Background: It is unclear whether Legionella pneumophila possesses phospholipase C (PLC) activity and thereby generates 1,2-diacylglycerol.
Results: L. pneumophila possesses three secreted enzymes with PLC activity, PlcA, PlcB, and PlcC, and a plcABC mutant was attenuated in host killing.
Conclusion: L. pneumophila encodes three members of a novel PLC family contributing to virulence.
Significance: We determined PLC activity for L. pneumophila and defined the characteristics of a novel PLC family present in Legionella, Pseudomonas, and fungi.
Keywords: Bacteria, Bacterial Pathogenesis, Diacylglycerol, Phospholipase C, Virulence Factors, Galleria mellonella, Legionella, type II (Lsp) Secretion, Type IVB (Dot/Icm) Secretion, Zinc Metallophospholipase C
Abstract
Legionella pneumophila is a water-borne bacterium that causes pneumonia in humans. PlcA and PlcB are two previously defined L. pneumophila proteins with homology to the phosphatidylcholine-specific phospholipase C (PC-PLC) of Pseudomonas fluorescens. Additionally, we found that Lpg0012 shows similarity to PLCs and has been shown to be a Dot/Icm-injected effector, CegC1, which is designated here as PlcC. It remained unclear, however, whether these L. pneumophila proteins exhibit PLC activity. PlcC expressed in Escherichia coli hydrolyzed a broad phospholipid spectrum, including PC, phosphatidylglycerol (PG), and phosphatidylinositol. The addition of Zn2+ ions activated, whereas EDTA inhibited, PlcC-derived PLC activity. Protein homology search revealed that the three Legionella enzymes and P. fluorescens PC-PLC share conserved domains also present in uncharacterized fungal proteins. Fifteen conserved amino acids were essential for enzyme activity as identified via PlcC mutagenesis. Analysis of defined L. pneumophila knock-out mutants indicated Lsp-dependent export of PG-hydrolyzing PLC activity. PlcA and PlcB exhibited PG-specific activity and contain a predicted Sec signal sequence. In line with the reported requirement of host cell contact for Dot/Icm-dependent effector translocation, PlcC showed cell-associated PC-specific PLC activity after bacterial growth in broth. A PLC triple mutant, but not single or double mutants, exhibited reduced host killing in a Galleria mellonella infection model, highlighting the importance of the three PLCs in pathogenesis. In summary, we describe here a novel Zn2+-dependent PLC family present in Legionella, Pseudomonas, and fungi with broad substrate preference and function in virulence.
Introduction
Phospholipases are important enzymes that bacteria utilize to modulate the host environment into one well suited for the requirements of the pathogen at specific stages of infection. Various types of phospholipases hydrolyze unique phospholipid ester bonds. Phospholipases A (PLA)2 and B (PLB) release fatty acids from the phospholipid molecule, thereby generating lysophospholipids or glycerophosphorylcholine. The phosphodiesterases, phospholipase C (PLC) and phospholipase D (PLD), hydrolyze phosphodiester bonds to generate either 1,2-diacylglycerol (1,2-DG) and a phosphoryl alcohol or phosphatidic acid and an alcohol, respectively (1–3).
Bacteria can employ these phospholipases to accomplish manifold tasks, including the induction of host disintegration and bacterial exit or to fine-tune the host milieu (1–5). For example, Listeria monocytogenes can destroy its host membrane via the secreted PLCs PlcA and PlcB to egress from the phagosome and spread intercellularly (6–8). Another example is the type III-secreted PLA ExoU, a patatin-like protein (PLP) found in Pseudomonas aeruginosa that can act as a cytotoxin or modulate host cell signaling on a minute to minute basis. ExoU in particular also has been shown to trigger an arachidonic acid-dependent inflammatory cascade in vivo, activate several transcription factors, and promote the production of proinflammatory cytokines (5, 9). In the case of PLCs, both Clostridium perfringens α-toxin and nonhemolytic Bacillus cereus PC-PLC can trigger the arachidonic acid cascade and stimulate thromboxane or prostaglandin production to result in inflammation (10, 11). Furthermore, the Pseudomonas aeruginosa hemolytic PLC analogously activates the lipoxygenase pathway that contributes to increased vascular permeability (12). Given the fact that protein kinase C, which is activated by the PLC reaction product 1,2-DG, influences several processes including cell proliferation, it is plausible that several bacterial PLCs, such as B. cereus PLC and C. perfringens α-toxin, analogously can influence diverse cellular processes (13, 14).
Like the latter two, Legionella pneumophila, a bacterium known to cause life-threatening pneumonia, also possesses phospholipase activity. Most of its identified enzymes are PLAs that belong to three families consisting of a total of at least 15 proteins, including the GDSL enzymes (three members), the PLPs (depending upon the strain, 10–11 members), and PlaB (15, 16). L. pneumophila employs a variety of protein secretion mechanisms important to the virulence of the pathogen and a multitude of exported proteins, including the PLPs VipD, VpdA, VpdB, VpdC, which are secreted by the type IV Dot/Icm secretion system, and the GDSL proteins PlaA and PlaC, both secreted by the type II Lsp secretion system (17–30).
In addition to PLA enzymes, secreted hydrolytic activity acting upon the water-soluble phosphodiester substrate para-nitrophenylphosphorylcholine (p-NPPC) was found in L. pneumophila, which may indicate the presence of PLC activity (31). Although this activity is presumably of PLC origin, conversion of a phospholipid substrate into typical cleavage products such as 1,2-DG or phosphoryl alcohol has not yet been proven (32–35). The signal peptide-containing protein PlcA, responsible for up to ∼70% of the secreted L. pneumophila p-NPPC-hydrolase activity, was identified due to its homology to the phosphatidylcholine (PC)-specific PLC PlcC of Pseudomonas fluorescens (32, 36–38). Furthermore, an L. pneumophila Lsp type II secretion mutant is about 80–90% defective in secreted p-NPPC hydrolase activity, with export of p-NPPC hydrolase activity partially dependent (up to 30%) upon the Tat pathway (32, 36). Because of the observed difference (70 versus 30%), it is likely that PlcA is also exported via the sec system (36). The virulence factor peptidylprolyl cis-trans-isomerase Mip promotes p-NPPC hydrolase activity as well, albeit independently of PlcA (39). These data suggest that additional Lsp- and/or Mip-dependent enzymes contributing to p-NPPC hydrolysis exist in L. pneumophila. A protein homologous to PlcA was recently identified and designated PlcB (40). Whether PlcB, which contains a putative signal peptide akin to PlcA, also contributes to secreted p-NPPC hydrolase activity is currently unknown. L. pneumophila plcA and plcB single and double knock-out mutants replicate intracellularly in a manner comparable with that of wild type bacteria in macrophage, epithelial cell, and/or amoeba infection models, indicating that these enzymes are not necessary for these in vitro virulence characteristics (32, 40). Moreover, L. pneumophila plcA mutants display no attenuation compared with wild type bacteria in in vivo mouse infections (30).
To summarize, it is still unclear whether L. pneumophila possesses one or several secreted PLC activities that target phospholipids. We therefore screened the L. pneumophila genome sequence for potentially encoded PLC enzymes and found, in addition to PlcA and PlcB described previously (32, 40), one further homologous protein that we designated PlcC. In earlier studies, PlcC was found in another context and described as a cytotoxic type IVB-secreted L. pneumophila effector protein when expressed in yeast, and it was named CegC1 (29, 41–43). These three L. pneumophila genes were interesting candidates for further analysis because all of them are transcriptionally induced during host cell infection (44–46). Here we describe our observation that all three proteins exhibit PLC activity and demonstrate the importance of zinc ions for activity. Conserved amino acid residues in all of the L. pneumophila enzymes and PlcC of P. fluorescens were targeted for mutagenesis, with the finding that 15 residues are essential for PLC activity of PlcC. These residues also were completely conserved in other homologs of fungal proteins that have not yet been characterized. This set of properties, namely (a) lack of significant homology to enzymes of other PLC families, (b) considerable homology between the respective enzymes and conservation of specific essential amino acids embedded within conserved protein motifs, and (c) the necessity of Zn2+ for enzymatic activity, distinguishes these enzymes from other defined PLC groups. Therefore, we propose that the enzymes belong to a novel PLC family present in Legionella, some Pseudomonas spp., and fungi.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Growth Conditions, and Preparation of Cell Lysates and Culture Supernatants
L. pneumophila sg1 strains JR32 (47) (kindly provided by H. Shuman, Columbia University, New York) and Corby (kindly provided by K. Heuner, Robert Koch-Institut, Germany) (48) were used as wild type controls. Characteristics of isogenic L. pneumophila JR32 as well as strain Corby mutants and complementing strains are listed in supplemental Table 1. Further experimental procedures for the mutants and complementing strains generated in this study are described below. All cloning experiments and plasmid propagations were performed in Escherichia coli strain DH5α or Top10 (Invitrogen); recombinant expression of PLC proteins was done in E. coli BL21. The strains used are listed in supplemental Table 1 and were grown on buffered charcoal yeast extract (BCYE) agar and in buffered yeast extract (BYE) broth (both for Legionella) or on Luria Bertani (LB) agar and in LB broth (both for E. coli) as described previously (49).
L. pneumophila was grown routinely on BCYE agar for 2–3 days at 37 °C (50). For growth in liquid laboratory medium, L. pneumophila was inoculated at an OD660 = 0.2–0.3 and was cultured in BYE broth at 37 °C with continuous shaking at 250 rpm. Bacterial growth was checked by determining the optical density of the culture at wavelength 660 nm (OD660) using a Beckman spectrophotometer DU520 (Beckman Coulter). When needed, media were supplemented with antibiotics at final concentrations as follows: kanamycin, 25 μg/ml (E. coli 50 μg/ml); chloramphenicol, 6 μg/ml (E. coli 30 μg/ml); gentamicin, 5 μg/ml (E. coli 10 μg/ml); and hygromycin, 100 μg/ml (E. coli 150 μg/ml).
For assessment of hydrolytic activities, L. pneumophila culture supernatants were harvested at the end of exponential growth (OD660 = 2.0) by centrifugation for 5 min at 6000 × g, with subsequent concentration by MicroconTM filter tubes (10.000 NMWL (nominal molecular weight limit), Millipore) to obtain concentrated culture supernatants. Cell lysates were produced as described previously (27). Cell culture supernatants and cell lysates were stored at 4 °C overnight or at −20 °C before being subjected to activity assays.
DNA Techniques and Sequence Analysis
E. coli DH5α or BL21 were used for the propagation of recombinant plasmid DNA with backbones consisting of the following vectors: pMMB2002 (21), pLAW344 (51) (kindly provided by H. Shuman), pET160GW/D/TOPO (Invitrogen), pBCKS (Stratagene), pGEM-T Easy (Promega), and pGP172 (52) (kindly provided by S. Halbedel). Genomic and plasmid DNA were prepared, amplified, and sequenced according to standard protocols. Primers were obtained from Eurofins MWG Operon (see supplemental Table 2). Restriction enzymes were purchased from New England Biolabs. Foreign DNA was introduced into L. pneumophila strains by electroporation with an Invitrogen cell porator according to the manufacturer's specifications as described previously (27). Nucleotide and translated protein sequences were analyzed using the DNASTAR package, the NCBI website (www.ncbi.nlm.nih.gov/), ExPASy (www.expasy.ch), ClustalW2 multiple alignment, and the SignalP 4.0 server.
Cloning of L. pneumophila plcA, plcB, and plcC into Vectors for Recombinant Expression in E. coli and Complementation of L. pneumophila Mutants
To recombinantly overexpress N-terminally His6- or Strep-tagged versions of PlcA, PlcB, and PlcC, the corresponding genes were amplified using a proofreading polymerase (Pfu polymerase, Fermentas; see supplemental Table 2 for primers). The purified PCR products were ligated into the TOPO site of pET160GW/D/TOPO (Invitrogen), yielding pPA163 (plcA), pPA164 (plcB), and pMS2 (plcC). Additionally, the purified PCR products were ligated into the SacII site of pGP172 to express N-terminally Strep-tagged PlcA (pMS36), PlcB (pMS37), and PlcC (pMS38). plcA and plcB were cloned without the predicted signal peptides. To introduce amino acid exchanges into PlcC, pMS2 and pMS38 were mutated by means of site-directed PCR mutagenesis using Turbo-Pfu polymerase (Stratagene); primers are listed in supplemental Table 2. All plasmids mentioned were confirmed by sequencing prior to use.
For complementation studies of the L. pneumophila JR32 plcA, plcB, and plcC mutants, the respective PLC ORFs, including the native promoter sequences and signal peptide-encoding regions, were amplified using Pfu polymerase and cloned into the XbaI/KpnI sites of pMMB2002 (21). The resulting vectors, pMS18 (plcA), pMS19 (plcB), pMS12 (plcC), were electroporated into the respective mutant strain. All strains are listed in supplemental Table 1, and all primers and plasmids used for cloning are outlined in supplemental Table 2.
Expression of His6-tagged PlcC or Strep-tagged PlcA, PlcB, and PlcC from pET160GW/D/TOPO or pGP172
Miniprep DNA of plcA, plcB, or plcC expression plasmids was transformed into chemocompetent E. coli BL21 cells and grown in LB broth supplemented with 100 μg/ml ampicillin. After overnight incubation, cultures were diluted 100-fold in fresh broth, and the cells were grown to an OD600 of 0.6. After cooling to 18 °C, expression was induced by the addition of 1 mm isopropyl 1-thio-β-d-galactopyranoside and continued overnight. Cells were harvested by centrifugation and stored frozen until used.
For purification of Strep-tagged proteins, cells were disrupted by means of an Emulsi-Flex (Avestin). Strep-PlcC and its variants were subsequently partially purified using Strep-Tactin columns (IBA-lifesciences). Strep-PlcA/B were partially purified by using Strep-Tactin Superflow high capacity matrix (IBA-lifesciences) according to the manufacturer's recommendations.
L. pneumophila PLC Enzyme Knock-out Mutant Construction
PLC gene loci were cloned into pGEM-T Easy using primers outlined in supplemental Table 2. The resulting plasmids (pPA158 harboring the plcA locus and pMS29 harboring the plcB locus) were amplified subsequently by inverse PCR and Phusion polymerase (Finzymes) to generate XbaI recognition site-flanked linearized plasmids lacking the coding regions for plcA and plcB by using primers plcAmutXba_fw/rv and plcBmut_Xba_rf/rv. In the case of the plcC locus, SmaI restriction sites were inserted into the termini of the plcC ORF (on plasmid pPA111) by means of PCR-based site-directed mutagenesis. Using XbaI or SmaI sites, hygR (plcA), knR (plcB), and gmR (plcC) resistance cassettes were ligated into the linearized vector backbones, resulting in pPA159, pPA151, and pPA114, respectively. The gene replacement loci were then reamplified by PCR, and the resulting fragments were ligated into the EcoRV site of the suicide vector pLAW344 (51), yielding pPA160 (plcA::hygR), pPA155 (plcB::knR), and pPA127 (plcC::gmR), respectively. Knock-out mutants were generated as described elsewhere (51). In short, pPA160, pPA155, and pPA127 were electroporated into L. pneumophila JR32 and selected for CmS, SuccR, HygR, KnR, or GmR clones, respectively. For double and triple mutants, the procedure was repeated sequentially. Mutants were confirmed by PCR using internal and external flanking primer pairs.
Thin Layer Chromatography Assay for PLC Activity
10-Fold concentrated L. pneumophila supernatants, 10-fold diluted L. pneumophila cell lysates or undiluted cell lysate of E. coli expression strains (the latter adjusted to an OD 660 nm of 1) were incubated with equal volumes of a mixture containing 13.4 mm (all substrates for L. pneumophila, except when indicated for the PC substrate of E. coli preparations such as experiments shown in Fig. 1a) or 6.7 mm substrate (all substrates for E. coli unless stated otherwise; dipalmitoylphosphatidylcholine (DPPC) > 99% purity, dipalmitoylphosphatidylglycerol (DPPG) > 99% purity, dipalmitoylphosphatidylethanolamine (DPPE) > 99% purity, dipalmitoylphosphatidylserine (DPPS) > 99% purity, phosphatidylinositol (PI) ≥ 98% purity, sphingomyelin (SM) ≥ 98% purity, and cardiolipin (CL) ≥ 98% purity), 3 mm NaN3, 0.5% (v/v) Triton X-100, and 40 mm Tris-HCl, pH 7.5 (25 °C). All lipids, including standards for thin layer chromatography (TLC), were obtained from Sigma Chemicals or Avanti Polar Lipids. Prior to incubation, the lipid substrates were incubated for 15 min at 37 °C with shaking at 250 rpm and then exposed to ultrasonication (Sonoplus, Bandelin, Berlin, Germany) for 3 × 15 s with power set at 65%. Enzyme-lipid mixtures were incubated for 5 or 20 h at 37 °C and agitated at 100 rpm. DPPC was supplemented with 1 mm (Legionella) or 10 mm (E. coli) ZnCl2. Hydrolysis of all other lipid substrates was performed without the addition of Zn2+ for L. pneumophila. For the determination of substrate specificity of E. coli cell lysates expressing plcC, all substrates contained 0.1 mm ZnCl2. The presence of 1,2-DG as a marker of phospholipase C activity was determined after lipid extraction and TLC using the running solvent n-hexane-diethyl ether-glacial acetic acid (70:30:4 (v/v/v)) and staining with 0.2% naphthol blue-black (Sigma-Aldrich) as described previously (33, 53, 54). BYE broth or 40 mm Tris-HCl, pH 7.5 (25 °C), was incubated, treated like the cultures, and subsequently used as a negative control.
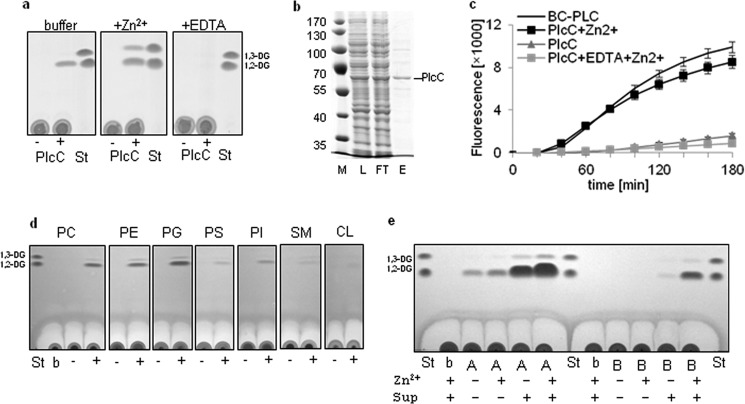
FIGURE 1.
L. pneumophila PlcC exhibits Zn2+-dependent PLC activity resulting in preferential hydrolysis of DPPC, DPPE, and DPPG. Incubation of PlcA and PlcB with L. pneumophila culture supernatant activates PLC activity. a, uninduced (−) and isopropyl 1-thio-β-d-galactopyranoside-induced (+) E. coli BL21 cell lysates expressing plcC (BL21(pMS2)) harboring an N-terminal His6 tag or buffer control (Tris-HCl) was incubated with 13.4 mm DPPC, DPPC supplemented with 10 mm ZnCl2, and DPPC supplemented with 10 mm EDTA for 20 h at 37 °C. Lipids subsequently were extracted and subjected to TLC analysis. b, lysate (L), column flow-through (FT), and eluted partially purified Strep-PlcC (E; 400 ng protein) were applied to reducing SDS-PAGE and stained with Coomassie Blue. c, 2 μg of partially purified Strep-PlcC was quantitatively assessed for PC-PLC activity compared with the positive control, 5 milliunits of B. cereus PC-PLC. Further PlcC incubations in parallel were supplemented with 0.1 mm ZnCl2 or 1 mm EDTA and incubated at 37 °C. d, cell lysates of the above mentioned isopropyl 1-thio-β-d-galactopyranoside-induced E. coli BL21 plcC expression clone were incubated with DPPC (PC), DPPE (PE), DPPG (PG), DPPS (PS), PI, SM, and CL for 20 h at 37 °C and analyzed as described in “Experimental Procedures.” All substrates were supplemented with 0.1 mm ZnCl2. e, recombinantly expressed and partially purified Strep-PlcA and Strep-PlcB (about 5 μg of protein) were incubated with DPPG for 5 h at 37 °C with or without the addition of L. pneumophila plcABC mutant culture supernatant and 0.1 mm ZnCl2. All results are representative of at least two additional experiments. St, standard; b, buffer; A, PlcA; B, PlcB.
Quantitative Assay for PC-PLC
To quantify PLC activity, the Amplex Red PC-specific PLC assay kit (Molecular Probes) was used according to the manufacturer's specifications, except that DPPC substrate (>99% purity) was prepared as described above with the modification that a final concentration of 0.2 mm was used. PC-PLC from B. cereus provided with the Amplex Red assay kit served as a positive control.
Assay for Hydrolysis of p-NPPC
The assay was performed using late logarithmic growth phase L. pneumophila JR32 wild type and PLC knock-out mutant culture supernatants (concentrated 3-fold) incubated with the same volume of 20 mm p-NPPC, 6 mm NaN3, 1% Triton X-100, and 20 mm MnCl2 in 40 mm Tris-HCl, pH 7.5 (25 °C), for up to 20 h. Subsequently, the release of yellow p-nitrophenol was read optically at a wavelength of 405 nm and compared with p-nitrophenol (Sigma-Aldrich) standard solutions (35, 55). BYE broth was used as a negative control.
Intracellular Infection of Acanthamoeba castellanii Amoebae and U937 Cells
A. castellanii amoebae (ATCC 30234) and U937 monocytes (CRL-1593.2; American Type Culture Collection, Manassas, VA), a human cell line that differentiates into macrophage-like cells upon treatment with phorbol esters (80 nm phorbol 12-mystrate 13-acetate (P-8139, Sigma) and incubation for 36–48 h), were used as hosts for in vitro infection by L. pneumophila. Amoebae and monocytes were maintained and infected as described previously (56, 57). To assess the intracellular growth of L. pneumophila, wells containing amoebae or U937 cells at concentrations of 105 amoebae/ml and 106 macrophages/ml were infected with wild type bacteria or isogenic mutants at multiplicities of infection of 0.1. Infections were performed as described previously (27).
Infection of Galleria mellonella Larvae
G. mellonella larvae were acquired from Livefoods UK and stored at room temperature in the dark until used. Bacteria were grown for 4 days on BCYE agar at 37 °C and then subsequently inoculated into BYE broth and cultured at 37 °C with shaking until late logarithmic growth phase as described by Harding et al. (58). The bacteria were then diluted in sterile PBS to an OD660 of 1.0, representing about 1 × 109 bacteria/ml. 10 μl of the suspension was used for larval inoculation as described by Harding et al. (58). 10 larvae were infected per strain, and uninfected controls and BYE/PBS-inoculated controls were incubated for up to 72 h at 37 °C. At set time points, survival of larvae was recorded.
RESULTS
Search for PLC in L. pneumophila, Homology Comparison, and General Properties of Detected Proteins
At the time this study was undertaken, it was not clear whether L. pneumophila exhibited PLC activity upon phospholipids. We proceeded to screen L. pneumophila genome sequences for potential PLC enzymes and found, in addition to the previously described PlcA and PlcB (32, 40), one additional protein, which we designated PlcC (Lpg0012). All three proteins showed homology to the PC-PLC PlcC of P. fluorescens, ranging from 21 to 43% sequence identity and 38 to 63% similarity, with PlcC being the most distant and PlcA the closest homolog. plcC was conserved in all Legionella genome sequences available to us, including six strains of L. pneumophila, two strains of Legionella longbeachae, one strain of Legionella drancourtii, and one strain of Legionella dumoffii. Interestingly, plcA was only found in L. pneumophila and plcB only in L. pneumophila and L. longbeachae (Table 1). L. pneumophila PlcC, which is annotated as a hypothetical protein bearing no conserved functional domains and shown in previous studies to be CpxR-co-regulated, was designated CegC1 (41). CegC1, which is injected into the host cell via the Dot/Icm type IVB secretion system, is toxic when expressed in yeast (29, 41–43). Important protein features and published data on the three L. pneumophila proteins are summarized in Table 2. The L. pneumophila PLC-like genes, especially plcC, were deemed good candidates for further analysis because all of them are transcriptionally induced during amoeba and macrophage infection (44–46) (Table 2).
TABLE 1.
Locus tags of PlcA-, PlcB-, PlcC-like genes in L. pneumophila and genome-sequenced non-pneumophila strains
| Species | PlcA | PlcB | PlcC |
|---|---|---|---|
| Lpn Philadelphia-1 | Lpg0502 | Lpg1455 | Lpg0012 |
| Lpn Corby | Lpc2843 | Lpc0870 | Lpc0013 |
| Lpn Lens | Lpl0541 | Lpl1573 | Lpl0012 |
| Lpn Paris | Lpp0565 | Lpp1411 | Lpp0012 |
| Lpn Alcoy | Lpa02121 | Lpa04089 | Lpa00759 |
| Lpn 130b | LPW_05821 | LPW_30711 | LPW_00111 |
| LI D-4968 | LLB_0340 | LLB_1422 | |
| Ll NSW 150 | LLO_1329 | LLO_0432 | |
| Ld LLAP12 | LDG_1325 | ||
| Fd Tex-KL | FdumT_13877 |
TABLE 2.
Protein properties and published data of the novel L. pneumophila PLC family members
| PlcA | PlcB | PlcC | |
|---|---|---|---|
| Locus tag | Lpg0502 | Lpg1455 | Lpg0012 |
| Protein size | 47, 97 kDa | 47, 23 kDa | 60, 09 kDa |
| pI | 6, 85 | 6, 74 | 6, 55 |
| Signal peptide predicted (SignalP 4.0) | Yes (22 aa) | Yes (21 aa) | No |
| Secretion type | II (Lsp), Tat/Sec | II (Lsp)? | IVB Dot/Icm |
| % GC | 38, 38 | 37, 62 | 41, 19 |
| Up-regulation during Amoeba infection | |||
| Brüggemann et al. (44) | No | Yes | Yes |
| Weissenmayer et al. (45) | Yes | Yes | Yes |
| Macrophage infection | |||
| Faucher et al. (46) | No | Yes | Yes |
| Regulated by | RpoS, Mip? (39) | CpxR-co-regulated | |
| Remarks | Not essential for in vivo mouse infection and in vitro intracellular replication in macrophages, epithelial cells, and amoebae | Not essential for host infection and in vitro intracellular replication in macrophages and epithelial cells | Toxic when expressed in yeast |
| References | (30, 32, 36, 40) | (39, 40, 96) | (29, 41–43) |
Recombinantly Expressed L. pneumophila plcC/cegC1 Exhibits Zn2+-dependent PLC Activity Selective for DPPC, DPPE, and DPPG
To determine whether the proteins exhibit PLC activity, we cloned plcA, plcB, and plcC and expressed the resulting N-terminally His-tagged variants in E. coli BL21. When cell lysates were incubated with DPPC substrate, formation of the PLC reaction product 1,2-DG was observed only for the plcC-expressing strain (Fig. 1a and data not shown). We also tested the influence of Zn2+, given that some PLC enzymes, including the PC-PLCs of C. perfringens, B. cereus, L. monocytogenes, and others, are known to harbor Zn2+ ions as co-factors (1, 59–63). As assumed, we observed an increased production of the major reaction product, 1,2-DG, and also of 1,3-DG for PlcC when Zn2+ was added (Fig. 1a). 1,3-DG likely results from intramolecular reorganization of the fatty acid chains (i.e. isomerization). Both forms of DG were observed previously due to the action of other PLCs, such as the PLC of Sinorhizobium meliloti or the PLC Rv3487c from Mycobacterium tuberculosis (64, 65). Complete inhibition of L. pneumophila PlcC enzymatic activity was noted when the chelator EDTA was added (Fig. 1a).
We further recombinantly expressed and partially purified Strep-PlcC (Fig. 1b) and analyzed the protein in a PC-based quantitative assay. Strep-PlcC showed PC-PLC activity comparable with that of the positive control B. cereus PC-PLC, and a linear increase in activity with increasing amounts of protein was found (data not shown). We confirmed that Zn2+ activated, whereas EDTA inhibited, PlcC-derived PLC activity (Fig. 1c).
Next, we evaluated the phospholipid substrate spectrum of recombinantly expressed PlcC. The majority of DG resulted from DPPC, DPPE, and DPPG substrates, with a faint band of 1,2-DG also resulting from incubation with PI, DPPS, SM, and CL. This illustrates that PlcC has a broad substrate spectrum (Fig. 1d). We additionally assessed whether PlcC shows activity toward PC with unsaturated fatty acids. 16:0/18:1-PC and 16:0/18:2-PC were hydrolyzed by PlcC with efficiency comparable to that of saturated 16:0/16:0-PC (data not shown). In summary, our results indicate that L. pneumophila PlcC possesses PLC activity largely toward phosphatidylcholine and phosphatidylethanolamine, as well as phosphatidylglycerol, and is enhanced by the co-factor Zn2+.
Recombinantly Expressed L. pneumophila plcA and plcB Exhibit Activatable PG-PLC Activity
PLC activity was not observed for either PlcA or PlcB under the conditions presented in Fig. 1a, although protein expression was confirmed (data not shown). This implied that these proteins do not exhibit PLC activity or require enzymatic activation or an unknown co-factor. Because acyltransferase/PLA activity of L. pneumophila PlaC is activated by a protease present in the bacterial culture supernatant (27, 66), we attempted to activate partially purified Strep-PlcA and -PlcB. Indeed, PlcB showed PLC activity toward PG after incubation with the L. pneumophila plcABC mutant culture supernatant. PlcA under those conditions exhibited activity without supernatant, but PLC activity was raised markedly with the addition of supernatant (Fig. 1e). We further observed that Zn2+ promoted 1,2-DG release (Fig. 1e). Only minimal activity toward PC was detected solely for PlcA (data not shown). Our results therefore show that both enzymes possess PLC activity but require activation by a so far unknown factor present in the L. pneumophila culture supernatant.
Detection of Conserved Amino Acids Important for PlcC/CegC1 PLC Activity
We next compared the L. pneumophila PlcA, PlcB, PlcC and P. fluorescens PlcC protein sequences and screened for conserved amino acid residues that may be important for PLC activity (Fig. 2). We identified and mutated the following 18 conserved residues in PlcC, with a focus on His, Phe, Asp, Arg, Tyr, Glu, and Ser because of their potential role in catalytic activity or co-factor binding: D63V, Y156A, H166N, F167A, H179N, F244A, H247N, F253A, D251V, H257N, R265Q, H284N, E286A, D314V, R326Q, S336A, R365Q, and H409N (Fig. 2) (59, 60, 62, 67, 68). After expression and partial purification of the different Strep-PlcC constructs in E. coli, 15 amino acids, namely Asp-63, Tyr-156, His-166, Phe-167, Phe-244, His-247, Asp-251, Phe-253, Arg-265, His-257, His-284, Glu-286, Asp-314, Arg-326, and Arg-385 were found to be necessary for PLC activity (Fig. 3). Expression of Strep-tagged PlcC with mutations H179N and S336A did not yield any detectable protein. When cell lysates of the His-tagged mutants were analyzed, no reduction in activity was detected, and therefore these residues were not considered essential for PlcC activity (data not shown).
FIGURE 2.
ClustalW2 protein alignment shows relatedness of L. pneumophila PlcA, PlcB, PlcC, and P. fluorescens PC-PLC and identifies conserved residues. Asterisks indicate conserved amino acid residues among the four proteins shown. Conserved residues that were analyzed experimentally are highlighted in gray. The position within the protein and the amino acid exchange in L. pneumophila PlcC is mentioned. Residues that are important for L. pneumophila PlcC PLC activity are shown in bold. (For experimental results see Fig. 3.)
FIGURE 3.
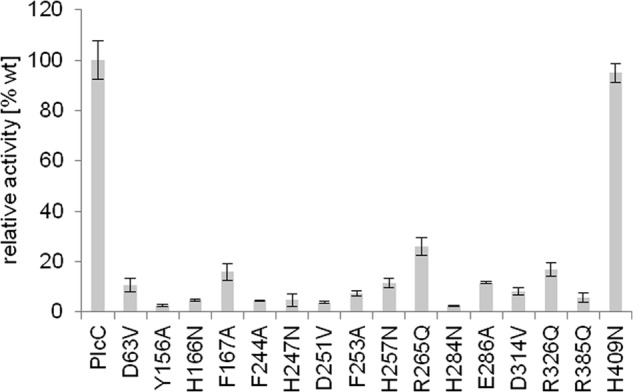
Site-directed mutagenesis of L. pneumophila PlcC identifies group-specific essential amino acids and regions for catalytic activity and/or co-factor binding. PC-PLC activity of Strep-PlcC wild type is compared with Strep-PlcC protein variants. 1 μg of each partially purified protein was incubated with 0.1 mm ZnCl2 and 0.2 mm DPPC for 90 min at 37 °C. Activities relative to wild type protein activities are shown.
The alignment shown in Fig. 2 further demonstrates that PlcC possesses a C-terminal protein extension of 107 amino acids, which spans beyond the last completely conserved amino acid, Trp-418. The extension in PlcA, PlcB, and P. fluorescens PC-PLC was only 41, 14, and 19 amino acids, respectively, which may point to the presence of a protein region specific for export of type IVB-secreted PlcC (Fig. 2). Significant protein homology to the PlcC C-terminal extension was not found in any other protein except the PlcC proteins of Legionella spp. As shown in Fig. 4, the essential amino acids were embedded in six blocks of amino acid homology with abundant conserved amino acids. Interestingly, central block III comprising the consensus signature motif F(A/T)XH(Y/F)(Y/L)XDXF(A/S)XGH, showed homology to a portion of the Zn2+-binding motif of the PC-PLCs from B. cereus, C. perfringens, and L. monocytogenes (Fig. 4) (1, 61, 62, 69–71). With the exception of block V, which is also present in C. perfringens PC-PLC, conservation of further protein stretches was not observed. Although block III might be involved in Zn2+ co-factor binding in the Legionella PLC proteins as well, a close relationship between these and the B. cereus, L. monocytogenes, or C. perfringens PLCs is not evident.
FIGURE 4.
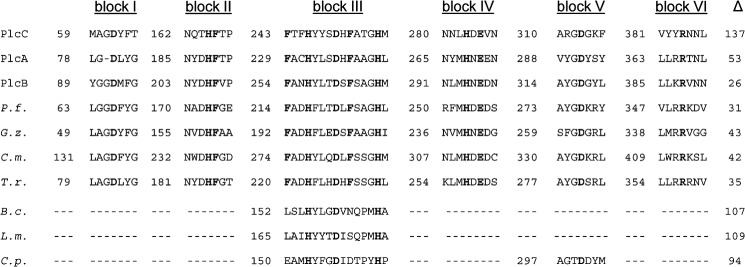
Partial amino acid sequence alignment of L. pneumophila PLC enzymes with P. fluorescens PlcC, including uncharacterized homologous proteins and protein domains/motifs essential for PLC activity. Upper panel, partial amino acid sequence alignment of L. pneumophila PlcC, PlcA, and PlcB with P. fluorescens PlcC (P.f., gi 11611251) and uncharacterized homologs of different pathogenic fungi: G. zeae (G.z., gi 46119808), C. militaris CM01 (C.m., gi 346326200), and T. rubrum (T.r., gi 327307586). Lower panel, homologous regions from characterized PLC enzymes of different bacterial species: B. cereus (B.c., gi 28414376), L. monocytogenes (L.m., gi 374922439), and C. perfringens C.p., gi 110675499). Experimentally determined essential amino acids for PlcC activity are shown in bold. Numerals indicate the number of amino acids before the start of the homologous region. The Δ column designates the length of the C-terminal domain proceeding after the last homology region.
Presence of PlcC-like Proteins in Fungi and Conservation of Essential Amino Acid Residues in Homologous Proteins
Because the presence of PlcC-like proteins among bacteria seemed limited to Legionella spp. and some Pseudomonas spp., we analyzed whether eukaryotes possessed such proteins. We found several uncharacterized fungal proteins, for example of the plant pathogen Gibberella zeae, the insect pathogen Cordiceps militaris, and the human pathogen Trychophyton rubrum, which showed conservation of the six homology blocks (Fig. 4).
After in Vitro Growth, L. pneumophila Exhibited Secreted DPPG-hydrolyzing PLC Activity Dependent upon PlcA and PlcB and Cell-associated DPPC-hydrolyzing PLC Activity conferred by PlcC/CegC1
After showing that the three recombinantly expressed PLCs were active, we tested L. pneumophila wild type and diverse PLC gene mutants for PLC activity. In pre-experiments, we examined which phospholipid substrates would uncover PLC activity and found that DPPC/Zn2+ was most appropriate for cell lysates and DPPG for culture supernatants. After incubation with the respective substrate, we detected 1,2-DG and 1,3-DG formation for wild type cell lysates as well as for plcA and plcB but not plcC knock-out mutants (Fig. 5a, left panel). This revealed that L. pneumophila indeed exhibits cell-associated PLC activity and that PlcC is responsible for that activity. Complementation of plcC knock-out mutants with plcC in trans restored the cell-associated PLC activity defect (Fig. 5a, right panel).
FIGURE 5.
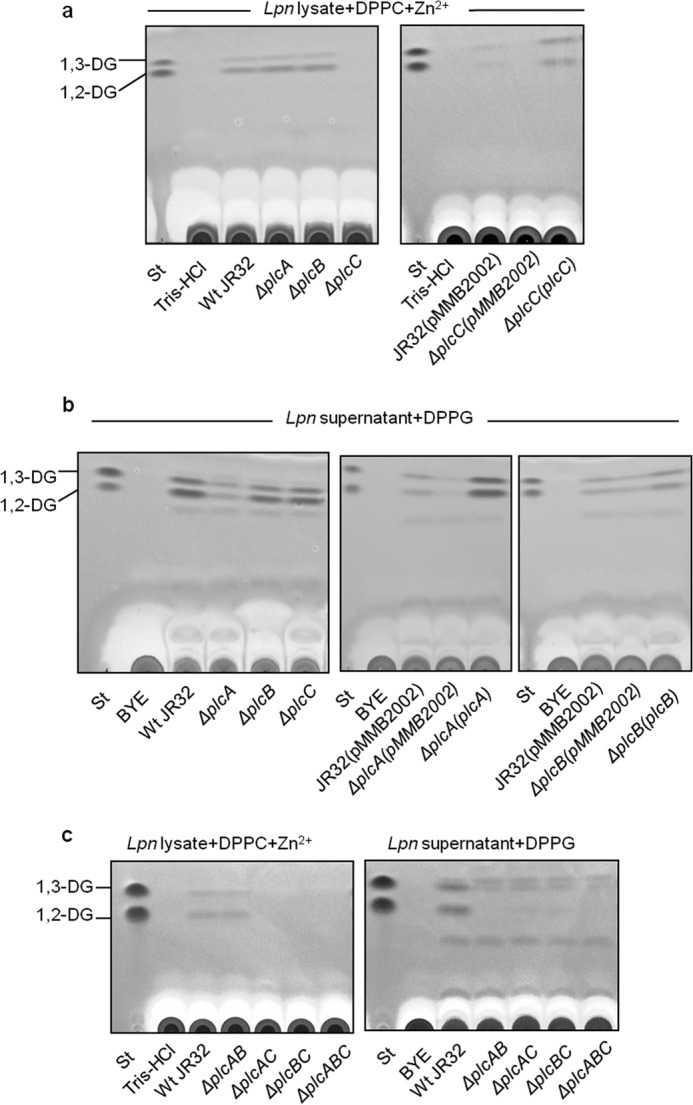
Both PlcA and PlcB contribute to the secreted DPPG-PLC activity of L. pneumophila, whereas PlcC accounts for total cell-associated DPPC-PLC. 10-Fold diluted cell lysates and 10-fold concentrated supernatants from late logarithmic phase L. pneumophila JR32 wild type and PLC single, double, and triple knock-out strains were analyzed for DG release. a, 10-fold diluted cell lysates of L. pneumophila JR32 and isogenic ΔplcA, ΔplcB, and ΔplcC mutants were incubated with DPPC/1 mm ZnCl2 for 20 h at 37 °C, and then lipids were extracted and subjected to TLC analysis. PC-PLC was completely abolished in the ΔplcC mutant (left panel). Loss of PC-PLC was complemented by introducing a vector-encoded copy of the plcC gene including its native promoter sequence (ΔplcC(plcC), right panel). b, analogous to a, corresponding 10-fold concentrated culture supernatants were incubated with DPPG, and the extracted lipids were subjected to TLC analysis. ΔplcA showed reduced PG-PLC activity, whereas secreted PG-PLC remained unaffected in the ΔplcB and ΔplcC strains. PG-PLC activity within the secreted protein fraction was increased in ΔplcA and ΔplcB strains with in trans complementation of the wild type plcA or plcB genes including the respective native promoters (right panel). c, 10-fold diluted cell lysates or 10-fold concentrated culture supernatants of double and triple mutants were assessed for PLC activity analogous to a and b. Cell-bound DPPC-PLC was abolished in ΔplcAC, ΔplcBC, and ΔplcABC stains. Within the supernatant fraction, DPPG-PLC was detected in neither ΔplcAB nor ΔplcABC, although residual 1,2-DG was found in the ΔplcAC and ΔplcBC strains. All results are representative of at least two additional experiments. St, standard.
We also detected PLC activity in L. pneumophila wild type culture supernatants. In an L. pneumophila plcA mutant, the amount of 1,2-DG generated was strongly reduced, indicating that PlcA is the most prominently secreted PLC under the conditions used (Fig. 5b, left panel). This was corroborated further by the observation that in trans complementation with plcA corrected the defect in the supernatant activity (Fig. 5b, middle panel). No clear reduction in activity was observed in plcB mutant culture supernatant (Fig. 5b, left panel). However, when we complemented a plcB mutant with plcB in trans, increased amounts of 1,2-DG and 1,3-DG were detected, indicating a positive correlation between PlcB levels and secreted PLC activity (Fig. 5b, right panel). In line with our observation that recombinantly expressed PlcA and PlcB yielded 1,2-DG generation from PG after incubation with L. pneumophila culture supernatant (Fig. 1e), our experiments in L. pneumophila further demonstrated PLC activity. Activity testing of L. pneumophila PLC double and triple knock-out mutants confirmed our earlier observations with the single mutants, namely that both secreted PlcA and PlcB contribute to generation of 1,2-DG in the supernatant and that PlcC alone is responsible for the observed DG release by cell lysate (Fig. 5c). Interestingly, a triple plcABC mutant still produced a small amount of 1,3-DG, suggesting that another unknown PLC enzyme may be responsible (Fig. 5c). In summary, we showed that L. pneumophila does indeed possess both secreted and cell-associated PLC activities, with contributions by all three PLC enzymes.
L. pneumophila-secreted PLC Activity Depends upon an Intact Type II Secretion System
Next, it was interesting to analyze the contribution of the different L. pneumophila protein secretion systems to export of PLC activity. On the one hand, we examined an L. pneumophila dotB knock-out mutant for PLC activity. Neither in the cell lysates nor in the culture supernatants did we observe a deficiency of PLC activity in the dotB mutant compared with the wild type (29, 41–43). Therefore, in line with the reported requirement of host cell contact for Dot/Icm-dependent translocation (72, 73), the effector PlcC showed cell-associated PLC activity after bacterial growth in broth. We nevertheless cannot exclude that minor PlcC-dependent PLC activity is exported by the Dot/Icm system but escaped detection, because acyl hydrolases (such as LipA or LipB (32)) may degrade the PLC reaction product, 1,2-DG. On the other hand, an L. pneumophila lspDE secretion mutant was impaired in PLC activity export, resulting in the accumulation of PLC activity as shown by the observed increase in cell-associated PLC activity (Fig. 6).
FIGURE 6.
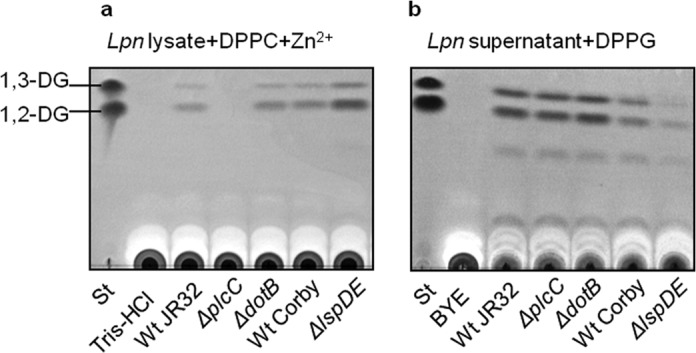
The Lsp type II secretion system is responsible for PG-PLC activity export. 10-Fold diluted cell lysates (a) and 10-fold concentrated culture supernatants (b) of late logarithmic phase L. pneumophila JR32 wild type and isogenic ΔplcC and ΔdotB mutants as well as L. pneumophila Corby and isogenic lspDE mutant were incubated with DPPC/1 mm ZnCl2 or DPPG, respectively, for 20 h at 37 °C. Lipids then were extracted and subjected to TLC analysis. A mixture of Tris-HCl buffer or BYE broth and the lipid was also incubated to serve as a negative control. The results are representative of at least two additional experiments. St, standard.
L. pneumophila plcA and plcB but Not plcC Mutants Show Reduced p-NPPC Hydrolase Activity
We were interested in how these proteins contribute to p-NPPC hydrolysis, which is often used as a proxy for the presence of PLC. Aragon et al. (32) had already demonstrated that PlcA contributes to L. pneumophila secreted p-NPPC hydrolase activity to a large extent. We found that among PLC single mutants, the plcA mutant had about 30% less secreted p-NPPC hydrolase, while the plcB mutant secreted about 20% less p-NPPC hydrolase. A PLC triple mutant showed about a 50% reduction in secreted activity (Fig. 7), suggesting the presence of p-NPPC hydrolases in addition to the PLCs described here. No significant reduction was detected for the plcC mutant. In conclusion, we demonstrated that both PlcA and PlcB contribute to secreted p-NPPC hydrolase activity, leaving open the possibility that uncharacterized p-NPPC hydrolases may exist in the culture supernatant.
FIGURE 7.
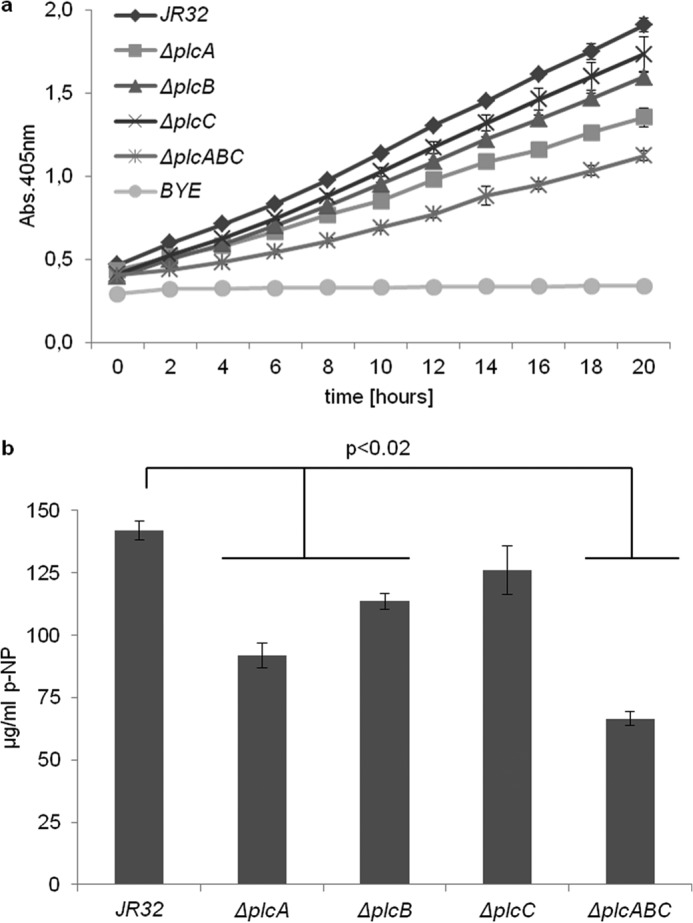
Reduced secreted p-NPPC hydrolase activities of L. pneumophila ΔplcA and ΔplcB strains. Late logarithmic culture supernatants of L. pneumophila JR32 wild type, ΔplcA, ΔplcB, ΔplcC, and ΔplcABC strains were incubated with p-NPPC for 20 h at 37 °C, and release of p-nitrophenol (p-NP) was determined. BYE broth was treated in the same way and served as the negative control. a, time course of p-nitrophenol release; b, corresponds to the 20-h time point. The results represent the means ± S.D. of triplicate samples and are representative of at least three independent experiments. ΔplcA, ΔplcB, and ΔplcABC strains were significantly different from the wild type in all experiments (p < 0.02; Student's t test, n 3).
PlcA, PlcB, and PlcC as Single Enzymes or in Combination Are Not Essential for Intracellular Replication in A. castellanii Amoebae and U937 Macrophages
Because PLC enzymes from a variety of bacterial pathogens contribute to pathogenesis, specifically intracellular survival and pathogen spread, we infected both A. castellanii amoebae and U937 macrophages with L. pneumophila wild type and isogenic plcA, plcB, and plcC single, double, and triple knock-out mutants. The wild type and all mutants replicated similarly in A. castellanii and U937 macrophages, whereas the dotA knock-out mutant exhibited delayed replication, as expected (Fig. 8 and data not shown for U937 infections). To summarize, although PlcC/CegC1 has been described as one cytotoxic effector among about 300 type IVB-secreted and more than 25 type II-secreted effector proteins (18–20, 29, 43), none of the three L. pneumophila PLCs was essential for host cell infection in the unicellular models.
FIGURE 8.
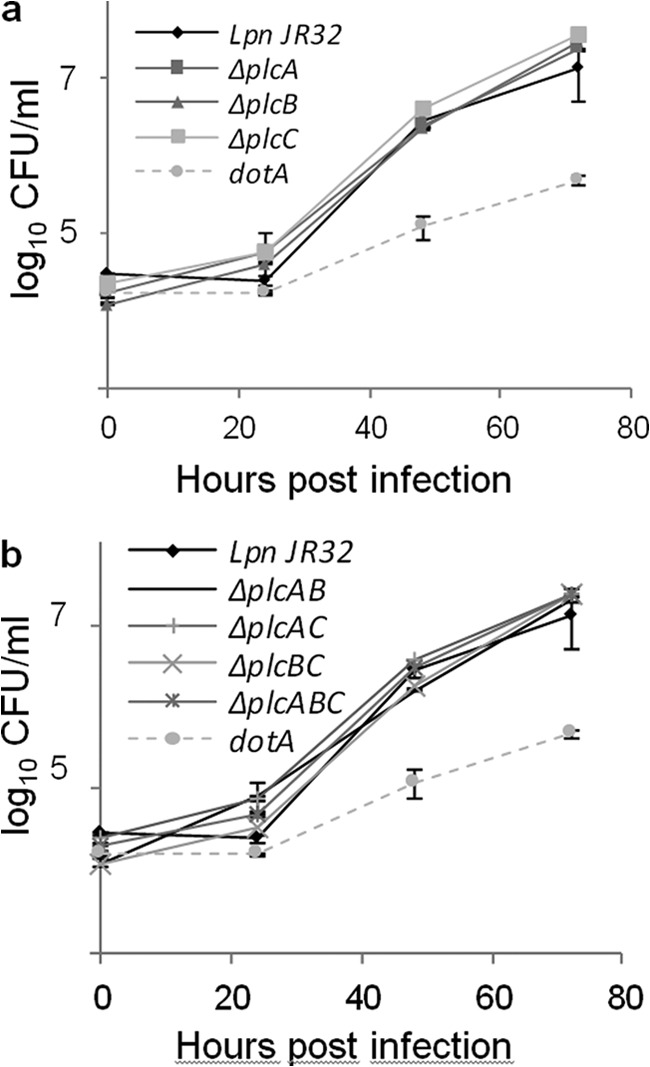
PlcA, PlcB, and PlcC, individually and in combination, are dispensable for intracellular replication of L. pneumophila in A. castellanii. a, L. pneumophila JR32 wild type as well as ΔplcA, ΔplcB, or ΔplcC strains, and b, the corresponding double and triple mutants were used to infect monolayers of A. castellanii amoebae at a multiplicity of infection of 0.1. An L. pneumophila JR32 ΔdotA strain was employed as a virulence-attenuated control. At various time points post-inoculation, bacteria were quantified by plating aliquots onto BCYE agar. Results are the means ± S.D. from duplicate samples and are representative of two independent experiments.
PLC Genes Have a Role in Host Killing in a G. mellonella Infection Model
Recently, a wax moth larvae (G. mellonella) infection model has been established for L. pneumophila. It has been demonstrated that the loss of important virulence determinants such as the Dot/Icm secretion system improves larvae survival and attenuated bacterial intracellular replication (58). Here, the triple L. pneumophila ΔplcABC mutant showed significant (p < 0.005) attenuation with respect to larvae mortality at 24 and 48 h post-infection, demonstrating that the three PLC genes have a role in the toxic effects observed with wild type L. pneumophila (Fig. 9). No defect in G. mellonella killing was observed during infection with the single or double mutants (data not shown).
FIGURE 9.
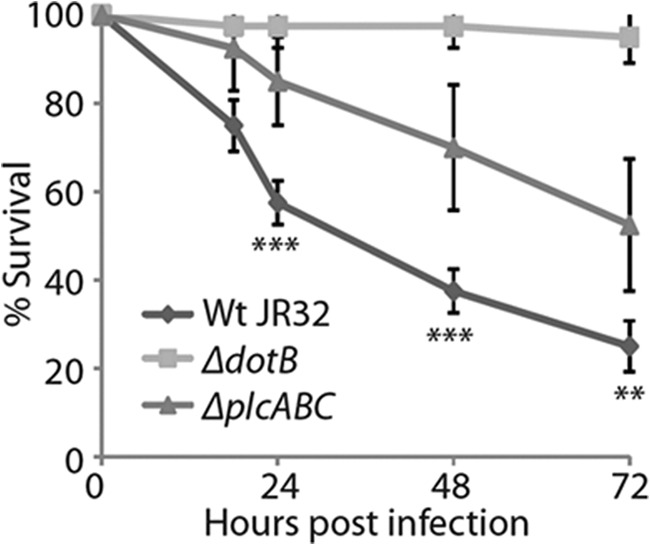
PLC activity has a role in killing of G. mellonella larvae by L. pneumophila. 107 CFU/larvae of L. pneumophila JR32 wild type and the triple ΔplcABC mutant were used to infect G. mellonella larvae, and survival of the larvae was monitored over 72 h. L. pneumophila JR32 ΔdotB strain was employed as a virulence-attenuated control. The ΔplcABC mutant was significantly (p < 0.005) attenuated in larval survival compared with the WT strain at 24 h post-infection. Results are means ± S.D. from four independent experiments. ***, p < 0.005; **, p < 0.05.
In summary, we have demonstrated for the first time PLC activity in L. pneumophila, which we attribute to three proteins, PlcA, PlcB, and PlcC/CegC1. We also describe here important conserved domains involved in their catalytic activity or co-factor binding. These three proteins additionally act as novel L. pneumophila virulence factors and represent a novel group of metallo-PLC enzymes present in some Gram-negative bacteria and fungi.
DISCUSSION
Here, we present evidence that L. pneumophila does indeed possess three PLC enzymes that release the signaling molecule 1,2-DG from phospholipids. Earlier descriptions of L. pneumophila PLC activity had left open the possibility that other enzymes, in addition to PLC, may cause the observed release of a water-soluble, tritium-labeled reaction product from phosphatidylcholine, such as PLD (release of choline) or PLA/LPLA (release of glycerophosphorylcholine) (34, 35). Indeed, the PLA/LPLA reaction product, glycerophosphorylcholine (but not phosphorylcholine) was found when phosphatidylcholine was incubated with L. pneumophila culture supernatants and may be responsible for some of the previous PLC descriptions (33). On the other hand, Aragon et al. (32) were unable to assay for PLC by monitoring 1,2-DG release due to the presence of multiple PLA, LPLA, and lipases potentially deacylating the PLC reaction product. We also hypothesized that the prominently secreted and cell-associated L. pneumophila PLA/LPLA prevented the detection of PLC reaction products. This is consistent with the observation that cell-associated PLA/LPLA PlaB knock-out mutants, when assayed for cell-associated phospholipase activity, released not only the PLA reaction products lysophosphatidylcholine and free fatty acids but also 1,2-DG. 1,2-DG was not detected, however, in wild type incubations (74).3 We attribute the detection of 1,2-DG in our study in part to the addition of Zn2+, which not only boosted L. pneumophila PLC activity but also seemed to inhibit bacterial PLA/LPLA activities.4 That Zn2+ may inhibit PLA activity has already been described for several snake venom PLA2 (75, 76). Moreover, the specific reaction conditions (e.g. choosing appropriate dilutions and reaction lengths) and the use of DPPG as a substrate further optimized PLC activity, thereby allowing detectable 1,2-DG development.
The L. pneumophila PLC enzymes described here display significant protein homology to enzymes in other Legionella species and P. fluorescens (Figs. 2 and 4 and Table 1) (32, 37, 40). Further bacterial protein homologs in addition to those found in Legionella spp. and some Pseudomonas spp. (no homolog in P. aeruginosa) were not found but were present, interestingly, in fungi, including G. zeae, C. militaris, and T. rubrum. None of these fungal proteins have been characterized yet, although they may prove to be interesting candidates for virulence factor analyses because of their potential PLC activity. The related bacterial and fungal PLC-like enzymes do not share significant homology to the well characterized family of Zn2+-dependent (broad spectrum) PC-preferring PLC (PC-PLC) enzymes of several Gram-positive bacteria, such as B. cereus, L. monocytogenes, and C. perfringens (Fig. 4) (1, 37, 61–63, 77). One important exception, however, is a short stretch of essential amino acids harboring the signature F(A/T)XH(Y/F)(Y/L)XDXF(A/S)XGH, where the histidines and the aspartate of the motif are also conserved in those Gram-positive bacterial PLCs (Fig. 4). This specific region includes residues involved in co-factor binding (1, 61, 62, 69–71). Furthermore, the PLCs described here possess no significant protein homology to the acidic phosphatase/PLC family of several Gram-negative bacteria that do not require additional metal ions for activity and that can effectively hydrolyze p-NPPC (e.g. P. aeruginosa PlcH and PlcN, Francisella tularensis AcpA, M. tuberculosis PlcA, PlcB, PlcC, and PlcD, and P. fluorescens CGDEase) (1, 37, 78–81). The lack of protein homology therefore supports the notion that these enzymes may belong to a novel family of PLC enzymes, as already suggested by Preuss et al. and Rossignol et al. (37, 38). This new family of PLCs possesses the following distinguishing characteristics: (a) it includes proteins from Gram-negative bacteria as well as fungi, (b) its members share defined blocks of amino acid homology, and (c) its members seem to require Zn2+.
Export into the bacterial supernatant, surface presentation, or injection into a eukaryotic cell is a common feature of host-targeting phospholipases. Clearly, bacteria utilize a variety of enzymes to cleave phospholipids, and the transport systems used are likewise multifaceted. For example, the pathogen P. aeruginosa employs different modes of phospholipase application, such as Sec- or Tat-dependent and subsequent Xcp type II-dependent secretion of PlcB, hemolytic PlcH, and non-hemolytic PlcN; the type III-dependent injection of the PLA cytotoxin ExoU (82–85); or type V autotransport for a second patatin-like PLA PlpD (84–89). This suggests that each specific function correlates with a corresponding unique mode of enzyme transport, which seems to be important in the case of Legionella. L. pneumophila PlcA and PlcB harbor predicted signal peptides and therefore are candidates for type II secretion after inner membrane crossing via Sec- or Tat-dependent processes (Table 2) (32, 36). It has been shown previously that PlcA secretion contributes about 50–70% to secreted p-NPPC hydrolase activity, whereas Tat- and Lsp-dependent secretion contribute 30 and 80–90%, respectively, to secreted p-NPPC hydrolase activity (32, 36). Type II-secreted enzymes such as zinc metalloproteinase ProA have been found in the Legionella phagosome (30, 90–93), so it is conceivable that PlcA and PlcB also may have a function in lipid hydrolysis within the phagosome. This may be important for phagosome remodeling, for release of signal transducers allowing intracellular replication, or even for the destruction of the membrane inclusion upon commencement of bacterial replication. It also may allow bacteria to manipulate host-signaling pathways for the purpose of directly injecting a PLC enzyme into the host cell cytosol, such as type IVB-secreted PlcC/CegC1. There are examples of bacterial phospholipases that are directly injected into the host cell, but this has been described only for PLA thus far, such as P. aeruginosa ExoU, which is injected into the host cell via type III secretion (84, 85, 94). Now that the PLC activity of the type IVB-secreted effector PlcC/CegC1 and the importance in virulence of the three PLC have been established, a variety of mechanisms of host cell modulation via the known effects of PKC or arachidonic acid cascade activation become possible (10–14, 95). However, although all three PLC enzymes have been found up-regulated during host cell infection (44–46), we did not observe an essential impact of the three PLC on infection and intracellular replication using macrophage and amoeba infection models. It remains to be elucidated in the future whether they play a role in in vivo infection models in addition to G. mellonella, as has been described for the four PLC of M. tuberculosis (77).
Why bacteria such as P. aeruginosa or M. tuberculosis express a multitude of PLC enzymes remains an unanswered question. Our work adds L. pneumophila, with its three PLC, to this list of PLC-expressing bacteria. Interestingly, these three PLC homologs were conserved in all L. pneumophila genomes, although only the type IVB-secreted PlcC was conserved in all other (currently accessible) Legionella genomes (Table 1). L. pneumophila therefore seems to harbor a variety of these enzymes, which conceivably could have related functions, although differences in secretion type and substrate preferences suggest distinct functions. This raises the question of whether type IVB effectors are more versatile than type II effectors in allowing L. pneumophila to adapt to diverse hosts and environments. The answer to this latter question may begin to shed light upon the means by which L. pneumophila ultimately causes the clinical manifestations of Legionnaires' disease.
Supplementary Material
Acknowledgments
We thank Susanne Karste and Simone Dumschat for excellent technical assistance.
This work was supported by the special research fund of the Robert Koch Institute, Germany, and by Grant DFG FL 359/6-1 from the German Research Foundation (DFG).

This article contains supplemental Tables 1 and 2.
S. Banerji and A. Flieger, unpublished observation.
P. Aurass, M. Schlegel, and A. Flieger, unpublished observation.
- PLA–D
- phospholipases A–D
- LPLA
- lysophospholipase A
- DG
- diacylglycerol
- PG
- phosphatidylglycerol
- PLP
- patatin-like protein
- p-NPPC
- para-nitrophenylphosphorylcholine
- PC
- phosphatidylcholine
- BCYE
- buffered charcoal yeast extract
- BYE
- buffered yeast extract
- DPPC
- dipalmitoylphosphatidylcholine
- DPPG
- dipalmitoylphosphatidylglycerol
- DPPE
- dipalmitoylphosphatidylethanolamine
- DPPS
- dipalmitoylphosphatidylserine
- PI
- phosphatidylinositol
- SM
- sphingomyelin
- CL
- cardiolipin.
REFERENCES
- 1. Titball R. W. (1993) Bacterial phospholipases C. Microbiol. Mol. Biol. Rev. 57, 347–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Songer J. G. (1997) Bacterial phospholipases and their role in virulence. Trends Microbiol. 5, 156–161 [DOI] [PubMed] [Google Scholar]
- 3. Schmiel D. H., Miller V. L. (1999) Bacterial phospholipases and pathogenesis. Microbes Infect. 1, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 4. Sato H., Frank D. W. (2004) ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53, 1279–1290 [DOI] [PubMed] [Google Scholar]
- 5. Sitkiewicz I., Stockbauer K. E., Musser J. M. (2007) Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 15, 63–69 [DOI] [PubMed] [Google Scholar]
- 6. Marquis H., Doshi V., Portnoy D. A. (1995) The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63, 4531–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith G. A., Marquis H., Jones S., Johnston N. C., Portnoy D. A., Goldfine H. (1995) The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63, 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camilli A., Tilney L. G., Portnoy D. A. (1993) Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8, 143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saliba A. M., Nascimento D. O., Silva M. C., Assis M. C., Gayer C. R., Raymond B., Coelho M. G., Marques E. A., Touqui L., Albano R. M., Lopes U. G., Paiva D. D., Bozza P. T., Plotkowski M. C. (2005) Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell. Microbiol. 7, 1811–1822 [DOI] [PubMed] [Google Scholar]
- 10. Fujii Y., Sakurai J. (1989) Contraction of the rat isolated aorta caused by Clostridium perfringens α toxin (phospholipase C): evidence for the involvement of arachidonic acid metabolism. Br. J. Pharmacol. 97, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levine L., Xiao D. M., Little C. (1987) Increased arachidonic acid metabolites from cells in culture after treatment with the phosphatidylcholine-hydrolyzing phospholipase C from Bacillus cereus. Prostaglandins 34, 633–642 [DOI] [PubMed] [Google Scholar]
- 12. Meyers D. J., Berk R. S. (1990) Characterization of phospholipase C from Pseudomonas aeruginosa as a potent inflammatory agent. Infect. Immun. 58, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz-Laviada I., Larrodera P., Diaz-Meco M. T., Cornet M. E., Guddal P. H., Johansen T., Moscat J. (1990) Evidence for a role of phosphatidylcholine-hydrolysing phospholipase C in the regulation of protein kinase C by ras and src oncogenes. EMBO J. 9, 3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parkinson E. K. (1987) Phospholipase C mimics the differential effects of phorbol-12-myristate-13-acetate on the colony formation and cornification of cultured normal and transformed human keratinocytes. Carcinogenesis 8, 857–860 [DOI] [PubMed] [Google Scholar]
- 15. Lang C., Flieger A. (2011) Characterisation of Legionella pneumophila phospholipases and their impact on host cells. Eur. J. Cell Biol. 90, 903–912 [DOI] [PubMed] [Google Scholar]
- 16. Banerji S., Aurass P., Flieger A. (2008) The manifold phospholipases A of Legionella pneumophila: identification, export, regulation, and their link to bacterial virulence. Int. J. Med. Microbiol. 298, 169–181 [DOI] [PubMed] [Google Scholar]
- 17. Hubber A., Roy C. R. (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26, 261–283 [DOI] [PubMed] [Google Scholar]
- 18. Cianciotto N. P. (2009) Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol. 4, 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isberg R. R., O'Connor T. J., Heidtman M. (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franco I. S., Shuman H. A., Charpentier X. (2009) The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell. Microbiol. 11, 1435–1443 [DOI] [PubMed] [Google Scholar]
- 21. Rossier O., Starkenburg S. R., Cianciotto N. P. (2004) Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72, 310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogel J. P., Andrews H. L., Wong S. K., Isberg R. R. (1998) Conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876 [DOI] [PubMed] [Google Scholar]
- 23. Segal G., Purcell M., Shuman H. A. (1998) Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. U.S.A. 95, 1669–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. VanRheenen S. M., Luo Z. Q., O'Connor T., Isberg R. R. (2006) Members of a Legionella pneumophila family of proteins with ExoU (phospholipase A) active sites are translocated to target cells. Infect. Immun. 74, 3597–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shohdy N., Efe J. A., Emr S. D., Shuman H. A. (2005) Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc. Natl. Acad. Sci. U.S.A. 102, 4866–4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flieger A., Neumeister B., Cianciotto N. P. (2002) Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect. Immun. 70, 6094–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerji S., Bewersdorff M., Hermes B., Cianciotto N. P., Flieger A. (2005) Characterization of the major secreted zinc metalloprotease-dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila. Infect. Immun. 73, 2899–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hales L. M., Shuman H. A. (1999) The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181, 4879–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu W., Banga S., Tan Y., Zheng C., Stephenson R., Gately J., Luo Z. Q. (2011) Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PloS One 6, e17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DebRoy S., Dao J., Söderberg M., Rossier O., Cianciotto N. P. (2006) Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. U.S.A. 103, 19146–19151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fliegera A., Gong S., Faigle M., Neumeister B. (2000) Critical evaluation of p-nitrophenylphosphorylcholine (p-NPPC) as artificial substrate for the detection of phospholipase C. Enzyme Microb. Technol. 26, 451–458 [DOI] [PubMed] [Google Scholar]
- 32. Aragon V., Rossier O., Cianciotto N. P. (2002) Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148, 2223–2231 [DOI] [PubMed] [Google Scholar]
- 33. Flieger A., Gong S., Faigle M., Deeg M., Bartmann P., Neumeister B. (2000) Novel phospholipase A activity secreted by Legionella species. J. Bacteriol. 182, 1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baine W. B. (1988) A phospholipase C from the Dallas 1E strain of Legionella pneumophila serogroup 5: purification and characterization of conditions for optimal activity with an artificial substrate. J. Gen. Microbiol. 134, 489–498 [DOI] [PubMed] [Google Scholar]
- 35. Baine W. B. (1985) Cytolytic and phospholipase C activity in Legionella species. J. Gen. Microbiol. 131, 1383–1391 [DOI] [PubMed] [Google Scholar]
- 36. Rossier O., Cianciotto N. P. (2005) The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect. Immun. 73, 2020–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossignol G., Merieau A., Guerillon J., Veron W., Lesouhaitier O., Feuilloley M. G., Orange N. (2008) Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preuss I., Kaiser I., Gehring U. (2001) Molecular characterization of a phosphatidylcholine-hydrolyzing phospholipase C. Eur. J. Biochem. 268, 5081–5091 [DOI] [PubMed] [Google Scholar]
- 39. Debroy S., Aragon V., Kurtz S., Cianciotto N. P. (2006) Legionella pneumophila Mip, a surface-exposed peptidylproline cis-trans-isomerase, promotes the presence of phospholipase C-like activity in culture supernatants. Infect. Immun. 74, 5152–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCoy-Simandle K., Stewart C. R., Dao J., DebRoy S., Rossier O., Bryce P. J., Cianciotto N. P. (2011) Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect. Immun. 79, 1984–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Altman E., Segal G. (2008) The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J. Bacteriol. 190, 1985–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heidtman M., Chen E. J., Moy M. Y., Isberg R. R. (2009) Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell. Microbiol. 11, 230–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang L., Boyd D., Amyot W. M., Hempstead A. D., Luo Z. Q., O'Connor T. J., Chen C., Machner M., Montminy T., Isberg R. R. (2011) The E Block motif is associated with Legionella pneumophila translocated substrates. Cell. Microbiol. 13, 227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brüggemann H., Hagman A., Jules M., Sismeiro O., Dillies M. A., Gouyette C., Kunst F., Steinert M., Heuner K., Coppée J. Y., Buchrieser C. (2006) Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8, 1228–1240 [DOI] [PubMed] [Google Scholar]
- 45. Weissenmayer B. A., Prendergast J. G., Lohan A. J., Loftus B. J. (2011) Sequencing illustrates the transcriptional response of Legionella pneumophila during infection and identifies seventy novel small non-coding RNAs. PloS One 6, e17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faucher S. P., Mueller C. A., Shuman H. A. (2011) Legionella Pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadosky A. B., Wiater L. A., Shuman H. A. (1993) Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61, 5361–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jepras R. I., Fitzgeorge R. B., Baskerville A. (1985) A comparison of virulence of two strains of Legionella pneumophila based on experimental aerosol infection of guinea pigs. J. Hyg. 95, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aurass P., Pless B., Rydzewski K., Holland G., Bannert N., Flieger A. (2009) bdhA-patD operon as a virulence determinant, revealed by a novel large-scale approach for identification of Legionella pneumophila mutants defective for amoeba infection. Appl. Environ. Microbiol. 75, 4506–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edelstein P. H. (1981) Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14, 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiater L. A., Sadosky A. B., Shuman H. A. (1994) Mutagenesis of Legionella pneumophila using Tn903 dlllacZ: identification of a growth phase-regulated pigmentation gene. Mol. Microbiol. 11, 641–653 [DOI] [PubMed] [Google Scholar]
- 52. Merzbacher M., Detsch C., Hillen W., Stülke J. (2004) Mycoplasma pneumoniae HPr kinase/phosphorylase. Eur. J. Biochem. 271, 367–374 [DOI] [PubMed] [Google Scholar]
- 53. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 54. Plekhanov A. Y. (1999) Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal. Biochem. 271, 186–187 [DOI] [PubMed] [Google Scholar]
- 55. Broich M., Rydzewski K., McNealy T. L., Marre R., Flieger A. (2006) The global regulatory proteins LetA and RpoS control phospholipase A, lysophospholipase A, acyltransferase, and other hydrolytic activities of Legionella pneumophila JR32. J. Bacteriol. 188, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moffat J. F., Tompkins L. S. (1992) A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60, 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cianciotto N. P., Fields B. S. (1992) Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc. Natl. Acad. Sci. U.S.A. 89, 5188–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harding C. R., Schroeder G. N., Reynolds S., Kosta A., Collins J. W., Mousnier A., Frankel G. (2012) Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. 80, 2780–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benfield A. P., Goodey N. M., Phillips L. T., Martin S. F. (2007) Structural studies examining the substrate specificity profiles of PC-PLC(Bc) protein variants. Arch. Biochem. Biophys. 460, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sakurai J., Nagahama M., Oda M. (2004) Clostridium perfringens α-toxin: characterization and mode of action. J. Biochem. 136, 569–574 [DOI] [PubMed] [Google Scholar]
- 61. Geoffroy C., Raveneau J., Beretti J. L., Lecroisey A., Vazquez-Boland J. A., Alouf J. E., Berche P. (1991) Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect. Immun. 59, 2382–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hough E., Hansen L. K., Birknes B., Jynge K., Hansen S., Hordvik A., Little C., Dodson E., Derewenda Z. (1989) High-resolution (1.5 A) crystal structure of phospholipase C from Bacillus cereus. Nature 338, 357–360 [DOI] [PubMed] [Google Scholar]
- 63. Krug E. L., Kent C. (1984) Phospholipase C from Clostridium perfringens: preparation and characterization of homogeneous enzyme. Arch. Biochem. Biophys. 231, 400–410 [DOI] [PubMed] [Google Scholar]
- 64. Zavaleta-Pastor M., Sohlenkamp C., Gao J. L., Guan Z., Zaheer R., Finan T. M., Raetz C. R., López-Lara I. M., Geiger O. (2010) Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc. Natl. Acad. Sci. U.S.A. 107, 302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Srinivas M., Rajakumari S., Narayana Y., Joshi B., Katoch V. M., Rajasekharan R., Balaji K. N. (2008) Functional characterization of the phospholipase C activity of Rv3487c and its localization on the cell wall of Mycobacterium tuberculosis. J. Biosci. 33, 221–230 [DOI] [PubMed] [Google Scholar]
- 66. Lang C., Rastew E., Hermes B., Siegbrecht E., Ahrends R., Banerji S., Flieger A. (2012) Zinc metalloproteinase ProA directly activates Legionella pneumophila PlaC glycerophospholipid:cholesterol acyltransferase. J. Biol. Chem. 287, 23464–23478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moser J., Gerstel B., Meyer J. E., Chakraborty T., Wehland J., Heinz D. W. (1997) Crystal structure of the phosphatidylinositol-specific phospholipase C from the human pathogen Listeria monocytogenes. J. Mol. Biol. 273, 269–282 [DOI] [PubMed] [Google Scholar]
- 68. Kubiak R. J., Yue X., Hondal R. J., Mihai C., Tsai M. D., Bruzik K. S. (2001) Involvement of the Arg-Asp-His catalytic triad in enzymatic cleavage of the phosphodiester bond. Biochemistry 40, 5422–5432 [DOI] [PubMed] [Google Scholar]
- 69. Naylor C. E., Eaton J. T., Howells A., Justin N., Moss D. S., Titball R. W., Basak A. K. (1998) Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 5, 738–746 [DOI] [PubMed] [Google Scholar]
- 70. Hansen S., Hansen L. K., Hough E. (1993) The crystal structure of tris-inhibited phospholipase C from Bacillus cereus at 1.9 A resolution. The nature of the metal ion in site 2. J. Mol. Biol. 231, 870–876 [DOI] [PubMed] [Google Scholar]
- 71. Vazquez-Boland J. A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J., Cossart P. (1992) Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kirby J. E., Vogel J. P., Andrews H. L., Isberg R. R. (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27, 323–336 [DOI] [PubMed] [Google Scholar]
- 73. Roy C. R., Berger K. H., Isberg R. R. (1998) Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28, 663–674 [DOI] [PubMed] [Google Scholar]
- 74. Flieger A., Rydzewski K., Banerji S., Broich M., Heuner K. (2004) Cloning and characterization of the gene encoding the major cell-associated phospholipase A of Legionella pneumophila, plaB, exhibiting hemolytic activity. Infect. Immun. 72, 2648–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mezna M., Ahmad T., Chettibi S., Drainas D., Lawrence A. J. (1994) Zinc and barium inhibit the phospholipase A2 from Naja naja atra by different mechanisms. Biochem. J. 301, 503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wells M. A. (1973) Spectral perturbations of Crotalus adamanteus phospholipase A 2 induced by divalent cation binding. Biochemistry 12, 1080–1085 [DOI] [PubMed] [Google Scholar]
- 77. Raynaud C., Guilhot C., Rauzier J., Bordat Y., Pelicic V., Manganelli R., Smith I., Gicquel B., Jackson M. (2002) Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 45, 203–217 [DOI] [PubMed] [Google Scholar]
- 78. Costas M. J., Pinto R. M., Cordero P. M., Cabezas A., Alves-Pereira I., Cameselle J. C., Ribeiro J. M. (2010) CGDEase, a Pseudomonas fluorescens protein of the PLC/APase superfamily with CDP-ethanolamine and (dihexanoyl)glycerophosphoethanolamine hydrolase activity induced by osmoprotectants under phosphate-deficient conditions. Mol. Microbiol. 78, 1556–1576 [DOI] [PubMed] [Google Scholar]
- 79. Stonehouse M. J., Cota-Gomez A., Parker S. K., Martin W. E., Hankin J. A., Murphy R. C., Chen W., Lim K. B., Hackett M., Vasil A. I., Vasil M. L. (2002) A novel class of microbial phosphocholine-specific phospholipases C. Mol. Microbiol. 46, 661–676 [DOI] [PubMed] [Google Scholar]
- 80. Felts R. L., Reilly T. J., Tanner J. J. (2006) Structure of Francisella tularensis AcpA. Prototype of a unique superfamily of acid phosphatases and phospholipases C. J. Biol. Chem. 281, 30289–30298 [DOI] [PubMed] [Google Scholar]
- 81. Reilly T. J., Baron G. S., Nano F. E., Kuhlenschmidt M. S. (1996) Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J. Biol. Chem. 271, 10973–10983 [DOI] [PubMed] [Google Scholar]
- 82. Phillips R. M., Six D. A., Dennis E. A., Ghosh P. (2003) In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278, 41326–41332 [DOI] [PubMed] [Google Scholar]
- 83. Sato H., Frank D. W., Hillard C. J., Feix J. B., Pankhaniya R. R., Moriyama K., Finck-Barbançon V., Buchaklian A., Lei M., Long R. M., Wiener-Kronish J., Sawa T. (2003) The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22, 2959–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Finck-Barbançon V., Goranson J., Zhu L., Sawa T., Wiener-Kronish J. P., Fleiszig S. M., Wu C., Mende-Mueller L., Frank D. W. (1997) ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25, 547–557 [DOI] [PubMed] [Google Scholar]
- 85. Hauser A. R., Kang P. J., Engel J. N. (1998) PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27, 807–818 [DOI] [PubMed] [Google Scholar]
- 86. Voulhoux R., Ball G., Ize B., Vasil M. L., Lazdunski A., Wu L. F., Filloux A. (2001) Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20, 6735–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ochsner U. A., Snyder A., Vasil A. I., Vasil M. L. (2002) Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 8312–8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barker A. P., Vasil A. I., Filloux A., Ball G., Wilderman P. J., Vasil M. L. (2004) A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol. Microbiol. 53, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 89. Salacha R., Kovaci F., Brochier-Armanet C., Wilhelm S., Tommassen J., Filloux A., Voulhoux R., Bleves S. (2010) The Pseudomonas aeruginosa patatin-like protein PlpD is the archetype of a novel Type V secretion system. Environ. Microbiol. 12, 1498–1512 [DOI] [PubMed] [Google Scholar]
- 90. Rechnitzer C., Williams A., Wright J. B., Dowsett A. B., Milman N., Fitzgeorge R. B. (1992) Demonstration of the intracellular production of tissue-destructive protease by Legionella pneumophila multiplying within guinea pig and human alveolar macrophages. J. Gen. Microbiol. 138, 1671–1677 [DOI] [PubMed] [Google Scholar]
- 91. Liles M. R., Edelstein P. H., Cianciotto N. P. (1999) The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31, 959–970 [DOI] [PubMed] [Google Scholar]
- 92. Aragon V., Kurtz S., Flieger A., Neumeister B., Cianciotto N. P. (2000) Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68, 1855–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rossier O., Cianciotto N. P. (2001) Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69, 2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Finck-Barbançon V., Yahr T. L., Frank D. W. (1998) Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J. Bacteriol. 180, 6224–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. de Felipe K. S., Glover R. T., Charpentier X., Anderson O. R., Reyes M., Pericone C. D., Shuman H. A. (2008) Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 4, e1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hovel-Miner G., Pampou S., Faucher S. P., Clarke M., Morozova I., Morozov P., Russo J. J., Shuman H. A., Kalachikov S. (2009) SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J. Bacteriol. 191, 2461–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.