Abstract
Purpose
To determine differences in the clinical characteristics and antifungal susceptibility patterns among molecularly characterized ocular Fusarium sp isolates.
Methods
58 Fusarium isolates obtained from 52 eyes of 52 patients were retrieved from the Bascom Palmer Eye Institute’s (BPEI) ocular microbiology laboratory and grown in pure culture. These isolates were characterized based on DNA sequence analysis of the ITS1/2 and rDNA regions. Antifungal susceptibilities were determined for each isolate using broth microdilution methods and the corresponding medical records were reviewed to determine clinical outcomes.
Results
Fusarium (F.) solani isolates had significantly higher voriconazole MIC90 values than F. non-solani organisms (16 and 4ug/ml, respectively). F. solani isolates also exhibited a significantly longer time to cure (65 vs 40.5 days), a worse follow up BCVA (20/118 vs 20/36), and increased need for urgent surgical management (7 vs 0 penetrating keratoplasties) when compared to F. non-solani isolates.
Conclusions
This is the first report to examine the correlation between ocular genotyped Fusarium species and clinical outcomes. It supports the overall worse prognosis for F. solani versus F. non-solani isolates, including higher voriconazole resistance by the former. The clinical implementation of molecular-based diagnostics and antifungal efficacy testing, may yield important prognostic and therapeutic information that could improve the management of fungal ocular infections.
Keywords: keratitis, Fusarium sp, genotyping, antifungal, clinical outcomes
Introduction
Following the recent global outbreak of contact lens associated Fusarium keratitis (2005–2006) there has been a renewed interest and significant focus of research establishing epidemiology, classification, diagnosis and treatment standards. It is now clear that the incidence of Fusarium keratitis has increased dramatically over the past 4 decades, accounting for up to 50% of all microbial keratitis cases in tropical climates. 1–9 This increasing incidence is multi-factorial and is believed to be due to increased awareness and changes in risk factor profiles throughout the global population, including an increase in the use of topical steroids and antibacterial agents, as well as an increase in surgical procedures, contact lens use, ocular trauma, chronic ocular surface diseases, and immune compromised patients 6, 10–13.
Among the Fusarium species that are pathogenic to the eye, F. solani is the most common, followed by F. oxysporum, F. dimerum, F. incaratum-equiseti, and Gibberella fujikuroi. 14, 15 Microbiologic techniques have long served as the diagnostic gold standard in the setting of fungal keratitis. Such techniques are capable of reliably differentiating among the different molds and yeasts causing keratitis. Although highly accurate at genus identification, (Fusarium, Aspergillus, Candida, ect.) morphologic classification of Fusarium isolates to the species level using microbiologic techniques (F. solani, F. oxysporum, etc) is problematic and inconsistent due to the large degree of morphologic variability demonstrated at different growth stages.15, 16 In light of the increasing incidence of Fusarium keratitis, this inconsistency in morphologic identification has generated significant interest in finding a more consistent and reliable basis for organism classification. Recent reports have demonstrated genotypic identification systems as a more accurate and reproducible means of properly identifying ocular Fusarium pathogens 14, 17 and a clear consensus has emerged 18–21 that DNA sequence-based methods will be essential for rapid species identification of the Fusarium genus in clinical laboratories22.
The question remains: is it clinically important to accurately differentiate between the different Fusarium species causing keratitis? Recent reports suggest that filamentous fungi harbor unique species-specific in vitro susceptibility profiles to the existing and emerging antifungal agents 23–25. Within the Fusarium genus and among isolates pathogenic to the eye, however, these species-specific antifungal susceptibility profiles have not been firmly established 26, 27. Furthermore, although the etiology and epidemiology of Fusarium keratitis has been well studied 3–5, 28, 29, very little has been revealed about the differences in clinical characteristics and outcomes associated with infections due to different Fusarium species.
In this study, we investigate and compare the in vitro susceptibility profiles and provide the first report of the clinical characteristics and outcomes among ocular pathogenic Fusarium isolates classified by genotypic analysis. Such information is useful from a prognostic, diagnostic and therapeutic viewpoint to determine the level of pathogen identification that has the potential to directly influence practice patterns and patient outcomes.
Methods
Isolates
Fifty-eight Fusarium sp isolates, representing 52 patients, were retrospectively selected from the Bascom Palmer microbiology isolate library based on the morphologic species classification to include approximately 20 isolates of each: F. solani, F. oxysporum and F. species without further designation. We included consecutive isolates from May 2005 to June 2007. In addition, this group of isolates was supplemented with samples dating back to April 2000 to achieve the desired equal distribution from each morphological designation. The source distribution of the isolates was cornea (41), aqueous humor (4), vitreous (1), contact lens (8), and contact lens case (4). A recent paper from the authors of this study 15, explains in detail the genotyping procedures, conforming to the most recent Fusarium species complexes classification system14, 21. The quality control (QC) reference strains Candida albicans ATCC 90028, Candida parapsilosis ATCC 22019, Candida krusei ATCC 6258 and Aspergillus flavus ATCC 204304 were included as control isolates for the Clinical and Laboratory Standards Institute (CLSI) (Formerly NCCLS) testing method.
Antifungal Agents
The methodology as described in the CLSI document M38-A reference was followed for broth dilution antifungal susceptibility testing of filamentous fungi. Additive drug dilutions were prepared to yield twice the final strength required for the test. Stock solutions of amphotericin B (AMB) and natamycin (NAT) at their final concentrations were frozen at −80°C until needed. The agents evaluated in this study have well-established microdilution MIC ranges for these QC strains (M38-A). Thirty isolates were also sent to the University of Texas fungal testing laboratory for voriconazole (VOR) susceptibility testing and identity confirmation (following CLSI M38-A methodology).
Medical records review
Following Institutional Review Board (IRB) approval, the medical records from 50 of the 52 affected patients were available for review. All patients included in the chart review had positive corneal culture results for Fusarium sp growth or positive culture from contact lens paraphernalia plus clinical and confocal microscopy appearance of filamentous fungal corneal infection.
The follow up BCVA of patients who underwent penetrating keratoplasty (PK), was the last BCVA measured prior to surgery. For this group of patients, the time to cure was assigned as an estimate, which was the longest time to cure among clinically treated patients.
The follow up BCVA of the patients treated with topical or oral antimicrobial agents, was the VA measured when the patient was considered cured (inactive corneal scar with intact epithelium).
Snellen visual acuities were converted to logMAR by the formula logMAR acuity = minus log (numerator Snellen/denominator Snellen) for the purpose of data analysis. Vision levels classified as count fingers, hand motion, light perception, and no light perception were assigned Snellen acuities of 1/200, .5/200, 20/20,000, and 20/200,000. The corresponding logMAR VAs were 2.3, 2.6, 3.0, 4.0 respectively, similar to a previously published scale 30.
Statistical analyses were performed using SPSS software version 15.0 (SPSS Inc, Chicago, Illinois, USA). Tests of significance were two-tailed with p ≤0.05 for all tests. Univariate comparisons between F. solani and F. non-solani were performed using the two-sided Student t-test for continuous variables. Non-parametric tests (Mann-Whitney Test) were used for analyzing the data which does not follow normal distribution. Chi-square or Fisher’s exact test was performed for categorical variables. Analysis of Variance (ANOVA) was performed for multiple comparisons.
Results
Microbiological and Molecular Identification
Identification of the organisms as belonging to the Fusarium genus took a mean of 3.6 (±2.9) days, with a range of 2 to 12 days, whereas identification to the species level using microbiological techniques required a mean of 8 (±3.7) days.
Molecular identification took up to 24 hours to identify the isolates to the species level. Based on genotype, the 58 isolates were classified into one of five groups: F. solani species complex (FSSC) (75%), F. oxysporum species complex (FOSC) (16%), F. incarnatum-equiseti species complex (FIESC) (5%), F. dimerum species complex (FDSC) (2%), and Fusarium species not otherwise identified (2%). Sequence analysis showed 15 distinct sequences among the 58 isolates. For the purposes of data analysis, and due to isolate characteristics homogeneity, the isolates were divided into 2 groups: F. solani and F. non-solani, which includes all species other than F. solani.
Clinical Characteristics and Epidemiology
The 58 samples were isolated from 52 eyes of 52 patients. The clinical characteristics are shown in table 1.
Table 1.
Clinical characteristics of Fusarium sp keratitis
|
Fusarium sp total (N=50) |
F. solani (N=38) |
F. non-solani (N=12) |
p-value | ||
|---|---|---|---|---|---|
| Gender | Male | 22 (44%) | 17 (45%) | 5 (42%) | 1.00* |
| Female | 28 (56%) | 21 (55%) | 7 (58%) | ||
| Age (mean(SD)) | 39 (19) | 39 (20) | 40 (18) | 0.97** | |
| CL wear | 33 (66%) | 24 (63%) | 9 (75%) | 0.51* | |
| Trauma | 9 (18%) | 7 (18%) | 2 (17%) | 1.00* | |
| Infiltrate’s location | 1.00* | ||||
| Central 6mm | 36 (72%) | 27 (71%) | 9 (75%) | ||
| Peripheral | 14 (28%) | 11 (29%) | 3 (25%) | ||
| Smear (+) results | |||||
| Cornea | 14/34 (41%) | 11/29 (38%) | 3/5 (60%) | 0.63* | |
| CL smear | 1/12 (8%) | 1/5 (20%) | 0/7 (0%) | 0.42* | |
| A/C | 0/3 (0%) | 0/3 (0%) | 0 | N/A | |
| Vitreous | 0/1 (0%) | 0/1 (0%) | 0 | N/A | |
| Total | 15/50 (30%) | 12/38 (32%) | 3/12 (25%) | 1.00* | |
| Time to (+) culture† (days) | 3.6 (2.9) (Range: 2–12) |
3.5 (3.1) (range: 2–12) |
3.8 (2.1) (range: 2–11) |
0.8** | |
| Treatment delay (days) | 9.7 (7) (range: 0–35) |
10.3 (7.6) (range: 0–35) |
7.2 (4.3) (range: 1–12) |
0.22** | |
| Time to cure (days) | 60 (range: 10–100) |
65 (range: 10–100) |
40.5 (range: 10–92) |
0.035*** | |
| Initial BCVA LogMAR (Snellen) | 0.83 (20/135) (range: 20/20-LP) |
0.89 (20/155) (range: 20/20-LP) |
0.65 (20/89) (range: 20/25-HM) |
0.38** | |
| Follow up BCVA LogMAR (Snellen) | 0.65 (20/89) (range: 20/20-LP) |
0.77 (20/118) (range: 20/20-LP) |
0.25 (20/36) (range:20/20–20/100) |
0.01** | |
| Penetrating Keratoplasty | 7 (14%) | 7 (18%) | 0 (0%) | 0.17* | |
| Follow up BCVA<20/200 | 9 (19%) | 9 (25%) | 0 (0%) | 0.09* | |
Treatment delay= time from the onset of symptoms to the initiation of antifungal agents.
Time to cure= duration of treatment necessary to eliminate evidence of clinically active infection.
Fisher’s exact test
t-test
Mann-Whitney test
Genus level (Fusarium sp)
The follow up time in this study had a mean of 7.3 (±6) months, ranging from 15 days to 24 months. The time from the onset of symptoms until the first evaluation at BPEI ranged from 1 to 33 days, with a mean of 6.6 (±7.1) days. No patients had additional pathology at baseline that accounted for decreased visual acuity at any time during the management.
The infiltrates involved the central 6mm of the cornea in 36 of 50 cases, with no difference observed between F. solani (71%) and F. non-solani isolates (75%). Compared to peripheral infiltrates, central infiltrates were associated with a statistically significant worse initial BCVA (20/235 and 20/33, respectively, p<0.001, t-test) and final BCVA (20/142 and 20/28, respectively, p=0.001, t-test) when compared to peripheral infiltrates.
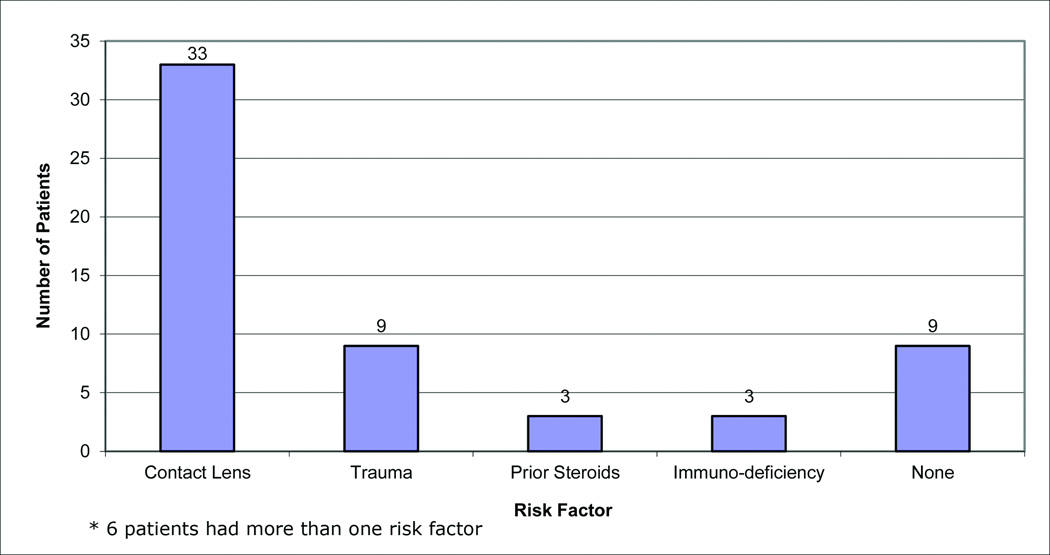
Risk factors
Risk factors for infectious keratitis were found in 49 patients (Figure 1). Among the 38 patients with F. solani infection, 24 were CL wearers (63%) and among the 12 patients with F. non-solani infection, 9 were CL wearers (75%). The frequency of trauma history between F. solani and F. non-solani infections was similar (20%) in both groups. Three patients had history of previous topical steroid use (2 in the F. solani and 1 in the F. non-solani group).
Figure 1.
Risk factors among Fusarium sp keratitis patients*.
*There were 6 patients that had more than 1 risk factor.
Management
Complete information of the treatment protocols was present in the records of 48 patients. Both topical and systemic antimicrobial agents were used for treatment of these infections. Topical natamycin 5% (Natacyn®, Alcon Labs, Fort Worth, TX) was used in 41 cases, and was used as monotherapy in 26 cases and in combination with other antimicrobial agents in 15 cases. Topical voriconazole 1% (compounded from Vfend®, Pfizer Inc, New York, NY) was prescribed in 7 cases, oral fluconazole 100mg (generic fluconazole, Cipla Pharmaceuticals, Mumbai, India) BID in 6 cases, topical amphotericin B 0.15% (compounded from generic amphotericin B, X-Gen Pharmaceuticals Inc, Big Flats, NY) in 4 cases, moxifloxacin 0.5% (Vigamox®, Alcon Labs, Fort Worth, TX) in 3 cases, combination of fortified cefazolin 5% (compounded from generic cefazolin, Abraxis BioScience, Los Angeles, CA) with tobramycin 1.4% (compounded from Ak-Tob® 0.3%, Akorn, Lake Forest, IL + generic tobramycin, Hospira Pharmaceuticals, Lake Forest, IL) in 3 cases each and oral valacyclovir 500mg (Valtrex®, GlaxoSmithKline, London, UK) 3x/day in 1 case.
Therapeutic penetrating keratoplasty (PK) was necessary in 7 cases, all of which were caused by F. solani isolates. The time from diagnosis to PK ranged from 4 to 57 days, with a mean of 28 (±22) days. The indications for PK were perforation in 4 cases and severe infiltrates non-responsive to clinical therapy in 3 cases. Four of the therapeutic grafts failed (three due to rejection and one to stromal melting) and repeat PK was successful in 2 cases.
Outcomes
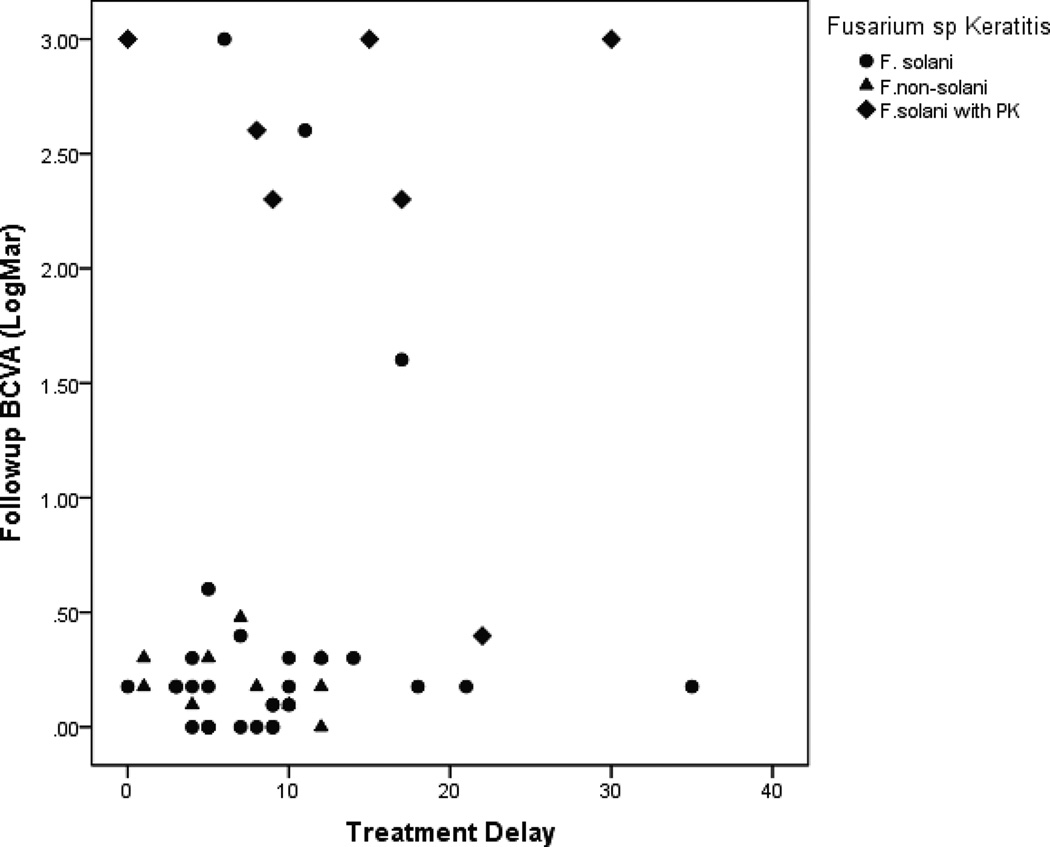
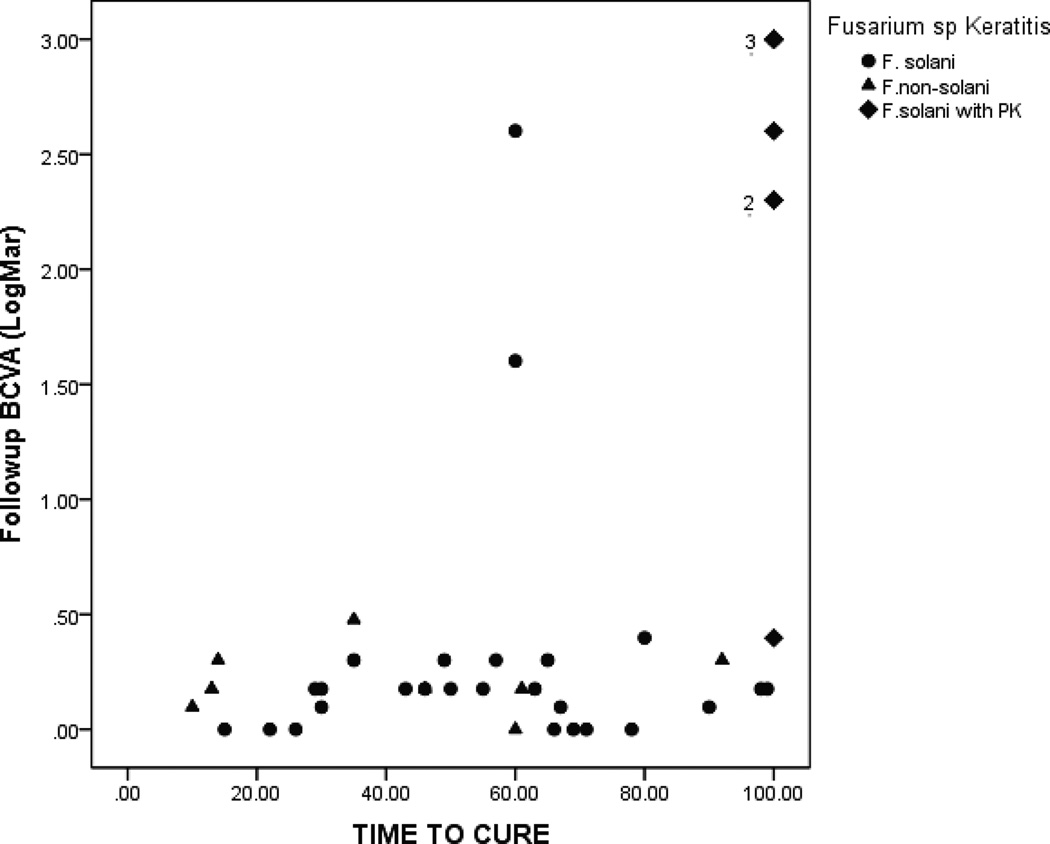
Treatment delay (Figure 2) and time to cure (Figure 3) had a significant correlation with the follow up BCVA of F. solani versus F. non-solani isolates.
Figure 2.
Correlation of treatment delay and follow up BCVA for F. solani vs F. non-solani isolates.
Figure 3.
Time to cure versus follow up BCVA in patients with Fusarium sp keratitis
Among the 26 patients that received natamycin as monotherapy, 22 (85%) ended with BCVA ≥ 20/40, and the other 4 patients (15%) had a follow up BCVA between 20/60 and 20/80 (Table 2).
Table 2.
Treatment regimen versus visual outcomes.
| Nata Only (N=26) |
Nata+Azoles (N=11) |
Nata+Others (N=4) |
Others (N=7) |
P-value | |
|---|---|---|---|---|---|
| Mean Initial BCVA | 0.66 (0.73) 20/91 |
1.27 (0.99) 20/372 |
0.54 (0.38) 20/69 |
0.92 (0.99) 20/166 |
0.20* |
| Mean Follow up BCVA | 0.24 (0.46) 20/35 |
1.74 (1.24) 20/1099 |
0.11 (0.08) 20/26 |
0.73 (1.12) 20/107 |
<0.001* |
| Mean BCVA improvement | 0.43 (0.67) | −0.47† (1.23) | 0.43 (0.31) | 0.32 (0.42) | 0.03* |
| Initial BCVA Better than or equal to 20/40 | 11 (42.3%) | 1 (9.1%) | 2 (50%) | 2 (28.6%) | 0.22** |
| Follow up BCVA Better than or equal to 20/40 | 22 (88%) | 3 (27.3%) | 4 (100%) | 4 (66.7%) | 0.001** |
| BCVA improve 2 or more lines | 14 (56%) | 2 (18.2%) | 4 (100%) | 3 (50%) | 0.032** |
Analysis of Variance (ANOVA) was performed.
Chi-Square Test
Mean follow up BCVA was worse than mean initial BCVA.
Natamycin was used as combined therapy in 15 cases, 11 used natamycin with azoles. Eight of these patients (73%) underwent PK or had final BCVA < 20/400. The other three patients (27%) had a good final outcome, with BCVA ≥ 20/40.
Among the 9 patients with a follow up BCVA <20/200, risk factors were identified in 8 cases (6 CL wearers, 2 used previous topical steroids, 2 immunosupressed patients) and PK was performed in 6 cases. The initial BCVA in this group was LogMAR 1.25 (20/355) and follow up BCVA 1.66 (20/915).
Antifungal Susceptibility Testing
Minimum inhibitory concentrations for 50 and 90% of the isolates (MIC50 and MIC90), medians, and MIC ranges for F. solani and F. non-solani isolates are listed in table 3.
Table 3.
Voriconazole, Natamycin and Amphotericin B MIC50’s, MIC90’s and medians (ug/ml).
| Voriconazole | Amphotericin B | Natamycin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 MIC90 (range) |
Median | p-value | MIC50 MIC90 (range) |
Median | p-value | MIC50 MIC90 (range) |
Median | p-value | |
| F. solani (n=44) | 16 16 (4- ≥16) n=15 |
16 | <0.001* | 2 2 (1-≥16) n=43 |
2 | 0.07* | 4.8 4.8 (2.4–9.6) n=44 |
4.8 | 0.37* |
| F. non-solani (n=14) | 4 4 (2-≥16) n=12 |
4 | 2 2 (1–2) n=14 |
2 | 2.4 4.8 (2.4–4.8) n=14 |
3.6 | |||
MIC50 and MIC90 values at 48 hours.
Mann-Whitney Test
In general, the isolates were equally susceptible to natamycin and amphotericin B. F.solani organisms had significantly higher voriconazole MIC values than F. non-solani organisms.
Discussion
A wide spectrum of Fusarium is known to be pathogenic in the human eye. Our improved understanding of this spectrum begs the questions we set forth to address in this study, namely to determine clinical characteristics and antifungal susceptibilities within the Fusarium genus. Currently, the literature describing these relationships is sparse. Defining these inherent qualities is critical to determining the clinical significance of accurate and rapid species identification.
Clinical Characteristics and Outcomes
In this study, F. solani isolates were associated with significantly longer treatment course (p=0.035, Mann-Whitney test), worse follow up BCVA (p=0.01, t-test), and higher necessity for PK compared to F. non-solani isolates. These observations suggest increased pathogenicity and differences between in vitro and in vivo antifungal susceptibility among F. solani isolates compared to other species within the genus.
Interestingly, F. solani isolates were associated with poorer outcomes compared to other species of Fusarium, despite slight differences between in vitro susceptibilities for the most commonly available and utilized topical antifungal agents (natamycin and amphotericin B). This observation suggests that other factors may be responsible for this discordance between in vitro and in vivo antifungal activities, like inter-species differences in pathogenicity.
Systemically, F. solani organisms are significantly more pathogenic and incur a higher risk of mortality compared to F. non-solani isolates 31. The suggestion of enhanced pathogenicity among F. solani isolates (compared to other Fusarium species) observed in our study, as well as other non-ocular studies, may be explained by species-specific differences in virulence strategies, including organism adherence to the ocular surface 32, invasion of the organism into the corneal stroma 33, or alteration of host and pathogen defense mechanisms 34.
Antifungal susceptibility
The increasing use of DNA-based identification schemes to accurately and rapidly identify fungal organisms to the species level has brought to light the importance of defining specific antifungal susceptibility profiles. This is particularly important for the Fusarium genus, for which minimum inhibitory concentrations (MIC’s) are higher and more variable than for other molds (e.g. Aspergillus sp) 23, 26, 27.
The results of the in vitro susceptibility tests from the isolates in this study (Table 2, 3) demonstrated high levels of in vitro resistance among Fusarium sp to available antifungal agents, particularly when compared to published susceptibility profiles of other filamentous fungi, including Aspergillus sp and Paecilomyces sp 26, 35–38. Across the Fusarium genus, amphotericin B had the lowest MIC values. Finally, different patterns of susceptibility were noted among the different Fusarium species. Specifically, this study demonstrates significantly higher MIC values (p<0.001, Mann-Whitney test) for voriconazole among F. solani isolates compared to F. non-solani isolates.
By the present date, six studies have explored in vitro antifungal susceptibility patterns among genetically characterized Fusarium isolates 26, 27, 37–40. These studies consistently demonstrate variable MICs and high levels of resistance across the spectrum of antifungal agents.
Repeatedly, F. solani isolates have been reported to exhibit greater resistance to antifungal agents than F. non-solani in studies using both morphologic identification 23, 25, 41–45 and molecular characterization 37–39. Among the studies utilizing reliable molecular identification techniques to characterize the isolates 37–39, F. solani isolates demonstrated higher levels of resistance to voriconazole, posaconazole, and pentamidine when compared to other members of the Fusarium genus.
Management
In the present study, 11 patients received a combined therapy of natamycin and azoles. A statistically significant amount of patients in this group experienced a worse outcome (follow up BCVA < 20/40 and loss of ≥2 lines of BCVA) when compared to the other groups (p<0.001 and 0.032, Chi-square test, respectively). Alternatively, the cases with combination therapy may have had more severe disease which was not reflected by the visual acuity. Of note, it has been demonstrated that combination treatment may produce antagonistic interactions in vitro, particularly when natamycin is used in combination with azoles 46.
Limitations of the study
The lower prevalence of F. non-solani when compared with F. solani infections, did not allow for selection of an even distribution of Fusarium species to be genotyped. In future studies, an equal distribution and larger number of isolates from all species would provide more power for further inter-species comparisons. Furthermore, due to the inherent limitations in a retrospective study, the clinical data was incomplete in some cases, specifically risk factor assessment and follow-up information.
Conclusions
This study demonstrates important differences in antifungal susceptibility profiles and clinical characteristics among Fusarium isolates causing keratitis, and suggests inter-species differences in virulence mechanisms yet to be explored. Future studies comparing in vitro and in vivo antifungal activity of the available antifungal agents alone and in combination will allow us to accurately assess drug interactions and optimize specific treatment strategies. Furthermore, this study suggests that accurate species identification, especially if performed with rapid PCR techniques, may yield important prognostic and therapeutic information that can influence management decisions and improve patient outcomes in the setting of Fusarium keratitis.
Acknowledgements
We would like to thank Richard Stratton from the BPEI imaging department, for helping with the image processing.
Sources of funding:
Institutional grants from Research to Prevent Blindness and National Eye Institute; University of Miami Wallace H. Coulter Center for Translational Research; Funding from the Coulter Center was provided by a matching grant from Bausch & Lomb Inc. and Alcon Labs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work has been presented at:
Award of Best Poster presented at the Cornea and External Disease Section, American Academy of Ophthalmology (AAO) meeting, 2008
Ocular Microbiology and Immunology Group (OMIG) meeting, 2008
Association for Research in Vision and Ophthalmology (ARVO) meeting, 2009
[None of the authors have any financial interests to disclose]
References
- 1.Xie L, Hu J, Shi W. Treatment failure after lamellar keratoplasty for fungal keratitis. Ophthalmology. 2008;115:33–36. doi: 10.1016/j.ophtha.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 2.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 3.Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101:1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 5.Godoy P, Cano J, Gene J, et al. Genotyping of 44 isolates of Fusarium solani, the main agent of fungal keratitis in Brazil. J Clin Microbiol. 2004;42:4494–4497. doi: 10.1128/JCM.42.10.4494-4497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 7.Garg P, Gopinathan U, Choudhary K, et al. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107:574–580. doi: 10.1016/s0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 8.Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117:2263–2267. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Balbuena AL, Vanzzini-Rosano V, Valadéz-Virgen Jde J, et al. Fusarium keratitis in Mexico. Cornea. 2009;28:626–630. doi: 10.1097/ICO.0b013e31819bc2ea. [DOI] [PubMed] [Google Scholar]
- 10.Tanure MA, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hofling-Lima AL, Forseto A, Duprat JP, et al. Laboratory study of the mycotic infectious eye diseases and factors associated with keratitis. Arq Bras Oftalmol. 2005;68:21–27. doi: 10.1590/s0004-27492005000100005. [DOI] [PubMed] [Google Scholar]
- 12.Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea. 2009;28:638–643. doi: 10.1097/ICO.0b013e318191695b. [DOI] [PubMed] [Google Scholar]
- 13.Wykoff CC, Flynn HW, Jr, Miller D, et al. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008;115:1501–1507. doi: 10.1016/j.ophtha.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell K, Sarver BA, Brandt M, et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J Clin Microbiol. 2007;45:2235–2248. doi: 10.1128/JCM.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oechsler RA, Feilmeier MR, Ledee D, et al. Utility of Molecular Sequence Analysis of the ITS rRNA Region for Identification of Fusarium spp from Ocular Sources. Invest Ophthalmol Vis Sci. 2009;50:2230–2236. doi: 10.1167/iovs.08-2757. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao CR, Huang L, Bouchara JP, et al. Identification of medically important molds by an oligonucleotide array. J Clin Microbiol. 2005;43:3760–3768. doi: 10.1128/JCM.43.8.3760-3768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang DC, Grant GB, O'Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell K, Kistler HC, Tacke BK, et al. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci USA. 2000;97:7905–7910. doi: 10.1073/pnas.130193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell K, Sutton DA, Rinaldi MG, et al. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J Clin Microbiol. 2004;42:5109–5120. doi: 10.1128/JCM.42.11.5109-5120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, O'Donnell K, Sutton DA, et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfonso EC. Genotypic identification of Fusarium species from ocular sources: comparison to morphologic classification and antifungal sensitivity testing (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:227–239. [PMC free article] [PubMed] [Google Scholar]
- 22.He D, Hao J, Zhang B, et al. Pathogenic spectrum of fungal keratitis and specific identification of Fusarium solani. Invest Ophthalmol Vis Sci. 2011;52:2804–2808. doi: 10.1167/iovs.10-5977. [DOI] [PubMed] [Google Scholar]
- 23.Arikan S, Lozano-Chiu M, Paetznick V, et al. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J Clin Microbiol. 1999;37:3946–3951. doi: 10.1128/jcm.37.12.3946-3951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dotis J, Simitsopoulou M, Dalakiouridou M, et al. Amphotericin B formulations variably enhance antifungal activity of human neutrophils and monocytes against Fusarium solani: comparison with Aspergillus fumigatus. J Antimicrob Chemother. 2008;61:810–817. doi: 10.1093/jac/dkn036. [DOI] [PubMed] [Google Scholar]
- 25.Lewis RE, Wiederhold NP, Klepser ME. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob Agents Chemother. 2005;49:945–951. doi: 10.1128/AAC.49.3.945-951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azor M, Gene J, Cano J, et al. Universal in vitro antifungal resistance of genetic clades of the Fusarium solani species complex. Antimicrob Agents Chemother. 2007;51:1500–1503. doi: 10.1128/AAC.01618-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell K, Sutton DA, Fothergill A, et al. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J Clin Microbiol. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfonso EC, Cantu-Dibildox J, Munir WM, et al. Insurgence of Fusarium keratitis associated with contact lens wear. Arch Ophthalmol. 2006;124:941–947. doi: 10.1001/archopht.124.7.ecs60039. [DOI] [PubMed] [Google Scholar]
- 29.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 30.Scott IU, Schein OD, West S, et al. Functional status and quality of life measurement among ophthalmic patients. Arch Ophthalmol. 1994;112:329–335. doi: 10.1001/archopht.1994.01090150059023. [DOI] [PubMed] [Google Scholar]
- 31.Mayayo E, Pujol I, Guarro J. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J Med Microbiol. 1999;48:363–366. doi: 10.1099/00222615-48-4-363. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Ahearn DG, Stulting RD, et al. Differences among strains of the Fusarium oxysporum-F. solani complexes in their penetration of hydrogel contact lenses and subsequent susceptibility to multipurpose contact lens disinfection solutions. Cornea. 2007;26:1249–1254. doi: 10.1097/ICO.0b013e318148bd9a. [DOI] [PubMed] [Google Scholar]
- 33.Hua X, Yuan X, Di Pietro A, et al. The molecular pathogenicity of Fusarium keratitis: a fungal transcriptional regulator promotes hyphal penetration of the cornea. Cornea. 2010;29:1440–1444. doi: 10.1097/ICO.0b013e3181d8383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasanthi M, Prajna NV, Lalitha P, et al. A pilot study on the infiltrating cells and cytokine levels in the tear of fungal keratitis patients. Indian J Ophthalmol. 2007;55:27–31. doi: 10.4103/0301-4738.29491. [DOI] [PubMed] [Google Scholar]
- 35.Espinel-Ingroff A, Boyle K, Sheehan DJ. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia. 2001;150:101–115. doi: 10.1023/a:1010954803886. [DOI] [PubMed] [Google Scholar]
- 36.Pradhan L, Sharma S, Nalamada S, et al. Natamycin in the treatment of keratomycosis: correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J Ophthalmol. 2011;59:512–514. doi: 10.4103/0301-4738.86328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, et al. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–809. doi: 10.1093/jac/dkn022. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal NJ, Boey A, Park BJ, et al. Determination of in vitro susceptibility of ocular Fusarium spp. isolates from keratitis cases and comparison of Clinical and Laboratory Standards Institute M38-A2 and E test methods. Diagn Microbiol Infect Dis. 2008;62:348–350. doi: 10.1016/j.diagmicrobio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Lionakis MS, Lewis RE, Samonis G, et al. Pentamidine is active in vitro against Fusarium species. Antimicrob Agents Chemother. 2003;47:3252–3259. doi: 10.1128/AAC.47.10.3252-3259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tortorano AM, Prigitano A, Dho G, et al. Species distribution and in vitro antifungal susceptibility patterns of 75 clinical isolates of Fusarium spp. from northern Italy. Antimicrob Agents Chemother. 2008;52:2683–2685. doi: 10.1128/AAC.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinel-Ingroff A. Comparison of In vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pujol I, Guarro J, Gene J, et al. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 44.Meletiadis J, Meis JF, Mouton JW, et al. Comparison of NCCLS and 3-(4,5-dimethyl-2-Thiazyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J Clin Microbiol. 2000;38:2949–2954. doi: 10.1128/jcm.38.8.2949-2954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Poeta M, Schell WA, Perfect JR. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Wang Z, Li R, et al. In vitro evaluation of combination antifungal activity against Fusarium species isolated from ocular tissues of keratomycosis patients. Am J Ophthalmol. 2008;146:724–728. doi: 10.1016/j.ajo.2008.06.008. [DOI] [PubMed] [Google Scholar]