Abstract
Bone marrow-derived multipotent mesenchymal stromal cells (MSCs) are the most frequently investigated cell type for potential regenerative strategies because they are relatively easy to isolate and are able to differentiate into several mesenchymal lineages. Unfortunately, during ex vivo culture, MSCs present gradual loss of differentiation potential and reduced clinical efficacy. Reactive oxygen species (ROS) are associated with oxidative damage and accumulate during MSC expansion. Because ROS are believed to be involved in the loss of multipotency, we hypothesized that compounds with antioxidant activity have the capacity to scavenge ROS, prevent cellular damage, and rescue culture-induced loss of multipotency. In this manuscript, we show that antioxidant supplementation can partially rescue the loss of alkaline phosphatase expression induced by oxidizing agents and increases the yield of hMSCs, when supplemented to a fresh bone marrow aspirate. Concomitantly, oxidative DNA damage and ROS levels in hMSCs were reduced by antioxidants. We conclude that antioxidant supplementation during MSC expansion reduces the DNA damage load and increases the MSC yield.
Introduction
Mesenchymal stromal cells (MSCs) are being investigated for a diverse range of clinical applications, including functional repair of bone defect,1,2 improving the engraftment of transplanted hematopoietic cells,3 and treatment of graft-versus-host disease.4,5 The possibility to apply MSCs for bone regeneration has gained considerable interest in the last decades due to their easy isolation, efficient in vitro proliferation capacity and broad differentiation potential.6 Preclinical proof of concept has been obtained in animal models,7 but clinical application has been hampered by large donor variability and the loss of MSC multipotency upon expansion in vitro. For instance, it has been demonstrated that the amount of ectopic bone formation in immune-deficient mouse models correlated to the extent cells were expanded in vitro before implantation.8 These observations are in line with our own observations showing that alkaline phosphatase (ALP) expression, mineralization, and adipogenesis of culture-expanded hMSCs decrease sharply between passage 3 and 4.9,10 Moreover, we observed that during in vitro culture of hMSCs, the level of DNA damage increases and the DNA damage signaling pathway is activated around passage 3–4.10
Based on this, we hypothesize that loss of multipotency is the consequence of the accumulation of cellular damage that cells face during culture. Potential sources of cellular damage are the so-called reactive oxygen species (ROS), such as hydroxyl radicals, superoxide anions, and hydrogen peroxide (H2O2). ROS can inflict a multitude of damages to cells resulting in protein misfolding, altered protein conformation or even fragmentation,11–13 and lipid peroxidation.14 Damage to nuclear DNA includes DNA fragmentation, oxidation of bases, such as 8-oxo-7,8-dihydroguanine (8-oxo-G), strand breakage,15 and DNA-protein crosslinks. Oxidative stress frequently results from an imbalance in redox signaling, for instance, due to the production of high level of oxidants during normal cellular metabolism (e.g., mitochondrial electron transport or NADPH oxidases) or from environmental stimuli (e.g., cytokines).16 Oxidative stress is implicated in the etiology of various degenerative diseases and the process of aging.16,17 Many mutations that prolong life provide a global increase in oxidative stress resistance and enhance antioxidant gene activities, for instance, in some long-lived strains of Drosophila.18,19 A correlation has been suggested between the metabolic rate of an organism, its production of ROS, and life span,20 and later evidence indicates that longevity correlates best to ROS production.21
ROS have been associated with the pathogenesis of bone loss-related diseases. For example, aged osteoporotic women present a marked decrease in plasma antioxidants.22 A biochemical link between increased oxidative stress and reduced bone density was also established in a study of 48 women and 53 men.23 Furthermore, osteoporosis has been noted in two mouse models of premature aging in which a role for oxidative damage has been suggested.24,25 Finally, we observed that osteogenic differentiation was inhibited when hMSCs were exposed to oxidative damage-inducing agents.10
With the documented link between oxidative damage and loss of functionality during ageing and MSC expansion, several studies have addressed the potential use of reagents with antioxidant properties to suppress this effect. For instance, in hematopoietic stem cells defective in the cell cycle checkpoint activator ataxia telangiectasia mutated, the self-renewal capacity can be enhanced through the supplementation of antioxidative substances such as N-acetyl-L-cysteine (NAC) to the culture medium.26 Recently, it was demonstrated that during normal cell culturing, MSCs present low antioxidant levels and, therefore, display high oxidative stress. Supplementation of the antioxidant selenium increased the antioxidant capacity of the cells and reduced cell damage.27 However, it remains unclear whether antioxidant supplementation is able to restore or prevent the loss of multipotency and proliferation observed during in vitro expansion and whether it could extend the period in which these cells would remain functionally viable for clinical applications. We, therefore, investigated the role of antioxidants during in vitro expansion and differentiation of hMSCs. We used a model system to mimic oxidative damage generated during hMSC in vitro expansion to further elucidate the effect of oxidative damage in the loss of differentiation potential, as well as the role of antioxidants to prevent or reduce damage accrual. Selected antioxidants were tested for their capability to prevent/restore the loss of multipotency that hMSCs face during normal in vitro culturing.
Materials and Methods
Isolation and culture of hMSCs
Bone marrow aspirates were obtained from the acetabulum of three donors (two males, one female, aged 65–66 years old) with written informed consent. In short, aspirates were resuspended using 20G needles, plated at a density of 5×105 cells/cm2, and cultured in the hMSC proliferation medium (PM) containing the α-minimal essential medium (Life Technologies), 10% fetal bovine serum (Cambrex), 0.2 mM ascorbic acid (ASAP; Life Technologies), 2 mM L-glutamine (Life Technologies), 100 U/mL penicillin (Life Technologies), 10 μg/mL streptomycin (Life Technologies), and 1 ng/mL basic fibroblast growth factor (bFGF; Instruchemie). Cells were grown at 37°C in a humid atmosphere with 5% CO2. The medium was refreshed twice a week and cells were used for further subculturing or cryopreservation when reaching 80%–90% confluence. After expansion, cells were characterized for surface marker expression, and presented the typical MSC expression profile [ >90% positive for CD73, CD90, and CD105; <2% positive for CD11b, CD19, CD34, CD45, and low expression of HLA-DR (around 6%)] as described previously.10 The hMSC basic medium (BM)/control medium was composed of hMSC PM without bFGF. The hMSC osteogenic medium (OM) was composed of hMSC BM supplemented with 10−8 M dexamethasone (dex; Sigma), and the hMSC mineralization medium (MM) was composed of BM supplemented with 10−8 M dex and 0.01 M β-glycerophosphate (Sigma). Oxidants (tert-butyl hydroperoxide (t-BHP) at 50 μM, peroxynitrite at 30 μM, ascorbate/Fe2+ at 250 μM, and H2O2 at 30 μM) and antioxidants (D-mannitol at 5 mM, Trolox at 50 μM, and sodium selenite [NaSel] at 100 nM) were supplemented to the medium when indicated for the duration of 6 days. The optimal concentration of each oxidant/antioxidant was predetermined based on assessing its effect on cell viability (see Results section). In every experiment, all the conditions were performed at the same time, using cells from the same donors, at the same population doubling, and using the same medium and the same culture conditions; sometimes, the conditions were split on the graphs for interpretation purposes only.
Proliferation and viability
To assess the effect of antioxidant supplementation on hMSC proliferation and viability, cells were seeded at 1000 cells/cm2 in PM until they reached 80%–90% confluence and were then counted using either a Coulter Counter or using the Alamar blue assay. Briefly, the Alamar blue solution was diluted 1:10 in a culture medium, and cells were incubated for 4 h. The Alamar blue solution from each well was then transferred into 96-well plates, and fluorescence was measured using a VICTOR3 luminometer (Perkin Elmer).
ALP activity
For the biochemical ALP assay, hMSCs were seeded at 1000 cells/cm2 and allowed to adhere for 10–15 h in BM, and then cells were grown in OM for 6 days. Each experiment was performed at least in triplicate with a negative control (cells grown in BM) and a positive control (cells grown in OM). Briefly, cells were washed twice with phosphate-buffered saline (PBS; Life Technologies) and lysed using a 0.2% Triton X-100 solution in 100 mM PBS pH 7.8, supplemented with a protease inhibitor cocktail (Roche Applied Science). The lysate was incubated in the dark at 25°C with CDP-Star substrate (Roche) and allowed to react for 30 min. Luminescence was measured using a VICTOR3 luminometer (Perkin Elmer) at 25°C. The total ALP luminescence was normalized for the cell number using Alamar blue as a readout. The data were then analyzed using one-way ANOVA and the Tukey post-test with a significance of 0.05.
Immunofluorescence staining of 53BP1 foci
Immunostaining for 53BP1, a P53 binding protein that relocates to the site of DNA strand breaks, was performed to measure the level of DNA damage in the cells. Cells were grown on glass coverslips for at least 48 h before immunostaining to minimize stress as a consequence of passaging. Cells were washed twice with PBS and fixed for 20 min with freshly prepared 4% paraformaldehyde in PBS. After permeabilization for 20 min with PBST-0.2% (PBS with 0.2% Triton X-100), cells were blocked in 3% bovine serum albumin (BSA) in PBST-0.1% for 1 h. The 53BP1 antibody (Novus) was diluted in a blocking buffer and incubated overnight at 4°C. Samples were then washed with Tris-buffered saline with Tween-20 (TBST), incubated with the Alexa-488 secondary antibody, and visualized by confocal laser scanning microscopy. At least 50 cells were analyzed for each condition except for one condition with a lower number (42 cells).
Oxidative stress determination (8-oxo-G and CM-H2DCFDA)
For the determination of oxidative stress load, two different methods were employed. The first method is based on the detection of 8-oxoguanine adducts in fixed permeabilized cells using flow cytometry. After fixation and permeabilization of hMSCs, a FITC-labeled protein conjugate specifically binding to the 8-oxyguanine moiety was added for 1 h, according to the manufacturer's instructions (Argutus Medical OxyDNA test; BD Biosciences). The presence of the oxidized DNA was then measured by FACS in at least 10,000 cells. The second method consisted in the direct detection of intracellular reactive oxygen metabolites as described previously.28 CM-H2DCFDA is a cell-permeant indicator for ROS that is nonfluorescent until removal of the acetate groups by intracellular esterases, and oxidation occurs within the cell.29 Shortly, adherent cells were harvested with trypsin and combined with cells floating in the medium. Cells were then washed and treated with 10 μM 5,-6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Molecular Probes) for 30 min at 37°C. Ten thousand cells were then analyzed on a BD FACScan.
ALP expression
The effect of oxidative damage inducers and antioxidants on ALP expression (as a readout for osteogenic differentiation potential) was also measured by flow cytometry. In short, cells were seeded at 5000 cells/cm2 in BM, allowed to attach for 10–15 h, and then cultured for 4 days. Each experiment consisted of a negative control (cells grown in BM), a positive control (cells grown in OM), and one or more experimental conditions. After 4 days of treatment, cells were trypsinized and incubated for 30 min in PBS containing 5% BSA, after which cells were further incubated in PBS-1% BSA containing the primary antibody (anti-ALP B4-78; Developmental Studies Hybridoma Bank) for 1 h. Cells were then washed and incubated with the secondary antibody (goat anti-mouse IgG conjugated with phycoerythrin) for 30 min. After incubation, cells were washed three times and resuspended in PBS-1% BSA. Viaprobe (BD Biosciences) was added for live/dead staining and allowed to incubate for 10 min. Cells were then analyzed using a FACScalibur (BD Biosciences), and ALP levels were determined on live cells only.
Mineralization and adipogenesis
The mineralization and adipogenesis assay were performed as described previously.9,30 For mineralization, hMSCs were grown in the MM for 28 days. Then total calcium deposition was assayed using a calcium assay kit (Sigma diagnostics; 587A). Briefly, the culture medium was aspirated, washed twice with calcium and magnesium-free PBS (Life Technologies), and incubated overnight with 0.5 N HCl on an orbital shaker at room temperature. The supernatant was collected and measured of absorbance at 620 nm (Bio-Tek instruments) and expressed as mg/dL. For adipogenesis, hMSCs were grown in the adipogenic medium for 21 days, then the cells were fixed overnight in formol (3.7% formalin plus CaCl2+2H2O [1 g/100 mL]), rinsed with water, incubated for 5 min in 60% isopropanol, and stained for 5 min in freshly filtered oil red O solution. Oil red O stain was quantified by extraction with 4% Igepal (Sigma) in isopropanol for 15 min and measurement of absorbance at 520 nm. Experiments were performed at least in triplicate using BM as the negative control.
Results
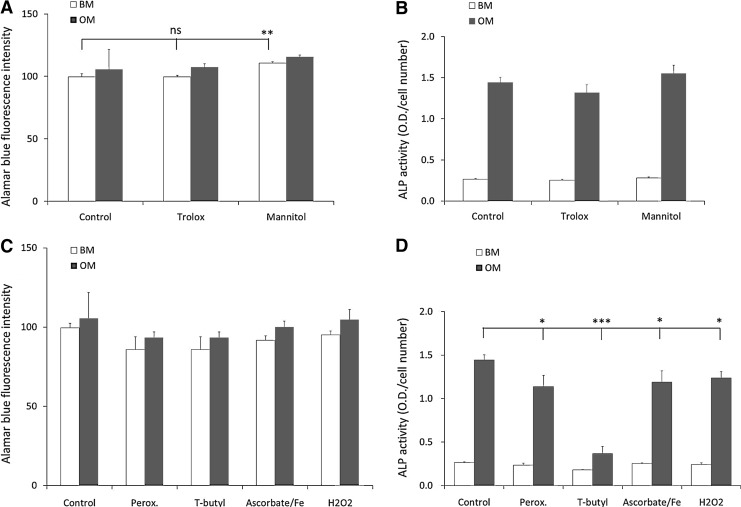
Selection of the optimal antioxidant and oxidative stress inducer concentrations
To investigate the effect of antioxidant supplementation on hMSCs (three donors), the optimal concentration of each inducer or antioxidant/scavenger was established by assessing their effects on cell viability (dose–response curve, see Supplementary Figs. S1 and S2; Supplementary Data are available online at www.liebertpub.com/tea). The highest concentration that did not elicit a profound effect on cell viability (arbitrary set at a reduction in cell number by 25% after 6 days of culture compared to control) was used for future experiments. With the selected concentration of each inducer or antioxidant/scavenger, we tested their effects on both the viability and differentiation of hMSCs (Fig. 1). Supplementation of the antioxidant D-mannitol (5 mM) had a mild, but significantly positive effect on hMSCs viability in BM, but no effect in OM (Fig. 1A). The antioxidant Trolox (50 μM) did not influence viability significantly in both BM and OM. The effect of antioxidant supplementation on dex-induced ALP activity was evaluated by exposing hMSCs in BM and OM to the concentrations indicated above for 6 days after which we did not observe a significant effect showing that these concentrations of antioxidants do not effect hMSC viability or differentiation (Fig. 1B). Similarly, we assessed concentrations in which a panel of oxidative damage inducers had minimal effects on viability (see Supplementary Fig. S2 and Fig. 1C). However, all the compounds significantly reduced dex-induced ALP activity (Fig. 1D). The effect was most profound when t-BHP was used, with a decrease from 1.44±0.06 (arbitrary units) in the control, to 0.37±0.08 in t-BHP-treated cells. Even in BM, t-BHP treatment resulted in a statistically significant decrease in ALP activity.
FIG. 1.
Effect of oxidative damage inducers and scavengers on proliferation and differentiation of human mesenchymal stromal cells (hMSCs). hMSCs were cultured in basic medium (BM) or osteogenic medium (OM) in the presence of antioxidants (Trolox 50 μM or D-mannitol 5 mM) (A, B) or oxidative damage inducers (peroxynitrite 30 μM; tert-butyl hydroperoxide (t-BHP) 50 μM, ascorbate/Fe2+ 250 μM and hydrogen peroxide 30 μM) (C, D). Proliferation (A, C) and alkaline phosphatase (ALP) expression (B, D) was measured after 6 days of culture. All conditions were performed at the same time, and the conditions were split for interpretation purposes only. For ALP, data are expressed as total ALP activity normalized for cell number. Proliferation is presented as the fluorescence intensity in arbitrary units (Alamar blue). Error bars represent standard deviation. Statistical analysis was performed using Student's t-test with a significance of p<0.05. Asterisks represent *p<0.05, **p<0.01, ***p<0.001. ns, not significant.
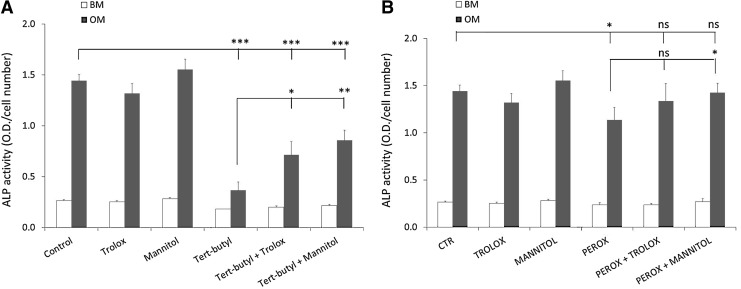
Antioxidants can rescue ROS damage-induced loss of differentiation
Next, we tested whether the negative effect of the oxidative damage inducers on dex-induced ALP expression could be rescued by cosupplementation of ROS scavengers. The addition of t-BHP to the medium resulted in a reduction in ALP activity from 1.44±0.06 to 0.37±0.08, which was partially rescued by the addition of the antioxidants Trolox and D-mannitol to 0.71±0.13 and 0.86±0.10, respectively (Fig. 2A). When peroxynitrite was used to induce oxidative damage, it resulted in the reduction in ALP levels from 1.44±0.06 in the control to 1.14±0.13. Cosupplementation of D-mannitol rescued the effect of peroxynitrite to 1.42±0.10. The effect of Trolox cosupplementation (1.34±0.19) was not significant (Fig. 2B). We conclude that both D-mannitol and Trolox are able to, at least, partially rescue oxidative damage-induced loss of differentiation potential. Several other oxidative damage inducers [2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH), H2O2, the pair ascorbate/Fe2+, and paraquat] and antioxidants [Taxifolin and manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP)] were tested as well, but for the sake of simplicity only two models of oxidative stress induction (t-BHP, Fig. 2A; peroxynitrite, Fig. 2B) were included in the manuscript.
FIG. 2.
Antioxidant supplementation prevents reactive oxygen species (ROS) induced loss of differentiation. ALP expression was measured after 6 days of culture in BM or OM and the antioxidant effect (Trolox 50 μM and D-mannitol 5 mM) was measured in two-model systems of oxidative stress induction: t-BHP (50 μM) (A) or peroxynitrite (30 μM) (B). All conditions were performed at the same time, and the conditions were split for interpretation purposes only. Data are expressed as total ALP activity normalized for cell numbers. Error bars represent standard deviation. Statistical analysis was performed using Student's t-test with a significance of p<0.05. Asterisks represent *p<0.05, **p<0.01, ***p<0.001. ns, not significant.
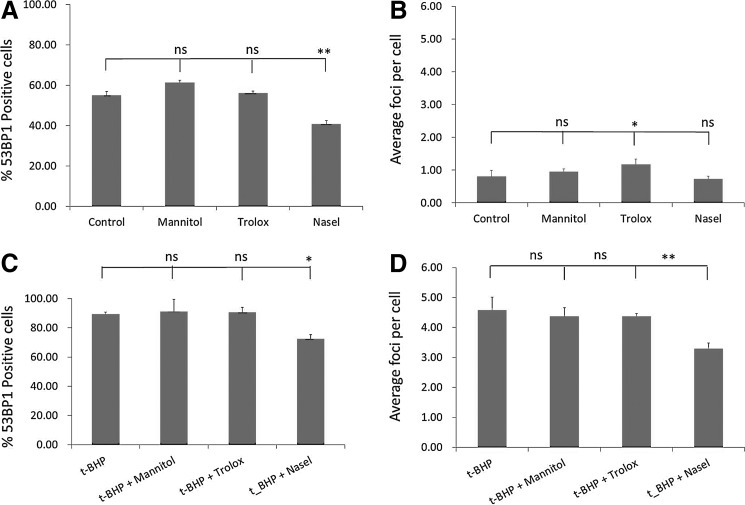
Selenium reduces oxidative damage load
Because DNA damage accumulation was previously suggested as a possible cause for loss of multipotency after extended in vitro culture, we determined whether antioxidant supplementation (D-mannitol and Trolox) could reduce the accumulation of DNA damage that hMSCs face during in vitro culture (Fig. 3). NaSel was used as a positive control because it was previously described to restore antioxidant capacity and prevent cell damage in hMSCs.27
FIG. 3.
Effect of antioxidant supplementation on DNA damage accrual. Effect of antioxidants (D-mannitol 5 mM, Trolox 50 μM, and sodium selenite [NaSel] 100 nM) on DNA damage load (A, B). DNA damage load was assessed by quantifying the percentage of 53BP1-positive cells (A, C) and the number of 53BP1 foci per cell (B, D) either in the absence of oxidative damage inducer (A, B) or in the presence of the oxidizing agent t-BHP (50 μM) (C, D). All conditions were performed at the same time, and the conditions were split for interpretation purposes only. Error bars represent standard deviation. Statistical analysis was performed using one-way ANOVA and Tukey's post-test with a significance of p<0.05. Asterisks represent *p<0.05, **p<0.01. ns, not significant.
DNA damage is presented as the percentage of 53BP1-positive cells or average foci per cell. When t-BHP is added to hMSCs, the percentage of 53BP1-positive cells increased significantly from 54.08±2.00 in the control (Fig. 3A) to 89.37±1.32 in t-BHP-treated cells (Fig 3C). Even more profound, the average number of 53BP1 foci per cell increased from 0.81±0.19 in the control (Fig. 3B) to 4.58±0.43 in the cells treated with t-BHP (Fig. 3C). Addition of NaSel to hMSCs treated with t-BHP resulted in a significant decrease in 53BP-positive nuclei from 89.37±1.32 to 72.44±2.94 in t-BHP and tBHP+NaSel, respectively. Similarly, NaSel treatment significantly reduced the number of foci per cell from 4.58±0.43 in the t-BHP-treated hMSCs to 3.30±0.18 when t-BHP and NaSel were added. D-mannitol and Trolox treatment resulted in a reduction in the average number of 53BP1 foci, but this was not statistically significant.
When hMSCs were grown under normal culture conditions, the addition of NaSel resulted in the reduction of 53BP1-positive cells from 54.08±2.00 to 40.67±1.82, with and without NaSel, respectively. Addition of D-mannitol and Trolox did not result in a significant change in the percentage of 53BP1-positive hMSCs under normal culture conditions (Fig. 3A), and in the case of Trolox, we even observed a mild increase in the average number of foci per cell, but not the percentage of 53BP-positive cells.
Supplementation of antioxidants enhances the yield of hMSCs
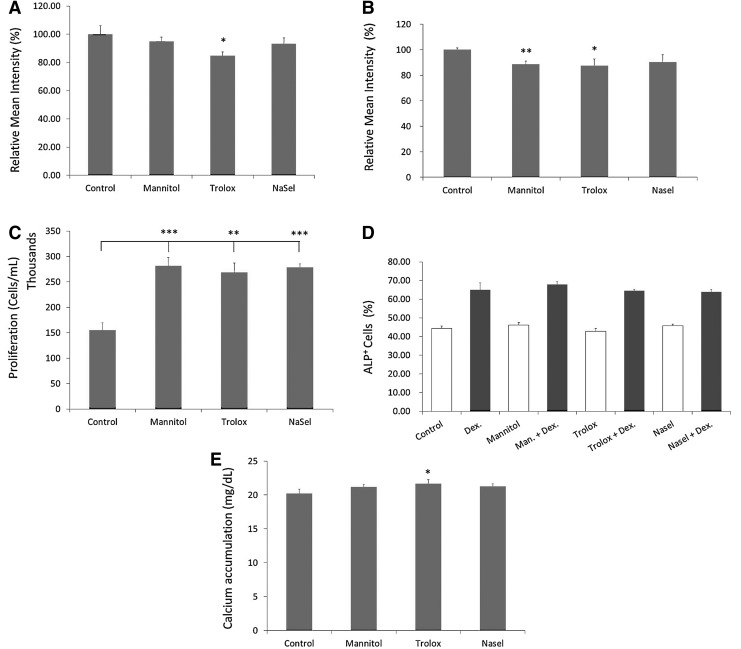
To investigate whether antioxidant supplementation reduces the oxidative damage load during in vitro culture, we exposed hMSCs to antioxidants from the moment that the bone marrow aspirate was placed in the tissue culture flasks.
To prove the efficacy of antioxidant supplementation, hMSCs were minimally expanded (passage 1), either in the presence or absence of antioxidants/scavengers, and then several parameters were evaluated (Fig. 4). Because 8-oxoguanine is the most common DNA adduct during oxidative damage and its production is linked to an increased risk of mutagenesis, the 8-oxo-G content was measured by flow cytometry. When the normal culture medium was supplemented with Trolox, we detected a significantly lower level of 8-oxoguanine adducts compared to hMSCs grown under normal conditions (from 100%±5.94% in the control to 84.71%±2.69%) (Fig. 4A). We also measured the intracellular ROS production because part of the oxidative DNA damage cells face is created by normal metabolic activity. As can be seen in Figure 4B, both D-mannitol and Trolox significantly reduced intracellular ROS formation (from control 100.10%±1.35% to 88.65%±2.39% and 87.53%±5.19%, for D-mannitol and Trolox, respectively). Selenium was able to reduce intracellular levels to 90.23%±5.75%, although the observed difference was not statistically significant.
FIG. 4.
Effect of antioxidant supplementation on cellular parameters of hMSCs (passage 1). The effect of antioxidant supplementation (D-mannitol 5 mM, Trolox 50 μM, and NaSel 100 nM) on DNA damage load (A, B), on proliferation in vitro (P0) (C) and differentiation potential (P1) (D, E) were evaluated. Oxidative damage accrual was assessed by quantifying 8-oxoguanine adducts (A) and intracellular ROS production (CM-H2DCFDA) (B) and expressed as percentage reduction of the mean intensity, relative to the control. Proliferation is expressed as thousands of cells/mL of culture medium (C). Percentage of ALP-positive cells was analyzed by FACS after 6 days, both in the presence or absence of the osteogenic inducer (dexamethasone) (D). Mineralization capacity was also measured and calcium accumulation was quantified and expressed as mg/dL (E). Error bars represent standard deviation. Statistical analysis was performed using Student's t-test with a significance of p<0.05. Asterisks represent *p<0.05, **p<0.01, ***p<0.001. Dex, dexamethasone.
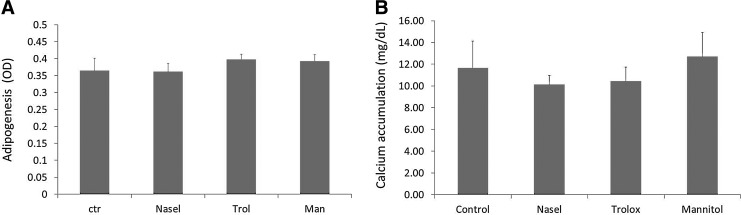
We then tested whether the reduction in oxidative damage by the antioxidants/scavengers was reflected in hMSC growth and differentiation parameters. The compounds were added when the fresh bone marrow aspirate was plated and cell numbers were determined when cells nearly reached confluence. In the donor tested (D 215), supplementation of all scavengers resulted in a significantly higher MSC yield (281,400±18,560 cells, 268,540±21,024 cells, and 278,720±7005 cells, for hMSCs supplemented with D-mannitol, Trolox, or NaSel, respectively), than control (154,898±16,220 cells) (Fig. 4C). When a mineralization experiment was performed with these cells (Fig. 4E), the hMSCs grown on the presence of Trolox displayed a small, but statistically significant enhancement of mineralization. In contrast, the antioxidants had no effect on dex-induced ALP expression (Fig. 4D). When the cells were expanded up to passage three in the three different antioxidants, we observed no beneficial effect in the differentiation assays (mineralization and adipogenesis) (Fig. 5).
FIG. 5.
Effect of continuous antioxidant supplementation on cellular parameters of expanded hMSCs (passage 3). Effect of antioxidants (D-mannitol 5 mM, Trolox 50 μM, and NaSel 100 nM) on differentiation potential of in vitro expanded hMSCs in the presence of antioxidants. Adipogenic differentiation (A) and mineralization (B) were assessed in passage 3 cells. Error bars represent standard deviation.
Discussion and Conclusions
During normal culturing conditions, hMSCs accumulate DNA damage and oxidative stress, and their antioxidant capacity is reduced.27 The in vivo performance of hMSCs is also dependent on the extent of expansion. Supplementation of the antioxidant selenium was shown to reduce the oxidative stress levels in hMSCs by restoring the antioxidant capacity of the cells.27 However, several questions still remained, for example, whether antioxidant supplementation could delay the functional senescence of MSCs after long-term in vitro culture. To investigate the effect of antioxidant supplementation in reducing the early aging of hMSCs in vitro, we tried to establish different model systems of induction of damage (oxidative stress), and therefore chemically induced loss of differentiation potential and concomitantly tested whether this process could be reversed by the usage of antioxidants.
There is a wide variety of compounds with known antioxidant activity with a broad range of action. Therefore, our first step was to screen a set of antioxidants and inducers. After testing first their effect on cell viability and then on the ALP levels, we concluded that the best antioxidants to test further were D-mannitol and Trolox and decided to use peroxynitrite and t-BHP as inducers.
D-mannitol is reported to be a potent hydroxyl-free radicals scavenger,31,32 while Trolox is a vitamin E hydrosoluble analogue, which is known to bind the cell plasma membrane, where it exerts its main function of protecting cells from lipid peroxidation.33 The oxidizing agent selected is t-BHP, a well-known compound and its toxicity is widely characterized.34–36 Peroxynitrite (ONOO−) is formed when nitric oxide (NO) reacts with the superoxide anion (O2−) and its generation can lead to oxidation and nitration of lipids, DNA, and amino acid residues on proteins.37,38 It is also responsible for the formation of other dangerous reactive species. This compound has the capacity to act in a hydroxyl radical-like manner to induce lipid and protein oxidation, readily reacts with CO2 to form nitroso peroxocarboxylate (ONOOCO2−), can become protonated as peroxonitrous acid (ONOOH), and can undergo homolysis to form either hydroxyl (•OH) radicals and nitrogen dioxide (•NO2) radicals, or be rearranged to nitrate (NO3).39
After establishing a model system to mimic the oxidative stress that hMSCs face in culture, we then assessed whether these antioxidants were able to reverse the chemically induced loss of differentiation potential. Indeed, we show that both Trolox and D-mannitol were able to partially decrease the chemically induced loss of differentiation potential, which lead us to test whether these antioxidants were also able to revert or prevent the loss of differentiation potential after in vitro expansion, since a similar process is believed to occur. For those experiments, selenium was used as a positive control since it has previously demonstrated its efficacy on restoring the antioxidant capacity of hMSCs.
Although selenium was able to reduce the chemically induced oxidative stress levels (the total percentage of positive cells and average foci per cell), the effect seems to be on prevention of oxidative stress rather than actually a reversal of the damage already accumulated, since it failed to rescue cells that had already lost their differentiation potential after extensive in vitro expansion. Therefore, we investigated whether long-term, continuous supplementation of different antioxidants was able to significantly prevent oxidative stress accumulation and enhance biological activity.
Interestingly, antioxidant supplementation had a beneficial reducing effect on the level ROS and 8-oxo-G in the cells and a positive effect on the total yield of cells that could be obtained from the bone marrow aspirate. Supplementation of antioxidants during the early expansion phase can be an easy way to increase the total yield of low passage cells. We can only speculate on the precise mechanism responsible for this observed increase in cell numbers, but some possibilities could be the suppression of cell death and apoptosis by reducing the oxidative stress levels, by affecting the attachment during the initial phase or by actively inducing proliferation. Furthermore, we showed that antioxidant supplementation (especially Trolox) was able to significantly reduce the oxidative damage cells face during the initial phase of culture. This is in line with other reports where authors show that Trolox and other antioxidants were able to markedly inhibit the formation of 8-oxoguanine adducts in a concentration-dependent manner.40 Intracellular vitamin C concentrations were shown to be negatively correlated with 8-oxo-deoxyguanosine concentrations in lymphocytes from 105 healthy volunteers.41 Furthermore, vitamin C has also been shown to act as an antioxidant in vivo42 and from the 44 published in vivo studies examined, 38 demonstrated a decrease in the number of markers of oxidative damage to DNA, 14 showed no differences, while only 6 reported an increase in oxidative damage after supplementation with vitamin C.
Next, we investigated the effects of oxidants and antioxidants on the differentiation potential of hMSCs. Recent studies support the hypothesis that ROS involves in the regulation of stem cell differentiation, although the underlying mechanism remains to be found. It has been suggested that stemness is characterized by the presence of structurally unsaturated metabolites whose levels decrease upon differentiation; and the activation of oxidation is a metabolic signature for the differentiation process.43 Ji et al. showed that differentiation of human embryonic stem cells into bi-potent mesendodermal cell lineage increased in ROS-inducing conditions; moreover, this ROS-inducing differentiation was decreased by free radical scavenger treatment.44 Liu and coworkers reported that using a standard adipogenic differentiation cocktail (IDII: insulin, dex, indomethacin, and 3-Isobutyl-1-methylanxthine), adipogenesis was induced in human adipose-derived stem cells, which was accompanied by ROS generation; when ROS was scavenged with NAC or EUK-8, this IDII-induced adipogenesis was inhibited.45 Smith et al. demonstrated that the intracellular redox state in dividing oligodendrocyte-type-2 astrocyte progenitor cells was strongly associated with their balance between self-renewal and differentiation; and that signaling molecules that promote pluripotency make the cells more reduced, whereas those that promote differentiation make the cells more oxidized.46
Addition of antioxidants revealed minor or no beneficial effects on the differentiation potential (ALP and mineralization) of early expanded hMSCs, which could be due to the fact that cells had been only minimally expanded and still presented their maximum differentiation potential. To further confirm this, we assessed the effect of antioxidant supplementation on further expanded hMSCs, since after this expansion period, cells are known to accumulate significantly higher levels of DNA damage, as previously described.10
Unfortunately, hMSCs still presented a decrease in differentiation potential and the addition of antioxidants was not able to prevent the oxidative damage load cells face during in vitro culture. One of the reasons behind this could be the fact that hMSCs are accumulating high amounts of oxidative damage and that the observed preventive effect of antioxidant supplementation was unable to fully prevent the accumulation of oxidative damage during culture, ultimately leading to the loss of differentiation potential and render these cells less optimal for clinical usage. In addition, there is a huge variety of oxidizing radicals and these antioxidants only manage to scavenge a subset of them. Their half-life is also limited while oxidative damage is being constantly produced. Unfortunately, higher concentrations of antioxidants cannot be used since they produce cytotoxic effects. The discovery of novel and more potent antioxidants with lower side effects would be highly interesting, and a combination of several antioxidants might be worth testing.
Here we show that continuous antioxidant supplementation during the early expansion phase of hMSCs, leads to an enhanced yield of hMSCs, which can be of high interest for clinical applications. We have also shown that Trolox supplementation can reduce the oxidative damage cells face during early culture periods. However, the beneficial effects shown by antioxidants were unable to rescue hMSC differentiation capacity after in vitro expansion, which could have been explained by several factors.
Oxidative damage to DNA, lipids, and proteins is a reality during in vitro culturing since hMSCs are expanded out of their natural niche. Although there is a constant improvement of the culture conditions, they are still far from optimal, and may be the cause for the early loss of functionality. This might be a consequence of epigenetic events among other factors, such as improper cell–cell contacts, improper surface area, or protein modifications by oxidative damage, all of which might change the gene expression profile of hMSCs, and lead to the loss of expression of important proteins and ultimately to loss of multipotency.
Further work still needs to be performed to understand the basic phenomenon behind loss of multipotency after in vitro expansion to prevent it for occurring during normal culturing conditions. Other antioxidants can be further tested and might present a higher preventive activity and therefore, the results obtained here, leave a window for further experimentation.
Supplementary Material
Acknowledgments
The Netherlands Technology Foundation (STW grant TGT.6745) is acknowledged for financial support.
Disclosure Statement
No competing financial interest exists.
References
- 1.Kadiyala S. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3:173. [Google Scholar]
- 2.Bruder S.P., et al. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;(355 Suppl):S247. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 3.Almeida-Porada G., et al. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620. [PubMed] [Google Scholar]
- 4.Tse W.T., et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 5.Ringden O., et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Petite H., et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 8.Agata H., et al. Characteristic change and loss of in vivo osteogenic abilities of human bone marrow stromal cells during passage. Tissue Eng Part A. 2010;16:663. doi: 10.1089/ten.TEA.2009.0500. [DOI] [PubMed] [Google Scholar]
- 9.Siddappa R., et al. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 10.Alves H., et al. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med. 2010;14:2729. doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagan J.M. Sleczka B.G. Sohar I. Quantitation of oxidative damage to tissue proteins. Int J Biochem Cell Biol. 1999;31:751. doi: 10.1016/s1357-2725(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 12.Kohen R. Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 13.Dalle-Donne I., et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 14.Niki E., et al. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338:668. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 15.Cadet J., et al. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T. Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Valko M., et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Dudas S.P. Arking R. A coordinate upregulation of antioxidant gene activities is associated with the delayed onset of senescence in a long-lived strain of Drosophila. J Gerontol A Biol Sci Med Sci. 1995;50:B117. doi: 10.1093/gerona/50a.3.b117. [DOI] [PubMed] [Google Scholar]
- 19.Harshman L.G. Haberer B.A. Oxidative stress resistance: a robust correlated response to selection in extended longevity lines of Drosophila melanogaster? J Gerontol A Biol Sci Med Sci. 2000;55:B415. doi: 10.1093/gerona/55.9.b415. [DOI] [PubMed] [Google Scholar]
- 20.Balaban R.S. Nemoto S. Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Ku H.H. Brunk U.T. Sohal R.S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 22.Maggio D., et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 23.Basu S., et al. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 24.de Boer J., et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 25.Tyner S.D., et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 26.Ito K., et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 27.Ebert R., et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24:1226. doi: 10.1634/stemcells.2005-0117. [DOI] [PubMed] [Google Scholar]
- 28.Hockenbery D.M., et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 29.Jakubowski W. Bartosz G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int. 2000;24:757. doi: 10.1006/cbir.2000.0556. [DOI] [PubMed] [Google Scholar]
- 30.De Boer J. Wang H.J. Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 31.Bors W. Saran M. Michel C. Pulse-radiolytic investigations of catechols and catecholamines. II. Reactions of Tiron with oxygen radical species. Biochim Biophys Acta. 1979;582:537. doi: 10.1016/0304-4165(79)90145-4. [DOI] [PubMed] [Google Scholar]
- 32.Ching T.L. Haenen G.R. Bast A. Cimetidine and other H2 receptor antagonists as powerful hydroxyl radical scavengers. Chem Biol Interact. 1993;86:119. doi: 10.1016/0009-2797(93)90116-g. [DOI] [PubMed] [Google Scholar]
- 33.Valko M., et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Joyeux M., et al. tert-Butyl hydroperoxide-induced injury in isolated rat hepatocytes: a model for studying anti-hepatotoxic crude drugs. Planta Med. 1990;56:171. doi: 10.1055/s-2006-960918. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes E.R., et al. Hepatoprotective activity of xanthones and xanthonolignoids against tert-butylhydroperoxide-induced toxicity in isolated rat hepatocytes—comparison with silybin. Pharm Res. 1995;12:1756. doi: 10.1023/a:1016230125496. [DOI] [PubMed] [Google Scholar]
- 36.Tseng T.H., et al. Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food Chem Toxicol. 1997;35:1159. doi: 10.1016/s0278-6915(97)85468-3. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 38.Nordberg J. Arner E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 39.Radi R., et al. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 40.Qi W., et al. Chromium(III)-induced 8-hydroxydeoxyguanosine in DNA and its reduction by antioxidants: comparative effects of melatonin, ascorbate, and vitamin E. Environ Health Perspect. 2000;108:399. doi: 10.1289/ehp.00108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenton K.J., et al. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am J Clin Nutr. 2003;77:189. doi: 10.1093/ajcn/77.1.189. [DOI] [PubMed] [Google Scholar]
- 42.Carr A. Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 43.Yanes O., et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji A.R., et al. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med. 2010;42:175. doi: 10.3858/emm.2010.42.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higuchi M. Dusting G.J. Peshavariya H. Jiang F. Hsiao S.T. Chan E. Liu G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0306. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith J., et al. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.