholar]
- 28. Zeller R (2004) It takes time to make a pinky: unexpected insights into how SHH patterns vertebrate digits. Sci STKE 2004: pe53. [DOI] [PubMed] [Google Scholar]
- 29. Merlo GR, Paleari L, Mantero S, Genova F, Beverdam A, et al. (2002) Mouse model of split hand/foot malformation type I. Genesis. 33: 97–101. [DOI] [PubMed] [Google Scholar]
- 30. Robledo RF, Rajan L, Li X, Lufkin T (2002) The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev 16: 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shamseldin HE, Faden MA, Alashram W, Alkuraya FS (2012) Identification of a novel DLX5 mutation in a family with autosomal recessive split hand and foot malformation. J Med Genet 49: 16–20. [DOI] [PubMed] [Google Scholar]
- 32. Lallemand Y, Nicola MA, Ramos C, Bach A, Cloment CS, et al. (2005) Analysis of Msx1; Msx2 double mutants reveals multiple roles for Msx genes in limb development. Development 132: 3003–3014. [DOI] [PubMed] [Google Scholar]
- 33. Lallemand Y, Bensoussan V, Cloment CS, Robert B (2009) Msx genes are important apoptosis effectors downstream of the Shh/Gli3 pathway in the limb. Dev Biol 331: 189–198. [DOI] [PubMed] [Google Scholar]
- 34. Bensoussan-Trigano V, Lallemand Y, Saint Cloment C, Robert B (2011) Msx1 and Msx2 in limb mesenchyme modulate digit number and identity. Dev Dyn 240: 1190–1202. [DOI] [PubMed] [Google Scholar]
- 35. Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, et al. (1999) Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 126: 3795–3809. [DOI] [PubMed] [Google Scholar]
- 36. Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, et al. (2002) Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis 34: 221–227. [DOI] [PubMed] [Google Scholar]
- 37. Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, et al. (1999) Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126: 3831–3846. [DOI] [PubMed] [Google Scholar]
- 38. Depew MJ, Simpson CA, Morasso M, Rubenstein JL (2005) Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat 207: 501–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houzelstein D, Cohen A, Buckingham ME, Robert B (1997) Insertional mutation of the mouse Msx1 homeobox gene by an nlacZ reporter gene. Mech Dev 65: 123–133. [DOI] [PubMed] [Google Scholar]
- 40. Satokata I, Ma L, Ohshima H, Bei M, Woo I, et al. (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24: 391–395. [DOI] [PubMed] [Google Scholar]
- 41. Satokata I, Maas R (1994) Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6: 348–356. [DOI] [PubMed] [Google Scholar]
- 42. Ferrari D, Sumoy L, Gannon J, Sun H, Brown AM, et al. (1995) The expression pattern of the Distal-less homeobox-containing gene Dlx-5 in the developing chick limb bud suggests its involvement in apical ectodermal ridge activity, pattern formation, and cartilage differentiation. Mech Dev 52: 257–264. [DOI] [PubMed] [Google Scholar]
- 43. Bendall AJ, Abate-Shen C (2000) Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 247: 17–31. [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Hu G, Wang H, Sciavolino P, Iler N, et al. (1997) Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol 17: 2920–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davidson D (1995) The function and evolution of Msx genes: pointers and paradoxes. Trends Genet 11: 405–411. [DOI] [PubMed] [Google Scholar]
- 46. Panganiban G, Rubenstein JL (2002) Developmental functions of the Distal-less/Dlx homeobox genes. Development 129: 4371–4386. [DOI] [PubMed] [Google Scholar]
- 47. Merlo GR, Zerega B, Paleari L, Trombino S, Mantero S, et al. (2000) Multiple functions of Dlx genes. Int J Dev Biol 44: 619–626. [PubMed] [Google Scholar]
- 48. Liu YH, Tang Z, Kundu RK, Wu L, Luo W, et al. (1999) Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol 205: 260–274. [DOI] [PubMed] [Google Scholar]
- 49. Odelberg SJ, Kollhoff A, Keating MT (2000) Dedifferentiation of mammalian myotubes induced by msx1. Cell 103: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 50. Hu G, Lee H, Price SM, Shen MM, Abate-Shen C (2001) Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128: 2373–2384. [DOI] [PubMed] [Google Scholar]
- 51. Ishii M, Merrill AE, Chan YS, Gitelman I, Rice DP, et al. (2003) Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 130: 6131–6142. [DOI] [PubMed] [Google Scholar]
- 52. Levi G, Puche AC, Mantero S, Barbieri O, Trombino S, et al. (2003) The Dlx5 homeodomain gene is essential for olfactory development and connectivity in the mouse. Mol Cell Neurosci 22: 530–543. [DOI] [PubMed] [Google Scholar]
- 53. Perera M, Merlo GR, Verardo S, Paleari L, Corte G, et al. (2004) Defective neuronogenesis in the absence of Dlx5. Mol Cell Neurosci 25: 153–161. [DOI] [PubMed] [Google Scholar]
- 54. Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, et al. (2007) Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci 27: 3230–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Merlo GR, Mantero S, Zaghetto AA, Peretto P, Paina S, et al. (2007) The role of Dlx homeogenes in early development of the olfactory pathway. J Mol Histol 38: 347–358. [DOI] [PubMed] [Google Scholar]
- 56. Muraglia A, Perera M, Verardo S, Liu Y, Cancedda R, et al. (2008) DLX5 overexpression impairs osteogenic differentiation of human bone marrow stromal cells. Eur J Cell Biol 87: 751–761. [DOI] [PubMed] [Google Scholar]
- 57. Lo Iacono N, Mantero S, Chiarelli A, Garcia E, Mills AA, et al. (2008) Regulation of Dlx5 and Dlx6 gene expression by p63 is involved in EEC and SHFM congenital limb defects. Development 135: 1377–1388. [DOI] [PubMed] [Google Scholar]
- 58. Radoja N, Guerrini L, Lo Iacono N, Merlo GR, Costanzo A, et al. (2007) Homeobox gene Dlx3 is regulated by p63 during ectoderm development: relevance in the pathogenesis of ectodermal dysplasias. Development 134: 13–18. [DOI] [PubMed] [Google Scholar]
- 59. Chung IH, Han J, Iwata J, Chai Y (2010) Msx1 and Dlx5 function synergistically to regulate frontal bone development. Genesis 48: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levi G, Mantero S, Barbieri O, Cantatore D, Paleari L, et al. (2006) Msx1 and Dlx5 act independently in development of craniofacial skeleton, but converge on the regulation of Bmp signaling in palate formation. Mech Dev 123: 3–16. [DOI] [PubMed] [Google Scholar]
- 61. Han J, Mayo J, Xu X, Li J, Bringas P Jr, et al. (2009) Indirect modulation of Shh signaling by Dlx5 affects the oral-nasal patterning of palate and rescues cleft palate in Msx1-null mice. Development 136: 4225–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pan ZZ, Kronenberg MS, Huang DY, Sumoy L, Rogina B, et al. (2002) MSX2 expression in the apical ectoderm ridge is regulated by an MSX2 and Dlx5 binding site. Biochem Biophys Res Commun 290: 955–961. [DOI] [PubMed] [Google Scholar]
- 63. Sumoy L, Wang CK, Lichtler AC, Pierro LJ, Kosher RA, et al. (1995) Identification of a spatially specific enhancer element in the chicken Msx-2 gene that regulates its expression in the apical ectodermal ridge of the developing limb buds of transgenic mice. Dev Biol 170: 230–242. [DOI] [PubMed] [Google Scholar]
- 64. Vieux-Rochas M, Coen L, Sato T, Kurihara Y, Gitton Y, et al. (2007) Molecular dynamics of retinoic acid-induced craniofacial malformations: implications for the origin of gnathostome jaws. PLoS One 2: e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jones CM, Lyons KM, Hogan BL (1991) Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111: 531–542. [DOI] [PubMed] [Google Scholar]
- 66. Paina S, Garzotto D, DeMarchis S, Marino M, Moiana A, et al. (2011) Wnt5a is a transcriptional target of Dlx homeogenes and promotes differentiation of interneuron progenitors in vitro and in vivo. J Neurosci 31: 2675–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ala U, Piro RM, Grassi E, Damasco C, Silengo L, et al. (2008) Prediction of human disease genes by human-mouse conserved coexpression analysis. PLoS Comput Biol 4: e1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Piro RM, Ala U, Molineris I, Grassi E, Bracco C, et al. (2011) An atlas of tissue-specific conserved coexpression for functional annotation and disease gene prediction. Eur J Hum Genet 19: 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, et al. (2008) Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Portales-Casamar E, Thongjuea S, Kwon AT, Arenillas D, Zhao X, et al. (2010) JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res 38: D105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, et al. (2000) A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci 20: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, et al. (2004) A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131: 5153–5165. [DOI] [PubMed] [Google Scholar]
- 73. Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM (2003) Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34: 303–307. [DOI] [PubMed] [Google Scholar]
- 74. Michos O, Panman L, Vintersten K, Beier K, Zeller R, et al. (2004) Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131: 3401–3410. [DOI] [PubMed] [Google Scholar]
- 75. Ma L, Lu MF, Schwartz RJ, Martin JF (2005) Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611. [DOI] [PubMed] [Google Scholar]
- 76. Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, et al. (2008) BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem 283: 29119–29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75: 45–58. [PubMed] [Google Scholar]
- 78. Marazzi G, Wang Y, Sassoon D (1997) Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol 186: 127–138. [DOI] [PubMed] [Google Scholar]
- 79. Bei M, Maas R (1998) FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125: 4325–4333. [DOI] [PubMed] [Google Scholar]
- 80. Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274: 19838–19845. [DOI] [PubMed] [Google Scholar]
- 81. Sirard C, Kim S, Mirtsos C, Tadich P, Hoodless PA, et al. (2000) Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor beta-related signaling. J Biol Chem 275: 2063–2070. [DOI] [PubMed] [Google Scholar]
- 82. Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, et al. (2001) Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27: 84–88. [DOI] [PubMed] [Google Scholar]
- 83. Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, et al. (2000) Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech Dev 99: 29–38. [DOI] [PubMed] [Google Scholar]
- 84. Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, et al. (2000) A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 85. Bei M, Kratochwil K, Maas RL (2000) BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development 127: 4711–4718. [DOI] [PubMed] [Google Scholar]
- 86. Chen Y, Bei M, Woo I, Satokata I, Maas R (1996) Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122: 3035–3044. [DOI] [PubMed] [Google Scholar]
- 87. Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, et al. (2002) Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129: 4135–4146. [DOI] [PubMed] [Google Scholar]
- 88. Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, et al. (2006) Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kraus P, Fraidenraich D, Loomis CA (2001) Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev 100: 45–58. [DOI] [PubMed] [Google Scholar]
- 90. Guerrini L, Costanzo A, Merlo GR (2011) A symphony of regulations centered on p63 to control development of ectoderm-derived structures. J Biomed Biotechnol 2011: 864904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Top. Location of predicted conserved Dlx binding sites in the Dlx5-Dlx6 intergenic genomic region. Sites are indicated with colour vertical bars (asterisk) and annotated with the species conservation. The chromosomal position and coordinates are also reported. The mammalian genomic conservation is reported on the bottom. The known i56i element is correctly predicted by the PWM bioinformatic approach we have adopted. Bottom. Same as above, relative to the Msx2 proximal promoter. Two known conserved Dlx binding sites are correctly predicted.
(PDF)
Quantification of the Msx2 mRNAs by qRT-PCR in the anterior and posterior halves of HLs from Msx1+/−;Dlx5−/−;Dlx6−/− embryos, relative to the corresponding WT samples (set = 1).
(PDF)
Quantification of the Fgf8 and Shh mRNAs by qRT-PCR in the HLs from Msx1−/− (top left), Dlx5−/−;Dlx6−/− (top right), Msx1+/−;Dlx5+/−;Dlx6+/− (bottom left) and Msx1+/−;Dlx5−/−;Dlx6−/− (bottom right) embryos, relative to the corresponding WT samples (set = 1).
(PDF)
Sequences of the oligonucleotides used for real-time qPCR on mouse embryonic tissues.
(PDF)
Sequences of the oligonucleotides used for ChIP analysis on the predicted Dlx elements near the human BMP2 and BMP4 loci.
(PDF)
The Dlx5 Position-Weight matrix and results of the prediction of Dlx5 binding sites based on genomic conservation.
(PDF)
Sequences of the mouse and human conserved genomic regions containing predicted Dlx5 binding sites near the BMP2 locus.
(PDF)
Sequences of the mouse and human conserved genomic regions containing predicted Dlx5 binding sites near the BMP4 locus.
(PDF)