Abstract
Lipid profiles in wheat leaves and the effects of tan spot on the profiles were quantified by mass spectrometry. Inoculation with Pyrenophora tritici-repentis significantly reduced the amount of leaf lipids, including the major plastidic lipids monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), which together accounted for 89% of the mass spectral signal of detected lipids in wheat leaves. Levels of these lipids in susceptible cultivars dropped much more quickly during infection than those in resistant cultivars. Furthermore, cultivars resistant or susceptible to tan spot displayed different lipid profiles; leaves of resistant cultivars had more MGDG and DGDG than susceptible ones, even in non-inoculated plants. Lipid compositional data from leaves of 20 non-inoculated winter wheat cultivars were regressed against an index of disease susceptibility and fitted with a linear model. This analysis demonstrated a significant relationship between resistance and levels of plastidic galactolipids and indicated that cultivars with high resistance to tan spot uniformly had more MGDG and DGDG than cultivars with high susceptibility. These findings suggest that lipid composition of wheat leaves may be a determining factor in the resistance response of cultivars to tan spot.
Introduction
Wheat is an important source of calories for humans and animals. In the U.S., wheat is consumed by humans in various products such as bread, pasta, and pizza. Worldwide wheat production in 2009 was estimated at 25 billion bushels and 2.2 billion bushels were produced in the U.S. Kansas produced 360 million bushels of wheat in 2010 (USDA, 2010) and over 400 million bushels in 12 of the last 33 years. However, exposure of wheat to abiotic stresses, such as drought, heat, and cold, can affect wheat production. Biotic agents, including insects, bacteria, and fungi, also reduce yields. Exposure to abiotic and biotic stresses can affect both wheat quality and quantity.
Tan spot, which is caused by the fungus Pyrenophora tritici-repentis (Died.) Drechsler, is one of the major foliar diseases of wheat worldwide (De Wolf et al., 1998), causing crop losses of up to 50% (Shabeer and Bockus, 1988; Singh and Hughes, 2005). Increases in disease incidence have been attributed to changes in cultural practices (Lamari and Bernier, 1989) such as shifts from conventional tillage to conservation- and zero-tillage, shorter crop rotations, continuous wheat cultivation, and the use of highly susceptible cultivars (Ciuffetti et al., 1998). The fungus overwinters as fruiting bodies called pseudothecia that develop on the previous season's infected wheat residue on and above the soil surface. Pseudothecia release sexual spores (ascospores) in the spring, inducing the first infections of the growing season. Asexual spores (conidia) are produced on crop residue and from leaf spots. Conidia are dispersed by wind and germinate to infect wheat in a wide range of temperatures, but infection requires continual leaf wetness for at least 6 hours (McMullen, 2010). During the growing season, many conidia can form in the lesions, serving as secondary inoculum to produce an epidemic (McMullen and Adhikari, 2009).
Tan spot produces two main phenotypic symptoms on wheat leaves, necrosis and chlorosis. These symptoms are induced by at least three host-specific toxins designated Ptr ToxA, Ptr ToxB, and Ptr Tox C (Strelkov and Lamari, 2003). These toxins are important in the tan spot/wheat pathosystem. The eight races of the fungus are classified based on virulence patterns, related to the putative production or non-production of these toxins, as deduced by effect of the fungi on a set of differential wheat lines/cultivars (Lamari and Strelkov, 2010). Ptr Tox A is the best characterized toxin and was the first to be isolated (Ballance et al., 1989; Tomas et al., 1990; Tuori et al., 1995). It is responsible for the necrotic symptom on sensitive wheat genotypes and is a 13.2-kDa protein encoded by the ToxA gene (Ballance et al., 1996; Ciuffetti et al., 1997). Ptr Tox B is also a small (6.6 kDa) protein molecule and is encoded by the ToxB gene; it induces chlorosis on sensitive wheat genotypes (Strelkov et al., 1998). Ptr Tox C also induces chlorosis but on different wheat lines/cultivars (Gamba et al., 1998). It is not proteinaceous like Ptr Tox A and Ptr Tox B but is a non-ionic, polar, low-molecular mass molecule (Effertz et al., 2002).
In successful infections, the fungal ascospores or conidia germinate by forming a germ tube under free moisture when they land on wheat leaves. The germ tube produces a penetration peg which facilitates penetration of the epidermal cell. Infection can be either direct or indirect, such as through stomata, with the penetration peg forming a vesicle inside the leaf. Intercellular fungal hyphae grow and expand among the epidermal and mesophyll cells. The toxins produced by P. tritici-repentis enter cells and induce damage of cellular organelles (Loughman and Deverall, 1986, http://www.apsnet.org/edcenter/intropp/lessons/fungi/ascomycetes/Pages/TanSpot.aspx). Manning and Ciuffetti (2005) suggest that Ptr ToxA is internalized in only sensitive wheat cultivars and, once internalized, it localizes to the chloroplasts. The Ptr ToxA protein is able to cross the plant plasma membrane from the apoplastic space to the interior of a plant cell, leading to cell death. Ciuffetti el al. (2010) report that Ptr ToxA leads to a light-dependent reactive oxygen species accumulation that correlates with the presence of necrosis and modifies photosystem I and photosystem II in the absence of light. In the development of chlorosis in response to Ptr ToxB, the chlorophyll molecule is photooxidized and illuminated thylakoid membranes become unable to dissipate excess excitation energy (Stelkov et al., 1998). Kim et al. (2010) report that Ptr ToxB inhibits photosynthesis in toxin-sensitive wheat lines and suggest that it induces alterations of the proteome level in host metabolism.
Lipids are often defined as biological compounds that are soluble in organic solvents (Buchanan et al., 2000; Voet et al., 2006). In practice, the term “lipids” also includes fatty acid-containing compounds, such as highly glycosylated glycolipids, that have limited solubility in organic solvents and excludes hydrophobic molecules, such as hydrophobic peptides, which can be otherwise classified. Lipids are important to plants for energy storage and as protective “waxy” surfaces on cells and plant leaves, stems, and roots (Graham et al., 2003). They form cell membranes, including those in which photosynthesis takes place, and serve as signal transduction messengers that influence plant growth, development, and response to stress (Shah, 2005). Polar glycerolipids are an important group of membrane-forming lipids that typically contain two long-chain fatty acids esterified to glycerol, with a polar headgroup also attached to the glycerol.
Plants are constantly exposed to both abiotic and biotic stresses. Plant membrane lipids, particularly polar glycerolipids, may change as plants respond to these stresses, and the lipid changes may be intimately involved in mediating plant stress responses. For example, levels of unsaturated fatty acyl chains, including linolenic acid, change in response to temperature, affecting membrane fluidity and the ability of a plant to tolerate extreme temperatures (e.g., Iba, 2002; Welti et al., 2002). Linolenic acid also is involved in protein modifications in plants under heat stress (Yamauchi et al., 2008). Freezing tolerance also is enhanced by the presence of an activity, encoded by the gene SENSITIVE TO FREEZING2 (SFR2), which remodels the outer chloroplast membrane (Moellering et al., 2010). The enzyme transfers a galactosyl residue from a monogalactosyldiacylglycerol to a galactolipid accepter to form an oligogalactolipid and diacylglycerol. The biochemical activity of SFR2 causes a compositional change in the membrane that is likely to increase bilayer physical stability during freezing (Moellering et al., 2010). Phosphatidic acid, formed from the hydrolysis of other phospholipids by the action phospholipase D (PLD) in plant stresses such as freezing and drought, can regulate the function of a number of proteins, for example ABI1, a protein phosphatase involved in absisic acid signaling (Wang et al., 2006; Zhang et al., 2004). Finally, membrane lipids undergo modifications that produce lipid-derived signal molecules, such as jasmonic acid, that are critical to plant wound and disease responses (Shah, 2005).
As described above, the toxins produced by P. tritici-repentis interact closely with host tissues. The toxins can cause necrotic cell death, inhibition of photosynthesis, and induction of chlorosis in wheat leaves. The interactions of the fungus and its toxins with plant leaves involve interactions with membranes. Thus, it is likely that there are interactions among the fungus, wheat lipids, and wheat proteins that regulate lipid composition. A working hypothesis for this research was that the fungus-host interaction affects wheat leaf lipid metabolism, resulting in different membrane lipid compositions between leaves of diseased and healthy wheat cultivars. Therefore, one goal of the research was to determine if infection by P. tritici-repentis affects the lipid profiles of wheat leaves. A second goal was to determine if there was a difference in leaf lipid profiles among wheat cultivars susceptible or resistant to tan spot.
MATERIALS AND METHODS
Experiment #1 plant material
Wheat (Triticum aestivum L.) seeds were planted and the resulting seedlings grown for one month in the greenhouse [20-28°C with supplemental light from high pressure sodium lights (400 w)]. Experiments were conducted between November and April. Seedlings were grown in racks holding 2.5 × 13 cm plastic tubes (Stuewe and Sons, Tangent, OR) filled with a mixture of steam-sterilized soil and vermiculite (50:50). There were 48 treatments arranged in a randomized complete block design with five replications. An experimental unit was a single plant growing in a tube. Treatments included three resistant and three susceptible cultivars, with or without inoculation with P. tritici-repentis, and four harvest times after inoculation. Resistant cultivars were Betty, Jagger, and Karl 92 and susceptible cultivars were Larned, Newton, and TAM 105. Tan spot susceptibility/resistance ratings are shown in Table 1. Harvest times were 2, 4, 6, or 8 days after inoculation. The experiment was conducted twice.
Table 1.
Reaction of 20 selected winter wheat cultivars to tan spot
| Entry No. | Cultivar | Tan spot ratinga |
|---|---|---|
| 1 | Red Chief | 2.60 |
| 2 | Heyne | 2.80 |
| 3 | Betty | 3.03 |
| 4 | Karl 92 | 3.27 |
| 5 | Jagger | 3.31 |
| 6 | 2137 | 3.89 |
| 7 | Victory | 4.10 |
| 8 | Overley | 4.64 |
| 9 | Wesley | 5.24 |
| 10 | Protection CL | 6.12 |
| 11 | Onaga | 6.45 |
| 12 | Jagalene | 6.57 |
| 13 | Abilene | 6.96 |
| 14 | Ike | 8.02 |
| 15 | 2180 | 8.40 |
| 16 | Newton | 8.48 |
| 17 | Larned | 8.68 |
| 18 | TAM 105 | 8.87 |
| 19 | Arkan | 9.01 |
| 20 | Stanton | 9.05 |
0.5-to-9.49 scale where 0.5 = highly resistant and 9.49 = highly susceptible. Values are the means of at least five replicated phenotyping experiments (see De Wolf et al., 2011 and 3 Bockus, unpublished).
Inoculum production and inoculation
Race 1 of P. tritici-repentis was used. This race is the most abundant in the Great Plains of the U.S., including Kansas, and produces ToxA and ToxC. Spores were produced by placing 0.5-cm2 mycelial plugs of P. tritici-repentis from one-fourth-strength potato-dextrose agar (1/4 PDA) in the center of plates of V-8 agar (150 ml V-8 juice, 3 g CaCO3, 15 g agar, 850 ml distilled water). Plates were incubated in the dark at 21-24°C for 5 days until the colony reached about 5 cm in diameter. Aerial mycelia were knocked down with a sterile, bent-glass rod and plates were incubated in the light (about 30 μE s-1 m-2) at 21-24°C for 12 h to produce conidiophores and then in the dark at 16°C for 12 h to produce conidia. Plants were inoculated when one month old. Spore suspensions were quantified using a hemacytometer and adjusted with distilled water to 10,000 spores per milliliter. Thirty five milliliters of a spore suspension were uniformly applied using a DeVilbis atomizer (Micromedics Inc., St Paul, MN) per 30 × 60 cm rack holding two replications (96 tubes). Leaves were allowed to dry to stick spores to the leaves and the plants then placed into a mist chamber to maintain continual leaf wetness for 48 h at 20-28°C. After the mist treatment, plants were returned to the greenhouse bench (20-28°C).
Disease rating, harvest, and processing
At each sample time, leaves were rated for percentage leaf area displaying chlorosis and/or necrosis (Raymond et al., 1985). After rating for leaf area affected by disease, lipid extraction was carried out according to the protocols published by the Kansas Lipidomics Research Center (Devaiah et al., 2006; Xiao et al., 2010) with slight modifications as stated below. The first and second leaves of each plant were removed, quickly cut with scissors into 1-cm pieces, and immersed in 6 ml preheated (75°C) isopropanol with 0.01% butylated hydroxytoluene (BHT). The extraction solvent was in a 50-ml glass tube with a Teflon-lined screw cap. Leaf pieces were incubated in the 75°C isopropanol for at least 30 min. Three milliliters chloroform and 1 ml water were then added to each tube and the tube was vortexed. Tubes were agitated in a shaking incubator at room temperature for 1 h. The lipid extracts were transferred to another glass tube using a glass pipette. Four more extractions of lipid using 4 ml chloroform/methanol (2:1) with 0.01% BHT were carried out with shaking for 5 h or overnight until the leaves of the sample became white. Every sample had 5 extractions, including the one with the isopropanol. Samples were backwashed by adding 1 ml 1 M KCl to the combined extract, vortexing, centrifuging (10 min at 1000 rpm), and removing the upper phase. A second backwash involved adding 2 ml water and repeating the backwash steps. All tubes were then evaporated under nitrogen. After complete evaporation, the extract was dissolved in 1 ml chloroform. All extracts were stored at -75°C until lipid analysis. The remaining plant tissues were dried in an oven (105°C) overnight and weighed (mg) to determine the dry extracted tissue mass.
Quantification of lipids using mass spectrometry
An aliquot of the dissolved extract in 1 ml chloroform was used for lipid analysis by mass spectrometry. For analysis, 150-300 μl extract, dependent upon leaf dry weight, were combined with chloroform/methanol/300 mM ammonium acetate in water and internal standards. Complete information about internal standard additions, solvents, and mass spectral parameters are published (Xiao et al., 2010; see supplemental data of that paper at http://www.plantcell.org/content/suppl/2010/04/21/tpc.110.075333.DC1/Supplemental_Data_Final.pdf). The lipid extracts were analyzed by a triple quadrupole mass spectrometer (API 4000, Applied Biosystem, Foster City, CA). Injections to the mass spectrometer were at the rate of 30 μl/min using an autosampler with a 1-ml sample loop (LC Mini PAL, CTC Analytics AG, Zwingen, Switzerland).
Lipid profiles in resistant and susceptible cultivars (experiment #2)
Twenty winter wheat cultivars were selected based on Kansas State University extension ratings, and data from unpublished phenotypic experiments, and ranged from resistant to susceptible to tan spot (De Wolf et al. 2011, Table 1). Seedlings were grown in the greenhouse as described above for 1 month. A single seed was sown in each tube. The design was a randomized complete block with 20 treatments (cultivars) and 5 replications (3 plants per replication).
For lipid extraction, the five extractions were performed as described above. After these five chloroform/methanol based extractions, 4 ml “solvent H” per tube were added and the tube incubated on a heating block at 60°C for 15 min. “Solvent H” was prepared by mixing isopropanol/hexane/water (55:20:25, v/v/v) for 30 min with a magnetic stirrer. The mixture was allowed to settle for 30 min, the upper phase was removed and discarded, the volume of the lower phase was measured, and BHT was added to a concentration of 0.01%. The clear lower phase with BHT 0.01% is “solvent H”. The “solvent H” extract of the wheat leaves was combined with previous chloroform/methanol extracts. The “solvent H” extraction, including the heating step, was repeated three more times, all extracts were combined, and no backwash was performed. Evaporation, drying, and weighing of leaves were as described above.
Data analysis
Data processing was carried out using a custom script and Applied Biosystems Analyst software. The amounts of lipid species were calculated using the software program Excel and the LipidomeDB Data Calculation Environment (http://lipidome.bcf.ku.edu:9000/Lipidomics). Values are presented as mass spectral signal (intensity), normalized to internal standards, per mg dry weight measured after lipid extraction, where a value of 1 refers to the same amount of mass spectral signal as 1 nmol of internal standards. There were no significant experimental repetition-by-treatment interactions that would preclude combining the two repetitions of experiment #1; therefore, the two repetitions were merged. Also, within a resistance class (“resistant” or “susceptible”), there were no significant cultivar-by-inoculation interactions that would preclude combining data from the three resistant cultivars and from the three susceptible cultivars. Therefore, data within a resistance class were pooled for experiment #1. To determine the effect of resistance, inoculation, and time, linear models were fit to data using SAS (SAS Institute, Cary, NC). For each of the galactolipids (MGDG and DGDG), four lines were compared. The four lines included resistant cultivars without inoculation, resistant cultivars with inoculation, susceptible cultivars without inoculation, and susceptible cultivars with inoculation. The amount of lipid was the independent variable and harvest time was the dependent variable. The resultant slopes of the lines were statistically compared (P=0.05) using SAS. When the slopes of two lines were not significantly different, the equal-slopes model was used to compare the estimates of the intercepts (P=0.05). For analysis of data from the experiment with 20 cultivars (experiment #2), linear regression was used to determine the relationship between the amount of lipid and cultivar rating for tan spot resistance/susceptibility.
RESULTS
Lipids detected in wheat leaves (experiment #1)
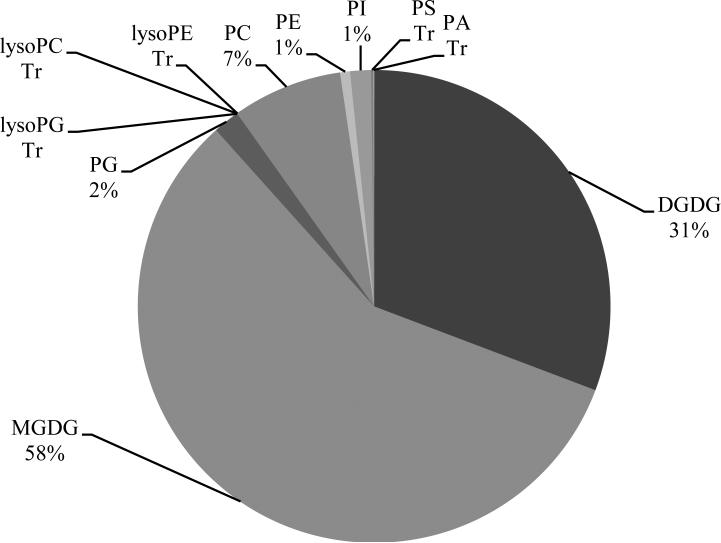
Two classes of galactolipids and nine classes of phospholipids in extracts from wheat leaves were detected by mass spectrometry (Fig. 1). The galactolipids were monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), and the phospholipids were phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), lysophosphatidylcholine (LysoPC), lysophosphatidylethanolamine (LysoPE), and lysophosphatidylglycerol (LysoPG). Galactolipids were the major lipid components in wheat leaves, accounting for 89% of all lipid mass spectral signal while the phospholipid classes were present in lesser amounts (Fig. 1). PC was the most abundant phospholipid class. In both galactolipid classes, MGDG and DGDG, the major molecular species was 36:6 which has two linolenic acid moieties (di18:3). Lipid analytical results in the repeated experiment were similar.
Fig. 1.
Percentage of each lipid class (normalized mass spectral signal) detected in noninoculated wheat leaves; average of plants that were 30, 32, 34, and 36 days old. Lipids with “Tr” indicate detection at very low levels (“Trace”). Abbreviations: MGDG = monogalactosyldiacylglycerol; DGDG = digalactosyldiacylglycerol; PC = phosphatidylcholine; PE = phosphatidylethanolamine; PG = phosphatidylglycerol; PI = phosphatidylinositol; PS = phosphatidylserine; PA = phosphatidic acid; lysoPC = lysophosphatidylcholine; lysoPE = lysophosphatidylethanolamine; and lysoPG = lysophosphatidylglycerol.
Lipid class composition for healthy vs. diseased and resistant vs. susceptible plants (experiment #1)
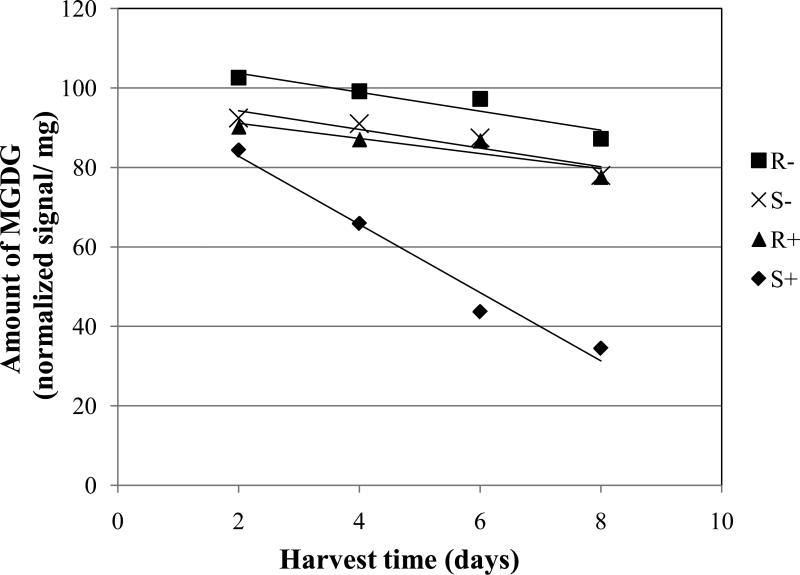
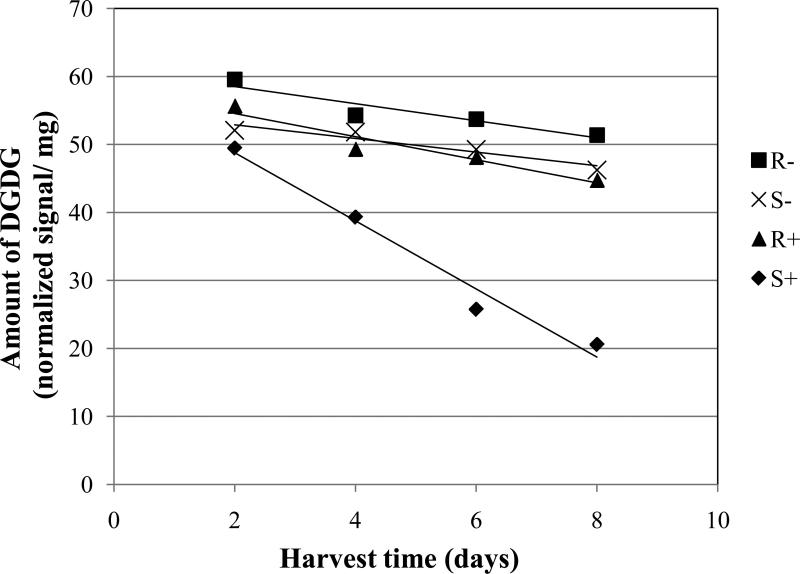
There were consistent differences in the amounts of galactolipids (MGDG and DGDG) between inoculated and non-inoculated treatments. The susceptible cultivars showed a significantly faster decline of MGDG and DGDG with inoculation when compared with the non-inoculated treatments for the same susceptible cultivars (Figs. 2 and 3, Table 2; S+ vs. S-). The negative slope of the linear model was 3.6 and 5.0 times steeper for the inoculated treatments vs. the non-inoculated treatments for MGDG and DGDG, respectively. Similar large differences in the rate of reduction of galactolipids were seen when comparing the slopes of the inoculated, susceptible cultivars with those of the inoculated or non-inoculated, resistant cultivars (Figs. 2 and 3, Table 2; S+ vs. R+ or R-).
Fig. 2.
Amount of monogalactosyldiacylglycerol (MGDG) in wheat leaves (normalized mass spectral signal per dry leaf mass) over time. One-month-old plants were inoculated or left non-inoculated at day 0. Abbreviations are: R- = average of three resistant wheat cultivars (Betty, Jagger, Karl 92) without inoculation; S- = average of three susceptible cultivars (Larned, Newton, TAM 105) without inoculation; R+ = resistant cultivars with inoculation; and S+ = susceptible cultivars with inoculation. Each data point is the mean of three cultivars each with five replications. Equations for the trend lines and P values for significance of the slopes different from zero are as follows R- , Y = -2.39 X + 108.53 (P = 0.0963); S-, Y = -2.37 X + 99.12 (P = 0.1054); R+, Y = -1.91 X + 94.95 (P = 0.1847); and S+, Y = -8.61 X + 100.10 (P = <0.0001).
Fig. 3.
Amount of digalactosyldiacylglycerol (DGDG) in wheat leaves (normalized mass spectral signal per dry leaf mass) over time. One-month-old plants were inoculated or left noninoculated at day 0. Abbreviations are: R- = average of three resistant wheat cultivars (Betty, Jagger, Karl 92) without inoculation; S- = average of three susceptible cultivars (Larned, Newton, TAM 105) without inoculation; R+ = resistant cultivars with inoculation; and S+ = susceptible cultivars with inoculation. Each data point is the mean of three cultivars each with five replications. Equations for the trend lines and P values for significance of the slopes different from zero are as follows: R- , Y = -1.25 X + 61.02 (P = 0.0367); S-, Y = -1.01 X + 54.95 (P = 0.0965); R+, Y = -1.70 X + 57.96 (P = 0.0047); and S+, Y = -5.02 X + 58.85 (P = <0.0001).
Table 2.
Statistical P values for the comparison of slopes (in parentheses) and estimates of the intercepts (in parentheses) for the amount of MGDGa or DGDG regressed against harvest time for wheat cultivars resistant and susceptible to tan spot (lines shown in Figs. 2 and 3).
| MGDG | |||
| Comparison of slopes | S-b (-2.37) | R+ (-1.91) | S+ (-8.61) |
| R- (-2.39) | 0.9896 | 0.8112 | 0.0025 |
| S- (-2.37) | - | 0.8227 | 0.0025 |
| R+ (-1.91) | - | - | 0.0011 |
| Comparison of interceptsc | |||
| R- vs. S- (96.5 vs. 87.3) | 0.0482 | - | - |
| R+ vs. R- (85.4 vs. 96.5) | 0.0172 | - | - |
| R+ vs. S- (85.4 vs. 87.3) | 0.6890 | - | - |
| DGDG | |||
| Comparison of slopes | S- (-1.01) | R+ (-1.70) | S+ (-5.02) |
| R- (-1.25) | 0.7771 | 0.5996 | <0.0001 |
| S- (-1.01) | - | 0.4215 | <0.0001 |
| R+ (-1.70) | - | - | 0.0001 |
| Comparison of interceptsc | |||
| R- vs. S- (54.7 vs. 49.9) | 0.0127 | - | - |
| R+ vs. R- (49.5 vs. 54.7) | 0.0064 | - | - |
| R+ vs. S- (49.5 vs. 49.9) | 0.8212 | - | - |
Abbreviations: MGDG = monogalactosyldiacylglycerol; and DGDG = digalactosyldiacylglycerol.
R- = wheat cultivars resistant to tan spot without inoculation; S- = susceptible cultivars without inoculation; R+ = resistant cultivars with inoculation; and S+ = susceptible cultivars with inoculation. Slopes (normalized signal/mg/day) and estimates of the intercepts are shown in parentheses.
The estimates of the intercepts were only compared for those pairings where the slopes of the lines were not significantly different.
For MGDG, the slopes of the lines for the non-inoculated, susceptible cultivars, the inoculated, resistant cultivars, and the non-inoculated, resistant cultivars were not significantly different from each other or from zero (Fig. 2, Table 2; S-, R+, R-). However, the line for the non-inoculated, resistant cultivars was significantly above the line for the non-inoculated, susceptible cultivars (Table 2, R- vs. S-).
For DGDG, the slopes of the lines for the non-inoculated, susceptible cultivars, the inoculated, resistant cultivars, and the non-inoculated, resistant cultivars were not significantly different from each other (Fig. 3, Table 2; S-, R+, R-). With this galactolipid, the slopes for the inoculated, resistant cultivars, and the non-inoculated, resistant cultivars were significantly different from zero but they were not very steep (Fig. 3, R+, R-). Although the slopes were not significantly different from each other, the line for the non-inoculated, resistant cultivars was significantly above the line for the non-inoculated, susceptible cultivars (Fig. 3, Table 2; R- vs. S-).
Correlation of lipid amount with tan spot resistance (experiment #2)
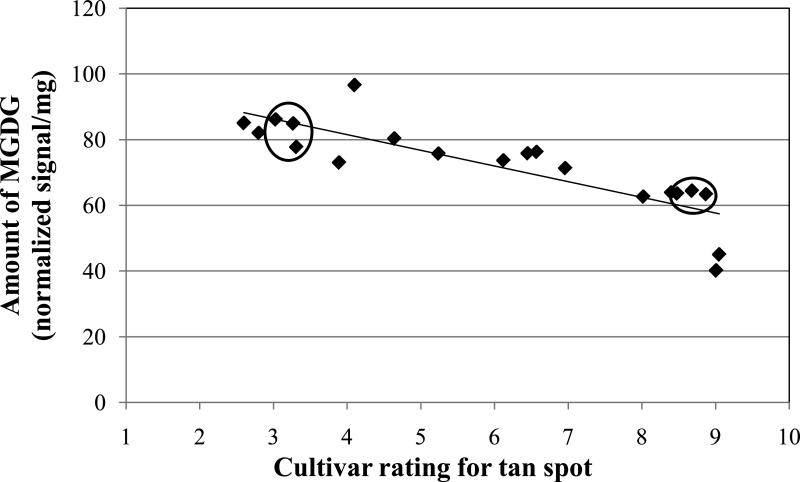
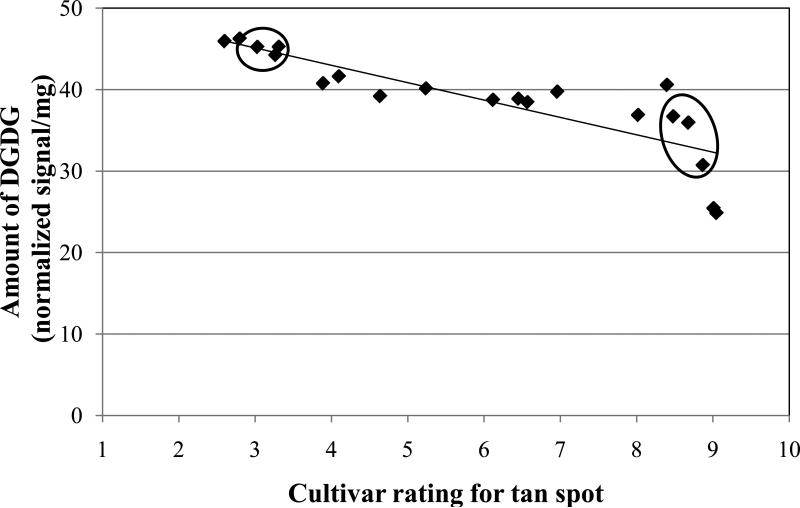
Results from experiment #1 with six cultivars showed that non-inoculated resistant cultivars had higher amounts of MGDG and DGDG compared to the non-inoculated susceptible cultivars (Figs. 2 and 3, Table 2). Therefore, a second, expanded experiment involving 20 cultivars was conducted to corroborate that preliminary finding. The amounts of the major lipids were regressed against the disease phenotype rating for the cultivars. The cultivars had a range of reaction to tan spot from resistant to highly susceptible (Table 1). Figure 4 shows a significant (P<0.0001) negative correlation between the amount of MGDG and the susceptibility rating. As the extension rating increased, the amount of MGDG decreased. There was a similar significant (P<0.0001) negative correlation between the amount of DGDG and tan spot extension rating; the higher the rating, the lower the amount of DGDG (Fig. 5).
Fig. 4.
Amount of monogalactosyldiacylglycerol (MGDG) versus the level of resistance to tan spot for non-inoculated, 28-day-old seedling leaves of 20 winter wheat cultivars. Rating values (see Table 1) are on a 0.5-9.49 scale where 0.5 = highly resistant to tan spot and 9.49 = highly susceptible. Each data point is the mean of five replications for a single cultivar. Linear equation is: Y = -4.77 X + 100.63 (Adjusted R2 = 0.6815, N = 20, and P < 0.0001). The three resistant and three susceptible cultivars that were used in experiment #1 are circled.
Fig. 5.
Amount of digalactosyldiacylglycerol (DGDG) versus the level of resistance to tan spot for non-inoculated, 28-day-old seedling leaves of 20 winter wheat cultivars. Rating values (see Table 1) are on a 0.5-9.49 scale where 0.5 = highly resistant to tan spot and 9.49 = highly susceptible. Each data point is the mean of five replications for a single cultivar. Linear equation is: Y = -2.13 X + 51.52 (Adjusted R2 = 0.7041, N = 20, and P < 0.0001). The three resistant and three susceptible cultivars that were used in experiment #1 are circled.
DISCUSSION
The results presented here give evidence that a biotic stress can profoundly affect lipid profiles in plants. The results are the first documentation of the influence of the wheat leaf spot disease tan spot on lipids in wheat leaves. When comparing lipid profiles in diseased vs. healthy plants, tan spot resulted in significant changes in lipid classes in some of the detected lipids (Table 2). Healthy wheat leaves had more of the lipids MGDG and DGDG. Furthermore, reductions of over 50% were observed for both lipids in inoculated, susceptible cultivars 8 days after inoculation (Figs. 2 and 3, S+). It is unknown whether the fungus itself degraded the lipids or whether it induced plant enzymes to degrade the lipids. Further research is needed to elucidate the answer to that question.
Plants interact with the biotic and abiotic environments and have systems to protect themselves against stresses. When exposed to stresses, their survival often depends on how fast they recognize and response to these stresses (Maffei et al., 2007). The plasma membrane of a plant cell is often the first component where plants interact with environmental stresses. Early events in the interaction between plants and environmental stresses can involve activities such as a kinase signal transduction pathway, phytohormones, and the production of reactive oxygen species in the plasma membrane (Maffei et al., 2007). During these interactions, the composition of lipids in the cell membrane is changed.
There have been many studies involving changes in lipid profiles in plant cell membranes (Iba, 2002, Moellering et al., 2010, Welti et al., 2002, Yamauchi et al., 2008). However, most previous studies have focused on changes in plant lipid composition due to abiotic stresses. Important findings in membrane biology concern the relationship between lipid composition and how plants adjust to temperature stress (Wolter et al., 1992). In this regard, unsaturated fatty acids are linked to biochemical and physiological changes in plants exposed to chilling injury. Murata et al. (1992) proposed a hypothesis that the level of unsaturated phosphatidylglycerol (PG) in chloroplast membranes determines the chilling sensitivity of plant species.
Results shown here are the first to correlate the amount of lipid moieties in wheat leaves with resistance level to tan spot. Wheat cultivars resistant or susceptible to tan spot showed different lipid profiles. For non-inoculated treatments, the slopes of the lines for the galactolipids MGDG and DGDG were the same for resistant and susceptible cultivars and most were not significantly different from zero (Figs. 2 and 3, Table 2, R- vs. S-). This indicates that the levels did not change over time in healthy leaves. However, the estimates of the intercepts showed that there were higher (P<0.05) levels of those lipids in the resistant cultivars (Table 2, 96.5 vs. 87.3 for MGDG and 54.7 vs. 49.9 for DGDG). Similarly, the rates of reduction due to tan spot for the galactolipids were significantly different between resistant and susceptible cultivars. Resistant cultivars had a significantly slower loss (larger slope) of MGDG and DGDG compared with susceptible cultivars (Figs. 2 and 3, Table 2, R+ vs. S+). Therefore, these results suggest that lipids in susceptible wheat cultivars are influenced by tan spot more than those in resistant cultivars and the disease results in faster degradation of galactolipids in susceptible cultivars.
The experiment using 20 cultivars corroborated the above finding of higher levels of the major lipids in resistant cultivars in non-inoculated plants. There was a significant (P<0.0001) linear relationship between the amounts of MGDG and DGDG in non-inoculated wheat leaves and the level of resistance to tan spot (Figs. 4 and 5). As the level of resistance increased (lower rating number), the level of MGDG and DGDG also increased. Using calculations from the linear equations, cultivars with a rating of 1 would have 66.1% more MGDG and 52.7% more DGDG than cultivars with a rating of 9 (Figs. 4 and 5). MGDG and DGDG are major membrane constituents of chloroplasts and most abundant in plant leaves. They are indispensible for efficiency of photosynthetic light reactions. For example, Jarvis et al. (2000) reported that MGDG synthase activity in the Arabidopsis mutant mgd1 was reduced by 50% relative to that of the wild-type, thus reducing the amount of MGDG and chlorophyll. Similarly, dgd 1 mutant plants contained 10% of the wild-type amount of DGDG and the mutant plants showed a strong reduction of photosynthetic capacity (Dörmann et al., 1995; Hartel et al., 1997; Reifarth et al., 1997).
Three kinds of host specific toxins are involved in the pathogenesis of tan spot diseases. These toxins move into chloroplasts and interact with thylakoid membranes causing inhibition of photosynthesis in wheat leaves (Stelkov et al., 1998; Manning and Ciuffetti, 2005; Kim et al., 2010). Therefore, it may be that the toxins are responsible for the change in lipid profiles documented in this research. However, further research is needed to document this possibility.
In conclusion, data presented here are the first to quantify the effects of tan spot on lipids in wheat leaves. Data from time-course experiments indicate that tan spot significantly reduced the amount of lipids including the major lipids MGDG and DGDG in leaves. This reduction was at a much higher rate for susceptible cultivars compared with resistant ones. Furthermore, data showed that cultivars resistant to tan spot have different lipid profiles when compared with susceptible cultivars. Resistant cultivars had more MGDG and DGDG than susceptible ones, even in non-inoculated leaves. These findings are indirect evidence that the amounts of some lipids in wheat leaves are a determinant in the resistance response of cultivars to tan spot. However, further research is needed to corroborate this conclusion.
ACKNOWLEDGEMENTS
We would like to acknowledge the technical assistance of Mary R. Roth and statistical advice from Sean Meier and Timothy C. Todd. This work was supported by the Kansas Wheat Commission and the Kansas Agricultural Experiment Station. Equipment acquisition at the Kansas Lipidomics Research Center was funded by the National Science Foundation (EPS 0236913 and DBI 0521587), Kansas Technology Enterprise Corporation, Kansas IDeA Networks of BiomedicalResearch Excellence (INBRE) of the National Institutes of Health (P20 RR16475), and Kansas State University. RJ was supported by Kansas State University's Targeted Excellence program. Contribution number KAES #12-320-J from the Kansas Agricultural Experiment Station.
Contributor Information
Dongwon Kim, Department of Plant Pathology, Kansas State University, Manhattan, KS 66506.
Richard Jeannotte, Kansas Lipidomics Research Center, Division of Biology, Kansas State University, Manhattan, KS 66506..
Ruth Welti, Kansas Lipidomics Research Center, Division of Biology, Kansas State University, Manhattan, KS 66506..
William W. Bockus, Department of Plant Pathology, Kansas State University, Manhattan, KS 66506
LITERATURE CITED
- Ballance GM, Lamari L, Bernier CC. Purification and characterization of a host-selective necrosis toxin from Pyrenophora tritici-repentis. Physiological and Molecular Plant Pathology. 1989;35:203–213. [Google Scholar]
- Ballance GM, Lamari L, Kowatsch R, Bernier CC. Cloning, expression and occurrence of the gene encoding the Ptr necrosis toxin from Pyrenophora tritici-repentis. Molecular Plant Pathology. 1996 http://www.bspp.org.uk/mppol/1996/1209ballance/
- Ciuffetti LM, Tuori RP, Gaventa JM. A single gene encodes a selective toxin causal to the development of tan spot of wheat. Plant Cell. 1997;9:135–144. doi: 10.1105/tpc.9.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffetti LM, Manning VA, Pandelova I, Betts MF, Martinez JP. Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora triticirepentis–wheat interaction. New Phytologist. 2010;187:911–919. doi: 10.1111/j.1469-8137.2010.03362.x. [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species fromorgans of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- De Wolf ED, Bockus WW, Whitworth JR. Wheat Variety Disease and Insect Ratings 2011. Kansas Cooperative Extension Service publication MF-991; 2011. p. 4. [Google Scholar]
- De Wolf ED, Effertz RJ, Al S, Francl LJ. Vistas of tan spot research. Canadian Journal of Plant Pathology. 1998;20:349–370. [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell. 1995;7:1801–1810. doi: 10.1105/tpc.7.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz RJ, Meinhardt SW, Anderson JA, Jordahl JG, Francl LJ. Identification of a chlorosis-inducing toxin from Pyrenophora tritici-repentis and the chromosomal location of an insensitivity locus in wheat. Phytopathology. 2002;92:527–533. doi: 10.1094/PHYTO.2002.92.5.527. [DOI] [PubMed] [Google Scholar]
- Gamba FM, Lamari L, Brule-Babel AL. Inheritance of race-specific necrotic and chlorotic reactions induced by Pyrenophora tritici-repentis in hexaploid wheats. Canadian Journal of Plant Pathology. 1998;20:401–407. [Google Scholar]
- Graham LE, Graham JM, Wilcox LW. Plant Biology. Pearson Education; New Jersey: 2003. Four types of primary compounds are the molecules of life. pp. 55–59. [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Grimm B, Benning C. Changes in the composition of the photosynthetic apparatus in the galactolipid-deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiology. 1997;115:1175–1184. doi: 10.1104/pp.115.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review Plant Biology. 2002;53:225–45. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- Jarvis P, Dörmann P, Peto CA, Lutes J, Benning C, Chory J. Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc. Natl. Acad. Sci. 2000;97:8175–8179. doi: 10.1073/pnas.100132197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Bouras N, Kav NN, Strelkov SE. Inhibition of photosynthesis and modification of the wheat leaf proteome by Ptr ToxB: a host-specific toxin from the fungal pathogen Pyrenophora tritici-repentis. Proteomics. 2010;10:2911–2926. doi: 10.1002/pmic.200900670. [DOI] [PubMed] [Google Scholar]
- Lamari L, Bernier CC. Evaluation of wheat lines and cultivars to tan spot (Pyrenophora tritici-repentis) based on lesion type. Canadian Journal of Plant Pathology. 1989;11:49–56. [Google Scholar]
- Lamari L, Strelkov SE. The wheat/Pyrenophora tritici-repentis interaction: progress towards an understanding of tan spot disease. Canadian Journal of Plant Pathology. 2010;32:4–10. [Google Scholar]
- Loughman R, Deverall BJ. Experiments with excised lesions from wheat leaves infected by Pyrenophora tritici-repentis. Australasian Plant Pathology. 1986;18:90–93. [Google Scholar]
- Maffei ME, Mithofer A, Boland W. Before gene expression: early events in plant–insect interaction. Trends in Plant Science. 2007;12:310–316. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Manning VA, Ciuffetti LM. Localization of Ptr ToxA produced by Pyrenophora tritici-repentis reveals protein import into wheat mesophyll cells. Plant Cell. 2005;17:3203–3212. doi: 10.1105/tpc.105.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen M, Adhikari T. Fungal leaf spot diseases of wheat: tan spot, stagonospora nodorum blotch and septoria tritici blotch. North Dakota State University Extension Service; 2009. p. 1249. [Google Scholar]
- McMullen MP. Tan spot (yellow leaf spot). In: Bockus WW, Bowden RL, Hunger RM, Morrill WL, Murray TD, Smiley RW, editors. Compendium of Wheat Diseases and Pests. American Phytopathological Society; St. Paul, MN: 2010. pp. 82–84. [Google Scholar]
- Moellering ER, Muthan B, Benning C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science. 2010;330:226–228. doi: 10.1126/science.1191803. [DOI] [PubMed] [Google Scholar]
- Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I. Genetically engineered alteration in the chilling sensitivity of plants. Nature. 1992;356:313–326. [Google Scholar]
- Raymond PJ, Bockus WW, Norman BL. Tan spot of winter wheat: Procedures to determine host response. Phytopathology. 1985;75:686–690. [Google Scholar]
- Reifarth F, Christen G, Seeliger AG, Dörmann P, Benning C, Renger G. Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry. 1997;36:11769–11776. doi: 10.1021/bi9709654. [DOI] [PubMed] [Google Scholar]
- Shabeer A, Bockus WW. Tan spot effects on yield and yield components relative to growth stage in winter wheat. Plant Disease. 1988;72:599–602. [Google Scholar]
- Shah J. Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annual Review Phytopathology. 2005;43:229–260. doi: 10.1146/annurev.phyto.43.040204.135951. [DOI] [PubMed] [Google Scholar]
- Singh PK, Hughes GR. Genetic control of resistance to tan necrosis induced by Pyrenophora tritici-repentis, races 1 and 2, in spring and winter wheat genotypes. Phytopathology. 2005;95:172–177. doi: 10.1094/PHYTO-95-0172. [DOI] [PubMed] [Google Scholar]
- Strelkov SE, Lamari L, Balance GM. Induced chlorophyll degradation by a chlorosis toxin from Pyrenophora tritici-repentis. Canadian Journal of Plant Pathology. 1998;20:428–435. [Google Scholar]
- Strelkov SE, Lamari L. Host-parasite interaction in tan spot [Pyrenophora tritici-repentis] of wheat. Canadian Journal of Plant Pathology. 2003;25:339–349. [Google Scholar]
- USDA-NASS . Wheat varieties. Online. USDA-NASS, Kansas Field Office, Dept. of Agric.; Topeka, KS: 2010. [Google Scholar]
- Tomas A, Feng GH, Reeck GR, Bockus WW, Leach JE. Purification of a cultivar-specific toxin from Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Mol. Plant Microbe Interact. 1990;3:221–224. [Google Scholar]
- Tuori RP, Wolpert TJ, Ciuffetti LM. Purification and immunological characterization of toxic components from cultures of Pyrenophora tritici-repentis. Mol. Plant Microbe Interact. 1995;8:41–48. doi: 10.1094/mpmi-8-0041. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. Wiley; 2006. Biological membranes and lipids. pp. 234–281. [Google Scholar]
- Xiao S, Gao W, Chen Q-F, Chan S-W, Zheng S-X, Ma J, Wang M, Welti R, Chye M-L. Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell. 2010;22:1463–1482. doi: 10.1105/tpc.110.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Welti R, Li W, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. Journal of Biochemistry. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Wolter FP, Schmidt R, Heinz E. Chilling sensitivity of Arabidopsis thaliana with genetically engineered membrane lipids. The European Molecular Biology Organization Journal. 1992;11:4685–4692. doi: 10.1002/j.1460-2075.1992.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Furutera A, Seki K, Toyoda Y, Tanaka K, Sugimoto Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiology Biochemistry. 2008;46:786–793. doi: 10.1016/j.plaphy.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhao J, Wang X. Phospholipase Da1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 2004;101:9508101, 95. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]