Abstract
The ST5 lineage of methicillin-resistant Staphylococcus aureus (MRSA) is one of the most globally disseminated hospital-associated MRSA (HA-MRSA) lineages. We isolated a new local variant (designated ST764) over at least 5 years that causes invasive infections, including necrotizing fasciitis, and is carried by medical students, as well as household members. Analysis of the genome sequence of one isolate compared to that of the reference ST5 strain revealed that ST764 had acquired virulence traits similar to those of community-associated MRSA (CA-MRSA) through the acquisition of two new mobile genetic elements, ACMEII and SaPInn54, which carried ACME arcA and the staphylococcal enterotoxin B gene (seb), respectively, and through enhanced expression of cytolytic peptide genes, although ST764 was negative for Panton-Valentine leukocidin. Other differences between ST764 and ST5 included the acquisition of an ACMEII-related cassette (cJR1), prophage φ2NN54, and streptococcal Tn5251 and decreased numbers of copies of Tn554. As for superantigen genes, although the two possessed seg, sei, sem, sen, and seo, ST764 lacked tst, sec, sel, and sep. The data suggest that ST764 MRSA is a novel hybrid variant of ST5 HA-MRSA with the characteristics of CA-MRSA and that the evolution of ST764 includes multiple steps, e.g., acquisition of novel or nonstaphylococcal mobile elements.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) was reported in 1961 and has continued to be a life-threatening multiple-drug-resistant bacterium in hospitals (1). MRSA is generated from methicillin-susceptible S. aureus (MSSA) by the acquisition of staphylococcal cassette chromosome mec (SCCmec) at the 3′ end (i.e., the 15-bp SCCmec insertion site, att) of orfX (2, 3). It is thought that this has occurred only a limited number of times, resulting in MRSA epidemics in hospital settings (4–6).
Among these epidemic MRSA strains, ST5 is one of the most globally disseminated lineages (4–6). The ST5/SCCmecII lineage was previously referred to as the New York/Japan clone (5–8). This type of MRSA became dominant in Tokyo in 1992 (9). The dominant Japanese MRSA clone was originally characterized by type II coagulase, a ribotype pattern similar to N315, a toxin type positive for toxic shock syndrome toxin 1 (TSST-1) and staphylococcal enterotoxin C (SEC), and multiple-antibiotic resistance, including ciprofloxacin, and emerged through replacement of the preexisting dominant MRSA strain in 1982, which was characterized by type IV coagulase.
In the United States, a dominant MRSA clone (the New York clone), which was characterized by mecA polymorph I, Tn554 pattern A, and pulsed-field gel electrophoresis (PFGE) pattern A (clonal type I:A:A), was isolated in 1994 (10) and 1998 (11). In 2000, the two major MRSA clones in Japan and the United States were shown to be the same, and transcontinental spread from Japan to the United States was suggested (12). The MRSA clone was then recognized as the New York/Japan clone, with genotype ST5/SCCmecII (5, 6, 8), and also as a global clone spreading to, for example, North and Central America, Asia, and Europe (13–17).
Since 1981 (18) or 1997 to 1999 (19), another class of MRSA (called community-associated MRSA [CA-MRSA]), which spreads in community settings, has also been noted; since then, the term “hospital-associated MRSA (HA-MRSA)” has been used for MRSA isolated in the nosocomial environment.
HA-MRSA is multidrug resistant, and infections occur most frequently among inpatients, for example, those who have undergone invasive medical procedures or those aged 50 to 60 years and older (20, 21). In contrast, CA-MRSA is resistant to β-lactam agents only or to some agents in restricted classes, although some recently successful CA-MRSA strains, such as USA300, have become resistant to multiple antibiotics, including levofloxacin (22, 23). Moreover, CA-MRSA infections occur through skin-to-skin contact in healthy individuals, especially children and adolescents, and are associated mainly with skin and soft tissue infections (SSTIs), including pyogenic skin infection, but occasionally with invasive infections, such as bacteremia (and sepsis) and necrotizing pneumonia (1, 21, 24, 25).
It is thought that CA-MRSA possesses unique features compared to HA-MRSA, possibly related to enhanced virulence. One such candidate is the arginine catabolic mobile element (ACME), carrying the arc and opp-3 regions, found in ST8 CA-MRSA USA300, the most common clone in the United States (22, 26). USA300 has an array (combination) of ACME and SCCmecIVa (a subtype [a] of SCCmecIV, a typical SCCmec type of CA-MRSA), which is supposed to be responsible for the community spread and colonization of MRSA (USA300) (27). ACME includes three types: type I (ACMEI), carrying both arcA and opp-3 regions; ACMEII, carrying only the arcA region; and ACMEIII, carrying only the opp-3 region (27, 28).
The enhanced virulence candidates of CA-MRSA also include the cytolytic peptide (or phenol-soluble modulin [PSM]) genes (e.g., psmα [encoding PSMα] or hld [encoding δ-hemolysin]), which can be expressed at higher levels than those of HA-MRSA (26, 29). All analyzed S. aureus genome sequences contain the genes encoding δ-hemolysin, PSMα, and PSMβ; of those, PSMα and δ-hemolysin induce potent cytolytic activity in human cells (29). The enhanced levels of PSMs are supposed to be involved in the pathogenesis of CA-MRSA infections, such as bacteremia and abscesses (26).
CA-MRSA often produces Panton-Valentine leukocidin (PVL) (26); USA300 (22) is an example. PVL causes apoptosis and necrosis in human polymorphonuclear cells or monocytes (30). In this study, we isolated a new local variant (designated ST764) of the ST5 lineage from hospitalized patients, medical students, and family members and characterized the ST764 variant by comparative genomics using a reference ST5 strain.
MATERIALS AND METHODS
Bacterial strains.
MRSA strain NN54 was isolated from a case of necrotizing faciitis occurring in a 60-year-old inpatient with a history of chronic kidney failure, hemodialysis, diabetes, and hospitalization and was cultured from blood, a biopsy specimen of the lesion, and abscesses. MRSA NN37 was isolated from the blood of a 54-year-old inpatient with bacteremia on the day of admission in Niigata, Japan, in 2005. He had no history of hospitalization, surgery, dialysis, or indwelling percutaneous medical devices or catheters in the past 1 year.
MRSA carriage in fifth-year medical students undergoing clinical practice in Niigata University Hospital was investigated for 5 years from 2006 through 2010. Two of 261 students were positive for MRSA; MRSA NN35A and NN35B were isolated from their hands in 2006 and 2009, respectively.
Independently of the above study on patients and medical students, nasal MRSA carriage by healthy family members in Niigata was investigated, and three MRSA strains, NN41F1G, NN41F1F, and NN41F1D, were isolated from three members, a 56-year-old grandfather, 34-year-old father, and 6-year-old daughter, of the same household in 2007.
The control strains used in this study include N315 (ST5 [31]), Mu50 (ST5 [31]), I6 (ST5 [32]), I8 (ST5 [32]), I10 (ST5 [this study]), BK2464 (ST5 [4]), USA300-0114 (ST8 [33]), NN36 (ST8 [34]), NN47 (ST8 [35]), 549 (ST8 [36]), PM1 (ST59 [37]), and COL (ST250 [5, 6]).
Molecular typing of MRSA.
Molecular characterization of MRSA was performed as described previously (37). Sequence type (ST) typing was conducted using seven housekeeping genes (38), and the ST was obtained from the MLST (multilocus sequence typing) website (http://www.mlst.net/). The spa type was analyzed by sequencing the PCR product of the spa gene (39) and determined using a public spa-type database (http://tools.egenomics.com/) or Ridom SpaServer (http://www.spaserver.ridom.de/). Typing of agr was carried out by PCR with previously reported primers (40, 41). The SCCmec types I to V (2, 42) were analyzed by PCR, as described by Kondo et al. (42), using reference strains. Coagulase (Coa) typing was conducted using a staphylococcal coagulase antiserum kit (Denka Seiken, Tokyo, Japan). Virulence genes were analyzed by PCR following previously published methods (27, 37, 43–46). The target genes in PCR included 49 genes: genes from three leukocidins (lukS-PV–lukF-PV, lukE-lukD, and lukM), 5 hemolysin genes (hla, hlb, hlg, hlgv, and hld), a peptide cytolysin gene (psmα), 18 staphylococcal enterotoxin (SE) genes (tst, sea, seb, sec, sed, see, seg, seh, sei, sej, sek, sel, sem, sen, seo, sep, seq, and ser), a putative staphylococcal enterotoxin gene (seu), 3 exfoliative toxin genes (eta, etb, and etd), a staphylococcal superantigen-like gene cluster (ssl), the epidermal cell differentiation inhibitor gene (edin), 14 adhesin genes (icaA, icaD, eno, fib, fnbA, fnbB, ebpS, clfA, clfB, sdrC, sdrD, sdrE, cna, and bbp), and ACME arcA and ACME opp-3C genes (27).
SEB assay.
The amount of staphylococcal enterotoxin B (SEB) in the supernatants of bacterial cultures at 2.0 × l09 CFU/ml was examined using an SET-RPLA kit (Denka Seiken) according to the instructions of the manufacturer.
Susceptibility testing.
Susceptibility testing of bacterial strains was carried out using the agar dilution method with Mueller-Hinton agar, according to previous procedures, by the Clinical and Laboratory Standards Institute (47).
Genome analysis.
The MRSA NN54 genome was analyzed by pyrosequencing using an FLX genome sequencer system with the assembler software GS De Novo Assembler version 2.0 (Roche Diagnostics, Branford, CT). In this study, 291,924 reads yielded 131 Mb of raw sequences (ca. 47 times the genome). The constructed contigs were mapped on the 2,814,816-bp complete genome of MRSA strain N315 (GenBank accession number FN433596) using In Silico MolecularCloning software version 4.2 (In Silico Biology, Yokohama, Japan). The gene or open reading frame (orf) was searched for using In Silico MolecularCloning software version 4.2. Homology analysis was performed using BLAST (http://blast.ncbi.nlm.nih.gov/).
Assay of mRNA expression levels.
The mRNA expression levels of the cytolytic peptide genes (psmα and hld), the ACME arcA gene, and the 16S rRNA genes were examined by reverse transcription (RT)-PCR assay with PCR primers reported previously (27, 45), essentially according to the experimental strategy described previously (48). Band intensity was determined using image-processing and analysis software, NIH Image (NIH, Bethesda, MD).
Statistical analysis.
Data were evaluated by analysis of variance with repeated measurement. The level of significance was defined as a P value of <0.05.
Nucleotide sequence accession number.
The NN54 genome was deposited in GenBank under accession number BAFI01000000.
RESULTS AND DISCUSSION
Isolation and genotying of ST764 MRSA.
The seven MRSA strains isolated in Niigata from 2005 to 2009 from invasive infections in patients, the hands of medical students undergoing clinical practice, and the nasal mucosa of healthy household members were typed, and the results showed that all were an ST764 variant of ST5/SCCmecII lineage (Table 1); ST764 is a single-locus variant of ST5. All seven strains were negative for PVL, similar to ST5; positive for the ACME arcA and seb genes, in contrast to ST5; and negative for SaPIm1/n1, in contrast to the ST5 Japanese type (but similar to the U.S. type strain BK2464). All ST764 strains were resistant to multiple antibiotics, including levofloxacin and fosfomycin, similar to ST5 (31, 32).
Table 1.
Molecular characterization of ST764 MRSA strains compared to ST5/SCCmecII MRSA in Japan
| Characteristic | Valuea |
|
|---|---|---|
| ST764 variants (n = 7) | ST5/SCCmecII control strains (n = 5) | |
| Type | ||
| ST | 764 | 5 |
| spa | 2 (t002) | 2 (t002) |
| agr | 2 | 2 |
| SCCmec | II | II |
| Coagulase | II | II |
| Virulence gene | ||
| Leukocidin genes | ||
| lukPVSF | − | − |
| lukE-lukD | + | + |
| Hemolysins | ||
| hla | + | + |
| hlg, hlgv | + (5/7) | + |
| hlb | (+)b | (+)b |
| Peptide cytolysin | ||
| psmα, hld | + | + |
| Enterotoxin | ||
| SaPIm1/n1 (tst, sec, sel) | − | + |
| SaPInn54 (seb) | + | − |
| egc (seg, sei, sem, sen, seo) | + | + |
| sep | − | + (2/5) |
| Adhesin | ||
| c12agc | + | + |
| ACME | ||
| arcA | + | − |
| opp-3C | − | − |
| Drug resistance (non-β-lactams)d | ERY, CLI, GEN, KAN, TET, FOS, LVX | ERY, CLI (3/5), GEN (4/5), KAN, TET, FOS (2/5), LVX, VAN (1/5)e |
ST764 (n = 7) included NN54, NN37, NN35A, NN35B, NN41F1G, NN41F1F, and NN41F1D; ST5/SCCmecII (n = 5) included type strains N315 and Mu50 and clinical isolates I6, I8, and I10. +, positive; −, negative. Unique characteristics of ST764 are shaded.
Split hlb gene.
c12ag, core 12 adhesin genes shared by all strains: icaA and icaD (for biofilm formation); eno (for laminin-adhesin); fnbA and fnbB (for fibronectin-adhesin); ebpS (for elastin-adhesin); and clfA, clfB, fib, sdrC, sdrD, and sdrE (for fibrinogen-adhesin).
ERY, erythromycin; CLI, clindamycin; GEN, gentamicin; KAN, kanamycin; TET, tetracycline; FOS, fosfomycin; LVX, levofloxacin; VAN, vancomycin.
Strain Mu50 was vancomycin intermediate (31).
Of two ST764 isolates from patients, strain NN37 met the Centers for Disease Control and Prevention (CDC) criteria for the CA-MRSA definition (20), while strain NN54 met the HA-MRSA definition. Regarding colonization, 5th-year medical students probably have a risk for MRSA colonization; however, although the ST5 MRSA is the predominant HA-MRSA in hospitals in Niigata (similar to Tokyo), no ST5 MRSA was isolated from the nares and hands of 5th-year medical students (n = 261), suggesting that ST764 MRSA may possess higher potential for colonization than ST5 MRSA. In the present screening test for household colonization in Niigata, ST764 MRSA was the second most frequent isolate, following ST8 CA-MRSA (data not shown).
Comparative genomics of ST764 MRSA (strain NN54).
The NN54 genome was estimated to be at least 2.8 Mb, showing approximately 99.7% homology with the N315 genome, albeit with marked divergence in mobile DNA structures (Fig. 1). There were four insertions in the NN54 genome (Fig. 1). One insert (23.5 kb) consisted of cassette JR1 (cJR1) and ACMEII, inserted in tandem between orfX (the att site) and SCCmecII (of N315); the entire structure is described below. The second insert was 13.5 kb, carried the seb gene, and was inserted into the noncoding region, located downstream of transfer-messenger RNA (tmRNA); this insert was designated SaPInn54 (Fig. 1), and the entire structure is described below.
Fig 1.
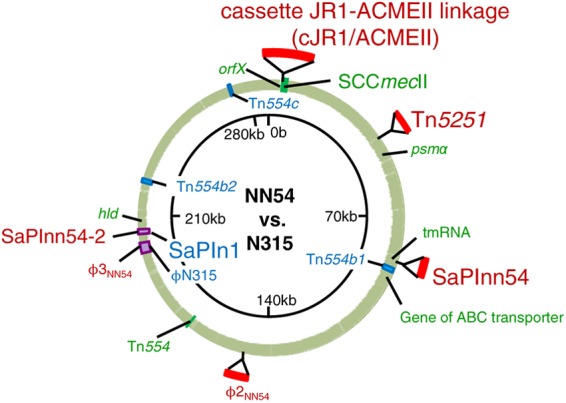
Comparative genomics of ST764 MRSA strain NN54. The NN54 genome contigs were mapped on the N315 (ST5) genome (shown as a circle). Information on the NN54 and N315 genomes is presented outside and inside the genome circle, respectively. Colored regions in NN54: green, highly homologous to N315; blue, deletion; red, insertion; purple, SaPI or prophage diversity.
The third insert was the 44.5-kb prophage φ2 (designated φ2NN54), which was inserted into the hypothetical protein gene (SA1320) (Fig. 1). The prophage φ2 group includes two types (49): one carrying the PVL gene (e.g., φSa2USA300 of USA300) (22) and the other without the PVL gene. NN54 carried the latter type. Compared to φ2 group phages, φ2NN54 showed the highest homology (85.3%) to φSa2USA300.
The fourth insert was the tetM-carrying Tn5251 of Streptococcus pneumoniae, which was inserted into the noncoding region, located downstream of the hypothetical protein gene (SA0368). The tetM-carrying Tn5251 (Tn916-like genetic element) is part of the composite conjugative transposon of S. pneumoniae (50). Tn5251 is inserted into Tn5252 to form the composite element Tn5253 (50); however, NN54 lacked Tn5252, consistent with the previous notion that Tn5251 behaves as an independent, fully functional conjugative transposon (51).
NN54 carried the pN315-like sequence, which is similar to the penicillinase plasmid (pN315) of N315 (31), albeit with some divergence; NN54 (pN315-like) carried the truncated β-lactamase gene (ΔblaZ) and fosfomycin resistance protein gene (fosD), for example.
As for deletions, although N315 possessed four copies (a, b1, b2, and c) of the erythromycin resistance-encoding Tn554, three copies (b1, b2, and c) were deleted in the NN54 genome; NN54 carried only a Tn554 copy, corresponding to Tn554a. Moreover, ST764 lacked superantigen genes tst, sec, and sel compared to N315; this was because ST764 carried SaPInn54-2, which lacked tst, sec, and sel, while N315 carried SaPIn1 with tst, sec, and sel (homology between SaPInn54-2 and SaPIn1 was 62.3%) (Fig. 1). ST764 also lacked sep; ST764 carried φ3NN54 without sep, while N315 carried φN315 with sep (homology between φ3NN54 and φN315 was 86.5%) (Fig. 1).
Comparative genomic analysis between NN54 and N315 demonstrated further divergences, e.g., a gene mutation causing ebhA-ebhB fusion (for a putative adhesin). More detailed comparative genomic analysis between ST764 and ST5 (including predicted gene products) is illustrated in Table S1 in the supplemental material.
Structure of cJR1-ACMEII-SCCmecII.
NN54 carried the three-cassette array cJR1-ACMEII-SCCmecII, each demarcated by the 15-bp att direct-repeat sequences, as shown in Fig. 2 (middle).
Fig 2.
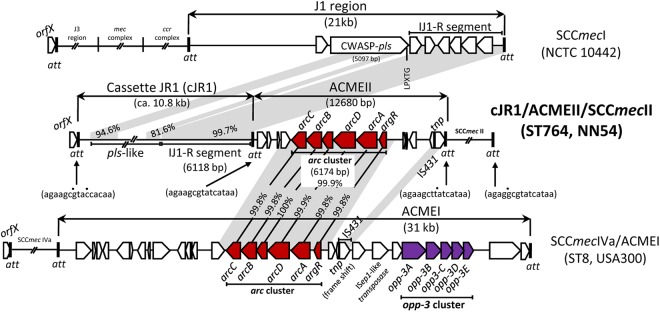
Structure of the cJR1-ACMEII-SCCmecII region on the NN54 genome. A novel att-mediated array (cJR1-ACMEII-SCCmecII) (middle) was compared to the J1 region of SCCmecI (top) and ACMEI (bottom); the GenBank accession numbers are AB687558, AB033763, and CP000255, respectively. Homologous regions are shaded. Dots above 15-bp att sequences show a divergent nucleotide (from orfX att).
ACMEII carried a 6.2-kb arc cluster very similar (99.9% homologous) to that of ACMEI (of USA300) but lacked the opp-3 cluster (unlike USA300) (Fig. 2, bottom). Moreover, ACMEII carried the intact transposase gene (tnp) of IS431, while IS431-tnp in ACMEI had a frameshift mutation.
ACME of CA-MRSA USA300, carrying ACME arcA and opp-3, exhibits multiple functions and is considered to enhance the growth and survival of USA300; USA300 with ACME outcompeted an isogenic strain without ACME in pathogenicity and fitness in a rabbit infection model (26, 27). It is also thought that ACME enhances colonization and survival on the skin (26). This model is also supported by previous observations that arginine deiminase, encoded by arcA, e.g., inhibits human peripheral blood mononuclear cell proliferation (22, 52) and protects bacteria against damage caused by acidic environments through the production of NH3 (53) and that oligopeptide permease (Opp), encoded by the opp-1 and opp-2 operons in S. aureus, is a member of the ABC transporter family and displays multiple functions, including bacterial growth in animal infection models (54). In contrast to the above-mentioned ACMEI, the role of ACMEII, which lacks the opp region of ACMEI, remains less understood.
As for cJR1, the right-side 6.1-kb region showed high homology (99.7%) to the right-side J1-joining region of SCCmecI (Fig. 2, top). Although the SCCmecI J1 region contained the 5,097-kb large orf (pls), encoding a cell-wall-anchored surface protein (CWASP) with the LPXTG motif (55), the pls-homologous sequence (pls-like) in cJR1 had a deletion; due to sequence repetitions, the pls-like sequence remained incomplete. cJR1 possessed no tnp gene.
The function of cJR1 remains unknown; however, it carries part of the J1 region, containing the putative surface protein gene (pls-like) and may act as an adhesin and contribute to students', as well as household members', carriage, in combination with ACMEII. Another example, in which the J1 region of SCCmec encodes a putative surface protein (a possible adhesin), is ST8 CA-MRSA/J, a successful CA-MRSA clone in Japan (56, 57). CA-MRSA/J is characterized by (i) genotype ST8/spa606(t1767)/agr1/CoaII; (ii) SCCmecIV of a novel subtype, which is expressed as IV.new.1.1 (now designated IVl), reflecting the differences in the three nonessential component regions of SCCmec (J1, J2, and J3), according to the guidelines (2); (iii) mosaic SaPIj50 with the tst, sec, and sel genes; and (iv) high expression of the psmα and hld genes, similar to USA300. CA-MRSA/J is negative for PVL and ACME.
In NN54, the cJR1-ACMEII array seems to have been inserted as a unit at the orfX att site. During the course of this study, Shore et al. described ACMEII in MRSA with ST22/SCCmecIVh (58); in their strain (M08/0126), the ACMEII-ΔJ1SCCmecI array is inserted at the att site between orfX and SCCmecIVh, resulting in the structure orfX-ACMEII-ΔJ1SCCmecI-SCCmecIVh (therefore, in opposite orientation in this study, orfX-cJR1-ACMEII-SCCmecII).
Structure of SaPInn54.
The entire structure of SaPInn54 is shown in Fig. 3 (middle). SaPIs are actually phage-related chromosomal islands and represent phage satellites producing phage-like infectious particles, and the integrase gene (int) and replication initiator gene (rep) play roles in SaPI transfer (59). The SaPI core region (9.5 kb), carrying the rep and int genes, of SaPInn54 was very similar to the corresponding region of SaPImw2, which is present in CA-MRSA USA400 strain MW2. They also shared the same 15-bp att sequence and insertion site on the chromosome (Fig. 3, top and middle); however, the virulence gene-carrying regions of SaPInn54 and SaPImw2 were divergent. SaPInn54 carried the seb gene, while SaPImw2 carried the sec and sel genes (although the two SaPIs shared similar ear genes, encoding a putative penicillin-binding protein [PBP]).
Fig 3.
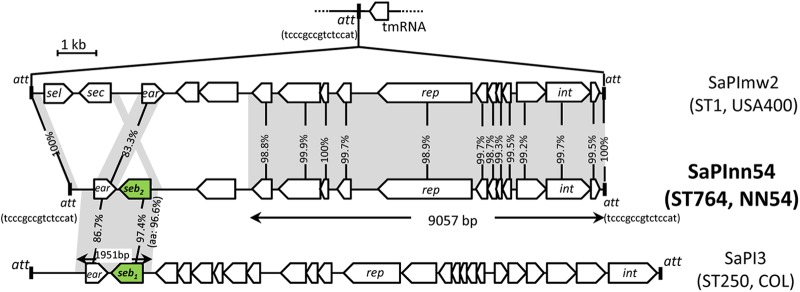
Structure of SaPInn54 carrying the SEB gene on the NN54 genome. A novel mosaic, SaPInn54 (middle), was compared to SaPImw2 (top) and SaPI3 (bottom); the GenBank accession numbers are AB690437, NC_003923, and SaPI3 CP000046, respectively. Homologous regions are shaded.
The 2.0-kb ear- and seb-carrying segment of SaPInn54 was very similar to that of SaPI3 in ST250 MRSA COL (Fig. 3, bottom) and ST59 MRSA PM1 (data not shown). The SaPI core region (carrying the rep and int genes) and att of SaPInn54 and SaPI3 were divergent (Fig. 3, bottom).
The seb genes of SaPInn54 and SaPI3 were not the same: homology was 97.4% at the nucleotide level and 96.6% at the amino acid level; mature SEB1, produced from SaPI3, and SEB2, produced from SaPInn54, differed by 5 amino acids (see Fig. S1 in the supplemental material). SEB1 was identical to purified SEB protein (Protein Data Bank [PDB] code ISE4A); therefore, SEB2 represents a new SEB variant. SEB production levels, immunologically determined, were 14.3 μg/ml for NN54, 14.3 μg/ml for COL, and 31.3 μg/ml for PM1.
SEB is a superantigen (60) and a potential biological weapon (category B bioterrorism agent) (61). Since SEB is also considered to play a role in immune evasion upon staphylococcal infection (62), SEB may contribute to community infection, similar to successful ST59 CA-MRSA with SaPI3 (e.g., strain PM1) in Taiwan (37).
mRNA expression levels of virulence genes.
As shown in Fig. 4, for the PSMα and δ-hemolysin genes (psmα and hld, respectively), mRNA expression levels of ST764 strains were similar to those of USA300 but significantly higher than those of ST5 (P < 0.05), suggesting that ST764 MRSA possessed enhanced cytolytic peptide genes (with high expression) of CA-MRSA. Similar to USA300 (26), high levels of PSMα (and δ-hemolysin) in ST764 MRSA may contribute to bacteremia.
Fig 4.
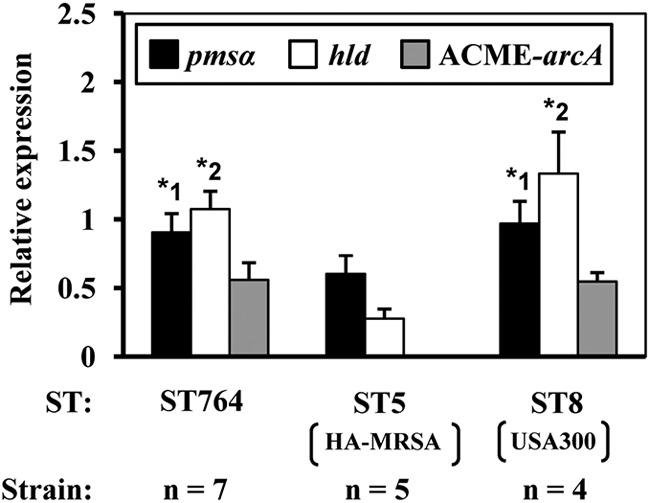
Levels of mRNA expression for cytolytic peptide genes (psmα and hld) and the ACME arcA gene of ST764 MRSA compared to those of ST5/SCCmecII MRSA and USA300. The ST764 and ST5 strains are described in Table 1. The ST8 strains included the type strain USA300-0114 and three Japanese isolates NN36, NN47, and 549. For each strain, mRNA expression levels were measured in triplicate. The experiment was independently repeated three times. The psmα and hld mRNA expression levels in ST764 and ST8 were similar, but their levels (*1 and *2) were significantly higher than in ST5 (P < 0.05). ACME arcA mRNA expression levels in ST764 and ST8 were similar (ST5 lacked the ACME arcA gene).
For the ACME arcA gene, the mRNA expression levels of ST764 MRSA (carrying ACMEII) and ST8 CA-MRSA USA300 (carrying ACMEI) were similar (Fig. 4). Further experiments in animal infection models are needed to evaluate the virulence of ST764 MRSA.
Origin of ST764 MRSA.
The origin of ST764 MRSA is not known; however, most probably it evolved from ST5/SCCmecII MRSA in Japan (Niigata), because its ST is a novel ST5 variant, and the unique genetic features of ST764, as described in this study, have not been reported previously.
Conclusions.
In summary, ST764 MRSA, a novel hybrid variant of the ST5 HA-MRSA lineage with the characteristics of CA-MRSA, has emerged in Niigata, Japan, causing invasive infections (necrotizing fasciitis and bacteremia) and carried by medical students in hospitals and also producing carriers in the community. The evolution of ST764 MRSA includes multiple steps involving the insertion of genetic structures, cJR1/ACMEII, SaPInn54 (with seb2), φ2NN54 (lacking the PVL gene), and streptococcal Tn5251 (carrying tetM), and the acquisition of enhanced expression of the psmα and hld genes, similar to CA-MRSA. Further divergence was observed in SaPI (at groEL), φ3, plasmid pN315, and several chromosomal genes.
ACKNOWLEDGMENTS
We thank H. de Lencastre for the BK2464 and COL strains, L. K. McDougal and L. L. McDonald for the USA300-0114 strain, and K. Hiramatsu for the N315 and Mu50 strains.
Footnotes
Published ahead of print 14 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01147-12.
REFERENCES
- 1. Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874–885 [DOI] [PubMed] [Google Scholar]
- 2. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noto MJ, Kreiswirth BN, Monk AB, Archer GL. 2008. Gene acquisition at the insertion site for SCCmec, the genomic island conferring methicillin resistance in Staphylococcus aureus. J. Bacteriol. 190:1276–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aires de Sousa M, Conceição T, Simas C, de Lencastre H. 2005. Comparison of genetic backgrounds of methicillin-resistant and -susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J. Clin. Microbiol. 43:5150–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. U. S. A. 99:7687–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliveira DC, Tomasz A, de Lencastre H. 2001. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb. Drug Resist. 7:349–361 [DOI] [PubMed] [Google Scholar]
- 7. Coombs GW, Van Gessel H, Pearson JC, Godsell MR, O'Brien FG, Christiansen KJ. 2007. Controlling a multicenter outbreak involving the New York/Japan methicillin-resistant Staphylococcus aureus clone. Infect. Control Hosp. Epidemiol. 28:845–852 [DOI] [PubMed] [Google Scholar]
- 8. Oliveira DC, Tomasz A, de Lencastre H. 2002. Secrets of success of a human pathogen: molecular evolution of pandemic clones of meticillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 2:180–189 [DOI] [PubMed] [Google Scholar]
- 9. Tanaka T, Okuzumi K, Iwamoto A, Hiramatsu K. 1995. A retrospective study of methicillin-resistant Staphylococcus aureus clinical strains in Tokyo University Hospital. J. Infect. Chemother. 1:40–49 [Google Scholar]
- 10. de Lencastre H, Severina EP, Roberts RB, Kreiswirth BN, Tomasz A, Initiative Pilot Study Group BARG 1996. Testing the efficacy of a molecular surveillance network: methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) genotypes in six hospitals in the metropolitan New York City area. Microb. Drug Resist. 2:343–351 [DOI] [PubMed] [Google Scholar]
- 11. Roberts RB, Chung M, de Lencastre H, Hargrave J, Tomasz A, Nicolau DP, John JF, Jr, Korzeniowski O, Tri-State Collaborative Study Group MRSA 2000. Distribution of methicillin-resistant Staphylococcus aureus clones among health care facilities in Connecticut, New Jersey, and Pennsylvania. Microb. Drug Resist. 6:245–251 [DOI] [PubMed] [Google Scholar]
- 12. Aires de Sousa M, de Lencastre H, Santos Sanches I, Kikuchi K, Totsuka K, Tomasz A. 2000. Similarity of antibiotic resistance patterns and molecular typing properties of methicillin-resistant Staphylococcus aureus isolates widely spread in hospitals in New York City and in a hospital in Tokyo, Japan. Microb. Drug Resist. 6:253–258 [DOI] [PubMed] [Google Scholar]
- 13. Cha HY, Moon DC, Choi CH, Oh JY, Jeong YS, Lee YC, Seol SY, Cho DT, Chang HH, Kim SW, Lee JC. 2005. Prevalence of the ST239 clone of methicillin-resistant Staphylococcus aureus and differences in antimicrobial susceptibilities of ST239 and ST5 clones identified in a Korean hospital. J. Clin. Microbiol. 43:3610–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conceição T, Aires-de-Sousa M, Füzi M, Tóth Á, Pászti J, Ungvári E, van Leeuwen WB, van Belkum A, Grundmann H, de Lencastre H. 2007. Replacement of methicillin-resistant Staphylococcus aureus clones in Hungary over time: a 10-year surveillance study. Clin. Microbiol. Infect. 13:971–979 [DOI] [PubMed] [Google Scholar]
- 15. Ip M, Yung RW, Ng TK, Luk WK, Tse C, Hung P, Enright M, Lyon DJ. 2005. Contemporary methicillin-resistant Staphylococcus aureus clones in Hong Kong. J. Clin. Microbiol. 43:5069–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simor AE, Ofner-Agostini M, Bryce E, McGeer A, Paton S, Mulvey MR, Canadian Hospital Epidemiology Committee and Canadian Nosocomial Infection Surveillance Program Health Canada 2002. Laboratory characterization of methicillin-resistant Staphylococcus aureus in Canadian hospitals: results of 5 years of National Surveillance, 1995–1999. J. Infect. Dis. 186:652–660 [DOI] [PubMed] [Google Scholar]
- 17. Velazquez-Meza ME, Aires de Sousa M, Echaniz-Aviles G, Solórzano-Santos F, Miranda-Novales G, Silva-Sanchez J, de Lencastre H. 2004. Surveillance of methicillin-resistant Staphylococcus aureus in a pediatric hospital in Mexico City during a 7-year period (1997 to 2003): clonal evolution and impact of infection control. J. Clin. Microbiol. 42:3877–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention 1981. Community-acquired methicillin-resistant Staphylococcus aureus infections—Michigan. MMWR Morb. Mortal. Wkly. Rep. 30:185–187 [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. MMWR Morb. Mortal. Wkly. Rep. 48:707–710 [PubMed] [Google Scholar]
- 20. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto T, Nishiyama A, Takano T, Yabe S, Higuchi W, Razvina O, Shi D. 2010. Community-acquired methicillin-resistant Staphylococcus aureus: community transmission, pathogenesis, and drug resistance. J. Infect. Chemother. 16:225–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 23. McDougal LK, Fosheim GE, Nicholson A, Bulens SN, Limbago BM, Shearer JE, Summers AO, Patel JB. 2010. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 54:3804–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak JM, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 25. Zetola N, Francis JS, Nuermberger EL, Bishai WR. 2005. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5:275–286 [DOI] [PubMed] [Google Scholar]
- 26. Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 197:1523–1530 [DOI] [PubMed] [Google Scholar]
- 28. Miragaia M, de Lencastre H, Perdreau-Remington F, Chambers HF, Higashi J, Sullam PM, Lin J, Wong KI, King KA, Otto M, Sensabaugh GF, Diep BA. 2009. Genetic diversity of arginine catabolic mobile element in Staphylococcus epidermidis. PLoS One 4:e7722 doi:10.1371/journal.pone.0007722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]
- 30. Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6:e1000715 doi:10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225–1240 [DOI] [PubMed] [Google Scholar]
- 32.Takizawa Y, Taneike I, Nakagawa S, Oishi T, Nitahara Y, Iwakura N, Ozaki K, Takano M, Nakayama T, Yamamoto T. 2005. A Panton-Valentine leucocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more typical PVL-negative MRSA strains found in Japan. J. Clin. Microbiol. 43:3356–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, Dunman PM. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shibuya Y, Hara M, Higuchi W, Takano T, Iwao Y, Yamamoto T. 2008. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in Japan. J. Infect. Chemother. 14:439–441 [DOI] [PubMed] [Google Scholar]
- 35. Yabe S, Takano T, Higuchi W, Mimura S, Kurosawa Y, Yamamoto T. 2010. Spread of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone among family members in Japan. J. Infect. Chemother. 16:372–374 [DOI] [PubMed] [Google Scholar]
- 36. Mine Y, Higuchi W, Taira K, Nakasone I, Tateyama M, Yamamoto T, Uezato H, Takahashi K. 2011. Nosocomial outbreak of multidrug-resistant USA300 methicillin-resistant Staphylococcus aureus causing severe furuncles and carbuncles in Japan. J. Dermatol. 38:1167–1171 [DOI] [PubMed] [Google Scholar]
- 37. Takano T, Higuchi W, Otsuka T, Baranovich T, Enany S, Saito K, Isobe H, Dohmae S, Ozaki K, Takano M, Iwao Y, Shibuya M, Okubo T, Yabe S, Shi D, Reva I, Teng LJ, Yamamoto T. 2008. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob. Agents Chemother. 52:837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilot P, Lina G, Cochard T, Poutrel B. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40:4060–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strommenger B, Cuny C, Werner G, Witte W. 2004. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in central Europe and agr specificity groups. Eur. J. Clin. Microbiol. Infect. Dis. 23:15–19 [DOI] [PubMed] [Google Scholar]
- 42. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, Hiramatsu K. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 51:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 193:1495–1503 [DOI] [PubMed] [Google Scholar]
- 44. Fluckiger U, Ulrich M, Steinhuber A, Doring G, Mack D, Landmann R, Goerke C, Wolz C. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 73:1811–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J. Infect. Dis. 202:1866–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omoe K, Hu DL, Takahashi-Omoe H, Nakane A, Shinagawa K. 2003. Identification and characterization of a new staphylococcal enterotoxin-related putative toxin encoded by two kinds of plasmids. Infect. Immun. 71:6088–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clinical and Laboratory Standards Institute 2012. Performance standard for antimicrobial susceptibility testing; 22nd informational supplement, M100–S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 48. Yamamoto T, Takano T, Yabe S, Higuchi W, Iwao Y, Isobe H, Ozaki K, Takano M, Reva I, Nishiyama A. 2012. Super-sticky familial infections caused by Panton-Valentine leukocidin-positive ST22 community-acquired methicillin-resistant Staphylococcus aureus in Japan. J. Infect. Chemother. 18:187–198 [DOI] [PubMed] [Google Scholar]
- 49. Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, Broker BM, Doskar J, Wolz C. 2009. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 191:3462–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Provvedi R, Manganelli R, Pozzi G. 1996. Characterization of conjugative transposon Tn5251 of Streptococcus pneumoniae. FEMS Microbiol. Lett. 135:231–236 [DOI] [PubMed] [Google Scholar]
- 51. Santoro F, Oggioni MR, Pozzi G, Iannelli F. 2010. Nucleotide sequence and functional analysis of the tet (M)-carrying conjugative transposon Tn5251 of Streptococcus pneumoniae. FEMS Microbiol. Lett. 308:150–158 [DOI] [PubMed] [Google Scholar]
- 52. Degnan BA, Palmer JM, Robson T, Jones CE, Fischer M, Glanville M, Mellor GD, Diamond AG, Kehoe MA, Goodacre JA. 1998. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect. Immun. 66:3050–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Degnan BA, Fontaine MC, Doebereiner AH, Lee JJ, Mastroeni P, Dougan G, Goodacre JA, Kehoe MA. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD, Westbrock-Wadman S, Hufnagle WO, Folger KR, Bayer AS, Stover CK. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30:393–404 [DOI] [PubMed] [Google Scholar]
- 55. Savolainen K, Paulin L, Westerlund-Wikstrom B, Foster TJ, Korhonen TK, Kuusela P. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iwao Y, Ishii R, Tomita Y, Shibuya Y, Takano T, Hung WC, Higuchi W, Isobe H, Nishiyama A, Yano M, Matsumoto T, Ogata K, Okubo T, Khokhlova O, Ho PL, Yamamoto T. 2012. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: associated infections, genetic diversity, and comparative genomics. J. Infect. Chemother. 18:228–240 [DOI] [PubMed] [Google Scholar]
- 57. Iwao Y, Takano T, Higuchi W, Yamamoto T. 2012. A new staphylococcal cassette chromosome mec IV encoding a novel cell-wall-anchored surface protein in a major ST8 community-acquired methicillin-resistant Staphylococcus aureus clone in Japan. J. Infect. Chemother. 18:96–104 [DOI] [PubMed] [Google Scholar]
- 58. Shore AC, Rossney AS, Brennan OM, Kinnevey PM, Humphreys H, Sullivan DJ, Goering RV, Ehricht R, Monecke S, Coleman DC. 2011. Characterization of a novel arginine catabolic mobile element (ACME) and staphylococcal chromosomal cassette mec composite island with significant homology to Staphylococcus epidermidis ACME type II in methicillin-resistant Staphylococcus aureus genotype ST22-MRSA-IV. Antimicrob. Agents Chemother. 55:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Novick RP, Christie GE, Penades JR. 2010. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 8:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlievert PM, Bohach GA. 2007. Staphylococcal and streptcoccal superantigens: an update, p 21–36 In Kotb M, Fraser JD. (ed), Superantigens: molecular basis for their role in human diseases. ASM Press, Washington, DC [Google Scholar]
- 61. Centers for Disease Control and Prevention 2000. Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup. MMWR Recommend. Rep. 49(RR-4):1–14 [PubMed] [Google Scholar]
- 62. Vojtov N, Ross HF, Novick RP. 2002. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc. Natl. Acad. Sci. U. S. A. 99:10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]


