Abstract
Ion channels are multimeric, transmembrane proteins that selectively mediate ion flux across the plasma membrane in a variety of cells including vascular smooth muscle cells (VSMCs). The dynamic interplay of Ca2+ and K+ channels on the plasma membrane of VSMCs plays a pivotal role in modulating the vascular tone of small arteries and arterioles. The abnormally-elevated arterial tone observed in hypertension thus points to an aberrant expression and function of Ca2+ and K+ channels in the VSMCs. In this short review, we focus on the three well-studied ion channels in VSMCs, namely the L-type Ca2+ (CaV1.2) channels, the voltage-gated K+ (KV) channels, and the large-conductance Ca2+-activated K+ (BK) channels. First, we provide a brief overview on the physiological role of vascular CaV1.2, KV and BK channels in regulating arterial tone. Second, we discuss the current understanding of the expression changes and regulation of CaV1.2, KV and BK channels in the vasculature during hypertension. Third, based on available proof-of-concept studies, we describe the potential therapeutic approaches targeting these vascular ion channels in order to restore blood pressure to normotensive levels.
Keywords: Hypertension, vascular smooth muscle cell, ion channel, calcium channel, potassium channel, gene therapy
1. Introduction
Hypertension or high blood pressure is a multi-factorial disease that plagues more than 30% of adult Americans and about one quarter of people worldwide [1,2]. Hypertension arises because of complex interactions of various genes with the environment, and is considered a leading risk factor for cardiovascular and kidney diseases [3,4]. Hypertension with unknown etiology, also known as essential, primary or idiopathic hypertension, accounts for 95% of all human hypertension [2]. Blood pressure is determined by two important physical parameters, cardiac output (CO) and total peripheral resistance (TPR). Multiple pathways including the autonomic nervous system, renin-angiotensin system, aldosterone and other vasoactive substances affect CO and TPR to tightly regulate blood pressure and thus ensure appropriate flow of blood to various organs in the body [5–7]. In most clinical cases, CO is normal whereas TPR is elevated due to an abnormal constriction of the small arteries and arterioles [8,9]. The diameter of small arteries and arterioles is maintained mainly by the dynamic interplay of Ca2+ and K+ channels expressed on the plasma membrane of vascular smooth muscle cells (VSMCs) [10]. The opening of K+ channels in response to endogenous stimuli or pharmacological agents results in an efflux of K+ from VSMCs, hyperpolarization of the plasma membrane, closure of Ca2+ channels, reduced intracellular Ca2+ levels and eventually vasodilation. Conversely, closure of K+ channels depolarizes the plasma membrane resulting in the opening of more Ca2+ channels, increased intracellular Ca2+ levels, and vasoconstriction. The elevated vascular tone observed in human hypertension and in several experimental models of hypertension thus points to abnormalities in the expression and function of Ca2+ and/or K+ channels in VSMCs [11]. Indeed, the VSMCs are more depolarized as a consequence of the ‘ion channel remodeling’ that occurs during chronic hypertension [12]. Several families of Ca2+ and K+ channels are expressed in VSMCs (reviewed in [13,14]). In this review, we will only focus on three of the channel types in VSMCs that are reported to be altered in animal models of hypertension: the L-type Ca2+ (CaV1.2) channels, the voltage-gated K+ (KV) channels, and the large-conductance, Ca2+-activated K+ (BK) channels. We will limit our discussion to three important aspects of these channels: 1) their physiological role in VSMCs, 2) the alteration of these channels in VSMCs during hypertension, and 3) their potential as therapeutic targets for the treatment of hypertension.
2. Physiological role of vascular ion channels
VSMCs express different types of ion channels at the sacroplasmic reticulum and the plasma membrane to closely regulate intracellular Ca2+ levels, resting membrane potential (Em) and cell contractility. In VSMCs, Em is primarily determined by K+ efflux through several plasma membrane K+ channels, including the voltage-gated K+ (KV) channels and the large-conductance Ca2+-activated K+ channels, often referred to as “Maxi-K” or “Big K” (BK) channels[10,15]. While there are also inwardly-rectifying K+ (KIR) channels [16,17], ATP-sensitive K+ (KATP) channels [17,18], and two pore domain K+ (K2P) [19,20] channels present in VSMCs that likely also contribute to the final Em, these channels are reviewed elsewhere [21]. Voltage-gated L-type Ca2+ (CaV1.2) channels open in response to membrane depolarization. Excitation of VSMCs results in depolarization, which leads to the voltage-dependent opening of CaV1.2 and KV channels. The opening of CaV1.2 channels allows Ca2+ influx, causing a rise in global Ca2+ and activation of the cellular contractile machinery. The depolarization and the corresponding increase in intracellular Ca2+ through CaV1.2 channels both lead to BK channel opening, which causes a compensatory hyperpolarizing current that closes CaV1.2 channels to buffer vasoconstriction. The opening of KV channels also allows K+ to flow out of the cell and hyperpolarizes the VSMCs [10,15]. In this section, we review the structure and physiological function of these ion channels in the VSMCs and when appropriate provide a brief comparison to the channels found in cardiomyocytes.
2.1. Voltage-gated L-type Ca2+ (CaV1.2) channels
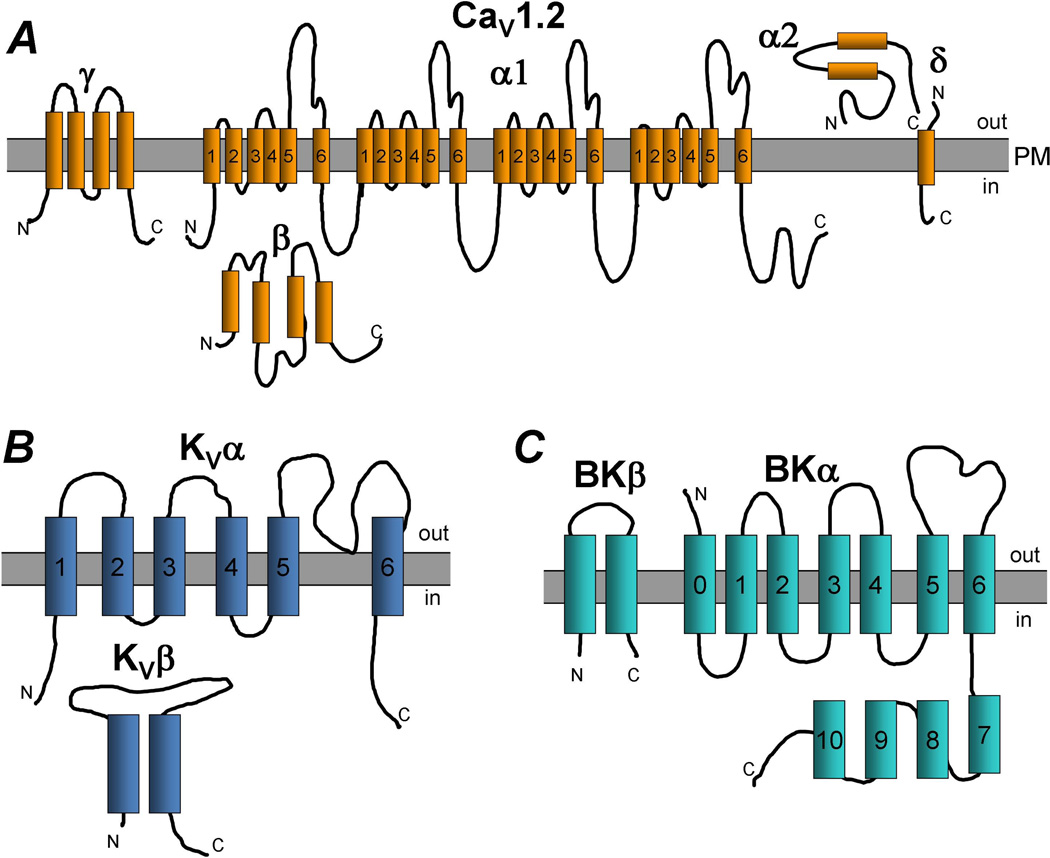
Voltage-gated, long-lasting “L”-type Ca2+ (CaV1.2) channels are opened by depolarization, show a unitary conductance of 20–30 pS, and are slowly inactivating. Structurally, CaV1.2 channels are multimeric protein complexes, comprised of pore-forming α1 subunits and auxiliary β, α2δ and γ subunits (Figure 1A). The α1 subunits have four repeat domains each of which has six transmembrane sections (S1-S6). The α1 subunits are responsible for voltage sensing, Ca2+ permeability, Ca2+-dependent inactivation, and inhibition by Ca2+ channel blockers. Cytosolic accessory β subunits, of which there are four isoforms (β1-4), interact with the α1 subunits to modulate channel properties such as plasma membrane targeting, channel inactivation and voltage-dependent gating [13,22–24]. The β subunits are classified as membrane-associated guanylate kinases, a class of scaffolding protein that has both guanylate kinase and Src homology 3 domains [25,26]. In VSMCs, both β2 and β3 subunits have been identified in bovine [27], mouse [28] and rabbit aortae [29]. More recently, β3 subunits have been identified in mouse mesenteric arteries as an important regulator of CaV1.2 subunits in hypertension [30]. Also present in the CaV1.2 complex are auxiliary α2δ (α2δ1-4) and γ subunits, though their functions are less clear. Bannister et al observed that inhibition of the α2δ-1 subunit caused vasodilation of pressurized rat cerebral arteries and reduced CaV1.2 currents in cerebral artery VSMCs [31]. Moreover, knockdown of α2δ-1 reduced plasma membrane expression of CaV1.2, suggesting that α2δ-1 is important for plasma membrane expression of functional CaV1.2 channels [31].
Figure 1.
Proposed topology of vascular ion channels. A. Voltage-gated L-type Ca2+ channel (CaV1.2) subunits α1, α2, β, γ, δ are depicted. PM: plasma membrane. B. Voltage-gated K+ channel (KV) α and β subunits. C. Large-conductance, Ca2+-activated K+ channel (BK) α and β subunits.
CaV1.2 channels open in response to depolarizing stimuli, which allows Ca2+ to flow inside the VSMC to initiate cell contraction and other Ca2+-dependent processes. Calcium-dependent inactivation occurs when increasing intracellular Ca2+ causes closure of CaV1.2 channels, even when depolarization is maintained [32–34]; CaV1.2 channels can also inactivate via voltage-dependent inactivation [35,36]. Both Ca2+-dependent inactivation and voltage-dependent inactivation limit the amount of Ca2+ entry into the VSMC, thus buffering constriction. The Ca2+-binding protein, calmodulin, is also important in modulating CaV1.2 activity. The C-terminal lobe of calmodulin binds to the cytoplasmic carboxyl terminus of the CaV1.2 channel to cause Ca2+-dependent inactivation. In addition, the amino-terminal lobe of calmodulin can bind to the carboxyl terminus of CaV1.2 to regulate Ca2+-dependent facilitation of CaV1.2 [37–39].
Besides being regulated by Ca2+, CaV1.2 channel activity can also be modulated by other proteins such as scaffolding proteins and signaling molecules [40]. Initially observed in neurons, a scaffolding protein, A-kinase anchoring protein (AKAP), was observed to bind several sites on the α1 subunit of CaV1.2, including the amino terminus, the transmembrane I-II linker and the carboxy terminus [41]. This observation was further confirmed in VSMCs where AKAP150 anchors protein kinase C (PKC) to the sarcolemma, and is required for PKC activation of Ca2+ sparklets in a subset of CaV1.2 channels that importantly contribute to Ca2+ influx and regulation of [Ca2+]i [42]. In addition to facilitating opening of CaV1.2 channels, AKAPs also regulate a Ca2+-dependent negative feedback mechanism of CaV1.2 channels via calcineurin (also known as protein phosphatase 2B) [42,43]. In summary, CaV1.2 channel activity is tightly regulated in VSMCs by several mechanisms to control [Ca2+]i and to carefully regulate vascular tone.
In cardiomyocytes, L-type Ca2+ channels play a major role in excitation-contraction coupling. CaV1.2 channels open in response to the depolarization of cardiomyocytes during an action potential. The increase in intracellular Ca2+ concentration via opening of CaV1.2 channels triggers Ca2+ release from sarcoplasmic ryanodine receptors (RyRs), which increases intracellular Ca2+ sufficiently to allow Ca2+ binding to troponin C and initiation of cardiomyocyte contraction. Structurally, cardiac voltage-gated Ca2+ channels have similar subunit composition to vascular voltage-gated Ca2+ channels, with the α1C (CaV1.2) subunit being the most abundant cardiac α subunit. Low voltage-activated CaV3 channels (T-type voltage-gated Ca2+ channels, α1G and α1H) are also expressed in the heart, but their expression is mainly limited to the sinoatrial pacemaker cells and Purkinje fibers, where they contribute to setting the frequency of action potential firing [44,45]. In cardiomyocytes, auxiliary β1, β2 and β3 subunits are expressed [46], and in the adult human myocardium, five β2 splice variants, β2a – β2e have been identified, with varying effects on channel activation and inactivation kinetics [47]. Other important auxiliary subunits that affect channel function are the α2δ and γ subunits. In cardiomyocytes, α2δ affects voltage dependence, current kinetics, and is required for normal cardiac excitation-contraction coupling. Differently from α2δ in VSMCs, cardiac α2δ is not involved in membrane targeting of CaV1.2 [48,49]. Three γ subunit isoforms are expressed in the rat heart (γ4, γ6, and γ7), while four isoforms (γ4, γ6, γ7, and γ8) have been detected in human cardiac tissue [50]. In heterologous expression systems, all of the γ isoforms in rat and human heart co-immunoprecipate with CaV1.2, but the effects of the γ subunit on channel activation and inactivation vary by isoform [50].
Similar to VSMCs, scaffolding proteins and other signaling molecules can modulate CaV1.2 channel function in cardiomyocytes. For example, calmodulin modulates CaV1.2 current in ventricular myocytes via binding to the C-terminus of CaV1.2 [51,52]. Similarly, AKAP15 directly anchors protein kinase A (PKA) to the CaV1.2 channels in cardiomyocytes and facilitate the increase in CaV1.2 current by β-adrenergic receptor activation [53,54]. Interestingly, K+ channel interacting proteins (KChIPs), small Ca2+-binding proteins that were originally identified as KV4 channel auxiliary subunits, can directly regulate cardiac CaV1.2 channel current [55]. There are no reports to date regarding KChIP modulation of vascular CaV1.2 channel activity.
2.2. Voltage-gated K+ (KV) channels
The voltage-gated K+ (KV) channels are multimeric complexes that are activated by depolarization and allow K+ conductance across the plasma membrane (Figure 1B). The pore-forming α subunits of KV channels are transmembrane proteins that contain six transmembrane domains (S1–S6). The transmembrane domains S1 through S4 form the voltage sensor of the KV channel, while the S5 and S6 domains form the channel pore. A functional KV channel is comprised of four α subunits, which may be the same or different isoforms from the same gene family, of which there are several different gene families. Since the KV channel α subunits can form homo- and hetero-tetramers, the biophysical properties, physiological regulation and pharmacological properties of these channels can vary. For example, while mRNA for KV1.1–1.6 was detected in rat cerebral arteries, only protein for KV1.2 and1.5 was detected suggesting that the functional KV channel is a KV1.2/1.5 heterotetramer in cerebral vasculature [56].
Physiologically, in rat mesenteric arteries, KV channels contribute to resting membrane potential and vessel tone [57]. Plane et al observed that after blockade of KV1 channels with either 4-aminopyridine (a selective KV channel blocker) or correolide (a selective KV1 channel blocker), the myogenic response, the constriction that occurs in response to increased intraluminal pressure, was greater compared to control conditions [58]. Similarly, the KV2 channel blocker stromatoxin constricted pressurized, perfused rat cerebral arteries, suggesting an influence of KV2 channels on cerebral myogenic tone [59]. In rat aortic VSMCs, KV2.1 channels are expressed and blockade of KV2 but not KV1 channels induces oscillatory contractions [60].
KV channels are also targets for phosphorylation by protein kinase A (PKA) and PKC [57,61–65]. PKC signaling leads to closure of KV channels and smooth muscle constriction [57,63–65], while PKA signaling via G-protein coupled-receptor activation and adenylyl cyclase or guanylyl cyclase, which increase cAMP and cGMP, respectively, can open KV channels to hyperpolarize the membrane and facilitate smooth muscle cell relaxation [57,61,62,65].
Recently, another class of KV channels, the KCNQ (KV 7) channels, were identified in VSMCs. The KCNQ family of K+ channels are outwardly-rectifying, voltage-gated K+ channels that regulate membrane excitability in cardiac, neuronal and inner ear cells. Initially identified in murine portal vein myocytes, KCNQ channels have since been identified in aorta [66], pulmonary [67], tibial [66], basilar [68,69], mid-cerebral [69] and mesenteric arteries [70,71] from rats, mice and humans. In rat cerebral arteries, the KCNQ channels contribute to the development of myogenic tone [69] and Mani et al suggest that KCNQ channel openers may be more effective for the treatment of cerebral vasospasm than the currently used calcium channel blocker nimodipine. Schleifenbaum et al suggest that H2S, which may be the adipocyte-derived relaxing factor released from perivascular fat, inhibits vascular contraction via opening of KCNQ channels [66]. Jackson-Weaver et al on the other hand found that in small mesenteric arteries endothelial production of H2S plays a major role in maintaining low vascular tone [72]. It is unknown if heritable mutations in KCNQ channels affect vascular function, possibly contributing to vascular dysfunction in disease states.
In non-vascular tissues and heterologous expression systems, the pore-forming α subunits of KV channels are known to interact with several ancillary proteins including the smaller β subunits, K+ channel interacting protein (KChIP) and accessory proteins (KChAP), the minimal K+ channel protein (minK), the minK-related peptide (MiRP) and scaffolding proteins such as post synaptic density 95 (PSD-95) (reviewed in [73]). However, only a few of these ancillary proteins have been identified to complex with KV channels in vascular tissues. Several vascular beds including the pulmonary, mesenteric and renal arteries express many β subunits either at the mRNA or protein level [74–76]. However, the physiological function(s) of β subunits in vascular tissues is yet to be identified. Our laboratory recently reported that PSD 95, a member of the membrane-associated guanylate kinases, complexes with the pore-forming α1.2 subunit of KV1 channels at the plasma membrane of VSMCs in cerebral arteries [77]. Using ex vivo gene knockdown in cerebral arteries, we demonstrated that PSD-95 regulates KV1 channel expression in cerebral VSMCs and modulate the membrane potential and diameter of cerebral arteries [77]. Clearly, future endeavors should be directed towards identifying the expression, and importantly the physiological function of ancillary proteins of KV channels in the vascular tissues.
Owing to the diversity of the KV channel gene family and many different combinations of subunits for heteromultimerization, the biophysical properties of KV channels and their responses to pharmacological intervention may vastly differ between different tissue types. For example, in cardiomyocytes, at least seven different KV channels have been cloned expressing two different outward currents, delayed rectifiers (KV1.1, KV1.2, KV1.5, KV2.1, KVLQT1 and hERG) and transient rectifiers (KV1.4, KV4.2 and KV4.3). In cardiomyocytes, potassium channels determine the magnitude and duration of action potentials. The diversity of types and levels of expression produce the heterogeneity of action potential configurations throughout the regions of the heart [78]. A consequence of this diversity on response to pharmacological intervention is demonstrated by hERG. Although hERG (KV11.1) is expressed in smooth muscle cells, brain and endocrine cells its function in the heart makes it central to drug safety since a wide variety of prescription medications can block KV11.1 causing drug-induced QT prolongation and an increased risk of sudden cardiac arrest [79]. A more thorough treatment of KV and other ion channels in cardiomyocytes can be found in other reviews [80,81].
2.3. High-conductance Ca2+-sensitive K+ (BK) channels
Another important ion channel for maintaining VSMC Em is the large-conductance, Ca2+-sensitive K+ channel, also known as the “Maxi-K” or “Big K” (BK) channel (Figure 1C). Compared to KV channels, BK channels have large single-channel conductance (100–300 pS). The pore-forming α subunits of BK channels have seven (S0–S6) transmembrane domains, which show partial homology with the six transmembrane domains, S1–S6, of KV channels. While voltage-sensitivity and pore-formation of the BK channel are determined by the transmembrane domains of the α subunit, Ca2+-sensitivity is conferred by four intracellular domains (S7–S10) and by the interaction of the first transmembrane domain (S0) of the α-subunit with an auxiliary β1 subunit. BK channels are encoded by the hSlo gene, and phenotypic diversity is generated by a high level of alternative splicing [82–84]. Similarly to KV channels, opening of BK channels leads to efflux of K+ out of VSMCs, hyperpolarizing the plasma membrane.
The BK channels are expressed in many vascular beds, including the small arteries and arterioles of the cerebral, coronary and renal circulations. The BK channels in some vascular beds, such as rat cerebral arteries, appear to be in close proximity to ryanodine receptors (RyRs) and inositol 1,4,5-trisphosphate receptors (IP3Rs) in the sarcoplasmic reticulum (SR). The Ca2+ released by the RyRs, also known as Ca2+ sparks, stimulates opening of BK channels since blockade of RyRs with either ryanodine or depletion of intracellular Ca2+ with thapsigargin reduced Ca2+ sparks and BK currents [85–89]. IP3Rs can also stimulate opening of BK channels, however this stimulation can be dependent on Ca2+ released from IP3Rs as in basilar VSMCs [90] or independent of Ca2+ released from IP3Rs as was observed in mouse and rat cerebral arteries [91]. In addition to interacting with SR Ca2+ release channels, BK channels couple to some plasma membrane ion channels, such as transient receptor potential (TRP) channels. For example, TRPC1 channels coupled to BK channels in cultured aortic VSMCs [92] and TRPV4 channels complex with RyRs and BK channels in rat cerebral artery VSMCs [93,94].
In addition to their voltage and Ca2+ sensitivity, BK channels are also regulated by signaling molecules such as PKA and PKC. PKA can both activate and inhibit BK channel opening, depending on the splice variant of the channel [95–98]. In the majority of arteries studied, activation of PKA and cGMP-dependent protein kinase leads to activation of BK channels [95]. However, the literature regarding the effect of PKC on BK channels is less clear and less studied. For example, in rat pulmonary arterial smooth muscle cells, forskolin, an activator of cAMP, induces PKC-mediated opening of BK channels [99] and PKC activators also open BK channels [100]. However, in rat tail artery smooth muscle cells, patch clamps studies demonstrate that the catalytic subunit of PKC significantly reduces single BK channel openings [101]. In addition, new signaling molecules continue to emerge such as endothelium-derived H2S that targets BK channels to maintain low myogenic tone [72]. Thus, BK channels are regulated by several cellular processes and these can vary between vascular beds of interest.
Compared to VSMCs, there are very few studies of BK channels in cardiomyocytes. It is generally believed that the involvement of BK channels is limited in cardiomyocyte, possibly due to the low levels of BK channel expression in the whole heart [102–104]. However, some BK channels are found in the inner mitochondrial membrane of cardiomyocytes and may protect the heart against ischemic injury [105,106]. Another recent study utilizing whole animal and perfused heart preparations reported that the heart rate is significantly reduced by inhibiting BK channels in wild-type mice but not in BK knockout mice [107]. Thus, BK channels may have important physiological functions in cardiomyocytes more than previously thought, and any therapies targeting vascular BK channels may have to consider cardiac effects as well.
3. Vascular ion channel remodeling during hypertension
Small arteries and arterioles undergo extensive biological and structural adaptation in response to elevated intraluminal perfusion pressure that occurs during chronic hypertension. The underlying pathophysiological processes appear to be complex, and likely involve vascular remodeling, endothelial dysfunction, smooth muscle cell hypertrophy, and changes in extracellular matrix composition and function [108,109]. The net effect of these adaptive changes is augmented vasoconstrictor responses and attenuated vasodilator responses to various physiological stimuli, resulting in elevated vascular tone in the arteries and arterioles that are exposed to persistent high blood pressure [110,111]. The elevated vascular tone observed in the small arteries of cerebral, coronary and renal circulations during hypertension is thought to buffer the transmission of the high systemic pressure to the fragile capillaries of the brain, heart and kidneys, respectively, to prevent pressure-induced damages [112,113]. However, in the case of small arteries and arterioles of the mesenteric and skeletal muscle beds that contribute substantially to total peripheral resistance, elevated vascular tone during hypertension would further accentuate the increase in systemic blood pressure. Similar to other components of the vasculature, during persistent high blood pressure, ion channels in the plasma membrane of VSMCs also undergo ‘electrical remodeling’ such that the arteries maintain a heightened vascular tone. In fact, evidence for ‘electrical remodeling in VSMC membranes exposed to high blood pressure was first observed in the early 1970’s when increased transmembrane Ca2+ and K+ flux was observed in arteries from different experimental models of hypertension [114,115]. Subsequently, in the early 1980’s, it was first reported that VSMCs from arteries of spontaneously hypertensive rats (SHR), a genetic rodent model for hypertension, were depolarized, and generated Ca2+-dependent tone [12,116]. Building on these pioneering studies, several important discoveries were reported regarding changes in ion channel expression in VSMCs during hypertension. In the following sections, we present a synopsis of our current understanding of the topic (summarized in Figure 2).
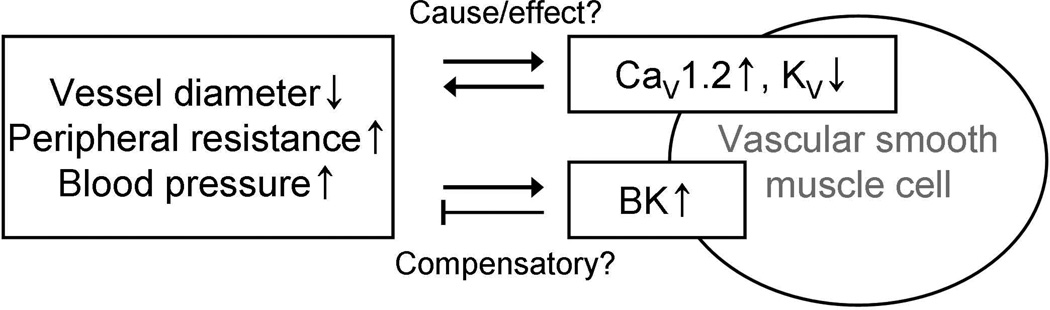
Figure 2.
Changes in vascular ion channel expression during hypertension. Voltage-gated L-type Ca2+ channels (CaV1.2) are upregulated and voltage-gated K+ channels (KV) are downregulated in hypertension. The changes in expression may be the cause of vasoconstriction and increased blood pressure or a result of sustained elevation in blood pressure. Large-conductance Ca2+ -activated K+ channels (BK) are upregulated during hypertension, possibly as a compensatory mechanism inhibiting further increases in blood pressure.
3.1 Upregulation of vascular Ca2+ channels during hypertension
Several years of research have conclusively demonstrated that an upregulation of L-type Ca2+ (CaV1.2) channel function in VSMCs is a hallmark feature of hypertension. A strong positive correlation exists between blood pressure and the number of functional CaV1.2 channels in the VSMCs in vivo. For example, systolic blood pressure was found to be linearly correlated to membrane densities of CaV1.2 channel currents in VSMCs from small mesenteric arteries of SHR and WKY rats [117]. Interestingly, a reduction in systolic blood pressure by treatment with ramipril, an angiotensin converting enzyme inhibitor, resulted in a concomitant decrease in CaV1.2 channel current densities in VSMCs of SHR [118]. A plethora of other studies have also shown that the profound increase in CaV1.2 channel function observed during hypertension is an abnormality shared among VSMCs of several vascular beds [13,117,119–123]. Electrophysiological studies have demonstrated elevated Ca2+ currents in freshly isolated VSMCs of cerebral, mesenteric, renal, skeletal and pulmonary arteries from various hypertensive animal models [13,117,119,120,123]. Single channel experiments and other studies have further shown that the elevated Ca2+ currents observed in VSMCs during hypertension are not the result of altered single channel conductance, open-time distribution or voltage sensitivity, but rather due to an increased number of CaV1.2 channel openings [124]. Complementing the electrophysiological studies, contractile studies in isolated arteries further proved that compared to normotensive animals, arteries from hypertensive animals develop more Ca2+-dependent spontaneous tone that was sensitive to CaV1.2 channel blockers [120]. For example, renal arteries from SHRs, a genetic model of hypertension, and aortic-banded rats, that develop hypertension due to elevated angiotensin II levels, develop more Ca2+-dependent spontaneous tone that was reversed by nifedipine (1µmol/L), a CaV1.2 channel specific blockers, compared to their normotensive counterparts [13].
The profound upregulation in vascular CaV1.2 channel function observed in hypertensive animals is largely attributed to increased expression of the channel. Immunoblot analyses show that the expression of the pore-forming α1C subunit of the CaV1.2 channel is elevated in arteries of hypertensive animals compared to age-matched normotensive animals [123,125]. For example, the protein expression of α1C was higher in mesenteric, renal and skeletal muscle arteries of SHR compared to age-matched WKY rats [13]. In a separate study, increased expression of vascular tissue–specific exon 1-encoded α1C protein was observed in aorta and mesenteric arteries of SHR compared to WKY rats [126]. Further, the increases in exon 1-encoded α1C protein was specific to vasculature, and was not observed in brain or visceral smooth muscles. Increased expression of pore-forming α1C subunit was also seen in renal arteries of aortic-banded rats compared to Sham rats, suggesting that the increased expression of vascular CaV1.2 channel is an anomaly shared between hypertensive animal models with different etiologies [120]. In the same study, the authors found that renal arteries that were exposed to high blood pressure for as little as 2 days exhibited increased expression of α1C subunit, implying that the abnormal increase in vascular CaV1.2 channels may be an early event occurring during the development of hypertension.
Although the paradigm for functional upregulation of vascular CaV1.2 channels during hypertension is now well established, there is a paucity of information regarding the mechanisms that lead to increased protein expression of the pore-forming α1C subunit. In this regard, even less information is available on cellular processes and proteins that regulate the expression of CaV1.2 channels in VSMCs during physiological conditions. Nevertheless, data available so far points to the involvement of multiple cellular mechanisms in the upregulated protein expression of vascular CaV1.2 channels during hypertension. Slight increases in α1C transcript level were observed in mesenteric arteries of SHR compared WKY rats [125]. However, in this study, a 1.53-fold increase in α1C transcript level was accompanied by an unmatched 3.4-fold increase in α1C protein level in mesenteric arteries of SHR. Similarly, in neonatal piglets with hypoxia-induced pulmonary hypertension, a profound increase in the expression of α1C protein in pulmonary arteries was accompanied by no change in transcript levels [123]. Thus, in addition to transcriptional activity, post-transcriptional mechanisms such as increased translation efficiency, increased trafficking of channel proteins to plasma membrane and increased stability of channel protein complex may also contribute to the upregulation of α1C protein in the vasculature during hypertension. A recent study indicated a potential role of microRNAs (miRNAs) in determining α1C protein expression at the post-transciptional level in the vasculature [127]. In this study, miR-328 was found to regulate hypoxic pulmonary hypertension by post-transcriptional repression of α1C subunits in pulmonary arterial smooth muscle cells. Consequently, mice overexpressing miR-328 had remarkably decreased right ventricular systolic pressure and pulmonary artery wall thickness [127]. Enhanced anterograde trafficking of the channel protein complex to the plasma membrane via the ancillary β subunits may also contribute to increased CaV1.2 channel activity in the vasculature during hypertension. In heterologous expression systems, the β subunit has been shown to promote plasma membrane expression of CaV1.2 channels by binding to the pore-forming α1C subunit and potentially masking an ER retention signal [128]. In a recent study, through the use of CaV β3 knockout (β3−/−) mice, Kharade et al demonstrated that in mesenteric arteries from angiotension II-induced hypertensive mice, the β3 subunit is required for the upregulation of CaV1.2 channels, increased calcium-dependent tone, and the development of high blood pressure [30]. Evidence from a recent study also points to clustering and coordinated gating of CaV1.2 channels in the plasma membrane of VSMCs during hypertension [129]. The authors in this study observed a higher frequency of coupled CaV1.2 channel gating events, measured as persistent Ca2+ sparklets, in VSMCs of angiotensin II-induced hypertensive animals, and raised the possibility of transient interaction of 2–6 adjacent CaV1.2 channels via their C-terminus during hypertension. Such a transient interaction of adjacent CaV1.2 channels and their simultaneous activation would result in enhanced Ca2+ influx per gating event and elevated myogenic tone during hypertension. Interestingly, persistent Ca2+ sparklet activity by CaV1.2 channels in VSMCs requires recruitment of PKCα by AKAP150 to the plasma membrane, suggesting that scaffolding proteins that ‘organize’ signaling molecules and ion channels may be important in mediating the vascular ion channel phenotype observed during hypertension [42]. Consistent with this hypothesis, AKAP150 knockout mice showed a lack of persistent Ca2+ sparklets, decreased myogenic tone, and did not develop angiotensin II-induced hypertension [42]. Although more mechanistic studies are needed in the future, information available thus far suggests the involvement of multiple regulatory pathways in the enhanced expression of vascular CaV1.2 channels during hypertension.
3.2 Alteration in vascular KV channels during hypertension
The KV channels are major contributors to resting membrane potential and the diameter of small arteries of several vascular beds including the cerebral, coronary, mesenteric and pulmonary circulations [14]. Pioneering electrophysiological studies by Harder et al. demonstrating that VSMCs from cerebral arteries of SHR are more depolarized than those from WKY rats prompted researchers to investigate the functional expression of vascular K+ channels during hypertension [116]. Our laboratory, in addition to others, is particularly interested in determining if vascular KV1 channels inactivate or down-regulate in response to elevated blood pressure, resulting in depolarized arteries and increased myogenic tone. In this regard, Post et al. initially reported that hypoxia-induced rises in cytosolic calcium inhibit KV channel currents in patch-clamped VSMCs isolated from canine pulmonary arteries [130]. Cox and Petrou also observed that Ca2+ influx through L-type Ca2+ channels decreases KV channel current in patch-clamped VSMCs isolated from rat mesenteric arteries [131]. These authors proposed that an elevated level of cytosolic calcium in the VSMCs of hypertensive rats may inactivate KV1 channels resulting in membrane depolarization and arterial constriction, independent of changes in channel expression. Thus, one mechanism suggested to reduce vascular KV1 channel function is through the inactivation of KV1 channels by intracellular calcium. Alternatively, a loss of KV1 channel function in VSMCs of arteries exposed to high blood pressure could be due to a reduced expression of channel proteins. Wang et al. reported that KV channel current was attenuated in a rat model of pulmonary arterial hypertension (PAH) induced by hypoxia [132]. In this study, reduced expression of α1.2 and α1.5 mRNA and protein was reported in small pulmonary arteries of rats with hypoxia-induced PAH. A down-regulation of KV1 channel function, mRNA and protein was also documented in small pulmonary arteries of humans with primary PAH [133]. In agreement with these findings, reduced densities of KV current also have been reported in VSMCs from small mesenteric arteries and renal interlobar arteries of the SHR [134,135]. Furthermore, the mesenteric arteries of Nω-nitro-L-arginine-induced hypertensive rats exhibit a reduced expression of α1.5 that is associated with depolarization and enhanced contractile sensitivity [136,137]. The authors in these studies further reported that in whole cell patch-clamp experiments using a Ca2+-free pipette solution, K+ current attributed largely to voltage-dependent K+ (KV) channels was reduced by 60% in VSMCs of the hypertensive rats [137]. In this context, studies done in our laboratory also demonstrated that depolarization and elevated vascular tone seen in small cerebral arteries of SHR and renal hypertensive rats were associated with a loss of functional KV1 channels [138]. We further demonstrated that the loss of KV1 channel function in cerebral arteries was due to depressed gene activity of the pore-forming α subunits. Many other studies revealed that in addition to KV1 channels, Shab-type voltage-gated K+ (KV2) channels also contribute to membrane hyperpolarization and vessel diameter. For example, Amberg and Santana reported that KV2 channels contribute to the resting diameter of rat middle cerebral arteries [59]. In their study, stromatoxin (ScTx) -induced block of the KV2 channels reduced resting diameter by 13% in arteries of control rats. In contrast, cerebral arteries of angiotensin II-induced hypertensive rats showed only a 6% constriction in response to ScTx treatment and had a lower density of ScTx-sensitive K+ current, suggesting attenuated KV2 channel function. A separate study further suggested that activation of the transcription factor NFATc3 by Ca2+ entry via CaV1.2 channels would result in decreased KV2 channel expression in cerebral arteries during hypertension pointing to an interdependency of functional ion channel expression [139]. Reduced gene expression of α2.1 and α2.2 leading to KV2 channel dysfunction was also observed in myocytes of vasospastic cerebral arteries in a canine model of subarachnoid hemorrhage, a disorder often associated with chronic hypertension [140]. Recently, studies reported that many members of the KV7 family are expressed in rodent and human blood vessels, and play an important role in controlling vascular tone [69–71]. Importantly, similar to KV1 channels, a depression of KV7 channel function was observed in aorta and mesenteric arteries of SHR and angiotensin II-induced mice, and the decreased KV7 channel function was accompanied by a reduced expression of KV7.4 protein levels [141]. Furthermore, a loss of KV7 channel function as well as KV7.4 protein expression levels were implicated in impaired β-adrenoceptor mediated vasodilation observed in renal arteries of SHR [142].
However, since the pore-forming α subunits of KV channels are encoded by several genes and the α subunits that complex to form the heterotetrameric channel vary with vascular beds, predictably, the functional expression of vascular KV channels in response to high blood pressure differs between hypertensive animal models [136,143]. For example, mesenteric arteries of Nω-nitro-L-arginine-induced hypertensive rats showed decreased expression of KV α1.5 [136]; however increased KV channel current and a negative shift in voltage dependence of activation accompanied by an increase in mRNA and/or protein levels of α1.2, α1.3, α1.5 and α2.1 was observed in mesenteric arteries of SHR [143,144]. Subtle differences in the biogenic process of KV channels between different hypertensive animal models also has been reported in the cerebral arteries [138]. Experiments in our laboratory found that the decrease in KV1 channel function in cerebral arteries of SHR is associated with reduced gene expression of α1.2 and not α1.5; however, depressed gene expression of α1.5 was observed as an additional abnormality in cerebral arteries of aortic-banded rats that had high blood pressure [138]. The expression of KV channels also differs between vascular beds exposed to high blood pressure. For example, in SHR, protein expression of α2.1 was increased in mesenteric arteries, decreased in tail arteries, and unchanged in thoracic aorta compared to control WKY rats [143]. A recent study showed that mesenteric arteries in a genetic mouse model of hypertension were associated with de novo protein synthesis of the KV6.3 subunit, and based on differences in current kinetics, the authors suggested that a switch from a homotetrameric KV2.1 channel to a heterotetrameric KV2.1/ KV6.3 channel occurs during hypertension [145]. Another ‘silent’ subunit, namely KV α9.3, was recently reported to complex with KV2.1 channels in rat middle cerebral arteries; however the fate of KV α9.3 during hypertension remains to be seen [146].
Even though several ancillary subunits are present for KV channels in brain, only a few among them have been reported in vascular tissues. Importantly, the expression of ancillary β subunit in the vasculature during hypertension has been a subject of interest. In rats exposed to chronic hypoxia and in patients with primary pulmonary hypertension, a loss of KV1 channel function was associated with a reduced gene activity of KV1 α subunits, but no change in KVβ transcript levels [147,148]. Similarly, exposing cultured pulmonary VSMCs to hypoxia for 60 hours resulted in reduced KV1 channel expression and function, but no changes in ancillary KVβ1, KVβ2 and KVβ3 mRNA levels [132]. However, the mRNA level of KVβ1.1 was increased in mesenteric and tail arteries, and thoracic aorta in SHR compared to normotensive WKY rats [144]. The mRNA levels of other KV1 channel-accessory proteins, namely KChIP3 and KChAP were found to be reduced in aorta in a genetic mouse model of hypertension; however only KChAP mRNA levels were reduced in mesenteric arteries [145]. Collectively, the functional expression of KV channels and associated proteins differs between vascular beds during hypertension, and highlights the complexities involved in the regulation of KV channel function and expression.
3.3 Changes in vascular BK channels during hypertension
The BK channels are an important family of potassium channels that are densely expressed in VSMCs, and are activated by membrane depolarization and increased cytosolic Ca2+ concentration. The physiological function of vascular BK channels acting as a homeostatic mechanism to counteract myogenic constriction due to elevated intraluminal pressure, and thereby ensuring perfusion to critical organs is well recognized [149]. Several laboratories have studied BK channels in resistance and conduit arteries in different experimental models of hypertension [135,150–158]. A majority of the studies report that the functional expression of BK channels in VSMCs increases during hypertension [135,150–155]. In fact, as early as the 1970’s, it was known that the smooth muscle cells from aorta, renal and caudal arteries of hypertensive rats had increased transmembrane K+ ion flux that was sensitive to depletion of extracellular Ca2+ and Ca2+ channel blockers [114,159,160]. Subsequently, using isometric tension recording and vascular reactivity techniques along with BK channel specific pharmacological blockers such as tetraethylammonium (TEA), charybdotoxin and iberiotoxin, several studies demonstrated enhanced function of BK channels in arteries of experimental hypertensive animals [152,161–164]. For example, compared to control tissues, treatment with TEA robustly contracted proximal aortic segments from aortic-banded hypertensive rats, implying an accentuated dilator function of BK channels in aorta of hypertensive rats. Similarly, aortic rings from SHR contracted strongly to TEA, whereas aortic rings from normotensive WKY rats showed little to no constriction [161]. Concurrently, studies done in other vascular beds, such as the cerebral, femoral, carotid, renal and mesenteric vasculature, also showed elevated constrictor responses to BK channel specific blockers [152,162–164]. For example, treatment of endothelium-denuded carotid, femoral and mesenteric arterial segments from SHR with charybdotoxin elicited a concentration-dependent contraction, whereas the arterial segments from normotensive WKY rats did not contract [163]. Subsequent studies using the patch clamp technique provided additional evidence regarding increased BK channel function in VSMCs during hypertension. In agreement with vessel reactivity studies, results published from several laboratories indicate that compared to control, whole cell K+ current through iberiotoxin-sensitive BK channels is elevated in VSMCs from aorta of SHR, stroke-prone SHR, and rats with renal hypertension [150,151,153,154,161]. Additionally, myocytes from mesenteric arteries of DOCA salt-induced hypertensive rats also exhibited elevated iberiotoxin-sensitive K+ current compared to myocytes from Sham animals [165]. In fact, the levels of whole cell K+ current through BK channels in VSMCs is positively correlated to blood pressure in hypertensive animals [152]. Lowering of blood pressure in SHR by the angiotensin-converting enzyme inhibitor ramipril normalized elevated BK current densities, in addition to abolishing TEA-induced contraction of aortic segments from SHR [152]. Single channel studies done by England et al clearly demonstrate that the accentuated iberiotoxin-sensitive K+ current seen in VSMCs of SHR is not a result of altered BK channel conductance, but rather is due to an increase in the number of BK channel openings [150]. Ultimately, in situ studies of cerebral arterioles from hypertensive rats provided additional evidence for upregulation of vascular BK channel function [155]. In this study, when iberiotoxin (10 nM) was topically applied to pial arterioles in situ by a cranial window preparation, arterioles from SHR constricted more compared to arterioles from normotensive WKY rats. Thus, the upregulation of vascular BK channel function seems to act as an adaptive mechanism to diminish elevated vascular excitability and vasospastic episodes in microcirculatory beds during hypertension, and thus ward off ischemic events of critical organs.
Biochemical and molecular biological studies further explored whether an increase in BK channel function in arteries during hypertension is due to elevated expression of the pore-forming subunit of the channel. Immunoblot analyses revealed that the densities of the 125-kDa immunoreactive band that represent the pore-forming α subunit is at least 2–3 times elevated in aorta of SHR compared to the normotensive WKY rats [154]. The expression of the pore-forming α subunit of BK channel is also increased in microcirculatory beds of SHR such the cerebral, mesenteric and coronary beds [11]. However, there is a scarcity of information regarding gene expression of the pore-forming α subunit of the BK channel in arteries during hypertension. Initial studies done in this regard suggest that the mRNA level of the α subunit of the BK channel is unchanged in aorta of SHR compared to WKY rats [154]. Thus, post transcriptional modifications and protein stability of the pore-forming α subunit of the BK channel, and/or its association with auxiliary regulatory proteins may play an important role in the increased functional expression of vascular BK channels during hypertension.
Even though the majority of the studies point to increased functional expression of BK channels in vasculature during hypertension, it is important mention that there are at least 3 studies to our knowledge that report decreased BK channel function in resistance arteries during hypertension [156–158]. Decreased BK channel current was reported in VSMCs of cerebral and mesenteric arteries from SHR and angiotensin II-infused hypertensive mice compared to normotensive Sprague Dawley rats and saline infused mice, respectively [156,157]. Similarly, compared to control rats, cerebral arteries from angiotensin II-infused hypertensive Sprague Dawley rats constricted less to iberiotoxin in isolated vessel reactivity studies [158]. The decreased BK channel function in these studies was explained as an effect of reduced mRNA expression of the ancillary BK β1 subunit due to the activation of calcineurin/NFATc3 signaling pathway by elevated circulating angiotensin II [156] . As mentioned before, BK β1 subunit confers Ca2+ sensitivity to BK channels, and mediates the coupling of Ca2+ sparks to BK channel activation [166]. Hence, reduced expression of the BK β1 subunit would uncouple BK channels from L-type Ca2+ channels that produce Ca2+ sparks in VSMCs, resulting in disruption of the BK channel-mediated homeostatic mechanism to counteract myogenic constriction during higher intraluminal pressure. Accordingly, using acute blood pressure measurement techniques such as carotid arterial catheterization and tail cuff plethysmography, the BK β1 knock out mice were initially reported to have a 10–20 mmHg increase in mean arterial pressure compared to wild-type age-matched controls [166–169]. However, a recent report using radiotelemetry to continuously measure blood pressure demonstrated that the 24-hour mean arterial pressure was similar between wild-type and BK β1 knock out mice [170]. Thus, more studies are necessary to further elucidate the role of the ancillary BK β1 subunit in the vasculature during hypertension. To add further complexity to the subject, intriguingly, the expression pattern of the ancillary BK β1 subunit differs from that of the pore-forming α subunit in the vasculature during hypertension. For example, reduced mRNA expression of the β1 subunit in cerebral and mesenteric arteries from SHR and angiotensin II-infused hypertensive mice was accompanied by no change in the expression of BK channel pore-forming α subunit mRNA in these animals [156–158]. Collectively, the information available from studies thus far points to the intricacies associated with the expression and regulation of vascular BK channels during hypertension.
4. Vascular ion channels as antihypertensive targets
The prevalence of hypertension among adults in the United States is approximately 30% and increases with age to nearly 70% among adults 65 years and older [171]. Pharmacologic treatment and control of blood pressure has increased in the last 10 years [172]. There are currently more than one hundred molecules approved for the treatment of hypertension, however only one third of treated patients have their blood pressure normalized [172,173]. This is mainly due to low patient compliance to drug regimens, which require daily dosing and often multi-drug therapy [174,175]. Using gene therapy to control vascular tone by altering the expression and function of ion channels is an approach that could reduce the impact of patient adherence by avoiding the need for daily drug administration and reducing side effects associated with the non-specific effects of small molecule drugs [173,176]. This also avoids blood pressure fluctuations caused by short drug half-lives and inconsistent patient dosing intervals [176]. This approach has been demonstrated in proof of concept studies targeting CaV1.2, KV1.5 and BK channels using knockdowns and virally mediated gene delivery of altered channel subunits [169,173,176–179]. Genetic epidemiological studies of BK subunit mutations also lend support to gene-targeted therapies for hypertension [180,181].
Calcium channels have been the main vascular ion channels to be directly targeted by drugs in the treatment of hypertension. Calcium channel blockers (CCB) have been extensively used for the control of hypertension for over two decades and were the most frequently prescribed antihypertensive as late as the 1990’s [182]. However, the usefulness of CCB is complicated by their contraindications and some side effects [183]. CCBs, especially non-dihydropyridines, are contraindicated in patients with heart failure because of potential adverse effects such as atrioventricular nodal block and depressed contractility [183]. Tachycardia and edema are common side effects of some CCB including dihydropyridines. The dihydropyridines that selectively block vascular CaV1.2 channels have less direct action on the heart and cause reflex tachycardia through increase sympathetic activity due to the rapid onset of channel blockade [176]. Edema of the lower extremities, particularly the ankles, is thought to result from venous resistance to CCB-induced dilation [184]. CCBs are often given in combination with other classes of antihypertensive drugs to achieve an adequate level of blood pressure control through synergistic effects and to counteract side-effects of each component. CCB-induced reflex tachycardia is reduced by the addition of a β-adrenergic receptor blocker. CCBs are often paired with an angiotensin receptor blockers (ARB) or angiotensin converting enzyme inhibitors (ACEI) and are common multi-drug fixed dose pill formulations. The sympathetic activation of the renin system induced by CCBs can be buffered by these drugs while the reduced sodium balance produced by the CCBs augment the antihypertensive effects of ARB and ACEI [185]. Several studies suggest that these antihypertensive drugs may affect vascular ion channel expression independent of their effect on blood pressure through mitigating Ang II levels. Cox et al. used ramipril, an ACEI, and showed inihibition of Ang II leads to downregulation of Ca2+ and K+ channels in mesenteric VSMCs in WKY and SHR [118]. A study involving aldosterone overexpressing transgenic mice showed aldosterone induced a concentration-dependent decrease in both BK-α and BK-β1 subunit mRNA levels suggesting the benefits of aldosterone antagonist treatment may be partially mediated through regulation of BK channel expression in VSMCs [186].
Gene therapy-based treatments aimed at reducing abnormal expression of L-type calcium channels in VSMCs may be a new way to treat hypertension with higher vascular selectivity and fewer side effects than attempting to block Ca2+ influx with CCB [176]. Several studies targeting CaV1.2 serve as proof of concept for gene therapy approach to hypertension. Marsh and Telemaque used a gene therapy approach designed to reduce the availability of β2-subunits required for CaV1.2 expression at the membrane. They conducted experiments using adenovirus delivery to establish dominant-negative mutated β2-subunits of the L-type calcium channel [173,177]. Recombinant adenovirus expressing the full or C- / N- terminus truncated β2-subunits were over expressed in HL-1 cells and used as α1C chaperon decoys [177]. The dominant-negative function demonstrated in HL-1 cells illustrated that the β2-subunit is a feasible molecular target for reducing the number of functional CaV1.2 channels in cardiomyocytes [177]. Studies focusing on reducing CaV1.2 expression selectively in noncardiac VSMCs have been conducted. Using a vascular smooth muscle specific enhancer/promoter, a viral vector mediated shRNA gene treatment selectively reduced the expression of CaV1.2 in A7r5 VSMCs with little effect in non-VSMCs including cultured cardiomyocytes [176]. Although siRNA is not a feasible long-term therapy due to its transient nature and systemic delivery, these studies demonstrate the proof of concept that vascular CaV1.2 expression and function can be selectively reduced in an aim to treat hypertension long-term without affecting cardiac CaV1.2 [176]. Bannister et al. targeted CaV1.2 e1b and CaV1.2 e1c subunit splice variants with shRNAs. They demonstrated in rat cerebral artery smooth muscle cells both CaV1.2 splice variants are located in the plasma membrane and shRNAs targeting each splice variant selectively reduces their expression. These knockdowns resulted in a decrease in CaV1.2 protein leading to vasodilation and reduced CaV1.2 currents suggesting exon 1 of CaV1.2 could be a novel antihypertensive target. Additionally, knockdowns of the α2δ subunit, which inserts both variants into the VSMC plasma membrane, reduce the surface expression of both CaV1.2 variants and currents making it an additional subunit target for gene therapy [178]. In particular, as CaV β3 knockout mice showed a blunted increase in blood pressure in response to angiotensin II infusion, CaV β3 subunits may also be targeted for potential antihypertensive therapy [30].
Potassium channels are another target for gene therapy for hypertension, especially for pulmonary hypertension. In chronic hypoxic pulmonary hypertension, the loss of KV1.5 expression appears to be important to the pathogenesis. Studies in rodent models of hypoxia relevant to pulmonary hypertension in humans demonstrated reduced pulmonary hypertension despite continued hypoxia upon administration of cloned Kv1.5 from human pulmonary arteries. The augmentation of KV1.5 was achieved by gene transfer to the pulmonary circulation via an adenovirus vector aerosol [179]. Large conductance potassium channels (BK) present another possible target for hypertensive gene therapy due to their proposed role as negative feedback regulators of vascular tone. In smooth muscle, an increase in BK channel activity is induced by local release of Ca2+ from the SR. This leads to K+ efflux and hyperpolarization of the membrane closing L-type calcium channels and preventing further influx of Ca2+ [180]. BKβ1 subunits confer increased sensitivity to membrane potential and Ca2+ level changes to BK channels. The potential of BK channels as a target for gene therapy can be seen in knockout animal studies and human epidemiological studies.
The in vivo function of the BK β subunit has been assessed in knockout mice with a disrupted BKβ1 gene. BKβ1−/− mice have abnormal Ca2+ spark/spontaneous transient outward K+ current coupling which is shifted to more depolarized potentials. The elevated blood pressure seen between BKβ1−/− and +/+ mice were maintained over a wide range of blood pressure levels and different genetic backgrounds suggesting that the formation of heteromultimeric BKα and BKβ1 subunits is fundamental to the BK channel’s negative feedback role in vascular tone [169]. Several genetic epidemiological studies have implicated variations in BK subunits in the prevalence of certain forms of hypertension. A single nucleotide substitution in the β1 gene (KCNMB1), corresponding to an E65K mutation in the protein conferred a gain of function correlated with low prevalence of diastolic hypertension. The mutation showed increased Ca2+ sensitivity compared to wild-type without changes in channel kinetics [180]. The effect of this mutation is sexually dimorphic, protecting women and is influenced by estradiol and age. The genetic evidence for the different impact of the BK channel in the control blood pressure points to the E65K polymorphism as one of the strongest genetic factors correlated with reduced incidence of myocardial infarction and stroke [181]. The gain of function of the E65K polymorphism and reduced prevalence of diastolic hypertension suggests gene therapy modification of the BK β1 subunit as a potential target for essential hypertension.
As described above, evidence for the role of ion channel subunits in modulating blood pressure and proof-of-concept experiments demonstrating targeted modification of ion channel expression and function have laid a basic foundation for gene therapy treatment of hypertension. Future endeavors must resolve problems of efficacy and safety inherent in gene therapy. An ideal gene therapy vector would have attributes which include high titer, immunologically inert, sustained or regulatable expression, size capacity to accommodate the largest genes plus regulatory regions, cell type-specific targeting and the ability to transduce non-dividing post-mitotic cells. A final high value property is site specific chromosome integration. This allows faithful replication and segregation of the gene therapy vector during cell division, eliminates the uncertainty of random integration and allows endogenous regulatory regions to control expression under physiological conditions [187]. Limitations and safety concerns arise from deviation from this ideal. Low titer and low expression of transgenes can result in therapeutic failure while replication in non-target cells, immune activation, insertion mutagenesis and latent reactivation of the vector represent serious safety concerns [187,188]. Adeno-associated virus (AAV) in particular seems to avoid most of these safety concerns and is used in several preclinical or clinical trials [189]. Low titer of AAV and low gene transduction in vascular tissue are major roadblocks in using AAV for a systemic gene transduction in VSMCs. Modification of AAV tropism to target VSMC may help in achieving adequate expression for therapeutic efficacy [190].
Some studies discussed in this review utilized targeted gene knockout mice [30,169]. In general, care must be taken in interpreting results from knockout mice. For example, genetic background of the mice, interference by a selection marker gene, flanking genes or compensatory genetic loci may all influence the final phenotype in addition to the deleted gene [191,192]. Also, since hypertension is a disease with complex etiologies involving multiple genes and environmental factors, it is difficult to distinguish a direct causation between a gene and the disease from a mere correlation [193]. Furthermore, in global knockout mice where the target gene is removed from all tissues, developmental changes and the effects from tissues other than VSMCs, such as cardiomyocytes or neurons, may well contribute to the observed phenotypes. In this regard, smooth muscle-selective knockouts [194,195] or inducible, smooth muscle-selective knockouts [196] are available and may provide a cleaner picture of the complex mechanisms involved in hypertension.
5. Conclusion
The vasculature is an important part of blood pressure regulation. As outlined in this review, evidence suggests that ion channel subunits play an important role in modulating blood pressure and that the expression of those subunits change during hypertension, perhaps as a cause/effect and also as a compensatory mechanism. We have limited ourselves to considering three channel types, CaV1.2, KV, and BK channels. There is a therapeutic potential for antihypertensive therapy targeting subunits or associated proteins of these channels pharmacologically or at the gene level. A better understanding of their regulation in hypertension and the interaction with associated proteins will guide our endeavor in utilizing these ion channels for therapeutic purposes.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health, F32 HL095284-03 (KMT) and 1R01-HL097107 (SWR) and by American Heart Association grant SDG 0830060N (SWR).
Abbreviations
- AAV
adeno-associated virus
- ACEI
angiotensin converting enzyme inhibitors
- AKAP
A-kinase anchoring protein
- ARB
angiotensin receptor blockers
- BK
large-conductance, Ca2+-activated K+ channel
- CaV1.2
L-type Ca2+ channel
- CCB
calcium channel blocker
- CO
cardiac output
- DOCA
deoxycorticosterone acetate
- Em
membrane potential
- IP3R
inositol 1,4,5-trisphosphate receptor
- KATP
ATP-sensitive K+ channel
- KChAP
K+ channel accessory protein
- KIR
inward-rectifying K+ channel
- KV
voltage-gated K+ channel
- PAH
pulmonary arterial hypertension
- PKA
protein kinase A
- PKC
protein kinase C
- RyR
ryonodine receptor
- ScTx
stromatoxin
- SHR
spontaneously hypertensive rat
- TEA
tetraethylammonium
- TPR
total peripheral resistance
- TRP
transient receptor potential
- VSMC
vascular smooth muscle cell
- WKY
Wistar-Kyoto rat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Biny K. Joseph, Email: bjoseph@venenumbiodesign.com.
Keshari M. Thakali, Email: kmthakali@uams.edu.
Christopher L. Moore, Email: cmoore3@uams.edu.
Sung W. Rhee, Email: rheesung@uams.edu.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK. Epidemiology of hypertension. Lancet. 1994;344:101–106. doi: 10.1016/s0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 5.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 6.Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 7.Blaustein MP. Endogenous ouabain: Role in the pathogenesis of hypertension. Kidney Int. 1996;49:1748–1753. doi: 10.1038/ki.1996.260. [DOI] [PubMed] [Google Scholar]
- 8.Beevers G, Lip GYH, O'Brien E. The pathophysiology of hypertension. BMJ. 2001;322:912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safar ME, Chau NP, Weiss YA, London GM, Milliez PL. Control of cardiac output in essential hypertension. The American Journal of Cardiology. 1976;38:332–336. doi: 10.1016/0002-9149(76)90175-2. [DOI] [PubMed] [Google Scholar]
- 10.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology - Cell Physiology. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 11.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 12.Harder DR, Brann L, Halpern W. Altered membrane electrical properties of smooth muscle cells from small cerebral arteries of hypertensive rats. Blood Vessels. 1983;20:154–160. doi: 10.1159/000158469. [DOI] [PubMed] [Google Scholar]
- 13.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph BK, Rhee SW, Hirenallur DK, Rusch NJ. Potassium channels in vascular smooth muscle: structure, function and experimental intervention. In: Savineau J-P, editor. New Frontiers in Smooth Muscle Biology and Physiology. Trivandrum, Kerala, India: Transworld Research Network; 2007. pp. 173–194. [Google Scholar]
- 15.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 16.Chrissobolis S, Sobey CG. Inwardly rectifying potassium channels in the regulation of vascular tone. Curr Drug Targets. 2003;4:281–289. doi: 10.2174/1389450033491046. [DOI] [PubMed] [Google Scholar]
- 17.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 18.Quayle JM, Standen NB. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994;28:797–804. doi: 10.1093/cvr/28.6.797. [DOI] [PubMed] [Google Scholar]
- 19.Goonetilleke L, Quayle J. TREK-1 K+ channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc Ther. 2012;30:e23–e29. doi: 10.1111/j.1755-5922.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 20.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 21.Thakali KM, Pathan AR, Kharade SV, Rusch NJ. Potassium, sodium and chloride channels in arterial smooth muscle cells. In: Hill J, editor. Muscle: Fundamental biology and mechanisms of disease. Elsevier; 2012. pp. 1133–1143. [Google Scholar]
- 22.Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel beta subunits. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- 23.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 24.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 25.Hanlon MR, Berrow NS, Dolphin AC, Wallace BA. Modelling of a voltage-dependent Ca2+ channel beta subunit as a basis for understanding its functional properties. FEBS Lett. 1999;445:366–370. doi: 10.1016/s0014-5793(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci U S A. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimer D, Huber IG, Garcia ML, Haase H, Striessnig J. βsubunit heterogeneity of L-type Ca2+ channels in smooth muscle tissues. FEBS Lett. 2000;467:65–69. doi: 10.1016/s0014-5793(00)01124-8. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Yamamura H, Suzuki T, Kang MG, Ohya S, Murakami A, Miyoshi I, Sasano H, Muraki K, Hano T, Kasai N, Nakayama S, Campbell KP, Flockerzi V, Imaizumi Y, Yanagisawa T, Iijima T. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel beta3 subunit. J Biol Chem. 2003;278:43261–43267. doi: 10.1074/jbc.M211380200. [DOI] [PubMed] [Google Scholar]
- 29.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, Flockerzi V. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. Embo J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharade SV, Sonkusare SK, Srivastava AK, Thakali KM, Fletcher TW, Rhee SW, Rusch NJ. The beta3 Subunit Contributes to Vascular Calcium Channel Upregulation and Hypertension in Angiotensin II-Infused C57BL/6 Mice. Hypertension. 2013;61:137–142. doi: 10.1161/HYPERTENSIONAHA.112.197863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckert R, Ewald D. Residual calcium ions depress activation of calcium-dependent current. Science. 1982;216:730–733. doi: 10.1126/science.6281880. [DOI] [PubMed] [Google Scholar]
- 33.Ganitkevich V, Shuba MF, Smirnov SV. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morad M, Soldatov N. Calcium channel inactivation: possible role in signal transduction and Ca2+ signaling. Cell Calcium. 2005;38:223–231. doi: 10.1016/j.ceca.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 38.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 40.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 42.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 43.Schuhmann K, Romanin C, Baumgartner W, Groschner K. Intracellular Ca2+ inhibits smooth muscle L-type Ca2+ channels by activation of protein phosphatase type 2B and by direct interaction with the channel. J Gen Physiol. 1997;110:503–513. doi: 10.1085/jgp.110.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cribbs L. T-type calcium channel expression and function in the diseased heart. Channels (Austin) 2010;4:447–452. doi: 10.4161/chan.4.6.12870. [DOI] [PubMed] [Google Scholar]
- 45.Ono K, Iijima T. Cardiac T-type Ca2+ channels in the heart. J Mol Cell Cardiol. 2010;48:65–70. doi: 10.1016/j.yjmcc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, Schwartz A, Herzig S. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating. Biophys J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu PJ, Best PM. Molecular cloning of calcium channel alpha(2)delta-subunits from rat atria and the differential regulation of their expression by IGF-1. J Mol Cell Cardiol. 2003;35:207–215. doi: 10.1016/s0022-2828(02)00313-9. [DOI] [PubMed] [Google Scholar]
- 49.Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L, Katchman A, Morrow JP, Doshi D, Marx SO. Cardiac L-type calcium channel (CaV1.2) associates with gamma subunits. FASEB J. 2011;25:928–936. doi: 10.1096/fj.10-172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nie HG, Hao LY, Xu JJ, Minobe E, Kameyama A, Kameyama M. Distinct roles of CaM and Ca2+/CaM - dependent protein kinase II in Ca2+-dependent facilitation and inactivation of cardiac L-type Ca2+ channels. J Physiol Sci. 2007;57:167–173. doi: 10.2170/physiolsci.RP000507. [DOI] [PubMed] [Google Scholar]
- 52.Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 54.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104:1382–1389. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric KV1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96:216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- 59.Amberg GC, Santana LF. KV2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–C356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 60.Tammaro P, Smith AL, Hutchings SR, Smirnov SV. Pharmacological evidence for a key role of voltage-gated K+ channels in the function of rat aortic smooth muscle cells. Br J Pharmacol. 2004;143:303–317. doi: 10.1038/sj.bjp.0705957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 62.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- 63.Coussin F, Scott RH, Nixon GF. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- 64.Park WS, Son YK, Han J, Kim N, Ko JH, Bae YM, Earm YE. Staurosporine inhibits voltage-dependent K+ current through a PKC-independent mechanism in isolated coronary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2005;45:260–269. doi: 10.1097/01.fjc.0000154370.57789.fe. [DOI] [PubMed] [Google Scholar]
- 65.Ko EA, Park WS, Firth AL, Hong da H, Choi SW, Heo HJ, Kim MH, Noh JH, Ko JH, Kim N, Earm YE, Song DK, Han J. Increased sensitivity of serotonin on the voltage-dependent K+ channels in mesenteric arterial smooth muscle cells of OLETF rats. Prog Biophys Mol Biol. 2010;103:88–94. doi: 10.1016/j.pbiomolbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 67.Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:368–376. doi: 10.1124/jpet.108.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mani BK, Brueggemann LI, Cribbs LL, Byron KL. Activation of vascular KCNQ (KV7) potassium channels reverses spasmogen-induced constrictor responses in rat basilar artery. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (KV7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588:3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162:42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 74.Fergus DJ, Martens JR, England SK. KV channel subunits that contribute to voltage-gated K+ current in renal vascular smooth muscle. Pflugers Arch. 2003;445:697–704. doi: 10.1007/s00424-002-0994-7. [DOI] [PubMed] [Google Scholar]
- 75.Platoshyn O, Remillard CV, Fantozzi I, Mandegar M, Sison TT, Zhang S, Burg E, Yuan JX. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L226–L238. doi: 10.1152/ajplung.00438.2003. [DOI] [PubMed] [Google Scholar]
- 76.Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- 77.Joseph BK, Thakali KM, Pathan AR, Kang E, Rusch NJ, Rhee SW. Postsynaptic density-95 scaffolding of Shaker-type K+ channels in smooth muscle cells regulates the diameter of cerebral arteries. J Physiol. 2011;589:5143–5152. doi: 10.1113/jphysiol.2011.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archer SL, Rusch NJ. Potassium channels in cardiovascular biology. xlv. New York: Kluwer Academic/Plenum; 2001. 899 pp. [Google Scholar]
- 79.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 80.Balse E, Steele DF, Abriel H, Coulombe A, Fedida D, Hatem SN. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol Rev. 2012;92:1317–1358. doi: 10.1152/physrev.00041.2011. [DOI] [PubMed] [Google Scholar]
- 81.Pongs O. Ins and outs of cardiac voltage-gated potassium channels. Curr Opin Pharmacol. 2009;9:311–315. doi: 10.1016/j.coph.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- 86.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- 88.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 89.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 90.Kim CJ, Weir BK, Macdonald RL, Zhang H. Erythrocyte lysate releases Ca2+ from IP3-sensitive stores and activates Ca2+-dependent K+ channels in rat basilar smooth muscle cells. Neurol Res. 1998;20:23–30. doi: 10.1080/01616412.1998.11740480. [DOI] [PubMed] [Google Scholar]
- 91.Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. The Journal of general physiology. 2010;136:283–291. doi: 10.1085/jgp.201010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BKCa channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 93.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 94.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 96.Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 98.Wu RS, Marx SO. The BK potassium channel in the vascular smooth muscle and kidney: alpha- and beta-subunits. Kidney Int. 2010;78:963–974. doi: 10.1038/ki.2010.325. [DOI] [PubMed] [Google Scholar]
- 99.Zhu S, White RE, Barman SA. Effect of PKC isozyme inhibition on forskolin-induced activation of BKCa channels in rat pulmonary arterial smooth muscle. Lung. 2006;184:89–97. doi: 10.1007/s00408-005-2567-y. [DOI] [PubMed] [Google Scholar]
- 100.Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1275–L1281. doi: 10.1152/ajplung.00259.2003. [DOI] [PubMed] [Google Scholar]
- 101.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- 102.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel beta-subunit genes: cloning and characterization. Genomics. 1999;55:57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- 104.Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–33609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- 105.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 106.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 107.Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. A role for BK channels in heart rate regulation in rodents. PLoS One. 2010;5:e8698. doi: 10.1371/journal.pone.0008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology - Cell Physiology. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 109.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 110.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 111.Heistad DD, Mayhan WG, Coyle P, Baumbach GL. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels. 1990;27:258–262. doi: 10.1159/000158817. [DOI] [PubMed] [Google Scholar]
- 112.Heistad DD. Protection of the blood-brain barrier during acute and chronic hypertension. Fed Proc. 1984;43:205–209. [PubMed] [Google Scholar]
- 113.Hayashi K, Epstein M, Saruta T. Altered myogenic responsiveness of the renal microvasculature in experimental hypertension. J Hypertens. 1996;14:1387–1401. doi: 10.1097/00004872-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 114.Jones AW. Reactivity of ion fluxes in rat aorta during hypertension and circulatory control. Fed Proc. 1974;33:133–137. [PubMed] [Google Scholar]
- 115.Jones AW, Hart RG. Altered ion transport in aortic smooth muscle during deoxycorticosterone acetate hypertension in the rat. Circ Res. 1975;37:333–341. doi: 10.1161/01.res.37.3.333. [DOI] [PubMed] [Google Scholar]
- 116.Harder DR, Smeda J, Lombard J. Enhanced myogenic depolarization in hypertensive cerebral arterial muscle. Circ Res. 1985;57:319–322. doi: 10.1161/01.res.57.2.319. [DOI] [PubMed] [Google Scholar]
- 117.Lozinskaya IM, Cox RH. Effects of age on Ca2+ currents in small mesenteric artery myocytes from Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1997;29:1329–1336. doi: 10.1161/01.hyp.29.6.1329. [DOI] [PubMed] [Google Scholar]
- 118.Cox RH, Lozinskaya I, Matsuda K, Dietz NJ. Ramipril treatment alters Ca2+ and K+ channels in small mesenteric arteries from Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens. 2002;15:879–890. [PubMed] [Google Scholar]
- 119.Simard JM, Li X, Tewari K. Increase in functional Ca2+ channels in cerebral smooth muscle with renal hypertension. Circ Res. 1998;82:1330–1337. doi: 10.1161/01.res.82.12.1330. [DOI] [PubMed] [Google Scholar]
- 120.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–e104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 121.Cox RH, Lozinskaya IM. Augmented calcium currents in mesenteric artery branches of the spontaneously hypertensive rat. Hypertension. 1995;26:1060–1064. doi: 10.1161/01.hyp.26.6.1060. [DOI] [PubMed] [Google Scholar]
- 122.Ohya Y, Abe I, Fujii K, Takata Y, Fujishima M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res. 1993;73:1090–1099. doi: 10.1161/01.res.73.6.1090. [DOI] [PubMed] [Google Scholar]
- 123.Hirenallur SD, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L915–L924. doi: 10.1152/ajplung.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ohya Y, Tsuchihashi T, Kagiyama S, Abe I, Fujishima M. Single L-type calcium channels in smooth muscle cells from resistance arteries of spontaneously hypertensive rats. Hypertension. 1998;31:1125–1129. doi: 10.1161/01.hyp.31.5.1125. [DOI] [PubMed] [Google Scholar]
- 125.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 126.Wang WZ, Saada N, Dai B, Pang L, Palade P. Vascular-specific increase in exon 1B-encoded CAV1.2 channels in spontaneously hypertensive rats. Am J Hypertens. 2006;19:823–831. doi: 10.1016/j.amjhyper.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 127.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 128.Herlitze S, Xie M, Han J, Hümmer A, Melnik-Martinez KV, Moreno RL, Mark MD. Targeting mechanisms of high voltage-activated Ca2+ channels. Journal of Bioenergetics and Biomembranes. 2003;35:621–637. doi: 10.1023/b:jobb.0000008027.19384.c0. [DOI] [PubMed] [Google Scholar]
- 129.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- 131.Cox RH, Petrou S. Ca2+ influx inhibits voltage-dependent and augments Ca2+-dependent K+ currents in arterial myocytes. Am J Physiol. 1999;277:C51–C63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- 132.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 134.Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens. 2001;14:897–907. doi: 10.1016/s0895-7061(01)02145-8. [DOI] [PubMed] [Google Scholar]
- 135.Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- 136.Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K+ channel proteins in vascular smooth muscle from rats made hypertensive with N-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1277–H1283. doi: 10.1152/ajpheart.01052.2004. [DOI] [PubMed] [Google Scholar]
- 137.Bratz IN, Swafford AN, Jr, Kanagy NL, Dick GM. Reduced functional expression of K+ channels in vascular smooth muscle cells from rats made hypertensive with N-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1284–H1290. doi: 10.1152/ajpheart.01053.2004. [DOI] [PubMed] [Google Scholar]
- 138.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular Shaker-type K+ channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297:H293–H303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates KV2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 140.Jahromi BS, Aihara Y, Ai J, Zhang ZD, Nikitina E, Macdonald RL. Voltage-gated K+ channel dysfunction in myocytes from a dog model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008;28:797–811. doi: 10.1038/sj.jcbfm.9600577. [DOI] [PubMed] [Google Scholar]
- 141.Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. Downregulation of KV7.4 channel activity in primary and secondary hypertension. Circulation. 2011;124:602–611. doi: 10.1161/CIRCULATIONAHA.111.032136. [DOI] [PubMed] [Google Scholar]
- 142.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 2012;59:877–884. doi: 10.1161/HYPERTENSIONAHA.111.187427. [DOI] [PubMed] [Google Scholar]
- 143.Cox RH, Fromme SJ, Folander KL, Swanson RJ. Voltage gated K+ channel expression in arteries of Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens. 2008;21:213–218. doi: 10.1038/ajh.2007.44. [DOI] [PubMed] [Google Scholar]
- 144.Cox RH, Folander K, Swanson R. Differential expression of voltage-gated K+ channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37:1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- 145.Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of KV6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol. 2009;587:625–640. doi: 10.1113/jphysiol.2008.165217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric KV2.1/KV9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588:4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- 148.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits KV channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 149.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 150.England SK, Wooldridge TA, Stekiel WJ, Rusch NJ. Enhanced single-channel K+ current in arterial membranes from genetically hypertensive rats. Am J Physiol. 1993;264:H1337–H1345. doi: 10.1152/ajpheart.1993.264.5.H1337. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, Jones AW, Sturek M. Increased barium influx and potassium current in stroke-prone spontaneously hypertensive rats. Hypertension. 1994;23:1091–1095. doi: 10.1161/01.hyp.23.6.1091. [DOI] [PubMed] [Google Scholar]
- 152.Rusch NJ, Runnells AM. Remission of high blood pressure reverses arterial potassium channel alterations. Hypertension. 1994;23:941–945. doi: 10.1161/01.hyp.23.6.941. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, Jones AW, Sturek M. Ca2+-dependent K+ current in arterial smooth muscle cells from aldosterone-salt hypertensive rats. Am J Physiol. 1995;269:H1246–H1257. doi: 10.1152/ajpheart.1995.269.4.H1246. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 155.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- 156.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 157.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 158.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones AW. Altered ion transport in vascular smooth muscle from spontaneously hypertensive rats. Influences of aldosterone, norepinephrine, and angiotensin. Circ Res. 1973;33:563–572. doi: 10.1161/01.res.33.5.563. [DOI] [PubMed] [Google Scholar]
- 160.Smith JM, Jones AW. Calcium antagonists inhibit elevated potassium efflux from aorta of aldosterone-salt hypertensive rats. Hypertension. 1990;15:78–83. doi: 10.1161/01.hyp.15.1.78. [DOI] [PubMed] [Google Scholar]
- 161.Rusch NJ, De Lucena RG, Wooldridge TA, England SK, Cowley AW., Jr A Ca2+-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992;19:301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- 162.Asano M, Masuzawa-Ito K, Matsuda T, Imaizumi Y, Watanabe M, Ito K. Functional role of Ca2+-activated K+ channels in resting state of carotid arteries from SHR. Am J Physiol. 1993;265:H843–H851. doi: 10.1152/ajpheart.1993.265.3.H843. [DOI] [PubMed] [Google Scholar]
- 163.Asano M, Masuzawa-Ito K, Matsuda T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol. 1993;108:214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kolias TJ, Chai S, Webb RC. Potassium channel antagonists and vascular reactivity in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 1993;6:528–533. doi: 10.1093/ajh/6.6.528. [DOI] [PubMed] [Google Scholar]
- 165.Xu H, Bian X, Watts SW, Hlavacova A. Activation of vascular BK channel by tempol in DOCA-salt hypertensive rats. Hypertension. 2005;46:1154–1162. doi: 10.1161/01.HYP.0000186278.50275.fa. [DOI] [PubMed] [Google Scholar]
- 166.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 167.Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKbeta1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol. 2009;297:F420–F428. doi: 10.1152/ajprenal.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 170.Xu H, Garver H, Galligan JJ, Fink GD. Large-conductance Ca2+-activated K+ channel beta1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol. 2011;300:H476–H485. doi: 10.1152/ajpheart.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yoon PW, Gillespie CD, George MG, Wall HK. Control of hypertension among adults--National Health and Nutrition Examination Survey, United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):19–25. [PubMed] [Google Scholar]
- 172.Vital signs: prevalence, treatment, and control of hypertension--United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–108. [PubMed] [Google Scholar]
- 173.Marsh JD, Telemaque S, Rhee SW, Stimers JR, Rusch NJ. Delivery of ion channel genes to treat cardiovascular diseases. Trans Am Clin Climatol Assoc. 2008;119:171–182. discussion 182–173. [PMC free article] [PubMed] [Google Scholar]
- 174.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 175.Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007;15:257–263. doi: 10.1097/CRD.0b013e3180cabbe7. [DOI] [PubMed] [Google Scholar]
- 176.Rhee SW, Stimers JR, Wang W, Pang L. Vascular smooth muscle-specific knockdown of the noncardiac form of the L-type calcium channel by microRNA-based short hairpin RNA as a potential antihypertensive therapy. J Pharmacol Exp Ther. 2009;329:775–782. doi: 10.1124/jpet.108.148866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Telemaque S, Sonkusare S, Grain T, Rhee SW, Stimers JR, Rusch NJ, Marsh JD. Design of mutant beta2 subunits as decoy molecules to reduce the expression of functional Ca2+ channels in cardiac cells. J Pharmacol Exp Ther. 2008;325:37–46. doi: 10.1124/jpet.107.128215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bannister JP, Thomas-Gatewood CM, Neeb ZP, Adebiyi A, Cheng X, Jaggar JH. CaV1.2 channel N-terminal splice variants modulate functional surface expression in resistance size artery smooth muscle cells. J Biol Chem. 2011;286:15058–15066. doi: 10.1074/jbc.M110.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel KV1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 180.Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Senti M, Fernandez-Fernandez JM, Tomas M, Vazquez E, Elosua R, Marrugat J, Valverde MA. Protective effect of the KCNMB1 E65K genetic polymorphism against diastolic hypertension in aging women and its relevance to cardiovascular risk. Circ Res. 2005;97:1360–1365. doi: 10.1161/01.RES.0000196557.93717.95. [DOI] [PubMed] [Google Scholar]
- 182.Manolio TA, Cutler JA, Furberg CD, Psaty BM, Whelton PK, Applegate WB. Trends in pharmacologic management of hypertension in the United States. Arch Intern Med. 1995;155:829–837. [PubMed] [Google Scholar]
- 183.Eisenberg MJ, Brox A, Bestawros AN. Calcium channel blockers: an update. Am J Med. 2004;116:35–43. doi: 10.1016/j.amjmed.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 184.Thakali KM, Kharade SV, Sonkusare SK, Rhee SW, Stimers JR, Rusch NJ. Intracellular Ca2+ silences L-type Ca2+ channels in mesenteric veins: mechanism of venous smooth muscle resistance to calcium channel blockers. Circulation research. 2010;106:739–747. doi: 10.1161/CIRCRESAHA.109.206763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kalra S, Kalra B, Agrawal N. Combination therapy in hypertension: An update. Diabetol Metab Syndr. 2010;2:44. doi: 10.1186/1758-5996-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, Samuel JL, Richard V, Delcayre C. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- 187.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 188.Tomanin R, Scarpa M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr Gene Ther. 2004;4:357–372. doi: 10.2174/1566523043346011. [DOI] [PubMed] [Google Scholar]
- 189.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- 191.Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol. 2009;530:423–433. doi: 10.1007/978-1-59745-471-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thyagarajan T, Totey S, Danton MJ, Kulkarni AB. Genetically altered mouse models: the good, the bad,and the ugly. Crit Rev Oral Biol Med. 2003;14:154–174. doi: 10.1177/154411130301400302. [DOI] [PubMed] [Google Scholar]
- 193.Smithies O. Quantitative genetic variations and essential hypertension. Harvey Lect. 1999;95:1–20. [PubMed] [Google Scholar]
- 194.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Regan CP, Manabe I, Owens GK. Development of a smooth muscle-targeted cre recombinase mouse reveals novel insights regarding smooth muscle myosin heavy chain promoter regulation. Circ Res. 2000;87:363–369. doi: 10.1161/01.res.87.5.363. [DOI] [PubMed] [Google Scholar]
- 196.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]