Abstract
The phylogenetically ancient SLC26 gene family encodes multifunctional anion exchangers and anion channels transporting a broad range of substrates, including Cl−, HCO3−, sulfate, oxalate, I−, and formate. SLC26 polypeptides are characterized by N-terminal cytoplasmic domains, 10–14 hydrophobic transmembrane spans, and C-terminal cytoplasmic STAS domains, and appear to be homo-oligomeric. SLC26-related SulP proteins of marine bacteria likely transport HCO3− as part of oceanic carbon fixation. SulP genes present in antibiotic operons may provide sulfate for antibiotic biosynthetic pathways. SLC26-related Sultr proteins transport sulfate in unicellular eukaryotes and in plants. Mutations in three human SLC26 genes are associated with congenital or early onset Mendelian diseases: chondrodysplasias for SLC26A2, chloride diarrhea for SLC26A3, and deafness with enlargement of the vestibular aqueduct for SLC26A4. Additional disease phenotypes evident only in mouse knockout models include oxalate urolithiasis for Slc26a6 and Slc26a1, non-syndromic deafness for Slc26a5, gastric hypochlorhydria for Slc26a7 and Slc26a9, distal renal tubular acidosis for Slc26a7, and male infertility for Slc26a8. STAS domains are required for cell surface expression of SLC26 proteins, and contribute to regulation of the cystic fibrosis transmembrane regulator in complex, cell- and tissue-specific ways. The protein interactomes of SLC26 polypeptides are under active vestigation.
Keywords: SLC26, bicarbonate, chloride, sulfate, oxalate, STAS domain, Cystic Fibrosis Transmembrane Regulator
1. Introduction
Bicarbonate is transported across cell plasma membranes by anion channels of several gene families, and by anion exchangers of the SLC4 and the SLC26 gene families. The phylogenetically older SLC26-SulP gene family (2.A.53.1–2.A.53.3 in the Transporter Classification System of Saier (www.tcdb.org) is part of the APC gene superfamily, and encodes polypeptides that operate as electroneutral or electrogenic anion exchangers of monovalent and divalent anions, and in some cases and conditions as anion channels. Bacterial SLC26-related SulP proteins may contribute importantly to the oceanic carbon cycle, to sulfate transport, and to biosynthesis of fatty acids and antibiotics. SLC26-related Sultr proteins are major contributors to sulfate transport by yeast, algae, and plants. The eleven mammalian SLC26 genes are expressed throughout the body, some with widespread expression patterns and others with more limited tissue distributions. Human SLC26 mutations underlie autosomal recessive chondrodysplasias, chloride diarrhea, and deafness. Additional phenotypes exhibited by mice deficient for individual SLC26 polypeptides include urolithiasis, hepatotoxicity, distal renal tubular acidosis, and male infertility. These phenotypes reflect tissue-specific defects in transepithelial Cl− absorption and secretion, HCO3− absorption and secretion, oxalate secretion, I− secretion, and SO42− uptake, along with their downstream sequellae Additional reviews on aspects of the SLC26 gene family include (Aronson, 2010; Dorwart et al., 2008b; Kere, 2006; Markovich, 2011; Ohana et al., 2009; Price and Howitt, 2011; Sharma et al., 2011a)
2. General Features of SLC26/SulP Structure and Function
The mammalian SLC26 gene family comprises 11 genes, SLC26A1-A11 (Figure 1, Table 1). SLC26 and SLC26-related SulP (sulfate permease) genes have been described in all nearly all organisms studied. These genes encode polypeptides with cytoplasmic N- and C-termini flanking a transmembrane domain of unknown structure, modeled to span the lipid bilayer 10–14 times (Figure 2). The C-terminal cytoplasmic region of all SLC26 and most SulP proteins includes a "sulfate transporter and anti-sigma factor antagonist (STAS) domain" (Aravind and Koonin, 2000). Anti-sigma factor antagonists counter the activity of repressors (anti-sigma factors) of sigma (transcription) factors in the bacterial sporulation stress response pathway (Masuda et al., 2004). The SpoIIAA anti-sigma factor antagonist of B. subtilis is the prototype STAS domain (Aravind and Koonin, 2000; Sharma et al., 2011a). SpoIIAA-like STAS domain structures have been solved by X-ray crystallography from the putative HCO3− transporter YchM from E. coli (Babu et al., 2010) and from rat SLC26A5/prestin (Pasqualetto et al., 2010). NMR solution structures have been solved for the STAS domain of putative sulfate transporter Rv1739c from M. tuberculosis (Sharma et al., 2011b). Mammalian STAS domains differ from anti-sigma factor antagonists in the nominally unstructured "intervening seqence" (IVS) inserted between helix α1 and β3. No function has yet been reported for the IVS, and its deletion was required for production of the first STAS domain crystals diffracting to high resolution (Pasqualetto et al., 2010). The mammalian and bacterial STAS domains reported to date have been monomeric in solution. A small number of bacterial SulP transporters lack a C-terminal STAS domain, but possess in its place an enzymatically active (Nishimori et al., 2010) β-carbonic anhydrase domain (Felce and Saier, 2004). The transport activity of these holoproteins has not been expressed, but they are presumed to serve as HCO3− or CO32− transporters.
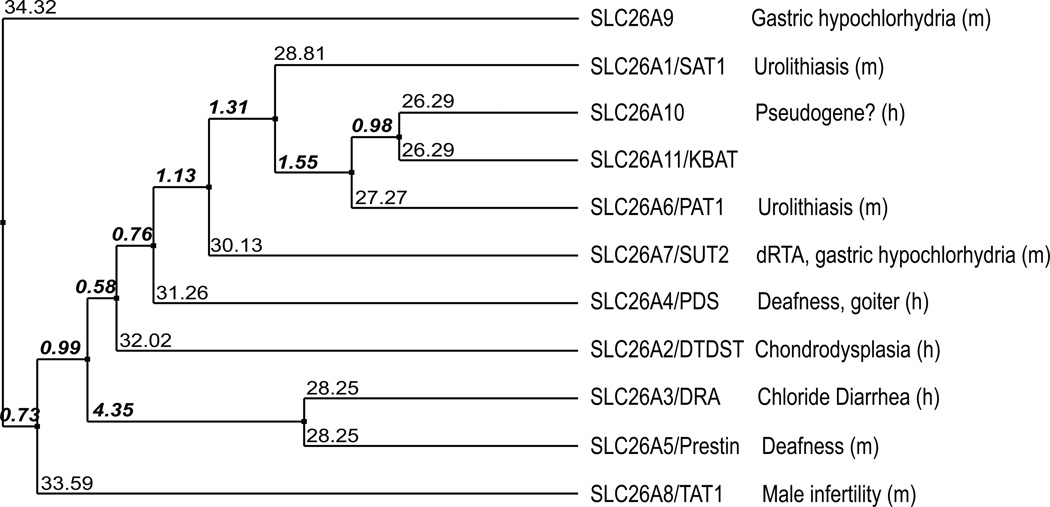
Figure 1.
Phylogenetic relationship of human SLC26 polypeptides generated with Jalview (http://www.jalview.org) using NCBI protein sequences listed in Table 1. Distance matrices were calculated from % sequence identity using average distance algorithm (UPGMA). Average relative distances are shown in bold italics. Sequence identities are shown without italics.
Table 1.
The SLC26 Multifunctional Anion Exchanger /Anion Channel gene Family
| Human gene name |
Protein names | Predominant Substrates1 |
Tissue distribution2 (Subcellular localization) |
Human Mendelian Disease (KO mouse phenotype) |
Human Chr. Locus3 |
RefSeq ID4 | Splice variants5 |
|---|---|---|---|---|---|---|---|
| SLC26A1 | SAT1/SLC26A1 | E: SO42−, oxalate, glyoxylate | Hepatocytes Renal ProxTubule Intestine (basolateral) |
(oxalate urolithiasis, nephrocalcinosis, urinary sulfate wasting, hepatotoxicity) | 4p16.3 rev NC_000004.11 972861–987224 |
NM_022042.2 NP_071325.2 |
3 mRNAs 2 ORFs |
| SLC26A2 | DTDST/SLC26A2 | E: SO42−, oxalate, Cl− | Chondrocytes Renal Prox Tubule Intestine, Panc duct (apical) |
Diastrophic dysplasia Diastrophic dysplasia broad bone-platyspondylic variant Atelosteogenesis II Achondrogenesis IB Multiple epiphyseal dysplasia type 4 De la Chapelle dysplasia |
5q32 NC_000005.9 149340300–149366963 |
NG_007147.1 NM_000112.3 NP_000103.2 |
1 mRNA 1 ORF |
| SLC26A3 | DRA/CLD/SLC26A3 | E: Cl−, HCO3−, oxalate | Enterocytes, sperrm Epididymis (apical) |
Congenital Chloride Diarrhea |
7q31 rev NC_000007.13 107405912–107443678 |
NG_008046.1 NM_000111.2 NP_000102.1 |
1 mRNA 1 ORF |
| SLC26A4 | PDS/Pendrin/SLC26A4 | E: I−, Cl−, HCO3− | Cochlear, vestibular Epithelial cellls, Thyrocytes Type B intercalated cell Airway epithelial cell (apical) |
Pendred Syndrome Deafness (DFNB4) + enlargement of the vestibular acqueduct |
7q31 NC_000007.13 107301080–107358254 |
NG_008489.1 NM_000441.1 NP_000432.1 |
1 mRNA 1 ORF |
| SLC26A5 | Prestin/SLC26A5 | E: (Cl−, formate, oxalate, SO42− | Cochlear hair cells | (deafness) | 7q22.1 rev NC_000007.13 102993177–103086624 |
NG_023055.1 NM_001167962.1 NP_001161434.1 |
5 mRNAs 5 ORFs |
| SLC26A6 | CFEX/PAT1/SLC26A6 | E: Cl−, HCO3−, oxalate, OH−, formate | Enterocytes Pancreatic duct Renal Prox Tubule Cardiac myocytes Sperm |
(grossly normal: loss or reduction of cAMP-stimulated HCO3− secretion in GI epithelia) | 3p21.3 rev NC_000003.11 48663156–48672926 |
NM_001040454.1 NP_001035544.1 |
4 mRNAs 4 ORFs |
| SLC26A7 | SUT2/SLC26A7 | E: Cl−, HCO3−, OH−, SO42− Ch: Cl− | Gastric Parietal Cells Type A Intercalated Cells Endothelial cells (apical, lysosome) |
(gastric hypochlorhydria, distal renal tubular acidosis) | 8q23 NC_000008.10 92261516–92410378 |
NM_052832.2 NP_439897.1 |
2 mRNAs 2 ORFs (Cterm var) |
| SLC26A8 | TAT1/SLC26A8 | E Cl−, HCO3−, OH− | Male germ cells Sperm |
(male infertility) | 6p21 rev NC_000006.11 35911291–35992413 |
NM_001193476.1 NP_001180405.1 |
3 mRNAs 2ORFs +/− 2 exons |
| SLC26A9 | SLC26A9 | E: Cl−, HCO3, Ch: Cl−, HCO3− | Airway epithelial cells Gastric parietal cells Kidney (?cell type) Brain (?apical) |
(gastric hypochlorhydria) | 1q32.1 rev NC_000001.10 205882176–205912588 |
NM_052934.3 NP_443166.1 |
2 mRNAs 2 ORFs Cterm var |
| SLC26A10 | (SLC26A10) | Widespread (? Transcribed Pseudogene) |
Not reported | 12q13 NC_000012.11 58013693–58019934 |
NM_133489.2 NP_597996.2 |
1 mRNA 1 ORF |
|
| SLC26A11 | SUT1/KBAT/SLC26A11 | E: Cl−, HCO3−, SO42−, Oxalate ?Ch: Cl− | Renal Intercalated cells (apical), panc duct Endothelial cells Brain, Widespread |
Not reported | 17q25.3 NC_000017.10 78194200–78227308 |
NG_008229 NM_001166347.1 NP_001159819.1 |
4 mRNAs 1 ORF |
E: anion exchanger activity; Ch: anion channel activity
Partial list of tissues in which expression has been doumented
Human chromosomal localization data from reference GRCh37.p5 Primary Assembly
RefSeq data includes gene sequence (NG) when available, as well as first listed mRNA (NM) and first listed polypeptide (NP) sequences.
# transcripts and # encoded polypeptides from RefSeq as of 3/11/2012.
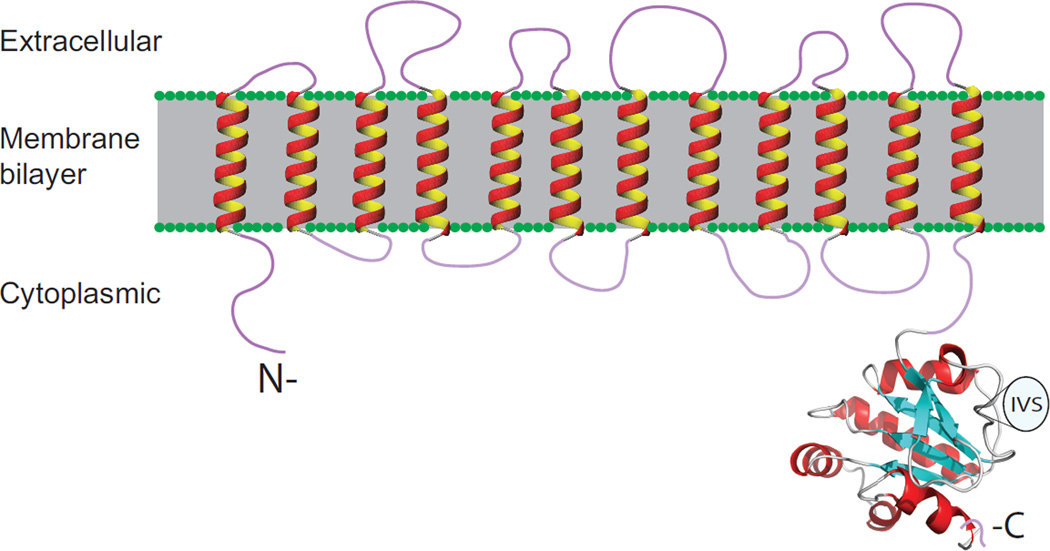
Figure 2.
Structural topology model of SLC26 polypeptides showing the short cytoplasmic N-terminal region followed by a transmembrane domain with 12 putative membrane-spanning α-helices, and the C-terminal cytoplasmic region, largely comprising the STAS domain. The STAS domain shown is the backbone structure of the human pendrin STAS domain encompassing aa 515–734 (excluding the intervening sequence (IVS) region of aa 566–653 between helix α1 and strand β3), as modeled with PyMOL from the X-ray crystal structure of rat SLC26A5/prestin (PDB ID 3LLO).
The transmembrane domain of SLC26/SulP polypeptides is believed to have both N- and C-termini located at the cytosolic membrane face. The number of transmembrane spans in cyanobacterial SulP protein BicA has been estimated at twelve by topographical testing of phoA/lacZ fusion proteins (Price et al., 2011) (Figure 2). Despite minimal overall sequence homology, modeling and functional mutagenesis data are consistent with the interesting hypothesis that the SLC26 transmembrane structural fold resembles, at least in part, that of the Clc Cl−/H+ exchanger/anion channel proteins (Ohana et al., 2009). No crystal structure or electron diffraction structure for a SulP or SLC26 transmembrane domain has been reported, but multiple lines of evidence support homo-oligomeric (Currall et al., 2011; Navaratnam et al., 2005; Zheng et al., 2006) or, more specifically, dimeric (Compton et al., 2011; Detro-Dassen et al., 2008) or tetrameric structures (Hallworth and Nichols, 2012; Mio et al., 2008; Wang et al., 2010b; Zheng et al., 2006). Each subunit is thought to constitute its own anion translocation pathway (Ohana et al., 2009), but co-expression of two mutant prestin polypeptides with distinct voltage-dependence properties yielded a novel electrical signature, suggesting interdependence of protomer function within the oligomer (Detro-Dassen et al., 2008). Regulated modulation of oligomeric state has not been reported.
SLC26 polypeptides have been characterized as anion exchangers and anion channels. They have been shown to transport halides (Cl−, I−, Br−), thiocyanate (SCN−), monovalent oxyanions (OH−, HCO3−, NO3−, formate, glyoxylate), and divalent oxyanions (SO42−, oxalate) with narrow or broad anion selectivities characteristic of each gene product (Chernova et al., 2005; Ohana et al., 2011; Ohana et al., 2009). Anion exchange activity has been reported for most SLC26 polypeptides, but only SLC26A7 and SLC26A9 have been shown to function additionally or exclusively as anion channels. SLC26-mediated anion exchange has been observed to be electroneutral (Chernova et al., 2005; Heneghan et al., 2010; Ohana et al., 2012; Ohana et al., 2011; Shcheynikov et al., 2008) or electrogenic (Clark et al., 2008; Shcheynikov et al., 2006), depending on the SLC26 polypeptide and its cis- and trans-substrates, and perhaps also on the tissue or expression system (Alper et al., 2011). Evidence for Na+ cotransport has been presented for SLC26A9 (Chang et al., 2009b) and for a bacterial SulP (Price and Howitt, 2011). The Sultr sulfate transporters of yeast and plants are likely electrogenic H+/SO42− cotransporters (Takahashi, 2012). Recent mutagenesis and ion substitution experiments that alter transport stoichiometry and selectivity (Ohana et al., 2011) are consistent with a diversity of transport mechanisms mediated by the (presumably uniform but as yet undefined) structural fold of the SLC26/SulP superfamily.
3. Prokaryotic SulP/SLC26 Transporters
Marine cyanobacteria constitute through their photosynthetic activity a major component of oceanic carbon fixation and a major contributor to oceanic pH regulation. Cyanobacteria deploy at least five inorganic carbon uptake mechanisms across a range of substrate affinities and transport capacities to maintain a cytosol:ocean HCO3− gradient as high as 103. One of these mechanisms is mediated by the SLC26-related SulP protein BicA, a high-flux, low affinity Na+-dependent HCO3− transporter (Price and Howitt, 2011; Price et al., 2004). BicA and other inorganic carbon transporters provide cytosolic HCO3− for carboxysomal uptake and generation of 3-phosphoglycerate, for subsequent return to the cytosol for anabolic biosynthetic processes.
E. coli has a single SulP polypeptide, YchM. Attempts to crystallize the YchM STAS domain led to spontaneous co-crystallization with acyl carrier protein (ACP) liganded with malonyl coA (Babu et al., 2010). Intact E coli deleted in YchM or rescued with YchM lacking the STAS domain exhibited 25–40% reduction in Na+-dependent [14C]HCO3− incorporation into acid-stable material, consistent with YchM function as a Na+-dependent HCO3− transporter. Engineered YchM deletion exhibited synthetic lethality with multiple fatty acid biosynthesis genes, and the YchM polypeptide physically interacted with many proteins of the fatty acid biosynthetic pathway. YchM was thus proposed to supply HCO3− to a membrane-scaffolded complex of fatty acid biosynthetic enzymes (Babu et al., 2010).
ACP and fatty acyl-coA transferases are also required at multiple stages of microbial polyketide synthesis. Indeed, STAS domain-encoding SulP genes have been found as components of antibiotic operons, including the BLM bleomycin biosynthesis operon of Streptomyces verticillus (Du et al., 2000), and as components of penicillin biosynthesis operons in S. coelicolor, S avermitilis and other antibiotic-producing strains of Streptomyces. These yet unstudied proteins may thus provide HCO3− or SO42− to the antibiotic biosynthetic pathways.
The genome of M. tuberculosis includes three open reading frames encoding SulP polypeptides. Overexpression of one of these, Rv1739c, led to increased [35S]-SO42− uptake into intact E. coil that was not inhibited by added Cl−, HCO3−, oxalate, or formate (Zolotarev et al., 2008). Mycobacterial incorporation of SO42− into outer membrane sulfolipid is important to pathogenicity (Hatzios and Bertozzi, 2011), but complementation with Rv1739c did not suffice to rescue SO42− auxotrophy in a mutant of the ABC sulfate transporter complex. Nonetheless, deletion in mycobacteria of ABC sulfate transporter complex subunits does not prevent infection. Indeed, expression of SulP genes Rv1739c and Rv1707 was upregulated in activated macrophages 24 hrs post-infection, and so may play a role in mycobacterial survival, latency, or pathogenesis in hypoxic or other stress conditions. The STAS domain of Rv1739c binds and changes conformation in response to guanine nucleotides (Sharma et al., 2012; Sharma et al., 2011b). Associated GTPase activity was much lower than that of SpoIIAA (Sharma et al., 2011b), and may require auxiliary proteins or different conditions for expression.
M. tuberculosis Rv3273 encodes a SulP transporter fused not to a C-terminal STAS domain, but rather to an enzymatically active (Nishimori et al., 2010) β-carbonic anhydrase domain (Felce and Saier, 2004). SulP-βCA proteins are encoded in the genomes of multiple other bacterial species, as well, including the pathogens Legionella and Leptospira, but constitute a small minority of the numerous bacterial SulP proteins. Although likely encoding HCO3− transporters, the regulation and function of SulP-βCA genes and proteins remain unknown.
Synechococcus SulP LtnT encodes a (likely inactive) C-terminal cNMP-binding domain in place of a STAS domain. LtnT rescued low affinity nitrate uptake in a mutant lacking nitrate uptake by the nrtABCD system, and the inhibitory cNMP domain was disinhibited by a His kinase relay system (Maeda et al., 2006). Additional bacterial SulP genes are predicted to encode cNMP domains situated C-terminal to a STAS domain. Other domains predicted in rare ORFs to be situated C-terminal to SulP STAS domains include a Rhodanese domain, an ADP-ribosyl glycohydrolase domain, and a t-SNARE-like domain. A few ORFs predict a nuclear pore complex component domain between the SulP transmembrane and STAS domains. Bacterial SulP transport functions have not yet been reconstituted from isolated proteins into lipid vesicles or bilayers, or functionally expressed in bacterial membrane vesicles.
4. Yeast and Plant Sultr/SLC26 Transporters
The yeast S. cerevisiae exhibits minimal Cl− transport, but can vary SO42− transport rates by 105-fold between replete and starved states (Jennings and Cui, 2012). Plasmalemmal SO42− uptake into yeast is mediated by two SLC26-type gene products, Sul1p and Sul2p (Cherest et al., 1997). In conditions of sulfur starvation, Sul2p mediates 80% of influx. The catalytic turnover of the transport process, rather than cytosolic or extracelllar [SO42−], governs the rate of Sul2p degradation, likely protecting the cell from the toxic environmental substrate, chromate, and the potentially toxic micronutrients selenate and molybdate, as well as from metabolic SO42− excess. Sul1p and Sul2p mediate SO42− influx as cotransport with H+, with still uncertain stoichiometry that may itself be subject to regulation (Jennings and Cui, 2012).
Isolated S. cerevisiae vacuoles also mediate SO42− accumulation against a SO42− gradient. The non-essential gene product Ygr125w is a SulP-STAS protein with a C-terminal cNMP-bdg domain under GATA factor regulation. The Ygr125w-GFP fusion protein localizes to the vacuole, suggesting this protein as a candidate vacuolar sulfate transporter. CDART also predicts SulP-βCA-encoding genes in S. cerevisiae, Neurospora, Aspergillis, Candida, Penicillium, Trichophyton, the plant fungus Ustilago, and the algae Chlorella and Volvox. Experimental demonstration of these proteins and their putative roles as HCO3− transporters remain to be investigated.
The genome of the weed Arabidopsis thaliana encodes at least 14 SulP/Sultr genes in 5 clades. These transporters are expressed in distinct subcellular membranes of specific tissues and with developmental stage-specific patterns. Although most are SO42− transporters, some (such as SHST1 from the tropical plant Stylosanthes hamata exhibit preferential nM affinity uptake of molybdate (Fitzpatrick et al., 2008), and at least one regulates cellular levels of phytate (Ye et al., 2011). The most extensively studied are plasmalemmal SO42− transporters Sultr1;1 and Sultr1;2. Deletion of both transporters produces greater sulfate transport deficiency and selenate tolerance than of either alone (Barberon et al., 2008). Experiments in plant cells and with yeast mutant complementation suggest a transport stoichiometry of 3H+/SO42− (Buchner et al., 2004). The STAS domain of Sultr1;2 is essential to holoprotein plasmalemmal targetting and influences both transport kinetics and protein stability (Shibagaki and Grossman, 2006). This STAS domain also binds and acivates the enzyme O-acetylserinelyase/Cysteine synthase (OASTL). Conversely, OASTL coexpression downregulates Sultr1;2-mediated SO42− uptake (Shibagaki and Grossman, 2010). Thus Sultr1;2 and STAS domain-bound OASTL may constitute a SO42− transport metabolon.
5. SLC26 transporters of animals
5.1. SLC26A1 (Sat-1)
SLC26A1/Sat1 was cloned as a hepatic sulfate transporter (Bissig et al., 1994), and subsequently characterized as an anion exchanger also transporting oxalate, glyoxylate, and Cl− (Schnedler et al., 2011; Xie et al., 2002). SO42− uptake is increased by acid pHo and greatly stimulated (through an unknown mechanism) by low concentrations of cis Cl− or other monovalent anions (Satoh et al., 1998; Xie et al., 2002). The transporter resides at the basolateral membrane of hepatocytes, enterocytes and proximal tubular epithelial cells (Figure 3A), accumulating there by a mechanism that relies in part on a dileucine sorting motif (Regeer and Markovich, 2004). Female sex steroids post-transcriptionally reduce levels of SLC26A1 in liver, but not in kidney, perhaps contributing to the elevated serum oxalate levels and urinary oxalate excretion observed preferentially in males (Brzica et al., 2009). The Slc26a1 promoter includes response elements regulated by thyroxine and transcription factor AP-1 (Markovich, 2011), but in vivo transcriptional regulation has not been reported.
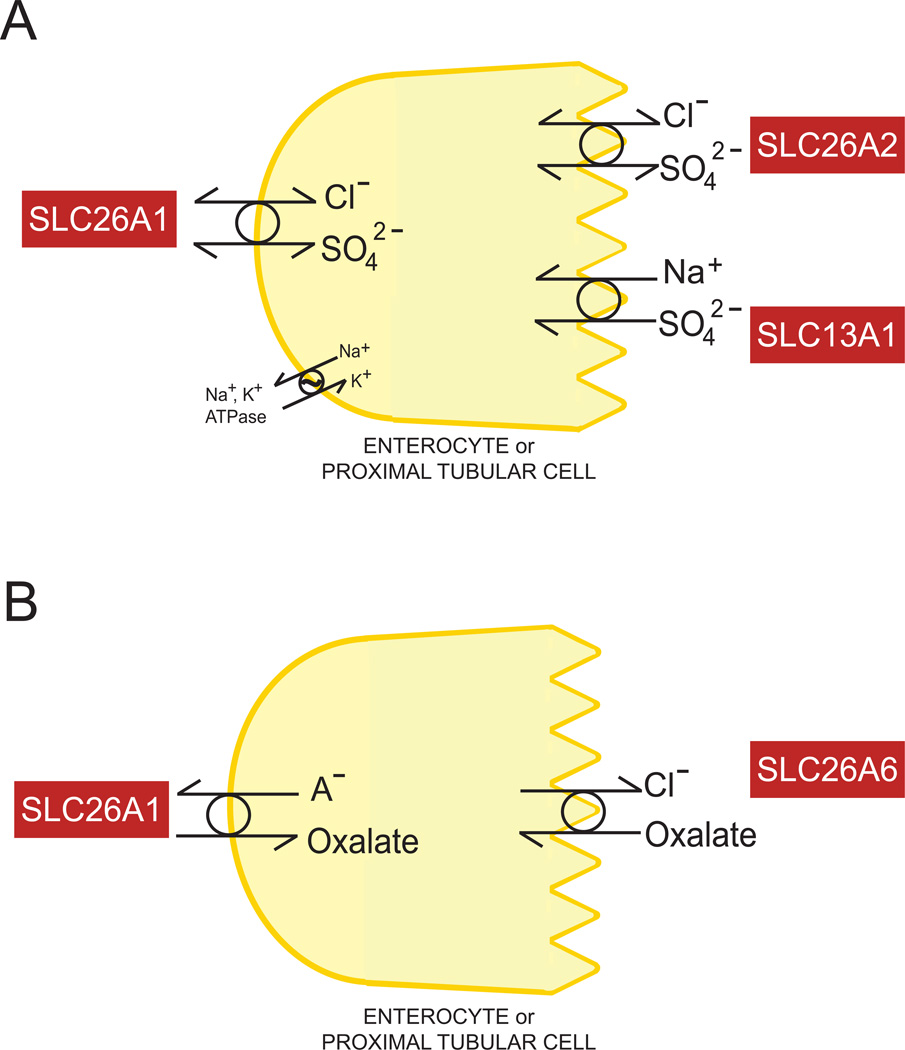
Figure 3.
A. Schematic diagram of enterocyte or proximal tubular SO42− absorption and secretion involving apical SLC26A2 and SLC13A1, and basolateral SLC26A1. B. Schematic diagram of enterocyte or proximal tubular oxalate secretion involving basolateral SLC26A1 and apical SLC26A6.
No human disease has been linked to mutations or polymorphisms in the SLC26A1 gene. However, the Slc26a1−/− mouse exhibits hyposulfatemia, hypersulfaturia, and urolithiasis and nephrocalcinosis in the setting of hyperoxaluria (Figure 3B). Hepatic oxalate production appeared unchanged, but increased acetaminophen-induced hepatotoxicity may have reflected reduced availability of intracellular SO42− for drug conjugation reactions (Dawson et al., 2010; Markovich, 2011). SLC26A1 is localized to the basolateral membrane of the P2 proximal tubule in euryhaline eels, where it likely contributes to renal sulfate adaptation responses (Watanabe and Takei, 2011).
5.2. SLC26A2 (DTDST)
SLC26A2 was first identified by positional cloning as the gene underlying autosomal recessive diastrophic dysplasia (Hastbacka et al., 1994). The encoded protein was named DTDST (diastophic dysplasia sulfate transporter) after test of its transport function was inspired by homology with SLC26A1 and a previously identified Neurospora SO42− permease. Numerous SLC26A2 mutations have since been identified in five additional phenotypically related human recessive chondrodysplasia syndromes (Table 1) (Jackson et al., 2012). An SLC26A2 chondrodysplasia has been partially phenocopied in the Slc26a2 A386V knock-in mouse, which exhibited post-natal growth retardation, reduced mobility, and 50% mortality by P21 associated with skeletal abnormalities and reduced epiphyseal growth plate thickness. Chondrocyte proliferation and differentiation were delayed, and cultured chondrocytes were nearly devoid of SO42− uptake (Forlino et al., 2005), reflecting in vivo chondrocyte inability to utilize extracellular cysteine as a source of SO42− for macromolecular sulfation (Pecora et al., 2006), and leading to undersulfated proteoglycans of cells and matrix, disorganization of collagen fibers, premature onset of mineralization (Cornaglia et al., 2009) and failure of fibronectin matrix assembly (Galante and Schwarzbauer, 2007). BMP-2 transcriptionally up-regulated SLC26A2 in C3H10T1/2 chondrocytes through interactions with a xenobiotic response element, and binding sites for SP-1 and CFBA1 in the SLC26A2 promoter (Kobayashi et al., 1997).
SLC26A2 is widely expressed in other tissues during development (Haila et al., 2001), in the apical membrane of renal proximal tubules (Chapman and Karniski, 2011), and in the intestinal tract (Haila et al., 2001; Heneghan et al., 2010) (Figure 3A). SLC26A2 also provides SO42− for sulfation of sialyl groups of the Lewis(x) antigen in normal colonic epithelial cells. In contrast, Lewis(x) antigen sialyl groups are unsulfated in colon cancer cells, associated with epigenetic suppression of SLC26A2 expression reversible by histone deacetylase inhibitors. SLC26A2 expression was reduced in colon cancer biopsies (Galamb et al., 2008), and knockdown of SLC26A2 in culture cancer cells increased proliferation (Yusa et al., 2010), highlighting a role for SLC26A2 in colon cancer biology. SLC26A2 was upregulated in Crohn's colitis (Comelli et al., 2009), and has been proposed as part of a diagnostic index distinguishing Crohn's from ulcerative colitis (von Stein et al., 2008).
SLC26A2 mediates electroneutral anion exchange, accepting as substrates SO42−, oxalate, Cl− (Heneghan et al., 2010) and OH−, with additional capacity to transport I−, Br− and NO3−. The electroneural exchange mechanism accommodates SO42−/oxalate exchange or divalent exchange with two monovalent anions (SO42−/2Cl− or SO42−/(Cl− + OH−) (Ohana et al., 2012). The contribution of SLC26A2 to intestinal and renal oxalate transport remains unknown (Figure 3B), but neither hyperoxaluria nor increased nephrocalcinosis or lithiasis has been noted among mice or patients with SLC26A2 chondrodysplasias. Mutation of conserved transmembrane domain residue E417 of mouse SLC26A2 abolished transport without reducing cell surface expression. Mutation of F368 differentially altered affinities for extracellular SO42− and Cl−, while leaving unchanged the SLC26A1-like positive regulation by extracellular Cl− (Ohana et al., 2012).
5.3. SLC26A3 (DRA, CLD)
SLC26A3 was first identified as a candidate tumor suppressor gene (Down-regulated in adenoma, DRA) (Schweinfest et al., 1993). However, its identification by positional cloning as the gene mutated in recessive congenital chloride-losing diarrhea (CLD) (Hoglund et al., 1996) led to the demonstration of its Cl−/HCO3− exchange activity (Chernova et al., 2003; Melvin et al., 1999). SLC26A3 expression is predominantly in the intestine, where it is most abundant in colon and duodenum, and less abundant in ileum and jejunum. In duodenum, SLC26A3 is relatively enriched at the crypt and lower villus, whereas mouse SLC26A6 predominates at the upper villus, but both are detectable along the entire crypt-villus axis. Apical membrane SLC26A3-mediated Cl−/HCO3− exchange functions in a coupled manner with NHE3-mediated Na+/H+ exchange to generate electroneutral NaCl reabsorption across the intestinal mucosa (Walker et al., 2009; Walker et al., 2008) (Figure 4A). In some regions of the intestine, however, these anion and cation transporters are differentially expressed and can function independently (Talbot and Lytle, 2010).
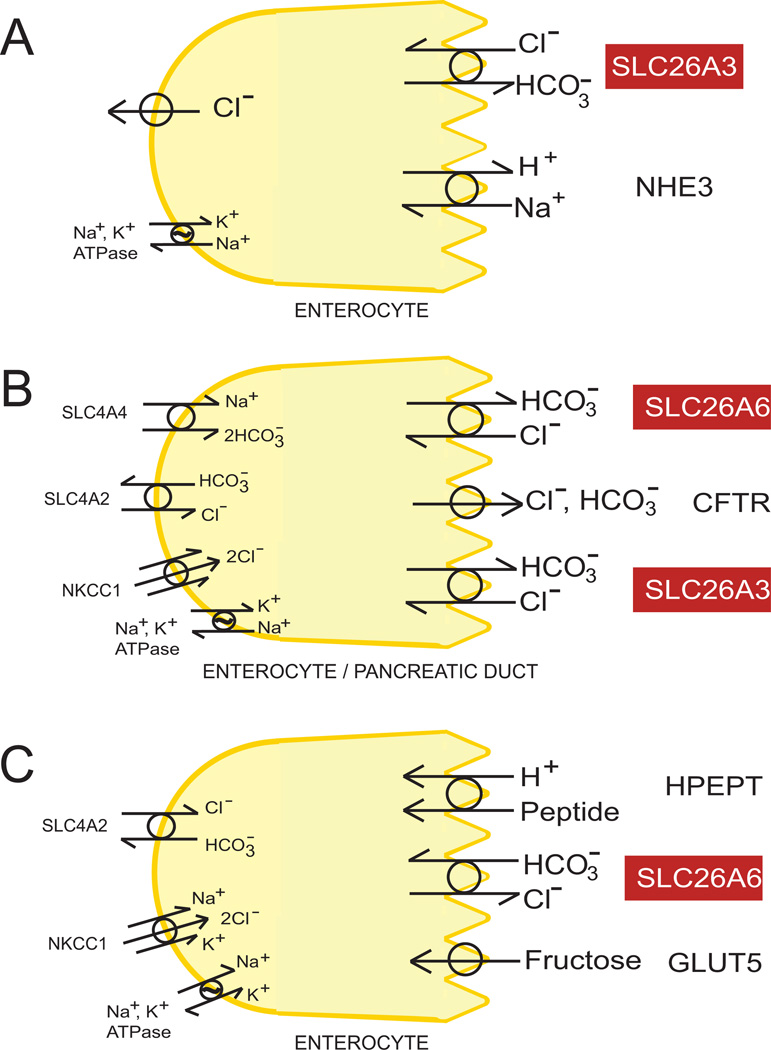
Figure 4.
A. Schematic diagram of enterocyte NaCl absorption mediated by coupled function of SLC26A3 and NHE3. The basolateral Cl− exit pathways may be multiple. B. Schematic of enterocyte or pancreatic duct cell secretion of HCO3− and Cl− via coordinated action of apical CFTR, SLC26A6, and SLC26A3. C. Schematic of enterocyte apical SLC26A6 facilitating H+-peptide cotransport by apical HPEPT, and being faciliated by apical GLUT5-mediated fructose transport. Carbonic anhydrases are required for activities in all panels (not shown).
Nearly 60 independent mutations in SLC26A3 have been associated with congental chloride diarrhea in more than 250 patients (Wedenoja et al., 2011), including one of the first patients whose diagnosis resulted from whole exome sequencing (Choi et al., 2009b). Distinct founder mutations have been characterized in Finland, Poland, and the Arab Middle East, and the majority of mutations are missense. Functional studies of SLC26A3 disease missense mutations have revealed loss-of-function, largely due to destabilization or trafficking abnormalities, without evident genotype/phenotype correlation. Disease mutant ΔY527 is remarkable for partial retention of Cl−/HCO3− exchange activity in heterologous expression systems (Chernova et al., 2003; Dorwart et al., 2008a). Modifier genes likely influence CLD, since siblings with identical SLC26A3 mutations can exhibit different disease severity (Hoglund et al., 2001). The Slc26a3−/− mouse also exhibits chloride-losing diarrhea and systemic volume depletion (Schweinfest et al., 2006).
Integrity of the SLC26A3 STAS domain is required for surface expression in mammalian cells (Dorwart et al., 2008a) and oocytes, but the region C-terminal to the cytoplasmic STAS domain, including the PDZ recognition motif, is dispensable for oocyte surface expression (Chernova et al., 2003; Lamprecht et al., 2009). N-glycosylation of SLC26A3 is important for optimal surface expression and protein stabilization, but is not required for anion exchange activity (Hayashi and Yamashita, 2012).
Several reports (Ko et al., 2002; Shcheynikov et al., 2006; Xie et al., 2002) documented electrogenic 2Cl−/1HCO3− exchange by mouse SLC26A3 expressed in Xenopus oocytes, as detected by simultaneous measurement of intracellular [Cl−] and pH in tandem with membrane potential or currents. This conclusion was supported by modification of SLC26A3 Cl−/HCO3− exchange stoichiometry from 2:1 to 1:1 by the conserved residue mutation E367A, without changing associated uncoupled SCN− current (Ohana et al., 2011).
However, the electroneutral nature of intestinal NaCl reabsorption (Musch et al., 2009) and the electroneutral mechanism of Na+/H+ exchange (Orlowski and Grinstein, 2004; Zachos et al., 2005) are consistent with multiple reports of electroneutral SLC26A3-mediated Cl−/HCO3− exchange in multiple recombinant expression systems (summarized in (Alper et al., 2011), including for multiple species in Xenopus oocytes (Chernova et al., 2003; Stewart et al., 2011)). SLC26A3 is responsible for nearly all electroneutral Cl− reabsorption across mouse jejunum (Walker et al., 2008). In Slc26a3−/− duodenum, basal and cAMP-stimulated HCO3− secretion are reduced by 50 and 60%, respectively, without any change in basal or stimulated short circuit current (Walker et al., 2009). Similarly, stimulation of duodenal bicarbonate secretion by specific pharmacological inhibition of apical NHE3 elicited no significant increase in short-circuit current (Singh et al., 2010). Moreover, in the setting of the systemic dehydration associated with reduced NaCl reabsorption in Slc9a3−/− mice, duodenal HCO3− secretion is elevated up to three-fold without significant increase in electrogenic HCO3−-dependent secretory short-circuit current (Hoover, 2010). In addition, the large mucosal Cl−-stimulated increase in mouse cecal HCO3− secretion was unaccompanied by change in short-circuit current (Kawamata et al., 2006). In addition, Cl−/HCO3− exchange and 36Cl− transport were completely abolished in Slc26a3−/− cecal mucosa without change in short circuit current, whereas lumen Cl− removal-induced depolarization of the apical membrane was Slc26a3-independent, but required the presence of CFTR (Alper et al., 2011). These observations are consistent with mouse cecum's abundant expression of SLC26A3 in the presence of very low levels of SLC26A6. Since cecum also expresses minimal NHE3 (Talbot and Lytle, 2010), SLC26A3 in mouse intestine appears to mediate electroneutral Cl−/HCO3− exchange not only when functionally coupled with NHE3 to produce NaCl reabsorption, but also during HCO3− secretion in the absence of NHE3.
The C-terminal PDZ recognition motif in SLC26A3 binds the PDZ domain adaptor proteins PDZK1, E3KARP, and IKEPP in lipid rafts. Pharmacological disruption of lipid rafts leads to decreased SLC26A3 surface expression and function, in a manner that requires the C-terminal PDZ recognition motif. The PDZ recognition motif is also required for acute inhibition of SLC26A3 by purinergic or other elevation of intracellular Ca2+ (Lamprecht et al., 2009) and by PI3-kinase (Lissner et al., 2010), but not for acute regulatory inhibition of SLC26A3 by acidic pHi (Hayashi et al., 2009). However, the increased Cl−/base exchange activity in Caco-2 cells induced by lysophosphatidic acid (LPA), accompanied by increased SLC26A3 surface accumulation, was also mediated by PI-3 kinase, consistent with the antidiarrheal effect of LPA (Singla et al., 2010).
SLC26A3 interacts directly and functionally with CFTR (Figure 4B). cAMP activated SLC26A3 only in the presence of coexpressed CFTR (Chernova et al., 2003; Ko et al., 2002). Combined overexpression of SLC26A3 and CFTR in heterologous systems revealed that each protein stimulated activity of the other through direct interaction of the SLC26A3 STAS domain with the R domain of CFTR, as well as indirectly through mediation of PDZ domain proteins (Ko et al., 2002; Ko et al., 2004). The interaction with STAS domain required R domain phosphorylation in HEK-293 cells (Ko et al., 2004), but not when studied in solution with purified recombinant protein domains (Dorwart et al., 2008a). In contrast to overexpression studies in cell culture, experiments with Slc26a3−/− duodenum revealed an unaltered cAMP-stimulated short circuit current, suggesting no effect of SLC26A3 on Cftr-mediated electrogenic HCO3− secretion, despite SLC26A3-mediated electroneutral HCO3− secretion accounting for half of cAMP-stimulated HCO3− secretion (Walker et al., 2009). SLC26A3 is expressed in pancreatic duct, but the effects of SLC26A3 knockdown in cultured duct cells or of knockout in perfused or resealed pancreatic duct segments have not been reported.
SLC26A3 expression in the Hnf1a−/− mouse is reduced by 40% in the small intestine but not colon. Conditional deletion of both Hnf1a and Hnf1b completely abrogated Slc26a3 expression, producing a chloride-losing diarrhea accompanied by 20% weight loss within one week. Stimulatory Hnf1 binding sites were documented in the proximal promoter and intron 8 of the Slc26a3 gene (D'Angelo et al., 2010). Basal transcriptional activity of the SLC26A3 gene in colon carcinoma cells also required HNF-4. Butyrate, a product of endogenous colonic flora, stimulated SLC26A3 transcription through interaction of transcription factor YY1 and an unidentified GATA factor. SLC26A3 transcription was also stimulated by Lactobacilli through unknown mechanisms (Malakooti et al., 2011).
In contrast, inflammatory mediators suppressed SLC26A3 transcription. IL-1β and IFN-γ both suppressed transcription of SLC26A3 in Caco-2 cells. IFN-γ signalled through JAK1 and JAK2 kinases to activate cytosolic STAT1 and promote its nuclear translocation to a GAS element in the SLC26A3 promoter (Malakooti et al., 2011). Slc26a3 mRNA levels exhibited circadian variation in rat colon in parallel with changes in nutrient transport (Sotak et al., 2011).
Intestinal SLC26A3 mRNA was downregulated in patients with ulcerative colitis, and in the IL-10 transgenic colitis model (Yang et al., 1998), but SLC26A3 protein levels in patients were unchanged (Lohi et al., 2002b). Ileocolonic pH may be abnormally reduced in inflammatory bowel disease (IBD). In the IBD model of TNFα-overexpressing mice, ileal and colonic pH values were also low, with selective reduction of Slc26a3 mRNA and protein despite normal levels of Cftr and Slc9a3 (Xiao et al., 2012). Colonic crypt Cl−/HCO3− exchange activity was also reduced in ulcerative colitis patients in parallel with decreased SLC26A3 mRNA (Farkas et al., 2011). SLC26A3 polymorphisms were associated with increased ulcerative colitis in Japanese (Asano et al., 2009). Polymorphisms in the regulator of SLC26A3, HNF4A, associated in the Korean population with ulcerative colitis but not Crohn's disease (Yang et al., 2011). The cytopathic diarrheas of infection with enteropathogenic E. coli (Gill et al., 2007) and of C. rodentium colitis (Borenshtein et al., 2008) (Borenshtein et al., 2009) were also associated with reduced mucosal expression of SLC26A3.
5.4. SLC26A4 (PDS/Pendrin)
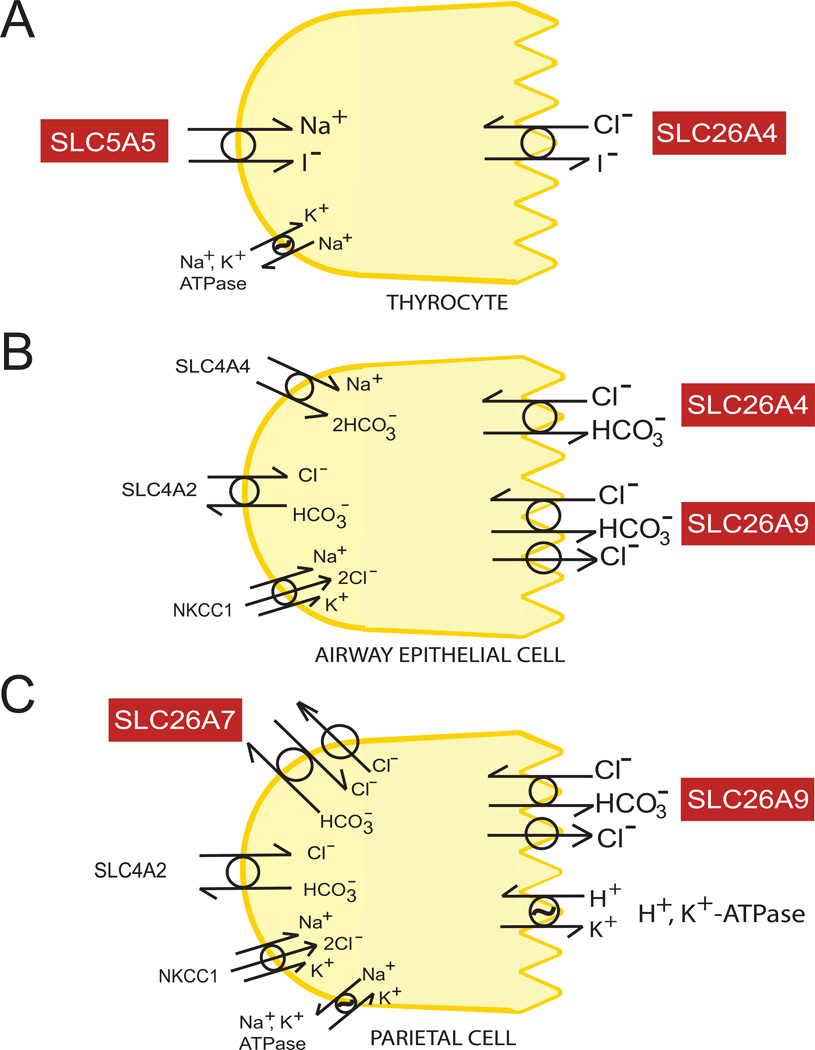
SLC26A4/PDS was identified by positional cloning (Sheffield et al., 1996) as the disease gene for autosomal recessive non-syndromic deafness DFNB4 and for Pendred Syndrome (deafness with enlargement of the vestibular aqueduct and variably penetrant, often euthyroid goiter). The thyroid can show impaired organification of iodide, without reduction of iodide uptake or TSH signaling. The SLC26A4 gene product, pendrin, is expressed in cochlear epithelial cells of the spiral prominence, root cells, and in spindle cells of the stria vascularis. Pendrin is also expressed in the apical membrane of epithelial cells of the endolymphatic sac, and in epithelial cells surrounding the hair cells of the saccule, utricle, and ampulla (Choi et al., 2011). In addition, pendrin is localized in the apical membrane of the thyrocyte (Bidart et al., 2000; Royaux et al., 2000) (Figure 6A), the apical membrane of the renal collecting duct Type B intercalated cell (Royaux et al., 2001) (Figure 5A), and in the apical membrane of airway epithelia (Nakao et al., 2008; Pedemonte et al., 2007) (Figure 5B) and salivary duct (Shcheynikov et al., 2008).
Figure 6.
A. Schematic of a thyrocyte secreting I− into the thyroid follicle by coordinated action of basolateral SLC5A5 (Na+/I− symporter) and apical SLC26A4. B. Airway epithelial cell secreting anions by activity of apical SLC26A4 and SLC26A9. (Apical CFTR and Ca2+-activated Cl− channels not shown). C. Gastric parietal cell secreting H+ by combined basolateral activities of SLC26A7, SLC4A2, and NKCC1, and apical activity of H+,K+-ATPase, with contribution from SLC26A9 (predominantly expressed in surface mucosal cells). Carbonic anhydrases are required for activities in panels B and C (not shown).
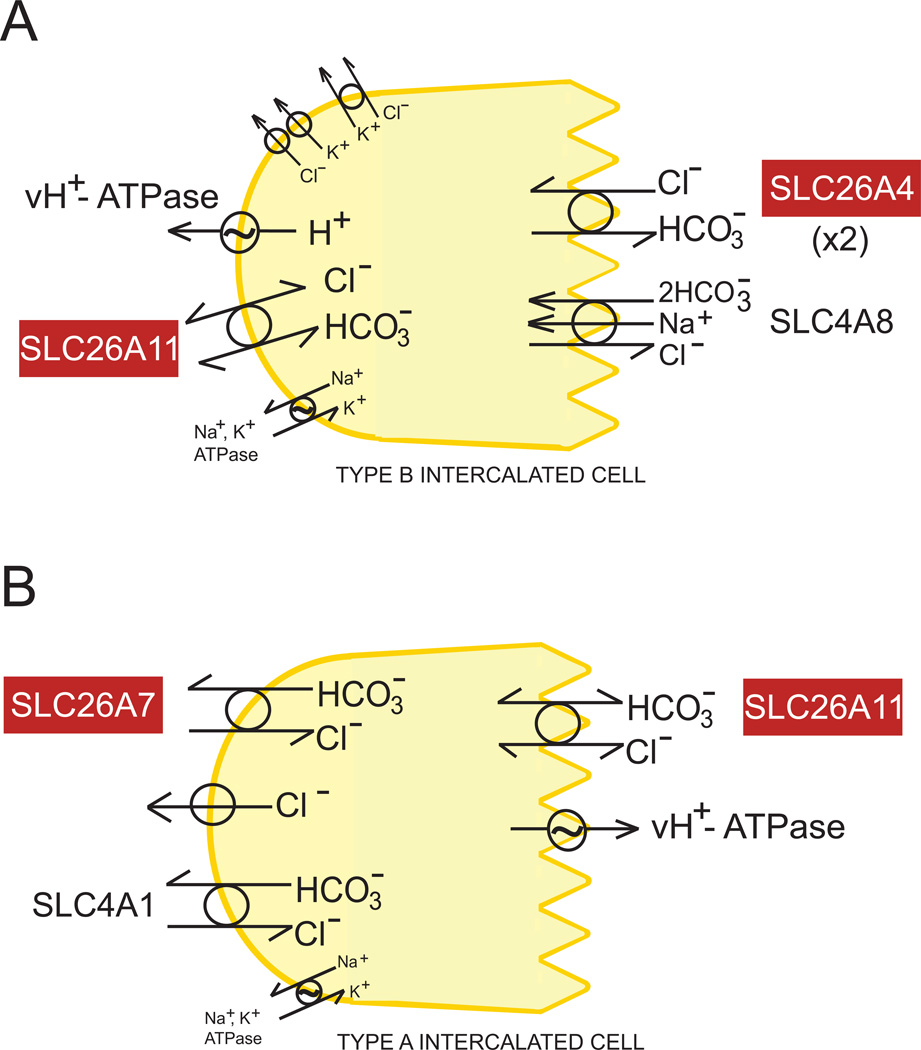
Figure 5.
A. Schematic of a renal cortical collecting duct Type B intercalated cell absorbing NaCl via coupled apical function of SLC26A4 and SLC4A8 (with contribution from basolateral vH+-ATPase), and secreting HCO3− via apical SLC26A4 and basolateral vH+-ATPase, with possible contribution of basolateral SLC26A11 (which also facilitates or may itself mediate anion conductance). B. Schematic of a renal collecting duct Type A intercalated cell secreting acid via the apical vH+-ATPase and basolateral SLC4A1 and SLC26A7, with possible contribution of apical SLC26A11. Carbonic anhydrases are required for activities in both panels (not shown).
Pendrin is an electroneutral anion exchanger (Reimold et al., 2011; Shcheynikov et al., 2008) with a broad anion substrate selectivity, including Cl−, HCO3−, I−, formate, nitrate, and SCN−. Most deafness-associated pendrin missense mutations lead to loss of function with intracellular retention, but a minority are hypofunctional at the cell surface (Choi et al., 2009a; Dai et al., 2009; Yoon et al., 2008). Some trafficking mutants can be rescued to the cell surface by chemical chaperones such as TMAO (Shepshelovich et al., 2005) or by low temperature incubation (Yoon et al., 2008). Loss of Cl−/HCO3− exchange with resultant acidification of endolymph (Wangemann et al., 2007) is thought to inhibit Ca2+ reabsorption via Trpv5 and Trpv6 (Nakaya et al., 2007). The elevated endolymph [Ca2+] ultimately leads to otoconial malformation (Everett et al., 2001) and in at least one type of Slc26a4−/− mouse to mega-otolith formation (Dror et al., 2010). The altered endolymphatic ion homeostasis is associated with reduced endocochlear potential reflecting reduced stria vascularis K+ channel activity (Wangemann et al., 2007). Enlargement of the stria vascularis displaces adjacent fibrocytes, with delayed induction of deiodinase activity, suggesting possible local hypothytroidism (Wangemann et al., 2009). The absence of pendrin function in mouse cochlea causes profound deafness only during the developmental period between E16.5 and P2. Loss of pendrin initiated at E18.5 produced a partial hearing loss phenotype phenotypically closer to the human disorder. Graded temporal changes in hearing paralleled changes in endolympatic potential and pH, and enlargement of the vestibular aqueduct (Choi et al., 2011).
The Slc26a4−/− mouse has no thyroid phenotype (Everett et al., 2001), even after dietary iodide deficiency (Iwata et al., 2011). Increasing evidence suggests that the pendrin is not a principal contributor to apical I− secretion into the thyroid follicle for thyroxine biosynthesis (Fong, 2011), despite its ability to mediate Cl−/I− or I−/HCO3− exchange in salivary duct (Shcheynikov et al., 2008), thyrocytes (Pesce et al., 2012), and renal collecting duct (Kim et al., 2009), where it reclaims filtered iodide from the urine.
In the renal Type B intercalated cell, apical pendrin is also responsible for a major component of electroneutral Cl− reabsorption coupled (at least in mouse) to the apical membrane Na+-dependent Cl−/HCO3− exchanger, SLC4A8 (Figure 5A). This pair of transporters mediates nearly half of CCD NaCl reaborption by a thiazide-sensitive mechanism independent of the classical thiazide receptor, NCC (Leviel et al., 2010). Slc26a4−/− mice are normotensive at baseline, but exhibit a greatly blunted hypertensive response to administration of DOCA-salt (Verlander et al., 2003) or of angiotensin II (Verlander et al., 2011), implicating the pendrin/SLC4A8 pathway in these models of hypertension. Absence of pendrin function also impairs ENaC activity through inhibition of mineralocorticoid-depedent increases in protein expression (Kim et al., 2007) and through reduced HCO3−-stimulated increases in ENaC activity and abundance (Pech et al., 2010). Pendrin is thus a target for novel diuretic development, although not yet identified as a risk modifier in human hypertension. The acidic urine of Slc26a4−/− mice resulting from decreased collecting duct HCO3− secretion is associated with hypercalciuria, likley reflecting downregulation of distal nephron TRPV5 and NCX1 (Barone et al., 2011).
Pendrin in the airway epithelial cell apical membrane (Figure 6B) mediates IL4-stimulated Cl−/SCN− exchange to provide substrate to lactoperoxidase to generate the anti-microbial, hypothiocyanite (Pedemonte et al., 2007). Pendrin is also induced in airways by allergy and rhinovirus infection, and IL-13, leading to increased thickness of airway surface liquid (Nakagami et al., 2008). Interferon-γ is thought to mediate part of pendrin regulation by cytokines. Mouse models of asthma and chronic obstructive lung disease exhibit pendrin upregulation, and consequent increased levels of MUC5AC, a major component of airway mucus. Pendrin overexpression in airways led to mucus overproduction and neutrophil recruitment (Nakao et al., 2008). However, genome-wide association studies in asthmatics have not yet identified pendrin as a candidate risk modifier gene.
Pendrin trafficking to the apical membrane is activated by cAMP in microperfused mouse corical collecting duct (CCD) and in polarized renal OKP cells via phosphorylation of S49 in the N-terminal intracellular domain of mouse pendrin (Azroyan et al., 2012). This response likely underlies in part the isoproterenol-induced increased NaCl reabsorption in CCD. cAMP activation of PKA-mediated phosphorylation at T717 of human pendrin has been implicated in TSH-stimulated pendrin trafficking in polarized thyrocytes (Pesce et al., 2012). These cAMP effects were not evident in oocytes overexpressing human pendrin (Reimold et al., 2011). Deletion of Slc26a4 had no effect on CFTR fuction in parotid duct, a tissue in which pendrin contributes to I− but not to HCO3− transport (Shcheynikov et al., 2008), whereas overexpressed CFTR activated pendrin in HEK-293 cells (Ko et al., 2002). Pendrin-mediated Cl−/Cl− exchange activity was pHi-independent in Xenopus oocytes (Reimold et al., 2011), but pendrin-mediated Cl−/HCO3− exchange activity in cultured cells was activated by both acidic pHi and acidic pHo (Azroyan et al., 2011).
Pendrin in kidney is regulated by systemic Cl− load, acid-base status or loading, aldosterone and angiotensin. pH, aldosterone, and uroguanylin all regulate pendrin at the transcriptional level in kidney (summarized in (Rozenfeld et al., 2012). Uroguanylin regulation is mediated at least in part by HSF-1. Transcription factor FOXI1 regulates pendrin expression in kidney and cochlea, and TTF-1 factors regulate pendrin transcription in thryoid. Pendrin is also regulated in thyroid cells by thyroglobulin, TSH, and iodide, and influenced by insulin and PKC-ε.
5.5. SLC26A5 (Prestin)
Cochlear outer hair cells (OHC) change their length and stiffness at frequencies of at least 70 kHz upon alteration of their membrane potential during auditory transduction (Frank et al., 1999). This electromotility arises from the piezoelectric effect of rapid conformational changes in SLC26A5/prestin of the OHC lateral membrane (Zheng et al., 2000), and is generally considered responsible at least in part for cochlear OHC amplification of incoming auditory signals sensed by the stereociliary mechanotransduction mechanism (Dallos, 2008). The Slc26a5−/− mouse exhibited a hearing threshold shifted by ~50 decibels (Cheatham et al., 2004; Liberman et al., 2002), as did a mutant prestin knockin mouse with other OHC mechanical properties closer to those of wildtype cells (Dallos et al., 2008). However, SLC26A5's early status as a human deafness gene (Liu et al., 2003) has been re-evaluated since discovery of the few deafness-associated coding variants among individuals with normal hearing (Minor et al., 2009).
Prestin-mediated electromotility is usually demonstrated in OHC or in prestin-transduced heterologous cells by measurement of nonlinear capacitance (NLC). NLC was proposed to arise from the ability of prestin to bind Cl− (in a salicylate-sensitive manner) and then move it across the bilayer electric field in a voltage-sensitive manner without releasing it on the extracellular side (Oliver et al., 2001). In contrast to the electromotility and lack of anion transport of mammalian prestin, prestins from chicken and zebrafish lack electromotility, but mediate 1:1 electrogenic exchange of Cl− with oxalate or SO42− (Schaechinger and Oliver, 2007). This transport function is of yet unknown significance to the OHC. Zebrafish prestin also exhibits some voltage-dependent charge movement, and so constitutes an intermediate form. Indeed, later investigations detected transport of oxalate and formate by mammalian prestin in CHO cells (but not in Xenopus oocytes), and discovered missense mutations that can abrogate anion transport but preserve NLC (Bai et al., 2009).
Systematic evaluation of chimeric rat/zebrafish prestins has defined two core regions that recapitulate most of the differences between mammalian and non-mammalian prestins: three residues within the first two putative transmembrane spans (rat aa 93, 101, and 136), and rat residues 381–438 comprising putative transmembrane spans 9–10. These changes in combination were necessary and sufficient to confer a form of fast NLC onto zebrafish prestin. However, the anion transport properties of zebrafish prestin were preserved, and substrate-induced activation of oxalate/Cl− exchange potentiated NLC amplitude, in contrast to intracellular oxalate's reduction of NLC in wildtype rat prestin (Schaechinger et al., 2011). Similar chimeras of gerbil prestin with chicken or zebrafish prestin highlighted the important contribution of gerbil prestin residues 158–168 (conserved across mammals) in the fast NLC response. Again, NLC was successfully transferred into non-mammalian prestin without loss of anion exchange function (Tan et al., 2012). The findings strengthen the hypothesis that wildtype prestin function involves an alternating access cycle of conformational change.
The three-dimensional structure of the rat prestin STAS domain has been solved by X-ray crystallography, but deletion of the IVS intervening sequence was required to achieve crystallization (Pasqualetto et al., 2010). Prestin can be co-immunoprecipitated with CFTR from cochlear extracts with or without prior cAMP treatment, and prestin colocalizes with CFTR in cochlear OHC. CFTR enhances prestin NLC in HEK-293 cells, but prestin does not alter CFTR conductance (Homma et al., 2010). The prestin STAS domain has also been reported to bind MAP1S, VAP-33, and to additional candidate interactor proteins (summarized in (Sharma et al., 2011a)).
5.6. SLC26A6 (Pat-1, CFEX)
SLC26A6 was first cloned by homology from human (Lohi et al., 2000), then later also identified as a mouse kidney protein with Cl−/formate exchange (Cfex) activity (Knauf et al., 2001). SLC26A6 expression was also noted in intestine, pancreas, heart, muscle, stomach, esophagus, and placenta. Subsequent functional characterization of recombinant SLC26A6 documented a broad range of exchange substrates, including HCO3−, OH−. SO42−, oxalate, and nitrate (Alvarez et al., 2004; Chernova et al., 2005; Wang et al., 2002; Xie et al., 2002), as well as uncoupled channel activity with SCN− and NO3− (Shcheynikov et al., 2006). Tightly coupled anion exchange was influenced by a critical, conserved Glu residue (Ohana et al., 2011). Mouse SLC26A6-mediated Cl−/formate exchange is electroneutral (Ohana et al., 2011), whereas Cl−/oxalate and Cl−/SO42− exchange are electrogenic (Chernova et al., 2005; Jiang et al., 2002; Ohana et al., 2011) (although electrogenicity of Cl−/oxalate exchange by human SLC26A6 has not been uniformly detected (Chernova et al., 2005). Mouse SLC26A6 expressed in Xenopus oocytes has been demonstrated to mediate electrogenic 1Cl−/2HCO3− exchange in Xenopus oocytes (Ko et al., 2002; Shcheynikov et al., 2006). Charge reversal at conserved E357 abolished ion transport, whereas the E357A substitution abolished only exchange, but left uncoupled SCN− current intact (Ohana et al., 2011). However, electrogenic Cl−/HCO3− exchange by SLC26A6 was not detected in ooocytes by others under conditions which easily detected electrogenic Cl−/oxalate exchange (Chernova et al., 2005). CFTR complementation of CFPAC-1 cells normally lacking CFTR expression activated apparently electroneutral apical Cl−/HCO3− exchange with pharmacological properties of SLC26A6 (Rakonczay et al., 2008). Guinea pig cardiomyocyte Cl− /OH− exchange (likely reflecting activity of SLC26A6 since SLC4A3 mediates minimal Cl−/OH− exchange (Alvarez et al., 2004)) was voltage-independent over a 160 mV range and exhibited the reduced DIDS-sensitivity and an extracellular [Cl− ]-dependence (Niederer et al., 2008) consistent with electroneutral exchange by recombinant guinea pig SLC26A6 (Stewart et al., 2011). In addition, CFTR overexpression in HEK-293 cells and NIH-3T3 cells activated endogenous, depolarization-insensitive Cl−/HCO3− exchange that was, respectively DIDS-sensitive (SLC26A6-like) or DIDS-insensitive (Lee et al., 1999b), whereas in T84 cells this CFTR-elicted Cl−/HCO3− exchange was activated further by depolarization (Lee et al., 1999a).
Recombinant coexpression of SLC26A6 and CFTR led to stimulation of both activities, and to the conclusion that SLC26A6 mediates electrogenic anion exchange (Ko et al., 2002; Ko et al., 2004; Shcheynikov et al., 2006). However, studies in mouse intestinal mucosa have questioned the electrogenicity of SLC26A6-mediated Cl−/HCO3− exchange (Figure 4B). Baseline HCO3− secretion by Slc26a6−/− duodenal mucosal was reduced by 20–30% without detectable change in short circuit current. Whereas Cl−-dependent HCO3− secretion stimulated by PGE2 and by carbachol were respectively decreased by 59% and 35%, the forskolin-stimulated, Cl−-independent, electrogenic secretion of HCO3− was undiminished (Tuo et al., 2006; Wang et al., 2005). Moreover, PGE2 stimulated Cl−-dependent HCO3− secretion in Cftr−/− duodenum without accompanying increase in short circuit current (Tuo et al., 2006). Lumenal glucose-stimulated Cl− absorption across mouse jejunum was reduced in the absence of SLC26A6, despite increased glucose-induced (likely SGLT-mediated) short-circuit current (Seidler et al., 2008). These data together demonstrate selectivity of SLC26A6 regulatory pathways, and are consistent with electroneutral SLC26A6-mediated Cl−/HCO3− exchange mechanisms in duodenum and jejunum of mouse. Interestingly, jejunal fructose absorption by the electroneutral apical membrane GLUT5 transporter also stimulated SLC26A6-mediated Cl− absorption, increased jejunal biosynthesis of both GLUT5 and SLC26A6, and led to hypertension (Figure 4C). Dietary fructose-induced hypertension and natriuresis were attenuated in the Slc26a6−/− mouse (Singh et al., 2008).
Electroneutral HCO3− secretion by mouse duodenum (Figure 4B) was increased by genetic deletion of Slc9a3 or Nhe3 inhibition by S1611, and stimulation of HCO3− secretion by S1611 required Nhe3 expression. In addition, S1611-stimulated duodenal HCO3− secretion was reduced in the absence of SLC26A3 or SLC26A6 in proportion to their ~70/30 baseline functional ratio in duodenum. The resultant imbalances should have allowed detection of opposing electrogenic Cl−/HCO3− exchange activities, if present. CFTR activity is required for SLC26-mediated duodenal HCO3− secretion (but not Cl− absorption) (Singh et al., 2010), complicating evaluation of the electrogenicity of Slc26-mediated Cl− /HCO3− exchange. But duodenal villus SLC26A6 also facilitates electrogenic H+/oligopeptide absorptive cotransport, probably by pHi buffering in its HCO3− import mode (Simpson et al., 2010). In the presence of mannose (taken up by an electroneutral pathway), and by monitoring exchange of intracellular HCO3− for extracellular Cl−, SLC26A6 activity in duodenal lower villus mucosal membrane was detected for the first time, and was not detectably inhibited by high K+ membrane depolarization except in the presence of Cftr (Walker et al., 2011).
The stimulated pancreas of human and guinea pig secretes up to 140 mM HCO3− (Lee et al., 2012; Steward and Ishiguro, 2009; Steward et al., 2005; Stewart et al., 2011), which serves to neutralize gastric acid in the duodenum, and also serves in the pancreatic duct itself to prevent premature activation of acinar proenzymes and to neutralize protons co-secreted from exocytosed acinar cell granules (Hegyi et al., 2011). SLC26A3, SLC26A6, and SLC26A11 have been identified in the pancreatic duct (Stewart et al., 2011). SLC26A6 and SLC26A3, in addition to CFTR, are believed to contribute to the ductal HCO3− secretion and Cl− reabsorption essential for normal ductal fluid secretion. CFTR activation can suffice to explain at least 60%, and likely nearly all of maximal stimulated HCO3− secretion in isolated perfused pancreatic duct of guinea pig (Ishiguro et al., 2009; Ishiguro et al., 2002), reflecting the CFTR anion selectivity switch from Cl− to HCO3−, likely through WNK kinase stimulation by low ductal intracellular [Cl−] (Park et al., 2010). Secretion of 140 mM HCO3− by electroneutral Cl−/HCO3− exchange or by the 2Cl−/1HCO3− exchange mechanism proposed for SLC26A3 (Shcheynikov et al., 2006) is thermodynamically not possible (Lee et al., 2012; Steward et al., 2005). Indeed, measurements of guinea pig pancreatic duct membrane potential and HCO3− secretion were consistent with 1Cl−/2HCO3− apical exchange stoichiometry in the presence of CFTR (Stewart et al., 2011; Stewart et al., 2009; Yamaguchi et al., 2009), but also allowed for other interpretations. However, recombinant guinea pig SLC26A6 expressed in Xenopus oocytes displayed electroneutral transport (Stewart et al 2011). Thus, SLC26A6-mediated Cl−/HCO3− exchange in pancreatic duct may be regulated by factors or transport pathways absent from some heterologous expression systems that confer electrogenic transport. Maximal ductal HCO3− secretion would also require inhibition of any lumenal electroneutral or 2:1 Cl−/HCO3− exchange (Hegyi and Rakonczay, 2007; Pallagi et al., 2011), potentially by WNK4 (Kahle et al., 2004) or other regulatory pathways.
Unlike in guinea pig and human pancreatic duct, peak stimulated HCO3− secretion by mouse pancreatic duct reaches only ~40–50 mM (Steward et al., 2005), concentrations achievable by electroneutral Cl−/HCO3− exchange. Nonetheless, lumenal forskolin-stimulated Cl−/HCO3− exchange in mouse submandibular duct was activated by depolarization (Lee et al., 1999a). Knockout of mouse SLC26A6 by two groups altered pancreatic ductal HCO3− secretion in two very different ways. Ishiguro et al. found no change in pancreatic juice volume or pH in intact mice or in isolated perfused ducts, in the setting of 5-fold increased Slc26a3 mRNA (Ishiguro et al., 2007). In contrast, Wang et al. observed in Slc26a6−/− pancreatic duct greatly upregulated ductal basal fluid secretion and loss of secretin-stimulated incremental secretion, without change in Slc26a3 mRNA (Wang et al., 2006), suggesting that SLC26A6 may inhibit CFTR function in unstimulated duct, but enhance CFTR function in stimulated conditions. In isolated, resealed mouse pancreatic duct fragments, SLC26A6 and IRBIT were both essential to forskolin-stimulated HCO3− secretion (Yang et al., 2009). Thus the functional roles of SLC26A6 in pancreatic HCO3− secretion and the functional coupling of SLC26A6-mediated Cl−/HCO3− exchange may vary among species and among different cell types in heterologous and endogenous settings.
The Slc26a6−/− mouse exhibits hyperoxaluria (Figure 3B). Two knockout strains differing several-fold in urinary Ca2+ excretion differed also in presence (Jiang et al., 2006) or apparent absence of urolithiasis (Freel et al., 2006). Hyperoxaluria resulted from 50–75% reduction in enterocyte oxalate secretion, without change in enteric oxalate absorption (Jiang et al., 2006). The associated hyperoxalemia and increase filtered oxalate load is thus more important phenotypically than the predicted decrease in proximal tubule oxalate secretion. Proximal tubular lumenal oxalate-dependent Cl− reabsorption was also abolished in Slc26a6−/− mice. However, lumenal formate-dependent Cl− reabsorption in perfused proximal tubules did not statistically differ from wildtype (Wang et al., 2005), despite the robust Cl−/formate exchange activity of heterologous SLC26A6 (Knauf et al., 2001).
The identification of Slc26a6 as an oxalate lithiasis gene suggested a hypothesis for the contrasting human susceptibility and murine refractoriness to oxalate kidney stone disease. Comparison in Xenopus oocytes of oxalate/Cl− exchange by mouse Slc26a6 and human SLC26A6 revealed respective values of K1/2 for extracellular Cl− of 8 mM and 62 mM (Clark et al., 2008) (but see (Freel et al., 2009) for contrasting data). These divergent values suggest, in the context of chyme [Cl−] in human upper ileum (Fordtran and Locklear, 1966) and in mouse intestine (Talbot and Lytle, 2010), that mouse SLC26A6 is always saturated with respect to lumenal Cl−, whereas most human SLC26A6 in the enterocyte apical membrane is exposed to sub-K1/2 lumenal [Cl−]. Indeed, the most common SLC26A6 variant cSNP V206M/V185M lowered transport rate (Clark et al., 2008; Monico et al., 2008), suggesting it as a lithiasis risk modifier. However, the presence of this variant did not correlate with plasma [oxalate] or urinary oxalate excretion either in patients with primary hyperoxaluria (Monico et al., 2008) or in patients with primary hyperparathyroidism. Interestingly, hyperparathyroid patients with the V206M allele were more frequently hypertensive, and hyperparathyroid stone-formers exhibited attenuated hypercalciuria (Corbetta et al., 2009).
In the setting of nephrolithiasis, therapeutic enhancement of enteric oxalate secretion remains a therapeutic goal, especially since (at least in mice) intestinal oxalate absorption is entirely paracellular (Knauf et al., 2011). One approach may be to antagonize the muscarinic downregulaton of SLC26A6 (Hassan et al., 2012) mediated by protein kinase C-delta through reduction of transporter surface expression (Hassan et al., 2007). This downregulation by protein kinase C differed mechanistically from that reported for SLC26A6-mediated Cl−/HCO3− exchange enhanced by carbonic anhydrase II (Alvarez et al., 2005).
D. melanogaster expresses an SLC26A6 polypeptide related equally in sequence to SLC26A5/prestin and SLC26A6/Pat1/Cfex, designated dPrestin. This transporter is highly expressed in gut epithelial cells, in which it mediates electrogenic oxalate and sulfate transport (Hirata et al., 2012). Dietary administration of ethylene glycol leads to Ca oxalate crystal deposition in the fly malphigian tubule (Chen et al., 2011). More recent preliminary data has shown that increased dietary oxalate suffices to promote appearance of Ca oxalate crystals in the malphigian tubules, and that dPrestin knockdown in tubule principal cells reduces the rate of stone formation (Dow, 2012), establishing D. melanogaster as a versatile experimental model for oxalate lithiasis, amenable to high throughput drug screens for SLC26A6 antagonists.
5.7. SLC26A7
SLC26A7 was first cloned by homology screening from kidney (Lohi et al., 2002a) and from high endothelial venule cells of lymphoid tissue, in search of sulfate transporters (Vincourt et al., 2002). SLC26A7 has been reported to exhibit both a Cl−/HCO3− exchange activity (Petrovic et al., 2004; Petrovic et al., 2003) and an anion channel of linear I/V relationship, with increased Cl− selectivity produced by cytosolic acidic pHi, minimal HCO3−/OH− permeability, and broad inhibitor sensitivity (Kim et al., 2005; Kosiek et al., 2007). Mouse Slc26a7 was localized in gastric parietal cell basolateral membrane (Petrovic et al., 2003) (Figure 6C), where it appeared to contribute an increased proportion of cellular Cl− loading during histamine stimulation (Kosiek et al., 2007). Kidney SLC26A7 was localized in the basolateral membrane of renal collecting duct Type A intercalated cells (Petrovic et al., 2004) (Figure 5B). However, two different SLC26A7 antibodies localized SLC26A7 to the subapical region of proximal tubule cells and the basolateral membrane of thick ascending limb cells (Dudas et al., 2006).
The Slc26a7−/− mouse was remarkable for distal renal tubular acidosis and gastric hypochlorhydria. (Xu et al., 2009). Isolated perfused outer medullary collecting ducts revealed severely decreased basolateral Cl−/HCO3− exchange in hypertonic medium, but only minimal decrease in isotonic medium. Type A intercalated cells of water deprived rats with presumed medullary interstitial hypertonicity upregulated SLC26A7 three-fold while downregulating SLC4A1/kAe1 two-fold, whereas SLC26A7 was unchanged in renal cortex and in stomach (Barone et al., 2004). SLC26A7 polypeptide was undetectable in Type A intercalated cells of Brattleboro rats, but treatment with the vasopressin analog dDAVP restored SLC26A7 to normal levels without alteration in Slc26a7 mRNA abundance. AQP2 polypeptide increased in parallel, whereas kAE1 was slightly decreased and vH+-ATPase was unchanged (Petrovic et al., 2006). GFP-SLC26A7 overexpressed in MDCK cells was localized in a diffusely vesicular pattern, but accumulated in plasma membrane after overnight exposure to either hypertonic medium or to reduced [K+] medium. C-terminally truncated SLC26A7 failed to relocalize in response to hypertonicity (Xu et al., 2006). Chronic dietary K+ depletion increased outer medulla SLC26A7 polypeptide level without altering mRNA levels, and failed to increase cortical SLC26A7 protein or mRNA. In contrast, kAE1 mRNA increased five-fold in cortex, and 70% in medulla (Barone et al., 2007). Changes in both gene products could contribute to the alkalosis associated with systemic K+ depletion. Slc26a7 mRNA and protein in medulla were also increased by four days' acid loading, and in one study also in distal renal tubular acidosis setting of the Slc4a1−/− mouse (Sun and Petrovic, 2008), but not in another (Stehberger et al., 2007). Thus, the different regulatory responses of SLC26A7 and SLC4A1 in mouse renal intercalated cells and of SLC26A7 and SLC4A2 in rat parietal cells appear complementary.
5.8. SLC26A8 (Tat-1)
The STAS domain of SLC26A8 was discovered as a yeast two-hybrid screen interaction partner of the male germ cell-specific, Rnd2-associated GTPase accelerating protein, MgcRacGap (Toure et al., 2001) (Naud et al., 2003), related to the fly male fertility gene, RotundRacGap. SLC26A8 was independently cloned by homology (Lohi et al., 2002a). The SLC26A8 polypeptide exhibited modest transport of Cl−, SO42−, and oxalate (Lohi et al., 2002a; Toure et al., 2001).
Mouse SLC26A8 polypeptide is expressed in spermatocytes (Toure et al., 2001) and in mature sperm at the annulus connecting midpiece to principal piece. The slc26a8−/− mouse exhibited male infertility due to immotile sperm characterized by decreased ATP content and reduced fertilization capacity (Toure et al., 2007). SLC26A8 normally accumulates at the annulus after prior assembly of the septin complex earlier in spermatogenesis (Toure et al., 2011). The Slc26a8−/− sperm ultrastructure phenocopied that of the septin 4 knockout, with disorganized annulus structure, hairpin bending of the sperm tail with disrupted axial structures, an abnormal mitochondrial sheath assembly (Toure et al., 2007), and reduced sperm levels of cAMP. cAMP supplementation partially rescued sperm motility in a PKA-dependent manner. SLC26A8 also colocalized with CFTR in sperm, and interacted directly with and stimulated CFTR anion channel activity in heterologous cells (Rode et al., 2012).
A survey of 83 infertile men with spermatogenic dysfunction failed to identify cosegregating polymorphisms of SLC26A8 (Makela et al., 2005). However, the sperm from one of 75 asthenozoospermic patients were remarkable for absence of SLC26A8, Septin 4, and Septin 7 from a morphologically normal annulus without mutations or altered protein levels (Lhuillier et al., 2009).
5.9. SLC26A9
SLC26A9 (Lohi et al., 2002a) is expressed widely, but most prominently in brain and on apical membranes of airway epithelial cells (Figure 6B), and gastric mucosa (Figure 6C). SLC26A9-mediated Cl−/HCO3− exchange is inhibited by NH4+ and, in vivo, by lumenal acidic pHo. A component of Cl−-independent HCO3− transport was also noted (Xu et al., 2005). SLC26A9 was later reported as a highly selective Cl− channel with minimal HCO3− permeability, and insensitive to regulation by OH− or HCO3−. SLC26A9-mediated Cl− conductance was inhibited by WNK1, WNK3, and WNK4 through a kinase activity-independent reduction in surface expression (Dorwart et al., 2007). However, others reported HCO3−-stimulation of SLC26A9 Cl− channel activity (Loriol et al., 2008), SLC26A9-mediated electrogenic Cl−/HCO3− exchange, and (unique to date among mammalian SLC26 polypeptides) Na+/anion cotransport (Chang et al., 2009b). Human bronchial epithelial cells expressed a native, constitutively active, CFTR-dependent Cl− conductance of distinct pharmacological properties that was attributed to SLC26A9 (Bertrand et al., 2009). Heterologous SLC26A9 increased CFTR levels and forskolin-stimulated currents in human bronchial epithelial cells, but did not confer SLC26A9-associated constitutive current. SLC26A9 also enhanced CFTR currents in Xenopus oocytes (Avella et al., 2011b). The L683P STAS domain mutant of SLC26A9 expressed in oocytes reduced SLC26A9 Cl− currents and reduced CFTR stimulation (Avella et al., 2011a). In contrast, SLC26A9 expressed in Xenopus oocytes was inhibited by phosphorylated R domain of CFTR through an interaction with the STAS domain (Chang et al., 2009a). The functional consequences of SLC26A9 coexpression with wildtype CFTR were recently shown to differ in polarized airway epithelial cells and in nonpolarized HEK-293 cells (Ousingsawat et al., 2012). Thus SLC26A9 function and regulation has been highly context-dependent.
Gastric SLC26A9 was detected by immunoblot in parietal cell tubulovesicles of wildtype mice (Xu et al., 2008), in addition to the earlier reported cytochemically predominant localization in gastric surface epithelial cells (Xu et al., 2005). The Slc26a9−/− mouse exhibited gastric hypochlorhydria. Loss of tubulovesicles from reduced numbers of parietal cells was accompanied by concentrated apical localization of H+,K+-ATPase. Slc26a9−/− gastric mucosa differed from wildtype in failure to alkalinize the lumenal surface in response to PGE2 or in response to two-photon photodamage (Demitrack et al., 2010).
5.10. SLC26A10
SLC26A10 has been considered an expressed pseudogene. No human open reading frame that includes an intact STAS domain has been noted, and no attempts have been reported to detect the encoded human SLC26A10 polypeptide of a predicted 563 aa in length. Although the genomes of many other organisms are predicted to encode apparently full-length SLC26A10 polypeptides with intact STAS domains, no functional studies of these putative SLC26A10 polypeptides have been reported. However, Slc26a10 mRNA is abundantly expressed in heart (Alvarez et al., 2004) and has been evaluated as a biomarker of congestive heart failure (Hare JM and Heidecker B, US Patent application 12/610,529; http://www.freepatentsonline.com/y2010/0112587.html). The SLC26A10 gene is predicted "with high confidence" to be under imprinting regulation (Luedi et al., 2007), and is within a transcribed 12q14–15 amplicon present in soft tissue sarcoma and atypical lipoma (Francis et al., 2007) (Trombetta et al., 2009) and in neuroblastoma and glioma (Lo et al., 2007; Su et al., 2004). SLC26A10 mRNA was downregulated 10-fold in gastric carcinoma cells by knockdown of PTP-1B. (Wang et al., 2010a). A genome-wide association study of determinants of plasma polyunsaturated fatty acid concentrations found the strongest association of docosahexanoic acid levels with a polymorphism in the SLC26A10 gene, and additional significant SLC26A10 associations with levels of eicosapentanoic and arachidonic acids (Tanaka et al., 2009). The finding is intriguing in light of the association of SLC26-related ychM of E. coli with fatty acid biosynthesis (Babu et al., 2010).
5.11. SLC26A11 (KBAT)
SLC26A11 was first characterized as a SO42− transporter cloned from human lymphoid high endothelial venules and expressed in kidney, brain and placenta, and at lower levels in other tissues (Vincourt et al., 2003), including pancreatic duct (Stewart et al., 2011). Guinea pig SLC26A11 mediated exchange of Cl−, SO42−, and oxalate in pHo-regulated manners, was not a strong Cl−/HCO3− exchanger, and was remarkable for its relative resistance to H2-DIDS accompanied by strong sensitivity to block by nominally CFTR-specific inhibitors (Stewart et al., 2011). SLC26A11 mRNA in fibroblasts was reduced by ~60% by knock-down of caveolin-1 (Lim et al., 2010). SLC26A11 has also been identified in several proteome catalogs of the lysosomal membrane (summarized in (Stewart et al., 2011)), where it may serve as a lysosomal SO42− transporter. Interestingly, the SLC26A11 gene closely abuts in a head-to-head manner the gene encoding sulfamidase (SGSH). SGSH mutations cause the lysosomal storage disease San Filippo Syndrome Type A (mucopolysaccharidosis IIIA). A single MPSIIIA patient has been reported with a genomic deletion spanning 5 exons of SGSH and the first two exons of SLC26A11 (Ouesleti et al., 2011), although the patient phenotype was not reported to differ from that with other severe SHSG mutations.
Recently, mouse SLC26A11 was named KBAT (kidney brain anion transporter) as it colocalized with renal vH+-ATPase to the apical membrane of Type A intercalated cells (Figure 5B), and in both apical and basolateral membranes of non-A non-B intercalated cells (pictured as a Type B cell in Figure 5A), as well as in the ruffled border of osteoclasts (Xu et al., 2011). Expresson studies in COS7 cells suggested that mouse SLC26A11 mediated Cl−/HCO3− exchange and either mediated or activated a Cl− conductance. SLC26A11 also activated in a Cl−-dependent manner the vH+-ATPase-mediated recovery from acid load that was only partially sensitive to DIDS or to nominal voltage clamping (Xu et al., 2011). The mechanism by which the exchange and conductance activities of SLC26A11 might be regulated in different subcellular locales, cell types or physiological conditions remains unknown. SLC26A11 mutations have not been reported as a cause of human distal renal tubular acidosis.
6. Prospects
Investigation of SLC26 and related SulP and Sultr genes and proteins has accelerated greatly in recent years. Studies of the mammalian biology of the SLC26 gene family have encompassed the gastrointestinal, renal, endocrine, auditory, skeletal, and reproductive systems, providing new insights into the biology of Cl−, HCO3−, SO42−, oxalate, I−, and SCN−. Functional expression studies have revealed remarkably varied mechanisms among paralogs and between orthologs of different species, with considerable plasticity of transport modes and mechanisms for individual gene products. These differences appear to reflect SLC26 responses to different anion substrates, to different heterologous expression systems, to profound differences in regulation between endogenous and heterologous settings, and between different tissues and cell types in vivo. SLC26 polypeptides may operate in the context of species-specific redundancies, as reflected in the overlapping but distinct spectrum of pathologies that characterize SLC26 mutations or genetic deletions in humans, mice, and other model organisms. Within species, these redundancies can be tissue-specific, as well. Differences in knockout phenotypes between nominally similar mouse strains produced and studied in different labs suggest phenotypic susceptibility to yet uncharacterized modifier gene effects.
Future work will advance the discovery of SLC26-interacting proteins, defining consequences of these interactions on trafficking, localization, signaling, and degradation, and integrating SLC26 anion transport functions with cell type-specific cell biological pathways. The widespread expression of SulP proteins in microbes may yield insights into stress pathways and latency pathologies of intracellular pathogens not elucidated by early generation screens for bacterial pathogenesis genes. Elaboration of the roles of SLC26 proteins in plants, insects, and fish will enhance our understanding of environental adaptation to variations in salinity, tonicity, nutrient supply, and fluctuations of environmental toxins. Finally, application of this growing body of knowledge should lead to novel diagnostics and therapeutics for SLC26-related disease, and may provide chemical and genetic tools to increase agricultural yields and, more speculatively, to increase global CO2 sequestration.
Acknowledgements
We thank Dr. Andrew K. Stewart for helpful discussion. This work was supported by NIH DK43495 (SLA), NIH DK34854 (Harvard Digestive Diseases Center), and the US-Israel Binational Science Foundation (SLA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL. Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl−/1HCO3− exchange. Am. J. Physiol. Cell Physiol. 2011;300(2):C276–C286. doi: 10.1152/ajpcell.00366.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J. Physiol. 2004;561(Pt 3):721–734. doi: 10.1113/jphysiol.2004.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J. 2005;24(14):2499–2511. doi: 10.1038/sj.emboj.7600736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. The STAS domain - a link between anion transporters and antisigma-factor antagonists. Curr. Biol. 2000;10(2):R53–R55. doi: 10.1016/s0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Aronson PS. Role of SLC26A6-mediated Cl-oxalate exchange in renal physiology and pathophysiology. J. Nephrol. 2010;23(Suppl. 16):S158–S164. [PubMed] [Google Scholar]
- Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, Matsumoto T, Matsui T, Kakuta Y, Kinouchi Y, Shimosegawa T, Hosokawa M, Arimura Y, Shinomura Y, Kiyohara Y, Tsunoda T, Kamatani N, Iida M, Nakamura Y, Kubo M. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat. Genet. 2009;41(12):1325–1329. doi: 10.1038/ng.482. [DOI] [PubMed] [Google Scholar]
- Avella M, Borgese F, Ehrenfeld J. Characterization of the L683P mutation of SLC26A9 in Xenopus oocytes. Biochim. Biophys. Acta. 2011a;1810(6):577–583. doi: 10.1016/j.bbagen.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Avella M, Loriol C, Boulukos K, Borgese F, Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J. Cell Physiol. 2011b;226(1):212–223. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- Azroyan A, Laghmani K, Crambert G, Mordasini D, Doucet A, Edwards A. Regulation of pendrin by pH: dependence on glycosylation. Biochem. J. 2011;434(1):61–72. doi: 10.1042/BJ20101411. [DOI] [PubMed] [Google Scholar]
- Azroyan A, Morla L, Crambert G, Laghmani K, Ramakrishnan S, Edwards A, Doucet A. Regulation of pendrin by cAMP: possible involvement in beta adrenergic-dependent NaCl retention. Am. J. Physiol. Renal Physiol. 2012 doi: 10.1152/ajprenal.00403.2011. in press. [DOI] [PubMed] [Google Scholar]
- Babu M, Greenblatt JF, Emili A, Strynadka NC, Reithmeier RA, Moraes TF. Structure of a SLC26 anion transporter STAS domain in complex with acyl carrier protein: implications for E. coli YchM in fatty acid metabolism. Structure. 2010;18(11):1450–1462. doi: 10.1016/j.str.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Bai JP, Surguchev A, Montoya S, Aronson PS, Santos-Sacchi J, Navaratnam D. Prestin's anion transport and voltage-sensing capabilities are independent. Biophys. J. 2009;96(8):3179–3186. doi: 10.1016/j.bpj.2008.12.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian JC, Gosti F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol. 2008;180(3):608–619. doi: 10.1111/j.1469-8137.2008.02604.x. [DOI] [PubMed] [Google Scholar]
- Barone S, Amlal H, Kujala M, Xu J, Karet F, Blanchard A, Kere J, Soleimani M. Regulation of the basolateral chloride/base exchangers AE1 and SLC26A7 in the kidney collecting duct in potassium depletion. Nephrol. Dial. Transplant. 2007;22(12):3462–3470. doi: 10.1093/ndt/gfm486. [DOI] [PubMed] [Google Scholar]
- Barone S, Amlal H, Xu J, Kujala M, Kere J, Petrovic S, Soleimani M. Differential regulation of basolateral Cl−/HCO3− exchangers SLC26A7 and AE1 in kidney outer medullary collecting duct. J. Am. Soc. Nephrol. 2004;15(8):2002–2011. doi: 10.1097/01.ASN.0000135060.83250.07. [DOI] [PubMed] [Google Scholar]
- Barone S, Amlal H, Xu J, Soleimani M. Deletion of the Cl−/HCO3− exchanger pendrin downregulates calcium-absorbing proteins in the kidney and causes calcium wasting. Nephrol. Dial. Transplant. 2012;27(4):1368–1379. doi: 10.1093/ndt/gfr505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J. Gen. Physiol. 2009;133(4):421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidart JM, Mian C, Lazar V, Russo D, Filetti S, Caillou B, Schlumberger M. Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues. J. Clin. Endocrinol. Metab. 2000;85(5):2028–2033. doi: 10.1210/jcem.85.5.6519. [DOI] [PubMed] [Google Scholar]
- Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J. Biol. Chem. 1994;269(4):3017–3021. [PubMed] [Google Scholar]
- Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol. 2008;9(8):R122. doi: 10.1186/gb-2008-9-8-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infection Immun. 2009;77(9):3639–3650. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzica H, Breljak D, Krick W, Lovric M, Burckhardt G, Burckhardt BC, Sabolic I. The liver and kidney expression of sulfate anion transporter sat-1 in rats exhibits male-dominant gender differences. Pflugers Arch. 2009;457(6):1381–1392. doi: 10.1007/s00424-008-0611-5. [DOI] [PubMed] [Google Scholar]
- Buchner P, Takahashi H, Hawkesford MJ. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J. Exp. Botany. 2004;55(404):1765–1773. doi: 10.1093/jxb/erh206. [DOI] [PubMed] [Google Scholar]
- Chang MH, Plata C, Sindic A, Ranatunga WK, Chen AP, Zandi-Nejad K, Chan KW, Thompson J, Mount DB, Romero MF. Slc26a9 is inhibited by the R-region of the cystic fibrosis transmembrane conductance regulator via the STAS domain. J. Biol. Chem. 2009a;284(41):28306–28318. doi: 10.1074/jbc.M109.001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MH, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9--anion exchanger, channel and Na+ transporter. J. Membr. Biol. 2009b;228(3):125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JM, Karniski LP. Protein localization of SLC26A2 (DTDST) in rat kidney. Histochem. Cell Biol. 2011;133(5):541–547. doi: 10.1007/s00418-010-0694-x. [DOI] [PubMed] [Google Scholar]
- Cheatham MA, Huynh KH, Gao J, Zuo J, Dallos P. Cochlear function in Prestin knockout mice. J. Physiol. 2004;560(Pt 3):821–830. doi: 10.1113/jphysiol.2004.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Liu HP, Chen HY, Tsai FJ, Chang CH, Lee YJ, Lin WY, Chen WC. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 2011;80(4):369–377. doi: 10.1038/ki.2011.80. [DOI] [PubMed] [Google Scholar]
- Cherest H, Davidian JC, Thomas D, Benes V, Ansorge W, Surdin-Kerjan Y. Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics. 1997;145(3):627–635. doi: 10.1093/genetics/145.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol. Chem. 2005;280(9):8564–8580. doi: 10.1074/jbc.M411703200. [DOI] [PubMed] [Google Scholar]
- Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J. Physiol. 2003;549(Pt 1):3–19. doi: 10.1113/jphysiol.2003.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY, Kim HM, Ito T, Lee KY, Li X, Monahan K, Wen Y, Wilson E, Kurima K, Saunders TL, Petralia RS, Wangemann P, Friedman TB, Griffith AJ. Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J. Clin. Invest. 2011;121(11):4516–4525. doi: 10.1172/JCI59353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, Kittles R, Eisenman D, Kim HJ, Niparko J, Thomsen J, Arnos KS, Nance WE, King KA, Zalewski CK, Brewer CC, Shawker T, Reynolds JC, Butman JA, Karniski LP, Alper SL, Griffith AJ. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum. Mutat. 2009a;30(4):599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloglu A, Ozen S, Sanjad S, Nelson-Williams C, Farhi A, Mane S, Lifton RP. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl. Acad. Sci. USA. 2009b;106(45):19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL. Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J. Physiol. 2008;586(5):1291–1306. doi: 10.1113/jphysiol.2007.143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli EM, Lariani S, Zwahlen MC, Fotopoulos G, Holzwarth JA, Cherbut C, Dorta G, Corthesy-Theulaz I, Grigorov M. Biomarkers of human gastrointestinal tract regions. Mamm Genome. 2009;20(8):516–527. doi: 10.1007/s00335-009-9212-7. [DOI] [PubMed] [Google Scholar]
- Compton EL, Karinou E, Naismith JH, Gabel F, Javelle A. Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. J. Biol. Chem. 2011;286(30):27058–27067. doi: 10.1074/jbc.M111.244533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta S, Eller-Vainicher C, Frigerio M, Valaperta R, Costa E, Vicentini L, Baccarelli A, Beck-Peccoz P, Spada A. Analysis of the 206M polymorphic variant of the SLC26A6 gene encoding a Cl− oxalate transporter in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 2009;160(2):283–288. doi: 10.1530/EJE-08-0623. [DOI] [PubMed] [Google Scholar]
- Cornaglia AI, Casasco A, Casasco M, Riva F, Necchi V. Dysplastic histogenesis of cartilage growth plate by alteration of sulphation pathway: a transgenic model. Conn. Tissue Res. 2009;50(4):232–242. doi: 10.1080/03008200802684623. [DOI] [PubMed] [Google Scholar]
- Currall B, Rossino D, Jensen-Smith H, Hallworth R. The roles of conserved and nonconserved cysteinyl residues in the oligomerization and function of mammalian prestin. J. Neurophysiol. 2011;106(5):2358–2367. doi: 10.1152/jn.00496.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137(9):1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- Dai P, Stewart AK, Chebib F, Hsu A, Rozenfeld J, Huang D, Kang D, Lip V, Fang H, Shao H, Liu X, Yu F, Yuan H, Kenna M, Miller DT, Shen Y, Yang W, Zelikovic I, Platt OS, Han D, Alper SL, Wu BL. Distinct and novel SLC26A4/Pendrin mutations in Chinese and U.S. patients with nonsyndromic hearing loss. Physiol. Genomics. 2009;38(3):281–290. doi: 10.1152/physiolgenomics.00047.2009. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Cur. Opin. Neurobiol. 2008;18(4):370–376. doi: 10.1016/j.conb.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58(3):333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J. Clin. Invest. 2010;120(3):706–712. doi: 10.1172/JCI31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demitrack ES, Soleimani M, Montrose MH. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl(−)/HCO(3)(−) Am. J Physiol. Gastrointest. Liver Physiol. 2010;299(1):G255–G264. doi: 10.1152/ajpgi.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J. Biol. Chem. 2008;283(7):4177–4188. doi: 10.1074/jbc.M704924200. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Baker JM, Forman-Kay JD, Muallem S, Thomas PJ. Congenital chloride-losing diarrhea causing mutations in the STAS domain result in misfolding and mistrafficking of SLC26A3. J. Biol. Chem. 2008a;283(13):8711–8722. doi: 10.1074/jbc.M704328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl(−) channel regulated by the WNK kinases. J. Physiol. 2007;584(Pt 1):333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiol. 2008b;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Dow JA, Cabrero P, Hirata T, Romero MF. Organotypic models for kidney disease: Drosophila get kidney stones, too. 2012 http://www.drosophila-conf.org/cgi-bin/dros12-cgi/dros12s?author=romero&sort=ptimes&sbutton=Detail&absno=70092&sid=487008. [Google Scholar]
- Dror AA, Politi Y, Shahin H, Lenz DR, Dossena S, Nofziger C, Fuchs H, Hrabe de Angelis M, Paulmichl M, Weiner S, Avraham KB. Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J. Biol. Chem. 2010;285(28):21724–21735. doi: 10.1074/jbc.M110.120188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Sanchez C, Chen M, Edwards DJ, Shen B. The biosynthetic gene cluster for the antitumor drug bleomycin from Streptomyces verticillus ATCC15003 supporting functional interactions between nonribosomal peptide synthetases and a polyketide synthase. Chem. & Biol. 2000;7(8):623–642. doi: 10.1016/s1074-5521(00)00011-9. [DOI] [PubMed] [Google Scholar]
- Dudas PL, Mentone S, Greineder CF, Biemesderfer D, Aronson PS. Immunolocalization of anion transporter Slc26a7 in mouse kidney. Am. J. Physiol. Renal. Physiol. 2006;290(4):F937–F945. doi: 10.1152/ajprenal.00197.2004. [DOI] [PubMed] [Google Scholar]
- Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum. Molec. Genet. 2001;10(2):153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- Farkas K, Yeruva S, Rakonczay Z, Jr, Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm. Bowel Dis. 2011;17(4):884–898. doi: 10.1002/ibd.21432. [DOI] [PubMed] [Google Scholar]
- Felce J, Saier MH., Jr Carbonic anhydrases fused to anion transporters of the SulP family: evidence for a novel type of bicarbonate transporter. J. Molec. Microbiol. Biotechnol. 2004;8(3):169–176. doi: 10.1159/000085789. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KL, Tyerman SD, Kaiser BN. Molybdate transport through the plant sulfate transporter SHST1. FEBS Lett. 2008;582(10):1508–1513. doi: 10.1016/j.febslet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Fong P. Thyroid iodide efflux: a team effort? J. Physiol. 2011;589(Pt 24):5929–5939. doi: 10.1113/jphysiol.2011.218594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Digest. Dis. 1966;11(7):503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Forlino A, Piazza R, Tiveron C, Della Torre S, Tatangelo L, Bonafe L, Gualeni B, Romano A, Pecora F, Superti-Furga A, Cetta G, Rossi A. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum. Molec. Genet. 2005;14(6):859–871. doi: 10.1093/hmg/ddi079. [DOI] [PubMed] [Google Scholar]
- Francis P, Namlos HM, Muller C, Eden P, Fernebro J, Berner JM, Bjerkehagen B, Akerman M, Bendahl PO, Isinger A, Rydholm A, Myklebost O, Nilbert M. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics. 2007;8:73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Hemmert W, Gummer AW. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. USA. 1999;96(8):4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(4):G719–G728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297(5):G918–G929. doi: 10.1152/ajpgi.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galamb O, Sipos F, Solymosi N, Spisak S, Krenacs T, Toth K, Tulassay Z, Molnar B. Diagnostic mRNA expression patterns of inflamed, benign, and malignant colorectal biopsy specimen and their correlation with peripheral blood results. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2835–2845. doi: 10.1158/1055-9965.EPI-08-0231. [DOI] [PubMed] [Google Scholar]
- Galante LL, Schwarzbauer JE. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J. Cell Biol. 2007;179(5):999–1009. doi: 10.1083/jcb.200707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J. Clin. Invest. 2007;117(2):428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U. SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem. Cytochem. 2001;49(8):973–982. doi: 10.1177/002215540104900805. [DOI] [PubMed] [Google Scholar]
- Hallworth R, Nichols MG. Prestin in HEK cells is an obligate tetramer. J. Neurophysiol. 2012;107(1):5–11. doi: 10.1152/jn.00728.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HA, Cheng M, Aronson PS. Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am. J. Physiol. Cell Physiol. 2012;302(1):C46–C58. doi: 10.1152/ajpcell.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am. J. Physiol. Cell Physiol. 2007;292(4):C1485–C1492. doi: 10.1152/ajpcell.00447.2006. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78(6):1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Hatzios SK, Bertozzi CR. The regulation of sulfur metabolism in Mycobacterium tuberculosis. PLoS Pathogens. 2011;7(7) doi: 10.1371/journal.ppat.1002036. e1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Suruga K, Yamashita Y. Regulation of intestinal Cl−/HCO3− exchanger SLC26A3 by intracellular pH. Am. J. Physiol. Cell Physiol. 2009;296(6):C1279–C1290. doi: 10.1152/ajpcell.00638.2008. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Yamashita Y. Role of N-glycosylation in cell surface expression and protection against proteolysis of the intestinal anion exchanger SLC26A3. Am. J. Physiol. Cell Physiol. 2012;302(5):C781–C795. doi: 10.1152/ajpcell.00165.2011. [DOI] [PubMed] [Google Scholar]
- Hegyi P, Maleth J, Venglovecz V, Rakonczay Z., Jr Pancreatic ductal bicarbonate secretion: challenge of the acinar Acid load. Front. Physiol. 2011;2:36. doi: 10.3389/fphys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi P, Rakonczay Z., Jr The inhibitory pathways of pancreatic ductal bicarbonate secretion. Int. J. Biochem. Cell Biol. 2007;39(1):25–30. doi: 10.1016/j.biocel.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL. Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am. J. Physiol. Cell Physiol. 2010;298(6):C1363–C1375. doi: 10.1152/ajpcell.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Czapar A, Brin L, Haritonova A, Bondeson DP, Linser P, Cabrero P, Thompson J, Dow JA, Romero MF. Ion and solute transport by Prestin in Drosophila and Anopheles. J. Insect Physiol. 2012;58(4):563–569. doi: 10.1016/j.jinsphys.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat. Genet. 1996;14(3):316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Holmberg C, Sherman P, Kere J. Distinct outcomes of chloride diarrhoea in two siblings with identical genetic background of the disease: implications for early diagnosis and treatment. Gut. 2001;48(5):724–727. doi: 10.1136/gut.48.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Miller KK, Anderson CT, Sengupta S, Du GG, Aguinaga S, Cheatham M, Dallos P, Zheng J. Interaction between CFTR and prestin (SLC26A5) Biochim. Biophys. Acta. 2010;1798(6):1029–1040. doi: 10.1016/j.bbamem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover EE, Walker NM, Brazill J, Clarke LL. Dehydration increases electroneutral HCO3− secretion in the Nhe3 knockout duodenum. Gastroenterol. 2010:A261. [Google Scholar]
- Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(1):G447–G455. doi: 10.1152/ajpgi.00286.2006. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J. Gen. Physiol. 2009;133(3):315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J. Gen. Physiol. 2002;120(5):617–628. doi: 10.1085/jgp.20028631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Yoshida T, Teranishi M, Murata Y, Hayashi Y, Kanou Y, Griffith AJ, Nakashima T. Influence of dietary iodine deficiency on the thyroid gland in Slc26a4-null mutant mice. Thyroid Res. 2011;4(1):10. doi: 10.1186/1756-6614-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GC, Mittaz-Crettol L, Taylor JA, Mortier GR, Spranger J, Zabel B, Le Merrer M, Cormier-Daire V, Hall CM, Offiah A, Wright MJ, Savarirayan R, Nishimura G, Ramsden SC, Elles R, Bonafe L, Superti-Furga A, Unger S, Zankl A, Briggs MD. Pseudoachondroplasia and multiple epiphyseal dysplasia: a 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution. Hum. Mutat. 2012;33(1):144–157. doi: 10.1002/humu.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Cui J. Inactivation of Saccharomyces cerevisiae Sulfate Transporter Sul2p: Use It and Lose It. Biophys. J. 2012;102(4):768–776. doi: 10.1016/j.bpj.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nature Genet. 2006;38(4):474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J. Biol. Chem. 2002;277(37):33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc. Natl. Acad. Sci. USA. 2004;101(7):2064–2069. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata K, Hayashi H, Suzuki Y. Chloride-dependent bicarbonate secretion in the mouse large intestine. Biomed. Res. 2006;27(1):15–21. doi: 10.2220/biomedres.27.15. [DOI] [PubMed] [Google Scholar]
- Kere J. Overview of the SLC26 family and associated diseases. Novartis Fdn. Symp. 2006;273:261–264. 2–11; discussion 11–18. [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J. Biol. Chem. 2005;280(8):6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am. J. Physiol. Renal Physiol. 2007;293(4):F1314–F1324. doi: 10.1152/ajprenal.00155.2007. [DOI] [PubMed] [Google Scholar]
- Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, Wall SM. Role of pendrin in iodide balance: going with the flow. Am. J. Physiol. Renal Physiol. 2009;297(4):F1069–F1079. doi: 10.1152/ajprenal.90581.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J. Am. Soc. Nephrol. 2011;22(12):2247–2255. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc. Natl. Acad. Sci. USA. 2001;98(16):9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(−) transport in cystic fibrosis. EMBO J. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat. Cell Biol. 2004;6(4):343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Sugimoto T, Saijoh K, Fujii M, Chihara K. Cloning and characterization of the 5'-flanking region of the mouse diastrophic dysplasia sulfate transporter gene. Biochem. Biophys. Res. Comm. 1997;238(3):738–743. doi: 10.1006/bbrc.1997.7380. [DOI] [PubMed] [Google Scholar]
- Kosiek O, Busque SM, Foller M, Shcheynikov N, Kirchhoff P, Bleich M, Muallem S, Geibel JP. SLC26A7 can function as a chloride-loading mechanism in parietal cells. Pflugers Arch. 2007;454(6):989–998. doi: 10.1007/s00424-007-0254-y. [DOI] [PubMed] [Google Scholar]
- Lamprecht G, Hsieh CJ, Lissner S, Nold L, Heil A, Gaco V, Schafer J, Turner JR, Gregor M. Intestinal anion exchanger down-regulated in adenoma (DRA) is inhibited by intracellular calcium. J. Biol. Chem. 2009;284(29):19744–19753. doi: 10.1074/jbc.M109.004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. J. Biol. Chem. 1999a;274(21):14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012;92(1):39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, Muallem S. Regulation of Cl−/ HCO3− exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J. Biol. Chem. 1999b;274(6):3414–3421. doi: 10.1074/jbc.274.6.3414. [DOI] [PubMed] [Google Scholar]
- Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J. Clin. Invest. 2010;120(5):1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhuillier P, Rode B, Escalier D, Lores P, Dirami T, Bienvenu T, Gacon G, Dulioust E, Toure A. Absence of annulus in human asthenozoospermia: case report. Hum. Reprod. 2009;24(6):1296–1303. doi: 10.1093/humrep/dep020. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419(6904):300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lim JS, Choy HE, Park SC, Han JM, Jang IS, Cho KA. Caveolae-mediated entry of Salmonella typhimurium into senescent nonphagocytotic host cells. Aging Cell. 2010;9(2):243–251. doi: 10.1111/j.1474-9726.2010.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner S, Nold L, Hsieh CJ, Turner JR, Gregor M, Graeve L, Lamprecht G. Activity and PI3-kinase dependent trafficking of the intestinal anion exchanger downregulated in adenoma depend on its PDZ interaction and on lipid rafts. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(4):G907–G920. doi: 10.1152/ajpgi.00191.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, Webb BT, Yan D, Arnos KS, Corey D, Dallos P, Nance WE, Chen ZY. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum. Molec. Genet. 2003;12(10):1155–1162. doi: 10.1093/hmg/ddg127. [DOI] [PubMed] [Google Scholar]
- Lo KC, Rossi MR, LaDuca J, Hicks DG, Turpaz Y, Hawthorn L, Cowell JK. Candidate glioblastoma development gene identification using concordance between copy number abnormalities and gene expression level changes. Genes Chrom Cancer. 2007;46(10):875–894. doi: 10.1002/gcc.20474. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70(1):102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Makela S, Lehtonen E, Kestila M, Saarialho-Kere U, Markovich D, Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J. Biol. Chem. 2002a;277(16):14246–14254. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- Lohi H, Makela S, Pulkkinen K, Hoglund P, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002b;283(3):G567–G575. doi: 10.1152/ajpgi.00356.2001. [DOI] [PubMed] [Google Scholar]
- Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J. Characterization of SLC26A9, facilitation of Cl(−) transport by bicarbonate. Cell Physiol. Biochem. 2008;22(1–4):15–30. doi: 10.1159/000149780. [DOI] [PubMed] [Google Scholar]
- Luedi PP, Dietrich FS, Weidman JR, Bosko JM, Jirtle RL, Hartemink AJ. Computational and experimental identification of novel human imprinted genes. Genome Res. 2007;17(12):1723–1730. doi: 10.1101/gr.6584707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Sugita C, Sugita M, Omata T. Latent nitrate transport activity of a novel sulfate permease-like protein of the cyanobacterium Synechococcus elongatus. J. Biol. Chem. 2006;281(9):5869–5876. doi: 10.1074/jbc.M513196200. [DOI] [PubMed] [Google Scholar]
- Makela S, Eklund R, Lahdetie J, Mikkola M, Hovatta O, Kere J. Mutational analysis of the human SLC26A8 gene: exclusion as a candidate for male infertility due to primary spermatogenic failure. Molec. Hum. Reprod. 2005;11(2):129–132. doi: 10.1093/molehr/gah140. [DOI] [PubMed] [Google Scholar]
- Malakooti J, Saksena S, Gill RK, Dudeja PK. Transcriptional regulation of the intestinal luminal Na and Cl transporters. Biochem. J. 2011;435(2):313–325. doi: 10.1042/BJ20102062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovich D. Physiological roles of renal anion transporters NaS1 and Sat1. AmJ. Physiol. Renal Physiol. 2011;300(6):F1267–F1270. doi: 10.1152/ajprenal.00061.2011. [DOI] [PubMed] [Google Scholar]
- Masuda S, Murakami KS, Wang S, Anders Olson C, Donigian J, Leon F, Darst SA, Campbell EA. Crystal structures of the ADP and ATP bound forms of the Bacillus anti-sigma factor SpoIIAB in complex with the anti-anti-sigma SpoIIAA. J. Mol. Biol. 2004;340(5):941–956. doi: 10.1016/j.jmb.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(−)/HCO(3)(−) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J. Biol. Chem. 1999;274(32):22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- Minor JS, Tang HY, Pereira FA, Alford RL. DNA sequence analysis of SLC26A5, encoding prestin, in a patient-control cohort: identification of fourteen novel DNA sequence variations. PloS One. 2009;4(6):e5762. doi: 10.1371/journal.pone.0005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio K, Kubo Y, Ogura T, Yamamoto T, Arisaka F, Sato C. The motor protein prestin is a bullet-shaped molecule with inner cavities. J. Biol. Chem. 2008;283(2):1137–1145. doi: 10.1074/jbc.M702681200. [DOI] [PubMed] [Google Scholar]
- Monico CG, Weinstein A, Jiang Z, Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ, Milliner DS, Aronson PS. Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am. J. Kidney Dis. 2008;52(6):1096–1103. doi: 10.1053/j.ajkd.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(2):G202–G210. doi: 10.1152/ajpgi.90350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Favoreto S, Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC, Erle DJ. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J. Immunol. 2008;181(3):2203–2210. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED, Izuhara K. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J.. Immunol. 2008;180(9):6262–6269. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC. Lack of pendrin HCO3− transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am. J. Physiol. Renal Physiol. 2007;292(5):F1314–F1321. doi: 10.1152/ajprenal.00432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naud N, Toure A, Liu J, Pineau C, Morin L, Dorseuil O, Escalier D, Chardin P, Gacon G. Rho family GTPase Rnd2 interacts and co-localizes with MgcRacGAP in male germ cells. Biochem. J. 2003;372(Pt 1):105–112. doi: 10.1042/BJ20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys. J. 2005;89(5):3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer SA, Swietach P, Wilson DA, Smith NP, Vaughan-Jones RD. Measuring and modeling chloride-hydroxyl exchange in the Guinea-pig ventricular myocyte. Biophys. J. 2008;94(6):2385–2403. doi: 10.1529/biophysj.107.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori I, Minakuchi T, Maresca A, Carta F, Scozzafava A, Supuran CT. The beta-carbonic anhydrases from Mycobacterium tuberculosis as drug targets. Curr. Pharm. Design. 2010;16(29):3300–3309. doi: 10.2174/138161210793429814. [DOI] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Park M, Muallem S. Solute Carrier Family 26 Member a2 (Slc26a2) Protein Functions as an Electroneutral SOFormula/OH−/Cl− Exchanger Regulated by Extracellular Cl. J. Biol. Chem. 2012;287(7):5122–5132. doi: 10.1074/jbc.M111.297192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J. Gen. Physiol. 2011;137(2):239–251. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J. Physiol. 2009;587(Pt 10):2179–2185. doi: 10.1113/jphysiol.2008.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292(5525):2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447(5):549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- Ouesleti S, Brunel V, Ben Turkia H, Dranguet H, Miled A, Miladi N, Ben Dridi MF, Lavoinne A, Saugier-Veber P, Bekri S. Molecular characterization of MPS IIIA, MPS IIIB and MPS IIIC in Tunisian patients. Clin Chim. Acta. 2011;412(23–24):2326–2331. doi: 10.1016/j.cca.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Schreiber R, Kunzelmann K. Differential contribution of SLC26A9 to Cl(−) conductance in polarized and non-polarized epithelial cells. J. Cell. Physiol. 2012;227(6):2323–2329. doi: 10.1002/jcp.22967. [DOI] [PubMed] [Google Scholar]
- Pallagi P, Venglovecz V, Rakonczay Z, Jr, Borka K, Korompay A, Ozsvari B, Judak L, Sahin-Toth M, Geisz A, Schnur A, Maleth J, Takacs T, Gray MA, Argent BE, Mayerle J, Lerch MM, Wittmann T, Hegyi P. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl channels and luminal anion exchangers. Gastroenterol. 2011;141(6):2228 e2226–2239 e2226. doi: 10.1053/j.gastro.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterol. 2010;139(2):620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Pasqualetto E, Aiello R, Gesiot L, Bonetto G, Bellanda M, Battistutta R. Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. J. Mol. Biol. 2010;400(3):448–462. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3. J. Am. Soc. Nephrol. 2010;21(11):1928–1941. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecora F, Gualeni B, Forlino A, Superti-Furga A, Tenni R, Cetta G, Rossi A. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochem. J. 2006;398(3):509–514. doi: 10.1042/BJ20060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J. Immunol. 2007;178(8):5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- Pesce L, Bizhanova A, Caraballo JC, Westphal W, Butti ML, Comellas A, Kopp P. TSH regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Endocrinol. 2012;153(1):512–521. doi: 10.1210/en.2011-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic S, Amlal H, Sun X, Karet F, Barone S, Soleimani M. Vasopressin induces expression of the Cl−/HCO3− exchanger SLC26A7 in kidney medullary collecting ducts of Brattleboro rats. Am. J. Physiol. Renal Physiol. 2006;290(5):F1194–F1201. doi: 10.1152/ajprenal.00247.2005. [DOI] [PubMed] [Google Scholar]
- Petrovic S, Barone S, Xu J, Conforti L, Ma L, Kujala M, Kere J, Soleimani M. SLC26A7: a basolateral Cl−/HCO3− exchanger specific to intercalated cells of the outer medullary collecting duct. Am. J. Physiol. Renal Physiol. 2004;286(1):F161–F169. doi: 10.1152/ajprenal.00219.2003. [DOI] [PubMed] [Google Scholar]
- Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl−/HCO3− exchanger specific to gastric parietal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(6):G1093–G1103. doi: 10.1152/ajpgi.00454.2002. [DOI] [PubMed] [Google Scholar]
- Price GD, Howitt SM. The cyanobacterial bicarbonate transporter BicA: its physiological role and the implications of structural similarities with human SLC26 transporters. Biochemistry and cell biology = Biochim Biol. Cell. 2011;89(2):178–188. doi: 10.1139/o10-136. [DOI] [PubMed] [Google Scholar]
- Price GD, Shelden MC, Howitt SM. Membrane topology of the cyanobacterial bicarbonate transporter, SbtA, and identification of potential regulatory loops. Molec. Membr. Biol. 2011;28(5):265–275. doi: 10.3109/09687688.2011.593049. [DOI] [PubMed] [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc. Natl. Acad. USA. 2004;101(52):18228–18233. doi: 10.1073/pnas.0405211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakonczay Z, Jr, Hegyi P, Hasegawa M, Inoue M, You J, Iida A, Ignath I, Alton EW, Griesenbach U, Ovari G, Vag J, Da Paula AC, Crawford RM, Varga G, Amaral MD, Mehta A, Lonovics J, Argent BE, Gray MA. CFTR gene transfer to human cystic fibrosis pancreatic duct cells using a Sendai virus vector. J. Cell. Physiol. 2008;214(2):442–455. doi: 10.1002/jcp.21220. [DOI] [PubMed] [Google Scholar]
- Regeer RR, Markovich D. A dileucine motif targets the sulfate anion transporter sat-1 to the basolateral membrane in renal cell lines. Am. J. Physiol. 2004;287(2):C365–C372. doi: 10.1152/ajpcell.00502.2003. [DOI] [PubMed] [Google Scholar]
- Reimold FR, Heneghan JF, Stewart AK, Zelikovic I, Vandorpe DH, Shmukler BE, Alper SL. Pendrin function and regulation in Xenopus oocytes. Cell. Physiol. Biochem. 2011;28(3):435–450. doi: 10.1159/000335106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P, Lores P, Jollivet M, Melin P, Zvetkova I, Bienvenu T, Becq F, Planelles G, Edelman A, Gacon G, Toure A. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum. Molec. Genet. 2012;21(6):1287–1298. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinol. 2000;141(2):839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc. Natl. Acad. USA. 2001;98(7):4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld J, Tal O, Kladnitsky O, Adler L, Efrati E, Carrithers SL, Alper SL, Zelikovic I. The pendrin anion exchanger gene is transcriptionally regulated by uroguanylin: a novel enterorenal link. Am. J. Physiol. Renal Physiol. 2012;302(5):F614–F624. doi: 10.1152/ajprenal.00189.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y. Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J. Biol. Chem. 1998;273(20):12307–12315. doi: 10.1074/jbc.273.20.12307. [DOI] [PubMed] [Google Scholar]
- Schaechinger TJ, Gorbunov D, Halaszovich CR, Moser T, Kugler S, Fakler B, Oliver D. A synthetic prestin reveals protein domains and molecular operation of outer hair cell piezoelectricity. EMBO J. 2011;30(14):2793–2804. doi: 10.1038/emboj.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc. Natl. Acad. USA. 2007;104(18):7693–7698. doi: 10.1073/pnas.0608583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnedler N, Burckhardt G, Burckhardt BC. Glyoxylate is a substrate of the sulfate-oxalate exchanger, sat-1, and increases its expression in HepG2 cells. J. Hepatol. 2011;54(3):513–520. doi: 10.1016/j.jhep.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc. Natl. Acad. USA. 1993;90(9):4166–4170. doi: 10.1073/pnas.90.9.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 2006;281(49):37962–37971. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhoft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns MP, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflugers Arch. 2008;455(4):757–766. doi: 10.1007/s00424-007-0318-z. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Rigby AC, Alper SL. STAS domain structure and function. Cell. Physiol. Biochem. 2011a;28(3):407–422. doi: 10.1159/000335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Ye L, Alper SL, Rigby AC. Guanine nucleotides differentially modulate backbone dynamics of the STAS domain of the SulP/SLC26 transport protein Rv1739c of Mycobacterium tuberculosis. FEBS J. 2012;279(3):420–436. doi: 10.1111/j.1742-4658.2011.08435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Ye L, Baer CE, Shanmugasundaram K, Alber T, Alper SL, Rigby AC. Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. J. Biol. Chem. 2011b;286(10):8534–8544. doi: 10.1074/jbc.M110.165449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J. Gen. Physiol. 2006;127(5):511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J. Physiol. 2008;586(16):3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield VC, Kraiem Z, Beck JC, Nishimura D, Stone EM, Salameh M, Sadeh O, Glaser B. Pendred syndrome maps to chromosome 7q21–34 and is caused by an intrinsic defect in thyroid iodine organification. Nat. Genet. 1996;12(4):424–426. doi: 10.1038/ng0496-424. [DOI] [PubMed] [Google Scholar]
- Shepshelovich J, Goldstein-Magal L, Globerson A, Yen PM, Rotman-Pikielny P, Hirschberg K. Protein synthesis inhibitors and the chemical chaperone TMAO reverse endoplasmic reticulum perturbation induced by overexpression of the iodide transporter pendrin. J. Cell Sci. 2005;118(Pt 8):1577–1586. doi: 10.1242/jcs.02294. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Grossman AR. The role of the STAS domain in the function and biogenesis of a sulfate transporter as probed by random mutagenesis. J. Biol. Chem. 2006;281(32):22964–22973. doi: 10.1074/jbc.M603462200. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Grossman AR. Binding of cysteine synthase to the STAS domain of sulfate transporter and its regulatory consequences. J. Biol. Chem. 2010;285(32):25094–25102. doi: 10.1074/jbc.M110.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL. Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in duodenal villous epithelium. Am. J. Physiol. Gastrointest Liver Physiol. 2010;298(5):G683–G691. doi: 10.1152/ajpgi.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008;74(4):438–447. doi: 10.1038/ki.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function. Am. J. Physiol. Cell Physiol. 2010;298(5):C1057–C1065. doi: 10.1152/ajpcell.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla A, Dwivedi A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl−/OH− exchange. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298(2):G182–G189. doi: 10.1152/ajpgi.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak M, Polidarova L, Musilkova J, Hock M, Sumova A, Pacha J. Circadian regulation of electrolyte absorption in the rat colon. Am. J Physiol Gastrointest Liver Physiol. 2011;301(6):G1066–G1074. doi: 10.1152/ajpgi.00256.2011. [DOI] [PubMed] [Google Scholar]
- Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3− exchanger (slc4a1) J. Am. Soc. Nephrol. 2007;18(5):1408–1418. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H. Molecular and cellular regulation of pancreatic duct cell function. Curr/ Opin. Gastroenterol. 2009;25(5):447–453. doi: 10.1097/MOG.0b013e32832e06ce. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am. J. Physiol. Cell Physiol. 2011;301(2):C289–C303. doi: 10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(6):G1307–G1317. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WT, Alaminos M, Mora J, Cheung NK, La Quaglia MP, Gerald WL. Positional gene expression analysis identifies 12q overexpression and amplification in a subset of neuroblastomas. Cancer Genet. Cytogenet. 2004;154(2):131–137. doi: 10.1016/j.cancergencyto.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Sun X, Petrovic S. Increased acid load and deletion of AE1 increase Slc26a7 expression. Nephron. 2008;109(3):29–35. doi: 10.1159/000145465. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Buchner P, Yohimoto N, Hawkesford MJ, Shiu SH. Evolutionary relationships and functional diversity of plant sulfate transporters. Frontiers in Plant Physiology. 2012;2:119. doi: 10.3389/fpls.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(2):G358–G367. doi: 10.1152/ajpgi.00151.2010. [DOI] [PubMed] [Google Scholar]
- Tan X, Pecka JL, Tang J, Lovas S, Beisel KW, He DZ. A motif of eleven amino acids is a structural adaptation that facilitates motor capability of eutherian prestin. J. Cell Sci. 2012;125(4):1039–1047. doi: 10.1242/jcs.097337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5(1) doi: 10.1371/journal.pgen.1000338. e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure A, Lhuillier P, Gossen JA, Kuil CW, Lhote D, Jegou B, Escalier D, Gacon G. The testis anion transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum. Molec. Genet. 2007;16(15):1783–1793. doi: 10.1093/hmg/ddm117. [DOI] [PubMed] [Google Scholar]
- Toure A, Morin L, Pineau C, Becq F, Dorseuil O, Gacon G. Tat1, a novel sulfate transporter specifically expressed in human male germ cells and potentially linked to rhogtpase signaling. J. Biol. Chem. 2001;276(23):20309–20315. doi: 10.1074/jbc.M011740200. [DOI] [PubMed] [Google Scholar]
- Toure A, Rode B, Hunnicutt GR, Escalier D, Gacon G. Septins at the annulus of mammalian sperm. Biol. Chem. 2011;392(8–9):799–803. doi: 10.1515/BC.2011.074. [DOI] [PubMed] [Google Scholar]
- Trombetta D, Mertens F, Lonoce A, D'Addabbo P, Rennstam K, Mandahl N, Storlazzi CT. Characterization of a hotspot region on chromosome 12 for amplification in ring chromosomes in atypical lipomatous tumors. Genes Chrom Cancer. 2009;48(11):993–1001. doi: 10.1002/gcc.20700. [DOI] [PubMed] [Google Scholar]
- Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3− secretion. Gastroenterol. 2006;130(2):349–358. doi: 10.1053/j.gastro.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42(3):356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am. J Physiol. Renal Physiol. 2011;301(6):F1314–F1325. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincourt JB, Jullien D, Amalric F, Girard JP. Molecular and functional characterization of SLC26A11, a sodium-independent sulfate transporter from high endothelial venules. FASEB J. 2003;17(8):890–892. doi: 10.1096/fj.02-0787fje. [DOI] [PubMed] [Google Scholar]
- Vincourt JB, Jullien D, Kossida S, Amalric F, Girard JP. Molecular cloning of SLC26A7, a novel member of the SLC26 sulfate/anion transporter family, from high endothelial venules and kidney. Genomics. 2002;79(2):249–256. doi: 10.1006/geno.2002.6689. [DOI] [PubMed] [Google Scholar]
- von Stein P, Lofberg R, Kuznetsov NV, Gielen AW, Persson JO, Sundberg R, Hellstrom K, Eriksson A, Befrits R, Ost A, von Stein OD. Multigene analysis can discriminate between ulcerative colitis, Crohn's disease, and irritable bowel syndrome. Gastroenterol. 2008;134(7):1869–1881. doi: 10.1053/j.gastro.2008.02.083. [DOI] [PubMed] [Google Scholar]
- Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterol. 2009;136(3):893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NM, Simpson JE, Hoover EE, Brazill JM, Schweinfest CW, Soleimani M, Clarke LL. Functional activity of Pat-1 (Slc26a6) Cl(−)/HCO(−) exchange in the lower villus epithelium of murine duodenum. Acta Physiol. 2011;201(1):21–31. doi: 10.1111/j.1748-1716.2010.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterol. 2008;135(5):1645 e1643–1653 e1643. doi: 10.1053/j.gastro.2008.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen X, Liu B, Zhu Z. Suppression of PTP1B in gastric cancer cells in vitro induces a change in the genome-wide expression profile and inhibits gastric cancer cell growth. Cell Biol. Int. 2010a;34(7):747–753. doi: 10.1042/CBI20090447. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang S, Jia S, He DZ. Prestin forms oligomer with four mechanically independent subunits. Brain Res. 2010b;1333:28–35. doi: 10.1016/j.brainres.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25(21):5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl(−)/HCO3(−) exchanger in the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(3):G573–G579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am. J. Physiol. Cell Physiol. 2005;288(4):C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Kim HM, Billings S, Nakaya K, Li X, Singh R, Sharlin DS, Forrest D, Marcus DC, Fong P. Developmental delays consistent with cochlear hypothyroidism contribute to failure to develop hearing in mice lacking Slc26a4/pendrin expression. Am. J. Physiol. Renal Physiol. 2009;297(5):F1435–F1447. doi: 10.1152/ajprenal.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am. J. Physiol. Renal Physiol. 2007;292(5):F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Takei Y. Molecular physiology and functional morphology of SO(2) excretion by the kidney of seawater-adapted eels. J. Exp. Biol. 2011;214(Pt 10):1783–1790. doi: 10.1242/jeb.051789. [DOI] [PubMed] [Google Scholar]
- Wedenoja S, Pekansaari E, Hoglund P, Makela S, Holmberg C, Kere J. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum. Mutat. 2011;32(7):715–722. doi: 10.1002/humu.21498. [DOI] [PubMed] [Google Scholar]
- Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3(−) secretion in murine ileocolonic inflammation. Inflamm. Bowel Dis. 2012;18(1):101–111. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am. J. Physiol. Renal Physiol. 2002;283(4):F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M. Slc26a11, a chloride transporter, localizes with the vacuolar H(+)-ATPase of A-intercalated cells of the kidney. Kidney Int. 2011;80(9):926–937. doi: 10.1038/ki.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+ Am. J. Physiol. Cell Physiol. 2005;289(2):C493–C505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc. Natl. Acad. USA. 2008;105(46):17955–17960. doi: 10.1073/pnas.0800616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M. Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J. Biol. Chem. 2009;284(43):29470–29479. doi: 10.1074/jbc.M109.044396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, Soleimani M. Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion. J. Am. Soc. Nephrol. 2006;17(4):956–967. doi: 10.1681/ASN.2005111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Ishiguro H, Steward M, Sohma Y, Yamamoto A, Shimouchi A, Kondo T. Apical Cl−/HCO3− exchanger stoichiometry in the modeling of HCO3− transport by pancreatic duct epithelium. J. Med. Invest. 2009;56(Suppl):325–328. doi: 10.2152/jmi.56.325. [DOI] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J. Clin. Invest. 2009;119(1):193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol Gastrointest. Liver Physiol. 1998;275(6 Pt 1):G1445–G1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- Yang SK, Jung Y, Kim H, Hong M, Ye BD, Song K. Association of FCGR2A, JAK2 or HNF4A variants with ulcerative colitis in Koreans. Dig. Liver Dis. 2011;43(11):856–861. doi: 10.1016/j.dld.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Ye H, Zhang XQ, Broughton S, Westcott S, Wu D, Lance R, Li C. A nonsense mutation in a putative sulphate transporter gene results in low phytic acid in barley. Funct. Integr. Genom. 2011;11(1):103–110. doi: 10.1007/s10142-011-0209-4. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Park HJ, Yoo SY, Namkung W, Jo MJ, Koo SK, Park HY, Lee WS, Kim KH, Lee MG. Heterogeneity in the processing defect of SLC26A4 mutants. J. Med. Genet. 2008;45(7):411–419. doi: 10.1136/jmg.2007.054635. [DOI] [PubMed] [Google Scholar]
- Yusa A, Miyazaki K, Kimura N, Izawa M, Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70(10):4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham M. Analysis of the oligomeric structure of the motor protein prestin. J. Biol. Chem. 2006;281(29):19916–19924. doi: 10.1074/jbc.M513854200. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405(6783):149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zolotarev AS, Unnikrishnan M, Shmukler BE, Clark JS, Vandorpe DH, Grigorieff N, Rubin EJ, Alper SL. Increased sulfate uptake by E. coli overexpressing the SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. Comp. Biochem. Physiol. 2008;149(3):255–266. doi: 10.1016/j.cbpa.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]