Abstract
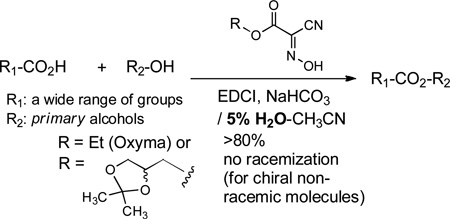
Oxyma and an oxyma derivative, (2,2-dimethyl-1,3-dioxolan-4-yl)methyl 2-cyano-2-(hydroxyimino)acetate (5b), displayed remarkable effect on selective esterifications of primary alcohols. A wide range of carboxylic acids could be esterified with primary alcohols by using EDCI, NaHCO3, and Oxyma or an Oxyma derivative 5b in 5% H2O-CH3CN. An Oxyma derivative 5b is particularly useful since it could be removed after the reaction via a simple basic or an acidic aqueous work-up procedure.
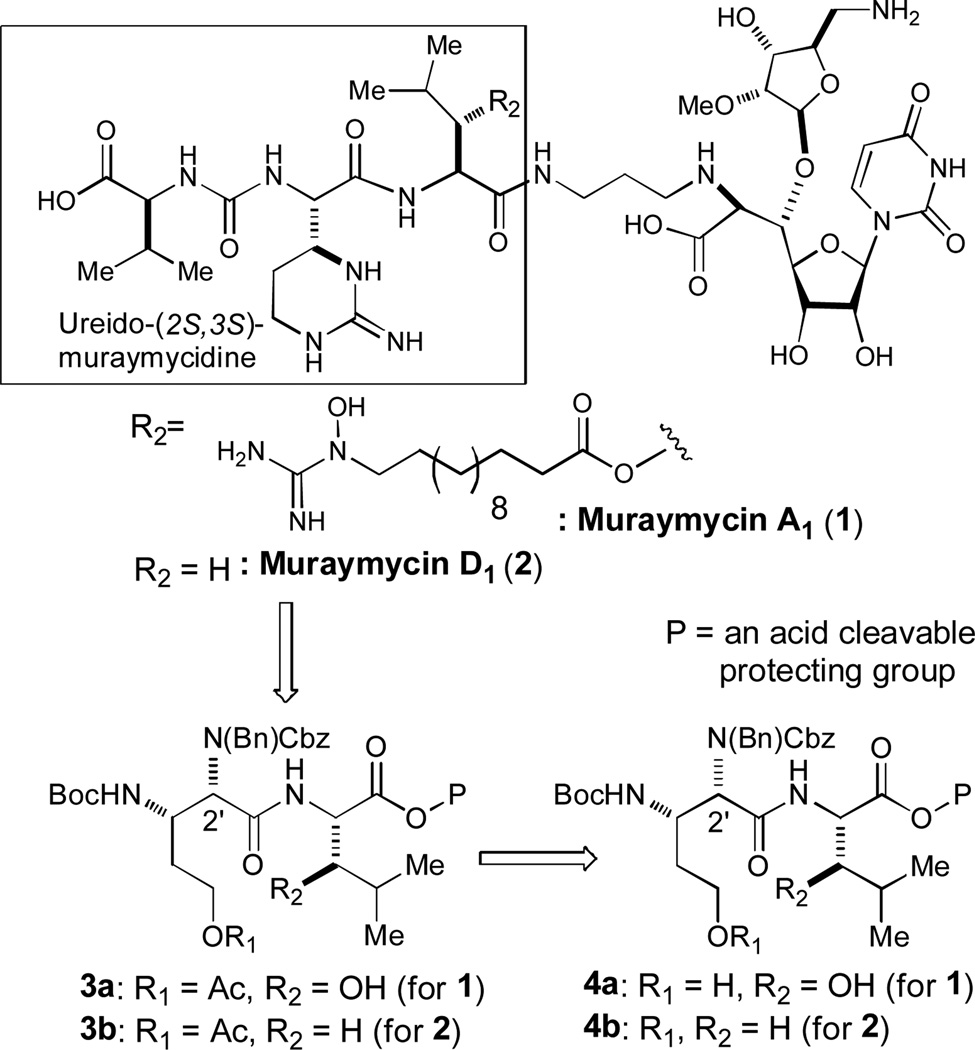
In our efforts on total synthesis of muraymycins A1 (1) and D1 (2), and their analogs for structure-activity relationship studies against Gram-positive bacteria including M. tuberculosis, it is crucial to develop an efficient synthesis of the dipeptide 3a and 3b (Figure 1).1 We have recently reported an efficient synthesis of the ureido-muraymycidine derivatives (the partial structure highlighted in a box in Figure 1).1b In the synthesis of muraymycin A1 selective acetylation of the primary alcohol is necessary to accomplish an efficient synthesis of the left half of 1. We have screened reported esterification conditions for 4a to form the mono-acetate 3a. Although several acetylation conditions with the controlled amounts of reagents and at lowered temperatures provided the mono-acetate at the primary alcohol, the selectivity of mono- and di-acetate was not satisfactory. For example, acetylation of 4a with Ac2O (5 equiv) and pyridine (10 equiv) in CH2Cl2 at 0 °C gave a mixture of 3a and the di-acetate (3/1) in less than 40% yield. DCC-mediated acetylations under anhydrous conditions yielded the diacetate as a major product. Thus, we commenced optimizing esterification conditions that protect the primary alcohol of 4a with AcOH to yield 3a exclusively.
Figure 1.
Syntheses of muraymycins A1 (1) and D1 (2).
In our recent finding of amide-forming reactions with the ethyl 2-cyano-2-(hydroxyimino)acetate (Oxyma)2 derivative (glyceroacetonide-Oxyma, 5b in Table 1) in water media, it was observed that the 5b-esters of amino acids (e.g. 9) are stable during amide-forming reactions in water. Typically, glyceroacetonide-Oxyma catalyzed amide-forming reactions could be achieved with EDCI (1.5 equiv), NaHCO3 (3–6 equiv) in water (0.2–0.3 M) to yield the corresponding peptides in greater than 90% without detectable diastereomers.3 It has been reported that nucleophilicity of the oxygen atom of alcohols is slightly stronger than that of water.4 Thus, we expected that selective coupling of the oxime-esters 9 (Table 1) with alcohols could be achieved in water media in the presence of a weak base. Gratifyingly, acetylation of 4a with excess AcOH (10 equiv), glyceroacetonide-Oxyma 5b (5 equiv), NaHCO3 (10 equiv) in water (0.2 M) provided the mono-acetate 3a in 85% yield without the formation of the diacetate (R2 = OAc in 3a in Figure 1). Herein, we report the optimization of selective esterifications of primary alcohols with Oxyma 5a or glyceroacetonide-Oxyma 5b, EDCI, and NaHCO3 in water-containing solvent systems.
Table 1.
Amide- or ester-forming reactions in water media.
 | ||||
|---|---|---|---|---|
| entry | additive / solvent |
7 or 8 | product (10 or 11) |
yield (%)d |
| 1a |
5b H2O |
HCl•H-L-Ala- OMe(7a) |
Boc-Phe-Ala- OMe (10a)c |
93 |
| 2a |
5a H2O |
7a | 10ac | 25 |
| 3b |
5b H2O |
MeOH | Boc-Phe-OMe (11a)c |
45 |
| 4b |
5b 50% H2O- CH3CN |
MeOH | 11a | 55 |
| 5b |
5b 5%H2O- CH3CN |
MeOH | 11ac | >95 |
| 6b |
5a 5%H2O- CH3CN |
MeOH | 11ac | >95 |
| 7b |
5a 5%H2O- dioxane |
MeOH | 11ac | 90 |
| 8b |
5a 5%H2O- acetone |
MeOH | 11ac | 85 |
| 9b |
5a 5%H2O- CH3CN |
iPrOH | Boc-Phe-OiPr (11b)c |
0 |
| 10b |
5a 5%H2O- CH3CN |
tBuOH | Boc-Phe-OtBu (11c)c |
0 |
6 (1.0 equiv), 7 (1.5 equiv), additive (1.5 equiv), EDCI (1.5 equiv), NaHCO3 (6 equiv) in H2O (0.2 M concentrations), 2 h;
6 (1.0 equiv), 8 (2.0 equiv), additive (1.5 equiv), EDCI (1.5 equiv), NaHCO3 (6 equiv) (0.2 M concentrations);
de or ee was determined to be >99% via HPLC analysis;
isolated yield.
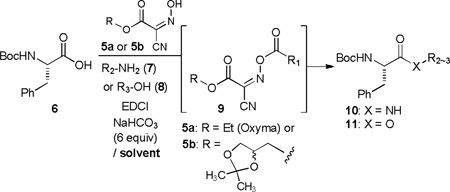
Although an acetylation of 4a to form 3a could be achieved in water with excess reagents, high-yielding esterifications of alcohols using limited amount of carboxylic acids or alcohols are considered to be challenging transformations in aqueous media. Uronium-based reagents have previously been applied to introduce esters on primary alcohols under non-aqueous conditions.5 To the best of our knowledge, no practical esterification reaction has been developed in water-containing solvent systems. We have observed that glyceroacetonide-Oxyma 5b is beneficial in high-yielding amide-forming reactions in water with wide range of amino acid derivatives.3 Reactivity difference between Oxyma 5a and 5b in amide-forming reactions in water is attributed to the fact that water solubility of 5b is improved 2.1 times greater than that of 5a at pH 8.3 (entries 1 and 2 in Table 1).6 The esterification reactions of Boc-L-Phe-OH (6, 1 equiv) with alcohols (2 equiv), 5a or 5b (1.5 equiv), EDCI (1.5 equiv), and NaHCO3 (6 equiv) were examined in water and water-containing solvent systems, and these data are summarized in Table 1. Esterification of 6 with MeOH in water furnished Boc-L-Phe-OMe (11a) in 45% yield in 2 h (entry 3). This low-yielding reaction in entry 3 was attributed to a slower reaction rate of the esterification compared to the amide-forming reaction in entry 1. In addition, it was realized that the oxime-ester intermediate 9 has a half-life of approximately 6 h in water at pH 8.3.7 Thus, we examined the effect of a co-solvent to increase nucleophilicity of alcohol and a half-life of 9. The same reaction in H2O-CH3CN (1/1) improved the isolated yield of 11a to 55% after 2 h (entry 4). Significant improvement of methyl esterification of 6 was observed when the reaction was performed in 5% H2O-CH3CN (entry 5); the isolated yield of 11a was greater than 95%. Oxyma 5a could effectively serve as a coupling additive for an esterification reaction in the solvent system (5% H2O-CH3CN) (entry 6). Thus, further studies of selective esterifications of primary alcohols were performed using Oxyma 5a. Although several solvents such as 5% H2O-dioxane and 5% H2O-acetone could be utilized for effective methyl esterification of 6 (entries 7 and 8), the esterifications in 5% H2O-CH3CN were superior to those in the other solvent system tested. Under the optimized conditions [acid (1 equiv), alcohol (2 equiv), 5a or 5b (1.5 equiv), EDCI (1.5 equiv), and NaHCO3 (6 equiv)], isopropanol and tert-butanol did not form the corresponding esters with 6 even after a prolonged reaction time.8
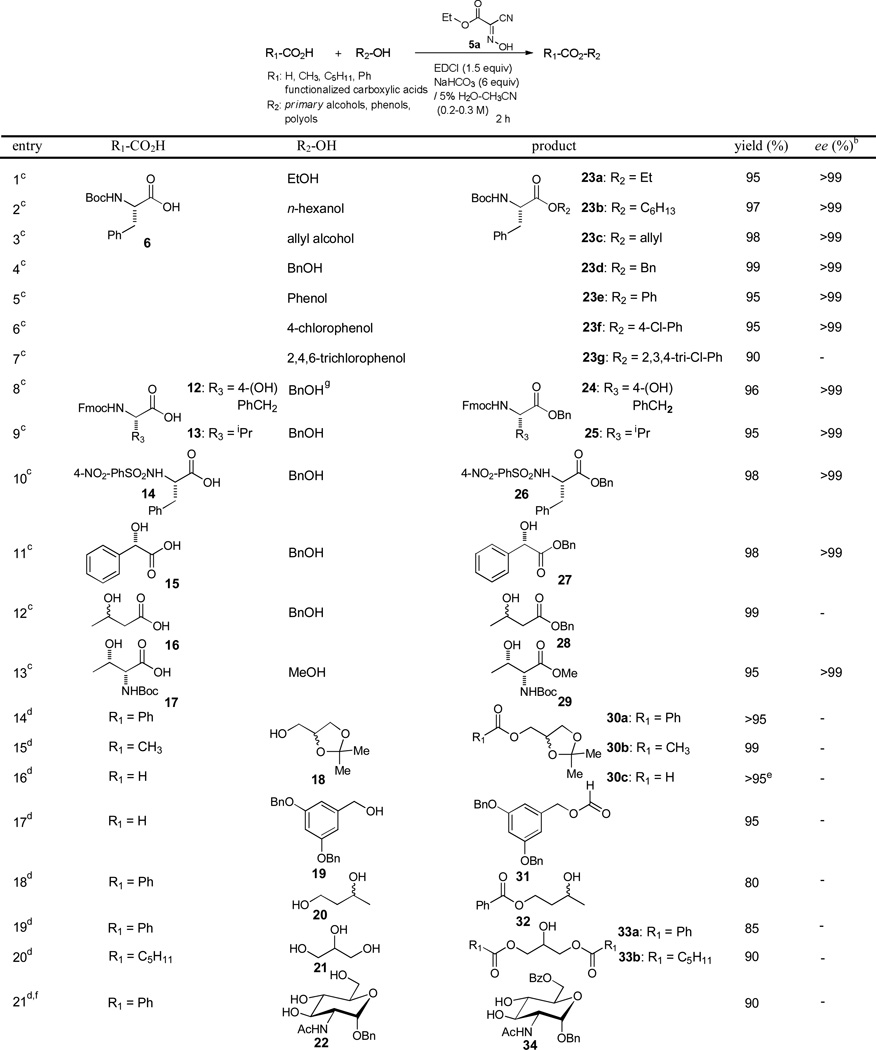
In order to understand the scope and limitations of the selective esterification reactions of primary alcohols with EDCI, Oxyma 5a, and NaHCO3 in 5% H2O-CH3CN, these conditions were applied to esterifications of a wide variety of acids with alcohols. Selected examples are summarized in Table 2. Esterifications of 6 with methanol, primary alcohols and phenols furnished the corresponding esters in greater than 90% yield without detectable racemization (entries 1–7). Significantly, allyl alcohol could be esterified to provide 23c in 98% yield. It is worth pointing out that esterifications of carboxylic acid with allyl alcohols have never been successfully performed using carbodiimide-mediated reaction conditions (entry 3).9 Unlike 4-(dialkylamino)pyridine-catalyzed DCC-mediated esterification conditions, the Fmoc-group was not cleaved during the benzyl esterifications of the Fmoc-protected amino acids, 12 and 13 (entries 8 and 9).10 Esterifications of N-sulfonylated α-amino acids using carbodiimide coupling reagents often result in low conversion with significant racemization. However, under the conditions in Table 2, the benzyl esterification of 14 furnished 26 in 98% yield with >99% ee (entry 10). The chiral carboxylic acids possessing secondary alcohols, 15, 16, and 17 could be esterified efficiently with the primary alcohols. Benzyl esterifications of (S)-mandelic acid (15) and 3-hydroxybutanoic acid (16) furnished the corresponding benzyl esters 27 and 28 in 98% and 99% yields, respectively (entries 11 and 12). Methyl esterification of Boc-L-Thr-OH (17) gave rise to Boc-L-Thr-OMe (29) in 95% yield (entry 13). Benzoylation, acetylation, and formylation reactions of DL-1,2-isopropylideneglycerol (18) provided the corresponding esters 30a-c in greater than 95% yields (entries 14–16). It should be noted that (2,2-dimethyl-1,3-dioxolan-4-yl)methyl formate (30c) was not stable to silica gel, thus, its yield was determined based on 1H-NMR analysis of the crude product. On the other hand, formylation of (3,5-bis(benzyloxy)phenyl)methanol (19) afforded 31 in 95% yield after silica gel chromatography (entry 17). Selective esterifications of diols were also demonstrated, and selected examples are summarized in Table 2. The primary alcohol of butane-1,3-diol (20) was selectively benzoylated to afford 32 in 80% yield. Esterifications of glycerol (21) with benzoic acid and n-hexanoic acid furnished the corresponding diesters 33a and 33b in 85% and 90% yield, respectively (entries 19 and 20). Benzoylation of benzyl 2-(acetylamino)-2-deoxy-α-D-glucopyranoside (22) was achieved selectively at the C6-position to afford the mono-benzoate 34 in 90% yield (entry 21).
Table 2.
Selective esterifications of primary alcohols using EDCI, Oxyma 5a, and NaHCO3 in 5% H2O-CH3CNa
 |
All reactions were carried out using 5a (1.5 equiv) at rt except where noted;
ee was determined by HPLC (Daicel Chiralcel OD-Hcolumn);
R1-CO2H (1 equiv) and R2-OH (2 equiv) were used;
R1-CO2H (2 equiv) and R2-OH (1 equiv) were used;
yield was determined via 1H-NMR;
the reaction was carried out at 0 °C;
R1-CO2H (1 equiv) and R2-OH (8 equiv) were used.
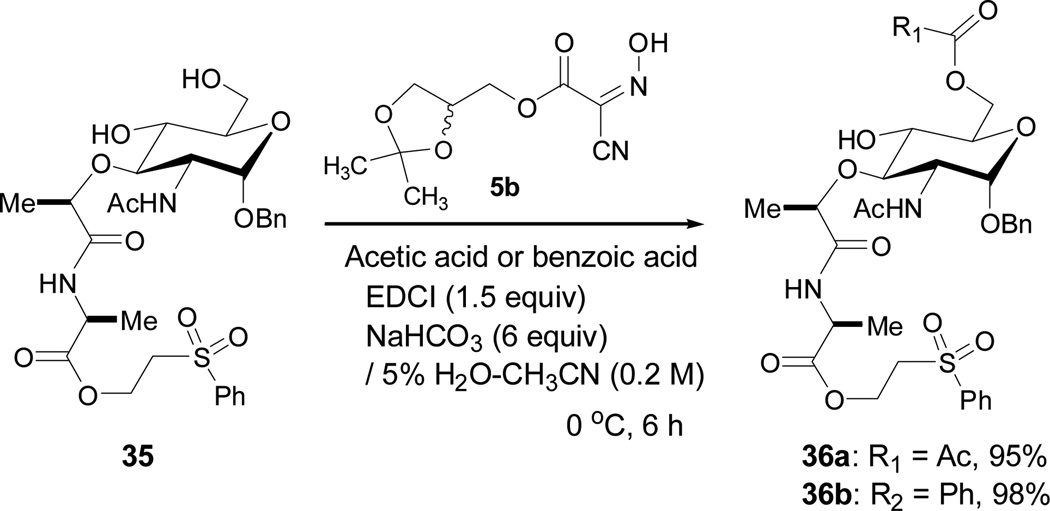
Finally, acylations of the diol of a complex muramic acid derivative 35 were demonstrated as selective esterifications of primary alcohols (Scheme 1).11 Acetylation and benzoylation of 35 using 5b (1.5 equiv), acid (2 equiv), EDCI (1.5 equiv), and NaHCO3 (6 equiv) at 0 °C gave rise to the primary acetate 36a and benzoate 36b in greater than 95% without the formation of diacylated product. In the reactions summarized in Scheme 1, it is a significant benefit to use glyceroacetonide-Oxyma 5b. Although the same reaction with Oxyma 5a gave equal conversion yield as observed in Scheme 1, separation of 5a from the product was extremely difficult via silica gel chromatography. On the other hand, 5b could be removed completely via standard acidic and basic work-ups.
Scheme 1.
Selective acylations of 35.
In conclusion, we have optimized selective esterifications of primary alcohols using Oxyma 5a or glyceroacetonide-Oxyma 5b, EDCI, and NaHCO3 in 5% H2O-CH3CN. The selective esterification conditions described here do not require the strict anhydrous condition necessary for ordinal esterification reactions. The coupling additive 5b can be removed easily after the reactions via acidic and basic work-ups. The new esterification conditions reported here should be a valuable asset in organic synthesis and for selective modifications of polyol molecules.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (NIAID grant AI AI084411) and University of Tennessee for generous financial support.
Footnotes
Supporting Information Available Experimental procedures and copies of NMRs. This is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Kurosu M, Li K. J. Org. Chem. 2008;73:9767–9770. doi: 10.1021/jo801408x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Aleiwi BA, Schneider CM, Kurosu M. J. Org. Chem. 2012;77:3859–3867. doi: 10.1021/jo300205b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Aleiwi BA, Kurosu M. Tetrahedron Lett. 2012;53:3758–3762. doi: 10.1016/j.tetlet.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Khattab SN. Bull. Chem. Soc. Jpn. 2010;83:1374–1379. [Google Scholar]; (b) El-Faham A, Subiro's-Funosas R, Albericio F. Chem. Eur. J. 2010;19:3641–3649. [Google Scholar]; (c) Subiro's-Funosas R, Prohens R, Barbas R, El-Faham A, Albericio F. Chem. Eur. J. 2009;15:9394–9403. doi: 10.1002/chem.200900614. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Wang Y, Kurosu M. Org. Lett. 2012;14:3372–3375. doi: 10.1021/ol3013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Pearson RG, Songstad J. J. Am. Chem. Soc. 1967;89:1827–1836. [Google Scholar]; (b) Pearson RG, Sobel H, Songstad J. J. Am. Chem. Soc. 1968;90:319–326. [Google Scholar]
- 5.(a) Twibanire JK, Grindley TB. Org. Lett. 2011;13:2988–2991. doi: 10.1021/ol201005s. [DOI] [PubMed] [Google Scholar]; (b) Twibanire JK, Omran RP, Grindley TB. Org. Lett. 2012;14:3909–3911. doi: 10.1021/ol301697c. [DOI] [PubMed] [Google Scholar]
- 6.Six equivalents of NaHCO3 in water (0.2 M) shows pH of 8.3.
- 7.The acetate and benzoate of 5b have a half-life of over 12 h.
- 8.Esterifications of 6 with (+)-menthol and cholesterol also did not provide the corresponding esters.
- 9.(a) Monagle JJ. J. Org. Chem. 1962;27:3851–3855. [Google Scholar]; (b) Steglich W, Höfle G. Angew. Chem. Int. Ed. Engl. 1969;8:981. [Google Scholar]; (c) Boden EP, Keck GE. J. Org. Chem. 1985;50:2394–2395. [Google Scholar]
- 10.Spivey AC, Arseniyadis S. Angew. Chem. Int. Ed. 2004;43:5436–5441. doi: 10.1002/anie.200460373. [DOI] [PubMed] [Google Scholar]
- 11.Under the optimized conditions, acetylation of 4a furnished 3a in greater than 95% without the formation of the diacetate (Figure 1)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.