Abstract
Background
Breakdown of humoral tolerance to RBC antigens may lead to autoimmune hemolytic anemia, a severe and sometimes fatal disease. The underlying mechanisms behind the breakdown of humoral tolerance to RBC antigens are poorly understood.
Design and Methods
In order to study the pathogenesis of autoimmune hemolytic anemia, we developed a murine model with RBC-specific expression of a model antigen carrying epitopes from hen egg lysozyme and ovalbumin.
Results
Humoral tolerance was observed; this was not broken even by strong immunogenic stimulation (lysozyme or ovalbumin with adjuvant). Autoreactive CD4+ T cells were detected by tetramer enrichment assays, but failed to activate or expand despite repeat stimulation, indicating a nonresponsive population rather than deletion. Adoptive transfer of autoreactive CD4+ T cells (OT-II mice) led to autoantibody (anti-lysozyme) production by B cells in multiple anatomic compartments, including the bone marrow.
Conclusions
These data demonstrate that B cells autoreactive to RBC antigens survive in healthy mice with normal immune systems. Furthermore, autoreactive B cells are not centrally tolerized and are receptive to T-cell help. As the autoreactive T cells are present but non-responsive, these data indicate that factors that reverse T-cell non-responsiveness may be central to the pathogenesis of autoimmune hemolytic anemia.
Key words: tolerance, B cells, T cells, erythrocyte-specific, self-antigen
Introduction
Autoimmune hemolytic anemia (AIHA) consists of loss of tolerance to self-antigens on red blood cells (RBCs) in the humoral compartment.1 When this occurs, hemolysis may ensue, leading to substantial morbidity and mortality. Response to pharmacological immunesuppression and/or surgical splenectomy is variable, and in extreme cases, patients fail to respond to any of the established interventions.2 Because autoantigens are often ubiquitous epitopes found on essentially 100% of blood donors, transfusion support of AIHA patients may not be feasible, as all units of RBCs will be incompatible.3 In most cases, the above factors lead to AIHA resulting in a chronic and debilitating disease; in unusually severe cases, death can occur due to profound autohemolysis.
Some forms of AIHA are known to be secondary to infectious disease or immune dysregulation as a result of neoplasia.4-6 However, in the primary form of AIHA, no inciting cause is identified.7 The basic pathogenesis of primary AIHA is poorly understood, but clearly results from a failure of tolerance mechanisms. However, whether this failure is central versus peripheral and at the level of T and/or B cells remains unresolved. Approximately 9,000 cases of clinically significant AIHA are observed annually in the US.1 However, the frequency of AIHA grossly underestimates the frequency of humoral autoimmunity to RBC antigens, as many anti-RBC autoantibodies do not induce hemolysis, although the reasons for this are not known.8 Based upon large scale analysis of blood donors, the frequency of autoantibodies to RBCs in asymptomatic patients is as high as 0.1%. Likewise, approximately 3% of hospitalized adults have RBC autoantibodies, also often in the absence of hemolysis.8,9 Therefore, baseline humoral tolerance to RBC antigens appears to fail in up to 1-3/1,000 humans, indicating that tolerance mechanisms to RBC antigens are lost with considerable frequency.
The relative inefficiency of humoral tolerance to RBC antigens can not be predicted, given the known characteristics of central B-cell tolerance. Central tolerance in the Bcell compartment occurs as a result of exposure to autoantigens at several checkpoints during B-cell development.10 Establishment of tolerance can lead to deletion, anergy, or receptor editing such that the immunoglobulin is no longer autoreactive.11,12 Like B cells, erythrocyte precursors mature into RBCs in the bone marrow, and blood group antigens are expressed on RBCs during their development.13-15 As such, B cells undergo central tolerance induction in close proximity to a rich source of RBC antigens; therefore, it is a reasonable hypothesis that central B-cell tolerance to RBC antigens would normally be an efficient and robust process. However, the transfusion of rat RBCs into mouse results in AIHA, presumably by linking foreign helper T-cell epitopes to B-cell epitopes that are cross-reactive between mice and rats; in other words, linked recognition of T-cell epitopes to humoral auto-antigens.16,17 The induction of autoantibodies to RBCs in this case provides strong evidence that B-cell tolerance to RBC antigens is incomplete in the baseline state. Although dysregulation of central education of newly forming B cells by the introduction of rat RBCs cannot be ruled out.
Additional studies of B cells autoreactive to RBC antigens, carried out by Honjo et al., have made extensive use of immunoglobulin transgenic mice expressing a B-cell receptor (BCR) that is reactive to a murine RBC autoantigen.18 While deletion of some autoreactive B cells does occur in these mice, there is substantial breakthrough of autoreactive B cells and synthesis of autoantibody.19 The resulting mice develop clinical AIHA, with a range of severity, regulated in part by baseline innate immune activation and interaction with gut flora.20,21 The use of BCR transgenic mice to model AIHA has been a highly innovative and fruitful approach to analyzing tolerance/autoimmunity to RBC antigens. However, there are also limitations to this strategy. These include lack of a negative control in which the autoantigen is absent, an extremely high B-cell precursor frequency, affinity matured immunoglobulin, and potential biological confounders from the pathophysiology as a result of chronic hemolysis.
So as to build on the current mechanistic understanding, we report the engineering and use of a new model of tolerance/autoimmunity to RBC antigens that does not depend upon immunoglobulin transgenic mice and does not involve the pathophysiology co-incident with clinically significant AIHA. We have recently described the HOD mouse, which expresses an erythrocyte-specific transgene consisting of hen egg lysozyme (HEL) fused to ovalbumin (OVA) fused to the human Duffy blood group antigen [HEL-OVA-Duffy (HOD)].22 The use of the HOD model antigen allows for analysis of tolerance at both the CD4+ Tcell and B-cell levels to an RBC-specific antigen in the context of a natural immune system (i.e. not using BCR transgenic mice). We found that RBC autoreactive B cells survive central tolerance mechanisms and are capable of producing autoantibodies if given T-cell help. Likewise, autoreactive CD4+ T cells survive thymic education, but persist in an anergic state, and represent a stopgap to autoimmunity. To the best of our knowledge, this represents the first report of B-cell tolerance biology to RBC antigens in the absence of BCR transgenic mice, the first assessment of RBC autoreactive CD4+ T cells in healthy animals, and provides a unique insight into baseline immunological tolerance to RBC antigens and potential induction points for pathogenesis of autoimmune hemolytic anemia.
Design and Methods
Mice
B6 and Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). B6-[TG]TCR-OTII-RAG1 tm1Mom mice (OTII/RAG1ko) were obtained from Taconic Farms, Inc. through the NIAID Exchange program, NIH mouse line #4234. B6.HOD, mHEL, mOVA, OT-IIxThy1.1, TCR75, SMARTA, and SwHEL mice were bred at the Emory University Department of Animal Resources. All procedures were approved by the institutional Animal Care and Use Committee.
The Online Supplementary Appendix provides information on transfusion and immunization, immunofluorescence histology, ELISA for anti-HEL and anti-OVA humoral response, staining leukocytes, ELISPOT, flow cytometric crossmatching, CD4+ T-cell purification and adoptive transfer of cells, and MHCII tetramer-based enrichment of antigen-specific T cells.
Statistical analysis
Statistical significance was determined using PRISM software and performing a Student's t-test for comparison of 2 samples or two-way repeated measure analysis of variance (ANOVA) with a Bonferroni post-test for 3 or more samples with multiple conditions. P<0.05 was considered statistically significant.
Results
Expression of the HOD antigen is restricted to erythroid cells in the B6.HOD mouse
The HOD mouse expresses a triple fusion transgene containing two well-characterized antigens [hen egg lysozyme (HEL) fused to a portion of ovalbumin (OVA)], which is in turn fused to an authentic human blood group antigen (Duffy) (HOD antigen). The HOD gene is expressed under an RBC-specific regulatory element utilizing a beta-globin promoter and enhancer.22 Previous characterization of peripheral blood in FVB.HOD mice revealed HOD expression on RBCs but not on platelets or leukocytes.22 For the current studies, the HOD transgene was backcrossed 10 generations onto a B6 background (B6.HOD), and tissue expression of HOD was characterized. Similar to what was observed on the FVB background, the HOD antigen is detected on RBCs in peripheral blood, with no detectable expression on leukocytes or platelets (Online Supplementary Figure S1A). mHEL transgenic mice that express HEL under a ubiquitous MHCI promoter were used as a positive control for HEL staining on leukocytes.23
HOD antigen expression was assessed during erythropoiesis by staining bone marrow with anti-CD71 and erythrocyte-specific anti-TER119 to delineate the stages of erythrocyte development (Online Supplementary Figure S1B). Cells were also stained with anti-HEL (clone 4B7) directly conjugated to Alexafluor 647.24 CD71hiTER119lo (RI) basophilic erythroblasts had low levels of detectable anti-HEL staining above control B6 mice. Both CD71hiTER119+ (RII) late basophilic erythroblasts and CD71moderateTER119+ (RIII) polychromatophilic erythroblasts had higher levels of anti-HEL reactivity, which decreased but remained detectable on CD71- TER119+ (RIV) orthochromatophilic erythrobasts (Online Supplementary Figure S1B). No HEL expression was detected on non-erythroid elements of the bone marrow (TER119-).
Anti-HEL staining was also carried out on frozen sections of the spleen, kidney, lung, liver, draining lymph nodes, mesenteric lymph nodes, Peyer's patches, small and large intestine, heart, pleural and peritoneal viscera. In lymphatic tissues, anti-B220 and anti-Thy1.2 were used to delineate B- and T-cell zones, respectively. No anti-HEL staining was detected above background signal seen in wild-type B6 controls in any of the tissues examined (representative spleen shown in Online Supplementary Figure S1C). This was not due to the inability to detect HEL expression by immunofluorescence, as staining of mHEL mice gave a strong signal in both the B- and T-cell zones. RBCs are destroyed by the freezing process and are, therefore, not visible on the splenic sections shown in either B6.HOD or mHEL mice. However, staining of slide of peripheral blood smears using the same reagents showed positive signal on HOD but not B6 RBCs (data not shown). Furthermore, no anti-HEL expression above that of B6 control mice was noted in any non-lymphatic tissue of the B6.HOD mice (data not shown). Taken together, these data demonstrate that the HOD antigen is expressed throughout multiple stages of erythropoiesis in B6.HOD bone marrow and mature RBCs, but is not detectable on platelets, leukocytes, or the parenchyma of other analyzed tissues.
B-cell tolerance to the HOD antigen is incomplete
We hypothesized that HOD antigen expressed in the bone marrow would induce tolerance of HOD reactive B cells. To test this hypothesis, B6.HOD mice were immunized with HEL protein emulsified with complete Freund's adjuvant (HEL/CFA). The combined results of 3 experiments demonstrated that no anti-HEL IgG was detected in the B6.HOD mice, whereas high titer anti-HEL IgG was seen in B6 mice (Figure 1A). No antibody was detected prior to immunization.
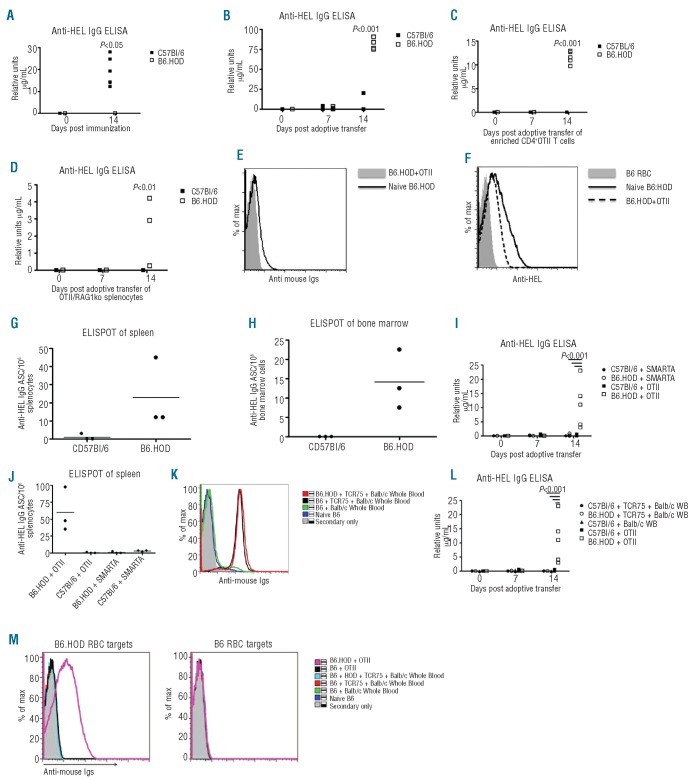
Figure 1.
B6.HOD mice make anti-RBC autoantibodies when T-cell tolerance is circumvented. (A) B6 and B6.HOD mice were immunized with HEL/CFA. Serum was collected and analyzed two weeks after immunization for anti-HEL IgG by ELISA. B6 and B6.HOD mice were adoptively transferred with (B) 10x106 OT-II splenocytes, (C) enriched CD4+ OT-II splenocytes, or (D) OTII/Rag1ko splenocytes. Two weeks later, sera were analyzed for anti-HEL IgG by ELISA. RBCs from indicated mice were stained with either anti-IgG to assess binding of auto-antibodies to RBCs (E) or with anti-HEL followed by anti-IgG to assess levels of antigen (F). Anti-HEL IgG antibody secreting cells were quantified in (G) spleen and (H) bone marrow by ELISPOT. (I) Enriched CD4+ T cells from SMARTA mice or OTII/Rag1ko mice were adoptively transferred into B6 and B6.HOD mice. Sera were collected seven and 14 days post adoptive transfer and analyzed for anti-HEL IgG by ELISA. (J) Anti-HEL IgG antibody secreting cells from splenocytes were quantified by ELISPOT. (K) Enriched CD4+ T cells from splenocytes of TCR75 or splenocytes from OTII/Rag1ko mice were adoptively transferred into B6 and B6.HOD mice. Recipient mice that received TCR75 CD4+ T cells were transfused with Balb/c blood. One group of B6 mice received Balb/c whole blood in the absence of TCR75 T cells. Sera were analyzed by flow crossmatch with Balb/c splenocytes. (L) Anti-HEL antibodies were assessed by ELISA and (m) by flow crossmatch with (left) B6.HOD RBCs and (right) B6 RBCs. Antibodies that reacted with antigens on target cells were visualized by anti-mouse immunoglobulins conjugated to APC. All data are representative of at least 3 independent experiments (3-5 mice/group/experiment) with similar results.
Whereas the above data indicate tolerance to HEL in B6.HOD mice, they do not distinguish between B- and T-cell tolerance. To directly assess B-cell tolerance, existing CD4+ T-cell tolerance was broken by adoptive transfer of OT-II CD4+ T cells into B6.HOD mice. OT-II mice express a transgenic TCR specific for OVA323-339 presented by I-Ab. As the HOD transgene contains OVA323-339 and B6 mice express I-Ab, OT-II CD4+ T cells are anti-self for B6.HOD mice. We hypothesized that if B-cell tolerance were complete, then even in the presence of OVA-specific CD4+ T-cell help, no HOD-specific autoantibodies would be produced. We have previously reported that OT-II CD4+ T cells enhance anti-HEL antibody production in B6 mice transfused with HOD RBCs, presumably by recognizing OVA323-339 peptide presented by self I-Ab 25 B6.HOD mice that received OT-II splenocytes made high levels of anti-HEL IgG compared to background signal in control B6 mice receiving the same number of OT-II CD4+ T cells (P<0.001 at 1:50 dilution; Figure 1B). Because of the concern of transferring HEL-reactive B cells along with OT-II T cells, CD4+ T-cell enrichments were carried out. These eliminated most B-cell contamination, with similar results (Figure 1C). Finally, to rule out passive transfer of HEL specific B cells from OT-II donors, OT-II/RAG1ko donors were utilized, which gave similar results (Figure 1D). All subsequent studies were validated using OT-II/RAG1ko CD4+ T cells and all data shown are from experiments using OT-II/RAG1ko mice. Together, these data show that B-cell tolerance to HOD is incomplete.
At baseline, B6.HOD animals do not have detectable anti-HOD antibodies, nor do they have evidence of hemolysis (e.g. no anemia or reticulocytosis; data not shown). Furthermore, B6.HOD mice that were induced to make autoantibodies (through OT-II transfer) do not have evidence of hemolysis (data not shown; Hudson et al., manuscript in preparation). To test if the autoantibodies were binding to RBCs, peripheral blood from B6.HOD mice that made anti-HEL after receiving OT-II T cells were stained with anti-Ig and analyzed by flow cytometry. There was a very small shift observed compared to RBCs from naïve B6.HOD mice (Figure 1E). The same RBCs were also stained with anti-HEL and then anti-IgG; both naïve B6.HOD and B6.HOD that had received OT-II T cells shifted compared to wild-type B6 (Figure 1F). Interestingly, RBCs from B6.HOD mice that received OT-II T cells had lower staining with anti-HEL than naïve B6.HOD mice, but still significantly higher than wild-type. These findings are consistent with our previous observations that anti-HEL does not induce hemolysis in mHEL RBCs24,26,27 and with the observation in humans that the vast majority of antibodies to RBCs (both autoantibodies and transfusion induced alloantibodies) do not result in hemolysis. Therefore, the model is not only consistent with the behavior of many RBC autoantibodies in humans, but also prevents confounding effects of the process of hemolysis on the underlying immunobiology being studied.
ELISPOTs were performed on cells from the spleen, bone marrow and peritoneal cavity to enumerate anti-HEL secreting plasma cells. B6.HOD mice that received OT-II/RAG1ko T cells had a significant number of HEL-specific antibody secreting cells (ASCs) in the spleen, compared to background signal in control B6 mice that received the same number of OT-II splenocytes (Figure 1G). The positive events in B6 mice receiving OT-II/RAG1ko T cells were no greater in number than naïve mice, and in no case did B6 or B6.HOD mice have significant levels of anti-HEL signal or significant numbers of HEL specific ASCs in the absence of adoptive transfer of OT-II cells (data not shown). Similar to spleen, significant anti-HEL ASCs were detected in the bone marrow of B6.HOD but not B6 mice (Figure 1H). No HEL-specific ASCs were detected in the peritoneal cavity from either the B6 or the B6.HOD mice receiving OT-II/RAG1ko adoptive transfer (data not shown).
To assess the antigen-specificity of anti-HEL induction by OT-II transfer, LCMV-specific CD4+ T cells from TCR transgenic SMARTA mice were adoptively transferred into B6 and B6.HOD mice. SMARTA CD4+ T cells are TCR transgenic with specificity to an epitope derived from a murine pathogen not contained within the HOD transgene.28 In 4 of 4 experiments, no anti-HEL IgG was detected after transfer of SMARTA CD4+ T cells (P<0.001 at 1:50 dilution; Figure 1I). Similarly, ELISPOTs of spleen and bone marrow showed no difference with transfer of SMARTA CD4+ T cells (Figure 1J). These data indicate that the anti-HEL IgG observed with OT-II adoptive transfer was not an artifact of increased non-specific CD4+ T cells.
Although the SMARTA T cells control for CD4+ T-cell number, unlike the OT-II T cells, their antigen is not present in the system and, therefore, they do not control for T-cell activation and effector function. To test whether anti-HEL antibodies were generated non-specifically in response to bystander activation of CD4+ T cells, TCR75 mice were utilized as T-cell donors. TCR75 mice are transgenic for a TCR that recognizes a peptide derived from H-2kd MHCI presented in I-Ab MHCII.29 B6 and B6.HOD mice were adoptively transferred with enriched CD4+ T cells from TCR75 splenocytes. To introduce the antigen recognized by TCR75, mice were then transfused with 100 μL of packed Balb/c whole blood 24 h after adoptive transfer. Adoptive transfer of TCR75 CD4+ T cells induced high levels of BALB/c reactive antibodies that were not detected in recipients of BALB/c whole blood only, indicating that the TCR75 cells actively provided help. Both B6 and B6.HOD mice that received TCR75 and BALB/c whole blood made similar amounts of anti-Balb/c antibodies (Figure 1K). In contrast, neither group made anti-HEL antibodies (by ELISA) or HOD reactive antibodies (by flow crossmatch) (Figure 1L and M, respectively). Taken together, these data demonstrate that HOD autoreactive B cells exist at baseline in B6.HOD mice, and that they differentiate into antibody secreting plasma cells when provided with CD4+ T-cell help that recognize cognate peptide derived from HOD presented by self MHCII.
Antigen expression on RBCs influences central tolerance of autoreactive B cells
To test the extent to which expression of RBC antigen tolerizes developing naïve B cells, B6.HOD mice were bred with SwHEL mice, which express a transgenic BCR specific for HEL that is capable of normal class switching.30 Femoral marrow was harvested from B6.HOD x SwHEL F1 mice (F1 mice). HEL-specific B cells were visualized by staining with tetramerized HEL linked to allophycocyanin. B-cell lineage and stage of development was determined using a panel of antibodies (Online Supplementary Figure S2A). Isotype matched antibody controls were used to establish specificity of staining for antibodies (data not shown). Tetramerized mouse albumin (conjugated to a separate fluorochrome from HEL) was used as a specificity control for the tetramerized HEL; ‘non-specific’ cells reacting with albumin were excluded as part of the gating strategy (Online Supplementary Figure S2A). Consistent with previous reports, 40-60% of IgM+IgD+ B cells (mature naïve population) in SwHEL mice were specifically reactive with tetramerized HEL, indicating BCR HEL specificity (Online Supplementary Figure S2B, fraction F, bottom row).30 However, there was significant decrease in HEL-reactive mature naïve B cells in bone marrow of F1 mice, demonstrating that expression of HOD antigen on RBCs resulted in decreased overall development of autoreactive B cells (Online Supplementary Figure S2B, fraction F, top row). In SwHEL mice, HEL reactivity was detected in large pre-B cells and immature B cells, consistent with BCR expression during known stages of B-cell development. At both of these stages (both known checkpoints in B-cell development31), the HEL-reactive B cells are decreased in F1 mice. However, a percentage of HEL-reactive B cells survive this process. F1 mice also displayed a 2-fold reduction in overall IgM+IgD+ naïve B cells, regardless of specificity (Online Supplementary Figure S2C), potentially as a result of altered B-cell developmental environment due to extensive ongoing education of auto-reactive B cells.
T-cell tolerance to the HOD antigen is complete due to non-responsiveness not deletion
To test the hypothesis that T-cell tolerance to the HOD antigen is complete, B6 and B6.HOD mice were immunized with OVA/CFA and then boosted with OVA/IFA. Similar to responses to HEL/CFA (Figure 1A), B6 but not B6.HOD mice made detectable anti-HOD antibodies (Figure 2A). To test the hypothesis that HOD-specific T cells are deleted in B6.HOD mice, endogenous OVA-specific T cells were quantified by the tetramer enrichment assay described by Moon et al.32 Pooled leukocytes from spleen and lymph nodes were stained with a mixture of OVA329-337 and OVA326-334 I-Ab tetramers. Cells were also stained with an I-Ab tetramer presenting a peptide derived from the glycoprotein of LCMV (LCMV GP66-77) as a specificity control. Tetramer-bound cells were then stained with antibodies against CD44, CD3, CD4, CD8, CD11b, CD11c, B220, and F4/80.32 In 4 independent experiments, B6 and B6.HOD mice had similar numbers of OVA-specific CD4+ T cells (Figure 2B, mean numbers were 40 cells and 56 cells, respectively; P=0.3). This precursor frequency is of a similar magnitude as previously reported for naïve B6 mice using these tetramer reagents.22 The identified populations were antigen specific, as they reacted with OVA tetramers but not control LCMV tetramer (Figure 2C). The endogenous OVA tetramer positive CD4+ T cells from naïve mice were CD44lo, suggesting they had not previously been activated (Figure 2D, lower right hand quadrant).
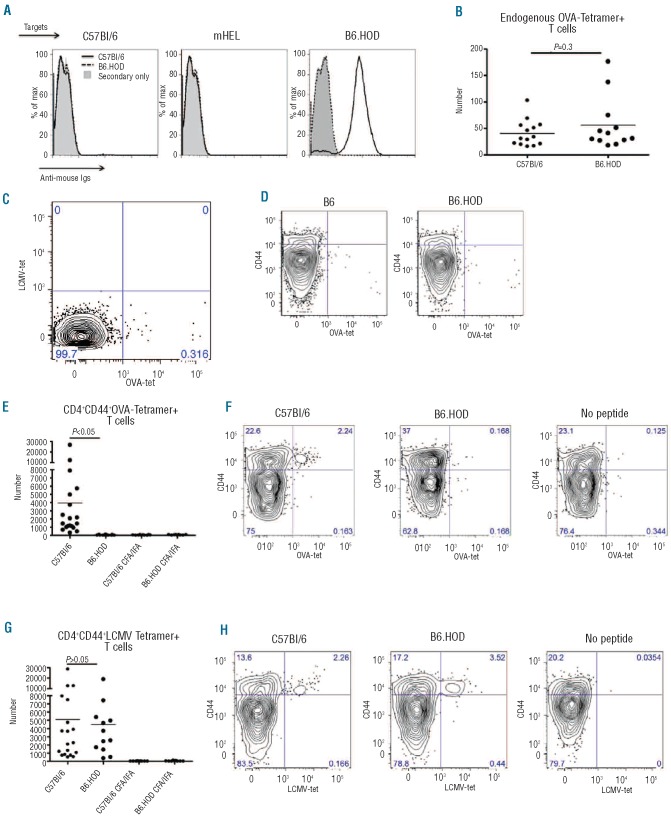
Figure 2.
OVA-specific T cells in B6.HOD mice are present but do not respond to antigenic challenge. B6 and B6.HOD mice were immunized with OVA/CFA followed by OVA/IFA boost. Anti-OVA IgG were analyzed by (A) flow crossmatch against B6, mHEL, and B6.HOD targets. (B) Total leukocytes from B6 or B6.HOD mice were enumerated by tetramer enrichment assay (C) were analyzed for antigen specificity by comparing tetramer staining (OVA323-339 and OVA326-334 vs. LCMV GP66-77), and (D) were evaluated for activation by anti-CD44 staining. (E) To assess the ability to expand upon challenge, B6 and B6.HOD mice were immunized with OVA323-339 and LCMV61-80 peptides in CFA and subsequently boosted with peptides in IFA. OVA-specific CD4+CD44+ T cells were enumerated and (F) representative flow plots are provided. (G) LCMV-specific CD4+CD44+ T cells were also enumerated and (H) activation was assessed by anti-CD44 staining. Representative flow plots are shown. Control CFA-IFA immunizations in the absence of peptides were included as controls. All data are representative of 4 independent experiments with similar results; at least 12 mice were analyzed per group.
To test the function of the visualized OVA-specific populations, mice were immunized with OVA323-339 and LCMV GP61-80 peptides emulsified in CFA and boosted two weeks later with both peptides in IFA. Spleen and draining lymph nodes were harvested 7-10 days post boost and stained with the same OVA and LCMV-specific tetramers. B6 but not B6.HOD mice had a significant expansion of OVA-specific CD4+ T cells upon immunization (Figure 2E, mean 3943 and 53, respectively; P<0.05). OVA-specific T cells were CD44hi, indicating antigen experience (Figure 2F). In contrast to OVA, B6 and B6.HOD mice had similar numbers of LCMV-specific CD44+CD4+ T cells (Figure 2G-H, mean 5085 and 4498, respectively), demonstrating normal reactivity to a third-party antigen (not self), and thus indicating that decreased anti-OVA responses were antigen specific and not a general property of B6.HOD mice (Figure 2H, mean 4.7 and 3.7). No significant OVA or LCMV-tetramer positive CD4+ T-cell populations were observed with adjuvant alone (Figure 2E-H). Therefore, the tetramer data presented (Figure 2B) demonstrate that while OVA autoreactive T cells are present in the HOD mouse, immunization data (Figure 2E-F) demonstrate that they are non-responsive to cognate antigen, indicating an anergic phenotype.
Discussion
Autoreactive B cells that encounter their cognate antigen in the bone marrow tend to undergo deletion, anergy, or receptor editing to establish central tolerance to self-antigen. Due to the close juxtaposition of developing B cells and RBCs, we hypothesized that RBC-specific B cells would be tolerized to erythrocyte self-antigens. Adoptive transfer of OT-II T cells into HOD mice clearly demonstrates that whatever B-cell tolerance occurs, it is insufficient to remove RBC autoreactive B cells. Because B6 mice are not prone to get AIHA spontaneously, we conclude that such RBC autoreactive B cells represent the normal state for healthy mice. This leads us to the conclusion that immune dysregulation need not impair B-cell tolerance mechanisms in order for AIHA to occur, as sufficient numbers of functional RBC autoreactive B cells survive in the normal state. These findings are consistent with the observation that infusion of rat RBCs into healthy mice induces autoantibodies to RBC antigens; it is assumed that conserved B-cell epitopes are linked to foreign T-cell epitopes in this setting.33 These findings are consistent with models of spontaneous loss of humoral tolerance to RBC antigens in mice prone to develop AIHA.34 In the current model, whatever the tolerance mechanisms may be, the RBC autoreactive B cells represent an intrinsically dangerous population, as all it takes is T-cell help to launch a full autoimmune antibody response.
Most RBC antigens are not expressed on platelets or leukocytes, but can have varied expression on non-hematopoietic tissues (e.g. expression of Duffy in kidney and Kell in skeletal muscle).35,36 Because B cells are tolerized to native antigen encountered during development in the bone marrow or in the periphery, widespread expression of antigens in multiple compartments is hypothesized to result in tighter regulation of autoreactive B cells.12 Consistent with this hypothesis, of the autoantibodies to RBCs in humans that have identifiable specificities, RhD or RhD associated proteins are a common target; however, many additional specificities have also been noted.37-39 The RhD antigen has not been detected on non-RBC elements in either hematopoietic or non-hematopoietic tissues.40,41 In contrast, autoantibodies are seldom seen against more ubiquitous antigens, such as ABO antigens, and experimental expression of autoantigen in liver results in increased tolerance in the B-cell compartment.42,43 Therefore, the HOD model directly tests the effects of antigen-restricted expression on RBCs on establishment of B- and T-cell tolerance, so as to most closely model one pattern of tolerance breakdown and subsequent pathogenesis of AIHA observed in humans (i.e. Rh group antigens).
Mechanistically, it is unclear why all RBC autoreactive B cells are not deleted. It is possible that autoreactive B cells develop in a compartment where they do not encounter RBC antigens (e.g. extramedullary development).44,45 However, the adoptive transfer experiments also indicate that HOD autoreactive B cells are presenting OVA peptide on MHCII, thus demonstrating they have encountered their cognate antigen. Furthermore, data generated with the B6.HODxSwHEL F1 demonstrate that at least some B cells developing in the bone marrow do encounter RBC antigens and are removed. Therefore, we can rule out antigenic ignorance due to anatomical sequestration. While SwHEL mice have a lower precursor frequency than most BCR transgenic mice, the numbers of autoreactive B cells are not physiological.30,46-48 and may overcome deletion mechanisms. However, incomplete deletion of autoreactive B cells is also observed with adoptive transfer of OT-II CD4+ T cells into B6.HOD mice, therefore, demonstrating that central tolerance mechanisms were insufficient to eliminate naturally small numbers of RBC autoreactive B cells. It is still possible that the surviving autoreative B cells represent a population with decreased affinity for the autoantigen, but upon loss of tolerance, can increase immunoglobulin binding through affinity maturation.
It is worth noting that these data do not distinguish between B-cell development in the bone marrow (in the presence of RBC autoantigens) and extramedullary development (absence of RBC autoantigens) with subsequent trafficking to areas where the antigens are present. Nevertheless, B cells appear to be encountering their antigen but not progressing to plasma cells due to lack of T-cell help. Indeed, neither immunization with HEL/CFA nor OVA/CFA induced antibodies in the HOD mice. Because T-cell help is sufficient to induce autoantibodies (i.e. OT-II adoptive transfer), we interpret lack of response to HEL/CFA or OVA/CFA to indicate robust T-cell tolerance. Interestingly, use of CFA can break self-tolerance to self-antigens in other compartments (e.g. MOG/CFA induced autoimmune encephalomyelitis).49 Despite lack of a T-cell response, HEL/CFA and OVA/CFA would still lead to strong signaling to the autoreactive B-cell population in HOD mice, without leading to antibody secretion. These data are in agreement with previous reports in humans that demonstrate anti-RBC B cells do not respond to polyclonal B-cell activation and are, therefore, tightly regulated.50 Thus, CD4+ T-cell tolerance appears to be a critical checkpoint to prevent AIHA in healthy animals.
An unanticipated finding in the current study is that autoantibodies to HOD appear to be restricted to HEL epitopes. No antibodies were detected against OVA or Duffy antigens (data not shown), nor did epitope spreading result in autoantibodies against normal mouse RBC antigens. The reasons for this observation remain unclear. It is possible that HEL is so dominant a B-cell antigen that its response outcompetes B cells specific for other epitopes; however, this is unlikely to prevent all epitope spreading over an extended time period. It is worth noting that mice have an ortholog to human Duffy; thus B-cell responses may be blunted a priori against this part of HOD due to additional self-tolerance. However, this does not explain the absence of anti-OVA. These findings are anomalous in the context of the biology of epitope spreading and requries further study to address this issue.
The nature of the CD4+ T-cell tolerance appears not to be thymic deletion. Rather tetramer enrichment assays demonstrate that numbers of HOD reactive T cells do not differ significantly between B6 and B6.HOD mice. In contrast, peptide immunization demonstrated the OVA reactive CD4+ T-cell population in B6.HOD (but not B6) was non-reactive to antigen, and thus appears to be in an anergic state. This is not the result of some general immunological change as a result of expressing the HOD transgene; CD4+ T cells specific for a third party antigen activate and expand normally in B6.HOD mice (i.e. LCMV peptide). Our data argue that thymic dysregulation, such that normal deletion of autoreactive T cells fails, is not an essential component of AIHA induction. Rather the dangerous autoreactive CD4+ T cells are present in the natural state. Unlike the autoreactive B cells, which activate and differentiate if given their natural stimulus (CD4+ T-cell help), the autoreactive CD4+ T cells fail to activate when given their natural stimulus (antigen plus activation of innate immunity, HEL/CFA or OVA/CFA). A thorough understanding of specific factors that can reverse non-responsiveness of RBC-reactive CD4+ T cells is likely to be key in further clarification of AIHA pathogenesis, as is further characterization of the phenotype of autoreactive CD4+ T cells, which may include anergy and/or regulatory T cells (Treg) that actively suppress immune responses. Should these findings translate into the human setting, they serve to focus hypotheses of human AIHA pathogenesis on tolerance and regulation of the CD4+ T-cell compartment.
Supplementary Material
Acknowledgments
the SwHEL mice were a generous gift from Dr. Robert Brink. We also thank the Winship Cancer Institute Cell Imaging and Microscopy Core and the Pediatric Flow Cytometry Core.
Funding: this work was supported in part by NIH grant P01HL086773-03. We thank Drs. Justin Taylor and Marc Jenkins for OVA326-334 and OVA329-337 tetramers, the NIH tetramer core for the LCMV GP66-77 tetramer, and Dr. Brian Evavold for SMARTA mice and peptides (OVA323-339 and LCMV GP61-80).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hemato. 2002;69(4):258-71 [DOI] [PubMed] [Google Scholar]
- 2.Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116(11):1831-8 [DOI] [PubMed] [Google Scholar]
- 3.Vos GH, Petz LD, Hugh Fudenberg H. Specificity and Immunoglobulin Characteristics of Autoantibodies in Acquired Hemolytic Anemia. J Immunol. 1971;106(5):1172-6 [PubMed] [Google Scholar]
- 4.Meite M, Léonard S, Idrissi ME, Izui S, Masson PL, Coutelier JP. Exacerbation of autoantibody-mediated hemolytic anemia by viral infection. J Virol. 2000;74(13):6045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efremov D, Ivanovski M, Burrone O. The pathologic significance of the immunoglobulins expressed by chronic lymphocytic leukemia B-cells in the development of autoimmune hemolytic anemia. Leuk Lymphoma. 1998;28:285-93 [DOI] [PubMed] [Google Scholar]
- 6.Coutelier JP, Detalle L, Musaji A, Meite M, Izui S. Two-Step Mechanism of Virusinduced Autoimmune Hemolytic Anemia. Ann NY Acad Sci. 2007;1109:151-7 [DOI] [PubMed] [Google Scholar]
- 7.Petz LD, Garratty G. Immune Hemolytic Anemias. 2nd ed. Philadelphia: Churchill Livingstone, 2004 [Google Scholar]
- 8.Klein HG, Anstee DJ. Mollison's Blood Transfusion in Clinical Medicine. 11th ed. Oxford: Blackwell Publishing, 2005 [Google Scholar]
- 9.Branch D, Petz L. Detecting alloantibodies in patients with autoantibodies. Transfusion. 1999;39(1):6-10 [DOI] [PubMed] [Google Scholar]
- 10.Ding C, Yan J. Regulation of autoreactive B cells: checkpoints and activation Archivum Immunologiae et Therapiae Experimentalis. 2006;55(2):83-9 [DOI] [PubMed] [Google Scholar]
- 11.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6(10):728-40 [DOI] [PubMed] [Google Scholar]
- 12.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19(1):595-621 [DOI] [PubMed] [Google Scholar]
- 13.Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12(2):106-14 [DOI] [PubMed] [Google Scholar]
- 14.Bony V, Gane P, Bailly P, Cartron JP. Timecourse expression of polypeptides carrying blood group antigens during human erythroid differentiation. Br J Haematol. 1999;107(2):263-74 [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106(41):17413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker RN, Casswell KM, Elson CJ. Identification of murine erythrocyte autoantigens and cross-reactive rat antigens. Immunology. 1993;78(4):568-73 [PMC free article] [PubMed] [Google Scholar]
- 17.Naysmith JD, Ortega-Pierres MG, Elson CJ. Rat erythrocyte-induced anti-erythrocyte autoantibody production and control in normal mice. Immunol Rev. 1981;55:55-87 [DOI] [PubMed] [Google Scholar]
- 18.Okamoto M, Murakami M, Shimizu A, Ozaki S, Tsubata T, Kumagai S, et al. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992;175(1):71-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami M, Honjo T. Anti-red blood cell autoantibody transgenic mice: murine model of autoimmune hemolytic anemia. Sem Immunol. 1996;8(1):3-9 [DOI] [PubMed] [Google Scholar]
- 20.Murakami M, Nakajima K, Yamazaki K, Muraguchi T, Serikawa T, Honjo T. Effects of Breeding Environments on Generation and Activation of Autoreactive B-1 Cells in Anti-red Blood Cell Autoantibody Transgenic Mice. J Exp Med. 1997;185(4):791-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami M, Tsubata T, Shinkura R, Nisitani S, Okamoto M, Yoshioka H, et al. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180(1):111-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114(11):2315-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676-82 [DOI] [PubMed] [Google Scholar]
- 24.Zimring JC, Cadwell CM, Chadwick TE, Spitalnik SL, Schirmer D, Wu T, et al. Nonhemolytic antigen loss from red blood cells requires cooperative binding of multiple antibodies recognizing different epitopes. Blood. 2007;110(6):2201-8 [DOI] [PubMed] [Google Scholar]
- 25.Hudson KE, Lin E, Hendrickson JE, Lukacher AE, Zimring JC. Regulation of primary alloantibody response through antecedent exposure to a microbial T-cell epitope. Blood. 2010;115(19):3989-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimring JC, Hair GA, Chadwick TE, Deshpande SS, Anderson KM, Hillyer CD, et al. Nonhemolytic antibody-induced loss of erythrocyte surface antigen. Blood. 2005;106(3):1105-12 [DOI] [PubMed] [Google Scholar]
- 27.Cadwell CM, Zimring JC. Cross-linking induces non-haemolytic antigen-loss from transfused red blood cells: a potential role for rheumatoid factor. Vox Sang. 2008;95(2):159-62 [DOI] [PubMed] [Google Scholar]
- 28.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific major MHC class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28(1):390-400 [DOI] [PubMed] [Google Scholar]
- 29.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77(3):452-5 [DOI] [PubMed] [Google Scholar]
- 30.Phan TG, Amesbury M, Gardam S, Crosbie J, Hasbold J, Hodgkin PD, et al. B Cell Receptor-independent Stimuli Trigger Immunoglobulin (Ig) Class Switch Recombination and Production of IgG Autoantibodies by Anergic Self-Reactive B Cells. J Exp Med. 2003;197(7):845-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11(1):14-20 [DOI] [PubMed] [Google Scholar]
- 32.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4+ T Cell Frequency Varies for Different Epitopes and Predicts Repertoire Diversity and Response Magnitude. Immunity. 2007;27(2):203-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman PC. Immune hemolytic anemia— selected topics. ASH Education Program Book. 2009;2009(1):80-6 [DOI] [PubMed] [Google Scholar]
- 34.Hoyer KK, Kuswanto WF, Gallo E, Abbas AK. Distinct roles of helper T-cell subsets in a systemic autoimmune disease. Blood. 2009;113(2):389-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausen H, Hakomori S-i.ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56(1):1-20 [DOI] [PubMed] [Google Scholar]
- 36.Russo D, Wu X, Redman CM, Lee S. Expression of Kell blood group protein in nonerythroid tissues. Blood. 2000;96(1):340-6 [PubMed] [Google Scholar]
- 37.Barker RN, Casswell KM, Reid ME, Sokol RJ, Elson CJ. Identification of autoantigens in autoimmune haemolytic anaemia by a non-radioisotope immunoprecipitation method. Br J Haematol. 1992;82(1):126-32 [DOI] [PubMed] [Google Scholar]
- 38.Leddy JP, Falany JL, Kissel GE, Passador ST, Rosenfeld SI. Erythrocyte membrane proteins reactive with human (warm-reacting) anti-red cell autoantibodies. J Clin Invest. 1993;91(4):1672-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos GH, Petz LD, Fudenberg HH. Specificity and immunoglobulin characteristics of autoantibodies in acquired hemolytic anemia. J Immunol. 1971;106(5):1172-6 [PubMed] [Google Scholar]
- 40.Rojewski MT, Schrezenmeier H, Flegel WA. Tissue distribution of blood group membrane proteins beyond red cells: Evidence from cDNA libraries. Transf Apher Sci. 2006;35(1):71-82 [DOI] [PubMed] [Google Scholar]
- 41.Reid M, Lomas-Francis C. The Blood Group Antigen Facts Book. 2nd ed. Amsterdam: ElsevierAcademic Press,2004 [Google Scholar]
- 42.Ota T, Ota M, Duong BH, Gavin AL, Nemazee D. Liver-expressed Igkappa superantigen induces tolerance of polyclonal B cells by clonal deletion not kappa to lambda receptor editing. J Exp Med. 2011;208(3):617-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins WM. The ABO blood group system: historical background. Transfus Med. 2001;11(4):243-65 [DOI] [PubMed] [Google Scholar]
- 44.Kantor AB, Stall AM, Adams S, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89(8):3320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20(1):253-300 [DOI] [PubMed] [Google Scholar]
- 46.Goodnow CC. Transgenic Mice and Analysis of B-Cell Tolerance. Ann Rev Immunol. 1992;10(1):489-518 [DOI] [PubMed] [Google Scholar]
- 47.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562-6 [DOI] [PubMed] [Google Scholar]
- 48.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant Autoantibody Production by Early Human B Cell Precursors. Science. 2003;301(5638):1374-7 [DOI] [PubMed] [Google Scholar]
- 49.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative Selection and Peptide Chemistry Determine the Size of Naive Foreign Peptide–MHC Class II-Specific CD4+ T Cell Populations. J Immunol. 2010;185(8):4705-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieben R, Tucci A, Nydegger UE, Zubler RH. Self tolerance to human A and B histoblood group antigens exists at the B cell level and cannot be broken by potent polyclonal B cell activation in vitro. Eur J Immunol. 1992;22(10):2713-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.