Abstract
Cancer cells display numerous abnormal characteristics which are initiated and maintained by elevated mutation rates and genome instability. Chromosomal DNA is continuously surveyed for the presence of damage or blocked replication forks by the DNA Damage Response (DDR) network. The DDR is complex and includes activation of cell cycle checkpoints, DNA repair, gene transcription, and induction of apoptosis. Duplicating a damaged genome is associated with elevated risks to fork collapse and genome instability. Therefore, the DNA Damage Tolerance (DDT) pathway is also employed to enhance survival and involves the recruitment of translesion DNA synthesis (TLS) polymerases to sites of replication fork blockade or single stranded DNA gaps left after the completion of replication in order to restore DNA to its double stranded form before mitosis. TLS polymerases are specialized for inserting nucleotides opposite DNA adducts, abasic sites, or DNA crosslinks. By definition, the DDT pathway is not involved in the actual repair of damaged DNA, but provides a mechanism to tolerate DNA lesions during replication thereby increasing survival and lessening the chance for genome instability. However this may be associated with increased mutagenesis. In this review, we will describe the specialized functions of Y family polymerases (Rev1, Polη, Polι and Polκ) and DNA polymerase ζ in lesion bypass, mutagenesis, and prevention of genome instability, the latter due to newly appreciated roles in DNA repair. The recently described role of the Fanconi anemia pathway in regulating Rev1 and Polζ-dependent TLS is also discussed in terms of their involvement in TLS, interstrand crosslink repair, and homologous recombination.
Keywords: POLH, POLI, POLK, REV1, REV3, REV7, postreplication repair, Fanconi anemia, Translesion DNA Synthesis, chemoresistance
1. Introduction
Cancer cells display numerous abnormal characteristics including loss of differentiated phenotypes, uncontrolled cell proliferation, and escape from apoptosis, all of which are initiated and maintained by increased mutation rates and genome instability [1]. Mutations in DNA arise from multiple mechanisms including replication errors, spontaneous decay of DNA, or damage created by endogenous reactive metabolites or environmental sources. The continuous accumulation of mutated genes, which leads to turning ‘ON’ or ‘OFF’ of crucial regulatory genes, creates pools of heterogeneous cancer cells possessing selective advantages for survival, duplication, and metastasis [2]. As a means to counteract carcinogenesis, a complex and interactive cellular response to damaged DNA, the DNA Damage Response (DDR), detects DNA lesions and initiates signal transduction cascades that lead to ‘protective’ cell cycle arrests at various positions in the cell cycle along with accurate DNA repair [3–6]. The importance of the DDR in genome maintenance is underscored by the elevated risk of cancer in patients harboring inactivating mutations in genes belonging to these pathways. Hereditary forms of cancer originating in the colon, breast, ovary, and skin are definitively linked to mutations in mismatch repair (MLH1), homologous recombination (BRCA1 and BRCA2), and nucleotide excision repair (Xeroderma Pigmentosum proteins) [7, 8]. Thus, a deficiency in a single gene can establish the conditions for carcinogenesis, by negatively impacting the fidelity of DNA repair.
The ability to duplicate the entire genome in an error-free manner is vital for limiting the risk for cancer. High fidelity nature of genome duplication is largely accredited to the proofreading capabilities of the DNA polymerase δ and ε, which ensure that a correct nucleotide is incorporated at each step. However, the properties of high fidelity and processivity can become limiting when a DNA polymerase encounters an altered template due to covalent adducts and/or distortions in the secondary structure of DNA. DNA polymerase δ and ε stall cannot accommodate bulky lesions or deformed structures within the template and will stall, ultimately leading to replication fork regression and collapse posing a risk for chromosomal translocations and aberrations. Thus, a second response to damaged DNA, termed DNA damage tolerance (DDT), has evolved to promote replication through and beyond an altered template. This highly conserved process, known as translesion DNA synthesis (TLS), invokes the activities of specialized DNA polymerases (TLS polymerases) that can accommodate lesions within template DNA, but in a potentially error-prone manner due to the fact that incorporating nucleotides opposite non-coding bases in the template is inherently mutagenic [9–12]. For these reasons, the DDT pathway is tightly regulated to prevent erroneous TLS during genome duplication. Despite the error-prone nature of the DDT pathway, the ability to replicate through polymerase blocking lesions is important for maintaining genome stability by lowering the overall risk replication fork stalling and degradation. In this review, we will describe the specialized functions of Y family TLS polymerases and DNA polymerase ζ in both mutagenesis and promotion of genome stability, the latter due to recently described roles in DNA repair.
2. TLS polymerases – tolerant enzymes that can use a damaged template to copy DNA
Exposure to ultraviolet (UV) light creates cyclobutane pyrimidine dimers and pyrimidine(6–4)pyrimidone photoproducts in DNA, both of which impose a substantial block to normal replicative DNA polymerases. Early studies in UV-treated prokaryotes brought forth the idea that mechanisms exist that fill in daughter strand gaps left behind after incomplete replication of UV-damaged DNA [13]. In other words, cells possess a unique ability to ‘repair’ gaps left behind where replication could not be completed due to the presence of polymerase blocking lesions (hence the term ‘postreplication repair). DNA damage tolerance became linked with the active process of mutagenesis after the identification of mutant bacteria and yeast exhibiting non-mutable or ‘reversionless’ phenotypes post-UV treatment [14, 15]. The rev1, rev3, and rev7 (for “defective mutation reversion”) genes were discovered using genetic screens that searched for mutants that are hypomutable in response to UV [15, 16]. Elimination of either Rev gene resulted in the same hypomutagenic phenotype suggesting they worked together to influence the rate of mutation. Even background mutation rates were reduced in the absence of rev1, rev3, or rev7 gene function. During the same period, human cell lines derived from patients with the variant form of Xeroderma Pigmentosum (XP-V), a hereditary disorder associated with sensitivity to sunlight and high incidence of skin cancer, were characterized as being deficient in filling in gaps in DNA left behind after replication of DNA damaged by UV light [17]. These findings implied that similar pathways exist in human cells and can influence the process of carcinogenesis, in addition to the development of human disease.
2.1 Rev1 and DNA polymerase ζ
Cloning and characterization of Rev3 revealed that mutagenesis is due to the activities of a DNA polymerase sharing sequence similarities within the catalytic domain within Polδ, Polε and Polα, all members of the B family of DNA polymerases [18–22]. Rev7 and Rev3 were later shown to form a DNA polymerase complex named Polζ [23]. Polζ possesses lower processivity and is devoid of the 3′→ 5′ proofreading exonuclease activity present in most B-family DNA polymerases [23]. Although, Rev3 alone is capable of polymerization, association of Rev3 with Rev7 has been shown to stabilize and significantly enhance the polymerase activity of Rev3 by 20–30 fold, suggesting that Rev7 functions as a processivity factor for Polζ [23]. Recently, the two accessory subunits (Pol31 and Pol32) which associate with the catalytic subunit (Pol3) of yeast Polδ, were also identified in a complex with Polζ and the presence of Pol31 and Pol32 is needed for efficient TLS in yeast [24, 25][CC1]. Thus, Polζ is now considered to be a four subunit polymerase. These recent findings suggest that accessory subunit ‘swapping’ between Polδ and ζ may facilitate the exchange of DNA polymerases during the transition from normal replication to TLS [26].
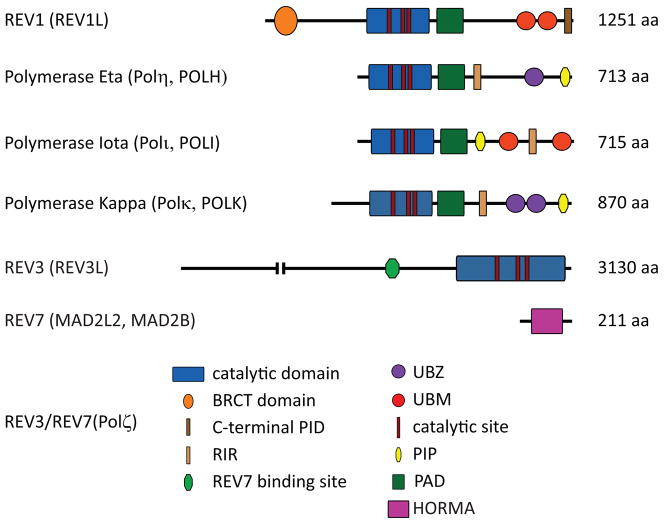
Compared to other TLS polymerases, Polζ is not very efficient in replicating through most DNA lesions, the exception being certain adducts like thymine glycol, where it has been reported to perform both the insertion and extension steps opposite the thymine glycol lesion in an error-free manner [27]. Rather than inserting nucleotides opposite DNA adducts, Polζ appears to be particularly specialized to extend distorted base pairs, such as mismatches that might result from an inaccurate base insertion by a TLS polymerase or a base pair involving a bulky DNA lesion [28, 29]. Therefore, the primary role of Polζ in TLS is performing extension beyond nucleotides inserted opposite the lesion by another TLS polymerase [30]. In addition, Polζ has recently been shown to be essential for bypassing ribonucleotides misincorporated into DNA and promotes tolerance to the presence of these toxic lesions [31]. The overall proficiency in extending insertions opposite mismatches and DNA adducts with a relatively high error rate for base substitutions is associated with the mutagenic properties of Polζ [32, 33]. Mammalian Rev3 (encoded by the Rev3l gene) is twice as large as the yeast protein, expressed at extremely low levels, and has yet to be characterized at the biochemical level [19, 20, 22]. Yeast can grow and thrive without Rev3 activity and suffer some survival disadvantage when cells are exposed to genotoxic stress. This is in direct contrast to mice where knocking out Rev3l leads to embryonic lethality as well as genome instability [34–39] Embryonic cells. lacking Rev3 show high levels of apoptosis and display elevated numbers of spontaneous chromosomal aberrations and translocations The embryonic lethality observed in Rev3−/− mice strongly suggests that at least during early development, loss of Polζ activity cannot be compensated for by other TLS polymerases The structural domains of mammalian Rev1, Rev3,. and Rev7 are detailed in Figure 1.
Figure 1. The structural domains of Polζ (comprised of REV3 and REV7) and the Y family of DNA polymerases.
BRCT, BRCA1 C-terminus like domain; PIP, PCNA interacting peptide; PAD, polymerase-associated domain; UBZ, ubiquitin-binding Zinc finger domain; UBM, ubiquitin-binding motif; RIR, REV1-interacting region; PID, C-terminal polymerase-interacting domain; HORMA; HOP1, REV7 and MAD2.
Yeast and mammalian Rev1 (Rev1l) are related to the E. coli UmuC protein and not considered processive DNA polymerases. Instead Rev1 is a deoxycytidyl (dCMP) transferase, whose activity appears to be restricted to inserting dCMPs opposite guanines and abasic sites in template DNA [40–46]. Additional insights made from the crystal structure of the polymerase domain of S. cerevisiae Rev1 bound to a primer template and an incoming dCTP revealed that Rev1 uses a novel means of catalysis whereby the incoming dCTP pairs with an arginine within the active site and the template base is flipped out of the catalytic site [47]. This limited dCMP transferase activity does not seem to be important for most functions of Rev1 since catalytic inactive Rev1 can still promote Rev3-dependent mutagenesis in yeast [48, 49]. Instead, Rev1 appears to play a structural role in regulating Polζ-dependent TLS.
2.2 DNA polymerase eta
The gene responsible for XP-V (also referred to as RAD30A or POLN) turned out to be a DNA polymerase that replicates DNA templates containing thymine dimers efficiently and with high fidelity and has been named DNA polymerase eta (Polη) [50–53]. In fact, Polη appears to be specifically designed for accurate replication of these lesions [54, 55]. In the absence of Polη, cells replicate DNA poorly following UV irradiation and are UV hypermutable emanating from the loss of error-free Polη activity past UV photoadducts [17, 50, 56–61]. In this scenario, TLS is presumably carried out by more error prone polymerases, as has been observed in yeast where deletion of rad30 reveals the well-characterized mutator phenotype dependent upon rev1, rev3, and rev7 [48] The increase in error-prone TLS in XP-V patients most likely. contributes to carcinogenesis of the skin Consistent with these observations, REV1, REV3, and. REV7 have all been associated with UV-induced mutagenesis in human cells [41, 62–65].
2.3 DNA polymerase iota
It is unclear whether REV1 and REV3 are solely responsible for UV-induced hypermutablity observed in XP-V patient cells since vertebrates express two additional Y-family TLS polymerases, Polι and Polκ. Polι is a paralog of Polη, and thus named RAD30B, and has been characterized as the most error-prone polymerase within the Y family, incorporating dGMP opposite thymine 10 times more frequently than dAMP, but accurately incorporating dTMP opposite adenine [29, 66, 67]. This unique property is explained by the fact that Polι utilizes Hoogsteen base paring for efficient and correct dNMP incorporation opposite altered purines, such as 8-oxoguanine, which would otherwise be complementary for dA or dC [68, 69]. The unique structure of Polι restricts the template 8-oxoG in the syn confirmation thereby promoting the correct insertion of dCMP [70]. On this note, Polι may play a specialized role in the tolerance of oxidative damage for which 8-oxoG is a major product of oxidative DNA damage by chemicals such as hydrogen peroxide [71]. Unlike Polη, Polι is capable of inserting the correct nucleotide opposite highly distorting (6–4) photoproducts, but does not have the capacity to extend beyond the initial insertion [28, 29]. Other TLS polymerases are required to extend beyond the first insertion opposite this adduct (e.g. Rev3 and Polκ). In the absence of Polη, a significant amount of UV-induced mutagenesis has been attributed to Polι activity and may therefore also contribute to carcinogenesis in XP-V patients [72–76].
2.4 DNA polymerase kappa
Polκ is the orthologue of the E. coli dinB (DNA Pol IV) in higher eukaryotes [77–79] Polκ is specialized in performing error-free bypass of bulky minor groove N2-deoxyguanine adducts among other lesions, including mispaired primers, and plays a critical role in limiting mutagenesis from specific bulky lesions such as benzo(a)pyrene adducts, an environmental carcinogen present in tobacco smoke [80–88]. In the absence of Polκ, mice exhibit elevated mutation frequencies suggesting that Polκ is needed to execute error-free lesion bypass of DNA damaged by reactive metabolites that form bulky adducts [89]. On the other hand, Polκ is highly error-prone when replicating a normal template and therefore can be a notable source for mutagenesis in mammalian cells [77, 86, 90, 91]. The crystal structure of the ternary complex of the Polκ catalytic core with DNA and an incoming nucleotide reveals that the DNA is completely encircled by the polymerase, with an N-clasp augmenting the thermodynamic stability of the catalytic complex. This locked structure leads to increased polymerase residence on the template, thus allowing the efficient extension of distorted primer termini [92, 93]. Polκ is also involved in nucleotide excision repair (NER) after UV irradiation, especially under conditions of deoxynucleotide imbalances, highlighting recent observations that TLS polymerases can be recruited to facilitate actual DNA repair processes [94, 95].
3. Regulation of TLS – ‘polymerase switching’
3.1 PCNA ubiquitination
In S. cerevisiae, genetic studies have shown that genes belonging to the rad6 epistasis group are involved in the replication of damaged DNA, a process termed postreplication repair [96–98]. Of these, rad6 and rad18 are required for both error-free and error-prone replicative bypass of UV-induced DNA lesions, while rad5, mms2, and ubc13 are important for regulating error-free lesion bypass using a TLS polymerase-independent mechanism that involves template switching [99]. Rad6 and the Mms2-Ubc13 complex function as E2 ubiquitin-conjugating enzymes in association with the Rad18 and Rad5 E3 ubiquitin ligases respectively, and regulate the ubiquitination state of the homotrimeric sliding clamp, proliferating cell nuclear antigen (PCNA) [96, 97, 100]. Replication fork stalling uncouples the coordinated efforts of DNA polymerase and DNA helicase resulting in the generation of long stretches of single-stranded DNA (ssDNA) [101, 102]. Replication factor A (RPA) rapidly binds to ssDNA and serves as a recruitment factor for Rad18 and other DNA damage response proteins such as ATR and ATRIP [103, 104]. Current models suggest that Rad6 and Rad18 are recruited to these regions via RPA to monoubiquitinate PCNA on lysine-164 (K164) triggering TLS (Figure 2). The first ubiquitin moiety conjugated to PCNA on K164 can then be further extended by Mms2–Ubc13 and Rad5 forming a poly-K63-linked ubiquitin chain which signals the error-free template switching mechanism [100, 105–107]. However, this view has recently been challenged with new data suggesting that the human homologue of Rad5, HTLF, transfers polyubiquitin chains to RAD6, permitting RAD18 to attach polyubiquitin K-63-linked ubiquitin chains to PCNA [108]. Thus, RAD18 may directly control both states of PCNA ubiquitination. Regardless of the precise mechanism of ubiquitin chain elongation on PCNA, it is generally believed that monoubiquitination of PCNA mediates the switch to TLS, whereas polyubiquitination of PCNA channels the postreplication repair pathway into an uncharacterized error-free damage avoidance pathway that involves template switching [9].
Figure 2. A model for the regulation of the TLS pathway.
Replication forks may inevitably encounter DNA adducts that are not repaired (such as a 6–4 photoproduct). These lesions block high fidelity polymerases, potentially leading to replication fork stalling, gaps in replicated DNA, and the generation of DNA double-strand breaks (DSBs). Current models suggest that replication fork stalling uncouples the replicative helicase from normal high fidelity DNA polymerases resulting in further unwinding of the double helix and the creation of abnormal large stretches of single stranded DNA (ssDNA). These single-stranded, unreplicated regions become coated with the ssDNA binding protein Replication protein A (RPA) which serves to initiate ATR signaling, as well as recruit the RAD18 E3 ubiquitin ligase to trigger the postreplication repair pathway which includes TLS. Together, the E3 ubiquitin ligase RAD18 and the RAD6 E2 ubiquitin conjugating enzyme monoubiquitinate PCNA on Lys-164, the latter a homotrimeric protein that functions as an auxiliary factor for DNA polymerases. This event is thought to operate as a molecular switch from normal DNA replication to the TLS. In this example of the two polymerase model for lesion bypass across a 6–4 photoproduct, Polymerase eta (Polη) inserts a nucleotide directly opposite the lesion and requires an additional TLS polymerase, such as DNA polymerase zeta (Polζ), to extend beyond the insertion. REV1 facilitates the exchange from Polη to Polζ which subsequently performs the extension step beyond the inserted nucleotide opposite the damaged base. Lesion bypass of DNA adducts may be executed with high fidelity or can lead to mutations that promote carcinogenesis. The Fanconi anemia (FA) core complex plays an important role localizing REV1 to stalled replication forks via interaction with the FA core complex-associated FAAP20 protein. The deubiquitinase protein USP1 maintains PCNA in a non-ubiquitinated state thereby minimizing TLS activity in the cell under normal conditions. USP1 proteins levels are reduced following UV exposure thereby shifting the balance towards PCNA ubiquitination by RAD18 and RAD6. ELG1 is an alternative component of the replication factor C complex (which loads PCNA onto DNA) and associates with the USP1-UAF1 complex to down regulate PCNA monubiquitination and TLS. Potential outcomes of TLS activity or lack of TLS activity are illustrated.
One potential outcome of attaching a single ubiquitin moiety to PCNA is mutagenic lesion bypass by Rev1 and Polζ. It is important to note here that Rev1 and Polζ-dependent TLS not only occurs during replication, but also after DNA replication has completed, being responsible for filling in gaps left behind after stalled replication forks resume DNA synthesis downstream of a replication-blocking lesion [109–113]. Hence, significant TLS activity occurs in G2 after replication has completed. Alternatively, Polη can accurately insert nucleotides opposite thymine dimers and reduce UV-induced mutagenesis, and hence Polη’s activity is often referred to as being responsible for error-free lesion bypass within the postreplication repair pathway, at least in the more simplified model described for yeast.
All eukaryotic Y-family polymerases possess ubiquitin binding motifs (UBM) or ubiquitin-binding zinc finger (UBZ) domains which increase their affinity for ubiquitinated PCNA [114–119]. Any DNA adduct that poses a block to replication induces the formation of TLS polymerase-containing foci, and this event is driven by PCNA monoubiquitination [120–128]. It is unclear why such a high concentration of TLS polymerases is needed within the region surrounding stalled replication forks such that they form visible foci via immunofluorescence microscopy. Polymerase foci colocalize with PCNA and γH2AX, a marker of DNA damage and replication stress, implying these are regions of chromatin undergoing postranslational modifications by both the DDR and DDT pathways [127, 129].
Although ubiquitination of PCNA is absolutely necessary for TLS in yeast, recent studies suggest that alternative pathways may regulate at least some polymerase switching in vertebrates. Analysis of the replication of damaged DNA in chicken DT40 cells demonstrated a predominant role for PCNA ubiquitination only in promoting the filling in of post-replication gaps rather than TLS occurring at a stalled replication fork [130]. This latter activity requires Rev1 and its ability to contact ubiquitin and other TLS polymerases (see section 3.2). In addition, Rev1-dependent TLS across a pyrimidine 6–4 photoproduct in DT40 cells carrying a PCNA K164 mutation appears to be normal as measured by a plasmid system [131]. Recent work from the Livneh group utilized mouse embryonic fibroblasts in which specific TLS genes associated with ubiquitination of PCNA were manipulated. These studies showed that eliminating expression of Rev3, Polη or Rev1 in PCNAK164R/K164R mouse embryo fibroblasts further increased their sensitivity to UV-radiation indicating the existence of a TLS pathway that is independent of PCNA ubiquitination [132]. At least in DT40 cells, this non-canonical TLS pathway appears to be largely dependent on Rev1 [130, 131]. Recent studies have identified the Fanconi anemia core complex as also being an important regulator of Rev1 localization to stalled replication forks resulting from UV or cisplatin treatment [127, 133, 134].
As with all posttranslational modifications, the presence of ubiquitinated PCNA is tightly regulated by both its addition and removal. The deubiquitinase (DUB), ubiquitin specific protease 1 (USP1), is a key regulator of this pathway by maintaining PCNA in a ubiquitin-free form [135]. USP1 protein levels are reduced following UV exposure thereby shifting the balance towards PCNA ubiquitination by Rad18 and Rad6. The mechanism for this decrease in response to UV involves an autocleavage event followed by proteasomal degradation of the cleaved products. However, this appears to be a unique response to UV damage and not other genotoxic agents. Studies conducted in cells lacking USP1 reveal that these cells exhibit increased TLS activity as well as increased mutation rates due to erroneous TLS [132, 135]. Two recent studies revealed that a major function of USP1 and its partner UAF1 is to suppress sporadic monubiquitination of PCNA during normal DNA replication [136, 137]. Reduced replication fork speed and genomic instability observed in USP1-deficient cells has been attributed to increased levels of PCNA monoubiquitination and hyperaccumulation of Polκ to replication forks [138]. USP1−/− mice also display increases in PCNA monoubiquitylation and exhibit high rates of perinatal lethality, depletion of male germ cells, infertility, hypersensitivity to the crosslinking agent mitomycin C, and chromosome instability [139]. Thus, inappropriate recruitment of TLS polymerases can interfere with the maintenance of genome stability during DNA replication.
Other factors have been found to regulate the state of PCNA ubiquitination. Elg1 comprises an alternative Replication Factor C (RFC) complex when substituted for the RFC1 subunit. Human ELG1 (which is also called ATAD5) interacts with PCNA, colocalizes with the sliding clamp at stalled replication forks, and also associates with the USP1-UAF1 complex which removes ubiquitin moieties from PCNA [137]. Similar to USP1-depleted cells, cells with reduced ELG1 function display hyper-PCNA monoubiquitylation, elevated mutation rates, and chromosomal instability. [137, 140]. Furthermore, deletion of Elg1 in mice leads to chromosomal instability and a strong predisposition to cancer [141]. These results indicate that inappropriate recruitment of TLS polymerases to replication forks can have detrimental consequences to genome stability and therefore, their activities must be tightly regulated.
3.2 Rev1 and Polζ: completing TLS across many DNA lesions
Today, it is clear that for most DNA adducts, more than one TLS polymerase is needed to accomplish a single lesion bypass event [30, 127, 142]. Current models suggest a two step mechanism where a Y family polymerase inserts the first nucleotide opposite a damaged base and a second polymerase (notably Polζ) performs the extension step, even if the resulting primer/template pair is highly distorted (Figure 2). Rev1 is thought to be an essential regulator of TLS polymerase switching between the first TLS polymerase and Polζ [143, 144]. Rev1 has several protein-protein interactions domains that facilitate its function as a TLS polymerase scaffold protein. Rev1 is the only Y family polymerase that contains an N-terminal BRCA1 C-terminal homology (BRCT) domain which is capable of making direct contact with PCNA and DNA, the latter with a preference towards 5′-phosphorylated primer/template junctions that mimic a postreplication gap in DNA [145, 146]. Important to its overall function, Rev1 possesses a unique TLS polymerase interacting domain that makes direct contact with Rev7 of Polζ and a variety of TLS DNA polymerases including Polη and Rev3 [128, 143, 144, 147–150]. Disruption of the polymerase-interaction domain essentially eliminates Rev1-mediated mutagenesis [151, 152]. A Rev1-interacting region (RIR) has been identified in all Y family polymerases (Polη, Polι and Polκ) and is required for efficient binding to the c-terminus of Rev1. This motif is defined by short sequences in which the presence of two consecutive phenylalanine (F) residues is critical [153–155]. The ability to interact with Rev1 via the RIR motif is necessary for Polκ function in mouse embryonic fibroblasts [153].
We are now beginning to understand how mammalian Rev1 can interact with Polζ and other Y family polymerases simultaneously to regulate multi-polymerase TLS. The similarity of phenotypes in cells depleted of either Rev1 or Rev3 has strongly suggested direct interactions between Rev1 and Polζ to form a functional complex [127, 156–160]. At least in yeast, protein interactions with the c-terminal domain of Rev1 appear to be limited to Polζ via the Rev7 subunit [143, 150]. Recently, the crystal structure between human REV7 and the REV7 binding site on REV3 (amino acids 1847–1898) has been solved [161]. Based on this data, Hara et al suggest that REV7 binds both REV3 and REV1 simultaneously where binding of REV7 to REV3 alters the conformation of REV7 in a way that reveals an additional REV1 binding site [161]. This is an intriguing result since it has always been assumed that REV7 interactions with REV1 and REV3 were mutually exclusive. Over the past year, several structural and biochemical analyses of protein fragments belonging to REV1, REV7, plus the RIR from different Y family polymerases now indicate that the REV1 C-terminal domain can simultaneously contact both insertion and extension TLS polymerases [154, 155, 162, 163]. Future studies will be needed to fully disclose how REV1 performs its function as a TLS polymerase ‘switcher’ during complex lesion bypass events.
4. TLS polymerases directly implicated in DNA repair and genome maintenance
4.1 Rev1 and Polζ promote resistance to many forms of DNA damage
Deletion of Rev7, Rev3 or Rev1 in chicken DT40 cells or mammalian cells leads to remarkable hypersensitivity to a wide variety of genotoxic stresses including UV, methyl-methanesulfonate (MMS), DNA crosslinking agents, and DNA double-strand breaks (DSBs) [35, 127, 156, 157, 159, 164–170]. This has lead to the idea that Rev1 and Polζ may have additional roles in promoting survival, genome stability, and DNA repair that is not compensated for by other TLS polymerases and beyond the conical postreplication repair pathway. An open question is whether lack of Polζ-dependent extension beyond most if not all DNA adducts explains this profound hypersensitivity to such a variety of DNA lesions, especially ionizing radiation (IR), where one would presume most damage is the result of ssDNA breaks which are rapidly repaired. Increased radiosensitivity, chromosomal aberrations, and slow resolution of γ-H2AX foci after IR suggest insufficient or slow repair of DSBs, as has been observed in irradiated nasopharyngeal carcinoma cells expressing shRNA specific for REV7 (MAD2B) as well as other tumor cell lines cells depleted of REV1, REV3 or REV7 [156, 164, 171]. Recently, direct evidence has been obtained demonstrating that Polζ plays a direct role in executing homologous recombination repair, potentially explaining this extreme hypersensitivity to DSBs and replication stress induced by various genotoxic agents [156, 169].
4.2 Are Rev1 and Polζ part of the fanconi anemia pathway?
Fanconi anemia (FA), first described in 1927 by the Swiss pediatrician Guido Fanconi, is a genome instability syndrome characterized by bone marrow failure, developmental abnormalities and an increased incidence of developing cancers [172, 173]. At the cellular level, FA cells display increases in chromosomal aberrations, particularly of the type referred to as radial chromosomes, and exhibit extreme hypersensitivity to DNA interstrand crosslink (ICL) inducing agents, such as mitomycin C, cisplatin and diepoxybutane [174–176]. At least 15 FA or FA-interacting genes have been identified and these ‘FA’ proteins have been shown to form several complexes that regulate or perform ICL repair [134, 172, 177]. Eight of these proteins (FANCA, -B, -C, -E, -F, -G, -L and -M) along with five FA-associated proteins (FAAP100, FAAP24, HES1, MHF1, MHF2 and the newly identified FAAP20) form the nuclear core complex [173]. The core complex functions as an E3 ubiquitin ligase by monoubiquitinating the FANCD2-FANCI heterodimer in response to DNA damage (Figure 3) [178–181]. Monoubiquitination takes place constitutively in S-phase and is dramatically increased upon DNA damage [181, 182]. In response to DNA damage, the monoubiquitinated FANCD2-I complex is recruited to chromatin where it interacts with downstream FA proteins (FANCD1/BRCA2, FANCJ/BACH1, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, and FA-associated nuclease 1 (FAN1)), all of which are involved in promoting homologous recombination repair [183–192]. Monoubiquitinated FANCD2 provides binding sites for SLX4, a protein scaffold that binds multiple structure-specific endonucleases including the FA-associated nuclease, FAN1. This complex is believed to unhook an ICL, participate in HR repair of the resulting replication associated break, and potentially facilitate TLS across the unhooked crosslink [191, 193–195]. Deficiencies in any of these proteins results in the characteristic deficiency in DSB resolution following exposure to DNA crosslinking agents, as well as reductions in gene conversion efficiencies, identical to what has have observed in REV1 or Polζ-depleted cells [127, 156].
Figure 3. Interactions of the Fanconi anemia (FA) pathway with TLS pathway.
High fidelity DNA polymerases stall upon encounter of an interstrand crosslink (ICL) in DNA. This event triggers the Fanconi anemia (FA) pathway, whereby the FA core complex detects an ICL and functions as an E3 ubiquitin ligase to monoubiquitinate the FANCD2-FANCI heterodimer. The monoubiquitinated FANCD2-I complex is recruited to the chromatin where it promotes ‘unhooking’ of the ICL and further nucleolytic processing of DNA by structure specific endonucleases - MUS81-EME1, SNM1A, FAN1, XPF-ERCC1 and SLX1-SLX4. Replication-associated DSBs are generated during the repair process and become substrates for homologous recombination (HR) to ultimately complete ICL repair. FAAP20 interacts with the FA core complex and binds to monoubiquitinated REV1. FAAP20 may direct REV1 and Polζ to the unhooked crosslink where they perform lesion bypass across the adduct creating a substrate for HR repair. We speculate that REV1 and Polζ are not only involved in TLS across the ‘unhooked ICL’ in preparation HR repair, but also perform DNA synthesis during HR repair when the template is incompatible for normal replicative DNA polymerases and may still contain unhooked ICL lesions or other polymerase blocking lesions. Both the FA pathway and REV1/Polζ have been implicated in facilitating HR repair of DSBs separate from the ICL repair pathway.
Ketan Patel first demonstrated an epistatic relationship between the FANCC gene, which encodes one of the fanconi anemia core proteins, and Rev1 or Rev3 in chicken DT40 cells treated with cisplatin, thus directly implicating Rev1 and Rev3 in ICL repair [160]. The authors found little difference in the sensitivities of FANCC−/− DT40 cells compared to FANCC−/−/Rev1 −/− cells or FANCC−/−/Rev3−/− cells to loss in viability or the accumulation of chromosomal aberrations following cisplatin treatment, thus establishing an epistatic relationship between these genes. Sonoda’s laboratory confirmed these important observations [166]. Direct evidence implicating the involvement of the FA pathway and Polζ in ICL repair was obtained using the Xenopus leavis egg extract system, wherein it was shown FANCD2-FANCI ubiquitination promotes both the incision and TLS steps of ICL repair [196, 197]. This work led to the proposal that like PCNA ubiquitination, FANCD2-I ubiquitination plays a role in recruiting TLS polymerases to ICLs via their ubiquitin-binding domains. However to date, no evidence exists suggesting a direct interaction between FANCD2 and Rev1. Regardless, these studies brought forth the most current model for ICL repair that involves the cooperation between the FA pathway, translesion DNA synthesis by Rev1 and Polζ, and homologous recombination (Figure 3).
Early studies into the molecular defects of FA patient cell lines discovered that these cells are hypomutagenic in response to UV, a phenotype very similar to Rev1 and Rev3-deficient cells [198]. Similarly, FANCG−/− hamster CHO cells show reduced generation of viable HPRT mutations, thereby pointing towards a defect in TLS at the HPRT locus during DNA replication [199]. Also, FANCC-deleted DT40 cells have a lower frequency of spontaneous point mutations, suggesting that an intact Fanconi anemia pathway is required for Rev1-mediated error prone TLS [160]. Overall, these results suggest that Rev1 and Rev3 are regulated by the FA pathway in executing lesion bypass in two different scenarios: bypass of UV-generated photoproducts or cisplatin intrastrand crosslinks and repair of ICLs. Of importance, there appears to be specific differences in the requirement of FA proteins for TLS in response to replication blockade versus ICL repair. The recruitment of Rev1 into nuclear foci post UV and cisplatin treatment requires an intact Fanconi anemia core complex but is independent of FANCD2 monoubiquitination, thereby suggesting that FA core complex alone regulates TLS in response to replication fork stalling by UV or cisplatin [127, 133]. Accordingly, FA core-deficient patient cells are not as efficient in generating spontaneous and UV-induced point mutations as cDNA-corrected cells [133]. FAAP20 was recently identified by several studies to be an integral part of the FA core complex which plays a role in maintaining the integrity of the core complex, and promotes FANCD2 monoubiquitination [134, 200, 201]. Recent work from the D’Andrea group discovered that FAAP20 directly binds to FANCA and contains a c-terminal UBZ4 domain that associates with ubiquitinated Rev1, thereby providing a physical link between Rev1 and the FA pathways [134]. Together, these studies suggest that FAAP20 specifically promotes Rev1-dependent TLS across replication stalling lesions like thymine dimers and bulky cisplatin adducts and may also directly promote Rev1-dependent TLS during ICL repair.
The identification of BRCA2 and RAD51C mutations causing fanconi anemia-like syndromes provided a direct link between the FA pathway and regulation of homologous recombination repair [183, 190]. Other members of the FA pathway have also been implicated in regulating or participating in HR repair [160, 178, 202–206]. Deficiencies in the FA pathway are typically associated with partial defects in HR repair, the exception being BRCA2 deficiency since this protein is essential for RAD51 loading. We have also discovered that depletion of REV1, REV3, or REV7 in human cells is associated with a reduction in HR efficiency by approximately 50%, similar to FA cells, as opposed to 90–95% seen in cells deficient in the RAD51 protein [156]. This same reduction in HR repair is observed in cells depleted of another DNA polymerase implicated in ICL repair and HR, Polν, and no additive reductions in gene conversion efficiencies are observed when FANCD2 is co-depleted along with Polν, suggesting that Polν participates in the same FA pathway regulating HR repair [207]. Since these studies are measuring repair of a site specific DSB by HR, they imply that alternative DNA polymerases may be needed to perform a subset of these reactions and are not confined to preparing the sister chromatid for HR repair after ICL unhooking. This idea has been recently confirmed in genetic studies using Drosophila melanogaster as a model system, demonstrating a specific requirement for Polζ during HR repair [169]. Together, these observations suggest that Rev1 and Polζ (possibly in collaboration with Polν) may be required to synthesize DNA during a subset of HR reactions that involve extension of distorted or mispaired primer templates that would otherwise cause stalling of normal DNA polymerases. It is of interest to note that the RAD51D gene has been linked to mutation rates in mammalian cells thus HR repair may not always ‘error-free’ [208]. At least in mammalian cells, the Fanconi anemia pathway may be important for regulating TLS during HR repair and rescues problematic HR templates by recruiting the TLS pathway (Figure 3).
5. Deregulated TLS and cancer
5.1 Lack of TLS activity → genome instability
Polη is the only known TLS polymerase that is directly linked to preventing cancer in humans. Inactivating mutations in Polη have always been thought to result in the substitution of alternative error prone polymerases that leads to a constant state of hypermutability upon exposure to sunlight. It is interesting to note that one of the emerging hallmark phenotypes of cancer cells is increased hypermutability [209–216]. However, cancer progression in XP-V patients may be more complex than just more mutations resulting in more risk for carcinogensis. At the cellular level, the absence of Polη leads to enhanced genome instability, especially when p53 function is disrupted - a frequent occurrence during the progression of skin cancer [217–219]. The fact that increases in DNA double stranded breaks marked by DNA damage response proteins like MRE11 occur after exposure to UV irradiation, suggests that replication forks collapse more frequently in XP-V cells. The inability to rapidly engage in TLS following replication fork stalling at DNA adducts, fragile sites, repetitive sequences, or secondary structures formed in template DNA can lead to ‘replication stress’, ultimately leading to replication fork breakage (Figure 4) [220–223]. Incorrect repair of replication-associated DSBs is directly implicated in chromosomal translocations and chromosomal aberrations [205, 224–227].
Figure 4. Consequences of TLS polymerase imbalance.
A) Overactive or inappropriate Translesion DNA synthesis (TLS) polymerases activity (yellow circle) may lead to error-prone DNA replication, resulting in a hypermutable phenotype, carcinogenesis and chemoresistance. B) Balanced activity between TLS polymerases and high delity polymerases (blue circle) leads to normal replication of an undamaged template or appropriate TLS of a damaged template, both routes maintain genomic stability, the latter by preventing replication fork collapse. C) Cells with deficient TLS polymerase activity may not e ectively deal with replication stress, leading to an increased frequency of DSBs and chromosome instability, that in turn can result in cell death or cancer.
Absence of Polζ has been shown to influence tumorigenesis in mouse models [228]. Both thymic lymphomas and mammary gland tumors form more rapidly in a p53 null background. These results indicate that even under conditions of ‘reduced mutagenesis’, absence of Polζ creates a state of increased genomic instability [39, 228]. One would have expected that absence of Polζ-dependent mutagenesis would reduce the rate of carcinogenesis. Instead, lack of the essential extender polymerase likely leads to greater instability by incapacitating the TLS pathway, thereby influencing the rate of replication fork collapse and DSB formation during S phase. A deficiency in HR repair could also be responsible for increased chromosome instability in cells lacking Polζ and the rapid onset of cancer observed in mice [156, 169]. Thus far it has been difficult to correlate a lack of REV1 and Polζ to the incidence of cancer in humans. However, recent studies indicate that both the levels of REV1 and REV3 can be modulated by specific miRNAs that may be dysregulated in cancer [229, 230].
5.2 Too much TLS activity → genome instability
The ‘overuse’ of TLS polymerases during DNA replication can clearly promote carcinogenesis due to their error prone nature on a non-damaged template (Figure 4). Multiple studies have searched for unusual DNA polymerase expression patterns in cancer cell lines and patient tumor samples, as well as, characterized the consequences of polymerase overexpression on genome stability [231–241]. Thus far, studies have focused on identifying differences in mRNA levels or the presence of variant alleles when comparing normal and cancer tissue. It is not surprising that there is little evidence thus far indicating that TLS polymerases are grossly up- or downregulated in cancer cells. Large changes in expression levels may be incompatible with cell survival and hence selected against [242, 243]. Therefore, subtle modifications to DDT signaling must also be considered when attempting to identify events that may alter TLS polymerase activity in cancer cells [137, 138, 244–246]. Any change in the steady state level of PCNA ubiquitination can significantly affect the fidelity of DNA replication and upset the balance between normal versus TLS polymerase activity. On the other hand, reduced levels of TLS activity would in turn lead to higher incidences of replication-associated DSBs or frank deficiencies in DNA repair, such as when lacking a specific polymerase like Polζ. The damage tolerance pathway is in all likelihood much more delicately balanced than currently realized.
6. Conclusion
The past two decades have seen extraordinary advances in our understanding of how mutations may occur and potentiate carcinogenesis. Some of the same polymerases that have been characterized as facilitators of mutagenesis, are now implicated in suppressing tumorigenesis by maintaining genomic stability (e.g. Polζ). Recent evidence suggests that cancer cells may be particularly sensitive to inhibition of the DDR pathway that has evolved to promote replication fork stability [247]. Interfering with the ATR-Chk1 kinase pathway has been shown to be selectively toxic in cancer cells that have become dependent upon this pathway to cope with constant replication stress [248]. If it holds true, that cancer cells are more dependent upon Polζ for growth and survival (and perhaps HR repair) as suggested by Knobel et al [243], we anticipate that a potential new Achilles heal may have been identified. Suppressing the expression of TLS polymerases, such as REV1 and Polζ, has been shown to limit mutagenesis and sensitize tumor cells to cancer therapy suggesting that it may be fruitful to explore this avenue for drug development [167, 249–252]. Blocking the TLS pathway to sensitize tumor cells to conventional treatment is rational, and may be even more effective when combined with an inhibitor of Chk1 or ATR. Of course, understanding the implications for inhibiting TLS polymerases on overall genome maintenance in normal somatic cells is paramount before such an undertaking can be considered.
Acknowledgments
The work in our laboratory is supported by NIH grant CA133046 to CEC and the Cancer Biology Training Program (NIH T32 CA009676-20) to CMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer. 2011;11:450–457. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccia A, Elledge SJ. The DNA Damage Response: Making It Safe to Play with Knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kee Y, D’Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24:1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleaver JE. Cancer in Xeroderma Pigmentosum and related disorders of DNA repair. Nat Rev Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 8.O’Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 9.Chang DJ, Cimprich KA. DNA damage tolerance: when it’s OK to make mistakes. Nat Chem Biol. 2009;5:82–90. doi: 10.1038/nchembio.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic Translesion Polymerases and Their Roles and Regulation in DNA Damage Tolerance. Microbiol Mol Biol R. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedberg EC. Suffering in silence: the tolerance of DNA damage. Nature Rev Mol Cell Biol. 2005;6:943–953. doi: 10.1038/nrm1781. [DOI] [PubMed] [Google Scholar]
- 12.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 13.Rupp WD, Howard-flanders P. Discontinuities in the DNA synthesized in an Excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968;31:291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Shinoura Y. Isolation and characterization of mutants of Escherichia-coli deficient in induction of mutations by ultraviolet-light. Molecular & General Genetics. 1977;156:121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- 15.Lemontt JF. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics. 1971;68:21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence C, Das G, Christensen R. REV7, a new gene concerned with UV mutagenesis in yeast. Mol Gen Genet. 1985;200:80–85. doi: 10.1007/BF00383316. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann AR, Kirk-Bell S, Arelett CF, Paterson MC, Lohman PHM, de Weerd-Kastelein EA, Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci USA. 1975;72:219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison A, Christensen RB, Alley J, Beck AK, Bernstine EG, Lemontt JF, Lawrence CW. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Wu X, Wang Z. A full-length cDNA of hREV3 is predicted to encode DNA polymerase zeta for damage-induced mutagenesis in humans. Mutat Res. 1999;433:89–98. doi: 10.1016/s0921-8777(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 21.Morelli C, Mungall AJ, Negrini M, Barbanti-Brodano G, Croce CM. Alternative splicing, genomic structure, and fine chromosome localization of REV3L. Cytogenet Cell Genet. 1998;83:18–20. doi: 10.1159/000015157. [DOI] [PubMed] [Google Scholar]
- 22.Van Sloun PP, Romeijn RJ, Eeken JC. Molecular cloning, expression and chromosomal localisation of the mouse Rev3l gene, encoding the catalytic subunit of polymerase zeta. Mutat Res. 1999;433:109–116. doi: 10.1016/s0921-8777(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JR, Lawrence CW, Hinkle DC. Thymine-Thymine Dimer Bypass by Yeast DNA Polymerase zeta. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RE, Prakash L, Prakash S. Pol31 and Pol32 subunits of yeast DNA polymerase δ are also essential subunits of DNA polymerase ζ. Proc Natl Acad Sci USA. 2012;109:12455–12460. doi: 10.1073/pnas.1206052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarova AV, Stodola JL, Burgers PM. A four-subunit DNA polymerase ζ complex containing Pol δ accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langston LD, O’Donnell M. Subunit sharing among high- and low-fidelity DNA polymerases. Proc Natl Acad Sci USA. 2012;109:12268–12269. doi: 10.1073/pnas.1209533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RE, Yu SL, Prakash S, Prakash L. Yeast DNA polymerase zeta (α) is essential for error-free replication past thymine glycol. Genes Dev. 2003;17:77–87. doi: 10.1101/gad.1048303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RE, Haracska L, Prakash S, Prakash L. Role of DNA Polymerase α in the Bypass of a (6–4) TT Photoproduct. Mol Cell Biol. 2001;21:3558–3563. doi: 10.1128/MCB.21.10.3558-3563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 30.Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- 31.Lazzaro F, Novarina D, Amara F, Watt Danielle L, Stone Jana E, Costanzo V, Burgers Peter M, Kunkel Thomas A, Plevani P, Muzi-Falconi M. RNase H and Postreplication Repair Protect Cells from Ribonucleotides Incorporated in DNA. Mol Cell. 2012;45:99–110. doi: 10.1016/j.molcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence CW. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv Protein Chem. 2004;69:167–203. doi: 10.1016/S0065-3233(04)69006-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Garg P, Stith CM, Nick McElhinny SA, Kissling GE, Burgers PM, Kunkel TA. The fidelity of DNA synthesis by yeast DNA polymerase zeta alone and with accessory proteins. Nucleic Acids Res. 2006;34:4731–4742. doi: 10.1093/nar/gkl465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Kajiwara K, Kawamura K, Kimura M, Miyagishima H, Koseki H, Tagawa M. An essential role for REV3 in mammalian cell survival: absence of REV3 induces p53-independent embryonic death. Biochem Biophys Res Commun. 2002;293:1132–1137. doi: 10.1016/S0006-291X(02)00341-8. [DOI] [PubMed] [Google Scholar]
- 35.Van Sloun PP, Varlet I, Sonneveld E, Boei JJ, Romeijn RJ, Eeken JC, de Wind N. Involvement of mouse Rev3 in tolerance of endogenous and exogenous DNA damage. Mol Cell Biol. 2002;22:2159–2169. doi: 10.1128/MCB.22.7.2159-2169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittschieben J, Shivji MKK, Lalani E, Jacobs MA, Marini F, Gearhart PJ, Rosewell I, Stamp G, Wood RD. Disruption of the developmentally regulated Rev3l gene causes embryonic lethality. Curr Biol. 2000;10:1217–1220. doi: 10.1016/s0960-9822(00)00725-9. [DOI] [PubMed] [Google Scholar]
- 37.Bemark M, Khamlichi AA, Davies SL, Neuberger MS. Disruption of mouse polymerase α (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr Biol. 2000;10:1213–1216. doi: 10.1016/s0960-9822(00)00724-7. [DOI] [PubMed] [Google Scholar]
- 38.Esposito G, Godin I, Klein U, Yaspo ML, Cumano A. Rajewsky, Disruption of the Rev3l -encoded catalytic subunit of polymerase α in mice results in early embryonic lethality. Curr Biol. 2000;10:1221–1224. doi: 10.1016/s0960-9822(00)00726-0. [DOI] [PubMed] [Google Scholar]
- 39.Wittschieben JP, Reshmi SC, Gollin SM, Wood RD. Loss of DNA Polymerase α Causes Chromosomal Instability in Mammalian Cells. Cancer Res. 2006;66:134–142. doi: 10.1158/0008-5472.CAN-05-2982. [DOI] [PubMed] [Google Scholar]
- 40.Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 41.Gibbs PE, Wang XD, Li Z, McManus TP, McGregor WG, Lawrence CW, Maher VM. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc Natl Acad Sci USA. 2000;97:4186–4191. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda Y, Takahashi M, Tsunekuni N, Minami T, Sumii M, Miyagawa K, Kamiya K. Deoxycytidyl Transferase Activity of the Human REV1 Protein Is Closely Associated with the Conserved Polymerase Domain. J Biol Chem. 2001;276:15051–15058. doi: 10.1074/jbc.M008082200. [DOI] [PubMed] [Google Scholar]
- 43.Haracska L, Prakash S, Prakash L. Yeast Rev1 Protein Is a G Template-specific DNA Polymerase. J Biol Chem. 2002;277:15546–15551. doi: 10.1074/jbc.M112146200. [DOI] [PubMed] [Google Scholar]
- 44.Masuda Y, Kamiya K. Biochemical properties of the human REV1 protein. FEBS Letters. 2002;520:88–92. doi: 10.1016/s0014-5793(02)02773-4. [DOI] [PubMed] [Google Scholar]
- 45.Lin W, Xin H, Zhang Y, Wu X, Yuan F, Wang Z. The human REV1 gene codes for a DNA template-dependent dCMP transferase. Nucleic Acids Res. 1999;27:4468–4475. doi: 10.1093/nar/27.22.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 2002;30:1630–1638. doi: 10.1093/nar/30.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. Rev1 Employs a Novel Mechanism of DNA Synthesis Using a Protein Template. Science. 2005;309:2219–2222. doi: 10.1126/science.1116336. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence CW. Cellular roles of DNA polymerase α and Rev1 protein. DNA Repair. 2002;1:425–435. doi: 10.1016/s1568-7864(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 49.Nelson JR, Gibbs PE, Nowicka AM, Hinkle DC, Lawrence CW. Evidence for a second function for Saccharomyces cerevisiae Rev1. Mol Microbiol. 2000;37:549–554. doi: 10.1046/j.1365-2958.2000.01997.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RE, Prakash S, Prakash L. Efficient Bypass of a Thymine-Thymine Dimer by Yeast DNA Polymerase, Polη. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 51.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 52.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon J-H, Prakash L, Prakash S. Highly error-free role of DNA polymerase η in the replicative bypass of UV-induced pyrimidine dimers in mouse and human cells. Proc Natl Acad Sci USA. 2009;106:18219–18224. doi: 10.1073/pnas.0910121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biertümpfel C, Zhao Y, Kondo Y, Ramón-Maiques S, Gregory M, Lee JY, Masutani C, Lehmann AR, Hanaoka F, Yang W. Structure and mechanism of human DNA polymerase [eegr] Nature. 2010;465:1044–1048. doi: 10.1038/nature09196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silverstein TD, Johnson RE, Jain R, Prakash L, Prakash S, Aggarwal AK. Structural basis for the suppression of skin cancers by DNA polymeraseα. Nature. 2010;465:1039–1043. doi: 10.1038/nature09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonald JP, Levine AS, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roush AA, Suarez M, Friedberg EC, Radman M, Siede W. Deletion of the Saccharomyces cerevisiae gene RAD30 encoding an Escherichia coli DinB homolog confers UV radiation sensitivity and altered mutability. Mol Gen Genetics. 1998;257:686–692. doi: 10.1007/s004380050698. [DOI] [PubMed] [Google Scholar]
- 58.Cordonnier AM, Fuchs RP. Replication of damaged DNA: molecular defect in xeroderma pigmentosum variant cells. Mutat Res. 1999;435:111–119. doi: 10.1016/s0921-8777(99)00047-6. [DOI] [PubMed] [Google Scholar]
- 59.Maher VM, Ouellette LM, Curren RD, McCormick JJ. Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature. 1976;261:595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- 60.Boyer JC, Kaufmann WK, Brylawski BP, Cordeirostone M. Defective postreplication repair in xeroderma-pigmentosum variant fibroblasts. Cancer Res. 1990;50:2593–2598. [PubMed] [Google Scholar]
- 61.Myhr BC, Turnbull D, Dipaolo JA. Ultraviolet mutagenesis of normal and xeroderma-pigmentosum variant human-fibroblasts. Mutat Res. 1979;62:341–353. doi: 10.1016/0027-5107(79)90089-7. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Zhang H, McManus TP, McCormick JJ, Lawrence CW, Maher VM. hREV3 is essential for error-prone translesion synthesis past UV or benzo[a]pyrene diol epoxide-induced DNA lesions in human fibroblasts. Mutat Res. 2002;510:71–80. doi: 10.1016/s0027-5107(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 63.Clark DR, Zacharias W, Panaitescu L, McGregor WG. Ribozyme-mediated REV1 inhibition reduces the frequency of UV-induced mutations in the human HPRT gene. Nucleic Acids Res. 2003;31:4981–4988. doi: 10.1093/nar/gkg725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diaz M, Watson NB, Turkington G, Verkoczy LK, Klinman NR, McGregor WG. Decreased frequency and highly aberrant spectrum of ultraviolet-induced mutations in the hprt gene of mouse fibroblasts expressing antisense RNA to DNA polymerase zeta. Mol Cancer Res. 2003;1:836–847. [PubMed] [Google Scholar]
- 65.McNally K, Neal JA, McManus TP, McCormick JJ, Maher VM. hRev7, putative subunit of hPol zeta, plays a critical role in survival, induction of mutations, and progression through S-phase, of UV(254nm)-irradiated human fibroblasts. DNA Repair. 2008;7:597–604. doi: 10.1016/j.dnarep.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tissier A, McDonald JP, Frank EG, Woodgate R. polι, a remarkably error-prone human DNA polymerase. Genes Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Yuan F, Wu X, Wang Z. Preferential incorporation of G opposite template T by the low-fidelity human DNA polymerase ι. Mol Cell Biol. 2000;20:7099–7108. doi: 10.1128/mcb.20.19.7099-7108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nair DT, Johnson RE, Prakash S, Prakash L, Aggarwal AK. Replication by human DNA polymeraseα occurs by Hoogsteen base-pairing. Nature. 2004;430:377–380. doi: 10.1038/nature02692. [DOI] [PubMed] [Google Scholar]
- 69.Kirouac KN, Ling H. Structural basis of error-prone replication and stalling at a thymine base by human DNA polymerase α. EMBO J. 2009;28:1644–1654. doi: 10.1038/emboj.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirouac KN, Ling H. Unique active site promotes error-free replication opposite an 8-oxo-guanine lesion by human DNA polymerase ι. Proc Natl Acad Sci USA. 2011;108:3210–3215. doi: 10.1073/pnas.1013909108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petta TB, Nakajima S, Zlatanou A, Despras E, Couve-Privat S, Ishchenko A, Sarasin A, Yasui A, Kannouche P. Human DNA polymerase iota protects cells against oxidative stress. EMBO J. 2008;27:2883–2895. doi: 10.1038/emboj.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohkumo T, Kondo Y, Yokoi M, Tsukamoto T, Yamada A, Sugimoto T, Kanao R, Higashi Y, Kondoh H, Tatematsu M, Masutani C, Hanaoka F. UV-B radiation induces epithelial tumors in mice lacking DNA polymerase eta and mesenchymal tumors in mice deficient for DNA polymerase iota. Mol Cell Biol. 2006;26:7696–7706. doi: 10.1128/MCB.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gueranger Q. Role of DNA polymerases η, ι and ζ in UV resistance and UV-induced mutagenesis in a human cell line. DNA Repair. 2008;7:1551–1562. doi: 10.1016/j.dnarep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y. Evidence that in xeroderma pigmentosum variant cells, which lack DNA polymerase α, DNA polymeraseα αcauses the very high frequency and unique spectrum of UV-induced mutations. Cancer Res. 2007;67:3018–3026. doi: 10.1158/0008-5472.CAN-06-3073. [DOI] [PubMed] [Google Scholar]
- 75.Dumstorf CA, Clark AB, Lin Q, Kissling GE, Yuan T, Kucherlapati R, McGregor WG, Kunkel TA. Participation of mouse DNA polymeraseα in strand-biased mutagenic bypass of UV photoproducts and suppression of skin cancer. Proc Natl Acad Sci USA. 2006;103:18083–18088. doi: 10.1073/pnas.0605247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziv O, Geacintov N, Nakajima S, Yasui A, Livneh Z. DNA polymerase ζ cooperates with polymerases κ and ι in translesion DNA synthesis across pyrimidine photodimers in cells from XPV patients. Proc Natl Acad Sci USA. 2009;106:11552–11557. doi: 10.1073/pnas.0812548106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogi T, Kato T, Jr, Kato T, Ohmori H. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein dinB. Genes Cells. 1999;4:607–618. doi: 10.1046/j.1365-2443.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 78.Johnson RE, Prakash S, Prakash L. The human DINB1 gene encodes the DNA polymerase Poltheta. Proc Natl Acad Sci USA. 2000;97:3838–3843. doi: 10.1073/pnas.97.8.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerlach VL, Feaver WJ, Fischhaber PL, Friedberg EC. Purification and characterization of pol kappa, a DNA polymerase encoded by the human DINB1 gene. J Biol Chem. 2001;276:92–98. doi: 10.1074/jbc.M004413200. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Wu X, Guo D, Rechkoblit O, Wang Z. Activities of human DNA polymerase kappa in response to the major benzo[a]pyrene DNA adduct: error-free lesion bypass and extension synthesis from opposite the lesion. DNA Repair. 2002;1:559–569. doi: 10.1016/s1568-7864(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 81.Ogi T, Shinkai Y, Tanaka K, Ohmori H. Polkappa protects mammalian cells against the lethal and mutagenic effects of benzo[a]pyrene. Proc Natl Acad Sci USA. 2002;99:15548–15553. doi: 10.1073/pnas.222377899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avkin S, Goldsmith M, Velasco-Miguel S, Geacintov N, Friedberg EC, Livneh Z. Quantitative analysis of translesion DNA synthesis across a benzo[a]pyrene-guanine adduct in mammalian cells: the role of DNA polymerase kappa. J Biol Chem. 2004;279:53298–53305. doi: 10.1074/jbc.M409155200. [DOI] [PubMed] [Google Scholar]
- 83.Choi JY, Angel KC, Guengerich FP. Translesion synthesis across bulky N2-alkyl guanine DNA adducts by human DNA polymerase kappa. J Biol Chem. 2006;281:21062–21072. doi: 10.1074/jbc.M602246200. [DOI] [PubMed] [Google Scholar]
- 84.Jarosz DF, Godoy VG, Delaney JC, Essigmann JM, Walker GC. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature. 2006;439:225–228. doi: 10.1038/nature04318. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Yuan F, Wu X, Wang M, Rechkoblit O, Taylor JS, Geacintov NE, Wang Z. Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro. Nucleic Acids Res. 2000;28:4138–4146. doi: 10.1093/nar/28.21.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Washington MT, Johnson RE, Prakash L, Prakash S. Human DINB1-encoded DNA polymerase kappa is a promiscuous extender of mispaired primer termini. Proc Natl Acad Sci USA. 2002;99:1910–1914. doi: 10.1073/pnas.032594399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki N, Ohashi E, Kolbanovskiy A, Geacintov NE, Grollman AP, Ohmori H, Shibutani S. Translesion Synthesis by Human DNA Polymerase κ on a DNA Template Containing a Single Stereoisomer of dG-(+)- or dG-(−)-anti-N2-BPDE (7,8-Dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene)†. Biochemistry. 2002;41:6100–6106. doi: 10.1021/bi020049c. [DOI] [PubMed] [Google Scholar]
- 88.Bi X, Slater DM, Ohmori H, Vaziri C. DNA polymerase κ is specifically required for recovery from the benzo[a]pyrene-dihydrodiol epoxide (BPDE)-induced S-phase checkpoint. J Biol Chem. 2005;280:22343–22355. doi: 10.1074/jbc.M501562200. [DOI] [PubMed] [Google Scholar]
- 89.Stancel JNK, McDaniel LD, Velasco S, Richardson J, Guo CX, Friedberg EC. Polk mutant mice have a spontaneous mutator phenotype. DNA Repair. 2009;8:1355–1362. doi: 10.1016/j.dnarep.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Yuan F, Xin H, Wu X, Rajpal DK, Yang D, Wang Z. Human DNA polymerase kappa synthesizes DNA with extraordinarily low fidelity. Nucleic Acids Res. 2000;28:4147–4156. doi: 10.1093/nar/28.21.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohashi E, Bebenek K, Matsuda T, Feaver WJ, Gerlach VL, Friedberg EC, Ohmori H, Kunkel TA. Fidelity and processivity of DNA synthesis by DNA polymerase kappa, the product of the human DINB1 gene. J Biol Chem. 2000;275:39678–39684. doi: 10.1074/jbc.M005309200. [DOI] [PubMed] [Google Scholar]
- 92.Uljon SN, Johnson RE, Edwards TA, Prakash S, Prakash L, Aggarwal AK. Crystal structure of the catalytic core of human DNA polymerase kappa. Structure. 2004;12:1395–1404. doi: 10.1016/j.str.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Lone S, Townson SA, Uljon SN, Johnson RE, Brahma A, Nair DT, Prakash S, Prakash L, Aggarwal AK. Human DNA polymerase kappa encircles DNA: implications for mismatch extension and lesion bypass. Mol Cell. 2007;25:601–614. doi: 10.1016/j.molcel.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 94.Ogi T, Lehmann AR. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8:640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 95.Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R, Nakazawa Y, Niimi A, Miki Y, Jaspers NG, Mullenders LHF, Yamashita S, Fousteri MI, Lehmann AR. Three DNA Polymerases, Recruited by Different Mechanisms, Carry Out NER Repair Synthesis in Human Cells. Mol Cell. 2010;37:714–727. doi: 10.1016/j.molcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Jentsch S, McGrath JP, Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987;329:131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- 97.Bailly V, Lauder S, Prakash S, Prakash L. Yeast DNA Repair Proteins Rad6 and Rad18 Form a Heterodimer That Has Ubiquitin Conjugating, DNA Binding, and ATP Hydrolytic Activities. J Biol Chem. 1997;272:23360–23365. doi: 10.1074/jbc.272.37.23360. [DOI] [PubMed] [Google Scholar]
- 98.Broomfield S, Chow BL, Xiao W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc Natl Acad Sci USA. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao W, Chow BL, Broomfield S, Hanna M. The Saccharomyces cerevisiae RAD6 group is composed of an error-prone and two error-free postreplication repair pathways. Genetics. 2000;155:1633–1641. doi: 10.1093/genetics/155.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 101.Byun TS, Pacek M, Mc Yee, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang DJ, Lupardus PJ, Cimprich KA. Monoubiquitination of Proliferating Cell Nuclear Antigen Induced by Stalled Replication Requires Uncoupling of DNA Polymerase and Mini-chromosome Maintenance Helicase Activities. Journal of Biological Chemistry. 2006;281:32081–32088. doi: 10.1074/jbc.M606799200. [DOI] [PubMed] [Google Scholar]
- 103.Davies AA, Huttner D, Daigaku Y, Chen S, Ulrich HD. Activation of Ubiquitin-Dependent DNA Damage Bypass Is Mediated by Replication Protein A. Mol Cell. 2008;29:625–636. doi: 10.1016/j.molcel.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zou L, Elledge SJ. Sensing DNA Damage Through ATRIP Recognition of RPA-ssDNA Complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 105.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 106.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 107.Andersen PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 2008;18:162–173. doi: 10.1038/cr.2007.114. [DOI] [PubMed] [Google Scholar]
- 108.Masuda Y, Suzuki M, Kawai H, Hishiki A, Hashimoto H, Masutani C, Hishida T, Suzuki F, Kamiya K. En bloc transfer of polyubiquitin chains to PCNA in vitro is mediated by two different human E2–E3 pairs. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopes M, Foiani M, Sogo JM. Multiple Mechanisms Control Chromosome Integrity after Replication Fork Uncoupling and Restart at Irreparable UV Lesions. Mol Cell. 2006;21:15–27. doi: 10.1016/j.molcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 110.Diamant N, Hendel A, Vered I, Carell T, Reißner T, de Wind N, Geacinov N, Livneh Z. DNA damage bypass operates in the S and G2 phases of the cell cycle and exhibits differential mutagenicity. Nucleic Acids Res. 2012;40:170–180. doi: 10.1093/nar/gkr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jansen JG, Tsaalbi-Shtylik A, Hendriks G, Gali H, Hendel A, Johansson F, Erixon K, Livneh Z, Mullenders LHF, Haracska L, de Wind N. Separate Domains of Rev1 Mediate Two Modes of DNA Damage Bypass in Mammalian Cells. Mol Cell Biol. 2009;29:3113–3123. doi: 10.1128/MCB.00071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jansen JG, Tsaalbi-Shtylik A, Hendriks G, Verspuy J, Gali H, Haracska L, de Wind N. Mammalian polymerase α is essential for post-replication repair of UV-induced DNA lesions. DNA Repair. 2009;8:1444–1451. doi: 10.1016/j.dnarep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Temviriyanukul P, van Hees-Stuivenberg S, Delbos F, Jacobs H, de Wind N, Jansen JG. Temporally distinct translesion synthesis pathways for ultraviolet light-induced photoproducts in the mammalian genome. DNA Repair. 2012;11:550–558. doi: 10.1016/j.dnarep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I. Ubiquitin-Binding Domains in Y-Family Polymerases Regulate Translesion Synthesis. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- 115.Plosky BS, Vidal AE, Fernandez de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, Woodgate R. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 2006;25:2847–2855. doi: 10.1038/sj.emboj.7601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bomar MG, Pai MT, Tzeng SR, Li SS, Zhou P. Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase η. EMBO Rep. 2007;8:247–251. doi: 10.1038/sj.embor.7400901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bomar MG, D’Souza S, Bienko M, Dikic I, Walker GC, Zhou P. Unconventional ubiquitin recognition by the ubiquitin-binding motif within the Y family DNA polymerases iota and Rev1. Mol Cell. 2010;37:408–417. doi: 10.1016/j.molcel.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui G, Benirschke RC, Tuan H-F, Juranic N, Macura S, Botuyan MV, Mer G. Structural Basis of Ubiquitin Recognition by Translesion Synthesis DNA Polymerase ι. Biochemistry. 2010;49:10198–10207. doi: 10.1021/bi101303t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burschowsky D, Rudolf F, Rabut G, Herrmann T, Peter M, Wider G. Structural analysis of the conserved ubiquitin-binding motifs (UBMs) of the translesion polymerase iota in complex with ubiquitin. J Biol Chem. 2011;286:17398. doi: 10.1074/jbc.M110.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kannouche P, Broughton BC, Volker M, Hanaoka F, Mullenders LHF, Lehmann AR. Domain structure, localization, and function of DNA polymerase η, defective in xeroderma pigmentosum variant cells. Genes Dev. 2001;15:158–172. doi: 10.1101/gad.187501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kannouche P, Fernandez de Henestrosa AR, Coull B, Vidal AE, Gray C, Zicha D, Woodgate R, Lehmann AR. Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells. EMBO J. 2002;21:6246–6256. doi: 10.1093/emboj/cdf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ogi T, Kannouche P, Lehmann AR. Localisation of human Y-family DNA polymerase α: relationship to PCNA foci. J Cell Sci. 2004 doi: 10.1242/jcs.01603. [DOI] [PubMed] [Google Scholar]
- 123.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 124.Bi X, Barkley LR, Slater DM, Tateishi S, Yamaizumi M, Ohmori H, Vaziri C. Rad18 Regulates DNA Polymerase α and Is Required for Recovery from S-Phase Checkpoint-Mediated Arrest. Mol Cell Biol. 2006;26:3527–3540. doi: 10.1128/MCB.26.9.3527-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murakumo Y, Mizutani S, Yamaguchi M, Ichihara M, Takahashi M. Analyses of ultraviolet-induced focus formation of hREV1 protein. Genes Cells. 2006;11:193–205. doi: 10.1111/j.1365-2443.2006.00938.x. [DOI] [PubMed] [Google Scholar]
- 126.Guo C, Tang TS, Bienko M, Parker JL, Bielen AB, Sonoda E, Takeda S, Ulrich HD, Dikic I, Friedberg EC. Ubiquitin-Binding Motifs in REV1 Protein Are Required for Its Role in the Tolerance of DNA Damage. Mol Cell Biol. 2006;26:8892–8900. doi: 10.1128/MCB.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hicks JK, Chute CL, Paulsen MT, Ragland RL, Howlett NG, Gueranger Q, Glover TW, Canman CE. Differential Roles for DNA Polymerases Eta, Zeta, and REV1 in Lesion Bypass of Intrastrand versus Interstrand DNA Cross-Links. Mol Cell Biol. 2010;30:1217–1230. doi: 10.1128/MCB.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tissier A, Kannouche P, Reck M-P, Lehmann AR, Fuchs RPP, Cordonnier A. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase polη and REVl protein. DNA Repair. 2004;3:1503–1514. doi: 10.1016/j.dnarep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 129.Ward IM, Chen J. Histone H2AX Is Phosphorylated in an ATR-dependent Manner in Response to Replicational Stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 130.Edmunds CE, Simpson LJ, Sale JE. PCNA Ubiquitination and REV1 Define Temporally Distinct Mechanisms for Controlling Translesion Synthesis in the Avian Cell Line DT40. Mol Cell. 2008;30:519–529. doi: 10.1016/j.molcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 131.Szüts D, Marcus AP, Himoto M, Iwai S, Sale JE. REV1 restrains DNA polymerase ζ to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo. Nucleic Acids Res. 2008;36:6767–6780. doi: 10.1093/nar/gkn651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hendel A, Krijger PH, Diamant N, Goren Z, Langerak P, Kim J, Reissner T, Lee KY, Geacintov NE, Carell T, Myung K, Tateishi S, D’Andrea A, Jacobs H, Livneh Z. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 2011;7:e1002262. doi: 10.1371/journal.pgen.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mirchandani KD, McCaffrey RM, D’Andrea AD. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair. 2008;7:902–911. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim H, Yang K, Dejsuphong D, D’Andrea AD. Regulation of Rev1 by the Fanconi anemia core complex. Nat Struct Mol Biol. 2012;19:164–170. doi: 10.1038/nsmb.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D’Andrea AD. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 136.Yang K, Moldovan G-L, Vinciguerra P, Murai J, Takeda S, D’Andrea AD. Regulation of the Fanconi anemia pathway by a SUMO-like delivery network. Genes Dev. 2011;25:1847–1858. doi: 10.1101/gad.17020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee K-y, Yang K, Cohn MA, Sikdar N, D’Andrea AD, Myung K. Human ELG1 Regulates the Level of Ubiquitinated Proliferating Cell Nuclear Antigen (PCNA) through Its Interactions with PCNA and USP1. J Biol Chem. 2010;285:10362–10369. doi: 10.1074/jbc.M109.092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jones MJK, Colnaghi L, Huang TT. Dysregulation of DNA polymerase kappa recruitment to replication forks results in genomic instability. Embo J. 2012;31:908–918. doi: 10.1038/emboj.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim JM, Parmar K, Huang M, Weinstock DM, Ruit CA, Kutok JL, D’Andrea AD. Inactivation of Murine Usp1 Results in Genomic Instability and a Fanconi Anemia Phenotype. Dev Cell. 2009;16:314–320. doi: 10.1016/j.devcel.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sikdar N, Banerjee S, Lee K-y, Wincovitch S, Pak E, Nakanishi K, Jasin M, Dutra A, Myung K. DNA damage responses by human ELG1 in S phase are important to maintain genomic integrity. Cell Cycle. 2009;8:3199–3207. doi: 10.4161/cc.8.19.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bell DW, Sikdar N, Lee K-y, Price JC, Chatterjee R, Park H-D, Fox J, Ishiai M, Rudd ML, Pollock LM, Fogoros SK, Mohamed H, Hanigan CL, Zhang S, Cruz P, Renaud G, Hansen NF, Cherukuri PF, Borate B, McManus KJ, Stoepel J, Sipahimalani P, Godwin AK, Sgroi DC, Merino MJ, Elliot G, Elkahloun A, Vinson C, Takata M, Mullikin JC, Wolfsberg TG, Hieter P, Lim D-S, Myung K, Program NCS. Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian Atad5. PLoS Genet. 2011;7:e1002245. doi: 10.1371/journal.pgen.1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shachar S, Ziv O, Avkin S, Adar S, Wittschieben J, Reiszner T, Chaney S, Friedberg EC, Wang Z, Carell T, Geacintov N, Livneh Z. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. EMBO J. 2009;28:383–393. doi: 10.1038/emboj.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Acharya N, Johnson RE, Prakash S, Prakash L. Complex Formation with Rev1 Enhances the Proficiency of Saccharomyces cerevisiae DNA Polymerase α for Mismatch Extension and for Extension Opposite from DNA Lesions. Mol Cell Biol. 2006;26:9555–9563. doi: 10.1128/MCB.01671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, Kisker C, Friedberg EC. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Groote FH, Jansen JG, Masuda Y, Shah DM, Kamiya K, de Wind N, Siegal G. The Rev1 translesion synthesis polymerase has multiple distinct DNA binding modes. DNA Repair. 2011;10:915–925. doi: 10.1016/j.dnarep.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 146.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, Ulrich HD, Friedberg EC. REV1 Protein Interacts with PCNA: Significance of the REV1 BRCT Domain In Vitro and In Vivo. Mol Cell. 2006;23:265–271. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 147.Murakumo Y, Ogura Y, Ishii H, Numata S, Ichihara M, Croce CM, Fishel R, Takahashi M. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J Biol Chem. 2001;276:35644–35651. doi: 10.1074/jbc.M102051200. [DOI] [PubMed] [Google Scholar]
- 148.Masuda Y, Ohmae M, Masuda K, Kamiya K. Structure and Enzymatic Properties of a Stable Complex of the Human REV1 and REV7 Proteins. J Biol Chem. 2003;278:12356–12360. doi: 10.1074/jbc.M211765200. [DOI] [PubMed] [Google Scholar]
- 149.Ohashi E, Murakumo Y, Kanjo N, Akagi J, Masutani C, Hanaoka F, Ohmori H. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells. 2004;9:523–531. doi: 10.1111/j.1356-9597.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 150.D’Souza S, Walker GC. Novel Role for the C Terminus of Saccharomyces cerevisiae Rev1 in Mediating Protein-Protein Interactions. Mol Cell Biol. 2006;26:8173–8182. doi: 10.1128/MCB.00202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ross AL, Simpson LJ, Sale JE. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005;33:1280–1289. doi: 10.1093/nar/gki279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.D’Souza S, Waters L, Walker G. Novel conserved motifs in Rev1 C-terminus are required for mutagenic DNA damage tolerance. DNA Repair. 2008;7:1455–1470. doi: 10.1016/j.dnarep.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohashi E, Hanafusa T, Kamei K, Song I, Tomida J, Hashimoto H, Vaziri C, Ohmori H. Identification of a novel REV1-interacting motif necessary for DNA polymerase κ function. Genes Cells. 2009;14:101–111. doi: 10.1111/j.1365-2443.2008.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pozhidaeva A, Pustovalova Y, D’Souza S, Bezsonova I, Walker GC, Korzhnev DM. NMR Structure and Dynamics of the C-Terminal Domain from Human Rev1 and Its Complex with Rev1 Interacting Region of DNA Polymerase η. Biochemistry. 2012;51:5506–5520. doi: 10.1021/bi300566z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wojtaszek J, Liu J, D’Souza S, Wang S, Xue Y, Walker GC, Zhou P. Multifaceted recognition of vertebrate Rev1 by translesion polymerases ζ and κ. J Biol Chem. 2012;287:26400–26408. doi: 10.1074/jbc.M112.380998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma S, Hicks JK, Chute CL, Brennan JR, Ahn J-Y, Glover TW, Canman CE. REV1 and polymerase ζ facilitate homologous recombination repair. Nucleic Acids Res. 2012;40:682–691. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Simpson LJ, Sale JE. Rev1 is essential for DNA damage tolerance and non-templated immunoglobulin gene mutation in a vertebrate cell line. EMBO J. 2003;22:1654–1664. doi: 10.1093/emboj/cdg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen X, Jun S, O’Neal LE, Sonoda E, Bemark M, Sale JE, Li L. REV3 and REV1 Play Major Roles in Recombination-independent Repair of DNA Interstrand Cross-links Mediated by Monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) J Biol Chem. 2006;281:13869–13872. doi: 10.1074/jbc.C600071200. [DOI] [PubMed] [Google Scholar]
- 159.Okada T, Sonoda E, Yoshimura M, Kawano Y, Saya H, Kohzaki M, Takeda S. Multiple Roles of Vertebrate REV Genes in DNA Repair and Recombination. Mol Cell Biol. 2005;25:6103–6111. doi: 10.1128/MCB.25.14.6103-6111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 161.Hara K, Hashimoto H, Murakumo Y, Kobayashi S, Kogame T, Unzai S, Akashi S, Takeda S, Shimizu T, Sato M. Crystal Structure of Human REV7 in Complex with a Human REV3 Fragment and Structural Implication of the Interaction between DNA Polymerase ζ and REV1. J Biol Chem. 2010;285:12299–12307. doi: 10.1074/jbc.M109.092403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wojtaszek J, Lee C-J, D’Souza S, Minesinger B, Kim H, D’Andrea AD, Walker GC, Zhou P. Structural Basis of Rev1-mediated Assembly of a Quaternary Vertebrate Translesion Polymerase Complex Consisting of Rev1, Heterodimeric Polymerase (Pol) ζ, and Pol κ. J Biol Chem. 2012;287:33836–33846. doi: 10.1074/jbc.M112.394841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kikuchi S, Hara K, Shimizu T, Sato M, Hashimoto H. Structural Basis of Recruitment of DNA Polymerase ζ by Interaction between REV1 and REV7 Proteins. J Biol Chem. 2012;287:33847–33852. doi: 10.1074/jbc.M112.396838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheung HW, Chun ACS, Wang Q, Deng W, Hu L, Guan XY, Nicholls JM, Ling MT, Chuan Wong Y, Wah Tsao S, Jin DY, Wang X. Inactivation of Human MAD2B in Nasopharyngeal Carcinoma Cells Leads to Chemosensitization to DNA-Damaging Agents. Cancer Res. 2006;66:4357–4367. doi: 10.1158/0008-5472.CAN-05-3602. [DOI] [PubMed] [Google Scholar]
- 165.Sonoda E, Okada T, Zhao GY, Tateishi S, Araki K, Yamaizumi M, Yagi T, Verkaik NS, van GDC, Takata M, Takeda S. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22:3188–3197. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Ohzeki M, Ishiai M, Yamamoto K, Takata M, Arakawa H, Buerstedde JM, Yamazoe M, Kawamoto T, Araki K, Takahashi JA, Hashimoto N, Takeda S, Sonoda E. Multiple Repair Pathways Mediate Tolerance to Chemotherapeutic Cross-linking Agents in Vertebrate Cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 167.Wu F, Lin X, Okuda T, Howell SB. DNA polymerase zeta regulates cisplatin cytotoxicity, mutagenicity, and the rate of development of cisplatin resistance. Cancer Res. 2004;64:8029–8035. doi: 10.1158/0008-5472.CAN-03-3942. [DOI] [PubMed] [Google Scholar]
- 168.Roos WP, Tsaalbi-Shtylik A, Tsaryk R, Güvercin F, de Wind N, Kaina B. The Translesion Polymerase Rev3L in the Tolerance of Alkylating Anticancer Drugs. Mol Pharmacol. 2009;76:927–934. doi: 10.1124/mol.109.058131. [DOI] [PubMed] [Google Scholar]
- 169.Kane DP, Shusterman M, Rong Y, McVey M. Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila. PLoS Genet. 2012;8:e1002659. doi: 10.1371/journal.pgen.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sharma S, Shah NA, Joiner AM, Roberts KH, Canman CE. DNA Polymerase ζ Is a Major Determinant of Resistance to Platinum-Based Chemotherapeutic Agents. Mol Pharmacol. 2012;81:778–787. doi: 10.1124/mol.111.076828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao J, Liu S, Wang H, Zhang X, Kang T, Li Z, Deng H, Yue W, Cao S. Mitotic arrest deficient protein MAD2B is overexpressed in human glioma, with depletion enhancing sensitivity to ionizing radiation. J Clinical Neuroscience. 2011;18:827–833. doi: 10.1016/j.jocn.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 172.Moldovan G-L, D’Andrea AD. How the Fanconi Anemia Pathway Guards the Genome. Annu Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim H, D’Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26:1393–1408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sasaki MS. Is Fanconi’s anaemia defective in a process essential to the repair of DNA cross links? Nature. 1975;257:501–503. doi: 10.1038/257501a0. [DOI] [PubMed] [Google Scholar]
- 175.German J, Schonberg S, Caskie S, Warburton D, Falk C, Ray JH. A test for Fanconi’s anemia. Blood. 1987;69:1637–1641. [PubMed] [Google Scholar]
- 176.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, 3rd, Hurov KE, Luo J, Ballif BA, Gygi SP, Hofmann K, D’Andrea AD, Elledge SJ. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sims AE, Spiteri E, Sims RJ, 3rd, Arita AG, Lach FP, Landers T, Wurm M, Freund M, Neveling K, Hanenberg H, Auerbach AD, Huang TT. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat Struct Mol Biol. 2007;14:564–567. doi: 10.1038/nsmb1252. [DOI] [PubMed] [Google Scholar]
- 180.Dorsman JC, Levitus M, Rockx D, Rooimans MA, Oostra AB, Haitjema A, Bakker ST, Steltenpool J, Schuler D, Mohan S, Schindler D, Arwert F, Pals G, Mathew CG, Waisfisz Q, de Winter JP, Joenje H. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell Oncol. 2007;29:211–218. doi: 10.1155/2007/151968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D’Andrea AD. Interaction of the Fanconi Anemia Proteins and BRCA1 in a Common Pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 182.Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D’Andrea AD. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 183.Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, D’Andrea AD. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 184.Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, Wang W, Livingston DM, Joenje H, de Winter JP. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2007;39:159–161. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- 185.Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 186.Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, Andreassen PR, Cantor SB. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8:255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 187.Shen X, Do H, Li Y, Chung WH, Tomasz M, de Winter JP, Xia B, Elledge SJ, Wang W, Li L. Recruitment of fanconi anemia and breast cancer proteins to DNA damage sites is differentially governed by replication. Mol Cell. 2009;35:716–723. doi: 10.1016/j.molcel.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stoepker C, Hain K, Schuster B, Hilhorst-Hofstee Y, Rooimans MA, Steltenpool J, Oostra AB, Eirich K, Korthof ET, Nieuwint AW, Jaspers NG, Bettecken T, Joenje H, Schindler D, Rouse J, de Winter JP. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet. 2011;43:138–141. doi: 10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
- 189.Crossan GP, van der Weyden L, Rosado IV, Langevin F, Gaillard PH, McIntyre RE, Gallagher F, Kettunen MI, Lewis DY, Brindle K, Arends MJ, Adams DJ, Patel KJ. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet. 2011;43:147–152. doi: 10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, Fraternali F, Freund M, Hartmann L, Grimwade D, Roberts RG, Schaal H, Mohammed S, Rahman N, Schindler D, Mathew CG. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42:406–409. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- 191.Liu T, Ghosal G, Yuan J, Chen J, Huang J. FAN1 Acts with FANCI-FANCD2 to Promote DNA Interstrand Cross-Link Repair. Science. 2010;329:693–696. doi: 10.1126/science.1192656. [DOI] [PubMed] [Google Scholar]
- 192.Smogorzewska A, Desetty R, Saito TT, Schlabach M, Lach FP, Sowa ME, Clark AB, Kunkel TA, Harper JW, Colaiacovo MP, Elledge SJ. A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol Cell. 2010;39:36–47. doi: 10.1016/j.molcel.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sengerova B, Wang AT, McHugh PJ. Orchestrating the nucleases involved in DNA interstrand cross-link (ICL) repair. Cell Cycle. 2011;10:3999–4008. doi: 10.4161/cc.10.23.18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cybulski KE, Howlett NG. FANCP/SLX4: a Swiss army knife of DNA interstrand crosslink repair. Cell Cycle. 2011;10:1757–1763. doi: 10.4161/cc.10.11.15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yamamoto KN, Kobayashi S, Tsuda M, Kurumizaka H, Takata M, Kono K, Jiricny J, Takeda S, Hirota K. Involvement of SLX4 in interstrand cross-link repair is regulated by the Fanconi anemia pathway. Proc Natl Acad Sci USA. 2011;108:6492–6496. doi: 10.1073/pnas.1018487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Knipscheer P, Räschle M, Smogorzewska A, Enoiu M, Ho TV, Schärer OD, Elledge SJ, Walter JC. The Fanconi Anemia Pathway Promotes Replication-Dependent DNA Interstrand Cross-Link Repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Raschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC. Mechanism of Replication-Coupled DNA Interstrand Crosslink Repair. Cell. 2008;134:969–980. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Papadopoulo D, Guillouf C, Mohrenweiser H, Moustacchi E. Hypomutability in Fanconi Anemia Cells is Associated with Increased Deletion Frequency at the HPRT Locus. Proc Natl Acad Sci USA. 1990;87:8383–8387. doi: 10.1073/pnas.87.21.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hinz JM, Nham PB, Salazar EP, Thompson LH. The Fanconi anemia pathway limits the severity of mutagenesis. DNA Repair. 2006;5:875–884. doi: 10.1016/j.dnarep.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 200.Ali AM, Pradhan A, Singh TR, Du C, Li J, Wahengbam K, Grassman E, Auerbach AD, Pang Q, Meetei AR. FAAP20: a novel ubiquitin-binding FA nuclear core-complex protein required for functional integrity of the FA-BRCA DNA repair pathway. Blood. 2012;119:3285–3294. doi: 10.1182/blood-2011-10-385963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leung JW, Wang Y, Fong KW, Huen MS, Li L, Chen J. Fanconi anemia (FA) binding protein FAAP20 stabilizes FA complementation group A (FANCA) and participates in interstrand cross-link repair. Proc Natl Acad Sci USA. 2012;109:4491–4496. doi: 10.1073/pnas.1118720109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakanishi K, Yang YG, Pierce AJ, Taniguchi T, Digweed M, D’Andrea AD, Wang ZQ, Jasin M. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc Natl Acad Sci USA. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yamamoto K, Hirano S, Ishiai M, Morishima K, Kitao H, Namikoshi K, Kimura M, Matsushita N, Arakawa H, Buerstedde JM, Komatsu K, Thompson LH, Takata M. Fanconi Anemia Protein FANCD2 Promotes Immunoglobulin Gene Conversion and DNA Repair through a Mechanism Related to Homologous Recombination. Mol Cell Biol. 2005;25:34–43. doi: 10.1128/MCB.25.1.34-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang N, Liu X, Li L, Legerski R. Double-strand breaks induce homologous recombinational repair of interstrand cross-links via cooperation of MSH2, ERCC1-XPF, REV3, and the Fanconi anemia pathway. DNA Repair. 2007;6:1670–1678. doi: 10.1016/j.dnarep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pace P, Mosedale G, Hodskinson MR, Rosado IV, Sivasubramaniam M, Patel KJ. Ku70 Corrupts DNA Repair in the Absence of the Fanconi Anemia Pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 206.Kratz K, Schopf B, Kaden S, Sendoel A, Eberhard R, Lademann C, Cannavo E, Sartori AA, Hengartner MO, Jiricny J. Deficiency of FANCD2-associated nuclease KIAA1018/FAN1 sensitizes cells to interstrand crosslinking agents. Cell. 2010;142:77–88. doi: 10.1016/j.cell.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 207.Moldovan GL, Madhavan MV, Mirchandani KD, McCaffrey RM, Vinciguerra P, D’Andrea AD. DNA polymerase POLN participates in cross-link repair and homologous recombination. Mol Cell Biol. 2010;30:1088–1096. doi: 10.1128/MCB.01124-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hinz JM, Urbin SS, Thompson LH. RAD51D- and FANCG-dependent base substitution mutagenesis at the ATP1A1 locus in mammalian cells. Mutat Res. 2009;665:61–66. doi: 10.1016/j.mrfmmm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burch LH, Yang Y, Sterling JF, Roberts SA, Chao FG, Xu H, Zhang L, Walsh J, Resnick MA, Mieczkowski PA, Gordenin DA. Damage-induced localized hypermutability. Cell Cycle. 2011;10:1073–1085. doi: 10.4161/cc.10.7.15319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen JM, Férec C, Cooper DN. Transient hypermutability, chromothripsis and replication-based mechanisms in the generation of concurrent clustered mutations. Mutation Research - Reviews in Mutation Research. 2012;750:52–59. doi: 10.1016/j.mrrev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 211.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, Raine K, Jones D, Marshall J, Ramakrishna M, Shlien A, Cooke SL, Hinton J, Menzies A, Stebbings LA, Leroy C, Jia M, Rance R, Mudie LJ, Gamble SJ, Stephens PJ, McLaren S, Tarpey PS, Papaemmanuil E, Davies HR, Varela I, McBride DJ, Bignell GR, Leung K, Butler AP, Teague JW, Martin S, Jönsson G, Mariani O, Boyault S, Miron P, Fatima A, Langerod A, Aparicio SAJR, Tutt A, Sieuwerts AM, Borg Å, Thomas G, Salomon AV, Richardson AL, Borresen-Dale AL, Futreal PA, Stratton MR, Campbell PJ. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roberts SA, Sterling J, Thompson C, Harris S, Mav D, Shah R, Klimczak LJ, Kryukov GV, Malc E, Mieczkowski PA, Resnick MA, Gordenin DA. Clustered Mutations in Yeast and in Human Cancers Can Arise from Damaged Long Single-Strand DNA Regions. Mol Cell. 2012;46:424–435. doi: 10.1016/j.molcel.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin M-L, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, Menzies A, Martin S, Leung K, Chen L, Leroy C, Ramakrishna M, Rance R, Lau KW, Mudie LJ, Varela I, McBride DJ, Bignell GR, Cooke SL, Shlien A, Gamble J, Whitmore I, Maddison M, Tarpey PS, Davies HR, Papaemmanuil E, Stephens PJ, McLaren S, Butler AP, Teague JW, Jönsson G, Garber JE, Silver D, Miron P, Fatima A, Boyault S, Langerod A, Tutt A, Martens JWM, Aparicio SAJR, Borg Å, Salomon AV, Thomas G, Borresen-Dale AL, Richardson AL, Neuberger MS, Futreal PA, Campbell PJ, Stratton MR. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, Ha C, Johnson S, Kennemer MI, Mohan S, Nazarenko I, Watanabe C, Sparks AB, Shames DS, Gentleman R, de Sauvage FJ, Stern H, Pandita A, Ballinger DG, Drmanac R, Modrusan Z, Seshagiri S, Zhang Z. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–477. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 216.Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, Lin M-L, Beare D, Lau KW, Greenman C, Varela I, Nik-Zainal S, Davies HR, Ordonez GR, Mudie LJ, Latimer C, Edkins S, Stebbings L, Chen L, Jia M, Leroy C, Marshall J, Menzies A, Butler A, Teague JW, Mangion J, Sun YA, McLaughlin SF, Peckham HE, Tsung EF, Costa GL, Lee CC, Minna JD, Gazdar A, Birney E, Rhodes MD, McKernan KJ, Stratton MR, Futreal PA, Campbell PJ. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cleaver JE, Afzal V, Feeney L, McDowell M, Sadinski W, Volpe JPG, Busch DB, Coleman DM, Ziffer DW, Yu Y, Nagasawa H, Little JB. Increased Ultraviolet Sensitivity and Chromosomal Instability Related to P53 Function in the Xeroderma Pigmentosum Variant. Cancer Res. 1999;59:1102–1108. [PubMed] [Google Scholar]
- 218.Limoli CL, Giedzinski E, Morgan WF, Cleaver JE. Inaugural Article: Polymerase eta deficiency in the xeroderma pigmentosum variant uncovers an overlap between the S phase checkpoint and double-strand break repair. Proc Natl Acad Sci USA. 2000;97:7939–7946. doi: 10.1073/pnas.130182897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Limoli CL, Giedzinski E, Bonner WM, Cleaver JE. UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, gamma -H2AX formation, and Mre11 relocalization. Proc Natl Acad Sci USA. 2002;99:233–238. doi: 10.1073/pnas.231611798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rey L. Human DNA polymerase η is required for common fragile site stability during unperturbed DNA replication. Mol Cell Biol. 2009;29:3344–3354. doi: 10.1128/MCB.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sarkies P, Reams C, Simpson LJ, Sale JE. Epigenetic instability due to defective replication of structured DNA. Mol Cell. 2010;40:703–713. doi: 10.1016/j.molcel.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Halazonetis TD, Gorgoulis VG, Bartek J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 223.Hile SE, Wang X, Lee MYWT, Eckert KA. Beyond translesion synthesis: polymerase κ fidelity as a potential determinant of microsatellite stability. Nucleic Acids Res. 2012;40:1636–1647. doi: 10.1093/nar/gkr889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bunting SF, Callén E, Wong N, Chen H-T, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng C-X, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A. 53BP1 Inhibits Homologous Recombination in Brca1-Deficient Cells by Blocking Resection of DNA Breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Murai J, Yang K, Dejsuphong D, Hirota K, Takeda S, D’Andrea AD. The USP1/UAF1 Complex Promotes Double-Strand Break Repair through Homologous Recombination. Mol Cell Biol. 2011;31:2462–2469. doi: 10.1128/MCB.05058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, Martinez-Perez E, Boulton SJ, La Volpe A. Preventing Nonhomologous End Joining Suppresses DNA Repair Defects of Fanconi Anemia. Mol Cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 228.Wittschieben JP, Patil V, Glushets V, Robinson LJ, Kusewitt DF, Wood RD. Loss of DNA Polymeraseα Enhances Spontaneous Tumorigenesis. Cancer Res. 2010;70:2770–2778. doi: 10.1158/0008-5472.CAN-09-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang Y, Huang J-W, Calses P, Kemp CJ, Taniguchi T. MiR-96 downregulates REV1 and RAD51 to promote cellular sensitivity to cisplatin and PARP inhibition. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang S, Chen H, Zhao X, Cao J, Tong J, Lu J, Wu W, Shen H, Wei Q, Lu D. REV3L 3′ UTR 460 T>C polymorphism in microRNA target sites contributes to lung cancer susceptibility. Oncogene. 2012 doi: 10.1038/onc.2012.32. [DOI] [PubMed] [Google Scholar]
- 231.Wang J, Kawamura K, Tada Y, Ohmori H, Kimura H, Sakiyama S, Tagawa M. DNA Polymerase α, Implicated in Spontaneous and DNA Damage-induced Mutagenesis, Is Overexpressed in Lung Cancer. Cancer Res. 2001;61:5366–5369. [PubMed] [Google Scholar]
- 232.Bergoglio V, Canitrot Y, Hogarth L, Minto L, Howell SB, Cazaux C, Hoffmann JS. Enhanced expression and activity of DNA polymerase beta in human ovarian tumor cells: impact on sensitivity towards antitumor agents. Oncogene. 2001;20:6181–6187. doi: 10.1038/sj.onc.1204743. [DOI] [PubMed] [Google Scholar]
- 233.Canitrot Y, Frechet M, Servant L, Cazaux C, Hoffmann JS. Overexpression of DNA polymerase beta: a genomic instability enhancer process. FASEB J. 1999;13:1107–1111. doi: 10.1096/fasebj.13.9.1107. [DOI] [PubMed] [Google Scholar]
- 234.Srivastava DK, Husain I, Arteaga CL, Wilson SH. DNA polymerase β expression differences in selected human tumors and cell lines. Carcinogenesis. 1999;20:1049–1054. doi: 10.1093/carcin/20.6.1049. [DOI] [PubMed] [Google Scholar]
- 235.O-Wang J, Kawamura K, Tada Y, Ohmori H, Kimura H, Sakiyama S, Tagawa M. DNA polymerase kappa, implicated in spontaneous and DNA damage-induced mutagenesis, is overexpressed in lung cancer. Cancer Res. 2001;61:5366–5369. [PubMed] [Google Scholar]
- 236.Bergoglio V, Pillaire MJ, Lacroix-Triki M, Raynaud-Messina B, Canitrot Y, Bieth A, Gares M, Wright M, Delsol G, Loeb LA, Cazaux C, Hoffmann JS. Deregulated DNA polymerase beta induces chromosome instability and tumorigenesis. Cancer Res. 2002;62:3511–3514. [PubMed] [Google Scholar]
- 237.Pillaire MJ, Selves J, Gordien K, Gouraud PA, Gentil C, Danjoux M, Do C, Negre V, Bieth A, Guimbaud R, Trouche D, Pasero P, Méchali M, Hoffmann JS, Cazaux C. A DNA replication signature of progression and negative outcome in colorectal cancer. Oncogene. 2010;29:876–887. doi: 10.1038/onc.2009.378. [DOI] [PubMed] [Google Scholar]
- 238.Albertella MR, Lau A, O’Connor MJ. The overexpression of specialized DNA polymerases in cancer. DNA Repair. 2005;4:583–593. doi: 10.1016/j.dnarep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 239.Yang J, Chen Z, Liu Y, Hickey RJ, Malkas LH. Altered DNA polymerase iota expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res. 2004;64:5597–5607. doi: 10.1158/0008-5472.CAN-04-0603. [DOI] [PubMed] [Google Scholar]
- 240.Wang YQ, Seimiya M, Kawamura K, Yu L, Ogi T, Takenaga K, Shishikura T, Nakagawara A, Sakiyama S, Tagawa M, O-Wang J. Elevated expression of DNA polymerase kappa in human lung cancer is associated with p53 inactivation: Negative regulation of POLK promoter activity by p53. Int J Oncol. 2004;25:161–165. [PubMed] [Google Scholar]
- 241.Canitrot Y, Capp JP, Puget N, Bieth A, Lopez B, Hoffmann JS, Cazaux C. DNA polymerase beta overexpression stimulates the Rad51-dependent homologous recombination in mammalian cells. Nucleic Acids Res. 2004;32:5104–5112. doi: 10.1093/nar/gkh848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lange SS, Wittschieben JP, Wood RD. DNA polymerase zeta is required for proliferation of normal mammalian cells. Nucleic Acids Res. 2012;40:4473–4482. doi: 10.1093/nar/gks054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Knobel P, Kotov I, Felley-Bosco E, Stahel R, Marti T. Inhibition of REV3 Expression Induces Persistent DNA Damage and Growth Arrest in Cancer Cells. Neoplasia. 2011;13:961–970. doi: 10.1593/neo.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Avkin S, Sevilya Z, Toube L, Geacintov N, Chaney SG, Oren M, Livneh Z. p53 and p21 Regulate Error-Prone DNA Repair to Yield a Lower Mutation Load. Mol Cell. 2006;22:407–413. doi: 10.1016/j.molcel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 245.Soria G, Podhajcer O, Prives C, Gottifredi V. P21Cip1//WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation. Oncogene. 2006;25:2829–2838. doi: 10.1038/sj.onc.1209315. [DOI] [PubMed] [Google Scholar]
- 246.Fox JT, Lee K-y, Myung K. Dynamic regulation of PCNA ubiquitylation/deubiquitylation. FEBS Letters. 2011;585:2780–2785. doi: 10.1016/j.febslet.2011.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bartek J, Mistrik M, Bartkova J. Thresholds of replication stress signaling in cancer development and treatment. Nat Struct Mol Biol. 2012;19:5–7. doi: 10.1038/nsmb.2220. [DOI] [PubMed] [Google Scholar]
- 248.Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montaña MF, D’Artista L, Schleker T, Guerra C, Garcia E, Barbacid M, Hidalgo M, Amati B, Fernandez-Capetillo O. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol. 2011;18:1331–1335. doi: 10.1038/nsmb.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lin X, Okuda T, Trang J, Howell SB. Human REV1 Modulates the Cytotoxicity and Mutagenicity of Cisplatin in Human Ovarian Carcinoma Cells. Mol Pharmacol. 2006;69:1748–1754. doi: 10.1124/mol.105.020446. [DOI] [PubMed] [Google Scholar]
- 250.Okuda T, Lin X, Trang J, Howell SB. Suppression of hREV1 expression reduces the rate at which human ovarian carcinoma cells acquire resistance to cisplatin. Mol Pharmacol. 2005;67:1852–1860. doi: 10.1124/mol.104.010579. [DOI] [PubMed] [Google Scholar]
- 251.Xie K, Doles J, Hemann MT, Walker GC. Error-prone translesion synthesis mediates acquired chemoresistance. Proc Natl Acad Sci USA. 2010;107:20792–20797. doi: 10.1073/pnas.1011412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Doles J. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc Natl Acad Sci USA. 2010;107:20786–20791. doi: 10.1073/pnas.1011409107. [DOI] [PMC free article] [PubMed] [Google Scholar]