Abstract
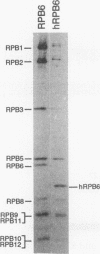
We isolated the cDNA encoding the homolog of the Saccharomyces cerevisiae nuclear RNA polymerase common subunit RPB6 from hamster CHO cells. Alignment of yeast RPB6 with its mammalian counterpart revealed that the subunits have nearly identical carboxy-terminal halves and a short acidic region at the amino terminus. Remarkably, the length and amino acid sequence of the hamster RPB6 are identical to those of the human RPB6 subunit. The conservation in sequence from lower to higher eukaryotes also reflects conservation of function in vivo, since hamster RPB6 supports normal wild-type yeast cell growth in the absence of the essential gene encoding RPB6.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker J., Wintzerith M., Vigneron M., Kedinger C. Structure of the gene encoding the 14.5 kDa subunit of human RNA polymerase II. Nucleic Acids Res. 1993 Nov 25;21(23):5345–5350. doi: 10.1093/nar/21.23.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. D., Kelley J. M., Gocayne J. D., Dubnick M., Polymeropoulos M. H., Xiao H., Merril C. R., Wu A., Olde B., Moreno R. F. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991 Jun 21;252(5013):1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Amegadzie B. Y., Ahn B. Y., Moss B. Characterization of a 7-kilodalton subunit of vaccinia virus DNA-dependent RNA polymerase with structural similarities to the smallest subunit of eukaryotic RNA polymerase II. J Virol. 1992 May;66(5):3003–3010. doi: 10.1128/jvi.66.5.3003-3010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Valenzuela P., Rutter W. J. Phosphorylation of yeast DNA-dependent RNA polymerases in vivo and in vitro. Isolation of enzymes and identification of phosphorylated subunits. J Biol Chem. 1977 May 10;252(9):3082–3091. [PubMed] [Google Scholar]
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Buhler J. M., Iborra F., Sentenac A., Fromageot P. The presence of phosphorylated subunits in yeast RNA polymerases A and B. FEBS Lett. 1976 Nov 15;72(1):37–41. doi: 10.1016/0014-5793(76)80893-9. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Sharp P. A., Guarente L. Function of a yeast TATA element-binding protein in a mammalian transcription system. Nature. 1988 Jul 7;334(6177):37–42. doi: 10.1038/334037a0. [DOI] [PubMed] [Google Scholar]
- Cavallini B., Huet J., Plassat J. L., Sentenac A., Egly J. M., Chambon P. A yeast activity can substitute for the HeLa cell TATA box factor. Nature. 1988 Jul 7;334(6177):77–80. doi: 10.1038/334077a0. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Khalili K., Zandomeni R., Weinmann R. The gene encoding the large subunit of human RNA polymerase II. J Biol Chem. 1985 Dec 5;260(28):15204–15210. [PubMed] [Google Scholar]
- Comai L., Tanese N., Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992 Mar 6;68(5):965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- Cormack B. P., Strubin M., Ponticelli A. S., Struhl K. Functional differences between yeast and human TFIID are localized to the highly conserved region. Cell. 1991 Apr 19;65(2):341–348. doi: 10.1016/0092-8674(91)90167-w. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dillon P. J., Rosen C. A. A rapid method for the construction of synthetic genes using the polymerase chain reaction. Biotechniques. 1990 Sep;9(3):298–300. [PubMed] [Google Scholar]
- Flanagan P. M., Kelleher R. J., Feaver W. J., Lue N. F., LaPointe J. W., Kornberg R. D. Resolution of factors required for the initiation of transcription by yeast RNA polymerase II. J Biol Chem. 1990 Jul 5;265(19):11105–11107. [PubMed] [Google Scholar]
- Freund E., McGuire P. M. Characterization of RNA polymerase type II from human term placenta. J Cell Physiol. 1986 Jun;127(3):432–438. doi: 10.1002/jcp.1041270312. [DOI] [PubMed] [Google Scholar]
- Gill G., Tjian R. A highly conserved domain of TFIID displays species specificity in vivo. Cell. 1991 Apr 19;65(2):333–340. doi: 10.1016/0092-8674(91)90166-v. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kelleher R. J., 3rd, Flanagan P. M., Chasman D. I., Ponticelli A. S., Struhl K., Kornberg R. D. Yeast and human TFIIDs are interchangeable for the response to acidic transcriptional activators in vitro. Genes Dev. 1992 Feb;6(2):296–303. doi: 10.1101/gad.6.2.296. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Woychik N., Liao S. M., Young R. A. RNA polymerase II subunit composition, stoichiometry, and phosphorylation. Mol Cell Biol. 1990 May;10(5):1915–1920. doi: 10.1128/mcb.10.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kolodziej P., Young R. A. RNA polymerase II subunit RPB3 is an essential component of the mRNA transcription apparatus. Mol Cell Biol. 1989 Dec;9(12):5387–5394. doi: 10.1128/mcb.9.12.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKune K., Richards K. L., Edwards A. M., Young R. A., Woychik N. A. RPB7, one of two dissociable subunits of yeast RNA polymerase II, is essential for cell viability. Yeast. 1993 Mar;9(3):295–299. doi: 10.1002/yea.320090309. [DOI] [PubMed] [Google Scholar]
- Pati U. K., Weissman S. M. Isolation and molecular characterization of a cDNA encoding the 23-kDa subunit of human RNA polymerase II. J Biol Chem. 1989 Aug 5;264(22):13114–13121. [PubMed] [Google Scholar]
- Pati U. K., Weissman S. M. The amino acid sequence of the human RNA polymerase II 33-kDa subunit hRPB 33 is highly conserved among eukaryotes. J Biol Chem. 1990 May 25;265(15):8400–8403. [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Aronson D. B., Burgess R. R. Purification of eukaryotic RNA polymerase II by immunoaffinity chromatography. Elution of active enzyme with protein stabilizing agents from a polyol-responsive monoclonal antibody. J Biol Chem. 1990 Apr 25;265(12):7069–7077. [PubMed] [Google Scholar]
- Treich I., Carles C., Riva M., Sentenac A. RPC10 encodes a new mini subunit shared by yeast nuclear RNA polymerases. Gene Expr. 1992;2(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- Woychik N. A., Lane W. S., Young R. A. Yeast RNA polymerase II subunit RPB9 is essential for growth at temperature extremes. J Biol Chem. 1991 Oct 5;266(28):19053–19055. [PubMed] [Google Scholar]
- Woychik N. A., Liao S. M., Kolodziej P. A., Young R. A. Subunits shared by eukaryotic nuclear RNA polymerases. Genes Dev. 1990 Mar;4(3):313–323. doi: 10.1101/gad.4.3.313. [DOI] [PubMed] [Google Scholar]
- Woychik N. A., McKune K., Lane W. S., Young R. A. Yeast RNA polymerase II subunit RPB11 is related to a subunit shared by RNA polymerase I and III. Gene Expr. 1993;3(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Woychik N. A., Young R. A. RNA polymerase II subunit RPB10 is essential for yeast cell viability. J Biol Chem. 1993 Jun 5;268(16):12230–12230. [PubMed] [Google Scholar]
- Woychik N. A., Young R. A. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol Cell Biol. 1989 Jul;9(7):2854–2859. doi: 10.1128/mcb.9.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]