Abstract
ToxR facilitates TcpP-mediated activation of the toxT promoter in Vibrio cholerae, initiating a regulatory cascade that culminates in cholera toxin secretion and toxin coregulated pilus expression. ToxR binds a region from −104 to −68 of the toxT promoter, from which ToxR recruits TcpP to the TcpP-binding site from −53 to −38. To precisely define the ToxR-binding site within the toxT promoter, promoter derivatives with single-base-pair transversions spanning the ToxR-footprinted region were tested for transcription activation and DNA binding. Nine transversions between −96 to −83 reduced toxT promoter activity 3-fold or greater, and all nine reduced the relative affinity of the toxT promoter for ToxR at least 2-fold, indicating that activation defects were due largely to reduced binding of ToxR to the toxT promoter. Nucleotides important for ToxR-dependent toxT activation revealed a consensus sequence of TNAAA-N5-TNAAA extending from −96 to −83, also present in other ToxR-regulated promoters. When these consensus nucleotides were mutated in the ompU, ompT, or ctxA promoters, ToxR-mediated regulation was disrupted. Thus, we have defined the core ToxR-binding site present in numerous ToxR-dependent promoters and we have precisely mapped the binding site for ToxR to a position three helical turns upstream of TcpP in the toxT promoter.
INTRODUCTION
The gastrointestinal disease cholera is due primarily to the secretion of cholera toxin (CT) by ingested Vibrio cholerae and is facilitated by the toxin coregulated pilus (TCP) (1). The expression of CT and TCP, encoded by the ctx and tcp operons, are both positively and negatively regulated at the transcriptional level. Positive regulation of ctx and tcp requires ToxT (2, 3), the expression of which is initiated by the combined actions of ToxR and TcpP at the toxT promoter (4–7). While toxR expression is generally considered to be constitutive, tcpP expression is regulated by AphA, AphB, cAMP receptor protein (CRP), and HapR according to environmental conditions (8–13). Moreover, TcpP is degraded under noninducing conditions (14, 15). Thus, positive regulation of the transcription cascade culminating in CT secretion and TCP production is mediated by the sensing and integration of environmental signals by AphA, AphB, and cAMP receptor protein (CRP) at the tcpPH promoter and possible additional signals sensed by ToxR/ToxS and TcpP/TcpH. Furthermore, activity of the downstream regulator, ToxT, responds to the presence of bile and bicarbonate (16, 17), and ToxT itself is degraded in order to shut down virulence gene expression under noninducing conditions (18). Negative regulation of CT and TCP expression is mediated by H-NS, which binds and represses the activities of the ctxAB, tcpA, and toxT promoters (19, 20), and by the CRP-cAMP complex, which plays a role in HapR activation. HapR in turn represses aphA and tcpPH expression (9, 10, 12, 21).
TcpP and ToxR are inner membrane proteins with C-terminal periplasmic domains lacking homology to other proteins and N-terminal cytoplasmic domains with strong homology to the OmpR/PhoB family of winged helix-turn-helix transcriptional activators (22). The DNA-binding domains of OmpR/PhoB family proteins generally interact as dimers with direct repeat DNA sequences (23, 24), suggesting that these domains dimerize in a head-to-tail configuration. We have recently shown that TcpP also binds an RNA polymerase-proximal direct repeat element from −53 to −38 on the toxT promoter (25). However, the specific ToxR-binding site is undefined.
toxT expression requires that membrane-localized ToxR be coexpressed with TcpP (4, 6, 7, 26, 27), and we hypothesize that ToxR recruits TcpP to what appears to be a weak TcpP-binding site (relative to ToxR-binding affinity) (6, 28). Once recruited to the toxT promoter, TcpP activates toxT transcription (29). The ability of ToxR to facilitate TcpP-mediated toxT activation requires that ToxR binds a poorly defined DNA-binding site containing sequences from an inverted repeat element that lies upstream of the TcpP-binding site (Fig. 1A) (5, 6). ToxR-dependent recruitment of TcpP to the promoter may increase the local concentration of TcpP, facilitating TcpP binding to its weak binding site. This could occur while maintaining a ToxR-TcpP interaction, or ToxR may release TcpP upon DNA binding to allow TcpP to bind its adjacent binding site. Finally, it is possible that although ToxR and TcpP can establish a protein-protein interaction (28, 29), the main role of ToxR is to simply recruit the toxT promoter to the membrane, where membrane-localized TcpP has easier access to its toxT promoter-binding site.
Fig 1.
DNA sequence of the V. cholerae classical strain O395 promoter-proximal region of the toxT promoter and ToxR-dependent activation of single-base-pair substitutions. (A) Nucleotides are numbered relative to the toxT transcription start site (5). The region of ToxR-dependent DNase I protection is indicated above the DNA sequence (6). The solid gray arrows above the sequence indicate the position of the putative 5′-TNAAA-N5-TNAAA-3′ direct repeat motif important for ToxR binding. An inverted repeat sequence (5, 50) is indicated by the black convergent arrows between −93 and −58. A promoter-proximal degenerate ToxR-binding site is indicated by dashed gray arrows from −69 to −56. The boxed nucleotides indicate the pentameric direct repeat motif recognized by TcpP (25). Single-nucleotide substitutions generated within the toxT promoter region from −100 to −57 are indicated on the bottom line in italics. (B) Effects of ToxR-binding site mutations on toxT-lacZ activity in wild-type V. cholerae strain O395. Strains carrying a plasmid-borne wild-type toxT-lacZ fusion (−172 to +45), single-base-pair substitution toxT promoter mutants, promoter deletion derivatives, or empty vector (promoterless lacZ vector, pTG24) were assessed for β-galactosidase activity. The positions of substitutions and endpoints are indicated relative to the toxT transcription start site. Error bars represent the standard deviations for each data set. *, P < 0.005 as assessed using the Student t test, n = 6 or more measurements.
Although there are a large number of genes comprising the ToxR regulon (30), only a select few are known to be directly regulated by ToxR. In addition to facilitating the TcpP-dependent activation of the toxT promoter, ToxR directly activates the ompU promoter and represses the ompT promoter (31, 32). Furthermore, when overexpressed, ToxR can directly activate the ctxA promoter (33), although under physiological conditions the ctxA promoter is activated by ToxT (2, 3, 20, 34, 35). The binding sites for ToxR at the ompU, ompT, ctxA, and toxT promoters have been defined by DNase I footprinting (6, 31, 32); however, comparisons of these footprinted regions has not identified a clear consensus ToxR-binding sequence found at all ToxR-footprinted promoters.
At the toxT promoter, the ToxR footprint spans the region from −104 to −68, partially overlapping an inverted repeat sequence (Fig. 1A, black arrows) (5, 6). Plasmid-borne lacZ fusion and mobility shift studies using toxT promoter deletion derivatives indicate that at least some sequences important for ToxR binding and ToxR-dependent promoter activation lie between −114 and −73 (5). Subsequently, a screen for toxT promoter mutants defective in ToxR-dependent activation identified single nucleotide substitutions in the toxT promoter at positions −86 and −84 in the upstream half of the inverted repeat sequence that reduced both ToxR-dependent promoter activation and the affinity of the toxT promoter for ToxR (5). Moreover, substitutions at positions −67 and −65 within the downstream half of the inverted repeat that are complementary to the −86 and −84 substitutions in the upstream half of the inverted repeat had little effect on ToxR-dependent fusion activity (5). These results suggest that the inverted repeat sequence does not represent a symmetrical binding site for ToxR at the toxT promoter. Thus, some other sequence motif containing nucleotides −86 and −84 is likely to strongly influence ToxR binding or ToxR-dependent recruitment of TcpP to the toxT promoter.
In this report, systematic transversion mutagenesis of the ToxR-footprinted region of the toxT promoter was used to identify nucleotides that were critical for promoter activation. These studies defined the sequence TNAAA-N5-TNAAA from −96 to −83 as the ToxR-binding site in the toxT promoter. Transversions altering these critical nucleotides reduced the affinity of the promoter for ToxR and defined a minimal region of the toxT promoter that was essential for ToxR-dependent toxT activation. Furthermore, mutation of this repeat element in the ompU, ompT, and ctxA promoters resulted in loss of ToxR responsiveness by those promoters as well.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All V. cholerae strains used in this study are derived from O1 serotype classical biotype strain O395 (36). V. cholerae, Escherichia coli, and plasmids used in this study are listed in Table S1 in the supplemental material. Strains were routinely grown in Luria-Bertani (LB) medium containing 10 g/liter NaCl at 37°C or Vibrio cholerae LB (Vc LB, containing 5 g/liter NaCl). Unless otherwise indicated, antibiotics were used at the following concentrations: streptomycin, 100 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; and kanamycin, 30 μg/ml.
DNA manipulations.
Cloning procedures and transformation of E. coli strains were carried out using standard protocols (37). pTG24-based fusion plasmids were transferred to V. cholerae by electroporation (2.2 kV) using an E. coli Pulsor (Bio-Rad), and pMMB207-based plasmids were transferred to V. cholerae by triparental mating using mobilization plasmid pRK2013 (38).
Generation of promoter mutants.
The wild-type toxT promoter, toxTpro, was amplified using purified V. cholerae strain O395 chromosomal DNA as the template, the toxTpro−172 BamHI and toxTpro+45 EcoRI primers (see Table S2 in the supplemental material), and the Expand High Fidelity PCR system (Roche). The amplified DNA fragment was gel purified, digested with EcoRI and BamHI, and ligated into EcoRI/BamHI-digested pBluescript SK(+), generating pTG3. Nucleotide substitutions within the ToxR-binding region of the toxT promoter region were generated by a one-step process in which the entire plasmid is amplified using complementary mutagenic primers, pTG3 as the template, and Pfu Turbo DNA polymerase (Stratagene), followed by DpnI cleavage for enrichment for PCR-amplified plasmids or the two-step SOEing PCR amplification technique (39) using complementary mutagenic primers, the exterior primers toxTpro−172 BamHI and toxTpro+45 EcoRI (see Table S2), pTG3 as the template, and the Expand High Fidelity PCR system (Roche), followed by PCR product purification, digestion with EcoRI and BamHI, and ligation into EcoRI/BamHI-digested pBluescript SK(+). Deletion derivatives of toxTpro were generated using PCR amplification using pTG3 as the template, the toxTpro+45 EcoRI primer, and either the Δ−101 BamHI, Δ−82 BamHI, or Δ−47 BamHI primer (see Table S2). The DNA sequences of all PCR-generated V. cholerae DNA fragments were determined at The University of Michigan Core sequencing facility to verify the mutations and confirm the absence of additional nucleotide changes. DNA fragments carrying the wild-type, deleted, and substituted toxT promoters were excised from pBluescript-based constructs as NotI/SalI fragments and recloned into NotI/SalI-digested pTG24 (25), generating lacZ transcriptional fusions.
ompU promoter DNA from −211 to +22 relative to the transcription start site was PCR amplified in plasmid pBluescript SK(+)-ompU using mutagenic primer pairs listed in Table S2 in the supplemental material. Following DpnI digestion and DH5α transformation, candidate ompU mutants were confirmed by sequencing prior to excision with EcoRI and BamHI and ligation into the promoterless lacZ vector pTL61T (40).
ctx-lacZ fusions published previously (20) were PCR amplified using mutagenic primers listed in Table S2 in the supplemental material, DpnI digested, and transformed into DH5α. Candidate ctxA promoter mutants were confirmed by sequencing. Previously described ompT-lacZ fusions (32) were PCR amplified using mutagenic primers listed in Table S2 in the supplemental material, DpnI digested, and transformed into DH5α. Candidate ompT promoter mutants were confirmed by sequencing.
Measurement of lacZ fusion activity.
Cultures of toxT-lacZ reporter strains carrying both pTG24 and pMMB207 or their derivatives were grown overnight in LB broth containing 5 g/liter NaCl (Vc LB) at 30°C, diluted 1:50 in LB broth which had been adjusted to an initial pH of 6.5, and supplemented with chloramphenicol, ampicillin, streptomycin, and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) where required. After incubation for 4 h at 30°C (ToxR-inducing conditions), the optical densities at 600 nm (OD600) of the cultures were determined, and 5- to 100-μl samples were used in a standard β-galactosidase assay (41). For ompU-lacZ fusion constructs, β-galactosidase activity was measured on overnight cultures of V. cholerae grown at 30°C in Vc LB, pH 7. For ctxA-lacZ and ompT-lacZ fusions, β-galactosidase assays were performed on overnight cultures grown at 30°C in Vc LB, pH 6.5.
Mobility shift assays.
DNA gel mobility shift assays were performed essentially as previously described (25) using membrane preparations obtained either from V. cholerae strain TG128 (ToxR−) or TG129 (ToxR+; O395 ΔtoxR ΔtcpP expressing hemagglutinin [HA]-tagged ToxR from the plasmid pSK-toxR-HA; see Table S1 in the supplemental material) grown in Vc LB broth supplemented with 1 mM IPTG, streptomycin, chloramphenicol, and ampicillin. Protein concentrations were determined using the Quick-Start Bradford dye reagent (Bio-Rad). DNA fragments carrying either the entire region from −172 to +45, relative to the toxT transcription start site, or upstream deletion derivatives thereof, were excised from pBluescript clones using NruI and SalI, gel purified, and end labeled by Klenow DNA polymerase (Invitrogen) in the presence of [32P]dCTP or [32P]dATP (MP Biomedicals) as previously described (25). Increasing amounts of membrane preparations were mixed with the end-labeled DNA targets in a solution containing 10 mM Tris (pH 7.4), 1 mM EDTA, 5 mM NaCl, 50 mM KCl, 50 μg/ml bovine serum albumin (BSA), and 10 μg/ml sheared salmon sperm DNA. Binding reactions were performed at 30°C for 30 min, and the free and membrane-associated DNA target samples were separated by electrophoresis on a 6% polyacrylamide-TBE gel prerun with 5% thioglycolic acid as previously described (25). After electrophoresis, the gels were dried, the extents of DNA migration were recorded by autoradiography, and in some cases the relative intensities of the recorded signals were determined using a Biospectrum image analyzer (UVP, LLC) or using ImageJ (http://rsbweb.nih.gov/ij/).
RESULTS
Specific mutations in the ToxR-binding region of toxT disrupt ToxR-dependent promoter activation.
The ToxR-binding site within the toxT promoter has been defined previously by DNase I footprinting analysis as extending from −104 to −68 (6); however, the specific nucleotides within the ToxR-protected region important for toxT activation have not been systematically determined. To identify these nucleotides, a collection of toxT promoter derivatives with transversions at each base pair in the region from −100 to −57 were constructed (Fig. 1A). Transversions were generated using the toxT promoter region from −172 to +45 fused to a promoterless lacZ reporter gene (25, 40). In O395 (V. cholerae classical strain), 13 transversions reduced toxT promoter activity greater than 2-fold, and 12 of 13 mutations affect nucleotides in the region from −97 to −82 (Fig. 1B). Likewise, a previously identified A(−84)T substitution (42) also dramatically reduced fusion activity (Fig. 1B). Of the transversions in the region from −81 to −57, only that at A(−74)C reduced the activity of the fusion greater than 2-fold (Fig. 1B). Thus, nucleotides important for toxT-lacZ fusion activity were clustered within the promoter-distal portion of the ToxR-footprinted region, while nucleotides in the promoter-proximal portion of the footprint contributed little to promoter activity.
Transversions that reduced toxT promoter activity most dramatically identified the 5′-CTNAAAAAANNTNAAA-3′ nucleotide sequence (−97 to −82) as critical for ToxR-dependent toxT activation. Within this sequence is a direct repeat motif of (5′-TNAAA-N5-TNAAA-3′) composed of two half-sites that are centered one turn of the DNA helix apart. These features are consistent with the notion that two ToxR monomers bind in a head-to-tail configuration to two 5′-TNAAA-3′ half-sites. Thus, the motif 5′-TNAAA-N5-TNAAA may represent a minimally defined ToxR-binding site.
toxT promoter transversion mutations do not affect ToxR-independent toxT activation by overexpressed TcpP.
To rule out the possibility that transversion-dependent changes in toxT expression are due to defects in TcpP interaction with the toxT promoter, the wild-type toxT-lacZ fusion and mutant derivatives in the region from −100 to −80 were moved into an O395 ΔtoxR ΔtcpP/pEK41 background (EK459/pEK41) to assess the effects on ToxR-independent toxT activation in response to TcpP overexpression (pEK41 encodes an herpes simplex virus [HSV] epitope-tagged version of TcpP in vector pMMB207) (6). Previous studies have shown overexpressed TcpP can efficiently activate the toxT promoter, even in the absence of ToxR (4, 6).
In the EK459/pEK41 background, all 22 transversion mutants tested had less than a 30% decrease in TcpP-mediated toxT activation (Fig. 2A, black bars). More importantly, none of the tranversions in the TNAAA-N5-TNAAA putative ToxR-binding site had more than a 20% decrease in toxT activation (Fig. 2A). Thus, the effects of these promoter mutations on toxT activation are most likely due to defects in ToxR-dependent toxT activation.
Fig 2.
ToxR-binding site mutations do not affect toxT activation by overexpressed TcpP. (A) toxT-lacZ fusions with toxT promoter transversions from −100 to −80 were tested in a strain lacking ToxR (EK459 = O395 ΔtoxR ΔtcpP) but overexpressing TcpP from plasmid pEK41. Strains were grown for 4 h at 30°C, pH 6.5, in the presence of 1 mM IPTG, and β-galactosidase activities were determined for strains carrying either a promoterless lacZ fusion vector (vector) or its derivatives carrying either the wild-type toxT-lacZ fusion (wt) or single-base-pair substitutions. Black bars represent a ΔtoxR ΔtcpP background carrying the TcpP overexpression plasmid, pEK41. White bars represent a ΔtoxR ΔtcpP background carrying the empty vector expression plasmid, pMMB207. (B) Enhanced activation by coexpression of ToxR and overexpressed TcpP is lost when mutations in the ToxR-binding site are present. toxT-lacZ activation was measured in the ΔtoxR ΔtcpP strain EK459 harboring the TcpP-expressing vector pEK41 (black bars, same data as in panel A) or wild-type O395 (ToxR+) harboring pEK41 (gray bars). Error bars represent the standard deviation. *, P ≤ 0.05; P values for ToxR+ strains are significantly higher than those for ToxR− strains. #, P < 0.0001; P value for the ToxR+ strain is significantly lower than the ToxR− strain. All assessed using the Student t test. n = 6 or more measurements.
Consistent with the interpretation that the TNAAA-N5-TNAAA direct repeat element responds to ToxR, introduction of the toxT promoter mutants into a wild-type O395 strain (ToxR+) overexpressing TcpP (+pEK41) results in a strain with 50% higher levels of β-galactosidase expression (∼30,000 Miller units; Fig. 2B), but this level drops to the level of activation mediated by overexpressed TcpP alone, when mutations in the TNAAA-N5-TNAAA repeat element are encountered (Fig. 2B). Thus, the maximal level of toxT activation afforded by ToxR and overexpressed TcpP are not achieved when the ToxR-binding site is mutated.
In an EK459/pMMB207 background (O395 ΔtoxR ΔtcpP plus empty vector), the transversions did not dramatically alter the basal activity of the toxT-lacZ fusion (Fig. 2A, white bars).
Mutations in the putative ToxR-binding site of toxT disrupt ToxR-toxT interactions.
To determine whether mutations in the TNAAA-N5-TNAAA putative ToxR-binding site disrupt ToxR binding to the toxT promoter, electrophoretic mobility shift assays were performed. 32P-labeled toxT promoter targets were mixed with increasing concentrations of ToxR-containing V. cholerae membranes or negative-control membranes lacking ToxR.
In the presence of 0.77 mg/ml ToxR-containing membranes, approximately half of the input wild-type toxT promoter probe was shifted (see Fig. S1D in the supplemental material, lanes 1 and 25), while probes bearing mutations in the putative ToxR-binding site (TNAAA-N5-TNAAA, from −96 to −83) were shifted with 10 to 30% efficiency (see Fig. S1D, lanes 2 to 23). Thus, mutation of the putative ToxR-binding site led to a defect in ToxR binding, confirming the identity of the ToxR-binding site. Experiments with increasing concentrations of ToxR-containing membranes (see Fig. S1B to H in the supplemental material) were used to determine the concentration leading to an ∼50% shift for each toxT promoter mutant probe (Table 1; see also Fig. S1). toxT promoter probes bearing transversions in the ToxR-binding site required 2- to 5-fold more ToxR protein to reach 50% shifting (Table 1). Comparison of transversion-dependent effects on relative affinity and toxT-lacZ fusion activation indicates that reductions in relative affinity correlate well with reductions in promoter activation. The fact that the C(−95)A mutation at the N position of TNAAA consensus had no significant defect in transcription (Fig. 1B) or ToxR binding (Table 1; see also Fig. S1) supports the conclusion that this nucleotide position is not recognized by ToxR.
Table 1.
Relative affinities of toxT promoter mutants for membranes containing (ToxR+) or lacking (ToxR−) ToxR
| Targeta | Amt (mg/ml) of protein required for a 50% shift |
Fold increase relative to wild type | |
|---|---|---|---|
| ToxR+ | ToxR− | ||
| Wild type (−172 to +45) | 0.76 | > 5.6 | 1.0 |
| T(−100)G | 0.57 | > 5.6 | 0.8 |
| A(−99)C | 0.96 | > 5.6 | 1.3 |
| T(−98)G | 0.74 | > 4.2 | 1.0 |
| C(−97)A | 0.99 | > 5.6 | 1.3 |
| T(−96)G | 1.55 | > 5.6 | 2.0 |
| C(−-95)A | 0.44 | > 5.6 | 0.6 |
| A(−94)C | 2.01 | > 5.6 | 2.6 |
| A(−93)C | 2.33 | > 5.6 | 3.1 |
| A(−92)C | 2.02 | > 5.6 | 2.7 |
| A(−91)C | 2.12 | > 5.6 | 2.8 |
| A(−90)C | 1.83 | > 5.6 | 2.4 |
| A(−89)C | 1.48 | > 5.6 | 1.9 |
| C(−88)A | 0.77 | > 4.2 | 1.0 |
| A(−87)C | 0.74 | > 5.6 | 1.0 |
| T(−86)G | 3.42 | > 5.6 | 4.5 |
| A(−85)C | 1.23 | > 5.6 | 1.6 |
| A(−84)C | 4.12 | > 5.6 | 5.4 |
| A(−83)C | 1.98 | > 5.6 | 2.6 |
| A(−82)C | 1.39 | > 5.6 | 1.8 |
| T(−81)G | 0.67 | > 4.2 | 0.9 |
| A(−80)C | 0.74 | > 4.2 | 1.0 |
| T(−60)G | 0.83 | > 5.6 | 1.1 |
| A(−84)T | 2.39 | > 4.2 | 3.1 |
| toxT −100 to +45 | 0.74 | > 5.6 | 1.0 |
Numbers represent the position of the promoter mutation or the endpoints of deletions, relative to the toxT transcription start site. Bold nucleotides indicate the position of the ToxR-binding site direct repeat TNAAA-N5-TNAAA (−96 to −82).
Finally, further evidence that the TNAAA-N5-TNAAA sequence from −96 to −82 represents the ToxR-binding site within the toxT promoter is that a double-stranded oligonucleotide from that region can compete with the full-length toxT promoter for ToxR-mediated gel shifting activity, and mutations within the TNAAA-N5-TNAAA consensus binding site within these oligonucleotides disrupt inhibition activity (data not shown).
In the presence of 4.4 mg/ml negative-control membranes (lacking ToxR), less than 50% of the target promoters were shifted (see Fig. S2 in the supplemental material), while a few targets shifted greater than 50% in the presence of 5.6 mg/ml negative-control membranes (see Fig. S1I and Fig. S2 in the supplemental material), indicating that at high-membrane concentrations, one begins to detect increased background binding to toxT promoter probes.
The region from −82 to −68 of the toxT promoter, while containing a partially conserved ToxR-binding site, does not contribute to toxT activation.
Now that we had identified the ToxR-binding site in the toxT promoter, we recognized that the toxT promoter also contains an imperfect ToxR-binding site (ANAAA-N4-TNAAG) from −56 to −69 on the opposite strand from our recently defined ToxR-binding site (from −96 to −83). Thus, we sought to determine whether this imperfect ToxR-binding site (Fig. 1A, dashed gray arrow) supported any detectable ToxR binding or ToxR-dependent toxT activation.
In O395, the activity of the wild-type fusion was not altered by deletion of toxT promoter sequences upstream of −100 (Fig. 1B), indicating that the region from −172 to −101 does not significantly contribute to toxT promoter activation. In contrast, the deletions removing sequences upstream of −81 (or −47) reduced fusion activity by about 10-fold (Fig. 1B), indicating that the region from −100 to −82 strongly contributes to toxT promoter activity, as expected since this region contains the ToxR-binding site TNAAA-N5-TNAAA. Previous studies by Higgins et al. also demonstrated that while toxT promoter truncations lacking sequences from −172 to −114 maintained wild-type levels of activation, deleting the region from −114 to −73 resulted in a toxT promoter with just 10% activation (5). A toxT promoter fragment from −73 to +45 was also not bound by ToxR (5).
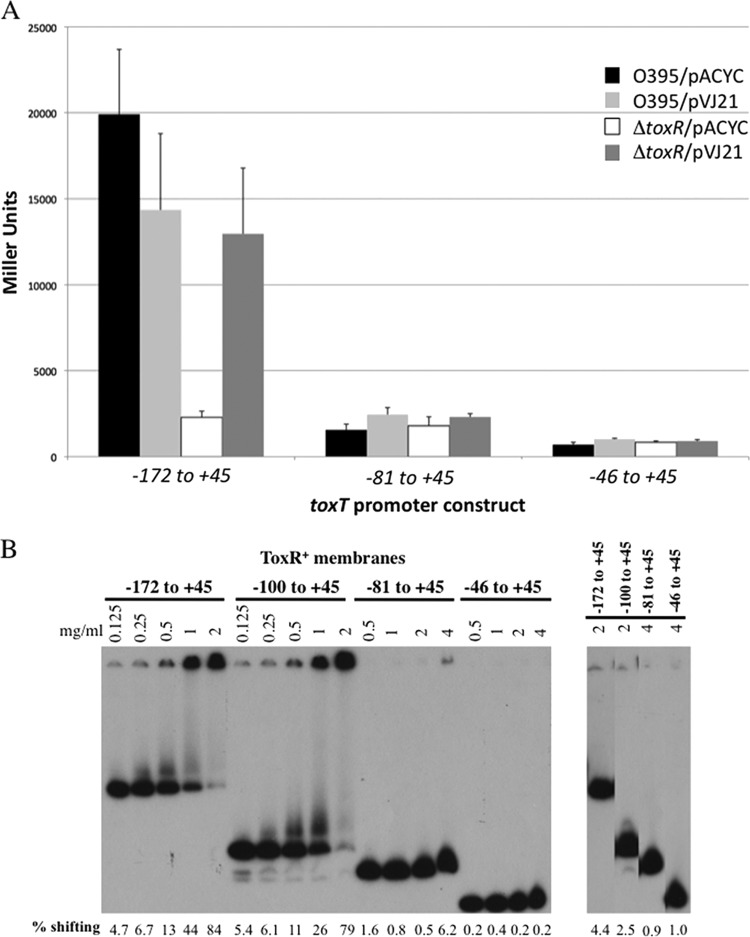
Since there is an imperfect ToxR-binding site from −69 to −56 of the toxT promoter, we assessed whether that region of the promoter has the potential for ToxR-dependent activation. As both our results with the −81 to +45 toxT-lacZ reporter construct and the −73 to +45 reporter construct described previously (5) showed that ToxR could not activate these promoter fragments, we hypothesized that the imperfect ToxR repeat from −69 to −56 of the toxT promoter may have a low-affinity ToxR-binding site. Thus, we tested the ability of overexpressed ToxR to restore activation to the −81 to +45 toxT-lacZ reporter plasmid. Even overexpression of toxR (from pVJ21) (43) was unable to restore activation to this promoter, as it showed β-galactosidase levels only slightly above O395 expressing the empty vector (Fig. 3A). This low level of ToxR responsiveness is similar to the negative control −46 to +45 reporter construct, which lacks the imperfect ToxR-binding site (Fig. 3A). Overexpressed ToxR in the ΔtoxR strain EK307 was able to activate the full-length toxT promoter construct from −172 to +45 (Fig. 3A). Gel-shift analysis also indicated that ToxR is largely unable to bind this imperfect repeat element, as a toxT promoter fragment from −81 to +45 showed nearly undetectable ToxR binding (Fig. 3B).
Fig 3.
ToxR fails to bind or activate a toxT-lacZ derivative containing the degenerate ToxR-binding site from −69 to −56. (A) toxT promoter derivatives driving lacZ expression were tested for activation in wild-type V. cholerae (O395) or the toxR mutant strain EK307 with or without overexpression of ToxRS from plasmid pVJ21. n = 6. (B) Electrophoretic mobility shift analysis of full-length (−172 to +45), −100 to +45, −81 to +45, and −46 to + 45 toxT derivatives with increasing concentrations of ToxR-containing membranes shows the degenerate ToxR-binding site from −69 to −56 has weak ToxR binding capacity. Negative-control gel shifting with membranes lacking ToxR (ToxR−) was also tested and showed minimal background. DNA bound by membrane-localized ToxR is retained in the well of the gel. The percentage of shifting by membranes is indicated under each lane as determined by ImageJ.
These data indicate that ToxR binds the imperfect ToxR-binding site from −69 to −56 in the toxT promoter poorly and that this specific DNA sequence does not contribute to ToxR-dependent toxT activation. This conclusion is also supported by the fact that the ToxR-footprinted region of the toxT promoter extends to only −68 (6).
The newly identified ToxR-binding site in the toxT promoter is also required for ToxR-mediated activation of ompU and ctxA and repression of ompT.
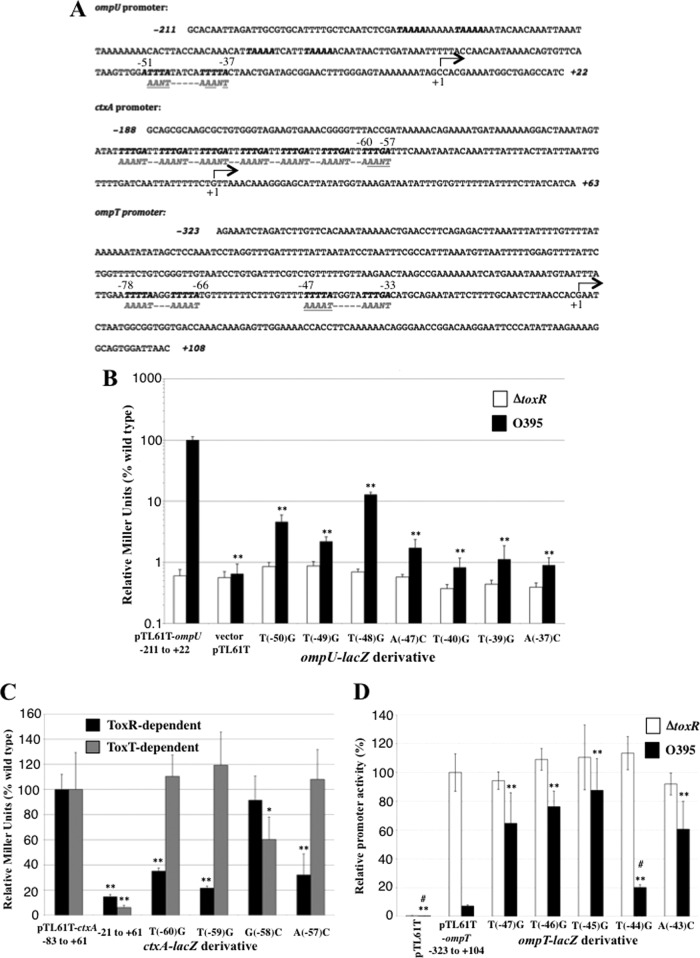
In addition to facilitating TcpP-mediated activation of the toxT promoter, ToxR can directly activate the ompU promoter and repress the ompT promoter (31, 32). Furthermore, while ctxA activation is usually accomplished by ToxT (3, 20, 34), when ToxR is expressed at high levels it can directly activate the ctxA promoter (2, 33). Thus, we examined the promoter sequences of the ompU, ompT, and ctxA genes for elements similar to the TNAAA-N5-TNAAA sequence identified in the toxT promoter. In the ompU promoter, we identified a similar sequence, 5′-TNAAA-N5-TNAAT-3′, located from −51 to −37 relative to the transcription start site (on the opposite strand from the ToxR-binding site in the toxT promoter), a position appropriate for direct activation of the ompU promoter by ToxR (Fig. 4A).
Fig 4.
The ToxR consensus-binding site is required for ToxR-mediated activation of the ompU and ctxA promoters and repression of the ompT promoter. (A) Location of consensus ToxR-binding sites in the ompU, ctxA, and ompT promoters. Nucleotides comprising potential ToxR-binding sites are in bold, while the opposite strand sequences, matching the toxT promoter consensus ToxR-binding site, are shown in gray. Those nucleotides targeted for mutagenesis are highlighted in gray and underlined. (B) Effects of transversion mutations on ToxR-mediated activation of the ompU promoter in wild-type V. cholerae or the toxR mutant strain, EK307. (C) Effect of mutations in the consensus ToxR-binding site within the promoter-proximal heptad repeat of the ctxA promoter. ctxA-lacZ expression was measured in a ΔtoxT strain (ToxR dependent) or wild-type V. cholerae O395 (ToxT dependent). (D) Effects of ompT transversion mutations on ToxR-mediated repression of the ompT promoter in wild-type V. cholerae or the toxR mutant strain, EK307. *, P < 0.05; **, P < 0.001 relative to the wild-type promoter; #, P < 0.001 relative to the ompT-lacZ T(−47)G mutant. All assessed using the Student t test, n = 6 or more measurements.
Transversion mutations introduced at positions −50, −49, −47, −40, −39, and −37 (conserved nucleotides) all resulted in a >10-fold decrease in ompU-lacZ activation, with promoter proximal mutations at −40, −39, and −37 resulting in ∼100-fold decreases in promoter activity (Fig. 4B). These decreases were not due to disruption of the RNA polymerase-binding site, as activity of these promoters in the absence of ToxR was comparable to the wild-type ompU-lacZ promoter (Fig. 4B, white bars). Thus, the TNAAA-N5-TNAA element in the ompU promoter contributed to ToxR-dependent activation as it did in the toxT promoter, confirming this as a minimal ToxR-responsive element of V. cholerae. Transversion mutation of the nonconsensus nucleotide at position −48, T(−48)G, also had an effect on ompU promoter activation, although it was the least defective (8-fold decrease) of all the mutations tested (Fig. 4B).
The ctxA promoter has an architecture made up of heptad repeats of TTTTGAT upstream of the basal promoter element. As such, it also contains a TNAAA repeat (on the opposite strand), but the spacing of this element does not provide the typical spacing, 10 to 11 base pairs, corresponding to one turn of the DNA helix. We hypothesize that this may explain why high levels of ToxR are required for activation of the ctxA promoter by ToxR. To assess ToxR-mediated ctxA-lacZ activation, we used a ΔtoxT strain, VJ740 (2), overexpressing ToxRS from plasmid pVJ21 (43). When the most promoter-proximal ToxR-binding site in the ctxA promoter (−60 to −57) (Fig. 4A) is mutated by transversion mutagenesis, ctxA-lacZ promoter activity is reduced 3- to 5-fold (Fig. 4C), indicating that this sequence in the ctxA promoter is ToxR responsive, like in toxT and ompU. We also mutated the nonconsensus nucleotide G(−58)C in the ctxA promoter and found it to have no effect on ToxR-mediated activation (Fig. 4C). Finally, as the ctxA promoter is typically directly activated by the ToxT protein, rather than ToxR (20, 34), we tested the effect of these promoter mutations on ToxT-mediated ctxA-lacZ activation in the wild-type strain, O395. The activation defects in O395 (ToxT dependent) were inversely related to those in the ΔtoxT mutant strain VJ740 overexpressing ToxR (EK3166) (Fig. 4C). Thus, ToxR and ToxT have overlapping but nonidentical binding sites in the ctxA promoter.
Finally, the ompT promoter, which is repressed by ToxR, also contains two consensus ToxR-binding sites, one from −78 to −66, and the other from −47 to −33 (Fig. 4A). Since mutation of the promoter-proximal ToxR-binding half-site would likely also affect RNA polymerase (RNAP) binding, we mutated the promoter distal ToxR-binding half-site of the ompT promoter from −47 to −43 (Fig. 4A). Transversion mutation of nucleotides −47, −46, −45, and −43 representing the TTTNA consensus binding site (opposite strand relative to toxT) resulted in loss of ToxR-mediated ompT repression, as ompT expression in the presence of ToxR increased 8- to 12-fold (Fig. 4D). Alternatively, mutation T(−44)G in the nonconsensus nucleotide resulted in a <3-fold increase in ompT expression (Fig. 4D).
Thus, all promoters directly regulated by ToxR contain a consensus TTTNA-N5-TTTNA ToxR-binding site (or near consensus), and mutation of that ToxR-binding site in each promoter leads to loss of ToxR responsiveness.
The ompU, ctxA, and ompT promoters all contain multiple ToxR-binding sites (Fig. 4A). Thus, while mutations of promoter-proximal ToxR-binding site nucleotides affected gene expression (Fig. 4), they did not affect ToxR binding to the promoter, as these mutations did not affect binding to the more promoter-distal ToxR-binding sites (see Fig. S3 in the supplemental material).
DISCUSSION
The purpose of this study was to define nucleotides within the ToxR-binding site of the toxT promoter that influence ToxR-dependent toxT promoter activation. Using plasmid-based toxT-lacZ fusion vectors, nine transversions in the region of −96 to −83 reduced toxT promoter activity 3-fold or greater, with those at −90, −86, −84, and −83 reducing this activity more than 6-fold (Fig. 1B). Transversions that altered promoter activity 3-fold or greater were located within the ToxR-footprinted region (−104 to −68) (6) and led to the identification of a TNAAA-N5-TNAAA consensus ToxR-binding site. Nucleotides within the second pentameric repeat from −86 to −82 may represent the more critical ToxR recognition site, as mutations in three of four conserved nucleotides resulted in a greater than 6-fold decrease in transcription activity, whereas none of the mutations in the ToxR recognition site from −96 to −92 had such strong effects on toxT activation (Fig. 1B).
Substitutions at −86 and −84, which were found to strongly affect ToxR-mediated toxT promoter activation, were previously identified in a screen for the loss of ToxR-mediated toxT promoter activation (Fig. 1B) (42). Furthermore, transversion mutations at these two nucleotides resulted in the greatest reduction in ToxR binding affinity (Table 1; see also Fig. S1 and S2 in the supplemental material). Substitutions at −67 and −65 (the complementary nucleotides of −86 and −84 in an inverted repeat within the toxT promoter) had little influence on ToxR-mediated promoter activation both in this report and a previous report (Fig. 1B) (42). Thus, substitutions occupying symmetrical positions with respect to that inverted repeat within the toxT promoter have differential effects on toxT activation, demonstrating that nucleotides critical to ToxR-mediated toxT promoter activation are not defined by the inverted repeat but rather by the TNAAA-N5-TNAAA direct repeat element overlapping the upstream half of the inverted repeat (Fig. 1A). As readthrough transcription is known to occur from the upstream tcpA promoter, transversions within the region from −100 to −60 can alter the sequence of the inverted repeat within the mRNA initiated from the tcpA promoter and may influence transcription attenuation in the tcpF-toxT intergenic region in the context of a chromosomally located toxT promoter, imposing an additional layer of control on toxT transcription levels (35, 42).
Based on ToxR-mediated DNA mobility shift experiments in this study, several transversions within the −96 and −83 region reduced the relative affinity of the toxT promoter for ToxR at least 2-fold, with those at −86 and −84 reducing this affinity more than 4-fold, again supporting the hypothesis that the −86 to −82 ToxR recognition site is more critical for ToxR interaction and toxT activation (Table 1; see also Fig. S1 and S2 in the supplemental material). It is notable that several adenosine nucleotides in the N5 spacer region were also required for efficient activation (Fig. 1B) and two, A(−91)C and A(−90)C, reduced ToxR binding affinities more than 2-fold (Table 1). Thus, the N5 spacer region also contributes to ToxR binding, possibly through wing domain-DNA interactions (23). This leads us to propose a modified (asymmetric) ToxR-binding site on the toxT promoter of TNAAAAA-N3-TNAAA. Alternatively, as poly(A) tracts have been shown to induce bends in the DNA helix (44), it is possible that A-to-C transversions within the linker region alter the spatial orientation of the two ToxR-binding half-sites, indirectly altering its interactions with the ToxR molecules. Thus, the motif 5′-TNAAA-N5-TNAAA-3′ represents a minimally defined ToxR-binding site, with nucleotides between the two half-sites providing structural information or, potentially, direct interactions with ToxR.
Transcription activation assays on truncated toxT promoter fragments demonstrated that the ToxR-binding site from −96 to −82 is required for binding and toxT activation and that deletion of this region from our −81 to +45 promoter derivative or the previously described −73 to +45 derivative results in a promoter with greatly reduced transcription activation (Fig. 1B and Fig. 3A) (5). Gel shift analysis with the −81 to +45 toxT promoter construct also demonstrated nearly undetectable levels of binding by ToxR (Fig. 3B), in agreement with previous studies using a truncated promoter from −73 to +45 (5). Thus, the promoter-proximal degenerate ToxR-binding site from −69 to −56 with two substitutions and altered spacing between the repeats (ANAAA-N4-TNAAG; hashed gray arrows in Fig. 1A) is unable to support efficient ToxR binding or toxT promoter activation. One surprising finding with the −81 to +45 toxT promoter construct lacking the ToxR-binding site was that activation by overexpressed TcpP was dramatically impeded if ToxR was coexpressed along with this promoter truncation (Fig. 2B). Since ToxR binds poorly to this promoter fragment (Fig. 4B), we propose this loss of activation is due to a previously established ToxR-TcpP interaction (28, 29) and diversion of TcpP away from the toxT promoter (perhaps toward the ompU and ompT promoters) by ToxR. Alternatively, the weak ToxR-binding activity of the −81 to +45 toxT promoter fragment observed in Fig. 3B may be sufficient to allow ToxR binding inside bacterial cells, and this binding may interfere with TcpP binding to its binding site from −53 to −38. According to this second hypothesis, binding of ToxR to its consensus ToxR-binding site from −96 to −82 would displace the weakly bound ToxR from the −81 to +45 region. This would be similar to PhoB repression of the phoBR promoter and derepression by PhoB binding to a neighboring upstream PhoB-binding site (45).
The motif 5′-TNAAA-N5-TNAAA-3′, or its complement (5′-TTTNA-N5-TTTNA-3′), occurs three times in the ToxR-footprinted region of the V. cholerae ompU promoter (31), twice in the ToxR-footprinted region of the V. cholerae ompT promoter (32), and within a heptad repeat element (TTTTGAT) in the ctxA promoter (46) (Fig. 4A). Mutation of the promoter-proximal ToxR-binding site in both the ompU and ctxA promoters dramatically reduced ToxR-dependent activation (Fig. 4B and C), and similar mutation in the ompT promoter prevented ToxR-mediated repression of the ompT expression (Fig. 4D). These results provide more evidence that we have identified the consensus ToxR-binding site that controls numerous ToxR-regulated promoters in V. cholerae. A recent study by Dittmer et al. using different point mutations in the ctxA promoter indicated some nucleotides within the TNAAA ToxR consensus-binding site may also contribute to ToxT binding (47). Differences in our results regarding nucleotides required for ToxT responsiveness of the ctxA promoter may reflect differences in the specific mutations tested, the way in which the cells were grown prior to assaying ctxA-lacZ expression or other factors.
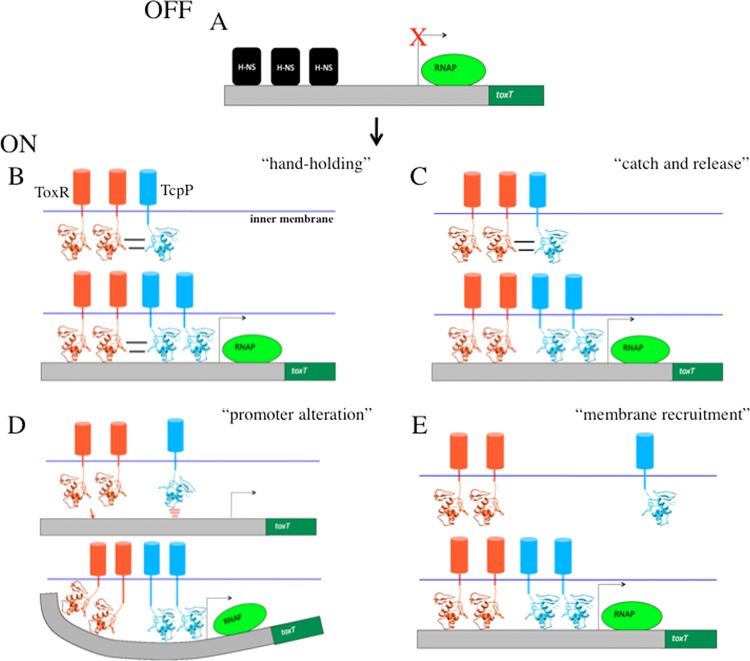
These studies provide us with a working model of toxT promoter activation that involves the binding of two ToxR molecules to the region from −96 to −83, allowing ToxR to displace H-NS (Fig. 5A) (19) and recruit two molecules of TcpP to bind the region from −53 to −38 (25). Whether ToxR releases TcpP upon DNA binding so TcpP can engage its binding site 30 nucleotides closer to the RNA polymerase-binding site (“catch and release” model; Fig. 5C) or ToxR and TcpP maintain interaction while bound to the toxT promoter (“hand-holding” model; Fig. 5B) remains to be determined. The argument against the “hand-holding” model is that the ToxR-binding site is three helical turns of the DNA upstream of TcpP, a distance that would require dramatic DNA-bending to maintain this protein-protein interaction. Furthermore, the ToxR-binding site can be moved an additional two helical turns upstream from the TcpP-binding site and maintain strong ToxR- and TcpP-dependent toxT activation (S. J. Morgan and E. S. Krukonis, unpublished data).
Fig 5.
Models for the role of ToxR in TcpP-mediated toxT activation. (A) As previously described, the toxT promoter is repressed by H-NS (19). (B) In the “hand-holding” model, ToxR and TcpP interact in the inner membrane of V. cholerae as previously described (29), and then ToxR escorts TcpP to the toxT promoter where ToxR relieves H-NS repression and maintains interaction with TcpP while TcpP stimulates transcription. (C) In the “catch and release” model, ToxR also interacts with TcpP and recruits TcpP to the toxT promoter, but upon DNA binding by ToxR, H-NS is displaced and ToxR releases TcpP so TcpP can bind the TcpP-binding site 30 nucleotides downstream of the ToxR-binding site (25). (D) In the “promoter alteration” model, interaction between ToxR and TcpP is not required for toxT activation; rather, ToxR binding to the toxT promoter displaces H-NS and alters the toxT promoter architecture such that a normally weak TcpP-binding site is altered in some way to facilitate enhanced TcpP binding, thus allowing TcpP-mediated activation of the toxT promoter. (E) In the “membrane recruitment” model, again interaction between ToxR and TcpP is not required, but the role of ToxR is to simply recruit the toxT promoter to the membrane where TcpP has easier access to its DNA-binding site. This model takes into account the fact that TcpP binding to the toxT promoter requires higher concentrations of V. cholerae membranes than ToxR binding (6) and the fact that membrane localization was previously shown to be required for ToxR to facilitate TcpP-mediated toxT activation (26).
In an alternative activation model, ToxR binding to the toxT promoter may alter the promoter architecture such that TcpP binding is facilitated, even without any direct contact between ToxR and TcpP (“promoter alteration” model; Fig. 5D). This could be due to ToxR removing the repressor H-NS from the toxT promoter and/or ToxR inducing DNA bending that allows TcpP better access to its DNA-binding site (Fig. 5D). Although, removal of H-NS alone does not account for full toxT activation as in an H-NS mutant, toxT is expressed to just 20% of the level expressed under ToxR- and TcpP-induced conditions (19). Evidence supporting the “promoter alteration” model comes from the fact that when ToxR binds the toxT promoter, a DNase I hypersensitivity site is revealed overlapping the TcpP-binding site (6, 25). This suggests that ToxR binding results in DNA bending or unwinding that might allow TcpP better access to its toxT promoter-binding site. However, this role alone cannot be sufficient for promoting TcpP-mediated activation, as a soluble form of ToxR that binds the same DNA-binding site does not facilitate TcpP-mediated toxT activation (26). Finally, it is possible that the main role of ToxR is to recruit the toxT promoter to a membrane-proximal location where TcpP can more efficiently interact with its relatively weak DNA-binding site (“membrane recruitment” model; Fig. 5E) (6, 25). According to this model, ToxR should be able to facilitate TcpP-mediated toxT activation from a considerable distance (so long as it still displaces H-NS binding), a model to be tested in the future. Most likely, aspects from several of these models contribute to how ToxR facilitates activation of the toxT promoter, including membrane recruitment, H-NS displacement, alterations to the promoter architecture, and possibly ToxR-TcpP interaction.
This study defines a minimal ToxR-responsive site, TNAAA-N5-TNAAA, in the toxT, ompU, ompT, and ctxA promoters. Based on the direct repeat nature of this ToxR-binding site, we hypothesize that two ToxR molecules bind this repeat element in a head-to-tail fashion, consistent with the structure determined for the E. coli PhoB-DNA cocrystal (23). The fact that the ToxR-binding site in the toxT promoter is in the opposite orientation from the promoter-proximal ToxR-binding sites of other ToxR-regulated promoters (ompU, ctxA, and ompT; Fig. 1A and 4A) suggests that ToxR favors this inverted orientation when playing a supporting role in TcpP-mediated toxT activation.
By defining the ToxR-binding site, we can compare the recognition sequences for a number of OmpR/PhoB family regulators in V. cholerae, including ToxR, TcpP, and PhoB. All three proteins have very similar recognition sequences: TTTNA-N5-TTTNA (ToxR), TGTAA-N6-TGTAA (TcpP) (25), and TGTCA-N6-TGTCA (PhoB) (45). This raises the question of how V. cholerae avoids cross talk among these closely related binding sites and what determines sequence-specific recognition of DNA within each protein. Previous studies on winged-helix-turn-helix proteins suggest that rather than differences in residues in the α3 DNA recognition helix, sequence specificity may be dictated by the preceding α2 helix and loop domain, which influence the positioning of the α3 helix relative to the rest of the molecule (48, 49). Future experiments will test whether this hypothesis holds true for ToxR and TcpP in V. cholerae as well.
Supplementary Material
ACKNOWLEDGMENTS
We thank Victor DiRita for providing ctx-lacZ constructs and James Kaper for providing the ompT-lacZ constructs.
This work was supported by NIH NIAID R01 AI075087 to E.S.K. and the Frederick G. Novy Fellowship from the University of Michigan Department of Microbiology and Immunology to S.J.M.
Footnotes
Published ahead of print 7 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00889-12.
REFERENCES
- 1.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the ToxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champion GA, Neely MN, Brennan MA, DiRita VJ. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323–331 [DOI] [PubMed] [Google Scholar]
- 3.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88:5403–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häse CC, Mekalanos JJ. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 95:730–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins DE, DiRita VJ. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol. Microbiol. 14:17–29 [DOI] [PubMed] [Google Scholar]
- 6.Krukonis ES, Yu RR, Dirita VJ. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67–84 [DOI] [PubMed] [Google Scholar]
- 7.Murley YM, Carroll PA, Skorupski K, Taylor RK, Calderwood SB. 1999. Differential transcription of the tcpPH operon confers biotype-specific control of the Vibrio cholerae ToxR virulence regulon. Infect. Immun. 67:5117–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacikova G, Lin W, Skorupski K. 2004. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol. Microbiol. 53:129–142 [DOI] [PubMed] [Google Scholar]
- 9.Kovacikova G, Skorupski K. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB, and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393–407 [DOI] [PubMed] [Google Scholar]
- 10.Kovacikova G, Skorupski K. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135–1147 [DOI] [PubMed] [Google Scholar]
- 11.Kovacikova G, Skorupski K. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skorupski K, Taylor RK. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 94:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skorupski K, Taylor RK. 1999. A new level in the Vibrio cholerae virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763–771 [DOI] [PubMed] [Google Scholar]
- 14.Beck NA, Krukonis ES, DiRita VJ. 2004. TcpH influences virulence gene expression in Vibrio cholerae by inhibiting degradation of the transcription activator TcpP. J. Bacteriol. 186:8309–8316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matson JS, DiRita VJ. 2005. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 102:16403–16408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abuaita BH, Withey JH. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect. Immun. 77:4111–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuhmacher DA, Klose KE. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abuaita BH, Withey JH. 2011. Termination of Vibrio cholerae virulence gene expression is mediated by proteolysis of the major virulence activator, ToxT. Mol. Microbiol. 81:1640–1653 [DOI] [PubMed] [Google Scholar]
- 19.Nye MB, Pfau JD, Skorupski K, Taylor RK. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu RR, DiRita VJ. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119–134 [DOI] [PubMed] [Google Scholar]
- 21.Liang W, Silva AJ, Benitez JA. 2007. The cyclic AMP receptor protein modulates colonial morphology in Vibrio cholerae. Appl. Environ. Microbiol. 73:7482–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Hackert E, Stock AM. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269:301–312 [DOI] [PubMed] [Google Scholar]
- 23.Blanco AG, Sola M, Gomis-Ruth FX, Coll M. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701–713 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Qin L, Egger LA, Inouye M. 2006. Transcription regulation of ompF and ompC by a single transcription factor, OmpR. J. Biol. Chem. 281:17114–17123 [DOI] [PubMed] [Google Scholar]
- 25.Goss TJ, Seaborn CP, Gray MD, Krukonis ES. 2010. Identification of the TcpP-binding site in the toxT promoter of Vibrio cholerae and the role of ToxR in TcpP-mediated activation. Infect. Immun. 78:4122–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford JA, Krukonis ES, DiRita VJ. 2003. Membrane localization of the ToxR winged-helix domain is required for TcpP-mediated virulence gene activation in Vibrio cholerae. Mol. Microbiol. 47:1459–1473 [DOI] [PubMed] [Google Scholar]
- 27.Ottemann KM, Mekalanos JJ. 1995. Analysis of Vibrio cholerae ToxR function by construction of novel fusion proteins. Mol. Microbiol. 15:719–731 [DOI] [PubMed] [Google Scholar]
- 28.Morgan SJ, Felek S, Gadwal S, Koropatkin NM, Perry JW, Bryson AB, Krukonis ES. 2011. The two faces of ToxR: activator of ompU, co-regulator of toxT in Vibrio cholerae. Mol. Microbiol. 81:113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krukonis ES, DiRita VJ. 2003. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol. Cell 12:157–165 [DOI] [PubMed] [Google Scholar]
- 30.Bina J, Zhu J, Dziejman M, Faruque S, Calderwood S, Mekalanos J. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. U. S. A. 100:2801–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford JA, Kaper JB, DiRita VJ. 1998. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol. Microbiol. 29:235–246 [DOI] [PubMed] [Google Scholar]
- 32.Li CC, Crawford JA, DiRita VJ, Kaper JB. 2000. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol. Microbiol. 35:189–203 [DOI] [PubMed] [Google Scholar]
- 33.Miller VL, Mekalanos JJ. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by ToxR. Proc. Natl. Acad. Sci. U. S. A. 81:3471–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Withey JH, DiRita VJ. 2006. The toxbox: specific DNA sequence requirements for activation of Vibrio cholerae virulence genes by ToxT. Mol. Microbiol. 59:1779–1789 [DOI] [PubMed] [Google Scholar]
- 35.Yu RR, DiRita VJ. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J. Bacteriol. 181:2584–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller VL, Taylor RK, Mekalanos JJ. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271–279 [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higuchi R. 1990. Recombinant PCR, p 177–183 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, CA [Google Scholar]
- 40.Linn T, Pierre RS. 1990. Improved vector system for constructing transcriptional fusions that ensure independent translation of lacZ. J. Bacteriol. 172:1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 42.Higgins DE, DiRita VJ. 1996. Genetic analysis of the interaction between Vibrio cholerae transcription activator ToxR and toxT promoter DNA. J. Bacteriol. 178:1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller VL, DiRita VJ, Mekalanos JJ. 1989. Identification of toxS, a regulatory gene whose product enhances ToxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koo HS, Wu HM, Crothers DM. 1986. DNA bending at adenine-thymine tracts. Nature 320:501–506 [DOI] [PubMed] [Google Scholar]
- 45.Diniz MM, Goulart CL, Barbosa LC, Farache J, Lery LM, Pacheco AB, Bisch PM, von Kruger WM. 2011. Fine-tuning control of phoBR expression in Vibrio cholerae by binding of PhoB to multiple pho boxes. J. Bacteriol. 193:6929–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekalanos JJ, Swartz DJ, Pearson GD, Harford N, Groyne F, de Wilde M. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557 [DOI] [PubMed] [Google Scholar]
- 47.Dittmer JB, Withey JH. 2012. Identification and characterization of the functional toxboxes in the Vibrio cholerae cholera toxin promoter. J. Bacteriol. 194:5255–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsden I, Chen Y, Jin C, Liao X. 1997. Evidence that the DNA binding specificity of winged helix proteins is mediated by a structural change in the amino acid sequence adjacent to the principal DNA binding helix. Biochemistry 36:13248–13255 [DOI] [PubMed] [Google Scholar]
- 49.Overdier DG, Porcella A, Costa RH. 1994. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino-acid residues adjacent to the recognition helix. Mol. Cell. Biol. 14:2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins DE, Nazareno E, DiRita VJ. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974–6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.