Abstract
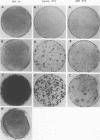
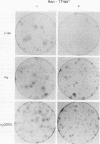
Expression of GTPase-deficient Gi2 alpha subunit (alpha i2) mutant polypeptides and overexpression of the wild-type alpha i2 polypeptide in Rat 1a, Swiss 3T3, and NIH 3T3 fibroblasts altered normal growth regulation and induced a loss of contact inhibition. In Rat 1a cells (but not in NIH 3T3 or Swiss 3T3 cells), expression of the GTPase-deficient alpha i2 mutant polypeptides allowed colony formation in soft agar, which correlated with a loss in anchorage dependence and a decreased serum requirement. The altered growth regulatory properties of Rat 1a cells induced by expression of alpha i2 mutant polypeptides was not significantly inhibited by cotransfection with a dominant negative Ha-ras mutant polypeptide (Asn-17rasH), indicating that the activated Gi2 membrane signal transduction protein is uniquely capable of altering the regulation of Rat 1a cell growth by a predominantly c-ras-independent mechanism. The results show that GTPase-deficient alpha i2 mutant polypeptides have the properties of an oncogene that can induce the phenotypic characteristics of transformation in Rat 1a cells but that only a subset of these changes is observed with NIH 3T3 and Swiss 3T3 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Cai H., Szeberényi J., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990 Oct;10(10):5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. E., Jauniaux J. C., Roger P. P. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci. 1989 Feb;14(2):67–71. doi: 10.1016/0968-0004(89)90046-7. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Identification of a 42-kilodalton phosphotyrosyl protein as a serine(threonine) protein kinase by renaturation. Mol Cell Biol. 1990 Jun;10(6):3020–3026. doi: 10.1128/mcb.10.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S., Scolnick E. M., Dixon R. A., Vogel U. S. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J Biol Chem. 1990 Nov 25;265(33):20437–20442. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson T. H., Roeber J. F., Johnson G. L. Conformational changes of adenylate cyclase regulatory proteins mediated by guanine nucleotides. J Biol Chem. 1981 Feb 10;256(3):1459–1465. [PubMed] [Google Scholar]
- Johnson G. L., Dhanasekaran N. The G-protein family and their interaction with receptors. Endocr Rev. 1989 Aug;10(3):317–331. doi: 10.1210/edrv-10-3-317. [DOI] [PubMed] [Google Scholar]
- Kirschmeier P. T., Housey G. M., Johnson M. D., Perkins A. S., Weinstein I. B. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988 Apr;7(3):219–225. doi: 10.1089/dna.1988.7.219. [DOI] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Kohno M., Pouysségur J. Alpha-thrombin-induced tyrosine phosphorylation of 43,000- and 41,000-Mr proteins is independent of cytoplasmic alkalinization in quiescent fibroblasts. Biochem J. 1986 Sep 1;238(2):451–457. doi: 10.1042/bj2380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Burgess A. W. Autocrine growth factors and tumourigenic transformation. Immunol Today. 1990 Jul;11(7):244–249. doi: 10.1016/0167-5699(90)90098-t. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Lowndes J. M., Gupta S. K., Osawa S., Johnson G. L. GTPase-deficient G alpha i2 oncogene gip2 inhibits adenylylcyclase and attenuates receptor-stimulated phospholipase A2 activity. J Biol Chem. 1991 Aug 5;266(22):14193–14197. [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., Ui M. Possible involvement of a GTP-binding protein, the substrate of islet-activating protein, in receptor-mediated signaling responsible for cell proliferation. J Biol Chem. 1987 Sep 15;262(26):12463–12467. [PubMed] [Google Scholar]
- Neer E. J., Pulsifer L., Wolf L. G. The amino terminus of G protein alpha subunits is required for interaction with beta gamma. J Biol Chem. 1988 Jun 25;263(18):8996–8970. [PubMed] [Google Scholar]
- Osawa S., Dhanasekaran N., Woon C. W., Johnson G. L. G alpha i-G alpha s chimeras define the function of alpha chain domains in control of G protein activation and beta gamma subunit complex interactions. Cell. 1990 Nov 16;63(4):697–706. doi: 10.1016/0092-8674(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Nakamura S., Kaziro Y. Platelet-derived growth factor stimulates formation of active p21ras.GTP complex in Swiss mouse 3T3 cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5993–5997. doi: 10.1073/pnas.87.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Kahan C., Hartmann T., Pouyssegur J. Strong and persistent activation of inositol lipid breakdown induces early mitogenic events but not Go to S phase progression in hamster fibroblasts. Comparison of thrombin and carbachol action in cells expressing M1 muscarinic acetylcholine receptors. J Biol Chem. 1990 Dec 25;265(36):22292–22299. [PubMed] [Google Scholar]
- Strathmann M. P., Simon M. I. G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann M., Simon M. I. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Wong Y. H., Federman A., Pace A. M., Zachary I., Evans T., Pouysségur J., Bourne H. R. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991 May 2;351(6321):63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- Woon C. W., Soparkar S., Heasley L., Johnson G. L. Expression of a G alpha s/G alpha i chimera that constitutively activates cyclic AMP synthesis. J Biol Chem. 1989 Apr 5;264(10):5687–5693. [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]