Abstract
Reelin is a glycoprotein that serves important roles both during development (regulation of neuronal migration and brain lamination) and in adults (maintenance of synaptic function). A number of neuropsychiatric disorders including autism, schizophrenia, bipolar disorder, major depression, Alzheimer’s disease and lissencephaly share a common feature of abnormal Reelin expression in the brain. Altered Reelin expression has been hypothesized to impair neuronal connectivity and synaptic plasticity, leading ultimately to the cognitive deficits present in these disorders. The mechanisms for abnormal Reelin expression in some of these disorders is currently unknown although possible explanations include early developmental insults, mutations, hypermethylation of the promoter for the Reelin gene (RELN), miRNA silencing of Reelin mRNA, and Reelin processing abnormalities. Increasing Reelin expression through pharmacological therapies may help ameliorate symptoms resulting from Reelin deficits.
Keywords: Reelin, schizophrenia, autism, bipolar disorder, major depression, lissencephaly, treatment
1. Chemistry of Reelin
Reelin glycoprotein plays a number of important roles in the central nervous system (CNS) developmentally including neuronal cell migration and proper brain lamination, while in the mature brain it is involved in modulating synaptic function. The gene for Reelin (RELN) in humans is located on chromosome seven (DeSilva et al. 1997) and its protein product is a secreted extracellular matrix protein (DeBergeyck et al. 1998). On SDS-PAGE, Reelin appears as multiple protein bands: Reelin 410 kDa (which corresponds to full length Reelin), Reelin 330 kDa, Reelin 180 kDa along with several lower molecular weight bands (Jossin, 2008; Fatemi, 2005; Ignatova et al. 2004; Smallheiser et al. 2000). Reelin contains a signal peptide, followed by an N-terminal sequence and hinge region, eight Reelin repeats of 350–390 amino acids, and ends with a highly basic C-terminus (DeBergeyck et al. 1998). Reelin in cleaved during processing at two locations: between repeats 2 and 3 (site N-t) and between repeats 6 and 7 (site C-t) (de Rouvroit et al. 1999). Reelin 330 kDa is produced from cleavage at site C-t while Reelin180 kDa is produced from cleavage site N-t. Little is known about the enzymes responsible for the proteolytic cleavage of Reelin beyond that it is likely one or more metalloproteinase (de Rouvroit et al. 1999; Jossin et al. 2004; Kohno et al. 2009). Recent work has shown that cleavage at the N-t site reduces Reelin’s signaling ability by 100 fold, as measured by the ability to induce disabled 1 (Dab1) phosphorylation (Kohno et al. 2009).
2. Genetics
The gene for Reelin (RELN) is located at 7q22.1 in humans (D’Arcangelo et al. 1995; DeSilva et al. 1997) and at chromosome 5 in mice (D’Arcangelo et al. 1995). The mouse RELN gene was found to contain 65 exons, spanning 450 kb of DNA (Royaux et al. 1997). Consequences of RELN mutation were first characterized in the homozygous reeler mouse which phenotypically exhibited an ataxic gait (Curran and D’Arcangelo, 1998; Falconer, 1951; Tissir and Goffinet, 2003). A number of brain abnormalities have been characterized in the brains of homozygous reeler mice. These include a non-foliated cerebellum and deficits in lamination of the hippocampus (Boyle et al. 2011; Hamburgh, 1963; Tissir and Goffinet, 2003). Most strikingly, in homozygous reeler mice, the cortex has been characterized as a laminar inversion of the typical inside-out pattern of development (Tissir and Goffinet, 2003). A recent analysis of neocortex of homozygous reeler mice found an even more complex pattern with evidence of a mirror-image laminar structure and rostrocaudal cell-type-specific differences in laminar phenotype (Boyle et al. 2011). Disorganization of the hippocampus and amygdala were also observed, suggesting pervasive disruption of brain cytoarchitecture as a result of reduced Reelin expression. Variants of RELN have been identified as risk factors for multiple neuropsychiatric disorders including autism, schizophrenia, bipolar disorder, and lissencephaly (as described in sections 6–8).
3. Reelin Receptors
Reelin binds two main receptors, including apolipoprotein E receptor 2 (ApoER2) and very-low-density lipoprotein receptor (VLDLR) (D’Arcangelo et al., 1999; Hiesberger et al., 1999). Recent work has elucidated the important roles of these receptors in mediating cell migration and the establishment of proper cytoarchitecture of the brain. Evidence from Vldlr and Apoer2 knockout mice suggest that the receptors may have divergent roles in neuronal migration with APOER2 being required to enable migration while VLDLR may act as a stop signal for migrating neurons (Hack et al. 2007). Moreover, Reelin’s receptors have an important role in neurotransmission. In rat and nonhuman primate cortex, Reelin and α3β1 integrin were found to colocalize at synaptic structures (Rodriguez et al. 2000) pointing to α3β1 integrin as a potential receptor for Reelin (Dulabon et al. 2000; Schmid et al. 2004). Moreover, mice deficient in APOER2 and VLDLR have defects in long term potentiation (LTP) and memory formation (Weeber et al. 2002; see section 5).
4. Reelin Signaling
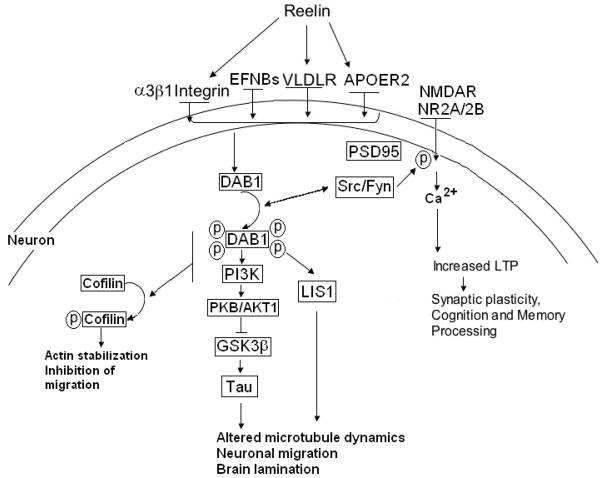
The Reelin signaling pathway has been characterized through the work of multiple laboratories (Herz and Chen, 2006; Jossin, 2011). Extracellular Reelin glycoprotein is secreted by Cajal-Retzius cells; certain cortical and hippocampal gamma-aminobutyric acid (GABA)ergic cells; and cerebellar granule cells (Del Río et al. 1997; Curran and D’Arcangelo, 1998; Frotscher, 1998). Reelin has also been identified as present in glial somata and astrocytic processes at much lower levels than in neurons (Roberts et al. 2005). Reelin binds its receptors VLDLR, APOER2, and potentially α3β1 integrin, initiating the signaling system in the effector cells, i.e., cortical pyramidal cells (Figure 1) (D’Arcangelo et al. 1999; Dong et al. 2003; Hiesberger et al. 1999; Strasser et al. 2004). Reelin induction of the cascade leads to clustering of the receptors resulting in recruitment and activation of Src-tyrosine kinase family/Fyn-kinase leading to tyrosine phosphorylation of the cytoplasmic adaptor protein DAB1 (Figure 1) (Bock and Herz, 2003; Jossin et al. 2003; Kuo et al. 2005). Activation of DAB1 results in multiple effects including activation of a kinase cascade including phosphatidylinositol-3-kinase (PI3K), protein kinase B (PKB/AKT), ultimately leading to the inhibition of glycogen synthase kinase (GSK3β) which normally phosphorylates the microtubule stabilizing protein tau (Figure 1) (Beffert et al. 2002; Bock et al. 2003; D’Arcangelo et al. 1999; Hiesberger et al. 1999). The ultimate effect of this cascade is on microtubule dynamics, promoting neuronal migration. Phosphorylation of DAB1 also results in the recruitment of lissencephaly 1 (LIS1) complex which is involved in neuronal migration and cortical lamination (Figure 1) (Assadi et al. 2003). Phosphorylation of a subpopulation of DAB1 molecules causes degradation of DAB1 via ubiquitination, resulting in termination of Reelin signaling cascade (Suetsugu et al. 2004).
Figure 1.
The Reelin signaling system. Extracellular Reelin glycoprotein binds to its receptors APOER2, VLDLR, and α3β1 integrin directly, initiating the signaling system in the effector cells. Reelin induction of the cascade leads to clustering of the receptors and ephrin B proteins causing clustering of DAB1 proteins and activation of Src-tyrosine kinase family/Fyn-kinase which phosphorylates DAB1 on four tyrosine residues. This leads to the recruitment and activation of kinase cascade including PI3K, PKB/AKT1 leading to inhibition of GSK3β. GSK3β normally phosphorylates tau, which promotes microtubule stability. DAB1 activation also leads to the recruitment of LIS1 which also affects microtubule dynamics. These processes promote neuronal migration and brain lamination. APOER2, PI3K, and DAB1 contribute to phosphorylation of cofilin which in turn stabilizes the actin cytoskeleton preventing cell migration, suggesting that Reelin may act as a stop signal. Finally, Reelin has a direct effect on enhancement of long term potentiation (LTP), via direct involvement of its receptors VLDLR and ApoER2. APOER2, associates with scaffolding protein PSD95, which helps couple the Reelin signaling complex with the NMDA receptor (NMDAR). Tyrosine phosphorylation of NMDAR subunits NR2A and NR2B by Fyn kinase results in Ca2+ influx through NMDAR, and ultimately induction of LTP and modulation of synaptic plasticity; potentially converging on Reelin’s role in cognition and memory processing. Reprinted in a modified form by permission from Nature Publishing Group, a division of MacMillan Publishers, LTD.: Fatemi, S.H. Molecular Psychiatry 10, 251–257, copyright 2005.
The importance of DAB1 phosphorylation to the Reelin signaling pathway is shown in Dab1 knock-in mice where the four Reelin-responsive tyrosine sites Y198, 220, 232, 185 are substituted with phenylalanine, resulting in mice that are indistinguishable from homozygous reeler mice, mice lacking Reelin receptors, or DAB1 (D’Arcangelo et al. 1995; Howell et al. 1999, 2000; Sheldon et al. 1997; Trommsdorf et al. 1999). Moreover, embryos of mice that express a nonphosphorylated Dab1 mutation or Src(−/−) Fyn(−/−) double knockout embryos exhibit deficits in neuronal migration of sympathetic preganglionic neurons that are similar to those of the reeler mouse, showing that tyrosine phosphorylation of DAB1 is required for proper neuronal migration (Yip et al. 2007).
Reelin interaction with ephrin B proteins (EFNBs) may also be important in neuronal migration (Sentürk et al. 2011). EFNBs associate with both APOER2 and VLDLR. Clustering of EFNBs leads to recruitment and phosphorylation of DAB1 (Figure 1) (Sentürk et al. 2011). Conversely, the authors showed that loss of function of EFNBs reduced DAB1 phosphorylation and resulted in neuronal migration deficits commonly seen in the reeler mouse. Others have found that Reelin, itself, may act as a stop signal for migrating neurons (Chai et al. 2009). Chai et al (2009) found that Reelin promoted phosphorylation of cofilin which prevents it from performing its normal role as an actin-depolymerizing protein, which in turn stabilized the cytoskeleton in the leading processes of migrating neurons (Figure 1). Moreover, Reelin has been previously shown to be highly expressed at the final destination of neuronal migration (Rossel et al. 2005), further providing evidence that it may be a stop signal for neuronal migration.
Reelin may also mediate cell migration to sites of brain injury (Courtès et al. 2011; Massalini et al. 2009). Recently, using a mouse model of brain injury, it was shown that Reelin expression increased at sites of lesions (induced either by intracerebral injection of lysolecitin or by thermocoagulation of the meninges) and that Reelin induced migration of progenitor cells in both healthy and lesioned animals (Courtès et al. 2011). In contrast, in mice that lacked Reelin signaling, lesions exhibited reduced recruitment of progenitor cells. Neural stem cells (NSC) from reeler mice have been shown to lack the ability to migrate in chains, which is the common mode of migration towards a site of injury and, in vivo fail to migrate towards gliomas (Massalini et al. 2009). The authors found that ectopic Reelin administration was able to partly correct for errors in migration. Taken together, these reports provide preliminary evidence that Reelin, in addition to regulating cell migration during development, may have a role in regulating cell migration in response to injury.
5. Reelin and Cognition
During development, there is a shift in Reelin expression from Cajal-Retzius cells to GABAergic interneurons in the neocortex and hippocampus. Additionally, Reelin is expressed by granule cells in the cerebellum, pyramidal cells in the entorhinal cortex in adults, and glial cells (Abraham and Meyer, 2003; Alcántara et al. 1998; Chin et al. 2007; Doehner and Knuesel, 2010; Lacor et al. 2000; Miettinen et al. 2005; Pesold et al. 1998; Roberts et al. 2005). Recent evidence has shown that the Reelin signaling system contributes to synapse formation and acts as a modulator of synaptic transmission and synaptic plasticity by regulating Ca2+ entry through N-methyl-d-aspartate (NMDA) receptors (Figure 1) (Beffert et al. 2005; Chen et al. 2005; Groc et al. 2007; Herz and Chen, 2006; Qiu and Weeber, 2007; Venturi et al. 2011; Weeber et al. 2002). Cultured neurons from mice deficient in either APOER2 or VLDLR display normal synaptic transmission, however, long-term potentiation (LTP) deficits are observed in response to high frequency stimulation (Weeber et al. 2002). Further, Reelin binding to its receptors results in phosphorylation of NMDA receptor subunits NR2A and NR2B which is mediated by DAB1 and SRC/FYN activation (Chen et al. 2005). Mutations of APOER2 that prevent its binding to DAB1 or postsynaptic density protein 95 (PSD95) prevent Reelin mediated synaptic plasticity (Beffet et al. 2005, 2006). In the reeler mouse, there is reduced expression of PSD-95, NR2A and NR2B, and phosphatase and tensin homolog (PTEN) which potentially contributes to impaired LTP in this animal model (Ventruti et al. 2011). Moreover, in cultured hippocampal cells from reeler mice, reduced clustering of 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) receptor subunit ionic glutamate receptor 1 (GluR1) and NMDA receptor subunit NR1 has been observed when compared with cultured hippocampal cells from wild-type mice (Qiu and Weeber, 2007). This difference was eliminated by supplementing Reelin in the reeler cultures. Moreover, when Reelin was supplemented in wild-type cultures there was an increase in AMPA-mediated excitatory postsynaptic currents; a reduction in the number of silent synapses; and altered expression of NMDA and AMPA receptor subunits with a reduction of NR2B expression and increased expression of NR2A and AMPA receptors. Further experiments using cultured hippocampal neurons have shown that postnatally, there is a decrease in the NR1/NR2B contribution to NMDA-mediated synaptic currents (Groc et al. 2007). This change was concomitant with an increased amount of Reelin at active synapses and was blocked by rendering Reelin non-functional, suggesting that Reelin plays a role in synaptic NMDA receptor assembly.
Animal models, in which Reelin expression is reduced, have provided important information on the impact of Reelin on LTP and cognition. Reeler mice display altered LTP and deficits in active avoidance tasks (Goldowitz and Koch, 1986; Marrone et al. 2006). Heterozygous reeler mice (HRM), which have been used as an animal model of schizophrenia (although some have questioned its validity; see Podhorna and Didriksen, 2004), display an approximately 50% reduction in Reelin expression and deficits in LTP (Tueting et al. 2006; Qiu et al. 2006). HRMs also display deficits in a number of cognitive measures including the acquisition of operant tasks (Krueger et al. 2006), executive function (Brigman et al. 2006), fear conditioned learning (Ammassari-Teule et al. 2009; Qiu et al. 2006), olfactory-conditioned learning (Larson et al. 2003), neophobic behavior on the elevated plus maze (Tueting et al. 1999), latent inhibition (Ammassari-Teule et al. 2009) and anxiety and motor impulsivity (Ognibene et al. 2007). Knock down of Reelin signaling in the lateral entorhinal cortex of rats results in impaired performance on the hippocampus-dependent water maze task (Stranahan et al. 2011). However, not all experimental models have resulted in impaired cognition. Prenatal application of 5-metoxytryptamine (5MT) to pregnant rats resulted in reduced Reelin expression in the exposed offspring, however, their performance on the Morris water maze as a measure of spatial cognition was no different from untreated animals (Lakatosova et al. 2011).
A number of these behavioral and cognitive deficits resulting from loss or reduction of Reelin expression are observed in individuals with autism and schizophrenia. Deficits in prepulse inhibition (PPI), a sensorimotor gating behavior, are also common to individuals with a number of psychiatric disorders including autism and schizophrenia (Geyer et al. 1999; McAlonan et al. 2002; Turetsky et al. 2007). Studies of PPI in HRMs have been equivocal. An initial study found an age-dependent reduction in PPI in HRMs (Tueting et al. 1999). Other groups, however, using a unimodal acoustic PPI paradigm, found no differences between wild type and HRMs (Barr et al. 2008; Podhorna and Didriksen, 2004; Teixeira et al. 2011). However, when using a crossmodal acoustic or tactile PPI paradigm, deficits have been observed (Barr et al. 2008). Other animal models including use of prenatal viral infection or a viral mimic have shown reduced Reelin expression and deficits in PPI (Fatemi et al. 1999; Harvey and Boksa, 2012; Meyer et al. 2008; Shi et al. 2003; please see Section 9).
6. The Role of Reelin in Schizophrenia
Multiple biological theories have been proposed to explain the origins of schizophrenia including neurodegenerative changes or disruptions of the dopaminergic, serotonergic, or glutamatergic signaling systems (Fatemi, 2008). Social factors including migration and urban birth and upbringing have also been implicated (Kneeland and Fatemi, 2012). Additionally, abuse of drugs such as cannabis, amphetamines or LSD can produce psychotic symptoms and may lead to the development of schizophrenia (Arsenault et al. 2004; Javitt and Laruelle, 2006). However, most of the available evidence points to a neurodevelopmental origin of schizophrenia in which early developmental insults (late first or second trimester) lead to activation of pathological neural circuits during adolescence and young adulthood resulting in the positive (i.e., hallucinations) and negative (i.e., poor to nonexistent social functioning, apathy) symptoms of schizophrenia (reviewed by Compton and Walker, 2009; Fatemi and Folsom, 2009; Rapoport et al., 2005, 2012; Tenyi et al., 2011). Evidence to support the neurodevelopmental hypothesis comes from: 1) increased rates of schizophrenia due to prenatal viral or bacterial infections (Brown, 2006; Kneeland and Fatemi, 2012); 2) increased risk of schizophrenia resulting from obstetric complications such as hypoxia, ischemia, or periventricular hemorrhages (Gilmore and Murray, 2006; Keshavan et al. 2006); 3) brain abnormalities such as enlargement of the cerebroventricular system, abnormal brain lamination, and changes in gray and white matter that indicate early developmental insult (Akbarian et al. 1993; Arnold and Trojanowski, 1997; Davis et al., 2003; Georgieva et al., 2007; Ritter et al., 2004; Tenyi et al., 2011); 4) Presence of congenital abnormalities and neurological soft signs in children that later develop schizophrenia (Lloyd et al. 2008; Barkus et al. 2006); and 5) Abnormal expression of genes involved in neuronal and glial migration, cell proliferation, and synaptogenesis (Hakak et al. 2001; Le-Niculescu et al. 2007; Mirnics et al. 2001; Sullivan et al. 2006; Vawter et al. 2002). Similar to autism, the brain and behavioral deficits associated with schizophrenia support a role for Reelin in the pathology of this disorder (Fatemi et al. 2008).
6.1. Reelin Expression in Plasma, Cerebrospinal Fluid, and Brain of Subjects with Schizophrenia
Reelin expression has been examined in blood and cerebrospinal fluid (CSF) of subjects with schizophrenia. In a study comparing serum Reelin across ethnic groups, Reelin 410 kDa was shown to be significantly elevated in plasma from Caucasian, Hmong, Laotian, and Vietnamese subjects with schizophrenia (Fatemi et al. 2001b). The 330 kDa Reelin fragment was also elevated in sera of Caucasian subjects with schizophrenia (Table 1). More recently, in a study of peripheral lymphocytes, drug-naïve subjects with schizophrenia displayed reduced expression of VLDLR but not APOER2 (Suzuki et al. 2008). After six months of drug treatment, there was increased expression of VLDLR, while APOER2 expression was reduced. Importantly, there was a negative correlation between VLDLR levels and severity of clinical symptoms (Suzuki et al. 2008). In contrast to altered Reelin expression in blood, a study by Ignatova et al (2004) which compared CSF levels of Reelin in subjects with schizophrenia, various neurological diseases, and cancer vs. healthy controls found no difference among disease states (Table 1). The elevated expression of Reelin in plasma contrasts with reduced Reelin expression in brains of subjects with schizophrenia.
Table 1.
Reelin expression in selected Neuropsychiatric disorders
| Disorder | CSF | Blood | Brain | Reference |
|---|---|---|---|---|
|
| ||||
| Autism | Reduced expression of Reelin 410 kDa | Keller et al. 2000; Fatemi et al. 2002 | ||
| Reduced expression of Reelin 330 kDa | Lugli et al. 2003 | |||
| Reduced expression of Reelin 180 kDa in frontal cortex and cerebellum | Fatemi et al. 2001a, 2005a | |||
| Reduced expression of Reelin 410 kDa in frontal cortex and cerebellum | Fatemi et al. 2005a | |||
|
| ||||
| Schizophrenia | No obvious correlation between CSF Reelin expression and schizophrenia | Ignatova et al. 2004 | ||
| Increased expression of Reelin 410 kDa and 330 kDa | Fatemi et al. 2001b | |||
| Reduced mRNA expression in PFC | Eastwood and Harrison, 2006; Guidotti et al. 2000; Impagnatiello et al. 1998 | |||
| Reduced mRNA expression and IR in hippocampus | Eastwood and Harrison, 2006; Fatemi et al. 2000 | |||
| Reduced expression of Reelin 180 kDa in cerebella | Fatemi et al. 2005b | |||
|
| ||||
| Bipolar disorder | Reduced expression of Reelin 330 kDa and 180 kDa | Fatemi et al. 2001b | ||
| Reduced mRNA expression in PFC | Guidotti et al. 2000 | |||
| Reduced expression of Reelin 410 kDa and 180 kDa in cerebella | Fatemi et al. 2005b | |||
| Reduced Reelin IR in hippocampus | Fatemi et al. 2000 | |||
|
| ||||
| Major Depressive Disorder | Reduced expression of Reelin 180 kDa | Fatemi et al. 2001 | ||
| No change in Reelin IR in hippocampus | Fatemi et al. 2000 | |||
| No change in Reelin mRNA in PFC | Guidotti et al. 2000 | |||
| No change in Reelin protein in cerebella | Fatemi et al. 2005b | |||
|
| ||||
| Lissencephaly | Absence of serum Reelin | Hong et al. 2000 | ||
| Absence of serum Reelin | Chang et al. 2007 | |||
|
| ||||
| Alzheimer’s Disease | Increased expression of Reelin 180 kDa | Botella López et al. 2006; Sáez Valero et al. 2003 | ||
| No change in Reelin expression | Increased expression of Reelin 180 kDa in frontal cortex but not cerebellum; altered glycosylation of Reelin | Botella López et al. 2006 | ||
| Reduced expression of Reelin in FC | Herring et al. 2012 | |||
IR, immunoreactivity; PFC, prefrontal cortex
Reduced Reelin expression has been observed in multiple brain regions of subjects with schizophrenia (Eastwood and Harrison, 2003, 2006; Fatemi et al. 2000, 2005b; Guidotti et al. 2000; Impagnatiello et al. 1998) (Table 1). Impagnatiello et al. (1998) observed reduced levels of Reelin mRNA expression in layers I and II of the prefrontal cortex (PFC) of subjects with schizophrenia when compared with controls, a finding that has been confirmed by others (Eastwood and Harrison, 2006; Guidotti et al. 2000). In contrast to these earlier findings, a recent study of Reelin mRNA expression in the PFC of subjects with schizophrenia found no difference between total expression of Reelin when compared with controls (Ovadia and Shifman, 2011). However, measurement of allelic expression involving single nucleotide polymorphism (SNP) rs2229864, revealed greater allelic expression imbalance in subjects with schizophrenia in PFC which they hypothesize to be due possibly to epigenetic factors (Ovadia and Shifman, 2011). In addition to PFC, Reelin expression has been shown to be reduced in interstitial white matter neurons in the superior temporal cortex of subjects with schizophrenia (Eastwood and Harrison, 2003). Reduction in Reelin expression has also been observed in hippocampus (Eastwood and Harrison, 2006; Fatemi et al. 2000) and cerebella (Fatemi et al. 2005b) of subjects with schizophrenia. Importantly, Fatemi et al. (2005b) found that Reelin deficits were not restricted to individuals with psychosis, but extended to non-psychotic subjects with bipolar disorder as well (Fatemi et al. 2000, 2005b). The observed reduction in Reelin expression may account for the multiple brain abnormalities associated schizophrenia. Less is known regarding other members of the Reelin signaling pathway.
6.2. Gene Association Studies
Positive associations between RELN and increased risk of schizophrenia have been identified when RELN variants have been combined with those of additional genes (Hall et al. 2007) or in gender-specific manner (Ben-David et al. 2010; Kuang et al. 2011; Li et al. 2011; Liu et al. 2011; Pisanté et al. 2009; Shifman et al. 2008). Hall et al. (2007) has demonstrated that SNPs of Reelin were associated with schizophrenia when combined with variants for additional genes, including brain-derived neurotrophic factor, neuropeptide Y, neuregulin 1, and synapsin 3, but not alone (Hall et al. 2007). Variants of RELN have been associated with increased risk of schizophrenia for women (Ben-David et al. 2010; Kuang et al. 2011; Li et al. 2011; Liu et al. 2011; Pisanté et al. 2009; Shifman et al. 2008). Shifman et al (2008) found that the RELN SNP rs7341475 was associated with increased risk of schizophrenia among Ashkenazi Jewish women. Their findings have recently been confirmed in three separate large population sample of Ashkenazi women (Ben-David et al. 2010; Liu et al. 2011; Pisanté et al. 2009), however, Ben-David et al. (2010) found the significance was less than previously reported. A recent study identified a RELN SNP (rs12705169) that associated with schizophrenia in a Han Chinese population sample (Li et al. 2011). When stratified by gender, significance remained only for females, suggesting a gender-specific effect. Similarly, rs362719 has also been associated with an increased risk of schizophrenia in female but not male Han Chinese (Kuang et al. 2011).
A number of negative gene association studies between RELN and schizophrenia have been published as well (Akahane et al. 2002; Chang et al. 2011; Goldberger et al. 2005; Huang and Chen, 2006). Because of its association with increased risk of autism, the 5′ untranslated region of the RELN gene has been investigated for conferring risk for the development of schizophrenia. However, this region has not been found to be associated with schizophrenia (Akahane et al. 2002; Goldberger et al. 2005; Huang and Chen, 2006). SNPs in RELN’s promoter region have shown no association with schizophrenia in a Han Chinese population sample (Chang et al. 2011).
6.3. Relationship between Reelin and Schizophrenia
Links between RELN and specific behavioral deficits in schizophrenia have been demonstrated (Greenbaum et al. 2010; Wedenoja et al. 2008, 2010). Quantifiable trait component analyses have revealed positive associations between an intragenic short tandem repeat (STR) with impaired visual working memory, and executive functioning (Wedenoja et al. 2008). A further study of this variant using a separate population sample confirmed the earlier finding of cognitive impairment and in addition, the allele was associated with more severe positive and negative symptoms (Wedenoja et al. 2010). The rs7341475 SNP of RELN has been shown to be associated with deficits in PPI (Greenbaum et al. 2010). A separate study, however, found that RELN SNP rs7341475 was not associated with deficits in working memory, Reelin mRNA expression in brain, or brain structure (Tost et al. 2010). Finally, a recent study found significant associations between SNPs of APOE, APOER2, VLDLR, and DAB1 with cognitive performance of subjects with schizophrenia while there was no such association for RELN (Verbrugghe et al. 2012). Additionally, in a second cohort of normal aging males, the authors found associations between SNPs of APOE and APOER2 with memory performance.
Animal models provide further evidence of the contribution of Reelin deficits to symptoms of schizophrenia. Knockdown of Reelin in medial PFC of rats during either puberty or adulthood results in PPI deficits (Brosda et al. 2011). Knockdown during puberty, but not adulthood, also resulted in impaired spatial working memory and object recognition. In a mouse model of prenatal restraint stress (PRS), offspring displayed reduced expression of Reelin in early life as well as adulthood, an effect that occurred with increased DNA methyltransferase (DNMT) expression (Matrisciano et al. 2012). Behaviorally, adult PRS mice displayed impaired PPI, social interaction, and fear conditioning. Finally Texeira et al. (2011) showed that a transgenic mouse model bred to increase Reelin production showed resistance to developing psychosis or depression (Fatemi, 2011; Texeira et al. 2011)..
7. Reelin and Autism
Autism is a debilitating neurodevelopmental disorder characterized by deficits in cognition, communication, and social interaction. Brains from subjects with autism display multiple pathologies including brain volume abnormalities including macrocephaly, minicolumnar structural abnormalities in the neocortex, and white and gray matter abnormalities (Bailey et al. 1993; Bauman and Kemper 2005; Casanova et al. 2002; Courchesne et al. 2003; Fatemi et al. 2012; Palmen et al. 2004; Schumann and Nordahl, 2010). Due to its important roles in brain development, Reelin has been studied as a potential biomarker for autism.
7.1. Plasma, Cerebrospinal Fluid, and Brain Expression of Reelin in Subjects with Autism
Reduced levels of Reelin have been observed in plasma from subjects with autism. Keller et al (2000) found reduced expression of Reelin 410 kDa in sera of subjects with autism as well as their unaffected siblings (Table 1). Similarly, Fatemi et al (2002) also found a significant reduction in plasma levels of Reelin 410 kDa in subjects with autism when compared with healthy controls (Fatemi et al. 2002) (Table 1). Transmission of “long” triplet alleles of Reelin has been correlated with a 25% reduction in expression of the Reelin 330 kDa fragment in blood (Lugli et al. 2003) (Table 1). The relative contributions of brain, liver or other sites of Reelin production to serum levels of Reelin are currently unclear although the reduction in Reelin 410 kDa in sera mirrors the reduction of Reelin 410 kDa in brain of subjects with autism. The relevance of serum levels of Reelin to autism is also unclear. However, hyperserotonemia has been associated with Reelin deficits both in autism and in a potential animal model for autism (Janusonis, 2008). Future experiments could examine whether there is a correlation between serum levels of Reelin and specific autistic behavioral phenotypes.
A series of postmortem studies have demonstrated reductions in Reelin protein in multiple brain sites of subjects with autism (Fatemi et al. 2001, 2005a) (Table 1). An initial study found reduction of Reelin 180 kDa fragment in cerebella of subjects with autism (Fatemi et al. 2001). A more extensive study found reduced Reelin protein levels in superior frontal cortex, parietal cortex and cerebella of subjects with autism when compared with controls. Reelin 410 kDa/β-actin values were reduced by 67%, 72%, and 39% in superior frontal cortex (p<0.035), parietal cortex, and cerebellum (p<0.016) of brains from subjects with autism vs. controls, respectively (Fatemi et al. 2005a). Reelin 330 kDa/β-actin values were reduced by 80%, 45%, and 25% in frontal (p<0.035), parietal, and cerebellar areas of brains from subjects with autism vs. controls, respectively (Fatemi et al. 2005a). Reelin 180 kDa/β-actin values were also reduced by 50%, 36%, and 27% in frontal (p<0.005), parietal, and cerebella (p<0.008) from subjects with autism vs. controls, respectively (Fatemi et al. 2005a). Moreover, mRNA for RELN was significantly reduced in frontal cortex and cerebella of subjects with autism, consistent with the protein data as was mRNA for DAB1 (Fatemi et al. 2005a). In contrast, mRNA for VLDLR was significantly increased in both areas, which may be a compensatory mechanism for reduced expression of Reelin (Fatemi et al. 2005a). While there is a reduction in mRNA and protein expression for Reelin in multiple brain regions in autism, less is known about other components of the Reelin signaling pathway in this disorder. Expansion of these studies to include multiple components of the Reelin signaling pathway, as well as testing additional brain regions that are relevant to autism such as the amygdala and hippocampus would provide a clearer picture of the role of Reelin signaling in autism.
7.2. Gene Association Studies
Gene association studies of RELN and autism have been inconsistent. Persico et al. (2001) demonstrated a significant association between autism and the length of a GGC repeat located immediately 5′ of the RELN gene initiation codon. This finding has been confirmed by in North American population samples (Skaar et al. 2005; Zhang et al. 2002), an Indian population sample (Dutta et al. 2007) and a Slovakian population sample (Kelemenova et al. 2010). Furthermore, preferred transmission of “long” triplet repeat alleles (more than 11 repeats) to subjects with autism has been observed (Lugli et al. 2003). Preferential expression of long triplet repeat alleles may impact Reelin expression. An in vitro study examining the effect of long triplet repeat alleles on Reelin expression revealed that 12- and 13-repeat alleles result in 50–60% less Reelin expression when compared with the more common 8–10 repeat alleles (Persico et al. 2006).
Other studies have demonstrated association of various single nucleotide polymorphisms (SNPs) and autism (Ashley-Koch et al. 2007; Li et al. 2008; Serajee et al. 2006). Li et al. (2008), found an association with autism for an SNP of RELN (located on intron 59) in a sample of Han Chinese, a finding that was verified by haplotype analysis. In an investigation of 34 Reelin SNPs, altered transmission of rs736707 from intron 59 and rs362691 from exon 22 was found in subjects with autism (Serajee et al. 2006). An association between an SNP (rs2073559) and autism has been identified (Ashley-Koch et al. 2007). In contrast, the authors found no significant association of SNPs for APOE with autism, nor was there a joint RELN-APOE contribution to autism risk. A separate study involving North American and Italian families with autistic probands found preferential transmission of APOE2, over APOE3 and APOE4 to autistic offspring (Persico et al. 2004). APOE2 displays reduced binding affinity for APOER2 (Rall et al. 1982) and the preferential transmission of this isoform may impair signaling.
In contrast, several studies have found no association between RELN polymorphisms and autism (Bonora et al. 2003; Devlin et al. 2004; Dutta et al. 2008; He et al. 2011; Krebs et al. 2002; Li et al. 2004). For example, several studies did not report an association between GGC repeats in the 5′ untranslated region of RELN and autism in various population samples (Bonora et al. 2003; Devlin et al. 2004; Krebs et al. 2002; Li et al. 2004). Additionally, Dutta et al. (2008) failed to find association between any of six RELN SNPs (rs727531, rs2072403, rs2072402, rs362691, rs362719, rs736707) and autism in an Indian population sample. Similarly, He et al. (2011) found no association between four RELN SNPs (rs736707, rs2229864, rs362691, and rs2073559) and autism in a Han Chinese population sample. Due to the heterogeneous nature of autistic spectrum disorders, it is not surprising that some studies show an association between RELN and autism while others do not. More research is needed to determine the impact of specific RELN mutations on Reelin expression and on brain and behavioral pathologies of autism.
7.3. Relationship between Reelin and Autism
Structural abnormalities observed in key brain regions including the amygdala, frontal, temporal, and cerebellar cortices, could potentially explain the cognitive and motor deficits observed in subjects with autism (Amaral et al. 2008; Bauman and Kemper, 2005; Courchesne et al. 2007; Fatemi et al. 2012). Reelin deficiency may contribute to these structural abnormalities as well as with abnormal synaptic connectivity. Cognitive deficits of HRM related to learning and memory show an impact of reduced Reelin expression on autistic symptomology. PPI deficits, which are associated with autism, have been observed in animal models deficient in Reelin expression (Shi et al. 2003).
8. Reelin in Other Neuropsychiatric Disorders
Altered expression of Reelin has been associated with additional brain disorders including Alzheimer’s disease (AD), lissencephaly, bipolar disorder, and major depression. Alzheimer’s disease (AD) is the most common form of senile dementia. It is characterized by progressive cognitive impairment. In brain, AD is characterized the presence of extracellular deposits consisting mainly of beta amyloid (Aβ) peptides and intracellular neurofibrillary tangles consisting mainly of phosphorylated tau protein. While AD is characterized as a neurodegenerative disease, a number of intracellular signaling pathways associated with neurodevelopment including Reelin, Notch, and Wnt have also been associated with the pathology of AD (Bothwell and Gingier, 2000; da Cruz e Silva et al. 2010; Grilli et al. 2003; Woo et al. 2009). In aged mice, Reelin has been observed to form plaques in hippocampus (Knuesel et al. 2009; Doehner et al. 2010) and Aβ peptides associate with these plaques (Doehner et al. 2010). Polymorphisms of RELN have been associated with AD (Antoniades et al. 2011; Kramer et al. 2011; Seripa et al. 2008). In CSF from subjects with AD, increased expression of the Reelin 180 kDa fragment has been observed (Botella-López et al. 2006; Sáez-Valero et al. 2003) while in plasma, there were no significant changes in Reelin expression (Botella-López et al. 2006) (Table 1). The researchers also found that Reelin mRNA and Reelin 180 kDa fragment were also increased in frontal cortex but not cerebellum of subjects with AD (Table 1). Furthermore, altered glycosylation of Reelin was observed which may interfere with its ability to interact with APOER2 (Bótella-Lopez et al. 2010). In contrast with these findings, Herring et al. (2012) found reduced Reelin expression in human frontal cortex from a pre-clinical stage of AD while VLDLR, APOER2, and DAB1 showed no change. Furthermore, in a mouse model of AD, depletion of Reelin in the hippocampus preceded the formation of Aβ plaques (Herring et al. 2012).
Lissencephaly is a neurodevelopmental disorder resulting from defective neuronal migration during the 12th through 24th week of gestation resulting in brain structural deficits including lack of gyral and sulcal development and improper lamination. Affected individuals display psychomotor deficits, seizures, and muscle spasticity. Lissencephaly is a heterozygous disorder with mutations within multiple genes resulting in the lissencephalic phenotype. Mutations in platelet activating factor acetylhydrolase, isoform 1B, alpha subunit (PAFAH1B1; also known as LIS1), results in lissencephaly associated with Miller-Dieker syndrome (Ledbetter et al. 1992; Reiner et al. 1993). The LIS1 complex binds to VLDLR (but not APOER2) (Zhang et al. 2007) and phosphorylated DAB1 (Assadi et al. 2003). Recruitment of the LIS1 complex contributes to regulation of microtubule dynamics and ultimately, regulation of neuronal migration, and proper brain lamination. Mutations in the RELN gene itself are associated with lissencephaly with cerebellar hypoplasia (LIS-CH) (Chang et al. 2007; Hong et al. 2000; Kono et al. 2008). A case study has demonstrated that an individual with LIS-CH lacked serum Reelin (Chang et al. 2007) (Table 1). Further studies are needed to determine whether this lack of serum Reelin is a common feature of LIS-CH.
Bipolar disorder is a major debilitating disease currently affecting between 0.4% – 1.6% of the world’s population (APA, 2000). Subjects with bipolar disorder present manic, hypomanic, mixed, and depressive episodes. Reduced Reelin expression has been observed in blood and brain of subjects with bipolar disorder. Reduced levels of the 330 kDa and 180 kDa fragments of Reelin have been shown to be significantly reduced in sera of subjects with bipolar disorder (Fatemi et al., 2001b) (Table 1). Guidotti et al (2000) found reduction of Reelin mRNA in PFC of subjects with bipolar disorder with psychosis (Guidotti et al. 2000). Reelin protein levels have also been found to be reduced in hippocampus and cerebella of subjects with bipolar disorder, regardless of presence of or absence of psychosis (Fatemi et al. 2000, 2005b). Ovadia and Shifman (2011) found no difference in total Reelin mRNA in PFC of subjects with bipolar disorder, however, there was a significant reduction of the relative amount of the short RELN isoform which is missing the C-terminal region. Other brain regions including the caudate nucleus and putamen have shown no difference in Reelin expression in subjects with bipolar disorder when compared with controls (Veldic et al. 2007). A recent gene association study found overtransmission of the C allele of RELN SNP rs362719 to offspring with bipolar disorder in a large family sample and that this association was almost entirely due to transmission to affected females (Goes et al. 2010).
A further psychiatric disorder that has demonstrated abnormal Reelin expression is major depressive disorder (MDD). MDD is characterized by depressed mood, sleep and appetite changes, loss of energy, feelings of worthlessness or guilt and suicidal ideation that persist for periods of two weeks or longer (Dunner, 2008). There is some evidence that Reelin expression is altered in subjects with MDD. The 180 kDa fragment of Reelin is significantly reduced in sera from subjects with major depressive disorder (MDD) (Fatemi et al. 2001b) (Table 1). In contrast to the significant reductions of Reelin mRNA and protein expression in cerebella, prefrontal cortex, and hippocampus of subjects bipolar disorder and schizophrenia, no significant reductions of Reelin have been observed in these regions for subjects with MDD (Fatemi et al. 2000, 2005b; Guidotti et al. 2000) (Table 1).
9. Potential Mechanisms for Reduced Reelin Expression in Neuropsychiatric Disorders
Reduced Reelin expression may be the result of multiple mechanisms. Spontaneous mutations of the RELN gene itself could result in reduced or absent Reelin expression as shown with the homozygous reeler mouse. As mentioned in the previous section, a mutation in RELN leads to LIS-CH and an absence of serum Reelin (Hong et al. 2000; Chang et al. 2007).
Reelin haploinsufficiency may be a second potential mechanism to cause altered Reelin expression (Figure 2). As discussed in section 5, the HRM has only one copy of the RELN gene which results in a number of behavioral abnormalities. In addition to the behavioral deficits observed in HRMs, Ammasari-Teule et al. (2009) observed reduced expression of parvalbumin-positive GABAergic interneurons in the dorsomedial and ventromedial intermediate striatum and caudal striatum while there was no loss in rostral striatum when compared with wild type mice.
Figure 2.
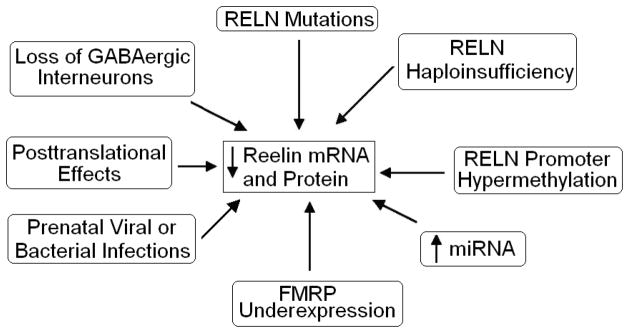
Multiple mechanisms to account for altered Reelin expression in neuropsychiatric disorders. Please see section 9 for further discussion.
Early developmental events such as prenatal viral or bacterial infections constitute a third mechanism of Reelin reduction (Figure 2). Prenatal viral infection of mice on embryonic day nine (E9) resulted in reduced Reelin expression in neocortex and hippocampus of pups at P0 (birth) (Fatemi et al. 1999). Similarly, the offspring of pregnant mice treated with the viral mimic polyriboinosinic-polyribocytidilic acid (PolyI:C), show reduced Reelin expression in both hippocampus and prefrontal cortex (Meyer et al. 2006, 2008). The offspring of mice infected or treated with viral mimic at E9 also display behavioral deficits reminiscent of both autism and schizophrenia including deficits in PPI (Meyer et al. 2008; Shi et al. 2003) and deficits in social interaction (Shi et al. 2003). Additionally, treatment of pregnant mice at E9 resulted in reduced number of Reelin positive cells in the dorsal striatum oriens at P28 (but not P14) in exposed offspring (Harvey and Boksa, 2012). Stimulation of the maternal immune system in rodents via injection of lipopolysaccharide (LPS) can also reduce Reelin expression in the offspring (Ghiani et al. 2011; Nouel et al. 2012). Injection of pregnant rats with LPS at E15 resulted in reduced expression of Reelin in forebrains of embryos at E18 (Ghiani et al. 2011). More recently, injection of mice with LPS on E9 resulted in a reduction of Reelin positive cells in the dentate gyrus at P14 (Nouel et al. 2012). However, western blotting revealed no difference in Reelin expression for multiple brain regions. Other investigations, however, have not reported any effects of prenatal insults on Reelin expression. For example, Matricon et al. (2010) found that prenatal treatment with methylazoxymethanol, which results in schizophrenia-like symptoms and brain abnormalities in exposed offspring, had no effect on Reelin expression.
A fourth potential mechanism is epigenetic silencing of the RELN gene through hypermethylation of the RELN promoter (Figure 2). DNA-methyltransferase 1 (DNMT1) activity results in methylation of the promoter region of a number of genes preventing transcription. Increased expression of DNMT1 mRNA in cortical GABAergic neurons from brains of subjects with schizophrenia has been associated with reduced Reelin mRNA (Veldic et al. 2004). Treatment of mouse primary cultures of cortical neurons with methionine has been shown to reduce Reelin and glutamic acid decarboxylase 67 kDa (GAD67) expression, which was accompanied by increased methylation of the Reln promoter (Noh et al. 2005). Using an antisense approach to reduce Dnmt1 resulted in increased Reelin and GAD67 mRNA expression and blocked methionine-induced reductions of Reelin and GAD67 suggesting that Dnmt1 methylation of the Reln and Gad67 promoters results in reduced Reelin and GAD67 mRNA levels. Furthermore, increased methylation of the RELN promoter in brains of subjects with schizophrenia has been observed in two studies (Abdolmaleky et al. 2005; Grayson et al. 2005). Recent studies, however, have failed to replicate the hypermethylation data mentioned above (Mill et al. 2008; Tochigi et al. 2008) and bring into question the assertion that Reelin deficits in schizophrenia are due to hypermethylation. A preliminary study has found that in normal individuals the human RELN promoter shows very little methylation prior to puberty while postpubertal individuals display heavy methylation, suggesting that sex hormones could potentially stimulate methylation (Lintas and Persico, 2010). The authors hypothesize that the timing of hypermethylation during adolescence may contribute to the emergence of schizophrenic symptoms and the worsening of autistic behaviors. However, while this theory appears acceptable with respect to schizophrenia, it does not parallel with emergence of autistic behavior in early childhood (ages 1–3), a time long before onset of puberty and emergence of male sex hormones. Thus, similar postmortem studies using tissue from subjects with schizophrenia or autism from various age groups need to be performed in order to test whether the same pattern holds true in both disorders. More investigation is needed to determine to what extent, if any, epigenetic mechanisms such as hypermethylation play in abnormal reductions of Reelin observed in schizophrenia or other disorders.
A fifth mechanism involving microRNAs (miRNAs) may also explain reduced Reelin expression in neurodevelopmental disorders (Figure 2). miRNAs are small, non-coding, RNA transcripts that regulate gene transcription post-translationally. miRNA expression is altered in both schizophrenia and autism (Ghahramani et al. 2011; Moreau et al. 2011) although it is currently unknown if miRNAs regulate Reelin expression. Preliminary experiments to determine miRNA expression in brains of subjects with autism has been performed (Fatemi and Kalscheuer, unpublished observations). Microarray analyses have thus far revealed changes in a number of miRNAs in cerebellum, parietal cortex (BA40), and frontal cortex (BA9) of subjects with autism (Fatemi and Kalscheuer, unpublished observations; Table 2). Significant changes have been observed for miR-214 in cerebellum, which was verified by qRT-PCR (Fatemi and Kalscheuer, unpublished observations; Table 2). Interestingly, Reelin is a predicted target of miR-214 (Target Scan 5.0). qRT-PCR revealed a significant downregulation of Reelin mRNA in the same brain region (Fatemi and Kalscheuer, unpublished observations). It may be the case that miR-214 negatively regulates Reelin mRNA, resulting in downregulated Reelin protein in subjects with autism.
Table 2.
Altered Expression of Selected miRNA in Subjects with Autism vs. Control.
| Brain Region | microRNA | Microarray Fold Change | p | qRT-PCR Fold Change | p |
|---|---|---|---|---|---|
| Cerebellum | miR-107 | 0.74 | 0.063 | ||
| miR-181b | 0.69 | 0.125 | 2.91 | 0.07 | |
| miR-184 | 1.62 | 0.09 | 4.17 | 0.01 | |
| miR-202 | 1.37 | 0.017 | |||
| miR-214 | 1.92 | 0.007 | 1.81 | 0.04 | |
| miR-410 | 0.64 | 0.029 | |||
| miR-634 | 0.59 | 0.058 | |||
|
| |||||
| Parietal Cortex (BA40) | miR-214 | 1.7 | 0.077 | 1.67 | 0.3 |
| miR-23a | 2.5 | 0.03 | |||
| miR-29a | 2.33 | 0.07 | |||
| miR-29b | 2.07 | 0.03 | 2.09 | 0.03 | |
| miR-30b | 1.84 | 0.08 | |||
| miR-491 | 0.33 | 0.067 | |||
| miR-9 | 1.87 | 0.07 | 1.44 | 0.45 | |
| miR-99b | 1.6 | 0.03 | |||
|
| |||||
| Frontal Cortex (BA9) | miR-20b | 0.36 | 0.1 | ||
| miR-29a | 0.39 | 0.16 | 0.14 | 0.22 | |
| miR-214 | 1.73 | 0.14 | 2.32 | 0.08 | |
| miR-326 | 2.32 | 0.02 | |||
| miR-342 | 3.39 | 0.087 | |||
(Fatemi and Kalscheuer, unpublished observations)
A sixth potential mechanism involves disruption of Reelin translation by fragile X mental retardation protein (FMRP) (Figure 2). FMRP acts as a translational repressor (De Rubeis and Bagni, 2010) and targets approximately 4% of all mRNAs in brain (Bassell and Warren, 2008). Reelin mRNA has recently been identified as a downstream target of FMRP (Darnell et al. 2011). In fragile X syndrome (FXS), mutations in the fragile X mental retardation 1 gene (FMR1) leads to an absence of FMRP which results ultimately in cognitive impairment and behavioral deficits. Reduced FMRP protein has been identified in several psychiatric disorders including autism (Fatemi and Folsom, 2010, 2011, Fatemi et al. 2011) as well as schizophrenia, bipolar disorder, and MDD (Fatemi et al. 2010). Abnormal FMRP production either directly or indirectly via miRNA could be linked to Reelin abnormalities in these disorders.
Dysfunction of Reelin processing may also play a role (Figure 2). Altered glycosylation of Reelin has been observed in cerebrospinal fluid (CSF), plasma, and frontal cortex extracts from subjects with Alzheimer’s disease (Botella-Lopez et al. 2006, 2010). However, this altered expression pattern was accompanied by increased Reelin expression in CSF (Botella-Lopez et al 2006). It is possible that other errors in Reelin processing such as improper folding or splicing (as documented by altered expression of Reelin 330 and 180 kDa) occurs in neurodevelopmental disorders.
Finally, abnormalities or loss of GABAergic interneurons may contribute to dysfunctional Reelin expression in schizophrenia and autism. HRMs display reduced colocalization of Reelin and neuronal nitric oxide synthase (nNOS) in interneurons located in the subgranular zone and molecular layer of the dentate gyrus when compared with wild-type mice and may contribute to behavioral deficits of this model (Romay-Tallon et al. 2010). However, Fatemi et al. (1999) did not report a change in nNOS in cortical layer I or calretinin, despite decreased Reelin expression, in a mouse model for schizophrenia and autism. An animal model of tuberous sclerosis (a condition which includes autism, seizures, and developmental delay as its symptoms) in which the tuberous sclerosis 1 gene (Tsc1) is deleted results in reduced expression of GABAergic interneurons in the cortex and a lower seizure threshold (Fu et al. 2011). The authors did not test for Reelin levels but it is possible that the reduction in GABAergic interneurons could result in reduced brain expression of Reelin.
10. Potential Involvement of Reelin in Pharmacotherapeutics
Therapies that increase Reelin expression may ameliorate some of the symptoms of these disorders. As mentioned in Section 5, Reelin supplementation to cultured neurons has been shown to enhance LTP. In a recent study, injection of purified Reelin into the ventricles of wild-type mice has been shown to result in increased hippocampal CA1 LTP, and improved performance on tasks measuring associative and spatial learning and memory (Rogers et al. 2011).
Chronic treatment with a typical antipsychotic drug (haloperidol), two atypical antipsychotic drugs (clozapine and olanzapine), an antidepressant (fluoxetine), and two mood stabilizers (valproic acid (VPA) and lithium), have been shown to alter mRNA and protein expression of Reelin and its signaling molecules in rat frontal cortex (Table 3; Fatemi et al. 2006, 2009). Olanzapine increased both Reelin mRNA and protein while both lithium and haloperidol had the opposite effect (Table 3). Olanzapine has been shown to successfully treat schizophrenia, bipolar disorder and as an augmentive treatment for major depression. It may do so by increasing expression of Reelin in brain, potentially stabilizing synapses and improving brain circuitry. Clozapine reduced Reelin protein while fluoxetine increased Reelin mRNA (Table 3). VLDLR protein expression was increased following treatment with fluoxetine, olanzapine, and haloperidol while VPA reduced both VLDLR mRNA and protein. Dab1 mRNA was increased following chronic treatment with clozapine and VPA (Table 3). DAB1 protein expression was increased by fluoxetine while it was reduced by olanzapine and VPA (Table 3). GSK3β mRNA and protein were increased by haloperidol and lithium while fluoxetine and clozapine increased GSK3β protein expression only (Table 3). In contrast, VPA reduced GSK3β mRNA expression (Table 3). GAD65 and GAD67 protein were reduced by both olanzapine and haloperidol. GAD65 protein was increased by fluoxetine while Gad65 mRNA was reduced by VPA (Table 3). More recently, the antidepressant citalopram has been shown to increase Reelin mRNA and protein levels (Jaako et al. 2011). These results suggest that drugs used to treat schizophrenia, autism, MDD, and bipolar disorder alter expression of members of the Reelin and GABAergic signaling systems which may help to explain their therapeutic efficacies.
Table 3.
Comparison of mRNA and Protein Expression of Reelin Signaling System Following Chronic Treatment With Psychotropic Agents in Rat Frontal Cortex
| Gene/Protein of Interest | Fluoxetine | Olanzapine | Clozapine | Haloperidol | Lithium | VPA | |
|---|---|---|---|---|---|---|---|
| Reelin | mRNA | ↑ | ↑ | NS | ↓ | ↓ | NS |
| Protein | NS | ↑ | ↓ | ↓ | ↓ | NS | |
|
| |||||||
| Vldlr | mRNA | NS | NS | NS | NS | NS | ↓ |
| Protein | ↑ | ↑ | NS | ↑ | NS | ↓ | |
|
| |||||||
| Dab-1 | mRNA | NS | NS | ↑ | NS | NS | ↑ |
| Protein | ↑ | ↓ | NS | NS | NS | ↓ | |
|
| |||||||
| Gsk3β | mRNA | NS | NS | NS | ↑ | ↑ | ↓ |
| Protein | ↑ | NS | ↑ | ↑ | ↑ | NS | |
|
| |||||||
| Gad65 | mRNA | NS | NS | NS | NS | NS | ↓ |
| Protein | ↑ | ↓ | NS | ↓ | NS | NS | |
|
| |||||||
| Gad67 | mRNA | NS | NS | NS | NS | NS | NS |
| Protein | NS | ↓ | NS | ↓ | NS | NS | |
↑, significant increase (p<0.05); ↓, significant decrease (p<0.05); NS, not significant; Reprinted from Schizophrenia Research, 111(1–3), Fatemi, S.H., Reutiman, T.J., Folsom, T.D. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats, 138–152, Copyright (2009) with permission from Elsevier
As mentioned in section 9, prenatal viral infection at E9 results in reduced Reelin expression and PPI deficits (Fatemi et al. 1999, Shi et al. 2003). The deficit in PPI was corrected following administration of either the atypical APD clozapine or the typical APD chlorpromazine (Shi et al. 2003). However, this effect of clozapine is likely to be independent of Reelin expression as chronic treatment with clozapine results in reduced expression of Reelin in frontal cortex of rats (Fatemi et al. 2009).
Drugs that affect the epigenetic regulation of Reelin may also prove useful treatments to increase Reelin expression. Drugs that reduce or inhibit DNMT1 activity, have been shown to increase Reelin mRNA expression (Kundadovic et al. 2007, 2009; Tremolizzo et al. 2005). Direct inhibitors of DNMT1 such as doxorubicin (DOXO), 5-aza-2′-deoxycytidine (AZA), and zebularine (ZEB) have been shown to increase expression of Reelin mRNA (Kundadovic et al. 2007). Histone deacetylases inhibit transcription by removing acetyl groups from the histone which in turn leads to increased binding between the histone and DNA. Inhibitors of histone deacetylases including MS-275, trichostatin A (TSA), and VPA reverse this effect. Histone deacetylation has been hypothesized to be facilitated by hypermethylation (Kundadovic et al. 2009). Indeed, MS-275, TSA, and VPA reduce expression of DNMT1 and reduce DNMT1 activity and MS-275 treatment increases Reelin expression (Kundadovic et al. 2009). Both MS-275 and DOXO decrease the methylation status of the RELN promoter (Kundadovic et al. 2007, 2009). In a mouse model of schizophrenia, treatment with VPA resulted in inhibition of Reln promoter hypermethylation, increased Reelin mRNA, and reversed PPI deficits (Tremolizzo et al. 2005). VPA’s effect on demethylation is potentiated when combined with clozapine or sulpride but not with haloperidol or olanzapine (Guidotti et al. 2009, 2011). Taken together, these results suggest that selectively reducing the methylation state of the RELN promoter could result in increased Reelin expression and ameliorate symptoms caused by Reelin deficits.
11. Conclusions
Reelin protein is required for proper brain development and synaptic plasticity. Disrupted Reelin expression has been identified in a number of neurodevelopmental disorders including autism, schizophrenia, as well as other neuropsychiatric disorders such as lissencephaly, Alzheimer’s disease, bipolar disorder, and major depression. The clinical phenotypes of these diverse conditions may be partially the result of improper neuronal migration, aberrant brain cytoarchitecture, and impaired synapse formation and stability, all of which are regulated by Reelin. Reduced Reelin expression may result from epigenetic effects such as hypermethylation of the Reelin promoter, early developmental events such as activation of the maternal immune system during gestation. Therapies that increase Reelin expression such as the use of olanzapine or inhibitors of histone deacetylases and DNA methyltrasferases may provide effective means of ameliorating symptoms of these disorders.
Highlights.
Reelin plays important roles in neuronal migration and brain development.
Reelin is an important modulator of synaptic function.
Reelin expression is altered in disorders such as schizophrenia and autism.
Reelin is an important potential therapeutic target.
Acknowledgments
Grant support by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD052074-01A2) and the National Institute of Mental Health (5R01MH086000-01A2) to SHF is gratefully acknowledged. Dr. Fatemi has several United States patents (7341844) on the use of Reelin as a diagnostic marker in psychiatric disorders but has not derived any financial gains from these patents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy D. Folsom, Email: folso013@umn.edu.
S. Hossein Fatemi, Email: fatem002@umn.edu.
References
- Abdolmaleky HM, Smith CL, Farone SV, Shafa R, Stone W, Glatt SJ, Tsuang MT. Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet. 2004;127B:51–59. doi: 10.1002/ajmg.b.20142. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Abraham H, Meyer G. Reelin-expressing neurons in the postnatal and adult human hippocampal formation. Hippocampus. 2003;13:715–727. doi: 10.1002/hipo.10125. [DOI] [PubMed] [Google Scholar]
- Akahane A, Kunugi H, Tanaka H, Nanko S. Association analysis of polymorphic CGG repeat in 5′ UTR of the reelin and VLDLR genes with schizophrenia. Schizophr Res. 2002;58:37–41. doi: 10.1016/s0920-9964(01)00398-x. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Bunney WE, Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Alcántara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;3:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing Inc; Washington, DC: 2000. [Google Scholar]
- Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- Antoniades D, Katopodi T, Pappa S, Lampropoulos A, Konsta V, Frydas E, Mpalogiannis S, Hatzistilianou M. The role of reelin gene polymorphisms in the pathogenesis of Alzheimer’s disease in a Greek population. J Biol Regul Homeost Agents. 2011;25:351–358. [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Recent advanced in defining the neuropathology of schizophrenia. Acta Neuropath. 1997;92:217–231. doi: 10.1007/s004010050512. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Ashley-Koch AE, Jaworski J, de Ma Q, Mei H, Ritchie MD, Skaar DA, Robert Delong G, Worley G, Abramson RK, Wright HH, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA. Investigation of potential gene-gene interactions between APOE and RELN contributing to autism risk. Psychatr Genet. 2007;17:221–226. doi: 10.1097/YPG.0b013e32809c2f75. [DOI] [PubMed] [Google Scholar]
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Bolton P, Le Couteur A, Rutter M, Harding B. Autism and megalencephaly. Lancet. 1993;341:1225–1226. doi: 10.1016/0140-6736(93)91065-t. [DOI] [PubMed] [Google Scholar]
- Barkus E, Stirling J, Hopkins R, Lewis S. The presence of neurological soft signs along the psychosis proneness continuum. Schizophr Bull. 2006;32:573–577. doi: 10.1093/schbul/sbj037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Fish KN, Markou A. The reelin receptors VLDLR and ApoER2 regulate sensorimotor gating in mice. Neuropharmacology. 2007;52:1114–1123. doi: 10.1016/j.neuropharm.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci. 2008;27:2568–2574. doi: 10.1111/j.1460-9568.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Structural Brain Anatomy in Autism: What is the Evidence? In: Bauman ML, Kemper TL, editors. The Neurobiology of Autism. Johns Hopkins University Press; Baltimore MD: 2005. pp. 121–135. [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signalling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem. 2002;51:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Ben-David E, Shiffman S International Schizophrenia Consortium. Further investigation of the association between rs7341475 and rs17746501 and schizophrenia. Am Med Genet B Neuropsychitr Genet. 2010;153B:1244–1247. doi: 10.1002/ajmg.b.31093. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Bonora E, Beyer KS, Lamb JA, Parr JR, Klauck SM, Benner A, Paolucci M, Abbott A, Ragoussis I, Poustka A, Bailey AJ, Monaco AP. International Molecular Genetic Study of Autism (IMGSAC). Analysis of reelin as a candidate gene for autism. Mol Psychiatry. 2003;10:885–892. doi: 10.1038/sj.mp.4001310. [DOI] [PubMed] [Google Scholar]
- Botella-López A, Burgaya F, Gavín R, García-Ayllón MS, Gómez-Tortosa E, Peña-Casanova J, Ureña JM, Del Río JA, Blesa R, Soriano E, Sáez-Valero J. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella-López A, Cuchillo-Ibáñez I, Cotrufo T, Mok SS, Li QX, Barquero MS, Dierssen M, Soriano E, Sáez-Valero J. Beta amyloid controls Reelin expression and processing in Alzheimer’s disease. Neurobiol Dis. 2010;37:682–691. doi: 10.1016/j.nbd.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Bothwell M, Giniger E. Alzheimer’s disease: neurodevelopment converges with neurodegeneration. Cell. 2000;102:271–273. doi: 10.1016/s0092-8674(00)00032-5. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, Hawrylycz MJ, Jones AR, Hevner RF, Lein ES. Cell-type-specific consequences of Reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J Comp Neurol. 2011;519:2061–2089. doi: 10.1002/cne.22655. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Padukiewicz KE, Sutherland ML, Rothblat LA. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006;120:984–988. doi: 10.1037/0735-7044.120.4.984. [DOI] [PubMed] [Google Scholar]
- Brosda J, Dietz F, Koch M. Impairment of cognitive performance after reelin knockdown in the medial prefrontal cortex of pubertal or adult rats. Neurobiol Dis. 2011;44:239–247. doi: 10.1016/j.nbd.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Chai X, Förster E, Zhao S, Bock HH, Frotscher M. Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun Integr Biol. 2009;2:375–377. doi: 10.4161/cib.2.4.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Duzcan F, Kim S, Cinbis M, Aggarwal A, Apse KA, Ozdel O, Atmaca M, Zencir S, Bagci H, Walsh CA. The role of RELN in lissencephaly and neuropsychiatric disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:58–63. doi: 10.1002/ajmg.b.30392. [DOI] [PubMed] [Google Scholar]
- Chang LH, Li M, Luo XJ, Liu XY, Yin LD, Yang SY, Diao HB, Su B, Pu XF. Association of RELN promoter SNPs with schizophrenia in the Chinese population. Dongwuxue Yanjiu. 2011;32:504–508. doi: 10.3724/SP.J.1141.2011.05504. [DOI] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Walker EF. Physical manifestations of neurodevelopmental disruption: are minor physical anomalies part of the syndrome of schizophrenia? Schizophr Bull. 2009;35:425–436. doi: 10.1093/schbul/sbn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia; potential for pharmacological intervention? Mol Interv. 2003;3:220–229. doi: 10.1124/mi.3.4.220. [DOI] [PubMed] [Google Scholar]
- Courtès S, Vernerey J, Pujadas L, Magalon K, Cremer H, Soriano E, Durbec P, Cayre M. Reelin controls progenitor cell migration in the healthy and pathological adult mouse brain. PLoS One. 2011;6:e20430. doi: 10.1371/journal.pone.0020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, D’Arcangelo G. Role of reelin in control of brain development. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins detected in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva OA, Henriques AG, Domingues SC, da Cruz e Silva EF. Wnt signalling is a relevant pathway contributing to amyloid beta-peptide-mediated neuropathology in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2011;9:720–726. doi: 10.2174/187152710793237458. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- DeBergeyck V, Naerhuyzen B, Goffinet AM, deRouvroit C. A panel of monoclonal antibodies against Reelin, the extracellular matrix protein defective in reeler mutant mice. J Neurosci Meth. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- Del Río JA, Heimrich B, Borrell V, Förster E, Drakew A, Alcántara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- de Rouvroit CL, DeBergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet AM. Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- De Rubeis S, Bagni C. Fragile X mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability. Mol Cell Neurosci. 2010;43:43–50. doi: 10.1016/j.mcn.2009.09.013. [DOI] [PubMed] [Google Scholar]
- DeSilva U, D’Arcangelo G, Braden VV, Chen J, Miao GG, Curran T, Green ED. The human reelin gene: isolation, sequencing, and mapping on chromosome 7. Genome Res. 1997;7:157–164. doi: 10.1101/gr.7.2.157. [DOI] [PubMed] [Google Scholar]
- Devlin B, Bennett P, Dawson G, Figlewicz DA, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD CPEA Genetics Network. Alleles of a reelin CGG repeat do not convey liability to autism in a sample from the CPEA network. Am J Med Genet. 2004;126B:46–50. doi: 10.1002/ajmg.b.20125. [DOI] [PubMed] [Google Scholar]
- Doehner J, Knuesel I. Reelin-mediated Signaling during Normal and Pathological Forms of Aging. Aging Dis. 2010;1:12–29. [PMC free article] [PubMed] [Google Scholar]
- Doehner J, Madhusudan A, Konietzko U, Fritschy JM, Knuesel I. Co-localization of Reelin and proteolytic AbetaPP fragments in hippocampal plaques in aged wild-type mice. J Alzheimers Dis. 2010;19:1339–1357. doi: 10.3233/JAD-2010-1333. [DOI] [PubMed] [Google Scholar]
- Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, Guidotti A. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci USA. 2003;100:5479–5484. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha 3 beta 1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Dunner DL. Major depressive disorder. In: Fatemi SH, Clayton P, editors. Medical Basis of Psychiatry. 3. Humana; New York: 2008. pp. 73–84. [Google Scholar]
- Dutta S, Guhathakurta S, Sinha S, Chatterjee A, Ahmed S, Ghosh S, Gangopadhyay PK, Singh M, Usha R. Reelin gene polymorphisms in the Indian population: a possible paternal 5′UTR-CGG-repeat-allele effect on autism. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:106–112. doi: 10.1002/ajmg.b.30419. [DOI] [PubMed] [Google Scholar]
- Dutta S, Sinha S, Ghosh S, Chatterjee A, Ahmed S, Usha R. Genetic analysis of reelin gene (RELN) SNPs: no association with autism spectrum disorder in the Indian population. Neurosci Lett. 2008;441:56–60. doi: 10.1016/j.neulet.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Cellular basis for reduced cortical reelin expression in schizophrenia. Am J Psychiatry. 2006;163:540–542. doi: 10.1176/appi.ajp.163.3.540. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Two new mutants, Trembler and ‘Reeler’, with neurological actions in the house mouse. J Genetics. 1951;1(50):82–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin glycoprotein: structure, biology and roles in health and disease. Mol Psychiatry. 2005;10:251–257. doi: 10.1038/sj.mp.4001613. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, editor. Reelin Glycoprotein, Structure, Biology and Roles in Health and Disease. Springer; New York: 2008. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin, a marker of stress resilience in depression and psychosis. Neuropsychopharmacology. 2011;36:2371–2372. doi: 10.1038/npp.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. FMRP is reduced in cerebella of subjects with autism. Ir J Psychiatr Behav Neurosci. 2010;4:56–57. [Google Scholar]
- Fatemi SH, Folsom TD. Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of individuals with autism: a postmortem brain study. Mol Autism. 2011;2:6. doi: 10.1186/2040-2392-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder, and major depression. Mol Psychiatry. 2000;5:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Halt AR, Realmuto GR. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord. 2001a;6:529–535. doi: 10.1023/a:1013234708757. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Kroll JL, Stary JM. Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. Neuroreport. 2001b;12:3209–3215. doi: 10.1097/00001756-200110290-00014. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Egan EA. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol Neurobiol. 2002;22:139–152. doi: 10.1023/a:1019857620251. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Brooks AI, Pearce DA, Reutiman TJ, Lee S. Reelin signaling is impaired in autism. Biol Psychiatry. 2005a;57:777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Egan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schiz Res. 2005b;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Bell C, Nos L, Fried P, Pearce DA, Singh S, Siderovski DP, Willard FS, Fukuda M. Chronic olanzapine treatment causes differential expression of genes in frontal cortex of rats as revealed by DNA microarray technique. Neuropsychopharmacology. 2006;31:1888–1899. doi: 10.1038/sj.npp.1301002. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Sidwell RW. The role of cerebellar genes in pathology of autism and schizophrenia. Cerebellum. 2008;7:279–294. doi: 10.1007/s12311-008-0017-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr Res. 2009;111:138–152. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res. 2010;124:246–247. doi: 10.1016/j.schres.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken) 2011;294:1635–1645. doi: 10.1002/ar.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum. 2012 doi: 10.1007/s12311-012-0355-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31:1511–1518. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr Opin Neurobiol. 1998;8:570–575. doi: 10.1016/s0959-4388(98)80082-2. [DOI] [PubMed] [Google Scholar]
- Fu C, Cawthon B, Clinkscales W, Bruce A, Winzenburger P, Ess KC. GABAergic Interneuron Development and Function Is Modulated by the Tsc1 Gene. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr300. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva V, Sivkov ST, Akabaliev VH, Koeva YA, Angelova RY, Deneva TI. Computertomographic findings of structural brain anomalies in schizophrenic patients in support of the neurodevelopmental hypothesis of the disorder. Folia Med (Plovdiv) 2007;49:11–15. [PubMed] [Google Scholar]
- Ghahramani Seno MM, Hu P, Gwadry FG, Pinto D, Marshall CR, Casallo G, Scherer SW. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011;1380:85–97. doi: 10.1016/j.brainres.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Mattan NS, Nobuta H, Malvar JS, Boles J, Ross MG, Waschek JA, Carpenter EM, Fisher RS, de Vellis J. Early effects of lipopolysaccharide-induced inflammation on foetal brain development in rat. ASN Neuro. 2011;3:e00068. doi: 10.1042/AN20110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Murray RM. Prenatal and perinatal factors. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American Psychiatric Publishing Textbook of Schizophrenia. American Psychiatric Publishing Inc; Washington DC: 2006. pp. 55–68. [Google Scholar]
- Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, Schweizer B, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB National Institute of Mental Health Genetics Initiative Bipolar Disorder Consortium. Sex-specific association of the Reelin gene with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:549–553. doi: 10.1002/ajmg.b.31018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger C, Gourion D, Leroy S, Schürhoff F, Bourdel MC, Leboyer M, Krebs MO. Population-based and family-based association study of 5′UTR polymorphism of the reelin gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:51–55. doi: 10.1002/ajmg.b.30191. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Koch J. Performance of normal and neurological mutant mice on radial arm maze and active avoidance tasks. Behav Neural Biol. 1986;46:216–226. doi: 10.1016/s0163-1047(86)90696-5. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M, Sharma RP. The human reelin gene: transcription factors (+), repressors (−), and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Greenbaum L, Levin R, Lerer E, Alkelai A, Kohn Y, Heresco-Levy U, Ebstein RP, Lerer B. Association of reelin (RELN) single nucleotide polymorphism rs7341475 with prepulse inhibition in the Jewish Israeli population. Biol Psychiatry. 2011;69:e17–18. doi: 10.1016/j.biopsych.2010.09.059. [DOI] [PubMed] [Google Scholar]
- Grilli M, Ferrari Toninelli G, Uberti D, Spano P, Memo M. Alzheimer’s disease linking neurodegeneration with neurodevelopment. Funct Neurol. 2003;18:145–148. [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–3891. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Lawyer G, Sillén A, Jönsson EG, Agartz I, Terenius L, Arnborg S. Potential genetic variants in schizophrenia: a Bayesian analysis. World J Biol Psychiatry. 2007;8:12–22. doi: 10.1080/15622970600892004. [DOI] [PubMed] [Google Scholar]
- Hamburgh M. Analysis of the postnatal developmental effects of “reeler,” a neurological mutation in mice. A study in developmental genetics. Dev Biol. 1963;19:165–185. doi: 10.1016/0012-1606(63)90040-x. [DOI] [PubMed] [Google Scholar]
- Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology. 2012;62:1767–1776. doi: 10.1016/j.neuropharm.2011.11.022. [DOI] [PubMed] [Google Scholar]
- He Y, Xun G, Xia K, Hu Z, Lv L, Deng Z, Zhao J. No significant association between RELN polymorphisms and autism in case-control and family-based association study in Chinese Han population. Psychiatry Res. 2011;187:462–464. doi: 10.1016/j.psychres.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct biding of Reelin to VLDL receptor and ApoE receptor 2 induces tyroene phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Honda T, Kobayashi K, Mikoshiba K, Nakajima K. Regulation of cortical neuron migration by the Reelin signaling pathway. Neurochem Res. 2011;36:1270–1279. doi: 10.1007/s11064-011-0407-4. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;20:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chen CH. Absence of association of a polymorphic GGC repeat at the 5′ untranslated region of the reelin gene with schizophrenia. Psychiatry Res. 2006;142:89–92. doi: 10.1016/j.psychres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Ignatova N, Sindic CJM, Goffinet AM. Characterization of the various farms of the Reelin protein in the cerebrospinal fluid of normal subjects and in neurological diseases. Neurobiol Dis. 2004;15:326–330. doi: 10.1016/j.nbd.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting T, Sharma RP, Costa E. A decrease of Reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaako K, Aonurm-Helm A, Kalda A, Anier K, Zharkovsky T, Shastin D, Zharkovsky A. Repeated citalopram administration counteracts kainic acid-induced spreading of PSA-NCAM-immunoreactive cells and loss of reelin in the adult mouse hippocampus. Eur J Pharmacol. 2011;666:61–71. doi: 10.1016/j.ejphar.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Janusonis S. Origin of the blood hyperserotonemia in autism. Theor Biol Med Model. 2008;5:10. doi: 10.1186/1742-4682-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Laruelle M. Neurochemical theories. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American Psychiatric Publishing Textbook of Schizophrenia. American Psychiatric Publishing Inc; Washington, DC: 2006. pp. 85–116. [Google Scholar]
- Jossin Y. Chemistry of Reelin. In: Fatemi SH, editor. Reelin Glycoprotein: Structure, Biology and Roles in Health and Disease. Springer; New York: 2008. pp. 37–48. [DOI] [PubMed] [Google Scholar]
- Jossin Y. Polarization of migrating cortical neurons by Rap1 and N-cadherin: Revisiting the model for the Reelin signaling pathway. Small Gtpases. 2011;2:322–328. doi: 10.4161/sgtp.18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Ogawa M, Metin C, Tissir F, Goffinet AM. Inhibition of SRC family kinases and non-classical protein kinases C induce a reeler-like malformation of cortical plate development. J Neurosci. 2003;23:9953–9959. doi: 10.1523/JNEUROSCI.23-30-09953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–521. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemenova S, Schmidtova E, Ficek A, Celec P, Kubranska A, Ostatnikova D. Polymorphisms of candidate genes in Slovak autism patients. Psychiatr Genet. 2010;20:137–139. doi: 10.1097/YPG.0b013e32833a1eb3. [DOI] [PubMed] [Google Scholar]
- Keller F, Persico AM, Zelante L, Gasperial P, D’Agruma N, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt K-L, Conciatori M, Marino R, Baldi A, Quattrocchi CC. Reelin gene alleles and haplotypes are associated with autistic disorder. Society for Neuroscience. 2000;26:77(31.11). [Google Scholar]
- Keshavan MS, Gilbert AR, Diwadkar VA. Neurodevelopmental theories. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American Psychiatric Publishing Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing Inc; 2006. pp. 69–84. [Google Scholar]
- Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;134:211–218. doi: 10.1016/j.pnpbp.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Nyffeler M, Mormède C, Muhia M, Meyer U, Pietropaolo S, Yee BK, Pryce CR, LaFerla FM, Marighetto A, Feldon J. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging. 2009;30:697–716. doi: 10.1016/j.neurobiolaging.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kohno S, Kohno T, Nakano Y, Suzuki K, Ishii M, Tagami H, Baba A, Hattori M. Mechanism and significance of specific proteolytic cleavage of Reelin. Biochem Biophys Res Comm. 2009;380:93–97. doi: 10.1016/j.bbrc.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Kono T, Moriyama N, Tanaka R, Iwasaki N, Arai J. Tigroid pattern of the white matter: a previously unrecognized MR finding in lissencephaly with cerebellar hypoplasia. Pediatr Radiol. 2008;38:1105–1108. doi: 10.1007/s00247-008-0909-7. [DOI] [PubMed] [Google Scholar]
- Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, Erten-Lyons D, Kaye JA, Welsh-Bohmer KA, Troncoso JC, Markesbery WR, Petersen RC, Turner RS, Kukull WA, Bennett DA, Galasko D, Morris JC, Ott J. Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiol Aging. 2011;32:2113–2122. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs MO, Betancur C, Leroy S, Bourdel MC, Gillberg C, Leboyer M. Paris Autism Research International Sibpair (PARIS) study. Absence of association between a polymorphic GGC repeat in the 5′ untranslated region of the reelin gene and autism. Mol Psychiatry. 2002;7:801–804. doi: 10.1038/sj.mp.4001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang WJ, Sun RF, Zhu YS, Li SB. A new single nucleotide mutation (rs362719) of the reelin (RELN) gene associated with schizophrenia in female Chinese Han. Genet Mol Res. 2011;10:1650–1658. doi: 10.4238/vol10-3gmr1343. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Costa E, Grayson DR. DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol. 2007;71:644–653. doi: 10.1124/mol.106.030635. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor P, Grayson DR, Auto J, Sugaya I, Costa E, Guidotti A. Reelin secretion from glutamatergic neurons in culture is independent from neurotransmitter regulation. Proc Natl Acad Sci USA. 2000;97:3556–3561. doi: 10.1073/pnas.050589597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatosova S, Celec P, Schmidtova E, Kubranska A, Durdiakova J, Ostatnikova D. The impact of serotonergic stimulation on reelin and glutamate decarboxylase gene expression in adult female rats. Bratisl Lek Listy. 2011;112:58–62. [PubMed] [Google Scholar]
- Larson J, Hoffman JS, Guidotti A, Costa E. Olfactory discrimination learning deficit in heterozygous reeler mice. Brain Res. 2003;97:40–46. doi: 10.1016/s0006-8993(03)02353-9. [DOI] [PubMed] [Google Scholar]
- Ledbetter SA, Kuwano A, Dobyns WB, Ledbetter DH. Microdeletions of chromosome 17p13 as a cause of isolated lissencephaly. Am J Hum Genet. 1992;50:182–189. [PMC free article] [PubMed] [Google Scholar]
- Le Niculescu H, Balaraman Y, Patel S, Tan J, Sidhu K, Jerome RE, Edenberg HJ, Kuczenski R, Geyer MA, Nurnberger JI, Jr, Faraone SV, Tsuang MT, Niculescu AB. Towards understanding the schizophrenia code: an expanded convergent functional genomics approach. Am J Med Genet B Neuropsychiatr Genet. 2007;144:129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- Li H, Li Y, Shao J, Li R, Qin Y, Xie C, Zhao Z. The association analysis of RELN and GRM8 genes with autistic spectrum disorder in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:194–200. doi: 10.1002/ajmg.b.30584. [DOI] [PubMed] [Google Scholar]
- Li J, Nguyen L, Gleason C, Lotspeich L, Spiker D, Risch N, Myers RM. Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. Am J Med Genet. 2004;126B:51–57. doi: 10.1002/ajmg.b.20122. [DOI] [PubMed] [Google Scholar]
- Li W, Song X, Zhang H, Yang Y, Jiang C, Xiao B, Li W, Yang G, Zhao J, Guo W, Lu L. Association study of RELN polymorphisms with autism in Han Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1505–1511. doi: 10.1016/j.pnpbp.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Lintas C, Persico AM. Neocortical RELN promoter methylation increases significantly after puberty. Neuroreport. 2010;21:114–118. doi: 10.1097/WNR.0b013e328334b343. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen PL, McGrath J, Wolyniec P, Fallin D, Nestadt G, Liang KY, Pulver A, Valle D, Avramopoulos D. Replication of an association of a common variant of the Reelin gene (RELN) with schizophrenia in Ashkenazi Jewish women. Psychiatr Genet. 2011;20:184–186. doi: 10.1097/YPG.0b013e32833a220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T, Dazzan P, Dean K, Fearon P, Doody GA, Tarrant J, Morgan KD, Morgan C, Hutchinson G, Leff J, Harrison G, Murray RM, Jones PB. Minor physical anomalies in patients with first-episode psychosis: their frequency and diagnostic specificity. Psychol Med. 2008;38:71–77. doi: 10.1017/S0033291707001158. [DOI] [PubMed] [Google Scholar]
- Lugli G, Krueger JM, Davis JM, Persico AM, Keller F, Smalheiser NR. Methodological factors influencing measurement and processing of plasma reelin in humans. BMC Biochemistry. 2003;4:9. doi: 10.1186/1471-2091-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, Van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russel A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, Bernardi G, Mercuri NB. Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci. 2006;24:2061–2070. doi: 10.1111/j.1460-9568.2006.05083.x. [DOI] [PubMed] [Google Scholar]
- Massalini S, Pellegatta S, Pisati F, Finocchiaro G, Farace MG, Ciafrè SA. Reelin affects chain-migration and differentiation of neural precursor cells. Mol Cell Neurosci. 2009;42:341–349. doi: 10.1016/j.mcn.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Matricon J, Bellon A, Frieling H, Kebir O, Le Pen G, Beuvon F, Daumas-Duport C, Jay TM, Krebs MO. Neuropathological and Reelin deficiencies in the hippocampal formation of rats exposed to MAM; differences and similarities with schizophrenia. PLoS ONE. 2010;5:e10291. doi: 10.1371/journal.pone.0010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.04.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Miettinen R, Riedel A, Kalesnykas G, Kettunen HP, Puoliväli J, Soininen H, Arendt T. Reelin-immunoreactivity in the hippocampal formation of 9-month-old wildtype mouse: effects of APP/PS1 genotype and ovariectomy. J Chem Neuroanat. 2005;30:105–118. doi: 10.1016/j.jchemneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bourchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenetic profiling reveals DNA-methylation changes associated with major psychosis. Am J Human Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A, Grayson DR. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci USA. 2005;102:1749–1754. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouel D, Burt M, Zhang Y, Harvey L, Boksa P. Prenatal exposure to bacterial endotoxin reduces the number of GAD67- and reelin-immunoreactive neurons in the hippocampus of rat offspring. Eur Neuropsychopharmacol. 2012;22:300–307. doi: 10.1016/j.euroneuro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Granstrem O, Pieretti S, Laviola G. Impulsivity-anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res. 2007;113:173–180. doi: 10.1016/j.brainres.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ovadia G, Shiffman S. The genetic variation of RELN expression in schizophrenia and bipolar disorder. PLoS ONE. 2011;6:e19955. doi: 10.1371/journal.pone.0019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Persico AM, D’Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Conciatori M, Marino R, Quattrocchi CC, Baldi A, Zelante L, Gasparini P, Keller F Collaborative Linkage Study of Autism. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6:150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- Persico AM, D’Agruma L, Zelante L, Militerni R, Bravaccio C, Schneider C, Melmed R, Trillo S, Montecchi F, Elia M, Palermo M, Rabinowitz D, Pascucci T, Puglisi-Allegra S, Reichelt KL, Muscarella L, Guarnieri V, Melgari JM, Conciatori M, Keller F. Enhanced APOE2 transmission rates in families with autistic probands. Psychiatr Genet. 2004;14:73–82. doi: 10.1097/01.ypg.0000128768.37838.17. [DOI] [PubMed] [Google Scholar]
- Persico AM, Levitt P, Pimenta AF. Polymorphic GGC repeat differentially regulates human reelin gene expression levels. J Neural Transm. 2006;113:1373–1382. doi: 10.1007/s00702-006-0441-6. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-ambiobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanté A, Bronstein M, Yakir B, Darvasi A. A variant in the reelin gene increases the risk of schizophrenia and schizoaffective disorder but not bipolar disorder. Psychiatr Genet. 2009;19:212. doi: 10.1097/YPG.0b013e32832cebe6. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Qiu S, Weeber SJ. Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol. 2007;97:2312–2321. doi: 10.1152/jn.00869.2006. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rall SC, Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci USA. 1982;79:4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.23. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Meador-Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res. 2004;68:65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Xu L, Roche JK, Kirkpatrick B. Ultrastructural localization of reelin in the cortex in post-mortem human brain. J Comp Neurol. 2005;482:294–308. doi: 10.1002/cne.20408. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Pesold C, Liu WS, Kribo V, Guidotti A, Pappas GD, Costa E. Colocalization of integrin receptors and reelin in dendritic spine post-synaptic densities of adult non-human primate cortex. Proc Natl Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romay-Tallón R, Dopeso-Reyes IG, Lussier AL, Kalynchuk LE, Caruncho HJ. The coexpression of reelin and neuronal nitric oxide synthase in a subpopulation of dentate gyrus neurons is downregulated in heterozygous reeler mice. Neural Plast. 2010;2010:130429. doi: 10.1155/2010/130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel M, Loulier K, Feuillet C, Alonso S, Carroll P. Reelin signaling is necessary for a specific step in the migration of hindbrain efferent neurons. Development. 2005;132:1175–1185. doi: 10.1242/dev.01683. [DOI] [PubMed] [Google Scholar]
- Royaux I, Lambert de Rouvroit C, D’Arcangelo G, Demirov D, Goffinet AM. Genomic organization of the mouse reelin gene. Genomics. 1997;46:240–250. doi: 10.1006/geno.1997.4983. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2010;1380:175–186. doi: 10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentürk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472:356–360. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Zhong H, Mahbubul Huq AH. Association of Reelin gene polymorphisms with autism. Genomics. 2006;87:75–83. doi: 10.1016/j.ygeno.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Seripa D, Matera MG, Franceschi M, Daniele A, Bizzarro A, Rinaldi M, Panza F, Fazio VM, Gravina C, D’Onofrio G, Solfrizzi V, Masullo C, Pilotto A. The RELN locus in Alzheimer’s disease. J Alzheimers Dis. 2008;14:335–344. doi: 10.3233/jad-2008-14308. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O’Donovan M, O’Neill FA, Owen MJ, Walsh D, Weinberger DR, Sun C, Flint J, Darvasi A. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563–571. doi: 10.1038/sj.mp.4001614. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–1386. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu S, Tezuka T, Morimura T, Hattori M, Mikoshiba K, Yamamoto T, Takenawa T. Regulation of actin cytoskeleton by mDab1 through N-WASP and ubiquitination of mDab1. Biochem J. 2004;384:1–8. doi: 10.1042/BJ20041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Owen MJ, O’Donovan MC, Freedman MD. Genetics. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American Psychiatric Publishing Textbook of Schizophrenia. American Psychiatric Publishing Inc; Washington DC: 2006. pp. 39–54. [Google Scholar]
- Suzuki K, Nakamura K, Iwata Y, Sekine Y, Kawai M, Sugihara G, Tsuchiya KJ, Suda S, Matsuzaki H, Takei N, Hashimoto K, Mori N. Decreased expression of reelin receptor VLDLR in peripheral blood lymphocytes of drug-naïve schizophrenic patients. Schizophr Res. 2008;98:148–156. doi: 10.1016/j.schres.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Martín ED, Sahún I, Masachs N, Pujadas L, Corvelo A, Bosch C, Rossi D, Martinez A, Maldonado R, Dierssen M, Soriano E. Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology. 2011;36:2395–2405. doi: 10.1038/npp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenyi T. Neurodevelopment and schizophrenia: data on minor physical anomalies and structural brain imaging. Neuropsychopharmacol Hung. 2011;13:229–232. [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and Brain Development. Nature Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N, Kato T. Methylation status of reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Tost H, Lipska BK, Vakkalanka R, Lemaitre H, Callicott JH, Mattay VS, Kleinman JE, Marenco S. No effect of a common allelic variant in the reelin gene on intermediate phenotype measures of brain structure, brain function, and gene expression. Biol Psychiatry. 2010;68:105–107. doi: 10.1016/j.biopsych.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. NeuroReport. 1999;10:1327–1334. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Tueting P, Doueiri MS, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventruti A, Kazdoba TM, Niu S, D’Arcangelo G. Reelin deficiency causes specific defects in the molecular composition of the synapses in the adult brain. Neuroscience. 2011;189:32–42. doi: 10.1016/j.neuroscience.2011.05.050. [DOI] [PubMed] [Google Scholar]
- Verbrugghe P, Bouwer S, Wiltshire S, Carter K, Chandler D, Cooper M, Morar B, Razif MF, Henders A, Badcock JC, Dragovic M, Carr V, Almeida OP, Flicker L, Montgomery G, Jablensky A, Kalaydjieva L. Impact of the Reelin signaling cascade (Ligands-Receptors-Adaptor Complex) on cognition in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2012 doi: 10.1002/ajmg.b.32042. In Press. [DOI] [PubMed] [Google Scholar]
- Wedenoja J, Loukola A, Tuulio-Henriksson A, Paunio T, Ekelund J, Silander K, Varilo T, Heikkilä K, Suvisaari J, Partonen T, Lönnqvist J, Peltonen L. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol Psychiatry. 2008;13:673–684. doi: 10.1038/sj.mp.4002047. [DOI] [PubMed] [Google Scholar]
- Wedenoja J, Tuulio-Henriksson A, Suvisaari J, Loukola A, Paunio T, Partonen T, Varilo T, Lönnqvist J, Peltonen L. Replication of association between working memory and Reelin, a potential modifier gene in schizophrenia. Biol Psychiatry. 2010;67:983–991. doi: 10.1016/j.biopsych.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- Woo HN, Park JS, Gwon AR, Arumugam TV, Jo DG. Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun. 2009;390:1093–1097. doi: 10.1016/j.bbrc.2009.10.093. [DOI] [PubMed] [Google Scholar]
- Yip YP, Kronstadt-O’Brien P, Capriotti C, Cooper JA, Yip JW. Migration of sympathetic preganglionic neurons in the spinal cord is regulated by Reelin-dependent Dab1 tyrosine phosphorylation and CrkL. J Comp Neurol. 2007;502:635–643. doi: 10.1002/cne.21318. [DOI] [PubMed] [Google Scholar]
- Zhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, Herz J, Clark GD, D’Arcangelo G. The Pafah1b complex interacts with the reelin receptor VLDLR. PLoS One. 2007;2:e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, Guidotti AR, Holden JJ. Reelin gene alleles and susceptibility to autism spectrum disorders. Mol Psychiatry. 2002;7:1012–1017. doi: 10.1038/sj.mp.4001124. [DOI] [PubMed] [Google Scholar]