Abstract
Development of nasal immunization for human use is hindered by the lack of acceptable adjuvants. Although CT is an effective adjuvant, its toxicity will likely prevent its use in nasal vaccines. This study compared non-toxin adjuvants to CT for their ability to induce protective antibody responses with nasal immunization. C3H/HeN and C57BL/6 mice were immunized with rPA formulated with the following adjuvants: CT, IL-1α, LPS, CpG, Pam3CSK4, 3M-019, resiquimod/R848 or c48/80. Serum and nasal wash cytokine concentrations were monitored 6 hours post-vaccination as biomarkers for acute activation of the innate immune system. Not all of the adjuvants induced significant changes in innate serum or nasal wash cytokines, but when changes were observed, the cytokine signatures were unique for each adjuvant. All adjuvants except Pam3CSK4 induced significantly increased anti-rPA serum IgG titers in both strains of mice, while only IL-1α, c48/80 and CpG enhanced mucosal anti-rPA IgA. Pam3CSK4 was the only adjuvant unable to enhance the induction of serum LeTx-neutralizing antibodies in C3H/HeN mice while c48/80 was the only adjuvant to induce increased serum LeTx-neutralizing antibodies in C57BL/6 mice. Only CT enhanced total serum IgE in C3H/HeN mice while IL-1α enhanced total serum IgE in C57BL/6 mice. The adjuvant influenced antigen-specific serum IgG subclass and T cell cytokine profiles, but these responses did not correlate with the induction of LeTx-neutralizing activity. Our results demonstrate the induction of diverse innate and adaptive immune responses by non-toxin nasal vaccine adjuvants that lead to protective humoral immunity comparable to CT and that these responses may be influenced by the host strain.
Introduction
Mucosal immunization requires the use of adjuvants for the induction of protective antigen-specific immune responses and to prevent the induction of tolerance [1]. Cholera toxin (CT) and labile toxin are known to be potent mucosal adjuvants for nasal immunization [2] but, their associated adverse effects [3-6] will likely prevent their use in humans. Safe, non-toxin adjuvants that induce protective immunity are needed for nasal immunization in humans.
Cytokines [7, 8] and toll-like receptor (TLR) ligands are potential non-toxin adjuvants that can be combined with antigens to enhance immune responses when administered intranasally [9-12]. Synthetic TLR4 ligands [9] and immunostimulatory DNA TLR9 ligands [10] have been used as adjuvants in mouse nasal immunization studies. Recently, nasal immunization was shown to be a safe and effective route of immunization in humans through use of the MPL-adjuvanted intranasal Norwalk (virus-like particle) vaccine [13]. The mast cell activator compound 48/80 (c48/80) is another potential non-toxin candidate that has demonstrated effective adjuvant activity in intranasally immunized mice [14, 15] and rabbits [16, 17].
In this study, we compared the non-toxin adjuvants IL-1α [7, 18], TLR ligands (LPS, CpG, Pam3CSK4, 3M-019 and resiquimod/R848 [8-12, 19-22]) and c48/80 [14, 15, 23] to CT for their ability to induce antigen-specific serum IgG, mucosal IgA and serum lethal toxin (LeTx)-neutralizing antibody responses in two strains of mice using nasal immunization with anthrax recombinant protective antigen (rPA) as a model system. We hypothesized that different nasal vaccine adjuvants provide unique activation of the innate and adaptive immune systems which correlate with the induction of LeTx-neutralizing antibodies. Additionally, we expected that the relative adjuvant activity would be dependent on the mouse strain used to perform the immunization studies.
Materials and Methods
Animals
Female C3H/HeN mice were purchased from the National Cancer Institute (Frederick, MD). Female C57BL/6J mice were purchased from Jackson Laboratory (stock # 000664, Bar Harbor, Maine). C57BL/6J mice were used as a research mouse strain that is commonly used to develop genetically-altered mice that may be used in future mechanistic studies. C3H/HeN mice were used as a TLR4+/+ mouse strain that will allow comparison to previous studies performed in both C3H/HeN and the C3H/HeJ TLR4−/− mouse strain used to rule out contaminating LPS influencing adjuvant activity (supplemental Figure 5 in [14]). All animal procedures were approved by Duke University’s Institutional Animal Care and Use Committee.
Antigens/adjuvants
All reagents were purchased from the following vendors: rPA, recombinant lethal factor (rLF) and CT (List Biologicals, Campbell, CA); recombinant mouse IL-1α (R&D Systems, Minneapolis, MN); c48/80 (Sigma, St. Louis, MO); LPS (from E.coli), Pam3CSK4, CpG (ODN 1826) and R848 (InVivogen, San Diego, CA). 3M-019 and resiquimod were obtained from 3M Pharmaceuticals (St. Paul, MN). All adjuvants were reconstituted in sterile water except for 3M-019 and resiquimod (DMSO stocks) before dilution in PBS in the vaccine formulation.
Nasal immunization and sample collection
Mice (5 - 10 per group per experiment; n = 5 - 15 per adjuvant) were intranasally immunized on days 0, 7 and 21 with rPA (2.5 μg) alone [14] or rPA formulated with 5 μg IL-1α [18], 1 μg CT [18], 15 μg c48/80 [14], 1 μg LPS (TLR4) [24], 10 μg CpG (TLR9) [19], 25 μg Pam3CSK4 (TLR1/2) [20], 10 μg 3M-019 (TLR7) or 10 μg resiquimod/R848 (TLR7/8) [21, 22]. All vaccine formulations were delivered in a 15 μl volume under isoflurane anesthesia (IsoFlo, USP; SOLVAY Animal Health, Mendota Heights, MN). Serum was collected 6 hours after the first immunization (day 0) as well as on days 14 and 42 as previously described [25]. Spleens, fecal material and vaginal lavage were collected on day 42 and processed as previously described [8]. Nasal wash was collected at 6 hours and on day 42 as described in [25].
ELISA
ELISA was used to determine the presence of rPA-specific IgG and IgA antibodies in serum and mucosal samples as previously described [26].
LeTx neutralization assay
A cytotoxicity assay using J774A.1 mouse macrophages (ATCC #TIB-67; Manassas, VA) was performed as previously described [26] except that the final concentration for both rPA and rLF was 187.5 ng/ml.
Splenocyte cultures
Splenocytes were collected on day 42 and re-stimulated as previously described [23]. Briefly, spleens cells were cultured in 48-well culture plates (Costar; Lowell, MA) at 1.25 × 106 cells per well (in 1 mL) for 5 days with T cell media ± rPA (5 μg/ml) to induce recall cytokine secretion by antigen-specific T cells. Supernatants were collected and stored at −8° C until tested for cytokine content by multiplex bead assay.
Cytokine/chemokine and total serum IgE
Cytokine/chemokine levels were measured in 6 hour serum and nasal wash samples using a mouse multi-plex kit (Bio-Rad; Hercules, CA). T cell cytokine levels were measured in splenocyte culture supernatants using a flourokine MAP mouse (C3H/HeN mice, R&D Systems; Minneapolis, MN) or a mouse multi-plex kit (C57BL/6 mice, Bio-Rad; Hercules, CA). Total IgE was measured in day 42 serum using a mouse IgE single plex kit (C3H/HeN mice; Millipore; Billerica, MA) or an IgE ELISA (C57BL/6 mice; Biolegend, Mouse IgE ELISA MAX™, San Diego, CA).
Statistics
One-way ANOVA/Tukey’s multiple comparisons and paired Student’s T-test were performed using GraphPad PRISM 5 software (LaJolla, CA). Cytokine concentrations (innate, T cell cytokines) were log10 transformed prior to statistical analysis. Data values are reported as mean ± standard deviation for all figures while mean, lower and upper confidence intervals are presented for innate cytokine responses described in Table 1. A p value < 0.05 was considered statistically significant.
Table 1. Non-toxin nasal adjuvants differentially influence the activation of the innate immune system in vivo.
C3H/HeN or C57BL/6 mice were nasally immunized with rPA (2.5 μg) alone (none) or rPA formulated with CT (1 μg), IL-1α (5 μg), c48/80 (15 μg), LPS (1 μg), CpG (10 μg), Pam3CSK4 (25 μg), 3M-019 (10 μg; C3H mice only) or resiquimod/R848 (10 μg) on day 0. Serum was collected 6 hours after nasal immunization of C3H/HeN mice and assayed using Bio-Plex to identify protein levels for 23 different cytokines and chemokines (IL-2, 3, 4, 5, 6, 9, 10, 12 (p40), 12 (p70), 13, 17, MIP-1α, MIP-1β, RANTES, TNFα, Eotaxin, G-CSF, GM-CSF, IFNγ, KC and MCP-1). Only cytokines/chemokines with significant differences between mice immunized with antigen alone or antigen combined with adjuvant are included in the table for C3H/HeN mice. For the repeat study performed in C57BL/6 mice, only IL-5, IL-6, G-CSF, KC, MCP-1, MIP-1β and RANTES were measured in serum and nasal wash samples. Only cytokines/chemokines showing significant differences in nasal wash samples are included in the table for C57BL/6 mice. Mean cytokine/chemokine values (pg/ml) with lower and upper 95% confidence intervals are included. Statistical analysis was performed using log10 transformed data followed by ANOVA and Tukey’s multiple comparison test.* = p < 0.05 vs antigen alone.
| Sample | Cytokine | rPA alone | CT | IL-1α | C48/80 | LPS | CpG | Pam3CKS4 | 3M-019 | Res/R848 |
|---|---|---|---|---|---|---|---|---|---|---|
| C3H/HeN Serum |
IL-5 | 11.5 4.45, 18.44 |
N.S. | 75.6 * 39.69, 111.4 |
N.S. | N.S. | 102.8 * 54.81, 150.8 |
N.S. | N.S. | |
| IL-6 | 40.0 −2.614, 82.64 |
N.S. | 589* 62.84, 1115 |
N.S. | N.S. | N.S. | 87.8 * 63.99, 111.6 |
N.S. | N.S. | |
| G-CSF | 106.6 40.3, 172.9 |
N.S. | 230813* 21317, 440309 |
N.S. | N.S. | N.S. | 1874* 559.9, 3188 |
483.2* 219.8, 746.6 |
858.8* 414, 1304 |
|
| KC | 40.1 18.89, 61.28 |
N.S. | 1562* 239.8, 2883 |
N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | |
| MCP-1 | 195.2 102.6, 287.8 |
N.S. | 1106* 479, 1734 |
N.S. | N.S. | N.S. | N.S. | 2407* 993, 3821 |
2,096* 794, 3399 |
|
| MIP-1β | 68.6 35.55, 101.7 |
N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | 543.3* 396.7, 689.8 |
304.1* 181.5, 426.6 |
|
| RANTES | 174.2 133.7, 214.7 |
N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | 3073* 1766, 4381 |
4,132* 2270, 5994 |
|
| C57BL/6 Serum |
IL-5 | 18.04 9.236, 26.85 |
54.58* 25.56, 83.6 |
N.S. | 481.9* −66.7, 1031 |
N.S. | N.S. | 65.32* 30.74, 99.9 |
N.S. | |
| IL-6 | 2.005 1.513, 2.49 |
54.36* 18.29, 90.43 |
2344* −1983, 6670 |
21.08* 1.505, 40.65 |
16.95* 6.999, 26.9 |
N.S. | 115.3* 10.57, 220 |
25.57* −0.7384, 51.8 |
||
| G-CSF | 83.91 45.81, 122 |
N.S. | 29209* 8182, 50236 |
N.S. | 517.7* 300.4, 735 |
N.S. | 2813* 1083, 4542 |
1604* −724.7, 3933 |
||
| KC | 68.88 53.29, 84.46 |
486.1* 270.9, 701.4 |
32621* −42281, 107524 |
N.S. | N.S. | N.S. | 647.6* 33.84, 1261 |
N.S. | ||
| MCP-1 | 80.36 33.57, 127 |
N.S. | 29504* −34050, 93059 |
N.S. | N.S. | N.S. | 4924* 757.7, 9090 |
5249* 4022, 6475 |
||
| MIP-1β | 7.883 4.832, 10.9 |
N.S. | 32.22* 21.07, 43.38 |
N.S. | N.S. | N.S. | 111.1* 39.23, 182.9 |
130.8* 62.43, 199.1 |
||
| RANTES | 197.4 134.8, 260.1 |
N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | 3326* 2247, 4405 |
||
| C57BL/6 Nasal Wash |
IL-6 | 2.323 −0.4118, 5.05 |
113* 12.84, 213.2 |
143.1* 84.22, 201.9 |
20.36* 0.2509, 40.47 |
36.85* 15.26, 58.43 |
N.S. | 20.44* 8.576, 32.2 |
15.14* −4.257, 34.5 |
|
| G-CSF | 3.983 2.461, 5.5 |
N.S. | 451.8* 306.6, 597.1 |
N.S. | 93.68* 45.81, 141.5 |
N.S. | 24.98* 5.355, 44.6 |
20.59* 5.78, 35.39 |
||
| KC | 3.763 1.391, 6.1 |
N.S. | 147* 96.83, 197.1 |
N.S. | 31.83* 13.35, 50.31 |
N.S. | 21.26* 3.814, 38.71 |
N.S. |
Results
Non-toxin nasal adjuvants differentially activate the innate immune system in vivo
Adjuvants are known to activate the innate immune system to produce elevated serum cytokines rapidly after vaccination [25, 27-29]. Nasal immunization studies were performed to compare non-toxin adjuvants to CT for their ability to activate the innate immune system in vivo and to determine if effective nasal adjuvants exhibit a common cytokine signature that correlated with adjuvant activity. LPS was used as a positive control due to its ability to rapidly induce cytokine production after nasal delivery[30]. Mice were nasally immunized with rPA alone or formulated with CT, IL-1α, c48/80, LPS, CpG, Pam3CSK4, 3M-019 (C3H/HeN mice only), resiquimod(C3H/HeN mice) or R848 (C57BL/6 mice). The dose of each adjuvant used was chosen based on previously-described mouse immunization studies [14, 18-22, 24]. Serum (C3H/HeN and C57BL/6) and nasal wash (C57BL/6 only) were collected 6 hours post-immunization and assayed for cytokine/chemokine levels (Table 1). Although CT is a classic nasal vaccine adjuvant, it did not induce changes in serum cytokines after nasal delivery to C3H/HeN mice. However, nasal delivery of CT to C57BL/6 mice induced elevated serum IL-5, IL-6 and KC 6 hours after nasal delivery. In C3H/HeN mice, IL-1α significantly increased G-CSF, KC, IL-6 and MCP-1 when compared to mice immunized with rPA alone. Similar results were observed with C57BL/6 mice except that serum MIP-1β levels were also elevated after nasal delivery of IL-1α and the magnitude of the cytokine elevations were influenced by the mouse strain. An increase in IL-5 was observed with c48/80 in both strains of mice while c48/80 also induced increased IL-6 production in C57BL/6 mice. Although LPS did not induce elevated serum cytokines in C3H/HeN mice, LPS induced significant elevations of IL-6 and G-CSF after nasal delivery in C57BL/6 mice. Pam3CSK4 induced elevated serum IL-5, IL-6 and G-CSF in both strains of mice and also induced elevated serum KC, MCP-1 and MIP-1β in C57BL/6 mice. 3M-019 and resiquimod both induced significantly increased serum levels of G-CSF, MCP-1, MIP-1β and RANTES in C3H/HeN mice. Nasal delivery of R848 induced serum cytokines in C57BL/6 mice similar to resiquimod but also induced increased serum IL-6. Nasal delivery of CpG as a vaccine adjuvant did not induce increases in any cytokine/chemokine tested in either strain of mice. These results demonstrate that nasal vaccine adjuvants provide unique activation of the innate immune system, based on serum cytokine and chemokine production, and that the adjuvant activity is significantly influenced by the host strain.
Nasal wash cytokine and chemokine concentrations were monitored in C57BL/6 mice to determine if local activation of the innate immune system could be observed after nasal immunization and to determine if nasal cytokine/chemokine profiles mirrored serum profiles. Cytokines and chemokines that were elevated in the serum after nasal immunization (IL-5, IL-6, G-CSF, KC, MCP-1, MIP-1β and RANTES) were monitored in nasal wash samples. However, only IL-6, G-CSF and KC were significantly elevated in nasal wash samples. IL-1α, LPS, and Pam3CK4 induced elevated IL-6, G-CSF and KC in nasal wash 6 hours after nasal immunization. R848 induced elevated IL-6 and G-CSF while CT and c48/80 induced elevated IL-6 in nasal wash after vaccination. As observed in the serum, CpG used as a nasal vaccine adjuvant did not increase any cytokine/chemokine tested. These results demonstrate that nasal vaccine adjuvants may induce local cytokine/chemokine production after nasal delivery but the profile and magnitude of cytokine/chemokine production is dependent upon the adjuvant used.
Nasal immunization with rPA formulated with non-toxin adjuvants induces antigen-specific serum IgG comparable to CT
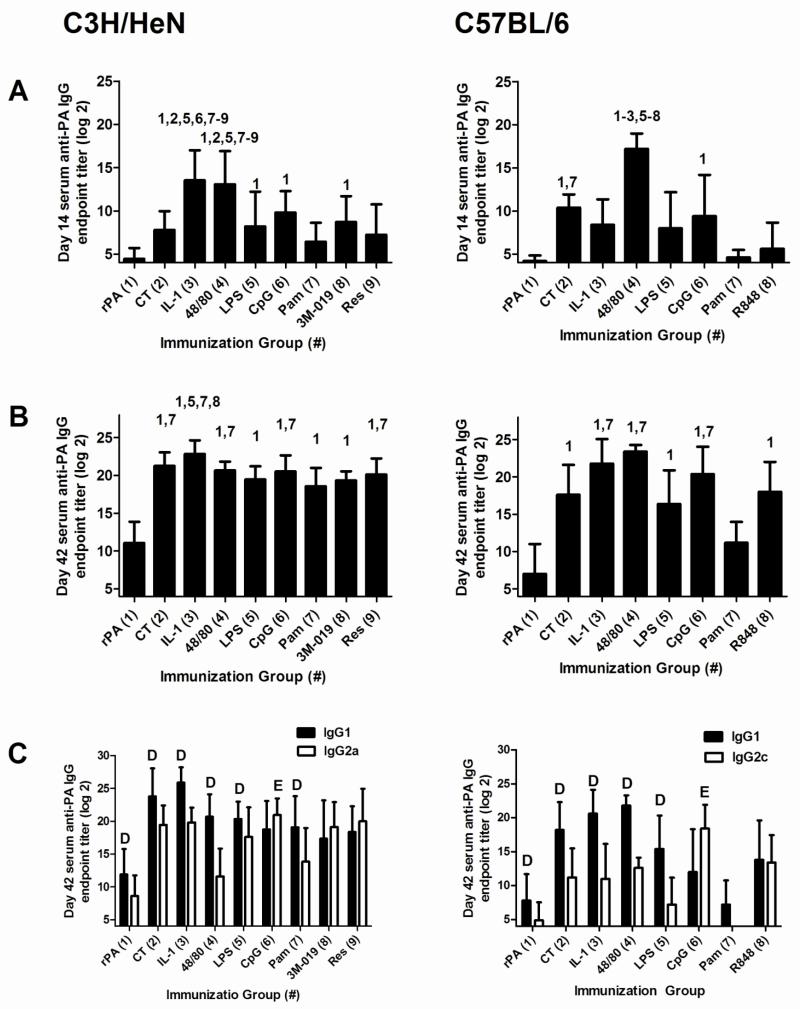
Serum was collected from nasally-immunized mice on days 14 and 42 to determine the influence of the adjuvants on the kinetics of serum anti-rPA IgG antibody responses (Figure 1A and B). On day 14 in C3H/HeN mice, CT (7.8 ± 2.1) did not significantly enhance anti-rPA IgG compared to immunization with rPA alone (4.5 ± 1.2). However, serum anti-rPA IgG log2 endpoint titers induced by IL-1α (13.5 ± 3.5) and c48/80 (13.1 ± 3.8) were significantly greater than the titers induced by all other adjuvants with the exception of CpG (9.8 ± 2.5). LPS, CpG and 3M-019 were the only TLR ligands which enhanced anti-rPA IgG (8.2 ± 4.0, 9.8 ± 2.5 and 8.7 ± 2.9, respectively) on day 14 compared to rPA alone. In C57BL/6 mice, CT (10.4 ± 1.5), c48/80 (17.2 ± 1.8) and CpG (9.4 ± 4.8) induced serum anti-rPA IgG titers greater than those induced by rPA alone (4.2 ± 0.6). c48/80 induced the highest anti-rPA IgG titers in C57BL/6 mice which were significantly higher than anti-rPA IgG titers induced by any other adjuvant.
Figure 1. Nasal immunization with rPA formulated with non-toxin adjuvants induces antigen-specific serum IgG comparable to CT.
C3H/HeN or C57BL/6 mice were nasally immunized with rPA (2.5 μg) alone or rPA plus CT (1 μg), IL-1α (5 μg), c48/80 (15 μg), LPS (1 μg), CpG (10 μg), Pam3CSK4 (25 μg), 3M-019 (10 μg; C3H/HeN mice only) or resiquimod/R848 (10 μg) on days 0, 7 and 21. Serum was collected on days 14 (A) and 42 (B) and assayed by ELISA to determine anti-rPA IgG log2 endpoint titers. The data represents 2-3 combined experiments for C3H/HeN mice (n = 15 per group, except for the 3M-019 and resiquimod groups in which n = 10) and one experiment for C57BL/6 mice (10 mice in the antigen alone group, 5 mice in each adjuvant group). Bars represent mean ± standard deviation. For day 14 and 42 IgG titers, statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparison test. The numbers above the error bars indicate which groups (1-9) are significantly different from the indicated group. Day 42 samples were also tested for anti-rPA IgG1 and IgG2a/c responses (C). For day 42 IgG1 and IgG2a/c titers, paired Student’s T-test was used to compare IgG1 and IgG2a/c titers within each adjuvant group. D=IgG1 titer significantly greater than IgG2a/c titer within the same adjuvant group. E=IgG2a/c titer significantly greater than IgG1 titer within the same adjuvant group. Samples that had no detectable anti-rPA antibody were assigned a log2 value of one less than the starting log2 dilution for statistical analysis.
Three weeks after the final immunization (day 42) in C3H/HeN mice, serum anti-rPA IgG titers in all adjuvanted groups were significantly increased compared to mice immunized with rPA alone (11.1 ± 2.8). CT induced anti-rPA IgG titers (21.3 ± 1.8) significantly greater than those induced by Pam3CSK4 (18.6 ± 2.4) while titers induced by IL-1α (22.9 ± 1.8) were enhanced compared to LPS (19.5 ± 1.8), Pam3CKS4 (18.6 ± 2.4) and 3M-019 (19.3 ± 1.2). Day 42 serum anti-rPA IgG titers were not significantly different between c48/80 (20.7 ± 1.2) and the TLR ligands (LPS, CpG; 20.5 ± 2.1, Pam3CSK4, 3M-019, and resiquimod; 20.1 ± 2.1). At day 42 in C57BL/6 mice, all adjuvants except Pam3CSK4 provided significant adjuvant activity and induced the production of elevated serum anti-rPA IgG titers compared to immunization with antigen alone. IL-1α (17.6 ± 4.0), c48/80 (23.4 ± 0.9) and CpG (20.4 ± 3.7) induced anti-rPA IgG responses that were significantly greater than anti-rPA IgG titers induced by rPA alone (7.0 ± 4) and rPA plus Pam3CSK4 (11.2 ± 2.8).
IgG anti-rPA subclasses at day 42 were monitored to determine the influence of adjuvant on IgG subclass responses (Figure 1C). In C3H/HeN mice, CT and the non-toxin adjuvants IL-1α, c48/80, LPS and Pam3CSK4 induced significantly higher serum anti-rPA IgG1 titers than IgG2a; whereas CpG was the only adjuvant to induce significantly higher IgG2a titers than IgG1. 3M-019 and resiquimod induced a balanced anti-rPA IgG1/IgG2a response. In C57BL/6 mice, CT, IL-1α, c48/80 and LPS induced significantly higher serum anti-rPA IgG1 titers than IgG2c. As with C3H/HeN mice, CpG was the only adjuvant to induce significantly higher IgG2c titers than IgG1 while R848 induced a balanced anti-rPA IgG1/IgG2c response.
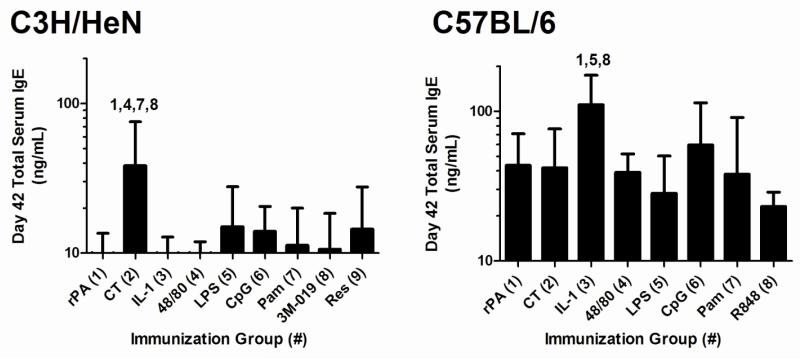
None of the non-toxin adjuvants induced elevated total serum IgE in C3H/HeN mice at day 42, in contrast to CT (Figure 2). In C57BL/6 mice, only IL-1α induced significantly elevated total serum IgE at day 42. Collectively, these results demonstrate that non-toxin adjuvants may be selected that have the capacity to induce significantly elevated antigen-specific serum IgG, preferentially induce antigen-specific IgG1, IgG2a/c or a balanced IgG subclass response while not influencing serum IgE levels, which is a potential adverse effect.
Figure 2. Total serum IgE after nasal immunization with rPA formulated with non-toxin adjuvants.
Mice were immunized as described in Figure 1. Total IgE was measured in day 42 serum. Bars represent mean ± standard deviation Statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparison test. The numbers above the error bars indicate which groups (1-9) are significantly different from the indicated group.
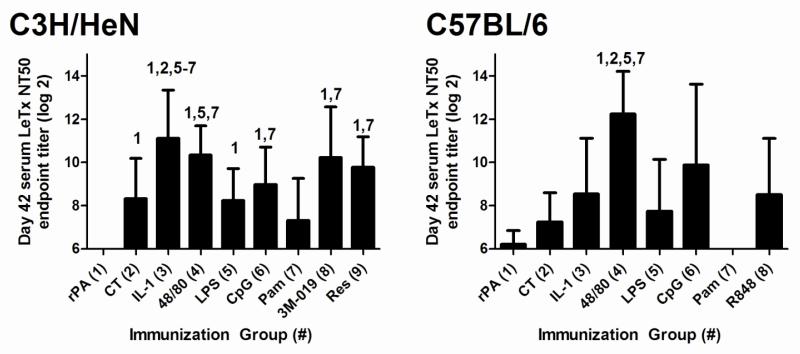
Non-toxin nasal adjuvants differentially induce serum LeTx-neutralizing activity
A LeTx neutralization assay was used to determine if adjuvant-induced anti-rPA serum antibodies also possessed protective LeTx-neutralizing activity (Figure 3) since binding antibodies measured by ELISA may not predict the protective capacity of vaccine-induced antibodies. At day 42 in C3H/HeN mice, all adjuvants except Pam3CSK4 (7.3 ± 1.9) exhibited LeTx-neutralizing activity (CT; 8.3 ± 1.9, IL-1α; 11.1 ± 2.2, c48/80; 10.3 ± 1.3, LPS; 8.2 ± 1.5, CpG; 9.0 ± 1.7, 3M-019; 10.2 ± 2.3 and resiquimod; 9.8 ± 1.4) significantly greater than immunization with rPA alone (undetectable). In C57BL/6 mice, only c48/80 induced significantly elevated serum LeTx-neutralizing antibodies (12.2 ± 1.9) that were significantly greater than mice immunized with rPA alone (6.2 ± 0.6), or rPA combined with CT (7.2 ± 1.3), LPS (7.7 ± 2.4) or Pam3CSK4 (undetectable). Our results indicate that nasal immunization with rPA formulated with non-toxin adjuvants has the potential to induce high serum titers of anti-rPA antibodies with LeTx-neutralizing activity comparable or superior to CT and that the ability of an adjuvant to induce a protective immune response is influenced by the host strain.
Figure 3. Non-toxin adjuvants differentially influence the induction of serum LeTx-neutralizing activity after nasal immunization with rPA.
Mice were immunized as described in Figure 1. A lethal toxin (LeTx) neutralization assay with J774A.1 macrophages was used to measure functional anti-rPA antibody responses in serum collected on days 42 of the nasal immunization regimen. The percent neutralization was plotted versus serum log2 dilutions and the linear range was used to calculate the serum log2 endpoint titer required for a 50% inhibition of LeTx-induced J774A.1 cell death (LeTx NT50). Data is expressed as mean ± standard deviation LeTx NT50. Statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparison test. The numbers above the error bars indicate which groups (1-9) are significantly different from the indicated group. Samples that had no detectable LeTx-neutralizing activity were assigned a value of one less than the starting serum log2 dilution for statistical analysis.
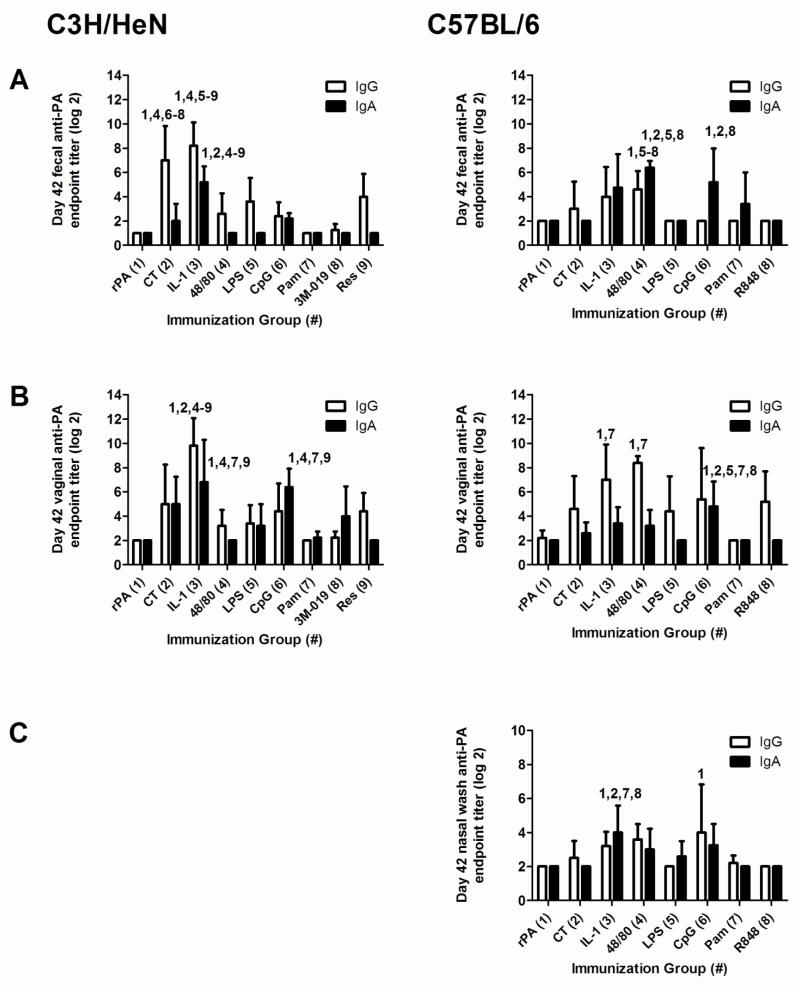
Non-toxin nasal adjuvants differentially induce antigen-specific mucosal IgA
IgA is the principal antibody isotype found in mucosal secretions and nasal immunization is capable of inducing humoral immunity at mucosal tissues such as the gastrointestinal and genitourinary tracts [8]. To evaluate the ability of the non-toxin adjuvants to enhance the induction of antigen-specific IgG and IgA in mucosal compartments, fecal (C3H/HeN and C57BL/6; Figure 4A) vaginal (C3H/HeN and C57BL/6; Figure 4B) and nasal wash (C57BL/6; Figure 4C) samples were collected on day 42 and assayed for anti-rPA IgA and IgG (Figure 4). Day 42 rPA-specific fecal IgA titers were significantly increased in the IL-1α group (5.2 ± 1.3) compared to all other groups in C3H/HeN mice. In C57BL/6 mice, fecal anti-rPA fecal IgA titers were significantly elevated in the c48/80 (6.4 ± 0.5) and CpG (5.2 ± 2.8) adjuvant groups.
Figure 4. Nasal immunization with rPA formulated with non-toxin adjuvants induces antigen-specific mucosal IgA and IgG.
Mice were immunized as described in Figure 1. The data represents one experiment in C3H/HeN mice and one experiment in C57BL/6 mice (4 – 5 mice per group). Day 42 fecal (A), vaginal (B) and nasal wash (C) samples were collected and tested for the presence of anti-rPA IgG and IgA by ELISA to determine anti-rPA IgA and IgG log2 endpoint titers. Bars represent mean ± standard deviation. Statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparison test. The numbers above the error bars indicate which groups (1-9) are significantly different from the indicated group. Samples that had no detectable anti-rPA IgG or IgA titers were assigned a log2 value of one less than the starting log2 dilution for statistical analysis.
In C3H/HeN mice, vaginal anti-rPA IgA titers were significantly increased in the IL-1α (6.8 ± 3.5) and CpG (6.4 ± 1.5) adjuvant groups compared to rPA alone (undetectable) or rPA plus c48/80, Pam3CKS4 and 3M-019. With C57BL/6 mice, CpG was the only adjuvant to induce elevated vaginal anti-rPA IgA titers (4.5 ± 2.0).
Nasal wash samples were only collected in C57BL/6 mice and IL-1α was the only adjuvant to induce significantly elevated nasal wash anti-rPA IgA responses (4.0 ± 1.6). Although many adjuvants provided effective nasal adjuvant activity as demonstrated by elevated antigen-specific serum IgG, the ability of an adjuvant to induce antigen-specific mucosal IgA did not correlate with its ability to induce antigen-specific serum IgG ELISA or LeTx-neutralizing antibody responses.
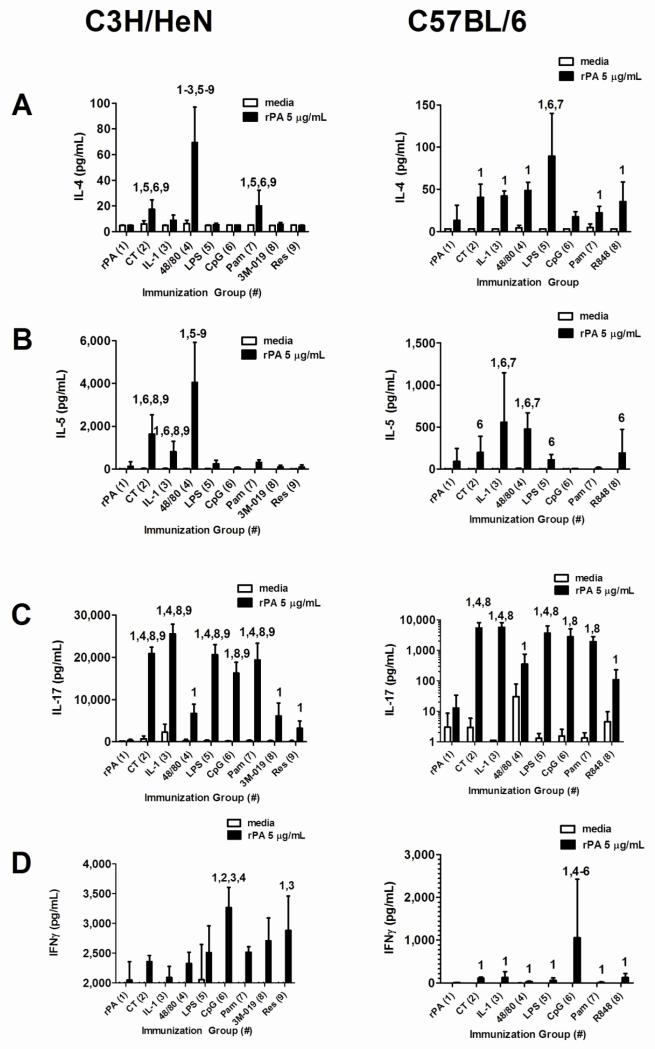
Non-toxin nasal adjuvants differentially induce antigen-specific cytokine responses
Antigen-specific cytokine responses were monitored to determine if nasal adjuvants induced unique adaptive cellular responses (Figure 5). For example, CT and CpG are known to induce Th2 and Th1-biased immune responses, respectively [31-33]. In C3H/HeN mice, antigen-stimulated cells isolated from mice immunized with rPA + c48/80 had the highest IL-4 (Figure 5A) production (69.2 ± 27.8 pg/ml) while CT (17.3 ± 7.4) and Pam3CSK4 (20.0 ± 12.1) also induced antigen-stimulated IL-4 production that was significantly elevated when compared to rPA-stimulated IL-4 production in cells isolated from mice immunized with rPA alone. In C57BL/6 mice, all adjuvants except CpG induced significantly elevated IL-4 levels when compared to mice immunized with rPA alone. LPS induced the highest antigen-stimulated IL-4 production (89.32 ± 50.8) in C57BL/6 mice.
Figure 5. Non-toxin adjuvants differentially influence the induction of antigen-specific lymphocyte cytokine responses after nasal immunization with rPA.
Mice were immunized as described in Figure 1. Splenocytes were harvested on day 42 and re-stimulated in culture at 1.25 × 106 cells per well (48 well plate) with or without rPA (5 μg/ml) to induce recall cytokine secretion by antigen-specific T cells. Supernatants were collected after 5 days of re-stimulation and assayed using Bio-Plex to identify protein levels of (A) IL-4, (B) IL-5, (C) IL-17 and (D) IFNγ. This data represents one experiment for each mouse strain (n = 5 per group). Each bar represents mean pg/ml ± standard deviation. Statistical analysis was performed using log10 transformed cytokine concentrations in the rPA stimulated cultures followed by ANOVA and Tukey’s multiple comparison test. The numbers above the error bars indicate which groups (1-9) are significantly different from the indicated group.
Antigen-stimulated IL-5 (Figure 5B) production was significantly increased in C3H/HeN mice with the use of CT (1,636 ± 903), IL-1α (807 ± 485) and c48/80 (4046 ± 1877) as nasal vaccine adjuvants with c48/80 inducing the greatest IL-5 production. rPA-induced IL-5 production in C57BL/6 mice was significantly elevated by the use of CT (198 ± 191), IL-1α (558 ± 556), c48/80 (477 ± 191), LPS (110 ± 63) and R848 (191 ± 282) with IL-1α inducing the greatest IL-5 production.
In both strains of mice, antigen-stimulated IL-17 (Figure 5C) production was significantly elevated by all adjuvants when compared to IL-17 production in cells isolated from mice immunized with rPA alone. In C3H/HeN mice, CT (20885 ± 1479), IL-1α (25520 ± 2278), LPS (20570 ± 2373) and Pam3CSK4 (19362 ± 3944) induced statistically similar antigen-stimulated IL-17 responses. In C57BL/6 mice, CT (5437 ± 2733), IL-1α (5704 ± 2436) and LPS (3709 ± 2653) induced statistically similar antigen-stimulated IL-17 responses.
Antigen-stimulated IFNγ (Figure 5D) production was the greatest for CpG in both C3H/HeN (3265 ± 341) and C57BL/6 (1059 ± 1365) mice. However, resiquimod also induced significantly elevated antigen-stimulated IFNγ production in C3H/HeN mice (2883 ± 575) and all adjuvants induced significantly increased antigen-stimulated IFNγ production in C57BL/6 mice. These results demonstrate that non-toxin nasal adjuvants induce diverse antigen-specific cytokine profiles and that the host utilized may influence the magnitude and profile of antigen-stimulated T cell cytokine production observed.
Discussion
In this study we have demonstrated that non-toxin adjuvants (IL-1α, c48/80 and TLR ligands) coadministered with anthrax rPA have the ability to provide adjuvant activity comparable or superior to the classic mucosal adjuvant CT for the induction of antigen-specific serum IgG, mucosal IgA and serum LeTx-neutralizing antibody responses after nasal immunization. Adjuvant-induced innate and adaptive immune signatures were unique to each adjuvant, were influenced by the mouse strain used as the host and did not predict in vivo adjuvant performance. This study provides useful data to allow comparison of the adjuvant activity of select non-toxin adjuvants for nasal delivery. Additional studies are needed to determine mechanistic differences between these nasal vaccine adjuvants.
It is important to emphasize that our nasal immunization studies utilized a very low dose of recombinant protective antigen (rPA) as the vaccine antigen. In our studies, we utilized 2.5 μg of rPA in each vaccine dose. This is in contrast to doses of 10, 25 and 40 μg utilized by others [31]. As others have reported[32], induction of LeTx neutralizing antibodies after nasal immunization is dose dependent with higher antigen doses inducing higher LeTx neutralizing antibody responses. We intentionally selected a low dose of antigen to provide a more sensitive assay to compare the efficacy of the test adjuvants using the induction of LeTx neutralizing antibodies as the indicator of effective adjuvant activity. We expected that the use of a low dose of rPA would provide an experimental system where some adjuvants would induce LeTx neutralizing antibody responses statistically different than other adjuvants within or between strains of mice and allow for a more robust comparison of adjuvant activity.
Although the adjuvant doses chosen were based on previously-published studies [18] [24] [14, 19-22], the adjuvant dose used may influence the induction of antigen-specific responses. Adjuvant dose-response studies are thus needed to determine the potential of the adjuvants to maximize induction of LeTx-neutralizing antibodies. Furthermore, the nasal vaccines were in an aqueous formulation and other formulations (e.g., dry powder [17, 34, 35]) may alter adjuvant activity and vaccine immunogenicity.
C3H/HeN mice have been shown to be less responsive to the adjuvant effect of orally-delivered CT compared to other strains of mice [36] suggesting that the nasal adjuvant activity of CT may also be impaired in C3H/HeN mice used in the present study. However, we observed that CT was statistically-equivalent to the most potent non-toxin adjuvant (IL-1α) on day 42 for the induction of antigen-specific serum IgG in C3H/HeN mice. While CT failed to provide significant adjuvant activity based on day 14 serum IgG titers in C3H/HeN mice, CT provided effective adjuvant activity in C57BL/6 mice at day 14 suggesting that the adjuvant activity of CT was influenced by the mouse strain. In contrast, although CT induced significantly elevated LeTx neutralizing antibodies in C3H/HeN mice at day 42, CT did not induce significantly elevated LeTx neutralizing antibodies in C57BL/6 mice under the conditions tested. Despite the differences observed with the use of CT in the two mouse strains, some consistencies were also observed between the mouse strains. Pam3CSK4 failed to induce serum LeTx-neutralizing activity after three immunizations in both strains of mice while c48/80 was the only adjuvant to induce LeTx-neutralizing activity in both C3H/HeN and C57BL/6 mice. Our results highlight the importance of testing adjuvants and vaccine regimens in different host strains to determine if the adjuvants provide reproducible adjuvant activity in hosts with different genetic backgrounds.
Serum anti-rPA IgG ELISA titers at day 42 did not correlate with day 42 serum LeTx neutralizing antibody responses. For example, in C3H/HeN mice, Pam3CSK4 induced significantly elevated rPA-specific IgG ELISA responses but did not induce LeTx-neutralizing antibody responses. In C57BL/6 mice, IL-1α, c48/80 and CpG induced statistically similar serum anti-rPA IgG ELISA responses while c48/80 was the only adjuvant that induced LeTx-neutralizing antibody responses at day 42. Potential explanations for the discrepancy between day 42 serum rPA-specific IgG ELISA titers and LeTx-neutralizing activity may include adjuvant-induced early effects on innate cytokine signaling. Our results confirm the ability of vaccine adjuvants to rapidly induce cytokine production by activation of the innate immune system as reported by others for parenteral [28, 29] and nasal immunization [25]. However, there were no obvious correlations between any individual cytokine/chemokine measured in 6 hour serum or nasal wash samples and day 42 serum LeTx-neutralizing activity (data not shown). Despite exhibiting significant nasal adjuvant activity for the induction of serum anti-rPA IgG responses, CpG did not induce significant changes in 6 hour serum or nasal wash cytokines in either strain of mice suggesting that the timing of sample collection may not have been optimal for this adjuvant. Alternatively, adjuvant-induced serum and/or nasal wash cytokine changes at 6 hours post-immunization as biomarkers of activation of the innate immune system are simply not reliable indicators of adjuvant effectiveness. Indeed, we have recently reported that IL-1α adjuvant-induced secretion of similar innate cytokines (including IL-6, G-CSF and KC) after nasal immunization was not required for the induction of maximal LeTx-neutralizing activity [25].
The differential induction of antigen-specific adaptive immune responses may also explain the discrepancy between day 42 serum rPA-specific IgG ELISA titers and LeTx-neutralizing activity. However, serum IgG subclass data suggested that LeTx-neutralizing activity did not favor anti-rPA IgG1 or IgG2a in C3H/HeN mice since adjuvants that induced greater IgG1 responses (CT, IL-1α, c48/80, and LPS), greater IgG2a responses (CpG) or balanced IgG1/IgG2a responses (3M-019 and resiquimod) were all able to induce significantly elevated LeTx-neutralizing antibody responses. In C57BL/6 mice, c48/80 was the only adjuvant to induce significantly elevated serum LeTx-neutralizing antibodies. However, IL-1α induced similar antigen-specific IgG subclass responses and T cell cytokine profiles to c48/80 but did not induce elevated serum LeTx neutralizing antibody responses. Our findings are consistent with previous reports showing that CpG, 3M-019 and resiquimod/R848 adjuvants favor the induction of Th1 cytokine-biased immune responses and IgG2a/c production (a Th1-dependent IgG subclass) [22, 32, 33, 37, 38] in contrast to Th2-skewing adjuvants like CT [31]. Our results indicate that antigen-specific serum IgG subclass and T cell cytokine responses are influenced by the adjuvant utilized, but diverse adaptive responses are equally capable of supporting the induction of effective LeTx-neutralizing activity.
Mucosal rPA-specific IgA and IgG were significantly increased on day 42 within the CT, IL-1α and CpG adjuvant groups in C3H/HeN mice and in the IL-1α, c48/80, and CpG groups in C57BL/6 mice. Our expectation was that mucosal IgA and IgG titers would be low relative to serum IgG due to the low dose of rPA used and that the mucosal responses induced in the current study would lack LeTx-neutralizing activity. Future studies are needed to determine if increasing the dose of rPA and/or adjuvant in the vaccine formulation improves mucosal antigen-specific IgA responses as described by Boyaka et al. [31]. Although the induction of serum LeTx-neutralizing anti-rPA IgG is relevant to protection against inhalation anthrax [35, 39], additional studies are needed using other model antigen systems to determine the efficacy of these adjuvants in the induction of protective mucosal immunity.
In addition to being highly efficacious, any vaccine adjuvant candidate must also be evaluated for safety. The use of CT as a vaccine adjuvant is associated with the induction of allergic sensitization that includes elevated antigen-specific and total IgE production, anaphylactic reactions and local inflammation in mouse models of mucosal immunization [3, 6, 40-42]. While the non-toxin adjuvants did not induce elevated IgE responses in C3H/HeN mice, IL-1α induced significantly elevated serum IgE responses in C57BL/6 mice. However, the significance of the elevated IgE responses after immunization with IL-1α is not clear since the total IgE concentrations induced by IL-1α were not significantly different than the total IgE concentrations induced by CpG, an adjuvant associated with Th1-type immune responses and acceptable safety in humans[43]. CT induced elevated total serum IgE responses in C3H/HeN mice but not in C57BL/6 mice, again demonstrating the influence of the host strain on the responses observed. Regardless of the total serum IgE responses measured, immediate hypersensitivity responses were absent in all mice in this study (data not shown). Previous studies in humans using some of these non-toxin compounds suggest that they may be suitable for human use. For example, c48/80 has been safely used in humans for dermal applications [44]. Also, the TLR agonists MPL [45, 46], CpG [47], LPS [48] have been safely administered in humans nasally or by lung instillation. Furthermore, resiquimod [49] has been well-tolerated as vaccine and therapeutic adjuvants, respectively, in cancer patients.
In conclusion, this study highlights the potential of non-toxin nasal vaccine adjuvants to induce serum LeTx-neutralizing antibody responses comparable or superior to CT without the adverse effects associated with CT. Although one goal of this study was to identify adjuvant-induced innate and adaptive factors that could serve as a biomarkers for effective adjuvant activity, our results suggest that the adjuvants tested induced unique innate cytokine profiles and Th1/Th2/Th17 responses which did not predict the ability of the adjuvants to induce high serum antibody titers with LeTx-neutralizing activity. Future studies are required to identify the mechanisms of action of non-toxin nasal vaccine adjuvants.
Highlights.
Non-toxin adjuvants were compared to cholera toxin for nasal adjuvant activity
Adjuvant-dependent innate and adaptive cytokine responses were monitored as biomarkers of adjuvant activity
Non-toxin adjuvants were as effective as cholera toxin when used as nasal adjuvants
Each adjuvant exhibited a unique biomarker signature
Acknowledgments
The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Anthrax Protective Antigen (PA), Recombinant from Bacillus anthracis, NR-140 and Anthrax Lethal Factor (LF), Recombinant from Bacillus anthracis, NR-570. The project described was supported by award numbers R01AI064879, R21AI059591 and U19AI056572 from the National Institute Of Allergy And Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Xiao BG, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85(2):119–28. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- [2].Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19(17-19):2534–41. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- [3].Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153(2):647–57. [PubMed] [Google Scholar]
- [4].van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting Edge: The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- [5].Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N Engl J Med. 2004 Feb 26;350(9):860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- [6].Hodge LM, Marinaro M, Jones HP, McGhee JR, Kiyono H, Simecka JW. Immunoglobulin A (IgA) Responses and IgE-Associated Inflammation along the Respiratory Tract after Mucosal but Not Systemic Immunization. Infect Immun. 2001 Apr 1;69(4):2328–38. doi: 10.1128/IAI.69.4.2328-2338.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Staats HF, Ennis FA., Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J Immunol. 1999 May 15;162(10):6141–7. [PubMed] [Google Scholar]
- [8].Bradney CP, Sempowski GD, Liao HX, Haynes BF, Staats HF. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol. 2002 Jan;76(2):517–24. doi: 10.1128/JVI.76.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Przetak M, Chow J, Cheng H, Rose J, Hawkins LD, Ishizaka ST. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine. 2003 Feb 14;21(9-10):961–70. doi: 10.1016/s0264-410x(02)00737-5. [DOI] [PubMed] [Google Scholar]
- [10].Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. Journal of Allergy & Clinical Immunology. 2004 Mar;113(3):448–54. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- [11].Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine. 2008 Dec 9;26(52):6777–83. doi: 10.1016/j.vaccine.2008.09.045. [DOI] [PubMed] [Google Scholar]
- [12].Caproni E, Tritto E, Cortese M, Muzzi A, Mosca F, Monaci E, et al. MF59 and Pam3CSK4 Boost Adaptive Responses to Influenza Subunit Vaccine through an IFN Type I-Independent Mechanism of Action. J Immunol. 2012 Feb 20; doi: 10.4049/jimmunol.1101764. [DOI] [PubMed] [Google Scholar]
- [13].Anonymous Technology to save millions, extends vaccine outreach programs. Vaccine management. Vaccine Weekly. 1996:16. [PubMed] [Google Scholar]
- [14].McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008 May;14(5):536–41. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- [15].Meng S, Liu Z, Xu L, Li L, Mei S, Bao L, et al. Intranasal immunization with recombinant HA and mast cell activator C48/80 elicits protective immunity against 2009 pandemic H1N1 influenza in mice. PLoS One. 6(5):e19863. doi: 10.1371/journal.pone.0019863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, et al. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS ONE. 2011;6(1):e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang SH, Kirwan SM, Abraham SN, Staats HF, Hickey AJ. Stable dry powder formulation for nasal delivery of anthrax vaccine. J Pharm Sci. 2012 Jan;101(1):31–47. doi: 10.1002/jps.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gwinn WM, Kirwan SM, Wang SH, Ashcraft KA, Sparks NL, Doil CR, et al. Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1. Vaccine. 2010 Oct 4;28(42):6901–14. doi: 10.1016/j.vaccine.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosset MB, Ballerini C, Gregoire S, Metharom P, Carnaud C, Aucouturier P. Breaking immune tolerance to the prion protein using prion protein peptides plus oligodeoxynucleotide-CpG in mice. J Immunol. 2004 May 1;172(9):5168–74. doi: 10.4049/jimmunol.172.9.5168. [DOI] [PubMed] [Google Scholar]
- [20].Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, et al. Cutting Edge: Activation of Toll-Like Receptor 2 Induces a Th2 Immune Response and Promotes Experimental Asthma. J Immunol. 2004 Mar 1;172(5):2739–43. doi: 10.4049/jimmunol.172.5.2739. 2004. [DOI] [PubMed] [Google Scholar]
- [21].Quarcoo D, Weixler S, Joachim RA, Stock P, Kallinich T, Ahrens B, et al. Resiquimod, a new immune response modifier from the family of imidazoquinolinamines, inhibits allergen-induced Th2 responses, airway inflammation and airway hyper-reactivity in mice. Clin Exp Aller. 2004 Aug;34(8):1314–20. doi: 10.1111/j.1365-2222.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- [22].Otero M, Calarota SA, Felber B, Laddy D, Pavlakis G, Boyer JD, et al. Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine. 2004 Apr 16;22(13-14):1782–90. doi: 10.1016/j.vaccine.2004.01.037. [DOI] [PubMed] [Google Scholar]
- [23].McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009 Jun 2;27(27):3544–52. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Jonge MI, Hamstra HJ, Jiskoot W, Roholl P, Williams NA, Dankert J, et al. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine. 2004 Sep 28;22(29-30):4021–8. doi: 10.1016/j.vaccine.2004.03.047. [DOI] [PubMed] [Google Scholar]
- [25].Thompson AL, Johnson BT, Sempowski GD, Gunn MD, Hou B, DeFranco AL, et al. Maximal adjuvant activity of nasally delivered IL-1alpha requires adjuvant-responsive CD11c(+) cells and does not correlate with adjuvant-induced in vivo cytokine production. J Immunol. 2012 Mar 15;188(6):2834–46. doi: 10.4049/jimmunol.1100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, et al. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infect Immun. 2007 Nov;75(11):5443–52. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Valensi JP, Carlson JR, Van Nest GA. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol. 1994 Nov 1;153(9):4029–39. [PubMed] [Google Scholar]
- [28].Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. J Immunol. 2008 Apr 15;180(8):5402–12. doi: 10.4049/jimmunol.180.8.5402. 2008. [DOI] [PubMed] [Google Scholar]
- [29].Korsholm KS, Petersen RV, Agger EM, Andersen P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology. 2010 Jan;129(1):75–86. doi: 10.1111/j.1365-2567.2009.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Danuser B, Rebsamen H, Weber C, Krueger H. Lipopolysaccharide-induced nasal cytokine response: a dose-response evaluation. Eur Arch Otorhinolaryngol. 2000 Dec;257(10):527–32. doi: 10.1007/s004050000285. [DOI] [PubMed] [Google Scholar]
- [31].Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective Mucosal Immunity to Anthrax: Neutralizing Antibodies and Th Cell Responses Following Nasal Immunization with Protective Antigen. J Immunol. 2003 Jun 1;170(11):5636–43. doi: 10.4049/jimmunol.170.11.5636. 2003. [DOI] [PubMed] [Google Scholar]
- [32].Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848) Vaccine. 2005 Nov 1;23(45):5263–70. doi: 10.1016/j.vaccine.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [33].Ghosh TK, Mickelson DJ, Fink J, Solberg JC, Inglefield JR, Hook D, et al. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006 Sep;243(1):48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [34].Wang SH, Thompson AL, Hickey AJ, Staats HF. Dry Powder Vaccines for Mucosal Administration: Critical Factors in Manufacture and Delivery. Curr Top Microbiol Immunol. 2011 Aug 6; doi: 10.1007/82_2011_167. [DOI] [PubMed] [Google Scholar]
- [35].Huang J, Mikszta JA, Ferriter MS, Jiang G, Harvey NG, Dyas B, et al. Intranasal administration of dry powder anthrax vaccine provides protection against lethal aerosol spore challenge. Hum Vaccines. 2007 May-Jun;3(3):90–3. doi: 10.4161/hv.3.3.4011. [DOI] [PubMed] [Google Scholar]
- [36].Elson CO. Cholera toxin as a mucosal adjuvant: effects of H-2 major histocompatibility complex and lps genes. Infect Immun. 1992;60(7):2874–9. doi: 10.1128/iai.60.7.2874-2879.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Durand V, Wong SY, Tough DF, Le Bon A. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-alpha/beta. Immunology & Cell Biology. 2004 Dec;82(6):596–602. doi: 10.1111/j.0818-9641.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- [38].Johnston D, Zaidi B, Bystryn JC. TLR7 imidazoquinoline ligand 3M-019 is a potent adjuvant for pure protein prototype vaccines. Cancer Immunol Immunother. 2007 Aug;56(8):1133–41. doi: 10.1007/s00262-006-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004 Jan 2;22(3-4):422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [40].Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995 Nov 15;155(10):4621–9. [PubMed] [Google Scholar]
- [41].Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Yamamoto M, Fujihashi K, et al. A Nontoxic Mutant of Cholera Toxin Elicits Th2-Type Responses For Enhanced Mucosal Immunity. Proc Natl AcadSci (USA) 1997;94(10):5267–72. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, et al. Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. 2005 Dec 1;167 doi: 10.1016/S0002-9440(10)61246-1. 2005. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, et al. Marked enhancement of the immune response to BioThrax(R) (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine. 2011 Aug 26;29(37):6313–20. doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- [44].Goldsmith P, Bunker C, Leslie T, Foreman J, Dowd PM. The effect of topical steroid on the actions of vasoconstrictor and vasodilator peptides in human skin. Skin Pharmacology. 1996;9(5):289–97. doi: 10.1159/000211427. [DOI] [PubMed] [Google Scholar]
- [45].Couch RB, Atmar RL, Cate TR, Quarles JM, Keitel WA, Arden NH, et al. Contrasting effects of type I interferon as a mucosal adjuvant for influenza vaccine in mice and humans. Vaccine. 2009 Aug 27;27(39):5344–8. doi: 10.1016/j.vaccine.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010 Dec 1;202(11):1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mansson A, Bachar O, Adner M, Cardell LO. Nasal CpG oligodeoxynucleotide administration induces a local inflammatory response in nonallergic individuals. Allergy. 2009 Sep;64(9):1292–300. doi: 10.1111/j.1398-9995.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- [48].Virtala R, Ekman AK, Jansson L, Westin U, Cardell LO. Airway inflammation evaluated in a human nasal lipopolysaccharide challenge model by investigating the effect of a CXCR2 inhibitor. Clin Exp Allergy. Apr;42(4):590–6. doi: 10.1111/j.1365-2222.2011.03921.x. [DOI] [PubMed] [Google Scholar]
- [49].Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. Jul 15;17(14):4844–53. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]