Abstract
Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem-cell disorders, characterized phenotypically by the abnormal accumulation of mature-appearing myeloid cells. Polycythemia vera, essential thrombocythemia, primary myelofibrosis (also known as ‘BCR-ABL1-negative’ MPNs), and chronic myeloid leukemia (CML) are the primary types of MPNs. After the discovery of the BCR-ABL1 fusion protein in CML, several oncogenic tyrosine kinases have been identified in ‘BCR-ABL1-negative’ MPNs, most importantly, JAK2V617F mutation. The similarity in the clinical characteristics of the BCR-ABL1-negative MPN patients along with the prevalence of the Janus kinase mutation in this patient population provided a strong rationale for the development of a new class of pharmacologic inhibitors that target this pathway. The first of its class, ruxolitinib, has now been approved by the food and drug administration (FDA) for the management of patients with intermediate- to high-risk myelofibrosis. Ruxolitinib provides significant and sustained improvements in spleen related and constitutional symptoms secondary to the disease. Although noncurative, ruxolitinib represents a milestone in the treatment of myelofibrosis patients. Other types of JAK2 inhibitors are being tested in various clinical trials at this point and may provide better efficacy data and safety profile than its predecessor. In this article, we comprehensively reviewed and summarized the available preclinical and clinical trials pertaining to JAK inhibitors.
Keywords: primary myelofibrosis, polycythemia vera, essential thrombocythemia, Janus kinase 2
Introduction
Myeloproliferative neoplasms (MPNs) include a diverse and heterogeneous group of clonal stem cell disorders, which are phenotypically characterized by the abnormal accumulation of mature-appearing myeloid cells [Tefferi, 2010]. Chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are considered ‘classic’ MPNs [Dameshek, 1951], while‘BCR-ABL1-negative’ MPN is an operational term that is used in reference to PV, ET, and PMF [Tefferi and Vardiman, 2008].
After the discovery of the BCR-ABL1 fusion antigen in CML [Bartram et al. 1983], several oncogenic tyrosine kinases have been identified, including protein kinases that result from the fusion of platelet growth factor receptor-b (PDGFRb) gene with its corresponding partner gene as exemplified by TEL-PDGFRB in patients with chronic myelomonocytic leukemia, interstitial deletions that give rise to the FIP1L1-PDGFRA fusion in chronic eosinophilic leukemia [Golub et al. 1994; Cools et al. 2003], the activating KIT-D816V allele in 90% of systemic mastocytosis [Nagata et al. 1995], and 8p11 stem cell myeloproliferative disorder (MPD), respectively [Golub et al. 1994; Carroll et al. 1996; Xiao et al. 1998; Chen et al. 2004]. One of the most important discoveries was the identification of JAK2V617F in 2005 and its occurrence in the majority of patients with PV, ET, and PMF [Baxter et al. 2005; James et al. 2005; Kralovics et al. 2005; Levine et al. 2005].
The JAK family and JAK/STAT pathway
The Janus family of kinases (JAK) include JAK1, JAK2, JAK3 and TYK2, and are required for the physiologic signaling of cytokines and growth factors that intrinsically lack kinase activity (erythropoietin [Epo], granulocyte–macrophage colony stimulating factor [GM-CSF], interleukin [IL]-3, IL-5, thrombopoietin, growth hormone and prolactin-mediated signaling) [Ihle et al. 1995; Pesu et al. 2008; Vainchenker et al. 2008]. The STAT (signal transducers and activators of transcription) family on the other hand is a downstream pathway that is activated upon the initiation of JAK signaling. It includes a number of latent transcription factors that, when phosphorylated on Y residues by the JAKs, drive the expression of genes involved in proliferation, apoptosis, migration, differentiation as well as the production of angiogenic and/or inflammatory proteins [Shuai and Liu, 2003; O’Shea et al. 2004; Fridman et al. 2011]. Each member of the JAK family has a primary role in mediating a signaling process with some overlap between them [Pesu et al. 2008]. JAK1 plays a crucial role in the signaling of many proinflammatory cytokines such as IL-1, IL-6 and tumor necrosis factor alpha (TNFα). JAK2 is important for hematopoietic growth factors signaling such as Epo, GM-CSF, thrombopoietin, IL-3, IL-5, growth hormone and prolactin-mediated signaling [Ihle et al. 1995]. JAK3 plays a role in mediating immune function (deficient JAK3 signaling in humans and mice was found to cause severe combined immunodeficiency [SCID]) [Nosaka et al. 1995], and TYK2 functions in association with JAK2 or JAK3 to transduce signaling of cytokines, such as IL-12 [Pesu et al. 2008; Vainchenker et al. 2008]. Bearing the aforementioned functions in mind, it is interesting to point out that it has been shown that patients with PMF have very high levels of circulating inflammatory cytokines [Schmitt et al. 2000; Panteli et al. 2005; Xu et al. 2005; Wang et al. 2006], a phenomenon that might be responsible for the hypercatabolic state and constitutional symptoms in such patients [Tefferi, 2000].
In addition to its involvement in the JAK/STAT pathway, JAK2 has been also identified in the nucleus of myeloid cell lines [Dawson et al. 2009]. It has been suggested that activated JAK2 phosphorylates histone H3 at tyrosine-41(H3Y41), resulting in the inhibition of the binding of the transcriptional repressor heterochromatin protein-1α (HP1 α), thus enhancing gene expression. The genetic deletion of JAK2 is lethal in embryonic mice owing to a lack of definitive erythropoiesis resulting from the absence of response of JAK2-deficient hematopoietic progenitors to erythropoietin stimulation [Parganas et al. 1998].
Biological and clinical relevance of JAK-STAT-relevant mutations
JAK2V617F mutation
A gain-of-function mutation that leads to a substitution of valine for phenylalanine at codon 617 of JAK2(JAK2V617F) has been identified in BCR-ABL1-negative MPN patients, with a frequency of 65–97% in PV, 23–57% in ET and 34–57% in PMF [Baxter et al. 2005; James et al. 2005; Kralovics et al. 2005; Levine et al. 2005]. This mutation occurs in the JAK2 pseudokinase domain and generates a constitutively active molecule resulting from a loss of the autoinhibitory effect of the pseudokinase domain on the kinase domain. Cells expressing JAK2V617F acquire cytokine-independent growth ability and/or cytokine hyper-responsiveness [James et al. 2005; Levine et al. 2005]. Most patients with MPN are heterozygous for JAK2V617F. However, there are a few homozygous cases which are seen more frequently in PV and PMF patients compared with ET. Homozygosity in this context is a product of mitotic recombination and duplication of the mutant allele, a mechanism known as uniparental disomy rather than loss of the remaining functional wild-type allele as is observed in certain tumor suppressor genes [Baxter et al. 2005; James et al. 2005; Kralovics et al. 2005; Levine et al. 2005]. The effect of JAK2V617F allele burden in MPNs has been demonstrated in several studies. It has been found that the higher JAK2V617F allele burden in PV patients correlates with an increased risk of MF transformation [Passamonti et al. 2010], more advanced myelofibrosis, greater splenomegaly, higher white blood counts, increased frequency of thrombosis including major cardiovascular events [Silver et al. 2011], and increased need for chemotherapy treatment [Vannucchi et al. 2007]. Interestingly, PMF patients with low JAK2V617F allele burden had a worse overall and leukemia-free survival when compared with patients with either a high allele burden or wild-type status [Tefferi et al. 2008].
Activation of the STAT family of transcription factors is important in JAK2V617F-mediated transformation as it has been suggested that the JAK2V617F may induce endogenous erythroid colonies (EECs) with an erythropoietin-independent differentiation EEC (which is a hallmark of human PV) via the STAT5/Bcl-xL pathway [Garcon et al. 2006].
The role of JAK2 activation in the pathogenesis of MPN was illustrated in murine bone marrow transplant (BMT) experiments. Data have shown that the expression of JAK2V617F, but not wild-type JAK2, in a murine BMT assay resulted in significant erythrocytosis in recipient mice 28 days after transplantation [James et al. 2005; Levine et al. 2005]. Further studies have shown that the expression of JAK2V617F in mice lead to the development of a disease that is similar to PV, which eventually progressed to myelofibrosis [Lacout et al. 2006; Wernig et al. 2006].
JAK2 exon 12 mutations
JAK2 exon 12 mutations are a group of mutations that are specifically found in the small proportion of JAK2V617F-negative PV patients with a frequency of 2–3% of PV patients [Pardanani et al. 2007; Scott et al. 2007; Tefferi, 2011; Verstovsek et al. 2011a]. The most frequently occurring mutations are the N542-E543del (23% of the combined group) and E543-D544del (11%) [Scott et al. 2007; Passamonti et al. 2011; Verstovsek et al. 2011a]. When compared with JAK2V617F-positive PV patients, those with JAK2 exon 12 mutations had significantly higher hemoglobin level and lower platelet and leukocyte counts at diagnosis but similar rates of thrombosis, myelofibrosis, leukemia, and death [Tefferi, 2011].
MPL mutations
MPL is located in chromosome 1p34 and encodes for the thrombopoietin receptor. It has been reported in 5–9% of PMF patients [Pardanani et al. 2006; Pikman et al. 2006] and 1–3% of ET patients but not in patients with PV or other myeloid disorders [Pardanani et al. 2006]. MPLW515L is one of the somatic mutations in exon 10 in the transmembrane region of MPL and the most frequent MPN-associated MPL mutation (1.4% of PMF patients [Pardanani et al. 2006]). Its expression results in cytokine-independent proliferation of hematopoietic cells and results in further activation of JAK-STAT signaling. In murine BMT assay, the expression of MPLW515L induced myeloproliferation characterized by splenomegaly, leukocytosis, marked thrombocytosis, extramedullary hematopoiesis, and myelofibrosis [Pardanani et al. 2006; Pikman et al. 2006; Vannucchi et al. 2008].
MPLW515K, MPLW515S, and MPLS505N are other MPL mutations at exon 10 which have been described in ET and PMF patients with an incidence of 0.4–3% [Pardanani et al. 2006; Pikman et al. 2006; Guglielmelli et al. 2007; Beer et al. 2008; Tefferi, 2012]. ET patients with MPL mutation were found to have the following characteristics: older age, lower hemoglobin level, higher platelet count, microvascular symptoms, and a higher risk of postdiagnosis arterial thrombosis [Beer et al. 2008; Vannucchi et al. 2008]. However, MPL mutation does not appear to affect survival, fibrotic or leukemic transformation [Beer et al. 2008].
When compared with MPL wild-type PMF patients, those with MPLW515L/K were more frequently female, were older, had lower hemoglobin level, and were more likely to require regular transfusional support. These data indicate that MPL mutation in myelofibrosis may predict for patients with more severe anemic phenotype [Guglielmelli et al. 2007].
LNK mutations
LNK, also known as Src homology 2 B3 (SH2B3), is an adaptor protein that negatively affects the JAK–STAT signaling [Takaki et al. 2002; Velazquez et al. 2002; Tong and Lodish, 2004].
LNK-deficient mice showed a phenotype that is similar to that seen in MPN: splenomegaly, thrombocytosis, an exaggerated response to cytokines and extramedullary hematopoiesis [Velazquez et al. 2002].
Loss of function mutations of LNK at exon2 have been reported in MPN patients and were found to be more prevalent in blast-phase MPNs compared with chronic phase MPNs. These mutations are more likely to affect exon 2 in the Pleckstrin homology (PH) domain spanning residues E208-D234 [Lasho et al. 2010; Oh et al. 2010; Pardanani et al. 2010].
The deregulated signaling of the JAK/STAT pathway and the resulting aberrant gene expression play an important role in the pathogenesis of MPNs. However, mutations involving genes that are important in other cellular pathways including those involved in epigenetic regulation are also found in MPNs and also likely contributing to the pathogenesis of MPNs. This suggests that JAK inhibition alone may insufficiently address the burden of disease.
JAK inhibitors
The clinical issues confronting patients with myelofibrosis have changed little with time. Clinical manifestations related to anemia, thrombocytopenia, extramedullary hematopoiesis, constitutional symptoms, and leukemic transformation remain the primary sources of morbidity and mortality in myelofibrosis patients. The disease course can also vary greatly from survival measured in decades to just several months. In the pre-JAK2 inhibitor era, nontransplant options included immunomodulatory agents, hydroxyurea, erythropoiesis-stimulating agents, androgenic steroids, and transfusions. Most myelofibrosis patients with anemia are primarily managed using immunomodulatory agents (lenalidomide or thalidomide ± prednisone), androgenic steroids (danazol), steroids, erythropoiesis-stimulating agents, and pegylated interferon. When constitutional symptoms and symptoms related to extramedullary hematopoiesis are present, hydroxyurea, immunomodulatory agents, splenectomy, and splenic irradiation are considered with only marginal and temporary success. The possibility of cure in myelofibrosis patients remains limited to a small subset of patients who are eligible to undergo allogeneic hematopoietic stem cell transplant (Allo-HSCT). However, there are several challenges encountered with this type of treatment approach including the limited number of suitable donors, presence of multiple comorbidities usually as a function of advanced age, difficulty in deciding at which time point during the disease course is it best to perform Allo-HSCT and lastly the choice of conditioning regimen.
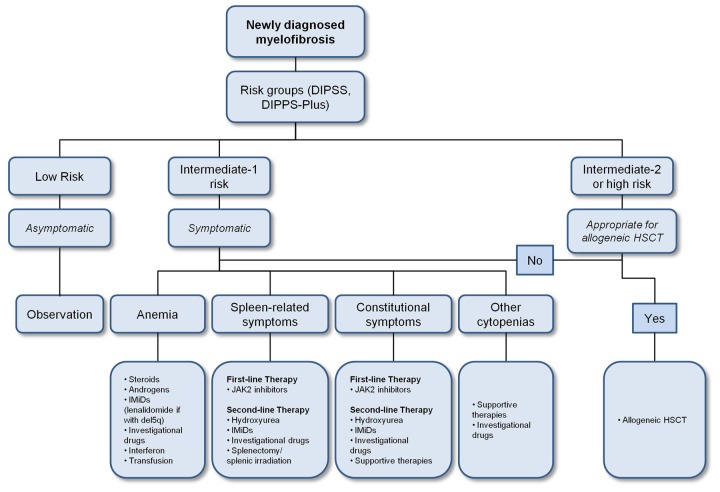
Various prognostic scoring schemes have been developed to help stratify patients into specific risk groups with designated estimates of their survival outcomes and also risk for acute myelogenous leukemia (AML) transformation to help provide guidance on when to initiate more intensive therapies that includes Allo-HSCT. The most commonly used risk scoring system in MF is the International Prognostic Scoring System (IPSS) which takes into account 5 different clinicopathologic parameters namely age >65 years old, presence of constitutional symptoms, hemoglobin level <10 g/dl, white blood cell count >25 × 109/l, and presence of circulating peripheral blood blasts. The IPSS, which is used at the time of diagnosis, has since undergone further refinements. The Dynamic IPSS was developed and allows for prognosis prediction at any time during the disease course. Finally, the Dynamic IPSS-plus takes into account three additional adverse prognostic factors, including unfavorable cytogenetic abnormalities, platelet counts <100 × 109/l and red blood cell transfusion dependence. The higher the score, the worse the risk groupings and associated outcomes. The prevailing expert opinion and clinical data support the potential benefit of Allo-HSCT in myelofibrosis patients whose disease are classified as either intermediate-2 or high risk, transfusion dependent, and those who have unfavorable cytogenetics [McLornan et al. 2012]. Data supporting Allo-HSCT in low-risk and intermediate-1-risk myelofibrosis patients are less established (Figure 1).
Figure 1.
Proposed treatment algorithm for primary myelofibrosis [Tefferi, 2011].
Risk stratification is according to DIPSS-plus [Gangat et al. 2011]. Very-high-risk group includes patients with monosomal karyotype, inv(3)/i(17q) abnormalities, or any two of circulating blasts >9%, leukocytes ≥ 40 × 109/L or other unfavorable karyotype [Tefferi et al. 2011].
Abbreviations: DIPSS, Dynamic International Prognostic Scoring System; HSCT, hematopoietic stem cell transplantation.
Given the high number of myelofibrosis patients who are ineligible for Allo-HSCT and who remain symptomatic despite conventional therapies, there was a need for novel therapies that can produce greater efficacy while targeting important disease-relevant pathophysiologic pathways. The similarity in clinical characteristics of the BCR-ABL1-negative MPN patients along with the prevalence of the JAK mutation in this population provided a strong rationale for the development of a new class of pharmacologic inhibitors of the JAK-STAT pathway. The optimism was further emphasized when taking into consideration the success that imatinib and other BCR-ABL1 (Philadelphia chromosome) directed agents have made in CML, with a hope that JAK inhibitors would have analogous effects in BCR-ABL1-negative patients [Kumar et al. 2009]. Although JAK2 mutations, in conjunction with other genetic/epigenetic abnormalities, can contribute to the initiation and progression of MPNs, it is very important to mention that recent evidence has shown that none of the JAK-STAT activating mutations (including JAK2V617F) in MPNs can be considered a causal event [Tefferi, 2010], in contrast to the role of BCR-ABL1 mutation in CML (see Tables 1 and 2).
Table 1.
General description of JAK inhibitors.
| JAK inhibitor | Anti-JAK2 IC50 [nM] | JAK selectivity (IC50 [nM]) | Non-JAK targets | Current phase | Tested diseases | Unique effects |
|---|---|---|---|---|---|---|
| INCB018424 (ruxolitinib) | 7.2 | JAK1 (1) JAK3 (98) TYK2 (9.3) |
– | III | PMF, PV, ET, cutaneous inflammation, leukemia, rheumatoid diseases | Response rates are similar regardless of MF subtype and regardless of JAK2V617F mutation presence |
| CEP701 (lestaurtinib) | 1 | JAK3 (3) | FLT3, TrkA | I/II | PMF, PV, ET, Hodgkin lymphoma, solid tumors, hematological malignancies, autoimmune diseases | Well tolerated; no decrease in JAK2V617F allele burden |
| SB1518 | 22 | JAK1 (58) JAK3 (24) |
FLT3 | I/II | PMF, lymphoma | Well tolerated; promising efficacy in symptomatic MF patients with splenomegaly |
| SAR302503 | 3 | JAK1 (35) JAK3 (332) TYK2 (135) |
FLT3, RET | I/II | PMF, mast cell leukemia | Improvement of baseline constitutional symptoms |
| XL019 | 2 | JAK1 (105) JAK3 (996) |
– | Discontinued | PMF, PV, post-PV/ET MF | Clinical studies discontinued due to high rate of neurotoxicity |
| CYT387 | 18 | JAK1 (0.6) JAK3 (8.6) |
JNK1, CDK2 | I/II | PMF, post-PV/ET MF | Significant improvement rates in anemia and splenomegaly, and it has a favorable toxicity profile |
| AZD1480 | 0.26 | JAK1 (5) JAK3 (15) |
TrkA, Aurora A, FGFR1 | I/II | PMF, post-PV/ET MF, glioblastoma, solid tumors | The first trial evaluating AZD1480 in humans is still ongoing |
| INCB028050 | 5.7 | JAK1 (5.9) | – | Preclinical | Rheumatoid arthritis (in rodent models) | – |
| INCB16562 | 0.3 | JAK1 (2.2) JAK3 (10.1) TYK2 (2.7) |
– | Preclinical | PMF, multiple myeloma | – |
| CP-690550 (Tasocitinib) | 250 | – | – | Preclinical | Rheumatic and autoimmune diseases, kidney transplantation, pulmonary eosinophilia, organ transplant rejection | – |
| NVP-BSK805 | – | – | – | Preclinical | PV | – |
ET, essential thrombocythemia; MF, myelofibrosis; PMF, primary myelofibrosis; PV, polycythemia vera.
Table 2.
Description of JAK inhibitor trials.
| JAK inhibitors | Study ID | Phase | Patients (n) | Dose | Splenomegaly improvement | Transfusion independency | MF-related symptoms improvement | JAK2V617F allele burden decrease | Hematological SE | Nonhematological SE | Reduced dose/discontinued therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| INCB018424 (Ruxolitinib) | Verstovsek et al. [2010] | I/II | 153 (81 PMF, 49 post-PV MF, 23 post-ET MF) | 20–100 mg daily | 44% (61/140); ≥50% reduction | – | 68.8% [75/109] according to MFSAF score | 14.7% (5/34) | Anemia (23%, new onset), thrombocytopenia (20%, Gr 3/4) | 49% Gr 1–4, 7% Gr 3/4 (the most common: diarrhea (5.9% Gr 1/2) and fatigue (4.3%; 1.3 Gr 3/4) | 75% were still taking the therapy at time of writing |
| Verstovsek et al. [2010] | II | 73 (34 PV, 39 ET) | PV: 10 mg BiD ET: 25 mg BiD | PV: 60% (13/21); all ≥50% reduction; 57%: nonpalpable spleen ET: 100% (4/4); all ≥50% reduction | phlebotomy independence among PV: 100% (24/24) | Marked improvement was noted in pruritus (100%) bone pain, night sweats, weakness and fever | – | PV versus ET: anemia (12% versus 18%, Gr ≥2), thrombocytopenia (6% versus 0%, Gr ≥2), neutropenia (0% versus 6%, Gr ≥2); all were reversible. | ET: gastrointestinal disorder (1 patient), and peripheral neuropathy (1 patient) | – | |
| Verstovsek et al. [2012] [COMFORT-I] | III | 309 with intermediate-2 to high risk MPN (ruxolitinib: 155; placebo: 154) | 15–20 mg BiD | Ruxolitinib: 41.9%; placebo: 0.7% (all ≥35% reduction) | – | Ruxolitinib: 45.9%; placebo: 5.3% (all ≥50% improvement) | – | Ruxolitinib versus placebo: grade 3/4 thrombocytopenia (12.9% versus 1.3%), grade 3/4 anemia (45.2% versus 19.2%) | Ruxolitinib versus placebo: abdominal pain (10.3% versus 41.1%), fatigue (25.2% versus 33.8%), diarrhea (23.2% versus 21.2%) and peripheral edema (18.7% versus 22.5%) | Ruxolitinib versus placebo: 11.0% versus 10.6% due to adverse events | |
| Harrison et al. [2012] [COMFORT-II] | III | 219 with intermediate-2 to high-risk MPN (ruxolitinib: 146; BAT: 73) | 15-20 mg BiD | Ruxolitinib: 28%; placebo: 0% (all ≥35% reduction) | – | At week 48, patients receiving ruxolitinib had marked reductions in myelofibrosis-associated symptoms, including appetite loss, dyspnea, fatigue, insomnia, and pain, whereas patients receiving the BAT had worsening symptoms | – | Thrombocytopenia and anemia occurred more frequently in the patients receiving ruxolitinib than in those receiving BAT | Ruxolitinib versus BAT: diarrhea (24.0% versus 12.0%), and peripheral edema (22% versus 26.0%) | Discontinuation of treatment owing to adverse events (8% in the ruxolitinib group versus 5% in the BAT group) | |
| Verstovsek, NCT01243944 [RESPONSE] | III (ongoing) | Enrolling PV patients resistant/intolerant to hydroxyurea | – | – | – | – | – | – | – | – | |
| CEP701 (Lestaurtinib) | Moliterno et al. [2009] | I/II | 39 ( 27 PV and 12 ET) | 80-120 mg BiD | 83% (15/18); all had >5 cm reduction in spleen volume | – | – | 20% (3/15) | Thrombotic events (13%) | Gastrointestinal (constitutional in nature) | 13% discontinued therapy (1: disease progression; 1: leg cramps; 3: GI SE) |
| Santos et al. [2010] | II | 22 (15 PMF, 4 post-PV MF, 3 post-ET MF) | 80 mg BiD | 18%; 13.6% response according to IWG criteria | 9.1% | – | None | Anemia (14%, all Gr 3/4); thrombocytopenia (23%) | Diarrhea (72%; 9% Gr 3/4,); nausea (50%, all Gr 1,2); vomiting (37%, all Gr 1,2) | – | |
| SB1518 | Verstovsek et al. [2009] | I | 43 (36 MF, 7 AML) | 100-600 mg daily | 28% (7/25); all had ≥50% reduction, and a clinical improvement according to IWG | – | – | – | Thrombocytopenia (4%, all Gr 3/4) | Diarrhea (30%; 7% Gr 3), nausea (12%, all Gr 1/2) | 37% discontinued therapy (7: disease progression; 4: toxicity; 5: other reasons) |
| Seymour et al. [2010] | I | 20 (85% were JAK2 positive) | 100-600 mg daily | Confirmed clinical improvement (rate not mentioned) | Confirmed improvement | – | – | – | Diarrhea (89%; 11% Gr 3); nausea and vomiting (39%; 6% Gr 3 nausea; all Gr 1/2 vomiting), abdominal pain (22%), fatigue (22%), dysgeusia and rash (17%; all Grade 1/2) | 30% discontinued therapy | |
| Komrokji 2011 | II | 34 (PMF, post-ET and post-PV MF) | 400 mg/day | 88% had reduction in palpable splenomegaly; 32% had a ≥35% reduction in splenic volume | 1 patient | Significant improvement (>2 points) in: abdominal pain, bone pain, early satiety, worst fatigue, inactivity, night sweats and pruritus | – | – | Increased bilirubin, allergic reaction and intermittent nausea | 50% discontinued therapy; 29% required dose reduction for SE | |
| Deeg 2011 | II | 33 with MF | Dose given QD | 96.7%; 56.7%: ≥25% reduction; 39%: ≥50% reduction; 23%: 100% reduction | – | 40-65% | – | – | Diarrhea (81%; 6% Gr 3), nausea (41%; all Gr 1/2), vomiting (22%; all Gr 1/2), and fatigue (9%; all Gr 1/2) | 24% reduced the dose; 64% remained on therapy at 6 months | |
| CYT387 | Pardanani et al. [2011a] | I/II | 166 (106 PMF, 24 post-ET MF, 36 post-PV MF) | 150 or 300 mg QD or 150 mg BiD | 31% improvement according to IWG-MRT criteria | 54% | Most patients had clinical improvement of constitutional symptoms | – | Thrombocytopenia (17%; Gr 3/4) | Dizziness, flushing and hypotension after the first dose (20% of patients) | 32(19%) during the core study and 16 (21%) during extension phase |
| SAR302503 | Pardanani et al. [2011b] | I/II | 59 (44 PMF, 12 post-PV MF, 3 post-ET MF) | 680 mg/dl | 6 cycles: 39%; 12 cycles: 47% (all ≥ 50% reduction) | – | Improvement in early satiety, night sweats, fatigue, pruritus, and cough. | 86% (38% had >20% decrease) | Anemia and thrombocytopenia | Increased amylase/lipase (DLT), nausea/vomiting, diarrhea, increased transaminases | 23% stopped by the sixth cycle |
| AZD1480 | Verstovsek, NCT00910728 | I/II (ongoing) | Enrolling patients with PMF, post-PV MF, post-ET MF | 2.5 mg, 10 mg, 100 mg | – | – | – | – | – | – | – |
| XL019 | Shah et al. [2008] | I/II | 30 (17 PMF, 13 post-PV/ET MF) | 25–50 mg QD, 25 mg three times per week | 100% [12/12] (42% had ≥50% reduction) | – | 90% [10/11] improvements in constitutional symptoms (pruritus/ fatigue) | – | – | peripheral neuropathy (23%, Gr 1/2; in 7 receiving ≥100 mg daily) | 70% discontinued XL019 due to neurotoxicity |
AML, acute myelogenous leukemia; BAT, best available therapy; DLT, dose-limiting toxicity; ET, essential thrombocythemia; Gr, grade; MF, myelofibrosis; MPN, myeloproliferative neoplasm; PMF, primary myelofibrosis; PV, polycythemia vera; SE, side effects.
Preclinical and clinical studies involving JAK2 inhibitors
INCB018424 (ruxolitinib)
INCB018424, also known as ruxolitinib, is a potent and selective inhibitor against both JAK1 and JAK2. It is orally bioavailable and has been studied extensively in the phase I, II and III clinical trial setting. It is the first US Food and Drug Administration (FDA)-approved JAK2 inhibitor for the treatment of myelofibrosis.
In phase I/II study, 153 patients with PMF, post-PV and post-ET myelofibrosis were studied [Verstovsek et al. 2010]. One 28-day cycle of ruxolitinib therapy induced dramatic reduction in multiple fibrogenic, pro-inflammatory and angiogenic growth factors that were markedly elevated prior to therapy, except for leptin and erythropoietin, which increased during therapy. After 1 month of therapy, total or individual symptom scores using the Myelofibrosis Symptom Assessment Form (MF-SAF) scores were improved in more than 50% of patients. The most significant improvements in MF-SAF scores were reported by patients experiencing abdominal discomfort, night sweats, pruritus, and fever. Overall, 61 (44%) of the 140 patients with splenomegaly showed clinical improvement ≥50% within the first 3 months of therapy, according to the International Working Group for Myelofibrosis Research and Treatment (IWG). Response rates were similar among patients with PMF, post-PV and post-ET myelofibrosis (49% versus 45% versus 62%), and regardless of the presence or absence of the JAK2V617F mutation (51% versus 45%, respectively). Although JAK2 was the intended target, JAK2V617F allele burden was only minimally decreased (13% after 12 cycles) [Verstovsek et al. 2010]. JAK2 inhibition is potentially responsible for the abrogation of neoplastic cell proliferation in the spleen, which results in a reduction in splenomegaly; interestingly, tumor lysis is not typically seen. Nonhematological toxicity occurred in less than 10% of patients, while the main adverse events were treatment-emergent anemia and thrombocytopenia; three patients developed AML [Verstovsek et al. 2010].
Results of the two randomized, multicenter, double-blind, placebo-controlled phase III trials in the United States and Europe were recently published [Harrison et al. 2011, 2012; Verstovsek et al. 2011b, 2012]; the Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT)-I trial assessed the activity of ruxolitinib at 15 or 20 mg orally twice daily in 309 patients with PMF, or with post-PV or -ET MF, whereas COMFORT-II trial compared the activity of ruxolitinib in 219 patients with PMF or post-PV or -ET MF against the best available therapy (BAT): the most common therapies used were antineoplastic agents, most frequently hydroxyurea (47%), and glucocorticoids (16%) or no therapy in intermediate-risk/high-risk myelofibrosis patients [Harrison et al. 2011, 2012; Verstovsek et al. 2011b, 2012].
The proportion of patients with at least a 35% reduction in spleen volume was detected by either MRI or computed tomography at 24 (COMFORT-I) or 48 (COMFORT-II) weeks of therapy (see Figure 2). In COMFORT-I, the reduction in spleen volume was observed in 41.9% of patients taking ruxolitinib compared with 0.7% taking placebo; the proportion of patients with a reduction of 50% or more in the total symptom score from baseline to week 24 was 45.9% in the ruxolitinib group versus 5.3% in the placebo group [Verstovsek et al. 2011b]. In COMFORT-II, the reduction in spleen volume was observed in 28% of patients on ruxolitinib compared with 0% on BAT after a 48-week follow-up period [Harrison et al. 2011, 2012; Verstovsek et al. 2012].
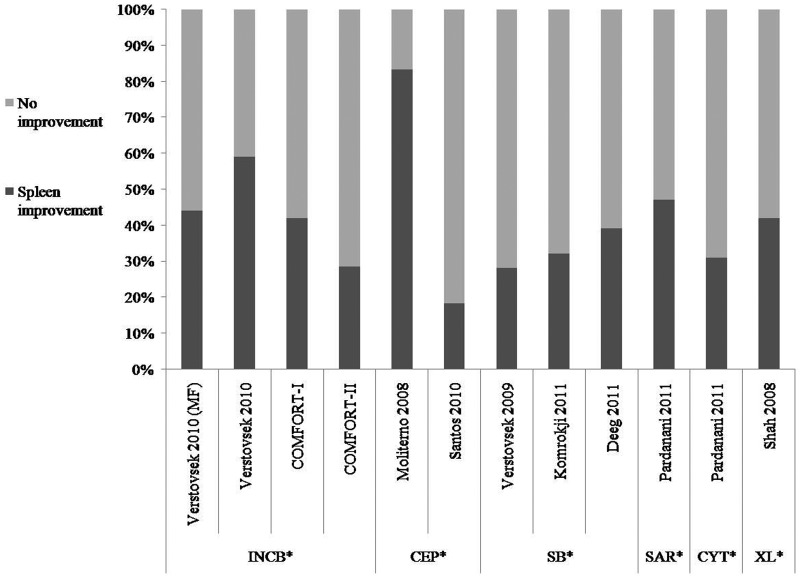
Figure 2.
Improvement in splenomegaly across trials testing JAK inhibitors in patients with myeloproliferative neoplasms.
Percentage of patients with myeloproliferative neoplasms who showed improvement of splenomegaly; INCB018424 [Verstovsek et al. 2010a] and [Verstovsek et al. 2010b] (≥50% reduction of palpable splenomegaly according to IWG criteria in Verstovsek et al. [2010a]); COMFORT-I and COMFORT-II (all ≥ 35% reduction in splenic volume based on IWG criteria); CEP-701 [Moliterno et al. 2009] (>5 cm reduction in spleen volume to nonpalpable spleen), [Santos et al. 2010] (spleen response according to IWG criteria); SB1518 [Verstovsek et al. 2009; Deeg et al. 2011] (all ≥ 50% reduction by physical exam), [Komrokji et al. 2011] (all ≥35% reduction in splenic volume); SAR302503 [Pardanani et al. 2011a] (all ≥50% reduction by palpation according to IWG criteria); CYT387 [Pardanani et al. 2011a] (improvement according to IWG criteria); XL019 [Shah et al. 2008] (≥50% reduction of palpable splenomegaly according to IWG criteria).
*INCB: INCB018424; CEP: CEP-701; SB: SB1518; SAR: SAR302503; CYT: CYT387; XL: XL019.
In the COMFORT-I study, the most common adverse events of any grade seen in >20% of patients on either arm of the study were (treatment versus PB) abdominal pain (10.3% versus 41.1%), grade 3/4 thrombocytopenia (12.9 % versus 1.3 %), fatigue (25.2% versus 33.8%), grade 3/4 anemia (45.2 % versus 19.2 %), diarrhea (23.2% versus 21.2%), and peripheral edema (18.7% versus 22.5%). Anemia and thrombocytopenia were manageable and rarely (one patient in each study group for each event) led to withdrawal from the study [Verstovsek et al. 2011b, 2012; Harrison et al. 2012].
In the COMFORT-II study, the most frequently reported nonhematologic adverse events of any grade in the ruxolitinib group was diarrhea (24% versus 11% in BAT). On the other hand, peripheral edema was the most frequently reported adverse event in the BAT group (26% in BAT group versus 21.9% in ruxolitinib group). Thrombocytopenia and anemia occurred more frequently in the patients receiving ruxolitinib than in those receiving BAT. However, these events rarely led to treatment discontinuation (one patient in each group discontinued the study owing to thrombocytopenia) and were generally manageable [Harrison et al. 2011, 2012].
In terms of ruxolitinib’s influence on survival, analysis at the initial data cutoff point of 24 months in the COMFORT-I study showed no difference in survival benefit with 10 (6.5%) deaths in the ruxolitinib group compared with 14 (9.1%) deaths in the placebo group (hazard ratio, 0.67; p = 0.33). However, a subsequent survival analysis with 4 additional months of follow-up showed a significant survival advantage in the ruxolitinib arm with 13 deaths (8.4%) compared with 24 (15.6%) deaths in the placebo arm (hazard ratio, 0.50; p = 0.04) [Verstovsek et al. 2011b, 2012].
When comparing ruxolitinib with BAT in the COMFORT-II study, no overall survival difference was observed between the two groups at 12 months of follow up. However, there are two important caveats to the interpretation of this survival data. First, approximately 25% of patients assigned to the BAT arm crossed over to ruxolitinib therapy and another 12% of patients withdrew consent with no further survival follow-up data available. Second, the study was not powered to detect differences in time-to-event endpoints [Harrison et al. 2012].
Ruxolitinib was further studied in patients with ET and PV. In a phase II study, the established dose was 10 and 25 mg twice daily as starting doses for expansion cohorts in PV and ET, respectively. For the PV patients, after a median follow up of 15 months, 97% of enrolled subjects achieved hematocrit control to <45% in the absence of phlebotomy, and all continued to maintain phlebotomy independence at the time of last follow-up visit. Splenomegaly was present in 74% of subjects at study entry: 59% of those achieved a ≥50% reduction in palpable spleen length, or the spleen became nonpalpable with all maintaining spleen response at the time of the last follow-up visit. Leukocytosis >15 × 109/l was present in 47% of subjects and improved (≤15 × 109/l) or normalized (≤ upper limit of normal) in 88% and 63%, respectively. Thrombocytosis >600 × 109/l was present in 38% of subjects and improved (≤600 × 109/l) or normalized (≤ upper limit of normal) in 92% and 69%, respectively. A total of 59% of subjects achieved a complete response as indicated by phlebotomy independence, resolution of splenomegaly and normalization of leukocytosis and thrombocytosis. Grade 3 adverse events potentially related to study medication included thrombocytopenia (2 patients), neutropenia (1 patient), renal tumor (1 patient), asthenia (1 patient), viral infection (1 patient), and atrial flutter (1 patient). For the ET patients (n = 39; median 84 months from diagnosis); after a median follow up of 15 months, 49% of enrolled subjects normalized platelet counts to ≤ upper limit of normal after a median of 0.5 months. A total of 88% maintained normal white blood cell (WBC) count. Palpable spleens resolved in 3 of 4 subjects; 49% of subjects achieved normalization of WBC and platelet counts in the presence of nonpalpable splenomegaly. Grade 3 adverse events potentially related to study medication included leukopenia (2 patients), gastrointestinal disorder (1 patient), and peripheral neuropathy (1 patient). Both patient groups demonstrated reductions in patient-reported symptom scores for pruritus, night sweats, and bone pain. Of 26 PV patients reporting pruritus at baseline (median score of 6 on a 10-point scale), 24 reported scores of 0 after a median duration of 1 month. A total of 42% of PV and 56% of ET patients had at least a 20% decrease in JAK2V617F allele burden. Clinical responses were unrelated to the presence/absence of JAK2V617F mutation at study entry or to the allele burden changes following treatment [Verstovsek et al. 2010].
The rapid and durable clinical benefits (normalization of hematological parameters, resolution of splenomegaly and alleviation of symptoms) in this phase II study, along with the tolerability of the drug led to the development of a phase III study [Verstovsek et al. 2010]. A global, open-label phase III trial is designed to compare the efficacy and safety of ruxolitinib to BAT in adult patients with PV who are resistant to or intolerant of hydroxyurea. Primary endpoints, assessed after 32 weeks of treatment, are based on achieving both phlebotomy independence and a ≥35% reduction in spleen volume as measured by imaging. Patients randomized to BAT may be eligible to cross over to receive ruxolitinib after week 32. Enrollment is now open globally with a target of 300 patients to be randomized 1:1 to ruxolitinib or BAT [Verstovsek, 2011a, 2011b].
CEP-701 (lestaurtinib)
CEP-701 is a small-molecule inhibitor of TRKA (tropomyosin-receptor kinase A) which was initially developed for use in prostate cancer, but because of its properties as a FLT3 and JAK2-inhibitor, it was primarily studied in AML and MPN [Levis et al. 2001]. CEP-701 inhibits both wild-type and mutant JAK2 in an in vitro kinase assay and also inhibits the proliferation of rogenitor cells from myeloproliferative disease patients in vitro [Hexner et al. 2008; Santos et al. 2010]; it strongly inhibits the phosphorylation of JAK2V617F and its downstream targets STAT5 and STAT3 [Hexner et al. 2008]. In clinical studies, CEP-701 has been relatively well tolerated and associated with improvements in splenomegaly and other symptoms, with the most common toxicities being nausea, vomiting, anorexia and diarrhea [Smith et al. 2004; Marshall et al. 2005; Santos et al. 2010].
In a study that examined the proliferation of primary erythroid cells from patients with MPNs, higher doses of CEP-701 were used, and it showed that the growth of 15 out of 18 samples from subjects with MPNs was inhibited more than 50% compared with the untreated cells. By specific MPN subtype, 3 of 4 samples from 3 subjects with PMF were inhibited, 9 of 10 samples from subjects with ET were inhibited, and 3 of 4 samples from subjects with a history of PV were inhibited; it markedly inhibited STAT5 and AKT phosphorylation in all MPN samples [Hexner et al. 2008].
In one phase II trial, 22 JAK2V617F -positive, -intermediate/high-risk patients were given 80 mg of CEP-701 twice daily, by solution, and six (27%) responded by IWG criteria for clinical improvement. Of these six respondents, three had a reduction in spleen volume, two became transfusion independent, and one had an improvement in both spleen volume and cytopenias. No changes in bone marrow fibrosis or JAK2V617F allele burden were reported. Phosphorylated STAT3 levels decreased from baseline in responders while on therapy. Myelosuppression and gastrointestinal symptoms were the most common adverse effects (grade 3/4 anemia or thrombocytopenia: 14% and 23%, respectively) [Santos et al. 2010].
CEP-701 was the first JAK2 inhibitor to be studied in a phase1/2 safety and efficacy study in high risk JAK2V617F positive ET and PV patients [Moliterno et al. 2009]. The primary endpoint was reduction in JAK2V617F neutrophil allele burden and the secondary endpoints included reduction in phlebotomy rates; improvement in hemoglobin, WBC and platelet counts; reduction in hydroxyurea dose and spleen volume; and the pharmacokinetics and pharmacodynamics of CEP-701 were also evaluated. The study enrolled 39 JAK2V617F-positive subjects, 27 PV and 12 ET, 22 females and 17 males. The median neutrophil JAK2V617F allele burden was 40%. More than half of the patients had had their disease for 5 years or longer. Within 18 weeks, responses included a reduction in spleen volume of >5 cm or to nonpalpable in 15/18 (83%) subjects and amelioration of pruritus in 5/5 patients studied. While reduction in phlebotomy requirement occurred in a number of phlebotomy-dependent patients (3/5 evaluable at time of report), this effect was not evident in these patients until 6 months of therapy, and was not associated with concomitant reductions in WBC or platelet count. A reduction in the JAK2V617F allele burden of 15% or more was observed in 3 of the 15 patients at the 18-week assessment. Dose-related gastrointestinal symptoms were the most common adverse effects. Serious adverse effects included thrombotic events (five overall, three venous [one deep vein thrombosis, one deep vein thrombosis/pulmonary embolism, one portal vein thrombosis], and two arterial) and one nonserious deep vein thrombosis.
In summary, CEP-701 is a multikinase inhibitor that showed a modest efficacy and mild but frequent gastrointestinal toxicity in myelofibrosis patients [Santos et al. 2010]. Moreover, it has been shown to be effective in improving the substantial symptoms in JAK2V617F-positive PV and ET patients. However, it did not prevent ET and PV patients from developing thrombotic events which are believed to be disease-specific interactions since thrombosis has not been a frequent complication of CEP-701 therapy in other malignancies [Moliterno et al. 2009].
SB1518 (pacritinib)
SB1518 is an oral JAK and FLT3 inhibitor, with a high selectivity against JAK2 and JAK2V617F, from S*Bio (Singapore); it has been proven to be active against leukemia cell lines that are dependent on JAK2 activation for their growth [Verstovsek et al. 2009]. Phase I/II trials in both the United States and Australia have shown the efficacy and safety of this agent [Verstovsek et al. 2009; Seymour et al. 2010].
In a phase I trial, SB1518 was given to 43 patients with either myelofibrosis (n = 36) or AML (n = 7) who had failed standard therapy. SB1518 was well tolerated; 7 out of 25 (28%) patients had a consistent decrease in spleen volume greater than 50% and met the criteria for clinical improvement according to the IWG. Marked inhibition of JAK2 and STAT5 was observed in blood samples collected 2 hours after the administration of the first dose of SB1518. The dosing ranged from 100 to 600 mg daily; the recommended phase II dose was 400 mg daily [Verstovsek et al. 2009; Quintas-Cardama et al. 2011]. In another phase I study originated from Australia and involving 20 patients with myelofibrosis, similar safety and efficacy results have been reported [Seymour et al. 2010].
SB1518 was evaluated in a phase II trial, and its results were reported recently. A total of 33 patients with myelofibrosis were involved [Deeg et al. 2011]. The primary objective was to evaluate spleen volume reduction by MRI in those patients with splenomegaly. A total of 17(57%) patients had a reduction in spleen volume by 25% or more. Symptom improvement was also reported in 40–65% of patients treated for 6 months [Deeg et al. 2011].
SB1518 was not associated with significant myelosuppression; there was no grade 3 or 4 neutropenia or thrombocytopenia [Verstovsek et al. 2009; Deeg et al. 2011]. Gastrointestinal side-effects, including nausea, diarrhea, vomiting, and abdominal pain, were common [Verstovsek et al. 2009; Seymour et al. 2010; Deeg et al. 2011]. In one of the trials, 16/43 (40%) discontinued SB1518 treatment due to toxicity, as a result of disease progression and due to other reasons [Verstovsek et al. 2009].
In another recent report of a phase II study, 34 primary, post-ET, or post-PV myelofibrosis patients were enrolled [Komrokji et al. 2011]. The primary endpoint of the study was to assess the spleen response rate, defined as a 35% reduction in MRI-measured spleen volume between baseline and week 24. A total of 17 patients (50%) have discontinued, including eight (24%) due to adverse events (one each for nausea, sepsis, increased bilirubin, subdural hematoma, allergic reaction, gastrointestinal bleed, and two due to thrombocytopenia), five for disease progression, and two for lack of response. Of the adverse events leading to discontinuation, only increased bilirubin, allergic reaction and intermittent nausea were considered possibly drug related. Ten patients required dose reduction for adverse events. One patient required drug discontinuation associated with decreased neutrophils and platelets. The most common treatment-related AEs were gastrointestinal, which were generally low grade and easily managed. Gastrointestinal adverse events of grade >2 included grade 3 diarrhea in two patients (6%). Only one patient discontinued for gastrointestinal toxicity. SB1518 produced meaningful reductions in splenomegaly. A total of 30 patients (88%) showed reductions in palpable splenomegaly. Eleven patients (32%) had a 35% reduction in splenic volume as measured by MRI. All spleen responses are ongoing; consequently a median duration of response has not been reached at the time of writing. Two patients met IWG-MRT criteria for clinical improvement in hemoglobin including one patient who became transfusion independent. At the 6-month visit, a significant reduction (>2 point improvement) was observed for MF-associated symptoms, including abdominal pain, bone pain, early satiety, worst fatigue, inactivity, night sweats and pruritus.
In summary, SB1518 shows promising efficacy in alleviating myelofibrosis-associated splenomegaly and constitutional symptoms at a dose that induces minimal myelosuppression. Once-daily dosing is well tolerated, with manageable gastrointestinal toxicity as the main side effect. Given the low frequency of myelosuppression with SB1518, this JAK2 inhibitor is of particular importance for myelofibrosis patients with impaired hematopoiesis [Komrokji et al. 2011].
SAR302503
SAR302503, previously known as TG101348, is a potent selective JAK inhibitor [Wernig et al. 2008]. The inhibitory activity of SAR 302503 was profiled in 223 different kinases, with JAK2 being among those significantly inhibited with it. In one of the studies on JAK2V617F-induced PV murine models, SAR302503 treatment resulted in marked reductions of hematocrit and spleen volume, and in some instances attenuation of myelofibrosis [Wernig et al. 2008].
Results of a multicenter phase I/II trial have been published recently [Pardanani et al. 2011b]. A total of 59 patients with intermediate or high risk for PMF (44 patients), post-PV (12 patients) or post-ET (3 patients) were involved; 86% of them were JAK2V617F positive. The maximum tolerated dose was found to be 680 mg/dl in a dose escalation on 28 of the 59 overall patients. The dose limiting toxicity was a reversible and asymptomatic increase in the serum amylase level. The median exposures to SAR302503 for the overall (n = 59) cohort was 155 days. The onset of spleen response was seen within the first two cycles. By 6 and 12 cycles of treatment, 39% and 47% of patients, respectively, had achieved a spleen response per IWG-MRT (≥50% decrease in palpable spleen volume persistent for at least 8 weeks). A significant decrease in JAK2V617F allele burden was observed at 6 months in 51 mutation-positive patients with a median allele burden of 20% at baseline. After 6 and 12 cycles of treatment, the median allele burdens were 17% and 19%, respectively. There was a significant and more pronounced decrease particularly in the subgroup with allele burden greater than 20% (n = 23). The decrease was durable at 12 months. The majority of patients with leukocytosis or thrombocytosis at baseline (n = 28 and n = 10, respectively) achieved normalization of blood counts; 57% and 56% of patients achieved a normal WBC count after 6 and 12 cycles, respectively, while 90% and 88 achieved a normal platelet count after 6 and 12 cycles. Adverse events included nausea, vomiting, diarrhea, anemia and thrombocytopenia. Despite having only a modest effect on cytokine levels, greater than half of the patients with early satiety, night sweats, fatigue, pruritus, and cough achieved rapid and durable improvement in these symptoms.
Although most patients improved or experienced resolution of baseline constitutional symptoms, there were no observed changes in pro-inflammatory cytokines (e.g. IL-6 and TNFα) during SAR302503 therapy, and this may be attributable to the higher selectivity of SAR302503 for JAK2.
CYT387
CYT387, an aminopyrimidine derivative, is a small-molecule ATP-competitive inhibitor with high selectivity for JAK1 and JAK 2 versus other closely related kinases (e.g. JAK3) [Pardanani et al. 2009]. This selectivity profile resembles some (INCB018424, CEP-701), but not other (SAR302503, XL019), small-molecule JAK inhibitors [Pardanani et al. 2009].
CYT387 inhibited in vitro JAK2V617F mutation harboring human erythroleukemia (HEL) cells as well as Ba/F3-JAK2V617F cells by decreasing ERK1/2, STAT-5, and STAT-3 phosphorylation [Pardanani et al. 2009]. It also inhibited the proliferation of erythropoietin-independent erythroid colonies from PV patients [Bumm et al. 2008].
CYT387 trials on mice revealed normalization of blood counts, pro-inflammatory cytokine levels and reduction in extramedullary hematopoiesis including spleen volume. However, fewer effects on the bone marrow were noticed, as hypercellularity persisted [Tyner et al. 2010]. It also failed to eliminate JAK2V617F-expressing cells.
These preclinical data provide a rationale for the use of CYT387 in MPN, and the most recent report from a multicenter phase I/II trial on 166 intermediate/high-risk myelofibrosis patients [Pardanani et al. 2011a] showed that CYT387 is well tolerated orally either once daily at 150 or 300 mg or twice daily at 150 mg. The study was conducted in three phases: dose-escalation, dose-confirmation, and dose-expansion phases. Oral CYT387 was administered at the previously mentioned dose levels for 9 months. Patients who maintained at least stable disease were permitted to continue CYT387 treatment beyond nine cycles in an extension phase of the study. The maximum tolerated dose was 300 mg/day. About 20% of the patients experienced a first-dose effect (dizziness, flushing and hypotension), which was self-limited. Grade 3/4 hematologic and nonhematologic adverse events were infrequent with the exception of thrombocytopenia, which occurred in approximately 17% of patients. Grade 3/4 nonhematologic laboratory adverse events include hyperlipasemia (4%) and increase in liver enzymes (1% grade 3 and less than 1% grade 4 increase in aspartate aminotransferase; 2% grade 3 increase in ALT). The overall anemia response rate was 54% in transfusion-dependent patients with a median time to confirm anemia response of 12 weeks (range 84 to 293 days). Spleen response rate by IWG-MRT criteria was approximately 31% (median time to response of 15 days) whereas the majority of patients experienced resolution of constitutional symptoms including pruritus, night sweats, fever, cough and bone pain at 6 months [Pardanani et al. 2011a].
In summary, CYT387 appears to result in a significant, durable response in anemia, splenomegaly and constitutional symptoms at 150 mg QD, 300 mg QD, and 150 mg BID dose levels.
AZD1480
The pyrazolyl pyrimidine, also known as (AZD1480), is a small-molecule potent ATP competitive inhibitor of JAK2 kinase. The antiproliferative activity has been shown to be tightly correlated with the inhibition of pSTAT5 in Ba/F3 TEL-JAK2 cells [Ioannidis et al. 2011]. STAT3 phosphorylation has also been inhibited by AZD1480 which is a dose-dependent inhibition of STAT3 nuclear translocation and STAT3-dependent tumor growth [Hedvat et al. 2009; Scuto et al. 2011; Xin et al. 2011]. Moreover, targeting STAT3 by AZD1480 directly inhibits the function of endothelial cells. IL-6-driven stimulation of STAT3 tyrosyl phosphorylation, which plays a role in tumorigenesis, can be completely blocked by AZD1480 [Guschin et al. 1995].
AZD1480 demonstrated significant cellular selectivity for JAK2 versus the antiproliferative activity of Aurora A/B, JAK3, and Tyk2 and to a smaller extent against JAK1 [Ioannidis et al. 2011]. Further in vivo studies in dogs and mice revealed excellent pharmacokinetic profile with long half-life and excellent oral bioavailability, suggestive of full absorption and minimal first-pass metabolism [Ioannidis et al. 2011].
AZD1480 has been further studied on myeloma cells [Scuto et al. 2011] and found out to be a dual JAK/FGFR inhibitor for targeting these cells. Its activity on JAK2 and FGFR3 is even greater than other JAK2 and FGFR3 inhibitors. It inhibited the growth and survival of human myeloma cells in vitro and in vivo. The lack of inhibition of proliferation and viability of bone marrow stromal cells and peripheral blood mononuclear cells derived from healthy donors suggests that the drug may spare normal cells.
There is an ongoing phase I\II clinical trial on oral AZD1480 (2.5, 10, 100 mg) for patients with PMF and post-PV/-ET myelofibrosis [Verstovsek et al. 2011a].
XL019
XL019 is a potent, reversible and highly selective inhibitor of JAK2 compared with other JAK family kinases (JAK1, JAK3, and TYK2). This selectivity was clearly observed in primary human cell assays. EPO-stimulated pSTAT5 in primary erythroid cells showed high sensitivity to XL019.
XL019 was discontinued while under two phase I\II studies in PMF, PV, post-PV, and post-ET myelofibrosis [Paquette et al. 2008; Shah et al. 2008].
Although the preliminary data showed that XL019 caused reduction in spleen volume, blasts count and WBC count [Paquette et al. 2008], the rate of neurological toxicity were unacceptable and reached 70% among patients who discontinued XL019 therapy [Shah et al. 2008]; this has precluded further development of XL019 for the treatment of patients with MPNs, and both ongoing studies were terminated [Quintas-Cardama et al. 2011].
JAK-inhibitors under investigations
CP-690,550 (tasocitinib)
This JAK inhibitor has been studied preclinically in human PV cells [Manshouri et al. 2008]. CP-690,550 has greater antiproliferative and pro-apoptotic activity against cells harboring JAK2V617F compared with wild-type JAK2. It caused cell growth inhibition and pro-apoptotic effect in murine factor-dependent cell Patersen-erythropoietin receptor (FDCP-EpoR) cells harboring human wild-type or JAK2V617F. This activity was paralleled with inhibition of phosphorylation of STAT3, STAT5, and v-akt murine thymoma viral oncogene homolog (AKT). Moreover, CP-690,550 expressed antiproliferative and pro-apoptotic activity on ex vivo expanded erythroid progenitors from JAK2V617F-positive PV patients. In contrast, expanded progenitors from healthy controls were less sensitive. The antiproliferative effect on the patient progenitors was coupled by a decrease in JAK2V617F mutant allele frequency, particularly in a patient homozygous for JAK2V617F. CP-690,550 is still in preclinical stages of development.
NVP-BSK805
NVP-BSK805 is a potent inhibitor of JAK2V617F and JAK2 wild-type enzymes by acting as an ATP-competitive inhibitor. It shows high selectivity against JAK2. It has an antiproliferative and pro-apoptotic effect on JAK2V617F-bearing cells by blocking STAT5 phosphorylation. In vivo studies [Baffert et al. 2010] show that NVP-BSK805 has a long half-life and a good oral bioavailability. In a Ba/F3 JAK2V617F mouse model, it decreased leukemic cell spreading and splenomegaly. Furthermore, NVP-BSK805 caused a potent suppression of recombinant human erythropoietin-induced polycythemia and extramedullary erythropoiesis in mice and rats [Baffert et al. 2010]. NVP-BSK805 has yet to enter clinic phase of development.
INCB16562
INCB16562 is a potent inhibitor of cell lines and primary cells from PV patients carrying the JAK2 and JAK1 mutations [Liu et al. 2009]; it works by blocking JAK-STAT signaling and inducing apoptosis. INCB16562 reduced malignant cell burden, reversed splenomegaly, extended survival [Liu et al. 2009], normalized WBC counts and platelet counts, markedly reduced extramedullary hematopoiesis and bone marrow fibrosis [Koppikar et al. 2010]. INCB16562 has been shown to be effective and beneficial in the treatment of myeloma cells as it inhibits IL-6-induced phosphorylation of STAT3 and the proliferation and survival of myeloma cells dependent on IL-6 for growth [Li et al. 2010]. INCB16562 has not been tested clinically in patients with MPNs.
Conclusion
Clinical trials using various pharmacologic inhibitors that target the JAK-STAT pathway in MF have resulted in meaningful and significant improvements in splenomegaly, associated clinical manifestations, and disease related constitutional symptoms. The JAK2 inhibitor ruxolitinib has successfully completed phase III trials and achieved FDA approval status on 16 November 2011. Ruxolitinib is the first FDA-approved drug in myelofibrosis. The early success of this class of agents also raised many important issues about JAK-STAT pathway and its relevance in myelofibrosis pathophysiology. It is now apparent that the importance of this pathway is shared between JAK2 wild-type and JAK2 mutated cases as illustrated by the efficacy of JAK2 inhibitors in both types of myelofibrosis patients. Knowing that patients with myelofibrosis regardless of JAK2 V617F mutational status may benefit from ruxolitinib therapy is important to practicing clinicians who treat patients with myelofibrosis. JAK2 allele burden, a frequently used biomarker of disease burden was not significantly affected by treatment with JAK2 inhibitors in earlier studies. This demonstrates the pathophysiologic complexity of myelofibrosis which we know may be driven by several molecular drivers such as mutations involving ASXL1, TET2, CBL unlike CML which is caused by a single primary molecular defect involving an aberrant chromosomal translocation and subsequent BCR-ABL1 fusion that results in a constitutively active fusion protein.
Based on currently available data, there are several limitations to the use of JAK2 inhibitors in myelofibrosis patients. First, some patients with myelofibrosis present with platelet counts between 50 × 109/l to 100 × 109/l or even <50 × 109/l, since ruxolitinib and other JAK2 inhibitors have platelet lowering properties, the safety and clinical efficacy of ruxolitinib or other JAK2 inhibitors in these groups of patients are unclear although the subject of current investigation. Second, some patients with myelofibrosis present with transfusion-dependent anemia, since ruxolitinib can lead to anemia in some patients, the utility and safety of ruxolitinib or other JAK2 inhibitors in these groups of patients are uncertain and currently being studied. Third, it does not cure the disease and requires continuing therapy to maintain response. Fourth, early JAK inhibitors, exemplified by ruxolitinib, have thus far not conclusively shown a long-term survival benefit, but review of more mature data will provide additional insight into this issue. Fifth, there are no data showing reversal of cytogenetic, molecular and other pathomorphologic disease features such as reticulin fibrosis. Lastly, in some patients, the response may be short lived and there are some reports of adverse events occurring at the time of withdrawal [Verstovsek, 2011b]. MPNs are a heterogeneous group of disorders and unlike BCR-ABL1-positive CML, are unlikely to be driven by a single mutation. Further investigation to evaluate other types of JAK2 inhibitors whether alone or in combination with other therapies such as immunomodulatory agents, histone deacetylase inhibitors, DNA methyltransferase inhibitors and other targeted agents may help improve outcomes in myelofibrosis and may help resolve some of the currently observed limitations in sole JAK2 inhibitor therapy.
Footnotes
Funding: This work was supported partially or in full by the Cleveland Clinic Institutional Seed Grant (RVT).
Conflict of interest statement: None declared.
Contributor Information
Mohamad Bassam Sonbol, Faculty of Medicine, Damascus University, Damascus, Syria.
Belal Firwana, Department of Internal Medicine, University of Missouri, Columbia, MO, USA.
Ahmad Zarzour, Faculty of Medicine, Damascus University, Damascus, Syria.
Mohammad Morad, Faculty of Medicine, Damascus University, Damascus, Syria.
Vishal Rana, Division of Hematology, Mayo Clinic, Rochester, MN, USA.
Ramon V. Tiu, Department of Translational Hematology and Oncology Research, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Avenue R40, Cleveland, OH 44195, USA
References
- Baffert F., Regnier C., De Pover A., Pissot-Soldermann C., Tavares G., Blasco F., et al. (2010) Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol Cancer Ther 9: 1945–1955 [DOI] [PubMed] [Google Scholar]
- Bartram C., De Klein A., Hagemeijer A., Van Agthoven T., Geurts Van Kessel A., Bootsma D., et al. (1983) Translocation of C-Ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 306: 277–280 [DOI] [PubMed] [Google Scholar]
- Baxter E., Scott L., Campbell P., East C., Fourouclas N., Swanton S., et al. (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365: 1054–1061 [DOI] [PubMed] [Google Scholar]
- Beer P., Campbell P., Scott L., Bench A., Erber W., Bareford D., et al. (2008) MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 112: 141–149 [DOI] [PubMed] [Google Scholar]
- Bumm T., Tyner J., Deininger J., Loriaux M., Vandyke J., Druker B., et al. (2008) Effects of CYT387, a potent novel JAK2 inhibitor on JAK2-V617F induced MPD [abstract]. ASH Annual Meeting Abstracts 112: 856 [Google Scholar]
- Carroll M., Tomasson M., Barker G., Golub T., Gilliland D. (1996) The TEL/platelet-derived growth factor beta receptor (PDGF Beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF Beta R kinase-dependent signaling pathways. Proc Natl Acad Sci U S A 93: 14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Deangelo D., Kutok J., Williams I., Lee B., Wadleigh M., et al. (2004) PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc Natl Acad Sci U S A 101: 14479–14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools J., Deangelo D., Gotlib J., Stover E., Legare R., Cortes J., et al. (2003) A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 348: 1201–1214 [DOI] [PubMed] [Google Scholar]
- Dameshek W. (1951) Some speculations on the myeloproliferative syndromes. Blood 6: 372–375 [PubMed] [Google Scholar]
- Dawson M., Bannister A., Gottgens B., Foster S., Bartke T., Green A., et al. (2009) JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 461: 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg H., Odenike O., Scott B., Estrov Z., Cortes J., Thomas D., et al. (2011) Phase II study of SB1518, an orally available novel JAK2 inhibitor, in patients with myelofibrosis J Clin Oncol 29: Abstract 6515. [Google Scholar]
- Fridman J., Scherle P., Collins R., Burn T., Neilan C., Hertel D., et al. (2011) Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J Invest Dermatol 131: 1838–1844 [DOI] [PubMed] [Google Scholar]
- Gangat N., Caramazza D., Vaidya R., George G., Begna K., Schwager S., et al. (2011) DIPSS Plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 29: 392–397 [DOI] [PubMed] [Google Scholar]
- Garcon L., Rivat C., James C., Lacout C., Camara-Clayette V., Ugo V., et al. (2006) Constitutive activation of STAT5 and BCL-XL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood 108: 1551–1554 [DOI] [PubMed] [Google Scholar]
- Golub T., Barker G., Lovett M., Gilliland D. (1994) Fusion of PDGF receptor Beta to a novel ETS-like gene, TEL, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 77: 307–316 [DOI] [PubMed] [Google Scholar]
- Guglielmelli P., Pancrazzi A., Bergamaschi G., Rosti V., Villani L., Antonioli E., et al. (2007) Anaemia characterises patients with myelofibrosis harbouring MPL mutation. Br J Haematol 137: 244–247 [DOI] [PubMed] [Google Scholar]
- Guschin D., Rogers N., Briscoe J., Witthuhn B., Watling D., Horn F., et al. (1995) A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J 14: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C., Kiladjian J., Al-Ali H., Gisslinger H., Waltzman R., Stalbovskaya V., et al. (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366: 787–798 [DOI] [PubMed] [Google Scholar]
- Harrison C., Kiladjian J., Al-Ali H., Gisslinger H., Waltzman R., Stalbovskaya V., et al. (2011) Results of a randomized study of the JAK inhibitor ruxolitinib (INC424) versus best available therapy (BAT) in primary myelofibrosis, postpolycythemia vera-myelofibrosis (PPV-MF), or postessential thrombocythemia-myelofibrosis (PET-MF) J Clin Oncol 29 (Suppl): Abstract LBA6501. [Google Scholar]
- Hedvat M., Huszar D., Herrmann A., Gozgit J., Schroeder A., Sheehy A., et al. (2009) The JAK2 inhibitor AZD1480 potently blocks STAT3 signaling and oncogenesis in solid tumors. Cancer Cell 16: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexner E., Serdikoff C., Jan M., Swider C., Robinson C., Yang S., et al. (2008) Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood 111: 5663–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J., Witthuhn B., Quelle F., Yamamoto K., Silvennoinen O. (1995) Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol 13: 369–398 [DOI] [PubMed] [Google Scholar]
- Ioannidis S., Lamb M., Wang T., Almeida L., Block M., Davies A., et al. (2011) Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-Yl)ethyl]-N4-(5-methyl-1H-pyrazol -3-Yl)pyrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the JAK/STAT pathway. J Med Chem 54: 262–276 [DOI] [PubMed] [Google Scholar]
- James C., Ugo V., Le Couedic J., Staerk J., Delhommeau F., Lacout C., et al. (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144–1148 [DOI] [PubMed] [Google Scholar]
- Komrokji R., Wadleigh M., Seymour J., Roberts A., To L., Zhu H., et al. (2011) Results of a phase 2 study of pacritinib (SB1518), a novel oral JAK2 inhibitor, in patients with primary, post-polycythemia vera, and post-essential thrombocythemia myelofibrosis [abstract]. ASH Annual Meeting Abstracts 118: 282 [Google Scholar]
- Koppikar P., Abdel-Wahab O., Hedvat C., Marubayashi S., Patel J., Goel A., et al. (2010) Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood 115: 2919–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R., Passamonti F., Buser A., Teo S., Tiedt R., Passweg J., et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779–1790 [DOI] [PubMed] [Google Scholar]
- Kumar C., Purandare A., Lee F., Lorenzi M. (2009) Kinase drug discovery approaches in chronic myeloproliferative disorders. Oncogene 28: 2305–2313 [DOI] [PubMed] [Google Scholar]
- Lacout C., Pisani D., Tulliez M., Gachelin F., Vainchenker W., Villeval J. (2006) JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood 108: 1652–1660 [DOI] [PubMed] [Google Scholar]
- Lasho T., Pardanani A., Tefferi A. (2010) LNK mutations in JAK2 mutation-negative erythrocytosis. N Engl J Med 363: 1189–1190 [DOI] [PubMed] [Google Scholar]
- Levine R., Wadleigh M., Cools J., Ebert B., Wernig G., Huntly B., et al. (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7: 387–397 [DOI] [PubMed] [Google Scholar]
- Levis M., Tse K., Smith B., Garrett E., Small D. (2001) A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood 98: 885–887 [DOI] [PubMed] [Google Scholar]
- Li J., Favata M., Kelley J., Caulder E., Thomas B., Wen X., et al. (2010) INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia 12: 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Caulder E., Li J., Waeltz P., Margulis A., Wynn R., et al. (2009) Combined inhibition of Janus kinase 1/2 for the treatment of JAK2V617F-driven neoplasms: selective effects on mutant cells and improvements in measures of disease severity. Clin Cancer Res 15: 6891–6900 [DOI] [PubMed] [Google Scholar]
- Manshouri T., Quintas-Cardama A., Nussenzveig R., Gaikwad A., Estrov Z., Prchal J., et al. (2008) The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci 99: 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Kindler H., Deeken J., Bhargava P., Vogelzang N., Rizvi N., et al. (2005) Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs 23: 31–37 [DOI] [PubMed] [Google Scholar]
- McLornan D.P., Mead A.J., Jackson G., Harrison C.N. (2012) Allogeneic stem cell transplantation for myelofibrosis in 2012. Br J Haematol 157(4): 413–25 [DOI] [PubMed] [Google Scholar]
- Moliterno A., Hexner E., Roboz G., Carroll M., Luger S., Mascarenhas J., et al. (2009) An open-label study of CEP-701 in patients with JAK2 V617F-positive PV and ET: update of 39 enrolled patients [abstract]. ASH Annual Meeting Abstracts 114: 753 [Google Scholar]
- Nagata H., Worobec A., Oh C., Chowdhury B., Tannenbaum S., Suzuki Y., et al. (1995) Identification of a point mutation in the catalytic domain of the protooncogene c-Kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A 92: 10560–10564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka T., Van Deursen J., Tripp R., Thierfelder W., Witthuhn B., McMickle A., et al. (1995) Defective lymphoid development in mice lacking JAK3. Science 270: 800–802 [DOI] [PubMed] [Google Scholar]
- O’Shea J., Pesu M., Borie D., Changelian P. (2004) A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov 3: 555–564 [DOI] [PubMed] [Google Scholar]
- Oh S., Simonds E., Jones C., Hale M., Goltsev Y., Gibbs K., et al. (2010) Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 116: 988–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteli K., Hatzimichael E., Bouranta P., Katsaraki A., Seferiadis K., Stebbing J., et al. (2005) Serum interleukin (IL)-1, IL-2, SIL-2RA, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol 130: 709–715 [DOI] [PubMed] [Google Scholar]
- Paquette R., Sokol L., Shah N., Silver R., List A., Clary D., et al. (2008) A phase I study of XL019, a selective JAK2 inhibitor, in patients with polycythemia vera [abstract]. ASH Annual Meeting Abstracts 112: 2810 [Google Scholar]
- Pardanani A., Caramazza D., George G., Lasho T., Hogan W., Litzow M., et al. (2011a) Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor for the treatment of myelofibrosis. In 2011 American Society of Hematology (ASH) Annual Meeting, Poster #3849.
- Pardanani A., Gotlib J., Jamieson C., Cortes J., Talpaz M., Stone R., et al. (2011b) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A., Lasho T., Finke C., Hanson C., Tefferi A. (2007) Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 21: 1960–1963 [DOI] [PubMed] [Google Scholar]
- Pardanani A., Lasho T., Finke C., Oh S., Gotlib J., Tefferi A. (2010) LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 24: 1713–1718 [DOI] [PubMed] [Google Scholar]
- Pardanani A., Lasho T., Smith G., Burns C., Fantino E., Tefferi A. (2009) CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 23: 1441–1445 [DOI] [PubMed] [Google Scholar]
- Pardanani A., Levine R., Lasho T., Pikman Y., Mesa R., Wadleigh M., et al. (2006) MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 108: 3472–3476 [DOI] [PubMed] [Google Scholar]
- Parganas E., Wang D., Stravopodis D., Topham D., Marine J., Teglund S., et al. (1998) JAK2 is essential for signaling through a variety of cytokine receptors. Cell 93: 385–395 [DOI] [PubMed] [Google Scholar]
- Passamonti F., Elena C., Schnittger S., Skoda R., Green A., Girodon F., et al. (2011) Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 117: 2813–2816 [DOI] [PubMed] [Google Scholar]
- Passamonti F., Rumi E., Pietra D., Elena C., Boveri E., Arcaini L., et al. (2010) A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 24: 1574–1579 [DOI] [PubMed] [Google Scholar]
- Pesu M., Laurence A., Kishore N., Zwillich S., Chan G., O’Shea J. (2008) Therapeutic targeting of Janus kinases. Immunol Rev 223: 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikman Y., Lee B., Mercher T., McDowell E., Ebert B., Gozo M., et al. (2006) MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 3: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A., Kantarjian H., Cortes J., Verstovsek S. (2011) Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov 10: 127–140 [DOI] [PubMed] [Google Scholar]
- Santos F., Kantarjian H., Jain N., Manshouri T., Thomas D., Garcia-Manero G., et al. (2010) Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood 115: 1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A., Jouault H., Guichard J., Wendling F., Drouin A., Cramer E. (2000) Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood 96: 1342–1347 [PubMed] [Google Scholar]
- Scott L., Tong W., Levine R., Scott M., Beer P., Stratton M., et al. (2007) JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 356: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuto A., Krejci P., Popplewell L., Wu J., Wang Y., Kujawski M., et al. (2011) The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 25: 538–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour F., To B., Goh A., Meadows L., Ethirajulu A., Wood A., et al. (2010) First report of the phase-I study of the novel oral JAK2 inhibitor SB1518 in patients with myelofibrosis Haematologica 95 (Suppl. 2): Abstract 1144. [Google Scholar]
- Shah N., Olszynski P., Sokol L., Verstovsek S., Hoffman R., List A., et al. (2008) A phase I study of XL019, a selective JAK2 inhibitor, in patients with primary myelofibrosis, post-polycythemia vera, or post-essential thrombocythemia myelofibrosis [abstract]. ASH Annual Meeting Abstracts 112: 98 [Google Scholar]
- Shuai K., Liu B. (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3: 900–911 [DOI] [PubMed] [Google Scholar]
- Silver R., Vandris K., Wang Y., Adriano F., Jones A., Christos P., et al. (2011) JAK2(V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk Res 35: 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Levis M., Beran M., Giles F., Kantarjian H., Berg K., et al. (2004) Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 103: 3669–3676 [DOI] [PubMed] [Google Scholar]
- Takaki S., Morita H., Tezuka Y., Takatsu K. (2002) Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, LNK. J Exp Med 195: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. (2000) Myelofibrosis with myeloid metaplasia. N Engl J Med 342: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Tefferi A. (2010) Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 24: 1128–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. (2011) Primary myelofibrosis: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 86: 1017–1026 [DOI] [PubMed] [Google Scholar]
- Tefferi A. (2012) Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 87: 285–293 [DOI] [PubMed] [Google Scholar]
- Tefferi A., Jimma T., Gangat N., Vaidya R., Begna K., Hanson C., et al. (2011) Predictors of greater than 80% 2-year mortality in primary myelofibrosis: a Mayo Clinic study of 884 karyotypically annotated patients. Blood 118: 4595–4598 [DOI] [PubMed] [Google Scholar]
- Tefferi A., Lasho T., Huang J., Finke C., Mesa R., Li C., et al. (2008) Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 22: 756–761 [DOI] [PubMed] [Google Scholar]
- Tefferi A., Vardiman J.W. (2008) Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 22: 14–22 [DOI] [PubMed] [Google Scholar]
- Tong W., Lodish H. (2004) LNK inhibits TPO-MPL signaling and TPO-mediated megakaryocytopoiesis. J Exp Med 200: 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner J., Bumm T., Deininger J., Wood L., Aichberger K., Loriaux M., et al. (2010) CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 115: 5232–5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W., Dusa A., Constantinescu S. (2008) JAKs in pathology: role of Janus kinases in hematopoietic malignancies and immunodeficiencies. Semin Cell Dev Biol 19: 385–393 [DOI] [PubMed] [Google Scholar]
- Vannucchi A., Antonioli E., Guglielmelli P., Longo G., Pancrazzi A., Ponziani V., et al. (2007) Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia 21: 1952–1959 [DOI] [PubMed] [Google Scholar]
- Vannucchi A., Antonioli E., Guglielmelli P., Pancrazzi A., Guerini V., Barosi G., et al. (2008) Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood 112: 844–847 [DOI] [PubMed] [Google Scholar]
- Velazquez L., Cheng A., Fleming H., Furlonger C., Vesely S., Bernstein A., et al. (2002) Cytokine signaling and hematopoietic homeostasis are disrupted in LNK-deficient mice. J Exp Med 195: 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S. (2011a) Response: a randomized, open label, phase III study of INC424 in polycythemia vera (PV) patients resistant to or intolerant of hydroxyurea (HU). J Clin Oncol 29: Abstract TPS203. [Google Scholar]
- Verstovsek S. (2011b) Study of efficacy and safety in polycythemia vera subjects who are resistant to or intolerant of hydroxyurea: JAK inhibitor INC424 (INCB018424) tablets versus best available care: the Response trial. http://clinicaltrials.gov/ct2/show/NCT01243944
- Verstovsek S., Hoffman R., Ribrag V. (2011a) Study to assess the safety of AZD1480 in patients with myeloproliferative diseases. http://clinicaltrial.gov/ct2/show/NCT00910728
- Verstovsek S., Kantarjian H., Mesa R., Pardanani A., Cortes-Franco J., Thomas D., et al. (2010a) Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 363: 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S., Mesa R., Gotlib J., Levy R., Gupta V., Dipersio J., et al. (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366: 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S., Mesa R., Gotlib J., Levy R., Gupta V., Dipersio J., et al. (2011b) Results of COMFORT-1, a randomized double-blind phase III trial of INCB18424 versus placebo for patients with myelofibrosis. J Clin Oncol 29 (Suppl): Abstract 6500. [Google Scholar]
- Verstovsek S., Odenike O., Scott B., Estrov Z., Cortes J., Thomas D., et al. (2009) Phase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/polycythemia vera myelofibrosis. Blood 114: Abstract 3905. [Google Scholar]
- Verstovsek S., Passamonti F., Rambaldi A., Barosi G., Rosen P., Levy R., et al. (2010b) Durable responses with the JAK1/ JAK2 inhibitor, INCB018424, in patients with polycythemia vera (PV) and essential thrombocythemia (ET) refractory or intolerant to hydroxyurea (HU) [abstract]. ASH Annual Meeting Abstracts 116: 313 [Google Scholar]
- Wang J., Chang T., Goldberg A., Novetsky A., Lichter S., Lipton J. (2006) Quantitative analysis of growth factor production in the mechanism of fibrosis in agnogenic myeloid metaplasia. Exp Hematol 34: 1617–1623 [DOI] [PubMed] [Google Scholar]
- Wernig G., Kharas M., Okabe R., Moore S., Leeman D., Cullen D., et al. (2008) Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 13: 311–320 [DOI] [PubMed] [Google Scholar]
- Wernig G., Mercher T., Okabe R., Levine R., Lee B., Gilliland D. (2006) Expression of JAK2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107: 4274–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Nalabolu S., Aster J., Ma J., Abruzzo L., Jaffe E., et al. (1998) FGFR1 is fused with a novel zinc-finger gene, ZnF198, in the t(8;13) leukaemia/lymphoma syndrome. Nat Genet 18: 84–87 [DOI] [PubMed] [Google Scholar]
- Xin H., Herrmann A., Reckamp K., Zhang W., Pal S., Hedvat M., et al. (2011) Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res 71: 6601–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Bruno E., Chao J., Huang S., Finazzi G., Fruchtman S., et al. (2005) Constitutive mobilization of CD34+ cells into the peripheral blood in idiopathic myelofibrosis may be due to the action of a number of proteases. Blood 105: 4508–4515 [DOI] [PubMed] [Google Scholar]