Abstract
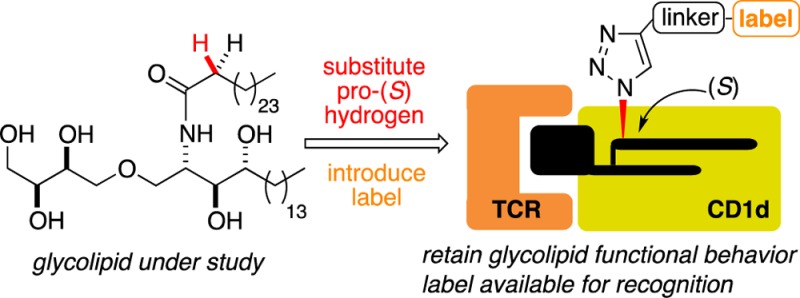
Invariant natural killer T cells (iNKT cells) are restricted by CD1d molecules and activated upon CD1d-mediated presentation of glycolipids to T cell receptors (TCRs) located on the surface of the cell. Because the cytokine response profile is governed by the structure of the glycolipid, we sought a method for labeling various glycolipids to study their in vivo behavior. The prototypical CD1d agonist, α-galactosyl ceramide (α-GalCer) 1, instigates a powerful immune response and the generation of a wide range of cytokines when it is presented to iNKT cell TCRs by CD1d molecules. Analysis of crystal structures of the TCR−α-GalCer–CD1d ternary complex identified the α-methylene unit in the fatty acid side chain, and more specifically the pro-S hydrogen at this position, as a site for incorporating a label. We postulated that modifying the glycolipid in this way would exert a minimal impact on the TCR–glycolipid–CD1d ternary complex, allowing the labeled molecule to function as a good mimic for the CD1d agonist under investigation. To test this hypothesis, the synthesis of a biotinylated version of the CD1d agonist threitol ceramide (ThrCer) was targeted. Both diastereoisomers, epimeric at the label tethering site, were prepared, and functional experiments confirmed the importance of substituting the pro-S, and not the pro-R, hydrogen with the label for optimal activity. Significantly, functional experiments revealed that biotinylated ThrCer (S)-10 displayed behavior comparable to that of ThrCer 5 itself and also confirmed that the biotin residue is available for streptavidin and antibiotin antibody recognition. A second CD1d agonist, namely α-GalCer C20:2 4, was modified in a similar way, this time with a fluorescent label. The labeled α-GalCer C20:2 analogue (11) again displayed functional behavior comparable to that of its unlabeled substrate, supporting the notion that the α-methylene unit in the fatty acid amide chain should be a suitable site for attaching a label to a range of CD1d agonists. The flexibility of the synthetic strategy, and late-stage incorporation of the label, opens up the possibility of using this labeling approach to study the in vivo behavior of a wide range of CD1d agonists.
Introduction
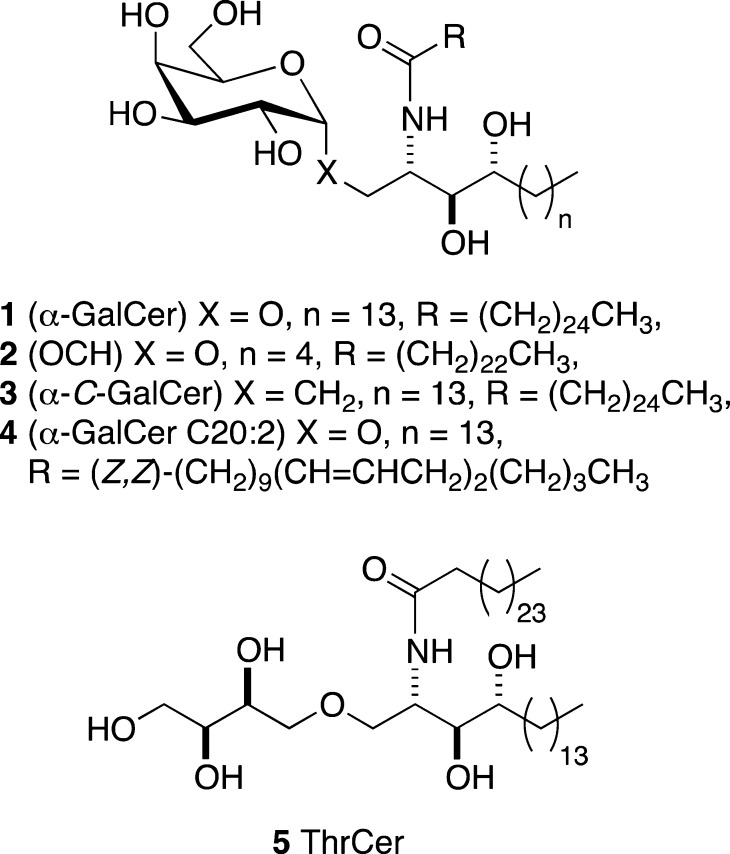
Invariant natural killer T (iNKT) cells are a distinctive subset of T lymphocytes that express an invariant αβ T cell receptor (TCR)1−3 and play an important role in infectious diseases,4,5 tumors,6,7 and autoimmune diseases,8 such as type I diabetes9,10 and lupus.11 In contrast to conventional CD4+ and CD8+ T lymphocytes, which recognize foreign peptides bound to the major histocompatibility complex (MHC) class I or MHC class II molecule, iNKT cells recognize a range of foreign and endogenous lipids bound to the nonpolymorphic CD1d molecule.12,13 α-Galactosyl ceramide (α-GalCer) 1 is the prototypical iNKT cell agonist (Figure 1). Its presentation by CD1d molecules to TCRs located on iNKT cells results in a powerful immune response involving the secretion of both pro-inflammatory cytokines, including interferon-γ (IFNγ) (Th1-type), and regulatory cytokines, including interleukin-4 (IL-4) (Th2-type).14 α-GalCer 1 has served as a valuable template for preparing structural analogues,15 such as compound OCH 2, a more Th2 cytokine-biasing CD1d agonist,16,17 and the C-glycosyl analogue of α-GalCer, 3(18,19) (Figure 1), which causes a more Th1 cytokine-biasing immune response upon iNKT cell activation.20−22 Threitol ceramide (ThrCer) 5 (Figure 1) also successfully activates iNKT cells and, significantly, overcomes the problematic iNKT cell activation-induced anergy, which is associated with iNKT cell stimulation by α-GalCer.23−25 Moreover, we have recently shown that amide isosteres of this attractive nonglycosidic CD1d agonist can also be used to bias the cytokine response toward Th1-type cytokines.26
Figure 1.
Glycolipid agonists of CD1d.
All of the aforementioned glycolipids activate iNKT cells in a similar fashion, namely through CD1d-mediated presentation to TCRs located on these cells; however, the origins of the different immune responses observed in each case remain poorly understood.27 Thus, while CD1d and TCR binding affinity likely play a role,28,29 other factors, such as the cellular location of glycolipid loading on the CD1d molecule, may also be important in determining the ensuing immune response.30 For example, the Th2 cytokine-biasing α-GalCer analogues, 2 and 4, are loaded rapidly onto CD1d molecules located at the surface of antigen-presenting cells.22,31 In contrast, the disaccharide Gal(α1→2)α-GalCer requires cellular internalization and lysosomal processing before loading can take place.32 α-GalCer 1 also exhibits a requirement for endosomal loading before surface presentation can take place, principally within lipid rafts.22,33 To investigate the cellular behavior of different CD1d agonists, we sought a method for labeling glycolipids, which would allow us to study the mechanisms that control their uptake by professional antigen-presenting cells (APC). Herein, we show that the methylene unit, and more specifically the pro-S hydrogen, α to the amide functionality provides a new site for appending a label to this class of molecules. We describe a general and flexible synthesis strategy that allows structural variation in the glycolipid as well as late-stage introduction of the label.
Experimental Procedures
General Experimental Procedures
Melting points were determined using open capillaries and are uncorrected. Infrared spectra were recorded either neat or as thin films between NaCl disks. The intensity of each band is described as s (strong), m (medium), or w (weak), with the prefix v (very) and suffix br (broad) where appropriate. 1H NMR spectra were recorded at 500, 400, or 300 MHz. 13C NMR spectra were recorded at 125, 100, or 75 MHz. Chemical shifts are reported as δ values (parts per million) referenced to the following solvent signals: CHCl3, δH 7.26; CDCl3, δC 77.0; CH3OH, δH 3.31; CD3OD, δC 49.9. For spectra recorded in a 1:2 CD3OD/CDCl3 mixed solvent system, chemical shifts are referenced to the residual methanol peak. The term “stack” is used to describe a region in which resonances arising from nonequivalent nuclei are coincident and multiplet, m, to describe a resonance arising from a single nucleus (or equivalent nuclei) but where coupling constants cannot be readily assigned. Mass spectra were recorded utilizing electrospray ionization (and a MeOH mobile phase) and are reported as m/z (%). HRMS spectra were recorded using a lock mass incorporated into the mobile phase.
All reagents were obtained from commercial sources and used without further purification unless stated otherwise. Anhydrous solvents were stored over 4 Å molecular sieves and under an Ar atmosphere. All solutions are aqueous and saturated unless stated otherwise.
All reactions were monitored by TLC using precoated aluminum-backed ICN silica plates (60A F254) and visualized by UV detection (at 254 nm) and staining with 5% phosphomolybdic acid in EtOH (MPA spray). Column chromatography was performed on silica gel (particle size of 40–63 μm mesh).
(2″S)-Biotinylated ThrCer [(S)-10]
A CuSO4 solution (12 μL of a 0.5 M solution, 6 μmol) and a sodium ascorbate solution (26 μL of a 1.0 M solution, 26 μmol) were added to a solution of azide (S)-17 (25 mg, 0.029 mmol) and alkyne 18 (13 mg, 0.029 mmol) in a t-BuOH/H2O mixture (1 mL, 1:1) at room temperature. The reaction mixture was heated for 10 h at 50 °C, diluted with CHCl3 (10 mL), and washed with brine (3 mL). The phases were separated, and the aqueous layer was extracted with CHCl3 (2 × 5 mL). The combined organic layers were dried over Na2SO4, and the volatiles were removed under reduced pressure. Purification of the residue by flash column chromatography (10% MeOH in CHCl3) afforded triazole (S)-10 as a white paste (28 mg, 77%): Rf = 0.20 (10% CH3OH in CHCl3); the poor solubility of this amphiphilic compound at room temperature prevented us from obtaining reliable optical rotation data; νmax (film) 3332br (O–H, N–H), 1672s (C=O) cm–1; 1H NMR (500 MHz, CDCl3/CD3OD, 2:1) δ 0.84 (t, J = 6.9 Hz, 6H, 2 × CH2CH3), 1.17–1.36 (stack, 70H), 1.38–1.46 (stack, 2H), 1.52–1.75 [stack, 4H, C(12‴)H2 (middle of stack), C(14‴)HaHb (LHS of stack), C(14‴)HaHb (RHS of stack)], 2.01–2.09 [stack, 1H, C(3′′)HaHb], 2.10–2.22 [stack, 3H, C(3′′)HaHb, C(11‴)H2], 2.71 [d, J = 12.8 Hz, 1H, C(18‴)HaHb], 2.89 [dd, J = 12.8, 5.0 Hz, 1H, C(18‴)HaHb], 3.13–3.19 [m, 1H, C(15‴)H], 3.33–3.38 [stack, 2H, C(9‴)H2], 3.47–3.71 [stack, 18H, including C(1)HaHb, C(4‴)H2], 3.73 [dd, J = 10.0, 5.0 Hz, 1H, C(1)HaHb], 3.75–3.79 (m, 1H), 4.17–4.22 [m, 1H, C(2)H], 4.30 [dd, J = 7.8, 4.3 Hz, 1H, C(16‴)H], 4.48 [dd, J = 7.8, 4.8 Hz, 1H, C(17‴)H], 4.65 [s, 2H, C(3‴)H2], 5.27 [dd, J = 8.5, 6.5 Hz, 1H, C(2′′)H], 7.99 [s, 1H, C(1‴)H], exchangeable hydrogens not observed; 13C NMR (125 MHz, CDCl3/CD3OD, 2:1) δ 14.3 [CH3, C(18), C(26′′) (resonance overlap)], 23.2 (CH2), 26.1 [CH2, C(12‴)], 26.2 (CH2), 26.4 (CH2), 28.8 [CH2, C(14‴)], [29.1, 29.5, 29.87, 29.90, 30.1, 30.19, 30.22, 32.5 (CH2, alkyl chain, some resonance overlap)], 36.2 [CH2, C(11‴)], 39.8 [CH2, C(9‴)], 40.8 [CH2, C(18‴)], 51.3 [CH, C(2)], 56.3 [CH, C(15‴)], 60.9 [CH, C(17‴)], 62.6 [CH, C(16‴)], 63.9 [CH2, C(4′)], 64.7 [CH, C(2′′)], 64.8 [CH2, C(3‴)], 70.17 [CH2, C(4‴)], 70.24 (CH2), 70.5 (CH2), 70.6 (CH2), 70.8 (CH), 70.99 (CH2), 71.03 (CH2), 72.5 (CH), 72.8 (CH), 73.4 [CH2, C(1′)], 75.0 [CH, C(3)], 123.3 [CH, C(1‴)], 145.3 [C, C(2‴)], 165.1 (C, NHC=ONH), 169.1 [C, C(1′′)], 175.2 [C, C(10‴)]; MS (TOF ES+) m/z 1276.8 ([M + Na]+, 100%), 649.9 (7), 398.1 (5); HRMS (TOF ES+) calcd for C67H127N7O12SNa [M + Na]+ 1276.9161, found 1276.9166.
Fluor 488-Labeled α-GalCer (11) (1:1 mixture of regioisomers in the label)
A CuSO4 solution (6 μL of a 0.5 M solution, 3 μmol) and a sodium ascorbate solution (13 μL of a 1.0 M solution, 13 μmol) were added to a solution of azide 22 (1.4 mg, 1.7 μmol) and alkyne 23 (1.0 mg, 1.7 μmol) in a t-BuOH/H2O mixture (0.5 mL, 1:1) at room temperature. The reaction mixture was heated for 10 h at 50 °C, diluted with CHCl3 (5 mL), and washed with brine (1.5 mL). The phases were separated, and the aqueous layer was extracted with CHCl3 (2 × 2.5 mL). The combined organic layers were dried over Na2SO4, and the volatiles were removed under reduced pressure. Purification of the residue by flash column chromatography (CHCl3/MeOH/H2O, 65:25:4) afforded triazole 11 as a red solid (1.4 mg, 59%, 1:1, mixture of regioisomers in the label): Rf = 0.32 (CHCl3/MeOH/H2O, 65:25:4); 1H NMR (400 MHz, CDCl3/CD3OD, 2:1) δ 0.83 (t, J = 6.7 Hz, 3H), 0.84 (t, J = 6.7 Hz, 3H), 1.10–1.39 (stack, 44H), 1.40–1.65 (m, 1H), 1.65–1.89 (m, 1H), 1.92–2.03 (stack, 5H), 2.12–2.30 (1H, m), 2.66–2.79 (stack, 2H), 3.46–3.79 (stack, 22H), 3.86 (dd, J = 10.8, 4.8 Hz, 1H), 3.90 (br s, 1H), 4.12–4.22 (m, 1H), 4.55–4.67 (m, 2H), 4.88 (d, J = 3.6 Hz, 1H), 5.21–5.41 (stack, 5H), 6.63–6.77 (stack, 4H), 6.96–7.09 (stack, 2H), 7.31 (d, J = 9.0 Hz, 0.5H), 7.76 (br s, 0.5H), 7.92 (s, 1H), 8.13–8.24 (m, 1H), 8.31 (d, J = 9.0 Hz, 0.5H), 8.72 (br s, 0.5H); MS (TOF ES−) m/z 1396.8 ([M – H]−, 10%), 1218.8 (5, [M – galactose]−), 1004.6 (15), 915.6 (25), 850.7 (65), 815.7 (35), 451.5 (95), 423.5 (100), 179.1 (20, [galactose]−).
Experimental procedures and characterization data for compounds 25, 26, 13a, 13b, 14, (2S)-methyl 2-azidohexacosanoate, (S)-15, (S)-17, 18, (S)-10, (rac)-15, 17, 10, 11, 27–30, and 20 can be found in the Supporting Information.
Mice, Cell Lines, and Cultures
C57 BL/6 mice were used in accordance with the Animals (Scientific Procedures) Act 1986. iNKT cells were generated from healthy blood donors as previously described.29,34iNKT cell cultures were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640) supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 1% Pen/Strep, 5% human serum (Gibco), and 500 units of IL-2/mL. Functional assays and C1R CD1d lipid pulsing were performed in RPMI-1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 1% Pen/Strep (Gibco), and 10% fetal calf serum (FCS) (Sigma).
iNKT Cell TCR Tetramer Staining
The synthesis of soluble iNKT TCR heterodimers has been described previously.29 C1R CD1d cells were pulsed with different concentrations of the indicated iNKT cell agonists for 16 h. Cells were washed with a PBS/10% FCS mixture and incubated with 0.5–1.0 μg of iNKT cell TCR tetramer or equivalent amounts of streptavidin on ice for 30 min. Cells were also stained with an antibiotin FITC-conjugated antibody (Jackson Laboratories) for 30 min on ice. Cells were washed, and samples were analyzed on a FACScalibur instrument (BD). Data analysis was performed using Flowjo (Treestar).
iNKT Cell Activation and Cytokine Detection
A polyclonal human iNKT cell line, or a mouse iNKT cell hybridoma (DN32), was incubated for 36 h with monocyte-derived DCs in the presence or absence of iNKT cell agonists and the level of cytokines released by iNKT cells measured in the supernatants. The concentrations of human IFN-γ and mouse IL-2 were measured by an enzyme-linked immunosorbent assay (ELISA) as previously described.24 All samples were tested at the same time on a single ELISA plate for each assay. In addition, for in vitro activation of mouse iNKT cells, 5 × 105 splenocytes from C57 BL/6 mice were pulsed with various concentrations of lipids for 48 h. Supernatants were removed, and the presence of IFN-γ was determined by an ELISA.24
Results and Discussion
Chemical labeling studies of α-GalCer 1 have previously introduced the label at C(6)OH of the galactose residue;35,36 however, this strategy was rejected for a number of reasons. First, we desired a labeling methodology that could be employed generally for a range of CD1d agonists, including truncated sugar analogues such as ThrCer 5, which lack a C(6) tethering site.37 Second, crystal structures of C(6)-derivatized α-GalCer analogues reveal the functionality at C(6) can exploit a hydrophobic binding site in CD1d,38 which is not available to α-GalCer, and that this additional binding affects the biological activity,39 something to be avoided when trying to retain the activity of the agonist under investigation. Labels have also been appended to the terminus of the N-acyl chain of α-GalCer;40−42 however, we reasoned that a CD1d agonist modified in this fashion would result in the label being buried deep inside the A′ pocket of the protein molecule. This might significantly alter the binding conformation of the glycolipid under study, and analogues biotinylated in this way would mean the biotin label would likely be unavailable for streptavidin or antibiotin antibody recognition. Analysis of X-ray structures of the TCR−α-GalCer–CD1d complex,43 and similar ternary complexes involving α-GalCer analogues,40−42 reveals the glycolipid binds to the CD1d molecule in a similar fashion, with the N-acyl chain occupying the hydrophobic A′ pocket of the protein and the ceramide base the less voluminous F′ pocket, leaving the polar sugar residue surface-exposed for TCR recognition. For our purposes, this analysis allowed us to identify the α-methylene unit of the amide chain, and specifically the pro-S hydrogen at this position, as being amenable to substitution because this group is directed out toward bulk solvent in the ternary complex (Figure 2). We postulated that a label incorporated into this position would protrude away from the ternary TCR–glycolipid–CD1d complex without deleteriously affecting its conformation and, at the same time, also permit recognition of a tethered reporter group such as a biotin label.
Figure 2.
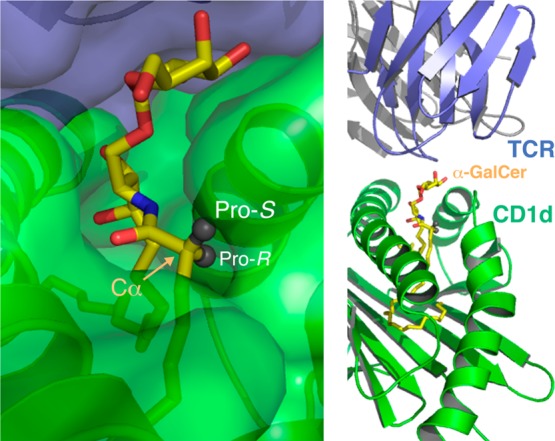
Substituents appended to the pro-S hydrogen substituent in the α position of the N-acyl chain (highlighted with the arrow) should extend beyond and away from the TCR–glycolipid–CD1d recognition site (structure taken from Protein Data Bank entry 2PO6(43)).
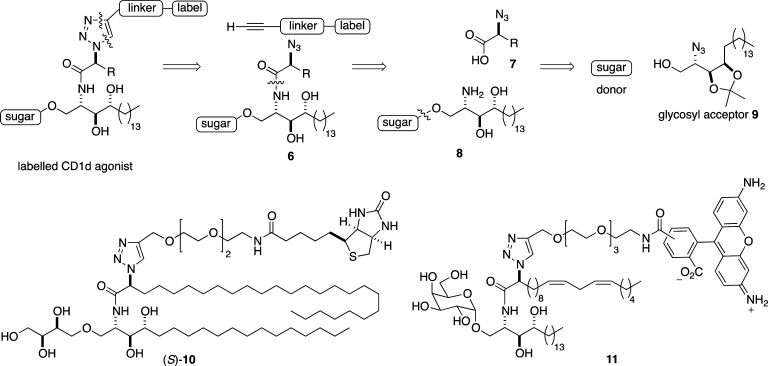
Because of the challenging practical issues associated with handling glycolipids, and the high cost of many labels, our chosen synthetic strategy for the two targets, (S)-10 [note that the (S) label denotes the absolute configuration of the stereogenic center located α to the amide carbonyl group] and 11, would incorporate the label at a late stage of the synthesis and use Click chemistry to assemble the components, ideally using an already fully deprotected glycolipid (Scheme 1). An oligo(ethylene glycol) spacer unit would be employed as a linker,44 to ensure the label extends sufficiently from the TCR recognition site so as not to interrupt antigen presentation. We expected such a linker might also impart favorable solubility properties on the final products.45,46 Further disconnection of amide 6 revealed α-azido acid 7, which would be coupled selectively with the amino functionality embedded in 8. Amine 8 would be assembled from phytosphingosine-derived glycosyl acceptor 9,25 and an appropriate glycosyl donor.
Scheme 1. General Retrosynthetic Strategy for the Assembly of Labeled Glycolipids and Target Molecules (S)-10 and 11.
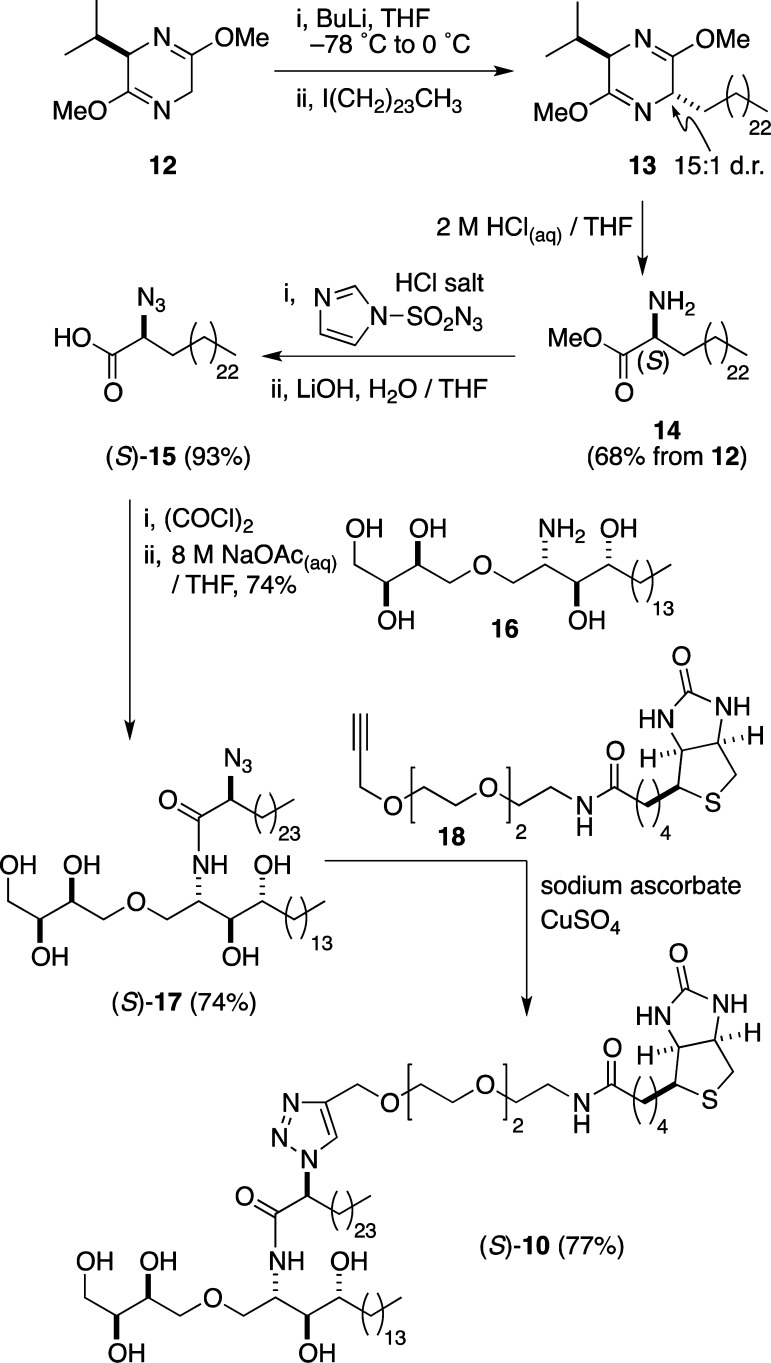
The synthesis of our first target, biotinylated ThrCer (S)-10, is detailed in Scheme 2. Enantiopure (S)-2-azidohexacosanoic acid (S)-15 was readily accessed from Schöllkopf’s auxiliary 12;47−49 thus, treatment of bis-lactim 12 with BuLi, followed by reaction of the resulting lithiated intermediate with 1-iodotetracosane,50 afforded the alkylation product as a separable 15:1 mixture of diastereoisomers. The desired major diastereoisomer 13 was then hydrolyzed to afford α-amino ester 14.49 Subsequent diazo transfer51 and ester hydrolysis49 afforded α-azido acid (S)-15, which was converted into the corresponding acid chloride and coupled with amine 16 (see the Supporting Information) under biphasic reaction conditions to afford amide (S)-17 [note that the (S) label denotes the absolute configuration of the stereogenic center located α to the amide carbonyl group] in good yield. While there is the potential for racemization of the α-azido acid chloride coupling partner, a comparison of the 13C NMR data of (S)-17 with those of (R/S)-17 [note that the (R/S) label denotes a 1:1 mixture of diastereoisomers epimeric at the stereogenic center located α to the amide carbonyl group in 17] showed that no epimerization had occurred at the α-stereogenic center under the acylation reaction conditions (see the Supporting Information). In the final step, Huisgen [3+2] dipolar cycloaddition52,53 of the azide in (S)-17 with alkyne 18 (see the Supporting Information) provided our target, biotinylated ThrCer (S)-10, in 77% isolated yield.
Scheme 2. Synthesis of Biotinylated ThrCer (S)-10.
Before progressing with biological analysis of biotinylated ThrCer (S)-10 and to assess the importance of obtaining the correct stereochemistry at the tethering site, we used racemic 2-azidohexacosanoic acid in the synthetic sequence summarized in Scheme 2 to access the diastereoisomeric end product (R)-10 [epimeric at the tethering site; the (R) label denotes the absolute configuration of the stereogenic center located α to the amide carbonyl group], which could be separated from its epimer (S)-10 by careful column chromatography.
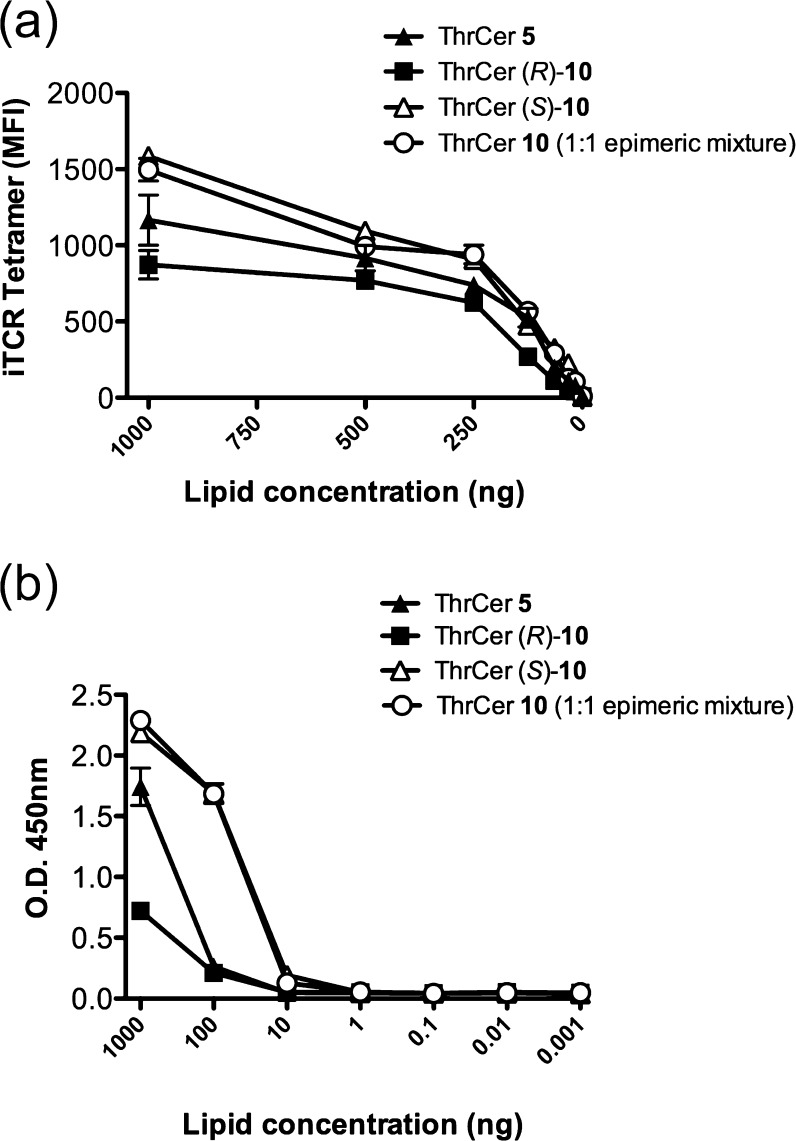
A 1:1 epimeric mixture (S/R)-10 and the single epimers of biotinylated ThrCer, (S)-10 and (R)-10, were next tested separately for their ability to be loaded onto human CD1d molecules as defined by staining with tetrameric human iNKT cell TCR29,54 (Figure 3a) and recognized by murine iNKT cells (Figure 3b).23 In the first experiment, C1R-human CD1d cells were loaded with ThrCer 5 and the corresponding biotinylated analogues and then incubated with fluorescent human iNKT cell TCR. Subsequent fluorescence-activated cell sorting (FACS) analysis revealed that (S)-10 and the epimeric mixture behaved like unlabeled ThrCer 5 in their ability to be recognized by the iNKT cell TCR, while the activity of the (R)-epimer, (R)-10, used on its own, was slightly attenuated (Figure 3a). In a second assay, C1R-mouse CD1d cells were first loaded with ThrCer 5 and the corresponding biotinylated analogues and then cultured overnight in the presence of iNKT cell hybridoma (DN32). iNKT cell activation was assessed by measuring interleukin-2 (IL-2) production by an ELISA.55,56 Once again, and in line with our predictions, the two epimeric biotinylated ThrCer analogues behaved differently in their ability to activate iNKT cells, and in accord with our hypothesis, the labeled analogue, possessing the (S)-configuration at the tethering site, behaved significantly better than its (R)-epimer (Figure 3b). In this experiment, the more active labeled epimer, (S)-10, appears to show greater activity than unlabeled ThrCer itself at high lipid concentrations. Modifying the structure of any agonist is likely to have some impact on the activity, and in this experiment, our labeled analogue is no different. The greater activity compared with that of its unlabeled analogue (ThrCer) in this assay is interesting, and we postulate that differences in lipid processing (i.e., uptake and presentation on CD1d molecules) of murine and human APCs and the subsequent iNKT cell TCR may explain these observations. Although there was a difference in activity between the two epimers, the biological results using a 1:1 epimeric mixture (in which the concentration of the more active epimer is halved) proved to be similar to those using the single epimer (S)-10, and for this reason, all subsequent experiments described below were performed with the more readily accessed epimeric mixture.
Figure 3.
iNKT cell recognition of ThrCer 5 and biotinylated ThrCer analogues, tested as single epimers (S)-10 and (R)-10 and as a 1:1 mixture. (a) Recognition of ThrCer and biotinylated analogues by human iNKT TCR assessed by FACS analysis following co-incubation of fluorescent human iNKT cell TCR and C1R cells loaded with indicated lipids. (b) Activation of iNKT cell hybridoma following overnight culture with dendritic cells loaded with ThrCer and biotinylated analogues as determined by IL-2 production in the supernatant. Data presented are means of triplicate wells and are representative of three independent experiments.
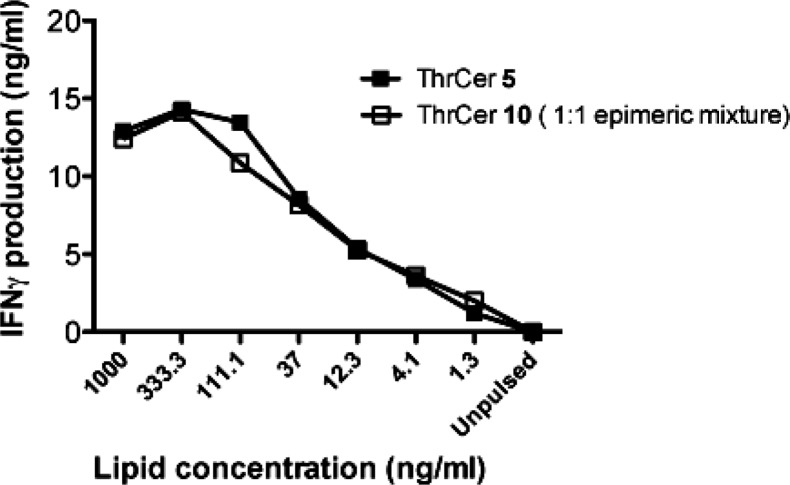
Further experiments using human iNKT cells confirmed that the presence of the biotin label does not affect optimal loading onto CD1d molecules and presentation to human iNKT cells. To this end, C1R-hCD1d cells were pulsed with labeled (10) and unlabeled ThrCer (5), and their ability to be recognized by iNKT cells was assessed by measuring the release of IFN-γ by an ELISA after the cells had been cultured for 36 h (Figure 4). The similar response profile observed with ThrCer 5 and its biotinylated analogue (10) indicated that human iNKT cells are sensitized to a similar level, and that the biotin label does not affect significantly CD1d loading or iNKT cell presentation.
Figure 4.
In vitro activation of human iNKT cells by ThrCer 5 and biotinylated ThrCer 10 (epimeric mixture). A human iNKT cell line was incubated with C1R CD1d cells pulsed either with ThrCer 5 (■) or with biotinylated ThrCer 10 (epimeric mixture) (□). IFN-γ secretion was analyzed after 36 h by an ELISA. The results are representative of three separate experiments.
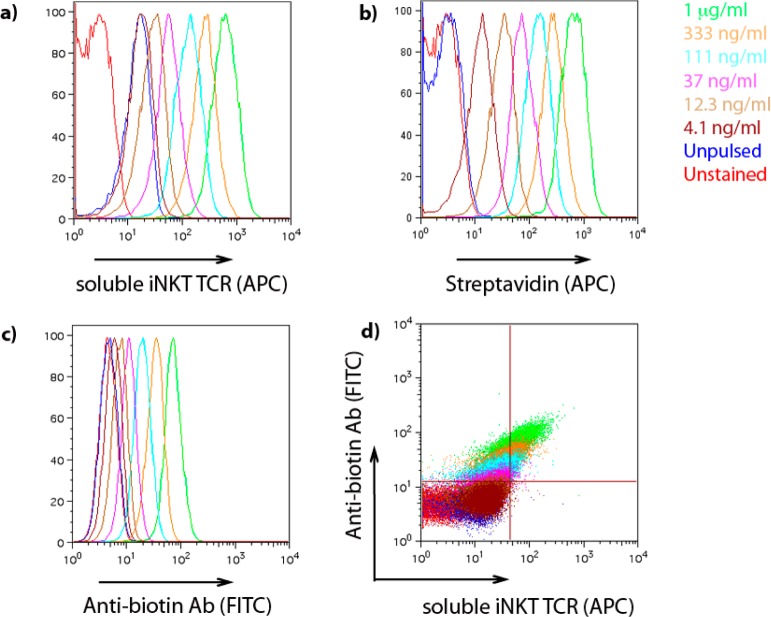
To further investigate the availability of the biotin on the cell surface of the APC, C1R CD1d cells were pulsed with biotinylated ThrCer 10 for 16 h before being stained with fluorescent streptavidin (Figure 5b), the antibiotin antibody (Figure 5), or soluble iNKT cell TCR (Figure 5a).29 These results demonstrate that (i) the label can be detected by these two commonly used detection methods, opening up the possibility of using these as staining reagents to identify biotinylated ThrCer-pulsed cells, (ii) the TCR recognizes the labeled glycolipid in the context of CD1d molecules, and (iii) we are able to perform double staining using both soluble iNKT cell TCR and antibiotin antibody showing that the glycolipid–CD1d complex and label can be detected at the same time (Figure 5d).
Figure 5.
Detection of biotinylated ThrCer 10 (1:1 epimeric mixture) on the surface of APCs. C1R CD1d cells were pulsed with the indicated concentrations of biotinylated ThrCer for 16 h and then stained with (a) soluble iNKT cell TCR (APC), (b) fluorescent streptavidin (APC), or (c) the antibiotin antibody [labeled with fluorescein isothiocyanate (FITC)]. Panels a–c depict histogram overlays of the indicated fluorescence intensities. The dot plot shown in panel d depicts a double staining with the soluble iNKT cell TCR (APC) and antibiotin antibody (FITC). The results are representative of two separate experiments.
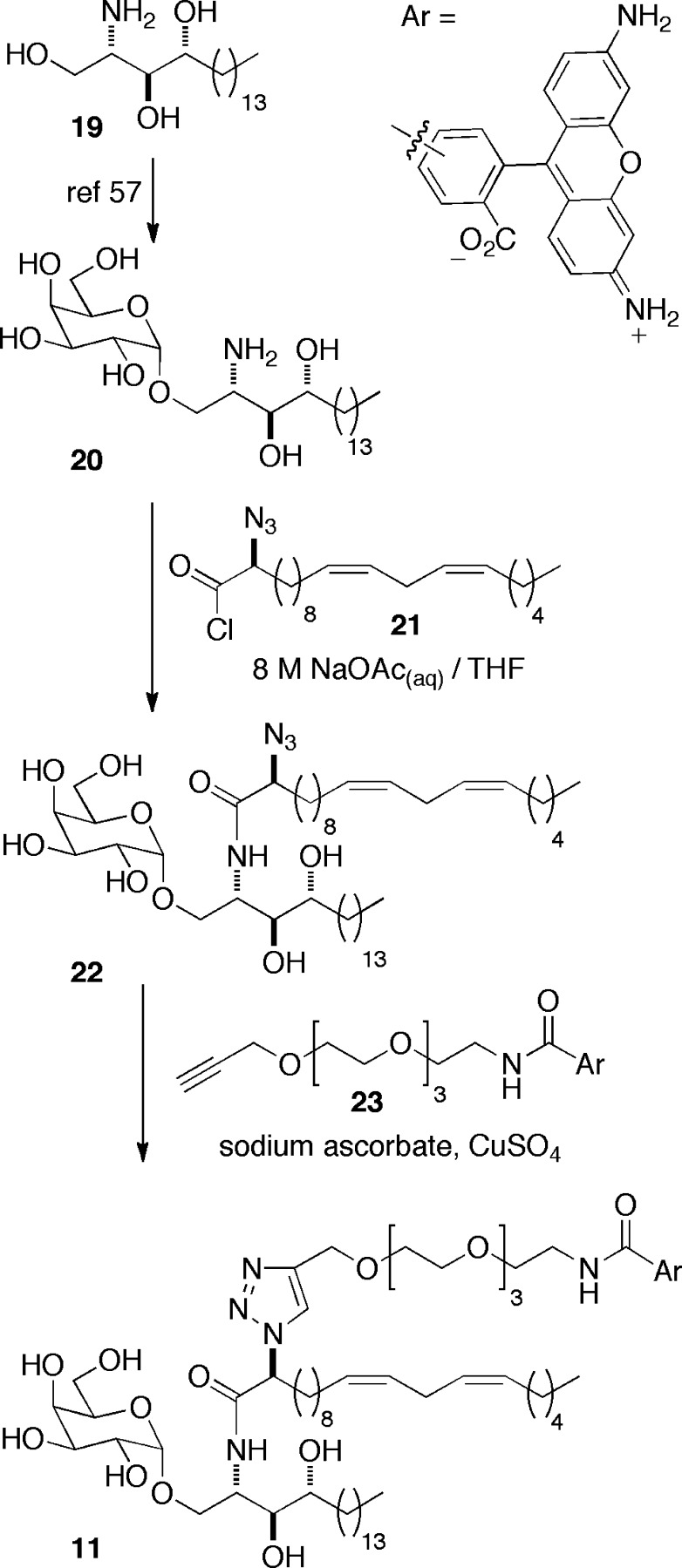
Next, to assess the generality of our glycolipid labeling strategy, we chose to introduce a fluorescent label into a second CD1d agonist, namely the Th2 cytokine-biasing CD1d agonist α-GalCer C20:2 (4). The synthesis of labeled α-GalCer C20:2, 11, is summarized in Scheme 3. α-Galactoside 20, prepared in six steps from phytosphingosine 19 using our recently developed methodology for accessing 6″-azido-6″-deoxy-α-GalCer analogues,57 was coupled with enantiomerically pure α-azido acid chloride 21, accessed from the Schöllkopf auxiliary and linoleyl bromide (see the Supporting Information), to provide amide 22 as an advanced intermediate in excellent yield. Subsequent Click reaction with alkyne 23 afforded our target, fluorescently labeled α-GalCer C20:2, 11.
Scheme 3. Synthesis of Fluor 488-Labeled α-GalCer C20:2 11.
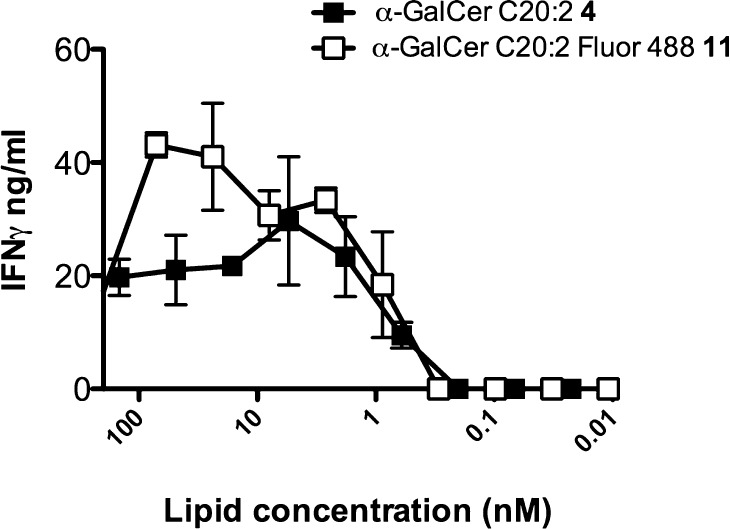
The ability of α-GalCer C20:2 (4) and labeled α-GalCer C20:2 (11) to activate murine iNKT cells was assessed (Figure 6).24 To this end, mouse splenocytes were cultured separately with various concentrations of α-GalCer C20:2 4, and the corresponding Fluor 488-labeled analogue 11, and then the presence of IFN-γ was detected in the culture supernatant by an ELISA. As for the biotinylated ThrCer analogue, these results showed that the label does not significantly affect the behavior of α-GalCer C20:2 in its ability to activate murine iNKT cells, especially at low biologically relevant concentrations of the glycolipid, opening up the possibility of using this labeled analogue to study the biology of the important Th2 cytokine-biasing CD1d agonist α-GalCer C20:2 4.
Figure 6.
In vitro activation of iNKT cells by Fluor 488-labeled α-GalCer C20:2 11. Splenocytes from C57 BL/6 mice were cultured in the presence of various concentrations of either unlabeled 4 or Fluor 488-labeled α-GalCer C20:2 11 for 48 h. The supernatants were then analyzed for the presence of IFN-γ by an ELISA. Data are means of duplicate wells and are representative of two independent experiments.
Conclusions
In summary, we have shown that the pro-S hydrogen site in the α-methylene of the N-acyl chain of two important CD1d agonists, namely, ThrCer 5 and α-GalCer C20:2 4, can be used to append a label. In the two examples targeted, the functional activity of the labeled molecules was comparable in the human and mouse systems to that displayed by the unlabeled molecules, which should allow us to use these labeled analogues to study their trafficking behavior in vivo. The synthesis of both epimers of the biotinylated ThrCer analogue confirmed the importance of choosing the correct configuration at the tethering site, which was in accord with our predictions based on X-ray crystallographic structure analyses of the TCR−α-GalCer–CD1d ternary complex. Moreover, we were able to show for the first time that a biotin label attached to ThrCer remains accessible even when the soluble iNKT TCR is bound to the CD1d–lipid complex, as demonstrated by the double staining with soluble iNKT TCR and an antibiotin antibody. Because our synthetic strategy allows the incorporation of any sugar headgroup and fatty acid acyl chain and involves the late-stage introduction of the label from an advanced intermediate, we expect other CD1d agonists (and potentially other bioactive glycolipids) can be labeled using this approach.
Acknowledgments
G.S.B. acknowledges support in the form of a Personal Research Chair from Mr. James Bardrick and a Royal Society Wolfson Research Merit Award and as a former Lister Institute-Jenner Research Fellow; The Wellcome Trust (084923/B/08/Z) for funding (to P.J.J., H.G., and J.-P.J.); and The University of Birmingham for studentships (to J.W. and Y.R.G.D.). The NMR spectrometers used in this research were funded in part through Birmingham Science City: Innovative Uses for Advanced Materials in the Modern World (West Midlands Centre for Advanced Materials Project 2), with support from Advantage West Midlands and part-funded by the European Regional Development Fund.
Glossary
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyante
- α-GalCer
α-galactosyl ceramide
- IFN
interferon
- IL
interleukin
- iNKT
invariant natural killer T
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- OD
optical density
- PBS
phosphate-buffered saline
- TCR
T cell receptor
- ThrCer
threitol ceramide
- Th1
type 1 helper T cell
- Th2
type 2 helper T cell
Supporting Information Available
Experimental procedures and full characterization data for compounds 25, 26, 13a, 13b, 14, (2S)-methyl 2-azidohexacosanoate, (S)-15, (S)-17, 18, (S)-10, (rac)-15, 17, 10, 11, 27–30, and 20; statistical analysis of biological data summarized in Figure 3; and scanned copies of 1H NMR and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
∥ J.W.: WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral St., Glasgow G1 1XL, U.K.
Author Present Address
⊥ Y.R.G.D.: School of Chemistry, Vanderbilt University, 7940 Stevenson Center, Stevenson Center Lane, Nashville, TN 37235.
Author Contributions
P.J.J. and P.P. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Stroynowski I. (1990) Molecules related to class-I major histocompatibility complex antigens. Annu. Rev. Immunol. 8, 501–530. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I.; Stankovic S.; Baxter A. G. (2010) Raising the NKT cell family. Nat. Immunol. 11, 197–206. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I.; MacDonald H. R.; Kronenberg M.; Smyth M. J.; van Kaer L. (2004) NKT cells: What’s in a name?. Nat. Rev. Immunol. 4, 231–237. [DOI] [PubMed] [Google Scholar]
- Brigl M.; Brenner M. B. (2010) How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 22, 79–86. [DOI] [PubMed] [Google Scholar]
- Tupin E.; Kinjo Y.; Kronenberg M. (2007) The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A.; Terabe M. (2009) The contrasting roles of NKT cells in tumor immunity. Curr. Mol. Med. 9, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M.; Berzofsky J. A. (2007) NKT cells in immunoregulation of tumor immunity: A new immunoregulatory axis. Trends Immunol. 28, 491–496. [DOI] [PubMed] [Google Scholar]
- Wu L.; Van Kaer L. (2009) Natural killer T cells and autoimmune diseases. Curr. Mol. Med. 9, 4–14. [DOI] [PubMed] [Google Scholar]
- Ly D.; Tohn R.; Rubin B.; Blumenfeld H.; Besra G. S.; Veerapen N.; Porcelli S. A.; Delovitch T. L. (2010) An α-galactosylceramide C20:2 N-acyl variant enhances anti-inflammatory and regulatory T-cell independent responses that prevent type 1 diabetes. Clin. Exp. Immunol. 160, 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Q. A.; Ly D.; Zucker P.; McGarry M.; Delovitch T. L. (2004) Interleukin-4 but not interleukin-10 protects against spontaneous and recurrent type 1 diabetes by activated CD1d-restricted invariant natural killer T-cells. Diabetes 53, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Forestier C.; Molano A.; Im J. S.; Dutronc Y.; Diamond B.; Davidson A.; Illarionov P. A.; Besra G. S.; Porcelli S. A. (2005) Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black × New Zealand White) F1 mice. J. Immunol. 175, 763–770. [DOI] [PubMed] [Google Scholar]
- Kobayashi E.; Motoki K.; Yamaguchi Y.; Uchida T.; Fukushima H.; Koezuka Y. (1996) Enhancing effects of α-,β-monoglycosylceramides on natural killer cell activity. Bioorg. Med. Chem. 4, 615–619. [DOI] [PubMed] [Google Scholar]
- Balk S. P.; Bleicher P. A.; Terhorst C. (1989) Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc. Natl. Acad. Sci. U.S.A. 86, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T.; Cui J.; Koezuka Y.; Toura I.; Kaneko Y.; Motoki K.; Ueno H.; Nakagawa R.; Sato H.; Kondo E.; Koseki H.; Taniguchi M. (1997) CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629. [DOI] [PubMed] [Google Scholar]
- For a recent review, see:Banchet-Cadeddu A.; Hénon E.; Dauchez M.; Renault J.-H.; Monneaux F.; Haudrechy A. (2012) The stimulating adventure of KRN7000. Org. Biomol. Chem. 9, 3080–3104. [DOI] [PubMed] [Google Scholar]
- Miyamoto K.; Miyake S.; Yamamura T. (2001) A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534. [DOI] [PubMed] [Google Scholar]
- Oki S.; Tomi C.; Yamamura T.; Miyake S. (2005) Preferential Th2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production inflammatory cytokines by bystander cells in vivo. Int. Immunol. 17, 1619–1629. [DOI] [PubMed] [Google Scholar]
- Schmieg J.; Yang G.; Franck R. W.; Tsuji M. (2003) Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J. Exp. Med. 198, 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.; Schmieg J.; Tsuji M.; Franck R. W. (2004) The C-glycoside analogue of the immunostimulant α-galactosylceramide (KRN7000): Synthesis and striking enhancement of activity. Angew. Chem., Int. Ed. 43, 3818–3822. [DOI] [PubMed] [Google Scholar]
- Araki M.; Miyake S.; Yamamura T. (2008) Synthetic glycolipid ligands for human iNKT cells as potential therapeutic agents for immunotherapy. Curr. Med. Chem. 15, 2337–2345. [DOI] [PubMed] [Google Scholar]
- Venkataswamy M. M.; Baena A.; Goldberg M. F.; Bricard G.; Im J. S.; Chan J.; Reddington F.; Besra G. S.; Jacobs W. R.; Porcelli S. A. (2009) Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 183, 1644–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im J. S.; Arora P.; Bricard G.; Molano A.; Venkataswamy M. M.; Baine I.; Jerud E. S.; Goldberg M. F.; Baena A.; Yu K. O.; Ndonye R. M.; Howell A. R.; Yuan W. M.; Cresswell P.; Chang Y.-T.; Illarionov P. A.; Besra G. S.; Porcelli S. A. (2009) Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 30, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. D.; Salio M.; Reddy B. G.; Shepherd D.; Gileadi U.; Brown J.; Masri S. H.; Polzella P.; Ritter G.; Besra G. S.; Jones E. Y.; Schmidt R. R.; Cerundolo V. (2008) Nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J. Immunol. 180, 6452–6456. [DOI] [PubMed] [Google Scholar]
- Reddy B. G.; Silk J. D.; Salio M.; Balamurugan R.; Shepherd D.; Ritter G.; Cerundolo V.; Schmidt R. R. (2009) Nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. ChemMedChem 4, 171–178.19160440 [Google Scholar]
- Garcia-Diaz Y. R.; Wojno J.; Cox L. R.; Besra G. S. (2009) Synthesis of threitol ceramide and [14C]threitol ceramide, non-glycosidic analogues of the potent CD1d antigen α-galactosyl ceramide. Tetrahedron: Asymmetry 20, 747–753. [Google Scholar]
- Wojno J.; Jukes J.-P.; Ghadbane H.; Shepherd D.; Besra G. S.; Cerundolo V.; Cox L. R. (2012) Amide analogues of CD1d agonists modulate iNKT-cell-mediated cytokine production. ACS Chem. Biol. 7, 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B. A.; Nagarajan N. A.; Wingender G.; Wang J.; Scott I.; Tsuji M.; Franck R. W.; Porcelli S. A.; Zajonc D. M.; Kronenberg M. (2010) Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 184, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S.; Chiba A.; Yamamura Y.; Miyake S. (2004) The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J. Clin. Invest. 113, 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C.; Shepherd D.; Fleire S.; Stronge V. S.; Koch M.; Illarionov P. A.; Bossi G.; Salio M.; Denkberg G.; Reddington F.; Tarlton A.; Reddy B. G.; Schmidt R. R.; Reiter Y.; Griffiths G. M.; Van Der Merwe P. A.; Besra G. S.; Jones E. Y.; Batista F. D.; Cerundolo V. (2007) The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 204, 1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataswamy M. M.; Porcelli S. A. (2010) Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin. Immunol. 22, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. O. A.; Im J. S.; Molano A.; Dutronc Y.; Illarionov P. A.; Forestier C.; Fujiwara N.; Arias I.; Miyake S.; Yamamura T.; Chang Y. T.; Besra G. S.; Porcelli S. A. (2005) Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl. Acad. Sci. U.S.A. 102, 3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozy T. I.; Naidenko O.; Qasba P.; Elewaut D.; Brossay L.; Khurana A.; Natori T.; Kozeuka Y.; Kulkarni A.; Kronenberg M. (2001) Glycolipid antigen processing for presentation by CD1d molecules. Science 291, 664–667. [DOI] [PubMed] [Google Scholar]
- Park Y.-K.; Lee J.-W.; Ko Y.-G.; Hong S.; Park S.-H. (2005) Lipid rafts are required for efficient signal transduction by CD1d. Biochem. Biophys. Res. Commun. 327, 1143–1154. [DOI] [PubMed] [Google Scholar]
- Salio M.; Shepherd D.; Dunbar P. R.; Palmowski M.; Murphy K.; Wu L. J.; Cerundolo V. (2001) Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J. Immunol. 167, 1188–1197. [DOI] [PubMed] [Google Scholar]
- Xia C.; Zhang W.; Zhang Y.; Woodward R. L.; Wang J.; Wang P. G. (2009) Facile synthesis of biotin labelled α-galactosylceramide as antigen for invariant natural killer T cells. Tetrahedron 65, 6390–6395. [Google Scholar]
- Zhou X.-T.; Forestier C.; Goff R. D.; Li C.; Teyton L.; Bendelac A.; Savage P. B. (2002) Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6″-amino-6″-deoxy-galactosylceramides. Org. Lett. 4, 1267–1270. [DOI] [PubMed] [Google Scholar]
- All three alcohol functionalities in ThrCer are important for TCR recognition (unpublished results).
- Aspeslagh S.; Li Y.; Yu E. D.; Pauwels N.; Trappeniers M.; Girardi E.; Decruy T.; Van Beneden K.; Venken K.; Drennan M.; Leybaert L.; Wang J.; Franck R. W.; Van Calenburgh S.; Zajonc D. M.; Elewaut D. (2011) Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 30, 2294–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappeniers M.; Van Beneden K.; Decruy T.; Hillaert U.; Linclau B.; Elewaut D.; Van Calenbergh S. (2008) 6′-Derivatised α-GalCer analogues capable of inducing strong CD1d-mediated Th1-biased NKT cell responses in mice. J. Am. Chem. Soc. 130, 16468–16469. [DOI] [PubMed] [Google Scholar]
- Sakai T.; Ehara H.; Koezuka Y. (1999) Synthesis of NBD-α-galactosylceramide and its immunologic properties. Org. Lett. 1, 359–361. [DOI] [PubMed] [Google Scholar]
- Sakai T.; Naidenko O. V.; Iijima H.; Kronenberg M.; Koezuka Y. (1999) Syntheses of biotinylated α-galactosylceramides and their effects on the immune system and CD1 molecules. J. Med. Chem. 42, 1836–1841. [DOI] [PubMed] [Google Scholar]
- Vo-Hoang Y.; Micouin L.; Ronet C.; Gachelin G.; Bonin M. (2003) Total enantioselective synthesis and in vivo biological evaluation of a novel fluorescent BODIPY α-galactosylceramide. ChemBioChem 4, 27–33. [DOI] [PubMed] [Google Scholar]
- Borg N. A.; Wun K. S.; Kjer-Nielsen L.; Wilce M. C.; Pellicci D. G.; Koh R.; Besra G. S.; Bharadwaj M.; Godfrey D. I.; McCluskey J.; Rossjohn J. (2007) CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49. [DOI] [PubMed] [Google Scholar]
- Poly(ethylene glycol) linkers are commonly used. See, for example:Natarajan A.; Du W.; Xiong C.-Y.; DeNardo G. L.; DeNardo S. J.; Gervay-Hague J. (2007) Construction of Di-scFv through a trivalent alkyne-azide 1,3-dipolar cycloaddition. Chem. Commun. 695–697. [DOI] [PubMed] [Google Scholar]
- Veronese F. M.; Pasut G. (2005) PEGylation, successful approach to drug delivery. Drug Discovery Today 10, 1451–1458. [DOI] [PubMed] [Google Scholar]
- Zalipsky S., and Harris J. M. (1997) in Introduction to chemistry and biological applications of poly(ethylene glycol), ACS Symposium Series, Vol. 680, pp 1–13, American Chemical Society, Washington, DC. [Google Scholar]
- Schöllkopf U.; Groth U.; Deng C. (1981) Enantioselective synthesis of (R)-amino acids using l-valine as a chiral agent. Angew. Chem., Int. Ed. 20, 798–799. [Google Scholar]
- Schöllkopf U.; Hartwig W.; Groth U. (1979) Enantioselective synthesis of α-methyl-α-aminocarboxylic acids by alkylation of the lactim ether of cyclo-(l-Ala-l-Ala). Angew. Chem., Int. Ed. 18, 863–864. [Google Scholar]
- Jones P.; Altamura S.; De Francesco R.; Paz O. P.; Kinzel O.; Mesiti G.; Monteagudo E.; Pescatore G.; Rowley M.; Verdirame M.; Steinkühler C. (2008) A novel series of potent and selective ketone histone deacetylase inhibitors with antitumor activity in vivo. J. Med. Chem. 51, 2350–2353. [DOI] [PubMed] [Google Scholar]
- Gensler W. J.; Alam I.; Prasad R. S.; Radhakrishna A. I.; Chaudhuri H. I. (1979) 3-Hydroxy-2-alkyl carboxylic acids related to mycolic acid. Tetrahedron 35, 2595–2680. [Google Scholar]
- Goddard-Borger E. D.; Stick R. V. (2007) An efficient, inexpensive and shelf-stable diazotransfer reagent: Imidazole-1-sulfonyl azide hydrochloride. Org. Lett. 9, 3797–3800. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. (2002) A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem., Int. Ed. 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. (2001) Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem., Int. Ed. 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- Salio M.; Speak A. O.; Shepherd D.; Polzella P.; Illarionov P. A.; Veerapen N.; Besra G. S.; Platt F. M.; Cerundolo V. (2007) Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. U.S.A. 104, 20490–20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O.; Bendelac A. (1994) An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J. Exp. Med. 180, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J.; Tatituri R. V. V.; Brigl M.; Kim E. Y.; Tuli A.; Sanderson J. P.; Gadola S. D.; Hsu F.-F.; Besra G. S.; Brenner M. B. (2011) Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12, 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis P. J.; Cox L. R.; Besra G. S. (2011) Synthesis of a versatile building block for the preparation of 6-N-derivatized α-galactosyl ceramides: Rapid access to biologically active glycolipids. J. Org. Chem. 76, 320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.