Abstract
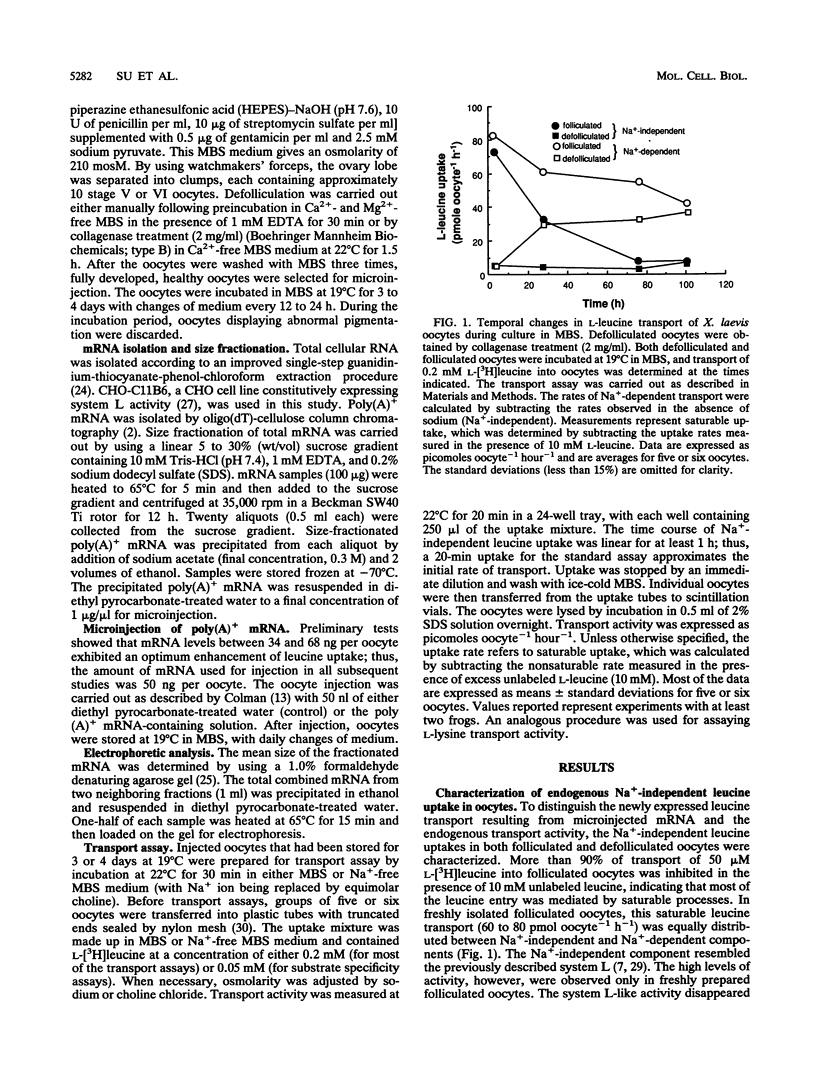
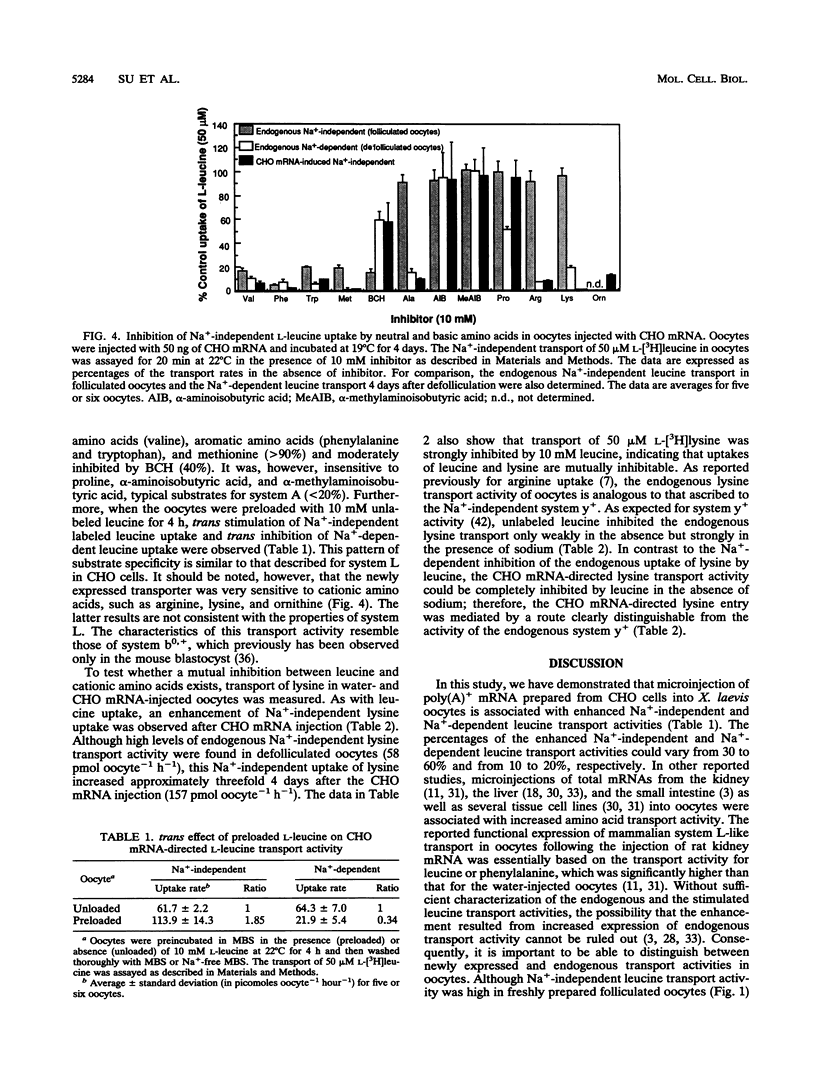
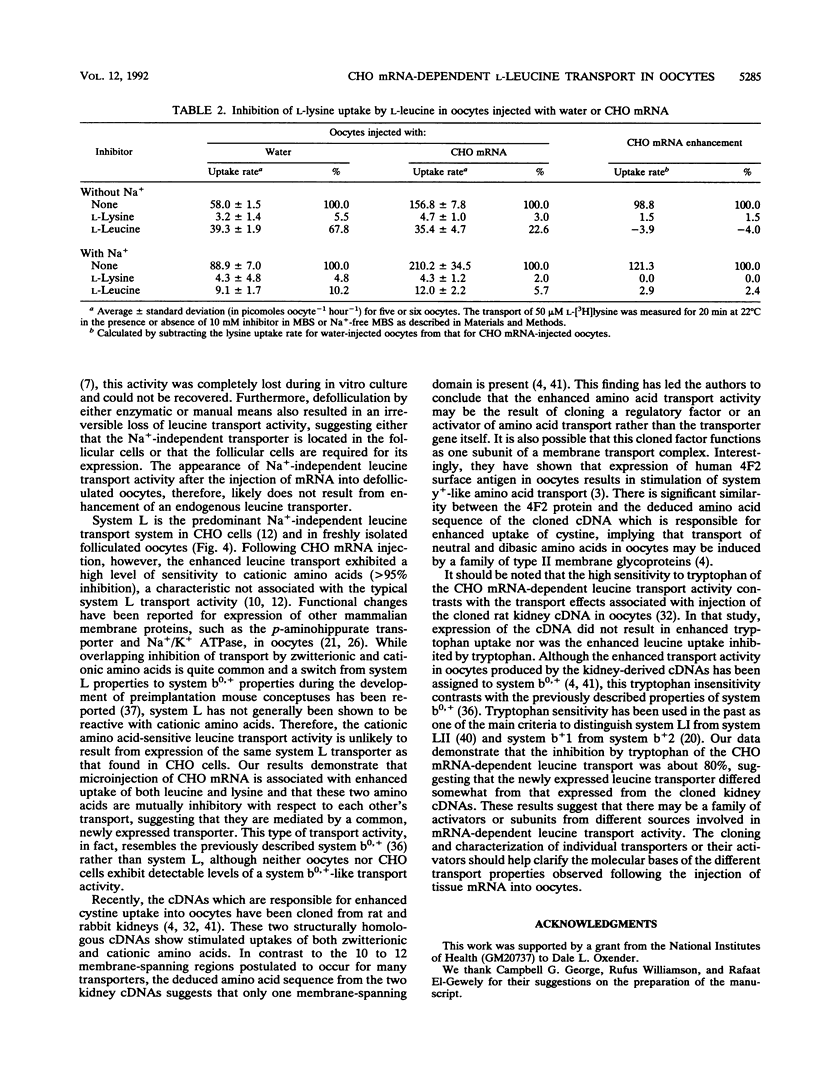
In freshly prepared uninjected folliculated oocytes, Na(+)-independent leucine uptake is mediated predominantly by a system L-like transport system. Removal of follicular cells, however, results in an irreversible loss of this transport activity. When total poly(A)+ mRNA derived from Chinese hamster ovary (CHO) cells was injected into prophase-arrested stage V or VI Xenopus laevis oocytes, enhanced expression of Na(+)-independent leucine transport was observed. The injected mRNAs associated with increased levels of leucine uptake were between 2 and 3 kb in length. The newly expressed leucine transport activity exhibited important differences from the known characteristics of system L, which is the dominant Na(+)-independent leucine transporter in CHO cells as well as in freshly isolated folliculated oocytes. The CHO mRNA-dependent leucine uptake in oocytes was highly sensitive to the cationic amino acids lysine, arginine, and and ornithine (> 95% inhibition). As with the leucine uptake, an enhanced lysine uptake was also observed in size-fractionated CHO mRNA-injected oocytes. The uptakes of leucine and lysine were mutually inhibitable, suggesting that the newly expressed transporter was responsible for uptakes of both leucine and lysine. The inhibition of uptake of lysine by leucine was Na+ independent, thus clearly distinguishing it from the previously reported endogenous system y+ activity. Furthermore, the high sensitivity to tryptophan of the CHO mRNA-dependent leucine transport was in sharp contrast to the properties of the recently cloned leucine transport-associated gene from rat kidney tissue, although leucine transport from both sources was sensitive to cationic amino acids. Our results suggest that there may be a family of leucine transporters operative in different tissues and possibly under different conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoshima H., Tomita K., Sugio S. Expression of amino acid transport systems in Xenopus oocytes injected with mRNA of rat small intestine and kidney. Arch Biochem Biophys. 1988 Aug 15;265(1):73–81. doi: 10.1016/0003-9861(88)90372-4. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Magagnin S., Werner A., Markovich D., Biber J., Testar X., Zorzano A., Kühn L. C., Palacin M., Murer H. Stimulation of system y(+)-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5606–5610. doi: 10.1073/pnas.89.12.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Werner A., Moore M. L., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5601–5605. doi: 10.1073/pnas.89.12.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely R. D., Berson H. E., Fremeau R. T., Jr, Caron M. G., Peek M. M., Prince H. K., Bradley C. C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991 Nov 7;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Boerner P., Evans-Laying M., U H. S., Saier M. H., Jr Polarity of neutral amino acid transport and characterization of a broad specificity transport activity in a kidney epithelial cell line, MDCK. J Biol Chem. 1986 Oct 25;261(30):13957–13962. [PubMed] [Google Scholar]
- Campa M. J., Kilberg M. S. Characterization of neutral and cationic amino acid transport in Xenopus oocytes. J Cell Physiol. 1989 Dec;141(3):645–652. doi: 10.1002/jcp.1041410324. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. On the strategy of kinetic discrimination of amino acid transport systems. J Membr Biol. 1985;84(2):97–103. doi: 10.1007/BF01872207. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984 Sep 3;779(3):255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Coady M. J., Pajor A. M., Toloza E. M., Wright E. M. Expression of mammalian renal transporters in Xenopus laevis oocytes. Arch Biochem Biophys. 1990 Nov 15;283(1):130–134. doi: 10.1016/0003-9861(90)90622-6. [DOI] [PubMed] [Google Scholar]
- Collarini E. J., Oxender D. L. Mechanisms of transport of amino acids across membranes. Annu Rev Nutr. 1987;7:75–90. doi: 10.1146/annurev.nu.07.070187.000451. [DOI] [PubMed] [Google Scholar]
- Guastella J., Brecha N., Weigmann C., Lester H. A., Davidson N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7189–7193. doi: 10.1073/pnas.89.15.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Ikeda T., Coady M., Gundersen C. B., Wright E. M. Expression of size-selected mRNA encoding the intestinal Na/glucose cotransporter in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2634–2637. doi: 10.1073/pnas.84.9.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilty J. E., Lorang D., Amara S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991 Oct 25;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kwon O., Kwon H. M., Hong S. K., Goldinger J. M. Size selected mRNA induces expression of P-aminohippurate transport in Xenopus oocytes. Proc Soc Exp Biol Med. 1989 Nov;192(2):205–208. doi: 10.3181/00379727-192-2-rc2. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Palacin M., Werner A., Dittmer J., Murer H., Biber J. Expression of rat liver Na+/L-alanine co-transport in Xenopus laevis oocytes. Effect of glucagon in vivo. Biochem J. 1990 Aug 15;270(1):189–195. doi: 10.1042/bj2700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Schwarz W., Gu Q. B. Characteristics of the Na+/K+-ATPase from Torpedo californica expressed in Xenopus oocytes: a combination of tracer flux measurements with electrophysiological measurements. Biochim Biophys Acta. 1988 Nov 22;945(2):167–174. doi: 10.1016/0005-2736(88)90479-8. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Collarini E. J., Mansukhani A., Hampel A. E., Oxender D. L. Isolation of Chinese hamster ovary cell mutants defective in the regulation of leucine transport. J Biol Chem. 1983 Jul 10;258(13):8183–8187. [PubMed] [Google Scholar]
- Sigel E. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol. 1990 Sep;117(3):201–221. doi: 10.1007/BF01868451. [DOI] [PubMed] [Google Scholar]
- Tarnuzzer R. W., Campa M. J., Qian N. X., Englesberg E., Kilberg M. S. Expression of the mammalian system A neutral amino acid transporter in Xenopus oocytes. J Biol Chem. 1990 Aug 15;265(23):13914–13917. [PubMed] [Google Scholar]
- Tate S. S., Urade R., Getchell T. V., Udenfriend S. Expression of the mammalian Na+-independent L system amino acid transporter in Xenopus laevis oocytes. Arch Biochem Biophys. 1989 Dec;275(2):591–596. doi: 10.1016/0003-9861(89)90405-0. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Yan N., Udenfriend S. Expression cloning of a Na(+)-independent neutral amino acid transporter from rat kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):1–5. doi: 10.1073/pnas.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Mackenzie B., Low S. Y., Rennie M. J. Expression of rat liver glutamine transporters in Xenopus laevis oocytes. J Biol Chem. 1992 Feb 25;267(6):3873–3877. [PubMed] [Google Scholar]
- Thomas E. L., Christensen H. N. Indications of spatial relations among structures recognizing amino acids and Na+ at a transport receptor site. Biochem Biophys Res Commun. 1970 Jul 27;40(2):277–283. doi: 10.1016/0006-291x(70)91006-5. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Shao T. C., Christensen H. N. Structural selectivity in interaction of neutral amino acids and alkali metal ions with a cationic amino acid transport system. J Biol Chem. 1971 Mar 25;246(6):1677–1681. [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Inhibition of transport system b0,+ in blastocysts by inorganic and organic cations yields insight into the structure of its amino acid receptor site. Biochim Biophys Acta. 1990 Jun 27;1025(2):215–224. doi: 10.1016/0005-2736(90)90100-3. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem. 1988 Mar 5;263(7):3150–3163. [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M., Weimer B. D. Changes in the activities of amino acid transport systems b0,+ and L during development of preimplantation mouse conceptuses. Biochim Biophys Acta. 1990 Jan 15;1021(1):77–84. doi: 10.1016/0005-2736(90)90387-4. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Christensen H. N., Campione A. L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem. 1985 Oct 5;260(22):12118–12123. [PubMed] [Google Scholar]
- Weissbach L., Handlogten M. E., Christensen H. N., Kilberg M. S. Evidence for two Na+-independent neutral amino acid transport systems in primary cultures of rat hepatocytes. Time-dependent changes in activity. J Biol Chem. 1982 Oct 25;257(20):12006–12011. [PubMed] [Google Scholar]
- Wells R. G., Hediger M. A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Christensen H. N. Cationic amino acid transport into cultured animal cells. II. Transport system barely perceptible in ordinary hepatocytes, but active in hepatoma cell lines. J Biol Chem. 1982 Apr 25;257(8):4450–4457. [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992 Jan 5;267(1):649–652. [PubMed] [Google Scholar]
- Young J. D., Mason D. K., Fincham D. A. Topographical similarities between harmaline inhibition sites on Na+-dependent amino acid transport system ASC in human erythrocytes and Na+-independent system asc in horse erythrocytes. J Biol Chem. 1988 Jan 5;263(1):140–143. [PubMed] [Google Scholar]