Abstract
Mycobacterium protein tyrosine phosphatase B (mPTPB) is essential for the survival and persistence of Mycobacterium in the host. Thus small molecule inhibitors of mPTPB are potential anti-TB agents. We developed an efficient organocatalytic multicomponent reaction (MCR) between pyrrole, formaldehyde and aniline, affording a potent and selective mPTPB inhibitor with IC50 at 1.5 µM and >50-fold specificity. Our studies provide a successful example of using organocatalysis as a discovery tool for the acquisition of PTP inhibitors.
Organocatalysis is a very useful tool for the preparation of various chiral and non chiral molecules, owing to the mild reaction conditions, low cost, and environmental consciousness.1,2,3 A recent trend in organocatalysis is organocatalyzed multicomponent reaction (MCRs)4 affording novel and complex molecules with multiple stereocenter controls, which is highly desirable in modern organic and medicinal chemistry.5 Examples in this subject include three-component domino condensations,6 Biginelli reactions,7 and Mannich reactions8 catalyzed by various organocatalysts to yield important novel amine building blocks and heterocycles. We are interested in applying these advanced synthetic strategies to the discovery of protein tyrosine phosphatase (PTP) inhibitors, which possess enormous potential therapeutic values for many human diseases.
Tuberculosis (TB) is a major worldwide threat to public health, with approximately 9 million new cases and 1.8 million deaths each year in the world.9 No new anti-TB drugs have been developed in close to 40 years.10 The inadequate efficacy, lengthy treatment, and multi-drug resistant TB underscore the urgency of developing new and more effective therapies.11 mPTPB has emerged as a novel anti-TB target. It is secreted by Mtb into the cytoplasm of macrophages, where it mediates mycobacterial survival in the host and serves as a virulence factor.12,13 Small molecules that inhibit mPTPB hence possess great potentials as novel anti-TB agents. Unfortunately, only a handful of mPTPB inhibitors have been reported,14 and many of them lack the required potency and selectivity, due to the challenge in acquiring selective PTP inhibitory agents targeting the conserved active site.15 Moreover, these molecules were acquired through multiple fragments appending procedures, which unavoidably introduce high molecular weight and lipophilicity, and thus are not appropriate as lead compounds.
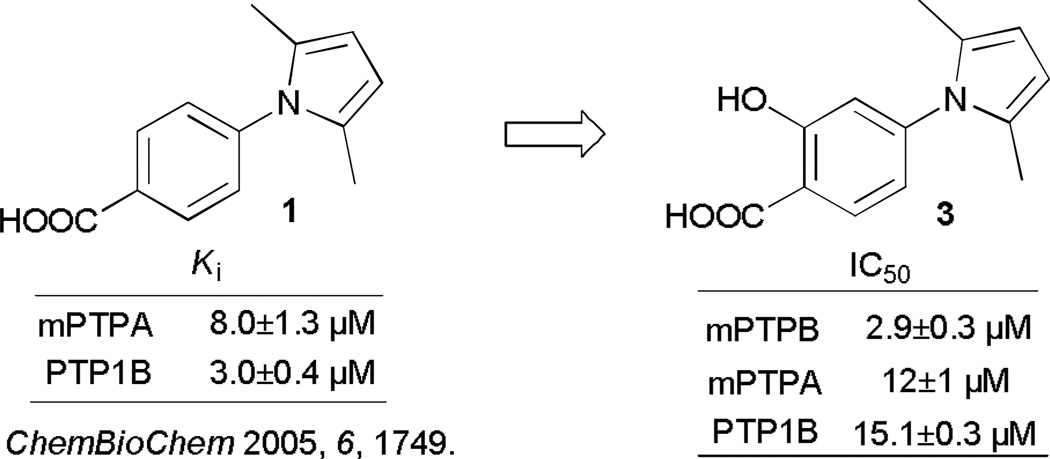
Pyrroles are favourable substrates in organic chemistry due to their high reactivity towards electrophilic aromatic substitutions and Diels-Alder reactions.16 Pyrrole is also a privileged structure motif that exists in various biologically active molecules such as drugs and natural products. Compound 1 and several analogues have been reported to inhibit PTP1B at micromolar range (Figure 1).17 Unfortunately, this class of compounds exhibited no selectivity against other PTPs, which is a common issue in the field due to the highly conserved active sites in over 100 PTP family members. In addition, compound 1 also exhibits poor stability. We envisaged that the poor stability is probably due to the high reactivity of the pyrrole ring, and that substitutions at the pyrrole reactive sites may mask its reactivity and hence increase its stability. More importantly, fragments added through the substitution reactions may not only enhance its binding affinity to PTPs, but also improve its specificity, as targeting both PTP active site and nearby peripheral site by two or more fragments is a proven strategy in acquiring potent and selective PTP inhibitors.15,18 To these ends, we sought to develop a pyrrole Mannich type reaction that couples the pyrrole, an amine and an aldehyde or ketone, which should be very useful for preparing pyrrole-based libraries that are potential PTP inhibitors with improved potency and specificity.
Fig. 1.
Structures and activities of N-Phenyl, 2,5-dimethyl pyrroles.
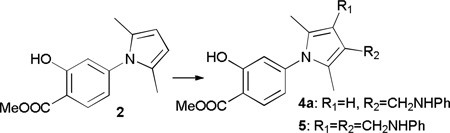
To begin our study, we designed 2 (Table 1) as the parent pyrrole compound, which, after hydrolysis, afforded compound 3 with a salicylic acid group serving as a nonhydrolazble p-Tyr mimetic.19 3 is a moderately selective inhibitor against mPTPB with an IC50 at 2.9 µM.20 Subsequently, MCR Mannich reaction between 2, formaldehyde and aniline was studied as the model reaction to probe the optimal conditions prior to the library generation. The reaction was first carried out in a range of solvents using HOAc as a catalyst. CH2Cl2 stands out as the most optimal solvent in affording both mono- and di-alkylated products in a combined 74% conversion (entry 1, Table 1). In exploring for alternative acids as catalysts, we found that this reaction was very sensitive to the acidity of catalysts. For example, TFA catalyzed reaction provided a complex mixture with the complete consumption of pyrrole (entry 2, Table 1), weaker acids such as proline, PTSA and benzoic acid, and inorganic acid HCl afforded products in zero to low conversions (entry 3–6, Table 1). In contrast, methoxyacetic acid catalyzed reaction slightly more efficiently than acetic acid, but with low selectivity for 4a (entry 7, Table 1). We also evaluated N,N-di[3,5-di(trifluoromethyl)phenyl]thiourea, a frequently used organocatalyst,21 and it showed no capability to catalyze this reaction (entry 8, Table 1). Increasing acetic acid from 20 mol% to 100 mol% did not show much improvement in total conversion, however, the selectivity for product 4a was increased by 1.7-fold (entry 1 vs entry 9, Table 1), and further increase of acetic acid in large excess resulted in a complex mixture with trace product. Finally, using 2 equiv. of pyrrole, 2 equiv. of HCHO, and 1 equiv. of aniline greatly improved the selectivity for 4a with 85% isolated yield (entry 10, Table 1). And reaction using 1 equiv. of pyrrole, 3 equiv. of HCHO, and 3 equiv. of aniline in the presence of methoxyacetic acid after extended time afford 5 as the sole product in 75% isolated yield. Thus we were able to obtain mono and di-alkylated products by fine-tuning the catalysts and the relative ratios of reactants.
Table 1.
MannichMCR between pyrrole, paraformaldehyde and aniline under various conditionsa
 | |||
|---|---|---|---|
| Entry | Catalyst | Ratio of 4a/5/2 %b |
Isolated yield % |
| 1 | HOAc (20 mol%) | 52//22/26 | |
| 2 | TFA (20 mol%) | NA | |
| 3 | Proline (20 mol%) | 7/0/93 | |
| 4 | PTSA (20 mol%) | 21/5/74 | |
| 5 | PhCO2H(20 mol%) | 28/13/59 | |
| 6 | 2 M HCl (20 mol%) | 26/4/70 | |
| 7 | MeOCH2CO2H (20 mol%) | 45/36/19 | |
| 8 | (3,5-(CF3)2-PhNH)2CS (20 mol%) | no reaction | |
| 9 | HOAc (100 mol%) | 59//17/24 | |
| 10c | HOAc (100 mol%) | 45/9/46 | 85 (4a)e |
| 11d | MeOCH2CO2H (100 mol%) | 0/100/0 | 75 (5) |
the reaction was carried out at rt for 24 h in 1 mL CH2Cl2 with 2 (0.1mmol), paraformaldehyde (0.12mmol), aniline (0.12mmol);
the ratio is based on UV absorption in LC-MS studies of crude reaction mixture.
2 equiv. of pyrrole, 2 equiv. of paraformaldehyde, and 1 equiv. of aniline, were used.
1 equiv. of pyrrole, 3 equiv. of formaldehyde, and 3 equiv. of aniline were used, reaction time was 48 h.
based on 1 equiv. aniline
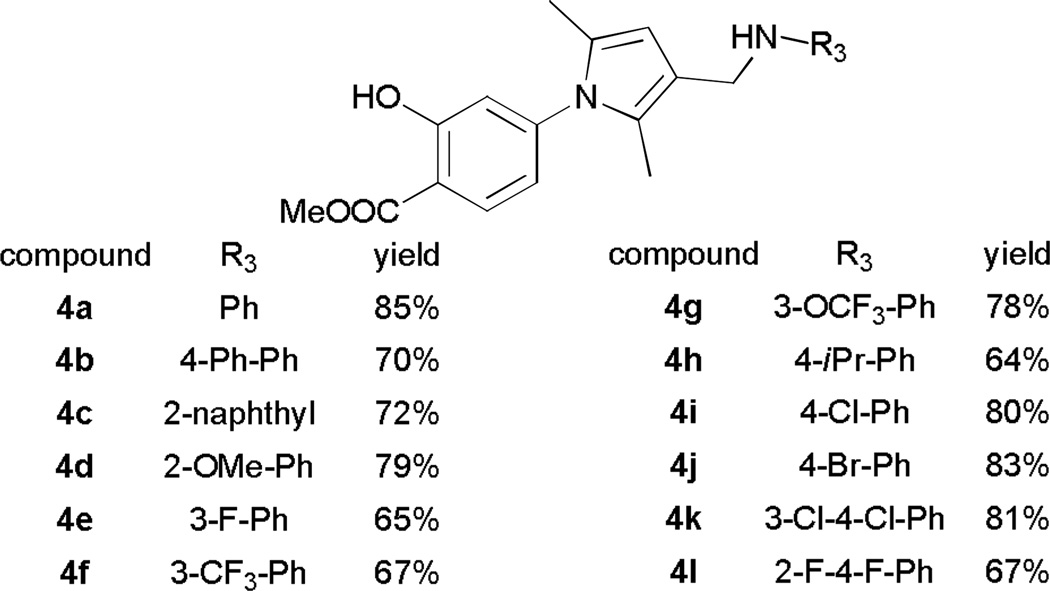
With reaction conditions towards either 4a or 5 at hand, we proceeded to expand the substrate scope by treating pyrrole 2 with paraformaldehyde and various amines. Generally, reactions went well with aromatic amines, such as aniline derivatives and naphthyl amines, and products can be received in moderate to good yields. For aniline derivatives, substituents at o, m, and p positions and with either electron donating or withdrawing properties are tolerated (Figure 2). Unlike many similar reactions where substituents at 2 position was not feasible,8 our studies indicate broader opportunity in choosing anilines for this reaction, and we attribute it to the small size of formaldehyde. Indeed, when we used larger aldehydes, i.e. acetaldehyde and benzaldehyde, the reaction did not proceed, even under elevated temperature, extended time, and with stronger acid catalysts. Aliphatic amines are usually not applicable in Mannich reaction,8 due to the sluggish imine formation. Thus it is not surprising that propyl amine, benzyl amine, diethyl amine and piperidine did not afford any product under our reaction conditions. Nevertheless, our goals are not limited to develop a Mannich reaction, but more importantly, to use it as a tool to develop more potent, selective, and stable PTP inhibitors. We anticipated that, after hydrolysis, the N-salicylic acid moiety would occupy the active site, while the added aniline moiety would target an adjacent secondary site, with the methylene from formaldehyde serving as a linker.18
Fig. 2.
Representative Mannich reaction products from 2, formaldehyde and aromatic amines.
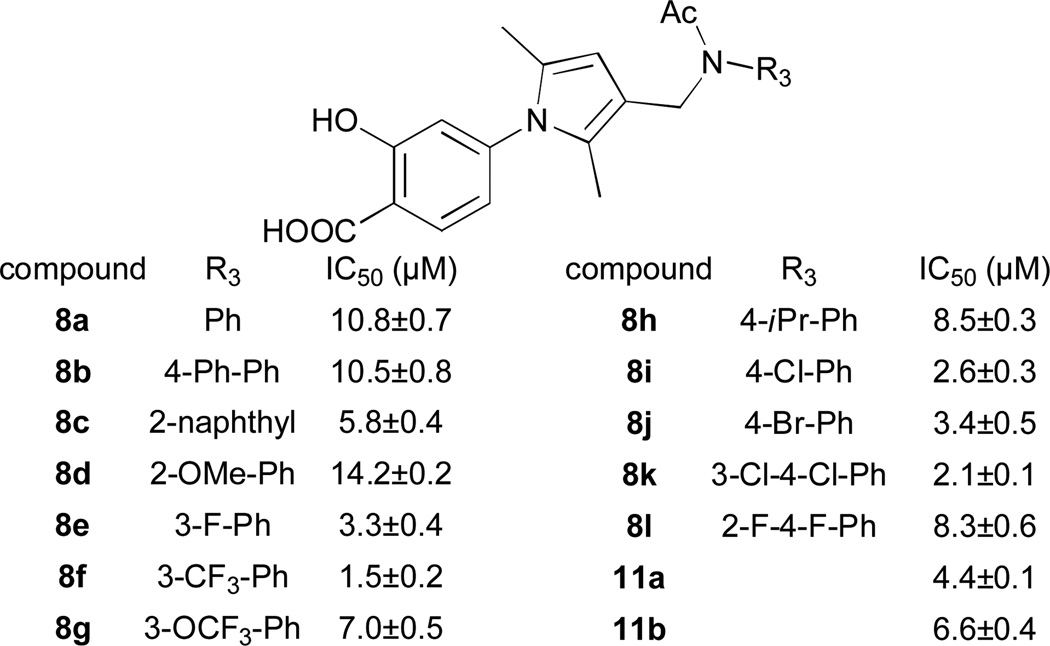
4a was converted to 6a under hydrolysis conditions (Scheme S1, Supplementary Information), which was observed in good yield by LC-MS analysis. However, once the reaction mixture was acidified by 2M HCl or NH4Cl prior to extraction, we were not able to observe the product, indicating that it decomposed rapidly even at weakly acidic conditions. The reason could be that the basic aniline nitrogen is easily protonated, and this internal proton donor at the right orientation could promote the degradation of pyrrole. To reduce the basicity of this nitrogen, we decided to protect it with a small group with electron withdrawing ability. Hence compound 4a was reacted with acetic anhydride to give compound 7a, and hydrolyzed to afford product 8a in excellent yield (Scheme S1, supplementary information). As expected, compound 8a indeed showed much improved stability.
Encouraged by the results, all of the Mannich adducts obtained in Figure 2 were protected by acetyl group, hydrolyzed and purified by reverse phase HPLC, to afford products in good yields and high purities. All hydrolyzed products display much improved stability compare to 3, and most of them exhibit good inhibitory activity under the same assay conditions (Figure 3).20 Although no profound influence on activity, substituents at the meta position are generally more beneficial than at ortho and para positions. Among them, 8f is the most potent mPTPB inhibitor, with an IC50 at 1.5 µM. Meanwhile bis Mannich adduct 9 exhibited no activity at 100 µM (page 19, Supplementary Information), suggesting that the second aniline fragment may disrupt the binding to mPTPB. Additionally, we reasoned that the introduction of one or two halogen atoms into the pyrrole ring may be helpful in improving potency, specificity and stability. Though brominations using either Br2 or NBS gave complex mixture, iodinations successfully afforded mono-iodinated 10a and di-iodinated 10b, by using I2 and ICl, respectively. The hydrolyzed compounds 11a and 11b (Scheme S2, Supplementary Information) indeed exhibited improved stability over parent compound 3, unfortunately they are several fold less potent than 8f from our MCR Mannich reactions as mPTPB inhibitors.
Fig. 3.
Structure of hydrolyzed products from Mannich reactions and their inhibition against mPTPB.20
Given the increased potency and stability, we proceeded to study compound 8f’s specificity to mPTPB over selected members of the PTP super family, including mPTPA, PTP1B, SHP2, CD45, PTPα, and MKP5. As shown in Table 2, 8f is highly selective for mPTPB, exhibiting a greater than 50-fold preference for mPTPB over all PTPs examined, marking compound 8f as one of the most selective mPTPB inhibitors reported to date. In comparison, compound 3 has only 4 and 5-fold selectivity against mPTPA and PTP1B, respectively. These results show that PTP inhibitors generated from the MCR Mannich reaction not only exhibit increased stability, potency, but also greatly improved specificity.
Table 2.
Specificity studies of compound 8f against a panel of PTPs.20
| Enzyme | IC50 (µM) |
| mPTPB | 1.5±0.2 |
| mPTPA | 180±30 |
| PTP1B | 200±30 |
| SHP2 | 86±7 |
| CD45 | 78±7 |
| PTPα | >> 100 |
| MKP5 | >> 100 |
In conclusion, we have successfully developed an efficient organocatalyzed MCR Mannich type reaction between pyrrole, paraformaldehyde, and anilines. By fine-tuning the catalysts and reactants ratios, we were able to obtain mono- and di-alkylated products in good yields. More importantly, these reactions enabled us to identify PTP inhibitors, which have increased stability, potency, and selectivity. In particular, compound 8f has an IC50value at 1.5 µM against mPTPB, with >50-fold selectivity over a large panel of PTPs. The low molecular weight and compact structure render 8f a good lead molecule for anti-TB drug discovery targeting mPTPB. Our studies provide a successful example in using organocatalysis as a tool to discover enzyme inhibitors. Given that a vast array of biologically active molecules containing pyrrole, furan, and indole moieties can serve as substrates in organocatalytic reactions, this study should have a broader impact on enzyme inhibitor discovery beyond the PTP target class.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grant CA152194.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
Notes and references
- 1.a) Ahrendt KA, Borths CJ, MacMillan DWC. J. Am. Chem. Soc. 2000;122:4243–4244. [Google Scholar]; b) List B, Lerner RA, Barbas CF., III J. Am. Chem. Soc. 2000;122:2395–2396. [Google Scholar]
- 2.a) Gaunt MJ, Johansson CCC, McNally A, Vo NT. Drug Discovery Today. 2007;12:8–27. doi: 10.1016/j.drudis.2006.11.004. [DOI] [PubMed] [Google Scholar]; b) Bertelsen S, Jørgensen KA. Chem. Soc. Rev. 2009;38:2178–2189. doi: 10.1039/b903816g. [DOI] [PubMed] [Google Scholar]
- 3.a) Dalko PI, editor. Enantioselective Organocatalysis. Weinheim, Germany: Wiley-VCH; 2007. [Google Scholar]; b) Pellissier H. Recent Developments in Asymmetric Organocatalysis. Royal Society of Chemistry; 2010. [Google Scholar]
- 4.a) Ramachary DB, Barbas CF., III Chem. Eur. J. 2004;10:5323–5331. doi: 10.1002/chem.200400597. [DOI] [PubMed] [Google Scholar]; b) Ramachary DB, Jain S. Org. Biomol. Chem. 2011;9:1277–1300. doi: 10.1039/c0ob00611d. [DOI] [PubMed] [Google Scholar]
- 5.Ramachary DB, Reddy YV. J. Org. Chem. 2010;75:74–85. doi: 10.1021/jo901799n. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z-J, Zhu D, Zeng X, Wang F, Tan B, Hou Y, Lv Y, Zhong G. Chem. Commun. 2010;46:2504–2506. doi: 10.1039/b924575h. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Chen X-H, Song J, Luo S-W, Fan W, Gong L-Z. J. Am. Chem. Soc. 2009;131:15301–15310. doi: 10.1021/ja905320q. [DOI] [PubMed] [Google Scholar]
- 8.a) Verkade JMM, van Hemert LJC, Quaedflieg PJLM, Rutjes FPJT. Chem. Soc. Rev. 2008;37:29–41. doi: 10.1039/b713885g. [DOI] [PubMed] [Google Scholar]; b) Cheng L, Wu X, Lu Y. Org. Biomol. Chem. 2007;5:1018–1020. doi: 10.1039/b701579h. [DOI] [PubMed] [Google Scholar]; c) Fu X, Loh W-T, Zhang Y, Chen T, Liu H, Wang J, Tan C-H. Angew. Chem. Int. Ed. 2009;40:7387–7390. doi: 10.1002/anie.200903971. [DOI] [PubMed] [Google Scholar]; d) Kumar A, Kumar Gupta M, Kumar M. Green Chem. 2012;14:290–295. [Google Scholar]
- 9.WHO Report 2010 on Global TB Control, 2010.
- 10.a) Fox W, Mitchison DA. Lancet. 1976;2:1349–1350. doi: 10.1016/s0140-6736(76)91989-9. [DOI] [PubMed] [Google Scholar]; b) Neff M. Am. Fam. Phys. 2003;68:1854, 1857–1858, 1861–1852. [Google Scholar]
- 11.Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Lancet. 2010;375:2100–2109. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- 12.Koul A, Choidas A, Treder M, Tyagi AK, Drlica K, Singh Y, Ullrich A. J. Bacteriol. 2000;182:5425–5432. doi: 10.1128/jb.182.19.5425-5432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Rao V, Shakila H, Gupta R, Khera A, Dhar N, Singh A, Koul A, Singh Y, Naseema M, Narayanan PR, Paramasivan CN, Ramanathan VD, Tyagi AK. Mol. Microbiol. 2003;50:751–762. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 14.a) Noren-Muller A, Reis-Correa I, Jr, Prinz H, Rosenbaum C, Saxena K, Schwalbe H, Vestweber D, Cagna G, Schunk S, Schwarz O, Schiewe H, Waldmann H. Proc. Natl. Acad. Sci. USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Soellner MB, Rawls KA, Grundner C, Alber T, Ellman JA. J. Am. Chem. Soc. 2007;129:9613–9615. doi: 10.1021/ja0727520. [DOI] [PubMed] [Google Scholar]; c) Tan LP, Wu H, Yang P-Y, Kalesh KA, Zhang X, Hu M, Srinivasan R, Yao SQ. Org. Lett. 2009;11:5102–5105. doi: 10.1021/ol9023419. [DOI] [PubMed] [Google Scholar]; d) Zhou B, He Y, Zhang X, Xu J, Luo Y, Wang Y, Franzblau SG, Yang Z, Chan R, Liu Y, Zheng J, Zhang Z-Y. Proc. Natl. Acad. Sci. USA. 2010;107:4573–4578. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Bialy L, Waldmann H. Angew. Chem. Int. Ed. 2005;44:3814–3839. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]; b) Combs AP. J. Med. Chem. 2010;53:2333–2344. doi: 10.1021/jm901090b. [DOI] [PubMed] [Google Scholar]
- 16.Estévez V, Villacampa M, Menéndez JC. Chem. Soc. Rev. 2010;39:4402–4421. doi: 10.1039/b917644f. [DOI] [PubMed] [Google Scholar]
- 17.Manger M, Scheck M, Prinz H, von Kries JP, Langer T, Saxena K, Schwalbe H, Frstner A, Rademann J, Waldmann H. Chem Bio Chem. 2005;6:1749–1753. doi: 10.1002/cbic.200500171. [DOI] [PubMed] [Google Scholar]
- 18.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang Z-Y. Proc. Natl. Acad. Sci. USA. 1997;94:13420–13425. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Zeng L-F, Yu Z-H, He R, Liu S, Zhang Z-Y. Bioorg. Med. Chem. 2012;20:1940–1946. doi: 10.1016/j.bmc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IC50 values were determined at pH 7 and 25 °C (for details, see Supplementary Information).
- 21.Schreiner PR, Wittkopp A. Org. Lett. 2002;4:217–220. doi: 10.1021/ol017117s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.