Abstract
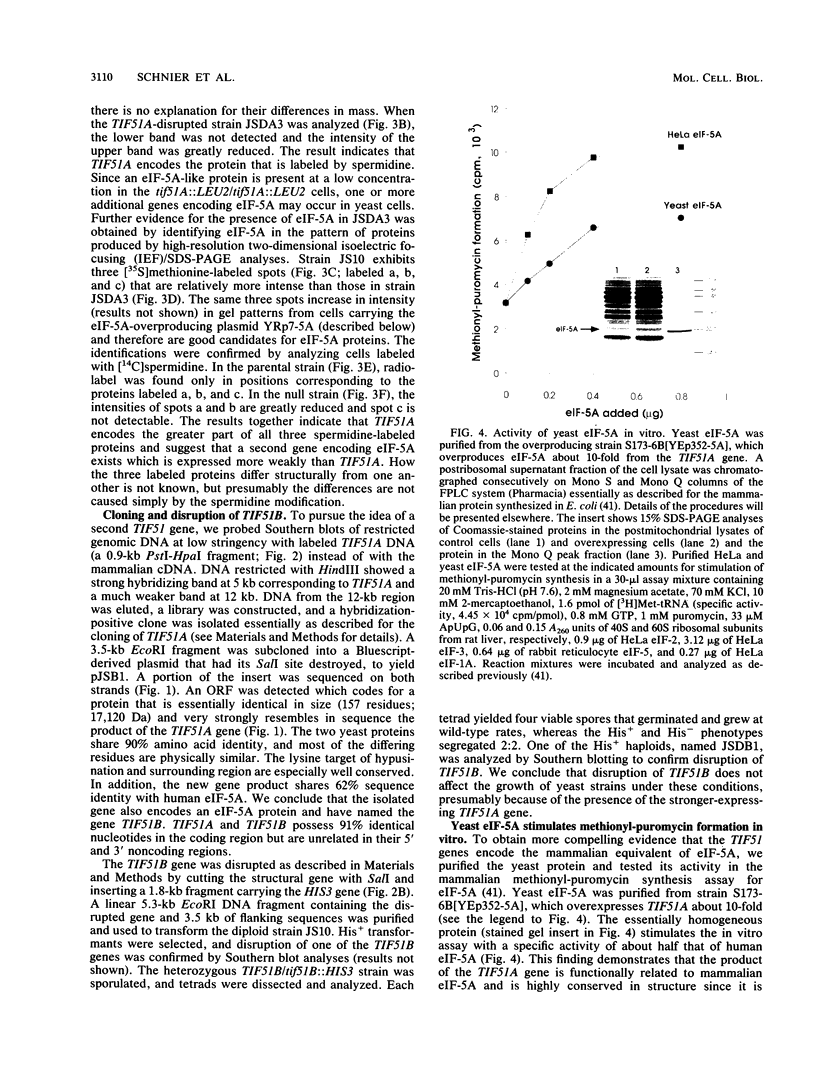
Translation intitiation factor eIF-5A (previously named eIF-4D) is a highly conserved protein that promotes formation of the first peptide bond. One of its lysine residues is modified by spermidine to form hypusine, a posttranslational modification unique to eIF-5A. To elucidate the function of eIF-5A and determine the role of its hypusine modification, the cDNA encoding human eIF-5A was used as a probe to identify and clone the corresponding genes from the yeast Saccharomyces cerevisiae. Two genes named TIF51A and TIF51B were cloned and sequenced. The two yeast proteins are closely related, sharing 90% sequence identity, and each is ca. 63% identical to the human protein. The purified protein expressed from the TIF51A gene substitutes for HeLa eIF-5A in the mammalian methionyl-puromycin synthesis assay. Strains lacking the A form of eIF-5A, constructed by disruption of TIF51A with LEU2, grow slowly, whereas strains lacking the B form, in which HIS3 was used to disrupt TIF51B, show no growth rate phenotype. However, strains with both TIF51A and TIF51B disrupted are not viable, indicating that eIF-5a is essential for cell growth in yeast cells. Northern (RNA) blot analysis shows two mRNA species, a larger mRNA (0.9 kb) transcribed from TIF51A and a smaller mRNA (0.8 kb) encoded by TIF51B. Under the aerobic growth conditions of this study, the 0.8-kb TIF51B transcript is not detected in the wild-type strain and is expressed only when TIF51A is disrupted. The TIF51A gene was altered by site-directed mutagenesis at the site of hypusination by changing the Lys codon to that for Arg, thereby producing a stable protein that retains the positive charge but is not modified to the hypusine derivative. The plasmid shuffle technique was used to replace the wild-type gene with the mutant form, resulting in failure of the yeast cells to grow. This result indicates that hypusine very likely is required for the vital in vivo function of eIF-5A and suggests a precise, essential role for the polyamine spermidine in cell metabolism.
Full text
PDF


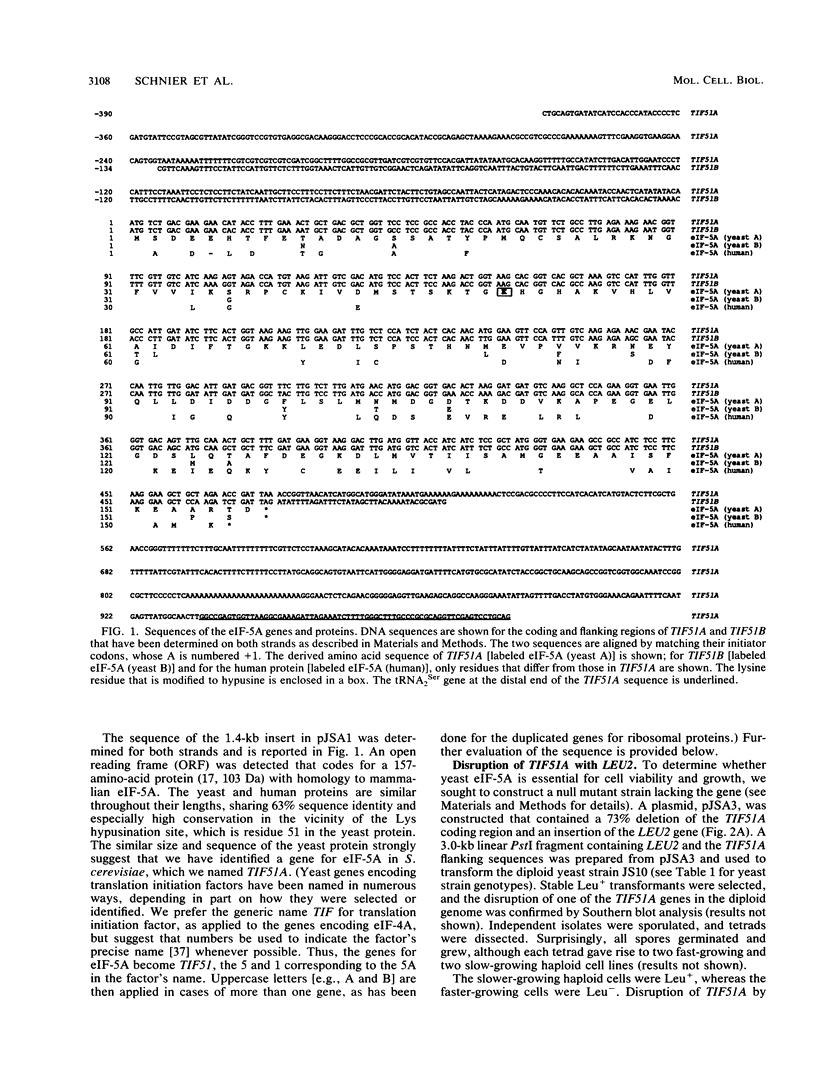
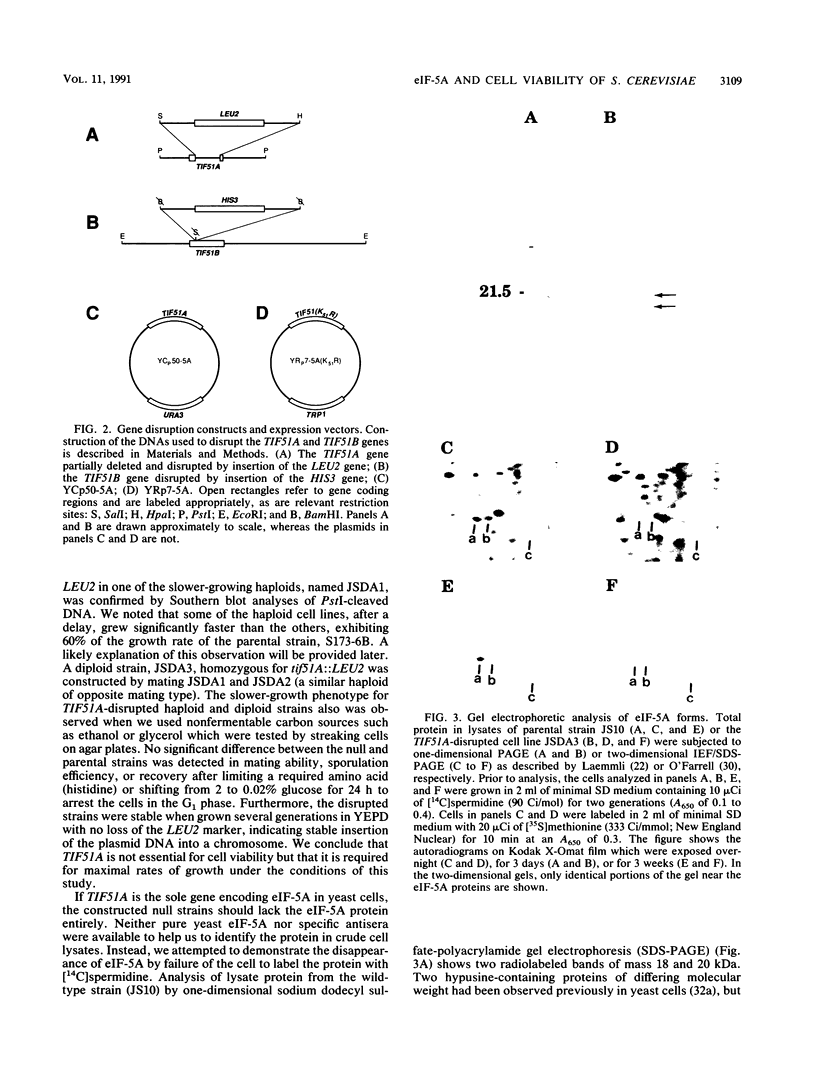




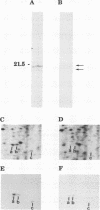
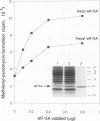
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann M., Trachsel H. Altered mRNA cap recognition activity of initiation factor 4E in the yeast cell cycle division mutant cdc33. Nucleic Acids Res. 1989 Aug 11;17(15):5923–5931. doi: 10.1093/nar/17.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Brown-Luedi M. L., Hershey J. W. Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3070–3077. [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cigan A. M., Donahue T. F. Sequence and structural features associated with translational initiator regions in yeast--a review. Gene. 1987;59(1):1–18. doi: 10.1016/0378-1119(87)90261-7. [DOI] [PubMed] [Google Scholar]
- Cigan A. M., Pabich E. K., Feng L., Donahue T. F. Yeast translation initiation suppressor sui2 encodes the alpha subunit of eukaryotic initiation factor 2 and shares sequence identity with the human alpha subunit. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2784–2788. doi: 10.1073/pnas.86.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E., Safer B., Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Cigan A. M., Pabich E. K., Valavicius B. C. Mutations at a Zn(II) finger motif in the yeast eIF-2 beta gene alter ribosomal start-site selection during the scanning process. Cell. 1988 Aug 26;54(5):621–632. doi: 10.1016/s0092-8674(88)80006-0. [DOI] [PubMed] [Google Scholar]
- Dorsman J. C., van Heeswijk W. C., Grivell L. A. Yeast general transcription factor GFI: sequence requirements for binding to DNA and evolutionary conservation. Nucleic Acids Res. 1990 May 11;18(9):2769–2776. doi: 10.1093/nar/18.9.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. D., Mora R., Meredith S. C., Lee C., Lindquist S. L. Eukaryotic initiation factor 4D, the hypusine-containing protein, is conserved among eukaryotes. J Biol Chem. 1987 Dec 5;262(34):16585–16589. [PubMed] [Google Scholar]
- Hershey J. W., Smit-McBride Z., Schnier J. The role of mammalian initiation factor eIF-4D and its hypusine modification in translation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):160–162. doi: 10.1016/0167-4781(90)90159-y. [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hashimoto S., Miyake A., Kashiwagi K., Hirose S. Increase of fidelity of polypeptide synthesis by spermidine in eukaryotic cell-free systems. Eur J Biochem. 1982 Nov 15;128(2-3):597–604. doi: 10.1111/j.1432-1033.1982.tb07006.x. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper W. M., Berry K. W., Merrick W. C. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem. 1976 Sep 25;251(18):5551–5557. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder P., Slonimski P. P. An essential yeast protein, encoded by duplicated genes TIF1 and TIF2 and homologous to the mammalian translation initiation factor eIF-4A, can suppress a mitochondrial missense mutation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2286–2290. doi: 10.1073/pnas.86.7.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Lieber R. H. Negative regulation of the Saccharomyces cerevisiae ANB1 gene by heme, as mediated by the ROX1 gene product. Mol Cell Biol. 1986 Dec;6(12):4145–4148. doi: 10.1128/mcb.6.12.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Weiss J. L., Walthall D. A., Zitomer R. S. Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc Natl Acad Sci U S A. 1983 Jan;80(1):151–155. doi: 10.1073/pnas.80.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K. D., Leung D., Lefebvre L., Smith M. The ANB1 locus of Saccharomyces cerevisiae encodes the protein synthesis initiation factor eIF-4D. J Biol Chem. 1990 May 25;265(15):8802–8807. [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Nomenclature of initiation, elongation and termination factors for translation in eukaryotes. Recommendations 1988. Nomenclature Committee of the International Union of Biochemistry (NC-IUB). Eur J Biochem. 1989 Dec 8;186(1-2):1–3. doi: 10.1111/j.1432-1033.1989.tb15169.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Page G. S., Hall B. D. Characterization of the yeast tRNA Ser genomic organization and DNA sequence. Nucleic Acids Res. 1981 Feb 25;9(4):921–934. doi: 10.1093/nar/9.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Liberato D. J., Yergey A. L., Folk J. E. The biosynthesis of hypusine (N epsilon-(4-amino-2-hydroxybutyl)lysine). Alignment of the butylamine segment and source of the secondary amino nitrogen. J Biol Chem. 1984 Oct 10;259(19):12123–12127. [PubMed] [Google Scholar]
- Park M. H., Liu T. Y., Neece S. H., Swiggard W. J. Eukaryotic initiation factor 4D. Purification from human red blood cells and the sequence of amino acids around its single hypusine residue. J Biol Chem. 1986 Nov 5;261(31):14515–14519. [PubMed] [Google Scholar]
- Park M. H. The essential role of hypusine in eukaryotic translation initiation factor 4D (eIF-4D). Purification of eIF-4D and its precursors and comparison of their activities. J Biol Chem. 1989 Nov 5;264(31):18531–18535. [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-McBride Z., Dever T. E., Hershey J. W., Merrick W. C. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989 Jan 25;264(3):1578–1583. [PubMed] [Google Scholar]
- Smit-McBride Z., Schnier J., Kaufman R. J., Hershey J. W. Protein synthesis initiation factor eIF-4D. Functional comparison of native and unhypusinated forms of the protein. J Biol Chem. 1989 Nov 5;264(31):18527–18530. [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Dix D. B., Gerson R. B., Karim A. M. Effect of Mg2+ concentration, polyamines, streptomycin, and mutations in ribosomal proteins on the accuracy of the two-step selection of aminoacyl-tRNAs in protein biosynthesis. J Biol Chem. 1981 Jul 10;256(13):6676–6681. [PubMed] [Google Scholar]
- Warner J. R. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989 Jun;53(2):256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]