Abstract
The physical distance between presynaptic Ca2+ channels and the Ca2+ sensors that trigger exocytosis of neurotransmitter-containing vesicles is a key determinant of the signalling properties of synapses in the nervous system. Recent functional analysis indicates that in some fast central synapses, transmitter release is triggered by a small number of Ca2+ channels that are coupled to Ca2+ sensors at the nanometre scale. Molecular analysis suggests that this tight coupling is generated by protein–protein interactions involving Ca2+ channels, Ca2+ sensors and various other synaptic proteins. Nanodomain coupling has several functional advantages, as it increases the efficacy, speed and energy efficiency of synaptic transmission.
Synaptic transmission involves a highly complex series of events. When an action potential invades a presynaptic terminal, Ca2+ inflow through voltage-gated Ca2+ channels leads to a rise in intracellular Ca2+ concentration. Next, Ca2+ binds to a presynaptic Ca2+ sensor, which subsequently triggers exocytosis of neurotransmitter-containing synaptic vesicles. Finally, the released transmitter diffuses across the synaptic cleft and binds to postsynaptic receptors. Thus, a voltage change in the presynaptic neuron (the action potential) is converted into two chemical signals (Ca2+ and transmitter) and then converted into an electrical response in the postsynaptic cell. Remarkably, what sounds like a lengthy sequence of slow biophysical and biochemical events takes place in less than a millisecond1-5.
How such a short synaptic delay can be achieved is not completely understood. According to the laws of physics, diffusion time is proportional to the square of distance6. Thus, the high speed of synaptic transmission requires tight packing of the relevant molecules. The hypothesis that there is tight coupling between Ca2+ channels and Ca2+ sensors of exocytosis received initial support from experiments on two ‘classical’ synapses in the peripheral nervous system: the frog neuromuscular junction7 (FIG. 1a) and the squid giant synapse8 (FIG. 1b). At the frog neuromuscular junction, high-resolution electron microscopy tomography revealed that the distance between putative Ca2+ channels and synaptic vesicles was only ~20 nm (REF. 9) and modelling combined with cooperativity measurements suggested that vesicle fusion results from the Ca2+ inflow through only one or two Ca2+ channels10. Similarly, at the squid giant synapse, functional analysis indicated that the Ca2+ source and Ca2+ sensor are tightly coupled at nanometre distance11 and only a few Ca2+ channels are required for release12,13. Evidence for both tight coupling and the involvement of a small number of channels has also been presented for the ciliary ganglion calyx synapses of the chick14,15. In this uniquely accessible synaptic preparation, simultaneous electrophysiological recording from the transmitter release face of the calyx terminal and biochemical detection of transmitter release demonstrated that the opening of a single presynaptic Ca2+ channel can trigger exocytosis14.
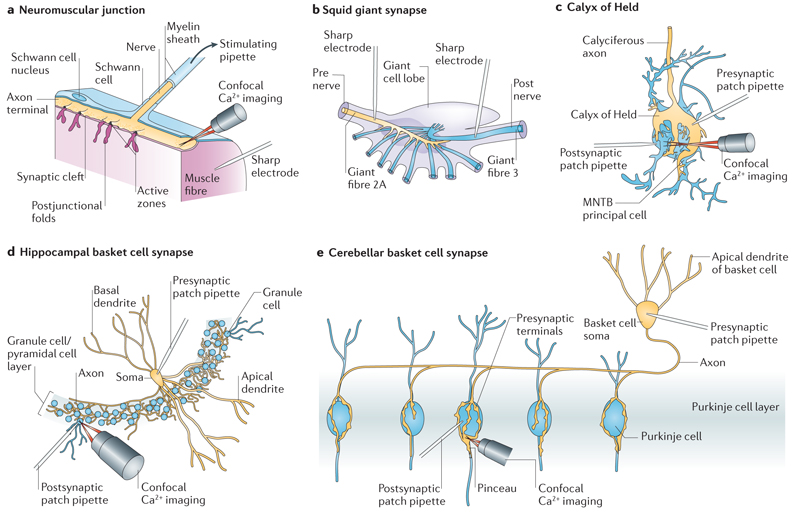
Figure 1. Model synapses used for the analysis of Ca2+ channel–sensor coupling.
a | The frog neuromuscular junction, which is a classical preparation for the analysis of synaptic transmission7. This synapse is formed between motor axons (yellow) and skeletal muscle fibres (pink). A technical advantage is the 1:1 innervation (1 motor axon:1 muscle fibre). Furthermore, the structure of this synapse has been studied extensively9. Presynaptic access, however, is not possible. b | The squid giant synapse8. This synapse is established between second and third order giant nerve fibres in the stellate ganglion of the squid136. A technical advantage is that presynaptic elements can be recorded directly with sharp microelectrodes. c | The calyx of Held in the auditory brainstem18,19. This synapse is formed between the globular bushy cells in the cochlear nucleus and the neurons of the medial nucleus of the trapezoid body (MNTB)19. A technical advantage of this synapse is that presynaptic terminals can be recorded directly with patch-clamp techniques. However, a disadvantage is that recordings from older animals (>postnatal day 8–10) are difficult. d | The hippocampal dentate gyrus basket cell synapse20. This synapse is established between fast-spiking, parvalbumin-expressing basket cells in the hippocampus (yellow) and postsynaptic target cells (in this case granule cells, blue). e | The cerebellar basket cell synapse21. This synapse is established between parvalbumin-expressing basket cells in the cerebellum (yellow) and postsynaptic target cells (in this case Purkinje cells, blue). In hippocampal and cerebellar basket cell synapses, paired recordings between presynaptic and postsynaptic neurons can be obtained with high success rates because of the relatively high connectivity. A disadvantage of these synapses is that presynaptic terminals cannot be routinely recorded. Part a is modified, with permission, from REF. 137 © (1992) Sinauer. Part b is modified, with permission, from REF. 136 © (1957) The Rockefeller University Press. Part c is modified, with permission, from REF. 19 © (2002) Macmillan Publishers Ltd. All rights reserved.
Notably, all of these synapses have highly specialized properties and belong to peripheral nervous systems of invertebrates or lower vertebrates. Does nanodomain coupling also occur at synapses in the mammalian CNS? This is an important question for several reasons. First, detailed knowledge about coupling is essential to understand the biophysical factors shaping the efficacy and speed of synaptic transmission. Second, knowledge about coupling is necessary to correctly interpret the mechanisms of presynaptic forms of plasticity16 and the action of Ca2+ buffers17. Finally, obtaining an answer is important for understanding the mechanisms underlying information processing and coding in the brain. A definitive answer has been obtained only recently, after a range of central synapses were made accessible to quantitative biophysical analysis. These include the young and mature calyx of Held (a glutamatergic synapse in the auditory system18,19 (FIG. 1c)) and GABAergic synapses in the hippocampus and the cerebellum20,21 (FIG. 1d,e) that mediate fast feedforward and feedback inhibition in neuronal microcircuits.
In this Review, we summarize recent evidence for tight coupling between Ca2+ channels and Ca2+ sensors of exocytosis at central synapses, address the molecular mechanisms involved and discuss the functional implications of this coupling configuration.
Tight coupling at fast central synapses
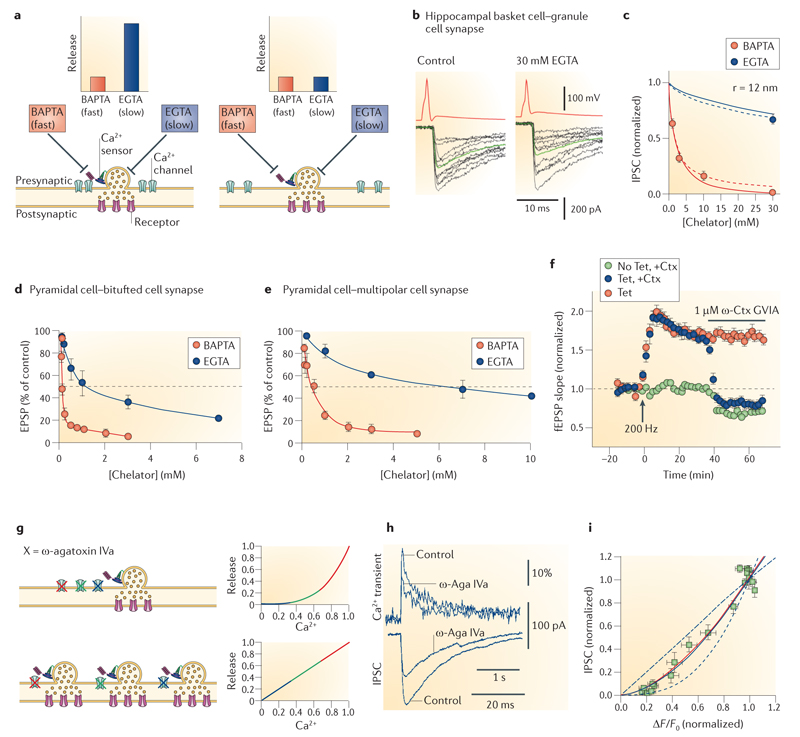
The coupling distance between Ca2+ channels and Ca2+ sensors can be probed using the intracellular application of two exogenous Ca2+ chelators that have different binding rates (kon), but comparable affinities (KD) (TABLE 1). The basic principle is simple11 (BOX 1). If the distance between Ca2+ channels and Ca2+ sensors of exocytosis is short (smaller than 100 nm), only the fast Ca2+ chelator BAPTA, but not the slow Ca2+ chelator EGTA, will have enough time to capture the Ca2+ on its way from the Ca2+ channels to the Ca2+ sensors and impair transmission in millimolar concentrations. By contrast, if the coupling distance is longer, both the fast and the slow Ca2+ chelator will be effective.
Table 1. Physicochemical properties of exogenous and endogenous Ca2+ buffers.
| Chelator/Ca2+-binding protein | Ca2+-binding rate (kon) | Ca2+-unbinding rate (koff) | Affinity (KD) | Refs |
|---|---|---|---|---|
| BAPTA* | 4 × 108 M−1 s−1 | 88 s−1 ‡ | 220 nM | 22,33,139 |
| EGTA* | 1 × 107 M−1 s−1 | 0.7 s−1 ‡ | 70 nM | 22,77 |
| Calbindin | 7.5 × 107 M−1 s−1 | 29.5 s−1 | 293 nM‡ | 77,79 |
| Calretinin§ | 1.8 × 106 M−1s−1 (T) | 1.29 s−1 (T) | 717 nM‡ | 78 |
| 3.1 × 108 M−1 s−1 (R) | 1.73 s−1 (R) | 5.6 nM‡ | ||
| Calmodulin N-lobe§ | 7.7 × 108 M−1 s−1 (T) | 1.6 × 105 s−1 (T) | 208 μM‡ | 79 |
| 3.2 × 1010 M−1 s−1 (R) | 2.2 × 104 s−1 (R) | 688 nM‡ | ||
| Calmodulin C-lobe§ | 8.4 × 107 M−1 s−1 (T) | 2.6 × 103 s−1 (T) | 31 μM‡ | 79 |
| 2.5 × 107 M−1 s−1 (R) | 6.5 s−1 (R) | 260 nM‡ |
For the exogenous chelators, the Ca2+-binding rate (on rate) is ~40 times higher for BAPTA than for EGTA. By contrast, the affinity values are comparable; in fact the affinity is threefold lower for BAPTA than for EGTA.
This value was calculated using KD = koff/kon.
For the Ca2+-binding proteins calretinin and calmodulin, Ca2+ binding is highly cooperative. Therefore, rates are given separately for tense (T) and relaxed (R) conformations of the protein.
Box 1. Probing nanodomains and microdomains with exogenous Ca2+ chelators.
The distance between Ca2+ source and Ca2+ sensor can be probed using Ca2+ chelators with different Ca2+-binding rates (kon), but comparable affinities (KD)11. Ca2+ chelators suppress synaptic transmission by intercepting the Ca2+ on its way from the Ca2+ source to the Ca2+ sensor (FIG. 2a). The exact amount of block depends on source-sensor distance, binding rate and concentration of the chelator. If the coupling distance is short, only the fast Ca2+ chelator will have an effect at millimolar concentrations. If the coupling distance is long, both fast and slow Ca2+ chelators will be effective, according to their affinity at equilibrium. This approach was first applied to the squid giant synapse11 using the fast chelator BAPTA and the slow chelator EGTA. BAPTA and EGTA are ideal experimental tools because they differ by a factor of ~40 in their on rates, but show comparable affinity values22,33,77,139 (TABLE 1).
The concentration dependence of the BAPTA and EGTA effects provides information about the average coupling distance between Ca2+ channels and Ca2+ sensors. Such data may be used to distinguish between nanodomain and microdomain coupling regimes. The concentration dependence of the chelator effects also provides information about the uniformity of the coupling distance. For example, at the young calyx of Held, the concentration dependence determined experimentally can only be described by theoretical models if significant non-uniformity in the coupling distance is assumed22.
Although the terms nanodomain and microdomain are widely used, they are not precisely defined. What is the distance limit between nanodomains and microdomains? One approach is to use the border between diffusion regimes and buffering regimes as a criterion (for example, by choosing a distance where buffering reduces the Ca2+ concentration to 50%). This can be roughly estimated from the length constant (λ) of endogenous buffers.
With , where DCa = 220 μm2 s−1 (REF. 17), kon = 108 M−1 s−1 (an on rate representative of endogenous buffers (TABLE 1)) and [B] = 100 μM, [Ca2+]50% is reached at a distance of 100 nm. Alternatively, the limit may be set according to vesicle size and active zone size. As the radius of synaptic vesicles is ~20 nm (REF. 64) and the radius of active zones is typically ~150 nm (REFS 26,40,64,65), the limit should be set in between. Throughout this Review, we define the border between nanodomain and microdomain at a distance of 100 nm.
This approach has been applied to several synapses in the mammalian CNS, leading to surprising results. In the young calyx of Held (~8–10 days after birth) and in neocortical glutamatergic synapses (~14–16 days after birth), evoked transmitter release is suppressed by ~1 mM intracellular BAPTA, but also by ~10 mM EGTA3,22-25 (TABLE 2; FIG. 2a–c). This implies that the distance between Ca2+ channels and Ca2+ sensors must be long. At the young calyx of Held, quantitative modelling suggests that the average coupling distance is ~100 nm (range from 30 to 300 nm)22. Thus, evoked transmitter release at these synapses is triggered by so-called ‘Ca2+ microdomains’.
Table 2. Sensitivity to BAPTA and EGTA distinguishes between nanodomain and microdomain coupling.
| Synapse | Age* and species | BAPTA IC50‡ or PSC amplitude | EGTA IC50‡ or PSC amplitude | Refs |
|---|---|---|---|---|
| Synapses with nanodomain coupling | ||||
| Squid giant synapse | Adult squid | 0.73 mM | >>80 mM | 11 |
| Mature calyx of Held | P16–18 mouse | 1.3 mM | 35.4 mM | 23 |
| Hippocampal basket cell–granule cell synapse |
P18–21 rat | 1.6 mM | 61.5 mM | 26 |
| Hippocampal basket cell–granule cell synapse |
P19–22 rat | 63.9 ± 4.3% in 100 μM BAPTA-AM§ |
No effect in 100 μM EGTA-AM§ | 43 |
| Cerebellar molecular layer interneuron–interneuron synapse |
P14–20 rat | Unknown | 97.5 ± 4.8% and 82.8 ± 11.3% in 20 μM EGTA-AM§ |
27 |
| Cerebellar climbing fibre–Purkinje cell synapse |
P8–20 rat | Unknown | 103 ± 5% in 20 μM EGTA-AM§ | 120 |
| Auditory hair cell ribbon synapse | P14–40 mouse | <<5 mM (almost complete block) | >>5 mM | 37 |
| Retinal bipolar cell ribbon synapse | P15–25 rat | 2.2 mM | >>5–10 mM | 140 |
| Synapses with microdomain coupling | ||||
| Young calyx of Held | P8–12 mouse | 1.3 mM | 7.5 mM | 23 |
| Young calyx of Held | P8–10 rat | 0.61 mM | 13.3 mM | 3,141 |
| Layer 5–layer 5 neocortical synapse | P14–16 rat | 0.7 mM | 7.9 mM | 24 |
| Layer 2/3 pyramidal cell synapse on bitufted interneuron |
P14–15 rat | 0.1 mM | 1 mM | 25 |
| Layer 2/3 pyramidal cell synapse on multipolar interneuron |
P14–15 rat | 0.5 mM | 7 mM | 25 |
| CCK interneuron–granule cell synapse |
P19–22 rat | Unknown | 6.8 ± 3.8% in 100 μM EGTA-AM§ | 43 |
| Cerebellar climbing fibre synapse, ectopic release on Bergmann glial cell |
P8–20 rat | Unknown | 67 ± 11% in 20 μM EGTA-AM§ | 120 |
EGTA-AM, EGTA acetoxymethyl ester; P, postnatal day; PSC, postsynaptic current.
For the calyx of Held, P12 is an important reference point because it represents the onset of hearing.
IC50 (concentration of an inhibitor at which 50% inhibition of the response is seen) values were either directly taken from references or calculated from the amount of block according to a Hill equation.
AM forms of EGTA permeate cell membranes easily. Once the intracellular compartment is reached, the AM residue is cleaved by endogenous esterases, and the Ca2+ chelator is trapped intracellularly. Although the precise EGTA concentration is not known, it is thought that this trapping mechanism leads to a ~100-fold enrichment in comparison to the extracellular concentration28.
Figure 2. Experimental determination of the coupling distance and the number of open Ca2+ channels that mediate transmitter release.
a | Ca2+ chelators with different on rates are used to probe the distance between Ca2+ channels and sensors. In a tight coupling regime (left), only the fast Ca2+ chelator BAPTA, but not the slow Ca2+ chelator EGTA, will capture the Ca2+ on its way from the source to the sensor. By contrast, in a loose coupling regime (right), both chelators will be effective, according to their affinity values, which are comparable. b | Effects of 30 mM EGTA on unitary inhibitory postsynaptic currents (IPSCs) at the hippocampal basket cell–granule cell synapse under steady-state conditions. Orange traces, presynaptic action potentials; black traces, IPSCs; green traces, averages. Note that EGTA has only minimal effects at this synapse. c | Concentration dependence of the effects of BAPTA and EGTA at the hippocampal basket cell–granule cell synapse. Lines represent predictions of a reaction–diffusion model simplified by linearization (continuous lines, predictions for a single Ca2+ channel; dashed lines, predictions for a cluster of multiple Ca2+ channels). The best description of the experimental data was obtained assuming a coupling distance of 12 nm. d,e | Target-cell-specific differences in the coupling distance. Concentration dependence of the effects of BAPTA and EGTA at glutamatergic synapses formed by pyramidal neurons in somatosensory cortex on bitufted interneurons (presumably representing somatostatin-positive subtypes) and multipolar interneurons (presumably representing parvalbumin-expressing subtypes). In the pyramidal neuron–multipolar interneuron synapses, synaptic transmission is only weakly sensitive to EGTA, suggesting tight coupling between Ca2+ channels and sensors. f | Presynaptic plasticity changes the contribution of N-type Ca2+ channels to transmitter release at glutamatergic perforant path synapses on hippocampal CA1 pyramidal neurons. After 200 Hz tetanic stimulation (arrow, Tet), inducing a presumably presynaptic form of long-term potentiation, the amount of block by ω-conotoxin GVIa (ω-Ctx GVIa), a selective N-type channel blocker, increases, suggesting that transmission becomes increasingly dependent on N-type channels. g | A slow calcium channel blocker can be used to estimate the number of open channels required for neurotransmission. In a multiple channel coupling scenario (upper panel), blocking Ca2+ channels with a slow blocker scales the Ca2+ transient at the vesicular Ca2+ sensor, reducing transmitter release supralinearly. In a single-channel scenario (lower panel), blocking Ca2+ channels sequentially eliminates channel–vesicle nanocomplexes, inhibiting transmitter release linearly. h | Ca2+ transients (upper traces) and IPSCs (lower traces) at the hippocampal basket cell–granule cell synapse before and after application of ω-agatoxin IVa (ω-Aga IVa). Corresponding scale bars are at the bottom. Note that the toxin reduces Ca2+ transients and IPSCs to a comparable extent. Presynaptic Ca2+ transients were measured as relative fluorescence changes (ΔF/F0) using the Ca2+ indicator dye Oregon Green BAPTA1. i | Plot of peak amplitudes of synaptic currents as a measure of exocytosis against ΔF/F0 as a measure of Ca2+ inflow (both normalized to the respective control value). The blue curves show the predictions of a binomial model of Ca2+ channel block with different numbers of open Ca2+ channels (n = 1, 2 or 10). The red curve shows free fit with a power function. Note that the best fit of the experimental observations can be obtained with a model assuming two or three Ca2+ channels. Parts b and c are reproduced, with permission, from REF. 26 © (2008) Elsevier. Parts d and e are reproduced, with permission, from REF. 25 © (2001) Wiley-Blackwell. Part f is reproduced, with permission, from REF. 48 © (2009) Elsevier. Parts h and i are reproduced, with permission, from REF. 56 © (2010) Macmillan Publishers Ltd. All rights reserved. EPSP, excitatory postsynaptic potential; fEPSP, field EPSP.
By contrast, at the output synapses of fast-spiking, parvalbumin-expressing GABAergic interneurons (basket cells) in the hippocampus (typically recorded ~18-21 days after birth), evoked transmitter release is inhibited by millimolar concentrations of BAPTA, but is largely unaffected by 30 mM EGTA26 (TABLE 2; FIG. 2a–c). Furthermore, at the output synapses of inhibitory cells in the cerebellum, intracellular application of 1 mM EGTA has no effect on the proportion of synaptic failures21. Likewise, at cerebellar basket cell synapses, bath application of 20 μM of membrane-permeable EGTA acetoxymethyl ester (EGTA-AM) has only minimal effects on evoked transmitter release following a single presynaptic action potential27. Although in the case of bath application of EGTA-AM the concentration of intracellular EGTA is only roughly known28, these results may suggest tight coupling between Ca2+ source and Ca2+ sensor. At the hippocampal basket cell-granule cell synapse, quantitative modelling reveals a uniform coupling distance in the range of 10–20 nm (REF. 26) (FIG. 2c). Thus, evoked transmitter release at fast hippocampal and cerebellar GABAergic synapses is triggered by ‘Ca2+ nanodomains’.
Although the terms nanodomain and microdomain are widely used, their definitions are not very precise and have undergone historic shifts. Originally, the term microdomain was used to describe the high concentration of Ca2+ found near an open Ca2+ channel29-32. Despite the name, these microdomains actually have spatial dimensions in the nanometre range (‘micro’ in ‘microdomain’ means ‘small’ in Greek). More recently, the terms nanodomain and microdomain have been widely applied to distinguish tight and loose coupling regimes. This definition is also confusing, as a limit of 50–150 nm is often used to separate between the two domains. Throughout this Review, we pragmatically refer to nanodomain coupling if the mean coupling distance is <100 nm, and to microdomain coupling if the distance is larger (BOX 1).
The Ca2+ chelator experiments not only suggest differences in the mean coupling distance but also in the uniformity of source–sensor coupling between synapses. In the young calyx of Held, 1 mM and 10 mM EGTA are almost equally effective3,22. Accordingly, there is no single distance value that describes the concentration dependence of the chelator’s effects at this synapse33. This suggests substantial non-uniformity in the coupling distance3,22. This hypothesis is corroborated by uncaging experiments, which indicate that a subpopulation of vesicles in the calyx is reluctantly released following Ca2+ channel opening, but rapidly released by Ca2+ uncaging34,35. By contrast, in the output synapses of hippocampal basket cells, a single coupling distance can adequately describe the effects of BAPTA and EGTA over a wide concentration range26 (FIG. 2c). This suggests that the coupling is substantially more uniform26. Consistent with this idea, the estimates of releasable pool sizes, as determined by action potential trains and sucrose application, differ at excitatory synapses but are comparable in inhibitory synapses36. Thus, the tightness and uniformity of coupling at different synapses seem to be related.
The finding that the calyx of Held uses microdomain signalling for transmitter release3,22 was puzzling for several reasons. First, it was difficult to accept that two synapses with calyx morphology (the calyx of Held and the ciliary ganglion calyx) would differ fundamentally in the coupling configuration. Second, if tight coupling served the purpose of improving the speed and precision of transmitter release, it may be surprising that it is not utilized in the auditory system, where the timing of signalling is critically important. Indeed, analysis of coupling at the auditory hair cell ribbon synapse (the first station in the auditory pathway) revealed that transmitter release was blocked by intracellular BAPTA, but not EGTA, suggesting nanodomain coupling37. Similar results were obtained at ribbon synapses in the visual system38,39. A resolution of this apparent paradox was provided when coupling at the calyx of Held was examined at different developmental stages40,41. In the mature calyx of Held (~16-18 days after birth), release is suppressed by millimolar concentrations of intracellular BAPTA, but is unaffected by 10 mM intracellular EGTA23,41 (TABLE 2). Modelling indicated that the coupling distance decreased to ~20 nm during development42, which is a similar distance to that at the hippocampal basket cell synapses. Thus, transmitter release at fast synapses in the mature auditory pathway is also triggered by Ca2+ nanodomains. Developmental processes may also regulate the tightness of coupling at glutamatergic24,25 and GABAergic synapses26,43 in the cortex. A systematic analysis of different synapses at different developmental stages is required to address this issue.
Specification and regulation of coupling
The results described above suggest that certain synapses in neuronal microcircuits (such as fast GABAergic output synapses of hippocampal or cerebellar basket cells) use nanodomain coupling, whereas others (such as glutamatergic synapses between layer 5 pyramidal neurons24) involve microdomain coupling. These results raise two important questions. What are the rules that lead to the use of nanodomain signalling in one case and microdomain signalling in the other case, and is the coupling distance regulated dynamically?
Several lines of evidence suggest that synapses formed by different presynaptic neurons on the same target cell can use different coupling configurations. One example is provided by the opposite properties of synapses of parvalbumin- and cholecystokinin (CCK)-expressing interneurons onto hippocampal granule cells and pyramidal cells43-45. The fast-spiking, parvalbumin-expressing interneurons exhibit tight coupling, as confirmed by the lack of effect of external EGTA-AM, whereas the CCK-expressing interneurons show loose coupling, as demonstrated by the large effect of EGTA-AM on evoked release under identical experimental conditions26,43 (TABLE 2).
Furthermore, synapses formed by the same presynaptic neuron on different postsynaptic target cells can differ in their coupling configuration. The diverging output from layer 2/3 pyramidal neurons in the neocortex onto two types of interneurons provides a clear example25. Layer 2/3 pyramidal neuron synapses on multipolar (presumably parvalbumin-expressing) interneurons are less sensitive to EGTA than synapses on bipolar (presumably somatostatin-expressing) interneurons (TABLE 2). These results may imply that a retrograde signalling mechanism regulates the tightness of the coupling in the presynaptic terminals.
Finally, the available results suggest that the use of nanodomain versus microdomain coupling may in some cases be pathway-specific. For example, both the input and the output synapses of parvalbumin-expressing interneurons use relatively tight coupling to trigger transmitter release25,26 (FIG. 2b,c,e). Likewise, both hair cells and mature calyces in the auditory system rely on nanodomain coupling23,46. Thus, the tightness of coupling appears to be regulated in a pathway-specific manner. This regulation may be activity-dependent47, but a more systematic analysis of different synapses, microcircuits and conditions will be needed to test this hypothesis.
An intriguing possibility is that the coupling between Ca2+ channels and Ca2+ sensors of exocytosis is not static, but is regulated dynamically. Recent results suggest that the induction of presynaptic long-term potentiation at distal perforant path synapses on CA1 pyramidal neurons is associated with an alteration in the dependence of transmitter release on P/Q- or N-type Ca2+ channels — resulting in an increased contribution of N-type Ca2+ channels after potentiation48 (FIG. 2f). It is possible that these changes are connected to changes in channel–sensor coupling. Thus, dynamic regulation of the coupling distance may contribute to presynaptic forms of plasticity at central synapses48.
In conclusion, the available evidence indicates that nanodomain coupling is regulated by both pre- and postsynaptic neurons, probably in a pathway-specific manner. Furthermore, recent results suggest that the coupling configuration is not static, but is regulated dynamically during presynaptic forms of synaptic plasticity. Further experiments will be needed to directly examine the dynamics of the coupling during presynaptic forms of plasticity.
How many Ca2+ channels for release?
Nanodomain coupling between Ca2+ channels and Ca2+ sensors places structural and functional constraints on the number of Ca2+ channels that can be involved in transmitter release. As voltage-gated Ca2+ channel proteins have a diameter of ~10 nm (REF. 49), the highest channel density that is physically possible is ~10,000 μm−2. Accordingly, the number of Ca2+ channels involved in transmitter release in nanodomain coupling regimes must be small. For example, only ~12 channels can be placed on a planar presynaptic membrane within 20 nm from a synaptic vesicle. Furthermore, if coupling is tight, only a small number of Ca2+ channels may be needed to reach effective Ca2+ concentrations at the sensor.
How can one experimentally determine the number of open Ca2+ channels necessary for transmitter release? A classical approach is based on an analysis of the relationship between presynaptic Ca2+ inflow and transmitter release during an experimental reduction in the number of active Ca2+ channels. Such a reduction of Ca2+ channel number can be achieved either by application of slow Ca2+ channel blockers, such as peptide toxins50, or by modifying the presynaptic voltage waveform that triggers exocytosis. The basic idea is relatively simple (BOX 2). If several open Ca2+ channels jointly trigger the release of a synaptic vesicle, the progressive reduction of Ca2+ inflow will lead to a supralinear reduction in transmitter release. This results from the so-called ‘intrinsic’ or ‘biochemical’ cooperativity51 of the Ca2+ sensor synaptotagmin, which has five binding sites for Ca2+ (REFS 52,53) and is expressed in multiple copies on each synaptic vesicle54. By contrast, in the extreme case when only a single open Ca2+ channel triggers release of a synaptic vesicle, the slow blocker will reduce Ca2+ inflow and release proportionally.
Box 2. Counting the number of release-relevant Ca2+ channels.
The number of open Ca2+ channels required for transmitter release can be determined from the shape of the relationship between release and presynaptic Ca2+ inflow12,13,56. In synapses where presynaptic voltage clamp is possible, the number of open channels can be manipulated by varying the amplitude and duration of the depolarization23. Under these conditions, the presynaptic Ca2+ current can be directly recorded. In other synapses where presynaptic voltage clamp is not possible, the number of Ca2+ channels can be changed by application of channel blockers50,56. Under these conditions, presynaptic Ca2+ inflow is quantified by Ca2+ imaging. The results from these measurements then give the relationship between transmitter release and presynaptic Ca2+ inflow. If a large number of open Ca2+ channels are required for transmitter release, the relationship will be supralinear, approaching the biochemical cooperativity of the Ca2+ sensor (FIG. 2g). By contrast, if a single open Ca2+ channel is sufficient to trigger transmitter release, the relationship will be linear because the blocker will sequentially eliminate channel–vesicle nanocomplexes (FIG. 2g). If the number of channels is small, but >1, the shape of the relationship will be intermediate between these two extremes.
Evidently, the power coefficient of the release-Ca2+ inflow relationship is not identical to the number of open Ca2+ channels necessary for transmitter release. To quantitatively determine this number, modelling has to be performed56. If blockers are used, a simple binomial model of Ca2+ channel block can be chosen. However, several factors must be considered. The properties of the blocker are crucial: the ideal blocker should have slow kinetics and block Ca2+ channels uniformly throughout the presynaptic terminal. Fast blockers that generate a flicker block130 or blockers that reduce the single-channel conductance cannot be used. The techniques for measuring presynaptic Ca2+ inflow and transmitter release have to be quantitative and linear. The modelling is based on several assumptions, such as uniform coupling distance and independent block of channels, which may not be valid in all cases. It must also be kept in mind that the method measures the number of open channels, not the total number of Ca2+ channels present. These two numbers can substantially differ because the maximal open probability of Ca2+ channels during presynaptic action potentials is significantly smaller than one59-62. This approach has been successfully applied to synapses where transmitter release exclusively relies on a single type of Ca2+ channel, such as the P/Q-type Ca2+ channel in GABAergic synapses43,56 or the L-type Ca2+ channel in auditory hair cell ribbon synapses46. At synapses where transmitter release relies on the concerted action of P/Q-, N- and R-type channels55,131-133, careful interpretation of the results is required. If release-[Ca2+] relationships are measured using subtype-specific blockers, the results will provide information about channel location rather than number. If channels are loosely coupled, they will contribute little to release (low power coefficient), whereas if they are tightly coupled, they will contribute more (high power coefficient)134. Thus, the power coefficients, although informative, are entirely unrelated to channel numbers. By contrast, non-additive blocker effects may provide indirect information about channel number. Evidence for non-additive blocker effects was reported at the young calyx of Held55, glutamatergic synapses in the hippocampus131,132,135 and glutamatergic parallel fibre synapses in the cerebellum133. In these synapses, the sum of the effects of individual blockers on transmitter release is larger than 100%, suggesting the involvement of a large number of channels.
This approach has recently been applied to various central synapses. At the young calyx of Held, the relationship between evoked transmitter release and presynaptic Ca2+ currents during slow Ca2+ channel block is highly supralinear, with a power coefficient (m) greater than 3, suggesting the involvement of a large number of open Ca2+ channels23,41,55. By contrast, at the output synapses of hippocampal basket cells, the relationship is only slightly supralinear, with a power coefficient of 1.6 (REF. 56) (FIG. 2h,i). Modelling of experimental data with a binomial model of Ca2+ channel block suggested that two or three open Ca2+ channels trigger transmitter release at this synapse. Furthermore, in the mouse calyx of Held, the power coefficient is markedly reduced during development23. Likewise, in the rat calyx, the power coefficient is slightly reduced during development and the relationship between transmitter release and Ca2+ charge is shifted to the left57. Collectively, these results suggest that during development the number of open channels required for transmitter release is reduced, while the tightness of the coupling of these channels to their Ca2+ sensors is increased. Modelling also suggested the involvement of a small number of open Ca2+ channels in the mature calyx42. Finally, in both auditory hair cell ribbon synapses and retinal ribbon synapses, the relationship between evoked transmitter release and presynaptic Ca2+ during slow Ca2+ channel block shows a power coefficient of 1.1–1.4, also suggesting the involvement of a small number of open Ca2+ channels39,46,58.
The involvement of a small number of open Ca2+ channels may be explained by two different configurations. In the first scenario, only a small number of Ca2+ channels are present at each active zone, but these channels are activated effectively by presynaptic action potentials. In the second scenario, the total Ca2+ channel number is large, but the efficacy of activation is low. In fast CNS synapses, the high efficacy of activation of P/Q- and N-type Ca2+ channels by action potentials (relative open probability 0.35–0.88 in different mammalian presynaptic terminals, including the calyx of Held)59-62 argues in favour of the first scenario. By contrast, in the auditory hair cell synapses, the lower efficacy of activation of L-type Ca2+ channels would be more consistent with the second scenario46.
These results converge towards a quantitative picture of signalling at fast central synapses. If an action potential invades a presynaptic structure, two or three Ca2+ channels near any given vesicle will open, generating a Ca2+ nanodomain. The Ca2+ concentration is high in the centre of the nanodomain, but steeply declines as a function of distance according to the laws of diffusion (Supplementary information S1 (box)). Thus, the Ca2+ sensor on the vesicle membrane would ‘see’ a Ca2+ transient with a high peak concentration and a fast time course, leading to vesicle fusion with high probability, short delay and high temporal precision. In this scenario, a ‘release site’63 would correspond to a channel–vesicle nanocomplex.
Ca2+ chelator experiments and cooperativity measurements provide additional constraints for the topographical arrangement of Ca2+ channels and vesicles in presynaptic terminals. First, they indicate that these nanocomplexes are sufficiently separated from their nearest neighbours so that their Ca2+ nanodomains do not overlap23,26,46,56. Second, they suggest that nanocomplexes must be sufficiently far away from isolated Ca2+ channels that are not coupled to any synaptic vesicles. Finally, they imply that nanocomplexes are distant from isolated fusion competent vesicles that are not coupled to any Ca2+ channels34. How could this segregation of Ca2+ channel–vesicle nanocomplexes be achieved? In basket cell synapses, which have small boutons with often a single active zone26, nanocomplexes could be allocated to different boutons. At mature calyx synapses, which have ~600 active zones40,64, or in auditory hair cells, which have ~15 active zones46,65, nanocomplexes could be placed into different active zones of the same presynaptic terminal. However, sufficient separation may also be possible if nanocomplexes are located in different subregions of the same active zone. Active zones have a mean area of ~0.1 μm2 (0.094 ± 0.01 μm2 in hippocampal basket cell synapses (A. Kulik, personal communication, and see REF. 26), 0.0996 μm2 in the young calyx64, 0.0548 μm2 in the mature calyx40 and 0.06 μm2 in auditory hair cells65), which corresponds to the area of a circle with ~150 nm radius. If channel–vesicle nanocomplexes were preferentially placed in the periphery (for example, via protein–protein interactions) several of these complexes could be accommodated in a single active zone.
Nanodomains and endogenous Ca2+ buffers
The defining feature of nanodomain coupling is that the fast exogenous buffer BAPTA interferes with release at millimolar concentrations, whereas the slow exogenous buffer EGTA is ineffective17. This raises the question of how endogenous buffers act in nanodomain coupling regimes. A large number of Ca2+ buffers are present in presynaptic terminals of fast signalling synapses. These include parvalbumin in GABAergic synapses in the hippocampus, the cerebellum and the calyx of Held66-69, calretinin in the mature calyx of Held and auditory or vestibular hair cells70,71, and calbindin in auditory hair cells66,72. In addition, several proteins in the active zone have binding sites for Ca2+. These include MUNC13 proteins, RAB3-interacting molecules (RIMs)105 and the Ca2+ sensor synaptotagmin52,53. Furthermore, several proteins enriched in the active zone contain binding sites for ubiquitously expressed Ca2+-binding proteins. For example, P/Q-type Ca2+ channels have binding sites for calmodulin73,74. Collectively, all of these proteins may contribute to the high endogenous buffer capacity of fast signalling neurons68,75.
Can these endogenous Ca2+-binding proteins affect nanodomain coupling? To address this question, information about concentration and Ca2+-binding rate is required17. Recent evidence suggests that endogenous Ca2+ buffers can reach high (millimolar) concentrations. Calibrated immunohistochemistry revealed that cerebellar basket cells express parvalbumin at a concentration of ~0.6 mM (REF. 76). Furthermore, single-cell protein content analysis demonstrated that vestibular hair cells contain calretinin at a concentration of ~1.2 mM (REF. 71). Finally, experiments with recombinant Ca2+ channels and tethered calmodulin mutants suggested a local calmodulin concentration as high as 2.5 mM (REF. 74). These Ca2+-binding proteins have 2–4 EF hand Ca2+ binding sites per molecule, resulting in high millimolar buffer concentrations in nanodomains. Recent results further suggest that the Ca2+-binding rate (kon) of endogenous buffers may be faster than previously thought. For several Ca2+-binding proteins, the kon values have now been quantified in Ca2+ uncaging experiments77-79. For both calretinin (relaxed form) and calbindin, kon values are comparable to those of BAPTA77-79 (TABLE 1). For the calmodulin C-lobe (relaxed form), kon is intermediate between BAPTA and EGTA, whereas for the calmodulin N-lobe, kon is 100-fold higher than that of BAPTA79 (TABLE 1). Finally, Ca2+ uncaging experiments suggest that kon of the Ca2+ sensor synaptotagmin is comparable to that of BAPTA80-82.
Taken together, these results indicate that many endogenous buffers are present at millimolar concentrations and have BAPTA-like binding properties, suggesting that they may interfere with nanodomain signalling. Several functional consequences are conceivable. First, fast endogenous buffers may reduce the amplitude of the Ca2+ transient, offering a mechanism to control the efficacy of synaptic transmission via regulation of buffer expression levels. Second, fast endogenous buffers will shorten the length constant of the buffer system, focusing the nanodomain in space. This effect may be particularly pronounced for fixed buffers (for example, calmodulin attached to presynaptic proteins), which will be saturated in the nanodomain, but unsaturated in the surround. This gradient of free buffer concentration may sharply focus the nanodomain in space83. Finally, buffers may contribute to the use-dependency of presynaptic Ca2+ signalling25,83-87. If presynaptic Ca2+ inflow during a first action potential saturates the buffer, the peak amplitude of a subsequent second Ca2+ transient will be facilitated relative to that of the first. Although facilitation of the Ca2+ transient is generally small, it will be amplified into a much larger facilitation of transmitter release because of intrinsic or biochemical cooperativity23,55,56,88. For example, with a power coefficient of 3.3 (REF. 56), a 1.1-fold (10%) increase would result in a (1.1)3.3 = 1.37-fold (37%) facilitation of release. Hence, endogenous Ca2+ buffers may regulate the amplitude, spatial extent and dynamics of Ca2+ nanodomains.
Among all Ca2+-binding proteins, parvalbumin appears to be a special case because its EF hand sites bind both Ca2+ and Mg2+ (REFS 89-91). Ca2+ binding exhibits fast on rate and high affinity, whereas Mg2+ binding is characterized by slower on rate and lower affinity. As the physiological cytoplasmic concentration of Mg2+ is high, Mg2+ has to unbind before Ca2+ can bind. Thus, parvalbumin may act as a slow buffer, in a similar way to the exogenous Ca2+ chelator EGTA90,91. Furthermore, parvalbumin exhibits a higher mobility than other Ca2+-binding proteins92,93. With all of these properties in mind, the tight correlation of parvalbumin expression with nanodomain signalling67-70 is highly perplexing. In some rapidly signalling synapses, the high total concentration of parvalbumin may provide a resolution to this apparent paradox. Although the fraction of free parvalbumin (the non-Mg2+-bound, non-Ca2+-bound state) under physiological conditions is <10%, the absolute concentration of the free buffer will be substantial under these conditions. This may have two consequences. First, parvalbumin may not exclusively act as a slow buffer (like EGTA)90; it may also act like a fast buffer (like BAPTA) under physiological conditions76. This explains how parvalbumin can affect synaptic transmission in tight coupling regimes21,68,76. Second, the Mg2+-bound parvalbumin fraction will not primarily slow the effective Ca2+-binding rate, but rather contribute to the regeneration of free buffer. Therefore, Mg2+ binding/unbinding may establish a ‘metabuffering’ (that is, buffering of buffering) mechanism, thus maintaining the concentration of free parvalbumin during repetitive activity in fast-spiking neurons. In parallel, the high mobility of parvalbumin will contribute to buffer regeneration in the nanodomain by rapid diffusion of free buffer from the periphery to the centre92,93.
From nanodomains to protein complexes
A distance between Ca2+ channels and sensors of exocytosis of ~20 nm (REFS 26,42) is consistent with the idea that tight coupling is achieved by protein-protein interactions. Active zones are comprised of several evolutionarily conserved proteins, including members of the SNARE, RIM, ELKS and septin families94. Recent results show that several of these proteins have a role in nanodomain coupling (FIG. 3).
Figure 3. Molecular mechanisms of nanodomain coupling.
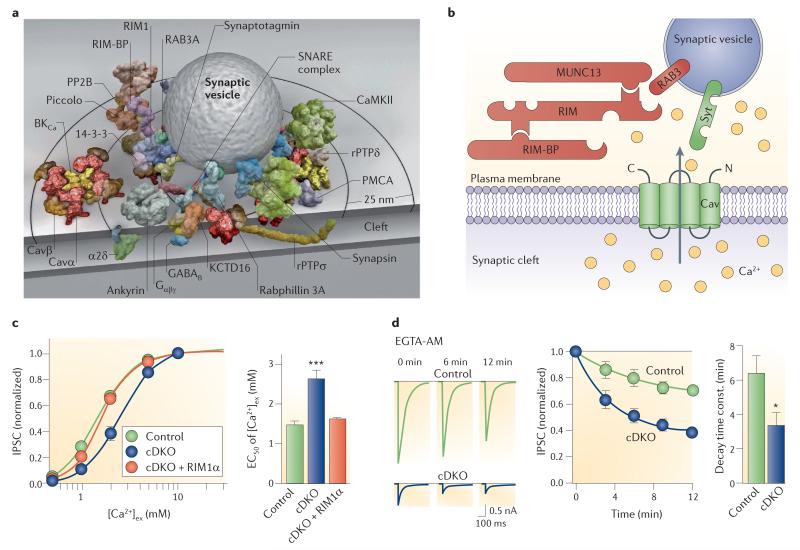
a | Space filling models of protein complexes in the active zone. A synaptic vesicle is surrounded by several proteins. Only a single copy of each protein is depicted94. b illustration of the proposed function of RAB3-interacting molecule (RIM) as a tether in the active zone. Both RIM and RIM binding protein (RIM-BP) bind to the C terminus (C) of the Ca2+ channel. Furthermore, the N terminus (N) of RIM binds to RAB3A. As RAB3A is a vesicular protein, the complex links Ca2+ channels to synaptic vesicles. c | Genetic elimination of RIMs changes the dependency of inhibitory postsynaptic current (IPSC) amplitudes on extracellular Ca2+ concentration at GABAergic synapses. Left, dose–effect curves in control synapses, RIM conditional double knockout (cDKO) synapses, and after rescue with recombinantly expressed RIM1α (cDKO + RIM1α). Right, summary bar graph of EC50 (the concentration of an agonist at which 50% of the response is seen) values in the three conditions. d | Genetic elimination of RIMs changes the coupling between Ca2+ source and Ca2+ sensor at GABAergic synapses. Left, IPSCs in control synapses (top) and in RIM double knockout synapses (bottom) at different times during application of EGTA acetoxymethyl ester (EGTA-AM). Centre, time course of inhibitory effects of EGTA-AM at control synapses (green) and double knockout synapses (blue). Right, time constants of the onset of the effects of EGTA-AM. EGTA-AM acts more rapidly in the RIM double knockout mouse, suggesting a looser coupling between Ca2+ channels and sensors of exocytosis105. Although the experiments were performed at cultured hippocampal inhibitory synapses, it is likely that at least a subset includes output synapses from parvalbumin-expressing, fast-spiking interneurons. Image in a is reproduced, with permission, from REF. 94 © (2010) PNAS. Part b is modified, with permission, from REF. 138 © (2011) Elsevier. Parts c and d are reproduced, with permission, from REF. 105 © (2011) Elsevier. BKCa, large conductance calcium-activated potassium channel; CaMKII, calcium/calmodulin-dependent protein kinase type II; Cav, voltage-gated calcium channel; KCTD16, K+ channel tetramerization domain-containing protein 16; PMCA, plasma membrane Ca2+ ATPase; PP2B, protein phosphatase 2B; Syt, synaptotagmin; RAB3A, small G protein localized on synaptic vesicles; rPTP, receptor protein tyrosine phosphatase.
The first presynaptic proteins shown to be involved in protein-protein interactions with presynaptic Ca2+ channels were the t-SNARE proteins, syntaxin and SNAP25. Both biochemical experiments (yeast two-hybrid experiments, co-immunoprecipitation and proteomic screens) and functional co-expression studies indicated that syntaxin and SNAP25 directly interact with voltage-gated Ca2+ channels at the intracellular loop between domains II and III of the channel protein, the so called ‘synprint’ site95-99. Synaptotagmin, the Ca2+ sensor that triggers exocytosis, also interacts with the synprint site in a Ca2+-dependent manner95-98. Intriguingly, the interactions between Ca2+ channels and SNARE proteins also affect Ca2+ channel function. Co-expression of syntaxin and SNAP25 with Ca2+ channels reduces the channel open probability, whereas additional co-expression of synaptotagmin reverses this effect98. These results suggest a dual function for protein-protein interactions between Ca2+ channels and SNAREs in nanodomain coupling. First, they link the individual molecular elements within the nanodomain. Second, they establish a regulatory switch by which presynaptic Ca2+ channels bound to Ca2+ sensors are functionally selected, whereas Ca2+ channels decoupled from Ca2+ sensors are disabled.
Another protein that is relevant for the Ca2+ channel–sensor coupling is the Drosophila melangoster protein Bruchpilot. Bruchpilot is a ~200 kDa active zone protein containing several coiled-coil domains100. In the neuromuscular junctions of Bruchpilot knockout flies, synaptic efficacy is reduced and sensitivity to EGTA-AM is increased, suggesting a conversion from nanodomain to microdomain coupling100. In mammalian synapses, two proteins homologous to Bruchpilot, ELKS/RAB6-interacting/CAST family member 1 (ERC1) and ERC2 are expressed. However, genetic elimination of ERC1 and ERC2 in mice has only moderate effects on synaptic function101,102. Further studies will be required to clarify the exact role of ELKS proteins in the regulation of coupling at mammalian synapses.
α-neurexins also appear to be involved in the regulation of coupling between Ca2+ channels and Ca2+ sensors of exocytosis103. Neurexins are 200 kDa polymorphic cell surface proteins with several epidermal growth factor (EGF) and laminin-neurexin-sex hormone binding globulin domains. They are encoded by three genes and expressed in ~1,000 isoforms. α-neurexins interact with neuroligins on the postsynaptic membrane and with both ELKS and synaptotagmin within the presynaptic terminal103,104. Deletion of all three neurexin genes reduces evoked transmitter release and the contribution of N-type Ca2+ channels to release at synapses in brainstem and cortex103, consistent with a role for α-neurexins in the regulation of Ca2+ channel–sensor coupling. Neurexin–neuroligin interactions may potentially explain the target cell specificity of coupling25. Ca2+ chelator experiments in neurexin knockout synapses will be needed to test this idea.
Recent results suggest that RIMs have a central organizing role in regulating the coupling between Ca2+ channels and Ca2+ sensors of exocytosis105,106 (FIG. 3b–d). RIMs are multidomain proteins that contain a PDZ domain that selectively interacts with the C terminus of P/Q- and N-type channels. RIMs also contain a binding site for the RIM-binding proteins (RIM-BPs), which in turn binds to several Ca2+ channel subtypes107. Thus, RIMs establish two links to voltage-gated Ca2+ channels: a direct and specific link, and an indirect and unselective link via RIM-BP. In inhibitory hippocampal synapses in culture, genetic elimination of RIM1 and RIM2 reduces the amplitude of evoked inhibitory postsynaptic currents, desynchronizes release, accelerates the onset of the blocking effects of EGTA-AM and shifts the dependence of release on extracellular Ca2+ concentration to higher values105 (FIG. 3c,d). Taken together, these results suggest that the coupling between Ca2+ channels and Ca2+ sensors of exocytosis is disrupted in RIM1 and RIM2 double knockout synapses. Similarly, in the calyx of Held, genetic elimination of RIM1 and RIM2 reduces both the presynaptic Ca2+ channel density and the amplitude of the Ca2+ transient at the Ca2+ sensor106. Additionally, RIM1 and RIM2 knockout may also affect the number of docked and primed vesicles105,106. Thus, at both inhibitory hippocampal synapses and the calyx of Held, RIMs seem to be crucially involved in the regulation of the coupling between Ca2+ channels and Ca2+ sensors of exocytosis.
Finally, the presynaptic GTP/GDP- and syntaxin-binding protein septin regulates the coupling between Ca2+ channels and Ca2+ sensors108,109. Septins are ~35 kDa proteins that form oligomers and higher order structures, such as filaments, rings and gauzes. Septins may form filaments between synaptic vesicles and active zones110. In the young calyx of Held, genetic elimination of septin 5 reduces the sensitivity to EGTA, suggesting a conversion from microdomain to nanodomain coupling109. Two aspects of the function of septin 5 are remarkable. First, unlike other presynaptic proteins, septin 5 increases the coupling distance, suggesting antagonistic control of coupling by presynaptic proteins. Second, the expression of septin 5 is downregulated during development, suggesting an involvement in the developmental switch from microdomain to nanodomain coupling at the calyx109.
Intriguingly, the tightness of the coupling not only depends on various release machinery proteins but also on the Ca2+ channel subtype. In basket cell output synapses of the hippocampus and cerebellum, as well as in the mature calyx of Held, tight coupling goes hand-in-hand with the nearly exclusive use of P/Q-type Ca2+ channels for transmitter release43,56,111-113. By contrast, loose coupling is often correlated with the involvement of N- or R-type Ca2+ channels43,55. Additionally, there is evidence that P/Q- and N-type Ca2+ channels populate partially non-overlapping ‘slots’ within the active zone of glutamatergic synapses114. Finally, L-type Ca2+ channels (rather than P/Q-, N- or R-type Ca2+ channels) are tightly coupled to their Ca2+ sensors in auditory hair cells37,46. Clearly, this coupling specificity cannot be mediated by the synprint site, which follows an efficacy sequence of N > P/Q > L115,116. Thus, the molecular mechanisms underlying this specificity remain unclear.
Nanodomain: advantage, bug or feature?
What are the functional consequences of nanodomain coupling? This question can be systematically addressed by modelling, combining simulation of buffered diffusion (Supplementary information S1 (box)) with previously established models of Ca2+ channel gating59,60 and Ca2+ sensor kinetics80-82,117,118 (Supplementary information S1 (box)).
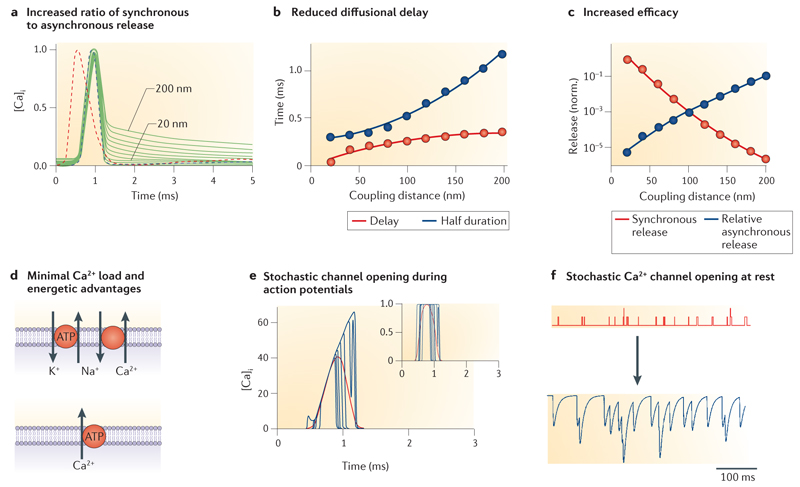
Modelling revealed that nanodomain coupling offers several functional advantages, but may also have disadvantages. The long list of obvious advantages includes increased efficacy and speed of synaptic transmission (FIG. 4a–c). First, tight coupling reduces the synaptic delay22,26. Although the reduction in delay is small for a monosynaptic connection (~100 μs), cumulative effects are expected in polysynaptic chains. Second, tight coupling reduces the duration of the release period, as the time course of the Ca2+ transient ‘seen’ by the Ca2+ sensor is faster in nanodomain than in microdomain coupling regimes. Third, tight coupling increases the ratio of peak Ca2+ to residual Ca2+ and hence the ratio of synchronous to asynchronous release26,43,119. Therefore, in relative terms, tight coupling reduces asynchronous release. This effect may be particularly important in small boutons, in which residual Ca2+ concentration after an action potential is higher than in large presynaptic terminals. Finally, another advantage of nanodomain coupling is that release outside the active zone (‘ectoptic release’) is minimized120,121.
Figure 4. Functional consequences of nanodomain coupling.
a | Tight coupling increases the ratio of synchronous to asynchronous release by increasing the ratio of peak Ca2+ to residual Ca2+. Traces show normalized action potential-evoked Ca2+ transients at distances between 20 nm and 200 nm (step size 20 nm). The fast component of the Ca2+ transient will drive synchronous release, whereas the slow component will initiate asynchronous release. The red dashed line represents the presynaptic action potential. b | Tight coupling reduces the component of the synaptic delay that is caused by diffusion of Ca2+ (red circles and curve) and, in parallel, increases the temporal precision of release in relation to the presynaptic action potential (‘half duration’; blue circles and curve). c | Tight coupling increases release probability and thus synaptic efficacy (red circles and curve) and, in relative terms, decreases asynchronous release (blue circles and curve). d | Tight coupling reduces the presynaptic Ca2+ load and thus introduces energetic advantages. Na+/K+-ATPase, Na+/Ca2+ exchanger and Ca2+-ATPase are depicted schematically. Na+/Ca2+ exchanger and Ca2+-ATPase are the main Ca2+ extrusion mechanisms in the presynaptic plasma membrane122. The Ca2+-ATPase is primary active, that is, directly dependent on the hydrolysis of ATP. The Na+/Ca2+ exchanger is secondary active. It exploits the Na+ ion gradient previously generated by the Na+/K+-ATPase, another primary active transport. Thus, both Ca2+ extrusion pathways require hydrolysis of ~1 ATP for the extrusion of 1 Ca2+ ion. e | Use of a small number of Ca2+ channels introduces stochastic components in Ca2+ channel opening and closing, without affecting the rising phase of corresponding Ca2+ transients. Main plot, simulated Ca2+ concentration 12 nm away from a single Ca2+ channel activated by an action potential. Inset, open probability of the single Ca2+ channel. Ten individual openings are shown superimposed. Red curves show a regime with an infinite number of Ca2+ channels for comparison. Note that the rising phases of the Ca2+ transients are similar, despite stochastic Ca2+ channel opening. Thus, the opening of the Ca2+ channels is stochastic, whereas the rising phase of the Ca2+ transients is largely deterministic. f | Use of a small number of Ca2+ channels may lead to excessive miniature release due to stochastic Ca2+ channel opening. Upper schematics, spontaneous opening of presynaptic Ca2+ channels; lower schematics, hypothetical ‘spontaneous’ release events driven by channel openings. Parts a–c are reproduced, with permission, from REF. 26 © (2008) Elsevier. Part e is reproduced, with permission, from REF. 56 © (2006) Macmillan Publishers Ltd. All rights reserved. In b and c, transmitter release was simulated using a previously established release model80-82.
As tight coupling of a small number of channels to the Ca2+ sensors reduces the total Ca2+ inflow into presynaptic terminals, this configuration is also favourable for the energetics of synaptic transmission (FIG. 4d). Ca2+ extrusion from the presynaptic terminal involves either Na+/Ca2+ exchangers or Ca2+-ATPases122. In both cases, the extrusion of one Ca2+ ion requires the hydrolysis of ~1 ATP molecule. A coupling configuration in which a small number of Ca2+ channels are tightly coupled to presynaptic Ca2+ sensors therefore reduces the metabolic cost of synaptic transmission. Such an energy-saving mechanism may be important at GABAergic synapses in the cortex and at glutamatergic synapses in the auditory pathway, which are active at high frequencies under physiological conditions in vivo.
A potential disadvantage of nanodomain coupling with a small number of Ca2+ channels could be an additional ‘jitter’ of evoked transmitter release caused by the stochastic opening of presynaptic Ca2+ channels15,22 (FIG. 4e). However, whereas the opening and closing of Ca2+ channels is stochastic, the rising phase of the corresponding Ca2+ transient evoked by an overshooting action potential is largely deterministic, governed by the increase in driving force during the repolarization phase56,123 (FIG. 4e). Thus, transmitter release remains tightly synchronized, even if evoked release is triggered by only a small number of Ca2+ channels.
Another potential disadvantage of nanodomain coupling is that stochastic openings of Ca2+ channels at rest might trigger spontaneous transmitter release15 (FIG. 4f). However, recent results in dentate gyrus granule cells suggest that blocking P/Q-type Ca2+ channels with ω-agatoxin IVa has no effect on miniature inhibitory postsynaptic current (IPSC) frequency, although evoked release at basket cell–granule cell synapses exclusively relies on P/Q-type Ca2+ channels124. Furthermore, BAPTA-AM and EGTA-AM reduce miniature IPSC frequency to the same extent, suggesting that microdomains rather than nanodomains trigger spontaneous release124. Thus, the high activation threshold and steep voltage dependence of P/Q-type Ca2+ channels, and the use of two or three open Ca2+ channels rather than a single channel, may protect the synapse from excessive spontaneous release generated by stochastic Ca2+ channel opening56,60.
Nanodomain coupling also has substantial implications for synaptic dynamics, promoting synaptic depression over synaptic facilitation for two reasons. First, for any given number of channels, it increases release probability and thus enhances depression owing to depletion of the releasable pool of synaptic vesicles. Second, it reduces facilitation by decreasing the relative weight of residual Ca2+ (REF. 125). Consistent with these effects, the fast signalling synapses that rely on nanodomain coupling often show depression during high-frequency stimulus trains, albeit to a different extent19-21.
Finally, nanodomain coupling will have implications for how neuromodulators affect the release of neurotransmitters and how they interact with synaptic dynamics. Previous studies suggested that presynaptic G-protein-coupled receptors (such as presynaptic GABAB receptors) reduce the activity of P/Q- and N-type Ca2+ channels via binding of G-protein β- and γ-subunits to Ca2+ channels126. In nanodomain coupling regimes, this will have two consequences. First, the reduction in transmitter release will be largely proportional to the degree of presynaptic receptor activation. This may allow a more precise regulation of synaptic efficacy than a highly supralinear relationship. Second, as presynaptic receptor activation will reduce the number of Ca2+ channel–vesicle nanocomplexes but will not affect release probability, the neuromodulators will not affect short-term dynamics, resulting in scaling of synaptic responses during repetitive stimulation, as observed in the hippocampus127 (but see REF. 142 for observations in the neocortex).
Conclusions
Twenty years after the original finding of nanodomain coupling at the squid giant synapse11, and after a subsequent decade of accumulating evidence for microdomain coupling at central synapses128, it has become clear that synapses in the mammalian CNS also make extensive use of nanodomain coupling for fast transmitter release. In particular, GABAergic interneuron output synapses and glutamatergic synapses in the auditory pathway rely on nanodomain coupling. Nanodomain coupling provides several functional advantages, including efficacy, speed and energy efficiency of synaptic transmission. How abundantly nanodomain coupling is used by different synapses in the mammalian CNS remains to be addressed. Furthermore, the rules of synapse specificity of nanodomain coupling remain to be determined. Finally, it will be interesting to see whether nanodomain coupling between Ca2+ channels and Ca2+ sensors of exocytosis is disrupted in neurological or psychiatric diseases129.
Supplementary Material
Acknowledgements
We thank D. Tsien and E. Neher for their comments on this Review, J. Guzmán and A. Pernía-Andrade for reading earlier versions and E. Kramberger for perfect editorial support. Work of the authors was funded by grants of the Deutsche Forschungsgemeinschaft to P.J. (grants SFB 780/A5, TR 3/B10 and the Leibniz programme), a European Research Council Advanced grant to P.J. and a Swiss National Foundation fellowship to E.E. We apologize that owing to space constraints, not all relevant papers could be cited.
Glossary
- Synaptic delay
The time interval between the presynaptic action potential and the postsynaptic response. A synaptic delay is comprised of several components: opening of presynaptic Ca2+ channels, diffusion of Ca2+ from the channels to the Ca2+ sensors, activation of Ca2+ sensors, exocytosis, diffusion of transmitter across the synaptic cleft and activation of postsynaptic receptors.
- Ca2+ chelators
Chemical substances that bind Ca2+. In synaptic physiology, BAPTA and EGTA are widely used Ca2+ chelators. Both chelators are also available in membrane-permeable acetoxymethyl ester (AM) forms.
- BAPTA
1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- Ca2+ microdomains
Domains of elevated Ca2+ concentration that extend over more than 100 nanometres. Note that this definition does not imply that the size of the domain is in the micrometre range (1 μm = 10−6 m).
- Basket cells
Types of perisomatic inhibitory GABAergic interneurons in the hippocampus and cerebellum. The name was given as the axon forms ‘baskets’ around somata of postsynaptic target cells.
- Ca2+ nanodomains
Domains of elevated Ca2+ concentration that extend over less than 100 nanometres (1 nm = 10−9 m).
- Intrinsic or biochemical cooperativity
Nonlinear dependence of transmitter release on the intracellular Ca2+ concentration, presumably owing to multiple Ca2+-binding sites on the Ca2+ sensor synaptotagmin and multiple copies of synaptotagmin on individual synaptic vesicles.
- Rab3-interacting molecules (RIMs)
Active zone proteins that serve as central organizers, tethering presynaptic Ca2+ channels and Ca2+ sensors of exocytosis. RIMs are encoded by four genes, which drive the expression of seven known isoforms. For synaptic transmission, only the long RIM versions are relevant.
- Length constant
The distance in which a quantity declines to the fraction 1/e. In the case of buffered diffusion of Ca2+, the length constant represents the mean distance Ca2+ diffuses before it is captured by the buffer.
- Fixed buffers
Fixed buffers always remain at the same location. In contrast to mobile buffers, fixed buffers can only be regenerated by Ca2+ unbinding, not by diffusion.
- SNARE
Soluble N-ethylmaleimide-sensitive-factor attachment protein (SNAP) receptor.
- ELKS
Glutamic acid, leucine, lysine and serine-rich protein (also known as cytomatrix of the active zone-associated structural protein (CAST)).
- Synaptic depression
Decrease in efficacy of synaptic transmission during and after stimulation of the presynaptic neuron. Synaptic depression is often interpreted as a depletion of the releasable pool of synaptic vesicles, although other mechanisms such as changes in presynaptic action potential shape and inactivation of presynaptic Ca2+ channels may also contribute.
- Synaptic facilitation
Short-lasting increase in efficacy of synaptic transmission during and after repetitive stimulation. Synaptic facilitation is often attributed to residual Ca2+ following the action potential, although other mechanisms such as saturation of endogenous buffers may also contribute.
Footnotes
Competing interests statement: The authors declare no competing financial interests.
FURTHER INFORMATION: Peter Jonas’s homepage : http://www.ist.ac.at
SUPPLEMENTARY INFORMATION: See online article: S1 (box)
References
- 1.Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc. R. Soc. Lond. B. 1965;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- 2.Llinás R, Sugimori M, Simon SM. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc. Natl Acad. Sci. USA. 1982;79:2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 5.Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 6.Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Annalen der Physik. 1905;17:549–560. [Google Scholar]
- 7.Katz B. The Release of Neural Transmitter Substances. Liverpool Univ. Press; Liverpool: 1969. [Google Scholar]
- 8.Llinás RR. The Squid Giant Synapse. Oxford Univ. Press; New York: 1999. [Google Scholar]
- 9.Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [A classical electron microscopy tomography study of the active zone at the frog neuromuscular junction. Four rows of presynaptic Ca2+ channels are opposed to two rows of synaptic vesicles, with ~20 nm between the individual elements.] [DOI] [PubMed] [Google Scholar]
- 10.Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J. Neurosci. 2006;26:13240–13249. doi: 10.1523/JNEUROSCI.1418-06.2006. [The authors cleverly exploit the advantage of the Monte-Carlo simulation to monitor individual Ca2+ ions. By backtracing the Ca2+ from the vesicle to the Ca2+ channels through which they entered, the authors conclude that only one or two open Ca2+ channels contribute to transmitter release at the frog neuromuscular junction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [A classical paper that uses exogenous Ca2+ chelators with different binding rates to probe the distance between Ca2+ source and Ca2+ sensor at the squid giant synapse. Based on the lack of effects of the slow Ca2+ chelator EGTA, the authors suggest that nanodomain coupling exists between Ca2+ channels and Ca2+ sensors at this invertebrate synapse.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augustine GJ. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J. Physiol. 1990;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann. NY Acad. Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- 14.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [The first direct evidence that a single Ca2+ channel triggers exocytosis at a chick calyx synapse. In enzymatically treated preparations, the release face of the calyx (that is, the side that is normally attached to the postsynaptic ciliary neuron) is sometimes partially separated from the postsynaptic cell, allowing simultaneous electro-physiological recording of presynaptic Ca2+ channel activity and chemoluminescent detection of acetylcholine release.] [DOI] [PubMed] [Google Scholar]
- 15.Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- 16.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nature Rev. Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 17.Neher E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 1998;24:345–357. doi: 10.1016/s0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- 18.Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J. Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Gersdorff H, Borst JGG. Short-term plasticity at the calyx of Held. Nature Rev. Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- 20.Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J. Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caillard O, et al. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc. Natl Acad. Sci. USA. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinrenken CJ, Borst JGG, Sakmann B. Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography. J. Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. J. Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [This paper demonstrates a developmental decrease of sensitivity of evoked transmitter release to EGTA at the calyx of Held, indicating a tightening of Ca2+ channel–sensor coupling. Furthermore, it shows a reduction in Ca2+ current cooperativity during development. Although the power coefficients in this study cannot be correlated to the number of open channels required for release (they exceed the upper bound of biochemical cooperativity), the results may indicate a reduction of this number during development.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J. Physiol. 1998;513:135–148. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J. Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [This paper reports that the Ca2+ chelator BAPTA induces pseudofacilitation. Careful quantitative analysis reveals buffer saturation as the underlying mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Christie JM, Chiu DN, Jahr CE. Ca2+-dependent enhancement of release by subthreshold somatic depolarization. Nature Neurosci. 2011;14:62–68. doi: 10.1038/nn.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J. Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chad JE, Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of Ca-dependent responses. Biophys. J. 1984;45:993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llinás R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 31.Fogelson AL, Zucker RS. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys. J. 1985;48:1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J. Neurosci. 1994;14:3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Moulder KL, Mennerick S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons. J. Neurosci. 2005;25:3842–3850. doi: 10.1523/JNEUROSCI.5231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc. Natl Acad. Sci. USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 39.Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J. Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taschenberger H, Leão RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J. Neurosci. 2008;28:14450–14458. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LY, Fedchyshyn MJ, Yang YM. Action potential evoked transmitter release in central synapses: insights from the developing calyx of Held. Mol. Brain. 2009;2:36. doi: 10.1186/1756-6606-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nature Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 44.Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nature Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J. Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J. Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [Evidence that a few Ca2+ channels trigger exocytosis at auditory hair cell ribbon synapses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erazo-Fischer E, Striessnig J, Taschenberger H. The role of physiological afferent nerve activity during in vivo maturation of the calyx of Held synapse. J. Neurosci. 2007;27:1725–1737. doi: 10.1523/JNEUROSCI.4116-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed MS, Siegelbaum SA. Recruitment of N-type Ca2+ channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses. Neuron. 2009;63:372–385. doi: 10.1016/j.neuron.2009.07.013. [This paper shows that distal perforant path synapses on CA1 pyramidal neurons exhibit a presynaptic form of long-term potentiation dependent on Ca2+ channel recruitment. This may suggest that the coupling between Ca2+ channels and transmitter release is altered during presynaptic forms of plasticity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pumplin DW, Reese TS, Llinás R. Are the presynaptic membrane particles the calcium channels? Proc. Natl Acad. Sci. USA. 1981;78:7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann. NY Acad. Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
- 51.Matveev V, Bertram R, Sherman A. Ca2+ current versus Ca2+ channel cooperativity of exocytosis. J. Neurosci. 2009;29:12196–12209. doi: 10.1523/JNEUROSCI.0263-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 53.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [A classical paper that quantitatively determines the protein content of synaptic vesicles. Among other proteins, 15 synaptotagmin copies and ten RAB3A copies are present per vesicle.] [DOI] [PubMed] [Google Scholar]
- 55.Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nature Neurosci. 2010;13:19–21. doi: 10.1038/nn.2461. [DOI] [PubMed] [Google Scholar]
- 57.Kochubey O, Han Y, Schneggenburger R. Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held. J. Physiol. 2009;587:3009–3023. doi: 10.1113/jphysiol.2009.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- 59.Borst JGG, Sakmann B. Calcium current during a single action potential in a large presynaptic terminal of the rat brainstem. J. Physiol. 1998;506:143–157. doi: 10.1111/j.1469-7793.1998.143bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J. Neurosci. 2007;27:13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang YM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of Held synapse. J. Neurosci. 2006;26:5698–5708. doi: 10.1523/JNEUROSCI.4889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin KH, Oleskevich S, Taschenberger H. Presynaptic Ca2+ influx and vesicle exocytosis at the mouse endbulb of Held: a comparison of two auditory nerve terminals. J. Physiol. 2011;589:4301–4320. doi: 10.1113/jphysiol.2011.209189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens CF. Neurotransmitter release at central synapses. Neuron. 2003;40:381–388. doi: 10.1016/s0896-6273(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 64.Sätzler K, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J. Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J. Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 67.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 68.Collin T, et al. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J. Neurosci. 2005;25:96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Müller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation. J. Neurosci. 2007;27:2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Felmy F, Schneggenburger R. Developmental expression of the Ca2+-binding proteins calretinin and parvalbumin at the calyx of Held of rats and mice. Eur. J. Neurosci. 2004;20:1473–1482. doi: 10.1111/j.1460-9568.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 71.Edmonds B, Reyes R, Schwaller B, Roberts WM. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nature Neurosci. 2000;3:786–790. doi: 10.1038/77687. [DOI] [PubMed] [Google Scholar]
- 72.Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci. 2005;25:7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee A, Zhou H, Scheuer T, Catterall WA. Molecular determinants of Ca2+/calmodulin-dependent regulation of Ca2.1 channels. Proc. Natl Acad. Sci. USA. 2003;100:16059–16064. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 75.Aponte Y, Bischofberger J, Jonas P. Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J. Physiol. 2008;586:2061–2075. doi: 10.1113/jphysiol.2007.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eggermann E, Jonas P. How the ‘slow’ Ca2+ buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nature Neurosci. 2011 Dec 4; doi: 10.1038/nn.3002. doi:10.1038/nn.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nägerl UV, Novo D, Mody I, Vergara JL. Binding kinetics of calbindin-D28k determined by flash photolysis of caged Ca2+ Biophys. J. 2000;79:3009–3018. doi: 10.1016/S0006-3495(00)76537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faas GC, Schwaller B, Vergara JL, Mody I. Resolving the fast kinetics of cooperative binding: Ca2+ buffering by calretinin. PLoS Biol. 2007;5:e311. doi: 10.1371/journal.pbio.0050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faas GC, Raghavachari S, Lisman JE, Mody I. Calmodulin as a direct detector of Ca2+ signals. Nature Neurosci. 2011;14:301–304. doi: 10.1038/nn.2746. [This paper measures the Ca2+-binding rates of different Ca2+-binding proteins directly, using fast Ca2+ uncaging in a cuvette. Surprisingly, the binding rate for the N-lobe of calmodulin (relaxed form) is even faster than that of BAPTA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 81.Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 82.Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- 83.Nowycky MC, Pinter MJ. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys. J. 1993;64:77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matveev V, Zucker RS, Sherman A. Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys. J. 2004;86:2691–2709. doi: 10.1016/S0006-3495(04)74324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson MB, Redman SJ. Calcium dynamics, buffering, and buffer saturation in the boutons of dentate granule-cell axons in the hilus. J. Neurosci. 2003;23:1612–1621. doi: 10.1523/JNEUROSCI.23-05-01612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 87.Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 88.Dodge FA, Rahamimoff R. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SH, Schwaller B, Neher E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: implications for [Ca2+] transients of neuronal dendrites. J. Physiol. 2000;525:419–432. doi: 10.1111/j.1469-7793.2000.t01-2-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- 91.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt H, Brown EB, Schwaller B, Eilers J. Diffusional mobility of parvalbumin in spiny dendrites of cerebellar Purkinje neurons quantified by fluorescence recovery after photobleaching. Biophys. J. 2003;84:2599–2608. doi: 10.1016/S0006-3495(03)75065-6. [This paper directly measures the diffusion coefficient of the Ca2+-binding protein parvalbumin from the time course of recovery of fluorescence after photobleaching. Unlike many other Ca2+-binding proteins, parvalbumin is highly mobile.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmidt H, Schwaller B, Eilers J. Calbindin D28k targets myo-inositol monophosphatase in spines and dendrites of cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA. 2005;102:5850–5855. doi: 10.1073/pnas.0407855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Müller CS, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl Acad. Sci. USA. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheng ZH, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc. Natl Acad. Sci. USA. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 97.Rettig J, et al. Isoform-specific interaction of the α1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc. Natl Acad. Sci. USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong H, Yokoyama CT, Scheuer T, Catterall WA. Reciprocal regulation of P/Q-type Ca2+ channels by SNAP-25, syntaxin and synaptotagmin. Nature Neurosci. 1999;2:939–941. doi: 10.1038/14721. [DOI] [PubMed] [Google Scholar]
- 99.Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J. Neurochem. 2001;77:972–985. doi: 10.1046/j.1471-4159.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 100.Kittel RJ, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Liu X, Biederer T, Südhof TC. A family of RIM-binding proteins regulated by alternative splicing: implications for the genesis of synaptic active zones. Proc. Natl. Acad. Sci. USA. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaeser PS, et al. ELKS2α/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64:227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Missler M, et al. α-neurexins couple Ca 2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 104.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to α- and β-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 105.Kaeser PS, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [This paper shows that RIM acts as a tethering molecule linking presynaptic Ca2+ channels to the exocytosis machinery.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hibino H, et al. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nature Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- 109.Yang YM, et al. Septins regulate developmental switching from microdomain to nanodomain coupling of Ca2+ influx to neurotransmitter release at a central synapse. Neuron. 2010;67:100–115. doi: 10.1016/j.neuron.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siksou L, et al. Three-dimensional architecture of presynaptic terminal cytomatrix. J. Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J. Physiol. 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forti L, Pouzat C, Llano I. Action potential-evoked Ca2+ signals and calcium channels in axons of developing rat cerebellar interneurones. J. Physiol. 2000;527:33–48. doi: 10.1111/j.1469-7793.2000.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stephens GJ, Morris NP, Fyffe REW, Robertson B. The Cav2.1/α1A (P/Q-type) voltage-dependent calcium channel mediates inhibitory neurotransmission onto mouse cerebellar Purkinje cells. Eur. J. Neurosci. 2001;13:1902–1912. doi: 10.1046/j.0953-816x.2001.01566.x. [DOI] [PubMed] [Google Scholar]
- 114.Cao YQ, Tsien RW. Different relationship of N- and P/Q-type Ca2+ channels to channel-interacting slots in controlling neurotransmission at cultured hippocampal synapses. J. Neurosci. 2010;30:4536–4546. doi: 10.1523/JNEUROSCI.5161-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mochida S, Westenbroek RE, Yokoyama CT, Itoh K, Catterall WA. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc. Natl Acad. Sci. USA. 2003;100:2813–2818. doi: 10.1073/pnas.262787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mochida S, et al. Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q-type calcium channels. Proc. Natl Acad. Sci. USA. 2003;100:2819–2824. doi: 10.1073/pnas.262787699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun J, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakaba T. Two Ca2+-dependent steps controlling synaptic vesicle fusion and replenishment at the cerebellar basket cell terminal. Neuron. 2008;57:406–419. doi: 10.1016/j.neuron.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 119.Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J. Neurosci. 1998;18:8214–8227. doi: 10.1523/JNEUROSCI.18-20-08214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [This paper shows that EGTA-AM leaves synaptic release on Purkinje cells unaffected, but inhibits ectopic release on Bergmann glial cells. This suggests that synaptic release is triggered by Ca2+ nanodomains, whereas ectopic release is driven by Ca2+ microdomains.] [DOI] [PubMed] [Google Scholar]
- 121.Matsui K, Jahr CE. Differential control of synaptic and ectopic vesicular release of glutamate. J. Neurosci. 2004;24:8932–8939. doi: 10.1523/JNEUROSCI.2650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J. Neurosci. 2005;25:6057–6065. doi: 10.1523/JNEUROSCI.0454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ribrault C, Sekimoto K, Triller A. From the stochasticity of molecular processes to the variability of synaptic transmission. Nature Rev. Neurosci. 2011;12:375–387. doi: 10.1038/nrn3025. [DOI] [PubMed] [Google Scholar]
- 124.Goswami SP, Jonas P, Bucurenciu I. Soc. Neurosci. Abstr. Washington DC: Nov 12-16, 2011. Differential dependence of miniature IPSC and EPSC frequency on presynaptic Ca2+ channels at hippocampal synapses; p. 446.07. [Google Scholar]
- 125.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 126.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 127.Hefft S, Kraushaar U, Geiger JRP, Jonas P. Presynaptic short-term depression is maintained during regulation of transmitter release at a GABAergic synapse in rat hippocampus. J. Physiol. 2002;539:201–208. doi: 10.1113/jphysiol.2001.013455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meinrenken CJ, Borst JGG, Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J. Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao YQ, et al. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43:387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 130.Lansman JB, Hess P, Tsien RW. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 132.Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 133.Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 134.Bertram R, Smith GD, Sherman A. Modeling study of the effects of overlapping Ca2+ microdomains on neurotransmitter release. Biophys. J. 1999;76:735–750. doi: 10.1016/S0006-3495(99)77240-1. [A detailed modelling paper that cleans up several misconceptions regarding the cooperativity of Ca2+ inflow and transmitter release.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu LG, Saggau P. Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J. Neurosci. 1994;14:5613–5622. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bullock TH, Hagiwara S. Intracellular recording from the giant synapse of the squid. J. Gen. Physiol. 1957;40:565–577. doi: 10.1085/jgp.40.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nicholls JG, Martin RA, Wallace, B G. From Neuron to Brain. Sinauer, Sunderland, Massachusetts, USA: 1992. [Google Scholar]
- 138.Pernía-Andrade A, Jonas P. The multiple faces of RIM. Neuron. 2011;69:185–187. doi: 10.1016/j.neuron.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 139.Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium. 1997;22:255–268. doi: 10.1016/s0143-4160(97)90064-6. [DOI] [PubMed] [Google Scholar]
- 140.Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J. Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J. Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.