Abstract
Balantidiasis is considered a neglected zoonotic disease with pigs serving as reservoir hosts. However, Balantidium coli has been recorded in many other mammalian species, including primates. Here, we evaluated the genetic diversity of B. coli in non-human primates using two gene markers (SSrDNA and ITS1-5.8SDNA-ITS2). We analyzed 49 isolates of ciliates from fecal samples originating from 11 species of captive and wild primates, domestic pigs and wild boar. The phylogenetic trees were computed using Bayesian inference and Maximum likelihood. Balantidium entozoon from edible frog and Buxtonella sulcata from cattle were included in the analyses as the closest relatives of B. coli, as well as reference sequences of vestibuliferids. The SSrDNA tree showed the same phylogenetic diversification of B. coli at genus level as the tree constructed based on the ITS region. Based on the polymorphism of SSrDNA sequences, the type species of the genus, namely B. entozoon, appeared to be phylogenetically distinct from B. coli. Thus, we propose a new genus Neobalantidium for the homeothermic clade. Moreover, several isolates from both captive and wild primates (excluding great apes) clustered with B. sulcata with high support, suggesting the existence of a new species within this genus. The cysts of Buxtonella and Neobalantidium are morphologically indistinguishable and the presence of Buxtonella-like ciliates in primates opens the question about possible occurrence of these pathogens in humans.
Author Summary
Balantidium coli is a pathogenic ciliate occurring in various hosts, including primates. Balantidiasis is considered a neglected disease with zoonotic potential and it is associated with pigs as reservoirs. Although it is considered to be rare, a high prevalence of B. coli persists in tropical and subtropical areas. The infection can cause symptoms ranging from mild diarrhea to fulminating dysentery. Recently, balantidiasis has appeared to be a serious problem in immunocompromised persons. Although a chapter about balantidiasis is included in almost every parasitology textbook, many aspects of the occurrence of these ciliates in mammals, including humans, remain unknown. As balantidiasis can pose a serious health problem, a better understanding of this organism has important practical implications. We performed an extensive evaluation of genetic diversity in B. coli based on samples from non-human primates, together with comparative material from pigs and reference sequences. We find that the diversity of the cyst-forming ciliates in non-human primates is broader than expected. This discovery poses novel questions about the real spectrum of intestinal ciliates occurring in humans, and might explain unknown factors in the epidemiology and pathogenicity of ciliate infections.
Introduction
Balantidium coli (Vestibuliferida: Balantidiidae) is a cosmopolitan ciliate colonizing the intestine of many mammalian hosts. However, domestic pigs and wild boars are considered to be the principal host and major reservoir [1], [2]. Balantidiasis is considered a zoonotic disease and human clinical cases in developed countries were typically associated with close contact with pigs in the past [2], [3]. Nowadays, localities with a high prevalence of B. coli infection in humans persist mostly in tropical and subtropical areas [4], [5]. Apart from humans, B. coli is also commonly reported to infect both captive and free-living non-human primates [6], [7], [8].
The clinical importance of B. coli varies. Presently, human populations living in close proximity with domestic pigs are naturally resistant and mostly without any clinical manifestation [5]. However, the infection can cause disease, with symptoms ranging from mild diarrhea to fulminating dysentery. On rare occasions these organisms may also invade other organs [2], [9], [10], [11], which is more frequently observed in immunocompromised individuals afflicted with AIDS or leukemia [12], [13].
The Balantidium taxonomy is somewhat controversial due to the pleomorphism of its trophozoites [2], [14], [15] and range of its hosts. Balantidium coli observed in dysenteric patients was originally described as Paramecium coli by Malmstein (1857) [16]. Subsequently, Stein (1863) [17] reclassified the ciliate into the genus Balantidium, which was erected a few years earlier by Claparède and Lachmann (1858) [18] for the newly described Balantidium entozoon from frogs. A great majority of the taxa included in this genus were isolated from amphibian, fish or insect hosts [19]. In mammals, all balantidia are currently referred to as B. coli, despite that several other species of Balantidium have recently been described based on slight morphological differences in trophozoites. The broad synonymy of B. coli includes twelve other species of mammalian balantidium, specifically from primates, pigs, guinea pigs and camels [14], [20], [21]. Apparently, trophozoite morphology alone is insufficient for taxonomical purposes.
The worldwide distribution of B. coli in various hosts, together with the zoonotic potential and unclear epidemiology of human balantidiasis, calls for further study addressing the genetic diversity of these pathogens. Currently, fairly extensive and congruent molecular phylogenies have been obtained based on small ribosomal subunits of free-living and commensal ciliates in recent years [22], [23], [24]. The SSrRNA is a gene of taxonomic relevance at the genus level and, nowadays, it is broadly used for taxonomic studies in combination with morphological features [e.g. 25]. Currently, only few SSrRNA sequences of Balantidium are available in the GenBank and their comparison across various hosts, pigs, ostriches and gorillas, has revealed little variability [26]; however, the sequence of B. entozoon differs by 5% from those of B. coli [27].
Recently, the molecular diversity of B. coli at the species/subspecies level has been explored based on the hypervariable gene marker ITS1-5.8S rRNA-ITS2 [21], leading to designation of two different genotypes, A and B, in isolates from domestic pigs and ostriches. However, a later study by the same authors demonstrated that the “genotypes” are de facto genetic variants, representing at least two separate micronuclear rRNA genes, which can be present even within a single cell of B. coli [28]. Moreover, they distinguished five types of these main variants (A0, A1, A2, B0 and B1) on the basis of the ITS1 helix II characteristics. Such ambiguous polymorphism questions the applicability of internal transcribed spacers as markers for analyzing the intraspecific genetic variability of B. coli and any taxonomical or epidemiological implications.
Our previous research on the occurrence of B. coli in African great apes revealed the common presence of trophozoites and/or cysts in captive animals from European facilities and African sanctuaries, in contrast to its absence in wild populations [7]. Although balantidiasis is a neglected disease, molecular diversity of Balantidium in humans has not yet been addressed. Based on the samples from captive and wild non-human primates, together with comparative material from domestic pigs in Europe and tropical Africa, we performed an extensive evaluation of the genetic diversity of B. coli using two nuclear gene markers: SSrDNA and ITS1-5.8SrDNA-ITS2. Our phylogenetic analyses also comprise sequences from type species of the genus ( = B. entozoon), and for the first time also the closely related ciliate from cattle, Buxtonella sulcata, to address the potential polyphyletic character of the genus Balantidium.
Materials and Methods
Fecal samples
Our study was completely non-invasive including only examination of fecal samples. The only invasive sampling was the use of cloacal lavage in edible frogs. This material was collected prior to our research during the field course for undergraduate students. As such, it was approved by Administratia Rezervatiei Biosferei Delta Dunarii (for details about the permits and collaborating authorities see Text S1).
Fecal samples from captive primates were obtained in collaboration with (i) several European Zoos and (ii) African sanctuaries during routine diagnostic check-ups. Every facility was provided with the results, which they later used for antiparasitic control. In European countries, our research was approved by particular Zoos (which follow their respective national animal care regulations or guidelines). To our knowledge, and based on long-term research on primate parasites, such non-invasive fecal sampling has no other regulations in the EU. Thus, this study complies with individual national animal care regulations or guidelines.
Fecal samples of primates (n = 35) containing B. coli-like ciliates were selected based on coproscopic analysis performed in previous pilot studies [7], [29]; these samples and their origin are listed in Tables 1 and 2. Other material included fecal samples from domestic pigs containing B. coli, cattle containing Buxtonella sulcata (mostly cysts and few trophozoites based on coproscopic analysis) and edible frogs positive for Balantidium entozoon (the samples and their origin are listed in Table 3). In captive facilities, fecal samples of primates, pigs and cattle were collected individually and by animal keepers during routine daily cleaning of cages/sleeping quarters. In the wild, fecal samples were obtained during distance tracking of the animals. Fecal samples of edible frogs were obtained by cloacal lavage. All samples were analyzed coproscopically using Sheather's flotation and Merthiolate-iodine-formaldehyde (MIFC) sedimentation [7].
Table 1. The summary of isolates from captive African great apes.
| Site (State) | Host species | Origin | S. No. | A. No. 18S | A. No. ITS |
| Antwerp Zoo (Belgium) | chimpanzees (Pan troglodytes) | captivity | 1 | JQ073313 | JQ073351 |
| Dierenpark Amersfoort (Netherlands) | chimpanzees (Pan troglodytes) | captivity | 1 | JQ073311 | JQ073349 |
| La Vallée des Signes (France) | chimpanzees (Pan troglodytes) | captivity | 1 | JQ073309 | JQ073347 |
| Limbe Wildlife Centre (Cameroon) | chimpanzees (Pan troglodytes) | captivity | 3 | JQ073315-17 | JQ073353-55 |
| Ogród Zoologiczny w Opolu (Poland) | gorilla (Gorilla gorilla) | captivity | 1 | JQ073312 | JQ073350 |
| Sweetwaters Chimpanzee Sanctuary (Kenya) | chimpanzees (Pan troglodytes) | captivity | 3 | JQ073314, -31-32 | JQ073352, -76-77 |
| Chimps' Sanctuary, PN de Conkouati Douli (Republic of Congo) | chimpanzees (Pan troglodytes) | captivity | 3 | JQ073329 | JQ073372-74 |
| Twycross Zoo (GB) | chimpanzees (Pan troglodytes) | captivity | 2 | JQ073310 | JQ073348, -18 |
| Zoo Aquarium Madrid (Spain) | chimpanzees (Pan troglodytes) | captivity | 2 | JQ073307-08 | JQ073345-46 |
| Zoologischer Garten Leipzig (Germany) | bonobo (Pan paniscus) | captivity | 1 | JQ073306 | JQ073344 |
NR- National Reservation; PN-Park National; S. No – number of samples; A. No. 18S/ITS – accession numbers for 18S-rDNA/ITS1-5.8SrRNA-ITS2 sequences in the GenBank.
Table 2. The summary of isolates from captive and wild-ranging other primates.
| Site (State) | Host species | Origin | S. No. | A. No. SSU | A. No. ITS |
| Amsterdam Zoo (Nl) | mandrill (Mandrillus sphinx) | captivity | 1 | - | JQ073338 |
| celebes crested macaque (Macaca nigra) | captivity | 1 | - | JQ073382 | |
| AAP Sanctuary For Exotic Animals (Nl) | hamadrys baboon (Papio hamadryas) | captivity | 6 | JQ07333 | JQ073339-41,-75,-81,-84 |
| barbary macaque (Macaca sylvanus) | captivity | 1 | - | JQ073369 | |
| Safari Park Beekse Bergen (Nl) | rhesus macaque (Macaca mulatta) | captivity | 2 | JQ073327-28 | JQ073367-68 |
| Apenheul Primate Park (Nl) | lion-tailed macaque (Macaca silenus) | captivity | 3 | - | JQ073370-71,-83 |
| Sweetwaters Chimpanzee Sanctuary (K) | hamadrys baboon (Papio hamadryas) | captivity | 1 | JQ073305 | JQ73343 |
| Dzanga-Ndoki NP (CAR) | agile mangabey (Cercopithecus agilis) | wild | 2 | JQ073325,-36 | JQ073364,-85 |
Nl-Netherlands, K-Kenya, CAR-Central African Republic; S. No. – number of samples; A. No. 18S/ITS – accession numbers for 18S-rDNA/ITS1-5.8SrRNA-ITS2 sequences in the GenBank.
Table 3. The summary of isolates from domestic pigs, cattle and edible frogs.
| Site (State) | Host species | S. No. | A. No. 18S | A. No. ITS |
| Czech Republic | domestic pig (Sus scrofa domestica) | 5 | JQ073304,-21-24 | JQ073342,-60-63 |
| Madagascar | domestic pig (Sus scrofa domestica) | 3 | JQ073320 | JQ073357-59 |
| Cameroon | domestic pig (Sus scrofa domestica) | 2 | JQ073334 | JQ073379-80 |
| Kenya | domestic pig (Sus scrofa domestica) | 1 | JQ073333 | JQ073378 |
| Central African Rep. | domestic pig (Sus scrofa domestica) | 2 | JQ073326 | JQ073365-66 |
| Czech Republic | wild boar (Sus scrofa) | 1 | JQ073319 | JQ073356 |
| Belgium | cattle (Bos taurus) | 2 | JQ073337 | JQ073386-87 |
| Romania | edible frog (Pelophylax kl. esculentus) | 2 | JQ408692 | JQ408693-94 |
S. No.-number of samples; A. No. 18S/ITS – accession numbers for 18S-rDNA/ITS1-5.8SrRNA-ITS2 sequences in the GenBank.
DNA isolation, PCR, sequencing
Fecal samples were fixed in 96% ethanol. A portion of each sample was dried overnight at 37°C. Total DNA was extracted using the Invitrogen Stool Kit following the manufacturer's instructions. Primers for amplification of the SSrRNA gene of B. coli, B. sulcata and B. entozoon were designed based on the published sequences of SSrDNA in the GenBank and also of other ciliates that could be present in fecal samples. The partial sequence of SSrDNA with the sizes 1047 bp (B. coli, B. sulcata) and 1040 bp (B. entozoon) were amplified using the forward primer SSUf (5′-CGCAAATCGCGATTTTGTCGCG-3′) and either the reverse primer SSUrBB (5′-AAATACATAGTCCCTCTAAGAAGTC-3′) for B. coli and B. sulcata or SSUrBE (5′-CCCTCTAAGAAGCTAATACTC-3′) for B. entozoon. PCR was performed in a Biometra T-personal thermocycler (Schoeller) programmed as follows: (i) SSUf+SSUrBB: 10 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 59°C, 1 min at 72°C, and 5 min at 72°C, and (ii) SSUf+SSUrBE: 10 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 53°C, 1 min at 72°C, and 5 min at 72°C. The ITS1 - 5.8S-rRNA - ITS2 coding region was amplified using published primers B5D and B5R for all three species and the PCR program according to the protocol [21]. The PCR products were checked by electrophoresis using 12 µl of PCR reaction loaded into a 1% agarose gel run using the Vilber Lourmat electrophoresis system (Schoeller); PCR products were visualized by staining with Goldview and UV translumination. The amplicons were purified using the Qiagen extraction gel kit. Sequencing of these amplicons was performed by Macrogen, Korea. Each sample was sequenced in both directions and the sequence contigs were assembled using the program ChromasPro, version 1.5 (Technelysium Pty Ltd). BLAST analyses were performed for all new sequences.
Phylogenetic analyses
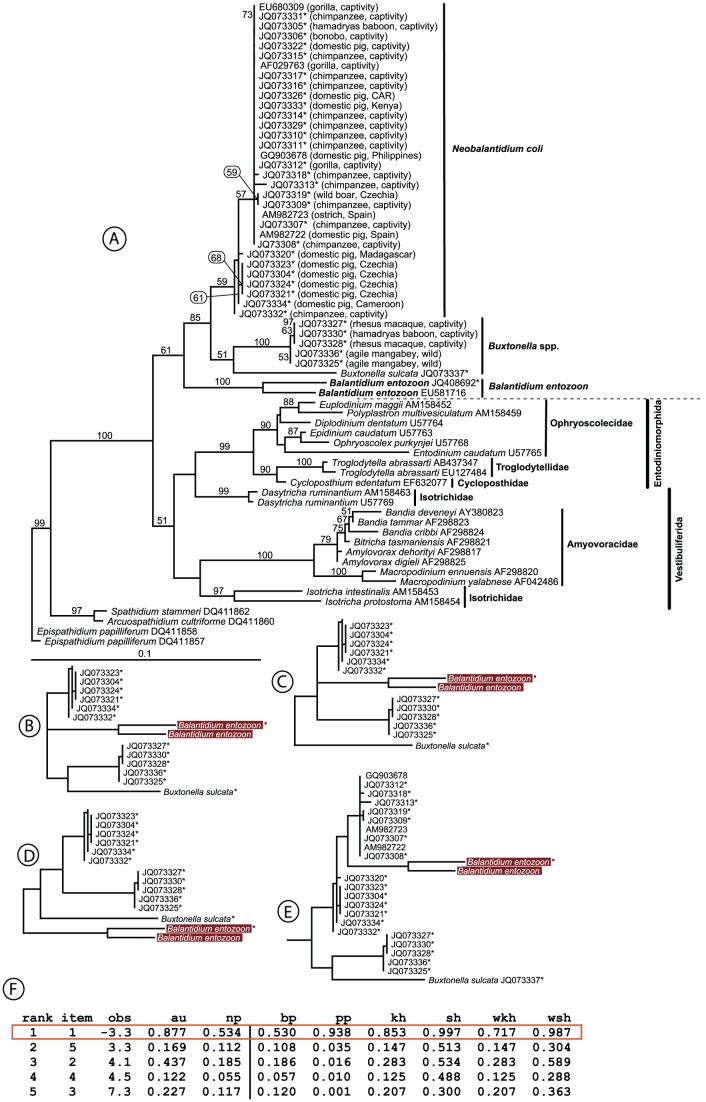
rRNA gene loci sequences coding for both SSU and ITS were aligned with available homologues from GenBank using the Kalign program at http://www.ebi.ac.uk/Tools/msa/kalign/ [30], [31]. Both alignments were manually edited in BioEdit [32] and ambiguously aligned. In the case of ITS sequences, the ambiguously aligned regions were omitted from further analyses. Alignments were analyzed individually and also in a combined data set. SSU and ITS data sets were analyzed using both Maximum likelihood (ML) and Bayesian inference (BI). Maximum likelihood topologies were computed using a gamma corrected GTR model of evolution and SPR heuristics as implemented in PhyML 3.0 [33]. Branching support was estimated from 1000 non-parametric bootstrap replicates using the above mentioned software and conditions. Bayesian topologies were estimated using Phylobayes 3.2 [34] under the GTR and/or GTR-CAT model of evolution. The latter is a combination of the empirical mixture model of 40 profiles (C40) sharing exchange rates as defined by the GTR model. For all data sets, two independent chains were run until they converged (i.e. their maximum observed discrepancy was lower than 0.1) and the effective sample size of all model characteristics was at least 100. Bayesian posterior probabilities (PP) provide branching support for the resulting topology (only branches with a PP higher than 0.95 were considered to be supported by the models). The possible hypotheses for the evolutionary history of the genus Balantidium were tested using the approximately unbiased (AU) test [35] on the SSrRNA gene dataset. Alternative topologies of the in-group (Fig. 1) were constrained and re-optimized using RAxML [36]. The site-specific log-likelihood scores of the resulting trees were obtained by the same software and further analyzed using the AU-test as implemented in CONSEL [37].
Figure 1. SSrDNA tree based on Trichostomatia SSrDNA sequences computed by RAxML.
A. The numbers above the branches represent Maximum likelihood bootstrap supports as computed from 1000 replicates. The scale bar represents 10 changes per 100 positions. B–E. The tree is complemented by an AU topology test, with all tested topologies shown below the main tree (topologies shown in reduced form). New sequences are marked with a star.
Results
The sequences of both above-mentioned markers obtained in this study from each host are available in GenBank under the accession numbers JQ73304-87, JQ408692-94 (Table 1–3).
Molecular phylogenies of SSrRNA genes/primary structure
Thirty-five partial SSrDNA sequences of ciliate species were examined and compared to other SSrDNA sequences representing the genus Balantidium (EU680309, AM982722-23, AF029763, GQ903678, EU581716) and related ciliates classified within the subclass Trichostomatia (AB437347, AF298817, AF298820-21, AF298823-25, AF042486, AM158452-54, AM158459, AM158463, AY380823, EF632077, EU127484, U57763-65, U57768-69) and Haptoria (DQ411862, DQ411860). This tree was rooted using Epispathidium papilliferum (DQ411857-8) as an outgroup.
The maximum likelihood (RAxML with GTR model) topology of our resulting tree shows that the subclass Trichostomatia is a monophyletic and highly supported group divided into two major clades. However, the order Vestibuliferida appears to be paraphyletic in its origin (Fig. 1). Clade I includes all the sequences of Balantidium and is further divided into two sub-clades: one that includes sequences of Balantidium coli, Buxtonella sulcata and Buxtonella-like ciliates from mammals, and the other represented by B. entozoon isolates only. Although the topology inferred from SSrRNA genes is unstable, it is very likely that the genus Balantidium is polyphyletic. Such topology is strongly preferred by the AU test (Fig. 1), although none of the alternative topologies could be fully rejected (see Fig. 1 for details). Generally, B. entozoon isolates jump to different positions in the tree, based on the particular method and substitutional model used. However, all obtained SSrDNA based topologies (RAxML with GTR model; PhyloBayes with CAT model; PhyloBayes with GTR model, see dataset S1, S2, S3 for details) result in the polyphyly of the genus Balantidium. All our SSrDNA sequences from pigs, wild boar, great apes and a single sequence from a captive baboon (JQ073305), together with reference sequences of Balantidium, were grouped into a single clade corresponding to B. coli. This clade displays internal polytomy, obviously due to the presence of a high number of similar sequences. Several ciliates from primates (agile mangabeys: JQ073325 and JQ073336; rhesus macaques: JQ073327-28; the hamadryas baboon: JQ0733330) formed a separate clade that branched together with sequences of B. sulcata from cattle. However, such a relationship is only very weakly supported by ML trees; trees computed by PhyloBayes placed B. sulcata on a long branch inside the B. coli clade (see dataset S1, S2).
All other trichostomatids are included in the main second clade—clade II—that is separated into two sub-clades with a weak bootstrap value (51%). One sub-clade contains mainly Australian intestinal ciliates and some isotrichids (Vestibuliferida) and with high support values of both branches (100%, 97%). The second sub-clade is represented by Ophryoscolecidae, Troglodytellidae and Cycloposthidae (Entodiniomorphida), plus one isotrichid ciliate (Vestibuliferida), with a support value of 99% (Fig. 1). Both ML and Bayesian inference showed similar clade II topologies, albeit with numerous polytomies in the Bayesian tree (see Fig. 1 and datasets S1, S2, S3 for details).
Molecular phylogenies of ITS1-5.8S rRNA-ITS2 marker
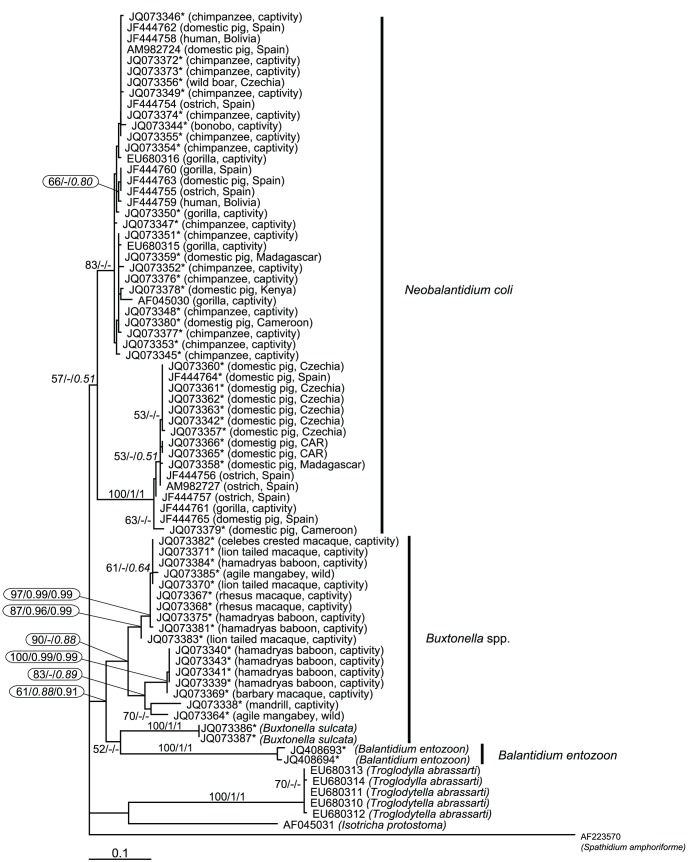
The phylogenetic trees based on the ITS1-5.8SrRNA-ITS2 sequences were estimated using Maximum likelihood phylogeny (PhyML) with the GTR model and Bayesian inference (PhyloBayes) with CAT and GTR models. This sequence data set includes our 52 sequences and reference sequences from GenBank that are comprised of five isolates of T. abrassarti (EU680310-14), and one from I. prostoma (AF045031); the haptorian ciliate Spathidium amphoriforme (AF223570) was used as an out-group. The resulting ML tree topology shows three major groups: (i) with T. abrassarti and I. prostoma, (ii) including B. entozoon, B. sulcata and isolates of ciliates from other primates and (iii) the “typical” B. coli clade (Fig. 2).
Figure 2. Maximum likelihood phylogenetic tree as inferred from the ITS1-5.8S-ITS2 DNA region.
The tree was computed using PhyML with the GTR model for nucleotide substitutions. Numbers above branches indicate ML bootstrap support from 1000 replicates/PhyloBayes posterior probabilities computed with CAT model/PhyloBayes posterior probabilities computed with GTR model. New sequences are marked with a star.
The majority of the supposed B. coli isolates were placed into a single clade, which was further divided into two highly supported sub-clades corresponding to the two major A and B genetic variants of B. coli as proposed [28]. The A-group includes 21 of our ciliate sequences and 11 reference sequences from captive great apes, two sequences from humans, one from wild boar, two from ostriches and six sequences from domestic pigs. The B-group contains 10 newly obtained sequences from ciliates from domestic pigs from different topodemes and four reference sequences (3 isolates from ostriches: AM982727, JF444756-7; captive gorilla: JF444761 and 2 domestic pigs). All obtained sequences originating from domestic pigs belong to type of genetic variant B. The sequence JQ073379 from the Cameroonian domestic pig branched separately in genetic variant B, which is most probably a new type of genetic variant. In three out of five populations of domestic pigs, both main types detected (Cameroon, Czech Republic, Madagascar), while in Kenya and CAR only one of them was found (either A or B, Fig. 2).
Importantly, the sequences of all 17 isolates of ciliates obtained from primates other than great apes and humans, together with B. sulcata and B. entozoon, formed a clade separated from B. coli. This group of isolates from primates is further divided into two strongly supported sub-groups that may represent a type of genetic variants. In this analysis, B. entozoon clustered as a sister taxon to B. sulcata, although with poor support. Bayesian trees inferred from ITS (CAT and GTR models) showed the same topology with the main clades forming polytomies in the tree. However, the ITS-based ML tree topology corresponds well to that of the SSrDNA-based tree, which was preferred by the AU test.
Eight chromatograms of the ITS1-5.8S rRNA-ITS2 sequences of B. coli revealed multiple sequence signals, specifically three chimpanzees (JQ 073346, JQ073348, JQ073354), three domestic pigs (JQ073357, JQ073378, JQ073380), a single bonobo (JQ073344) and a wild boar (JQ073356), suggesting the presence of more genetic variants within one isolate. The same situation is also seen in primates other than great apes, where five sequences with multiple signals were found. This situation, in agreement with results of a previous study [28], could not be resolved without cloning and is further discussed below.
Taxonomic summary
Our analyses have proven the polyphyletic character of the current genus Balantidium Claparède and Lachmann, 1858. The genus is well typified by its type species Balantidium entozoon from ranid frogs Pelophylax kl. esculentus. However, the historical placement of Paramecium coli Malmstein, 1857 into this genus by Stein (1863) is not supported by our results. As there is no available alternative in the complex synonymy of Balantidium coli, we erect a new genus to accommodate Paramecium coli Malmstein, 1857, together with other species previously described as members of the genus Balantidium from homeothermic hosts.
Neobalantidium gen. nov
Type species: Paramecium coli Malmstein, 1857.
Etymology: the new generic name is created by the prefix “neo-“, meaning new/novel. As there are no pathological signs associated with the presence of balantidia in amphibians, we suggest continuing to use the term “balantidiasis” for describing the clinical disease caused by species of Neobalantidium in homeothermic vertebrates including humans.
Diagnosis: vestibuliferid ciliate; trophozoite elongated or ovoid (Fig. 3a), rounded posteriorly and narrowing anteriorly, 30–300 µm long and 30–100 µm wide; surface covered with somatic cilia arranged in transverse field; interkinetal ridges present between longitudinal rows of cilia; vestibulum located apically and surrounded by closely packed, which are longer than the somatic ones [15], [26]. Under the pellicle, there are mucocysts that are involved in the synthesis of the paracrystalline and fibrous material utilized in cyst formation [26], [38]. The cyst is spherical or ovoid, 40–60 µm in diameter (Fig. 4a) with a thick and hyaline wall. The trophozoite is present inside the cyst with visible rows of cilia.
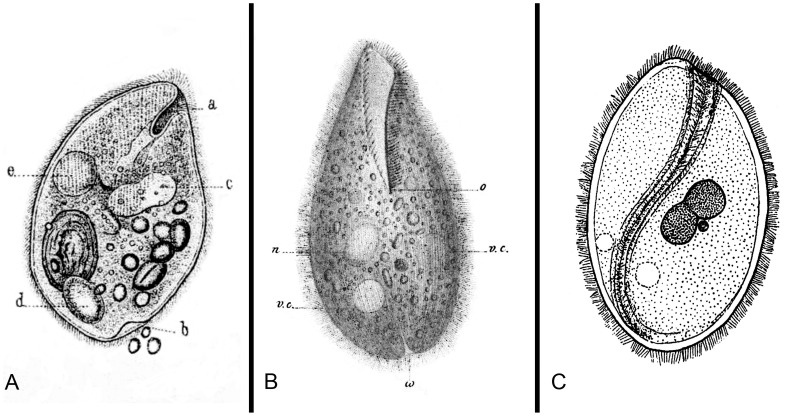
Figure 3. Reproduction of the original drawings of trophozoites of Paramecium coli, Balantidium entozoon and Buxtonella sulcata.
A. P. coli from Malmstein (1857); B. B. entozoon from Claparéde & Lachmann (1858); and C. B. sulcata from Jameson (1926).
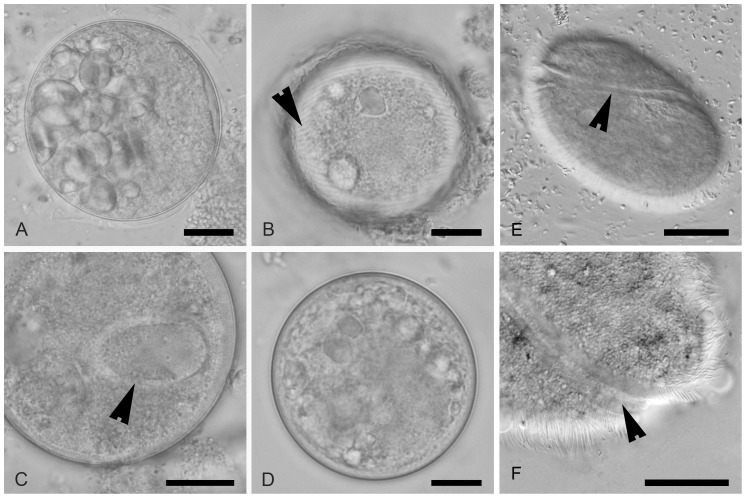
Figure 4. A–D: Comparison of cysts of Neobalantidium coli, Buxtonella sulcata and a Buxtonella-like ciliate; scale bars = 10 µm.
A. Cyst of N. coli from a domestic pig with visible ingested starch grains inside. B, D. Cysts of Buxtonella-like ciliate from an agile mangabey showing the trophozoite with visible rows of cilia (B, arrowhead). C. Cyst of B. sulcata from cattle with visible macronucleus (arrowhead). E. Trophozoite of Buxtonella sulcata with typical sulcus (arrowhead); scale bar = 20 µm. F. Detail of sulcus of Buxtonella sulcata (arrowhead); scale bar = 5 µm.
Differential diagnosis: among the vestibuliferid intestinal ciliates of vertebrates, Neobalantidium gen. nov. can be distinguished from most other ciliate genera (including Balantidium in poikilothermic vertebrates) by the presence of the cyst stage in the life cycle (Fig. 3a) [15]. The ability to form cysts corresponds with the presence of putative mucocysts. Buxtonella spp. also form the cysts that are indistinguishable from those of Neobalantidium (Fig. 4 a, c); however, the trophozoites of Buxtonella can be distinguished based on the vestibular groove running in a slight curve from the anterior to the posterior end of the body (Fig. 3c) [39]. The vestibular groove being at least as long as one-half of body length is a common feature distinguishing all the other genera within the family Pycnotrichidae [40].
Discussion
Balantidiasis is classified as a neglected disease [41] and a more or less identical chapter on balantidiasis is included in almost every parasitology textbook. However, many aspects of the infections caused by these ciliates in mammals remain unknown. Although a frequently diagnosed parasitosis of humans until recently [3], [42], balantidiasis is presently rare in developed countries. Most of the reported cases have been attributed to contact with domestic pigs [e.g. 2], but the epidemiology of B. coli infections in humans remains controversial. Balantidiasis has also been reported repeatedly in humans without any obvious contact with suids, such as patients from Islamic countries [43]. In contrast, people living in endemic areas with a high prevalence of infections in pigs are often negative or asymptomatic [e.g. 4], [5].
Recently, a high prevalence of B. coli has also been recorded in captive African great apes, in contrast to wild populations in which this ciliate is almost absent [7], [44]. Increased contact with reservoir hosts and the diet composition of captive apes were proposed to be the reasons for this discrepancy [7]. As in the situation for humans, the epidemiology of B. coli in non-human primates remains unclear.
In the present study, we analyzed our isolates together with reference sequences of B. coli-like ciliates from humans, non-human primates and domestic pigs using two phylogenetic markers: SSrRNA and ITS1-5.8SrRNA-ITS2. The most suitable SSrRNA tree having a topology most preferred by the AU test has two major clades, I and II. The latter included trichostomatid ciliates from five families within the orders Vestibuliferida and Entodiniomorphida, corroborating results in which vestibuliferid ciliates assigned as a paraphyletic group with B. coli, placed outside this clade [24], [45]. Our molecular phylogenies indicate a situation different from morphology-based taxonomy [39], showing the polyphyletic character of vestibuliferids, into which a number of entodiniomorphid ciliates is inserted. Furthermore, the tree generated from ITS1-5.8SrRNA-ITS2 sequences confirmed this polyphyletic trend at the genus level. Surprisingly, ciliates from mammals traditionally referred to as B. coli branched separately in our SSrRNA tree from B. entozoon - the nominotypic species of the genus Balantidium. The apparent polyphyly of the genus led us to erect a new genus Neobalantidium, which we use hereafter.
In the ITS1-5.8SrRNA-ITS2 tree, B. entozoon sequences branch as a sister group to Buxtonella sulcata. However, this poorly supported branching pattern (52% in the node for sub-clades B. sulcata and B. entozoon and 61% in the higher node shared by the previously mentioned and cyst-forming ciliates of primates other than apes) might be an artifact due to a low number of ITS sequences from trichostomatid ciliates. This is apparent when ITS and SSU tree topologies are compared. The genetic divergence of N. coli and B. entozoon is further supported by the formation of cysts in the former and their absence in the latter (and also in other Balantidium spp. from amphibians) [27], [46]. In contrast, cyst formation is typical for B. sulcata [47] and unidentified ciliates from other primates, such as those described in this study, that both group together with N. coli isolates (Fig. 2 b–d).
The phylogenetic analyses presented here represent the first attempt to clarify the position of B. sulcata. In the SSrRNA ML tree, B. sulcata formed a separate clade together with other yet unidentified ciliates originating from wild agile mangabeys and captive primates (other than apes), which were originally classified as Balantidium spp. Throughout the text, we refer to these cyst-forming ciliates as Buxtonella-like. However, morphological analyses of their trophozoites are necessary for proper taxonomic evaluation and confirmation of their classification within the genus Buxtonella or as a novel genus. Due to ethical considerations, invasive sampling of wild and Zoo primates is complicated, which is why we have been unable to obtain intestinal content.
To date, all cyst-forming ciliates diagnosed in both wild and captive non-human primates, including African great apes, were referred to as Balantidium coli [e.g. 6], [7], [48]. However, our data suggest that while captive apes are infected by N. coli via unknown means, non-human primates other than apes host Buxtonella-like ciliates. Such a scenario could explain the absence of cyst-forming ciliates in wild ranging populations of African great apes and their common presence in other syntopic primates [7], [8], [49]. The presence of Buxtonella-like ciliates in primates allows for hypothesizing the occurrence of these pathogens in human populations. The morphology of Neobalantidium and Buxtonella cysts is almost identical (see Fig. 2) [2], [47], making them indistinguishable from each other in coproscopic diagnostics.
We cannot exclude the possibility of co-infection with both types of cyst-forming ciliates. We most likely detected a mixed infection in an olive baboon from the Sweetwaters Chimpanzee Sanctuary in Kenya. The SSrDNA sequence obtained from this isolate was placed into the N. coli clade (JQ073305, see Fig. 1), whereas the ITS sequence branched within the clade of Buxtonella-like ciliates (JQ073343, see Fig. 2). N. coli was likewise detected in captive chimpanzees in the same sanctuary (SSrDNA: JQ073314, JQ073331-32; ITS regions: JQ073352, JQ073376-77; see Fig. 1, 2) and in domestic pigs from nearby localities (SSrDNA: JQ073333; ITS regions: JQ073378).
Until recently, ITS1-5.8S rRNA-ITS2 had appeared as a favorable marker for research of genetic diversity of trichostomatid ciliates [21], [50], [51]. However, by cloning the products obtained from PCR amplicons showing the multiple sequence signals in chromatograms from a single ciliate cell, Ponce-Gordo et al. [28] has suggested the co-existence of more genetic variants of micronuclear rRNA genes within the same cell of N. coli. Based on these facts, they established a novel terminology replacing the term “genotype” by “genetic variant A and B”. Our phylogenies inferred from the sequences of ITS1-5.8S rRNA-ITS2 are concordant with the previous study, as we detected main types A and B and four out of five “types of genetic variants” in our isolates. The presence of both main types was revealed in most of the populations of domestic pigs, whereas only main type A was detected in great apes. Obviously, results of PCR amplification can be biased by the existence of numbers of copies of main variants of ITS1-5.8S rRNA-ITS2 sequences. Similar to previous authors [21], [28], we revealed multiple signals in several chromatographs of N. coli sequences and also in Buxtonella-like ciliates. Taken together, the present data indicate that ambiguous genetic polymorphisms in the ITS regions of vestibuliferid cyst-forming ciliates are of no taxonomical importance and cannot be used to trace the epidemiology of the infection.
Interestingly, the level of ITS polymorphism differs among the trichostomatid ciliates. The ciliates Isotricha protostoma and Troglodytella abrassarti, distantly related to N. coli, exhibit rather low ITS polymorphism and high host specificity [50], [51]. Transmission of these ciliates is limited, due to the fragile nature of their trophozoites, to close contact between hosts [52], [53]. Higher genetic polymorphism of ITS genes of N. coli can result from transmissions facilitated by the resilient cysts of Neobalantidium.
Phylogenetically speaking, it is obvious that internal transcribed spacers are not a suitable tool for the study of genetic variability in the vestibuliferid cyst-forming ciliates. Unfortunately, no other hypervariable genes have been exploited in ciliates so far. The broader usage of other genes such as the large ribosomal subunit (LSrRNA), α-tubulin, phosphoglycerate kinase (PGK) and histone genes is unfortunately complicated by several factors: (i) they are highly conserved (histones) [54]; (ii) they provide a very congruent pattern of diversification at the genus level with SSrRNA (LSrRNA) [55]; (iii) sequence data are extremely sparse in GenBank (PGK) [56]; and/or, (iv) there exist multiple paralogs (α-tubulin) [57]. Thus, the necessity of searching for other markers of intraspecific polymorphism of studied cyst-forming ciliates arises, which is important for explaining host specificity and epidemiological aspects.
The proper determination of intestinal ciliates in mammals requires examination of morphological characteristics combined with molecular-phylogenetic data. Obviously, humans are not natural hosts for any intestinal ciliates, and the pathogens are maintained in populations of mammalian reservoir hosts. Our results demonstrate how misleading the coproscopic cyst morphology-based diagnostics of Neobalantidium and Buxtonella can be, which could also explain the prevailing ambiguity in the epidemiology of these infections (like the occurrence of balantidiasis in areas without pig rearing). So far, no molecular approaches have been applied, either in the diagnostics of balantidiasis in man, or in the broader determination of ciliates detected in clinical cases in different parts of the world. The discovery of novel Buxtonella-like ciliates in primates calls for broad molecular-based research addressing the diversity of the agents of balantidiasis in man, not only to describe the source of the infections, but also to set up proper control strategies. In this complex situation, the “old-fashion taxonomy” also matters.
Supporting Information
Bayesian phylogenetic tree (PhyloBayes, CAT model) based on SSrDNA sequences. The numbers above branches indicate Bayesian posterior probabilities (CAT model). New sequences are marked with a star.
(PDF)
Bayesian phylogenetic tree (PhyloBayes, GTR model) based on SSrDNA sequences. The numbers above branches indicate Bayesian posterior probabilities (CAT model)/Bayesian posterior probabilities (GTR model)/PhyML bootstrap computed from 1000 replicates. New sequences are marked with a star.
(PDF)
Maximum likelihood phylogenetic tree (PhyML, GTR model) based on SSrDNA sequences. The numbers above branches indicate PhyML bootstrap computed from 1000 replicates. New sequences are marked with a star.
(PDF)
Overview of research permits and collaborating authorities allowing work at localities in the wild mentioned in the Table 1–3.
(DOC)
Acknowledgments
This work could not have been done without the collaboration of zoos and African sanctuaries. We are grateful to all keepers, curators and veterinarians who provided us with samples of captive apes: Antwerp Zoo (Francis Vercammen); Twycross Zoo (Nie Masters); Zoo Aquarium Madrid (Eva Martinez Nevado); Dierenpark Amersfoort (Adrien van Zanten); La Vallée des Singes Zoo (Jan Vermeer); Zoologischer Garten Leipzig (Klaus Eulenberger); Ogród Zoologiczny w Opolu; Amsterdam Zoo (Mark J. Hoyer, Daphne Valk); AAP Sanctuary For Exotic Animals (Hester van Bolhuis, Imelda den Hartog, Jasper Iepema); Safari Park Beekse Bergen (Jaak Kaandorp); Apenheul Primate Park (Jeanette van der Wal, Frank Rietkerk); Chimps' Sanctuary, Parc National de Conkouati Douli, Republic of Congo (Laurien Bets, Lieze Rouffaer, Lynn Vandenberghe, Hilde Vanleeuwe). We would like to express our sincere thanks to Julius Lukeš and Jan Votýpka for collecting exotic pig samples, to Jiří Vávra for valuable consultation and to Cambridge Press for permitting use of the original picture from Jameson (1926). We are grateful to Andrew McIntosh from Kyoto University (Japan) for linguistic corrections and improvements.
Funding Statement
This work was supported by the project “CEITEC” – Central European Institute of Technology” (grant no. CZ.1.05/1.1.00/02.0068) from the European Regional Development Fund, by grant from the Grant Agency of the Czech Republic (grant no. 206/09/0927), and by institutional support of Institute of Vertebrate Biology Academy of Sciences of the Czech Republic (grant no. RVO:68081766) and by OPVK 2.3 project – Development of Scientific Team and Laboratory for Infectious Diseases Common to Humans and Great Apes (CZ.1.07/2.3.00/20.0300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nakauchi K (1999) The prevalence of Balantidium coli in fifty-six mammalian species. J Vet Med Sci 6: 63–65. [DOI] [PubMed] [Google Scholar]
- 2. Schuster FL, Ramirez-Avila L (2008) Current world status of Balantidium coli . Clin Microbiol Rev 21: 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Areán VM, Koppisch E (1956) Balantidiasis. A review and report of cases. Am J Pathol 22: 1089–1115. [PMC free article] [PubMed] [Google Scholar]
- 4. Esteban JG, Aguirre C, Angles R, Ash L R, Mas-Coma R (1998) Balantidiasis in Aymara children from the Northern Bolivian Altiplano. Am J Trop Med Hyg 59: 922–927. [DOI] [PubMed] [Google Scholar]
- 5. Owen IL (2005) Parasitic zoonosis in Papua New Guinea. J Helmint 79: 1–14. [DOI] [PubMed] [Google Scholar]
- 6. Levecke B, Dorny P, Geurden T, Vercammen F, Vercruysse J (2007) Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol 148: 236–246. [DOI] [PubMed] [Google Scholar]
- 7. Pomajbíková K, Petrželková KJ, Profousová I, Petrášová J, Modrý D (2010) Discrepancies in the occurrence of Balantidium coli between captive and African great apes. J Parasitol 96: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 8. Weyher AH, Ross C, Semple S (2006) Gastrointestinal parasites in crop raiding and wild foraging Papio anubis in Nigeria. Int J Primatol 27: 1519–1533. [Google Scholar]
- 9. Anargyrou K, Petrikkos GL, Suller MTE, Skiada A, Siakantaris MP, et al. (2003) Pulmonary Balantidium coli infection in a leukemic patient. Am J Hematol 73: 180–183. [DOI] [PubMed] [Google Scholar]
- 10. Ferry T, Bouhour D, De Monbrison F, Laurent F, Dumouchel-Champagne H, et al. (2004) Severe peritonitis due to Balantidium coli acquired in France. Eur J Clin Microbiol Infect Dis 23: 393–395. [DOI] [PubMed] [Google Scholar]
- 11. Maino A, Garigali G, Grande R, Messa P, Fogazzo GB (2010) Urinary balantidiasis: diagnosis at a glance by urine examination. J Nephrol 23: 732–737. [PubMed] [Google Scholar]
- 12. Cermeño JR, Hernández de Cuesta I, Uzcátegui O, Páez J, Rivera M, et al. (2003) Balantidium coli in an HIV-infected patient with chronic diarrhoea. AIDS 17: 941–2. [DOI] [PubMed] [Google Scholar]
- 13. Clyti E, Aznar C, Couppie P, El Guedi M, Carme B, et al. (1998) Un cas de co-infection par Balantidium coli et VIH en Guayane Française. Bull Soc Pathol Exot 91: 309–311. [PubMed] [Google Scholar]
- 14. Hegner R (1934) Specificity in the genus Balantidium based on size and shape of body and macronucleus, with descriptions of six new species. Am J Epidemiol 19: 38–67. [Google Scholar]
- 15.Zaman V (1978) Balantidium coli, In: J.PKreier, editors. In: Parasitic Protozoa, vol. 2. New York: Academic Press. pp. 633–653.
- 16. Malmstein PH (1857) Infusorien als Intestinal-Thiere beim Menschen. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin 12: 302–309. [Google Scholar]
- 17. Stein F (1863) Ueber Paramecium (?) coli Malmsten. Amtl Berl Dtsch Chem Ges 37: 165. [Google Scholar]
- 18.Claparède E, Lachmann J (1858) Balantidium entozoon. In: Études sur les infusoires et les Rhizopodes, Genève: Kossman. pp. 247.
- 19.Lynn D (2008) The ciliated protozoa. Characterization, classification and guide to the literature, 3rd ed. New York: Pergamon Press. 606 pp.
- 20. Quadri SS, Navarathnam S (1966) On a new species of Balantidium from an Indian monkey Macaca radiata . Riv Parassitol 27: 89–96. [PubMed] [Google Scholar]
- 21. Ponce-Gordo F, Jimenez_Ruiz E, Martínez-Díaz RA (2008) Tentative identification of the species of Balantidium from ostriches (Struthio camelus) as Balantidium coli-like by analysis of polymorphic DNA. Vet Parasitol 157: 41–49. [DOI] [PubMed] [Google Scholar]
- 22. Cameron SL, Wright AG, O'Donoghue PJ (2003) An expanded phylogeny of the Entodiniomorphida (Ciliophora: Litostomatea). Acta Protozool 42: 1–6. [Google Scholar]
- 23. Strüder-Kypke MC, Kornilova OA, Lynn DH (2007) Phylogeny of trichostome ciliates (Ciliophora, Litostomatea) endosymbiotic in the Yakut horse (Equus caballus). Eur J Protistol 43: 319–328. [DOI] [PubMed] [Google Scholar]
- 24. Vďačný P, Bourland WA, Orsi W, Epstein SS, Foissner W (2011) Phylogeny and classification of the Litostomatea (Protista, Ciliophora), with emphasis on free-living taxa and the 18S-rRNA gene. Mol Phylogenet Evol 59: 510–522. [DOI] [PubMed] [Google Scholar]
- 25. Cameron SL (2002) Taxonomy and phylogeny of endosymbiotic ciliates (Ciliophora∶Litostomatea) associated with Australian herbivorous marsupials. Int J Parasitol 33: 347–355. [DOI] [PubMed] [Google Scholar]
- 26. Nilles-Bije ML, Rivera WL (2010) Ultrastructural and molecular characterization of Balantidium coli isolated in the Philippines. Parasitol Res 106: 387–394. [DOI] [PubMed] [Google Scholar]
- 27. Grim JN, Buonanno F (2009) A re-desription of the ciliate genus and type species, Balantidium entozoon . Eur J Protistol 45: 147–182. [DOI] [PubMed] [Google Scholar]
- 28. Ponce-Gordo F, Fronseca-Salamanca F, Martínez-Díaz RA (2011) Genetic heterogeinity in internal transcribed spacer genes of Balantidium coli (Litostomatea, Ciliophora). Protist 162: 774–94. [DOI] [PubMed] [Google Scholar]
- 29.Levecke B (2010) The occurrence of gastrointestinal parasites in captive non-human primates. In: The importance of gastrointestinal protozoa in captive non-human primates [dissertation]. Ghent University. pp. 72–93.
- 30. Lassmann T, Sonnhammer EL (2005) Kalign-an accurate and fast multiple sequence alignment algorithm. BMC bioinformatics 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lassmann T, Sonnhammer EL (2006) Kalign, Kalignvu and Mumsa: web servers for multiple sequence alignment. Nucl Acids Res 34 Web Server issue: 596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95–98. [Google Scholar]
- 33. Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–21. [DOI] [PubMed] [Google Scholar]
- 34. Lartillot N, Lepage T, Blanquart S (2009) PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 25: 2286–2288. [DOI] [PubMed] [Google Scholar]
- 35. Shimodaira H (2004) Approximately unbiased tests of regions using multistep-multiscale bootstrap resampling. Ann Stat 32: 2616–2641. [Google Scholar]
- 36. Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 37. Shimodaira H, Hasegawa M (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17: 1246–1247. [DOI] [PubMed] [Google Scholar]
- 38. Skotarczak B (1999) Cytochemical identification of mucocysts in Balantidium coli trophozoites. Folia Biol (Krakow) 47: 61–65. [PubMed] [Google Scholar]
- 39. Jameson AP (1926) A ciliate, Buxtonella sulcata n. g., n. sp., from the caecum of cattle. Parasitology 18: 182–186. [Google Scholar]
- 40.Lynn DH, Small EB (2000) Ciliophora, Litostomatea. In: Lee JJ, Leedale GF, Bradbury P, editors. An illustrated guide to the protozoa, second edition, vol I. Lawrence, USA: Allen Press Inc. 493–5 p.
- 41.Garcia LS (2008) Balantidium coli; In: Khan NA, editor. Emerging protozoan pathogens. New York: Taylor & Francis. 353–366 p.
- 42. Kennedy CC, Stewart TRC (1957) Balantidial dysentery: A human case in Northern Ireland. Trans R Soc Trop Med Hyg 51: 549–558. [DOI] [PubMed] [Google Scholar]
- 43. Solaymani-Mohammadi S, Rezaian M, Anwar MA (2005) Human balantidiasis in Iran: an unresolved enigma? Trends Parasitol 21: 160–161. [DOI] [PubMed] [Google Scholar]
- 44. Bakuza JS, Nkwengulila G (2009) Variation over time in parasite prevalence among free-rangin chimpanzees at Gombe National Park, Tanzania. Int J Primatol 30: 43–53. [Google Scholar]
- 45. Strüder-Kypke MC, Wright AD, Foissner W, Chatzinotas A, Lynn DH (2006) Molecular phylogeny of litostome ciliates (Cilophora, Litostomatea) with emphasis on free-living haptorian genera. Protist 157: 261–278. [DOI] [PubMed] [Google Scholar]
- 46. Li M, Wang J, Zhang J, Gu Z, Ling F, et al. (2008) First report of two Balantidium species from the Chinese giant salamander, Andrias davidianus: Balantidium sinensis Nie 1935 and Balantidium andianusis n. sp. Parasitol Res 102: 605–611. [DOI] [PubMed] [Google Scholar]
- 47. Fox MT, Jacobs DE (1986) Patterns of infection with Buxtonella sulcata in British cattle. Res Vet Sci 41: 90–92. [PubMed] [Google Scholar]
- 48. Lilly AA, Mehlman PT, Doran D (2002) Intestinal parasites in gorillas, chimpanzees, and humans at Mondika Research Site, Dzanga-Ndoki National Park, Central African Republic. Int J Primatol 23: 555–573. [Google Scholar]
- 49. McGrew WC, Tutin CEG, Collins DA, File SK (1989) Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt. Assirik (Senegal). Am J Primatol 17: 147–155. [DOI] [PubMed] [Google Scholar]
- 50. Vallo P, Petrželková KJ, Profousová I, Petrášová J, Pomajbíková K, et al. (2012) Molecular diversity of entodiniomorphid ciliate Troglodytella abrassarti and evaluation of its coevolution with chimpanzees. Am J Phys Anthropol 148 4: 525–33. [DOI] [PubMed] [Google Scholar]
- 51. Wright AG (1999) Analysis of intraspecific sequence variation among eight isolate of the rumen symbiont, Isotricha protostoma (Ciliophora), from two continents. J Eukaryot Microbiol 46: 445–446. [DOI] [PubMed] [Google Scholar]
- 52. Modrý D, Petrželková KJ, Pomajbíková K, Tokiwa T, Křížek J, et al. (2009) The occurrence and ape-to-ape transmission of the entodiniomorphid ciliates Troglodytella abrassarti in captive gorillas. J Eukaryot Microbiol 56: 83–87. [DOI] [PubMed] [Google Scholar]
- 53.Williams AG, Coleman GS (1992) The rumen protozoa. New York: Springer Verlag. 441 p.
- 54. Bernhard D, Schlegel M (1998) Evolution of histone H4 and H3 genes in different ciliate lineages. J Mol Evol 46: 344–354. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Q, Simpson A, Song W (2012) Insigts into the phylogeny of systematically controversial haptorian ciliates (Ciliophora, Litostomatea) based on multigene analyses. Proc R Soc 279: 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tourancheau AB, Villalobo E, Tsao N, Torre A, Pearlman RE (1998) Protein coding gene trees in ciliates: comparison with rRNA-based phylogenies. Mol Phyl Evol 10: 299–309. [DOI] [PubMed] [Google Scholar]
- 57. Israel R, Kosakovsky Pond SL, Muse SV, Katz LA (2002) Evolution of duplicated alpha-tubulin genes in ciliates. Evol 56: 1110–1122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bayesian phylogenetic tree (PhyloBayes, CAT model) based on SSrDNA sequences. The numbers above branches indicate Bayesian posterior probabilities (CAT model). New sequences are marked with a star.
(PDF)
Bayesian phylogenetic tree (PhyloBayes, GTR model) based on SSrDNA sequences. The numbers above branches indicate Bayesian posterior probabilities (CAT model)/Bayesian posterior probabilities (GTR model)/PhyML bootstrap computed from 1000 replicates. New sequences are marked with a star.
(PDF)
Maximum likelihood phylogenetic tree (PhyML, GTR model) based on SSrDNA sequences. The numbers above branches indicate PhyML bootstrap computed from 1000 replicates. New sequences are marked with a star.
(PDF)
Overview of research permits and collaborating authorities allowing work at localities in the wild mentioned in the Table 1–3.
(DOC)