Abstract
Rationale
Most neuroactive drugs affect brain metabolism as well as systemic and cerebral blood flow, thus altering brain temperature. Although this aspect of drug action usually remains in the shadows, drug-induced alterations in brain temperature reflect their metabolic neural effects and affect neural activity and neural functions.
Objectives
Here, I review brain temperature changes induced by neuroactive drugs, which are used therapeutically (general anesthetics), as a research tool (dopamine agonists and antagonists), and self-administered to induce desired psychic effects (cocaine, methamphetamine, ecstasy). I consider the mechanisms underlying these temperature fluctuations and their influence on neural, physiological, and behavioral effects of these drugs.
Results
By interacting with neural mechanisms regulating metabolic activity and heat exchange between the brain and the rest of the body, neuroactive drugs either increase or decrease brain temperatures both within (35-39°C) and exceeding the range of physiological fluctuations. These temperature effects differ drastically depending upon the environmental conditions and activity state during drug administration. This state-dependence is especially important for drugs of abuse that are usually taken by humans during psycho-physiological activation and in environments that prevent proper heat dissipation from the brain. Under these conditions, amphetamine-like stimulants induce pathological brain hyperthermia (>40°C) associated with leakage of the blood-brain barrier and structural abnormalities of brain cells.
Conclusions
The knowledge on brain temperature fluctuations induced by neuroactive drugs provides new information to understand how they influence metabolic neural activity, why their effects depend upon the behavioral context of administration, and the mechanisms underlying adverse drug effects including neurotoxicity
Keywords: metabolism, cerebral blood flow, behavioral activation, general anesthesia, dopamine agonists and antagonists, cocaine, psychomotor stimulants, blood-brain barrier, brain edema, neurotoxicity
Introduction
When considering the neural mechanisms underlying behavioral, physiological, and subjective effects of neuroactive drugs, researchers rarely think about the changes in brain temperature induced by these drugs. While often viewed as a stable homeostatic parameter, brain temperature depends on brain metabolism and heat outflow from the brain, showing relatively large physiological fluctuations during changes in an organism's activity state, environmental stimulation, and performance of natural motivated behaviors (see for review in Kiyatkin 2010). Since most neuroactive drugs affect brain metabolism and the state of peripheral and cerebral blood vessels, they often decrease or increase brain temperature exceeding normal baselines (~37.0°C). In contrast to transient physiological fluctuations, changes in brain temperature induced by neuroactive drugs depend on the drug's dose and route of administration, with a potential to shift temperatures well beyond their physiological range, thus inducing robust brain hypothermia or hyperthermia. Although it is known that heat is toxic for neural cells and hyperthermia is a life-threatening complication of intoxication with psychomotor stimulants, drug-induced decreases in brain temperature also strongly affect neural activity and neural functions, contributing to the powerful inhibitory effects induced by sedative drugs and general anesthetics. Importantly, drugs interfere with ongoing physiological processes and their effects depend significantly on the environmental conditions and the activity state associated with drug use. This is especially important for addictive drugs that are self-administered by humans during psycho-physiological activation and under specific environmental conditions. In this work, I review changes in brain temperature induced by neuroactive drugs of several classes, consider their underlying mechanisms, and discuss the possible influence of these changes on the neural, physiological, and behavioral effects of these drugs. I will focus on several classes of drugs that are used therapeutically, abused by humans, and applied in research as pharmacological tools. Since I recently reviewed physiological brain temperature fluctuations (Kiyatkin 2010), this issue is considered with less detail. The major goal of this work is to demonstrate that the change in brain temperature induced by neuroactive drugs is an important reality, which could provide new valuable information on the mechanisms of drug action, drug therapeutics, and the prevention of harmful effects of addictive drugs.
1. Brain temperature as a physiological variable
While it is generally believed that brain temperature in healthy homeothermic organisms is stable and close to 37°C, abundant data obtained in animals suggest robust fluctuations in brain temperature following exposure to various environmental challenges and during different types of natural motivated behavior. By using miniature thermocouple sensors chronically implanted in different brain and peripheral locations, we showed that hypothalamic temperature in awake, freely moving male rats could fall to ~35°C during deep sleep (Smirnov and Kiyatkin 2010) and could phasically peak at ~39.5°C at the moment of ejaculation during copulatory behavior (Kiyatkin and Mitchum 2003). These limits obviously define the range of ‘normal’ brain temperature fluctuations (~4°C) within the entire physiological continuum. While the recording of brain temperature in rats is relatively simple, direct human data are limited and often restricted to neurological patients (Mariak et al. 1998, 1999, 2000; Rango et al. 2012; Rumana et al. 1998). Therefore, it is not definitely proven so far that similar, relatively large brain temperature fluctuations could occur in healthy humans. However, several observations suggest that this could be the case. First, monkeys that are much closer evolutionarily to humans also show robust physiological changes in brain temperature within the range observed in rats (Baker et al. 1973; Fuller and Baker 1983; Hayward and Baker 1968). Second, both rats and humans show similar pathological hyperthermia (>40-41°C) following exposure to psychomotor stimulants at large doses (Armenian et al. 2012; Dafters 1995; Kalant 2001; Nimmo et al. 1993). Finally, direct measurements from healthy human volunteers suggest that their brain temperature (assessed by venous outflow) could reach 39.5-40.0°C during a 30-min bicycle exercise while wearing water-impermeable cloth (Nybo et al. 2002; Nybo 2008) that impairs normal heat dissipation to the external environment. Importantly, the physical and mental states of these volunteers at these high temperatures remained within the normal range.
2. Basic mechanisms underlying brain temperature homeostasis
Brain temperature is determined by the balance of two opposing forces that tend either to increase it by metabolism-related intra-brain heat production or decrease it through heat dissipation by cerebral blood outflow to the rest of the body and then to the external environment. The brain, which represents ~2% of body weight in humans, accounts for ~20% of the organism's total energy consumption (Schmidt-Nielsen 1997; Siesjo 1978) and the heat continuously generated by brain tissue is removed by cerebral circulation due to a temperature differential between arterial blood inflowing to the brain and brain tissue. While it seems mechanistic, the cooling of an internal combustion engine appears to be a good analogy to brain temperature exchange. Similar to circulating coolant that continuously removes heat from a working engine, cool, oxygenated arterial blood removes heat from the brain via heat exchange and the now warmed venous blood returns to the heart to be cooled and oxygenated again in the lungs. Such an arrangement determines the critical role of cerebral blood flow in brain temperature homeostasis and the essential inter-dependence of temperature in the brain and the rest of the body. While brain temperature tends to increase due to metabolism-related intra-brain heat production, it also rises when brain-generated heat cannot be properly dissipated to the body and then to the external environment. Similarly, a decrease in cerebral metabolism tends to lower brain temperature but this effect could be strongly enhanced by peripheral vasodilatation that promotes heat loss to a cooler environment. Since most neuroactive drugs affect metabolism and the state of peripheral and cerebral blood vessels, drug-induced brain temperature responses depend significantly upon the ongoing organism's state and the environmental conditions. A drug at a certain dose could induce moderate brain hyperthermia under one set of environmental conditions, when adaptive mechanisms of heat loss are fully effective. However, the same drug in the same dose could induce pathological hyperthermia with possible lethality when used under conditions preventing proper heat dissipation. Since peripheral vasodilatation and perspiration are powerful means for heat loss in humans, drug-induced impairment of these adaptive mechanisms could be a very important factor determining robust increases in brain and body temperatures induced by certain drugs.
3. Brain hypothermia associated with general anesthesia
General anesthesia results in a robust decrease in body temperature and a significant impairment of temperature regulation. This makes the organism much more sensitive to external temperature influences (Lenhardt 2010; Sessler 2008). To counteract this impairment and prevent hypothermia, anesthesia is usually supplemented with body heating, thus helping to maintain temperatures within their physiological limits.
To examine how brain temperature is affected by general anesthesia, miniature thermocouple sensors were chronically implanted in two brain structures (medial-anterior hypothalamus and hippocampus), body core, and skin. Temperature recordings from these locations were conducted in freely moving rats that received a single intraperitoneal (ip) injection of sodium pentobarbital at a standard anesthetic dose (50 mg/kg). Measurements were conducted at standard ambient temperatures (22-23°C) and during body warming by a heating blanket set at 37°C (Kiyatkin and Brown 2005).
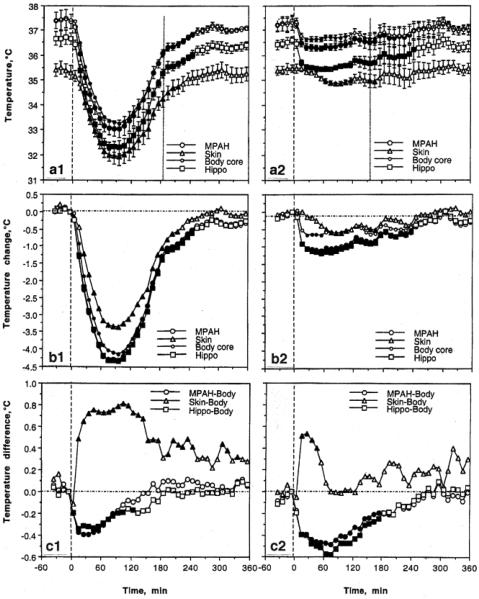
As shown in Fig 1a (left panel), an injection of pentobarbital results in robust temperature decreases (>4°C) in all recording locations that reached their nadir at ~90 min and slowly returned to baseline within the next 2.5-3.0 hours. However, the temperature dynamics differed in each location (b and c). In both brain sites, temperature decreases were stronger than in the body core, resulting in a significant drop in brain-body temperature differentials (c), suggesting relative brain cooling vs. the body core. In contrast, the temperature decrease in the skin was weaker than in the body core, resulting in a significant, ~0.7°C rise in skin-body differential (c). This finding indicates relative skin warming vs. the body core. While the relative brain cooling reflects the known inhibiting effect of pentobarbital on brain metabolism (Crane et al. 1978; Pierce 1962), relative skin warming reflects peripheral vasodilatation, a typical effect of anesthetic drugs that enhances heat loss to the external environment and makes the organism more sensitive to ambient temperatures.
Fig 1.
Changes in central and peripheral temperatures (medial preoptic-anterior hypothalamus or MPAH, hippocampus or Hippo, Body core, Skin) induced by sodium pentobarbital (50 mg/kg, ip at 0 min, first vertical hatched line) injected under standard ambient temperatures (22-23°C; left column) and during body warming by heating pad set at 37°C (right column). The second vertical hatched line on top graphs show the moment of awakening detected by first head movement. Top raw (a) shows absolute temperature changes, middle raw (b) shows temperature changes relative to pre-injection baseline (hatched horizontal line), and the bottom raw (c) shows between-site temperature differentials. Filled symbols mark values significantly different vs. baseline (F-test after one-way ANOVA with repeated measures). Original data were presented in Kiyatkin and Brown 2004. Reproduced by permission from Elsevier
When the same anesthetic drug was used during passive body warming (Fig 1; right panel), temperature decreases in each recording location were much smaller (1.0-1.5°C) and the between-site temperature differences changed. While temperatures in both brain sites also decreased vs. the body core, the decrease in this case was stronger and more prolonged than in un-warmed conditions (compare c1 and c2). Therefore, external body warming that counteracts hypothermia is more effective for the body than for the brain, making brain-body temperature differentials much larger. Although pentobarbital-induced brain hypothermia during body warming is weaker in terms of absolute change, the brain remains metabolically inhibited and its temperature remains lower than in the body core. Under conditions of body warming skin temperature showed the smallest change (a2 and b2), which was clearly weaker than that in un-warmed conditions. Moreover, body warming greatly decreased the temperature difference between the skin and the body core, confirming that body warming effectively counteracts heat loss to the external environment.
The present data may have important implications for neuronal and neurochemical evaluations conducted in anesthetized animals. In addition to the known direct actions of anesthetic drugs on neural cells, neural activity should also be profoundly affected by robust brain hypothermia typical of these preparations. Since most processes governing neuronal activity and excitability are temperature-dependent, even relatively small (1-3°C) temperature decreases affect the activity of single ionic channels and transmitter-receptor interactions, thus changing transmitter release and reuptake (see Kiyatkin 2010 for review) and distorting the drug-induced neuronal and neurochemical effects of tested drugs. Although body warming is often used in anesthetized animal preparations, it is quite difficult to maintain temperatures at stable levels (37°C) even using feedback-regulated heating devices. Even in this case, brain temperature remains 0.6-0.7°C cooler than that in the body core. Therefore, while effectively counteracting drug-induced hypothermia, body warming is unable to compensate fully for brain hypothermia typical of these conditions. Furthermore, external body warming during anesthesia inverts the normal direction of heat exchange between the brain and arterial blood, thus dramatically altering various metabolic and blood flow evaluations. For these reasons, work conducted with such preparations as well as in cold-maintained brain slices can produce an obscured, distorted view of the active brain (see Windels 2006; Windels and Kiyatkin 2006). Finally, the relative independence of brain temperature from body temperature could be of clinical importance in ischemic and traumatic brain damage in humans as well as for the development of neuroprotective and survival strategies. In contrast to normal physiological conditions where brain temperature is close to core body temperature, cortical temperature in patients with head trauma is about 1°C higher than and relatively independent of both rectal and jugular temperatures (Mariak et al. 1998, 1999, 2000; Rumana et al. 1998). Moreover, because of the known dorso-ventral brain temperature gradient (Delgado and Hanai 1966; Horvath et al. 1999; Mellergard and Nordstrom 1990; Schwab et al., 1997), deep brain structures may be 0.6-1.0°C warmer than the cortex, or almost 2°C warmer than body core. Since both traumatic and ischemic brain injury is strongly enhanced at higher temperatures, this relative brain hyperthermia makes these patients especially sensitive to fever and even modest environmental heating.
4. Alterations in brain temperature induced by selective stimulation and blockade of dopamine receptors
A large body of data suggests a tight link between dopamine (DA) activity and behavioral activation (see LeMoal and Simon 1991; Salamone et al. 2005 for review). DA antagonists that block DA receptors induce motor hypoactivity and greatly attenuate locomotor responses to natural arousing stimuli, while both direct and indirect DA agonists induce hyperlocomotion and stereotypy (Wise and Bozarth 1987). Since natural behavioral activation is consistently coupled with increases in brain temperature (Kiyatkin and Mitchum 2003; Smirnov and Kiyatkin, 2008), it is possible to hypothesize that pharmacological enhancement of DA neurotransmission that induces behavioral activation will also increase brain temperature. Similarly, the drugs that block DA neurotransmission and induce motor hypoactivity could be expected to decrease brain temperature. To clarify this issue, we examined how central (assessed in the nucleus accumbens or NAcc) and peripheral (temporal muscle and skin) temperatures are affected by pharmacological blockade and enhancement of DA neurotransmission in freely moving rats (Brown et al. 2007; Kiyatkin 2008). To block DA receptors, we used a mixture of D1-like (SCH23390) and D2-like selective (eticlopride) DA antagonists at 0.2 mg/kg subcutaneous (sc) doses (0.7 and 0.6 μM, respectively) that provide effective blockade of DA neurotransmission (for ~ 2-hours) as tested previously by the antagonism of striatal neuronal responses to iontophoretic DA (Kiyatkin and Rebec 1999). To activate DA receptors, we used apomorphine (APO), a directly acting DA agonist with high affinity for both D1-like and D2-like receptors (Jackson and Westlind-Danielsson 1994). This drug was used intravenously (iv) at a dose (250 μg/kg) that is known to induce powerful locomotor activation and stereotypy.
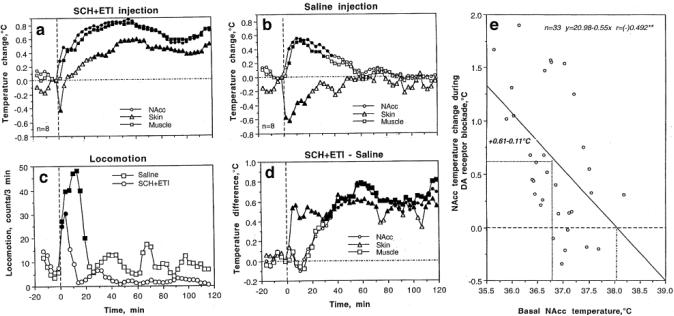
In contrast to our expectations, a mixture of DA antagonists that attenuated locomotor activity increased NAcc and muscle temperatures and induced a biphasic down-up change in skin temperature (Fig 2a). Since DA antagonists were delivered via sc injection, this procedure itself could be in part responsible for the effects induced by these drugs. As shown in Fig 2b and c, sc saline injection in fact induced transient locomotor activation, moderate increases in NAcc and muscle temperatures, and a rapid, transient fall in skin temperature. To reveal the pharmacological component of the temperature response, we analyzed drug-saline temperature differences (Fig. 2d). During this analysis, we found that DA antagonists slowly and moderately (0.6-9.8°C) increase brain and muscle temperatures from ~20 min post-injection for at least 2 hrs. These findings suggest that the pharmacological blockade of DA transmission induces some kind of metabolic brain activation with enhanced intra-brain heat production. This effect is accompanied by increases in skin temperature (d), pointing at peripheral vasodilatation, which promotes heat dissipation.
Fig 2.
Changes in temperature in the nucleus accumbens (NAcc), temporal muscle and skin induced by pharmacological blockade of DA transmission (SCH23390+eticlopride, sc, 0.2 mg/kg each). a, Relative temperature changes induced by drugs; b, Relative temperature changes induced by saline injection; c, Locomotor response induced by sc injection of drugs and saline; d, Temperature differences (drug-saline); e, Relationships between NAcc temperature change induced by DA antagonists and basal NAcc temperatures. Filled symbols show values significantly different from baseline (F-test after one-way ANOVA with repeated measures). Compiled from Brown et al. 2007
In this study, we also found that hyperthermic effects of DA antagonists are inversely related to basal brain temperatures, being stronger at lower temperatures and weaker at higher temperatures (Fig 2e). These relationships were evident within the full range of basal NAcc temperatures (mean±2SD or 35.7-37.9°C; see Fig. 2), but DA antagonists lost their ability to increase brain temperature at >38.0°C, the values seen during natural behavioral activation. Based on these data, it is possible to hypothesize that the effects of the DA receptor blockade on brain and body metabolism are state-dependent, with a stimulatory action present only at low levels of basal metabolic activity and lack of this action at high levels of basal metabolic activity when brain temperatures are already increased.
Although metabolic brain activation coupled with motor hypoactivity is an atypical combination for physiological conditions, several lines of evidence suggest that many central neurons become hyperactive during systemic DA receptor blockade. First, this procedure results in compensatory hyperactivity of DA neurons and increased DA release (Freeman et al. 1985; Imperato and DiChiara 1985). Second, striatal neurons lacking normal DA input have higher discharge rates and are more sensitive to glutamate than those in drug-free animals (Calabresi et al. 2000; Kiyatkin and Rebec 1999). Third, a DA receptor blockade results in heavy Fos expression in multiple structures that receive DA input (Ma et al. 2003; Wirtshafter and Asin 1995, 1999, 2003). Fos activation was evident within the entire system of basal ganglia and some of its important afferent and efferent structures, embracing massive numbers of neural cells. This cellular response may reflect the loss of the natural, restraining influence of tonic DA release on striatal activity, a factor important in mediating hypodynamia and behavioral hyporesponsiveness during various conditions associated with DA deficit. Importantly, it has been reported recently that Parkinsonian patients, who have a diminished DA transmission due to the loss of DA cells, also have higher brain temperatures than age-matched controls (Rango et al. 2012).
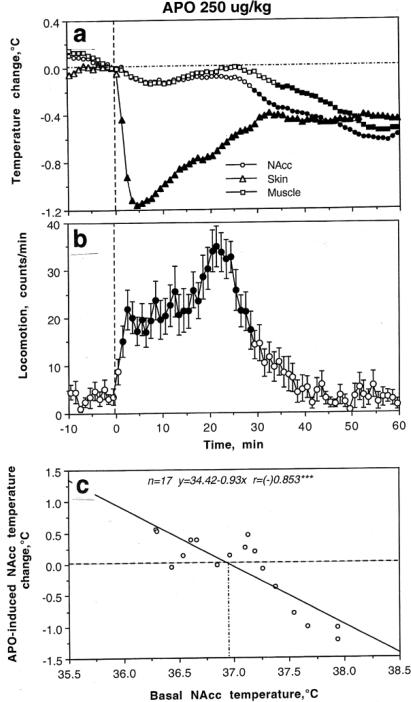
In contrast to DA antagonists, APO induced powerful locomotor activation and stereotypy for ~40 min post-injection and strongly decreased skin temperature, suggesting peripheral vasoconstriction (Fig 3a and b). However, mean temperatures in the NAcc and muscle showed a weak down-up-down fluctuation, with a significant decrease from ~35 min post-injection. This response pattern is atypical of natural behavioral activation. Similar to that with DA antagonists, the effects of APO on NAcc temperature were strongly dependent on baseline temperatures, with robust decreases at higher basal temperatures and no changes or weak increases at lower basal temperatures (Fig 3c). Therefore, APO at a dose that induces robust motor activation appears to induce weak metabolic inhibition coupled with strong peripheral vasoconstriction that prevents heat loss from skin surfaces. While the latter action is well known, explaining the high efficiency of DA as a therapeutic tool to increase arterial blood pressure, the dissociation between brain temperature and motor activation could be viewed as surprising. However, a dose-dependent body hypothermic effect of APO at doses that increase locomotion has been previously reported (Lapin and Samsonova 1968; Verma and Kulkarni 1993) and it has been shown that APO-induced hypothermia is in fact related to decreased metabolism and diminished metabolic heat production (Lin et al. 1979). Metabolic inhibition also occurs following direct APO injections in the ventricles, preoptic hypothalamus, striatum, and globus pallidus (Lin et al. 1982) and during electrical stimulation of substantia nigra, pars compacts (Lin et al. 1992) – a procedure that should selectively increase DA transmission. Body temperature also decreased following systemic injections of bromocriptine (Calne et al. 1975), another DA agonist, as well as intra-cerebral or intrahypothalamic injections of DA itself (Cox et al. 1978).
Fig 3.
Changes in nucleus accumbens (NAcc), temporal muscle, and skin temperatures induced by iv apomorphine (250 g/kg) in awake, freely moving rats. a, Relative temperature changes in each recording site; b, Changes in locomotion; c, Relationships between apomorphine-induced NAcc temperature change and basal NAcc temperatures. Filled symbols in a and b show values significantly different from baseline. Modified from Brown et al. 2007, where original data were presented
The present data could be relevant for understanding the regulatory role of DA with respect to other neurochemical systems involved in central activational processes. While DA transmission is enhanced under numerous conditions associated with brain activation, its primary role appears to be in regulating this activation, driving it at low levels and inhibiting it at higher levels. Our present data support this ‘homeostatic’ view on DA functions in relation to brain activation and brain metabolism. Particularly, they suggest that selective interruption of DA transmission, despite powerfully inhibiting spontaneous locomotion and behavioral responses to salient environmental stimuli, does not inhibit the brain. In contrast, the brain without functional DA appears to be metabolically more active and warmer despite the enhanced heat loss due to peripheral vasodilatation. This change parallels electrophysiological findings, suggesting that most DA-sensitive central neurons become more active, albeit disorganized, without DA input (Calabresi et al. 2000; Kiyatkin and Rebec 1999), and they are mostly inhibited by iontophoretic DA (Kiyatkin 2002). Because most cells that receive DA input are GABA-ergic, interruption of this restraining influence of DA and subsequent hyperactivity of these cells may be the primary factor determining behavioral inhibition following a DA deficit independently of its cause. In contrast, selective pharmacological stimulation of DA transmission by directly acting DA agonists appears to decrease brain metabolism despite intense hyperlocomotion and strong vasoconstriction that limits heat dissipation to the external environment. Importantly, this inhibiting effect of enhanced DA transmission on brain metabolism is evident only at higher basal brain temperatures, which under natural conditions are associated with behavioral activation and DA release. Therefore, it appears that DA has its specific modulatory effects on activational processes when activation is present and DA is released. Since APO has weak temperature-increasing effects at low basal temperatures that are consistently associated with deep inactivity or sleep, it may be suggested that DA under specific conditions could also increase brain metabolism. The relevance of this effect to natural conditions, however, is questionable because during behavioral inactivity and sleep, DA release is maintained at low levels.
The present data also suggest that the tight association between locomotor activation and increased brain metabolism that appears to exist under physiological and behavioral conditions (see Kiyatkin 2005 for review) does not hold for drug-induced behavioral activation. APO-induced hyperlocomotion appears to be related to the inhibition of brain metabolic activity, while locomotor inactivity following DA receptor blockade is associated with metabolic brain activation. While this uncoupling seems surprising, it is typical of other drugs. For example, iv heroin induces transient freezing and hypoactivity but brain and muscle temperatures strongly increase (Kiyatkin and Wise 2002). Transient episodes of hypoactivity consistently occur after each repeated heroin self-injection, but brain temperature remains strongly elevated without evident phasic changes. Therefore, it may be suggested that naturally occurring locomotor activation (searching, grooming, rearing) and inhibition (rest, sleep) differ in basic underlying mechanisms from similar behaviors induced by drugs.
5. State-dependent effects of iv cocaine on brain temperature
Cocaine is a widely used drug of abuse that acts on multiple neural substrates localized both in the CNS and periphery. Cocaine is a non-selective inhibitor of monoamine reuptake and cocaine's action on monoamine transporters, particularly the DA transporter, is usually viewed as the critical action in mediating its reinforcing properties (Kuhar et al. 1991; Wise and Bozarth 1987). Cocaine's action on uptake suggests that its effects should depend on the ongoing activity of monoamine neurons and release of monoamines, which are affected by an organism's functional state. This modulatory, activity-dependent pattern of cocaine's action on monoamine systems as well as other multiple actions of this drug in the brain and periphery (i.e., its interaction with ionic channels of different subtypes) should be considered as a whole to understand the highly variable and state-dependent physiological and behavioral effects of cocaine. Moreover, these effects also depend upon the drug's dose and route of administration. Since cocaine is interesting primarily as a drug of abuse, the effects induced by this drug in animal research should be always considered with respect to the doses and routes of administration used by humans. In contrast to animal research, when the effects of cocaine are typically assessed under quiet resting conditions, humans always take this drug during high activity levels when various physiological parameters are altered as a part of motivated drug-seeking behavior. This psycho-physiological activation appears to be an essential part of cocaine-taking behavior and it could dramatically alter the effects of this drug as seen in drug-naive, quietly resting animals.
In our studies of cocaine, all measurements were conducted in freely moving rats with thermocouple microsensors chronically implanted in different brain structures and several peripheral locations. Cocaine was delivered as iv injection at 1 mg/kg, a dose that is optimal for drug-self-administration in rats (Pickens and Thompson 1968). First, we examined how brain temperature is affected by cocaine administered to quietly resting animals. Second, we examined the pattern of temperature fluctuations during cocaine self-administration in drug-experienced, trained rats. Finally, to explore how the effects of cocaine are modulated by ongoing behavior, we conducted a yoked control experiment where cocaine was delivered passively at the dose/pattern that mimicked its self-administration.
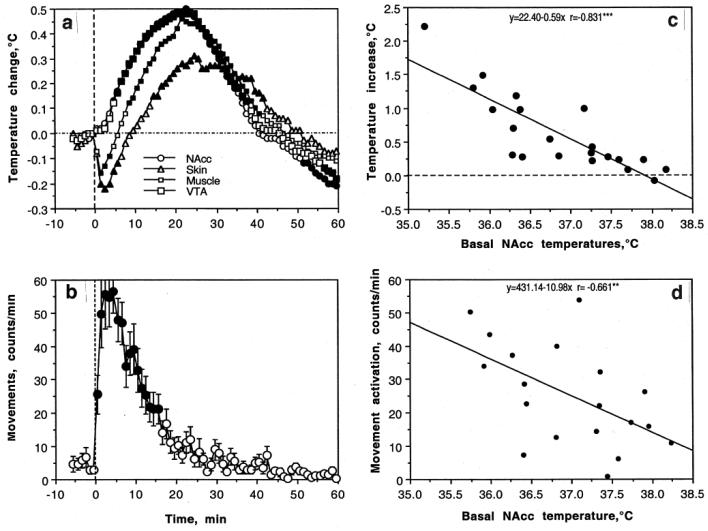
As shown in Fig 4, cocaine delivered to quietly resting rats induced strong locomotor activation (b), moderate increases in brain (NAcc and ventral tegmental area or VTA) and muscle temperatures, and a biphasic, down-up fluctuation in skin temperature (a). The temperature increase in both brain structures was similar and always more rapid and stronger than in temporal muscle that received the same arterial supply as the brain. This difference was maintained for ~20 min post-injection, suggesting preferential heat production in the brain as an obvious manifestation of drug-induced metabolic brain activation. Skin temperature decrease is consistent with peripheral vasoconstriction, a known effect of cocaine following its iv administration. Interestingly, the pattern of temperature response induced by iv cocaine under quiet resting conditions was similar to that induced by natural arousing stimuli, which also induced locomotor activation, increases in brain and muscle temperatures, and a rapid decrease in skin temperature (compare with Fig 2 for sc saline injection).
Fig 4.
Changes in brain (nucleus acumens or NAcc and ventral tegmental area or VTA), temporal muscle, and skin temperatures (a) as well as locomotion (b) induced by iv cocaine (1 mg/kg) administered to awake, freely moving rats under quiet resting conditions. c and d show the relationships between cocaine-induced changes in NAcc temperature (c) and locomotion (d) and basal NAcc temperature. Filled symbols in A and B show values significantly different from baseline
Similar to natural arousing stimuli, the hyperthermic effects of cocaine were strongly dependent upon baseline temperatures (Fig 4c). When basal NAcc temperatures were low (35.5-37.0°C), cocaine increased them strongly but the effect became progressively weaker when basal temperatures were higher [r=(-)0.831, p<0.001]. As shown by the regression analysis, the effect disappeared at ~38°C. Although correlation was weaker, locomotor response was also dependent on basal temperature (Fig 4d), being stronger at lower levels and weaker at higher levels [r=(-)0.661, p<0.01].
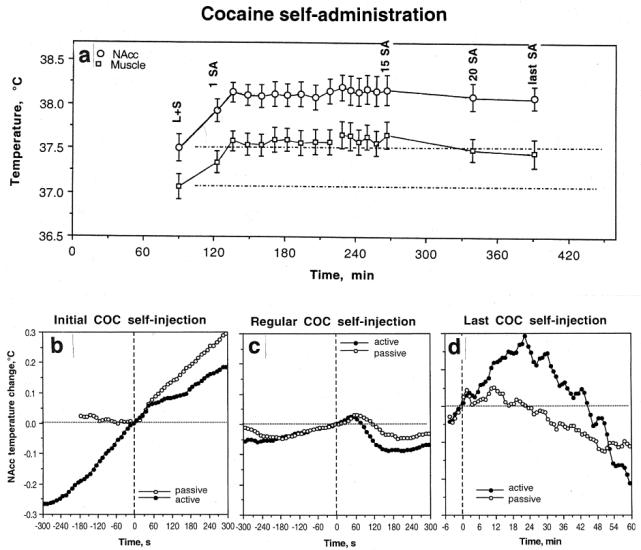
A strong dependence of cocaine's effects on brain temperature upon the behavioral context of drug administration was evident during drug self-administration (Kiyatkin and Brown 2003, 2004). We found that the first cocaine self-injection of a session induced in trained rats a strong increase in NAcc temperature (Fig 5a and b). This increase, however, was preceded by an equally strong temperature increase that occurred after the rat was exposed to a cocaine-related sensory cue that triggered drug-seeking behavior (a). This pre-injection increase was fully absent before the first passive cocaine injection in equally drug-experienced rats (yoked control) but in this case, NAcc temperature similarly increased after this passive injection. In sharp contrast to the initial self-injection of a session, clear biphasic fluctuations of much smaller magnitude (~0.1°C) were typical of all subsequent self-injections of the session (c). In these cases, NAcc temperature gradually increased before the lever-press for cocaine and transiently decreased after cocaine infusion. Despite their relatively small magnitude, these biphasic fluctuations were highly significant and they occurred at higher NAcc temperatures (~38.5°C or ~1.0-1.5°C above initial baseline; see a). As shown above, cocaine loses its ability to increase brain temperatures at these levels. These biphasic fluctuations also occurred during repeated passive cocaine injections but they were half as large in magnitude (c). Therefore, these biphasic changes are determined not only by behavior but also reflect the superimposition of repeated drug effects. Interestingly, NAcc temperatures began to decrease from 40-60 s after both active and passive cocaine injections, obviously reflecting the time necessary for the drug to travel to the brain, cross the blood-brain barrier (BBB), and diffuse to centrally located neural substrates. Although NAcc temperature rises for ~20 min after a single passive injection (see Fig 4), trained rats self-administer this drug with a mean inter-injection interval of 7-8 min, i.e., each subsequent injection occurs within the time of previous drug effect. Finally, NAcc temperature strongly increased after the last cocaine self-injection of the session, when the lever was blocked and the rat could not receive the drug despite multiple attempts to activate the blocked lever (Fig. 5d). This effect was seen only in behaving animals and was absent in rats that received cocaine passively. Therefore, this increase in brain temperature is primarily a correlate of drug-seeking behavior.
Fig 5.
a. Changes in nucleus accumbens (NAcc) and temporal muscle temperatures during cocaine self-administration behavior. Each value represents mean temperature ( standard error) immediately before the behavioral events. L+S, presentation of a light-sound cue. b, c and d, show rapid time-course dynamics of NAcc temperature associated with different critical events of self-administration behavior. Close circles show data obtained from self-administering animals and open circles represent data obtained from yoked-control rats. For clarity, standard errors are not shown in these graphs. Original data of this study were reported in Kiyatkin and Brown 2003, 2004
Therefore, the effects of iv cocaine at a low, self-administering dose on brain temperature are clearly state-dependent, showing increases when the drug is administered during quiet resting conditions at low basal temperatures and showing decreases when the drug is administered at high activity states when the pre-injection temperatures are already increased. This state-dependency determines the appearance of biphasic, down-up brain temperature fluctuations seen in drug-experienced rats self-administering cocaine. In this case, brain temperature is increased strongly in the beginning of the session after exposure to a cocaine-related sensory cue and the first self-injection of a session but then fluctuates biphasically within relatively stable but moderately increased levels during successive self-injections. Although these differences in the effects of cocaine depend upon the behavioral context of drug administration, our yoked control data suggest that some aspects of these differences are pharmacologically determined. Finally, this study clearly demonstrates that the effects of a drug that is self-administered differ substantially from the effects of the same drug administered passively in quiet resting conditions.
6. Brain hyperthermia induced by psychomotor stimulant drugs: State-dependency and environmental modulation
Methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA) are widely used psychomotor stimulant drugs. Both of these drugs increase metabolism and induce hyperthermia (Alberts and Sonsalla 1995; Freedman et al. 2005; Gordon et al., 1991; Green et al. 2003; Mechan et al. 2001; Sandoval et al. 2000), which is believed to be an important contributor to pathological changes associated with both acute intoxication with these drugs and their chronic abuse (Ali et al. 1996; Davidson et al. 2001; Kalant 2001; Kuhn and Geddes 2008; Schmued 2003; Seiden and Sabol 1996). Both METH and MDMA are often considered club drugs that are typically used by young adults under conditions of physical and emotional activation, often in a warm and humid environment. While the effects of any neuroactive drug are modulated by environmental conditions and specific activity states, these factors may be especially important for METH and MDMA because, in addition to metabolic activation, these drugs induce a strong peripheral vasoconstriction (Gordon et al. 1991; Pederson and Blessing 2001), thus diminishing heat dissipation from body surfaces and enhancing heat accumulation in the brain.
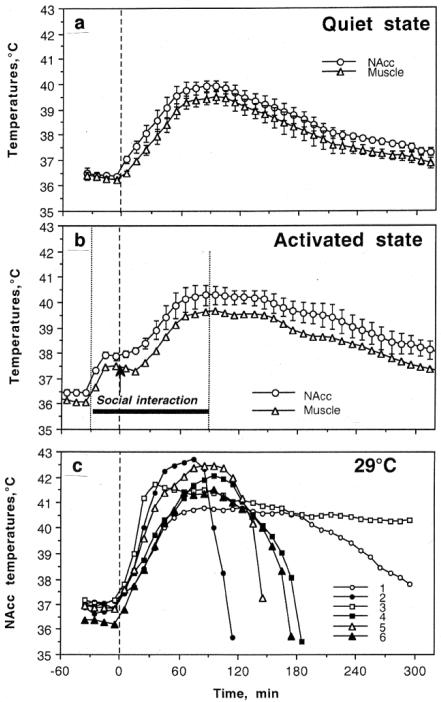
To assess how thermogenic effects of these drugs are modulated by activity state and environmental conditions that mimic human use, we examined temperature changes in the NAcc, hippocampus and temporal muscle induced in male rats by METH and MDMA (1-9 mg/kg, sc) in quiet resting conditions at normal laboratory temperature (23°C), during social interaction with a female, and at moderately warm ambient temperatures (29°C) (Brown et al. 2003; Brown and Kiyatkin 2004). While most experiments in rats are conducted at standard laboratory temperatures (22-23°C), 29°C is not a true hot temperature and is within the range of normothermy or a temperature comfort in rats (Romanovsky et al. 2002), where basal metabolism is maintained at its lowest levels.
As shown in Fig. 6, METH (9 mg/kg, sc) administered in quiet resting conditions strongly increased brain and muscle temperatures (a). The increase was more rapid and stronger in the NAcc than in temporal muscle, suggesting intra-brain heat production as the primary cause of brain hyperthermia and a factor behind a more delayed and weaker body hyperthermia. In contrast to moderate temperature increases (~1°C) elicited by natural stimuli, METH-induced hyperthermia was much stronger (3-4°C) and longer in its duration, exceeding 4-5 hours. When METH was injected during social interaction with another animal, it induced significantly stronger and more prolonged increases in NAcc and muscle temperatures (b). While thermogenic effects of social interaction and METH were not additive, under activated conditions NAcc temperature was maintained above 40°C for about 90 min, while it only touched this value under quiet resting conditions. The effects of METH were greatly potentiated by a slight increase in ambient temperature (c). When tested at 29°C, NAcc temperatures increased rapidly in all animals, rising to clearly pathological values (>41-42°C) and resulting in death of 5/6 animals within six hours post-injection. Similar data were obtained with MDMA (Brown and Kiyatkin 2004). In contrast to dose-dependent increases in brain temperature induced by METH, MDMA at a low dose (1mg/kg) slightly decreased brain temperature and high doses (9 mg/kg) induced increases that were about three-fold weaker (~1°C) than those with METH. However, brain temperature increases induced by MDMA were also potentiated during social interaction and most rats died (5/6) with robust hyperthermia when this drug was used at 29°C. Although 9 mg/kg of METH and MDMA exceeds the doses used by humans, these doses corresponds to only 1/6 and 1/5 of the LD50 in rats (55 and 49 mg/kg for ip injection, respectively; Davis et al. 1987; Yamamato 1963; Cole and Sumnall 2003) and does not induce lethality in normal environmental conditions. Therefore, the large LD50 determined in healthy rats in quiet resting conditions in a standard laboratory settings could be misleading and much smaller drug doses used under specific environmental conditions could induce life-threatening health complications.
Fig 6.
Changes in brain (NAcc) and temporal muscle temperatures induced by meth-amphetamine (9 mg/kg, sc) used under quiet resting conditions at 23°C (a), during social interaction with female at 23°C (b; -30 to 90 min), and in a warm (29°C) environment (c). Vertical hatched lines in each graph show the moment of drug injection. C shows post-METH dynamics of NAcc temperatures in each rat (n=6)
A powerful modulation of the toxic effects of drugs by environmental conditions seen in rats may help to explain the exceptionally strong, sometimes fatal, responses of some individuals to amphetamine-like substances under rave conditions. However, humans have much more sophisticated mechanisms of heat loss from body surfaces than do rats (Gordon 1990, 2007), thus making them more resistant to high environmental temperatures and thermogenic effects of psychomotor stimulants. In contrast to rats, humans have a well-developed ability to sweat and have a very high dynamic range of flow rates in the skin, thus allowing them to lose more metabolic heat (1 kW) than could be maximally produced in the body (Rowell 1983). These differences in the effector mechanisms of heat loss could explain weaker MDMA-induced body temperature increases and their lesser dependence on ambient temperatures found in monkeys (Banks et al 2007; Taffe et al. 2006; Von Hunen et al. 2007) and humans (Freedman et al. 2005). Despite their high efficiency, the compensatory mechanisms of heat loss in humans could be greatly impaired under specific conditions, resulting in progressive heat accumulation in the organism. A simple bicycle exercise that produces ~1°C brain temperature elevation under normal conditions produced strong hyperthermia (39.0-39.5°C) when the exercise is conducted in a special water-impermeable cloth that prevents heat dissipation to the external environment (Nybo et al. 2002). Therefore, pathological brain hyperthermia induced by overdose of psychomotor stimulants under rave conditions results not only from excessive heat production due to drug-induced and psycho-physiological activation but also from the impaired ability to dissipate metabolic heat due to environmental factors, powerful drug-induced peripheral vasoconstriction and a similar effect associated with psycho-physiological activation.
7. Adverse environmental conditions enhance histochemical and morphological perturbations induced by METH: Role of brain temperature
In addition to the social harms usually ssociated with drug addiction, the use of psychomotor stimulants could adversely influence human health, causing acute behavioral and physiological disturbances during intoxication and long-term neurological complications following chronic use (Kalant 2001). By inducing powerful and prolonged metabolic activation, psychomotor stimulants could be a co-factor in enhancing different latent pathological conditions, especially cardiovascular, neurological and psychiatric. By weakening the immune system, chronic drug use also increases the probability and severity of numerous viral and bacterial infections.
Considering the issue of neurotoxicity, it is usually assumed that METH and related drugs have direct toxic effects on neural cells, with relative selectivity towards specific cell groups, brain structures, and cellular organelles. In particular, METH preferentially affects midbrain DA neurons, damaging fine axonal terminals in the striatum (Ricaurte et al. 1980; Riddle et al. 2006; Woolverton et al. 1989) and resulting in health complications associated with pathologically altered DA transmission. Alterations in the activity and responsiveness of DA and other monoamine systems are important factors in psycho-emotional and psychiatric disorders including acute METH psychosis and severe depression following long-term METH use (Kalant 2001). However, METH and other psychomotor stimulant drugs also induce metabolic activation and hyperthermia. Enhanced metabolism is tightly related to oxidative stress, which is caused by an imbalance between the production of reactive oxygen and the ability of an organism to detoxify the reactive intermediates and repair the resulting damage. Disturbances in this normal red:ox state can cause toxic effects by the production of peroxides and free radicals that damage all components of the cell including proteins, lipids, and DNA. Oxidative stress as a consequence of brain hyper-metabolism is usually viewed as a primary factor of METH-induced neurotoxicity (Cadet et al. 2007; De Vito and Wagner 1989; Stephans and Yamamoto 1994). On the other hand, brain cells are exceptionally temperature-sensitive, with the appearance of structural abnormalities at ~40°C, i.e., only three degrees above a normal baseline (Chen et al. 2003; Iwagami 1996; Oifa and Kleshchnev 1985; Lepock 2003; Sharma and Hoopes 2003; Yamamoto and Zhu 1998). Due to the temperature dependence of most physico-chemical processes governing neural activity, hyperthermia could be a powerful factor to enhance the toxic effects of METH on brain cells.
In addition to the direct effects of high temperatures on brain cells and the potentiation of toxic effects of drug metabolites, brain hyperthermia appears to alter permeability of the BBB—an important border that maintains stability of the brain environment and protects neural cells from potentially dangerous ionic and chemical perturbations occurring in the body (Rapoport 1976; Zlokovic 2008). Although leakage of the BBB has been documented during environmental warming (Cervos-Navarro et al. 1998; Sharma et al. 1992), intense physical exercise (Watson et al., 2005), various types of stress (Esposito et al. 2001; Ovadia et al. 2001; Sharma and Dey 1986), and morphine withdrawal (Sharma and Ali 2006), data on drug-induced alterations in the BBB and its relationship to brain temperature are limited.
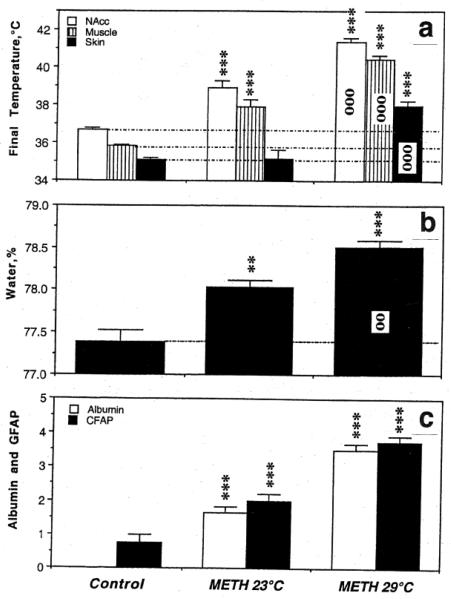
To clarify these issues, our physiological recordings were supplemented by histochemical and morphological examinations of brain tissue to determine acute changes in BBB permeability, glial activation, and alterations of brain cell morphology during acute METH intoxication in standard (23°C) and warm (29°C) ambient temperatures (Kiyatkin et al. 2007; Sharma and Kiyatkin 2009). The brains in this experiment were taken at different times after METH administration when NAcc temperature peaked or reached clearly pathological values (>41.5°C). The state of BBB permeability and edema were determined by albumin immunoreactivity and measuring brain water content. Albumin is a relatively large plasma protein (molecular weight 59 kDa, molecular diameter 70A) that is normally confined to the luminal side of the endothelial cells and is not present in the brain under normal conditions. Thus, the appearance of albumin immunoreactivity in brain cells or neuropil indicates a breakdown of the BBB. Glial fibrillary acidic protein (GFAP) is an intermediate filament protein that is expressed in glial cells (astrocytes) and the increased GFAP immunoreactivity (or astrocytic activation) is usually viewed as an index of gliosis or a relatively slow-developing correlate of neural damage (Finch 2003; Hausmann 2003). Normal brain tissue has only scattered GFAP-positive cells but rapid GFAP expression has been reported previously during environmental warming and brain trauma (Gordth et al. 2006; Sharma et al. 1992). Therefore, changes in GFAP immunoreactivity served as an index of acute METH-induced glial activation. Finally, brain slices were examined using light and electron microscopy to determine the extent and specifics of cellular abnormalities.
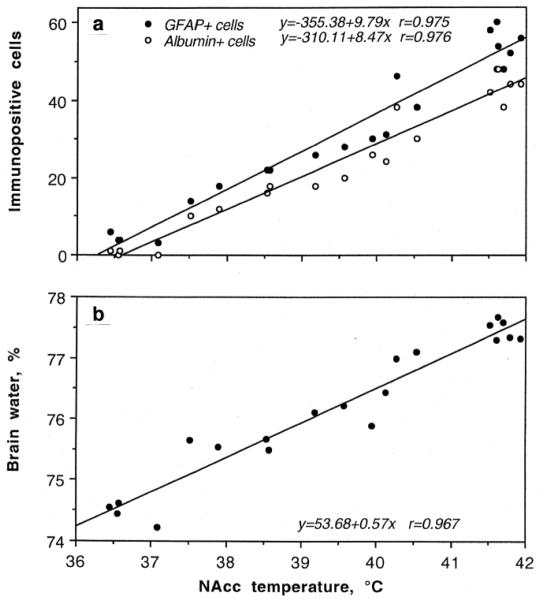
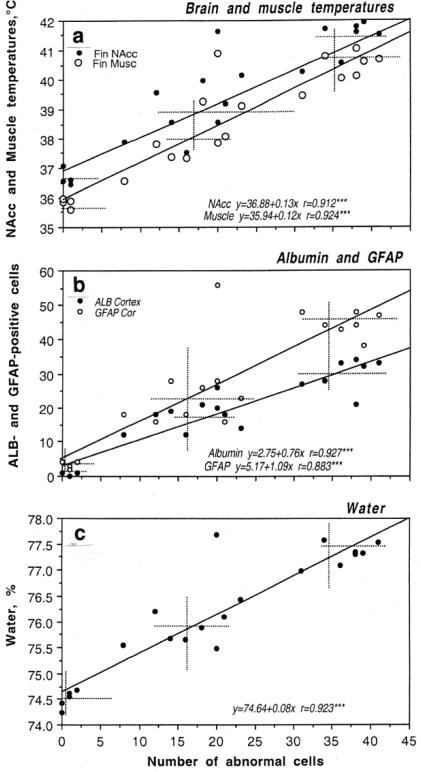
As shown in Fig 7, METH induced significant BBB leakage. Compared to saline-treated controls, albumin immunoreactivity increased strongly in both METH groups and the changes were significantly larger when the drug was administered at 29° than 23°C (Fig 7c). While rats in each condition showed significant increases in NAcc temperature, the increase was significantly stronger when METH was used at 29°C (Fig 7a). As shown in Fig.8a, albumin immunoreactivity was strongly dependent on brain temperature, with virtually no positive cells at low basal temperatures and a progressive increase at high temperatures. Similar differences were found with respect to astrocytic activation (Fig 7c). While only a few GFAP-positive astrocytes were scattered in the normal brains, their number was significantly larger in the METH-23°C group and almost doubled in the METH-29°C group. Similar to albumin, GFAP counts were also tightly correlated with brain temperatures (Fig 8a), suggesting that acute glial reaction is progressively stronger depending on the extent of brain temperature elevation. METH intoxication also strongly increased brain water content and this increase was enhanced when the drug at 29°C (Fig 7b). Tissue water content was directly related to brain temperatures (Fig 8b), suggesting tight relationships between brain hyperthermia and edema.
Fig 7.
Changes in several brain parameters (a, NAcc. Muscle and skin temperatures; b, brain tissue water content; c, albumin- and GFAP-positive cells) Induced by METH (9 mg/kg, sc) at normal (23°C) and warm (29°C) ambient temperatures. Control represents data obtained after saline injection. Asterisks mark values significantly different from control (**, p<0.01 and ***, p<0.001; Student's t-test) and circles mark significance of between-group (23°C vs. 29°C) differences. Original data were described in Kiyatkin et al. 2007
Fig 8.
The relationships between NAcc temperatures and the numbers of albumin- and GFAP-positive cells (a) and tissue water (b) during acute METH intoxication. There was a tight correlation (r is coefficient of correlation) between changes in these parameters. Histochemical data are shown for cortex. Original data were described in Kiyatkin et al., 2007
Acute METH exposure also induced structural abnormalities of brain cells (Fig 9). The number of abnormal cells assessed in the cortex tightly correlated with brain and muscle temperatures (a), albumin leakage and glial activation (b), and tissue water accumulation (c). Morphological cell abnormalities were virtually absent in saline-treated control, significantly increased in the METH-23°C group, and doubled again in the METH-29°C group. Correlations between all these parameters were linear within the entire range of their fluctuations.
Fig 9.
The relationships between the structural brain cell abnormalities (assessed in cortex) induced by methamphetamine (9 mg/kg, sc) and other physiological and brain parameters (a, Brain and muscle temperatures; b, Albumin and GFAP-positive cells; c, brain water content). Crosses of hatched lines show mean values of each parameter in control (saline), METH-23°C and METH-29°C groups. Each graph shows coefficients of correlation (r) and regression equations that describe the relationships between the parameters. Graphs were
Therefore, these data suggest that acute METH intoxication results in robust breakdown of the BBB, glial activation, and morphological abnormalities of brain cells. These effects are enhanced when the drug is used in a moderately warm environment, tightly correlating with drug-induced brain temperature elevation. Therefore, the neurotoxic effects of METH depend not only on direct drug action on brain cells and drug-induced metabolic activation but are also strongly modulated by environmental conditions associated with drug administration.
8. Conclusions and Functional implications
During their life span, humans are exposed to multiple neuroactive drugs, which could be administered and self-administered therapeutically to treat certain disease or taken voluntarily to induce desired subjective effects. While the direct action of these drugs on central neurons is commonly viewed as the basic mechanism underlying their behavioral, physiological and psycho-emotional effects, most of these drugs also alter brain metabolism as well as central and peripheral blood circulation, thus affecting brain temperature. Since most physico-chemical processes governing neural activity and neural functions are temperature-dependent, drug-induced temperature changes contribute to their direct central effects, thus affecting behavioral and physiological effects of drugs. Therefore, drug-induced change in brain temperature is simultaneously an important physiological parameter, which could provide new information on drug's actions in the CNS and a factor affecting drug-induced changes in neural activity and neural functions.
Data presented in this review suggest that brain temperature in awake, freely moving organisms is not stable but fluctuates within relatively large limits (~3°C), reflecting spontaneous and stimuli-induced fluctuations in activity state. Since drugs interfere with these variable activity states, their effects vary depending upon the environmental context and the organism's functional state at the moment of administration. This environment-state-drug interaction could be especially important for addictive drugs that are self-administered during psych-physiological activation under specific environmental conditions. This state dependency could play an important role in mediating the acute harmful effects of drugs, possibly contributing to more slowly developing neurotoxicity with their chronic use. As shown here, even relatively small changes in environmental conditions or activity state are able to dramatically amplify METH-induced functional and structural neural perturbations, often resulting in massive neural damage. Therefore, our knowledge on brain temperature and its physiological and drug-induced fluctuations could add a new dimension to understanding the mechanisms of drug action and adverse health effects induced by neuroactive drugs.
Acknowledgements
This study was supported by the Intramural Research Program of NIDA-NIH. I wish to thank Drs. Magalie Lenoir and Ken T. Wakabayashi for valuable comments on the matter of this manuscript.
Abbreviations
- APO
apomorphine
- DA
dopamine
- GFAP
glial fibrillary acidic protein
- iv
intravenous
- ip
intraperitoneal
- METH
methamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- NAcc
nucleus accumbens
- sc
subcutaneous
- VTA
ventral tegmental area
References
- Alberts DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–14. [PubMed] [Google Scholar]
- Ali SF, Newport GD, Holson RR, Slikker W, Bowyer JF. Low environmental temperatures or pharmacological agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–8. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- Armenian P, Mamantov TM, Tsutaoka BT, Gerona RR, Silman EF, Wu AH, Olson KR. Multiple MDMA (Ecstasy) overdoses at a rave event: A case series. J Intensive Care Med. 2012;XX:XX. doi: 10.1177/0885066612445982. (in press) [DOI] [PubMed] [Google Scholar]
- Baker MA, Frye FM, Millet VE. Origin of temperature changes evoked in the brain by sensory stimulation. Exp Neurol. 1973;38:502–19. doi: 10.1016/0014-4886(73)90172-6. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-Methylenedioxythamphetamine-induced thermodysregulation and pharmacokinetics in male monkey. Drug Metabolism Disposition. 2007;35:1840–5. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Gough B, Slikker W, Lipe GW, Wewport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44:87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- Brown PL, Wise RA, Kiyatkin EA. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J Neurosci. 2003;23:3924–9. doi: 10.1523/JNEUROSCI.23-09-03924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (“ecstasy”): modulation by environmental conditions. Eur J Neurosci. 2004;20:51–8. doi: 10.1111/j.0953-816X.2004.03453.x. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:930–8. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Brown PL, Bae D, Kiyatkin EA. Relationships between locomotor activation and alterations in brain temperature during selective blockade and stimulation of dopamine transmission. Neuroscience. 2007;145:335–43. doi: 10.1016/j.neuroscience.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci. 2000;23:S57–63. doi: 10.1016/s1471-1931(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Calne DB, Claveria LE, Reid JL. Hypothermic action of bromocriptine. Br J Pharmacol. 1975;54:123–4. doi: 10.1111/j.1476-5381.1975.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervos-Navarro J, Sharma HS, Westman J, Bongcum-Rudloff E. Glial cell reactions in the central nervous system following heat stress. Progr Brain Res. 1998;115:241–74. doi: 10.1016/s0079-6123(08)62039-7. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Xu RX, Huang QJ, Xu ZJ, Jiang XD, Cai YO. Effect of hyperthermia on tight junctions between endothelial cells of the blood-brain barrier model in vitro. Di Yi Jun Da Xue Xue Bao. 2003;23:21–4. [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The pre-clinical behavioral pharmacology of 3,4-methylenedioxymethamphetamine (MDMA). Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Cox B, Lee TF. Further evidence for a physiological role for hypothalamic dopamine in thermoregulation in the rat. J Physiol. 1980;300:7–17. doi: 10.1113/jphysiol.1980.sp013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PD, Braun LD, Cornford EM, Cremer JE, Glass JM, Oldendorf WH. Dose dependent reduction of glucose utilization by pentobarbital in rat brain. Stroke. 1978;9:12–8. doi: 10.1161/01.str.9.1.12. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol Beh. 1995;58:877–82. doi: 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Davis WM, Hatoum HT, Walters IW. Toxicity of MDA (2.4-methylenedioxyamphetamine) considered for relevance to hazards of MDMA (Ecstasy) abuse. Alcohol Drug Res. 1987;7:123–34. [PubMed] [Google Scholar]
- Delgado JMR, Hanai T. Intracerebral temperatures in free-moving cats. Am J Physiol. 1966;211:755–69. doi: 10.1152/ajplegacy.1966.211.3.755. [DOI] [PubMed] [Google Scholar]
- De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–50. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- Esposito P, Cheorghe D, Kendere K, Pang X, Connoly R, Jaconson S, Theodorides TC. Acute stress increases permeability of the blood-brain barrier through activation of brain must cells. Brain Res. 2001;888:117–27. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–7. doi: 10.1016/s0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Seiden LS. Role of hyperthermia in the mechanism of protection against serotoninergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J Pharmacol Exp Ther. 1995;272:868–75. [PubMed] [Google Scholar]
- Freedman RR, Johanson C-E, Tancer ME. Thermoregulatory effects of 3,4-metylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2005;183:248–56. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminegic neurons in freely moving rats. Life Sci. 1985;20:1983–94. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Fuller CA, Baker MA. Selective regulation of brain and body temperatures in the squirrel monkey. Am J Physiol. 1983;245:R293–7. doi: 10.1152/ajpregu.1983.245.2.R293. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Sjoqvist F. Hypothermic effect of apomorphine in the mouse. J Pharm Pharmacol. 1972;24:702–5. doi: 10.1111/j.2042-7158.1972.tb09093.x. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav. 1990;47:963–991. doi: 10.1016/0031-9384(90)90025-y. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermophysiological responses to hyperthermic drugs: extrapolating from rodent to human. Prog Brain Res. 2007;162:63–79. doi: 10.1016/S0079-6123(06)62005-0. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O'Callaghan PP, Miller DB. Effects of 3,4-Metylenedioxymetamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–44. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Gordth T, Chu H, Sharma HS. Spinal nerve lesion alters blood-spinal cord barrier functions and activates astrocytes in the rat. Pain. 2006;124:211–21. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”). Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–78. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Baker MA. Role of cerebral blood flow in the regulation of brain temperature in the monkey. Am J Physiol. 1968;215:389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain uncoupling protein 2: Uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J Neurosci. 1999;19:10417–27. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci. 1985;5:297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwagami Y. Changes in the ultrastructure of human cell related to certain biological responses under hyperthermic culture conditions. Human Cell. 1996;9:353–366. [PubMed] [Google Scholar]
- Jackson DM, Westlind-Dinielsson A. Dopamine receptors: Molecular biology, biochemistry and behavioral aspects. Pharmac Ther. 1994;65:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Ass J. 2001;165:917–28. [PMC free article] [PubMed] [Google Scholar]
- Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Can Med Assoc J. 1975;112:299–304. [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Dopamine in the nucleus accumbens: cellular actions, drug- and behavior-associated fluctuations, and a possible role in an organism's adaptive activity. Behav Brain Res. 2002;137:27–46. doi: 10.1016/s0166-4328(02)00283-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain hyperthermia as physiological and pathological phenomena. Brain Res Rev. 2005;50:27–56. doi: 10.1016/j.brainresrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature responses to salient stimuli persist during dopamine receptor blockade despite a blockade of locomotor responses. Pharmacol Biochem Behav. 2008;91:233–42. doi: 10.1016/j.pbb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci. 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Fluctuations in neural activity during cocaine self-administration: Clues provided by brain thermorecording. Neuroscience. 2003;116:525–38. doi: 10.1016/s0306-4522(02)00711-x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain temperature fluctuations during repeated passive vs. active cocaine administration: Clues for understanding the pharmacological determination of drug-taking behavior. Brain Res. 2004;1005:101–16. doi: 10.1016/j.brainres.2004.01.038. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav. 2005;84:563–70. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of blood-brain barrier during methamphetamine intoxication: Critical role of brain temperature. Eur J Neurosci. 2007;26:1242–53. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Mitchum R. Fluctuations in brain temperatures during sexual behavior in male rats: An approach for evaluating neural activity underlying motivated behavior. Neuroscience. 2003;119:1169–83. doi: 10.1016/s0306-4522(03)00222-7. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats. J Neurosci. 1999;19:3594–609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wise RA. Brain and body hyperthermia associated with heroin self-administration in rats. J Neurosci. 2002;22:1072–80. doi: 10.1523/JNEUROSCI.22-03-01072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JM. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Molecular footprints of neurotoxic amphetamine action. Ann NY Acad Sci. 2000;914:92–103. doi: 10.1111/j.1749-6632.2000.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Samsonova ML. Apomorphine-induced hypothermia in mice and the effect thereon of adrenergic and serotoninergic agents. Farmacol Toxicol. 1968;31:563–9. (in Russian) [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network--functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Lenhardt R. The effect of anesthesia on body temperature control. Front Biosci. 2010;15:1145–54. doi: 10.2741/s123. [DOI] [PubMed] [Google Scholar]
- Lepock JR. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia. 2003;19:252–66. doi: 10.1080/0265673031000065042. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chen YF, Wang Z, Wang HS. Effects of apomorphine on thermoregulatory responses of rats to different ambient temperatures. Can J Physiol Pharmacol. 1979;57:469–75. doi: 10.1139/y79-071. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chandra A, Tsay BL, Chern YF. Hypothalamic and striatal dopamine receptor activation inhibits heat production in the rat. Am J Physiol. 1982;242:R471–81. doi: 10.1152/ajpregu.1982.242.5.R471. [DOI] [PubMed] [Google Scholar]
- Lin MT, Ho MT, Young MS. Stimulation of the nigrostriatal dopamine system inhibits both heat production and heat loss mechanisms in rats. Naunyn Schmiedberg's Arch Pharmacol. 1992;346:504–10. doi: 10.1007/BF00169004. [DOI] [PubMed] [Google Scholar]
- Ma J, Ye N, Lange N, Cohen BM. Dynorphinergic GABA neurons are a target of both typical and atypical antipsychotic drugs in the nucleus accumbens shell, central amygdaloid nucleus and thalamic central medial nucleus. Neuroscience. 2003;121:991–8. doi: 10.1016/s0306-4522(03)00397-x. [DOI] [PubMed] [Google Scholar]
- Mariak Z, Jadeszko M, Lewko J, Lebkowski W, Lyson T. No specific brain protection against thermal stress in fever. Acta Neurochir. (Wien) 1998;140:585–90. doi: 10.1007/s007010050144. [DOI] [PubMed] [Google Scholar]
- Mariak Z, Lebkowski W, Lyson T, Lewko J, Piekarski P. Brain temperature during craniotomy in general anesthesia. Neurol Neurochir Pol. 1999;33:1325–7. [PubMed] [Google Scholar]
- Mariak Z, Lyson T, Peikarski P, Lewko J, Jadeszko M, Szydlik P. Brain temperature in patients with central nervous system lesions. Neurol Neurosurg Pol. 2000;34:509–22. [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O'Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenediomethamphetamine (MDMA, “ecstasy”) to rats. Br J Pharmacol. 2002;135:170–80. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellergard P, Nordstrom CH. Epidural temperatures and possible intracerebral temperature gradients in man. Br J Neurosurg. 1990;4:31–8. doi: 10.3109/02688699009000679. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 2003;92:48–53. doi: 10.1016/s0013-9351(02)00051-8. [DOI] [PubMed] [Google Scholar]
- Nimmo SM, Kennedy BW, Tullett WM, Blyth AS, Dougall JR. Drug-induced hyperthermia. Anesthesia. 1993;48:892–5. doi: 10.1111/j.1365-2044.1993.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Nybo L. Hyperthermia and fatigue. J Appl Physiol. 2008;104:871–7. doi: 10.1152/japplphysiol.00910.2007. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH, Nielson B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oifa AI, Kleshchnov VN. Ultrastructural analysis of the phenomenon of acute neuronal swelling. Zh Nevropatol Psikhiatr Im SS Korsakova. 1985;85:1016–20. (in Russian) [PubMed] [Google Scholar]
- Ovadia H, Abramsky O, Feldman S, Weidenfeld J. Evaluation of the effects of stress on the blood-brain barrier: critical role of the brain perfusion time. Brain Res. 2001;905:21–25. doi: 10.1016/s0006-8993(01)02361-7. [DOI] [PubMed] [Google Scholar]
- Pederson NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) on conscious rabbits. J Neurosci. 2001;21:8648–54. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed–ratio size. J Pharmacol Exp Ther. 1968;16:122–9. [PubMed] [Google Scholar]
- Pierce EC, Lambertsen CJ, Deautch S, Chase PE, Linde HW, Dripps RD, Price HL. Cerebral circulation and metabolism during thiopental anesthesia and hyper-ventilation in man. J Clin Invest. 1962;41:1664–71. doi: 10.1172/JCI104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rango M, Arighi A, Bonifati C, Bresolin N. Increased brain temperature in Parkinson's disease. Neuroreport. 2012;23:129–33. doi: 10.1097/WNR.0b013e32834e8fac. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Blood-brain barrier in physiology and medicine. Raven Press; New York: 1976. [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. The AAPS Journal. 2006;8(2) doi: 10.1007/BF02854914. Article 48 ( http://www.aapsj.orghttp://www.aapsj.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for expetiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–79. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res. 1983;52:367–76. doi: 10.1161/01.res.52.4.367. [DOI] [PubMed] [Google Scholar]
- Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperatures exceed systemic temperatures in head-injured patients. Clin Care Med. 1998;26:562–7. doi: 10.1097/00003246-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Cur Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Hanson GR, Fleckenstein AE. Methamphetamine decreases mouse striatal dopamine transport activity: roles of hyperthermia and dopamine. Eur J Pharmacol. 2000;409:265–71. doi: 10.1016/s0014-2999(00)00871-2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Adaptation and environment. 5th Edition Cambridge University Press; Cambridge: 1997. Animal physiology. [Google Scholar]
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–33. doi: 10.1016/s0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:62–7. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–38. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr. 1996;163:251276. [PubMed] [Google Scholar]
- Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and amphetamine. Ann NY Acad Sci. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Dey PK. Influence of long-term immobilization stress on regional blood-brain permeability, cerebral blood flow and 5-HT levels in conscious normotensive young rats. J Neurol Sci. 1986;72:61–76. doi: 10.1016/0022-510x(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Hoopes PJ. Hyperthermia-induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19:325–54. doi: 10.1080/0265673021000054621. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Kiyatkin EA. Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy. J Chem Neuroanat. 2009;37:18–32. doi: 10.1016/j.jchemneu.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Zimmer C, Westman J, Cervos-Navarro J. Acute systemic heat stress increases glial fibrillary acidic protein immunoreactivity in brain. An experimental study in the conscious normotensive young rats. Neuroscience. 1992;48:889–901. doi: 10.1016/0306-4522(92)90277-9. [DOI] [PubMed] [Google Scholar]
- Siesjo B. Brain Energy Metabolism. Wiley; New York: 1978. [Google Scholar]
- Smirnov MS, Kiyatkin EA. Fluctuations in central and peripheral temperatures associated with feeding behavior in rats. Amer J Physiol. 2008;295:R1414–24. doi: 10.1152/ajpregu.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: role for glutamate and dopamine influx. Synapse. 1994;17:203–9. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Teffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–81. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Kulkarni SK. Differential role of dopamine receptor subtypes in thermoregulation and stereotypic behavior in naïve and reserpinized rats. Arch Int Pharmacodyn Ther. 1993;324:17–32. [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/-)3,4-methylenedioxymethamphetamine in rhesus macaques. Neurophysichopharmacology. 2007;32:673–81. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Shirreffs SM, Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol. 2005;288:R1689–94. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- Windels F. Neuronal activity: from in vitro preparation to behaving animals. Mol Neurobiol. 2006;34:1–26. doi: 10.1385/mn:34:1:1. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. Stability of substantia nigra pars reticulata neuronal discharge rates during dopamine receptor blockade and its possible mechanisms. NeuroReport. 2006;17:1071–5. doi: 10.1097/01.wnr.0000221845.43126.7d. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Haloperidol induces Fos expression in the globus pallidus and substantia nigra of cynomolgus monkeys. Brain Res. 1995;835:154–61. doi: 10.1016/s0006-8993(99)01550-4. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Dopamine antagonists induce Fos-like immunoreactivity in the substantia nigra and entopeduncular nucleus of the rat. Brain Res. 1999;670:205–14. doi: 10.1016/0006-8993(94)01280-u. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Effects of haloperidol and clozapine on Fos expression in the primate striatum. NeuroReport. 2003;14:2429–32. doi: 10.1097/00001756-200312190-00028. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno L, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effect of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yamamoto H. The central effects of xylopinine in mice. Jap J Pharmacol. 1963;13:230–239. doi: 10.1254/jjp.13.230. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]