Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder that causes selective death of motor neurons followed by paralysis and death. A subset of ALS cases is caused by mutations in the gene for Cu, Zn superoxide dismutase (SOD1), which impart a toxic gain of function to this antioxidant enzyme. This neurotoxic property is widely believed to stem from an increased propensity to misfold and aggregate caused by decreased stability of the native homodimer or a tendency to lose stabilizing posttranslational modifications. Study of the molecular mechanisms of SOD1-related ALS has revealed a complex array of interconnected pathological processes, including glutamate excitotoxicity, dysregulation of neurotrophic factors and axon guidance proteins, axonal transport defects, mitochondrial dysfunction, deficient protein quality control, and aberrant RNA processing. Many of these pathologies are directly exacerbated by misfolded and aggregated SOD1 and/or cytosolic calcium overload, suggesting the primacy of these events in disease etiology and their potential as targets for therapeutic intervention.
I. ALS Is a Deadly Neurodegenerative Disorder
Amyotrophic lateral sclerosis (ALS) was first described by the noted French neurologist Jean-Martin Charcot in 1869, who connected the progressive paralytic syndrome with lesions in both white and gray matter of the central nervous system (CNS).1 Over 140 years later, ALS is the most common adult-onset motor neuron disorder, affecting approximately 1–2 per 100,000 people worldwide. Considering the short course of disease progression (death/tracheotomy typically within 2–5 years of diagnosis), 1 in every 800 individuals is expected to face ALS in his/her lifetime.2–4
As described by Charcot, ALS involves degeneration of the upper motor neurons (UMN) of the motor cortex and of the lower motor neurons (LMN), which extend through the brainstem and spinal cord to innervate skeletal muscle. Though the upper and lower motor systems are known to be interconnected, controlling voluntary muscle movement in concert, the primary site of dysfunction in ALS has long been a source of debate.5–7 Questions of UMN/LMN primacy aside, ALS is clearly specific for motor neurons and largely spares cognitive ability, sensation, and autonomic nervous functions. Muscles controlling eye movement and the pelvic floor are the only skeletal musculature left unaffected. However, in a minority of cases (5–10%), patients also develop frontotemporal lobar dementia (FTLD). It has been suggested that a greater percentage of patients experience some cognitive change (such as loss in executive function) without crossing the threshold required for a diagnosis of dementia.8
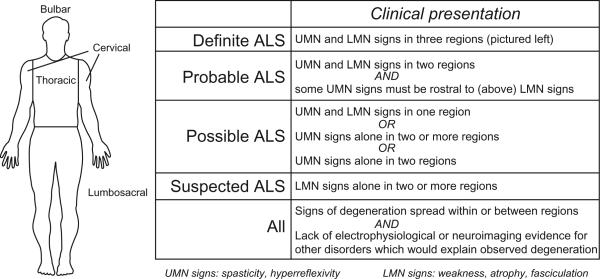
Clinical presentation varies but most commonly consists of weakness, fasciculations (twitching muscles), and/or hyperreflexivity of facial muscles (bulbar onset) or limbs (spinal onset). Interestingly, initial symptoms usually appear at a focal site and later spread along contiguous anatomic paths.9 Diagnosis is achieved by a combination of clinical examination and electromyography (EMG), in which positive, sharp waves and fibrillation potentials provide evidence for active denervation. The El Escorial criteria were developed in 1990 and are still utilized to diagnose and classify ALS cases as “possible,” “probable,” or “definite”10 (Fig. 1). Guidelines on implementation of the El Escorial criteria have been revised to place greater emphasis on electrophysiological abnormalities, which can be detected earlier and thus facilitate timely diagnosis.11
Fig. 1.
El Escorial criteria for diagnosis of ALS. UMN = upper motor neuron; LMN = lower motor neuron.
II. Etiology of ALS
The majority of ALS cases (≈82%) are sporadic (SALS), having no apparent heritability.9 Up to 5% of SALS cases are caused by mutations in the 43-kDa trans-activating response region DNA-binding protein (TDP-43). TDP-43 mutations have also been linked to ≈3% of inherited, or “familial” ALS (FALS).12 The most commonly occurring mutations in patients with FALS are found in the gene for Cu, Zn superoxide dismutase (SOD1) and account for approximately 20% of all FALS.13,14 Most of these mutations are missense mutations that cause autosomal dominant ALS, except the D90A polymorphism, which can also behave as a recessive mutation.15 FALS-causative mutations have also been found in genetic loci corresponding to alsin, a guanine exchange factor for Rac1 that plays a role in cytoskeletal dynamics16,17; senataxin, a DNA/RNA helicase that may be involved in RNA processing18,19; vesicle-associated membrane protein-associated protein B (VAPB), which facilitates intracellular vesicular trafficking20; and angiogenin (ANG)21–23 (Table I). Some polymorphisms found in patients with ALS do not segregate completely with disease and may represent genetic risk factors rather than causative mutations. Mutations in the neurofilament-heavy subunit,24,25 vascular endothelial growth factor (VEGF),26 and ciliary neurotrophic factor (CNTF)27,28 fall under this category. All genetic loci that have been reported as putative modifiers of ALS susceptibility are listed in the ALS Online Genetics Database (http://alsod.iop.kcl.ac.uk).
TABLE I.
Genetic Loci Associated with ALS
| Locus | Chromosome | Gene | Characteristics | |
|---|---|---|---|---|
| Classical ALS | ALS1 | 12q22.1 | Superoxide dismutase 1 (SOD1) | AD, adult onset |
| ALS2 | 2q33 | Alsin | AR, juvenile onset | |
| ALS3 | 18q21 | Unknown | AD, adult onset | |
| ALS4 | 9q34 | Senataxin (SETX) | AD, juvenile onset | |
| ALS5 | 15q15.1–21.1 | Unknown | AR, juvenile onset | |
| ALS6 | 16q12.1–12.2 | Fused in sarcoma (FUS) | AD, adult onset | |
| ALS7 | 20p13 | Unknown | AD, adult onset | |
| ALS8 | 20q13.33 | Vesicle-associated membrane protein-associated protein B (VAPB) | AD, adult onset | |
| ALS9 | 14q11 | Angiogenin (ANG) | AD, adult onset | |
| ALS10 | 1p36 | Tar DNA-binding protein (TARDBP) | AD, adult onset | |
| Atypical ALS | ALSX | X | Unknown | XD, adult onset |
| ALS/FTLD | 9q21–22 9p13.3–21.3 |
Unknown | XD, adult onset | |
| ALS–PDC | 17q21.1 | Membrane-associated protein tau (MAPT) | AD, adult onset |
AD, autosomal dominant; AR, autosomal recessive; XD, X-linked dominant; FTLD, frontotemporal lobar dementia; PDC, Parkinsonism-dementia complex.
Updated references for each locus at the ALS Online Genetics Database (http://alsod.iop.kcl.ac.uk).
Gray-shaded areas are alternated with unshaded rows to improve readability of the table
There is evidence to suggest that specific environmental factors play a prominent role in the etiology of some ALS cases. Geographically limited populations with dramatically increased ALS incidence, such as inhabitants of the Kii peninsula in Japan,29 the Chamorro people of Guam, Gulf War veterans,30,31 and Italian soccer players,32 certainly lead one to suspect the environment as a potential modifier of disease susceptibility. There also have been reports of ALS in individuals with intense exposure to particular stressors, such as harsh chemicals and heavy metals,33,34 viral infection,35 electrical shock,36 and traumatic nerve injury.37 Most of these reports, however, involve a very small number of cases and do not permit rigorous evaluation of these stressors as potential risk factors for ALS.
The most convincing instance of a causal link between ALS and toxin exposure is the case of the Chamorro population. A cycad indigenous to Guam produces the neurotoxin β-methylamino-L-alanine (BMAA) in its seeds, which are eaten by flying foxes as well as ground into flour by the Chamorro. While the dosage of BMAA resulting from a reasonable human consumption of cycad flour is far below the threshold necessary to provoke neurodegeneration in primates,38 this potent neurotoxin is enriched 100-fold in the tissues of the flying fox, a delicacy to the Chamorro.39,40 Furthermore, BMAA is found in the brain tissue from Chamorros who succumb to ALS, but not those who die of other causes, and the prevalence of ALS among this population dropped after overhunting thinned the flying fox population.39 While providing a convincing causal link between BMAA exposure and ALS, it is tempting to dismiss the case of the Chamorro as inapplicable to disease risk in the general population. However, BMAA-producing cyanobacteria are present in many ecosystems, and a recent study of Baltic Sea marine life revealed that BMAA is concentrated in the tissues of organisms at higher trophic levels, such as fish and mollusks, that are consumed by humans.41 BMAA also was found in cyanobacteria-containing sand from Qatar,42 raising the possibility that Gulf veterans may have been exposed to this toxin through inhalation. Furthermore, the incidence of ALS diagnosis is elevated 10–25–fold among residents of Enfield, New Hampshire, a town bordering Lake Mascoma, which is subject to frequent “blooms” of cyanobacteria.43 While no conclusive statements can be made from these few examples, the worldwide prevalence of cyanobacteria seems a compelling reason to investigate ALS risk associated with BMAA exposure.
III. SOD1-Related Pathology as a General Model for ALS
The discovery of SOD1's role in FALS14 offered the first insight into the molecular mechanisms of ALS, and the study of SOD1-mediated pathology has contributed much to our current understanding of the disease. The majority of in vivo work has utilized transgenic mice expressing FALS mutants of human SOD1, which develop a progressive motor neuron syndrome reminiscent of the human ALS phenotype (reviewed in Ref. 44). The sporadic disease differs little clinically from SOD1-related FALS, leading to the widespread supposition that all cases of ALS share some common mechanism(s) of pathology.2,45,46 In reviewing the proposed molecular bases of ALS, we focus on the contribution of SOD1, a well-studied cause of ALS that may exhibit pathogenic mechanisms common to other forms of the disease.
IV. Misfolding and Aggregation Is the Most Likely Source of SOD1 Toxicity
SOD1 is a ubiquitous cytosolic enzyme whose primary function is the dismutation of the superoxide radical to a less oxidizing species (H2O2) via a bound Cu2+ ion. Although this enzyme plays an important role as a cellular antioxidant, the ability of SOD1 mutants to selectively kill motor neurons is not linked to a loss of dismutase function. Not only do many FALS mutants retain enzymatic activity at or near wild-type levels,47–49 but SOD1 null mice do not exhibit neurodegeneration.50 Furthermore, the toxicity of SOD1 mutants cannot be reversed by coexpression of wild-type SOD1.51 This evidence has led to widespread acceptance of the hypothesis that SOD1 mutants acquire a novel toxic property independent of their enzymatic function.
Despite over 15 years of research, the mode(s) by which SOD1 mutants selectively kill motor neurons have not been clearly delineated. However, a large body of evidence implicates a common propensity to misfold and aggregate as the primary toxic gain of function. Destabilization of the native fold is an attractive hypothesis for SOD1 mutant pathogenicity, offering a plausible explanation for the common disease outcome of over 140 mutants spanning the sequence and structure. Early in silico studies by our laboratory predicted that a majority of SOD1 mutations would destabilize the native fold or quaternary structure,52 a trend that since has been verified experimentally.53–56 Especially severe destabilization caused by certain mutations could account for their inherently higher aggressiveness (short disease duration).57,58 Indeed, several recent analyses of in vitro SOD1 mutant behavior and FALS patient survival showed that protein instability and increased aggregation rate correlated with decreased survival time59,60 (Fig. 2). Furthermore, the presence of SOD1-immunoreactive proteinaceous aggregates in SALS patient motor neurons62–65 suggests that aberrant oligomerization of SOD1 could be a common feature of ALS, regardless of genotype. It thus appears that ALS is a protein conformational disorder, akin to other neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's.2
Fig. 2.
Protein instability and aggregation propensity correlate with shorter survival times in SOD1-related FALS. In all panels, data are weighted for linear regression analysis according to the number of patients for which survival data was available. (A) The aggregation propensities of FALS-causative SOD1 mutations are calculated using a rederivation of the Chiti–Dobson equation,61 which was validated by comparison with available experimental data, and normalized such that the least and most aggregation-prone proteins have values of 0 and 1, respectively. (B) Protein instability is taken as 1 minus the change in free energy of unfolding or change in melting temperature of the mutant protein compared to the wild type (from published in vitro data). Instability values are normalized such that the most and least stable proteins have values of 0 and 1, respectively. (C) Normalized sums of aggregation propensity and protein instability values plotted against patient survival. From Wang et al.60
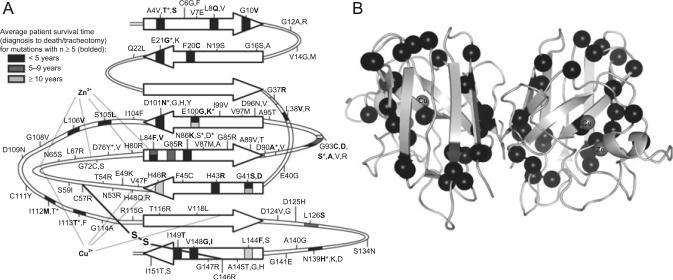
Though a primary role for SOD1 aggregation in FALS seems likely, deconstruction of the molecular determinants and mechanisms of this process is incomplete. SOD1 is an extremely stable enzyme in its fully mature, homodimeric form, remaining active in the presence of 6M guanidinium chloride or 8M urea.66,67 SOD1 owes its extraordinary stability largely to the coordination of Zn2þ, which constrains the relatively unstructured electrostatic and zinc-binding loops, “tethering” them together and protecting the protein core, an eight-stranded Greek key β-barrel53,68,69 (Fig. 3). The catalytic copper ion and an intrasubunit disulfide bridge between Cys-57 and Cys-146 appear to contribute relatively little to monomer thermodynamic stability, but the latter modification constrains loop mobility and facilitates dimer formation.66,68,74 Metal-bound, disulfide-oxidized SOD1 forms an exceptionally stable homodimer, with low nanomolar binding affinity.75,76 These maturation events are mutually interdependent—metal binding promotes disulfide bond formation, disulfide bond formation and metal binding promote dimerization, and dimeric SOD1 is more resistant to disulfide reduction/metal loss.68,75,77
Fig. 3.
Location of FALS-causative mutations on the SOD1 structure. (A) Map of SOD1 secondary structure showing locations of FALS missense mutations, residues that coordinate Cu2+ (His-46, His-48, His-120, His-63) and Zn2+ (His-63, His-71, His-80, and Asp-83) ions, and the intramolecular disulfide bridge. Arrows indicate β-strands. Epidemiological data were taken from Refs. 45,60,70–73; mutations with survival data for at least five patients are bolded, and the residue position is shaded to indicate the corresponding average survival time. For positions with more than one n≥5 mutation, the upper color corresponds to the first mutation listed. (B) Crystal structure (PDB code 1spd) of fully mature (metal-bound, disulfide-intact) homodimeric SOD1 with positions of aggressive (survival time <5 years) mutations indicated by black spheres.
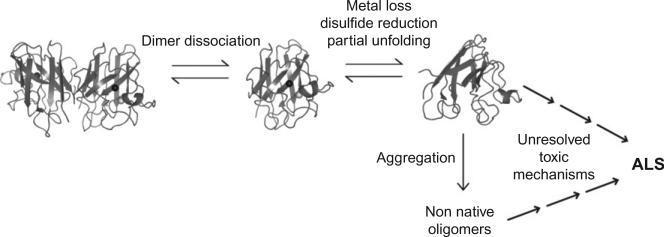
In vitro studies show that dimer dissociation is a necessary initiating step in SOD1 aggregation.76,78 The resultant monomeric SOD1 is more susceptible to the loss of the stabilizing zinc ion and disulfide bridge,79,80 leading to freer loop movement81 and exposure of β-barrel edge strands.68,82 Dynamical studies of wild-type and FALS mutant SOD1 revealed a transient “excited state” whose population is enhanced by mutations and zinc loss, but unaffected by disulfide status.83 Increased surface hydrophobicity of metal-free, disulfide-reduced mutant SOD1 was shown directly by Tiwari et al. using 1-anilinonaphthalene-8-sulfonic acid (ANS), a fluorescent dye that binds to hydrophobic surfaces.84 Munch et al. obtained similar results using a different hydrophobic dye, Sypro Orange, and found that increased exposure of hydrophobic regions precedes aggregation.85 A general model of SOD1 aggregation in ALS has emerged in which dimer dissociation and subsequent metal loss (and/or disulfide reduction) induce structural distortions that favor assembly into non-native oligomers (oligomers other than the native homodimer) (Fig. 4). FALS mutations promote aggregation by increasing the tendency of SOD1 to lose its stabilizing posttranslational modifications and/or by decreasing the intrinsic stability of the apo-monomer.52,54–56,68,86–88 Substantial gaps remain in our understanding of the relationship between SOD1 aggregation and ALS pathology. These pertain to aggregate structure, mechanism of formation, and toxicity.
Fig. 4.
General mechanism of SOD1 aggregation.
A. SOD1 Aggregate Structure
No high-resolution structural information is available for misfolded monomeric SOD1 or nonnative oligomers. The transient nature of many structurally-perturbed SOD1 species makes their isolation for study impractical. However, misfolded dimeric or monomeric SOD1 can be detected using an antibody specific for residues 145–151, which are normally buried within the native dimer interface.89 SOD1 monomers with a more substantially disrupted fold can be tracked using an antibody recognizing the natively buried residues of β strand 4 (residues 42–48).90 Chromatographic methods have also been utilized to isolate misfolded SOD1 using their affinity to hydrophobic resins.91 Continued study using these and similar methods will be useful in tracking the spatial and temporal distribution of misfolded SOD1 in cell culture, transgenic mouse models, and patients with ALS, providing insight into the molecular determinants and cellular consequences of SOD1 destabilization.
Electron microscopic, immunohistological, and biochemical studies have shed some light on the structural properties of SOD1 aggregates. Both insoluble, detergent-resistant aggregates and soluble oligomers have been noted in cell culture, transgenic mice, and in vitro.63,64,92–94 These species contain metal-free SOD1 that is full length and usually lacks the native disulfide bridge.95 Aggregates formed in vitro under near-physiological conditions are often fibrillar and bind thioflavin T (ThT+, suggestive of amyloid character),86,96–98 while in vivo aggregates sometime appear amorphous or pore-shaped and do not always bind amyloid-sensitive dyes.90,93,99–101 Soluble misfolded SOD1 populates a wide range of oligomeric states and also accumulates as non-native monomers, dimers, or trimers.62,91,96 The instability of some soluble oligomers may preclude the use of static structural techniques, such as X-ray crystallography, to determine structural details, but solution-state methods such as nuclear magnetic resonance (NMR) or limited proteolysis, especially coupled with computational structural modeling, may yield insights into their conformations.
B. Mechanism of SOD1 Aggregation
The likelihood that misfolded SOD1 samples a multitude of conformational states also complicates detailed mechanistic study of oligomer formation. However, it is clear that posttranslational modifications of the SOD1 polypeptide modulate oligomer formation to some extent. As discussed above, the native intramolecular disulfide bridge and metal binding both impart exceptional stability to SOD1 and, unsurprisingly, loss of these factors drives misfolding and aggregation. However, reduction of the native Cys-57–Cys-146 disulfide has been putatively linked to the initiation, but not elongation, of amyloid-like fibril formation in vitro.86,97 Disulfide-intact, but metal-free, SOD1 incubated at physiological pH and temperature can be induced to aggregate by disrupting noncovalent interactions with a chaotrope, but treatment with a reducing agent instead results in a 20-fold shorter lag period.97 Disulfide bond reduction, while apparently dispensable for fibril formation in vitro, may specifically accelerate nucleation. Indeed, the presence of a small amount of disulfide-reduced wild-type or mutant SOD1 appeared to “recruit” disulfide-intact wild-type SOD1 into fibrils without the need for additional reducing agent.97 The mechanism by which disulfide-reduced SOD1 facilitates fibril nucleation has not yet been demonstrated, although the requirement of Cys-57 and Cys-146 suggests that intermolecular cross-linking between these two residues may play a role.97 It is also unclear whether in vivo SOD1 aggregation, which is not always amyloid-like, proceeds by elongation of nuclei.
The two free cysteines in SOD1, at positions 6 and 111, also appear to be involved in SOD1 oligomer assembly. In vitro aggregation of metal-free wild-type SOD1 coincides with a loss of free cysteines and oligomer formation is attenuated by mutations at either or both sites,96,102 leading to the hypothesis that intermolecular disulfide cross-linking mediates oligomerization. However, more recent studies in mutant SOD1 transgenic mice show that aberrant disulfide linkages are present only in large-scale aggregates appearing late in the disease.103,104 A secondary role for intermolecular disulfide cross-linking in aggregation is unsurprising given the reducing environment of the cytosol and may be due to “trapping” of SOD1 in a misfolded state after an initial destabilizing trigger, such as Zn2+ loss or altered conformational dynamics resulting from mutation.87,88 Cell culture experiments reveal a key role for Cys-111 in the promotion of SOD1 oligomerization, as mutation of this residue, but not Cys-6, attenuated oligomer formation and protected cells from mutant SOD1-mediated toxicity.105 It could be that the higher solvent accessibility of Cys-111 (and thus, increased susceptibility to aberrant intermolecular disulfide cross-linking) accounts for its particular importance in SOD1 oligomerization. However, recent investigations by our laboratory offer an alternate interpretation of this phenomenon. We recently confirmed earlier reports that Cys-111 forms a mixed disulfide with glutathione and showed that this modification is abundant in human tissue. Interestingly, Cys-111 glutathionylation triggers dissociation of both wild-type and FALS mutant dimers in vitro, thus promoting the first step in SOD1 aggregation.105a The characterization of intermolecular disulfide formation as a nonessential late event in oligomerization suggests that Cys-111 may primarily promote aggregation by its ability to be glutathionylated, a modification that destabilizes the native homodimer. Treatments used to prevent Cys-111-mediated SOD1 aggregation in previous cell culture experiments,105 such as addition of a reducing agent and overexpression of glutaredoxin, would remove the glutathione moiety in addition to reducing intermolecular disulfides. Therefore, further study would be useful to resolve the contributions of Cys-111 glutathionylation and intermolecular disulfide bond formation in oligomer formation.
An emerging question in the study of mutant-mediated SOD1 aggregation is the extent of involvement of the wild-type protein. Since most FALS patients with SOD1 mutations are heterozygous, recent studies have utilized transgenic mice expressing both human wild-type and FALS mutant SOD1 to more accurately recapitulate SOD1 behavior in vivo. Coexpression of SOD1WT exacerbates the disease phenotypes of SOD1G93A106,107 SOD1G85R108 SOD1L126Z, and SOD1A4V mice,92 hastening the appearance of cellular pathologies and shortening survival times (Fig. 5). The effect of the wild-type protein on SOD1A4V mice is particularly dramatic; even though FALS patients with this mutation exhibit particularly rapid disease progression, mice expressing only SOD1A4V do not develop motor neuron disease within their lifetimes.48 The toxic effect of coexpressing wild-type protein may be a simple issue of protein copy number. An earlier study of G85R mice51 did not find any effect of human wild-type coexpression on survival, but both SOD1G85R and SOD1WT were expressed at lower levels than in the more recent model.108 The observation that mutant SOD1 toxicity depends heavily on protein abundance, while not surprising, is troubling since nearly all mutant SOD1 transgenic mice substantially overexpress the protein.44 However, mice overexpressing SOD1WT alone, while exhibiting minor deficits in motor function, do not experience paralysis or die prematurely.107 Thus, FALS mutants clearly possess intrinsic pathogenicity independent of gene dosage. Mutant-wild-type heterodimers and disulfide-linked aggregates containing both wild-type and mutant SOD1 have been observed,92,108 suggesting that wild-type SOD1 is “recruited” into non-native oligomers by pathogenic mutants, possibly under conditions of oxidative stress. These studies present an incomplete picture of the role of SOD1WT in aggregation but highlight the need for further scrutiny of the physiological relevance of commonly used transgenic mouse models.
Fig. 5.
Coexpression of wild-type SOD1 exacerbates the phenotype of FALS mutant transgenic mice. Survival and symptom onset are plotted versus age for mice expressing G93A, A4V, and L126Z (truncation) mutants of SOD1 with and without coexpression of the human wild-type enzyme (hwtSOD1). From Deng et al.92 Copyright 2006 National Academy of Sciences, USA.
C. Toxicity of SOD1 Aggregates
While misfolding and aggregation has been convincingly linked to ALS pathogenesis, the species responsible for motor neuron death has not been identified. Insoluble inclusion bodies appear in brain stem and spinal cord coincident with symptom onset and accumulate progressively in the terminal stages,109–113 leading to an initial belief that large-scale aggregates are themselves toxic. However, the ability to detect soluble misfolded SOD1 led to the discovery that these non-native forms are present from birth91,114 and selectively enriched in motor neurons89,91 of FALS transgenic mice. It thus appears that small misfolded SOD1 may be the actual toxic culprit(s), present throughout life but causing symptoms only when cells can no longer keep their deleterious effects in check. In such a scenario, assembly of soluble misfolded SOD1 into relatively inert inclusions is expected to be neuroprotective, a phenomenon that has been demonstrated for aggregation of Ab and huntingtin in Alzheimer's and Huntington's diseases, respectively.115–117 However, the relative toxicities of small soluble oligomers and large-scale aggregates of SOD1 remain to be directly proven. Similarly, no consensus has yet been reached on the mode(s) by which non-native SOD1 kills cells. The evidence at present, though sometimes contradictory, identifies a diverse set of targeted organelles, signaling pathways, and other cellular processes. In the remainder of this chapter, we will discuss the various pathological processes occurring in ALS, with special attention to a potential causal role for misfolded and/or aggregated SOD1.
V. Motor Neuron Death in ALS: Apoptotic Versus Necrotic, Cell-Autonomous Versus Non-Cell-Autonomous
Classification of motor neuron death in ALS remains controversial. Spinal cord motor neurons of ALS patients and transgenic mice overexpress the pro-apoptotic BH3-only protein Bax,118 and knocking out this protein in SOD1G93A mice results in delayed disease onset.119 However, activation of “executioner” caspases (caspase-3, caspase-6, and caspase-7) is not always seen120–122 and the morphology of dying motor neurons is often uncharacteristic of apoptotic bodies.123,124 The current model for neuronal death in ALS is the one that has characteristics of both apoptosis and necrosis, with “necrotic-like” and “apoptotic-like” processes dominating in different cell types and/or disease stages that have yet to be delineated.121,125
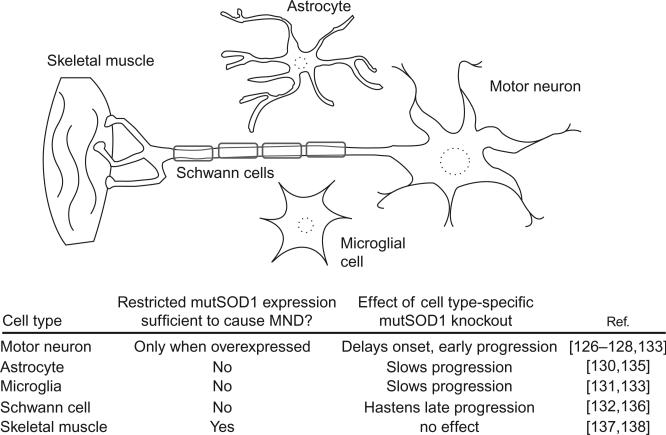
Another question pertaining to classification of cell death in ALS is the autonomy of this process in motor neurons. A cell-autonomous “dying forward” process was long assumed, in which dysfunction within motor neurons, independent of input from surrounding cells, leads to their death and a subsequent denervation of motor endplates. However, several studies using cell-specific expression of mutant SOD1 support a prominent role of non-neuronal cells in promoting cell death. The most striking evidence against cell-autonomous motor neuron death is the reported lack of ALS phenotype of transgenic mice expressing mutant SOD1 under a neuron-specific promoter.126,127 Mice with supraendogenous neuron-specific expression do experience neurodegeneration but show different pathological changes compared to transgenics ubiquitously expressing FALS mutant SOD1. Symptom onset occurs later, is diffuse rather than focal, and lacks certain morphological hallmarks such as mitochondrial vacuolization.128 Astrocytes, supporting cells that neighbor motor neurons, have also been proposed to turn deadly in ALS through defects in glutamate processing and other mechanisms (see below). While mutant SOD1-expressing astrocytes exert toxicity on motor neurons in coculture,129 astrocyte-specific expression failed to cause motor neuron disease in mice.130 Mutant SOD1 expression limited to microglia (phagocytic cells in the CNS) or Schwann cells, which myelinate motor axons, likewise produced no ALS phenotype.44,131,132 Although mutant SOD1 in neurons, astrocytes, and microglia appears insufficient to provoke ALS symptoms in isolation, knockdown in these cell types using Cre–Lox systems or siRNA delays disease onset and/or progression in transgenic mice with ubiquitous expression.133–135 Surprisingly, Schwann cell-specific knockout of mutant SOD1 was reported to accelerate disease progression.136 Taken together, these studies highlight the importance of non-neuronal cells in ALS pathogenesis and progression and suggest that the primary site of dysfunction may not be the motor neuron itself (Fig. 6).
Fig. 6.
Motor neuron death in ALS is not cell autonomous. Table below the figure summarizes findings of studies using transgenic mouse models with tissue-specific mutant SOD1 expression or Cre–Lox/siRNA knockdown of ubiquitously expressed mutant SOD1.
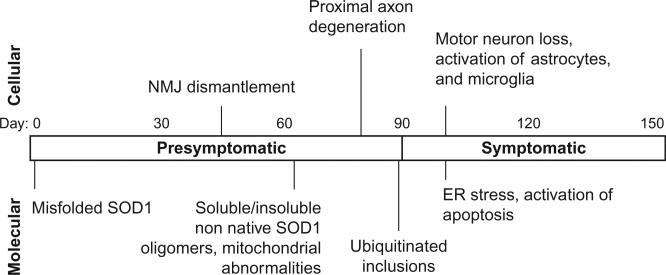
Interestingly, skeletal muscle-specific expression of mutant, and to a lesser extent, wild-type, SOD1 in mice was recently shown to cause early motor deficits, followed by neuromuscular junction (NMJ) dismantlement and late-onset motor neuron loss.137 This result is surprising in light of previous studies showing no effect of mutant SOD1 knockdown in muscle138,139 but is consistent with reports of muscular defects as the primary pathogenic events in ALS.140,141 The ability of muscle-restricted expression of mutant SOD1 to provoke motor neuron degeneration, as well as the precedence of neuromuscular denervation in the disease course (Fig. 7), suggests a “dying back” model of ALS where loss of the neuronal cell body is not the initiating event. The primacy of the NMJ in ALS pathogenesis is further supported by studies showing that inhibition of pro-apoptotic machinery, while completely preventing motor neuron loss in mice, did not prevent denervation and offered little functional improvement or lifespan extension.119,142,143 The mechanisms by which toxic signals are transmitted from muscle to NMJ to motor axons are unknown, but a recent study of SOD1G93A mice noted increased retrograde axonal transport of proteins related to cellular stress and death.144 Muscular overexpression of a mitochondrial uncoupling protein, which disrupts ATP synthesis, was also sufficient to induce progressive NMJ dismantlement and motor neuron loss in mice.145 Loss of compensatory reinnervation may also be involved in NMJ pathology. A skeletal muscle-specific microRNA was recently identified that slows disease progression in SOD1G93A mice by stimulating reformation of neuromuscular synapses with denervated muscles.146 Growing support for a “dying back” hypothesis of ALS highlights the need for additional investigation of skeletal muscle as a primary site of pathology.
Fig. 7.
Timeline of molecular and cellular pathologies in transgenic mice ubiquitously expressing SOD1G93A. The “symptomatic” stage denotes the period following initial onset of muscle weakness and wasting (80–100 days). Dates of pathology appearance taken from.44,91,114
VI. ALS Comprises a Spectrum of Pathologies
On a subcellular level, ALS pathology is staggeringly complex and includes abnormalities in nearly all cellular compartments. Many of these are undoubtedly secondary effects or compensatory mechanisms for an initial dysfunctional “trigger,” the identification of which has remained elusive despite nearly 20 years of research on the molecular bases of ALS. We will review some of the more notable and well-studied pathological processes and discuss their relevance to the initial stages of disease, when therapeutic intervention may still be possible.
A. Excitotoxic, Inflammatory, and Oxidative Insults
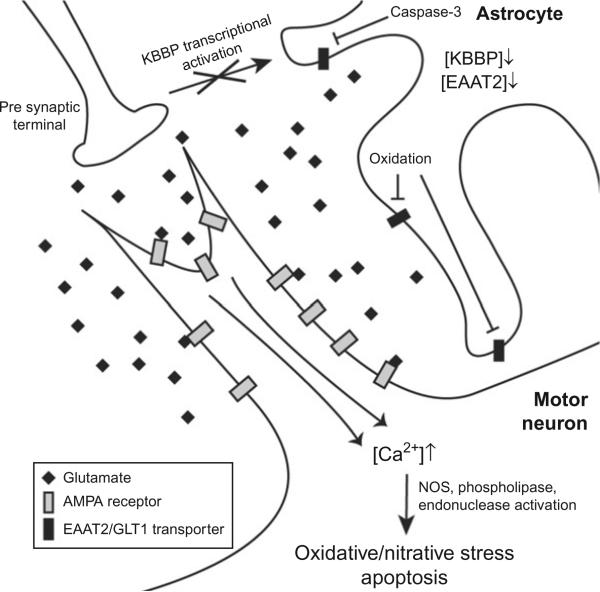
In 1992, Rothstein et al. found defects in glutamate signaling in neuronal tissue from patients who died of ALS but not Alzheimer's and Huntington's diseases,147 revealing a unique molecular basis for ALS. This phenomenon was later attributed to the selective loss of the astrocytic glutamate transporter EAAT2, which is crucial for prompt clearance of glutamate from the synaptic cleft after firing.148 Both FALS and SALS patients, and mutant SOD1 mice, have decreased levels of functional EAAT2 protein (also known as GLT1) and increased circulating glutamate in the cerebrospinal fluid (CSF).149–152 Work in transgenic mice confirms the importance of EAAT2/GLT1-mediated glutamate clearance to motor neuron health. Deletion of this gene is sufficient to induce progressive neurodegeneration,153 and genetically encoded154 or exogenously stimulated EAAT2/GLT1 overexpression155 delays symptom onset in ALS mouse models. The mechanism(s) by which EAAT2/GLT1 is downregulated in ALS are not yet understood, and it is not clear whether decreased mRNA synthesis/stability is a factor. Postmortem spinal cord from ALS patients had normal EAAT2/GLT1 mRNA levels.156 However, a later analysis of SOD1G93A mice using in situ hybridization and qRT-PCR revealed a substantial decrease in EAAT2/GLT1 promoter activity and transcript quantity concomitant with disease onset.157 EAAT2/GLT1 is directly affected by several deleterious processes that occur in the ALS-affected CNS, suggesting that deficiency of its transport function and subsequent glutamate overload may be a secondary event in ALS pathogenesis. Caspase-3 activation (which is itself a relatively late-occurring phenomenon158) results in a truncated, inactive version of EAAT2/GLT1,159 and oxidative damage to the C-terminus of EAAT2/GLT1 diminishes its ability to transport glutamate.160–162 EAAT2/GLT1 expression in astrocytes is also subject to modulation by neuronal signaling, via activation of the transcription factors κB motif binding phosphoprotein (KBBP) by presynaptic axons.157 Synapse loss and denervation in SOD1G93A mice results in decreased astrocytic KBBP and diminished expression of EAAT2/GLT1, revealing that the astrocytic glutamate transporter is downregulated in response to synaptic dysfunction.157 Taken together, these lines of evidence show that deficient glutamate reuptake by astrocytes is induced by preexisting neuronal stress, which is further exacerbated by the resultant excitotoxicity (Fig. 8).
Fig. 8.
Glutamate excitotoxicity causes influx of Ca2+ to motor neurons and activates apoptosis. ALS patients and mouse models have decreased levels of the astrocytic glutamate transporter EAAT2/GLT1, which clears glutamate from the synapse after firing. Dysfunction in the presynaptic motor axon disrupts activation of the EAAT2/GLT1 transcriptional activator κB motif binding phosphoprotein (KBBP). Deficient EAAT2/GLT1, combined with inactivation of the transporter by oxidative damage and caspase-3-mediated proteolysis, results in persistent glutamate stimulation of the Ca2+-permeable AMPA receptor, which is especially abundant in motor neurons. The resultant calcium influx activates several pro-oxidant and pro-apoptotic factors and prolonged Ca2+ excess results in motor neuron death.
Prolonged hyperstimulation by glutamate induces death primarily by allowing persistent calcium influx through the Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, which are specifically enriched in motor neurons.163–166 Excess Ca2+ floods into the mitochondria and overwhelms its natural buffering capacity, triggering reactive oxygen species (ROS) production, disrupting protein homeostasis, and eventually activating the apoptotic machinery.167,168 Cytosolic calcium overload further perpetuates itself by stimulating the opening of ryanodine receptors (RyR) on the endoplasmic reticulum (ER) membrane, allowing Ca2+ release from the luminal space into the cytosol.169 AMPA receptor-mediated calcium influx also increases mutant SOD1 aggregation in cultured motor neurons170 and mouse models of ALS,171 which produces additional ER and mitochondrial dysfunction (see below). Glutamate excitotoxicity thus acts synergistically with protein aggregation and mitochondrial/ER dysfunction to stress motor neurons and activate apoptosis in SOD1-related FALS (Fig. 10). Motor neurons are selectively vulnerable to excitotoxic stress due to their abundance of AMPA receptors and low calcium-buffering ability.172,173 Riluzole, the as-yet sole drug approved for the treatment of ALS, inhibits excitotoxic stress in neurons by slowing glutamate release and blocking AMPA receptors,174–177 but confers a survival benefit of only few months.178
Fig. 10.
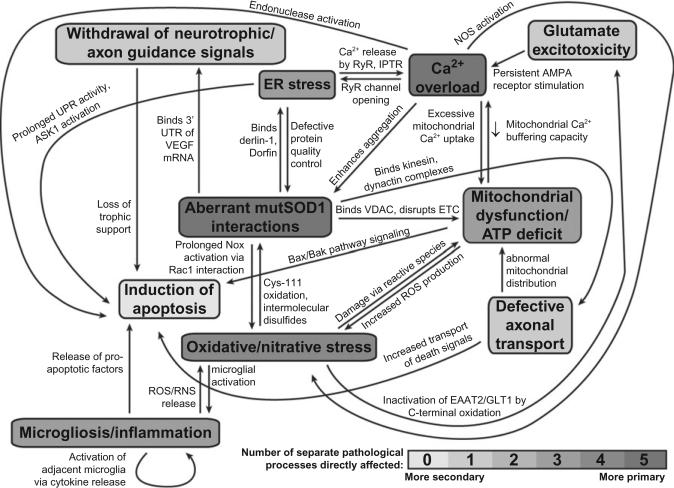
Diverse pathological processes in SOD1-related FALS are highly interrelated and many stem directly from SOD1 misfolding/aggregation and cytosolic calcium overload. Abbreviations: mutSOD1, mutant SOD1; UTR, untranslated region; VDAC, voltage-dependent anion channel; ETC, electron transport chain; UPR, unfolded protein response; ROS/RNS, reactive oxygen/nitrogen species.
In addition to excessive glutamate, secreted oxidative, nitrative, and inflammatory factors also contribute to motor neuron stress and death in ALS, as illustrated by the cytotoxic effect of ALS patient CSF on healthy rat spinal cord cultures.179 While it has become clear that neurons, astrocytes, and microglia are all capable of secreting pro-inflammatory cytokines and other inducers of cellular stress and death, the relative contribution of each cell type to the transmittance of stress signals in ALS is unresolved. Astrocytes expressing mutant SOD1 kill wild-type motor neurons in co-culture by secreting an unidentified soluble factor that activates the pro-apoptotic Bax protein.129 Activated microglia, which are pathologic hallmarks in the CNS of ALS patients and mouse models,180–184 release a host of inflammatory and proapoptotic factors. For example, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are activated, resulting in enhanced production of prostaglandins and nitric oxide (NO), respectively,185 and programmed death signals such as the Fas ligand (FasL) and tumor necrosis factor-alpha (TNF-α) are released.185,186 A great deal of crosstalk exists between the cellular responses to each individual cytokine. For example, COX-2 and iNOS are activated by TNF-α stimulation of astrocytes,187 and the transcription of FasL in motor neurons is activated by NO.186
In cases of SOD1-related FALS, the mutant protein directly contributes to production of extracellular stressors. First, mutant SOD1 disrupts redox regulation of NADPH oxidase (Nox), a membrane-bound producer of extracellular superoxide, through a direct interaction with Rac1. Oxidizing conditions normally promote the dissociation of the SOD1–Rac1 complex and cessation of Nox activation, but mutant SOD1 remains bound to Rac1 and allows persistent superoxide production under these conditions.188 Extracellular superoxide may then enhance neuroinflammation by stimulating microglia which are activated by ROS189 (Fig. 10). Furthermore, mutant SOD1 may itself be a secreted factor that contributes to neurotoxicity. Mutations in SOD1 confer an affinity for the secretory vesicle proteins chromogranins A and B.190 Localization of the mutant protein to secretory vesicles allows its transport from neurons and astrocytes to the extracellular space, resulting in activation of microglia and motor neuron death.190 Neuroinflammatory and excitotoxic insults from microglia and astrocytes are thought to primarily affect disease progression rather than representing a primary trigger of disease.191 However, some biochemical indicators of microglial activation, such their accumulation in the CNS and increased TNF-α production, are present prior to symptom onset in ALS mice.186,192 Although relief of excitotoxic stress with Riluzole offers limited survival benefit, the combination of this or similar drugs with anti-neuroinflammatory agents may result in more satisfactory functional outcomes.
B. Dysregulation of Neurotrophic Factors and Axon Guidance Proteins
Neural networks are not static entities that remain stable indefinitely after development; rather, they require the continuous input of neurotrophic factors and axon guidance cues secreted by glia and innervated muscle. Loss of survival-promoting neurotrophic signaling has therefore been proposed as a contributing factor to motor neuron demise in ALS. In support of this view, CNTF knockout produces progressive motor neuron death in mice193 and exacerbates neurodegeneration in the SOD1G93A model.194 Muscle-specific overexpression of glial cell-derived neurotrophic factor (GDNF) in the G93A mouse preserves NMJs and improves motor neuron survival,195 suggesting therapeutic potential of neurotrophic factor supplementation. However, deficits in neurotrophic factors are not seen in ALS patients; to the contrary, GDNF and CNTF are upregulated in muscle, CSF, and postmortem spinal cord from ALS patients.196–199 The over-abundance of these factors in symptomatic individuals suggests that their upregulation is part of a defensive response to existing pathology and is ultimately insufficient to halt disease progression. In line with this view, administration of CNTF200 or brain-derived neurotrophic factor (BDNF)201 showed no measurable benefit to ALS patients. It is possible that neurotrophic factors hold therapeutic potential if administered early and/or at intact NMJs (recapitulating the beneficial muscle-specific overexpression of GDNF reported by Li et al.195), but such a requirement likely precludes their usefulness to ALS patients.
VEGF and ANG, which are involved in maintenance of both neural networks and vasculature, have also been implicated in ALS.202,203 VEGF, in particular, has received significant attention as a disease-modifying factor since the discovery that diminished VEGF expression in transgenic mice is sufficient to cause late-onset neurodegeneration.204 Furthermore, ALS patients have decreased circulating levels of VEGF in CSF compared to healthy controls.205 Mutant SOD1 directly contributes to VEGF deficiency through binding of the 3’-untranslated region (UTR) of VEGF mRNA, destabilizing transcripts and downregulating expression206,207 (Fig. 10). Loss of VEGF function could also have a genetic basis independent of SOD1 mutations. Single-nucleotide polymorphisms in the VEGF promoter region were found to correlate with an increased risk of ALS,26 although a recent meta-analysis of available genotype data restricts this effect to males.208 Mutations in ANG were also linked to a small subset of ALS cases.21–23 ALS-associated ANG mutations occur at functionally important residues involved in catalysis and nuclear localization22 rather than hampering ANG expression, which is unchanged or even increased in ALS patients and mouse models.209,210 The common functional consequence of ALS-associated ANG mutations appears to be an inability to promote neural connectivity and survival.211
The relative contributions of these proteins’ neuroprotective and angiogenic properties to CNS health are unknown, but there is evidence that deficiencies in both functions could promote neurodegeneration. The neuroprotective effect of both VEGF and ANG in cell culture90,204,211 gives strong evidence for neurotrophic action of these proteins independent of vasculature. However, decreased cerebral blood flow in ALS patients212,213 and disruptions in the blood–spinal cord barrier of several mouse models214 indicate that vascular dysfunctions are indeed present in ALS. In mouse models of ALS, overexpression of VEGF or its receptors, or administration of purified VEGF directly to the CNS, resulted in neuroprotection and prolonged survival,215,216 leading to hope for VEGF administration as a therapeutic strategy. Restoration of ANG activity may also hold therapeutic potential. Further study is needed to explore this possibility and to resolve the molecular details of ANG and VEGF action in the CNS.
One possible mechanism for VEGF-mediated neuroprotection is its antagonism of the axon guidance protein Sema3A. Sema3A is a member of the semaphorin family of proteins, which guide axons to their targets during development and also play a role in the complex phenomena of neural network refinement and plasticity.217 Sema3A is a secreted glycoprotein that acts as an axonal chemorepellent through binding of a neuropilin-1/plexin-A coreceptor complex, which triggers downstream cytoskeletal reorganization and axon withdrawal.218 These receptor components are expressed throughout adulthood in spinal cord motor axons, a sensitivity which allows them to avoid Sema3A-producing scar tissue during post-injury regeneration.219–221 However, postnatal responsiveness to Sema3A may be a liability in individuals with SOD1 mutations. Terminal Schwann cells of SOD1G93A mice release abnormally high levels of Sema3A into the NMJ before symptom onset,219 which would be expected to induce axonal withdrawal from the synapse. Interestingly, VEGF also binds neuropilin-1, leading some to propose that it prevents denervation in ALS by competing with Sema3A for receptor binding.219
In addition to Sema3A, ALS patients show increased expression of other axonal chemorepellents, including ephrinA1 in motor neurons196 and Nogo-A in muscle.222 Muscle-specific overexpression of the secreted factor Nogo-A induced axon retraction from the NMJ in mice and higher Nogo-A expression in a subset of ALS patients correlated with disease severity.223 Although the dysregulation of axonal guidance proteins is clearly correlated with ALS pathology, there is no strong evidence for causation. In fact, Nogo-A upregulation has been reported to occur in response to neuromuscular denervation,224 not vice versa. However, overabundance of axonal chemorepellents at the NMJ following initial retraction would certainly inhibit compensatory reinnervation and may transform a minor insult into irreparable damage to the neuromuscular synapse (Fig. 9).
Fig. 9.
Aberrant expression of neurotrophic and axonal guidance factors at the neuromuscular junction of SOD1G93A mice. Expression of the neuroprotective vascular endothelial growth factor (VEGF) is decreased because of the binding of mutant SOD1 to the 3′-untranslated region of VEGF mRNA, while expression of the axonal chemorepellents Sema3A and Nogo-A is upregulated in terminal Schwann cells and muscle, respectively. Loss of trophic support and increased repulsive cues may induce withdrawal of the motor axon from the neuromuscular synapse.
C. Axonal Structure and Transport Defects
The combination of polarity, high energetic demand, and extreme axon length (up to 1m)225 makes axonal integrity paramount to motor neuron viability. Accumulation of neurofilaments, which maintain axonal diameter and structural integrity in motor neurons, is a long-recognized hallmark of ALS pathology in humans and mouse models and is thought to contribute to the selective vulnerability of long, large-caliber motor axons.2,48,226–228 Neurofilaments consist of light (NF-L), medium (NF-M), and heavy (NF-H) subunits, in equal proportion, and their proper assembly is crucial to the maintenance and extension of vulnerable large-caliber motor axons.229,230 Misassembly of neurofilaments due to over- or under-expression, mutation, or deficient transport of individual subunits results in their accumulation, further hindrance of axonal transport, and eventual motor neuron death.231–236 Hyperphosphorylation of neurofilaments also contributes to defective transport by causing their detachment from motor complexes and promoting aberrant self-association.237–240 In ALS, this phenomenon is attributable to overactivation of p38 MAP kinase and Cdk5, which phosphorylate NF-M and NF-H.241–243 Neurofilament accumulation appears to be selectively deleterious to axons. Overexpression of NF-H causes sequestration of neurofilaments within the cell body and perikarya and markedly delays disease onset in mouse models of ALS.244 In addition to relieving the axonal burden of neurofilament aggregates, thus facilitating transport, accumulated neurofilaments in the perikarya are thought to counter glutamate excitotoxicity by chelating excess calcium and/or binding the cytoplasmic domains of glutamate receptors.245,246
In addition to neurofilaments, axonal transport of many other cellular components is indispensable for motor neuron health and homeostasis. Transport between the cell body and neuromuscular synapse is mediated by the dynein/dynactin (retrograde) and kinesin (anterograde) motor protein complexes, which carry adaptor-bound cargo along axonal microtubules. Mutant SOD1 mice show presymptomatic defects in both anterograde and retrograde transport,144,247,248 with a particular retardation in the trafficking of mitochondria249,250 and cytoskeletal components such as neurofilament and tubulin subunits.248 Mitochondria are normally enriched near the neuromuscular synapse to meet the high energetic and calcium-buffering needs of the firing axon.251 Impaired anterograde transport may thus explain the early onset distal axonopathy observed in ALS mouse models. SALS patients show accumulation of mitochondria in proximal axons,252 which is further evidence for impaired anterograde transport as a fundamentally important mechanism of all ALS pathology. As with several other pathological processes in SOD1-related FALS, misfolding and aggregation directly impair axonal transport though aberrant interactions. Mutant SOD1 acquires the ability to bind motor complexes that are instrumental to both anterograde (kinesin-2253) and retrograde (dynein/dynactin254,255) axonal transport (Fig. 10). Mutant SOD1 also interacts directly with the 3’-UTR of NF-L mRNA,256 resulting in decreased expression that is observed in both FALS and SALS patients.257–259
Because of its prevalence in human patients and early onset in ALS mouse models, dysregulation of neurofilament transport and metabolism became an early candidate for a common mechanism of ALS pathogenesis. While this hypothesis is attractive due to its apparent specificity for motor axons, evidence for a primary role of axonal neurofilaments in ALS is contradictory. While the aforementioned work by Couillard-Despres et al. indicates that reducing axonal neurofilaments dramatically delays ALS pathology,244 a second study showed no benefit from sequestration of neurofilaments in the cell body and perikarya.260 It may be that neurofilament dysfunction modifies neurodegenerative severity in ALS but is not sufficient to cause disease, a possibility that does not preclude defective axonal transport as a primary mechanism of pathogenesis. The early retardation of both anterograde and retrograde trafficking in motor axons undoubtedly initiates a spectrum of deleterious effects such as energetic deficiencies at the distal synapse and impaired neuromuscular communication. Further study is crucial to reveal the underlying mechanisms and consequences of axonal transport malfunction in ALS and to identify targets for therapeutic intervention.
D. Mitochondrial Dysfunction
Mitochondrial abnormalities such as swelling and vacuolization are pathological hallmarks in spinal cords of ALS patients and most transgenic mouse models,49,107,121,261,262 leading to much interest in the mitochondrion's involvement in disease. Perturbed energy homeostasis and ATP deficits are observed in both SOD1G93A mice and skeletal muscle biopsies from ALS patients.263–268 One mechanism by which the FALS mutant G93A impairs cellular respiration is through a novel ability to bind cytosolic malate dehydrogenase, which disrupts the malate–aspartate shuttle.269 Misfolded and aggregated SOD1 mutants also accumulate on the cytoplasmic face of the outer mitochondrial membrane and bind directly to the voltage-dependent anion channel (VDAC), depolarizing the membrane and disrupting the normal functioning of the electron transport chain (ETC)266,270–272 (Fig. 10). ETC dysfunction is a notable convergence in the pathologies of sporadic and familial ALS, and ETC inhibition in SALS patients has been linked to mutations in mitochondrial DNA.267,273,274 Mitochondrial genome instability has been proposed (controversially) to play a central role in the natural aging process,275–279 which would offer a possible basis for the late onset of disease in SALS. Interestingly, SOD1G93A mice and a subset of sporadic ALS patients are hypermetabolic265,280 and administration of a high-fat diet modestly improved survival in mice.265 The cause(s) and significance of hypermetabolism are unknown, as are the mechanisms by which aberrant metabolic states mediate toxicity in ALS. The surprising finding that metabolic dysfunction in skeletal muscle can provoke motor neuron death145 suggests that mitochondrial defects may be central to the retrograde neurodegeneration seen in mouse models of ALS.
Mitochondria are also key players in the buffering of intracellular calcium, which in prolonged excess results in the activation of pro-oxidant and apoptotic factors such as nitric oxide synthase (NOS), phospholipases, and endonucleases.281,282 ALS mice show a CNS-specific decrease in mitochondrial calcium loading capacity that precedes motor deficits.283 Likewise, both ALS patients and mouse models have increased intracellular calcium concomitant with mitochondrial damage.283–285 It is not clear whether decreased mitochondrial buffering capacity precedes cytosolic Ca2+ overload or vice versa, since these processes reciprocally enhance each other167,286 (Fig. 10). Depletion of mitochondrial calcium-buffering ability is particularly deleterious to neurons and skeletal muscle, whose normal functioning involves frequent influxes of calcium to generate action potentials. This, combined with the enrichment of mutant SOD1 in mitochondria of motor neurons,266,271,272,287,288 muscle,137 and astrocytes,289 may account for the sensitivity of these cells to mutant SOD1-mediated toxicity. Disturbance of mitochondrial function may also directly cause cell death by activating the apoptotic cascade. Aberrant localization to the intermembrane space and matrix288,290,291 disrupts the structural integrity of the organelle, resulting in release of the apoptotic trigger cytochrome c.292 Misfolded SOD1 monomers and oligomers also provoke apoptosis by associating with the pro-survival factor Bcl-2. The normally anti-apoptotic Bcl-2 exposes a toxic BH3 domain upon mutant SOD1 binding, resulting in cell death and interference of synaptic transmission at the NMJ.293–295 Given the presymptomatic, cell type-specific recruitment of mutant SOD1 to mitochondria,271,287 dysfunctional changes in this organelle merit consideration as primary contributors to ALS pathogenesis.
E. Deficient Protein Quality Control
The presence of proteinaceous aggregates in spinal cords of FALS and SALS patients suggests that malfunction or overloading of protein quality control machinery is a common feature of neurodegeneration. The ubiquitin–proteasome system (UPS), in which ubiquitin-tagged proteins are targeted for proteasomal degradation, is one such mechanism of misfolded protein clearance. Degradation of misfolded mutant SOD1 proceeds via the UPS and impedes its functioning by sequestering proteasomal subunits and ubiquitin ligases such as Dorfin,296–300 while proteasomal inhibition produces a reciprocal enhancement of SOD1 aggregation93,301,302 (Fig. 10). Ubiquitin- and ubiquitin ligase-positive intraneuronal inclusion bodies are found in FALS mouse models47,303 and postmortem spinal cord of SALS patients,124,300,304–306 indicating UPS activity and sequestration in both forms of the disease. Studies of SOD1 mutant transgenic mice reveal proteasomal impairment in the disease-vulnerable spinal cord and brainstem only after the onset of symptoms307,308; so UPS dysfunction is unlikely to be an initiator of pathology in SOD1-related FALS. Interestingly, as disease progresses, constitutively active proteasomal components are replaced by inflammatory cytokine-responsive subunits to yield the inducible “immunoproteasome”, which degrades proteins into antigenic peptide fragments to be presented by the class 1 major histocompatibility complex.307,309,310 Inhibition of immunoproteasome formation using a small-molecule anti-inflammatory agent shortens survival in a rat model of ALS,311 but a more targeted genetic approach involving knockdown of the LMP2 immunoproteasomal subunit yielded no effect on survival in SOD1G93A mice.312 The role of the immunoproteasome in ALS pathology is yet to be precisely determined, but its upregulation may be a response to glia-mediated inflammation in the CNS.313,314
Protein quality control by ER-associated degradation (ERAD) is also impaired in ALS, leading to stress signaling that can directly induce motor neuron death via activation of apoptosis. During synthesis and maturation of nascent proteins in the ER, misfolded species are cleared from the luminal space by the ERAD pathway (reviewed in Ref. 315). Dysfunction or overloading of ERAD results in accumulation of unfolded proteins and triggers the unfolded protein response (UPR).316,317 Mutant SOD1 interferes directly with ERAD by binding to derlin-1, a transmembrane protein responsible for the translocation of misfolded proteins from the ER lumen,318 as well as the ER-luminal chaperone BiP319 (Fig. 10). Sustained ER stress in SOD1 mutant mice leads to the activation of ASK1, an apoptotic protein kinase, and survival can be prolonged by ASK1 ablation.318 Derlin-1 interaction was detected only after symptom onset,318 but multiple triggers of ER stress are clearly present in ALS, as evidenced by presymptomatic UPR activation in SOD1 mutant mouse models320 and upregulation of UPR components in SALS patients.306,321,322 Furthermore, mutations in the UPR protein VAPB have been linked to some ALS cases.20 Thus, ER stress may not be ruled out as a primary contributor to ALS pathogenesis nor may its involvement be limited to SOD1-related cases.
F. Aberrant RNA Processing
Malfunction and aggregation of two nucleic acid binding proteins was recently shown to be a common causal factor for some cases of both familial and sporadic ALS. Since the surprising observation that the 43-kDa trans-activating response region DNA-binding protein (TDP-43) is present in a majority of ubiquitinated proteinaceous inclusions in ALS and frontotemporal lobar degeneration (FTLD),323,324 over 35 dominant mutations in TDP-43 have been linked to ALS.309,325–343 TDP-43 is notably excluded from inclusions of patients with SOD1-related FALS,344 perhaps an indication of divergent pathological mechanisms. However, it was recently shown that the small heat shock protein B8 (HspB8) is involved in clearance of both SOD1 and TDP-43 aggregates,345 which is evidence that, despite differences in etiology, TDP-43 and SOD1-related ALS may respond to similar therapeutic approaches. Mutations in a second RNA/DNA-binding protein, fused in sarcoma (FUS) (also known as translocation in liposarcoma (TLS)), have also been linked to ALS and FTLD.346–360 This common genetic basis for ALS and FTLD blurs the distinction between these disorders and may account for their co-occurance in some patients.8
The study of TDP-43- and FUS/TLS-related proteinopathies is a burgeoning field, as even the normal functions of these proteins were not well understood prior to the revelation of their roles in neurodegenerative diseases. Both proteins are widely expressed, predominantly nuclear proteins in healthy cells361 and are involved in RNA processing events such as splicing and transcriptional regulation (reviewed in Ref. 362). Cytoplasmic aggregation and nuclear depletion are early, and perhaps independent, events in TDP-43-related ALS pathology363–366 and are accompanied by proteolytic cleavage,324,367,368 hyperphosphorylation,367,369,370 and ubiquitination.323,324 The combination of aberrant localization and posttranslational modification of TDP-43 in ALS raises the question of whether TDP-43 pathogenicity is a loss or gain of function. Does toxicity stem from the loss of normal TDP-43 nuclear function, or does cleaved, phosphorylated, or aggregated TDP-43 acquire cytotoxic properties? The TDP-43 C-terminal fragment is produced by caspase-3 cleavage371–374 and increases in abundance as symptoms progress, suggesting that proteolysis of TDP-43 may be secondary to activation of apoptosis. Cytoplasmic inclusions stain negative for several known binding partners of TDP-43,375 suggesting that aggregates do not exert toxicity by sequestering these components. However, the possibility that altered TDP-43 disrupts cellular homeostasis through novel aberrant interactions, as is the case with misfolded SOD1, has not been ruled out. FUS/TLS, while also aggregating in the cytoplasm,354,357,360 appears to retain a more normal pattern of localization in the ALS-affected CNS, and neither phosphorylation nor cleavage is significantly correlated with disease.354,357,360,370
Intense study is under way to clarify the cellular functions of TDP-43 and FUS/TLS and the role of mutations in ALS and other neurodegenerative disorders. Interestingly, defective RNA processing has been noted previously in ALS and shown to cause EAAT2 deficiency,376 a phenomenon that TDP-43 or FUS/TLS dysfunction may explain. Elucidation of the roles of TDP-43 and FUS/TLS in RNA metabolism has the potential to fill gaps in our understanding of numerous pathological deregulatory events in ALS.
VII. Concluding Remarks
The molecular biology of ALS is extraordinarily complex, and identification of the crucial initiating factors has remained elusive. However, a critical need exists for effective therapies to prevent loss of motor function and extend life. This effort should be focused on developing strategies for intervention at primary sites of dysfunction. In the case of SOD1-related FALS, protein misfolding and aggregation and calcium dysregulation drive many of the diverse pathological events in disease progression (Fig. 10) and should thus be considered prime candidates for therapeutic targeting. Mutant SOD1 transgenic mice will continue to be invaluable for mechanistic study of disease and development/evaluation of drug candidates. However, these models should be evaluated critically for relevance based on criteria such as copy numbers of wild-type and mutant SOD1 and presence of posttranslational modifications that affect stability.
References
- 1.Goetz CG. Amyotrophic lateral sclerosis: early contributions of Jean-Martin Charcot. Muscle Nerve. 2000;23:336. doi: 10.1002/(sici)1097-4598(200003)23:3<336::aid-mus4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS 1. Nat Rev Neurosci. 2001;2:806. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 4.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl. 1):S3. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 5.Chou SM, Norris FH. Amyotrophic lateral sclerosis: lower motor neuron disease spreading to upper motor neurons. Muscle Nerve. 1993;16:864. doi: 10.1002/mus.880160810. [DOI] [PubMed] [Google Scholar]
- 6.Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki Y, Mizutani T, Takasu T. Amyotrophic lateral sclerosis with marked neurological asymmetry: clinicopathological study. Acta Neuropathol. 1995;90:44. doi: 10.1007/BF00294458. [DOI] [PubMed] [Google Scholar]
- 8.Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- 9.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl.):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- 12.Beleza-Meireles A, Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph Lateral Scler. 2009;10:1. doi: 10.1080/17482960802585469. [DOI] [PubMed] [Google Scholar]
- 13.Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 14.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis 1. Nature. 1993;362:59. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 15.Al-Chalabi A, Andersen PM, Chioza B, Shaw C, Sham PC, Robberecht W, et al. Recessive amyotrophic lateral sclerosis families with the D90A SOD1 mutation share a common founder: evidence for a linked protective factor. Hum Mol Genet. 1998;7:2045. doi: 10.1093/hmg/7.13.2045. [DOI] [PubMed] [Google Scholar]
- 16.Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29:166. doi: 10.1038/ng1001-166. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 18.Chance PF, Rabin BA, Ryan SG, Ding Y, Scavina M, Crain B, et al. Linkage of the gene for an autosomal dominant form of juvenile amyotrophic lateral sclerosis to chromosome 9q34. Am J Hum Genet. 1998;62:633. doi: 10.1086/301769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet. 2004;74:1128. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenway MJ, Alexander MD, Ennis S, Traynor BJ, Corr B, Frost E, et al. A novel candidate region for ALS on chromosome 14q11.2. Neurology. 2004;63:1936. doi: 10.1212/01.wnl.0000144344.39103.f6. [DOI] [PubMed] [Google Scholar]
- 22.Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38:411. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Yu W, Kishikawa H, Folkerth RD, Iafrate AJ, Shen Y, et al. Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis. Ann Neurol. 2007;62:609. doi: 10.1002/ana.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Chalabi A, Andersen PM, Nilsson P, Chioza B, Andersson JL, Russ C, et al. Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet. 1999;8:157. doi: 10.1093/hmg/8.2.157. [DOI] [PubMed] [Google Scholar]
- 25.Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:1757. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 26.Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 27.Al-Chalabi A, Scheffler MD, Smith BN, Parton MJ, Cudkowicz ME, Andersen PM, et al. Ciliary neurotrophic factor genotype does not influence clinical phenotype in amyotrophic lateral sclerosis. Ann Neurol. 2003;54:130. doi: 10.1002/ana.10638. [DOI] [PubMed] [Google Scholar]
- 28.Giess R, Goetz R, Schrank B, Ochs G, Sendtner M, Toyka K. Potential implications of a ciliary neurotrophic factor gene mutation in a German population of patients with motor neuron disease. Muscle Nerve. 1998;21:236. doi: 10.1002/(sici)1097-4598(199802)21:2<236::aid-mus12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Kokubo Y, Kuzuhara S, Narita Y. Geographical distribution of amyotrophic lateral sclerosis with neurofibrillary tangles in the Kii Peninsula of Japan. J Neurol. 2000;247:850–2. doi: 10.1007/s004150070071. [DOI] [PubMed] [Google Scholar]
- 30.Haley RW. Excess incidence of ALS in young Gulf War veterans. Neurology. 2003;61:750. doi: 10.1212/wnl.61.6.750. [DOI] [PubMed] [Google Scholar]
- 31.Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, Harati Y, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61:742. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- 32.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128:472. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- 33.Sutedja NA, Fischer K, Veldink JH, Van Der Heijden GJ, Kromhout H, Heederik D, Huisman MH, Wokke JJ, Van den Berg LH. What we truly know about occupation as a risk factor for ALS: a critical and systematic review. Amyotroph Lateral Scler. 2008;10:295–301. doi: 10.3109/17482960802430799. [DOI] [PubMed] [Google Scholar]
- 34.Sutedja NA, Veldink JH, Fischer K, Kromhout H, Heederik D, Huisman MH, Wokke JH, Van den Berg LH. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2008;10:302–9. doi: 10.3109/17482960802455416. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP. Infectious agents and age-related neurodegenerative disorders. Ageing Res Rev. 2004;3:105. doi: 10.1016/j.arr.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jafari H, Couratier P, Camu W. Motor neuron disease after electric injury. J Neurol Neurosurg Psychiatry. 2001;71:265. doi: 10.1136/jnnp.71.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurtzke JF. Risk factors in amyotrophic lateral sclerosis. Adv Neurol. 1991;56:245. [PubMed] [Google Scholar]
- 38.Duncan MW, Steele JC, Kopin IJ, Markey SP. 2-Amino-3-(methylamino)-propanoic acid (BMAA) in cycad flour: an unlikely cause of amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Neurology. 1990;40:767. doi: 10.1212/wnl.40.5.767. [DOI] [PubMed] [Google Scholar]
- 39.Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurode-generative disease among the Chamorro people of Guam. Proc Natl Acad Sci USA. 2003;100:13380. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, et al. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc Natl Acad Sci USA. 2005;102:5074. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonasson S, Eriksson J, Berntzon L, Spacil Z, Ilag LL, Ronnevi LO, et al. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc Natl Acad Sci USA. 2010;107:9252. doi: 10.1073/pnas.0914417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox PA, Richer R, Metcalf JS, Banack SA, Codd GA, Bradley WG. Cyanobacteria and BMAA exposure from desert dust: a possible link to sporadic ALS among Gulf War veterans. Amyotroph Lateral Scler. 2009;10(Suppl 2):109. doi: 10.3109/17482960903286066. [DOI] [PubMed] [Google Scholar]
- 43.Caller TA, Doolin JW, Haney JF, Murby AJ, West KG, Farrar HE, et al. A cluster of amyotrophic lateral sclerosis in New Hampshire: a possible role for toxic cyanobacteria blooms. Amyotroph Lateral Scler. 2009;10(Suppl. 2):101. doi: 10.3109/17482960903278485. [DOI] [PubMed] [Google Scholar]
- 44.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Andersen PM, Nilsson P, Keranen ML, Forsgren L, Hagglund J, Karlsborg M, et al. Phenotypic heterogeneity in motor neuron disease patients with CuZn-superoxide dismutase mutations in Scandinavia. Brain. 1997;120(Pt. 10):1723. doi: 10.1093/brain/120.10.1723. [DOI] [PubMed] [Google Scholar]
- 46.Hand CK, Khoris J, Salachas F, Gros-Louis F, Lopes AA, Mayeux-Portas V, et al. A novel locus for familial amyotrophic lateral sclerosis, on chromosome 18q. Am J Hum Genet. 2002;70:251. doi: 10.1086/337945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 48.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 49.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 50.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury 1. Nat Genet. 1996;13:43. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 51.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1 1. Science. 1998;281:1851. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 52.Khare SD, Caplow M, Dokholyan NV. FALS mutations in Cu, Zn superoxide dismutase destabilize the dimer and increase dimer dissociation propensity: a large-scale thermodynamic analysis 1. Amyloid. 2006;13:226. doi: 10.1080/13506120600960486. [DOI] [PubMed] [Google Scholar]
- 53.Furukawa Y, O'Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo- and reduced form of SOD1, leading to unfolding and oxidative aggregation. J Biol Chem. 2005;280:17266. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 54.Hough MA, Grossmann JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA, et al. Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants 1. Proc Natl Acad Sci USA. 2004;101:5976. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez JA, Shaw BF, Durazo A, Sohn SH, Doucette PA, Nersissian AM, et al. Destabilization of apoprotein is insufficient to explain Cu, Zn-superoxide dismutase-linked ALS pathogenesis. Proc Natl Acad Sci USA. 2005;102:10516. doi: 10.1073/pnas.0502515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw BF, Valentine JS. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? 1. Trends Biochem Sci. 2007;32:78. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Radunovic A, Leigh PN. Cu/Zn superoxide dismutase gene mutations in amyotrophic lateral sclerosis: correlation between genotype and clinical features. J Neurol Neurosurg Psychiatry. 1996;61:565. doi: 10.1136/jnnp.61.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41:210. doi: 10.1002/ana.410410212. [DOI] [PubMed] [Google Scholar]
- 59.Bystrom R, Andersen PM, Grobner G, Oliveberg M. SOD1 mutations targeting surface hydrogen bonds promote amyotrophic lateral sclerosis without reducing apo-state stability. J Biol Chem. 2010;285:19544. doi: 10.1074/jbc.M109.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q, Johnson JL, Agar NY, Agar JN. Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival 1. PLoS Biol. 2008;6:e170. doi: 10.1371/journal.pbio.0060170. doi:10.1371/journal.pbio.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–8. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 62.Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104:12524. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibata N, Asayama K, Hirano A, Kobayashi M. Immunohistochemical study on superoxide dismutases in spinal cords from autopsied patients with amyotrophic lateral sclerosis 2. Dev Neurosci. 1996;18:492. doi: 10.1159/000111445. [DOI] [PubMed] [Google Scholar]
- 64.Shibata N, Hirano A, Kobayashi M, Sasaki S, Kato T, Matsumoto S, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis 1. Neurosci Lett. 1994;179:149. doi: 10.1016/0304-3940(94)90956-3. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto S, Kusaka H, Ito H, Shibata N, Asayama T, Imai T. Sporadic amyotrophic lateral sclerosis with dementia and Cu/Zn superoxide dismutase-positive Lewy body-like inclusions. Clin Neuropathol. 1996;15:41. [PubMed] [Google Scholar]
- 66.Bartnikas TB, Gitlin JD. Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J Biol Chem. 2003;278:33602. doi: 10.1074/jbc.M305435200. [DOI] [PubMed] [Google Scholar]
- 67.Forman HJ, Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973;248:2645. [PubMed] [Google Scholar]
- 68.Ding F, Dokholyan NV. Dynamical roles of metal ions and the disulfide bond in Cu, Zn superoxide dismutase folding and aggregation. Proc Natl Acad Sci USA. 2008;105:19696. doi: 10.1073/pnas.0803266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiwari A, Hayward LJ. Mutant SOD1 instability: implications for toxicity in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:115. doi: 10.1159/000089616. [DOI] [PubMed] [Google Scholar]
- 70.Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene 1. Curr Neurol Neurosci Rep. 2006;6:37. doi: 10.1007/s11910-996-0008-9. [DOI] [PubMed] [Google Scholar]
- 71.Esteban J, Rosen DR, Bowling AC, Sapp P, Kenna-Yasek D, O'Regan JP, et al. Identification of two novel mutations and a new polymorphism in the gene for Cu/Zn superoxide dismutase in patients with amyotrophic lateral sclerosis 1. Hum Mol Genet. 1994;3:997. doi: 10.1093/hmg/3.6.997. [DOI] [PubMed] [Google Scholar]
- 72.Nogales-Gadea G, Garcia-Arumi E, Andreu AL, Cervera C, Gamez J. A novel exon 5 mutation (N139H) in the SOD1 gene in a Spanish family associated with incomplete penetrance. J Neurol Sci. 2004;219:1–6. doi: 10.1016/j.jns.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease 1. Hum Mol Genet. 2009;18:3217. doi: 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banci L, Bertini I, Cantini F, D'Onofrio M, Viezzoli MS. Structure and dynamics of copper-free SOD: the protein before binding copper. Protein Sci. 2002;11:2479. doi: 10.1110/ps.0210802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, et al. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability 1. J Biol Chem. 2004;279:54558. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 76.Khare SD, Caplow M, Dokholyan NV. The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis 1. Proc Natl Acad Sci USA. 2004;101:15094. doi: 10.1073/pnas.0406650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. The unusually stable quaternary structure of human Cu, Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 78.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu, Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis 1. J Biol Chem. 2004;279:15499. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 79.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci USA. 2004;101:15893. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray SS, Nowak RJ, Strokovich K, Brown RH, Jr., Walz T, Lansbury PT., Jr. An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amytrophic lateral sclerosis 1. Biochemistry. 2004;43:4899. doi: 10.1021/bi030246r. [DOI] [PubMed] [Google Scholar]
- 81.Molnar KS, Karabacak NM, Johnson JL, Wang Q, Tiwari A, Hayward LJ, et al. A common property of amyotrophic lateral sclerosis-associated variants: destabilization of the copper/zinc superoxide dismutase electrostatic loop. J Biol Chem. 2009;284:30965. doi: 10.1074/jbc.M109.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durazo A, Shaw BF, Chattopadhyay M, Faull KF, Nersissian AM, Valentine JS, et al. Metal-free superoxide dismutase-1 and three different amyotrophic lateral sclerosis variants share a similar partially unfolded β-barrel at physiological temperature. J Biol Chem. 2009;284:34382. doi: 10.1074/jbc.M109.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teilum K, Smith MH, Schulz E, Christensen LC, Solomentsev G, Oliveberg M, et al. Transient structural distortion of metal-free Cu/Zn superoxide dismutase triggers aberrant oligomerization 1. Proc Natl Acad Sci USA. 2009;106:18273. doi: 10.1073/pnas.0907387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tiwari A, Liba A, Sohn SH, Seetharaman SV, Bilsel O, Matthews CR, et al. Metal deficiency increases aberrant hydrophobicity of mutant superoxide dismutases that cause amyotrophic lateral sclerosis. J Biol Chem. 2009;284:27746. doi: 10.1074/jbc.M109.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munch C, Bertolotti A. Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants. J Mol Biol. 2010;399:512. doi: 10.1016/j.jmb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furukawa Y, Kaneko K, Yamanaka K, O'Halloran TV, Nukina N. Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in the familial form of amyotrophic lateral sclerosis. J Biol Chem. 2008;283:24167. doi: 10.1074/jbc.M802083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khare SD, Ding F, Dokholyan NV. Folding of Cu, Zn superoxide dismutase and familial amyotrophic lateral sclerosis. J Mol Biol. 2003;334:515. doi: 10.1016/j.jmb.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 88.Khare SD, Dokholyan NV. Common dynamical signatures of familial amyotrophic lateral sclerosis-associated structurally diverse Cu, Zn superoxide dismutase mutants. Proc Natl Acad Sci USA. 2006;103:3147. doi: 10.1073/pnas.0511266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rakhit R, Robertson J, Vande VC, Horne P, Ruth DM, Griffin J, et al. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat Med. 2007;13:754. doi: 10.1038/nm1559. [DOI] [PubMed] [Google Scholar]
- 90.Kerman A, Liu HN, Croul S, Bilbao J, Rogaeva E, Zinman L, et al. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- 91.Zetterstrom P, Stewart HG, Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, et al. Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci USA. 2007;104:14157. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA. 2006;103:7142. doi: 10.1073/pnas.0602046103. doi:10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis 1. Proc Natl Acad Sci USA. 2000;97:12571. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY, et al. Intense superoxide - dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement 1. J Neuropathol Exp Neurol. 1996;55:481. doi: 10.1097/00005072-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Shaw BF, Lelie HL, Durazo A, Nersissian AM, Xu G, Chan PK, et al. Detergent-insoluble aggregates associated with amyotrophic lateral sclerosis in transgenic mice contain primarily full-length, unmodified superoxide dismutase-1. J Biol Chem. 2008;283:8340. doi: 10.1074/jbc.M707751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banci L, Bertini I, Durazo A, Girotto S, Gralla EB, Martinelli M, et al. Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: a possible general mechanism for familial ALS 1. Proc Natl Acad Sci USA. 2007;104:11263. doi: 10.1073/pnas.0704307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chattopadhyay M, Durazo A, Sohn SH, Strong CD, Gralla EB, Whitelegge JP, et al. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci USA. 2008;105:18663. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DiDonato M, Craig L, Huff ME, Thayer MM, Cardoso RM, Kassmann CJ, et al. ALS mutants of human superoxide dismutase form fibrous aggregates via framework destabilization 1. J Mol Biol. 2003;332:601. doi: 10.1016/s0022-2836(03)00889-1. [DOI] [PubMed] [Google Scholar]
- 99.Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Lindberg M, Oliveberg M, et al. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto G, Kim S, Morimoto RI. Huntingtin and mutant SOD1 form aggregate structures with distinct molecular properties in human cells. J Biol Chem. 2006;281:4477. doi: 10.1074/jbc.M509201200. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto G, Stojanovic A, Holmberg CI, Kim S, Morimoto RI. Structural properties and neuronal toxicity of amyotrophic lateral sclerosis-associated Cu/Zn superoxide dismutase 1 aggregates 1. J Cell Biol. 2005;171:75. doi: 10.1083/jcb.200504050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niwa J, Yamada S, Ishigaki S, Sone J, Takahashi M, Katsuno M, et al. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem. 2007;282:28087. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 103.Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR. Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci USA. 2009;106:7774. doi: 10.1073/pnas.0902505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:13528. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cozzolino M, Amori I, Pesaresi MG, Ferri A, Nencini M, Carri MT. Cysteine 111 affects aggregation and cytotoxicity of mutant Cu, Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:866. doi: 10.1074/jbc.M705657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105a.Redler RL, Wilcox KC, Proctor EA, Fee L, Caplow M, Dokholyan NV. Glutathionylation at Cys-111 Induces Dissociation of Wild Type and FALS Mutant SOD1 Dimers. Biochem. 2011;50:7057–66. doi: 10.1021/bi200614y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fukada K, Nagano S, Satoh M, Tohyama C, Nakanishi T, Shimizu A, et al. Stabilization of mutant Cu/Zn superoxide dismutase (SOD1) protein by coexpressed wild SOD1 protein accelerates the disease progression in familial amyotrophic lateral sclerosis mice. Eur J Neurosci. 2001;14:2032. doi: 10.1046/j.0953-816x.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- 107.Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7:623. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 108.Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Wild-type SOD1 overexpression accelerates disease onset of a G85R SOD1 mouse. Hum Mol Genet. 2009;18:1642. doi: 10.1093/hmg/ddp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sasaki S, Warita H, Murakami T, Shibata N, Komori T, Abe K, et al. Ultrastructural study of aggregates in the spinal cord of transgenic mice with a G93A mutant SOD1 gene 1. Acta Neuropathol. 2005;109:247. doi: 10.1007/s00401-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 110.Turner BJ, Lopes EC, Cheema SS. Neuromuscular accumulation of mutant superoxide dismutase 1 aggregates in a transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2003;350:132. doi: 10.1016/s0304-3940(03)00893-0. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, Xu G, Borchelt DR. High molecular weight complexes of mutant superoxide dismutase 1: age-dependent and tissue-specific accumulation. Neurobiol Dis. 2002;9:139. doi: 10.1006/nbdi.2001.0471. [DOI] [PubMed] [Google Scholar]
- 112.Wang J, Xu G, Gonzales V, Coonfield M, Fromholt D, Copeland NG, et al. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol Dis. 2002;10:128. doi: 10.1006/nbdi.2002.0498. [DOI] [PubMed] [Google Scholar]
- 113.Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, et al. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation. Hum Mol Genet. 2005;14:2335. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- 114.Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 115.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death 1. Nature. 2004;431:805. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 116.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 117.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer's disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 118.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 119.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 121.Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 122.Vukosavic S, Stefanis L, Jackson-Lewis V, Guegan C, Romero N, Chen C, et al. Delaying caspase activation by Bcl-2: a clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2000;20:9119. doi: 10.1523/JNEUROSCI.20-24-09119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guegan C, Przedborski S. Programmed cell death in amyotrophic lateral sclerosis. J Clin Invest. 2003;111:153. doi: 10.1172/JCI17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, et al. Lack of apoptosis in mice with ALS. Nat Med. 1999;5:966. doi: 10.1038/12381. [DOI] [PubMed] [Google Scholar]
- 125.Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 126.Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20:660. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:16021. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Turner BJ, Ackerley S, Davies KE, Talbot K. Dismutase-competent SOD1 mutant accumulation in myelinating Schwann cells is not detrimental to normal or transgenic ALS model mice. Hum Mol Genet. 2010;19:815. doi: 10.1093/hmg/ddp550. [DOI] [PubMed] [Google Scholar]
- 133.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 134.Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci USA. 2008;105:7594. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lobsiger CS, Boillee S, McAlonis-Downes M, Khan AM, Feltri ML, Yamanaka K, et al. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci USA. 2009;106:4465. doi: 10.1073/pnas.0813339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19:2284. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:19546. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Towne C, Raoul C, Schneider BL, Aebischer P. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther. 2008;16:1018. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- 140.Dobrowolny G, Aucello M, Molinaro M, Musaro A. Local expression of mIgf-1 modulates ubiquitin, caspase and CDK5 expression in skeletal muscle of an ALS mouse model. Neurol Res. 2008;30:131. doi: 10.1179/174313208X281235. [DOI] [PubMed] [Google Scholar]
- 141.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 142.Rouaux C, Panteleeva I, Rene F, Gonzalez de Aguilar JL, Echaniz-Laguna A, Dupuis L, et al. Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci. 2007;27:5535. doi: 10.1523/JNEUROSCI.1139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dewil M, dela Cruz VF, Van Den Bosch L, Robberecht W. Inhibition of p38 mitogen activated protein kinase activation and mutant SOD1(G93A)-induced motor neuron death. Neurobiol Dis. 2007;26:332. doi: 10.1016/j.nbd.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 144.Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, et al. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 148.Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 149.Fray AE, Ince PG, Banner SJ, Milton ID, Usher PA, Cookson MR, et al. The expression of the glial glutamate transporter protein EAAT2 in motor neuron disease: an immunohistochemical study. Eur J Neurosci. 1998;10:2481. doi: 10.1046/j.1460-9568.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- 150.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA. 2002;99:1604. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rothstein JD, Van KM, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 152.Sasaki S, Komori T, Iwata M. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol. 2000;100:138. doi: 10.1007/s004019900159. [DOI] [PubMed] [Google Scholar]
- 153.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 154.Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, et al. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 155.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 156.Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol. 1996;39:676. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- 157.Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pasinelli P, Houseweart MK, Brown RH, Jr., Cleveland DW. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:13901. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr., Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- 160.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 161.Trotti D, Nussberger S, Volterra A, Hediger MA. Differential modulation of the uptake currents by redox interconversion of cysteine residues in the human neuronal glutamate transporter EAAC1. Eur J Neurosci. 1997;9:2207. doi: 10.1111/j.1460-9568.1997.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 162.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bar-Peled O, O'Brien RJ, Morrison JH, Rothstein JD. Cultured motor neurons possess calcium-permeable AMPA/kainate receptors. Neuroreport. 1999;10:855. doi: 10.1097/00001756-199903170-00034. [DOI] [PubMed] [Google Scholar]
- 164.Van Den Bosch L, Vandenberghe W, Klaassen H, Van HE, Robberecht W. Ca(2+)-permeable AMPA receptors and selective vulnerability of motor neurons. J Neurol Sci. 2000;180:29. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 165.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lu YM, Yin HZ, Chiang J, Weiss JH. Ca(2+)-permeable AMPA/kainate and NMDA channels: high rate of Ca2+ influx underlies potent induction of injury. J Neurosci. 1996;16:5457. doi: 10.1523/JNEUROSCI.16-17-05457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J Neurosci. 2000;20:240. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 169.Jahn K, Grosskreutz J, Haastert K, Ziegler E, Schlesinger F, Grothe C, et al. Temporospatial coupling of networked synaptic activation of AMPA-type glutamate receptor channels and calcium transients in cultured motoneurons. Neuroscience. 2006;142:1019–29. doi: 10.1016/j.neuroscience.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 170.Kim HJ, Im W, Kim S, Kim SH, Sung JJ, Kim M, et al. Calcium-influx increases SOD1 aggregates via nitric oxide in cultured motor neurons 2. Exp Mol Med. 2007;39:574. doi: 10.1038/emm.2007.63. [DOI] [PubMed] [Google Scholar]
- 171.Tateno M, Sadakata H, Tanaka M, Itohara S, Shin RM, Miura M, et al. Calcium-permeable AMPA receptors promote misfolding of mutant SOD1 protein and development of amyotrophic lateral sclerosis in a transgenic mouse model. Hum Mol Genet. 2004;13:2183. doi: 10.1093/hmg/ddh246. [DOI] [PubMed] [Google Scholar]
- 172.Ince P, Stout N, Shaw P, Slade J, Hunziker W, Heizmann CW, et al. Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropathol Appl Neurobiol. 1993;19:291. doi: 10.1111/j.1365-2990.1993.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 173.Alexianu ME, Ho BK, Mohamed AH, La BV, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 174.Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2008;27:2501. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- 175.Hubert JP, Delumeau JC, Glowinski J, Premont J, Doble A. Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br J Pharmacol. 1994;113:261. doi: 10.1111/j.1476-5381.1994.tb16203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 177.Debono MW, Le GJ, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 178.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2007;(1):CD001447. doi: 10.1002/14651858.CD001447.pub2. [DOI] [PubMed] [Google Scholar]
- 179.Tikka TM, Vartiainen NE, Goldsteins G, Oja SS, Andersen PM, Marklund SL, et al. Minocycline prevents neurotoxicity induced by cerebrospinal fluid from patients with motor neurone disease. Brain. 2002;125:722. doi: 10.1093/brain/awf068. [DOI] [PubMed] [Google Scholar]
- 180.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 181.Almer G, Teismann P, Stevic Z, Halaschek-Wiener J, Deecke L, Kostic V, et al. Increased levels of the pro-inflammatory prostaglandin PGE2 in CSF from ALS patients. Neurology. 2002;58:1277. doi: 10.1212/wnl.58.8.1277. [DOI] [PubMed] [Google Scholar]
- 182.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 183.Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 184.Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140:691. [PMC free article] [PubMed] [Google Scholar]
- 185.Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- 186.Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, et al. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 187.Falsig J, Porzgen P, Lotharius J, Leist M. Specific modulation of astrocyte inflammation by inhibition of mixed lineage kinases with CEP-1347. J Immunol. 2004;173:2762. doi: 10.4049/jimmunol.173.4.2762. [DOI] [PubMed] [Google Scholar]
- 188.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model 1. J Clin Invest. 2008;118:659. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 190.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 191.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Graber DJ, Hickey WF, Harris BT. Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:8. doi: 10.1186/1742-2094-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 194.Giess R, Holtmann B, Braga M, Grimm T, Mnller-Myhsok B, Toyka KV, et al. Early onset of severe familial amyotrophic lateral sclerosis with a SOD-1 mutation: potential impact of CNTF as a candidate modifier gene. Am J Hum Genet. 2002;70:1277. doi: 10.1086/340427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 196.Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S, et al. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2005;57:236. doi: 10.1002/ana.20379. [DOI] [PubMed] [Google Scholar]
- 197.Grundstrom E, Askmark H, Lindeberg J, Nygren I, Ebendal T, Aquilonius SM. Increased expression of glial cell line-derived neurotrophic factor mRNA in muscle biopsies from patients with amyotrophic lateral sclerosis. J Neurol Sci. 1999;162:169. doi: 10.1016/s0022-510x(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 198.Grundstrom E, Lindholm D, Johansson A, Blennow K, Askmark H. GDNF but not BDNF is increased in cerebrospinal fluid in amyotrophic lateral sclerosis. Neuroreport. 2000;11:1781. doi: 10.1097/00001756-200006050-00037. [DOI] [PubMed] [Google Scholar]
- 199.Yamamoto M, Mitsuma N, Inukai A, Ito Y, Li M, Mitsuma T, et al. Expression of GDNF and GDNFR-alpha mRNAs in muscles of patients with motor neuron diseases. Neurochem Res. 1999;24:785. doi: 10.1023/a:1020739831778. [DOI] [PubMed] [Google Scholar]
- 200.A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology. 1996;46:1244. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 201.A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III). Neurology. 1999;52:1427. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 202.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Subramanian V, Feng Y. A new role for angiogenin in neurite growth and pathfinding: implications for amyotrophic lateral sclerosis. Hum Mol Genet. 2007;16:1445. doi: 10.1093/hmg/ddm095. [DOI] [PubMed] [Google Scholar]
- 204.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 205.Devos D, Moreau C, Lassalle P, Perez T, De SJ, Brunaud-Danel V, et al. Low levels of the vascular endothelial growth factor in CSF from early ALS patients. Neurology. 2004;62:2127. doi: 10.1212/01.wnl.0000129913.44351.a3. [DOI] [PubMed] [Google Scholar]
- 206.Li X, Lu L, Bush DJ, Zhang X, Zheng L, Suswam EA, et al. Mutant copper-zinc superoxide dismutase associated with amyotrophic lateral sclerosis binds to adenine/uridine-rich stability elements in the vascular endothelial growth factor 3’-untranslated region. J Neurochem. 2009;108:1032. doi: 10.1111/j.1471-4159.2008.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu L, Zheng L, Viera L, Suswam E, Li Y, Li X, et al. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J Neurosci. 2007;27:7929. doi: 10.1523/JNEUROSCI.1877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lambrechts D, Poesen K, Fernandez-Santiago R, Al-Chalabi A, Del BR, Van Vught PW, et al. Meta-analysis of vascular endothelial growth factor variations in amyotrophic lateral sclerosis: increased susceptibility in male carriers of the -2578AA genotype. J Med Genet. 2009;46:840. doi: 10.1136/jmg.2008.058222. [DOI] [PubMed] [Google Scholar]
- 209.Cronin S, Greenway MJ, Ennis S, Kieran D, Green A, Prehn JH, et al. Elevated serum angiogenin levels in ALS. Neurology. 2006;67:1833. doi: 10.1212/01.wnl.0000244466.46020.47. [DOI] [PubMed] [Google Scholar]
- 210.Sebastia J, Kieran D, Breen B, King MA, Netteland DF, Joyce D, et al. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009;16:1238. doi: 10.1038/cdd.2009.52. [DOI] [PubMed] [Google Scholar]
- 211.Subramanian V, Crabtree B, Acharya KR. Human angiogenin is a neuroprotective factor and amyotrophic lateral sclerosis associated angiogenin variants affect neurite extension/pathfinding and survival of motor neurons. Hum Mol Genet. 2008;17:130. doi: 10.1093/hmg/ddm290. [DOI] [PubMed] [Google Scholar]
- 212.Kobari M, Obara K, Watanabe S, Dembo T, Fukuuchi Y. Local cerebral blood flow in motor neuron disease: correlation with clinical findings. J Neurol Sci. 1996;144:64. doi: 10.1016/s0022-510x(96)00151-7. [DOI] [PubMed] [Google Scholar]
- 213.Waldemar G, Vorstrup S, Jensen TS, Johnsen A, Boysen G. Focal reductions of cerebral blood flow in amyotrophic lateral sclerosis: a [99mTc]-d,l-HMPAO SPECT study. J Neurol Sci. 1992;107:19. doi: 10.1016/0022-510x(92)90204-x. [DOI] [PubMed] [Google Scholar]
- 214.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 216.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27:304. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tannemaat MR, Korecka J, Ehlert EM, Mason MR, van Duinen SG, Boer GJ, et al. Human neuroma contains increased levels of semaphorin 3A, which surrounds nerve fibers and reduces neurite extension in vitro. J Neurosci. 2007;27:14260. doi: 10.1523/JNEUROSCI.4571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.De WF, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, et al. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32:102. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 220.Giger RJ, Pasterkamp RJ, Heijnen S, Holtmaat AJ, Verhaagen J. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory-hippocampal pathway and the motor system. J Neurosci Res. 1998;52:27. doi: 10.1002/(SICI)1097-4547(19980401)52:1<27::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 221.Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dupuis L, Gonzalez de Aguilar JL, di Scala F, Rene F, de Tapia M, Pradat PF, et al. Nogo provides a molecular marker for diagnosis of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:358. doi: 10.1006/nbdi.2002.0522. [DOI] [PubMed] [Google Scholar]
- 223.Jokic N, Gonzalez de Aguilar JL, Dimou L, Lin S, Fergani A, Ruegg MA, et al. The neurite outgrowth inhibitor Nogo-A promotes denervation in an amyotrophic lateral sclerosis model. EMBO Rep. 2006;7:1162. doi: 10.1038/sj.embor.7400826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Magnusson C, Libelius R, Tagerud S. Nogo (Reticulon 4) expression in innervated and denervated mouse skeletal muscle. Mol Cell Neurosci. 2003;22:298. doi: 10.1016/s1044-7431(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 225.Shaw PJ, Eggett CJ. Molecular factors underlying selective vulnerability of motor neurons to neurodegeneration in amyotrophic lateral sclerosis. J Neurol. 2000;247(Suppl. 1):I17. doi: 10.1007/BF03161151. [DOI] [PubMed] [Google Scholar]
- 226.Carpenter S. Proximal axonal enlargement in motor neuron disease. Neurology. 1968;18:841. doi: 10.1212/wnl.18.9.841. [DOI] [PubMed] [Google Scholar]
- 227.Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:471. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 228.Tu PH, Gurney ME, Julien JP, Lee VM, Trojanowski JQ. Oxidative stress, mutant SOD1, and neurofilament pathology in transgenic mouse models of human motor neuron disease. Lab Invest. 1997;76:441. [PubMed] [Google Scholar]
- 229.Julien JP. Neurofilaments and motor neuron disease. Trends Cell Biol. 1997;7:243. doi: 10.1016/S0962-8924(97)01049-0. [DOI] [PubMed] [Google Scholar]
- 230.Kawamura Y, Dyck PJ, Shimono M, Okazaki H, Tateishi J, Doi H. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1981;40:667. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- 231.Beaulieu JM, Robertson J, Julien JP. Interactions between peripherin and neurofilaments in cultured cells: disruption of peripherin assembly by the NF-M and NF-H subunits. Biochem Cell Biol. 1999;77:41. [PubMed] [Google Scholar]
- 232.Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 233.Cote F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993;73:35. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 234.Lee MK, Marszalek JR, Cleveland DW. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 1994;13:975. doi: 10.1016/0896-6273(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 235.Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA. Neurofilament transport in vivo minimally requires hetero-oligomer formation. J Neurosci. 2003;23:9452. doi: 10.1523/JNEUROSCI.23-28-09452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Millecamps S, Robertson J, Lariviere R, Mallet J, Julien JP. Defective axonal transport of neurofilament proteins in neurons overexpressing peripherin. J Neurochem. 2006;98:926. doi: 10.1111/j.1471-4159.2006.03932.x. [DOI] [PubMed] [Google Scholar]
- 237.Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, et al. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol. 2003;161:489. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jung C, Lee S, Ortiz D, Zhu Q, Julien JP, Shea TB. The high and middle molecular weight neurofilament subunits regulate the association of neurofilaments with kinesin: inhibition by phosphorylation of the high molecular weight subunit. Brain Res Mol Brain Res. 2005;141:151. doi: 10.1016/j.molbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 239.Shea TB, Zheng YL, Ortiz D, Pant HC. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res. 2004;76:795. doi: 10.1002/jnr.20099. [DOI] [PubMed] [Google Scholar]
- 240.Wagner OI, Ascano J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ackerley S, Grierson AJ, Banner S, Perkinton MS, Brownlees J, Byers HL, et al. p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofi-lament pathology in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2004;26:354. doi: 10.1016/j.mcn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 242.Guidato S, Tsai LH, Woodgett J, Miller CC. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J Neurochem. 1996;66:1698. doi: 10.1046/j.1471-4159.1996.66041698.x. [DOI] [PubMed] [Google Scholar]
- 243.Sun D, Leung CL, Liem RK. Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J Biol Chem. 1996;271:14245. doi: 10.1074/jbc.271.24.14245. [DOI] [PubMed] [Google Scholar]
- 244.Couillard-Despres S, Zhu Q, Wong PC, Price DL, Cleveland DW, Julien JP. Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc Natl Acad Sci USA. 1998;95:9626. doi: 10.1073/pnas.95.16.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18:720. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- 247.Warita H, Itoyama Y, Abe K. Selective impairment of fast anterograde axonal transport in the peripheral nerves of asymptomatic transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1999;819:120. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- 248.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 249.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Magrane J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1615. doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sasaki S, Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:535. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- 253.Tateno M, Kato S, Sakurai T, Nukina N, Takahashi R, Araki T. Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3. Hum Mol Genet. 2009;18:942. doi: 10.1093/hmg/ddn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Strom AL, Shi P, Zhang F, Gal J, Kilty R, Hayward LJ, et al. Interaction of amyotrophic lateral sclerosis (ALS)-related mutant copper-zinc superoxide dismutase with the dynein-dynactin complex contributes to inclusion formation. J Biol Chem. 2008;283:22795. doi: 10.1074/jbc.M800276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang F, Strom AL, Fukada K, Lee S, Hayward LJ, Zhu H. Interaction between familial amyotrophic lateral sclerosis (ALS)-linked SOD1 mutants and the dynein complex. J Biol Chem. 2007;282:16691. doi: 10.1074/jbc.M609743200. [DOI] [PubMed] [Google Scholar]
- 256.Ge WW, Wen W, Strong W, Leystra-Lantz C, Strong MJ. Mutant copper-zinc superoxide dismutase binds to and destabilizes human low molecular weight neurofilament mRNA. J Biol Chem. 2005;280:118. doi: 10.1074/jbc.M405065200. [DOI] [PubMed] [Google Scholar]
- 257.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME. Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol. 1994;53:221. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 258.Wong NK, He BP, Strong MJ. Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic amyotrophic lateral sclerosis (ALS). J Neuropathol Exp Neurol. 2000;59:972. doi: 10.1093/jnen/59.11.972. [DOI] [PubMed] [Google Scholar]
- 259.Menzies FM, Grierson AJ, Cookson MR, Heath PR, Tomkins J, Figlewicz DA, et al. Selective loss of neurofilament expression in Cu/Zn superoxide dismutase (SOD1) linked amyotrophic lateral sclerosis. J Neurochem. 2002;82:1118. doi: 10.1046/j.1471-4159.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 260.Eyer J, Cleveland DW, Wong PC, Peterson AC. Pathogenesis of two axonopathies does not require axonal neurofilaments. Nature. 1998;391:584. doi: 10.1038/35378. [DOI] [PubMed] [Google Scholar]
- 261.Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M, et al. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191:25. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 262.Martin LJ. Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration. Rev Neurosci. 2007;18:115. doi: 10.1515/revneuro.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- 263.Bowling AC, Schulz JB, Brown RH, Jr., Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61:2322. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 264.Browne SE, Yang L, DiMauro JP, Fuller SW, Licata SC, Beal MF. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol Dis. 2006;22:599. doi: 10.1016/j.nbd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 265.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA. 2004;101:11159. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 267.Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, et al. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. 2000;123:1339. doi: 10.1093/brain/123.7.1339. [DOI] [PubMed] [Google Scholar]
- 268.Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, et al. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1998;156:65. doi: 10.1016/s0022-510x(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 269.Mali Y, Zisapels N. Gain of interaction of ALS-linked G93A superoxide dismutase with cytosolic malate dehydrogenase. Neurobiol Dis. 2008;32:133. doi: 10.1016/j.nbd.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 270.Jung C, Higgins CM, Xu Z. A quantitative histochemical assay for activities of mitochondrial electron transport chain complexes in mouse spinal cord sections. J Neurosci Methods. 2002;114:165. doi: 10.1016/s0165-0270(01)00524-6. [DOI] [PubMed] [Google Scholar]
- 271.Liu J, Lillo C, Jonsson PA, Vande VC, Ward CM, Miller TM, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria 2. Neuron. 2004;43:5. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 272.Vande VC, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci USA. 2008;105:4022. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochondrial enzyme activity in amyotrophic lateral sclerosis: implications for the role of mitochondria in neuronal cell death. Ann Neurol. 1999;46:787. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 274.Swerdlow RH, Parks JK, Cassarino DS, Trimmer PA, Miller SW, Maguire DJ, et al. Mitochondria in sporadic amyotrophic lateral sclerosis. Exp Neurol. 1998;153:135. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- 275.de Grey AD. Mitochondrial mutations in mammalian aging: an over-hasty about-turn? Rejuvenation Res. 2004;7:171. doi: 10.1089/rej.2004.7.171. [DOI] [PubMed] [Google Scholar]
- 276.Khrapko K, Vijg J. Mitochondrial DNA mutations and aging: a case closed? Nat Genet. 2007;39:445. doi: 10.1038/ng0407-445. [DOI] [PubMed] [Google Scholar]
- 277.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 278.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 279.Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- 280.Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, et al. Hyper-metabolism in ALS patients: an early and persistent phenomenon. J Neurol. 2009;256:1236. doi: 10.1007/s00415-009-5100-z. [DOI] [PubMed] [Google Scholar]
- 281.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 282.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–71. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 284.Curti D, Malaspina A, Facchetti G, Camana C, Mazzini L, Tosca P, et al. Amyotrophic lateral sclerosis: oxidative energy metabolism and calcium homeostasis in peripheral blood lymphocytes. Neurology. 1996;47:1060. doi: 10.1212/wnl.47.4.1060. [DOI] [PubMed] [Google Scholar]
- 285.Siklos L, Engelhardt J, Harati Y, Smith RG, Joo F, Appel SH. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Ann Neurol. 1996;39:203. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- 286.Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–91. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 287.Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22:215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, et al. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jaarsma D, Rognoni F, Van DW, Verspaget HW, Haasdijk ED, Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. 2001;102:293. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 292.Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jonas EA. Molecular participants in mitochondrial cell death channel formation during neuronal ischemia. Exp Neurol. 2009;218:203. doi: 10.1016/j.expneurol.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, et al. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 295.Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH, Jr., Naniche N, et al. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19:2974. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Di NL, Whitson LJ, Cao X, Hart PJ, Levine RL. Proteasomal degradation of mutant superoxide dismutases linked to amyotrophic lateral sclerosis. J Biol Chem. 2005;280:39907. doi: 10.1074/jbc.M506247200. [DOI] [PubMed] [Google Scholar]
- 297.Hoffman EK, Wilcox HM, Scott RW, Siman R. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J Neurol Sci. 1996;139:15. [PubMed] [Google Scholar]
- 298.Niwa J, Ishigaki S, Hishikawa N, Yamamoto M, Doyu M, Murata S, et al. Dorfin ubiquitylates mutant SOD1 and prevents mutant SOD1-mediated neurotoxicity. J Biol Chem. 2002;277:36793–8. doi: 10.1074/jbc.M206559200. [DOI] [PubMed] [Google Scholar]
- 299.Urushitani M, Kurisu J, Tsukita K, Takahashi R. Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J Neurochem. 2002;83:1030. doi: 10.1046/j.1471-4159.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 300.Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis. 2001;8:933. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- 301.Hyun DH, Lee M, Halliwell B, Jenner P. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem. 2003;86:363. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 302.Puttaparthi K, Wojcik C, Rajendran B, DeMartino GN, Elliott JL. Aggregate formation in the spinal cord of mutant SOD1 transgenic mice is reversible and mediated by proteasomes 1. J Neurochem. 2003;87:851. doi: 10.1046/j.1471-4159.2003.02028.x. [DOI] [PubMed] [Google Scholar]
- 303.Bendotti C, Atzori C, Piva R, Tortarolo M, Strong MJ, Debiasi S, et al. Activated p38MAPK is a novel component of the intracellular inclusions found in human amyotrophic lateral sclerosis and mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2004;63:113. doi: 10.1093/jnen/63.2.113. [DOI] [PubMed] [Google Scholar]
- 304.Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE, et al. Ubiquitinimmunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain. 1991;114(Pt. 2):775. doi: 10.1093/brain/114.2.775. [DOI] [PubMed] [Google Scholar]
- 305.Mendonca DM, Chimelli L, Martinez AM. Expression of ubiquitin and proteasome in motorneurons and astrocytes of spinal cords from patients with amyotrophic lateral sclerosis. Neurosci Lett. 2006;404:315. doi: 10.1016/j.neulet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 306.Sasaki S. Endoplasmic reticulum stress in motor neurons of the spinal cord in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2010;69:346. doi: 10.1097/NEN.0b013e3181d44992. [DOI] [PubMed] [Google Scholar]
- 307.Cheroni C, Marino M, Tortarolo M, Veglianese P, De BS, Fontana E, et al. Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18:82. doi: 10.1093/hmg/ddn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sau D, De BS, Vitellaro-Zuccarello L, Riso P, Guarnieri S, Porrini M, et al. Mutation of SOD1 in ALS: a gain of a loss of function. Hum Mol Genet. 2007;16:1604. doi: 10.1093/hmg/ddm110. [DOI] [PubMed] [Google Scholar]
- 309.Kabashi E, Agar JN, Hong Y, Taylor DM, Minotti S, Figlewicz DA, et al. Proteasomes remain intact, but show early focal alteration in their composition in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2008;105:2353. doi: 10.1111/j.1471-4159.2008.05317.x. [DOI] [PubMed] [Google Scholar]
- 310.Puttaparthi K, Elliott JL. Non-neuronal induction of immunoproteasome subunits in an ALS model: possible mediation by cytokines. Exp Neurol. 2005;196:441. doi: 10.1016/j.expneurol.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 311.Ahtoniemi T, Goldsteins G, Keksa-Goldsteine V, Malm T, Kanninen K, Salminen A, et al. Pyrrolidine dithiocarbamate inhibits induction of immunoproteasome and decreases survival in a rat model of amyotrophic lateral sclerosis. Mol Pharmacol. 2007;71:30. doi: 10.1124/mol.106.028415. [DOI] [PubMed] [Google Scholar]
- 312.Puttaparthi K, Van KL, Elliott JL. Assessing the role of immuno-proteasomes in a mouse model of familial ALS. Exp Neurol. 2007;206:53. doi: 10.1016/j.expneurol.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 314.Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: sorting out the good from the evil. Neurobiol Dis. 2010;37:493. doi: 10.1016/j.nbd.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 317.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 318.Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1 1. Genes Dev. 2008;22:1451. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, et al. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci USA. 2006;103:6025. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 321.Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 322.Ilieva EV, Ayala V, Jove M, Dalfo E, Cacabelos D, Povedano M, et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- 323.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 324.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 325.Baumer D, Parkinson N, Talbot K. TARDBP in amyotrophic lateral sclerosis: identification of a novel variant but absence of copy number variation. J Neurol Neurosurg Psychiatry. 2009;80:1283. doi: 10.1136/jnnp.2008.166512. [DOI] [PubMed] [Google Scholar]
- 326.Corrado L, Ratti A, Gellera C, Buratti E, Castellotti B, Carlomagno Y, et al. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Hum Mutat. 2009;30:688. doi: 10.1002/humu.20950. [DOI] [PubMed] [Google Scholar]
- 327.Daoud H, Valdmanis PN, Kabashi E, Dion P, Dupre N, Camu W, et al. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J Med Genet. 2009;46:112. doi: 10.1136/jmg.2008.062463. [DOI] [PubMed] [Google Scholar]
- 328.Del BR, Ghezzi S, Corti S, Pandolfo M, Ranieri M, Santoro D, et al. TARDBP (TDP-43) sequence analysis in patients with familial and sporadic ALS: identification of two novel mutations. Eur J Neurol. 2009;16:727. doi: 10.1111/j.1468-1331.2009.02574.x. [DOI] [PubMed] [Google Scholar]
- 329.Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kamada M, Maruyama H, Tanaka E, Morino H, Wate R, Ito H, et al. Screening for TARDBP mutations in Japanese familial amyotrophic lateral sclerosis. J Neurol Sci. 2009;284:69. doi: 10.1016/j.jns.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 331.Kirby J, Goodall EF, Smith W, Highley JR, Masanzu R, Hartley JA, et al. Broad clinical phenotypes associated with TAR-DNA binding protein (TARDBP) mutations in amyotrophic lateral sclerosis. Neurogenetics. 2010;11:217. doi: 10.1007/s10048-009-0218-9. [DOI] [PubMed] [Google Scholar]
- 332.Kuhnlein P, Sperfeld AD, Vanmassenhove B, Van DV, Lee VM, Trojanowski JQ, et al. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol. 2008;65:1185. doi: 10.1001/archneur.65.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lemmens R, Race V, Hersmus N, Matthijs G, Van Den Bosch L, Van DP, et al. TDP-43 M311V mutation in familial amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:354. doi: 10.1136/jnnp.2008.157677. [DOI] [PubMed] [Google Scholar]
- 334.Luquin N, Yu B, Saunderson RB, Trent RJ, Pamphlett R. Genetic variants in the promoter of TARDBP in sporadic amyotrophic lateral sclerosis. Neuromuscul Disord. 2009;19:696. doi: 10.1016/j.nmd.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 335.Origone P, Caponnetto C, Bandettini Di PM, Ghiglione E, Bellone E, Ferrandes G, et al. Enlarging clinical spectrum of FALS with TARDBP gene mutations: S393L variant in an Italian family showing phenotypic variability and relevance for genetic counselling. Amyotroph Lateral Scler. 2010;11:223. doi: 10.3109/17482960903165039. [DOI] [PubMed] [Google Scholar]
- 336.Pamphlett R, Luquin N, McLean C, Jew SK, Adams L. TDP-43 neuropathology is similar in sporadic amyotrophic lateral sclerosis with or without TDP-43 mutations. Neuropathol Appl Neurobiol. 2009;35:222. doi: 10.1111/j.1365-2990.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 337.Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ticozzi N, Leclerc AL, van BM, Keagle P, McKenna-Yasek DM, Sapp PC, et al. Mutational analysis of TARDBP in neurodegenerative diseases. Neurobiol Aging. 2009;32:2096–9. doi: 10.1016/j.neurobiolaging.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histo-pathological analysis. Lancet Neurol. 2008;7:409. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Williams KL, Durnall JC, Thoeng AD, Warraich ST, Nicholson GA, Blair IP. A novel TARDBP mutation in an Australian amyotrophic lateral sclerosis kindred. J Neurol Neurosurg Psychiatry. 2009;80:1286. doi: 10.1136/jnnp.2008.163261. [DOI] [PubMed] [Google Scholar]
- 342.Xiong HL, Wang JY, Sun YM, Wu JJ, Chen Y, Qiao K, et al. Association between novel TARDBP mutations and Chinese patients with amyotrophic lateral sclerosis. BMC Med Genet. 2010;11:8. doi: 10.1186/1471-2350-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- 344.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 345.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet. 2010;19:3440. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- 346.Belzil VV, Valdmanis PN, Dion PA, Daoud H, Kabashi E, Noreau A, et al. Mutations in FUS cause FALS and SALS in French and French Canadian populations. Neurology. 2009;73:1176. doi: 10.1212/WNL.0b013e3181bbfeef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2010;81:639. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- 348.Chio A, Restagno G, Brunetti M, Ossola I, Calvo A, Mora G, et al. Two Italian kindreds with familial amyotrophic lateral sclerosis due to FUS mutation. Neurobiol Aging. 2009;30:1272. doi: 10.1016/j.neurobiolaging.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Corrado L, Del BR, Castellotti B, Ratti A, Cereda C, Penco S, et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J Med Genet. 2010;47:190. doi: 10.1136/jmg.2009.071027. [DOI] [PubMed] [Google Scholar]
- 350.Damme PV, Goris A, Race V, Hersmus N, Dubois B, Bosch LV, et al. The occurrence of mutations in FUS in a Belgian cohort of patients with familial ALS. Eur J Neurol. 2010;17:754. doi: 10.1111/j.1468-1331.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- 351.Dejesus-Hernandez M, Kocerha J, Finch N, Crook R, Baker M, Desaro P, et al. De novo truncating FUS gene mutation as a cause of sporadic amyotrophic lateral sclerosis. Hum Mutat. 2010;31:E1377. doi: 10.1002/humu.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Drepper C, Herrmann T, Wessig C, Beck M, Sendtner M. C-terminal FUS/TLS mutations in familial and sporadic ALS in Germany. Neurobiol Aging. 2011;32:548, e1–4. doi: 10.1016/j.neurobiolaging.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 353.Groen EJ, van Es MA, Van Vught PW, Spliet WG, van Engelen-Lee J, de Visser M, et al. FUS mutations in familial amyotrophic lateral sclerosis in the Netherlands. Arch Neurol. 2010;67:224. doi: 10.1001/archneurol.2009.329. [DOI] [PubMed] [Google Scholar]
- 354.Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 355.Lai SL, Abramzon Y, Schymick JC, Stephan DA, Dunckley T, Dillman A, et al. FUS mutations in sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:550, e1–4. doi: 10.1016/j.neurobiolaging.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Suzuki N, Aoki M, Warita H, Kato M, Mizuno H, Shimakura N, et al. FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusion. J Hum Genet. 2010;55:252. doi: 10.1038/jhg.2010.16. [DOI] [PubMed] [Google Scholar]
- 357.Tateishi T, Hokonohara T, Yamasaki R, Miura S, Kikuchi H, Iwaki A, et al. Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol. 2010;119:355. doi: 10.1007/s00401-009-0621-1. [DOI] [PubMed] [Google Scholar]
- 358.Ticozzi N, Silani V, Leclerc AL, Keagle P, Gellera C, Ratti A, et al. Analysis of FUS gene mutation in familial amyotrophic lateral sclerosis within an Italian cohort. Neurology. 2009;73:1180. doi: 10.1212/WNL.0b013e3181bbff05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Van LT, van der Zee J, Sleegers K, Engelborghs S, Vandenberghe R, Gijselinck I, et al. Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology. 2010;74:366. doi: 10.1212/WNL.0b013e3181ccc732. [DOI] [PubMed] [Google Scholar]
- 360.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Giordana MT, Piccinini M, Grifoni S, De MG, Vercellino M, Magistrello M, et al. TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol. 2010;20:351. doi: 10.1111/j.1750-3639.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Tatom JB, Wang DB, Dayton RD, Skalli O, Hutton ML, Dickson DW, et al. Mimicking aspects of frontotemporal lobar degeneration and Lou Gehrig's disease in rats via TDP-43 overexpression. Mol Ther. 2009;17:607. doi: 10.1038/mt.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2009;106:18809. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2010;107:3858. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, et al. Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J Biol Chem. 2009;284:8516. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, et al. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899. doi: 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 370.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Dormann D, Capell A, Carlson AM, Shankaran SS, Rodde R, Neumann M, et al. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- 372.Nishimoto Y, Ito D, Yagi T, Nihei Y, Tsunoda Y, Suzuki N. Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43. J Biol Chem. 2010;285:608. doi: 10.1074/jbc.M109.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2009;106:7607. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Neumann M, Igaz LM, Kwong LK, Nakashima-Yasuda H, Kolb SJ, Dreyfuss G, et al. Absence of heterogeneous nuclear ribonucleoproteins and survival motor neuron protein in TDP-43 positive inclusions in frontotemporal lobar degeneration. Acta Neuropathol. 2007;113:543. doi: 10.1007/s00401-007-0221-x. [DOI] [PubMed] [Google Scholar]
- 376.Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]