Abstract
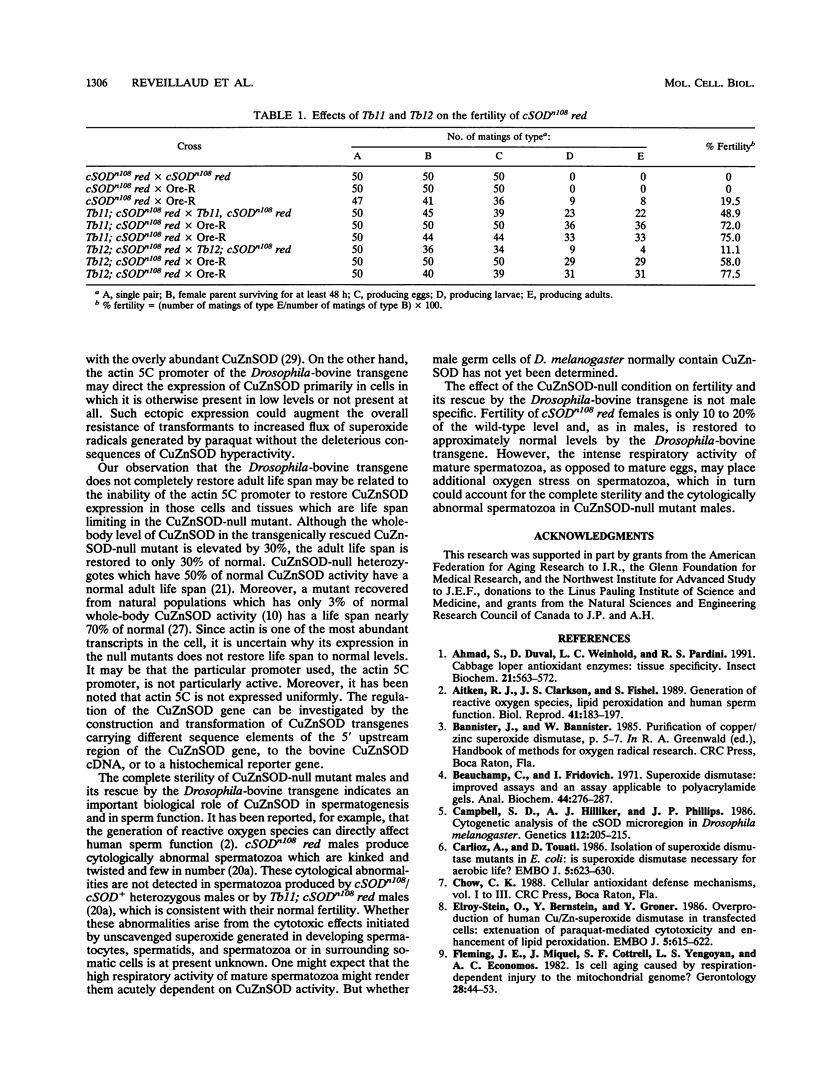
Null mutants for Cu/Zn superoxide dismutase (CuZnSOD) in Drosophila melanogaster are male sterile, have a greatly reduced adult life span, and are hypersensitive to paraquat. We have introduced a synthetic bovine CuZnSOD transgene under the transcriptional control of the D. melanogaster 5C actin promoter into a CuZnSOD-null mutant of D. melanogaster. This was carried out by P-element-mediated transformation of the Drosophila-bovine CuZnSOD transgene into a CuZnSOD+ recipient strain followed by genetic crossing of the transgene into a strain carrying the CuZnSOD-null mutation, cSODn108. The resulting transformants express bovine CuZnSOD exclusively to about 30% of normal Drosophila CuZnSOD levels. Expression of the Drosophila-bovine CuZnSOD transgene in the CuZnSOD-null mutant rescues male fertility and resistance to paraquat to apparently normal levels. However, adult life span is restored to only 30% of normal, and resistance to hyperoxia is 90% of that found in control flies. This striking differential restoration of pleiotropic phenotypes could be the result of a threshhold of CuZnSOD expression necessary for normal male fertility and resistance to the toxicity of paraquat or hyperoxia which is lower than the threshold required to sustain a normal adult life span. Alternatively, the differential rescue of fertility, resistance to active oxygen, and life span might indicate different cell-specific transcriptional requirements for these functions which are normally provided by the control elements of the native CuZnSOD gene but are only partly compensated for by the transcriptional control elements of the actin 5C promoter.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R. J., Clarkson J. S., Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989 Jul;41(1):183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Campbell S. D., Hilliker A. J., Phillips J. P. Cytogenetic analysis of the cSOD microregion in Drosophila melanogaster. Genetics. 1986 Feb;112(2):205–215. doi: 10.1093/genetics/112.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. E., Miquel J., Cottrell S. F., Yengoyan L. S., Economos A. C. Is cell aging caused by respiration-dependent injury to the mitochondrial genome? Gerontology. 1982;28(1):44–53. doi: 10.1159/000212510. [DOI] [PubMed] [Google Scholar]
- Graf J. D., Ayala F. J. Genetic variation for superoxide dismutase level in Drosophila melanogaster. Biochem Genet. 1986 Apr;24(3-4):153–168. doi: 10.1007/BF00502785. [DOI] [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988 Feb 5;263(4):1910–1914. [PubMed] [Google Scholar]
- Latter G. I., Burbeck S., Fleming J., Leavitt J. Identification of polypeptides on two-dimensional electrophoresis gels by amino acid composition. Clin Chem. 1984 Dec;30(12 Pt 1):1925–1932. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Hilliker A. J. Genetic analysis of oxygen defense mechanisms in Drosophila melanogaster. Adv Genet. 1990;28:43–71. doi: 10.1016/s0065-2660(08)60523-4. [DOI] [PubMed] [Google Scholar]
- Reveillaud I., Niedzwiecki A., Bensch K. G., Fleming J. E. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol. 1991 Feb;11(2):632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Seto N. O., Hayashi S., Tener G. M. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley B. E., Phillips J. P., Hilliker A. J. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990 Dec;33(6):867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]