Abstract
Heat shock proteins (Hsps) participate in the cellular response to stress and they are hiperexpressed in inflammatory conditions. They are also known to play a major role in immune modulation, controlling, for instance, autoimmune responses. In this study, we showed that oral administration of a recombinant Lactococcus lactis strain that produces and releases LPS-free Hsp65 prevented the development of experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. This was confirmed by the reduced inflammatory cell infiltrate and absence of injury signs in the spinal cord. The effect was associated with reduced IL-17 and increased IL-10 production in mesenteric lymph node and spleen cell cultures. Hsp65-producing-L. lactis-fed mice had a remarkable increase in the number of natural and inducible CD4+Foxp3+ regulatory T (Treg) cells and CD4+LAP+ (Latency-associated peptide) Tregs - which express the membrane-bound TGF-β - in spleen, inguinal and mesenteric lymph nodes as well as in spinal cord. Moreover, many Tregs co-expressed Foxp3 and LAP. In vivo depletion of LAP+ cells abrogated the effect of Hsp65-producing L. lactis in EAE prevention and worsened disease in medium-fed mice. Thus, Hsp65-L.lactis seems to boost this critical regulatory circuit involved in controlling EAE development in mice.
Keywords: Heat shock protein 65, Lactococcus lactis, Regulatory T cells, Experimental autoimmune, encephalomyelitis
1. Introduction
Heat shock proteins (Hsps) constitute a functional class of protein found in all living organisms, from bacteria to human beings, and whose expression becomes increased when cells are under stress [1]. These proteins are highly homologous among different species probably because they exert basic housekeeping functions in the cell. They act as intracellular chaperones, contribute to the cellular recycling of proteins favoring protein cleavage by proteasomes [2, 3], and participate in cell survival signaling pathways [4]. However, one of the most interesting functions of Hsps is related to their regulatory activities in the immune system [5]. The 60 kDa heat shock protein, for example, appears to affect immune inflammation by at least two different mechanisms: as a ligand for innate immune receptors, such as toll-like receptor (TLR)-2 [6], and as an antigen recognized by adaptive immune receptors [7]. After binding to TLR-2, Hsp60 may lead to a decrease in TNF-α and IFN-γ production and an increase of IL-10 release by T cells [8, 9]. As a protein recognized by clonal immune receptors, Hsps are considered as immunodominant antigens for self immune responses [10]. In this sense, self-Hsp-reactive T and B cell clones are described as part of a network of regulatory cells and molecules in the immune system engaged in homeostatic activities [11]. These activities would include tissue maintenance and repair, but also controlling clonal expansion and inflammation. Hsp60 has a strong effect in the survival and function of CD4+CD25+Foxp3+ regulatory T cells [6] and it was shown to efficiently drive the differentiation of CD4+CD25− T cell clones derived from juvenile idiopathic arthritis (JIA) patients into CD4+CD25high regulatory T cells [12, 13]. Hsp70 has also been demonstrated to exert immune-regulatory functions in animal models of arthritis [12]. Thus, Hsps may serve as promising candidates for the induction of self-reactive regulatory T cells (Tregs) and as alternative tools in the treatment of chronic inflammatory conditions and autoimmune diseases.
Multiple sclerosis (MS) is an autoimmune inflammatory neurodegenerative disorder characterized by nerve demyelination in the central nervous system. Symptoms depend on the site of the lesion but can include sensory loss, muscle weakness, speech difficulties, loss of coordination and dizziness. Treatments currently used in MS patients can only minimize the symptoms and retard disease development. Additionally, they are not efficient in severe forms of the disease and cause a myriad of side effects [14]. Therefore, therapies that aim to increase specific suppressor responses by the expansion of different types of regulatory T cells constitute the main focus of studies in this area [15].
Several types of T cells endowed with regulatory activities have been described in the past ten years. The main subset of regulatory T cells (Tregs) involved in self tolerance is the thymus-derived CD4+CD25+Foxp3+ T cell population [16]. However, other types of peripherally induced Tregs exist including CD4+CD25+Foxp3+ T cells induced in the intestinal mucosa [17], and CD4+ T cells characterized by their surface expression of the latency-associated peptide (LAP), which is the N-terminal propeptide of the TGF-β precursor [18]. Tregs exert suppressive activity by several mechanisms, namely modulation of antigen presenting cell (APC) function, metabolic disruption of target cells, cytotoxicity, production of inhibitory cytokines such as IL-10, TGF-β and IL-35 [19, 20] and IL-2 deprivation [21, 22]. CD4+CD25+LAP+ and CD4+CD25−LAP+ Tregs promote their suppressive functions mainly in a TGF-β-dependent fashion [18, 23, 24].
Several studies have shown that one of the best ways to induce specific regulatory T cells and tolerance peripherally is by the oral administration of antigens. Tolerance induced by feeding proteins, named oral tolerance, is a well known phenomenon that probably accounts for the robust balance that keeps the homeostasis of the gut mucosa to the daily challenge of microbiota and dietary antigens [25–27]. This is especially interesting regarding Hsps because bacterial components of our microbiota do express Hsps and they are likely to be involved in immune-regulatory networks in the gut.
Since Hsps are highly homologous among species and their expression is enhanced by inflammatory stress, these proteins are good antigen candidates for the therapeutic use of oral tolerance in autoimmune and other inflammatory diseases. Indeed, oral administration of Hsp65, a microbial 65 kDa Hsp (homolog to mammalian Hsp60) can induce tolerance and provide protection against adjuvant arthritis in rats [28] and atherosclerosis in mice [29, 30]. However, the mechanisms behind such effect remain elusive. Moreover, the proteolytic self-degradation of Hsp65 [31] leads to instability in its process of isolation, purification and storage. This usually hampers its usage as an immune-modulatory agent. To circumvent these caveats, we have developed a new strategy to deliver an endotoxin-free form of Mycobacterium leprae Hsp65 directly to the gut, without problems concerning separation and purification steps [32]. Such strategy involved the construction of a recombinant Lactococcus lactis strain, which is able to produce and secrete the endotoxin-free M. leprae Hsp65 to the extracellular medium, using a xylose-induced expression system (XIES). L. lactis, a lactic acid bacteria, is as an attractive and safe alternative, not only due to their “GRAS” (Generally Regarded As Safe) status granted by the U.S. Food and Drug Administration, but also because they are non-invasive, non-pathogenic, endotoxin free (gram-positive) and do not form inclusion bodies [33]. Moreover, L. lactis has been widely used for large-scale production of heterologous proteins for the last two decades [34].
Therefore, in the present study, we investigated the immunological effects of oral administration of M. leprae-Hsp65-producing L. lactis in the myelin oligodendrocyte glycoprotein (MOG35–55)-induced experimental autoimmune encephalomyelitis (EAE), a well characterized rodent model for multiple sclerosis (MS). We found that oral administration of M. leprae-Hsp65-producing L. lactis, but not the control (CT) L. lactis strain, prevented the development of MOG35–55-induced EAE in C57BL/6 mice. Moreover, EAE inhibition was associated with an anti-inflammatory cytokine milieu in lymph nodes and spleen, and an expansion of regulatory T cells in the peripheral lymphoid organs as well as within the spinal cord. In vivo depletion of LAP+ Tregs using an anti-mouse LAP mAb not only abolished the immune-modulatory effects of M. leprae-Hsp65-producing L. Lactis, but also worsened the EAE development in untreated diseased mice. Thus, M. leprae-Hsp65-producing L. lactis may constitute an important candidate for the treatment of multiple sclerosis.
2. Materials and methods
2.1. Construction of Hsp65-producing L. lactis
As described elsewhere [35], a recombinant L. lactis strain NCDO2118 able to secrete M. leprae Hsp65, using a xylose-inducible expression system (XIES), was constructed. The constructed vector (pSEC:hsp65) directed the expression of Hsp65 to the extracellular medium. L. lactis NCDO2118 harboring an empty vector (pXylT:SEC without hsp65) was used, as negative control, in all experiments.
2.2. Bacterial strains and growth conditions
The L. lactis NCDO2118 strains were grown in Difco M17 broth, supplemented with 0.5% glucose (GM17) or 1% xylose (XM17), at 30 °C, without agitation. When required, chloramphenicol (10 µg/ ml) was added to the media.
2.3. Conditions of xylose induction
On the first day, a single colony of recombinant L. lactis harboring an empty vector (harboring pXylT:SEC) or recombinant L. lactis NCDO2118 harboring pXylT:SEC:hsp65 was grown at 30 °C, without agitation, in 5 ml of GM17, containing chloramphenicol (Cm) (10 µg/ml). On the second day, the overnight culture was diluted 1:10,000 in 1% xylose fresh M17 (XM17), supplemented with Cm (10 µg/ml) to induce expression of the M. leprae hsp65 gene. On the third day, when a 2.0 optical density at 600 nm (OD600 nm) was reached, corresponding to 2.5 × 108 CFU/ml, protein extraction, Western blotting and the mice treatment were performed.
2.4. Protein extractions
Protein sample preparation from L. lactis cultures was performed as previously described [36], with some modifications. Samples were prepared from 2 ml of both induced and non-induced cultures. Next, they were centrifuged for 10 min at 4 °C, at 12,000 g. Later, supernatants and pellets were treated separately. Supernatants were run through a 0.2 µm pore-size filter and then 100 µl of 100% trichloroacetic acid (TCA) were added for protein precipitation. Dithiothreitol (DTT) at 10 mm and phenyl-methylsulphonyl fluoride (PMSF) at 1 mm were also added to the filtrate, which was then incubated for 1 h on ice and centrifuged (20 min, 12,000 g, 4 °C). Pellets were resuspended in 50 mm NaOH. The original cellular pellet was resuspended in 100 µl of TES-Lys buffer (25% sucrose, 1 mm EDTA, 50 mm Tris–HCl [pH 8.0] lysozyme [10 mg/ml]), PMSF 1 mm and DTT 10 mm. After that, it was incubated at 37 °C for 30 min and then, 50 µl of 20% SDS was added.
2.5. Protein quantification and Western blot analysis
The Bradford method [37] was used to determine the concentration of total proteins extracted from L. lactis. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed as described by [38].Western blot analyses were conducted according to [39], using anti-Hsp65 antibodies (1:2500) (Farmacore Biotecnologia Ltda) and rabbit anti-immunoglobulin (IgG), conjugated to alkaline phosphatase (Sigma). Immunodetection blots were scanned and the M. leprae Hsp65 signals were compared to those of known amounts of a purified Hsp65 produced in E. coli (Farmacore Biotecnologia Ltda).
2.6. Detection of viable Mycobacterium leprae Hsp65-producing L. lactis in the gut
Male and female C57BL/6 mice at 6–8 weeks of age were continuously fed M. leprae-Hsp65-producing L. lactis for four consecutive days. One day thereafter, intestinal lumen from cecum, small and large intestines was washed with phosphate-saline buffer (PBS) 1X and live M. leprae-Hsp65-producing L. lactis were counted by plating 10-fold dilution of the lavage in GM17E agar plates containing 10 µg/ml of chloramphenicol.
2.7. Animals
All animal procedures were approved by the University Ethical Committee for Animal Experimentation (CETEA-UFMG). Male and female C57BL/6 mice at 6–8 weeks of age were supplied by the Central Animal Facility of Universidade Federal de Minas Gerais (UFMG). C57BL/6 Foxp3-green fluorescence protein (GFP)-knock-in mice were kindly provided by Dr. Howard L. Weiner (Center for Neurologic Diseases – Brigham and Women’s Hospital, Boston, MA, USA). Mice were kept in the conventional, pathogen-free experimental animal facility of Laboratório de Imunobiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brasil.
2.8. L. lactis administration and EAE induction
During four days C57BL/6 or C57BL/6 Foxp3-GFP mice were continuously fed medium (control group), empty-vector-bearing L. lactis (CT-LL) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL). Daily, a fresh L. lactis total culture (bacteria plus supernatant obtained as described in item 2.3)was offered to mice. Since each mouse drank about 5 ml of culture per day (data no shown) containing 7 µg/ml [35] of M. leprae Hsp65, the total dose of bacteria per mouse was estimated to be 5 × 109 CFU and the total daily dose of M. leprae Hsp65-was about 35 µg per mouse. However, since live bacteria are fed, the effective dose is larger than that. Mice drink the bacteria solution throughout the day. Some of the fed bacteria (along with xylose-containing medium) reaches the small intestine and the colon alive and in conditions to release Hsp65. Previous data from our laboratories [49, 50] demonstrated that a continuous regimen of feeding is more efficient to induce oral tolerance. The Hsp65-producing L. lactis system imitates a continuous feeding regimen and that was ultimately the reason for choosing it. After 10 days mice were immunized in the base of tail with either 100 µg of myelin oligodendrocyte glycoprotein (MOG35–55; Proteimax, Brazil) (MEVGWYRSPFSRVVHLYRNGK) in CFA containing 4 mg/ml of Mycobacterium tuberculosis H37RA (Difco) or 500 µg of zymosan (Sigma) in IFA followed by i.p. injection of 300 ng of pertussis toxin (Sigma). At the indicated time points, animals were killed and draining lymph nodes, spleen and spinal cord were harvested for analysis (Fig. 1C). Clinical assessment of EAE was performed according to the following criteria: 0, no signs of disease; 0.5, partial tail paralysis; 1, tail paralysis or waddling gait; 1.5, partial tail paralysis and waddling gait; 2, tail paralysis and waddling gait; 2.5, partial limb paralysis; 3, paralysis of one limb; 3.5, paralysis of one limb and partial paralysis of another; 4, complete hind-limb paralysis; 4.5, complete hind-limb paralysis and front-limb weakness; 5, moribund.
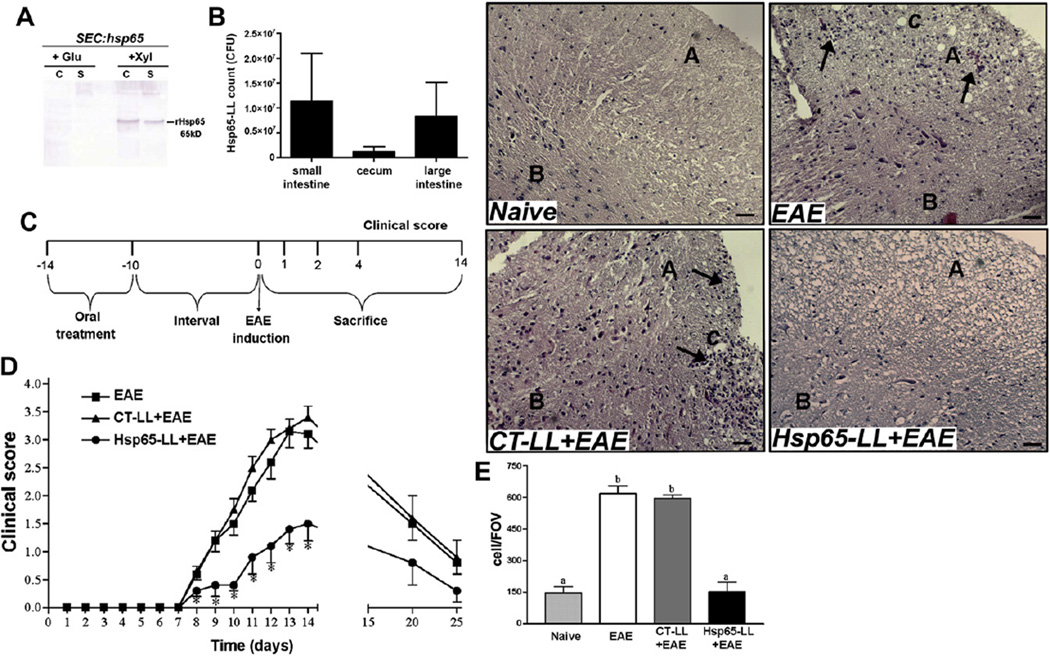
Fig. 1.
Extracellular rHsp65 production, viability of M. leprae-Hsp65-producing L. lactis in the gut and prevention of EAE development. (A) Extracellular rHsp65 production. Protein extracts of xylose induced (Xyl columns) and non-induced (Glu colums) culture samples of L. lactis NCDO2118 (SEC:hsp65) strain in stationary-phase (OD600 = 2.0) were prepared from cell (C columns) and supernatant (S columns) fractions. Extracts were analyzed by Western blotting using anti-hsp65 antibodies. (B) Viability of L. lactis in the gut. C57BL/6 mice were continuously fed M. leprae-Hsp65-producing L. lactis for four consecutive days. One day later, animals were killed and the intestinal lumen from cecum, small and large intestines was washed and plated in GM17E agar for 24 h at 37 °C. Colony-forming units (CFU) were then counted and plotted as mean ± standard error of the mean (SEM); n = 4 mice per group. ANOVA, post-test Tukey. (C) Experimental protocol for medium (EAE), control-(CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) pretreatment and EAE induction. (D) Pretreatment with Hsp65-LL prevented EAE development in mice. C57BL/6 mice were orally pretreated with medium (EAE), control (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. The course of EAE was followed daily and clinical scores were given as explained in material and methods section. Disease course is shown as mean EAE score ± SEM. n = 15 mice/group and data are the sum of three independent experiments; ANOVA, post-test Tukey. *Statistically different from EAE and CT-LL+EAE group; p < 0.05. (E) Spinal cords were removed 14 days after EAE induction and 5 µm serial section were stained with hematoxylin–eosin (HE). The inset graph represents the cells counting in a field of view (FOV) and bars are the mean of 3 mice/group ± SEM; ANOVA, post-test Tukey. Letter A indicates spinal cords’ white matter and B gray matter; C, white matter lesions; arrows indicate inflammatory cells infiltrate. Bars: 50 µm. Magnification: 100×.
2.9. Serum antibody response against Hsp65
To analyze the serum antibody response against Hsp65, ten days after EAE induction, sera of mice from all groups were collected and anti-Hsp65 antibodies were measured by ELISA. Briefly, 96-well microtiter plates (NUNC) were coated with 10 µg/ml of purified Hsp65 (MyBioSource). Plates were incubated with serum samples and bound antibodies were detected using alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology). Color reaction was developed at room temperature with orthophenyle-nediamine (OPD; 1 mg/ml), 0.04% H2O2 substrate in sodium citrate buffer. Reaction was interrupted by the addition of 20 µl/well of 2 N H2SO4. Absorbance was measured at 492 nm by an ELISA reader (Bio-Rad Model 450 Microplate Reader).
2.10. Mononuclear cell extraction from spinal cord
For mononuclear cell extraction, pool of 5 mice spinal cords were carefully removed, cut into pieces and put in 3 ml of RPMI 1640 (Gibco) medium containing 1.5 mg/ml of colagenase type IV (Sigma). Conic tubes containing the spinal cord samples were kept under agitation at 37 °C for 20 min and then more 2 ml of RPMI medium containing 1.5 mg/ml of colagenase type IV were added, being samples kept under agitation for more 20min. After that, tissues were homogenized, transferred to 50 ml conic tubes and volumes were completed for 45 ml with RPMI medium. Samples were centrifuged for 5 min at 4 °C, 220 g and then the supernatants were discarded. Pellets were resuspended with 3 ml of Percoll 35% and, carefully, added 3 ml of Percoll 65%. Samples were then centrifuged for 30 min at 4 °C, 300 g. The ring formed between the Percoll layers were removed, washed with 10 ml of RPMI medium at 4 °C, 220 g for 10 min and resuspended with 0.2 ml of complete RPMI medium. Leucocytes were then counted in a Neubauer chamber and stained for FACS analysis.
2.11. Antibodies and FACS analysis
Fluorescein isothiocyanate-conjugated (FITC) mAbs to CD4 and CD69; phycoerythrin (PE)-conjugated mAbs to CD4, CD25, CD44 and CTLA4; PE-Cy.5-conjugated mAbs to CD4, streptavidin-Cy.5-Chrome and streptavidin–allophycocyanin (APC), purified anti-CD3ε/CD28, rat IgG1 isotype control and rat IgG2a isotype control were purchased from BD Biosciences. PE-conjugated mAbs to TGF-β was purchased from IQ Products. Biotinylated anti-human LAP (TGF-β1) antibody was purchased from R&D System. PE-conjugated mAb anti-helios was purchased from Biolegend. Mouse anti-LAP mAb (clone TW716B4) was gently provided by Dr. Howard L. Weiner (Brigham & Women’s Hospital, Center for Neurologic Diseases, Harvard University). Surface staining was performed according to standard procedures at a density of 0.5–1 × 106 cells per 25 µl, and volumes were scaled up accordingly. Flow cytometric analysis was performed on a FACScan (BD Biosciences) or on a FACSCalibur (BD Biosciences) with the use of FlowJo software (Tree Star Inc).
2.12. Serum antibody response against Salmonella typhimurium
To verify whether M. leprae-Hsp65-producing L. lactis would have an immunosuppressive effect, mice were pretreated with medium or M. leprae-Hsp65-producing L. lactis for four consecutive days (as described before, item 2.8) and ten days later they were challenged with 105 Salmonella enterica serovar Typhimurium. Serum anti-Salmonella antibodies were measured by standard ELISA seven days after infection.
2.13. Cell preparation and cytokines assay
Spleen and mesenteric lymph nodes were removed and cells suspensions prepared using a tissue homogenizer, being gently centrifuged. Cells isolated from spleen (erythrocyte depleted) and mesenteric lymph nodes were cultured, at 5 × 106 cells/well for cytokine secretion analyses, in 24-well plates (NUNC) in complete RPMI and stimulated or not with 100 µg/ml of MOG35–55 or 1 µg/ml of anti-CD3. Supernatants were collected after 48 h to measure IL-10, IL-17 and IFN-γ. Plates were then coated with purified monoclonal antibodies reactive to IL-10, IL-17 or IFN-γ (BD Biosciences) overnight at 4 °C. In the following day, wells were washed and supernatants were added and left overnight at 4 °C. In the third day biotinylated monoclonal antibodies were added and incubated for 1 h at room temperature. Color reaction was developed at room temperature with 100 µl/well of orthophenyle-nediamine (OPD; 1 mg/ml), 0.04% H2O2 substrate in sodium citrate buffer. Reaction was interrupted by the addition of 20 µl/well of 2 N H2SO4. Absorbance was measured at 492 nm by an ELISA reader (Bio-Rad Model 450 Microplate Reader).
2.14. Intracellular TGF-β staining
Spleen, mesenteric and inguinal lymph nodes were removed 2 days after EAE induction and cells suspensions prepared using a tissue homogenizer, being gently centrifuged. Cells isolated from spleen (erythrocyte depleted), mesenteric and inguinal lymph nodes were cultured at 1 × 106 cells/well in 96-well plates (NUNC) in complete RPMI and stimulated with either 100 µg/ml of MOG35–55 or 1 µg/ml of anti-CD3 and 1 µg/ml of anti-CD28 or left unstimulated. Eighteen hours later, 10 µl of brefeldin A (1 mg/ml) was added and after 4 h cells were washed and FACS protocol started.
2.15. Histopathology
Spinal cord samples from medium, empty vector L. lactis or M. leprae-Hsp65-producing L. lactis pretreated mice were fixed with a solution containing 20% of dimethyl sulfoxide and 80% of methanol for six days at −80 °C, 24 h at −20 °C and then embedded in Paraplast® according to standard procedures. 5 µm of Paraplast® were cut and stained with hematoxylin and eosin. All sections were evaluated for histopathological changes, such as inflammatory cells infiltration and white matter lesions using an optical microscope (Olympus BX41) with a camera (Moticam 2500). For cell quantification, representative histological cuts were analyzed for cells in the field of view (FOV) using the ImageJ software and bar graphwas plotted through Prisma 5.0 software.
2.16. In vivo neutralization of LAP+ regulatory T cells
For in vivo neutralization of LAP regulatory T cells, C57BL/6 mice were immunized with 100 µg of MOG35–55 in CFA supplemented with 4 mg/ml of M. tuberculosis s.c. and 300 ng of pertussis toxin i.p. (as described above) one day after M. leprae-Hsp65-producing L. lactis or medium (control group) pretreatment. Mice received three i.p. injections of anti-LAP monoclonal antibody (20 µg/mouse/ day; clone TW716B4) or isotype control (IgG1) on alternating days beginning at the first day of L. lactis administration.
2.17. Statistical analysis
Results are presented as the mean values (±SEM) from groups of at least 6 animals for each condition and considered statistically significant when comparisons between groups, using one-way ANOVA and Tukey’s post hoc test, gave p-values less than 0.05.
3. Results
3.1. L. lactis produces and secretes LPS-free M. leprae Hsp65
Western blot experiments of xylose-induced and non-induced cultures were conducted to detect the production of M. leprae Hsp65 by L. lactis carrying the plasmid pSEC:Hsp65. Analysis of induced stationary-phase cell lysates and culture supernatants demonstrated that L. lactis pSEC:hsp65 were able to produce and secrete the recombinant protein. No signal of the protein was detected in the non-induced cultures (Fig. 1A). Analysis of protein content showed that the band migrated at the expected position (65 kDa), which is the size of M. leprae Hsp65 (Fig. 1A).
Azevedo and coworkers showed that L. lactis expressed Hsp65 preparations contained less endotoxin (lypopolysaccharide, LPS) than the limit set by the Food and Drug Administration (FDA) [35].
3.2. Viable L. lactis reaches the gut
Culture of intestinal lumen lavage of mice pretreated with M. leprae Hsp65-producing L. lactis showed a growth of 0.25–2 × 107 colony-forming units (CFU), suggesting that M. leprae-Hsp65-L. lactis survives gastric acid degradation and arrives viable at the cecum, small and large intestines (Fig. 1B).
3.3. Oral administration of M. leprae-Hsp65-producing L. lactis prevents EAE in mice
As described in the method section, M. leprae-Hsp65-producing L. lactis was continuously fed to C57BL/6 mice for four consecutive days. Ten days after the last feeding, EAE was induced (Fig. 1C). Fig. 1D shows that pretreatment of mice with M. leprae-Hsp65-producing L. lactis retarded the onset and decreased the severity of EAE when compared to the control group, i.e., mice that drank medium, and those pretreated with empty vector control L. lactis. Accordingly, as shown in Fig. 1E, fewer inflammatory cells infiltrated the spinal cord, and no white matter lesions were observed at the peak of the disease in M. leprae-Hsp65-L. lactis-fed mice.
3.4. Oral administration of M. leprae-Hsp65-producing L. lactis induces tolerance to Hsp65 and is associated with an anti-inflammatory profile of cytokine secretion
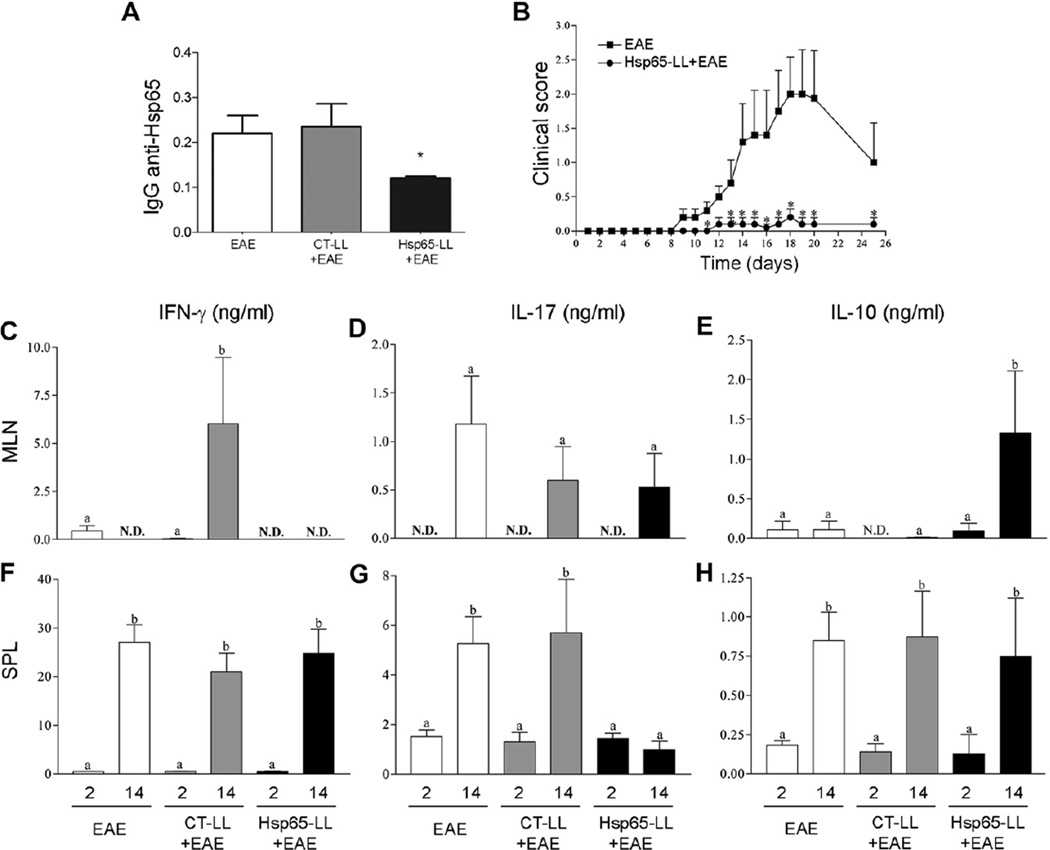
We started studying the mechanisms involved in EAE prevention by assessing whether oral administration of M. leprae-Hsp65-producing L. lactis could induce tolerance to Hsp65. Sera from mice treated with either medium (EAE), empty-vector-bearing L. lactis (CT-LL+EAE) and M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) were collected and an enzyme-linked immunosorbent assay (ELISA) for IgG anti-Hsp65 was performed. As shown in Fig. 2A, oral treatment with M. leprae-Hsp65-producing L. lactis induced suppression in antibody production to Hsp65, suggesting that mice fed M. leprae Hsp65 have reduced response against this antigen. It is also shown in Fig. 2C–H that this Hsp65-directed tolerance was spread to other antigens (cross tolerance) in the vicinity of the lesion (MOG35–55 in the case), since the production of inflammatory cytokines was reduced in spleen (SPL) and mesenteric lymph node (MLN) cell cultures stimulated with MOG35–55 (100 µg/ml) of M. leprae Hsp65-producing L. lactis fed mice. In fact, pretreatment of mice with M. leprae Hsp65-producing L. lactis led to non-detectable (N.D.) levels of IFN-γ in the MLN cell cultures at 2 and 14 days after EAE induction (Fig. 2C). Interestingly, however, the highest levels of IFN-γ were found in control-L. lactis-fed mice 14 days after EAE induction (Fig. 2C). No difference was found among groups in SPL cells for IFN-γ (Fig. 2F). When we measured IL-17, another pro-inflammatory cytokine involved in the pathogenesis of EAE and multiple sclerosis [37, 40, 41], reduced levels were found in cultures of MLN and in SPL cells from M. leprae-Hsp65-L. lactis-fed mice 14 days after EAE induction (Fig. 2D and G). IL-17 was not detected in MLN cell cultures 2 days post EAE induction. However, levels of IL-10, an anti-inflammatory cytokine, were increased only in supernatants of MLN cell cultures of M. leprae-Hsp65-L. lactis-fed mice 14 days after EAE induction (Fig. 2E and H). Of note, levels of IFN-γ, IL-17 and IL-10 measured at day 14 were higher than the levels found at day 2 post EAE induction in animals from all groups. Thus, M. leprae Hsp65 administration was associated with a decrease in the production of IL-17, and with an increase in the secretion of IL-10.
Fig. 2.
Oral pretreatment of mice with M. leprae-Hsp65-producing L. lactis decreases IgG response against Hsp65 and induces an anti-inflammatory cytokine profile during EAE development. (A) Oral tolerance to Hsp65. C57BL/6 mice were fed medium (EAE), control (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 10 days, sera were collected and anti-Hsp65 IgG antibodies were measured. ELISA scores were computed by running sums of ODs (492 nm) between 1:2 and 1:16 of serum dilutions in individual mice. Bar graphs are shown as a mean of 5 mice/group + SEM. ANOVA, post-test Tukey. *Statistically different from EAE and CT-LL+EAE groups; p < 0.05. (B) The effect of treatment with M. leprae-Hsp65-producing L. lactis in EAE development was independent of the adjuvant used for disease induction. C57BL/6 mice were fed either medium (EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days. EAE was induced ten days later using zymosan adsorbed in IFA and two injections of pertussis toxin. The course of EAE is shown as mean EAE score of 5 mice/group ± SEM and data are representative of two independent experiments; ANOVA, post-test Tukey. *Statistically different from EAE group; p < 0.05. (C–H) Anti-inflammatory cytokine profile induced by oral pretreatment of M. leprae-Hsp65-produncing L. lactis. Two and fourteen days after EAE induction, mesenteric lymph nodes (C, D and E) and spleen (F, G and H) were removed and cultured with or without MOG35–55 stimulus (100 µg/ ml) for two days to cytokine analysis. Data are shown as mean ± SEM of cytokine concentrations measured in supernatants of MOG-stimulated cultures minus the basal values measured in non-stimulated cultures. Bars are the mean of 5 mice/group and data are representative of two independent experiments; N.D. = not detected; ANOVA, post-test Tukey; p < 0.05.
3.5. The modulatory effect of M. leprae-Hsp65-producing L. lactis was independent of the adjuvant used for disease induction
Even with the results showing a typical bystander suppression phenomenon between Hsp65 and MOG35–55 (Fig. 2A and C–H), there was still a possibility that the adjuvant used for EAE induction was the main responsible for the suppressive effect observed after treatment with M. leprae Hsp65-producing L. lactis. The classical protocol of MOG-induced EAE involves immunization of mice with complete Freund’s adjuvant (CFA), which is rich in Hsp65 from M. tuberculosis. To further clarify this issue, we induced EAE using zymosan, a glucan prepared from yeast cell wall, which does not contain any kind of heat shock protein, in incomplete Freund adjuvant (IFA) together with MOG35–55 and pertussis toxin (see Material and methods section, item 2.8). Our results show that oral administration of M. leprae Hsp65-producing L. lactis was still able to prevent EAE induced by MOG adsorbed in zymosan (Fig. 2B), suggesting that M. leprae Hsp65 modulation of EAE was not due to tolerance induced to the adjuvant used for the immunization procedure.
3.6. Oral administration of M. leprae-Hsp65-producing L. lactis correlated with increase in activated T cells
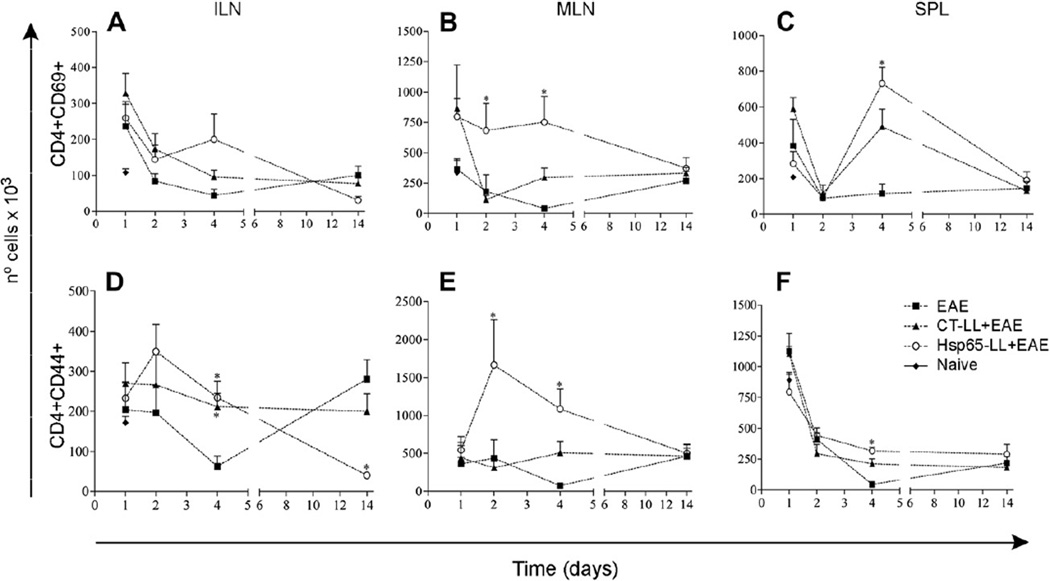
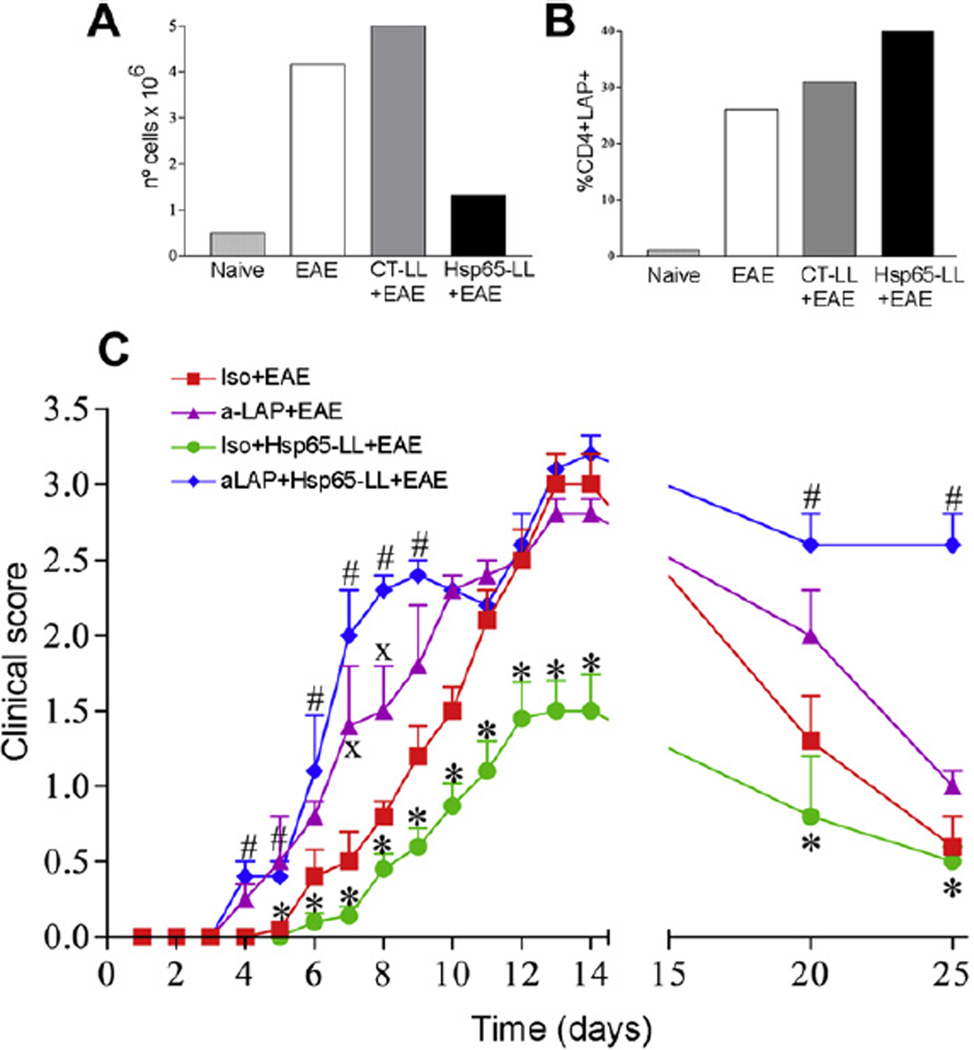
Since Hsp65 is widely found in bacteria and it is also a protein highly homologous to self Hsp60, the immune-modulatory effects observed in mice pretreated with M. leprae-Hsp65-producing L. lactis could be a result of a general state of immunosuppression that would interfere with protective responses against infection. To monitor the immunological status of M. leprae-Hsp65-L. lactis-fed mice, we sought for CD4+T cells expressing early and late markers of T cell activation. Numbers of CD4+ T cells expressing the earliest inducible cell surface glycoprotein acquired during lymphoid activation, CD69 (Fig. 3A–C), and the hyaluronic acid receptor CD44 (later activated T cells) (Fig. 3D–F) were analyzed 1, 2, 4 and 14 days after EAE induction. Interestingly, M. leprae-Hsp65-L. lactis-fed mice showed no reduction in the number of T cells expressing early and late activation markers. In fact, mice displayed higher numbers (Fig. 3) and frequencies (data not shown) of CD4+CD69+ and CD4+CD44+ T cells 2 and 4 days after EAE induction when compared with animals from the other two groups (medium and CTL. lactis-treated mice). Increase in CD4+CD69+ T cells was particularly observed in MLN (Fig. 3B) at days 2 and 4, and in the spleen at day 4 post EAE induction, although in this organ there was no difference between M. leprae Hsp65-L. lactis- and CT-L. lactis-treated mice (Fig. 3C).Numbers (Fig. 3E) and frequencies (data not shown) of CD4+CD44+ T cells were increased in MLN at days 2 and 4 post EAE induction in M. leprae-Hsp65-L. lactis-fed mice. However, CD4+CD44+ T cell numbers in inguinal lymph nodes and spleens of M. leprae-Hsp65-L. lactis-fed mice did not differ from the numbers found in CT-L. lactis-fed mice (Fig. 3D and F). At day 14, i.e., at the peak of the disease, numbers of CD4+CD69+ T cells were comparably low in all groups (Fig. 3A–C). However, numbers (Fig. 3D) and frequencies (data not shown) of CD4+CD44+ T cells in ILN of medium-fed mice were much higher than in M. leprae-Hsp65- or CT-L. lactis-fed animals. Thus, a global immunosuppression of T cells caused by oral administration of M. leprae-Hsp65-producing L. lactis seemed not to be the case in this study. Moreover, treatment with M. leprae Hsp65-producing L. lactis did not decrease immune response against S. enterica serovar Typhimurium (Supplemental Fig. 1), a pathogenic enteric bacterium, suggesting that the EAE suppression triggered by the treatment did not interfere with protective immune response to infection. Of note, M. leprae-Hsp65-producing L. lactis had an adjuvant effect on the antibody response against S. typhimurium.
Fig. 3.
Oral pretreatment of mice with M. leprae-Hsp65-producing L. lactis correlated with the increase in activated T cells in mice. C57BL/6 mice were fed or not (Naïve) medium (EAE), wild type (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 1, 2, 4 and 14 days, mice were killed and inguinal (ILN; A) and mesenteric lymph nodes (MLN; B) and spleen (SPL; C) removed. Cells were stained with either (A–C) PE-anti-CD4 and FITC-anti-CD69 or (D–F) Cy-anti-CD4 and PE-anti-CD44. Bar graphs are shown as mean ± SEM. ANOVA, post-test Tukey. *Statistically different from EAE group; p < 0.05.
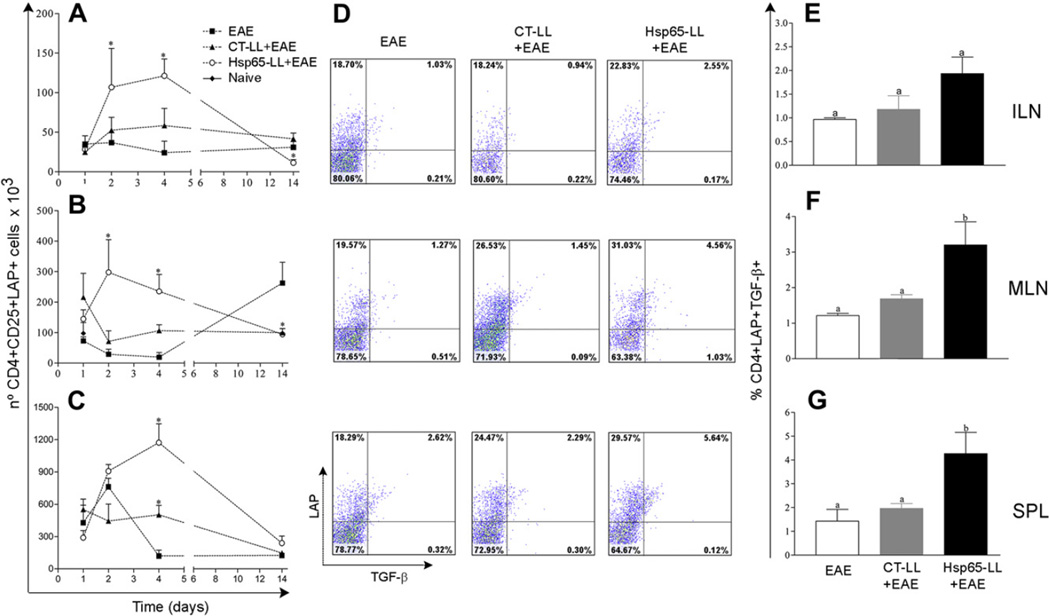
3.7. Administration of M. leprae-Hsp65-producing L. lactis led to an increase in regulatory T cells in secondary lymphoid organs
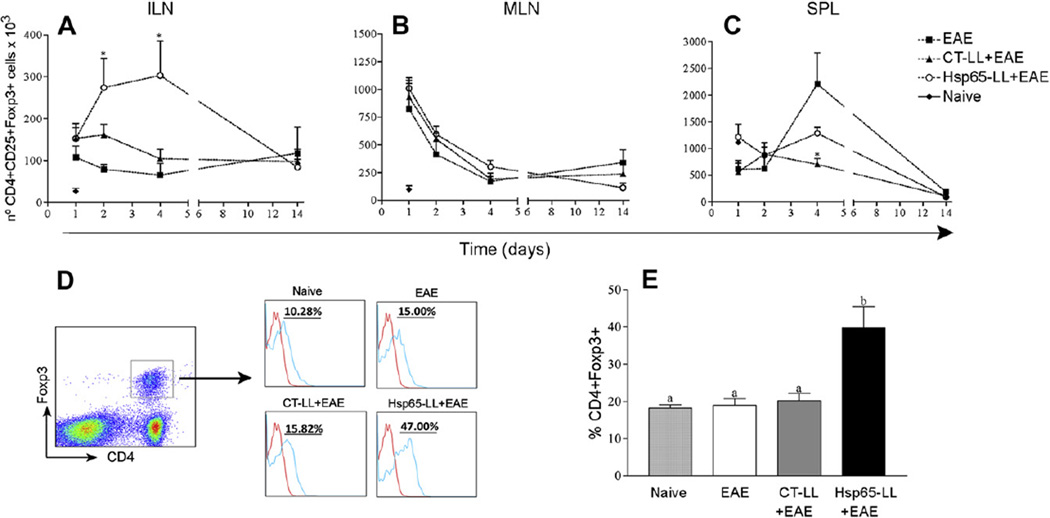
It has been described that Hsp65 modulates the regulatory T cell (Treg) compartment in mice [28, 42]. To verify whether the immune-modulatory effect of M. leprae-Hsp65-producing L. lactis in EAE correlated with expansion of T cells bearing a regulatory phenotype (Treg), we analyzed the numbers of two well-known Treg populations: CD4+CD25+Foxp3+ (Fig. 4) and CD4+CD25+LAP+ (Fig. 5) T cells. CD4+CD25+Foxp3+ Tregs were augmented only in ILN of M. leprae-Hsp65-L. lactis-fed mice at days 2 and 4 post EAE induction (Fig. 4A–C). At day 14, numbers (Fig. 4A–C) and frequencies (data not shown) of Foxp3+ Tregs were comparably low in all groups.
Fig. 4.
Oral pretreatment of mice with M. leprae-Hsp65-producing L. lactis lead to an increase in both natural and inducible CD4+Foxp3+ Treg cell populations in mice. C57BL/6 Foxp3-GFP Knock-in mice were fed or not (Naïve) medium (EAE), wild type (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 1, 2, 4 and 14 days, mice were killed and inguinal (ILN; A) and mesenteric lymph nodes (MLN; B) and spleen (SPL; C) removed. Cells were stained with Cy-anti-CD4 and PE-anti-CD25. CD4+ cells were gated. Bar graphs are shown as mean ± SEM. ANOVA, post-test Tukey. *Statistically different from EAE group; p < 0.05. (D and E) Increase in the natural regulatory T (nTreg) cells population in inguinal lymph node after oral administration of M. leprae-Hsp65-producing L. lactis. C57BL/6 Foxp3-GFP Knock-in mice were fed or not (Naïve) medium (EAE), control (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. Four days later, mice were killed and inguinal lymph nodes removed. Cells were stained with Cy-anti-CD4 and PE-anti-Helios. CD4+Foxp3+ cells were gated. Plots are representative of the mean of 4 mice/group and data are representative of three independent experiments. Bar graphs are shown as mean ± SEM. ANOVA, post-test Tukey. *Statistically different from EAE group; p < 0.05.
Fig. 5.
Oral pretreatment of mice with M. leprae-Hsp65-producing L. lactis was associated with an increased of CD4+LAP+ regulatory T cells expressing TGF-β in mice. C57BL/6 mice were fed or not (Naïve) medium (EAE), control (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 1, 2, 4 and 14 days, mice were killed and inguinal (ILN; A) and mesenteric lymph nodes (MLN; B) as well as spleen (SPL; C) removed. Cells were stained with FITC-anti-CD4, PE-anti-CD25, Bio-LAP and CY-STV. Graphs are shown as mean ± SEM. ANOVA, post-test Tukey. *Statistically different from EAE group; p < 0.05. (D–G) Oral administration of M. leprae-Hsp65-producing L. lactis increased TGF-β production by LAP+ Treg cells. C57BL/6 mice were fed medium (EAE), wild type (CT-LL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 2 days, mice were killed and inguinal (ILN; D and E) and mesenteric (MLN; D and F) lymph nodes as well as spleens (SPL; D and G) were removed and cultured with MOG35–55 (100 µg/ml) for 18 h and more 4 h with 10 µl of 1 mg/ml of brefeldin A. Cells were stained with FITC-anti-CD4, Bio-LAP and CY-STV and PE-anti-TGF-β. CD4+ cells were gated. Plots (D) are representative of the mean of 3 mice/group and bar graphs (E–G) are shown as mean ± SEM. Data are representative of three independent experiments; ANOVA, post-test Tukey; p < 0.05.
Since Hsp65 from bacteria is very homologous to murine Hsp60, it is plausible that auto-reactive natural regulatory T cells generated during thymus selection (nTregs) are part of the Tregs recruited or expanded by the treatment with M. leprae-Hsp65-producing L. lactis. The presence of Helios, a member of the Ikaros transcription factor family, together with Foxp3 has been recently described as a reliable marker for natural regulatory T cells (nTreg) [43]. Thus, we measured the expression of Helios in Foxp3+ Tregs found in ILN from mice pretreated with medium, control-L. lactis or M. leprae-Hsp65-producing L. lactis at day 2 post EAE induction when higher numbers of such cells were observed. About 50% of CD4+CD25+Foxp3+ T cells were Helios+ (nTregs) in M. leprae- Hsp65-L. lactis-fed mice, whereas only about 17–19% were nTregs in medium- and CT-L. lactis-fed mice (Fig. 4D and E). These results suggest that at least half of CD4+CD25+Foxp3+ T cells expanded in ILN after oral administration of M. leprae-Hsp65-producing L. lactis were natural regulatory T cells and therefore thymus derived.
When we looked for the mucosal-induced CD4+CD25+LAP+, an increase in the numbers (Fig. 5A–C) and frequencies (data not shown) of LAP+ Tregs were observed in ILN, MLN and SPL 2 and 4 days after EAE induction in mice that fed M. leprae-Hsp65-producing L. lactis when compared to animals from the other two experimental groups. Of note, the proportion of CD4+CD25−LAP+ Tregs was also increased in M. leprae-Hsp65-L. lactis-fed mice (data not shown). However, the numbers and frequencies of LAP+ T cells were much higher in the diseased group (EAE) than in the M. leprae- Hsp65-L. lactis- or CT-L. lactis-fed mice in MLN at day 14 (Fig. 5B and data not shown). Taken together, these data indicate that the prevention of EAE development by M. leprae-Hsp65-producing L. lactis correlates with an increase in the number of CD4+CD25+Foxp3+ and CD4+LAP+ regulatory T cells in secondary lymphoid tissues.
3.8. Regulatory T cells associated with the effect of M. leprae-Hsp65-producing L. lactis co-expressed Foxp3 along with LAP and produced TGF-β
Recently it was suggested that Foxp3 transcription factor is important for LAP expression [44]. Therefore, we next examined the percentages of LAP+ T cells co-expressing Foxp3 and vice-verse. Our results showed that more than 50% of the CD4+CD25+Foxp3+ Tregs co-expressed LAP, whereas only about 15% of the CD4+LAP+ Treg cells co-expressed Foxp3 in mice from all experimental groups. Moreover, mice pretreated with M. leprae-Hsp65-producing L. lactis showed slightly higher proportions of CD4+Foxp3+LAP+ Tregs than animals from the other groups analyzed (Supplemental Fig. 2A–F). The cytotoxic T-lymphocyte antigen-4 (CTLA-4, also known as CD152) is related to an activated state of Tregs and thereby is used as a marker of effector Tregs [45]. Thus, we next examined the expression of CTLA-4 on Tregs from ILN, MLN and spleen 2 days after EAE induction. Oral administration of M. leprae-Hsp65-producing L. lactis, but not medium or control L. lactis, led to a high expression of CTLA-4 on CD4+CD25+Foxp3+ Tregs (data not shown). However, the same was not observed for CD4+LAP+ Tregs (data not shown). This could be related to the fact that CD4+Foxp3+ Tregs produce their inhibitory effects mainly through cell–cell contact [20, 46, 47], whereas CD4+LAP+ Tregs control effectors T cells and antigen presenting cells (APCs) by releasing anti-inflammatory cytokines [15, 24]. In fact, when we measured intracellular TGF-β, we did find increased levels of this cytokine as well as high numbers of CD4+LAP+ Tregs expressing TGF-β in ILN, MLN and spleen stimulated with either MOG35–55 (Fig. 5D–G) or anti-CD3/CD28 or even with no stimulus (data not shown) in M. leprae-Hsp65-L. lactis-fed mice 2 days after EAE induction. Therefore, these data suggests that Foxp3+LAP+ Tregs correlated better with EAE suppression in our model than did Foxp3+LAP− Tregs.
3.9. Oral administration of M. leprae-Hsp65-producing L. lactis was associated with changes in the spinal cord T cell infiltrate
As shown above, oral administration of M. leprae-Hsp65-producing L. lactis in mice induced an increase in numbers of Tregs in secondary lymphoid tissues 2 and 4 days after EAE induction. After day 4, Tregs were found at low numbers in spleen, inguinal and mesenteric lymph nodes. Therefore, we examined whether CD4+CD25+Foxp3+ and CD4+LAP+ Treg cells could have reached the spinal cord of mice, justifying their decline in number and frequency in all these lymphoid organs at day 14. Leucocytes were isolated from the spinal cord of Foxp3-GFP-knock-in mice pretreated with medium, control L. lactis or M. leprae-Hsp65-producing L. lactis. These cells were stained with antibodies for CD4 and LAP. Flow cytometry analysis showed that, although fewer cells reached the spinal cord of M. leprae-Hsp65-L. lactis-fed mice (Fig. 6A), there were higher frequencies of LAP+ T cells (Fig. 6B), but not Foxp3+ Tregs (data not shown), in these mice when compared to animals pretreated with medium or control L. lactis. Thus, these results suggest that, after proliferation in secondary lymphoid tissues, LAP+ Tregs from M. leprae-Hsp65-L. lactis-fed mice were able to reduce the recruitment of encephalytogenic CD4+ T cells to the spinal cord perhaps by reaching themselves, in some extent, the central nervous system.
Fig. 6.
The modulatory effect of M. leprae-Hsp65-producing L. lactis in EAE development was dependent on LAP+ regulatory T cells. (A–B) M. leprae-Hsp65-L. lactis-fed mice had increased frequency of CD4+Foxp3+LAP+ Treg cells in the spinal cord. C57BL/6 Foxp3-GFP-knock-in mice were fed or not (Naïve) medium (EAE), control (CTLL+EAE) or M. leprae-Hsp65-producing L. lactis (Hsp65-LL+EAE) for four days and EAE was induced ten days later. After 14 days, mice were killed and spinal cords removed. (A) Bar graph is shown as counting of a pool of mononuclear cells from spinal cords of 5 mice/group in a Neubauer chamber. (B) Cells were stained with PE-anti-CD4, Bio-LAP and CY-STV. CD4+ cells were gated. Bar graph is shown as counting pool of lymphocytes expressing LAP and Foxp3 from spinal cords of 5 mice/group. (C) Pretreatment of M. leprae-Hsp65-producing L. lactis fed mice with anti-LAP dampens its prevention EAE. C57BL/6 mice were concomitantly fed medium or M. leprae-Hsp65-producing L. lactis for four days and 20 µg/mouse of intraperitoneally injected anti-LAP (aLAP+EAE, aLAP+Hsp65-LL+EAE) or isotype control rat anti-mouse IgG1; Iso+EAE, Iso+Hsp65-LL+EAE) at day 1, 3 and 5. EAE was induced 1 day later. The course of EAE is shown as mean EAE score ± SEM. n = 5 mice/group; ANOVA, post-test Tukey. *Statistically different from Iso+EAE (except at days 20 and 25), aLAP+EAE and aLAP+Hsp65-LL+EAE groups. #Statistically different from Iso + Medium + EAE group. XStatistically different from aLAP + EAE group; p < 0.05.
3.10. Depletion of LAP+ cells abolished the suppressive effect of M. leprae-Hsp65-producing L. lactis in EAE induction
Given that CD4+LAP+ T cells seemed to be the most relevant population of Tregs induced by oral administration of M. leprae-Hsp65-producing L. lactis, we decided to test whether depletion of LAP+ cells would abrogate its immune-modulatory effects on EAE development. As shown in Fig. 6C, pretreatment of mice with mouse anti-LAP mAb together with oral administration of M. leprae- Hsp65-producing L. lactis completely abolished the suppressive effect observed, confirming our hypothesis that LAP+ T cells mediated the immune-regulation of EAE by M. leprae-Hsp65-producing L. lactis. Strikingly, LAP+ cell depletion resulted in earlier onset and more severe disease in control medium-fed mice. It also prevented the natural remission of EAE observed at day 30. These data suggest that LAP+ Treg cells were also important to modulate EAE onset and severity even in untreated diseased mice.
4. Discussion
Since the revival of the regulatory T cells (Tregs) and their importance in maintaining the immune system homeostasis [16], much interest has been given to the discovery of therapies based on modulation of Tregs to treat autoimmune conditions such as multiple sclerosis (MS). However, no treatment used currently for MS has been confirmed to act by the action of these cells.
Peripheral tolerance induced by feeding proteins is an efficient way to specifically suppress inflammatory responses by the induction of different types of regulatory T cells. This approach has been proved successful in many animal models of autoimmune and other inflammatory diseases [48]. Among the feeding protocols already tested in oral tolerance experiments, continuous feeding of the antigen has been shown to be the most efficient one. Continuous feeding of specific antigens for one day is able to prevent the development of myelin basic protein (MBP)-induced experimental autoimmune encephalomyelitis (EAE) and to induce oral tolerance in senescent mice usually refractory to tolerance when fed by gavage [49, 50]. In this study, we tested the administration, by a continuous feeding protocol, of a recombinant L. lactis strain that is able to produce and release low-doses of LPS-free M. leprae Hsp65. M. leprae Hsp65-producing L. lactis survived gastric acid degradation and reached cecum, small and large intestines of fed mice (Fig. 1B). It is plausible that, in the intestine, both viable and non-viable bacteria release their cytoplasmic content of M. leprae Hsp65. In addition, M. leprae Hsp65 is released in the culture medium used for feeding at a concentration of 7 µg/ml (data not shown). Hsp65, amycobacterial homolog to the mammalian Hsp60, was chosen as a target antigen for tolerance induction because of its abundant expression in inflammatory conditions and its known regulatory activity in the immune system [5].
The influence of exposure to Hsp65 in the course of EAE has been previously reported in studies concerned about the role of infectious bacteria in the development of autoimmune diseases. With a transgenic mouse strain containing high frequencies of T cells specific for an encephalitogenic peptide of myelin basic protein (MBP), Goverman and coworkers noted that animals developed spontaneous EAE only when housed in conventional, as opposed to specific pathogen free (SPF) facilities [51]. Birnbaum and coworkers also showed that SJL mice housed in SPF facilities had more severe relapses of proteolypid-protein-peptide-139-151-(PLP)-induced EAE than did animals housed in conventional facilities. Proliferative T cell responses to human Hsp60 was decreased and skewed toward a Th2 profile in conventionally housed SJL mice [52]. The specific effects of parenteral vaccination with either Hsp65 peptides or the Hsp65 gene in the course of EAE induced in Lewis rats has also been tested with distinct outcomes. Gene vaccination had modulatory effects in brain and spinal cord inflammation, but it did not change the clinical development of the disease [53]. On the other hand, rats pre-immunized with Hsp65 peptide showed lower incidence and severity of disease, but the mechanism involved in the effect observed was unclear [54].
Oral administration of recombinant Hsp65 has been described to induce tolerance and protected rats against adjuvant arthritis [28] and mice against atherosclerosis [29, 30]. Since Hsps are hyperexpressed during inflammation, it is plausible that the inhibition of immune responses to Hsp65 in these disease models had the effect of promoting a bystander suppression of reactivities to other antigens involved in the pathological processes. Bystander suppression is a phenomenon associated with oral tolerance that spreads its inhibitory effect toward other neighbor antigens probably via the action of antigen presenting cells [27, 55, 56]. This cross-suppressive event is also called indirect effect of oral tolerance [57] and it precludes the need to identify the target antigen when a therapeutic use of oral tolerance protocols is planned.
In concert with these data, we showed that oral administration of M. leprae-Hsp65-producing L. lactis prevented MOG35–55-induced EAE (Fig. 1D–E), an effect associated with a decrease in anti-Hsp65 IgG response (Fig. 2A). Interestingly, there was also a reduction of IL-17 and increase of IL-10 in cultures of mesenteric lymph node and spleen cells stimulated with MOG35–55 (Fig. 2C–H). This suggests that oral administration of M. leprae-Hsp65-producing L. lactis induced both oral tolerance to Hsp65 and a bystander suppression of MOG35–55-specific T cell responses. These data also contribute to solve the problem concerning the use of complete Freund adjuvant (CFA) for EAE induction. As CFA contains Hsp65 from Mycobacterium sp., one may argue that the oral tolerance induced by administration of M. leprae-Hsp65-producing L. lactis halted the EAE immunization and thereby prevented EAE development only in CFA-related experimental models. However, oral administration of M. leprae-Hsp65-producing L. lactis was able to prevent MOG-induced EAE using zymosan, which does not contains Hsps (Fig. 2B). Therefore, M. leprae-Hsp65-L. lactis most probably prevented EAE by the oral tolerance and its bystander effects toward the myelin antigens involved in EAE inflammation.
Since Hsp65 is present in bacteria, the suppression observed after treatment with M. leprae-Hsp65-producing L. lactis may result from a general immunosuppressive state which would have the undesirable effect of compromising immune responses to infectious agents. To test this hypothesis, we examined the number of activated T cells in M. leprae-Hsp65-L. lactis-fed mice. Numbers and frequencies of CD4+ T cells expressing early (CD69) and late (CD44) activation markers were not reduced in these mice. In fact, they were increased in both mesenteric (MLN) lymph nodes and spleen of M. leprae-Hsp65-L. lactis-fed mice (Fig. 3). The high numbers of activated T cells found in these mice is not consistent with a general immunosuppressive state. Moreover, oral administration of M. leprae-Hsp65-producing L. lactis did not decrease immune response against Salmonella typhimurium (Supplemental Fig. 1), suggesting once again that immunosuppression was not the case seen in our study.
Conversely, as commented above, there was a suppression of inflammatory responses directed to MOG35–55 in M. leprae-Hsp65-L. lactis-fed mice. Levels of IL-17, one of the major pro-inflammatory cytokines involved in EAE development [37, 40, 41, 58] were reduced, and levels of the anti-inflammatory cytokine IL-10 were increase in supernatants of spleen and MLN cells stimulated with MOG35–55 (Fig. 2C–H). We also found that the frequency of CD4+LAP+TGF-β+ T cells (stimulated with either MOG35–55 or anti-CD3/CD28 or even without stimulation) were higher in cultures of spleen and MLN cells of M. leprae-Hsp65-L. lactis-fed mice (Fig. 5D–G) indicating that TGF-β can also be involved in regulation of EAE by the recombinant L. lactis. We cannot exclude the involvement of other regulatory cytokines such as IL-27, which has been shown to have significant inhibitory effects on IFN-γ- and IL-17-mediated neuro-inflammation [59]. IL-27 may be released by dendritic cells (DCs) conditioned by Tregs, which in turn can induce IL-10-secreting Tr1 regulatory cells [60].
In oral tolerance studies, 5–20 mg of fed antigen is considered a high dose whereas doses bellow 1 mg are considered low doses taking into account that digestion will cleave part of these antigens during their traffic through the gastrointestinal tract. It has been suggested that Tregs are involved in low-dose tolerance induction [61, 62]. This seemed to be the case in our study. The extracellular release of M. leprae Hsp65 by L. lactis reached 7 µg/ml (data not shown) in bacteria suspensions used for feeding and mice drank about 5 ml of bacteria-containing medium a day for four consecutive days. The intestinal mucosa is a privileged site for the generation of Tregs expressing LAP [18, 23, 24]. Therefore, the high numbers of the CD4+CD25+LAP+ (Fig. 5B) and CD4+CD25−LAP+ Tregs (data not shown) observed in MLN 2 and 4 days after EAE induction might have been induced or activated in the intestinal mucosa just after administration of M. leprae-Hsp65-producing L. lactis. From this site, they might have migrated to secondary lymphoid organs as well as to the spinal cord where they were found at high frequencies at day 14 (Fig. 6B). Therefore, one of the mechanisms involved in EAE suppression seemed to be the action of CD4+LAP+ Tregs generated or expanded in the gut after EAE induction (day 0) in M. leprae-Hsp65-L. lactis-fed mice that migrated to the spinal cord around day 14.
Regulatory T cells expressing CD25 and the transcription factor Foxp3 have been considered the main Tregs responsible for preventing and controlling autoimmune diseases [63]. CD4+CD25+Foxp3+ Tregs can be divided into two subgroups: natural Tregs (nTreg), which are generated in the thymus during the T cell maturation [64, 65] and express the negative regulator molecule CTLA-4 [66, 67]; and inducible Treg cells (iTreg), which are naïve CD4+CD25−Foxp3− converted to functional CD4+CD25+Foxp3+ Treg cells mainly at the gut-associated lymphoid tissue (GALT), through specialized dendritic cells in a TGF-β-and retinoic acid-dependent manner [17, 68]. After their formation in the thymus, nTregs go to secondary lymphoid organs where they control activation of naïve self-reactive T cells, thereby avoiding autoimmunity [69]. We did find high numbers (Fig. 4A) and proportions (data not shown) of CD4+CD25+Foxp3+ T cells in inguinal lymph nodes of M. leprae-Hsp65-L. lactis-fed mice at days 2 and 4 post EAE induction. This suggests that nTregs may be expanded in ILN just after MOG35–55-loaded dendritic cells (DCs) reached this lymph node coming from the base of the tail, where mice were immunized. In fact, measuring the expression of a recently described marker for nTregs, the Helios transcription factor [43], we could confirm that both nTregs and iTregs were part of the expanded population of Foxp3+Tregs present in ILNs. However, the frequencies of nTregs in M. leprae-Hsp65-fed mice were much higher than in animals from the other two groups (Fig. 4D and E). This indicates that somehow pretreatment with M. leprae-Hsp65-producing L. lactis either recruited natural CD4+CD25+Foxp3+ Tregs from the thymus or expanded preexisting nTregs in ILNs. Since Hsp65 is homologous to the murine Hsp60, and CD4+CD25+Foxp3+ nTregs bearing anti-Hsp60 TCRs are likely to be present in mouse lymph nodes. The iTregs found in ILN might have come from MLN. One day after EAE induction, the numbers and frequencies of such Tregs were increased in MLN of animals from all groups and their frequencies dropped at days 2 and 4 (Fig. 4B). We hypothesize that once Foxp3+ Treg cells were expanded in the ILN by MOG35–55-loaded DCs coming from the site of mice immunization, they were activated upregulating CTLA-4 expression (data not shown). This molecule is known to contribute to the suppressive function of Foxp3+ nTregs which is based on cell–cell contact [70]. However, CTLA-4 expression did not change in CD4+LAP+ Tregs (data not shown). This also suggests that TGF-β, the most important cytokine expressed and released by these cells, can be one of the immunosuppressive mediators triggered by treatment with M. leprae-Hsp65-producing L. lactis. One of the functions of the transcription factor Foxp3 in iTregs is to induce surface expression of LAP [44]. Indeed, more than fifty percent of Tregs expressing Foxp3 also co-expressed LAP in all organs analyzed. However, only about fifteen percent of LAP-expressing T cells co-expressed Foxp3 (Supplemental Fig. 2). TGF-β, a cytokine upregulated by M. leprae-Hsp65-L. lactis treatment, seems to be also responsible for LAP expression and therefore involved in the formation of Foxp3-independent LAP+ Tregs [44].
These results, along with the expansion of LAP+ Tregs in spleen, ILN and MLN (Fig. 5A–C), suggest that LAP+ Tregs are the most important regulatory T cells induced by Hsp65-producing L. lactis. To test this hypothesis, we injected mouse anti-LAP monoclonal antibodies into M. leprae-Hsp65-producing L. lactis pretreated and medium treated mice during EAE induction. In vivo depletion of LAP+ cells completely abrogated the suppressive effect of M. leprae- Hsp65-producing L. lactis administration. Moreover, anti-LAP treatment also worsened the clinical signs of EAE in medium-treated mice (Fig. 6C) indicating that LAP+ Tregs might be physiologically induced during the course of EAE and be involved in controlling disease severity. Indeed, MOG35–55-induced EAE in C57BL/6 mice displayed a progressive spontaneous remission of disease signs starting at day 14 up to day 25 post induction (Fig. 1D). This late remission of disease was also abrogated by anti-LAP treatment (Fig. 6C). In line with these results, we observed that although fewer cells reached the spinal cord in M. leprae-Hsp65-L. lactis-fed mice (Fig. 1E and Fig. 6A), frequencies of LAP+ Tregs, but not Foxp3+ Tregs, were higher in mice from this group (Fig. 6B). This result is consistent with finding that the spinal cord parenchyma of M. leprae-Hsp65-L. lactis-fed mice showed no signs of inflammatory damage (Fig. 1E).
Thus, it seems that expansion of Treg cells in secondary lymphoid organs after oral administration of M. leprae-Hsp65-producing L. lactis restrained the activation and migration of encephalitogenic T cells to the spinal cord, the site of the neurodegenerative lesions in EAE. Treatment with M. leprae-Hsp65-producing L. lactis led to an increase in the frequency of spleen CD4+LAP+ T cells and a decrease in the levels of MOG-induced IL-17 release by spleen cells. When Th17 cells reach the spinal cord is considered CNS and the central nervous system (CNS), they can induce the synthesis and release of the chemokine CCL20 which, in turn, attracts more CCR6-expressing Th17 cells to the CNS [71, 72]. Although there was not a dramatic effect in the levels of IFN-γ secreted by lymph node and spleen cells in M. leprae-Hsp65-L. lactis-fed mice, it is still possible that migration of Th1 cells to the CNS was also inhibited by this treatment. The number of leucocytes isolated from the spinal cord of these animals was comparable to the ones found in naïve mice (Fig. 6A).
Heat shock proteins (Hsps) have been implicated in the regulation of the immune system [5]. Since pretreatment with control L. lactis (containing an empty vector) did not affect EAE development in our study, we can assume that all regulatory effects observed after oral administration of M. leprae-Hsp65-producing L. lactis were dependent on M. leprae Hsp65. Nevertheless, it is also noteworthy that control L. lactis was not irrelevant in our model. It induced, for example, the production of IFN-γ by mesenteric lymph node cells stimulated with MOG (Fig. 2C). Therefore, it is possible that, since Hsps are self-antigens and thus naturally tolerated by the immune system, the presence of the L. lactis in a tolerogenic environment such as the gut, might have acted as an “alarm clock” for the immune system, facilitating M. leprae Hsp65 recognition and therefore its immunemodulatory action.
In summary, our results provide new insights into the mechanisms behind the immune-regulatory effects of M. leprae Hsp65 in a continuous feeding protocol. Moreover, the association between the probiotic L. lactis and the M. leprae heat shock protein 65 can be viewed as a novel therapeutic candidate for autoimmune diseases, particularly the multiple sclerosis.
Supplementary Material
Acknowledgments
We are thankful to Ilda Marçal de Souza for her excellent work taking care of the mice. This study had financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil (CNPq, 476961/2010-6), Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brasil (FAPEMIG, APQ-00575-09) and Instituto de Investigação em Imunologia (iii). Some of the authors received fellowships from FAPEMIG (R.M.R.) and CNPq (R.P.O., M.A.F.G., F.G.L., A.C.A., A.M.C.F.).
Footnotes
The authors have no conflicting financial interests.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2012.07.012.
References
- 1.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 2.Walter S, Buchner J. Molecular chaperones – cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Borges JC, Ramos CH. Protein folding assisted by chaperones. Protein Pept Lett. 2005;12:257–261. doi: 10.2174/0929866053587165. [DOI] [PubMed] [Google Scholar]
- 4.Chun JN, Choi B, Lee KW, Lee DJ, Kang DH, Lee JY, et al. Cytosolic Hsp60 is involved in the NF-kappaB-dependent survival of cancer cells via IKK regulation. PLoS One. 2010;5:e9422. doi: 10.1371/journal.pone.0009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354:23–27. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Quintana FJ, Mimran A, Carmi P, Mor F, Cohen IR. HSP60 as a target of anti-ergotypic regulatory T cells. PLoS One. 2008;3:e4026. doi: 10.1371/journal.pone.0004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- 9.Zanin-Zhorov A, Bruck R, Tal G, Oren S, Aeed H, Hershkoviz R, et al. Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol. 2005;174:3227–3236. doi: 10.4049/jimmunol.174.6.3227. [DOI] [PubMed] [Google Scholar]
- 10.Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 11.Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007;29:246–249. doi: 10.1016/j.jaut.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 12.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 13.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 14.Hohlfeld R, Barkhof F, Polman C. Future clinical challenges in multiple sclerosis: relevance to sphingosine 1-phosphate receptor modulator therapy. Neurology. 2011;76:S28–S37. doi: 10.1212/WNL.0b013e31820db40f. [DOI] [PubMed] [Google Scholar]
- 15.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 17.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 23.Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, et al. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 24.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 26.Vaz NM, de Faria AM, Verdolin BA, Silva Neto AF, Menezes JS, Carvalho CR. The conservative physiology of the immune system. Braz J Med Biol Res. 2003;36:13–22. doi: 10.1590/s0100-879x2003000100003. [DOI] [PubMed] [Google Scholar]
- 27.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobelens PM, Kavelaars A, van der Zee R, van Eden W, Heijnen CJ. Dynamics of mycobacterial HSP65-induced T-cell cytokine expression during oral tolerance induction in adjuvant arthritis. Rheumatology (Oxford) 2002;41:775–779. doi: 10.1093/rheumatology/41.7.775. [DOI] [PubMed] [Google Scholar]
- 29.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, et al. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 30.Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/s0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- 31.Portaro FC, Hayashi MA, De Arauz LJ, Palma MS, Assakura MT, Silva CL, et al. The Mycobacterium leprae hsp65 displays proteolytic activity. Mutagenesis studies indicate that the M. leprae hsp65 proteolytic activity is catalytically related to the HslVU protease. Biochemistry. 2002;41:7400–7406. doi: 10.1021/bi011999l. [DOI] [PubMed] [Google Scholar]
- 32.Pontes DS, de Azevedo MS, Chatel JM, Langella P, Azevedo V, Miyoshi A. Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems. Protein Expr Purif. 2011;79:165–175. doi: 10.1016/j.pep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6:349–362. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 35.de Azevedo MSP RC, Electo N, Pontes DS, Machado JBM, Gonçalves EDC, Azevedo V, Silva CL, Miyoshi A. Cytoplasmic and extracellular expression of pharmaceutical-grade mycobacterial 65 kDa heat shock protein in Lactococcus lactis. Genet Mol Res. 2012;11(2):1146–1157. doi: 10.4238/2012.April.27.14. [DOI] [PubMed] [Google Scholar]
- 36.Le Loir Y, Gruss A, Ehrlich SD, Langella P. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol. 1998;180:1895–1903. doi: 10.1128/jb.180.7.1895-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook JRD. Molecular cloning: a Laboratory manual. 3rd ed. New York: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 40.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 41.Basso AS, Cheroutre H, Mucida D. More stories on Th17 cells. Cell Res. 2009;19:399–411. doi: 10.1038/cr.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauet-Broere F, Wieten L, Guichelaar T, Berlo S, van der Zee R, Van Eden W. Heat shock proteins induce T cell regulation of chronic inflammation. Ann Rheum Dis. 2006;65(Suppl. 3):iii65–iii68. doi: 10.1136/ard.2006.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oida T, Weiner HL. TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One. 2011;5:e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 47.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–190. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 49.Faria AM, Ficker SM, Speziali E, Menezes JS, Stransky B, Silva Rodrigues V, et al. Aging affects oral tolerance induction but not its maintenance in mice. Mech Ageing Dev. 1998;102:67–80. doi: 10.1016/s0047-6374(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 50.Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 51.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 52.Birnbaum G, Kotilinek L, Miller SD, Raine CS, Gao YL, Lehmann PV, et al. Heat shock proteins and experimental autoimmune encephalomyelitis. II: environmental infection and extra-neuraxial inflammation alter the course of chronic relapsing encephalomyelitis. J Neuroimmunol. 1998;90:149–161. doi: 10.1016/s0165-5728(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 53.Zorzella-Pezavento SF, Chiuso-Minicucci F, Franca TG, Ishikawa LL, Martins DR, Silva CL, et al. Immunization with pVAXhsp65 decreases inflammation and modulates immune response in experimental encephalomyelitis. Neuroimmunomodulation. 2010;17:287–297. doi: 10.1159/000292018. [DOI] [PubMed] [Google Scholar]
- 54.Birnbaum G, Kotilinek L, Schlievert P, Clark HB, Trotter J, Horvath E, et al. Heat shock proteins and experimental autoimmune encephalomyelitis (EAE): I. Immunization with a peptide of the myelin protein 2’,3’ cyclic nucleotide 3’ phosphodiesterase that is cross-reactive with a heat shock protein alters the course of EAE. J Neurosci Res. 1996;44:381–396. doi: 10.1002/(SICI)1097-4547(19960515)44:4<381::AID-JNR10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 55.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho CR, Verdolin BA, de Souza AV, Vaz NM. Indirect effects of oral tolerance in mice. Scand J Immunol. 1994;39:533–538. doi: 10.1111/j.1365-3083.1994.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 58.Liu R, Bai Y, Vollmer TL, Bai XF, Jee Y, Tang YY, et al. IL-21 receptor expression determines the temporal phases of experimental autoimmune encephalomyelitis. Exp Neurol. 2008;211:14–24. doi: 10.1016/j.expneurol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Colgan J, Rothman P. All in the family: IL-27 suppression of T(H)-17 cells. Nat Immunol. 2006;7:899–901. doi: 10.1038/ni0906-899. [DOI] [PubMed] [Google Scholar]
- 60.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 61.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 63.Shevach EM, Piccirillo CA, Thornton AM, McHugh RS. Control of T cell activation by CD4+CD25+ suppressor T cells. Novartis Found Symp. 2003;252:24–36. [discussion 44, 106–14]. [PubMed] [Google Scholar]
- 64.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 65.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 66.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 68.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 70.Sakaguchi S, Wing K, Miyara M. Regulatory T cells – a brief history and perspective. Eur J Immunol. 2007;37(Suppl. 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 71.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–S23. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 72.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.