Localization of endosomal clathrin adaptors is regulated by glucose availability via an unknown mechanism. Studies in intact and permeabilized cells show that clathrin adaptor localization is precisely tuned by cellular ATP concentration. These data provide evidence for how membrane traffic is coordinated with overall proliferation rates.
Abstract
Glucose is a master regulator of cell behavior in the yeast Saccharomyces cerevisiae. It acts as both a metabolic substrate and a potent regulator of intracellular signaling cascades. Glucose starvation induces the transient delocalization and then partial relocalization of clathrin adaptors at the trans-Golgi network and endosomes. Although these localization responses are known to depend on the protein kinase A (PKA) signaling pathway, the molecular mechanism of this regulation is unknown. Here we demonstrate that PKA and the AMP-regulated kinase regulate adaptor localization through changes in energy metabolism. We show that genetic and chemical manipulation of intracellular ATP levels cause corresponding changes in adaptor localization. In permeabilized cells, exogenous ATP is sufficient to induce adaptor localization. Furthermore, we reveal distinct energy-dependent steps in adaptor localization: a step that requires the ADP-ribosylation factor ARF, an ATP-dependent step that requires the phosphatidyl-inositol-4 kinase Pik1, and third ATP-dependent step for which we provide evidence but for which the mechanism is unknown. We propose that these energy-dependent mechanisms precisely synchronize membrane traffic with overall proliferation rates and contribute a crucial aspect of energy conservation during acute glucose starvation.
INTRODUCTION
Many cells use nutrients as both the building blocks for new growth and signaling molecules to regulate developmental programs (Hundal and Taylor, 2009; Thevelein and Voordeckers, 2009; Grose and Rutter, 2010; Heublein et al., 2010). The yeast Saccharomyces cerevisiae responds to glucose with a distinct developmental program that maximizes biomass increase at the expense of surrounding cells (reviewed in Piskur et al., 2006). A key facet of this program is aerobic glycolysis. In aerobic glycolysis, glucose metabolism stops at the end of glycolysis, and glycolytic products are not metabolized to carbon dioxide in the mitochondria (reviewed in Vander Heiden et al., 2009). This program has two goals. First, cells use the carbon derived from glucose to promote biomass increase. Second, cells secrete the end product of glycolysis, ethanol, to inhibit growth of other microorganisms (reviewed in Wills, 1990). To achieve these goals, yeast must actively inhibit mitochondrial respiration when glucose is abundant. However, upon glucose starvation, yeast must shift out of this exploitative developmental program, reactivate the mitochondria, and produce proteins required to survive in the new environment of scarcity (Castelli et al., 2011).
The adaptation to glucose starvation is particularly challenging because the developmental program induced by glucose inhibits the synthesis of proteins that are required to use other energy sources. Furthermore, the cell does not retain reserve pools of glucose (Panek and Mattoon, 1977). Therefore, immediately upon glucose starvation, the cell experiences a severe energy deficit (Uesono et al., 2004), yet it must synthesize new proteins and activate inhibited pathways in order to be able to harness available energy sources such as ethanol, proteins, and lipids. The cell's immediate responses to glucose starvation must therefore facilitate the escape from this energy deficit.
A transient inhibition of endosomal protein traffic likely contributes to energy conservation to counteract the energy deficit immediately after acute glucose starvation (Aoh et al., 2011). In proliferating cells, membrane trafficking pathways consume large amounts of energy to maintain the steady-state localization of proteins within the endosomal system (reviewed in Luzio and Banting, 1993). Such trafficking pathways work via multiple rounds of anterograde traffic followed by retrograde traffic to retrieve components back to the original organelle. Clathrin is a major player in this repeated cycling of proteins between the trans-Golgi network (TGN) and endosomes (Stepp et al., 1997; Chu et al., 1999; Deloche et al., 2001). In this function, clathrin depends on clathrin adaptors that recruit it to the membrane. In yeast, there are at least five adaptors at the TGN and endosomes: the monomeric, Golgi-localized γ-adaptin proteins 1 and 2 (Gga1 and Gga2), the epsin-related proteins Ent3 and Ent5, and the clathrin adaptor protein complex 1 (AP-1; reviewed in Duncan and Payne, 2003). We previously found that clathrin adaptors immediately but transiently disassociate from membranes upon glucose starvation (Aoh et al., 2011). This delocalization causes a transient stoppage of clathrin-dependent traffic, which conserves energy. Although the functional significance of this transient stoppage of membrane traffic is apparent, it is unclear how the cell elicits it.
Protein kinase A (PKA) is the primary effector of the glucose-induced developmental program in yeast (Zaman et al., 2009). Our previous studies implicated PKA in the transient stoppage of endosomal traffic. However, its role appears to be indirect (Aoh et al., 2011). In cells with defects in PKA, adaptors remain membrane associated during glucose starvation. Surprisingly, acute chemical inhibition of PKA does not alter endosomal traffic. Only if PKA is inhibited for a prolonged period before starvation is the adaptor localization response altered. These results suggest that PKA acts indirectly on endosomal traffic (Aoh et al., 2011). Thus an unknown mechanism that is downstream of PKA must direct the transient stoppage of membrane traffic during glucose starvation.
To investigate the responses of endosomal traffic to glucose starvation in yeast, we took a genetic approach. This approach revealed that the AMP-activated kinase (AMPK) and glucose repression machinery are required for normal responses of clathrin adaptors to glucose starvation. We used chemical perturbations and an in vitro recruitment assay to demonstrate that ATP levels directly or indirectly regulate clathrin adaptor localization. Our findings suggest that endosomal traffic rates are coordinated with available cellular energy during the transition from proliferation to quiescence.
RESULTS
AMP-activated kinase is required for adaptor localization in prolonged starvation
In response to glucose starvation, clathrin-dependent traffic at the TGN and endosomes is transiently blocked. All clathrin adaptors at the TGN and endosomes immediately redistribute to the cytosol. Only after 30 min or more of continuous starvation do the adapters partially regain their normal punctate localization, reflecting association with membranes of the TGN and endosomes (Aoh et al., 2011; Supplemental Figure S1). We also demonstrated that these changes in localization reflect loss of adaptor association with membranes and not disassembly of the organelles (Aoh et al., 2011). Delocalization of adaptors prevents clathrin-dependent traffic and thus prevents energy consumption by the clathrin-dependent cycling of proteins between the TGN and endosomes. However, our previous findings did not reveal the molecular mechanism by which glucose starvation regulates adaptor function.
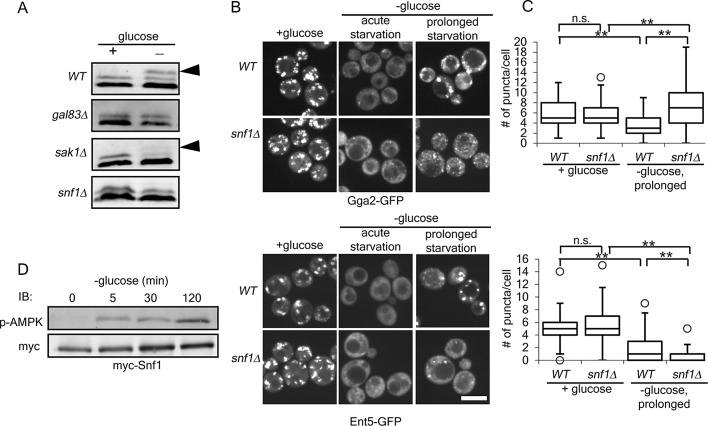
We previously found that Ent5 becomes hyperphosphorylated at the same time that adaptors relocalize to membranes during prolonged starvation (Aoh et al., 2011). This hyperphosphorylation is easily identified with immunoblot analysis (Figure 1A, arrowhead). Although the functional significance of this phosphorylation is unknown, mutations that prevented the immediate delocalization of adaptors during glucose starvation also prevented hyperphosphorylation (Aoh et al., 2011). Therefore, to identify additional regulators of adaptors, we screened for gene deletions that prevented Ent5 hyperphosphorylation during glucose starvation using a focused library of strains lacking kinases or kinase cofactors. We performed immunoblot analysis of lysates from cells from this library to identify gene deletions that altered Ent5 hyperphosphorylation after prolonged starvation (unpublished data).
FIGURE 1:
Snf1 is required for localization of the TGN–endosomal clathrin adaptors Gga2 and Ent5 during prolonged glucose starvation. (A) AMPK pathway proteins are required for Ent5 hyperphosphorylation. Cellular lysates were prepared from indicated cells before or 2 h after glucose starvation. The cell lysates were then probed with an antibody to Ent5. Arrowheads indicate hyperphosphorylated Ent5. (B) Snf1 is required for adaptor localization during prolonged glucose starvation. Wild-type (WT) and snf1∆ cells expressing Gga2-GFP (top) or Ent5-GFP (bottom) from their endogenous loci were imaged before (+glucose), within 10 min (acute starvation), and after 2 h of glucose starvation (prolonged starvation). Scale bar, 5 μm. (C) Quantification of the number of puncta per cell for cells in B. **p < 0.01; n.s., not significant. Charts show box plots of the data. The line indicates the median, the edges of the box indicate the 25 and 75% percentiles, and the whiskers extend to the extremes of the data not considered outliers. (D) Snf1 is activated within 5 min of glucose starvation. Cellular lysates were prepared from cells expressing Snf1-myc from its endogenous locus at the indicated time points before or after glucose starvation. Active Snf1 was detected with a phospho-AMPK, and total cellular Snf1 was detected with an anti-myc antibody.
Using this approach, we found that mutation of several proteins in the AMPK pathway gave strong defects in Ent5 hyperphosphorylation (Figure 1A). AMPK consists of a core catalytic α subunit and one of three partially redundant β subunits, which contribute substrate specificity (reviewed in Hedbacker and Carlson, 2008). AMPK also requires activating phosphorylation by one of three upstream kinases. In the screen, we found that deletion of GAL83, a β subunit, blocked Ent5 hyperphosphorylation. We also found that deletion of the primary upstream activating kinase, SAK1, caused partial defects (Figure 1A, arrowhead). In our initial screen, the core α catalytic subunit (SNF1) was not identified as required for hyperphosphorylation. However, strains from deletion library collection that we used to make the focused library might contain errors or have second-site suppressor mutations. Therefore we generated a strain carrying a complete deletion of SNF1. In this strain, Ent5 hyperphosphorylation was inhibited (Figure 1A). Thus the AMPK pathway is required for Ent5 hyperphosphorylation.
To determine whether Snf1 regulates adaptor localization responses, we investigated adaptor localization in snf1Δ cells. We chose Gga2 and Ent5 as representatives of the two functional modules of clathrin-dependent TGN-endosome traffic: the Gga/Ent3 module and the AP-1/Ent5 module. These two modules have different requirements for localization and act at different stages in transport within the TGN and endosomes (Boman et al., 2000; Costaguta et al., 2001, 2006; Fernandez and Payne, 2006; Daboussi et al., 2012; Hung et al., 2012). Furthermore, the Gga/Ent3 module promotes the recruitment of the Ent5/AP-1 module (Daboussi et al., 2012; Hung et al., 2012). Deletion of SNF1 did not dramatically alter adaptor localization in the presence of glucose or adaptor redistribution to the cytosol in the acute phase of glucose starvation (Figure 1, B and C). However, deletion of SNF1 caused dramatic changes in adaptor localization after prolonged starvation. In wild-type cells, Gga2 and Ent5 accumulate in large puncta after prolonged starvation. In cells lacking Snf1, Gga2 accumulated in many very small puncta, and Ent5 was found in only a few small puncta (Figure 1, B and C). These results indicate that Snf1 is required for proper localization of Gga2 and Ent5 during the prolonged phase of glucose starvation.
Snf1 is a major regulator of cell physiology during glucose starvation. It can phosphorylate several proteins involved in membrane traffic, raising the possibility that Snf1 may directly regulate adaptor localization (Ptacek et al., 2005). However, Snf1 should be activated rapidly upon glucose starvation, but relocalization of Gga2 and Ent5 is delayed until 30 min after the onset of starvation (Aoh et al., 2011; Mayer et al., 2011; Ruiz et al., 2011). A temporal difference in Snf1 activation and adaptor relocalization could mean that Snf1 acts indirectly in adaptor localization. To determine whether Snf1 activation coincided temporally with adaptor relocalization in our starvation conditions, we monitored Snf1 activation during glucose starvation. To monitor activation, we used an antibody that recognizes the active phosphorylated form of Snf1. Before glucose starvation, no active Snf1 was detected (Figure 1D). Within 5 min of starvation, Snf1 was phosphorylated and remained phosphorylated during the 2-h time course. Because Snf1 is activated substantially before adaptors relocalize to punctate structures, the effect of Snf1 on adaptors is likely indirect.
Glucose repression is required for adaptor relocalization in acute starvation
We previously identified another glucose-responsive kinase, PKA, as an indirect regulator of adaptor localization during glucose starvation (Aoh et al., 2011). PKA and Snf1 converge on the regulation of several glucose-responsive pathways, including opposing regulation of glucose repression (Zaman et al., 2009). Glucose repression is the suite of changes in transcription and cell physiology that optimizes the cell to exclusively derive energy from the glycolysis of glucose or fructose (Wilson and Roach, 2002). Glucose repression is induced by the activity of PKA (Thevelein and de Winde, 1999). During glucose starvation, glucose repression is inactivated by Snf1 (Carlson et al., 1981). We previously discovered that PKA activity in the presence of glucose is required for adaptor delocalization during the acute phase of glucose starvation (Aoh et al., 2011). Our finding that Snf1 is required for adaptor relocalization during the prolonged phase of glucose starvation suggested that the two pathways were playing opposing roles in adaptor localization. Because PKA and Snf1 play opposing roles in both adaptor localization and glucose repression, we hypothesized that adaptor localization was linked to the glucose repression program.
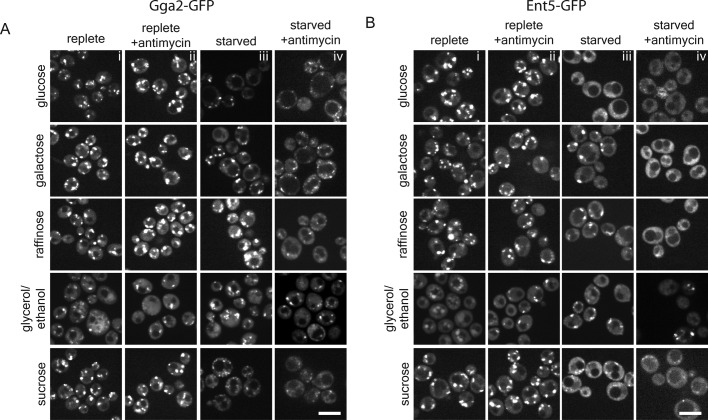
To investigate this hypothesis, we grew cells in carbon sources that activate glucose repression (repressing) or inactivate glucose repression (derepressing). In cells grown in the repressing carbon sources—glucose and sucrose—carbon starvation caused an immediate delocalization of Gga2 and Ent5 to the cytosol (Figure 2, A and B). In contrast, in cells grown in the derepressing carbon sources—galactose, raffinose, and glycerol/ethanol—starvation did not dramatically alter the localization of Gga2 or Ent5 (Figure 2, A and B). This result suggests that the starvation-induced redistribution of adaptors depends on glucose repression.
FIGURE 2:
Inhibition of cellular energy production induces adaptor redistribution. Wild-type cells expressing Gga2-GFP (A) or Ent5-GFP (B) from their endogenous loci were preadapted to different carbons sources by continuous logarithmic growth for 48 h. Cells were imaged during logarithmic phase growth (i), after treatment with 2 μg/ml antimycin (ii), immediately after carbon source withdrawal (iii), and immediately after carbon source withdrawal followed by treatment with 2 μg/ml antimycin (iv). Scale bar, 5 μm.
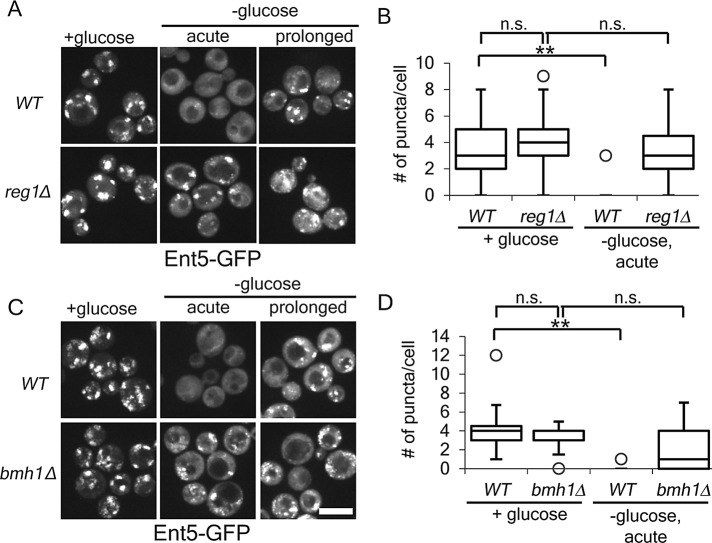
To further test the role of glucose repression in adaptor redistribution, we investigated adaptor redistribution in cells lacking proteins required to establish glucose repression. The phosphatase regulatory subunit, Reg1, and the 14-3-3 protein, Bmh1, are required to establish glucose repression (Dombek et al., 2004). We found that cells in lacking Reg1 or Bmh1, the number of punctate structures containing adaptors was unchanged by glucose starvation (Figure 3). This result further demonstrates that glucose repression is a necessary prerequisite for starvation-induced redistribution.
FIGURE 3:
Glucose repression genes are required for glucose starvation–induced redistribution of Ent5. (A,C). Indicated cells expressing Ent5-GFP from the endogenous locus were imaged before or immediately after glucose starvation. (B,D). Quantification of the number of puncta per cell for cells in A and C. **p < 0.01; n.s., not significant. Chart shows box plot of data as described in Figure 1. Scale bar, 5 μm.
Energy metabolism is required for adaptor localization
One of the major effects of glucose repression upon glucose starvation is on ATP production. The glucose repression program maintains aerobic glycolysis as the only pathway for energy generation, inhibiting all other modes of energy generation (Johnston, 1999). In the absence of parallel ATP generation pathways, ATP levels drop precipitously immediately after glucose starvation (Ashe et al., 2000). Because of the link between glucose repression and adaptor localization, we hypothesized that adaptor redistribution was linked to energy generation. To test this hypothesis, we inhibited respiration with the cytochrome C reductase inhibitor antimycin A in cells grown under different conditions. In the absence of mitochondrial function, cells can produce ATP via glycolysis with six carbon sugars (glucose, galactose, or raffinose). In cells grown in glucose, galactose, or raffinose, antimycin A had no dramatic effect on adaptor localization (Figure 2, A and B). This suggests that antimycin A does not influence adaptors in cells that can generate energy via glycolysis. In cells preadapted to glycerol/ethanol, addition of antimycin A did not cause redistribution of adaptors to the cytosol (Figure 2, A and B). This result may reflect the ability of these cells to store and metabolize carbohydrates such as glycogen and trehalose (Shi et al., 2010). Taken together, these results suggest that continued energy production is required for adaptor localization.
As a further test of this hypothesis, we treated cells that were preadapted and then starved for galactose or raffinose with antimycin A. Cells starved for galactose or raffinose generate ATP via respiration, and thus the addition of antimycin A reduces ATP levels in these cells. In cells that had been preadapted and then starved for either galactose or raffinose, adaptors redistributed to the cytosol immediately upon addition of antimycin A (Figure 2, A and B). In cells preadapted to glycerol/ethanol and then starved and treated with antimycin A, we saw a reduction in the number of adaptor puncta; however, delocalization was not as complete as with other conditions. This may reflect the ability of these cells to generate ATP by glycolysis of glycogen and trehalose. These results suggest that adaptor localization is closely correlated to steady-state maintenance of energy levels.
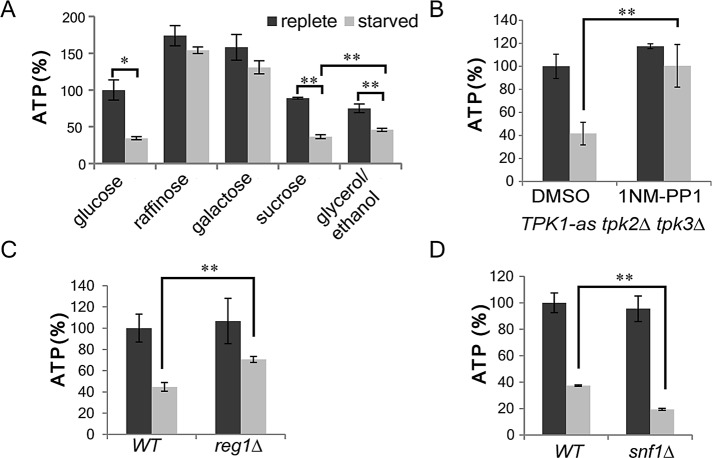
To verify that changes in ATP concentration paralleled the changes in adaptor localization, we monitored ATP concentration in cells grown in different carbon sources and then starved. As expected, ATP concentration dropped >50% in cells grown in glucose or sucrose and then starved for the carbon source (Figure 4A). In contrast, ATP concentration remained high upon starvation of cells grown in galactose or raffinose. This observation is consistent with the known effects of carbon source on oxidative phosphorylation.
FIGURE 4:
Cellular ATP concentration decrease significantly during glucose starvation and is regulated by glucose repression pathways. (A) Cellular ATP was measured in wild-type cells preadapted to different carbon sources (2% glucose, 2% galactose, 2% raffinose, 2% glycerol/3% ethanol, or 2% sucrose) before and after carbon source withdrawal. (B) TPK1-as tpk2∆ tpk3∆ cells were grown to mid–log phase, incubated with 2 μM 1NM-PP1 or DMSO for 1 h, and then starved. Cellular ATP was measured in cells before and after starvation. ATP concentrations were normalized to cells grown in glucose and treated with DMSO. (C) Cellular ATP was measured in reg1∆ cells before or after glucose starvation. The relative ATP concentrations were normalized to wild-type cells grown in glucose. (D) snf1Δ cells were processed as in C. **p < 0.01, *p < 0.05. Charts show average values and SD.
In cells grown in glycerol/ethanol, measured ATP concentrations were lower than in the other carbon sources. Furthermore, the levels after starvation were substantially lower than in unstarved cells. This was unexpected based on the continued localization of adaptors under these conditions. One possible explanation is that glycerol/ethanol-grown cells may have a lower volume of cytoplasm, and this causes an underestimation of actual ATP concentrations. Cytoplasmic volume depends on both the size of the cell and the volume of the cytoplasm occupied by the vacuole and other ATP-impenetrable organelles. We approximated total cell volume with optical density. Glycerol/ethanol-grown cells are smaller overall and have more mitochondria and larger vacuoles, and thus they are expected to have a lower cytoplasmic volume than glucose-grown cells (Jorgensen et al., 2002; Hughes and Gottschling, 2012). The ATP levels measured therefore likely underestimate ATP concentrations for the glycerol/ethanol-grown cells. However, even with this underestimation, the measured ATP concentration of glycerol/ethanol-starved cells were still 10% higher than those in cells starved for repressing sugars (Figure 4A; p < 0.01). Thus, overall these results show that adaptor delocalization coincides with low ATP concentration in cells.
We next examined ATP concentrations in cells with characterized defects in adaptor localization during glucose starvation. Cells lacking functional PKA or Reg1 fail to induce adaptor delocalization in the acute phase of glucose starvation (Aoh et al., 2011; Figure 3A). To modulate PKA activity, we used a strain carrying Tpk1-as, an analogue-sensitive allele of the PKA catalytic subunit Tpk1, in which PKA activity can be inhibited by the kinase inhibitor analogue 1NM-PP1. In this strain two of the three alternate catalytic subunits (TPK2 and TPK3) are deleted, and the third (TPK1) is mutated such that it can be inhibited by the kinase inhibitor analogue 1NM-PP1. When Tpk1-as cells were treated with 1NM-PP1 for 1 h before starvation, the ATP concentration did not change upon starvation (Figure 4B). This is in contrast to control dimethyl sulfoxide (DMSO)–treated cells, in which the ATP concentration dropped upon glucose starvation. Similarly, in cells lacking Reg1, ATP concentration dropped only 30%. This change was significantly smaller than the 50% reduction of ATP concentration in wild-type cells (Figure 4C; p < 0.01). These findings are consistent with the ability of 1NM-PP1 TPK1-as–treated cells and reg1Δ cells to generate ATP by mitochondrial respiration using nonglucose substrates. In contrast, in cells lacking Snf1, ATP concentration dropped 80% after glucose starvation, a level that was significantly lower than that of wild-type cells (Figure 4D; p < 0.01). Taken together, these results show that low ATP concentrations parallel changes in adaptor delocalization and that mutations that prevent adaptor delocalization prevent low ATP concentrations in the acute phase of glucose starvation.
To further investigate the coincidence between ATP concentration and adaptor localization, we monitored ATP concentration during a time course of starvation. In the first 5 min of glucose starvation, ATP concentration dropped to 20% of the level before starvation. The concentration rose to 35% within 10 min and continued to rise over the next 60 min (Figure 5A). By 30 min—the time point when adaptors return to membranes—ATP concentration was up to 40% of the prestarved level. In contrast, in cells lacking Snf1, ATP concentration dropped to 10% of prestarved level and never rose above 30% in the 60-min time course (Figure 5A). Thus the adaptor recruitment is coincident with ATP concentrations >40% of prestarved cells.
FIGURE 5:
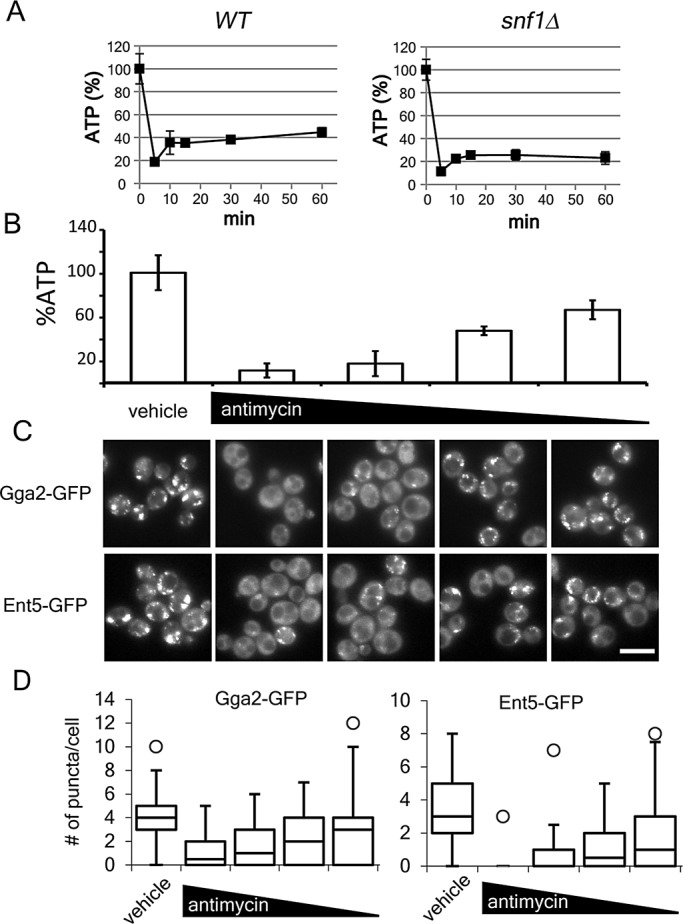
Cellular ATP concentrations correlate with the association of adaptors to membranes. (A) Cellular ATP measured in indicated cells before and after glucose starvation as described in Materials and Methods. (B) Wild-type cells were grown to mid–log phase in galactose and then starved. Various amounts of antimycin A were added to the cells. Cellular ATP measured as described. Charts show average values and SD. (C) Indicated cells were processed as in B and imaged. Scale bar, 5 μm. (D) Quantification of the number of puncta per cell for cells in C. Chart shows box plot of data as described in Figure 1.
Next we directly manipulated cellular ATP concentration and monitored adaptor localization. In derepressed starved cells, antimycin A caused a concentration-dependent reduction in ATP concentration (Figure 5B). Reduction of ATP concentration to <20% of replete concentration caused a nearly complete redistribution of adaptors to the cytosol (Figure 5C, second column). When ATP concentrations were at or >40% of replete, adaptors were recruited to membranes. Of interest, the effect was dose dependent. At lower concentrations of ATP, fewer punctate structures were observed than at higher concentrations of ATP (Figure 5D). Thus adaptor localization is closely correlated with cellular ATP concentration.
Addition of ATP is sufficient for adaptor localization
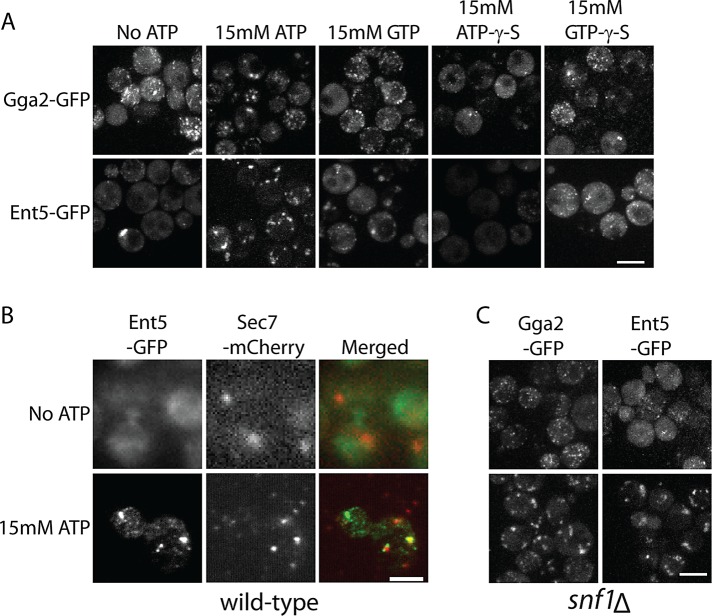
ATP is a ubiquitous molecule in cells. It is required for the proper functioning of motors, kinases, and other enzymes, for pH and ion homeostasis, and to maintain levels of GTP. Any one of these functions may directly or indirectly affect adaptor localization. To explore possible energy-dependent mechanisms, we developed a permeabilized cell assay of adaptor localization. To prevent ATP generation, we treated glucose-starved cells with antimycin A before permeabilizing the cells. After permeabilization, Gga2–green fluorescent protein (GFP) could be seen as many small puncta, similar to its appearance in glucose-starved snf1Δ cells. Ent5 was diffusely localized. Addition of ATP to permeabilized cells caused both adaptors to rapidly redistribute into a few large puncta that resembled their localization on organelles in intact cells (Figure 6A). Thus ATP can induce adaptor redistribution Furthermore, because permeabilized cells cannot maintain pH or ion gradients, cytosolic pH or ion concentrations can be excluded as the mechanism by which ATP regulates adaptors.
FIGURE 6:
ATP is sufficient to recruit adaptors in permeabilized cells. (A) Cells expressing Gga2-GFP or Ent5-GFP from the endogenous loci were permeabilized and incubated with or without indicated nucleotides for 5 min before imaging. (B) Ent5 is recruited to Sec7 containing organelles upon addition of ATP. Cells expressing Ent5-GFP and Sec7-mcherry were permeabilized and incubated with or without ATP. Cells were fixed, mounted for TIRF microscopy, and imaged. (C) Snf1 is not required for adaptor localization in the presence of exogenous ATP. Cells lacking Snf1 and expressing Gga2-GFP or Ent5-GFP from the endogenous loci were permeabilized and imaged with or without addition of exogenous ATP. Scale bar, 5 μm.
To determine whether adaptors were recruited to organelles, we performed colocalization analysis. Owing to difficulties in visualizing the weak mCherry fluorescence of mCherry-tagged proteins in permeabilized cells using a confocal microscope, we used total internal reflection microscopy (TIRF) for this analysis. Although TIRF only allows visualization of structures within close proximity of the cell surface, some Ent5-positive organelles are close enough to the cell surface to be captured via this method. Organelles were marked with Sec7-mCherry. Sec7 shows partial colocalization with Ent5 in intact cells in the presence of glucose and during prolonged starvation (Daboussi et al., 2012; Supplemental Figure S1). Of importance, Sec7 does not delocalize upon glucose starvation; therefore it is a reliable marker of organelles even when adaptors are delocalized (Aoh et al., 2011). When observed with TIRF microscopy, Ent5 appeared diffuse in starved, permeabilized cells. In contrast, Sec7 was apparent in bright puncta in the permeabilized cells (Figure 6B). On addition of ATP, Ent5 appeared in punctate structures. As in intact cells, some of the Ent5 punctate structures colocalized with Sec7. These results indicate that ATP directs adaptor recruitment to organelles in permeabilized cells.
The analysis of adaptor redistribution in intact cells suggested that Snf1 acts indirectly to regulate adaptor localization via energy metabolism. To further test this model, we performed ATP add-back experiments in permeabilized cells lacking Snf1. We found that ATP induced adaptor puncta even in permeabilized cells lacking Snf1 (Figure 6C). Because ATP can bypass the requirement for Snf1, this finding further supports our earlier conclusion that Snf1 regulates adaptor localization indirectly via its roles in energy metabolism.
We next examined the ability of other nucleotides to induce adaptor localization in permeabilized cells (Figure 6A). Addition of GTP caused minor changes in Gga2 and Ent5 localization. However, ATP-γ-S and GTP-γ-S caused little change in either Gga2 or Ent5 localization. This suggests that full adaptor localization requires ATP hydrolysis and suggests a possible role for GTP in adaptor localization.
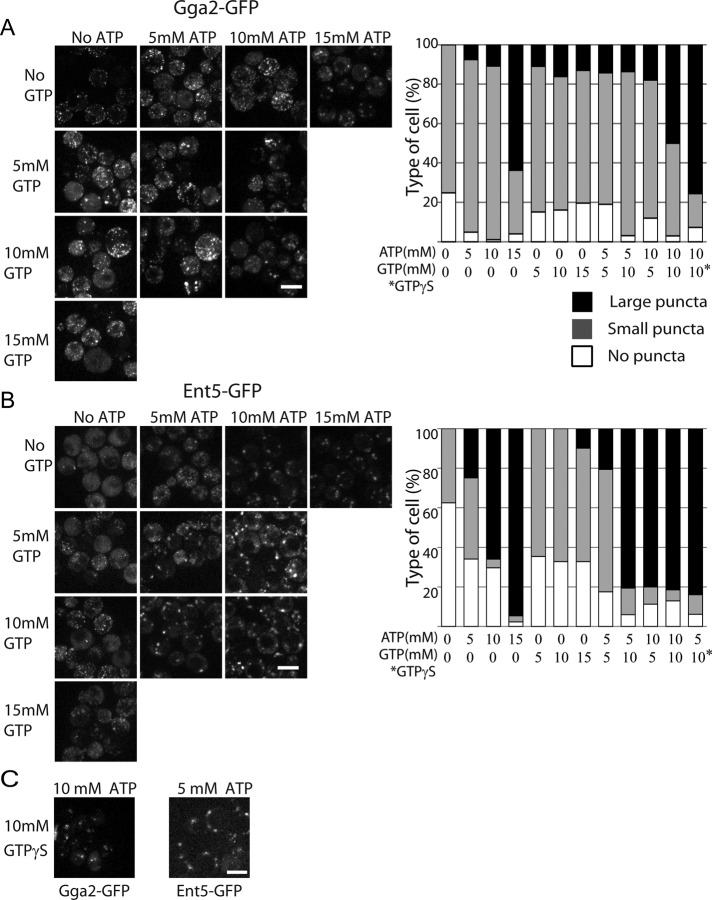
To further explore the role of nucleotides on adaptor localization, we examined adaptor localization in cells treated with different concentrations of ATP. For this analysis, we classified cells as having no puncta, small puncta, or large puncta. In cells expressing Gga2-GFP without exogenous ATP, no cells contained large puncta, 75% of cells had small puncta, and 25% had no puncta (Figure 7A). Addition of 5 or 10 mM ATP caused an increase in the number of cells with large puncta and in the number of cells with small puncta and a decrease of the number of cells with no puncta of Gga2. Increasing ATP from 10 to 15 mM caused a dramatic increase in the number of cells containing large puncta of Gga2. Thus, Gga2 shows a dose-dependent response to ATP.
FIGURE 7:
ATP and GTP both contribute to adaptor recruitment. (A, B) Right, addition of GTP reduces the amount of ATP required to induce adaptor localization in permeabilized cells. Cells were prepared as in Figure 6 and incubated with indicated amounts of nucleotides for 5 min before imaging. Left, quantification of adaptor localization in different conditions. Cells were classified as having no puncta, only small or dim puncta, or large puncta. Scale bar, 5 µm. Charts show representative data from one of three replicate experiments. n > 50 cells. (C) GTP-γ-S reduces the amount of ATP required to induce adaptor localization.
Ent5 also shows a dose-dependent response to ATP. In cells expressing Ent5-GFP without exogenous ATP, no cells contained large puncta, 38% of cells had small puncta, and 62% had no puncta (Figure 7B). Addition of 5 mM ATP caused an increase in the number of cells with large puncta and in the number of cells with small puncta and a corresponding decrease in the number of cells with no puncta. In the presence of 10 mM ATP, 65% of cells contained large puncta and only 4% contained small puncta. Increasing ATP from 10 to 15 mM ATP further increased the number of cells containing large puncta to 95%. Thus, similar to Gga2, Ent5 shows a dose-dependent response to ATP. Furthermore, ATP induces more cells to contain large puncta of Ent5 than large puncta of Gga2 at all concentrations of ATP tested. This difference suggests that, similar to intact cells, Gga2 and Ent5 have different requirements for localization in permeabilized cells.
We next examined the effects of different concentrations of GTP on adaptor localization. We found that addition of 5, 10, or 15 mM GTP caused 10–16% of cells to contain large puncta of Gga2 (Figure 7A). This increase in large puncta compared with cells with no nucleotide was coincident with a slight decrease in the number of cells with small puncta and in the number of cells with no puncta. In contrast, for Ent5, addition of 5 or 10 mM GTP only increased the number of cells with small puncta; it had no effect on the number of cells with large puncta of Ent5 (Figure 7B). However, increasing GTP from 10 to 15 mM caused 10% of the cells to contain large puncta of Ent5. This increase was coincident with a decrease in the number of cells with small puncta. Thus, although Gga2 and Ent5 show dose-dependent responses to GTP, the effect of GTP is weaker than the effect of ATP on both adaptors.
The finding that GTP can induce weak adaptor localization could be explained by a role of GTP in adaptor localization or secondary production of ATP by the transfer of the γ phosphate from GTP to ADP in the permeabilized cells. To distinguish between these two possibilities, we examined whether GTP and ATP acted synergistically. We treated cells with combinations of 5 or 10 mM ATP and 5 or 10 mM GTP. Gga2 localization in large puncta was increased by the addition of both GTP and ATP (Figure 7A). This effect was most pronounced for cells treated with 10 mM GTP and 10 mM ATP. This combination increased the number of cells containing large puncta of Gga2 from 16% in the presence of 10 mM ATP alone to 47% in the presence of 10 mM of ATP and GTP. GTP-γ-S was more effective than GTP in inducing large puncta of Gga2. In cells treated with 10 mM ATP and 10 mM GTP-γ-S, 75% of cells had large puncta of Gga2 (Figure 7, A, chart, and C). Because GTP-γ-S is a poor substrate for γ transfer reactions, this finding argues that the generation of ATP does not contribute to the synergy of GTP and ATP on Gga2 localization (Lacombe et al., 1990). Taken together, these results suggest that ATP and GTP act together to promote Gga2 localization.
The effect of GTP on Ent5 localization in ATP-treated cells was even more substantial. Addition of 10 mM GTP to cells treated with 5 mM ATP increased the number of cells containing large puncta of Ent5 from 34% to >80% (Figure 7B). Furthermore, 10 mM GTP-γ-S was as effective as 10 mM GTP on the formation of large Ent5 puncta in cells treated with 5 mM ATP (Figure 7, B, chart, and C). Taken together, these results suggest that as with Gga2, ATP and GTP act synergistically to promote Ent5 localization. Thus GTP and ATP both contribute to adaptor localization in permeabilized cells.
Although the concentration of nucleotide required for these effects was relatively high, the maximal ATP concentration used is only five times higher than present estimates of physiological ATP concentrations in yeast (Ozalp et al., 2011). Furthermore, without an ATP/GTP-regenerating system, nucleotides may be rapidly consumed. Thus the ATP responses observed may be induced by ATP concentrations equal to endogenous ATP concentrations. Alternatively, high nucleotide levels may be required because key factors such as GTP exchange factors are more dilute in the permeabilized cells. If the substrates or cofactors are more dilute, this would require higher concentrations of nucleotide for the same effect.
Upstream regulators of adaptors are differentially regulated by energy metabolism
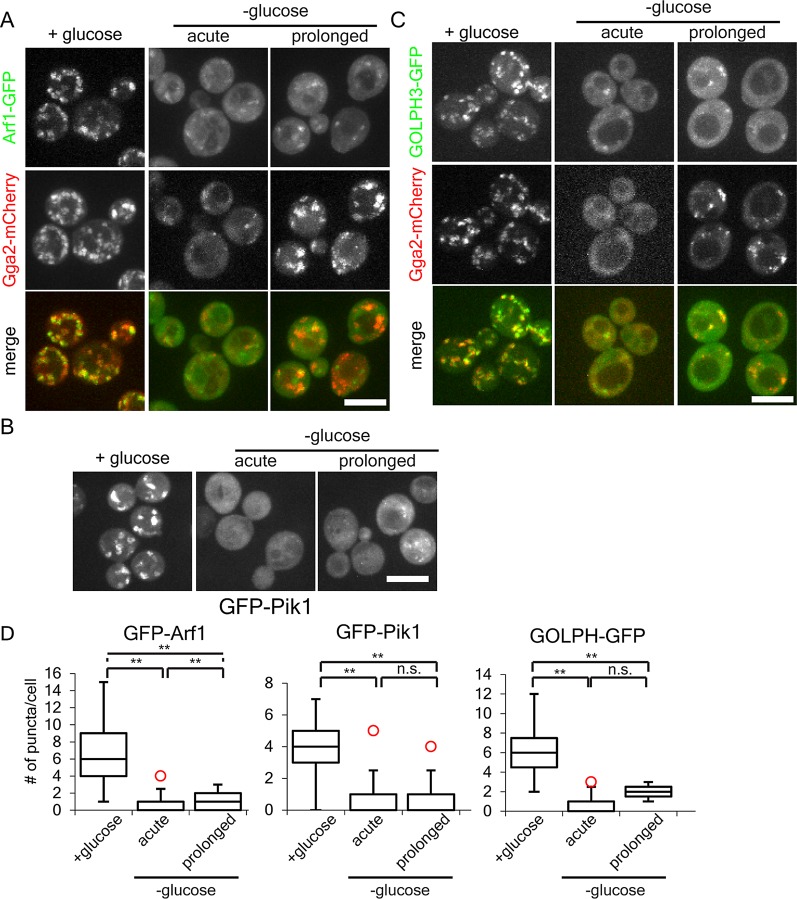
Neither Gga2 nor Ent5 has any obvious GTP- or ATP-sensing domains. Therefore the regulation of adaptor localization is likely mediated by one or more upstream factors. To begin to characterize these factors, we first determined whether known regulators of Gga2 and Ent5 respond to ATP concentration. Several nucleotide-binding proteins are known to act upstream of adaptors, including the functionally interchangeable small GTPases Arf1 and Arf2, the PI4 kinase Pik1, and its product PI4p. Although Pik1 is known to redistribute in cells starved for glucose, the kinetics of this delocalization were unknown (Faulhammer et al., 2007; Demmel et al., 2008). We investigated the changes in localization of the major Arf isoform (Arf1), Pik1, and the PI4p-binding domain from the human GOLPH3 proteins during glucose starvation. In the presence of glucose, Arf1-GFP, GFP-Pik1, and GFP-GOLPH3 all localized to many bright punctate structures in cells (Figure 8). Immediately upon glucose starvation, all three lost bright punctate staining. GFP-Pik1 became completely diffuse, whereas both Arf1 and GOLPH3 retained localization to dim punctate structures. These results show that the majority of Arf1 and Pik1 are rapidly delocalized during glucose starvation and that the levels of PI4p at the TGN and endosomes drop dramatically.
FIGURE 8:
Glucose starvation alters the localization of Arf1, Pik1, and PI4p. (A) Arf relocalizes to dim puncta during acute and prolonged starvation. Diploid cells heterozygous for ARF1-GFP and homozygous for GGA2-mCherry were imaged before, within 15 min, or after 2 h of glucose starvation. (B) Pik1 redistributes to the cytosol upon glucose starvation. Haploid wild-type cells expressing GFP-Pik1 from a plasmid were imaged before, within 15 min, or after 2 h of glucose starvation. (C) GOLPH3 relocalizes to dim puncta during acute and prolonged starvation. Haploid wild-type cells expressing Gga2-mCherry from the endogenous locus and the PI4P probe GOLPH3-GFP from a plasmid were imaged before, within 15 min, or after 2 h of glucose starvation. (D) Quantification of puncta per cell for cells grown in or acutely starved for glucose as described in A–C. **p < 0 .01; n.s., not significant. Charts show box plot of data as described in Figure 1.
On prolonged glucose starvation, when Gga2 relocalized to several bright punctate structures, Pik1 was not found in bright punctate structures. Arf1 and GOLPH3 punctate structures remained dim. Of note, the number of ARF structures went up only slightly, and GOLPH3 puncta did not change upon prolonged starvation (Figure 8D). These results show that unlike the adaptors, the localization of Arf1, Pik1, and GOLPH3 do not show adaptation in the form of increased membrane localization during prolonged glucose starvation.
We next investigated the colocalization of Arf1 and PI4p with Gga2. In the presence of glucose, Arf1 and GOLPH3 show partial colocalization with Gga2. During prolonged starvation, Arf1 and GOLPH3 also partially colocalized with Gga2. We observed three types of structures in ARF-expressing cells: those that contained both Arf1 and Gga2, those that contained only Arf1, and those that contained only Gga2 (Figure 8A). This partial colocalization is consistent with partial colocalization seen in glucose-replete cells. In contrast, for GOLPH3, we saw only two types of structures: those that contained both GOLPH3 and Gga2, and those that contained only Gga2 (Figure 8C). Gga2 is believed to activate the formation of PI4p in glucose-replete cells (Daboussi et al., 2012). Thus the partial colocalization of GOLPH3 and Gga2 in glucose-starved cells may indicate that Gga2 plays a similar role in influencing PI4p levels in glucose-starved cells. Taken together, these results show that glucose starvation clearly reduces the levels of Arf1 and PI4p in the endosomal system; however, their ability to localize to organelles that recruit Gga2 is unchanged.
We next investigated whether ATP induces changes in the localization of Arf1, Pik1, or PI4p in permeabilized cells. In glucose-starved permeabilized cells, Arf1 localized to a few dim puncta, whereas GOLPH3 and Pik1 were diffuse (Figure 9A). Addition of ATP caused a slightly higher number of dim Arf1 puncta. In contrast, ATP immediately induced formation of many bright GOLPH3 or Pik1 puncta. Thus ATP addition does not have a prominent effect on Arf1 localization, but it can direct dramatic changes in PI4p and Pik1 localization.
FIGURE 9:
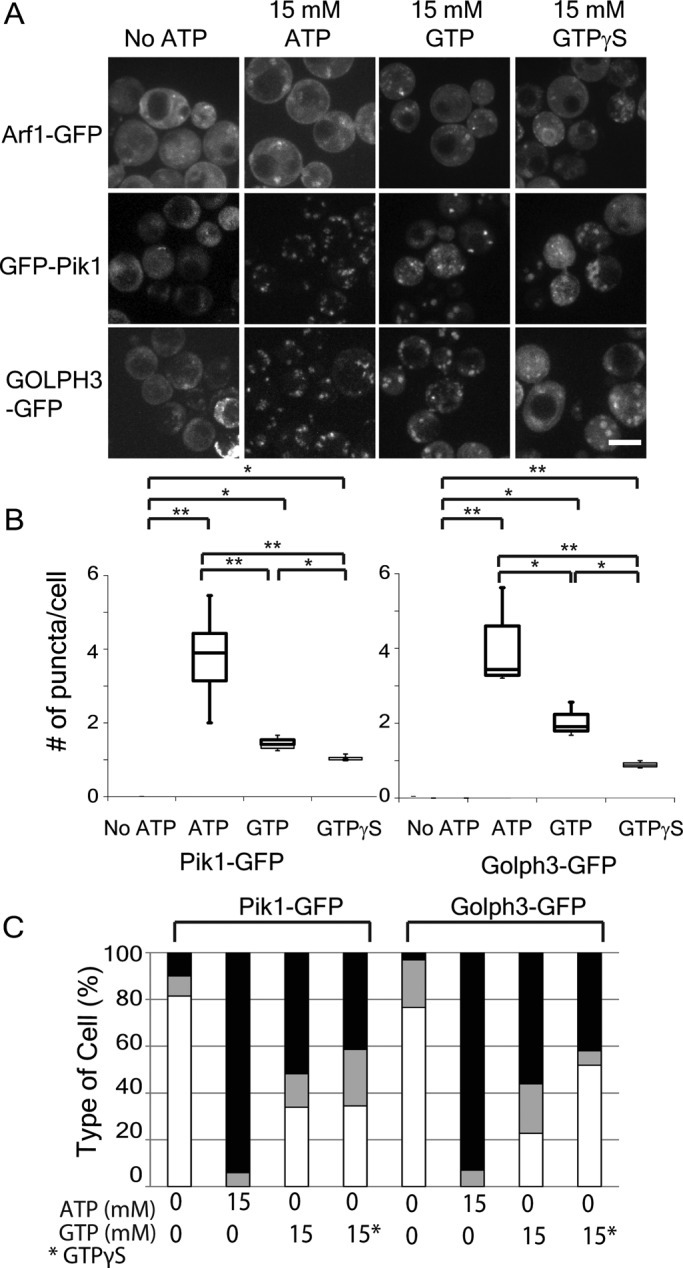
Arf1, Pik1, and PI4p show differential responses to exogenous nucleotides in permeabilized cells. (A) Diploid cells heterozygous for ARF1-GFP or haploid wild-type cells expressing GFP-Pik1 GOLPH3-GFP from plasmids were prepared as described in Figure 6A and imaged before or after addition of 15 mM indicated nucleotides. Scale bars, 5 μm. (B) Quantification of puncta per cell. **p < 0.01; *p < 0.05; n.s., not significant. Charts show box plot of data as described in Figure 1. (C) Quantification of adaptor localization in different conditions. Cells were classified as in Figure 7. Black portion of the bars indicates percentage of cells with large puncta; gray indicates small puncta; and white indicates no puncta.
We next investigated the effects of GTP and GTP-γ-S on Arf1, Pik1, and PI4p (Figure 9A). As with ATP, the localization of Arf1 was not substantially increased by either GTP or GTP-γ-S. In contrast, both Pik1 and GOLPH3 localized to puncta after the addition of GTP. However, the number of puncta per cell induced by GTP was less than the number induced by ATP (Figure 9B). GTP-γ-S was even less effective at recruiting Pik1 and GOLPH3 than was GTP. Both Pik1 and GOLPH3 formed fewer puncta in the presence of GTP-γ-S than in GTP. Furthermore, the number of cells with large puncta was lower in the presence of GTP-γ-S than in GTP (Figure 9C). Taken together, these results show that Arf1 localization is largely nonresponsive to nucleotides. In contrast, Pik1 and GOLPH3 localization is induced by ATP, partially by GTP, and weakly by GTP-γ-S.
Arf1 and PI4p are required for adaptor localization during glucose starvation
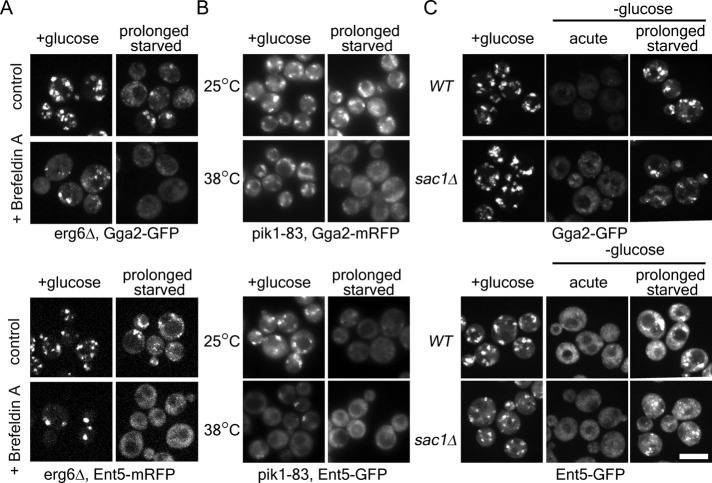
To investigate whether adaptor localization depends on Arf, Pik1, and PI4p during glucose starvation, we first tested whether Arf is required for adaptor localization in glucose starvation. To rapidly inhibit Arf function, we used the lactone antibiotic brefeldin A. Brefeldin A inhibits several Arf guanine nucleotide exchange factors (GEFs). Inhibition of these GEFs leads to rapid loss of active Arf at select locations (reviewed in Jackson and Casanova, 2000). In cells treated with brefeldin A in the presence of glucose, both Gga2 and Ent5 localized to a few large puncta per cell (Figure 10A and Supplemental Figure S2). This suggests that some Gga2 and Ent5 structures are independent of a brefeldin A–sensitive GEF in the presence of glucose. In contrast, during prolonged starvation both Gga2 and Ent5 became diffuse within minutes of brefeldin A treatment (Figure 10A). Thus the localization of both Gga2 and Ent5 depends on Arf during glucose starvation.
FIGURE 10:
Arf1, Pik1, and Sac1 modulate adaptor localization during glucose starvation. (A) Brefeldin A induces adaptor redistribution only during glucose starvation. erg6∆ cells expressing Gga2-GFP or Ent5-mCherry were imaged before or after 2 h of glucose starvation. Cells were treated with DMSO (control) or 150 μM brefeldin A for 5 min before imaging. (B) Adaptors show increased dependence on Pik1 during glucose starvation. pik1-83 cells expressing Gga2-mRFP or Ent5-GFP were imaged before or after 2 h of glucose starvation at the permissive temperature (25°C) or after a 30-min shift to nonpermissive (38°C) temperature. For temperature-shifted starved cells, cells were first starved for 1.5 h and then shifted to the nonpermissive temperature. (C) Gga2 but not Ent5 requires Sac1 for rapid redistribution during acute starvation. Wild-type and sac1∆ cells expressing Gga2-GFP or Ent5-GFP were imaged before or after 2 h of glucose starvation. Scale bar, 5 μm.
We next investigated whether Pik1 is required for adaptor localization during glucose starvation. To modulate Pik1 activity, we used a previously described temperature-sensitive pik1-83 allele (Hendricks et al., 1999). As previously described, in the presence of glucose, the pik1-83 allele did not alter Gga2 localization (Figure 10B and Supplemental Figure S2; Daboussi et al., 2012). In contrast, Gga2 became diffuse when cells were shifted to the nonpermissive temperature during prolonged starvation. These results suggest that Gga2 localization requires Pik1 activity during starvation.
Ent5 localization was highly dependent on Pik1 activity under all conditions tested (Figure 10B and Supplemental Figure S2). In the presence of glucose, Ent5 became diffuse upon shift to the nonpermissive temperature, confirming previous results that Ent5 requires Pik1 for localization (Daboussi et al., 2012). Of note, even at permissive temperature, Ent5 did not localize to bright puncta in the pik1-83 cells during prolonged glucose starvation. This result suggests that pik1-83 is not fully functional in glucose-starved cells and that Ent5 requires full Pik1 activity for localization during glucose starvation. Taken together, these results suggest that Ent5 localization depends on Pik1 activity both in the presence and in the absence of glucose.
PI4p levels are also regulated by the lipid phosphatase Sac1, which redistributes to the TGN during glucose starvation (Faulhammer et al., 2005, 2007). To investigate the role of Sac1 relocalization in adaptor localization during glucose starvation, we monitored Gga2 and Ent5 localization in cells lacking Sac1 (Figure 10C and Supplemental Figure S2). In these cells, Gga2 mostly became diffuse after glucose starvation, although some dim Gga2 puncta persisted. Such dim puncta were not seen in wild-type cells. In contrast, Ent5 became diffuse immediately after glucose starvation and then relocalized to bright puncta during prolonged starvation. These results suggest that Sac1 has a minor role in Gga2 relocalization but not Ent5 during glucose starvation.
DISCUSSION
Glucose repression machinery regulates adaptors indirectly
Glucose starvation causes immediate and transient responses in several cellular processes (Ashe et al., 2000; Uesono et al., 2004; Aoh et al., 2011). Until now, how the cell communicated glucose starvation to induce these transient responses was unknown. In this study, we characterized cellular energy as the mechanism leading to the transient delocalization of clathrin adaptors during glucose starvation. Our previous findings that PKA regulates adaptor localization in glucose starvation are explained by its role in cellular metabolism. Previously, we found that inhibition of PKA prevented adaptor redistribution in response to glucose starvation (Aoh et al., 2011). However, to alter adaptor redistribution, PKA had to be inhibited for an extended period before starvation. The need for extended inhibition suggested that PKA acts indirectly. Based on our present findings, the extended PKA inhibition allows glucose derepression and metabolic remodeling to occur even under glucose-replete conditions. Therefore ATP concentration does not drop low enough upon glucose starvation to cause adaptor delocalization. The role of Snf1 is similarly indirect. The delay between Snf1 activation and adaptor relocalization can be explained by the role of Snf1 in activation of respiration. Adaptors fail to relocalize in cells lacking Snf1 because, in the absence of both glycolysis and respiration, ATP concentration remain low. Thus the unknown “factor” that regulates adaptor localization downstream of PKA and Snf1 is mitochondrial function.
Energy regulates adaptor localization at multiple steps
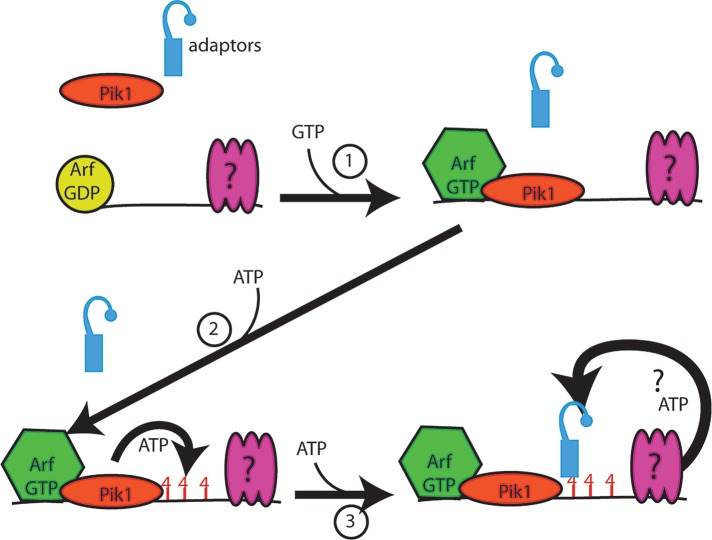
A multistep model of adaptor recruitment emerges from these studies (Figure 11). Our present findings are consistent with these steps occurring concurrently or in various orders. We propose that Arf acts early. Our finding that a 5-min treatment with brefeldin A eliminates adaptor localization during glucose starvation argues that Arf plays a key role in the process. However, Arf1 localization is not biphasic in response to glucose starvation and is unaffected by either ATP or GTP. This suggests that Arf localization is not regulated by energy levels. This finding is consistent with the ability of purified Arf1-GDP to associate with membranes (Liu et al., 2009). We propose that during glucose starvation a small pool of Arf remains associated with membranes; when energy levels rise, it is already positioned to help recruit adaptors.
FIGURE 11:
Model of energy-dependent steps in adaptor recruitment. The steps illustrated may occur concurrently or in a different sequence than illustrated. Before energy input, Arf is localized to membranes, but Pik1 and adaptors are cytosolic. 1) On availability of GTP, Pik1 becomes localized and activated. 2) On availability of ATP, Pik1 synthesizes PI4p; however, this is not sufficient for adaptor recruitment. 3) Adaptors are recruited only when a third, uncharacterized factor has access to sufficient ATP. All three activities—Arf1, Pik1 and this third factor—are required for localization of adaptors to membranes during glucose starvation.
A subsequent step is the Arf-GTP–dependent recruitment and activation of Pik1 (Figure 11, step 1). In permeabilized cells, we find that GTP induces robust recruitment of Pik1 in permeabilized cells. Although we do not see localization of GFP-Pik1 at membranes in starved cells, the requirement for Pik1 in intact cells strongly argues that Pik1 is active at the TGN and endosomes in glucose-starved cells. This apparent contradiction may be explained by competition from endogenous untagged Pik1 in the strains used. We propose that Arf1 likely helps to recruit and activate this pool of Pik1. This role is supported by reduced levels of PI4p in cells lacking Arf1 (Audhya et al., 2000) and the GTP responsiveness of Pik1 localization in permeabilized cells.
The next step is the ATP-dependent synthesis of PI4p (Figure 11, step 2). In permeabilized cells, GTP induces GOLPH3 recruitment. We propose that this reflects limited synthesis of PI4p by the GTP-activated Pik1 using the residual ATP in the permeabilized cells. Of importance, although GTP induces PI4p synthesis, it is not sufficient for adaptor recruitment in the absence of additional ATP. This suggests the existence of an additional ATP-dependent step (Figure 11, step 3). If Arf activation and PI4p synthesis were the only two processes required, we would expect substantial adaptor recruitment in cells treated with 15 mM GTP; in contrast, we see only minor changes in adaptor recruitment in GTP-treated permeabilized cells. The lack of adaptor recruitment in 15 mM GTP suggests the existence of an additional ATP-dependent factor. Alternatively, adaptor recruitment may require a very high concentration of PI4p not achieved with GTP addition. Although our present analysis cannot distinguish between these two alternatives, additional ATPases are implicated in clathrin function in replete cells (Maranda et al., 2001; Liu et al., 2008a). These ATPases—Drs2 and the V-ATPase—could constitute additional ATP-dependent requirements during glucose starvation. It also remains to be determined which of these steps precisely coordinates adaptor recruitment with energy levels in intermediate starvation conditions and how Arf1 localization is regulated by starvation.
Glucose starvation changes adaptor localization requirements
This study also reveals mechanistic changes in clathrin-dependent traffic during glucose starvation. We found that during glucose starvation, adaptor localization is more dependent on both Arf and Pik1 than it is in the presence of glucose. The increased importance of Arf and Pik demonstrates that the mechanistic underpinnings of clathrin-dependent traffic differ dramatically between glucose-replete and glucose-starved cells.
The mechanistic change in adaptor recruitment may be functionally important to the cell during glucose starvation. In the presence of glucose, the cell can afford to be wasteful if the wasteful process provides a competitive advantage. The cell synthesizes membrane proteins that it cannot use, only to degrade them in the vacuole. For example, when no siderophores are available, the cell makes siderophore transporters and then degrades them in the vacuole (Kim et al., 2007). Presumably, this process provides a competitive advantage in the wild through providing an enhanced ability to exploit a rare encounter with scarce siderophores. In the presence of glucose, the cell also allows waste via nonselective traffic. Instead of actively retaining proteins within an organelle, the cell relies on numerous retrieval systems to maintain steady-state localization of proteins in their correct localization (Black and Pelham, 2000). The changes in the mechanism of adaptor recruitment during prolonged glucose starvation likely reduces overall traffic rates to coincide with lower traffic volumes due to reduced synthesis of unneeded transmembrane proteins. This reduction in overall rates further reduces the volume of proteins requiring retrieval systems. The increased requirement for both Arf and Pik1 may be the mechanism that ensures that the reduction in overall traffic rates coincides with reduced volume. We can only speculate on whether the changes in adaptor recruitment mechanisms also act in increased surveillance to prevent the nonselective transport of cargo.
The regulation of adaptor localization by ATP explains how endosomal traffic rates are coordinated with available cellular energy. The productive traffic of proteins via clathrin-dependent pathways requires a large amount of ATP (Braell et al., 1984). Of importance, much of this energy is consumed in the disassembly of the clathrin coat, which must occur before fusion of the transport carrier with the target membrane (Bocking et al., 2011). By linking adaptor recruitment to available energy, the cell stops coat assembly at the first step and thus limits the amount of ATP consumed by clathrin-dependent traffic until energy levels rise. This mechanism preserves limited supplies of ATP for more critical activities such as reactivating the mitochondria. This regulatory mechanism is extremely rapid and reversible and, as our results in Figure 5 indicate, can tune traffic rates precisely with cellular ATP levels.
Energy appears to regulate diverse processes coordinately in yeast. In addition to endosomal traffic, translation initiation and cell polarity also transiently stop during glucose starvation and are dependent on the glucose repression machinery for this inhibition. These similarities suggest that these processes are coordinately regulated (Ashe et al., 2000; Uesono et al., 2004). However, at intermediate starvation levels, the cell appears to differentially regulate endosomal traffic and translation initiation. Translation initiation is strongly inhibited when cells are transferred to 0.5% glucose (Castelli et al., 2011). In contrast, we did not observe dramatic changes in adaptor localization in cells transferred to 0.5% glucose (unpublished data). The inhibition of translation initiation at intermediate concentrations of glucose allows the cell to prepare for imminent starvation (Castelli et al., 2011). The higher sensitivity of translation initiation to glucose depletion could reflect an additional, non–ATP-dependent regulation of translation initiation, or, alternatively, in cells cultured at 0.5% glucose, ATP levels may be reduced (Polakis and Bartley, 1966b, a; Wilson et al., 1996). Regardless of the mechanism of translation inhibition at intermediate levels, it is clear that the cell uses energy metabolism as a global tuning mechanism for endosomal traffic and likely for cell polarity and translation.
The ATP concentration–dependent changes in endosomal traffic are particularly striking. Intracellular ATP concentrations have long been correlated with growth rates in eukaryotic and prokaryotic cells (Crouch et al., 1993). Furthermore, metabolism alone can reprogram a differentiated cell to adopt stem cell characteristics or drive a stem cell to differentiate (Zhang et al., 2011). Our results suggest that metabolism, acting through endosomal traffic, may globally regulate cell behavior. Global changes in endosomal traffic would enhance or suppress the signaling and proliferating capacity of the cell by altering the protein content of the plasma membrane and endosomal organelles. Such a role for metabolic regulation in endosomes of multicellular organisms is yet to be explored.
MATERIALS AND METHODS
Yeast strains and plasmids
Yeast strains, sources, and plasmids are listed in Table 1 (Robinson et al., 1988; Liu et al., 2008b; Stephan et al., 2009; Wood et al., 2009). Fluorescent tags and gene deletions were introduced by standard PCR-based methods (Longtine et al., 1998). Strains containing multiple mutations were generated by standard yeast genetics. The GFP-GOLPH3 probe was graciously provided by Chris Burd (University of Pennsylvania, Philadelphia, PA). To construct the GFP-Pik1 plasmid pQA76, the genomic promoter and open reading frame (ORF) of Pik1 were amplified and ligated into the Kpn1/Sac1 site of pRS316. The ORF for GFP was amplified with flanking HindIII sites and ligated into a HindIII site in the N-terminus of the Pik1 ORF.
TABLE 1:
Strains and plasmids.
| Strain or plasmid | Description | Source |
|---|---|---|
| BY4741 | mat a his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | Invitrogen |
| BY4742 | mat α his3Δ1 leu2Δ0 ura3D0 lys2Δ0 | Invitrogen |
| DLY003 | BY4742 ENT5-GFP(S65T)-HIS3MX | Aoh et al. (2011) |
| DLY004 | BY4742 GGA2-GFP(S65T)-KANMX6 | Aoh et al. (2011) |
| DLY066 | BY4742 APL4-GFP(S65T)-KANMX6 | This study |
| Y1375 | Mat a ade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1 tpk2::KAN tpk3::TRP1 tpk1-M164G | Stephan et al. (2009) |
| DLY37 | BY4741 SEC7-MChERRY::HisMX ENT5-GFP::KANMX6 | This study |
| DLY067 | BY4742 reg1∆::KANMX4 | Invitrogen |
| DLY068 | BY4742 reg1∆::KANMX4 ENT5-GFP(S65T)-KANMX6 | This study |
| DLY070 | BY4742 snf1∆::HIS3MX | This study |
| DLY071 | BY4741 snf1∆::HIS3MX ENT5-GFP(S65T)-KANMX6 | This study |
| DLY072 | BY4742 snf1∆::HIS3MX GGA2-GFP (S65T)-KANMX6 | This study |
| DLY080 | Mat a/α his3∆1/his3∆1 leu2∆0/leu2∆0 met15∆0/met15∆0 LYS2/lys2∆0 ura3∆0/ura3∆0 GGA2-MCHERRY-KANMX/GGA2-MCHERRY-KANMX ARF1-GFP(S65T)-HIS3MX | This study |
| DLY100 | BY4742 gal83∆::KANMX4 | Invitrogen |
| DLY101 | BY4742 sak1∆::KANMX4 | Invitrogen |
| DLY102 | BY4742 Snf1-myc::His3MX::URA | This study |
| QAY632 | BY4742 ENT5-GFP(S65T)-KANMX6, KEX2-MCHERRY-HIS3MX | This study |
| QAY667 | BY4741 GGA2-GFP(S65T)-KANMX6 | This study |
| QAY768 | BY4741 SEC7-MCHERRY::HIS33MX APL4-GFP::KAMMX6 | This study |
| QAY769 | BY4742 SEC7-MCHERRY::HIS33MX GGA2-GFP::KAMMX6 | This study |
| QAY922 | BY4742 bmh1Δ-KANMX6 ENT5-GFP(S65T)-HIS3MX | This study |
| QAY1170 | Mat a/α his3∆1/his3∆1 leu2∆0/leu2∆0 met15∆0/MET15 LYS2/lys2∆0 ura3∆0/ura3∆0 ENT5-MCHERRY-KANMX/ENT5-MCHERRY-KANMX ARF1-GFP(S65T)-HIS3MX/ARF1 | This study |
| QAY1241 | BY4842 erg6Δ::KANMX GGA2-GFP(S65T)-HIS3MX | This study |
| QAY1244 | Mat α his3Δ1 leu2Δ0 ura3Δ0 met15Δ0erg6Δ::KANMX ENT5-MCHERRY-HIS3MX | This study |
| SEY6210 | Mat α ura3-52 lys2-801 ∆his3-200 leu2-3112, trp1-Δ901 | Robinson et al. (1988) |
| GPY404 | Mat a ura3-52 lys2-801 ∆his3-200 leu2-3112, trp1-Δ901 | Daboussi et al. (2012) |
| CTY244 | Mat a ura3-52 lys2-801 ∆his3-200 sac1∆1-354::HIS3 | Liu et al. (2008) |
| DLY 074 | CTY244 GGA2-GFP (S65T)-HIS3MX::URA | This study |
| DLY 075 | CTY244 ENT5-GFP(S65T)-HIS3MX::URA | This study |
| MDY655 | SEY6210 GGA2-GFP(S65T)-His3Mx::URA3 | Hung et al. (2012) |
| MDY399 | SEY6210 ENT5-GFP(S65T)-His3Mx::URA3 | Hung et al. (2012) |
| GPY4941 | GPY404 GGA2-mRFP::KANMX6 ENT5-GFP(S65T)::KANMX6 pik1Δ::HIS3 pRS314-pik1-83ts (TRP1 CEN6 pik1-83ts) | Daboussi et al. (2012) |
| GPY4940 | GPY404 GGA2-mRFP::KANMX6 ENT3-GFP(S65T)::KANMX6 pik1Δ::HIS3 pRS314-pik1-83ts (TRP1 CEN6 pik1-83ts) | Daboussi et al. (2012) |
| Y1375 | Mat a ade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1 tpk2::KAN tpk3::TRP1 tpk1-M164G | Stephan et al. (2009) |
| pCW26 | GFP-GOLPH3 | Wood et al. (2009) |
| pQA76 | pRS316-GFP-Pik1 | This study |
Media, antibodies, and reagents
Yeast cells were grown in yeast/peptone (YP) or supplemented synthetic media (SM) in the presence or absence of 2% glucose (dextrose), 2% galactose, 2% raffinose, or 2% glycerol/3% ethanol. Supplemented SM without dextrose was used for all starvation experiments. Yeast/peptone/dextrose (YPD) media is 1% bacto-yeast extract (Difco, Detroit, MI) and 2% bacto-peptone (Difco) supplemented with 2% dextrose and 20 μg/ml adenine, uracil, and l-tryptophan. Synthetic media with dextrose (SD) is 0.67% of yeast nitrogen base without amino acids (Difco) and 2% dextrose. SD media was supplemented with 100 μg/ml adenine, l-leucine, l-lysine, l-tryptophan; 50 μg/ml l-histidine and l-methionine; and 20 μg/ml uracil. For cells expressing GFP-GOLPH or GFP-Pik1, uracil was omitted to maintain plasmid selection. The following antibodies were used: anti-myc (UNC Immunology Core Facility, Chapel Hill, NC), anti–phospho AMPK (Cell Signaling, Danvers, MA), and anti-Ent5 (Aoh et al. 2011). Alexa Fluor secondary antibodies were from Invitrogen (Carlsbad, CA). Other reagents were obtained as follows: antimycin, ATP, ATP-γ-S, GTP, GTP-γ-S, and DMSO from Sigma-Aldrich (St. Louis, MO) and 1NM-PP1 from Calbiochem (San Diego, CA).
Immunoblotting
For whole-cell extracts to analyze most proteins, 2 OD600 of cells were resuspended in Laemmli sample buffer, boiled, and subjected to glass-bead disruption. The extracts were cleared by centrifugation. After SDS–PAGE, samples were transferred to nitrocellulose, blocked with 4% milk in Tris-buffered saline with Tween, and then probed with primary and fluorescent secondary antibodies. Fluorescence signals were detected on a Typhoon imaging system (Amersham Biosciences, Piscataway, NJ). For analysis of Snf1 activation, extractions were performed as described by Orlova et al. (2008). For kinase deletion library screen, individual cultures of each strain were grown to mid–logarithmic phase in SM with 2% glucose, washed three times into starvation media, and cultured overnight. Lysates were generated and processed as described.
Growth conditions and carbon starvation
For glucose starvation, yeast cells were grown overnight in YPD or SD media at 30°C and aerated by rotary shaking and then diluted into SD media and grown another 4–6 h to mid–log phase, as described previously (Aoh et al., 2011). To remove the glucose, the cells were washed three times with supplemented synthetic media and then sampled immediately for acute starvation or after 1–3 h for prolonged starvation. For assays of cells in different carbon sources, the cells were first preadapted to the carbon source by growing overnight in YPD, diluted into YP with 2% galactose, 2% raffinose, or 2% glycerol/3% ethanol, and grown overnight again. The cells were then diluted the next day and grown to mid–log phase (OD600 of ∼0.2–0.5). The cells were then starved as described. Antimycin A or an equivalent amount of vehicle (ethanol) was added to a final concentration of 2 μg/ml as indicated. For experiments using the tpk1-as tpk2∆ tpk3∆ strain, cells were grown in YPD as described and PKA was inhibited by incubating the cells with 2 μM 1NM-PP1 or an equivalent amount of DMSO after growth to mid–log phase for 1 h before starvation. To examine the effect of brefeldin A on adaptor localization, erg6Δ cells were grown in SD as described and brefeldin A was added to a final concentration of 150 μM for 5min before imaging at the indicated times. For experiments in pik1-83 cells, cells were grown overnight in YPD at room temperature on a rotary shaker and then diluted the next day into supplemented SD media and grown another 4–6 h at room temperature on a rotary shaker. The cells were shifted to the nonpermissive temperature by incubating them at 38°C with mixing in a heat block for 30 min.
Live microscopy, image processing, and quantitation
For carbon starvations, the cells were grown as described and then briefly pelleted and resuspended in 50–200 μl media before imaging. For experiments using antimycin A, the cells were grown as described and antimycin A was added for 5 min before imaging. Cells were mounted onto a clear coverslip and then imaged. For each field, Z-stack images were captured using a 100× oil objective (numerical aperture 1.4) on a spinning-disk confocal or epifluorescent microscope, as described previously (Aoh et al., 2011). The number of puncta per cells was quantitated by counting the number of foci in a single Z-stack from the middle of a cell (n > 40) for each condition specified. In micrographs of intact cells, Z-stacks were compressed into a single maximum-intensity image in ImageJ (National Institutes of Health, Bethesda, MD). Statistical significance was determined using a two-tailed Mann–Whitney U test for the null hypothesis that the medians were equal. For TIRF analysis, permeabilized cells were fixed using 4% paraformaldehyde for 15 min at room temperature. Cells were then washed three times with phosphate-buffered saline. Samples were mounted in Aqua Poly/Mount (Polysciences, Warrington, PA) and allowed to set at 4°C overnight before imaging. TIRF was performed using on a Nikon Eclipse Ti (Nikon, Melville, NY) using a 100× oil objective (numerical aperture 1.49). Image collection was on a Roper Scientific Cascade 512B camera (Roper, Tucson, AZ). We used 488- and 561-nm lasers to excite GFP and mCherry, respectively. The exposure time for both GFP and mCherry was 500 ms.
ATP measurements
ATP measurements were performed as described by Ashe et al. (2000). To measure ATP concentration in the presence of different carbons, cells were grown and washed as described, and ∼1 OD of cells was collected by centrifugation in the presence of the carbon source or 15 min after starvation. To measure ATP concentration in the presence of antimycin, the cells were grown in supplemented SD with 2% galactose as described and then starved for 30 min. Serially diluted antimycin A or an equivalent amount of vehicle (ethanol) was added to the cells for 5 min before collection. For all experiments, the cells were then resuspended in 2.5% trichloroacetic acid. The OD of the cells was measured. The cells were then diluted 1:20 with 25 mM Tris, pH 9.4. From 5 to 10 μl of the samples was assayed using the ATP Determination Kit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Luminescence was measured at 570 nm by using a SpectraMax Luminescence L microplate reader. Duplicate samples were taken for each condition, and the experiments were performed in triplicate. Relative ATP concentrations are expressed as the average percentage of control samples. Statistical significance was determined with a two-tailed Student's t test. Data are reported as ATP, with cell concentrations normalized to OD600.
Permeabilized cell assays
To generate permeabilized cells, ∼50 OD600 of cells grown to mid–log phase were pelleted and resuspended in supplemented SD media with 100 mM Tris-SO4 (pH 9.4) and 10 mM dithiothreitol (DTT) for 10 min at 30°C. The cells were then washed twice with supplemented SM and then incubated in supplemented SM with 1 M sorbitol, 10 mM Tris-HCl (pH 7.4), 2 μg/ml antimycin A, and 120 μg/ml lyticase for 15 min at 30ºC. To permeabilize the cells, the cells were pelleted and resuspended in 0.2 M sorbitol, 50 mM potassium acetate, 2 mM EDTA, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 6.0), 1 mM DTT, and protease inhibitor cocktail (Sigma-Aldrich). The cells were then dounced 20 times with a tight-fitting pestle. Cells were kept on ice for no more than 2 h before imaging. For the nucleotide treatments, nucleotides were added to the permeabilized cells and then incubated at room temperature without any agitation for 5 min before imaging.
Supplementary Material
Acknowledgments
We gratefully acknowledge A. P. Joglekar, K. Bloom, E. D. Salmon, and K. C. Slep for access to and help with microscopes used in this study; G. S. Payne, C. Burd, and V. Bankaitis for reagents; J. Y. Y. Martinez-Marquez, D. Buelto, and M. J. Lang for generating strains used in this study; and R. J. Duronio and A. P. Joglekar for comments and suggestions on the manuscript. This work was supported by National Institutes of Health Research Grant GM-092741 to M.C.D.
Abbreviations used:
- AMPK
AMP-activated kinase
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- mRFP
monomeric red fluorescent protein
- PKA
protein kinase A
- TGN
trans-Golgi network
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-10-0750) on January 23, 2013.
REFERENCES
- Aoh QL, Graves LM, Duncan MC. Glucose regulates clathrin adaptors at the TGN and Endosomes. Mol Biol Cell. 2011;22:3671–3683. doi: 10.1091/mbc.E11-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe MP, De Long SK, Sachs AB. Glucose depletion rapidly inhibits translation initiation in yeast. Mol Biol Cell. 2000;11:833–848. doi: 10.1091/mbc.11.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Foti M, Emr SD. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol Biol Cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocking T, Aguet F, Harrison SC, Kirchhausen T. Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating. Nat Struct Mol Biol. 2011;18:295–301. doi: 10.1038/nsmb.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA, Schlossman DM, Schmid SL, Rothman JE. Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol. 1984;99:734–741. doi: 10.1083/jcb.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Osmond BC, Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981;98:25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli LM, et al. Glucose depletion inhibits translation initiation via eIF4A loss and subsequent 48S preinitiation complex accumulation, while the pentose phosphate pathway is coordinately up-regulated. Mol Biol Cell. 2011;22:3379–3393. doi: 10.1091/mbc.E11-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DS, Pishvaee B, Payne GS. A modulatory role for clathrin light chain phosphorylation in Golgi membrane protein localization during vegetative growth and during the mating response of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:713–726. doi: 10.1091/mbc.10.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G, Duncan MC, Fernandez GE, Huang GH, Payne GS. Distinct roles for TGN/endosome epsin-like adaptors Ent3p and Ent5p. Mol Biol Cell. 2006;17:3907–3920. doi: 10.1091/mbc.E06-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta G, Stefan CJ, Bensen ES, Emr SD, Payne GS. Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol Biol Cell. 2001;12:1885–1896. doi: 10.1091/mbc.12.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat Cell Biol. 2012;19:239–248. doi: 10.1038/ncb2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche O, Yeung BG, Payne GS, Schekman R. Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol Biol Cell. 2001;12:475–485. doi: 10.1091/mbc.12.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmel L, et al. Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase Pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol Biol Cell. 2008;19:1046–1061. doi: 10.1091/mbc.E07-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek KM, Kacherovsky N, Young ET. The Reg1-interacting proteins, Bmh1, Bmh2, Ssb1, and Ssb2, have roles in maintaining glucose repression in Saccharomyces cerevisiae. J Biol Chem. 2004;279:39165–39174. doi: 10.1074/jbc.M400433200. [DOI] [PubMed] [Google Scholar]
- Duncan MC, Payne GS. ENTH/ANTH domains expand to the Golgi. Trends Cell Biol. 2003;13:211–215. doi: 10.1016/s0962-8924(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Faulhammer F, Kanjilal-Kolar S, Knodler A, Lo J, Lee Y, Konrad G, Mayinger P. Growth control of Golgi phosphoinositides by reciprocal localization of sac1 lipid phosphatase and pik1 4-kinase. Traffic. 2007;8:1554–1567. doi: 10.1111/j.1600-0854.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- Faulhammer F, Konrad G, Brankatschk B, Tahirovic S, Knodler A, Mayinger P. Cell growth-dependent coordination of lipid signaling and glycosylation is mediated by interactions between Sac1p and Dpm1p. J Cell Biol. 2005;168:185–191. doi: 10.1083/jcb.200407118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GE, Payne GS. Laa1p, a conserved AP-1 accessory protein important for AP-1 localization in yeast. Mol Biol Cell. 2006;17:3304–3317. doi: 10.1091/mbc.E06-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Rutter J. The role of PAS kinase in PASsing the glucose signal. Sensors (Basel) 2010;10:5668–5682. doi: 10.3390/s100605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–2420. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CW, Aoh QL, Joglekar AP, Payne GS, Duncan MC. Adaptor autoregulation promotes coordinated binding within the clathrin coat. J Biol Chem. 2012;287:17398–173407. doi: 10.1074/jbc.M112.349035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 2000;10:60–67. doi: 10.1016/s0962-8924(99)01699-2. [DOI] [PubMed] [Google Scholar]
- Johnston M. Feasting, fasting and fermenting. Glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M. Systematic identification of pathways that couple cell growth and division in yeast. Science. 2002;297:395–400. doi: 10.1126/science.1070850. [DOI] [PubMed] [Google Scholar]
- Kim Y, Deng Y, Philpott CC. GGA2- and ubiquitin-dependent trafficking of Arn1, the ferrichrome transporter of Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:1790–1802. doi: 10.1091/mbc.E06-09-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe ML, Wallet V, Troll H, Veron M. Functional cloning of a nucleoside diphosphate kinase from Dictyostelium discoideum. J Biol Chem. 1990;265:10012–10018. [PubMed] [Google Scholar]
- Liu K, Surendhran K, Nothwehr SF, Graham TR. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol Biol Cell. 2008a;19:3526–3535. doi: 10.1091/mbc.E08-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Boukhelifa M, Tribble E, Morin-Kensicki E, Uetrecht A, Bear JE, Bankaitis VA. The Sac1 phosphoinositide phosphatase regulates Golgi membrane morphology and mitotic spindle organization in mammals. Mol Biol Cell. 2008b;19:3080–3096. doi: 10.1091/mbc.E07-12-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kahn RA, Prestegard JH. Structure and membrane interaction of myristoylated ARF1. Structure. 2009;17:79–87. doi: 10.1016/j.str.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Banting G. Eukaryotic membrane traffic: retrieval and retention mechanisms to achieve organelle residence. Trends Biochem Sci. 1993;18:395–398. doi: 10.1016/0968-0004(93)90097-7. [DOI] [PubMed] [Google Scholar]
- Maranda B, Brown D, Bourgoin S, Casanova JE, Vinay P, Ausiello DA, Marshansky V. Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J Biol Chem. 2001;276:18540–18550. doi: 10.1074/jbc.M011577200. [DOI] [PubMed] [Google Scholar]
- Mayer FV, et al. ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell Metab. 2011;14:707–714. doi: 10.1016/j.cmet.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova M, Barrett L, Kuchin S. Detection of endogenous Snf1 and its activation state: application to Saccharomyces and Candida species. Yeast. 2008;25:745–754. doi: 10.1002/yea.1628. [DOI] [PubMed] [Google Scholar]
- Ozalp VC, Pedersen TR, Nielsen LJ, Olsen LF. Time-resolved measurements of intracellular ATP in the yeast Saccharomyces cerevisiae using a new type of nanobiosensor. J Biol Chem. 2011;285:37579–37588. doi: 10.1074/jbc.M110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek AD, Mattoon JR. Regulation of energy metabolism in Saccharomyces cerevisiae. Relationships between catabolite repression, trehalose synthesis, and mitochondrial development. Arch Biochem Biophys. 1977;183:306–316. doi: 10.1016/0003-9861(77)90444-1. [DOI] [PubMed] [Google Scholar]
- Piskur J, Rozpedowska E, Polakova S, Merico A, Compagno C. How did Saccharomyces evolve to become a good brewer. Trends Genet. 2006;22:183–186. doi: 10.1016/j.tig.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Polakis ES, Bartley W. Changes in dry weight, protein, deoxyribonucleic acid, ribonucleic acid and reserve and structural carbohydrate during the aerobic growth cycle of yeast. Biochem J. 1966a;98:883–887. doi: 10.1042/bj0980883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis ES, Bartley W. Changes in the intracellular concentrations of adenosine phosphates and nicotinamide nucleotides during the aerobic growth cycle of yeast on different carbon sources. Biochem J. 1966b;99:521–533. doi: 10.1042/bj0990521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438:679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Xu X, Carlson M. Roles of two protein phosphatases, Reg1-Glc7 and Sit4, and glycogen synthesis in regulation of SNF1 protein kinase. Proc Natl Acad Sci USA. 2011;108:6349–6354. doi: 10.1073/pnas.1102758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Sutter BM, Ye X, Tu BP. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol Biol Cell. 2010;21:1982–1990. doi: 10.1091/mbc.E10-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci USA. 2009;106:17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, Voordeckers K. Functioning and evolutionary significance of nutrient transceptors. Mol Biol Evol. 2009;26:2407–2414. doi: 10.1093/molbev/msp168. [DOI] [PubMed] [Google Scholar]
- Uesono Y, Ashe MP, Toh EA. Simultaneous yet independent regulation of actin cytoskeletal organization and translation initiation by glucose in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:1544–1556. doi: 10.1091/mbc.E03-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills C. Regulation of sugar and ethanol metabolism in Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol. 1990;25:245–280. doi: 10.3109/10409239009090611. [DOI] [PubMed] [Google Scholar]
- Wilson WA, Hawley SA, Hardie DG. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- Wilson WA, Roach PJ. Nutrient-regulated protein kinases in budding yeast. Cell. 2002;111:155–158. doi: 10.1016/s0092-8674(02)01043-7. [DOI] [PubMed] [Google Scholar]
- Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009;187:967–975. doi: 10.1083/jcb.200909063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S, Lippman SI, Schneper L, Slonim N, Broach JR. Glucose regulates transcription in yeast through a network of signaling pathways. Mol Syst Biol. 2009;5:245. doi: 10.1038/msb.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.