Abstract
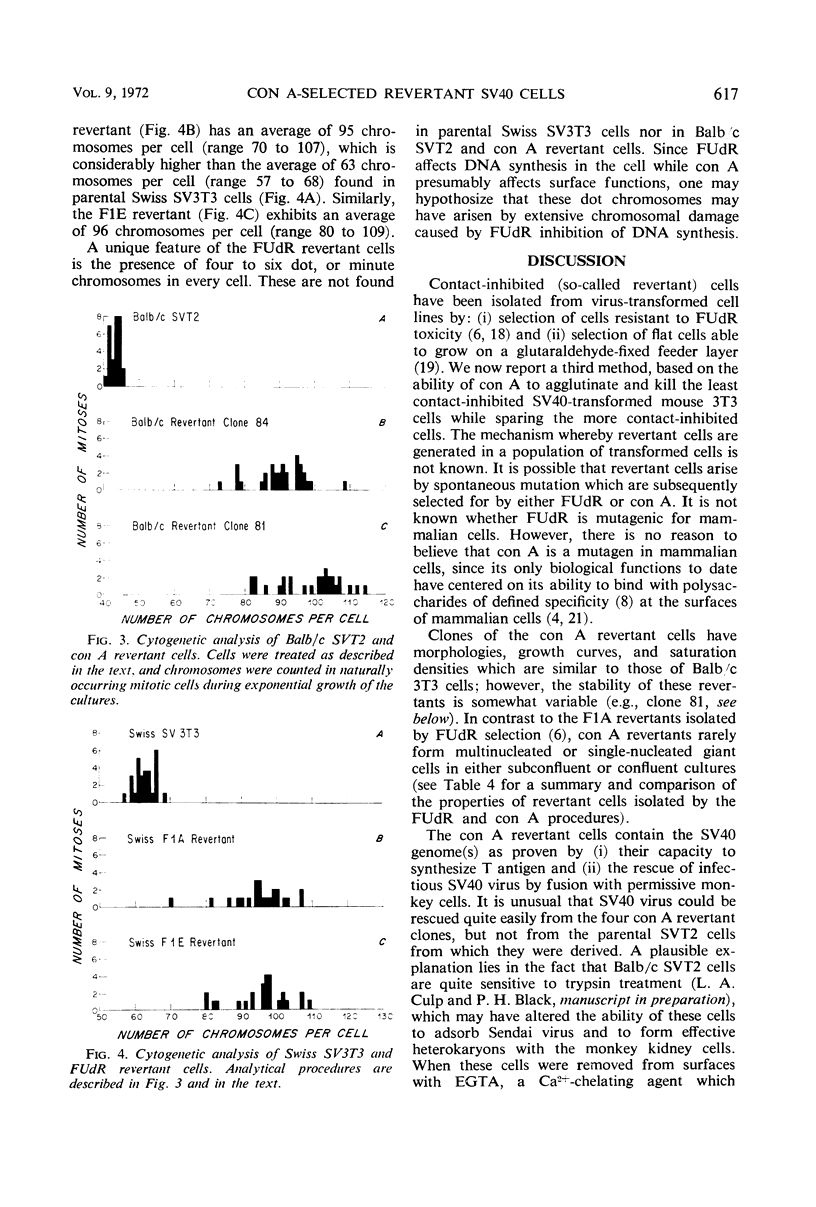
Contact-inhibited variants have been isolated by treatment of simian virus 40 (SV40)-transformed Balb/c 3T3 cells (SVT2) with the plant lectin concanavalin A. These con A revertant cells exhibit the following properties: (i) they resemble 3T3 cells morphologically and grow to saturation densities which are similar to that of 3T3 cells; (ii) they synthesize the SV40-specific T antigen and yield infectious virus after fusion with permissive monkey cells; (iii) they contain a high sialic acid content similar to that of 3T3 cells and not to that of SVT2 cells; sialic acid composition was found to be independent of serum concentration; (iv) they contain more chromosomes with the average number in the tetraploid range than the SVT2 cells from which they were derived; and (v) SVT2 and revertant cells, confluent or subconfluent, produce more collagen than Balb/3T3 cells. The relationship of surface membrane properties to contact inhibition of growth and the mechanisms for generating revertant cells are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnström G., Natarajan A. T. Mechanism of chromosome breakage--a new theory. Hereditas. 1966;54(3):379–388. doi: 10.1111/j.1601-5223.1966.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Codington J. F., Sanford B. H., Jeanloz R. W. Glycoprotein coat of the TA 3 cell. I. Removal of carbohydrate and protein material from viable cells. J Natl Cancer Inst. 1970 Oct;45(4):637–647. [PubMed] [Google Scholar]
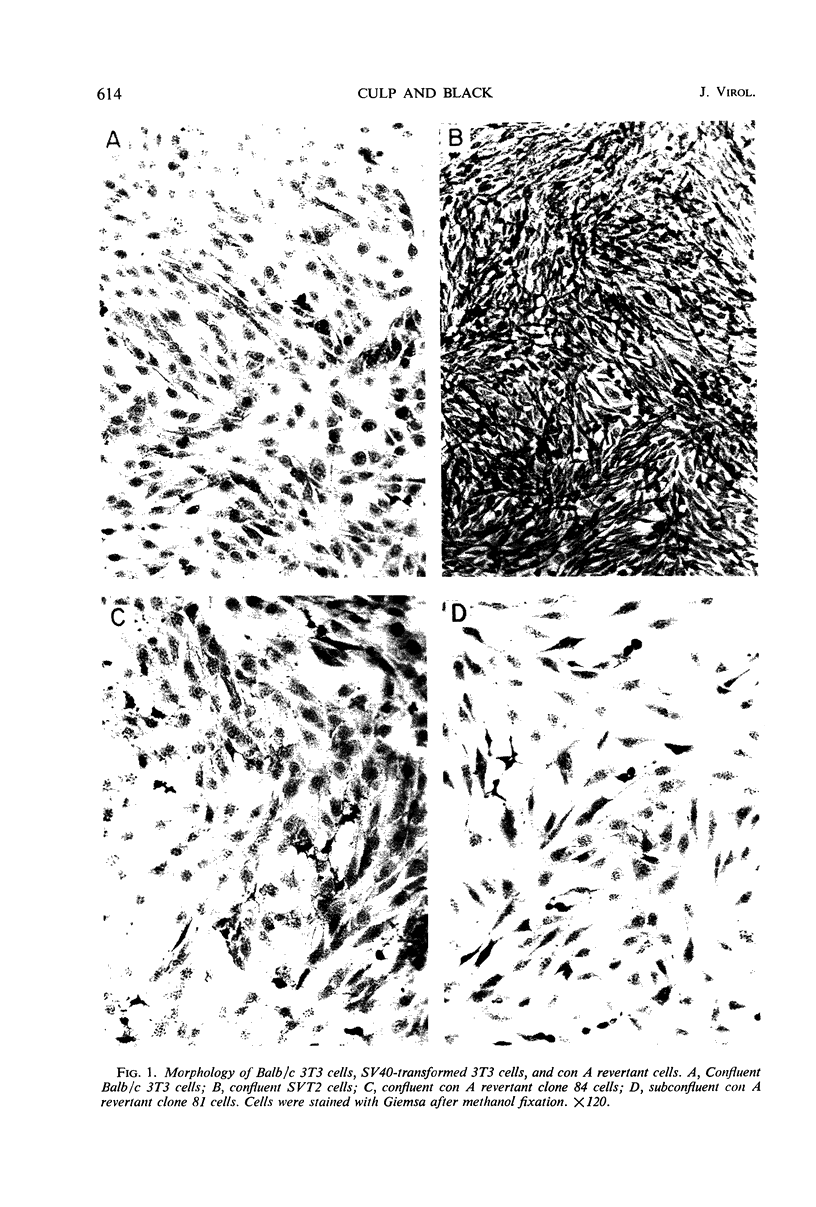
- Culp L. A., Grimes W. J., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. I. Biologic, virologic, and chemical properties. J Cell Biol. 1971 Sep;50(3):682–690. doi: 10.1083/jcb.50.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Glick M. C., Comstock C. A., Cohen M. A., Warren L. Membranes of animal cells. 8. Distribution of sialic acid, hexosamines and sialidase in the L cell. Biochim Biophys Acta. 1971 Apr 13;233(2):247–257. doi: 10.1016/0005-2736(71)90324-5. [DOI] [PubMed] [Google Scholar]
- Green H., Goldberg B. Synthesis of collagen by mammalian cell lines of fibroblastic and nonfibroblastic origin. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1360–1365. doi: 10.1073/pnas.53.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
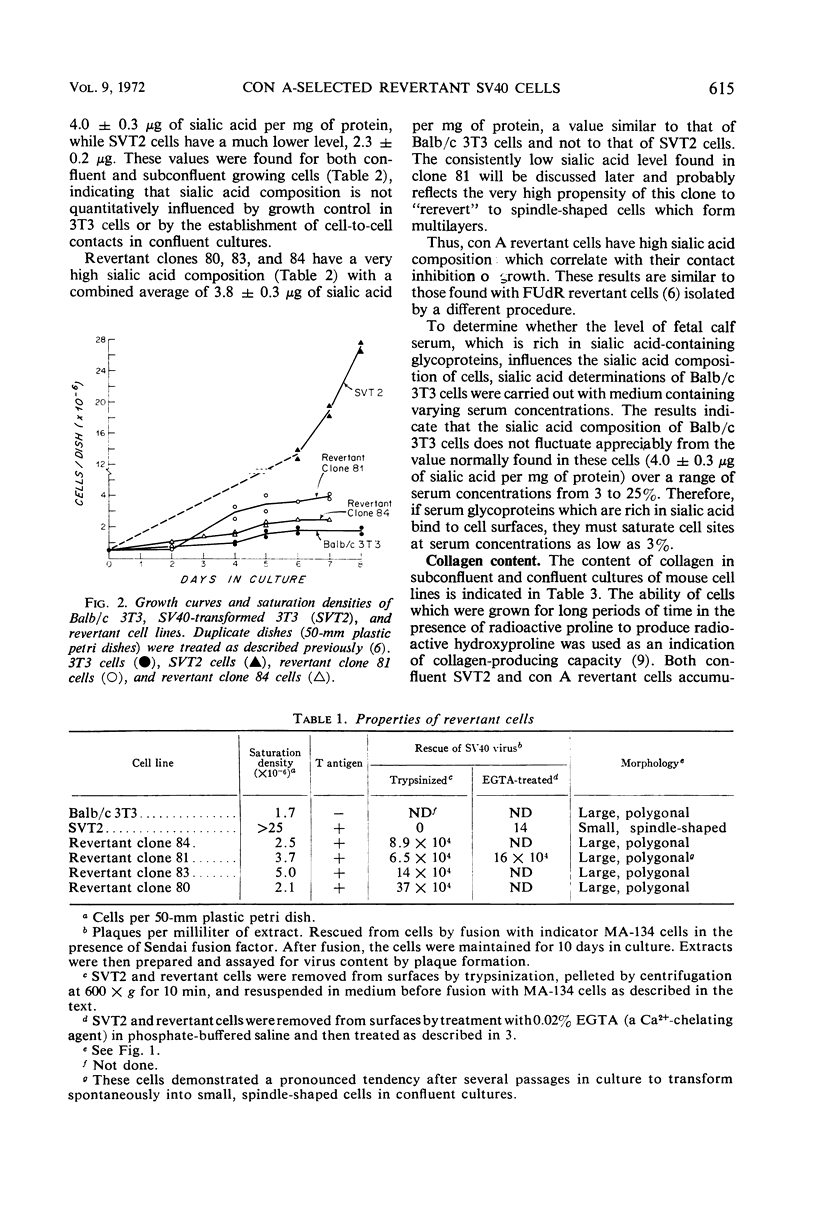
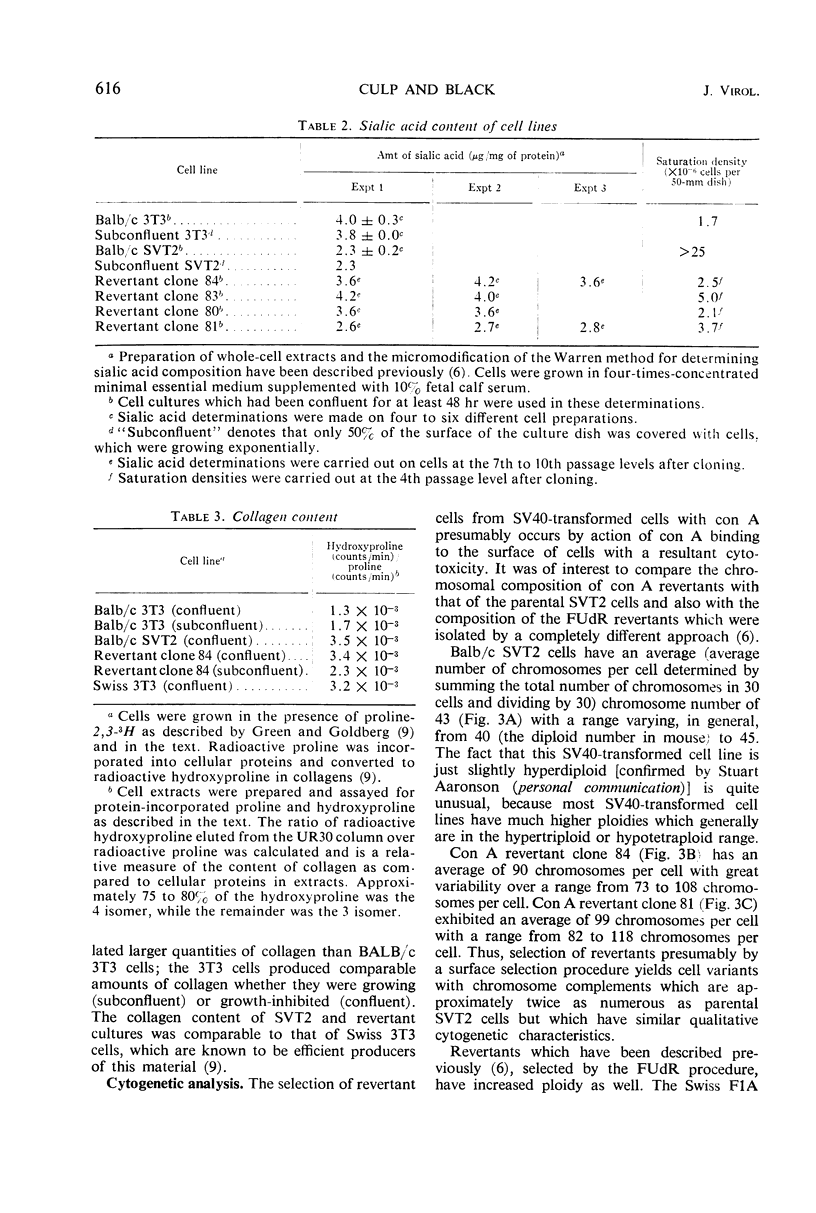
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- Inbar M., Rabinowitz Z., Sachs L. The formation of variants with a reversion of properties of transformed cells. 3. Reversion of the structure of the cell surface membrane. Int J Cancer. 1969 Sep 15;4(5):690–696. doi: 10.1002/ijc.2910040515. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADOFF S. Isolation and identification of PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:383–392. doi: 10.1111/j.1749-6632.1960.tb42702.x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. II. Ultrastructural study. J Cell Biol. 1971 Sep;50(3):691–708. doi: 10.1083/jcb.50.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R. M., Todd J., Gonzalez P., Gaffney E. V. Nucleoside incorporation into strain L cells: inhibition by pleuropneumonia-like organisms. Science. 1965 Sep 3;149(3688):1100–1101. doi: 10.1126/science.149.3688.1100. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz Z., Sachs L. Reversion of properties in cells transformed by polyoma virus. Nature. 1968 Dec 21;220(5173):1203–1206. doi: 10.1038/2201203a0. [DOI] [PubMed] [Google Scholar]
- Sanbe M., Aya T., Ikeuchi T., Sandberg A. A. Electron microscopic study of fused cells, with special reference to chromosome pulvrization. J Natl Cancer Inst. 1970 May;44(5):1079–1089. [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Haut W. F., Tung J. EFFECTS OF FLUORODEOXYURIDINE ON DNA REPLICATION, CHROMOSOME BREAKAGE, AND REUNION. Proc Natl Acad Sci U S A. 1962 Feb;48(2):190–198. doi: 10.1073/pnas.48.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]