Abstract
Despite the availability of antiviral chemotherapy, herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) infections remain a severe global health problem. Of particular concern is the growing incidence of drug resistance in immunocompromised patients, which stresses the urgency to develop new effective treatment alternatives. We have developed a humanized monoclonal antibody (mAb hu2c) that completely abrogates viral cell-to-cell spread, a key mechanism by which HSV-1/2 escapes humoral immune surveillance. Moreover, mAb hu2c neutralized HSV fully independent of complement and/or immune effector cell recruitment in a highly efficient manner. Prophylactic and therapeutic administration of mAb hu2c completely prevented infection-related mortality of severely immunodeficient mice being challenged with a lethal dose of HSV-1. The high neutralization capacity of mAb hu2c was fully maintained toward clinical HSV isolates being multiresistant to standard antiviral drugs, and infection was fully resolved in 7/8 nonobese diabetic/SCID mice being infected with a multidrug resistant HSV-1 patient isolate. Immunohistochemical studies revealed no significant cross-reactivity of the antibody toward human tissues. These features warrant further clinical development of mAb hu2c as an immunotherapeutic compound for the management of severe and particularly drug-resistant HSV infections.
Keywords: acyclovir resistance, immunocompromised mice, stem cell transplantation
Herpes simplex viruses type 1 and 2 (HSV-1 and HSV-2) infection-related diseases are considered a global health problem. After primary infection the virus persists in sensory and autonomic neural ganglia for the lifetime of its host. In immunocompetent individuals HSV may cause clinical recurrences with sometimes painful yet usually self-limiting orolabial or genital lesions. However, infections may become chronic and result in physical disabilities, social exclusion, and psychological distress over time (1). Moreover, severe and even life-threatening infections can particularly occur in newborns (2) and in immunocompromised individuals (3–5).
For more than two decades viral DNA targeting chemotherapeutics such as acyclovir (ACV) have been used as drugs for the prophylaxis and treatment of HSV infections. Viral resistance toward these compounds is, however, increasingly observed. ACV-refractory HSV keratitis, for example, is a leading cause of corneal morbidity and blindness in humans of industrialized countries (6). Not surprisingly, a particularly high prevalence of ACV resistance has been reported in immunocompromised patients being prophylactically treated with ACV for extended periods. A study conducted in the Netherlands analyzed 542 HSV isolates and found ACV resistance in only 0.27% of immunocompetent patients. In contrast, the prevalence of ACV-resistant virus in immunocompromised patients was 7%, and the highest frequency of resistance (14.3%) was observed among patients after hematopoietic stem cell transplantation (7). ACV resistance frequencies of even more than 25% have been reported for patients in the allogeneic stem cell or bone marrow transplantation setting (8, 9). Because resistance to ACV is mostly acquired through mutations in the HSV thymidine kinase (TK) gene, cross-resistance toward other viral TK-dependent nucleoside analogs, such as penciclovir and famciclovir, is not uncommon (10, 11). Although in nucleoside analog-resistant cases TK-independent drugs such as the viral DNA polymerase inhibitors foscarnet and cidofovir can be administered, these compounds frequently mediate severe toxic side effects, particularly in patients with high comorbidities. Most alarmingly, HSV strains being resistant to both TK-dependent drugs as well as polymerase inhibitors have been isolated from highly immunocompromised patients undergoing stem cell/bone marrow transplantation (12, 13).
Epidemiologic and biological studies have furthermore revealed HSV-2 as a cofactor for HIV transmission and disease progression (14). Although ACV therapy in HSV/HIV-coinfected patients has been shown to decrease HIV serum levels and may delay the requirement for initiation of antiretroviral therapy (15–17), it has recently been reported that HIV reverse transcriptase may be a direct molecular target of ACV in HIV-infected cells (18) that could result in the emergence of reverse transcriptase mutants being resistant toward approved anti-HIV inhibitors, such as lamivudine or emtricitabine (18, 19).
Like other pathogenic enveloped viruses HSV can spread either by releasing virions from infected cells (cell-free spread) or by moving directly between adjacent cells without diffusing through the extracellular space (cell-to-cell spread). Cell-to-cell spread enables HSV to bypass biophysical barriers and to circumvent classic neutralization by antibodies. The process by which extracellular virions enter cells or spread from cell to cell is mediated essentially by viral glycoproteins gD, gB, and gH/gL. Several monoclonal antibodies have been raised in mice against the viral glycoproteins gH, gD, and gB. We have previously shown that a gB-specific monoclonal antibody, designated mAb 2c, not only exhibited strong HSV-neutralizing capacity but was also capable of very effectively abrogating viral cell-to-cell spread both in vitro and in animal models (20). Interestingly, the particular sequence of the HSV-1/2 type-common gB epitope recognized by mAb 2c seemed to be indispensible for viral infectivity and fitness (21). Therefore, mutations in this epitope may render the virus noninfectious. This unique property suggested mAb 2c to also potently neutralize HSV strains being resistant toward currently used HSV drugs. On the basis of these considerations we have generated in the present study a humanized version of mAb 2c (mAb hu2c) for subsequent clinical development and investigated its efficacy toward nucleoside analog-resistant HSV strains in vitro and in vivo. Our studies provide a strong rationale for further clinical development of mAb hu2c as an antiherpetic compound.
Results
Generation of a HSV-Neutralizing Humanized Monoclonal Antibody for Therapeutic Applications.
We have recently demonstrated that binding of mAb 2c to a type-common gB epitope being indispensable for viral infectivity accounts for the therapeutic efficacy of this antibody (21). To exploit this unique epitope for therapeutic interventions in humans, we generated a humanized derivative of mAb 2c as well as a chimeric version as control.
For the vast majority of humanized antibodies retention of a set of potentially immunogenic murine residues within the human frameworks is usually required for maintaining the structural integrity of the grafted antigen-binding loops. To generate a humanized antibody with the lowest possible immunogenic potential we aimed in the present study to avoid any framework manipulations by careful selection of appropriate human germ-line sequences and simultaneous use of our previously described sequence multialignment approach (22). To identify appropriate human germ-line acceptor scaffolds for grafting the mAb 2c complementarity-determining regions (CDRs), variable domain framework sequences of mAb 2c were aligned to corresponding human sequences of the immunogenetics information system database (IMGT, www.imgt.org). The highest framework sequence identities to the corresponding murine mAb 2c variable light (VL) and variable heavy (VH) chain sequence showed the human germ-line sequences IGHV2-70 (88.5%) and IGKV2-40 (88.9%), respectively. Hence, CDR coding gene segments of the murine donor-antibody 2c (i.e., 2c VL-CDR1/2/3 and 2c VH-CDR1/2/3) were grafted into acceptor frameworks coding for IGHV2-70 and IGKV2-40, respectively. Humanized or murine variable domain encoding genes (20) were subsequently cloned into Ig expression vectors containing a human constant heavy γ1 chain, and a human constant κ chain, respectively. After stable cotransfection of vectors into Sp2/0-Ag14 cells, humanized IgG hu2c and chimeric IgG ch2c were produced and purified from cell culture supernatants.
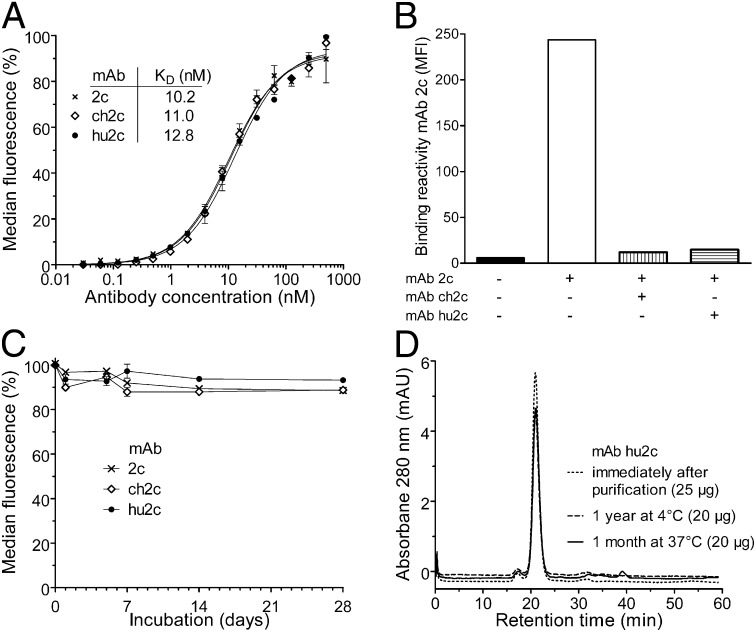
Using HSV-1–infected Vero cells we proved that the generated humanized antibody hu2c retained the high apparent affinity as the parental antibody mAb 2c and the chimeric control (Fig. 1A). Because in a previous study we demonstrated that cross-linking of a type-common discontinuous epitope within the extracellular domain I of gB through mAb 2c plays a decisive role for its antiviral activity (20), we compared the chimeric and humanized antibody with the murine mAb 2c for binding specificity toward the conformation-dependent epitope within this unique functional region of HSV gB and applied a cell-based competition assay. Fig. 1B shows that the parental mAb 2c, the chimeric mAb ch2c, and the humanized mAb hu2c competed with each other for binding to gB on the surface of HSV-1–infected Vero cells. Using peptide microarrays either spanning the extracellular gB region as 13mer overlapping peptides or displaying the consensus sequences of the discontinuous epitope as a peptide-duotope, we further confirmed that mAb hu2c exhibited the same restricted peptide reactivity and the same epitope fine specificity as its murine counterpart mAb 2c (Fig. S1 A and B). To ensure bioactivity of the recombinant antibodies after prolonged exposure to physiological temperatures, we assessed their antigen binding activities toward HSV-1–infected Vero cells by flow cytometry at several time points after incubation at 37 °C. Fig. 1C shows that the antigen-binding activity of the chimeric and humanized antibody remained fully stable even after incubation at 37 °C over a period of 4 wk. To further examine the biophysical stability of the humanized antibody as another important aspect for the development of therapeutic proteins, we analyzed its aggregation propensity by size exclusion chromatography after storage for 1 y at 4 °C and 1 mo at 37 °C. Although no stabilizing excipients have been used, mAb hu2c elution profiles and area under the curves remained unchanged for both storage conditions and were comparable to freshly purified mAb hu2c (Fig. 1D).
Fig. 1.
Humanized mAb hu2c retains the same specificity and biophysical properties of its parental murine mAb 2c. (A) Equilibrium-binding curves of mAbs 2c, ch2c, and hu2c as determined by flow cytometric analysis with HSV-1 infected Vero cells. (B) Binding of murine mAb 2c to glycoprotein B on the surface of HSV-1 F-infected Vero cells could be blocked either by mAb ch2c or mAb hu2c at 10-fold molar excess. Flow cytometric measurements are representative for at least two experiments performed in triplicate ± SEM. (C) Biological stability. Antigen-binding activity of antibodies (15 µg/mL, PBS) was assayed by flow cytometry after incubation for various time periods at 37 °C. (D) Same batch analysis of purified mAb hu2c (1 mg/mL in PBS) either stored at 4 °C for 1 y or 37 °C for 1 mo by size exclusion FPLC on a Superdex 200 column revealed single monomeric peaks with elution times (21 ± 0.07 min) identical to the profile of mAb hu2c analyzed immediately after production and purification.
Wild-Type and Nucleoside Analog-Resistant HSV Strains Are Highly Susceptible to mAb hu2c Neutralization.
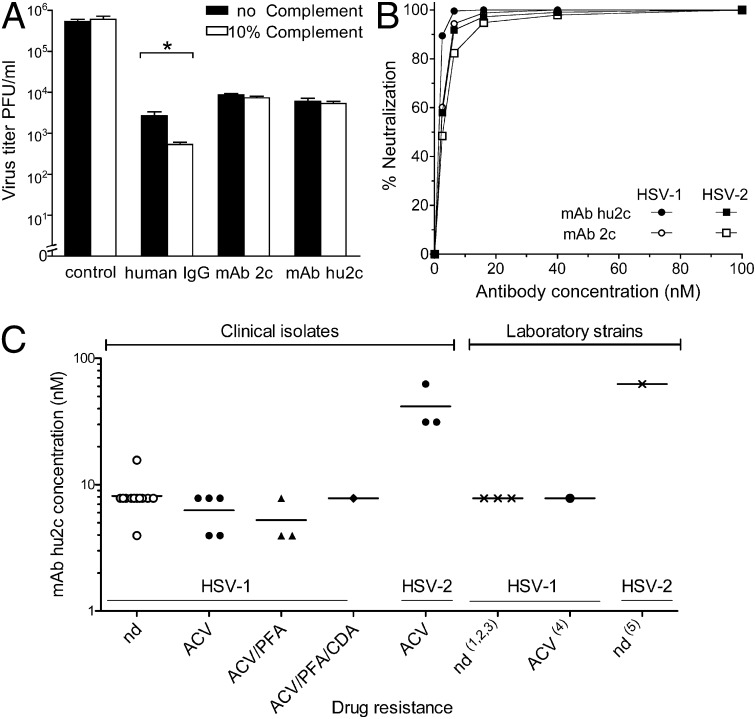
For assessing the antiviral potency of mAb hu2c we first analyzed its neutralization efficacy toward free virions using standard plaque reduction assays. Fig. 2A illustrates that both the murine and the humanized antibody neutralized free virions completely independent from complement activity. In contrast, a human IgG preparation (Cytotect; Biotest Pharmaceuticals) neutralized free virions in a clearly complement-dependent manner. The humanized antibody neutralized HSV-1 and HSV-2 as efficiently as its murine counterpart (Fig. 2B). The HSV-1 IC50 for mAb hu2c was 1.4 nM and for mAb 2c ws 2 nM. HSV-2 was neutralized by mAb hu2c and mAb 2c with IC50s of 2.2 nM and 2.7 nM, respectively. To further determine the antibody concentration being required for completely preventing HSV-1– and HSV-2–induced cytopathic effects, we carried out endpoint titration assays on confluent Vero cell monolayers grown in 96-well microtiter plates. Both the humanized and the murine antibody showed 100% neutralization of HSV-1 and HSV-2 at concentrations of 8 nM and 32 nM, respectively. Because mutations conferring viral resistance toward standard virustatics do not affect envelope glycoproteins we reasoned that mAb hu2c should also efficiently neutralize drug-resistant HSV strains. We therefore tested the activity of mAb hu2c for neutralizing patient HSV isolates with either unknown or known drug resistance (Fig. 2C). The neutralization assay revealed that clinical HSV-1 and HSV-2 isolates were indeed as susceptible to inactivation by mAb hu2c as nonresistant laboratory strains. Most importantly, neutralization efficiency of mAb hu2c was entirely independent from the clinical drug resistance status in all of the 12 tested patient HSV isolates. Even strains with TK-dependent and TK-independent drug cross-resistance were neutralized by mAb hu2c, with the same high efficacy as nonresistant laboratory strains or clinical isolates of unknown resistance. Complete neutralization of resistant HSV-2 isolates, however, consistently required 4- to 8-fold higher mAb hu2c concentrations than HSV-1 isolates. Because fusogenic loop regions of the gB protein play a most fundamental role for efficient HSV uptake into cells, we sequenced the regions encompassing the conformational epitope recognized by mAb hu2c from all tested drug-resistant clinical HSV isolates. The sequence comparison showed that the discontinuous epitope recognized by mAb hu2c remained unchanged in all isolates tested (Fig. S2) and explains the high efficacy of mAb hu2c also toward resistant and multiresistant HSV strains.
Fig. 2.
Neutralization of free HSV virions. (A) Complement-independent neutralization of mAbs 2c and hu2c. The neutralizing antibody activity of gB-specific mAbs or a human IgG preparation (Cytotect) toward a defined virus load of 5 × 105 pfu HSV-1 F was analyzed in the presence or absence of 10% human complement by plaque reduction assay on Vero cells 36 h after infection. The addition of complement significantly increased the neutralizing activity of polyclonal human antibodies (120 µg/mL; *P < 0.05; Mann Whitney U test). In contrast, both mAb 2c (0.5 µg/mL) and mAb hu2c (0.5 µg/mL) showed a complement-independent neutralization activity. (B) Humanized mAb displayed the same neutralization activity as the parental mAb against HSV-1 F and HSV-2 G. The neutralization activity was measured by plaque reduction assay from two individual experiments. (C) Clinical HSV-1 and HSV-2 isolates were neutralized by mAb hu2c irrespective of their resistance or cross-resistance toward standard antiviral drugs with the same efficiency as laboratory strains (1)HSV-1 F, (2)HSV-1 342 hv, (3)HSV-1 17 syn+, (4)HSV-1 thymidine kinase mutant strain TK−; and (5)HSV-2 G. ACV, acyclovir. CDV, cidofovir; nd, drug resistance not determined; PFA, foscarnet.
MAb hu2c Blocks HSV Cell-to-Cell Transmission with High Efficacy.
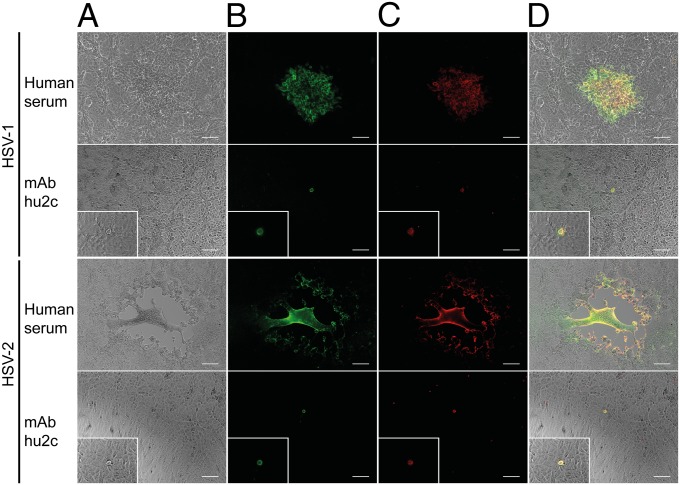
The presence of neutralizing antibodies does not necessarily prevent cell-to-cell spread of herpesviridae (23, 24). We therefore evaluated the impact of mAb hu2c on disruption of HSV-1 and HSV-2 cell-to-cell spread by mimicking this particular dissemination mode in vitro. To infect individual cells, confluent Vero cell monolayers were initially incubated with either HSV-1 or HSV-2 at low multiplicity of infection. To promote direct cell-to-cell transmission from individually infected cells but prevent viral spread through viral particles across the cell culture supernatant, Vero cell monolayers were treated with an excess of either pooled human polyclonal HSV-neutralizing sera, a purified human normal IgG preparation (Intratect; Biotest Pharmaceuticals), or purified mAb hu2c. After 48 h virus spread was detected by immunostaining with a mouse monoclonal antibody specific for a common epitope on glycoprotein D of HSV-1 and HSV-2. In the presence of pooled human polyclonal sera or purified human IgGs neither HSV-1 nor HSV-2 transmission could sufficiently be blocked, and extensive virus spread with plaque formation on Vero cell monolayers was observed (Fig. 3 A and B and Fig. S3 A and B). In contrast, mAb hu2c completely inhibited cell-to-cell spread of both viruses, as evidenced by detection of only single HSV-infected cells (Fig. 3B). Colocalization of human antibodies or humanized antibody with HSV-infected cells was demonstrated by staining with fluorescence-labeled anti-human IgG (Fig. 3 C and D and Fig. S3 C and D).
Fig. 3.
Blocking of viral cell-to-cell transmission by mAb hu2c. HSV-1 F- and HSV-2 G-infected Vero cells were incubated with either pooled human polyclonal HSV-neutralizing sera (1:40 for HSV-1 and 1:10 for HSV-2) or mAb hu2c (75 µg/mL for HSV-1 and 300 µg/mL for HSV-2). (B) Viral transmission was visualized 48 h after infection by immunostaining with an HSV-1/2 gD-specific murine antibody and an Alexa 488-conjugated secondary antibody. (C) Human and humanized IgGs were detected with an anti-human Cy3-conjugated antibody. (D) Colocalization of HSV-infection and antibodies as overlay of the phase-contrast pictures (A) with the fluorescence microscopic images (B and C). (Magnification, 100× and 400× for enlarged detail; scale bar, 100 μm.)
Off-target binding of therapeutic mAbs toward normal tissues can cause severe toxic side effects in patients. To evaluate cross-reactivity of mAb hu2c, we performed immunohistochemistry (IHC) analyses on 30 different human frozen tissues as recommended for antibody therapeutics by the US Food and Drug Administration. For this purpose mAb hu2c was both biotinylated for direct tissue staining as well as used in combination with a secondary antibody staining system. To first analyze the sensitivity, specificity, and reproducibility of IHC with mAb hu2c, infected Vero cells and brain sections from HSV-infected and uninfected mice were stained. Both the biotinylated as well as the nonbiotinylated humanized anti-HSV antibody produced a strong consistent staining in HSV-infected Vero cells as well as in infected brains of mice. Respective noninfected Vero cells as well as brains of noninfected animals were entirely negative. This proved the ability of mAb hu2c to reliably detect HSV in tissue (Fig. S4 A–D). We then probed for cross-reactivity toward a frozen normal human tissue array under identical experimental conditions. Weak to moderate immunoreactivity potentially representing low-affinity antibody binding was noted only in some thyreocytes and epithelial cells of salivary ducts (Fig. S4 E and F). When using the biotinylated antibody, we observed in addition some faint staining in hepatocytes, tubular kidney epithelium, and some bone marrow cells (Fig. S4 G–I). In contrast, we did not find this staining pattern in any of the investigated tissue when using the nonbiotinylated antibody with its respective detection system. Therefore, the faint staining pattern in liver, kidney, and bone marrow most likely indicates unspecific staining, which by our experiences is seen with a broad array of different antibodies on cryoslides and does not represent true antibody binding. All other normal tissues (adrenal, brain, breast, colon, esophagus, heart, kidney glomeruli, cholangial liver structures, lung, skeletal muscle, mesothel, nerve, ovary, pancreas, placenta, prostate, salivary glands, skin, small intestine, spleen, stomach, testis, thymus, thyroid, tonsil, uterus, and cervix) were entirely negative with both biotinylated and nonbiotinylated humanized antibodies.
Prophylactic and Therapeutic Treatment with mAb hu2c Protects Immunodeficient Mice from Lethal Herpes Simplex Encephalitis.
The increasing number of organ transplantations, administration of highly aggressive conditioning chemotherapy regimens in tumor patients before stem cell transplantation, and rising number of HIV infections over the last few decades have all resulted in a significantly higher number of immunocompromised patients worldwide. In those patients HSV reactivation is common and may cause most serious complications. For testing the in vivo efficacy of mAb hu2c we thus consequently applied an immunodeficient mouse model for HSV infection, resembling most closely the clinical situation of rapid HSV dissemination with eventual development of lethal encephalitis. In this set of experiments we evaluated (i) the efficacy of mAb hu2c for preventing the onset of HSV infection, and (ii) the capability of the humanized antibody for protecting animals with established HSV infection from a fatal course of disease.
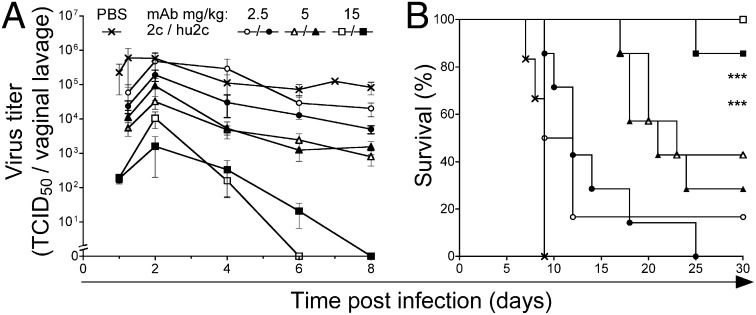
For disease protection experiments we investigated varying antibody concentrations (2.5–15 mg/kg). Nonobese diabetic (NOD)/SCID mice received mAb hu2c i.v. as a single dose 24 h before a vaginal challenge with HSV-1. Both the murine and the humanized mAb showed equivalent protection of NOD/SCID mice against HSV-1 infection (Fig. 4). Although antibody concentrations of 5 mg/kg did not significantly inhibit viral replication in the mucous membranes, 29% (2 of 7) of the mAb hu2c- and 43% (3 of 7) of the mAb 2c-treated animals were protected against virus-induced encephalitis (Fig. 4 A and B). Treatment of animals with a single dose of 15 mg/kg of either mAb 2c or mAb hu2c provided complete elimination of viral replication in the mucous membranes by days 6–8 after infection and protected animals against lethal encephalitis (Fig. 4 A and B). In the mAb 2c-treated group 100% of the animals survived (Fig. 4B). The single death in the mAb hu2c-treated group (1 of 8) was unrelated to HSV infection, because no signs of HSV-related symptoms were found, and no virus could be detected in the brain or bone marrow of the diseased mouse.
Fig. 4.
Antibody-mediated in vivo protection against herpes encephalitis. NOD/SCID mice received i.v. a single dose [15 mg/kg (squares); 5 mg/kg (triangles); 2.5 mg/kg (circles)] of either mAb 2c (open symbols) or mAb hu2c (closed symbols) 24 h before intravaginal challenge with a lethal dose of 1 × 106 TCID50 HSV-1 F. Control mice infected with HSV-1 F received PBS (cross). (A) Dose-dependent virus clearance from the vaginal mucosa, and (B) prevention of mortality. ***P < 0.001, log–rank test, Mantel-Cox. Error bars represent SEM.
To study the protective activity of the humanized antibody hu2c against neuronal spread from established peripheral infection, HSV-1–infected NOD/SCID mice received three therapeutic doses (15 mg/kg) of either mAb hu2c or the parental murine antibody mAb 2c, starting 1 d after infection. Postexposure administration of both antibodies resulted in clearance of vaginal virus shedding within 8 d and complete protection from HSV-1–associated death (Fig. 5A). In contrast, all mice of the control group treated with only PBS developed lethal encephalitis and died within 9 d (Fig. 5A). Death of one individual animal in each antibody treatment group was unrelated to HSV infection because after necropsy no infectious virus was detected in the brain or internal organs.
Fig. 5.
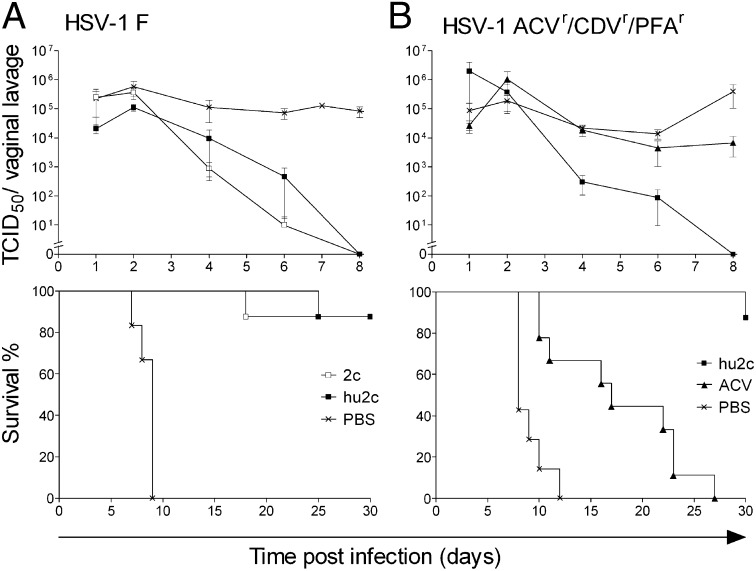
Protection of immunodeficient mice against dissemination of established HSV-1 infection by systemic treatment with mAb hu2c. NOD/SCID mice intravaginally infected with either (A) a laboratory HSV-1 strain (F), or (B) a multidrug-resistant clinical HSV-1 isolate (ACVR/CDVR/PFVR) were treated with antibodies i.v. 24 h, 40 h, and 56 h after infection at 15 mg/kg (n =8). Control groups received either (A and B) PBS (n = 7) or (B) ACV at 50 mg/kg every 12 h i.p. (n = 9). In contrast to the control groups, antibody-treated mice with established HSV-1 infections exhibited complete virus clearance from vaginal mucosa by day 8 independent of the viral drug resistance pattern (A and B, Upper) and were significantly protected from death (***P < 0.0001, log–rank test) (A and B, Lower). In mice infected with the multiresistant HSV-1 isolate ACV treatment had only minor effect on the virus load in the vaginal mucosa and could only delay the lethal outcome of the infection (***P = 0.0008, log–rank test) (B). Error bars represent SEM.
In immunocompromised patients the development of drug resistance, and particularly multidrug resistance, toward commonly used anti-HSV therapies could have fatal consequences and is therefore considered a most serious medical problem. To study whether mAb hu2c may become a therapeutic alternative in this clinical situation, we infected NOD/SCID mice with a clinically multidrug-resistant (ACVr/CDVr/PFAr) HSV-1 patient isolate 24 h before treatment with mAb hu2c or ACV (Fig. 5B). Drug resistance values of this isolate for 50% effective concentrations (EC50) against cytopathic effect were 53 µM for ACV, 14 µM for CDV, and 954 µM for foscarnet, respectively. Similar to the laboratory HSV strain, infection with the multidrug-resistant patient isolate caused lethal encephalitis in PBS-treated control animals between days 8 and 12 (Fig. 5B). Median survival time of mice receiving ACV standard therapy (50 mg/kg body weight) every 12 h was significantly longer than in the PBS control group (P = 0.0008; Fig. 5B). However, complete suppression of HSV-1 replication could not be achieved in this group, and all mice eventually developed progressive disease and lethal encephalitis. In contrast, infection was fully resolved in 7 of 8 infected mice after treatment with mAb hu2c (3× 15 mg/kg body weight). After treatment with mAb hu2c, virus shedding in the vaginal mucosa of mice was eliminated by day 8, and only one mouse developed clinical signs of HSV disease and eventually died from viral infection within the observation period of 30 d (Fig. 5B). Notably, this mouse displayed a significantly higher virus titer in the vaginal mucosa (1.6 × 107 median tissue culture infective dose, TCID50) 1 d after infection in comparison with the average virus titer level in vaginal lavages of the other animals (3–9 × 104 TCID50).
Discussion
ACV and related drugs are current standard of care for the treatment and suppression of both primary HSV infection and HSV reactivation. Risk groups such as immunocompromised patients or patients with herpetic keratitis who increasingly develop resistance and cross-resistance toward these drugs urgently require alternate options for preventing and treating drug-resistant HSV variants. Several attempts for developing a potent HSV vaccine have been made, but no such compound showed thus far sufficient clinical efficacy even in a large randomized trial (1). We have recently shown that the murine monoclonal antibody mAb 2c exhibits highly potent HSV-neutralizing activity through binding to a key fusogenic domain of the viral gB protein (20, 21). To exploit this property for therapeutic interventions in humans we have in the present study humanized mAb 2c and characterized its potential to prevent and treat HSV infection in vitro and in severely immunocompromised mice.
To develop a humanized antibody with the lowest possible immunogenic potential we grafted the murine mAb 2c CDRs into human germ-line acceptor frameworks. Generation of humanized antibodies has at times proven difficult, because simple grafting of the CDR into human acceptor frameworks was shown to be rarely sufficient for retaining appropriate antigen binding affinity (24, 25). This is in most cases caused by sterical constraints of the anchored human CDRs within the human frameworks. To preserve the grafted CDR conformations, retention of murine framework residues making key contacts to CDR loop residues is therefore usually required (26). For instance, the humanized anti-HSV mAbs Fd79 (gB) and Fd138-80 (gD) retain two and nine murine framework key contact residues, respectively, as well as seven or eight additional framework residue substitutions in the human acceptor frameworks (24). We show here that by careful selection of human acceptor germ-line frameworks in combination with our previously described sequence multialignment approach (22) a fully functional humanized antibody devoid of any potentially immunogenic murine framework residues could be generated. The humanized antibody mAb hu2c retained all essential properties of the parental mAb, namely specificity for the same type-common gB epitope, high affinity, and stability. We have also demonstrated that the humanized antibody exhibited the same efficient virus-neutralizing activity and potency for successful inhibition of viral transmission through cell-to-cell spread in vitro and in vivo. These features indicate retention of the same fine specificity of the humanized antibody in vivo and thus absence of sterical constraints of the grafted antigen-binding site and stable anchorage of the binding loops within the human acceptor frameworks.
To screen for potential off-target toxicity of the antibody we conducted a tissue cross reactivity study as a prerequisite for subsequent GLP-compliant toxicity studies in relevant animal models. Weak to moderate staining of some cells in very few nonvital organs revealed surprisingly little cross-reactivity of the antibody with human tissues compared with other approved antibody therapeutics (27–29).
Research investigating antibody-mediated protection in a mouse vaginal HSV-2 infection model demonstrated that polyclonal HSV-specific IgG antibodies largely rely on FcγR-dependent mechanisms (30). By using T-cell, B-cell, and natural killer cell function deficient NOD/SCID mice in the present study we could show that the protective mechanism conferred by mAb hu2c is entirely independent from FcγR-mediated effector functions. This feature has most important implications for the clinical use of the humanized antibody in highly immunocompromised situations such as, for example, in patients undergoing myeloablative chemotherapy followed by hematopoetic stem cell transplantation. When testing the neutralization capacity of mAb hu2c toward patient isolates of both HSV serotypes including drug- or multidrug-resistant HSV strains we observed that these isolates were neutralized with the same high in vitro efficacy as corresponding laboratory strains. These results provide evidence that on the basis of its specific mode of action (20) the humanized antibody is also highly active against HSV-1 and HSV-2 viruses that have become resistant or multiresistant toward the commonly used anti-HSV drugs. Remarkably, this capacity was fully retained in the in vivo situation: 7 of 8 NOD/SCID mice being infected with a multidrug-resistant HSV-1 patient isolate were cured by treatment with mAb hu2c. The only diseased animal in this group had an exceptionally high viral load after infection. The unique feature of overcoming drug resistance renders mAb hu2c also highly effective in the most desperate clinical situation if all available drugs for preventing viral dissemination are no longer effective.
We recently mapped the epitope of the mouse antibody 2c and analyzed its binding by homology modeling based on the solved crystal structure of the gB complex (20, 21). We found that the antibody binds to a discontinuous epitope comprising exposed residues 299–305 (upper section of domain I of gB) as well as residues of the sequence motif FEDF (also located in domain I). Surprisingly, the FEDF epitope is buried when mapped to the gB crystal structure and would be inaccessible to mAb 2c binding in the postfusion state. It therefore seems very likely that binding of mAb 2c at least to the FEDF motif must occur in the prefusion state and thereby prevent conformation transition to the postfusion state. Because monovalent derivatives of mAb 2c (scFv and Fab) were not able to control viral spread, we reasoned that cross-linking of gB through a bivalent molecule is a prerequisite for the antiviral activity of mAb 2c by stabilization of the gB prefusion conformation via immobilization of gB trimers that in turn prevents triggering the fusogenic machinery. This specific and unique feature of mAb 2c may well explain the extraordinary efficacy of the antibody for preventing cell-to-cell spread across even heavily infected tissues.
Complete neutralization of HSV-2 and inhibition of HSV-2 cell-to-cell transmission consistently required approximately fourfold higher antibody concentrations in comparison with the antiviral activity toward HSV-1. Because epitope alterations in HSV-2 vs. HSV-1 isolates could be excluded by epitope sequencing, we concluded that the different neutralization efficacy of mAb hu2c toward HSV-1 vs. HSV-2 isolates is the consequence of higher HSV-2 genome copy numbers, resulting in an increase of noninfectious virus particles, as we have reported earlier (20).
Other humanized or human anti-HSV mAbs with prophylactic and/or therapeutic potential have been described before. It has been shown that a humanized mAb specific for gB (24) or a fully human anti-gD specific mAb derived from a combinatorial human antibody phage display library (31) conferred protection against viral transmission in immunocompetent mice when topically applied almost simultaneously with a vaginal HSV-2 challenge (32, 33). Another study showed that i.p. treatment of T-cell–deficient nude mice with the aforementioned anti-gD human mAb either 24 h before, at the time of, or 24 h after intracutaneous HSV-1 infection protected 50%, 20%, and 11% of the mice from death (34). We are not aware, however, whether either one of these antibodies has been further developed for clinical applications.
In summary, we have generated a humanized monoclonal antibody (mAb hu2c) for immunotherapy of severe HSV infections particularly in immunocompromised patients. We have shown that this antibody very efficiently neutralizes clinical HSV isolates, including multidrug-resistant variants both in vitro and in severely immunocompromised mice, and exerts its anti-HSV activity fully independently of immune effector mechanisms. These features warrant the clinical development of mAb hu2c for treatment of severe HSV infections in immunocompromised and drug-resistant patients.
Methods
SI Methods provides a detailed description of experimental conditions.
Virus Neutralization and Cell-to-Cell Spread Assay.
Neutralizing activity of antibodies was determined either by endpoint titration assay or plaque reduction assay. For complement-dependent neutralization HSV-1 F (5 × 105 pfu) was incubated either with monoclonal antibodies mAb 2c or hu2c at 0.5 µg/mL or 120 µg/mL purified IgG from human donors with high CMV-neutralizing titers (Cytotect) in the presence or absence of 10% IgG-depleted human serum as a source of complement for 1 h at 37 °C before infection of Vero cells. Virus plaques were counted after 36 h of incubation. Neutralization experiments were performed in quadruplets; shown is the arithmetic mean ± SD. In cell-to-cell spread experiments either a human normal IgG preparation (Intratect) with a neutralizing titer of 125 µg/mL for HSV-1 and 500 µg/mL for HSV-2 or human polyclonal serum with high titers of anti-HSV-Ig were used as controls. Immunofluorescence images were acquired with a Zeiss Observer Z1 fluorescence microscope.
Mouse Experiments.
Intravaginal infection of female NOD/SCID (NOD.CB17-Prkdcscid/J) mice (Charles River Laboratories) with 1 × 106 TCID50 HSV-1 F or a multidrug-resistant clinical HSV-1 isolate was performed as previously described (20). Mice were treated by i.v. injection of purified mAb hu2c either 24 h before infection for immune prophylaxis or 24 h, 40 h, and 56 h after infection for therapeutic treatment. In the therapy experiment of multidrug-resistant virus infection an additional cohort of mice was treated i.p. with 50 mg/kg body weight ACV every 12 h during the course of the experiment starting on day 1 after infection. Therapy studies included exclusively animals with detectable HSV-1 infection (n = 7–8). All animal experiments were in compliance with institutional animal care guidelines and use committee-approved protocols.
Supplementary Material
Acknowledgments
We thank Anne Schlegelmilch, Armin Keller, Evelyn Exner, Miriam Dirks, and Tamana Karimi for excellent technical assistance, Henrike Reinhard for IgG-depleted human serum, and Prof. Dr. T. Mertens for kindly providing the clinical drug-resistant isolates. This work was supported by Deutsche José Carreras Leukämie Foundation Grant R06/14, the Jürgen Manchot Foundation, and by Deutsche Forschungsgemeinschaft Grant GK-1045.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220019110/-/DCSupplemental.
References
- 1.Belshe RB, et al. Herpevac Trial for Women Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimberlin DW, et al. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108(2):230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 3.Berrington WR, et al. Clinical correlates of herpes simplex virus viremia among hospitalized adults. Clin Infect Dis. 2009;49(9):1295–1301. doi: 10.1086/606053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooke AE, Eveson JW, Luker J, Oakhill A. Oral presentation of a novel variant of herpes simplex infection in a group of bone marrow transplant patients: A report of five cases. Br J Dermatol. 1999;141(2):381–383. doi: 10.1046/j.1365-2133.1999.03018.x. [DOI] [PubMed] [Google Scholar]
- 5.Yahav D, et al. Antiviral prophylaxis in haematological patients: Systematic review and meta-analysis. Eur J Cancer. 2009;45(18):3131–3148. doi: 10.1016/j.ejca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Remeijer L, Osterhaus A, Verjans G. Human herpes simplex virus keratitis: The pathogenesis revisited. Ocul Immunol Inflamm. 2004;12(4):255–285. doi: 10.1080/092739490500363. [DOI] [PubMed] [Google Scholar]
- 7.Stránská R, et al. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: Prevalence and characterization. J Clin Virol. 2005;32(1):7–18. doi: 10.1016/j.jcv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Danve-Szatanek C, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42(1):242–249. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morfin F, et al. HSV excretion after bone marrow transplantation: A 4-year survey. J Clin Virol. 2004;30(4):341–345. doi: 10.1016/j.jcv.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Sauerbrei A, et al. Novel resistance-associated mutations of thymidine kinase and DNA polymerase genes of herpes simplex virus type 1 and type 2. Antivir Ther. 2011;16(8):1297–1308. doi: 10.3851/IMP1870. [DOI] [PubMed] [Google Scholar]
- 11.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55(2):459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blot N, et al. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant. 2000;26(8):903–905. doi: 10.1038/sj.bmt.1702591. [DOI] [PubMed] [Google Scholar]
- 13.Darville JM, Ley BE, Roome AP, Foot AB. Acyclovir-resistant herpes simplex virus infections in a bone marrow transplant population. Bone Marrow Transplant. 1998;22(6):587–589. doi: 10.1038/sj.bmt.1701392. [DOI] [PubMed] [Google Scholar]
- 14.Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 15.Baeten JM, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: A randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198(12):1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delany S, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: A randomized placebo-controlled trial in South Africa. AIDS. 2009;23(4):461–469. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagot N, et al. ANRS 1285 Study Group Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356(8):790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 18.McMahon MA, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283(46):31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matamoros T, Kim B, Menéndez-Arias L. Mechanistic insights into the role of Val75 of HIV-1 reverse transcriptase in misinsertion and mispair extension fidelity of DNA synthesis. J Mol Biol. 2008;375(5):1234–1248. doi: 10.1016/j.jmb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Krawczyk A, et al. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J Virol. 2011;85(4):1793–1803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Däumer MP, et al. Characterisation of the epitope for a herpes simplex virus glycoprotein B-specific monoclonal antibody with high protective capacity. Med Microbiol Immunol (Berl) 2011;200(2):85–97. doi: 10.1007/s00430-010-0174-x. [DOI] [PubMed] [Google Scholar]
- 22.Krauss J, Arndt MA, Martin AC, Liu H, Rybak SM. Specificity grafting of human antibody frameworks selected from a phage display library: Generation of a highly stable humanized anti-CD22 single-chain Fv fragment. Protein Eng. 2003;16(10):753–759. doi: 10.1093/protein/gzg096. [DOI] [PubMed] [Google Scholar]
- 23.Hooks JJ, Burns W, Hayashi K, Geis S, Notkins AL. Viral spread in the presence of neutralizing antibody: Mechanisms of persistence in foamy virus infection. Infect Immun. 1976;14(5):1172–1178. doi: 10.1128/iai.14.5.1172-1178.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Co MS, Deschamps M, Whitley RJ, Queen C. Humanized antibodies for antiviral therapy. Proc Natl Acad Sci USA. 1991;88(7):2869–2873. doi: 10.1073/pnas.88.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Co MS, et al. Chimeric and humanized antibodies with specificity for the CD33 antigen. J Immunol. 1992;148(4):1149–1154. [PubMed] [Google Scholar]
- 26.Almagro JC, Fransson J. Humanization of antibodies. Front Biosci. 2008;13:1619–1633. doi: 10.2741/2786. [DOI] [PubMed] [Google Scholar]
- 27.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med (Berl) 1999;77(10):699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 28.Skartved NJ, et al. Preclinical pharmacokinetics and safety of Sym004: A synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res. 2011;17(18):5962–5972. doi: 10.1158/1078-0432.CCR-11-1209. [DOI] [PubMed] [Google Scholar]
- 29.Bussiere JL, Leach MW, Price KD, Mounho BJ, Lightfoot-Dunn R. Survey results on the use of the tissue cross-reactivity immunohistochemistry assay. Regul Toxicol Pharmacol. 2011;59(3):493–502. doi: 10.1016/j.yrtph.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Chu CF, et al. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J Reprod Immunol. 2008;78(1):58–67. doi: 10.1016/j.jri.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burioni R, Williamson RA, Sanna PP, Bloom FE, Burton DR. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci USA. 1994;91(1):355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitlin L, et al. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat Biotechnol. 1998;16(13):1361–1364. doi: 10.1038/4344. [DOI] [PubMed] [Google Scholar]
- 33.Zeitlin L, et al. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab’)2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225(1):213–215. doi: 10.1006/viro.1996.0589. [DOI] [PubMed] [Google Scholar]
- 34.Sanna PP, et al. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215(1):101–106. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.