Abstract
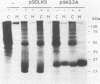
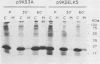
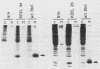
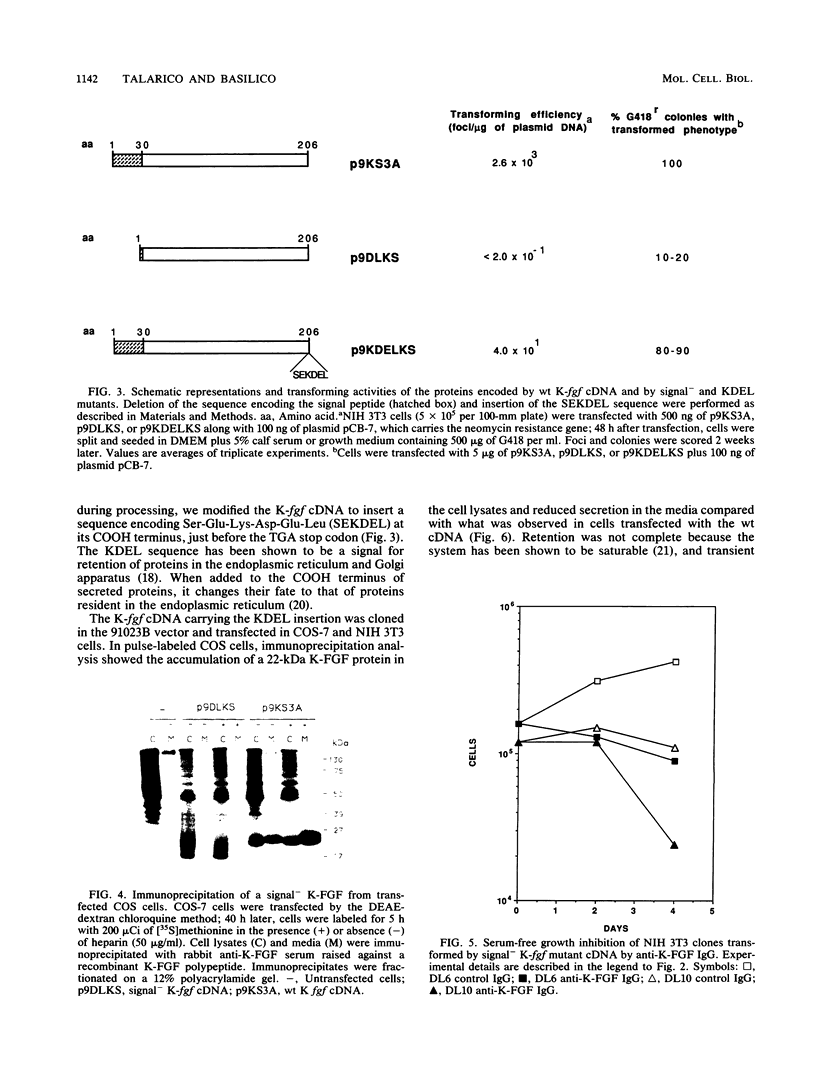
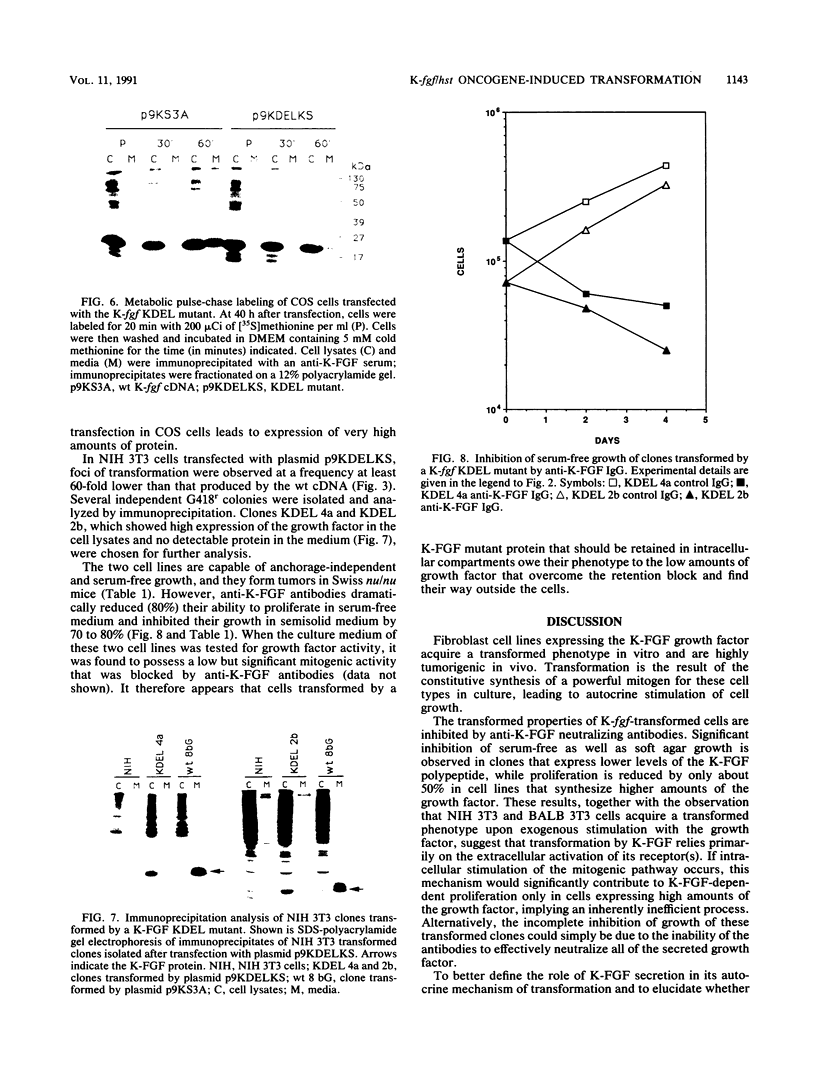
The K-fgf/hst oncogene encodes a secreted growth factor of the fibroblast growth factor (FGF) family. The ability of K-fgf-transformed cells to grow in soft agar and in serum-free medium is inhibited by anti-K-FGF neutralizing antibodies, consistent with an autocrine mechanism of transformation. The transformed properties of clones that express high levels of K-FGF are, however, only partially affected. To better define the autocrine mechanism of transformation by K-fgf and to determine whether receptor activation could occur intracellularly, we constructed two mutants of the K-fgf cDNA. Deletion of the sequences encoding the signal peptide suppressed K-fgf ability to induce foci in NIH 3T3 cells. A few morphologically transformed colonies were observed in cotransfection experiments, and they were found to express high levels of cytoplasmic K-FGF. However, their ability to grow in serum-free medium and in soft agar was inhibited by anti-K-FGF antibodies. Addition of a sequence encoding the KDEL endoplasmic reticulum and Golgi retention signal to the K-fgf cDNA led to accumulation of the growth factor in intracellular compartments. The ability of the KDEL mutant to induce foci in NIH 3T3 cells was much lower than that of the wild-type cDNA, and also in this case the transformed phenotype was reverted by anti-K-FGF antibodies. These and other findings indicate that the transformed phenotype of cells expressing a nonsecretory K-FGF is due to the extracellular activation of the receptor by the small amounts of growth factor that these cells still release. Thus, transformation by K-fgf appears to be due to an autocrine growth mechanisms that requires activation of the mitogenic pathway at the cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejcek B. E., Li D. Y., Deuel T. F. Transformation by v-sis occurs by an internal autoactivation mechanism. Science. 1989 Sep 29;245(4925):1496–1499. doi: 10.1126/science.2551043. [DOI] [PubMed] [Google Scholar]
- Blam S. B., Mitchell R., Tischer E., Rubin J. S., Silva M., Silver S., Fiddes J. C., Abraham J. A., Aaronson S. A. Addition of growth hormone secretion signal to basic fibroblast growth factor results in cell transformation and secretion of aberrant forms of the protein. Oncogene. 1988 Aug;3(2):129–136. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar C. E., Browder T. M., Abrams J. S., Nienhuis A. W. COOH-terminal-modified interleukin-3 is retained intracellularly and stimulates autocrine growth. Science. 1989 Sep 29;245(4925):1493–1496. doi: 10.1126/science.2789432. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Molloy C. J., Robbins K. C., Aaronson S. A. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Autocrine stimulation by the v-sis gene product requires a ligand-receptor interaction at the cell surface. J Cell Biol. 1988 Jul;107(1):287–298. doi: 10.1083/jcb.107.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin. Implications for the mechanism of autocrine transformation. J Biol Chem. 1988 Sep 5;263(25):12608–12618. [PubMed] [Google Scholar]
- Hébert J. M., Basilico C., Goldfarb M., Haub O., Martin G. R. Isolation of cDNAs encoding four mouse FGF family members and characterization of their expression patterns during embryogenesis. Dev Biol. 1990 Apr;138(2):454–463. doi: 10.1016/0012-1606(90)90211-z. [DOI] [PubMed] [Google Scholar]
- Jaye M., Lyall R. M., Mudd R., Schlessinger J., Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J. 1988 Apr;7(4):963–969. doi: 10.1002/j.1460-2075.1988.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kern F. G., Basilico C. Transcription from the polyoma late promoter in cells stably transformed by chimeric plasmids. Mol Cell Biol. 1985 Apr;5(4):797–807. doi: 10.1128/mcb.5.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Moscatelli D., Talarico D., Levytska V., Basilico C. A murine fibroblast growth factor (FGF) receptor expressed in CHO cells is activated by basic FGF and Kaposi FGF. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: reversal of morphological transformation and restoration of receptor number by suramin. J Cell Biol. 1989 Nov;109(5):2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Mitchell R., Ponte P., Gospodarowicz D. Expression of human basic fibroblast growth factor cDNA in baby hamster kidney-derived cells results in autonomous cell growth. J Cell Biol. 1988 Apr;106(4):1385–1394. doi: 10.1083/jcb.106.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada R., Kurokawa T., Iwane M., Igarashi K. Transformation of mouse BALB/c 3T3 cells with human basic fibroblast growth factor cDNA. Mol Cell Biol. 1988 Feb;8(2):588–594. doi: 10.1128/mcb.8.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico D., Ittmann M., Balsari A., Delli-Bovi P., Basch R. S., Basilico C. Protection of mice against tumor growth by immunization with an oncogene-encoded growth factor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4222–4225. doi: 10.1073/pnas.87.11.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Delli-Bovi P., Mansukhani A., Ziff E. B., Basilico C. Expression of the K-fgf protooncogene is repressed during differentiation of F9 cells. Oncogene Res. 1989;5(1):31–37. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]