Abstract
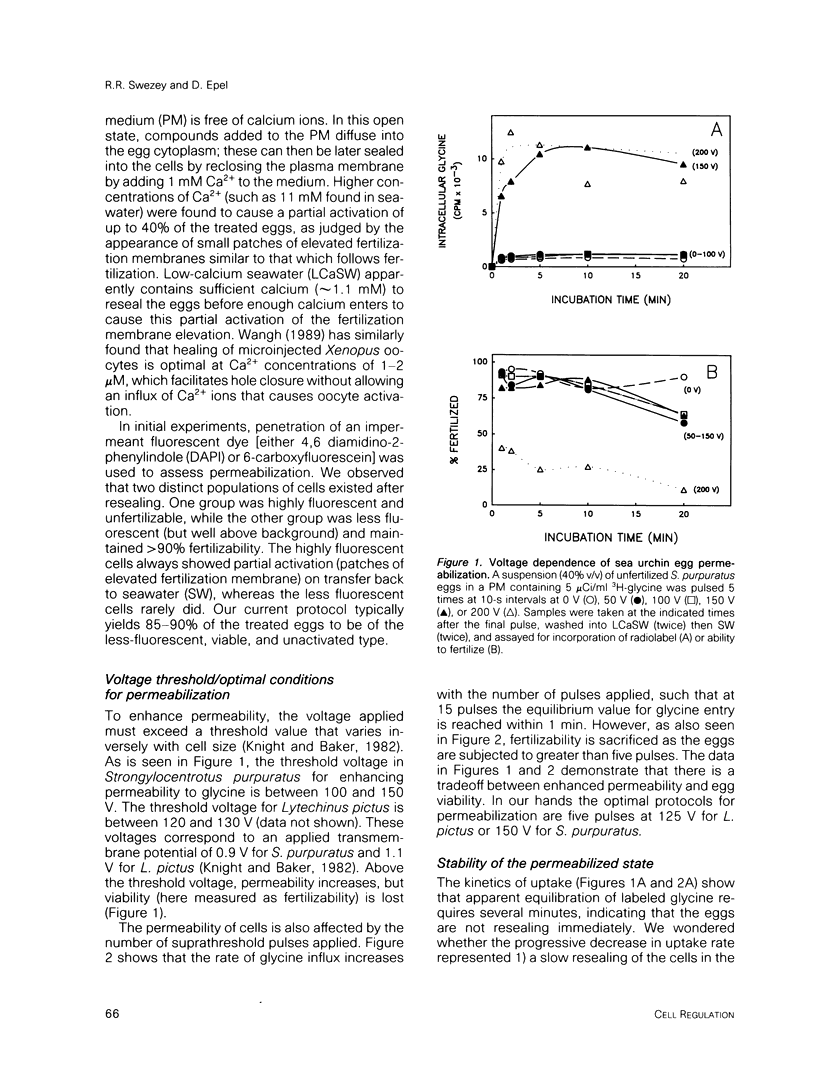
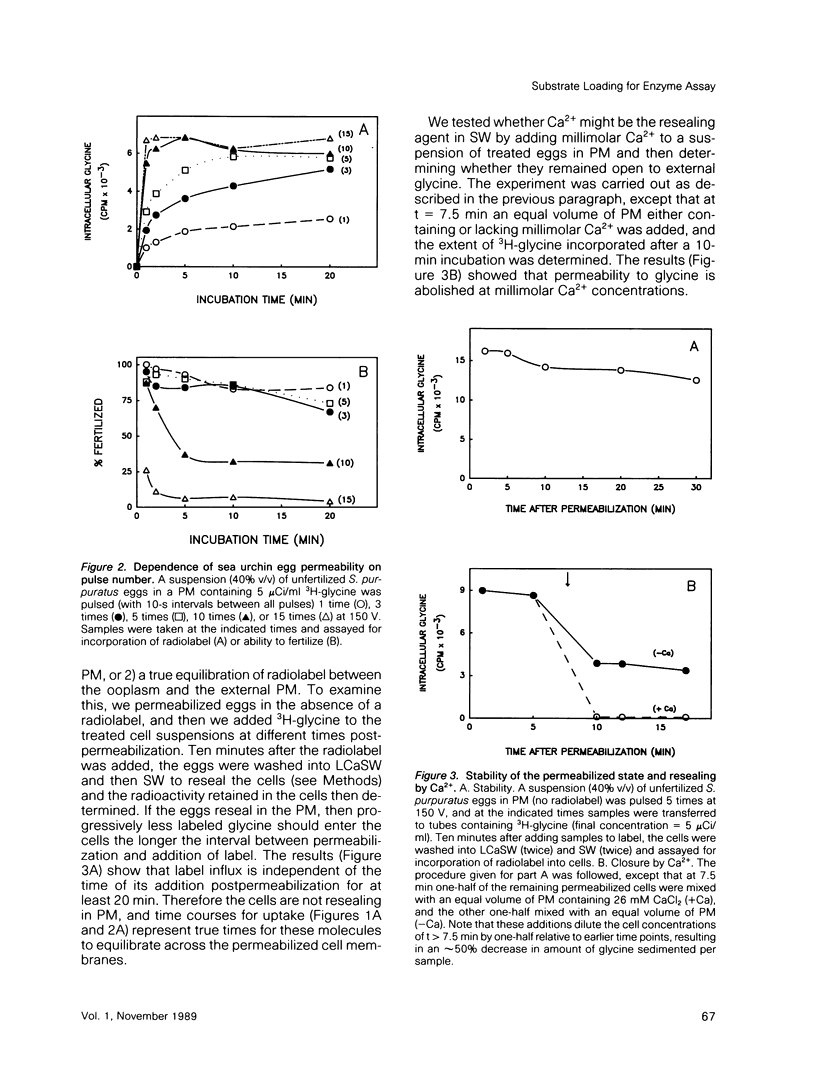
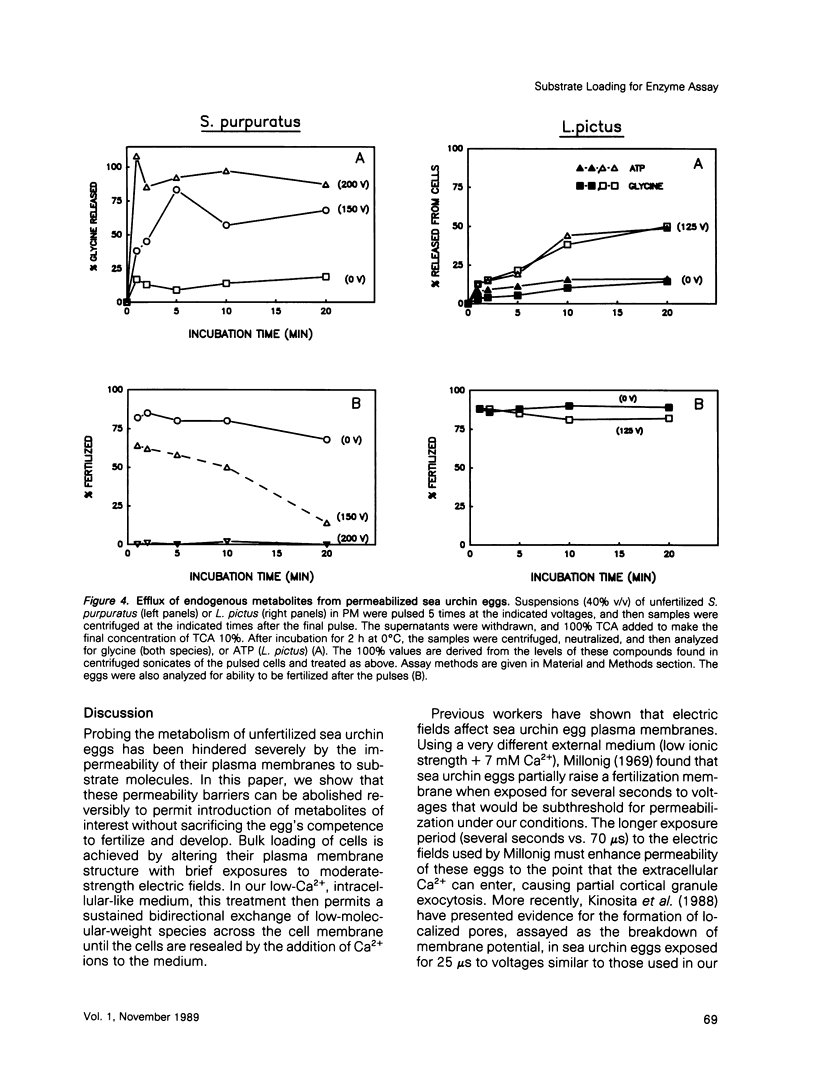
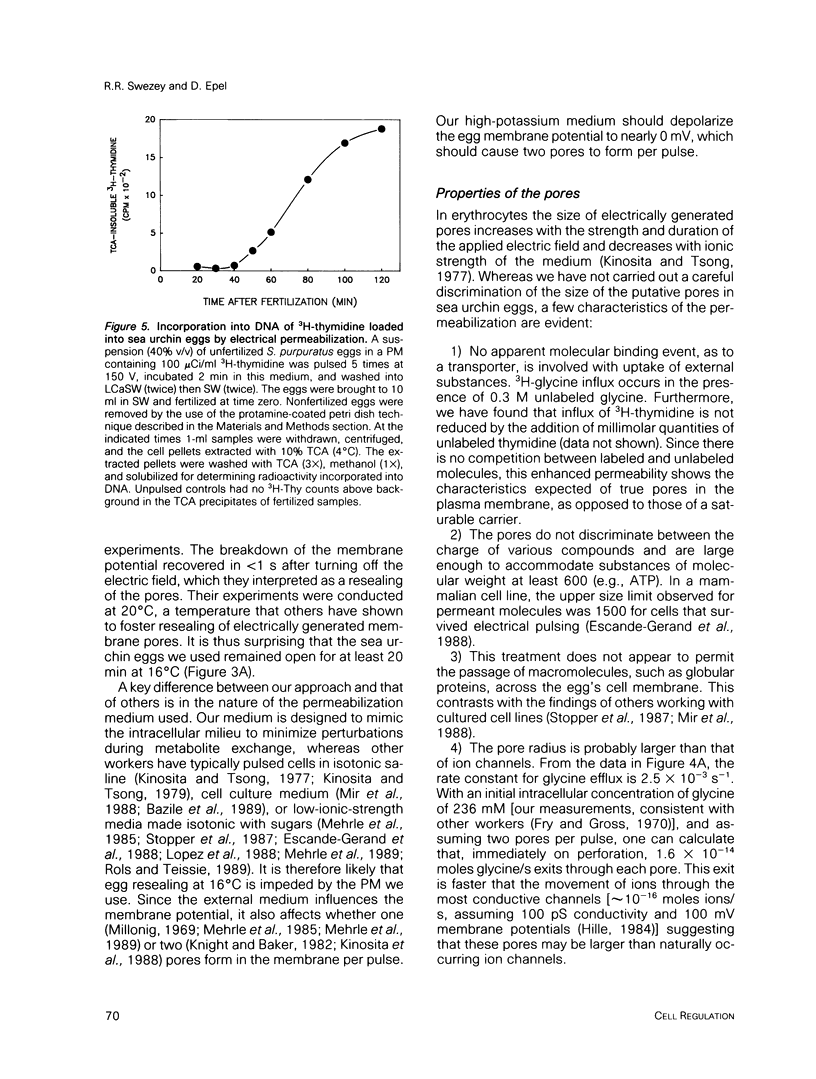
We describe a simple electroporation procedure for loading suspensions of unfertilized sea urchin eggs with impermeant small molecules under conditions that allow close to 90% successful fertilization and development. Poration is carried out in a low-Ca2+ medium that mimicks the intracellular milieu. The induced pores remain open for several minutes in this medium, allowing loading of the cells; resealing is achieved by adding back millimolar calcium ions to the medium. While the pores are open, an influx of exogenous molecules and efflux of endogenous metabolites takes place, and the eggs can lose up to 40% of their ATP content and still survive. Introduced metabolites are utilized by the cells, e.g., introduced 3H-thymidine is incorporated into DNA. This procedure will be useful for loading impermeant substrates into eggs, permitting in vivo assessment of metabolism, and also for introducing other interesting impermeant molecules, such as inhibitors, fluorescent indicators, etc. Though the details may differ, the principle of electroporation in an intracellular-like medium may prove to be useful for loading other cell types with minimal loss of viability.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Alder G. M., Graham J. M., Menestrina G., Pasternak C. A. Ion modulation of membrane permeability: effect of cations on intact cells and on cells and phospholipid bilayers treated with pore-forming agents. J Membr Biol. 1988 Jul;103(1):79–94. doi: 10.1007/BF01871934. [DOI] [PubMed] [Google Scholar]
- Bazile D., Mir L. M., Paoletti C. Voltage-dependent introduction of a d[alpha]octothymidylate into electropermeabilized cells. Biochem Biophys Res Commun. 1989 Mar 15;159(2):633–639. doi: 10.1016/0006-291x(89)90041-7. [DOI] [PubMed] [Google Scholar]
- Escande-Géraud M. L., Rols M. P., Dupont M. A., Gas N., Teissié J. Reversible plasma membrane ultrastructural changes correlated with electropermeabilization in Chinese hamster ovary cells. Biochim Biophys Acta. 1988 Apr 7;939(2):247–259. doi: 10.1016/0005-2736(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Esen A. A simple method for quantitative, semiquantitative, and qualitative assay of protein. Anal Biochem. 1978 Aug 15;89(1):264–273. doi: 10.1016/0003-2697(78)90749-2. [DOI] [PubMed] [Google Scholar]
- Fraley R., Subramani S., Berg P., Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980 Nov 10;255(21):10431–10435. [PubMed] [Google Scholar]
- Fry B. J., Gross P. R. Patterns and rates of protein synthesis in sea urchin embryos. II. The calculation of absolute rates. Dev Biol. 1970 Feb;21(1):125–146. doi: 10.1016/0012-1606(70)90065-5. [DOI] [PubMed] [Google Scholar]
- Grigor'ev N. G., Shmukler Iu B. Izuchenie reguliatsii tsitokeneza rannikh zarodyshei morskogo ezha s pomoshch'iu élektricheskogo proboia membrany. Dokl Akad Nauk SSSR. 1986;287(2):463–466. [PubMed] [Google Scholar]
- Heppel L. A., Weisman G. A., Friedberg I. Permeabilization of transformed cells in culture by external ATP. J Membr Biol. 1985;86(3):189–196. doi: 10.1007/BF01870597. [DOI] [PubMed] [Google Scholar]
- Hughes K., Crawford N. Reversible electropermeabilisation of human and rat blood platelets: evaluation of morphological and functional integrity 'in vitro' and 'in vivo'. Biochim Biophys Acta. 1989 Jun 6;981(2):277–287. doi: 10.1016/0005-2736(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Jastreboff M. M., Sokoloski J. A., Bertino J. R., Narayanan R. Use of electroporation to study the cytotoxic effects of fluorodeoxyuridylate in intact cells. Biochem Pharmacol. 1987 Apr 15;36(8):1345–1348. doi: 10.1016/0006-2952(87)90092-x. [DOI] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Kucera R., Paulus H. Studied on ribonucleoside-diphosphate reductase in permeable animal cells. I. Reversible permeabilization of mouse L cells with dextran sulfate. Arch Biochem Biophys. 1982 Mar;214(1):102–113. doi: 10.1016/0003-9861(82)90012-1. [DOI] [PubMed] [Google Scholar]
- Lemons R., Forster S., Thoene J. Protein microinjection by protease permeabilization of fibroblasts. Anal Biochem. 1988 Jul;172(1):219–227. doi: 10.1016/0003-2697(88)90435-6. [DOI] [PubMed] [Google Scholar]
- Lopez A., Rols M. P., Teissie J. 31P NMR analysis of membrane phospholipid organization in viable, reversibly electropermeabilized Chinese hamster ovary cells. Biochemistry. 1988 Feb 23;27(4):1222–1228. doi: 10.1021/bi00404a023. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Warder E. Glass beads load macromolecules into living cells. J Cell Sci. 1987 Dec;88(Pt 5):669–678. doi: 10.1242/jcs.88.5.669. [DOI] [PubMed] [Google Scholar]
- Mehrle W., Hampp R., Zimmermann U. Electric pulse induced membrane permeabilization. Spatial orientation and kinetics of solute efflux in freely suspended and dielectrophoretically aligned plant mesophyll protoplasts. Biochim Biophys Acta. 1989 Jan 30;978(2):267–275. doi: 10.1016/0005-2736(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Exogenous phospholipase C permeabilizes mammalian cells to proteins. Exp Cell Res. 1988 Jul;177(1):154–161. doi: 10.1016/0014-4827(88)90033-x. [DOI] [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Rols M. P., Teissie J. Ionic-strength modulation of electrically induced permeabilization and associated fusion of mammalian cells. Eur J Biochem. 1989 Jan 15;179(1):109–115. doi: 10.1111/j.1432-1033.1989.tb14527.x. [DOI] [PubMed] [Google Scholar]
- Schaefer-Ridder M., Wang Y., Hofschneider P. H. Liposomes as gene carriers: efficient transformation of mouse L cells by thymidine kinase gene. Science. 1982 Jan 8;215(4529):166–168. doi: 10.1126/science.7053567. [DOI] [PubMed] [Google Scholar]
- Sokoloski J. A., Jastreboff M. M., Bertino J. R., Sartorelli A. C., Narayanan R. Introduction of deoxyribonucleoside triphosphates into intact cells by electroporation. Anal Biochem. 1986 Nov 1;158(2):272–277. doi: 10.1016/0003-2697(86)90549-x. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature. 1988 Mar 24;332(6162):364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- Stopper H., Jones H., Zimmermann U. Large scale transfection of mouse L-cells by electropermeabilization. Biochim Biophys Acta. 1987 Jun 12;900(1):38–44. doi: 10.1016/0005-2736(87)90275-6. [DOI] [PubMed] [Google Scholar]
- Swezey R. R., Epel D. Enzyme stimulation upon fertilization is revealed in electrically permeabilized sea urchin eggs. Proc Natl Acad Sci U S A. 1988 Feb;85(3):812–816. doi: 10.1073/pnas.85.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swezey R. R., Epel D. Regulation of glucose-6-phosphate dehydrogenase activity in sea urchin eggs by reversible association with cell structural elements. J Cell Biol. 1986 Oct;103(4):1509–1515. doi: 10.1083/jcb.103.4.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T. Introduction of macromolecules into mammalian cells by cell fusion. Exp Cell Res. 1988 Sep;178(1):1–17. doi: 10.1016/0014-4827(88)90372-2. [DOI] [PubMed] [Google Scholar]
- Wangh L. J. Injection of Xenopus eggs before activation, achieved by control of extracellular factors, improves plasmid DNA replication after activation. J Cell Sci. 1989 May;93(Pt 1):1–8. doi: 10.1242/jcs.93.1.1. [DOI] [PubMed] [Google Scholar]


