Abstract
The minichromosome maintenance (or MCM) protein family is composed of six related proteins that are conserved in all eukaryotes. They were first identified by genetic screens in yeast and subsequently analyzed in other experimental systems using molecular and biochemical methods. Early data led to the identification of MCMs as central players in the initiation of DNA replication. More recent studies have shown that MCM proteins also function in replication elongation, probably as a DNA helicase. This is consistent with structural analysis showing that the proteins interact together in a heterohexameric ring. However, MCMs are strikingly abundant and far exceed the stoichiometry of replication origins; they are widely distributed on unreplicated chromatin. Analysis of mcm mutant phenotypes and interactions with other factors have now implicated the MCM proteins in other chromosome transactions including damage response, transcription, and chromatin structure. These experiments indicate that the MCMs are central players in many aspects of genome stability.
INTRODUCTION
In the early 1980s, Bik-Kwoon Tye's laboratory carried out a general screen for Saccharomyces cerevisiae mutants that had defects in maintaining a simple minichromosome. Several of the MCM genes showed were paralogues and shared a role in DNA replication. They were the founding members of a conserved protein family required for DNA replication that is now called by the name of the screen: the MCM (for “minichromosome maintenance”) proteins. Data at first suggested that the MCM proteins were replication initiation factors. More recent data suggest that they have an additional essential function as a helicase required for replication elongation (see extensive reviews in reference 12, 16, 58, 154, 231, and 321). They are also implicated in many other chromosome transactions including transcription, chromatin remodeling, and genome stability. MCMs thus recapitulate a pattern seen with other cell cycle genes, in which a simple model derived from analysis of a single process grows more complicated as additional functions are identified, which ultimately leads toward a holistic view of how multiple events are linked. In this review, I examine the history of MCMs and discuss recent insights into their function from structural studies. I describe evidence for their different roles in DNA replication and the identification of new functions. Finally, I highlight MCM puzzles and paradoxes that make these proteins of continuing interest 20 years after their first identification.
Original MCM Identification
The MCM protein family is named for the genetic screen in budding yeast from which the founding members were originally isolated. They were defective in minichromosome maintenance, showing a high rate of loss of plasmids that contained a cloned centromere and replication origin (201, 289). Thus, mutants isolated from this screen were defective in function of the replication origin or the centromere on the target plasmid (reviewed in reference 322). Early characterization of the mcm2 and mcm3 phenotypes indicated a role in replication, but the phenotypes varied with different replication origins on the test plasmid (93, 201, 289). Cloning showed that Mcm2 and Mcm3 proteins share a region of sequence similarity called the MCM box (346), and they eventually gave their name to a protein family. Importantly, the family we call MCM proteins include only the six proteins Mcm2 through Mcm7 (with the recent addition of an Mcm8 in some species [discussed below]). Other genes from the same screen, including MCM1 and MCM10, do not have similarity with MCM2 through MCM7 despite having an MCM name. Some of these other MCM genes also act during S phase, while others are involved in other aspects of chromosome metabolism.
Additional members of the MCM family in budding yeast were identified in screens for mutants with cell division cycle, or CDC phenotypes (33, 110, 111, 219). Once the proteins were identified as members of the MCM family, the genes were renamed; however, the original genetic names are still found in the literature. A guide to these synonyms is found in Table 1. Each MCM gene is essential for viability. Conditional mcm mutants showed an extensive network of genetic interactions with one another, as well as with other genes isolated in related screens (see, e.g., references 111, 219, and 346). This suggested that a large number of proteins work together in large complexes to regulate eukaryotic DNA replication.
TABLE 1.
Synonyms for conserved replication proteinsa
| Protein | Synonym in:
|
||||
|---|---|---|---|---|---|
| S. cerevisiae | S. pombe | Drosophila | Human | Xenopus | |
| Mcm2 | Mcm2 | Mcm2, Cdc19, Nda1 | DmMcm2 | BM28, Mcm2 | Mcm2 |
| Mcm3 | Mcm3 | Mcm3 | DmMcm3 | Mcm3 | Mcm3 |
| Mcm4 | Mcm4, Cdc54 | Mcm4, Cdc21 | DmMcm4 Dpa (disk proliferation abnormal) | Mcm4 | Mcm4 |
| Mcm5 | Mcm5, Cdc46 | Mcm5, Nda4 | DmMcm5, DmCdc46 | Mcm5 | Mcm5 |
| Mcm6 | Mcm6, | Mcm6, Mis5 | DmMcm6 | Mcm6 | Mcm6 |
| Mcm7 | Mcm7, Cdc47 | Mcm7 | DmMcm7 | Mcm7 | Mcm7 |
| Mcm8 | No homologue | No homologue | ? | Mcm8 | ? |
| Dbf4 | Dbf4, Dna52 | Dfp1, Him1 | Chiffon | Dbf4, ASK | Dbf4 |
| Alternative Dbf4 subunit | No homologue | No homologue | No homologue | Drf1 | Drf1 |
| Cdc6 | Cdc6 | Cdc18 | Cdc6 | Cdc6 | Cdc6 |
| Cdt1 | Tah11, Sid2 | Cdt1 | Dup (doubleparked) | Cdt1 | Cdt1 |
| Geminin | No homologue | No homologue | Geminin | Geminin | Geminin |
| ORC1-ORC6 | Orc1-Orc6 | Orp1-Orp6, Orc1-Orc6 | DmORC1-DmORC6 | ORC1-ORC6 | XOrc1-XOrc6 |
| Cdc45 | Cdc45 | Sna41, Cdc45 | Cdc45 | Cdc45 | Cdc45 |
| GINS | GINS | ||||
| PSF2 | Psf2, Cdc102 | Psf2, Bsh3 | ? | Psf2, Bsh3 | Psf2 |
| PSF3 | Psf3 | ? | ? | ? | Psf3 |
| SLD5 | Sld5, Cdc105 | ? | ? | ? | Sld5 |
| PSF1 | Psf1, Cdc101 | ? | ? | ? | Psf1 |
| Drc1 | Drc1, Sld2 | Drc1 | ? | ? | ? |
| Sld3 | Sld3 | Sld3 | ? | ? | ? |
| Dpb11 | Dpb11 | Rad4, Cut5 | Mus101 | TopBP1, Cut5 | Xmus101 |
| Mcm10 | Mcm10, Dna43 | Cdc23 | Mcm10 | Mcm10 | Mcm10 |
For references, see the text.
Licensing Factor Model
Using a Xenopus oocyte system, Blow and Laskey observed in the 1980s that sperm chromatin inside a nucleus could be replicated only once during each cycle but became competent again following mitosis (17). As in most eukaryotes (with the notable exception of the yeasts), the nuclear envelope in cycling Xenopus extracts breaks down during mitosis, providing access for the cytoplasmic components to the chromatin. Blow and Laskey speculated that the chromatin required an activity to mark it as capable of replicating: in effect, to license it for S phase. They further proposed that such a licensing activity would be used up or destroyed as a consequence of replication and therefore could be restored only when the nuclear envelope disintegrated during mitosis to allow fresh licensing factor access to the DNA. Licensing factor provided an appealing mechanism that could ensure that the genome was replicated once and only once in each cell cycle.
One of the most intriguing experiments early in the characterization of MCMs demonstrated that the S. cerevisiae Mcm5 (Cdc46) protein cycles in and out of the nucleus during each cell cycle (110). This observation was consistent with how a putative licensing factor would behave in an organism that maintains an intact nuclear envelope throughout the cell cycle (called a closed mitosis). To the replication community, this behavior of MCMs indicated that they would be key factors in regulating S-phase initiation. It also promised another example of synergy between cell cycle analyses in yeast and Xenopus, reminiscent of the groundbreaking studies that produced a universal model of cyclin-dependent kinase (CDK) activation in mitosis (237). This drove studies of MCM biology through the early 1990s.
As described below, cycling in and out of the nucleus is unique to budding-yeast MCMs, and further experiments eventually showed that the MCM proteins do not correspond to licensing factor as originally proposed. MCMs were nevertheless identified as key proteins in DNA replication initiation and shown to play a vital role in the licensing of origins for replication. While today we know much more about them (for example, in their role as replication proteins), in some regards we also know much less (as their roles expand beyond DNA replication). Such puzzles and paradoxes are a recurring theme in MCM biology.
THE MCM FAMILY
Conserved Protein Family
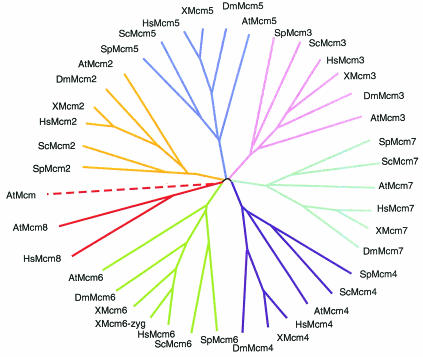
Members of the MCM family have been found in all eukaryotes by genetic and biochemical methods (Table 1; Fig. 1). In fission yeast, as in budding yeast, the mutants were originally identified in screens for cell cycle defects or for chromosome missegregation (201, 219, 227, 303, 316). In Drosophila, Mcm4 corresponds to the gene disc proliferation abnormal (76), while in Arabidopsis, Mcm7 is PROLIFERA (296), stressing their role in cell division. Human Mcm2 (BM28) was first identified as a nuclear protein (318), and human Mcm3 (P1) was isolated as a DNA polymerase alpha-associated protein (314). Related proteins were identified biochemically from a variety of systems, although it was not immediately apparent which were orthologues and which were paralogues (see e.g., references 75, 161, 294, and 314) (Table 1). With the completion of the S. cerevisiae genome sequence (96), it was evident that eukaryotes contain at least six distinct members of the family. The MCMs are not found in prokaryotes, but MCM-related proteins exist in Archaea (reviewed in references 152, 156, and 320). Our focus here is on the eukaryotic MCMs.
FIG. 1.
Phylogenetic tree of eukaryotic MCMs, assembled using ClustalX (ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/) and Phylip 3.6 (http://evolution.genetics.washington.edu/phylip.html) for Macintosh. Colors correspond to the seven MCM subfamilies. Dashed line, loose relationship. Accession numbers are as follows. S. pombe: SpMcm2, CAB58403; SpMcm3, P30666; SpMcm4, P29458, SpMcm5, CAA93299 and CAB61472; SpMcm6, CAB75412; SpMcm7, O75001. S. cerevisiae: ScMcm2, NP_009530; ScMcm3, NP_010882; ScMcm4, S56050; ScMcm5, A39631; ScMcm6, NP_011314; ScMcm7, S34027. Human: HsMcm2, P49736; HsMcm3, P25205; HsMcm4, NP_005905; HsMcm5, AAH03656; HsMcm6, NP_005906; HsMcm7, P33993; HsMcm8, NP_115874. Xenopus: Xmcm2, JC5085; Xmcm3, I51685; Xmcm4, T47223; Xmcm5, PC4225; Xmcm6Z, AAC41267; Xmcm6, T47222; Xmcm7, T47221. Arabidopsis: AtMcm2, NP_175112.1; AtMcm3, NP_199440.1; AtMcm4, NP_179236.2; AtMcm5, NP_178812.1; AtMcm6, NP_680393.1; AtMcm7, NP_192115.1; AtMcm8?, NP_187577.1; unknown Mcm, NP_179021.1. Drosophila: DmMcm2, AAF54207; DmMcm3, NP_511048.2; DmMcm4, S59872; DmMcm5, NP_524308.2; DmMcm6, NP_511065.1; DmMcm7, NP_523984.1.
Structure and Characteristics
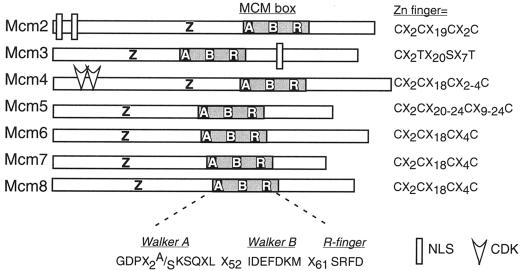
The MCMs are a distinct subgroup of the large AAA ATPasefamily, which has many cellular functions (reviewed in references 56, 187, 228, and 249). AAA ATPases generally form large ATP-dependent complexes, often heterohexamers. The MCMs are defined by a characteristic version of the ATPase domain, the MCM box, which spans about 200 residues (reviewed in references 113, 152, 164, 228, and 323) (Fig. 2). The MCM box includes two ATPase consensus motifs. The Walker A motif, including the P-loop of the active site, contains the invariant lysine residue found in all ATP-binding proteins. In the MCM family, the Walker A consensus has an alanine or serine in place of a glycine, along with several additional conserved residues, creating the MCM-specific consensus GDPxx(S/A)KS. The classic Walker B element D(D/E) is bulky and hydrophobic and is thought to contribute to ATP hydrolysis rather than binding. In the MCM proteins, the Walker B motif is part of the sequence IDEFDKM, which is conserved in all MCM proteins and defines the MCM family. Another short motif, SRFD, occurs approximately 70 residues after the Walker B element, and defines an “arginine finger.” All MCMs contain these sequences.
FIG. 2.
Structural features of the Mcm protein family, derived from the alignment used in Fig.1. Abbreviations: Z, zinc finger; A, Walker A ATPase motif; B, Walker B ATPase motif; R, arginine finger motif.
Outside the MCM box, the individual MCMs within a single species are not particularly closely related to each other. However, comparison of MCMs across species allowed the definition of distinct MCM classes, so that (for example) an Mcm2 protein from humans is more similar to Mcm2 from yeast (an orthologue) than to Mcm4 from humans (a paralogue). A phylogenetic tree of known MCMs from a range of eukaryotic species is shown in Fig. 1. (For a comparison to the archaeal MCMs, see the tree in reference 152.)
All eukaryotic MCMs have zinc-binding motifs of some sort, although the precise arrangement of the cysteines varies distinctly from family to family (Fig. 2). The Mcm3 class only weakly matches a classic zinc finger consensus, but it has been argued that the residues indicated in Fig. 2 are capable of chelating zinc (81). Mutational analysis of the yeasts, in which the mutant proteins are tested for their ability to rescue nonfunctional alleles, has shown that the zinc-binding motifs in several MCMs are required for viability (83, 347). Biochemical analysis suggests that the zinc motif also contributes to complex assembly and ATPase activity (81, 256, 284, 354). Studies of an archaeal MCM homohexamer indicate that the ability to chelate zinc is required for assembly of higher-order MCM complexes (described below). Several MCMs have weak homology to a leucine zipper consensus (LX6LX6LX6L) upstream of the zinc finger motif.
Only Mcm2 and Mcm3 families have sequences suggesting nuclear localization sequences (NLS). The NLS of Mcm2 has been identified functionally in Schizosaccharomyces pombe, S. cerevisiae, and mice (132, 230, 247). The Mcm3 NLS sequence has been molecularly characterized in S. cerevisiae and humans (309, 357).
There are other subunit-specific characteristics. Mcm2 family members have an extended N terminus rich in serine residues. Consensus sites (S/T)PX(K/R) for the CDKs are found in most Mcm4 family members. CDK consensus sequences in other MCMs vary from species to species. They are found in most metazoan Mcm2 family members, in Mcm3 in the yeasts, and sporadically amongst the other subunits. The role of CDK and other kinases in regulating MCMs is discussed in “Origin firing” (below).
Unusual MCMs.
With the completion of genome sequences of numerous eukaryotes, it is now apparent that fungi have just six MCMs but that there can be additional variants in more complicated organisms. One expected addition is the potential for developmentally regulated MCM subunits, forming isoforms of the known MCM subclasses. For example, there is a zygotic form of Mcm6 in Xenopus, which might be involved in the rapid embryonic divisions of a Xenopus egg (287). A sex-specific plasmodium Mcm4 has also been identified (189). These proteins are presumed to substitute for the “normal” MCM in appropriate developmental conditions.
More surprising was the recent identification of a human Mcm8 by two groups (100, 144). Comparison of its sequence to the others (Fig. 1) clearly indicates that it defines a separate MCM family, distinct from the canonical six. Intriguingly, while human Mcm8 shares all the classic MCM features including a putative zinc finger and the IDEKFM and arginine finger motifs, it is the only MCM that has a classic GKS motif in its Walker A sequence. It is widely expressed in a variety of tissues and may not be restricted to proliferating cells (100, 144). The protein is found in the nucleus, apparently chromatin associated during S phase (100). However, the two published studies disagree on whether this MCM interacts with other members of the family (100, 144). Mcm8 may be involved in negatively regulating the MCM hexamer (144). Alternatively, Mcm8 may form a homohexamer with a distinct function. An Mcm8-related sequence is also found in the mouse but has not been identified in Drosophila or Xenopus. Two additional MCMs beyond the canonical six are also found in the Arabidopsis sequence but have not been studied molecularly. One of them is closely related to human Mcm8 and also contains the GKS sequence in the Walker A motif. The tree in Fig. 1 suggests that this is the orthologue of Mcm8. The other is a more divergent sequence which changes the Walker B IDEKFM motif to IDEKSM and lacks the arginine finger (shown by the dashed line in Fig. 1).
Assembly of the MCM Complex
The first evidence that MCMs form a protein complex was provided by the discovery of genetic interactions between mcm mutants in the yeasts, including cosuppression and synthetic lethal interactions (see e.g., references 47, 83, 111, 216, and 346). Physical interactions were identified in vivo by using two-hybrid, coimmunoprecipitation, and biochemical purification (see, e.g., references 2, 28, 37, 45, 53, 129, 161, 185, 198, 224, 279, 284, and 315) and reconstituted using recombinant proteins (55, 128, 181, 258, 280). These studies suggested that the bulk of MCMs in vivo associate in a heterohexamer with 1:1:1:1:1:1 stoichiometry, although there are likely to be small amounts of single MCMs and MCM subcomplexes in the cell.
Mutational analysis of the yeasts has been a powerful tool to examine minimal requirements for MCM association in vivo. As expected, complex assembly is essential for function, since no mutants that disrupt complex assembly are viable (see e.g., references 53, 184, and 284). Importantly, however, assembly into an MCM complex is not sufficient for function. For example, fission yeast Mcm2 mutants lacking an NLS, mutants with ATPase domain defects in several of the MCMs, or certain alleles of budding yeast MCM3 still assemble into a complex but are not able to function in vivo (99, 184, 280, 284). Attempts to identify a minimal binding domain in Mcm2 showed that sequences outside the MCM box including, but not limited to, the zinc finger are essential for complex assembly. Only a few mutant Mcm2 proteins with very short amino-terminal deletions are proficient for complex assembly in either S. pombe or mouse (132, 284).
Nuclear regulation of complex assembly.
As discussed above, only Mcm2 and Mcm3 have identifiable nuclear localization sequences (NLS), leading to an early suggestion that these MCMs provide nuclear targeting to the other members of the family (160). In nearly all species, the bulk of MCMs are constitutively located in the nucleus throughout the entire cell cycle, with their chromatin association, rather than nuclear localization, subject to cell cycle regulation (see, e.g., references 86, 121, 153, 159, 199, 240, 247, 278, 300, and 318). However, there is still a role for the nuclear envelope in MCM complex assembly. This has been molecularly characterized using mutational analysis with the yeasts.
A series of experiments with S. pombe, using alleles of mcm2+, suggested that MCM complex assembly occurs in the cytoplasm and is necessary both for MCM localization and for retention in the nucleus (247). First, conditional mutations that disrupt the MCM complex lead to the relocalization of the wild-type MCMs from the nucleus to the cytoplasm. Second, an Mcm2 mutant lacking a functional NLS is still able to associate with the other members of the complex and, when overproduced, can actually trap the remaining wild-type MCMs in the cytoplasm. Third, a mutant form of Mcm2 that is defective in binding the other MCMs, but contains an intact NLS, is unable to localize in the nucleus even if export is blocked by mutating crm1, the nuclear export receptor. These data suggest that transport of Mcm2 or other MCMs into the nucleus requires complex assembly in the cytoplasm. Because unassociated MCM subunits can be exported, this codependence provides a way to ensure that the bulk of soluble MCMs within the nucleus are assembled together in a complex. This preserves the stoichiometry of the subunits, at least in the soluble fraction of the nucleoplasm, and is consistent with studies suggesting that the bulk of MCMs are in large protein complex.
Intriguingly, a recent report suggested that Crm1 (Exportin 1) may be actively involved in regulating MCM function, at least in Xenopus extracts (343). Association of the MCMs with Crm1 prevents rereplication within a single cell cycle. However, active export of the MCMs is not required for this effect (see “Chromatin binding and regulation of rereplication” below).
S. cerevisiae is a special case, because the MCM proteins cycle in and out of the nucleus during a single cell cycle, so that the bulk of the proteins are present only in the nucleus during S phase (54, 110, 347, 358). However, as in other species, localization depends on the NLS sequences of Mcm2 and Mcm3 (229, 357), and intact MCM complexes are required for MCMs to enter the nucleus and to remain there (177, 229). MCM export is regulated by CDK activity (176, 229); whether this also promotes complex disassembly is not known, but this could provide one way to facilitate export using the same mechanism observed in S. pombe.
Identification of the core.
Different MCM subunits have different relative affinities for one another, forming a distinct series of subcomplexes whether isolated from yeast, mouse, or Xenopus (45, 55, 118, 129, 181, 185, 224, 258, 280, 284). During purification, Mcm4, Mcm6, and Mcm7 subunits bind most tightly together to form a trimeric complex sometimes called the MCM core. Mcm2 binds to the core, but with reduced affinity. Mcm3 and Mcm5 together form a dimer and bind most weakly to the other MCMs, probably through Mcm7. In the absence of other MCMs during in vitro reconstitution experiments, the Mcm4,6,7 core will itself dimerize to form a dimer-trimer (Mcm4,6,7)2, which is disrupted by addition of Mcm2 (128, 181, 258).
Does this mini-MCM complex exists inside the cell, or is it an artifact of purification experiments? This question is extremely important, because only the (Mcm4,6,7)2 complex is associated with DNA helicase activity in vitro (discussed in more detail below [128, 182]). Addition of other MCMs abolishes this activity, so it has been suggested that Mcm2, Mcm3, and Mcm5 negatively regulate the active Mcm4,6,7 complex (130, 182, 355). However, the in vivo data indicate that the situation is much more complicated. As discussed above, assembly of the heterohexamer is required for proper localization in the nucleus. Most evidence suggests that the bulk of MCMs are assembled in the heterohexamer in vivo, both on and off the chromatin (see, e.g., references 2, 87, 160, 198, 258, 263, and 284). For example, Mcm3 and Mcm7 colocalize cytologically (see, e.g., reference 267). When MCM chromatin immunoprecipitation was followed by DNA-DNA hybridization to a yeast genomic DNA microarray, only about 40 sites, of a total of 600 to 700 sites with multiple MCMs, had a single MCM protein (341). One experiment with Xenopus suggests that MCMs may load independently on the chromatin (203), and individual MCMs can be differentially released from chromatin by nuclease treatment (118). However, interpretation of these results is complicated by the different relative affinities within the complex and questions of antibody accessibility within the intact complex.
Functional data obtained in vivo also suggest that the MCMs contribute to a common activity. Immunodepletion of any single MCM protein from Xenopus extracts has an equally negative effect on replication (see, e.g., references 198, 267, and 315). Mutation of any individual mcm gene in yeast yields phenotypes consistent with each subunit playing a positive role throughout replication (see, e.g., references 177, 178, 184, and 193), and there are no alleles of MCM2, MCM3, or MCM5 that identify a negative regulatory activity. There is also some inconsistency with in vivo and in vitro phenotypes of site-directed mutants. While specific mutation of the Walker A or B motif of Mcm6 has a strong, negative effect on helicase activity of the Mcm4,6,7 complex in vitro (355), mutational analysis of the yeasts suggests that Mcm6 is uniquely forgiving of mutations of these sites, and the mutant proteins are in several cases able to function in vivo (see below) (99, 280; T. Schwacha, personal communication).
These data do not preclude the possibility that a subset of MCMs rearrange inside the nucleus to form a modified complex on the chromatin, but they suggest that the (Mcm4,6,7)2 helicase is not the predominant form inside the cell. Again, this could indicate that there are functionally distinct pools of MCMs in the nucleus that play distinct roles, a model to which we will return. Further studies are be required to establish the role of (Mcm4,6,7)2 in vivo, but biochemical analysis is providing some tantalizing clues.
ATP-mediated complex assembly.
As described previously, the MCM proteins are members of the diverse AAA ATPase family, and ATP is known to be important for assembly of AAA ATPases into hexameric complexes (reviewed in references 56, 113, 249, and 323). Given their highly conserved Walker A and B motifs, it is not surprising that MCM complexes have ATPase activity. Attempts to reconstruct MCM complex assembly in vitro by monitoring ATPase activity suggest that individual MCMs are not active as ATPases by themselves (55, 181, 280, 354, 355). Here, too, the results suggest that there are two distinct classes of MCMs, one represented by the core subunits Mcm4,6,7 and the other represented by the peripheral subunits Mcm2, Mcm3, and Mcm5. Mutations in these different subunits differently affect ATPase activity of the intact complex.
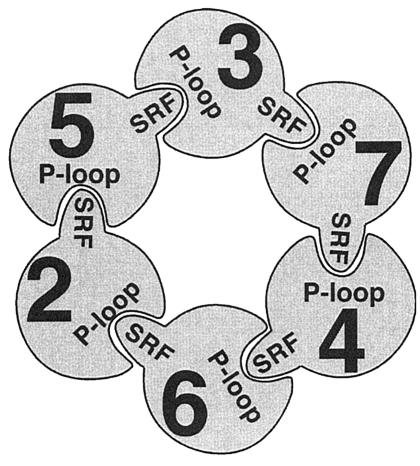
Reconstitution experiments using the intact complex from S. cerevisiae suggest that certain pairs of MCMs can associate to generate ATPase activity: Mcm3,7, Mcm4,7, and Mcm2,6 (55, 280). The order of subunits in the intact MCM complex may be inferred from determining these interactions (55) (Fig. 3); extensive two-hybrid studies using Drosophila MCMs, a more indirect approach, gives the same model (48). The biochemical data suggest that the ATP moiety binds at the interface of the two subunits, with one subunit providing the ATP-binding pocket of the P-loop, in the Walker A motif and the adjacent subunit providing a protruding arginine residue (the arginine finger) (55). Importantly, the sequence SRFD containing the arginine finger is conserved in all MCMs (Fig. 2). Together, these studies suggest a model where ATP hydrolysis occurs in pairs of subunits, with the intervening subunits being required for complex stabilization; thus, in the context of the intact structure, hydrolysis is linked around the ring to generate work (55, 280).
FIG. 3.
Pairwise interactions in vitro suggest this organization of the MCM heterohexamer. The P-loop (Walker A motif) and SRF motif (arginine finger) are proposed to act together as ATPase domains. In isolation, this complex does not have helicase activity in vitro (see the text). Reprinted from reference 55 with permission from the publisher.
This model implies that the Walker A and B motifs and the arginine fingers are not equally required in each subunit, since not every subunit is involved equally in hydrolysis. This can be tested by site-directed mutagenesis. Davey et al. showed that for the Mcm3,7 pair to have active ATPase activity in vitro, the Mcm3 subunit must have an intact arginine finger but the Walker A motif is not essential. In contrast, the Mcm7 subunit must have an intact Walker A motif but not an arginine finger (55).
Several studies have also examined the phenotypes of these mutants in vivo. Mutation of the conserved K to A in the Walker A motif of all the S. cerevisiae MCM subunits results in death in vivo, as do Walker B motif D-to-A mutations in all but S. cerevisiae Mcm2 (which has residual activity) and Mcm6 (280; Schwacha, personal communication). The arginine finger is likewise essential for viability in all MCMs (Schwacha, personal communication). There are slightly different phenotypes reported in S. pombe. The nonconservative Walker A mutation K to A is viable in S. pombe Mcm6, but the same change in S. pombe Mcm2, Mcm4, or Mcm7 is unable to complement a deletion (83, 99). Interestingly, a conservative change of K to R is tolerated in S. pombe Mcm2, Mcm6, and Mcm7 but not Mcm4. The Walker B mutation is similarly viable in S. pombe Mcm6 but lethal in Mcm2, Mcm4, and Mcm7. This suggests that the ATP-binding site of Mcm6 is dispensable, in contrast to predictions from studies of the helicase activity of the Mcm4,6,7 core in vitro (355). These in vivo results are consistent with the model proposed in Fig. 3: the arginine finger of Mcm6 should be required in combination with the Walker A (P-loop) motif of Mcm2, while the Walker A motif of both Mcm4 and Mcm7 should be essential for function.
MCM Helicase
The structure of the MCM complex and its arrangement as a heterohexameric ring suggest that the MCMs constitute the long-sought cellular replicative helicase. Electron microscopy analysis of the intact MCM hexamer from S. pombe (2) or of the (Mcm4,6,7)2 form of the helicase from mouse (275) suggests a globular complex with a central channel of 3 to 4 nm, similar to many multisubunit helicases from all kingdoms of life (reviewed in references 113 and 249. This channel is large enough to encircle either single- or double-stranded DNA. Although the structure of a eukaryotic, heterohexameric MCM complex has not been solved, striking insights are provided by three recently published studies.
The structure of the N-terminal region of the Methanobacterium thermoautotrophicum MCM protein (MtMCM) has been solved (81). This archaeal species has only one MCM protein, which assembles into two stacked homohexameric rings that have helicase activity (36, 157, 283). While the reported structure lacks the MCM homology domain including the ATPase motifs, it contains an extensive N-terminal domain that is sufficient to form a ring. This includes a zinc finger required for the head-to-head assembly of two hexamer rings (81). The central channel is 2 to 4 nm in diameter and is striking for its positive charge. Significantly, this study also demonstrates that the MtMCM is capable of binding double-stranded DNA and depends on the basic residues in the central channel to do so. Even though there is only limited primary sequence homology between the N termini of the eukaryotic MCMs and the N terminus of MtMCM, several of the charged residues that protrude into the central channel are conserved, and modification of these residues abolishes binding of the MtMCM complex to DNA (81).
The simian virus 40 large T antigen, a viral helicase, is another homohexamer ring that works in a head-to-head structure and is required for viral replication (reviewed in reference 288). The entire structure was solved (188), showing that the hexamer has two domains: one forming a smaller “head” that corresponds to the N-terminal domain solved for the MtMCM, and a larger “body” that corresponds to the ATPase domain. These two domains rotate around one another to open and close the central channel, leading the authors to propose an iris mechanism that grips double-stranded DNA to melt and unwind it. Here, too, the central channel is large enough to encompass a double strand of DNA, and a side channel provides an opening through which the unwound, single-stranded DNA may be extruded. The two linked hexamers are proposed to brace against each other and provide a ratchet mechanism to unwrap the DNA and give access to DNA replication proteins. This ratchet need not be adjacent to the replication fork but could pump open DNA from a distance; this could be consistent with theoretical speculations that MCM complexes act at a distance from replication forks (179), although there is solid evidence that MCMs are located at the fork (5, 40).
As discussed above, the reconstituted Mcm4,6,7 core complex has 3′-to-5′ DNA helicase activity in vitro, using a template with a single-stranded tail (128, 149, 182). Recent work suggests that the extended tail is occluded by steric exclusion from the center of the helicase ring (149). Intriguingly, the core helicase can also encircle double-stranded DNA that lacks an overhang and can translocate along the double-stranded DNA without unwinding it; this is sufficient to drive Holliday junction migration on naked DNA templates in vivo. Despite lacking sequence similarity to the MCMs, the bacterial 5′-to-3′ DnaB helicase shows similar characteristics and can even dislodge other proteins on the DNA (150). Thus, the Mcm4,6,7 core enzyme fulfills many of the expectations for a replicative helicase.
However, as described above, the MCM heterohexamer is the most common form in cells, while the Mcm4,6,7 core helicase is the only form purified that has activity in vitro. Thus, one of the most important puzzles to be solved regarding MCMs is the role of the core helicase in vivo. If this is the active form, how is it rearranged from the abundant heterohexamer? Does activation of proteins in the pre-replication complex by phosphorylation or binding of additional factors (discussed below) rearrange the MCM complex at the origin to generate the active helicase? This would suggest that the heterohexamer is an inert loading and spreading form of the MCM complex that moves out from the origin, leaving the modified core helicase alone at the fork to unwind the DNA. This simple model is inconsistent with in vivo data showing that the Mcm2 and Mcm3 subunits are required not only at initiation but also throughout the S phase, with phenotypes indistinguishable from Mcm4, Mcm6, or Mcm7 (178); if they were not required for activity of a rearranged helicase, this would not be the case. Perhaps other factors are required to adapt the full heterohexamer to helicase activity and their effect is mimicked in the structure of the core helicase in vitro. Supporting this model, the complete MCM complex immunoprecipitated from Xenopus lysates has processive helicase activity when Cdc45 is present (see below) (206). Clear resolution of these conflicting in vivo and in vitro results requires further experiments.
Expression and Abundance
For some MCM genes, there is evidence for increased transcription during the G1/S phase in actively dividing cells; however, despite this, MCM protein levels do not fluctuate during the cell cycle (see, e.g., references 54, 83, 161, 278, 319, and 358). MCMs are highly abundant proteins, estimated at approximately 30,000 copies/cell in S. cerevisiae (65, 185). With roughly 400 replication origins (259, 341), this suggests that MCMs exceed origins by approximately 75 to 1 in budding yeast. Assuming a genome size of approximately 14 Mb (96), this further suggests about two MCM complexes per kb. This level is apparently important: mutations that reduce levels of active MCMs cause defects in genome stability (80, 83, 185, 193), suggesting that a high threshold level of MCM is required for full activity. However, even during S phase, only a fraction of the MCMs are chromatin associated (see below). This leads to the hypothesis that the MCMs are not uniform in function: there may be pools of MCMs that are able to perform distinct roles, which could be distinguished by localization, modification, or binding to other factors.
MCMS ARE ESSENTIAL REPLICATION FACTORS
Evidence for a Role in Replication
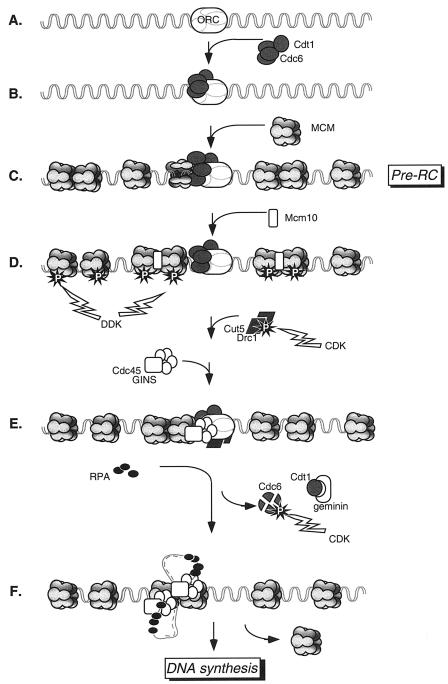
The primary models for the function of MCM proteins rely extensively on genetics in the case of S. cerevisiae and biochemistry in the case of Xenopus. Work with other systems has provided additional detail, evidence for variation, and important insights into function. Together, these studies have resulted in an increasingly complex model for the activation of individual replication origins in a single cell cycle. This section describes our current understanding of the role of MCMs in replication initiation; additional details may be found in numerous recent reviews (13, 16, 58, 154, 321). A diagram of the assembly and activation of the replication origin is presented in Fig. 4, and the identity of genes is given in Table 1.
FIG. 4.
Model for replication initiation driven by MCM proteins. Panels A to F show stages of replication that are described in the text.
Although many budding yeast mcm mutants are able to synthesize substantial amounts of DNA, their phenotypes clearly implicate them in replication, including nuclear and cellular morphology at the arrest point, their interactions with other replication mutants, and their origin-specific effects on the minichromosomes (see, e.g., references 54, 93, 111, 201, and 336). Similarly, temperature-sensitive fission yeast mcm strains synthesize significant amounts of DNA, but they arrest the cell cycle with a cdc phenotype and show the classic checkpoint-dependent arrest characteristic of other replication mutants (see, e.g., references 47, 82, 192, 216, and 303). The association of MCM proteins and DNA replication in Xenopus was made very clearly by showing that extracts depleted of MCMs were unable to support any DNA synthesis (see, e.g., references 37, 167, 198, 267, and 315). This led many investigators to conclude that the ability of most yeast mcm mutants to synthesize bulk DNA, without being able to complete the S phase, occurred because these conditional temperature-sensitive alleles incompletely inactivated the mutant proteins. This model predicted that most origins were able to fire with residual MCMs protein but that some were inactive and/or some regions of the genome were refractory to passive replication. Although we now know that this interpretation was incorrect, it focused considerable attention on the role of the MCMs in initiation, and it is that role that has been most closely examined.
Assembly of the Prereplication Complex
Sequential loading of replication factors.
Replication initiation depends on identification and activation of origins of replication distributed throughout the genome. Origin structure varies widely, ranging from small DNA elements with a defined consensus sequence (S. cerevisiae) to larger, degenerate elements without a consensus (S. pombe), to stochastically determined elements that may be established by spacing (Xenopus eggs) (reviewed in references 14 and 94). Regardless, the replication origin is marked by the binding of a complex of proteins called ORC (for “origin recognition complex”) (reviewed in references 12 and 295). It is useful to think of ORC as a marker of potential origins of replication. However, ORC function is not limited to replication initiation but has also been implicated in heterochromatin assembly, nucleosome remodeling, chromosome condensation, and transcriptional silencing (see, e.g., references 27, 59, 60, 84, 126, 195, 245, 253, and 257). Not surprisingly, ORC localization is not restricted to active replication origins, so it may serve a broader function as a landing platform for a range of chromosomal proteins (60, 84, 341).
At the replication origin, ORC is bound by Cdc6 and Cdt1 during G1 phase (fig. 4A to C). These proteins are required for subsequent loading of the MCM complex (42, 61, 65, 153, 191, 204, 232, 268, 311, 337, 340). Analysis of S. cerevisiae cdc6 or the equivalent S. pombe cdc18 mutants shows a range of phenotypes, depending on the allele: while some alleles result in a complete block to DNA synthesis, others allow significant accumulation of DNA (26, 49, 57, 112, 155, 190, 196, 227, 251, 255, 276, 334). This may be related to the observation that many mcm mutants synthesize substantial amounts of DNA. Evidence suggests that Cdc6 binds directly to ORC (191, 218). It has been suggested that Cdc6 functions as a clamp loader to assemble the MCM ring on the DNA, which is consistent with evidence suggesting that only a small fraction of chromatin-bound MCM is associated with Cdc6 (56, 88, 180, 210, 251, 334). The molecular contribution of Cdt1 to this activity is unclear. In metazoans, a small protein called geminin binds Cdt1 and prevents its association with MCMs in G2 or mitosis (302, 340, 348).
Once the MCMs are loaded, the origin is enabled for activation. This assembled origin complex is called the prereplication complex (pre-RC). Assembly of the pre-RC occurs at the end of mitosis, when CDK levels start to fall (11, 46, 153, 211, 300, 311). Although biochemical experiments suggest that the ORC and Cdc6 are dispensable once MCMs are bound (65, 122), in vivo experiments with the yeasts suggest that continued expression of Cdc6 throughout G1 is required to maintain the pre-RCs (11, 42, 255, 274).
Mcm10.
Despite its name, the Mcm10 protein is not a member of the MCM family, although it interacts physically and genetically with members of the MCM complex and other replication initiation factors (39, 43, 104, 119, 136, 151, 192). MCM10 was isolated in the same S. cerevisiae screen as the true MCM proteins and independently isolated in a screen for replication mutants (201, 293). Mcm10-containing complexes are extremely insoluble, especially during S phase, and the protein is part of a large complex (43, 104, 119, 136, 192). Although experiments with budding yeast suggested that Mcm10 is required for assembly of the pre-RC (119), studies with human cells, Xenopus extracts, and fission yeast indicate that Mcm10 acts after MCM association (101, 136, 339) (Fig. 4D). Additional data from S. pombe suggest that the S. pombe Cdc23 (Mcm10) protein may target the Dbf4-dependent kinase (DDK) to the MCM complex, which may facilitate Cdc45 binding (see below) (101, 183). There is also some evidence suggesting that S. cerevisiae Mcm10 may affect replication fork progression (213).
Origin Firing
Origin firing is controlled by two kinases: CDK and DDK (reviewed in references 109 and 145) (Fig. 4D). Genetics and biochemistry have at times conflicted over which kinase is required in order (see, e.g., references 140, 205, 236, 246, 282, 332, and 335). This may reflect the inherent limitations of genetic mutants or biochemical systems at recapitulating complex pathways or may indicate intrinsic differences in protein behavior between species. It is also possible that the kinases act at the same time on independent pathways which converge at a common point. Origin firing, culminating in DNA synthesis, results in loading an ever-expanding group of proteins. The order of these events is not completely worked out, and new players continue to be identified.
DDK and the bob1 mutation.
The conserved DDK consists of the catalytic subunit (Cdc7 or Hsk1) and a regulatory subunit (S. cerevisiae Dbf4; also called ASK, Dfp1, or Him1 in other systems [Table 1]) (reviewed in references 141, 145, and 281). While Dbf4 does not have the typical sequence motifs of a cyclin, it is transcriptionally and posttranslationally regulated to restrict the activity of its kinase partner to the S phase of the cell cycle (24, 35, 78, 137, 143, 173, 236, 243, 305, 335, 353). Like a cyclin, Dbf4 appears to provide Cdc7 kinase with substrate binding and specificity. Intriguingly, just as vertebrate cells have multiple cyclins, they may also separate DDK functions by using paralogous regulatory subunits: in humans and Xenopus, an alternative Dbf4 subunit called Drf1 has been identified that may be responsible for DDK activity outside of initiation (220, 350).
DDK is known from biochemical and genetic tests to associate at the origin of replication, suggesting that it must act at each pre-RC to initiate replication (64, 66, 183, 246, 264, 335). Several studies suggest that DDK binds both to ORC and to MCMs independently of ORC (48, 71, 140, 162). It phosphorylates a number of potentially relevant substrates including predominantly Mcm2 but also Mcm3, Mcm4, Mcm6, Mcm7, Cdc45, and DNA polymerase alpha (see, e.g., references 25, 143, 173, 186, 236, and 335). No consensus site for DDK phosphorylation has been identified, and despite much effort, no mcm2 phosphorylation site mutants have been identified in the yeasts, although the N terminus of Mcm2 is an excellent substrate in vitro. However, a new study has identified several sites in human Mcm2 phosphorylated by DDK that are apparently essential for DNA replication, although the sequences are not conserved in other Mcm2 proteins (T. Tsuji and W. Jiang, personal communication). Significantly, while DDK activity is limited temporally to the S phase, its function is not limited to replication: it has also been implicated in additional functions in heterochromatin assembly, gene silencing, repair, and recombination, (see, e.g., references 8, 9, 44, 90, 116, 117, 234, 290, and 306). These roles probably involve substrates distinct from the replication proteins; for example, the kinase also phosphorylates the heterochromatin protein Swi6/HP1 (9).
In vivo data linking DDK and MCMs were obtained in experiments with an S. cerevisiae mutant called mcm5-bob1, which is a recessive bypass allele of Mcm5 (103, 137). In an mcm5-bob1 mutant background, the DDK is no longer essential for viability. Interestingly, Mcm5 is the only MCM subunit that is not phosphorylated by DDK in vitro, so that this allele does not simply mimic a phosphorylation event, but has a more unusual effect. An attractive model is that the mcm5-bob1 mutation results a change in the shape of the MCM complex that mimics the effect of DDK phosphorylation on other subunits and therefore allows subsequent loading of Cdc45 (103, 282). The bob1 mutation is a proline-to-leucine change in a conserved residue of S. cerevisiae Mcm5 (103). The same proline is found in the archaeal MCM protein, and the structural effect of the bob1 mutation was tested in this context (81). It results in a modest but discernible shift in the position of one domain of the protein with respect to another, an effect referred to as a “domain push.” The authors postulated that this shift would also occur with other bulky amnio acid residues and, if so, that such residues should also cause a bob1 phenotype in S. cerevisiae Mcm5. This was tested in vivo in budding yeast; the experiments confirmed that small residues had no bob1 phenotype while large residues behaved similarly to the original bob1 mutation. Importantly, however, this effect appears to be Mcm5 specific; the same proline-to-leucine changes in S. cerevisiae Mcm2, Mcm3, or Mcm4 do not bypass cdc7 in S. cerevisiae (184; L. Pessoa-Brandão, R. Leon, and R. A. Sclafani, personal communication). The bob1 mutation has not yet been constructed into Mcm5 proteins outside of S. cerevisiae. In S. pombe, one of the two lesions in the mcm2+ temperature-sensitive allele called cdc19-P1 is also a proline-to-leucine change of this residue, which does not bypass the DDK kinase (83, 290).
CDK.
The CDKs are essential for replication initiation (see, e.g., references 18, 21, 62, 68, 69, 74, 122, 254, 282, and 311). Importantly, mcm5-bob1 is still dependent on CDK activity, indicating that this kinase either functions in parallel with or downstream of DDK (137, 282). If DDK provides the signal at individual origins, CDK appears to provide the link to the cell cycle.
This link may be through an essential but poorly understood protein called variously Cut5 or Dpb11 (Table 1) (Fig. 4D and E) (6, 107, 271, 344, 345; W. P. Dolan, D. A. Sherman, and S. L. Forsburg, submitted for publication). Genetic studies of the yeasts indicate that Dpb11/Cut5 is required both for initiation of DNA replication and, independently, for checkpoint responses to unreplicated or damaged DNA (6, 208, 270, 271). Temperature-sensitive S. pombe cut5 mutants are profoundly defective in DNA synthesis, this is one of the tightest initiation phenotypes of any temperature-sensitive mutant (see, e.g., references 77 and 271). S. cerevisiae Dpb11 associates with DNA polymerase epsilon, which also acts early in S phase (6). The yeast Dpb11/Cut5 binds to a CDK target protein called Drc1/Sld2, and CDK phosphorylation of Drc1 is required for replication initiation (146, 207, 235, 333).
Cdc45: a rate-limiting factor.
The most significant event in origin firing appears to be binding of a conserved protein called Cdc45, which is a rate-limiting factor for replication initiation (71, 214) (Fig. 4E). CDC45 was first identified in budding yeast by some of the same screens that identified MCM genes (219, 293). cdc45 and mcm mutants interact genetically, and the proteins interact physically (53, 102, 111, 120, 172, 214, 217, 269, 324, 325, 360, 361; Dolan et al., submitted). Cell cycle analysis of S. cerevisiae indicated that Cdc45 and DDK have a similar execution point, defined as the time at which a protein functions in the cell cycle (244). Combined with the data regarding the mcm5-bob1 mutant allele, a plausible model is that DDK phosphorylation of MCMs results in an allosteric change in the MCM complex that allows binding of Cdc45 at individual origins as they are activated. This suggests that the consequence of DDK activity is Cdc45 binding, consistent with biochemical analysis (see, e.g., references 71, 140, and 339). This is also consistent with localization showing that Cdc45 does not assemble at all origins all at once but apparently does so as they are fired (4, 214, 362). Cdc45 is itself a substrate of the DDK in vitro, but it is not clear whether this influences its function in vivo (236).
Assembly of the pre-RC is not sufficient for Cdc45 loading, however. CDK activity is also required (215, 362). This may be mediated at least in part by the Dpb11/Cut5 protein (described above), which is part of a CDK-regulated complex and is also required for Cdc45 loading (7, 107, 146, 207, 235, 304, 327, 333). However, athough MCMs may be liberally distributed along the chromatin and bound to Cdc7, only a fraction of MCM complexes will recruit Cdc45 (71). While the ratio of MCM complex to ORC in reconstitution experiments approaches 40:1, the ratio of Cdc45 to ORC is 2:1 (71). Thus, something limits Cdc45 binding to the MCMs at the origin.
Together, these data suggest that there may be two independent inputs into the replication initiation pathway: (i) the origin identification and pre-RC assembly of the MCMs, which is activated by DDK phosphorylation, and (ii) the cell cycle response provided by the CDK activation of Dpb11/Drc1. This is particularly satisfying because it provides a fail-safe mechanism for the initiation of replication by integrating two independent signals from converging pathways.
Although for many years the repertoire of players in replication initiation was thought to be complete, recent genetic experiments have identified new and unexpected players. Cdc45 functions with a protein called Sld3, which appears to be conserved (at least in the yeasts [147, 226]). Three very recent studies have identified an additional, completely new complex with a modified ring shape, called the GINS complex, which is mutually interdependent for origin binding with Cdc45 and Dpb11/Cut5 complexes (148, 169, 304) (Table 1). This complex is conserved in multiple eukaryotes and is essential for cell viability. Its role is likely to be more complicated than simply replication initiation: the S. pombe homologue of the Psf3 subunit of GINS, called Bsh3, was isolated in a screen for interactions with the passenger protein complex required for kinetochore function and was subsequently identified in human cells (H.-K. Huang and T. Hunter, personal communication). Whether this indicates a mechanistic link between replication and kinetochore assembly, or multiple independent functions associated with the GINS complex, remains to be determined.
The consequence of Cdc45 loading is the unwinding of DNA to open a single-stranded region (214, 330). Actual initiation (as measured by DNA synthesis) may be best scored by loading of replication protein A (RPA), which binds single-stranded DNA and is required for subsequent polymerase binding and activation (4, 214, 330, 361). Intriguingly, like all the MCMs, Cdc45 is required both to initiate and to complete DNA replication (178, 206, 312) (see “MCMs and replication in elongation” below).
Chromatin Binding and Regulation of Rereplication
As discussed above, MCMs in all organisms but S. cerevisiae are constitutively located in the nucleus throughout the cell cycle. MCM chromatin binding, rather than localization, is cell cycle regulated. MCMs bind chromatin only during the G1/S phase and are dislodged from the chromatin as the S phase proceeds (61, 87, 153, 159, 166, 168, 190, 199, 266, 279, 317, 347). Although there is always a soluble fraction of MCM protein in the nucleus throughout the cell cycle, access of the MCMs to chromatin is a key regulatory event necessary to control origin usage.
Are MCMs limited to the origin?
Cytological studies have indicated that MCMs are liberally distributed throughout the nucleus, associated with unreplicated chromatin, and are not concentrated at the origin (61, 166, 199). This suggests that only a subset of the MCMs are bound at the origin or with actively replicated DNA. This excess of MCMs distant from the origin (termed the MCM paradox [125, 179]) could suggest that MCMs load at the pre-RC and spread along the DNA, perhaps similarly to the translocating DnaB (150).
One possibility is that there is a qualitative difference in the binding of different pools of MCMs to the DNA. Early experiments suggested that MCM association with the chromatin could be stabilized by ATP (87). Genome-wide analysis using “ChIP on a chip” (chromatin immunoprecipitation and PCR, followed by microarray analysis of the products) suggests that there are 600 to 700 MCM sites per S. cerevisiae genome, of which approximately 10% do not colocalize with ORC (341). This is slightly more than the anticipated number of origins (259, 341) but substantially less than the 30,000 estimated MCM molecules per cell (65, 185). Even given the possibility that a significant fraction of the MCMs remain soluble in the nucleus, it would appear that this method underestimates the MCMs on the chromatin, either because they are not tightly associated or because they assemble into very large higher-order structures.
Several experiments suggest that there are multiple MCM complexes associated with a single origin (71, 239, 263). The ratio of MCMs bound to ORC at the origin were examined using a Xenopus in vitro system, in which origins are not defined (71). On an 80-bp fragment of DNA, ORC and MCM assembled at a 1:1 ratio. However, as the fragment length increased, the amount of MCM (but not of ORC) also increased, approaching the estimate of 20 to 40 MCMs per ORC observed for sperm chromatin in Xenopus extracts (71, 331). This observation suggests that MCMs spread away from ORC. Significantly, although Cdc7 kinase can associate with the distal MCMs, origin firing and Cdc45 loading does not occur at the position of all the MCM complexes. This implies that there is a difference between MCMs bound immediately adjacent to the origin at ORC and those distributed along the chromatin, although they seem to be bound equally tightly. Perhaps the spreading MCM complexes restrict the access of additional ORC complexes to the chromatin and thus ensure that potential origins are distributed some distance apart (71).
CDKs regulate MCM chromatin binding through multiple mechanisms.
MCM chromatin binding is regulated by phosphorylation; hypophosphorylation of Mcm2, Mcm3, and Mcm4 in various systems correlates with MCM chromatin binding (46, 89, 108, 224, 317, 358). However, mutation of CDK sites in a single MCM, at least in yeasts, is not sufficient to disrupt replication control, indicating the presence of multiple levels of redundant control (99, 230). Moreover, phosphorylation of MCMs may also affect enzymatic function apart from chromatin binding; in vitro studies indicate that CDK-dependent phosphorylation of Mcm4 also disrupts the helicase activity of the (Mcm4,6,7)2 form of the helicase (131, 134).
Importantly, CDKs negatively regulate pre-RC assembly overall (52, 79, 123, 176, 229) (Fig. 4E). In fission yeast, inactivation of CDK has the dramatic effect of causing massive rounds of rereplication; a similar, although less dramatic, effect is observed in budding yeast (22, 52, 221, 230). By manipulating CDK activity or Cdc6 levels to disrupt normal origin control, MCMs are reloaded onto the origins and cells rereplicate their DNA inappropriately in a single cell cycle (190, 230, 291, 351).
Other CDK targets include ORC and Cdc6 (23, 73, 79, 88, 108, 131, 134, 138, 176, 197, 225, 229, 233, 328). Phosphorylation of Cdc6 is associated with its degradation (in yeasts) or nuclear export (in mammals) (67, 72, 139, 163, 225, 233, 252, 272). Since Cdc6 is essential for MCMs to load onto chromatin (see above), this has the effect of restricting MCM loading to G1/S, when Cdc6 is present and active. Because high CDK activity blocks pre-RC assembly, this ensures that the firing of an origin also involves its inactivation, thus preventing repeated use of an origin within a single cell cycle.
Additional controls contribute to the prevention of MCM chromatin binding and inappropriate origin activation. A recent study suggests that association of a subset of MCMs with the Crm1 (exportin 1) nuclear export factor is required in Xenopus to prevent rereplication (343). However, this does not appear to involve actual MCM export, and it is likely that the Crm1 association involves only a fraction of the MCMs, which again suggests that there may be distinct pools of MCMs within the nucleus. This is a recurring theme in MCM analysis.
MCMs and Replication Elongation: from Assembly Factor to Helicase
Evidence for a postinitiation role.
The role of MCMs in origin activation is clear. Biochemical analysis of Xenopus unambiguously revealed an essential function in initiation of DNA synthesis. Execution point mapping of mcm mutants from yeasts clearly implicated them in replication initiation, and studies using synchronized yeast cultures clearly established that these proteins are required for assembly and activation of the pre-RC.
However, these experiments do not eliminate the possibility that MCMs have an additional function later in S phase. In the yeasts, many temperature-sensitive mcm mutants result in arrest at the restrictive temperature with a substantial amount of DNA synthesis, similar to the phenotype associated with mutations in proteins that act later in DNA replication (see, e.g., references 93, 193, 227, and 346). While the temperature-sensitive mutants were assumed to be “leaky” for activity, allowing a subset of origins to fire, this phenotype was also consistent with a later MCM function, a geneticist's argument that was subsequently proven correct. Early experiments offered several tantalizing clues. In fission yeast, spores in which mcm has been deleted germinate and proceed through a slow S phase. This reflects residual, or “maternal,” MCM packaged in the spore. Only if the residual protein is further inactivated with a temperature-sensitive allele do the deleted spores block replication initiation (193). This was interpreted to indicate that relatively little MCM is required for initiation and bulk DNA accumulation but that significant amounts are required for completion of DNA replication, arguing for an additional and essential role later in S phase. Consistent with this, a degron allele of S. pombe mcm4ts that results in rapid and irreversible protein inactivation is the only mcm4+ allele sufficiently stringent to causes initiation defects (194).
The most compelling evidence for a role in replication elongation was provided by an elegant experiment in which an MCM degron allele in synchronized S. cerevisiae cells allowed the protein to be selectively destroyed after replication initiation (178). These cells arrested during S phase with intermediate levels of DNA, showing that the MCMs indeed function after replication initiation; importantly, this was found for five of the six MCMs, only because a functional degron allele of Mcm5 could not be constructed for the experiment. Importantly, this shows that Mcm2 and Mcm3 are indistinguishable from the core complex Mcm4,6,7 even in the elongation stage of S phase. Similar observations using the same technique were made for Cdc45 (312). Thus, the MCMs convert from an assembly factor to an active participant in replication elongation along with Cdc45. Since the structure of the MCMs clearly suggests parallels to known DNA helicases (see above), the MCM complex became the obvious candidate for the cellular replicative helicase.
MCMs at the replication fork.
As described above, biochemical proof that the MCM heterohexamer functions as a helicase has been hard to obtain. Attempts to reconstitute an active helicase using purified proteins from several species have suggested that only the Mcm4,6,7 core complex has helicase activity in vitro. However, this activity is disrupted by addition of the other MCMs, although they are equally required in vivo. One possibility is that the MCM complex requires accessory components and that Cdc45 may be the missing factor (206). In that study, the investigators attempted to purify an active helicase from Xenopus extracts and found that the activity included all six MCM proteins and Cdc45. This is consistent with the evidence from ChIP, suggesting that Cdc45 and MCMs both travel with the replication fork (5). Using a set of nested PCR primers walking outward from an origin, it was determined that MCMs associate with the expanding replication fork with similar kinetics to DNA synthesis factors—and to Cdc45. Cytological studies with Drosophila agree with this, showing that MCMs specifically associate with the origin and then move away as the fork expands (40). These data are consistent with genetic and physical interactions between MCMs and Cdc45 and between RPA and DNA polymerase alpha (83, 172, 215, 324, 325, 361) and would place the MCMs at the fork. Figure 4F presents a speculative model of MCM function at the fork.
Cytological analysis of metazoans shows that MCMs liberally decorate unreplicated chromatin during S phase but are not particularly closely associated with the proteins of the replication fork (61, 166, 199), leading to a competing suggestion that an MCM helicase may function at a distance from the replication fork (179). However, since only a small fraction of the MCMs localize at the origin (MCMs outnumber ORC by approximately 40:1 in Xenopus extracts [71]), the majority of MCMs visualized cytologically are likely to be “remote MCMs” not located at the fork. Biochemical studies using Xenopus extracts suggest that these remote MCMs are bound equally tightly as the MCM complexes at the pre-RC (71), although they are not observed in ChIP experiments with yeast. Perhaps once the MCMs are activated by Cdc45 and the GINS complex, they become tightly associated with the replication fork, allowing their identification by ChIP.
What is the purpose of this vast excess of MCMs that cannot be placed at the fork? The cell requires the remote MCMs, because even modest reduction in MCM levels that do not particularly affect replication (71) can lead to genome instability (see, e.g., references 80, 185, and 193). Whatever role they play is probably limited to S phase, because bulk MCMs lose their chromatin association as S phase proceeds (see e.g., references 37, 46, 153, 159, 166, and 199) and in budding yeast they also leave the nucleus (see e.g., references 176 and 229).
MCM FUNCTIONS BEYOND REPLICATION
The abundance of MCMs and the evidence suggesting that they may affect chromatin functions outside the replication fork suggest that these proteins may have additional functions in the cell. Most evidence for this relies on physical interactions identified by coimmunoprecipitation or two-hybrid experiments. These may define functionally accessible regions of the MCMs; for example, the known interactions with Mcm2 all occur in the same region of its N terminus (27, 115, 130), while interactions with Mcm7 occur in the same region of its C terminus (31, 171, 298) (Fig. 5). Some phenotypes are suggestive of roles in chromosome structure, such as the recent observation that Drosophila mcm mutants have defects in chromosome condensation (39, 253). Increasingly, these observations suggest that MCMs, as is the case for other replication proteins, may have roles in a variety of chromosome transactions.
FIG. 5.
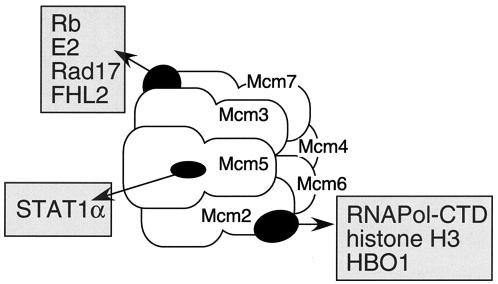
Associations between MCMs and other factors are grouped by function and the MCM subunit with which they interact. Interactions with Mcm7 occur through its C terminus, while interactions with Mcm2 occur through its N terminus. Abbreviations: E6, papillomavirus E6 protein; FHL2, heart-specific LIM-domain protein; Hbo1, MYST family HAT; Rad17, checkpoint protein; RNAPol-CTD; carboxy-terminal domain of RNA pol II. For references and discussion, see the text.
Transcription
Many studies have suggested interactions between transcription and replication apparatus (reviewed in references 209 and 223). Perhaps not surprisingly, the MCMs have been implicated in several aspects of transcriptional control. Is this a consequence of links between transcription and replication that coordinate cell proliferation, or is it evidence for an active role for MCMs in transcription? Some investigators point to simian virus 40 large T antigen, which is both a replicative helicase and a transcription factor, as a paradigm (reviewed in reference 288).
Several MCM proteins have been shown to associate with the carboxy-terminal domain (CTD) of RNA polymerase II (RNA pol II), and antibodies to Mcm2 inhibit transcription in vitro (349). Amino acids 168 to 212 of Mcm2 associate with the CTD, and point mutations in this region of Mcm2 disrupt the interaction (115). The CTD of RNA pol II is associated with the assembly of proteins involved in transcription initiation, elongation, and chromatin remodeling (reviewed in reference 105). A genetic interaction in S. cerevisiae between CTD domain mutants and an mcm5 mutant suggests that the interaction is required for replication, and the authors suggest that RNA pol II recruited to origins may influence chromatin structure (20, 91, 297).
MCMs may play a more direct role in transcription. Several lines of evidence suggest that the MCM proteins associate with specific transcription factors. During biochemical purification, the Mcm3-Mcm5 heterodimer associates with STAT1α, a transcription factor stimulated by gamma interferon (50, 359). This association is mediated by Mcm5. Residues 250 to 401 of Mcm5 are sufficient for interaction in vitro; although a point mutation at the extreme C terminus disrupts the interaction, it also disrupts the association of Mcm5 with other factors and may cause a structural change in the protein (50, 359). Intriguingly, increasing levels of Mcm5 in cells correlate with increased levels of transcriptional activation; additionally, levels of STAT1α-dependent transcription vary in the cell cycle, reaching a maximum at G1/S (359). The mechanism of this effect is not known. Loss of mcm5 activity in budding yeast leads to increased expression and nuclease sensitivity of genes at the telomeres (70), which may reflect a role in chromatin structure rather than transcription per se (see below).
Mcm7 has also been connected with several transcriptional regulators. In yeast, Mcm7 autoregulates its expression along with the transcription factor Mcm1 (80). Although MCM1 was isolated in the same minichromosome maintenance screen, it does not encode a member of the canonical MCM family; instead, it is a MADS-box transcription factor that is required for expression of a number of cell cycle-regulated genes (248). Interestingly, purified Mcm7 stimulated the binding of purified Mcm1 to promoters in vitro (80), indicating that this effect does not depend on the complete MCM complex, although it is not clear whether Mcm7 forms a stable complex with Mcm1. Moreover, despite the increased binding of Mcm1, Mcm7 in vivo appears to down-regulate its own transcription, acting as a transcriptional repressor. The functional significance of this interaction remains to be worked out.
Mcm7 also interacts with the retinoblastoma tumor suppressor protein (Rb) (298). A major role of Rb is in regulating the activity of the E2F transcription factor family, which is in turn required for the expression of a number of genes involved in the G1/S transition, including the MCMs (reviewed in reference 299). The carboxy-terminal 137 amino acids of Mcm7 also associate with the Rb-related factors p107 and p130 (298). Expression of the interacting fragment of Rb can inhibit DNA replication in vitro, presumably by interfering with the normal function of Mcm7 at the replication origin (298). Consistent with this, the interaction is disrupted by active cyclin D/CDK4 (95). This suggests that Rb family members may actively inhibit DNA replication by sequestering MCM proteins. Whether this association influences the activity of Rb remains to be determined. The C terminus of Mcm7 also interacts with the papillomavirus E6 oncoprotein (171), which may be involved in ubiquitylation (see below) (170).
Chromatin Remodeling
The structure of the chromatin and the pattern of histone modifications within it have been proposed to generate an epigenetic code that is crucial for regulated gene expression (reviewed in references 32, 98, 106, and 142). The replication community has only recently begun to explore the connections between histone acetylation and deacetylation and replication competence. For example, deleting the S. cerevisiae histone deacetylase (HDAC) RPD3 correlates with increased origin acetylation and earlier origin firing (329). A similar effect is observed if the Gcn5 histone acetyltransferase (HAT) is tethered next to an origin (329). Interestingly, tethering of PCAF/Gcn5 HAT adjacent to the polyomavirus replication origin also stimulates replication in animal cells (342). Early studies suggested that regions of the chromatin associated with MCM proteins are more susceptible to nuclease digestion, indicating that these domains may be less tightly compacted (118, 262). There is also a Cdc7-dependent change in origin topology, detected by genomic footprinting (92). These observations are consistent with a requirement for more open, histone-acetylated chromatin around a replication origin (reviewed in reference 209).
Budding yeast mcm5 mutants show increased expression and nuclease sensitivity of genes at telomeres (70). Telomeric gene expression in this system is modulated by the balance between the MYST family HAT called Sas2 and the HDAC called Sir2 (158, 301). Do mcm5 mutants disrupt this balance? Overexpression of a member of the Gcn5-containing SAGA complex partially suppresses the phenotypes associated with the mcm5 allele (70). This could indicate direct interactions such as those observed with polyomavirus large T antigen (342). Alternatively, MCMs may influence, or be sensitive to, the histone modifications mediated by this complex.
Evidence linking the MCMs directly to assembly or maintenance of chromatin structure is still incomplete but is nevertheless suggestive. Several interactions with chromatin-associated proteins occur through the amino terminus of Mcm2 (Fig. 5). Mcm2 binds histone H3 sufficiently tightly, that it is possible to purify the MCM complex on a histone affinity column (129); this association requires residues 63 to 153 of mouse Mcm2 (130). When incubated in vitro with histones H3 and H4 and plasmid DNA, Mcm2 by itself promotes the assembly of a putative nucleosome-like structure (132). Studies in vitro and in vivo show that human Mcm2 interacts with a protein called Hbo1, a member of the MYST family of HATs that also interacts with ORC (27, 126). This association occurs through residues 73 to 223 of Mcm2, and a point mutation in a conserved leucine in this region disrupts the interaction in two-hybrid assays. This mutation is likely to be specific to the interaction and does not generally disrupt the Mcm2 structure, because it can be suppressed in vitro by a corresponding mutation in the zinc finger of Hbo1 (27). Does this interaction indicate that MCMs target Hbo1 to the replication origin to acetylate histones? Or does Hbo1 target MCMs to specific regions to modulate silencing, which is linked to the S phase (reviewed in reference 10)? Could this be part of a larger role for MCMs in genome stability? Other members of the MYST family of HATs are implicated in DNA repair (15, 127). In addition, HATs are not limited to acetylating histones. The PCAF and Gcn5 HATs that stimulate polyomavirus replication do so by acetylating the polyomavirus large T antigen, which is required for origin binding and replication (342); in human cells, Mcm3 is also acetylated (see “Modifications of MCM” below). Thus, Hbo1 might directly modify MCMs. Interactions between MYST family HATs and MCMs are also observed in fission yeast (E. B. Gómez and S. L. Forsburg, unpublished observations), providing a genetic model for their study. Together, these data suggest that MCMs may be more active contributors to chromatin structure during S phase.
MCMs and Checkpoint Responses
Genetic analysis of the yeasts suggests that reduction of MCM activity is associated with genome instability and DNA damage (see, e.g., references 111, 185, and 193). Recent work suggests that MCMs may play a direct role as a target of the checkpoint response system. S-phase checkpoints consist of overlapping kinase cascades (reviewed in references 19, 29, 238, 242, and 261). The replication checkpoint is activated in response to replication arrest, such as that caused by the drug hydroxyurea (HU). The damage checkpoint is activated by DNA lesions or double-strand breaks (DSBs). The activated checkpoints cause cell cycle arrest, protect the replication fork from collapse, and activate repair.
Checkpoints may be active participants during S phase to identify and repair any lesions resulting from passage of the replication fork. For example, the Mrc1 protein, which acts with S. cerevisiae Rad53/S. pombe Cds1 as part of the replication checkpoint, has recently been shown to be required for efficient replication elongation, a function genetically separable from its role in checkpoint signaling (241). Its assembly at the origin requires DDK activity, reminiscent of Cdc45. In vertebrate cells, the Rad17 protein, a component of the checkpoint signaling apparatus, associates with early replication foci even in the absence of damage (51), and the Hus1 protein which acts with Rad17 is chromatin associated during normal S phase (356). Thus, interactions between replication and checkpoint proteins may be a normal response to the mutagenic potential of S-phase progression; this is consistent with observations suggesting that S. cerevisiae Mec1 (S. pombe Rad3/human ATR) prevents replication fork stalling (30).
When the replication checkpoint is activated by HU treatment, checkpoint proteins are recruited to the replication fork (51, 241). In the absence of the replication checkpoint kinase S. cerevisiae Rad53/S. pombe Cds1, cells treated with HU suffer replication fork collapse and replication proteins including polymerases are lost from the fork, leading to the model that the replication kinases recruited to the fork act on replication proteins to arrest replication and promote fork stabilization until DNA synthesis can continue (41, 292, 313).
In fission yeast, mcm mutants at the restrictive temperature suffer DSBs (J. M. Bailis and S. L. Forsburg, unpublished data) and activate the DNA damage checkpoint (192, 193, 202). If loss of MCMs causes damage, protecting the MCMs at the fork is a logical target of checkpoint activation. Mouse Mcm4 undergoes HU-specific phosphorylation by the checkpoint kinase ATR and by Cdk2 (133). This phosphorylation inactivates the Mcm4,6,7 helicase in vitro, consistent with the role of the checkpoint in shutting down replication when cells are starved for nucleotides following HU treatment (see, e.g., references 64, 273, and 285). Recent isolation of an HU-sensitive allele of mcm4 from fission yeast further suggests that Mcm4 may be a checkpoint target; other mcm4 alleles are not HU sensitive (T. Nakagawa and H. Masukata, personal communication). This effect may not be limited to Mcm4; there is now evidence that Mcm7 interacts with the Rad17 checkpoint protein, again through its C terminus (C.-C. Tsao and R. Abraham, personal communication). This could indicate that that MCMs recruit or anchor checkpoint proteins at the stalled replication forks.
MCMs in Cancer
As described above, dysregulation of MCMs by reducing or increasing the levels of a single MCM leads to disruptions in genome stability in yeast (see, e.g., references 185, 193, and 346). Since MCM activity is essential for DNA replication in dividing cells and is lost in quiescence (200), MCMs are obvious markers for proliferation. Molecular studies suggest that increased levels of MCMs mark not only proliferative malignant cells (85, 97, 114, 135, 212, 260, 265, 338) but also precancerous cells and the potential for recurrance (3, 124, 310). They thus may prove to be effective markers for tumor diagnosis.
Additionally, MCMs may be contributors to cancer. Proteins implicated directly in cancers are known to modulate MCM activity. As described above, Mcm7 physically interacts with Rb, presumably to reduce proliferative capacity (298), and with the papillomavirus transforming protein E6 (171). The MYCN transcription factor, amplified in neuroblastoma, upregulates Mcm7 expression, which could contribute to hyperproliferation in these cells (286). The same region of Mcm7 also interacts with a heart-specific LIM domain protein, FHL2, which is upregulated in various cancers (31). Given the range of new roles in which MCMs are now implicated, these interactions may not be limited to effects on replication.
MODIFICATION OF MCM: PHOSPHORYLATION, UBIQUITYLATION, AND ACETYLATION
MCMs are known to be modified by a variety of covalent attachments including phosphorylation, acetylation, and ubiquitylation. It is likely that additional modifications and the responsible enzymes will be identified in the future, providing additional levels of regulation. These modifications may provide the mechanisms for cells to distinguish between different pools of MCMs in the nucleus and to activate distinct functions of these proteins in vivo.
Phosphorylation
As described above, the function of at least two kinases is required for S-phase initiation: DDK, which phosphorylates primarily Mcm2 but also other MCM subunits, and CDK, which phosphorylates at least Mcm4 and perhaps other subunits. Other kinases may also be involved. There is evidence for phosphorylation of Mcm4 and other MCMs that is not CDK or DDK dependent (250). Additionally, a recent study suggests that Mcm4 may be a target of the ATR-Chk2 checkpoint kinase pathway in response to replication arrest caused by HU (133). Thus, the MCM proteins are targets of both positive and negative phosphorylation events.
The identity of the phosphatase(s) that dephosphorylates MCMs remains a mystery. We can infer that there is one, because Mcm4 phosphorylation is associated with its inactivation (see, e.g., references 46, and 108), and there is no evidence that the abundant MCMs turn over significantly during the cell cycle. The only evidence for a phosphatase associated with MCM function is the observation that protein phosphatase 2A is required for binding of Cdc45 to the pre-RC (38). Since this is a positive effect, it suggests that there is an inhibitory kinase. However, the identity and substrate(s) of this kinase are unknown.
Ubiquitylation
Ubiquitin is a small peptide that is covalently linked to lysine residues in the target proteins (reviewed in references 277 and 326). Chains of ubiquitin target proteins for degradation by the proteasome. More recent studies have indicated that ubiquitin and the related peptides SUMO and NEDD8 can also modify proteins to regulate them and can affect localization or protein association in addition to protein stability (reviewed in references 222, 277, and 352). Although there is not yet evidence for sumoylation or neddylation, it is likely that the MCMs will be substrates for a broad range of related modifications.
Genetic experiments with budding yeast isolated an allele of UBA1, a ubiquitin-conjugating enzyme, as a suppressor of an mcm3 mutant (34). This suggested that Mcm3 may be negatively regulated by ubiquitylation. Subsequent studies reveal that a fraction of wild-type S. cerevisiae Mcm3 is polyubiquitylated in vivo, although the consequences of this are not yet clear. Human Mcm7 is polyubiquitylated by the ubiquitin ligase E6-AP, which acts with the papillomavirus HPV-18E6 protein to form a virus-specific ubiquitin ligase (170). Data suggest that this targets Mcm7 for turnover by the proteosome. A homotypic binding site for the ligase was identified between residues 633 and 654 of Mcm7, defining an “L2G box,” (S/T)xxxLLG. In vivo studies show that Mcm7 is also polyubiquitylated in the absence of the E6-AP protein, suggesting that it is a substrate for ubiquitylation even in noninfected cells.
While the steady-state level of bulk MCMs remains fairly constant, as described above, it is possible that a fraction of MCMs are subject to regulated turnover. This could provide one mechanism to define functional pools within this abundant protein family.
Acetylation
Acetylation is now appreciated as a significant modification for many cellular proteins and is not limited to histones (reviewed in reference 165). As described above, the histone aceyltransferase PCAF/Gcn5 is reported to stimulate replication from a viral origin by contributing to the acetylation of polyomavirus T antigen, a viral helicase similar to MCMs (342). This suggests that the interaction of MCMs with other HATs such as Hbo1 may result in MCM acetylation. Therefore, the target of the HATs to which MCMs bind may not be histones but may be MCMs themselves (see “Chromatin remodeling” above).
Mcm3 protein is acetylated in mammalian cells by a protein called MCM3AP (308), which was originally isolated in a two-hybrid screen for Mcm3 interactors (309). The acetylated Mcm3 is associated with the chromatin and is not observed in cells arrested in G2/M, which suggests that acetylation is involved in regulating Mcm3 specifically during G1/S, when it is chromatin bound. Curiously, MCM3AP inhibits DNA replication in a cell-free system, suggesting that it is a negative regulator (307, 308); however, it clearly functions positively by promoting Mcm3 nuclear localization and chromatin binding (307, 309). This paradox has yet to be resolved. MCM3AP is distantly related to the PCAF/Gcn5 family of HATs (308), although it does not have a close homologue in the yeasts. It is a splice variant of a much larger protein called GANP that is found in B cells (1). GANP is associated with DNA primase activity, leading to the suggestion that it is specifically involved in the regulation of B-cell proliferation (174, 175).
CONCLUSIONS
The MCM proteins have traveled a long way from their identification nearly 20 years ago in a yeast screen for chromosome loss mutants. While for many years their role in replication initiation was thought to be their only function, it never explained the MCM paradox, i.e., their abundance and wide distribution in regions outside of replicating DNA. Thus it is gratifying that current studies implicate these factors in a range of additional chromosome transactions including transcription and chromatin remodeling. Insights derived from recent structural studies, combined with an expanding repertoire of MCM-interacting proteins, promise further advances in our understanding of this ubiquitous hexamer. However, puzzles and paradoxes remain. We still do not understand the relationship between the core helicase, which has been enzymatically characterized in vitro, and the larger heterohexamer, which is the predominant in vivo form. We have little insight into the way in which different pools of MCMs are distinguished inside the cell, whether by expression, location, modification, or activity. Finally, it is still unclear why these proteins need to be so abundant. While molecular mechanisms are still elusive, we can safely conclude that MCMs, through a variety of activities, are fundamental contributors to the integrity of the eukaryotic genome.
ADDENDUM IN PROOF
A distant MCM relative has recently been identified in Drosophila (H. Matsubayashi and M.-T. Yamamoto, Genes Genet. Syst. 78:363-371, 2003). However, unlike Mcm2-8, it is missing the highly conserved IDEFDKM motif and most other canonical sequences.
Acknowledgments
I am indebted to generous colleagues, including Robert Abraham, Steve Bell, Grant Brown, Brian Calvi, Mel DePamphilis, Tony Hunter, Yukio Ishimi, Wei Jiang, Tom Kelly, Hisao Masai, Hisao Masukata, Mike O'Donnell, Tony Schwacha, Robert Sclafani, Bruce Stillman, Bik Tye, and Teresa Wang, who shared new data, reprints, and helpful discussions. I also thank Julie Bailis, Eliana Gómez, and an anonymous reviewer for careful reading of the manuscript and many helpful comments.
Work in my laboratory is supported by grants from the American Cancer Society (RSG-00-132-04-CCG) and the National Institutes of Health (R01 GM59321).
REFERENCES
- 1.Abe, E., K. Kuwahara, M. Yoshida, M. Suzuki, H. Terasaki, Y. Matsuo, E. I. Takahashi, and N. Sakaguchi. 2000. Structure, expression, and chromosomal localization of the human gene encoding a germinal center-associated nuclear protein (GANP) that associates with MCM3 involved in the initiation of DNA replication. Gene 255:219-227. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, Y., J. Usukura, and M. Yanagida. 1997. A globular complex formation by Nda1 and the other five members of the MCM protein family in fission yeast. Genes Cells 2:467-479. [DOI] [PubMed] [Google Scholar]
- 3.Alison, M. R., T. Hunt, and S. J. Forbes. 2002. Minichromosome maintenance (MCM) proteins may be pre-cancer markers. Gut 50:290-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aparicio, O. M., A. M. Stout, and S. P. Bell. 1999. Differential assembly of CDC45p and DNA polymerases at early and late origins of DNA replication. Proc. Natl. Acad. Sci. USA 96:9130-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aparicio, O. M., D. M. Weinstein, and S. P. Bell. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: Redistribution of MCM proteins and Cdc45p during S phase. Cell 91:59-69. [DOI] [PubMed] [Google Scholar]
- 6.Araki, H., S. H. Leem, A. Phongdara, and A. Sugino. 1995. Dpb11, which interacts with DNA polymerase Ii(Epsilon) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc. Natl. Acad. Sci. USA 92:11791-11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arata, Y., M. Fujita, K. Ohtani, S. Kijima, and J. Y. Kato. 2000. Cdk2-dependent and -independent pathways in E2F-mediated S phase induction. J. Biol. Chem. 275:6337-6345. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod, A., and J. Rine. 1991. A role for CDC7 in repression of transcription at the silent mating-type locus HMR in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:1080-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailis, J. M., P. Bernard, R. Antonelli, R. Allshire, and S. L. Forsburg. 2003. Hsk1/Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat. Cell Biol. 5:1111-1116. [DOI] [PubMed] [Google Scholar]
- 10.Bailis, J. M., and S. L. Forsburg. 2003. It's all in the timing: linking S phase to chromatin structure and chromosome dynamics. Cell Cycle 2:303-306. [PubMed] [Google Scholar]
- 11.Baum, B., H. Nishitani, S. Yanow, and P. Nurse. 1998. Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J. 17:5689-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell, S. P. 2002. The origin recognition complex: from simple origins to complex functions. Genes Dev. 16:659-672. [DOI] [PubMed] [Google Scholar]
- 13.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 14.Bielinsky, A.-K., and S. A. Gerbi. 2001. Where it all starts: eukaryotic origins of DNA replication. J. Cell Sci. 114:643-651. [DOI] [PubMed] [Google Scholar]
- 15.Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qiu, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esal is required for DNA double-strand break repair. Nature 419:411-415. [DOI] [PubMed] [Google Scholar]
- 16.Blow, J. J. 2001. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 20:3293-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blow, J. J., and R. A. Laskey. 1988. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332:546-548. [DOI] [PubMed] [Google Scholar]
- 18.Blow, J. J., and P. Nurse. 1990. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell 62:855-862. [DOI] [PubMed] [Google Scholar]
- 19.Boddy, M. N., and P. Russell. 2001. DNA replication checkpoint. Curr. Biol. 11:R953-R956. [DOI] [PubMed] [Google Scholar]
- 20.Bodmer-Glavas, M., K. Edler, and A. Barberis. 2001. RNA polymerase II and III transcription factors can stimulate DNA replication by modifying origin chromatin structures. Nucleic Acids Res. 29:4570-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booher, R., and D. Beach. 1987. Interaction between cdc13+ and cdc2+ in the control of mitosis in fission yeast: dissociation of the G1 and G2 roles of the cdc2+ protein kinase. EMBO J. 6:3441-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broek, D., R. Bartlett, K. Crawford, and P. Nurse. 1991. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature 349:388-393. [DOI] [PubMed] [Google Scholar]
- 23.Brown, G. W., P. V. Jallepalli, B. J. Huneycutt, and T. J. Kelly. 1997. Interaction of the S phase regulator Cdc18 with cyclin-dependent kinase in fission yeast. Proc. Natl. Acad. Sci. USA 94:6142-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown, G. W., and T. J. Kelly. 1999. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. USA 96:8443-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown, G. W., and T. J. Kelly. 1998. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J. Biol. Chem. 273:22083-22090. [DOI] [PubMed] [Google Scholar]
- 26.Bruschi, C. V., J. N. McMillan, M. Coglievina, and M. S. Esposito. 1995. The genomic instability of yeast cdc6-1/cdc6-1 mutants involves chromosome structure and recombination. Mol. Gen. Genet. 249:8-18. [DOI] [PubMed] [Google Scholar]
- 27.Burke, T. W., J. Cooks-Gowen, M. Asano, and J. R. Nevins. 2001. Replication factors MCM2 and ORC1 interact with the histone acetyltransferase HBOI. J. Biol. Chem. 276:15397-15408. [DOI] [PubMed] [Google Scholar]
- 28.Burkhart, R., D. Schulte, B. Hu, C. Musahl, F. Gohring, and R. Knippers. 1995. Interations of human nuclear proteins P1Mcm3 and P1Cdc46. Eur. J. Biochem. 228:431-438. [PubMed] [Google Scholar]
- 29.Carr, A. M. 2002. DNA structure dependent checkpoints as regulators of DNA repair. DNA Repair 1:983-994. [DOI] [PubMed] [Google Scholar]
- 30.Cha, R. S., and N. Kleckner. 2002. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297:602-606. [DOI] [PubMed] [Google Scholar]
- 31.Chan, K. K., S. K. Tsui, S. M. Ngai, S. M. Lee, M. Kotaka, M. M. Waye, C. Y. Lee, and K. P. Fung. 2000. Protein-protein interaction of FHL2, a LIM domain protein preferentially expressed in human heart, with hCDC47. J. Cell. Biochem. 76:499-508. [PubMed] [Google Scholar]
- 32.Chen, H., M. Tini, and R. M. Evans. 2001. HATs on and beyond chromatin. Curr. Opin. Cell Biol. 13:218-224. [DOI] [PubMed] [Google Scholar]
- 33.Chen, Y. R., K. M. Hennessy, D. Botstein, and B.-K. Tye. 1992. Cdc46/MCM5, a yeast protein whose subcellular localization is cell cycle-regulated, is involved in DNA replication at autonomously replicating sequences. Proc. Natl. Acad. Sci. USA 89:10459-10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng, I. H., L. A. Roberts, and B. K. Tye. 2002. Mcm3 is polyubiquinated during mitosis before establishment of the pre-replication complex. J. Biol. Chem. 277:41706-41714. [DOI] [PubMed] [Google Scholar]
- 35.Cheng, L. A., T. Collyer, and C. F. J. Hardy. 1999. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol. Cell. Biol. 19:4270-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong, J. P. J., M. K. Hayashi, M. N. Simon, R.-M. Xu, and B. Stillman. 2000. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 97:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong, J. P. J., H. M. Mahbubani, C.-Y. Khoo, and J. J. Blow. 1995. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature 375:418-421. [DOI] [PubMed] [Google Scholar]
- 38.Chou, D. M., P. Petersen, J. C. Walter, and G. Walter. 2002. Protein phosphatase 2A regulates binding of Cdc45 to the prereplication complex. J. Biol. Chem. 277:40520-40527. [DOI] [PubMed] [Google Scholar]
- 39.Christensen, T. W., and B. K. Tye. 2003. Drosophila MCM10 interacts with members of the prereplication complex and is required for proper chromosome condensation. Mol. Biol. Cell 14:2206-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claycomb, J. M., D. M. MacAlpine, J. G. Evans, S. P. Bell, and T. L. Orr-Weaver. 2002. Visualization of replication initiation and elongation in Drosophila. J. Cell Biol. 159:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobb, J. A., L. Bjergbaek, K. Shimada, C. Frei, and S. M. Gasser. 2003. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 22:4325-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocker, J. H., S. Piatti, C. Santocanale, K. Nasmyth, and J. F. X. Diffley. 1996. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature 379:180-182. [DOI] [PubMed] [Google Scholar]
- 43.Cook, C. R., G. Kung, F. C. Peterson, B. F. Volkman, and M. Lei. 2003. A novel zinc finger is required for Mcm10 homocomplex assembly. J. Biol. Chem. 278:36051-36058. [DOI] [PubMed] [Google Scholar]
- 44.Costanzo, V., D. Shechter, P. J. Lupardus, K. A. Cimprich, M. Gottesman, and J. Gautier. 2003. An ATR- and Cdc7 dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11:203-213. [DOI] [PubMed] [Google Scholar]
- 45.Coue, M., F. Amariglio, D. Maiorano, S. Bocquet, and M. Mechali. 1998. Evidence for different MCM subcomplexes with differential binding to chromatin in Xenopus. Exp. Cell Res. 245:282-289. [DOI] [PubMed] [Google Scholar]
- 46.Coue, M., S. E. Kearsey, and M. Mechali. 1996. Chromatin binding, nuclear localization and phosphorylation of Xenopus cdc21 are cell-cycle dependent and associated with the control of initiation of DNA replication. EMBO J. 15:1085-1097. [PMC free article] [PubMed] [Google Scholar]
- 47.Coxon, A., K. Maundrell, and S. E. Kearsey. 1992. Fission yeast cdc21+ belongs to a family of proteins involved in an early step of chromosome replication. Nucleic Acids Res. 20:5571-5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crevel, G., A. Ivetic, K. Ohno, M. Yamaguchi, and S. Cotterill. 2001. Nearest neighbour analysis of MCM protein complexes in Drosophila melanogaster. Nucleic Acids Res. 29:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culotti, J., and L. H. Hartwell. 1971. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp. Cell Res. 67:389-401. [DOI] [PubMed] [Google Scholar]
- 50.DaFonseca, C. J., F. Shu, and J. J. Zhang. 2001. Identification of two residues in MCM5 critical for the assembly of MCM complexes and Stat1-mediated transcription activation in response to IFN-γ. Proc. Natl. Acad. Sci. USA 98:3034-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahm, K., and U. Hubscher. 2002. Colocalization of human Rad17 and PCNA in late S phase of the cell cycle upon replication block. Oncogene 21:7710-7719. [DOI] [PubMed] [Google Scholar]
- 52.Dahmann, C., J. F. X. Diffley, and K. A. Nasmyth. 1995. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr. Biol. 5:1257-1269. [DOI] [PubMed] [Google Scholar]
- 53.Dalton, S., and B. Hopwood. 1997. Characterization of Cdc47p-minichromosome maintenance complexes in Saccharomyces cerevisiae: identification of Cdc45p as a subunit. Mol. Cell. Biol. 17:5867-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalton, S., and L. Whitbread. 1995. Cell cycle-regulated nuclear import and export of CDC47, a protein essential for initiation of DNA replication in budding yeast. Proc. Natl. Acad. Sci. USA 92:2514-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davey, M. J., C. Indiani, and M. O'Donnell. 2002. Reconstitution of the Mcm2-7p heterohexamer, subunit arrangement and ATP site architecture. J. Biol. Chem. 278:4491-4499. [DOI] [PubMed] [Google Scholar]
- 56.Davey, M. J., D. Jeruzalmi, J. Kuriyan, and M. O'Donnell. 2002. Motors and switches: AAA+ machines within the replisome. Nat. Rev. Mol. Cell Biol. 3:826-835. [DOI] [PubMed] [Google Scholar]
- 57.DeRyckere, D., C. L. Smith, and G. S. Martin. 1999. The role of nucleotide binding and hydrolysis in the function of the fission yeast cdc18+ gene product. Genetics 151:1445-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diffley, J. F., and K. Labib. 2002. The chromosome replication cycle. J. Cell Sci. 115:869-872. [DOI] [PubMed] [Google Scholar]
- 59.Dillin, A., and J. Rine. 1998. Roles for ORC in M phase and S phase. Science 279:1733-1777. [DOI] [PubMed] [Google Scholar]
- 60.Dillin, A., and J. Rine. 1997. Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics 147:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimitrova, D. S., I. T. Todorov, T. Melendy, and D. M. Gilbert. 1999. Mcm2, but not RPA, is a component of the mammalian early G-1 phase prereplication complex. J. Cell Biol. 146:709-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirick, L., T. Bohm, and K. Nasmyth. 1995. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14:4803-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reference deleted.
- 64.Donaldson, A. D., W. L. Fangman, and B. J. Brewer. 1998. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 12:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donovan, S., J. Harwood, L. S. Drury, and J. F. X. Diffley. 1997. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl. Acad. Sci. USA 94:5611-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowell, S. J., P. Romanowski, and J. F. X. Diffley. 1994. Interaction of DBF4, the CDC7 protein-kinase regulatory subunit, with yeast replication origins in vivo. Science 265:1243-1246. [DOI] [PubMed] [Google Scholar]
- 67.Drury, L. S., G. Perkins, and J. F. X. Diffley. 2000. The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Curr. Biol. 10:231-240. [DOI] [PubMed] [Google Scholar]
- 68.Duncker, B. P., P. Pasero, D. Braguglia, P. Heun, M. Weinreich, and S. M. Gasser. 1999. Cyclin B-Cdk1 kinase stimulates ORC- and Cdc6-independent steps of semiconservative plasmid replication in yeast nuclear extracts. Mol. Cell. Biol. 19:1226-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dutta, A., and B. Stillman. 1992. cdc2 family kinases phosphorylate a human cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 11:2189-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dziak, R., D. Leishman, M. Radovic, B. K. Tye, and K. Yankulov. 2003. Evidence for a role of MCM (mini-chromosome maintenance)5 in transcriptional repression of sub-telomeric and Ty-proximal genes in Saccharomyces cerevisiae. J. Biol. Chem. 278:27372-27381. [DOI] [PubMed] [Google Scholar]
- 71.Edwards, M. C., A. V. Tutter, C. Cvetic, C. H. Gilbet, T. A. Prokhorova, and J. C. Walter. 2002. MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in xenopus egg extracts. J. Biol. Chem. 277:33049-33057. [DOI] [PubMed] [Google Scholar]
- 72.Elsasser, S., Y. Chi, P. Yang, and J. L. Campbell. 1999. Phosphorylation controls timing of Cdc6p destruction: a biochemical analysis. Mol. Biol. Cell 10:3263-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elsasser, S., F. Lou, B. Wang, J. L. Campbell, and A. Jong. 1996. Interaction between yeast Cdc6 protein and B-type cyclin/Cdc28 kinases. Mol. Biol. Cell 7:1723-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang, F., and J. W. Newport. 1991. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell 66:731-742. [DOI] [PubMed] [Google Scholar]
- 75.Feger, G. 1999. Identification and complete cDNA sequence of the missing Drosophila MCMs: DmMCM3, DmMCM6 and DmMCM7. Gene 227:149-155. [DOI] [PubMed] [Google Scholar]
- 76.Feger, G., H. Vaessin, T. T. Su, E. Wolff, L. Y. Jan, and Y. N. Jan. 1995. dpa, a member of the MCM family, is required for mitotic DNA replication but not endoreplication in Drosophila. EMBO J. 14:5387-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fenech, M., A. M. Carr, J. Murray, F. Z. Watts, and A. R. Lehmann. 1991. Cloning and characterization of the rad4 gene of Schizosaccharomyces pombe: a gene showing short regions of sequence similarity to the human XRCC1 gene. Nucleic Acids Res. 19:6737-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferreira, M. F., C. Santocanale, L. S. Drury, and J. F. Diffley. 2000. Dbf4p, an essential S-phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol. Cell. Biol. 20:242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Findeisen, M., M. El-Denary, T. Kapitza, R. Graf, and U. Strausfeld. 1999. Cyclin A-dependent kinase activity affects chromatin binding of ORC, Cdc6, and MCM in egg extracts of Xenopus lacvis. Eur. J. Biochem. 264:415-426. [DOI] [PubMed] [Google Scholar]
- 80.Fitch, M. J., J. J. Donato, and B. K. Tye. 2003. Mcm7, a subunit of the presumptive MCM helicase, modulates its own expression in conjunction with Mcm1. J. Biol. Chem. 278:25408-25416. [DOI] [PubMed] [Google Scholar]
- 81.Fletcher, R. J., B. E. Bishop, R. P. Leon, R. A. Sclafani, C. M. Ogata, and X. S. Chen. 2003. The structure and function of MCM from archaeal M. thermoautotrophicum. Nat. Struct. Biol. 10:160-167. [DOI] [PubMed] [Google Scholar]
- 82.Forsburg, S. L., and P. Nurse. 1994. The fission yeast cdc19+ gene encodes a member of the MCM family of replication proteins. J. Cell Sci. 107:2779-2788. [DOI] [PubMed] [Google Scholar]
- 83.Forsburg, S. L., D. A. Sherman, S. Ottilie, J. R. Yasuda, and J. A. Hodson. 1997. Mutational analysis of Cdc19p, a Schizosaccharomyces pombe MCM protein. Genetics 147:1025-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox, C. A., S. Loo, A. Dillin, and J. Rine. 1995. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev 9:911-924. [DOI] [PubMed] [Google Scholar]
- 85.Freeman, A., L. S. Morris, A. D. Mills, K. Stoeber, R. A. Laskey, G. H. Williams, and N. Coleman. 1999. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin. Cancer Res. 5:2121-2132. [PubMed] [Google Scholar]
- 86.Fujita, M., T. Kiyono, Y. Hayashi, and M. Ishibashi. 1996. Hcdc47, a human member of the MCM family—dissociation of the nucleus-bound form during S phase. J. Biol. Chem. 271:4349-4354. [DOI] [PubMed] [Google Scholar]
- 87.Fujita, M., T. Kiyono, Y. Hayashi, and M. Ishibashi. 1997. In vivo interaction of human MCM heterohexameric complexes with chromatin. Possible involvement of ATP. J. Biol. Chem. 272:10928-10935. [DOI] [PubMed] [Google Scholar]
- 88.Fujita, M., C. Yamada, H. Goto, and N. Yokoyama. 1999. Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human mcm complex, and CDC2 kinase-mediated hyperphosphorylation. J. Biol. Chem. 274:25927-25932. [DOI] [PubMed] [Google Scholar]
- 89.Fujita, M., C. Yamada, T. Tsurumi, F. Hanaoka, K. Matsuzawa, and M. Inagaki. 1998. Cell cycle- and chromatin binding state-dependent phosphorylation of human MCM heterohexameric complexes—a role for cdc2 kinase. J. Biol. Chem. 273:17095-17101. [DOI] [PubMed] [Google Scholar]
- 90.Fung, A., Ou, Bueler, and G. W. Brown. 2002. A conserved domain of S. pombe dfp1+ is uniquely required for chromosome stability following alkylation damage during S phase. Mol. Cell. Biol. 22:4477-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gauthier, L., R. Dziak, D. J. Kramer, D. Leishman, X. Song, J. Ho, M. Radovic, D. Bentley, and K. Yankulov. 2002. The role of the carboxyterminal domain of RNA polymerase II in regulating origins of DNA replication in Saccharomyces cerevisiae. Genetics 162:1117-11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geraghty, D. S., M. Ding, N. H. Heintz, and D. S. Pederson. 2000. Premature structural changes at replication origins in a yeast minichromosome maintenance (MCM) mutant. J. Biol. Chem. 275:18011-18021. [DOI] [PubMed] [Google Scholar]
- 93.Gibson, S. I., R. T. Surosky, and B.-K. Tye. 1990. The phenotype of the minichromosome maintenance mutant mcm3 is characteristic of mutants defective in DNA replication. Mol. Cell. Biol. 10:5707-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilbert, D. M. 2001. Making sense of eukaryotic DNA replication origins. Science 294:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gladden, A. B., and J. A. Diehl. 2003. The cyclin D1-dependent kinase associates with the pre-replication complex and modulates RB. MCM7 binding. J. Biol. Chem. 278:9754-9760. [DOI] [PubMed] [Google Scholar]
- 96.Goffeau, A., B. G. Barrell, H. Bussey, R. W. Davis, B. Dujon, H. Feldmann, F. Galibert, J. D. Hoheisel, C. Jacq, M. Johnston, E. J. Louis, H. W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin, and S. G. Oliver. 1996. Life with 6000 genes. Science 274:546-567. [DOI] [PubMed] [Google Scholar]
- 97.Going, J. J., W. N. Keith, L. Neilson, K. Stoeber, R. C. Stuart, and G. H. Williams. 2002. Aberrant expression of minichromosome maintenance proteins 2 and 5, and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett's mucosa. Gut 50:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goll, M. G., and T. H. Bestor. 2002. Histone modification and replacement in chromatin activation. Genes Dev. 16:1739-1742. [DOI] [PubMed] [Google Scholar]
- 99.Gómez, E. G., M. G. Catlett, and S. L. Forsburg. 2002. Different phenotypes in vivo are associated with ATPase motif mutations in Schizosaccharomyces pombe minichromosome maintenance proteins. Genetics 160:1305-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gozuacik, D., M. Chami, D. Lagorce, J. Faivre, Y. Murakami, O. Poch, E. Biermann, R. Knippers, C. Brechot, and P. Paterlini-Brechot. 2003. Identification and functional characterization of a new member of the human Mcm protein family: hMcm8. Nucleic Acids Res. 31:570-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gregan, J., K. Lindner, L. Brimage, R. Franklin, M. Namdar, E. A. Hart, S. J. Aves, and S. Kearsey. 2003. Fission yeast Cdc23/Mcm10 functions after pre-replicative complex formation to promote Cdc45 chromatin binding. Mol. Biol. Cell 14:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardy, C. F. J. 1997. Identification of Cdc45p, an essential factor required for DNA replication. Gene 187:239-246. [DOI] [PubMed] [Google Scholar]
- 103.Hardy, C. F. J., O. Dryga, S. Seematter, P. M. B. Pahl, and R. A. Sclafani. 1997. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc. Natl. Acad. Sci. USA 94:3151-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hart, E. A., J. A. Bryant, K. Moore, and S. J. Aves. 2002. Fission yeast Cdc23 interactions with DNA replication initiation proteins. Curr. Genet. 41:342-348. [DOI] [PubMed] [Google Scholar]
- 105.Hartzog, G. A., J. L. Speer, and D. L. Lindstrom. 2002. Transcript elongation on a nucleoprotein template. Biochim. Biophys. Acta 1577:276-286. [DOI] [PubMed] [Google Scholar]
- 106.Hasan, S., and M. O. Hottiger. 2002. Histone acetyl transferases: a role in DNA repair and DNA replication. J. Mol. Med. 80:463-474. [DOI] [PubMed] [Google Scholar]
- 107.Hashimoto, Y., and H. Takisawa. 2003. Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J. 22:2526-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hendrickson, M., M. Madine, S. Dalton, and J. Gautier. 1996. Phosphorylation of MCM4 by cdc2 protein kinase inhibits the activity of the minichromosome maintenance complex. Proc. Natl. Acad. Sci. USA 93:12223-12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henneke, G., S. Koundrioukoff, and U. Hubscher. 2003. Multiple roles for kinases in DNA replication. EMBO Rep. 4:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hennessy, K. M., C. D. Clark, and D. Botstein. 1990. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 4:2252-2263. [DOI] [PubMed] [Google Scholar]
- 111.Hennessy, K. M., A. Lee, E. Chen, and D. Botstein. 1991. A group of interacting yeast DNA replication genes. Genes Dev. 5:958-969. [DOI] [PubMed] [Google Scholar]
- 112.Herbig, U., C. A. Marlar, and E. Fanning. 1999. The Cdc6 nucleotide-binding site regulates its activity in DNA replication in human cells. Mol. Biol. Cell 10:2631-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hingorani, M. M., and M. O'Donnell. 2000. A tale of toroids in DNA metabolism. Nat. Rev. Mol. Cell Biol. 1:22-30. [DOI] [PubMed] [Google Scholar]
- 114.Hiraiwa, A., M. Fujita, T. Nagasaka, A. Adachi, M. Ohashi, and M. Ishibashi. 1997. Immunolocalization of hCDC47 protein in normal and neoplastic human tissues and its relation to growth. Int. J. Cancer. 74:180-184. [DOI] [PubMed] [Google Scholar]
- 115.Holland, L., L. Gauthier, P. Bell-Rogers, and K. Yankulov. 2002. Distinct parts of minichromosome maintenance protein 2 associate with histone H3/H4 and RNA polymerase II holoenzyme. Eur. J. Biochem. 269:5192-5202. [DOI] [PubMed] [Google Scholar]
- 116.Hollingsworth, R. E., R. M. Ostroff, M. B. Klein, L. A. Niswander, and R. A. Sclafani. 1992. Molecular genetic-studies of the cdc7 protein-kinase and induced mutagenesis in yeast. Genetics 132:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hollingsworth, R. E., and R. A. Sclafani. 1993. Yeast pre-meiotic DNA replication utilizes mitotic origin ARS1 independently of CDC7 function. Chromosoma 102:415-420. [DOI] [PubMed] [Google Scholar]
- 118.Holthoff, H. P., M. Baack, A. Richter, M. Ritzi, and R. Knippers. 1998. Human Protein MCM6 on HeLa Cell Chromatin. J. Biol. Chem. 273:7320-7325. [DOI] [PubMed] [Google Scholar]
- 119.Homesley, L., M. Lei, Y. Kawasaki, S. Sawyer, T. Christensen, and B. K. Tye. 2000. Mcm10 and the MCM2-7 complex interact to initiate DNA synthesis and to release replication factors from origins. Genes Dev. 14:913-926. [PMC free article] [PubMed] [Google Scholar]
- 120.Hopwood, B., and S. Dalton. 1996. Cdc45p assembles into a complex with Cdc46p/Mcm5p, is required for minichromosome maintenance, and is essential for chromosomal DNA replication. Proc. Natl. Acad. Sci. USA 93:12309-12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu, B., R. Burkhart, D. Schulte, C. Musahl, and R. Knippers. 1993. The P1 family—a new class of nuclear mammalian proteins related to the yeast MCM replication proteins. Nucleic Acids Res. 21:5289-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hua, X. H., and J. Newport. 1998. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J. Cell Biol. 140:271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hua, X. H., H. Yan, and J. Newport. 1997. A role for cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 137:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hunt, D. P., A. Freeman, L. S. Morris, N. G. Burnet, K. Bird, T. W. Davies, R. A. Laskey, and N. Coleman. 2002. Early recurrence of benign meningioma correlates with expression of mini-chromosome maintenance-2 protein. Br. J. Neurosurg. 16:10-15. [DOI] [PubMed] [Google Scholar]
- 125.Hyrien, O., K. Marheineke, and A. Goldar. 2003. Paradoxes of eukaryotic DNA replication: MCM proteins and the random completion problem. Bioessays 25:116-125. [DOI] [PubMed] [Google Scholar]
- 126.Iizuka, M., and B. Stillman. 1999. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 274:23027-23034. [DOI] [PubMed] [Google Scholar]
- 127.Ikura, T., V. V. Ogryzko, M. Grigoriev, R. Groisman, J. Wang, M. Horikoshi, R. Scully, J. Qin, and Y. Nakatani. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102:463-473. [DOI] [PubMed] [Google Scholar]
- 128.Ishimi, Y. 1997. A DNA helicase activity is associated with an MCM4, −6, and −7 protein complex. J. Biol. Chem. 272:24508-24513. [DOI] [PubMed] [Google Scholar]
- 129.Ishimi, Y., S. Ichinose, A. Omori, K. Sato, and H. Kimura. 1996. Binding of human minichromosome maintenance proteins with histone H3. J. Biol. Chem. 271:24115-24122. [DOI] [PubMed] [Google Scholar]
- 130.Ishimi, Y., Y. Komamura, Z. You, and H. Kimura. 1998. Biochemical function of mouse minichromosome maintenance 2 protein. J. Biol. Chem. 273:8369-8375. [DOI] [PubMed] [Google Scholar]
- 131.Ishimi, Y., and Y. Komamura-Kohno. 2001. Phosphorylation of Mcm4 at specific sites by cyclin-dependent kinase leads to loss of Mcm4,6,7 helicase activity. J. Biol. Chem. 276:34428-34433. [DOI] [PubMed] [Google Scholar]
- 132.Ishimi, Y., Y. Komamura-Kohno, K. Arai Ki, and H. Masai. 2001. Biochemical activities associated with mouse mcm2 protein. J. Biol. Chem. 276:42744-42752. [DOI] [PubMed] [Google Scholar]
- 133.Ishimi, Y., Y. Komamura-Kohno, H. J. Kwon, K. Yamada, and M. Nakanishi. 2003. Identification of MCM4 as a target of the DNA replication block checkpoint system. J. Biol. Chem. 278:24644-24650. [DOI] [PubMed] [Google Scholar]
- 134.Ishimi, Y., Y. Komamura-Kohno, Z. You, A. Omori, and M. Kitagawa. 2000. Inhibition of Mcm4,6,7 helicase activity by phosphorylation with cyclin A/Cdk2. J. Biol. Chem. 275:16235-16241. [DOI] [PubMed] [Google Scholar]
- 135.Ishimi, Y., L. Okayasu, C. Kato, H. J. Kwon, H. Kimura, K. Yamada, and S. Y. Song. 2003. Enhanced expression of Mcm proteins in cancer cells derived from uterine cervix. Eur. J. Biochem. 270:1089-1101. [DOI] [PubMed] [Google Scholar]
- 136.Izumi, M., K. Yanagi, T. Mizuno, M. Yokoi, Y. Kawasaki, K. Y. Moon, J. Hurwitz, F. Yatagai, and F. Hanaoka. 2000. The human homolog of Saccharomyces cerevisiae Mcm10 interacts with replication factors and dissociates from nuclease-resistant nuclear structures in G(2) phase. Nucleic Acids Res. 28:4769-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jackson, A. L., P. Pahl, K. Harrison, J. Rosamond, and R. A. Sclafani. 1993. Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol. Cell. Biol. 13:2899-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jallepalli, P. V., and T. J. Kelly. 1996. Rum1 and Cdc18 link inhibition of cyclin-dependent kinase to the initiation of DNA replication in Schizosaccharomyces pombe. Genes Dev. 10:541-552. [DOI] [PubMed] [Google Scholar]
- 139.Jallepalli, P. V., D. Tien, and T. J. Kelly. 1998. sud1+ targets cyclin-dependent kinase-phosphorylated Cdc18 and Rum1 proteins for degradation and stops unwanted diploidization in fission yeast. Proc. Natl. Acad. Sci. USA 95:8159-8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jares, P., and J. J. Blow. 2000. Xenopus Cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev. 14:1528-1540. [PMC free article] [PubMed] [Google Scholar]
- 141.Jares, P., A. Donaldson, and J. J. Blow. 2000. The Cdc7/Dbf4 protein kinase: target of the S phase checkpoint? EMBO Rep. 1:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 143.Jiang, W., D. McDonald, T. J. Hope, and T. Hunter. 1999. Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J. 18:5703-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson, E. M., Y. Kinoshita, and D. C. Daniel. 2003. A new member of the MCM protein family encoded by the human MCM8 gene, located contrapodal to GCD10 at chromosome band 20p12.3-13. Nucleic Acids Res. 31:2915-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnston, L. H., H. Masai, and A. Sugino. 1999. First the CDKs, now the DDKs. Trends Cell Biol. 9:249-252. [DOI] [PubMed] [Google Scholar]
- 146.Kamimura, Y., H. Masumoto, A. Sugino, and H. Araki. 1998. Sld2, which interacts with Dpb11 in Saccharomyces cerevisiae, is required for chromosomal DNA replication. Mol. Cell. Biol. 18:6102-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kamimura, Y., Y.-S. Tak, A. Sugino, and H. Araki. 2001. Sld3, which interacts with Cdc45 (Sld4) functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J. 20:2097-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanemaki, M., A. Sanchez-Diaz, A. Gambus, and K. Labib. 2003. Functional proteomic identification of DNA replication proteins by induced proteolysis in vivo. Nature 423:720-724. [DOI] [PubMed] [Google Scholar]
- 149.Kaplan, D. L., M. J. Davey, and M. O'Donnell. 2003. Mcm4,6,7 uses a “pump in ring” mechanism to unwind DNA by steric exclusion and actively translocate along a duplex. J. Biol. Chem. 278:49171-49182 [DOI] [PubMed] [Google Scholar]
- 150.Kaplan, D. L., and M. O'Donnell. 2002. DnaB drives DNA branch migration and dislodges proteins while encircling two DNA strands. Mol. Cell 10:647-657. [DOI] [PubMed] [Google Scholar]
- 151.Kawasaki, Y., S.-I. Hiraga, and A. Sugino. 2000. Interactions between Mcm10p and other replication factors are required for proper initiation and elongation of chromosomal DNA replication in Saccharomyces cerevisiae. Genes Cells 5:975-989. [DOI] [PubMed] [Google Scholar]
- 152.Kearsey, S. E., and K. Labib. 1998. MCM proteins: evolution, properties, and role in DNA replication. Biochim. Biophys. Acta 1398:113-136. [DOI] [PubMed] [Google Scholar]
- 153.Kearsey, S. E., S. Montgomery, K. Labib, and K. Linder. 2000. Chromatin binding of the fission yeast replication factor Mcm4 occurs during anaphase and requires ORC and Cdc18. EMBO J. 19:1681-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kelly, T. J., and G. W. Brown. 2000. Regulation of chromosome replication. Annu. Rev. Biochem. 69:829-880. [DOI] [PubMed] [Google Scholar]
- 155.Kelly, T. J., G. S. Martin, S. L. Forsburg, R. J. Stephen, A. Russo, and P. Nurse. 1993. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell 74:371-382. [DOI] [PubMed] [Google Scholar]
- 156.Kelman, Z., and J. Hurwitz. 2003. Structural lessons in DNA replication from the third domain of life. Nat. Struct. Biol. 10:148-150. [DOI] [PubMed] [Google Scholar]
- 157.Kelman, Z., J.-K. Lee, and J. Hurwitz. 1999. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum H contains DNA helicase activity. Proc. Natl. Acad. Sci. USA 96:14783-14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kimura, A., T. Umehara, and M. Horikoshi. 2002. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat. Genet. 32:370-377. [DOI] [PubMed] [Google Scholar]
- 159.Kimura, H., N. Nozaki, and K. Sugimoto. 1994. DNA polymerase alpha associated protein P1, a murine homolog of yeast MCM3, changes its intranuclear distribution during the DNA synthetic period. EMBO J. 13:4311-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kimura, H., T. Ohtomo, M. Yamaguchi, A. Ishii, and K. Sugimoto. 1996. Mouse MCM proteins: complex formation and transportation into the nucleus. Genes Cells 1:977-993. [DOI] [PubMed] [Google Scholar]
- 161.Kimura, H., N. Takizawa, N. Nozaki, and K. Sugimoto. 1995. Molecular cloning of cDNA encoding mouse Cdc21 and CDC46 homologs and characterization of the products: physical interaction between P1(MCM3) and CDC46 proteins. Nucleic Acids Res. 23:2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kneissl, M., V. Putter, A. A. Szalay, and F. Grummt. 2003. Interaction and assembly of murine pre-replicative complex proteins in yeast and mouse cells. J. Mol. Biol. 327:111-128. [DOI] [PubMed] [Google Scholar]
- 163.Kominami, K., and T. Toda. 1997. Fission yeast WD-repeat protein Popl regulates genome ploidy through ubiquitin-proteasome-mediated degradation of the CDK inhibitor Rum1 and the S-phase initiator Cdc18. Genes Dev. 11:1548-1560. [DOI] [PubMed] [Google Scholar]
- 164.Koonin, E. V. 1993. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucl. Acids Res. 21:2541-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kouzarides, T. 2000. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Krude, T., C. Musahl, R. A. Laskey, and R. Knippers. 1996. Human replication proteins hcdc21, hcdc46 and P1mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J. Cell Sci. 109:309-318. [DOI] [PubMed] [Google Scholar]
- 167.Kubota, Y., S. Mimura, S. Nishimoto, T. Masuda, H. Nojima, and H. Takisawa. 1997. Licensing of DNA replication by a multi-protein complex of MCM/P1 proteins in Xenopus eggs. EMBO J. 16:3320-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kubota, Y., S. Mimura, S.-I. Nishimoto, H. Takisawa, and H. Nojima. 1995. Identification of the yeast MCM3 related protein as a component of Xenopus DNA replication licensing factor. Cell 81:601-609. [DOI] [PubMed] [Google Scholar]
- 169.Kubota, Y., Y. Takase, Y. Komori, Y. Hashimoto, T. Arata, Y. Kamimura, H. Araki, and H. Takisawa. 2003. A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev. 17:1141-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuhne, C., and L. Banks. 1998. E3-Ubiquitin Ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G Box. J. Biol. Chem. 273:34302-34309. [DOI] [PubMed] [Google Scholar]
- 171.Kukimoto, I., S. Aihara, K. Yoshiike, and T. Kanda. 1998. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem. Biophys. Res. Commun. 249:258-262. [DOI] [PubMed] [Google Scholar]
- 172.Kukimoto, I., H. Igaki, and T. Kanda. 1999. Human CDC45 protein binds to minichromosome maintenance 7 protein and the p70 subunit of DNA polymerase alpha. Eur. J. Biochem. 265:936-943. [DOI] [PubMed] [Google Scholar]
- 173.Kumagai, H., N. Sato, M. Yamada, D. Mahony, W. Seghezzi, E. Lees, K. I. Arai, and H. Masai. 1999. A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells. Mol. Cell. Biol. 19:5083-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuwahara, K., S. Tomiyasu, S. Fujimura, K. Nomura, Y. Xing, N. Nishiyama, M. Ogawa, S. Imajoh-Ohmi, S. Izuta, and N. Sakaguchi. 2001. Germinal center-associated nuclear protein (GANP) has a phosphorylation-dependent DNA-primase activity that is up-regulated in germinal center regions. Proc. Natl. Acad. Sci. USA 98:10279-10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuwahara, K., M. Yoshida, E. Kondo, A. Sakata, Y. Watanabe, E. Abe, Y. Kouno, S. Tomiyasu, S. Fujimura, T. Tokuhisa, H. Kimura, T. Ezaki, and N. Sakaguchi. 2000. A novel nuclear phosphoprotein, GANP, is up-regulated in centrocytes of the germinal center and associated with MCM3, a protein essential for DNA replication. Blood 95:2321-2328. [PubMed] [Google Scholar]
- 176.Labib, K., J. F. X. Diffley, and S. E. Kearsey. 1999. G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus. Nat. Cell Biol. 1:415-422. [DOI] [PubMed] [Google Scholar]
- 177.Labib, K., S. E. Kearsey, and J. F. Diffley. 2001. MCM2-7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint. Mol. Biol. Cell 12:3658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Labib, K., J. A. Tercero, and J. F. X. Diffley. 2000. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 288:1643-1647. [DOI] [PubMed] [Google Scholar]
- 179.Laskey, R. A., and M. A. Madine. 2003. A rotary pumping model for helicase function of MCM proteins at a distance from replication forks. EMBO Rep. 4:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee, D. G., and S. P. Bell. 2000. ATPase switches controlling DNA replication initiation. Curr. Opin. Cell Biol. 12:280-285. [DOI] [PubMed] [Google Scholar]
- 181.Lee, J.-K., and J. Hurwitz. 2000. Isolation and characterization of various complexes of the minichromosome maintenance proteins of Schizosaccharomyces pombe. J. Biol. Chem. 275:18871-18878. [DOI] [PubMed] [Google Scholar]
- 182.Lee, J.-K., and J. Hurwitz. 2001. Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, 7 complex requires forked DNA structures. Proc. Natl. Acad. Sci. USA 98:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee, J.-K., Y.-S. Seo, and J. Hurwitz. 2003. The Cdc23 (Mcm10) protein is required for the phosphorylation of minichromosome maintenance comlex by the Dfp1-Hsk1 kinase. Proc. Natl. Acad. Sci. USA 100:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lei, M., I. H. Cheng, L. A. Roberts, M. A. McAlear, and B. K. Tye. 2002. Two mcm3 mutations affect different steps in the initiation of DNA replication. J. Biol. Chem. 277:30824-30831. [DOI] [PubMed] [Google Scholar]
- 185.Lei, M., Y. Kawasaki, and B. K. Tye. 1996. Physical interactions among MCM proteins and effects of MCM dosage on DNA replication in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:5081-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lei, M., Y. Kawasaki, M. R. Young, M. Kihara, A. Sugino, and B. K. Tye. 1997. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 11:3365-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leipe, D. D., L. Aravind, N. V. Grishin, and E. V. Koonin. 2000. The bacterial replicative helicase DnaB evolved from a RecA duplication. Genome Res. 1:5-16. [PubMed] [Google Scholar]
- 188.Li, D., R. Zhao, W. Lilyestrom, D. Gai, R. Zhang, J. A. DeCaprio, E. Fanning, A. Jochimiak, G. Szakonyi, and X. S. Chen. 2003. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 423:512-518. [DOI] [PubMed] [Google Scholar]
- 189.Li, J., and L. S. Cox. 2001. Identification of an MCM4 homologue expressed specifically in the sexual stage of Plasmodium falciparum. Int. J. Parasitol. 31:1246-1252. [DOI] [PubMed] [Google Scholar]
- 190.Liang, C., and B. Stillman. 1997. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 11:3375-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liang, C., M. Weinrich, and B. Stillman. 1995. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell 81:667-676. [DOI] [PubMed] [Google Scholar]
- 192.Liang, D. T., and S. L. Forsburg. 2001. Characterization of S. pombe mcm7+ and cdc23+ (MCM10) and interactions with replication checkpoints. Genetics 159:471-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liang, D. T., J. A. Hodson, and S. L. Forsburg. 1999. Reduced dosage of a single fission yeast MCM protein causes genetic instability and S phase delay. J. Cell Sci. 112:559-567. [DOI] [PubMed] [Google Scholar]
- 194.Lindner, K., J. Gregan, S. Montgomery, and S. Kearsey. 2002. Essential role of MCM proteins in pre-meiotic DNA replication. Mol. Biol. Cell 13:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lipford, J. R., and S. P. Bell. 2001. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 7:21-30. [DOI] [PubMed] [Google Scholar]
- 196.Liu, J., C. L. Smith, D. DeRyckere, K. DeAngelis, G. S. Martin, and J. M. Berger. 2000. Structure and function of Cdc6/Cdc18: implications for origin recognition and checkpoint control. Mol. Cell 6:637-648. [DOI] [PubMed] [Google Scholar]
- 197.Lopez-Girona, A., O. Mondesert, J. Leatherwood, and P. Russell. 1998. Negative regulation of Cdc18 DNA replication protein by Cdc2. Mol. Biol. Cell 9:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Madine, M. A., C.-Y. Khoo, A. D. Mills, and R. A. Laskey. 1995. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature 375:421-424. [DOI] [PubMed] [Google Scholar]
- 199.Madine, M. A., C. Y. Khoo, A. D. Mills, C. Musahl, and R. A. Laskey. 1995. The nuclear envelope prevents reinitiation of replication by regulating the binding of Mcm3 to chromatin in Xenopus egg extracts. Curr. Biol. 5:1270-1279. [DOI] [PubMed] [Google Scholar]
- 200.Madine, M. A., M. Swietlik, C. Pelizon, P. Romanowski, A. D. Mills, and R. A. Laskey. 2000. The roles of the MCM, ORC, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J. Struct. Biol. 129:198-210. [DOI] [PubMed] [Google Scholar]
- 201.Maine, G. T., P. Sinha, and B.-K. Tye. 1984. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics 106:365-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maiorano, D., G. Blom van Assendelft, and S. E. Kearsey. 1996. Fission yeast cdc21, a member of the MCM protein family, is required for onset of S phase and located in the nucleus throughout the cell cycle. EMBO J. 15:861-872. [PMC free article] [PubMed] [Google Scholar]
- 203.Maiorano, D., J. M. Lemaitre, and M. Mechali. 2000. Stepwise regulated chromatin assembly of MCM2-7 proteins. J. Biol. Chem. 275:8426-8431. [DOI] [PubMed] [Google Scholar]
- 204.Maiorano, D., J. Moreau, and M. Méchali. 2000. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature 404:622-625. [DOI] [PubMed] [Google Scholar]
- 205.Masai, H., E. Matsui, Z. You, Y. Ishimi, K. Tamai, and K.-I. Arai. 2000. Human Cdc7-related kinase complex: in vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a critical threonine residue of Cdc7 by Cdks. J. Biol. Chem. 275:29042-29052. [DOI] [PubMed] [Google Scholar]
- 206.Masuda, T., S. Mimura, and H. Takisawa. 2003. CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45. Genes Cells 8:145-161. [DOI] [PubMed] [Google Scholar]
- 207.Masumoto, H., S. Muramatsu, Y. Kamimura, and H. Araki. 2002. S-Cdk-dependent phosphorylation of Sld2 essential for chromosomal DNA replication in budding yeast. Nature 415:651-655. [DOI] [PubMed] [Google Scholar]
- 208.McFarlane, R. J., A. M. Carr, and C. Price. 1997. Characterisation of the Schizosaccharomyces pombe rad4/cut5 mutant phenotypes: dissection of DNA replication and G2 checkpoint control function. Mol. Gen. Genet. 255:332-340. [DOI] [PubMed] [Google Scholar]
- 209.Melendy, T., and R. Li. 2001. Chromatin remodeling and initiation of DNA replication. Front. Biosci. 6:D1048-D1053. [DOI] [PubMed] [Google Scholar]
- 210.Mendez, J., and B. Stillman. 2003. Perpetuating the double helix: molecular machines at eukaryotic DNA replication origins. Bioessays 25:1158-1167. [DOI] [PubMed] [Google Scholar]
- 211.Mendez, J., and B. Stillman. 2000. Chromatin association of human origin recognition complex, Cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20:8602-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Meng, M. V., G. D. Grossfeld, G. H. Williams, S. Dilworth, K. Stoeber, T. W. Mulley, V. Weinberg, P. R. Carroll, and T. D. Tisty. 2001. Minichromosome maintenance protein 2 expression in prostate: characterization and association with outcome after therapy for cancer. Clin. Cancer Res. 7:2712-2718. [PubMed] [Google Scholar]
- 213.Merchant, A. M., Y. Kawasaki, Y. R. Chen, M. Lei, and B.-K. Tye. 1997. A lesion in the DNA replication initiation factor Mcm10 induces pausing of elongation forks through chromosomal replication origins in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:3261-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mimura, S., T. Masuda, T. Matsui, and H. Takisawa. 2000. Central role for Cdc45 in establishing in initiation complex of DNA replication in Xenopus egg extracts. Genes Cells 5:439-452. [DOI] [PubMed] [Google Scholar]
- 215.Mimura, S., and H. Takisawa. 1998. Xenopus CDC45-dependent loading of DNA polymerase α onto chromatin under the control of S phase CDK. EMBO J. 17:5699-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Miyake, S., N. Okishio, I. Samejima, Y. Hiraoka, T. Toda, I. Saitoh, and M. Yanagida. 1993. Fission yeast genes nda1+ and nda4+, mutations of which lead to S-phase block, chromatin alteration and Ca2+ suppression, are members of the CDC46/MCM2 family. Mol. Biol. Cell 4:1003-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Miyake, S., and S. Yamashita. 1998. Identification of sna41+ gene, which is the suppressor of nda4 mutation and is involved in DNA replication in Schizosaccharomyces pombe. Genes Cells 3:157-166. [DOI] [PubMed] [Google Scholar]
- 218.Mizushima, T., N. Takahashi, and B. Stillman. 2000. Cdc6p modulates the structure and DNA binding activity of the origin recognition complex in vitro. Genes Dev. 14:1631-1641. [PMC free article] [PubMed] [Google Scholar]
- 219.Moir, D., S. E. Stewart, B. C. Osmond, and D. Botstein. 1982. Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100:547-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Montagnoli, A., R. Bosotti, F. Villa, M. Rialland, D. Brotherton, C. Mercurio, J. Berthelsen, and C. Santocanale. 2002. Drf1, a novel regulatory subunit for human Cdc7 kinase. EMBO J. 21:3171-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Moreno, S., and P. Nurse. 1994. Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature 367:236-242. [DOI] [PubMed] [Google Scholar]
- 222.Mueller, S., C. Hoege, G. Pyrowolakis, and S. Jentsch. 2001. SUMO, ubiuitin's mysterious cousin. Nat. Rev. Mol. Cell Biol. 2:202-210. [DOI] [PubMed] [Google Scholar]
- 223.Murakami, Y., and Y. Ito. 1999. Transcription factors in DNA replication. Front. Biosci. 4:D824-D833. [DOI] [PubMed] [Google Scholar]
- 224.Musahl, C., D. Schulte, R. Burkhart, and R. Knippers. 1995. A human homologue of the yeast replication protein Cdc21 interactions with other Mcm proteins. Eur. J. Biochem. 230:1096-1101. [DOI] [PubMed] [Google Scholar]
- 225.Muzi-Falconi, M., G. W. Brown, and T. J. Kelly. 1996. cdc18+ regulates initiation of DNA replication in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 93:1566-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nakajima, R., and H. Masukata. 2002. SpS1d3 is required for loading and maintenance of SpCdc45 on chromatin in DNA replication in fission yeast. Mol. Biol. Cell 13:1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nasmyth, K., and P. Nurse. 1981. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 182:119-124. [DOI] [PubMed] [Google Scholar]
- 228.Neuwald, A. F., L. Aravind, J. L. Spouge, and E. V. Koonin. 1999. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9:27-43. [PubMed] [Google Scholar]
- 229.Nguyen, V. Q., C. Co, K. Irie, and J. J. Li. 2000. Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr. Biol. 10:195-205. [DOI] [PubMed] [Google Scholar]
- 230.Nguyen, V. Q., C. Co, and J. J. Li. 2001. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411:1068-1073. [DOI] [PubMed] [Google Scholar]
- 231.Nishitani, H., and Z. Lygerou. 2002. Control of DNA replication licensing in a cell cycle. Genes Cells 7:523-534. [DOI] [PubMed] [Google Scholar]
- 232.Nishitani, H., Z. Lygerou, T. Nishimoto, and P. Nurse. 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404:625-628. [DOI] [PubMed] [Google Scholar]
- 233.Nishitani, H., and P. Nurse. 1995. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell 83:397-405. [DOI] [PubMed] [Google Scholar]
- 234.Njagi, G. D., and B. J. Kilbey. 1982. Cdc7-1 a temperature sensitive cell-cycle mutant which interferes with induced mutagenesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 186:478-481. [DOI] [PubMed] [Google Scholar]
- 235.Noguchi, E., P. Shanahan, C. Noguchi, and P. Russell. 2002. CDK phosphorylation of Drc1 regulates DNA replication in fission yeast. Curr. Biol. 12:599-605. [DOI] [PubMed] [Google Scholar]
- 236.Nougarede, R., D. Seta, P. Zarzov, and E. Schwob. 2000. Hierarchy of S-phase promoting factors: yeast Dbf4-Cdc7 kinase requires prior S-phase cyclin dependent kinase activation. Mol. Cell. Biol. 20:3795-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nurse, P. 1990. Universal control mechanism regulating onset of M phase. Nature 344:503-508. [DOI] [PubMed] [Google Scholar]
- 238.O'Connell, M. J., N. C. Walworth, and A. M. Carr. 2000. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 10:296-303. [DOI] [PubMed] [Google Scholar]
- 239.Ogawa, Y., T. Takahashi, and H. Masukata. 1999. Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol. Cell. Biol. 19:7228-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Okishio, N., Y. Adachi, and M. Yanagida. 1996. Fission yeast nda1 and nda4, MCM homologs required for DNA replication, are constitutive nuclear proteins. J. Cell Sci. 109:319-326. [DOI] [PubMed] [Google Scholar]
- 241.Osborn, A. J., and S. J. Elledge. 2003. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 17:1755-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Osborn, A. J., S. J. Elledge, and L. Zou. 2002. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 12:509-516. [DOI] [PubMed] [Google Scholar]
- 243.Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani, and J. J. Li. 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19:4888-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Owens, J. C., C. S. Detweiler, and J. J. Li. 1997. CDC45 is required in conjunction with CDC7/DBF4 to trigger the initiation of DNA replication. Proc. Natl. Acad. Sci. USA 94:12521-12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pak, D. T. S., M. Pflumm, I. Chesnokov, D. W. Huang, R. Kellum, J. Marr, P. Romanowski, and M. R. Botchan. 1997. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91:311-323. [DOI] [PubMed] [Google Scholar]
- 246.Pasero, P., B. P. Duncker, E. Schwob, and S. M. Gasser. 1999. A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication. Genes Dev. 13:2159-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pasion, S. G., and S. L. Forsburg. 1999. Nuclear localization of Schizosaccharomyces pombe Mcm2/Cdc19p requires MCM complex assembly. Mol. Biol. Cell 10:4043-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Passmore, S., R. Elble, and B. K. Tye. 1989. A protein involved in minichromosome maintenance in yeast binds a transcriptional enhancer conserved in eukaryotes. Genes Dev. 3:921-935. [DOI] [PubMed] [Google Scholar]
- 249.Patel, S. S., and K. M. Picha. 2000. Structure and function of hexameric helicases. Annu. Rev. Biochem. 69:651-697. [DOI] [PubMed] [Google Scholar]
- 250.Pereverzeva, I., E. Whitmire, B. Khan, and M. Coue. 2000. Distinct phosphoisoforms of the xenopus Mcm4 protein regulate the function of the Mcm complex. Mol. Cell. Biol. 20:3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Perkins, G., and J. F. X. Diffley. 1998. Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol. Cell 2:23-32. [DOI] [PubMed] [Google Scholar]
- 252.Petersen, B. O., J. Lukas, C. S. Sorensen, J. Bartek, and K. Helin. 1999. Phosphorylation of mammalian CDC6 by Cyclin A/CDK2 regulates its subcellular localization. EMBO J. 18:396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pflumm, M. F., and M. R. Botchan. 2001. Orc mutants arrest in metaphase with abnormally condensed chromosomes. Development 128:1697-1707. [DOI] [PubMed] [Google Scholar]
- 254.Piatti, S., T. Bohm, J. H. Cocker, J. F. X. Diffely, and K. Nasmyth. 1996. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 10:1516-1531. [DOI] [PubMed] [Google Scholar]
- 255.Piatti, S., C. Lengauer, and K. Nasmyth. 1995. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a ′reductional' anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 14:3788-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Poplawski, A., B. Grabowski, S. E. Long, and Z. Kelman. 2001. The zinc finger domain of the archaeal minichromosome maintenance protein is required for helicase activity. J. Biol. Chem. 276:49371-49377. [DOI] [PubMed] [Google Scholar]
- 257.Prasanth, S. G., K. V. Prasanth, and B. Stillman. 2002. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science 297:1026-1031. [DOI] [PubMed] [Google Scholar]
- 258.Prokhorova, T. A., and J. J. Blow. 2000. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J. Biol. Chem. 275:2491-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Raghuraman, M. K., E. A. Winzeler, D. Collingwood, S. Hunt, L. Wodicka, A. Conway, D. J. Lockhart, R. W. Davis, B. J. Brewer, and W. L. Fangman. 2001. Replication dynamics of the yeast genome. Science 294:115-121. [DOI] [PubMed] [Google Scholar]
- 260.Ramnath, N., F. J. Hernandez, D. F. Tan, J. A. Huberman, N. Natarajan, A. F. Beck, A. Hyland, I. T. Todorov, J. S. Brooks, and G. Bepler. 2001. MCM2 Is an independent predictor of survival in patients with non-small-cell lung cancer. J. Clin. Oncol. 19:4259-4266. [DOI] [PubMed] [Google Scholar]
- 261.Rhind, N., and P. Russell. 2000. Checkpoints: It takes more than time to heal some wounds. Curr. Biol. 10:R908-R911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Richter, A., M. Baack, H. P. Holthoff, M. Ritzi, and R. Knippers. 1998. Mobilization of chromatin-bound Mcm proteins by micrococcal nuclease. J. Biol. Chem. 379:1181-1187. [DOI] [PubMed] [Google Scholar]
- 263.Ritzi, M., M. Baack, C. Musahl, P. Romanowski, R. A. Laskey, and R. Knippers. 1998. Human minichromosome maintenance proteins and human origin recognition complex 2 protein on chromatin. J. Biol. Chem. 273:24543-24549. [DOI] [PubMed] [Google Scholar]
- 264.Roberts, B. T., C. Y. Ying, J. Gautier, and J. L. Maller. 1999. DNA replication in vertebrates requires a homologue of the Cdc7 protein kinase. Proc. Natl. Acad. Sci. USA 96:2800-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rodins, K., M. Cheale, N. Coleman, and S. B. Fox. 2002. Minichromosome maintenance protein 2 expression in normal kidney and renal cell carcinomas: relationship to tumor dormancy and potential clinical utility. Clin. Cancer Res. 8:1075-1081. [PubMed] [Google Scholar]
- 266.Romanowski, P., and M. A. Madine. 1996. Mechanisms restricting DNA replication to once per cell cycle—MCMs, pre-replicative complexes and kinases. Trends Cell Biol. 6:184-188. [DOI] [PubMed] [Google Scholar]
- 267.Romanowski, P., M. A. Madine, and R. A. Laskey. 1996. XMCM7, a novel member of the Xenopus MCM family, interacts with XMCM3 and colocalizes with it throughout replication. Proc. Natl. Acad. Sci. USA 93:10189-10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Romanowski, P., M. A. Madine, A. Rowles, J. J. Blow, and R. A. Laskey. 1996. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol. 6:1416-1425. [DOI] [PubMed] [Google Scholar]
- 269.Saha, P., K. C. Thome, R. Yamaguchi, Z. H. Hou, S. Weremowicz, and A. Dutta. 1998. The human homolog of Saccharomyces cerevisiae CDC45. J. Biol. Chem. 273:18205-18209. [DOI] [PubMed] [Google Scholar]
- 270.Saka, Y., P. Fantes, T. Sutani, C. McInerny, J. Creanor, and M. Yanagida. 1994. Fission yeast cut5 links nuclear chromatin and M phase regulator in the replication checkpoint control. EMBO J. 13:5319-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Saka, Y., and M. Yanagida. 1993. Fission yeast cut5+, required for S-phase onset and M-phase restraint, is identical to the radiation-damage repair gene rad4+. Cell 74:383-393. [DOI] [PubMed] [Google Scholar]
- 272.Sanchez, M., A. Calzada, and A. Bueno. 1999. The Cdc6 protein is ubiquitinated in vivo for proteolysis in Saccharomyces cerevisiae. J. Biol. Chem. 274:9092-9097. [DOI] [PubMed] [Google Scholar]
- 273.Santocanale, C., and J. F. X. Diffley. 1998. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395:615-618. [DOI] [PubMed] [Google Scholar]
- 274.Santocanale, C., and J. F. X. Diffley. 1996. ORC- and Cdc6-dependent complexes at active and inactive chromosomal replication origins in Saccharomyces cerevisiae. EMBO J. 15:6671-6679. [PMC free article] [PubMed] [Google Scholar]
- 275.Sato, M., R. Gotow, Z. You, Y. Komamura-Kohno, Y. Uchiyama, N. Yabuta, H. Nojima, and Y. Ishimi. 2000. Electron microscopic observation and single-stranded DNA binding activity of the Mcm4,6,7 complex. J. Mol. Biol. 300:421-431. [DOI] [PubMed] [Google Scholar]
- 276.Schepers, A., and J. F. Diffley. 2001. Mutational analysis of conserved sequence motifs in the budding yeast Cdc6 protein. J. Mol. Biol. 308:597-608. [DOI] [PubMed] [Google Scholar]
- 277.Schnell, J. D., and L. Hicke. 2003. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 278:35857-35860. [DOI] [PubMed] [Google Scholar]
- 278.Schulte, D., R. Burkhart, C. Musahl, B. Hu, C. Schlatterer, H. Hameister, and R. Knippers. 1995. Expression, phosphorylation and nuclear localization of the human P1 protein, a homoogue of the yeast Mcm 3 replication protein. J Cell Sci. 108:1381-1389. [DOI] [PubMed] [Google Scholar]
- 279.Schulte, D., A. Richter, R. Burkhart, C. Musahl, and R. Knippers. 1996. Properties of the human nuclear protein p85MCM—expression, nuclear localization and interaction with other MCM proteins. Eur. J. Biochem. 235:144-151. [DOI] [PubMed] [Google Scholar]
- 280.Schwacha, A., and S. P. Bell. 2001. Interactions between two catalytically distinct MCM subgroups are essential for coordinated ATP hydrolysis and DNA replication. Mol. Cell 8:1093-1104. [DOI] [PubMed] [Google Scholar]
- 281.Sclafani, R. A. 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113:2111-2117. [DOI] [PubMed] [Google Scholar]
- 282.Sclafani, R. A., M. Tecklenburg, and A. Pierce. 2002. The mcm5-bob1 bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdk1-dependent steps in Saccharomyces cerevisiae. Genetics 161:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shechter, D. F., C. Y. Ying, and J. Gautier. 2000. The intrinsic DNA helicase activity of Methanobacterium thermoautotrophicum delta H minichromosome maintenance protein. J. Biol. Chem. 275:15049-15059. [DOI] [PubMed] [Google Scholar]
- 284.Sherman, D. A., S. G. Pasion, and S. L. Forsburg. 1998. Multiple domains of fission yeast Cdc19p (MCM2) are required for its association with the core MCM complex. Mol. Biol. Cell 9:1833-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shirahige, K., Y. Hori, K. Shiraishi, M. Yamashita, K. Takahashi, C. Obuse, T. Tsurimoto, and H. Yoshikawa. 1998. Regulation of DNA-replication origins during cell-cycle progression. Nature 395:618-621. [DOI] [PubMed] [Google Scholar]
- 286.Shohet, J. M., M. J. Hicks, S. E. Plon, S. M. Burlingame, S. Stuart, S. Y. Chen, M. K. Brenner, and J. G. Nuchtern. 2002. Minichromosome maintenance protein MCM7 is a direct target of the MYCN transcription factor in neuroblastoma. Cancer Res. 62:1123-1128. [PubMed] [Google Scholar]
- 287.Sible, J. C., E. Erikson, M. Hendrickson, J. L. Maller, and J. Gautier. 1998. Developmental regulation of MCM replication factors in Xenopus laevis. Curr. Biol. 8:347-350. [DOI] [PubMed] [Google Scholar]
- 288.Simmons, D. T. 2000. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 55:75-134. [DOI] [PubMed] [Google Scholar]
- 289.Sinha, P., V. Chang, and B.-K. Tye. 1986. A mutant that affects the function of autonomously replicating sequences in yeast. J. Mol. Biol. 192:805-814. [DOI] [PubMed] [Google Scholar]
- 290.Snaith, H. A., G. Brown, and S. L. Forsburg. 2000. Schizosaccharomyces pombe Hsk1p is a potential Cds1p target required for genome integrity. Mol. Cell. Biol. 20:7922-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Snaith, H. A., and S. L. Forsburg. 1999. Rereplication in fission yeast requires MCM proteins and other S phase genes. Genetics 152:839-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sogo, J. M., M. Lopes, and M. Foiani. 2002. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297:599-602. [DOI] [PubMed] [Google Scholar]
- 293.Solomon, N., M. B. Wright, S. Chang, A. M. Buckley, L. B. Dumas, and R. F. Gaber. 1992. Genetic and molecular analysis of DNA43 and DNA52: two new cell-cycle genes in Saccharomyces cerevisiae. Yeast 8:273-289. [DOI] [PubMed] [Google Scholar]
- 294.Someya, A., M. Shioda, and A. Okuyama. 1995. The association and involvement of some members of the P1 protein family in a cell-free DNA replication of Xenopus eggs. Biochem. Biophys. Res. Commun. 209:823-831. [DOI] [PubMed] [Google Scholar]
- 295.Spradling, A. C. 1999. ORC binding, gene amplification, and the nature of metazoan replication origins. Genes Dev. 13:2619-2623. [DOI] [PubMed] [Google Scholar]
- 296.Springer, P. S., W. R. McCombie, V. Sundaresan, and R. A. Martienssen. 1995. Gene trap tagging of PROLIFERA, and essential MCM2-3-5 like gene in Arabidopsis. Science 268:877-880. [DOI] [PubMed] [Google Scholar]
- 297.Stagljar, I., U. Hubscher, and A. Barberis. 1999. Activation of DNA replication in yeast by recruitment of the RNA polymerase II transcription complex. Biol. Chem. 380:525-530. [DOI] [PubMed] [Google Scholar]
- 298.Sterner, J. M., S. Dew-Knight, C. Musahl, S. Kornbluth, and J. Horowitz. 1998. Negative regulation of DNA replication by the retinoblastoma protein Is mediated by its association with MCM7. Mol. Cell. Biol. 18:2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stevaux, O., and N. J. Dyson. 2002. A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol. 14:684-691. [DOI] [PubMed] [Google Scholar]
- 300.Su, T. T., and P. H. O'Farrell. 1997. Chromosome association of minichromosome maintenance proteins in Drosophila mitotic cycles. J. Cell Biol. 139:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Suka, N., K. Luo, and M. Grunstein. 2002. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine 16 and spreading of heterochromatin. Nat. Genet. 32:378-383. [DOI] [PubMed] [Google Scholar]
- 302.Tada, S., A. Li, D. Maiorano, M. Mechali, and J. J. Blow. 2001. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 3:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Takahashi, K., H. Yamada, and M. Yanagida. 1994. Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality. Mol. Biol. Cell 5:1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Takayama, Y., Y. Kamimura, M. Okawa, S. Muramatsu, A. Sugino, and H. Araki. 2003. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 17:1153-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Takeda, T., K. Ogino, E. Matsui, M. K. Cho, H. Kumagai, T. Miyake, K. Arai, and H. Masai. 1999. A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol. 19:5535-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Takeda, T., K. Ogino, K. Tatebayashi, H. Ikeda, K. Arai, and H. Masai. 2001. Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast. Mol. Biol. Cell 12:1257-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Takei, Y., M. Assenberg, G. Tsujimoto, and R. Laskey. 2002. The MCM3 acetylase MCM3AP inhibits initiation, but not elongation, of DNA replication via interaction with MCM3. J. Biol. Chem. 277:43121-43125. [DOI] [PubMed] [Google Scholar]
- 308.Takei, Y., M. Swietlik, A. Tanoue, G. Tsujimoto, T. Kouzarides, and R. Laskey. 2001. MCM3AP, a novel acetyltransferase that acetylates replication protein MCM3. EMBO Rep. 2:119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Takei, Y., and G. Tsujimoto. 1998. Identification of a novel MCM3-associated protein that facilitates MCM3 nuclear localization. J. Biol. Chem. 273:22177-22180. [DOI] [PubMed] [Google Scholar]
- 310.Tan, D. F., J. A. Huberman, A. Hyland, G. M. Loewen, J. S. Brooks, A. F. Beck, I. T. Todorov, and G. Bepler. 2001. MCM2— a promising marker for premalignant lesions of the lung: a cohort study. BMC Cancer 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tanaka, T., D. Knapp, and K. Nasmyth. 1997. Loading of an MCM protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell 90:649-660. [DOI] [PubMed] [Google Scholar]
- 312.Tercero, J. A., K. Labib, and J. F. Diffley. 2000. DNA synthesis at individual replication forks requires the essential initiation factor Cdc45p. EMBO J. 19:2082-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tercero, J. A., M. P. Longhese, and J. F. Diffley. 2003. A central role for DNA replication forks in checkpoint activation and response. Mol. Cell 11:1323-1336. [DOI] [PubMed] [Google Scholar]
- 314.Thömmes, P., R. Fett, B. Schray, R. Burkhart, M. Barnes, C. Kennedy, N. C. Brown, and R. Knippers. 1992. Properties of the nuclear P1 protein, a mammalian homologue of the yeast MCM3 replication protein. Nucleic Acids Res. 20:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Thömmes, P., Y. Kubota, H. Takisawa, and J. J. Blow. 1997. The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J. 16:3312-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Toda, T., K. Umesono, A. Hirata, and M. Yanagida. 1983. Cold sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J. Mol. Biol. 168:251-270. [DOI] [PubMed] [Google Scholar]
- 317.Todorov, I. T., A. Attaran, and S. E. Kearsey. 1995. BM28, a human member of the MCM2-3-5 family, is displaced from chromatin during DNA replication. J. Cell Biol. 129:1433-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Todorov, I. T., R. Pepperkok, R. Philipova, S. E. Kearsey, W. Ansorge, and D. Werner. 1994. A human nuclear protein with sequence homology to a family of early S phase proteins is required for entry into S phase and for cell division. J. Cell Sci. 107:253-265. [DOI] [PubMed] [Google Scholar]
- 319.Tsuruga, H., N. Yabuta, K. Hashizume, M. Ikeda, Y. Endo, and H. Nojima. 1997. Expression, nuclear localization and interactions of human MCM/P1 proteins. Biochem. Biophys. Res. Commun. 236:118-125. [DOI] [PubMed] [Google Scholar]
- 320.Tye, B. K. 2000. Insights into DNA replication from the third domain of life. Proc. Natl. Acad. Sci. USA 97:2399-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tye, B. K. 1999. MCM proteins in DNA replication. Annu. Rev. Biochem. 68:649-686. [DOI] [PubMed] [Google Scholar]
- 322.Tye, B. K. 1999. Minichromosome maintenance as genetic assay for defects in DNA replication. Methods 18:329-334. [DOI] [PubMed] [Google Scholar]
- 323.Tye, B. K., and S. Sawyer. 2000. The hexameric eukaryotic MCM helicase: building symmetry from nonidentical parts. J. Biol. Chem. 275:34833-34836. [DOI] [PubMed] [Google Scholar]
- 324.Uchiyama, M., K. Arai, and H. Masai. 2001. Sna41 goa1, a novel mutation causing G1/S arrest in fission yeast, is defective in a CDC45 homolog and interacts genetically with polalpha. Mol. Genet. Genomics 265:1039-1049. [DOI] [PubMed] [Google Scholar]
- 325.Uchiyama, M., D. Griffiths, K. Arai, and H. Masai. 2001. Essential role of Sna41/Cdc45 in loading of DNA polymerase alpha onto minichromosome maintenance proteins in fission yeast. J. Biol. Chem. 276:26189-26196. [DOI] [PubMed] [Google Scholar]
- 326.Ulrich, H. D. 2002. Degradation or maintenance: actions of the ubiquitin system on eukaryotic chromatin. Eukaryotic Cell 1:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Van Hatten, R. A., A. V. Tutter, A. H. Holway, A. M. Khederian, J. C. Walter, and W. M. Michael. 2002. The Xenopus Xmus 101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J. Cell Biol. 159:541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Vas, A., W. Mok, and J. Leatherwood. 2001. Control of DNA rereplication via Cdc2 phosphorylation sites in the origin recognition complex. Mol. Cell. Biol. 21:5767-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Vogelauer, M., L. Rubbi, I. Lucas, B. J. Brewer, and M. Grunstein. 2002. Histone acetylation regulates the time of replication origin firing. Mol. Cell 10:1223-1233. [DOI] [PubMed] [Google Scholar]
- 330.Walter, J., and J. Newport. 2000. Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha. Mol. Cell. 5:617-627. [DOI] [PubMed] [Google Scholar]
- 331.Walter, J., and J. W. Newport. 1997. Regulation of replicon size in Xenopus egg extracts. Science 275:993-995. [DOI] [PubMed] [Google Scholar]
- 332.Walter, J. C. 2000. Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J. Biol. Chem. 50:39773-39778. [DOI] [PubMed] [Google Scholar]
- 333.Wang, H., and S. J. Elledge. 1999. DRC1, DNA replication and checkpoint protein 1, functions with DPB11 control DNA replication and the S-phase checkpoint in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Weinreich, M., C. Liang, and B. Stillman. 1999. The Cdc6p nucleotide-binding motif is required for loading Mcm proteins onto chromatin. Proc. Natl. Acad. Sci. USA 96:441-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Weinreich, M., and B. Stillman. 1999. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18:5334-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Whitbread, L. A., and S. Dalton. 1995. Cdc54 belongs to the Cdc46/Mcm3 family of proteins which are essential for initiation of eukaryotic DNA replication. Gene 155:113-117. [DOI] [PubMed] [Google Scholar]
- 337.Whittaker, A. J., I. Royzman, and T. L. Orr-Weaver. 2000. Drosophilia Double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 14:1765-1776. [PMC free article] [PubMed] [Google Scholar]
- 338.Williams, G. H., P. Romanowski, L. Morris, M. Madine, A. D. Mills, K. Stoeber, J. Marr, R. A. Laskey, and N. Coleman. 1998. Improved cervical smear assessment using anitbodies against proteins that regulate DNA replication. Proc. Natl. Acad. Sci. USA 95:14932-14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wohlschlegel, J. A., S. K. Dhar, T. A. Prokhorova, A. Dutta, and J. C. Walter. 2002. Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Mol. Cell 9:1-20. [DOI] [PubMed] [Google Scholar]
- 340.Wohlschlegel, J. A., B. T. Dwyer, S. K. Dhar, C. Cvetic, J. C. Walter, and A. Dutta. 2000. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290:2309-2312. [DOI] [PubMed] [Google Scholar]
- 341.Wyrick, J. J., J. G. Aparicio, T. Chen, J. D. Barnett, E. G. Jennings, R. A. Young, S. P. Bell, and O. M. Aparicio. 2001. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science 294:2357-2360. [DOI] [PubMed] [Google Scholar]
- 342.Xie, A. Y., V. P. Bermudez, and W. R. Folk. 2002. Stimulation of DNA replication from the polyomavirus origin by PCAF and GCN5 acetyltransferases: acetylation of large T antigen. Mol. Cell. Biol. 22:7907-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yamaguchi, R., and J. Newport. 2003. A role for Ran-GTP and Crm1 in blocking re-replication. Cell 113:115-125. [DOI] [PubMed] [Google Scholar]
- 344.Yamamoto, R. R., J. M. Axton, Y. Yamamoto, R. D. Saunders, D. M. Glover, and D. S. Henderson. 2000. The Drosophila mus101 gene, which links DNA repair, replication and condensation of heterochromatin in mitosis, encodes a protein with seven BRCA1 C-terminus domains. Genetics 156:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yamane, K., and T. Tsuruo. 1999. Conserved BRCT regions of TopBP1 and of the tumor suppressor BRCA1 bind strand breaks and termini of DNA. Oncogene 18:5194-5203. [DOI] [PubMed] [Google Scholar]
- 346.Yan, H., S. Gibson, and B.-K. Tye. 1991. MCM2 and MCM3, two proteins important for ARS activity, are related in structure and function. Genes Dev. 4:968-977. [DOI] [PubMed] [Google Scholar]
- 347.Yan, H., A. M. Merchant, and B.-K. Tye. 1993. Cell cycle-regulated nuclear localization of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes Dev. 7:2149-2160. [DOI] [PubMed] [Google Scholar]
- 348.Yanagi, K., T. Mizuno, Z. You, and F. Hanaoka. 2002. Mouse geminin inhibits not only Cdt1-MCM6 interactions but also a novel intrinsic Cdt1 DNA binding activity. J. Biol. Chem. 277:40871-40880. [DOI] [PubMed] [Google Scholar]
- 349.Yankulov, K., I. Todorov, P. Romanowski, D. Licatalosi, K. Cilli, S. McCracken, R. Laskey, and D. L. Bentley. 1999. MCM proteins are associated with RNA polymerase II holoenzyme. Mol. Cell Biol. 19:6154-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yanow, S. K., D. A. Gold, H. Y. Yoo, and W. G. Dunphy. 2003. Xenopus Drf1, a regulator of Cdc7, displays checkpoint-dependent accumulation on chromatin during an S-phase arrest. J. Biol. Chem. 278:41083-41092. [DOI] [PubMed] [Google Scholar]
- 351.Yanow, S. K., Z. Lygerou, and P. Nurse. 2001. Expression of Cdc18/Cdc6 and Cdt1 during G2 phase induces initiation of DNA replication. EMBO J. 20:4648-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yeh, E. T., L. Gong, and T. Kamitani. 2000. Ubiquitin-like proteins: new wines in new bottles. Gene 248:1-14. [DOI] [PubMed] [Google Scholar]
- 353.Yoon, H.-J., S. Loo, and J. L. Campbell. 1993. Regulation of Saccharomyces cerevisiae CDC7 function during the cell cycle. Mol. Biol. Cell 4:195-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.You, Z., Y. Ishimi, H. Masai, and F. Hanaoka. 2002. Roles of Mcm7 and Mcm4 subunits in the DNA helicase activity of the mouse Mcm4/6/7 complex. J. Biol. Chem. 277:42471-42479. [DOI] [PubMed] [Google Scholar]
- 355.You, Z., Y. Komamura, and Y. Ishimi. 1999. Biochemical analysis of the intrinsic Mcm4-Mcm6-Mcm7 DNA helicase activity. Mol. Cell. Biol. 19:8003-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.You, Z., L. Kong, and J. Newport. 2002. The role of single-stranded DNA and polymerase α in establishing the ATR, Hus1 DNA replication checkpoint. J. Biol. Chem. 277:27088-27093. [DOI] [PubMed] [Google Scholar]
- 357.Young, M. R., K. Suzuki, H. Yan, S. Gibson, and B. K. Tye. 1997. Nuclear accumulation of Saccharomyces cerevisiae Mcm3 is dependent on its nuclear localization sequence. Genes Cells 2:631-643. [DOI] [PubMed] [Google Scholar]
- 358.Young, M. R., and B. K. Tye. 1997. Mcm2 and Mcm3 are constitutive nuclear proteins that exhibit distinct isoforms and bind chromatin during specific cell cycle stages of Saccharomyces cerevisiae. Mol. Biol. Cell 8:1587-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang, J. J., Y. Zhao, B. T. Chait, W. W. Lathem, M. Ritzi, R. Knippers, and J. E. Darnell, Jr. 1998. Ser727-dependent recruitment of MCM5 by Stat1alpha in IFN-gamma-induced transcriptional activation. EMBO J. 17:6963-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zou, L., J. Mitchell, and B. Stillman. 1997. CDC45, a novel yeast gene that functions with the origin recognition complex and MCM proteins in initiation of DNA replication. Mol. Cell. Biol. 17:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zou, L., and B. Stillman. 2000. Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication orgins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol. Cell. Biol. 20:3086-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zou, L., and B. Stillman. 1998. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280:593-596. [DOI] [PubMed] [Google Scholar]