Abstract
Proliferative vitreoretinopathy (PVR) exemplifies a disease that is difficult to predict, lacks effective treatment options, and substantially reduces the quality of life of an individual. Surgery to correct a rhegmatogenous retinal detachment fails primarily because of PVR. Likely mediators of PVR are growth factors in vitreous, which stimulate cells within and behind the retina as an inevitable consequence of a breached retina. Three classes of growth factors [vascular endothelial growth factor A (VEGF-A), platelet-derived growth factors (PDGFs), and non-PDGFs (growth factors outside of the PDGF family)] are relevant to PVR pathogenesis because they act on PDGF receptor α, which is required for experimental PVR and is associated with this disease in humans. We discovered that ranibizumab (a clinically approved agent that neutralizes VEGF-A) reduced the bioactivity of vitreous from patients and experimental animals with PVR, and protected rabbits from developing disease. The apparent mechanism of ranibizumab action involved derepressing PDGFs, which, at the concentrations present in PVR vitreous, inhibited non–PDGF-mediated activation of PDGF receptor α. These preclinical findings suggest that available approaches to neutralize VEGF-A are prophylactic for PVR, and that anti–VEGF-based therapies may be effective for managing more than angiogenesis- and edema-driven pathological conditions.
Proliferative vitreoretinopathy (PVR) is an example of a disease that remains difficult to manage despite multidecade efforts to improve treatment options.1–4 It is the primary reason for failure to correct a rhegmatogenous retinal detachment (RRD) and occurs in 5% to 10% of patients, although in cases involving penetrating ocular trauma, the incidence of disease approaches 50%.5–8 RRDs are detachment caused by a tear or other breaks in the retina, and can result in more severe retinal detachment (RD) or recurrent RD after surgical repair. The formation of membranes on the surface of the retina is associated with PVR and widely believed to promote this condition. Repeat surgery is the only treatment option; however, visual acuity better than 5/200 is achieved in less than half of the eyes.9 Vision loss is permanent and thereby substantially reduces the quality of life for these patients.
By using 2010 census data, an estimated 55,000 individuals in the United States experience an RRD annually, and by extrapolation, 2750 to 5550 people will develop PVR.5 Both the small size of this population and the difficulty in predicting which patient will succumb to this affliction make PVR a low priority for the pharmaceutical industry. Consequently, efforts to develop new therapies for PVR and evaluate them in clinical trials have languished.
The putative mediators of PVR pathogenesis (growth factors) also contribute to more common diseases, such as atherosclerosis and cancer.10–14 Consequently, the benefit of identifying culprits of PVR and their functional relationships is likely to extend beyond the scope of PVR.
Breaks in the retina expose cells within and beneath it to vitreous, which is a rich source of growth factors that are implicated in PVR pathogenesis.7,15–29 Although vitreous from both patients and experimental animals with PVR contain a wide spectrum of growth factors,29 those agents that act on the platelet-derived growth factor (PDGF) receptor α (PDGFRα) appear to be especially relevant to PVR pathogenesis because PDGFRα is associated with clinical PVR and is required for experimental PVR in the most commonly used model of this disease.15,30–34 These vitreal agents include PDGFs and two other classes of growth factors that influence activation of PDGFRα, namely, non-PDGFs [growth factors outside of the PDGF family (eg, insulin growth factor-1, EGF, human growth factor, and basic fibroblast growth factor) and vascular endothelial growth factor A (VEGF-A)].29,35–37
There are at least two ways to activate PDGFRα, and only the way that results in a decline in the level of p53 effectively induces PVR.35,36,38,39 Vitreous abounds with non-PDGFs that activate PDGFRα in the way that reduces p53.29,39 Although PDGFs are also present, their level in vitreous of patients and rabbits with PVR is insufficient to cause a substantial decline in the level of p53.38,39 Furthermore, PDGFs antagonize the ability of non-PDGFs to reduce the p53 level.37,39 The underlying mechanism appears to involve PDGF-mediated reduction in the amount of PDGFRα available to non-PDGFs.39 The results of these biochemical studies indicate that the key agent in vitreous that drives PVR is non-PDGFs, whereas PDGFs protect from PVR.
In vivo studies confirmed some, but not all, of these concepts. Neutralizing vitreal non-PDGFs protected rabbits from developing PVR, and thereby confirmed the idea that non-PDGFs were required for experimental PVR.29 In contrast, inhibiting vitreal PDGFs had no effect.36 In light of the fact that PDGFs antagonize non-PDGFs, one would have expected that neutralizing PDGFs should have promoted PVR. Although these findings indicated a requirement for non-PDGFs in experimental PVR, they also indicated that there were additional concepts that needed to be considered.
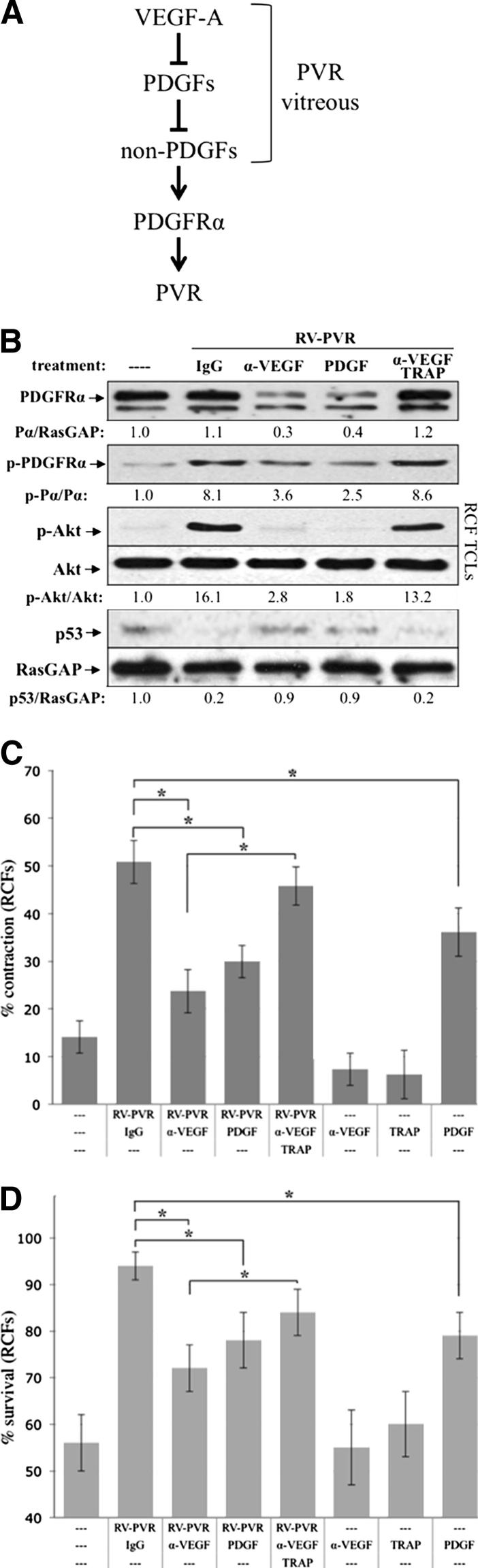
For instance, VEGF, which is also present in patients and experimental animals with PVR,16,20,29,40 competitively inhibited PDGF from binding and activating PDGFRα.37 Thus, a plausible explanation for why neutralizing PDGFs had no impact on PVR was because VEGF was already eliminating their contribution. Furthermore, because PDGFs antagonize non-PDGFs, then VEGF (by inhibiting PDGF) should promote the action of non-PDGFs (Figure 1A). This reasoning led to the prediction that neutralizing VEGF would inhibit PVR because it would allow PDGF-mediated inhibition of non-PDGFs, which activate PDGFRα in the way that leads to PVR.
Figure 1.
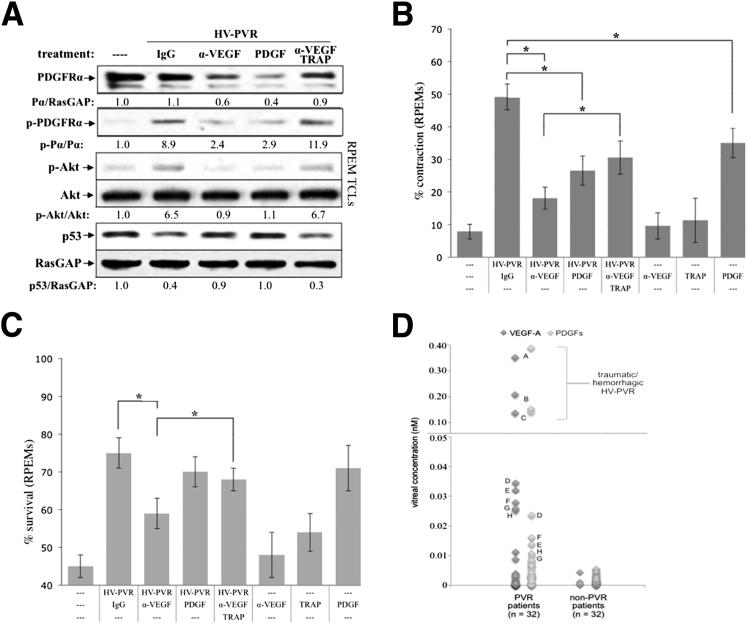
Vitreous-driven signaling events and cellular responses associated with PVR were potentiated by vitreal VEGF-A. A: The functional relationship between three classes of growth factors present in PVR vitreous. VEGF-A antagonizes the action of PDGFs, which block non-PDGFs (growth factors outside of the PDGF family) that activate PDGFRα indirectly and thereby drive experimental PVR.37,39B: Neutralizing vitreal VEGF-A prevented PVR vitreous-driven signaling events. Primary RCFs were serum starved overnight and either lysed immediately without treatment (—) or continuously treated for 48 hours with 400 μL RV-PVR supplemented with 10 μg/mL nonimmune IgG, 25 μg/mL neutralizing anti-VEGF antibody, ranibizumab (α-VEGF), 20 ng/mL PDGF-A, or a combination of 10 μg/mL α-VEGF and 2 μmol/L PDGF TRAP. After treatment, cells were lysed and the resulting TCLs were subjected to Western blot analysis using the indicated antibodies and quantified (see Materials and Methods). Ratios representing normalized band intensities are shown under each immunoblot. Blots shown are representative of three independent experiments. C: Neutralizing vitreal VEGF-A suppressed PVR vitreous-driven cell contraction. RCFs were preconditioned for 48 hours with serum-free medium alone (—) or 400 μL RV-PVR supplemented with 10 μg/mL nonimmune IgG, 25 μg/mL α-VEGF, 2 μmol/L α-VEGF + PDGF TRAP, or 20 ng/mL PDGF-A; in addition, cells were preconditioned with α-VEGF, PDGF TRAP, or PDGF-A alone as controls. After preconditioning, cells were transferred to collagen gels containing the same treatment and subjected to the collagen gel contraction assay. Gel area was measured after 24 hours. Data are presented as percentage contraction of collagen gels measured after 24 hours, and are represented as mean percentage contraction ± SDs obtained for three independent experiments. D: Neutralizing vitreal VEGF-A prevented PVR vitreous-driven cell survival. Near-confluent RCFs were placed in starvation medium (DMEM without serum) for 72 hours as an inducement of apoptosis, during which time they were conditioned with the same treatments as described in B. At 72 hours, surviving cells were quantified as those cells whose nuclei failed to stain positive for apoptosis (by TUNEL assay, see Materials and Methods). The graph presents data from three independent experiments showing the mean percentages of cells (± SD) surviving starvation. ∗P < 0.05 using a paired t-test. In each experiment, 12 randomly chosen fields were counted. Original magnification, ×100.
In the course of investigating this possibility, we discovered that ranibizumab, an anti–VEGF-A monoclonal antibody fragment, reduced the pathogenic bioactivity of vitreous from patients and experimental animals with PVR and protected rabbits from developing this disease. These preclinical findings suggest that one or more of the clinically approved approaches to neutralize VEGF-A are prophylactic for PVR. In addition, anti–VEGF-based therapies may be effective for managing more than the angiogenesis and vascular permeability-driven pathological conditions.
Materials and Methods
Growth Factors, Antibodies, and Major Reagents
Recombinant human PDGF-A, PDGF-AB, and PDGF-B were purchased from Peprotech Inc. (Rocky Hill, NJ). The following antibodies were raised in the laboratory, as referenced: anti-PDGFRα,41,42 anti–phospho-PDGFRα (Y742),43 and anti-RasGAP.44 Anti-Akt (9272S) and anti–phospho-Akt (pS473 and 9271L) were purchased from Cell Signaling (Danvers, MA). Anti–phospho-PDGFRα (pY720), anti-p53 (sc-126), PrA-agarose beads (sc-2001), and horseradish peroxidase–conjugated goat anti-rabbit and goat anti-mouse IgG secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescent substrate for horseradish peroxidase detection was purchased from Pierce (Rockford, IL). PDGF TRAP (which binds to and sequesters PDGF) is a chimera consisting of the extracellular domain of PDGFRα fused to human IgG Fc5, was provided by Dr. Debra Gilbertson at ZymoGenetics.42 The anti–VEGF-A Fab fragment, ranibizumab (GENENTECH, South San Francisco, CA), was a generous gift from Dr. Patricia D’Amore (Schepens Eye Research Institute, Boston, MA).
Cell Culture and Treatments
Primary rabbit conjunctival fibroblasts (RCFs) were isolated as described previously.45 These cells were used for analysis of rabbit vitreous bioactivity and for injection into rabbit eyes in the experimental PVR model. Primary mouse embryonic fibroblasts (MEFs) were obtained at third passage from ATCC (Manassas, VA) and used for the shRNA knockdown and receptor activation threshold experiments. R627 cells are immortalized fibroblasts derived from mouse embryos nullizygous for both PDGFR isoforms, in which a full-length kinase-inactive mutant PDGFRα is re-expressed.15,46 R627 cells were used in experiments to ascertain the role of PDGF-induced dimerization of PDGFRα in reducing the bioactivity of vitreous. ARPE-19α cells, derived from the human retinal pigment epithelial (RPE) cell line, ARPE-19 (ATCC), overexpress human PDGFRα.41 These cells were used to investigate the PDGFRα-inhibitory activity of human PVR vitreous. RPE cells from human PVR membranes (RPEM cells) were isolated from a surgically removed patient PVR membrane.47 RPEM cells (at passages 4 to 7) were used in experiments to assess human vitreous bioactivity. PAE-KDR cells are pig aortic endothelial (PAE) cells that overexpress human VEGF receptor 2 (VEGFR2), as previously described48; these cells were used to test whether heat functionally inactivates VEGF-A in PVR vitreous.
RCFs, MEFs, and R627 cells were maintained in high-glucose–containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, NY). ARPE-19α, RPEM, and PAE-KDR cells were maintained in a 1:1 mixture of high-glucose DMEM and Ham’s F12 medium (Gibco BRL). Cells were cultured in media supplemented with 10% fetal bovine serum (FBS), 500 U/mL penicillin, and 500 μg/mL streptomycin, and incubated at 37°C in a humidified 5% CO2 atmosphere.
Cells were grown to near confluence, serum starved overnight in DMEM with 0.1% FBS, and treated the next morning. Vitreous (RV-PVR or HV-PVR) that was used to stimulate cells consisted of an equal-volume pool of several individual samples. Vitreous or DMEM, supplemented with the indicated treatments, was added directly to cells after removal of media and rinsing with PBS. Treatments were performed under the same conditions as cells that were incubated (37°C in a humidified 5% CO2 atmosphere).
Preparation of Rabbit Vitreous
Vitreous was extracted from either PVR-positive rabbit eyes (RV-PVR) or healthy control rabbits eyes (RV) that were enucleated and frozen at −80°C. While still frozen, vitreous was removed, allowed to thaw to room temperature, and then centrifuged at 4°C for 5 minutes at 10,000 × g. The resultant clarified vitreous was used for subsequent analysis. Vitreous used for treatment is an equal-volume mix from several rabbit eyes of comparable clinical status (ie, PVR stage).
Western Blot Analysis
Cells were washed twice with ice-cold PBS after treatment, then lysed in sample buffer (50 mmol/L Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 1% β-mercaptoethanol, 10 mmol/L EDTA, and 0.02% bromophenol blue). Total cell lysates (TCLs) were incubated on ice for 15 minutes, heated to 95°C for 5 minutes, and clarified by centrifugation at 13,000 × g, 4°C for 15 minutes. Samples were then run on 8% or 10% acrylamide SDS-PAGE gels, and resolved proteins were transferred to polyvinylidene difluoride immunoblotting membranes by semidry transfer. Each immunoblot shown is representative of three independent experiments. Signal intensity was determined by densitometry using Quantity One software version 4.0 (Bio-Rad, Pinole, CA); signal quantities shown were standardized to background and normalized for loading.
Cell Contraction Assay
Cells were grown to near confluence and then preconditioned for 48 hours with the indicated treatment in DMEM before performing the contraction assay, as previously described.32,49 In brief, 1 × 106 cells/mL were suspended in a solution containing 1.5 mg/mL neutralized collagen I at pH 7.2 (INAMED, Fremont, CA) plus the indicated treatment and transferred to 24-well plates pre-incubated with PBS and 5 μg/μL bovine serum albumin for a minimum of 4 hours. After polymerization of the collagen gel (by incubation at 37°C for 90 minutes), gels were overlaid with 0.5 mL DMEM supplemented with the same indicated treatment. At 24 hours, collagen gel diameters were measured. At 0 hours, the diameter of the gel equals the diameter of the well. Triplicate gels were measured within each experiment. Values shown are means ± SD of at least three independent experiments.
Cell Survival Assay
Cells (RCFs or RPEMs) were seeded onto 60-mm dishes at 1 to 2 × 105 cells per dish, grown to 80% confluence in DMEM containing 10% FBS, then rinsed three times with PBS and cultured in starvation media (DMEM + 0.1% FBS) along with the indicated treatments. Media plus treatment was changed daily. On day 3 (at 72 hours), cells were rinsed in cold PBS and then apoptotic cells were quantified by the TUNEL assay using an apoptosis detection system, according to manufacturer’s instructions (Promega, Fitchburg, WI). The percentage of surviving cells was calculated as follows: (number of nonapoptotic nuclei/total nuclei analyzed) × 100. Because this assay does not account for dead cells that dislodged from the monolayer and were lost during the PBS rinses, the data presented likely underrepresent the actual percentage of cell survival.
Rabbit PVR Model
Dutch-belted adult (aged 3 to 6 months) female rabbits were purchased from Covance (Denver, PA). PVR was induced in the right eye of rabbits, as previously described.15,45 In brief, rabbits were acclimated for 1 week and then injected with 0.1 mL of perfluoropropane gas (Alcon, Fort Worth, TX) into the vitreous cavity 3 mm posterior to the limbus. One week later, each experimental eye was co-injected with 2 × 105 RCFs in 0.1 mL platelet-rich plasma (PRP) and 0.1 mL of balanced salt solution containing either 0.05 mg anti–VEGF-A (RBZ) or the same amount of isotype-matched control IgG. The PRP is included in the injection regimen to improve the severity and consistency of the pathological response to the injected fibroblasts.15
The retinal status was monitored using an indirect ophthalmoscope (Keeler Instruments, Broomall, PA) with a +30-diopter fundus lens (Volk Optical, Mentor, OH) at days 1, 3, 5, 7, 14, 21, and 28 after injection. Intraocular pressures were monitored daily for 3 days after gas injection and for 1 day after the co-injections (of cells + PRP and IgG/RBZ) using a tonometer. PVR was graded according to the five-stage scale of Fastenberg et al,50 described as follows: stage 0, no disease; 1, epiretinal-membrane formation; 2, vitreoretinal traction without retinal detachment; 3, localized retinal detachment of one to two quadrants; 4, extensive retinal detachment of two to four quadrants, without complete detachment; and 5, complete retinal detachment. Signs of toxicity, including intraocular inflammation and retinal hemorrhages, were assessed during each fundus examination and by histological analysis of retinas after sacrifice of animals. Retinal function was assessed by obtaining single-flash electroretinograms (ERGs) using a ColorDome stimulator (DIAGNOSYS, Westford, MA) of representative rabbits at day 26 after injection (both the injected right eye and the noninjected left eye were measured). Animals were sacrificed at day 28, and eyes were enucleated and frozen at −80°C to preserve their vitreous for later analysis. Surgical procedures were performed aseptically in conformance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The Schepens Animal Care and Use Committee approved the protocol used for these animal experiments.
Patient Vitreous
Undiluted patient vitreous was obtained by standard three-port vitrectomy with removal of vitreous before pars plana infusion.51 A total of 64 human vitreous samples were obtained: 32 with PVR and 32 with non-PVR retinal conditions (principally, macular holes, macular puckers, and non-rhegmatogenous retinal detachments). Individual sample volumes ranged from 0.6 to 1.3 mL.
Patient vitreous came from three independent sources: 32 (18 PVR and 14 non-PVR) from US Army military personnel donors at the Ophthalmology Service, Department of Surgery, Walter Reed Army Medical Center (Washington, DC); 16 (7 PVR and 9 non-PVR) from patient donors at the Queen Elizabeth II Hospital (Halifax, NS, Canada) and at the Vancouver Hospital in association with Capital Health (Halifax) and Vancouver Hospital, University of British Columbia (Vancouver, BC, Canada); and 16 (8 PVR and 8 non-PVR) from patient donors at the Ocular Angiogenesis Group, Department of Ophthalmology, Academic Medical Center, Meibergdreef (Amsterdam, The Netherlands). Research involving human specimens adhered to the tenets of the Declaration of Helsinki. Informed written consent was obtained from all patient donors. Before conducting any experiments, institutional review board approval was obtained to perform these studies (Schepens Eye Research Institute Institutional Review Board protocol S-226-0212, Vitreal Factors in Proliferative Vitreoretinopathy).
Quantifying VEGF-A and PDGFs in Human Vitreous
VEGF-A and PDGFs (comprising the A, AB, and B isoforms) were quantified in individual vitreous samples from humans with PVR (n = 32) or with non-PVR retinal conditions (n = 32, including macular holes, macular puckers, or non-rhegmatogenous retinal detachments). Multiplex bead analysis was performed as previously described.29 In brief, each vitreous sample (50 μL) was added in triplicate to a 96-well plate and then incubated overnight with a mixture of anti–VEGF-A, anti–PDGF-A, anti–PDGF-AB, and anti–PDGF-B monoclonal antibody–coated capture beads. Beads were washed and incubated with biotin-labeled anti-human polyclonal growth factor/cytokine antibodies for 1 hour, and then streptavidin-phycoerythrin was added for 30 minutes. Fluorescent emissions distinct to each growth factor were simultaneously measured using the BioPlex Detection System and the resulting data were analyzed using BioPlex multiplex software version 2.1 (BioRad). Growth factor concentrations were determined based on a series of standards run in parallel.
Statistical Analysis
All data were analyzed using the unpaired t-test or Mann-Whitney analysis; P < 0.05 was considered statistically significant.
Results
VEGF-A Potentiates the Bioactivity of PVR Vitreous
Previous studies (summarized in Figure 1A) predict that VEGF potentiates the bioactivity of vitreous (ie, ability to indirectly activate PDGFRα) by inhibiting PDGF-mediated suppression of non-PDGFs. This concept predicts that neutralizing VEGF would promote PDGF-mediated suppression of indirect activation of PDGFRα. We proceeded to test this prediction by assessing the impact of neutralizing VEGF on the bioactivity of vitreous and experimental PVR.
To maximize the translatability of these studies, we neutralized VEGF using ranibizumab, an Fab fragment of an antibody that neutralizes VEGF and an agent clinically approved for ocular use. Ranibizumab is designated α-VEGF for in vitro experiments and RBZ for in vivo experiments.
Figure 1B shows that α-VEGF diminished the ability of rabbit PVR vitreous (RV-PVR, which contains non-PDGFs, PDGFs, and VEGF-A)29 to drive the signature signaling events of PVR (phosphorylation of PDGFRα, prolonged activation of Akt, and suppression of p53)39 in primary RCFs. Varying the concentration of α-VEGF indicated that this phenomenon was dose dependent (Supplemental Figure S1). The competitive relationship between VEGF-A and PDGFs, diagrammed in Figure 1A, predicts that α-VEGF would derepress PDGFs and thereby promote a decline in the level of PDGFRα and antagonize non–PDGF-mediated signaling events.39 Indeed, neutralizing PDGFs by adding PDGF TRAP eliminated the effect of α-VEGF (Figure 1B), whereas TRAP alone had no effect (Supplemental Figure S2). Furthermore, boosting the concentration of PDGFs to a level that outcompetes VEGF-A for binding to PDGFRα37 had the same impact as α-VEGF (Figure 1B). These findings indicate that neutralizing VEGF-A diminished the ability of RV-PVR to stimulate signaling events associated with PVR, and that the mechanism involved derepressing vitreal PDGFs.
We also observed that α-VEGF attenuated the ability of RV-PVR to induce cellular events associated with PVR, such as contraction (Figure 1C) and survival (Figure 1D). As observed for the signaling events, PDGF TRAP counteracted the effect of α-VEGF, whereas increasing the level of PDGF mimicked it (Figure 1, C and D).
Because PDGF promotes many cellular responses associated with PVR,15,41 it was somewhat of a surprise that PDGF diminished the potency of RV-PVR (Figure 1, B–D). A comparison of PDGF and RV-PVR for their ability to induce contraction or survival revealed that PDGF was less potent than RV-PVR in promoting either response (Figure 1, C and D). Furthermore, because PDGF blocks non-PDGFs (Figure 1A), the maximum achievable response when both types of agonists are present and active (as is the case when α-VEGF derepresses PDGFs in RV-PVR) is the lower level that is attained with PDGF.
Taken together, these findings indicate that VEGF-A boosts the bioactivity of RV-PVR. These observations predict that neutralizing vitreal VEGF-A, and thereby diminishing the bioactivity of vitreous, will safeguard from PVR.
Neutralizing Vitreal VEGF-A Prevents Experimental PVR
To test if neutralizing VEGF-A protected from experimental PVR, we used the most common and aggressive model of this disease.30 Fibroblasts co-injected with PRP into vitreous assemble with extracellular matrix proteins to form a membrane that attaches to the surface of the retina. Contraction of this membrane causes retinal detachment. This animal model mimics those forms of clinical PVR in which the epiretinal membrane is responsible for retinal detachment.30
Experimental PVR was induced in 22 rabbits, randomly divided into two groups that received a single 0.05-mg injection of RBZ or IgG at PVR induction. The IgG-injected group developed PVR with the expected kinetics and severity.36 Within 1 week, 10 (91%) of 11 formed a PVR membrane (greater than stage 0) and 6 (55%) of 11 had developed at least partial retinal detachment (stage 3 or higher) (Supplemental Figure S3). By the end of the experiment (day 28) 9 (82%) of 11 rabbits had retinal detachment (Figure 2A). Previous studies document that injection of IgG does not influence the development of PVR.29,36
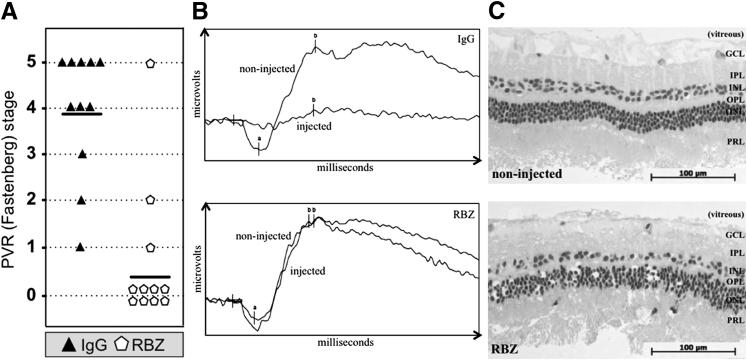
Figure 2.
Neutralizing vitreal VEGF-A safely and effectively prevented experimental PVR (A). One week after an intravitreal gas injection, rabbits received three separate 0.1-mL injections of PVR-inducing RCFs, PRP, and either 0.05 mg of α-VEGF ranibizumab (RBZ) or an equimolar amount of isotype control IgG. The concentration (calculated based on vitreous volume) of RBZ injected was 10-fold less than the amount typically used in human eyes.52,53 For each rabbit, only one eye was injected. Rabbits were examined and scored for development of PVR over a 4-week period; the results from the last time point (day 28) are shown, and the results from all other time points scored are shown in Supplemental Figure S3.Horizontal bars represent the mean PVR stage of each group (n = 11 for each group). Statistically significant differences at each time point were determined by Mann-Whitney analysis (P < 0.001). B: Treatment of rabbits with α-VEGF did not interfere with retinal function. Single-flash ERGs were obtained from rabbits on day 26 after injection; readouts were obtained for both injected and noninjected eyes of the same rabbit after dark adaptation. The ERGs shown span 100 milliseconds and are representative of three individual rabbits per group. The amplitude from the baseline to the a-wave trough (a) reflects the general physiological health of the outer retina (particularly the photoreceptors), while the amplitude from the a-wave trough to the b-wave peak (b) reflects the health of the inner retinal layers. RBZ-treated rabbit eyes elicited the same electrophysiological response as their noninjected counterpart eyes. Light-adapted, single-flash and light-adapted flicker ERGs also showed no significant difference between RBZ-injected and noninjected eyes (data not shown). C: Treatment of rabbits with RBZ did not cause major morphological changes in the retina. Eyes enucleated from a representative RBZ-treated rabbit (PVR stage 0) and a noninjected eye (PVR stage 0) were fixed in 10% formalin, embedded in methacrylate, divided into sections, and the resulting sections were stained with H&E. Representative IgG-treated rabbits had retinal detachments; thus, their morphological characteristics were not included in this analysis. A representative region of the neural retina is shown in each panel. Retinal layers are indicated: GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PRL, photoreceptor layer. These data indicate that α-VEGF/RBZ treatment did not adversely affect the retina.
A single injection of RBZ dramatically reduced PVR pathological characteristics. On day 5, 8 (73%) of 11 RBZ-injected rabbits showed no sign of pathological features compared with 1 (9%) of the 11 IgG-injected group (Supplemental Figure S3). The difference between experimental groups was statistically significant from day 5 to day 28, when 9 (82%) of 11 versus 0 (0%) of 11 rabbits remained disease free in the RBZ- and IgG-injected groups, respectively (Supplemental Figure S3).
Assessment of retinal function indicated that, although the retina was devoid of electrophysiological activity in eyes that developed severe PVR (stage 5, total retinal detachment), it was completely normal in eyes that were protected from PVR by RBZ (Figure 2B). The morphological characteristic of retinal sections from RBZ-injected eyes was overtly normal (Figure 2C). These results indicate that RBZ effectively and safely prevented PVR in this preclinical model of the disease.
PDGF-Mediated Dimerization Attenuates the Bioactivity of RV-PVR
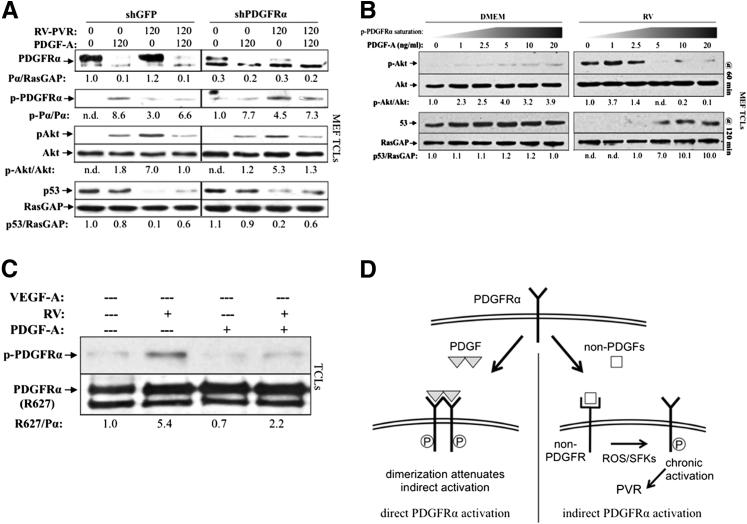
Figure 1B shows that derepressing PDGFs in RV-PVR resulted in a decline in the level of PDGFRα, which is the expected result given that PDGF promotes internalization and degradation of PDGFRα. Because this decline in the level of PDGFRα was associated with a nearly complete loss of the signature signaling events, it suggested that most of the pool of PDGFRα was required to trigger these signaling events. If this were true, then molecularly reducing the level of PDGFRα would also prevent RV-PVR–mediated signature signaling events. However, this is not what we observed. RV-PVR–induced activation of Akt and suppression of p53 were comparable in control cells and in cells expressing only 30% of the starting level of PDGFRα (Figure 3A). Although it is intuitive that reducing the pool of PDGFRα will reduce the number of receptors available for activation by RV-PVR, our results indicate that the mechanism by which PDGF attenuated the bioactivity of RV-PVR involved more than a reduction in the amount of PDGFRα.
Figure 3.
PDGF-mediated dimerization attenuated indirect activation of PDGFRα. A: Reduction of PDGFRα levels by shRNA did not attenuate indirect PDGFRα signaling. Lentiviruses were used to stably express shRNAs directed against green fluorescent protein (GFP; shGFP) or PDGFRα (shPDGFRα) in MEFs; shPDGFRα MEFs expressed approximately 70% less receptor than control shGFP MEFs. Cells were then starved and treated with 400 μL RV-PVR and/or 20 ng/mL PDGF-A, as indicated for 120 minutes at 37°C, then lysed and subjected to Western blot analysis using the indicated antibodies. Both immature and mature PDGFRα band intensities were normalized to RasGAP levels. These findings indicate that no more than 30% of the total pool of PDGFRα was required to trigger signaling events in response to RV-PVR. B: Near-confluent MEFs were serum starved overnight and treated with 400 μL DMEM or RV supplemented with an increasing concentration of PDGF-A. Cells were treated in parallel for 60 and 120 minutes, after which time they were harvested and TCLs were subjected to Western blot analysis using the indicated antibodies. Prolonged Akt phosphorylation and reduction in p53 levels (indicators of indirectly activated PDGFRα)39 were attenuated at saturating doses of PDGF-A, suggesting that PDGF-induced dimerization is a key component of PDGF-mediated attenuation of vitreous-driven indirect signaling of PDGFRα. C: R627 cells were serum starved overnight and either left alone or pre-incubated with 10 ng/mL PDGF-A for 30 minutes at 4°C (to ensure complete receptor dimerization on the cell surface), followed by treatment with or without 400 μL RV for 10 minutes at 4°C. After treatment, cells were lysed and subjected to Western blot analysis using anti–phospho-PDGFRα, followed by anti-pan PDGFRα, and normalized relative to the untreated control. Although indirect activation of PDGFRα still occurred at 4°C, receptor activation was approximately 2.5-fold lower compared with indirect receptor activation at 37°C (Supplemental Figure S4), demonstrating a correlation between dimerization and reduced capacity to undergo indirect activation, further suggesting that PDGF diminishes non–PDGF-mediated activation of PDGFRα by dimerizing PDGFRαs. D: Schematic showing the details and consequences of direct and indirect activation of PDGFRα. ROS, reactive oxygen species; SFK, Src-family kinases.
Given that RV-PVR activates PDGFRα monomers,37 whereas PDGF drives monomers into dimers,54,55 we considered whether PDGF-mediated dimerization of PDGFRα diminished its ability to be activated by RV. RV is vitreous from healthy rabbits and was used instead of RV-PVR because it contains little or no PDGFs or VEGF-A,29 which would complicate the interpretation of the results. A concentration of 5 ng/mL PDGF was the minimum dose required to achieve maximal activation of Akt, indicating that all receptors were dimerized (Figure 3B). This empirical finding was consistent with the fact that 2.5 × 1011 molecules of PDGF were required to saturate the approximately 1 × 1011 PDGFRαs that were present. As shown in Figure 3B, RV-mediated signaling events sharply declined as the concentration of PDGF approached saturation. These results support the idea that PDGF-mediated dimerization of PDGFRα reduced its ability to be activated by RV.
To begin to understand how dimerization could interfere with RV-dependent activation of PDGFRα, we considered whether dimerization influenced the capacity of PDGFRα to undergo RV-mediated phosphorylation, an event that is tightly associated with RV-dependent activation of PDGFRα.35,39 Indeed, PDGF diminished RV-dependent phosphorylation of PDGFRα (Figure 3C and Supplemental Figure S4), which supported the idea that monomeric receptors were more efficiently activated by the indirect route than dimerized receptors. These experiments were done with kinase-inactivated PDGFRα at 4°C to avoid PDGF-dependent autophosphorylation and internalization. We conclude that, in addition to reducing the overall level of PDGFRα on the cell surface, PDGF also attenuated RV-mediated activation of PDGFRα and subsequent signaling events by dimerizing PDGFRα (Figure 3D).
The VEGF-A/PDGF/Non-PDGF Relationship in RV-PVR Is Also Present in Human PVR Vitreous
To what extent does the pathogenesis of experimental PVR relate to clinical PVR? Vitreous is readily available from both experimental animals and patients, and we focused on this opportunity to begin to address this question. More specifically, we considered whether the growth factors and their relationships, which define the bioactivity of RV-PVR (Figure 1A), were comparable in RV-PVR and human PVR vitreous (HV-PVR).
As rabbits develop PVR, the composition of vitreous changes (ie, there is an increase in VEGF-A, PDGFs, and non-PDGFs).29 A similar difference is observed when comparing vitreous from patients with PVR versus vitreous of patients with retinal issues unrelated to PVR.16,20,29,40 Furthermore, PDGFs in both experimental and clinical PVR vitreous underperform.36 To test if this distinguishing feature of HV-PVR bioactivity was due to VEGF-A, as is the case for RV-PVR,37 we subjected HV-PVR to the following series of experiments.
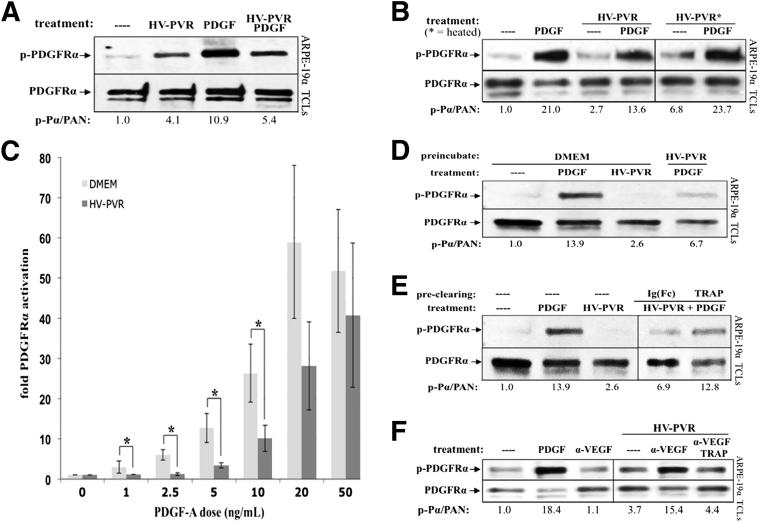
In addition to diminishing PDGF-dependent activation of PDGFRα by PDGFs that were present in vitreous,29,41 HV-PVR had a similar inhibitory impact on exogenously added, recombinant PDGF (Figure 4A). Because certain PDGFs are more thermally stable than VEGF-A,37,56 a simple way to begin to assess if the vitreal inhibitor was VEGF-A was to test if heating HV-PVR mitigated its ability to interfere with PDGF-dependent activation of PDGFRα. As shown in Figure 4B, heating HV-PVR increased the potency of the PDGFs in vitreous and diminished the ability of HV-PVR to interfere with activation of PDGFRα by recombinant PDGF. Heating HV-PVR also reduced its ability to promote phosphorylation of VEGFR2 (Supplemental Figure S5), which is consistent with the heat sensitivity of purified VEGF-A.37 Furthermore, increasing the concentration of PDGF in HV-PVR overcame the inhibitory action of vitreous (Figure 4C). These results supported the idea that VEGF-A was a heat-labile agent that antagonized PDGF-dependent activation of PDGFRα.
Figure 4.
PDGF-dependent activation of PDGFRα was inhibited by VEGF-A, a heat-labile, PDGFRα-associated agent in human PVR vitreous. A: Patient PVR vitreous contains inhibitory activity against PDGF-mediated PDGFRα activation. ARPE-19α cells were grown to near confluence, serum starved overnight, and then treated with serum-free medium alone (—), 200 μL human patient PVR vitreous (HV-PVR), 10 ng/mL PDGF-A, or both HV-PVR and PDGF-A for 5 minutes at 37°C. Cells were lysed and subjected to anti–phospho-PDGFRα (p-PDGFRα) and then anti-PDGFRα Western blot analysis. The p-PDGFRα immunoblot signal was normalized to total PDGFRα (PAN) and is presented as fold induction over the non-stimulated control. Blots shown are representative of three independent experiments. HV-PVR reduced PDGF-mediated activation of PDGFRα by approximately 50%, suggesting that patient PVR vitreous contains an inhibitor of PDGF-mediated PDGFRα activation. B: Inhibitory activity in human patient PVR vitreous (HV-PVR) is labile to heat. In a manner similar to A, cells were starved and treated with serum-free medium without treatment (—), 200 μL HV-PVR, 10 ng/mL PDGF-A, or both HV-PVR and PDGF-A; some HV-PVR treatments were first heat treated to 90°C for 5 minutes and then rapidly cooled on ice. Cells were treated for 5 minutes at 37°C and lysed, and the resulting TCLs were subjected to the same Western blot analysis as in A. Although PDGF-A largely survived the heat treatment, nearly all PDGF-inhibitory activity was eliminated from HV-PVR. Moreover, there were enough endogenous PDGFs in heat-treated HV-PVR to elicit a 3.5-fold activation of PDGFRα (over the non-stimulated control). Thus, heat treatment unmasked the ability of HV-PVR to activate PDGFRα, suggesting the presence of a heat-labile inhibitor that blocks vitreal PDGFs from functioning. C: HV-PVR–mediated inhibition of PDGF-dependent PDGFRα could be overcome by increasing the concentration of PDGF. Cells were cultured and starved as described in A. The indicated amount of PDGF-A was added to either 200 μL DMEM or HV-PVR and then used to treat cells for 5 minutes at 37°C. Cells were lysed and subjected to Western blot analysis, and the results were quantified as in Figure 1. Results from three independent experiments revealed that HV-PVR significantly inhibited PDGFRα phosphorylation at low doses of PDGF-A: 1, 2.5, 5, and 10 ng/mL. ∗P < 0.05 using a paired t-test. D: Cells preconditioned with patient PVR vitreous became resistant to subsequent treatment with PDGF-A. Cells were cultured and starved, as described in A, then pre-incubated for 15 minutes at 37°C with either DMEM or 200 μL HV-PVR. After incubation, the media/vitreous was removed and cells were extensively washed with PBS, after which they were treated with serum-free medium alone (—) or 10 ng/mL PDGF-A for 10 minutes at 37°C. Cells were subsequently lysed, and the resulting TCLs were subjected to Western blot analysis with the indicated antibodies. These data suggest that the inhibitor(s) present in HV-PVR acted at the level of cells. E: Preclearing HV-PVR vitreous with PDGF TRAP significantly reduced its ability to inhibit PDGFRα activation by exogenously added PDGF. HV-PVR (200 μL) was not manipulated or precleared with 2 μmol/L PDGF TRAP or an equimolar amount of a control IgG-Fc fragment (IgG-Fc). These clarified samples were then tested for their ability to block PDGFRα activation by exogenously added PDGF-A. To this end, cells were treated with clarified vitreous and 10 ng/mL PDGF-A for 10 minutes at 37°C. Serum-free media without treatment (—) and 10 ng/mL PDGF-A alone were used as negative and positive controls, respectively. Cells were lysed, and the resulting TCLs were subjected to the same Western blot analysis as used in A. The ability of PDGF TRAP to reduce PDGF-inhibitory activity from HV-PVR suggests that this inhibitor can associate with the extracellular domain of PDGFRα. F: Neutralizing VEGF-A in human PVR vitreous with ranibizumab enabled vitreal PDGFs to activate PDGFRα. Cells were serum starved overnight and either lysed immediately (—) or treated for 10 minutes at 37°C with 10 ng/mL PDGF-A, 10 μg/mL α-VEGF, or 200 μL HV-PVR supplemented with 10 μg/mL nonimmune IgG, 10 μg/mL α-VEGF, or a combination of 10 μg/mL α-VEGF and 2 μmol/L PDGF TRAP. After treatment, cells were lysed and the resulting TCLs were subjected to Western blot analysis using the indicated antibodies and quantified. Ratios representing normalized band intensities are shown under each immunoblot. Blots shown are representative of three independent experiments. These results show that neutralizing VEGF-A significantly enhanced the ability of vitreal PDGFs to activate PDGFRα.
In the context of RV-PVR, VEGF-A acted at the level of PDGFRα (ie, by competitively inhibiting vitreal PDGFs from binding and activating PDGFRα).37 We performed the following two experiments to test if the same was true for HV-PVR. First, pretreating cells with HV-PVR rendered them resistant to subsequent PDGF-dependent activation of PDGFRα (Figure 4D). Second, passing HV-PVR over a matrix coupled to a fusion protein that included the extracellular domain of PDGFRα diminished the ability of HV-PVR to suppress activation of PDGFRα by recombinant PDGF (Figure 4E). We concluded that HV-PVR acted at the level of PDGFRα (instead of at the level of PDGFs).
Finally, if the relationship between VEGF-A, PDGFs, and non-PDGFs in HV-PVR was the same as in RV-PVR (Figure 1A), then addition of α-VEGF to HV-PVR should derepress vitreal PDGFs to activate PDGFRα. This is what we observed (Figure 4F). Taken together, these studies demonstrate that the VEGF-A/PDGF/non-PDGF relationship that defines the bioactivity of RV-PVR (Figure 1A) is likely a critical element of HV-PVR bioactivity as well.
VEGF-A Potentiates the Bioactivity of Human PVR Vitreous
The results from Figure 4 predict that neutralizing VEGF-A will diminish the bioactivity of HV-PVR in a PDGF-dependent manner. Subsequent experiments were done with RPEM cells to further strengthen the relevance to clinical PVR.47 Similar to the results with RV-PVR (Figure 1), α-VEGF reduced HV-PVR–mediated activation of PDGFRα and the subsequent set of signature signaling events (Figure 5A). Neutralizing PDGFs (by adding PDGF TRAP) eliminated the effect of α-VEGF, and increasing the concentration of PDGF had the same effect as α-VEGF (Figure 5A). These findings indicate that α-VEGF diminished the ability of HV-PVR to stimulate signaling events associated with PVR by derepressing vitreal PDGFs.
Figure 5.
Neutralizing VEGF-A in human PVR vitreous prevented PVR-associated signaling events and cellular outcomes in RPE cells isolated from a human PVR membrane. Experimental data shown in A–C were performed similarly to those of Figure 1, B–D, with the exception that, in these experiments, HV-PVR was used (instead of RV-PVR) to stimulate PVR membrane-derived RPE cells (instead of RCFs). D: Comparison of VEGF-A and PDGF levels in the vitreous of patients with or without PVR. Vitreous from patients with PVR or non-PVR retinal diseases (macular holes or macular puckers) was subjected to multiplex analysis to determine the concentration of VEGF-A and PDGFs (total of A, AB, and B isoforms). Although 94% of PVR samples had a detectable level of VEGF-A, the same was true for only 34% of non-PVR samples. Molar amounts of VEGF-A and PDGFs in each sample were frequently detected at similar levels (symbols labeled with the same letter are the same sample). Most had low levels of both, whereas when PDGFs were present, there was also a matched (samples A to C) or slightly elevated (samples D to H) amount of VEGF-A. These observations indicate that the ratio of vitreal VEGF-A/PDGF correlates with clinical PVR and is a potential biomarker for PVR susceptibility. *P < 0.05 using a paired t-test.
We also observed that α-VEGF attenuated the ability of HV-PVR to induce cellular events associated with PVR, such as contraction (Figure 5B) and survival (Figure 5C). As observed for the signaling events (Figure 5A), PDGF TRAP mitigated the α-VEGF effect, whereas increasing the level of PDGF mimicked it (Figure 1, C and D). We conclude that, such as RV-PVR, VEGF-A potentiates the PVR potential of HV-PVR because of the VEGF-A/PDGF/non-PDGF relationship (Figure 1A). Clinical studies are the next logical step to test if neutralizing VEGF-A protects patients from PVR, as it does in the preclinical studies.
The VEGF-A/PDGF Ratio Is a Potential Biomarker for Susceptibility to PVR
If the VEGF-A/PDGF ratio is related to the pathogenesis of clinical PVR, then only certain ratios should be present in the vitreous of patients who develop PVR. Assuming that the level of non-PDGFs is sufficient to activate PDGFRα (a reasonable assumption given that vitreous of patients with PVR is a cornucopia of non-PDGFs),16,17,19,21–24,26,27,29,57 then vitreous from patients with PVR should either have matched levels of both VEGF-A and PDGFs or VEGF-A should be greater that PDGFs, because either scenario will be permissive for non-PDGFs to activate PDGFRα (Figure 1A). Analysis of the level of VEGF-A and PDGFs in vitreous from 32 patients with PVR showed that this was indeed the case (Figure 5D). Most had low levels of both, whereas when PDGFs were present, there was also a matched (samples A to C) or slightly elevated (samples D to H) amount of VEGF-A. These observations indicate that the ratio of vitreal VEGF-A/PDGFs correlates with clinical PVR and is a potential biomarker for PVR susceptibility. Perhaps the next step is to prospectively test if the VEGF-A/PDGFs ratio of the vitreous collected at the initial surgery for RD repair can predict subsequent PVR.
Discussion
We found that VEGF-A promoted the bioactivity of vitreous from patients and rabbits with PVR, and that the mechanism involved repressing PDGFs. Furthermore, neutralizing vitreal VEGF-A by intravitreally administering RBZ protected rabbits from PVR. Finally, the vitreal VEGF-A/PDGFs ratio is a potential indicator of PVR susceptibility.
TRAP (a fusion protein consisting of an antibody Fc domain and the extracellular domain of PDGFRα) binds to both PDGFs and VEGF-A.37 In many of the experiments described herein, TRAP was used to test if the increased bioactivity of vitreous, which was observed on neutralizing VEGF, was dependent on PDGF. In these types of experiments, the ability of TRAP to bind VEGF was irrelevant (because VEGF was already neutralized by α-VEGF) and, hence, did not preclude the interpretation of such experiments.
What is the likelihood that preclinical data presented herein are accurate guides for developing an effective PVR prophylaxis? The similarities between experimental and clinical PVR vitreous strongly support such a possibility. Furthermore, there appears to be a link between PVR and p53 in both experimental and clinical PVR. In rabbits, PDGFRα-mediated reduction of the level of p53 is required for PVR.38 The level of p53 in the retina of patients with PVR is lower than in individuals without PVR.58 Similarly, RRD patients harboring a single-nucleotide polymorphism in p53 are protected from PVR in certain populations.59 Taken together, these findings constitute a reasonable foundation for a clinical trial testing clinically approved anti–VEGF-A reagents, such as ranibizumab, to protect patients from developing PVR. The design of such a study should accommodate the fact that there are probably multiple forms of PVR, the putative existence of ill-defined genetic and/or environmental contributors to PVR, and lessons available from previous clinical trials.52,60
The relationship between vitreal growth factors (Figure 1A) reveals which of them are likely to be the best therapeutic targets. Neutralizing PDGFs should be ineffective, because they are already inhibited by VEGF-A. This prediction is consistent with our findings that several approaches to block PDGFs failed to affect PVR.36 Moreover, a mutant PDGFRα that lacks the extracellular domain and cannot be activated by PDGFs is fully capable of inducing PVR.36 In contrast, neutralizing non-PDGFs or VEGF-A is likely to be effective, and this is what we observed (Figure 2A).29 In light of the fact that many non-PDGFs are capable of indirectly activating PDGFRα, a combination of neutralizing agents is necessary to prevent PVR.29 In contrast, monotherapy directed at VEGF-A is simpler and more effectively protects from even the earliest stages of PVR (formation of a membrane) (Figure 2A and Supplemental Figure S3). Thus, VEGF-A appears to be the best vitreal target.
Not including a nonimmune Fab control group in the experiment shown in Figure 2 leaves open the question of whether any Fab fragment protects rabbits from developing PVR. The likelihood of this possibility seems low because the known function of Fab fragments is to bind antigens. Such as Fab fragments, intact antibodies bind antigens. The control group that was included in Figure 2 was injected with a nonimmune IgG, which failed to protect rabbits from PVR, despite the fact that each IgG molecule contained two Fabs. This observation suggests that individual Fab fragments would likewise fail to protect from PVR. Moreover, antibodies directed against growth factors other than VEGF-A were also unable to protect from PVR.36 These data, together with the knowledge of how antibodies function, strongly support the interpretation of Figure 2A that only Fab fragments capable of neutralizing VEGF-A prevented experimental PVR.
A single injection of RBZ, which effectively prevented rabbits from developing PVR, may be insufficient to protect patients. Unlike patients, rabbits do not undergo a mechanical vitrectomy and, therefore, RBZ clearance from the vitreous cavity will be slower because vitreous is present.1,3,53 In addition, susceptibility to PVR lasts several weeks after RRD repair. Multiple injections of RBZ are likely to be necessary to achieve a persistent level of RBZ sufficient to protect patients from developing PVR. Repeated intravitreal injections of ranibizumab are already being used in the treatment of ophthalmic diseases. They are considered safe and can be performed quickly and with minimal discomfort in the office.53,61
The published contributions of PDGFs in PVR include both promoting and suppressing PVR.30,36,37,39,62 This paradox can be resolved by considering that the dose of PDGF influences its effect. At a high level (500 ng/mL), PDGF begins to induce the types of signaling events that are triggered by non–PDGF-mediated activation of PDGFRα (namely, prolonged activation of Akt and suppression of p53).39 Publications indicating that PDGFs promote PVR typically used a high dose.30,62 In contrast, at the level observed in PVR vitreous (<100 ng/mL), PDGFs antagonize non–PDGF-mediated signaling events because they assemble PDGFRα into dimers, which are less capable of being activated by non-PDGFs. We conclude that PDGFs can either promote PVR (provided that the dose is sufficiently high) or attenuate it, which occurs when the dose is in the range observed in both experimental and clinical PVR vitreous.
The results presented herein indicate that inhibition of VEGF-A is potentially useful in treatment of diseases without angiogenesis or increased vascular permeability. In addition, because some of the mediators of PVR pathogenesis also contribute to more common diseases, such as atherosclerosis and cancer,10–14 identification and characterization of these factors in PVR and their functional relationships are likely to extend beyond the scope of this disease.
Acknowledgements
The authors thank Dr. Dean Eliott for reviewing the manuscript and providing constructive feedback, Dr. Debra Gilbertson for generously supplying PDGF TRAP, Dr. Patricia D'Amore for providing ranibizumab, and Natalie Doran (DIAGNOSYS, Westford, MA) for her technical assistance with rabbit ERGs.
Footnotes
Supported by Department of Defense grant WB1XWH-10-1-0392 and NIH grant EY012509 (A.K.), a Canadian Institutes of Health Research (CIHR) fellowship (S.P.), Mukai Fund and Massachusetts Eye and Ear Infirmary (Boston) gifts (S.M.), and CIHR grants IAO 77736 and MOP 97806 (J.M. and J.C.).
Supplemental Data
The effect of various doses of anti-VEGF on vitreal VEGF-A–driven p53 repression. Primary RCFs were serum starved overnight and either lysed immediately without treatment (—) or continuously treated for 48 hours with 400 μL RV-PVR supplemented with 10 μg/mL nonimmune IgG or the neutralizing anti-VEGF antibody, ranibizumab (α-VEGF), at the indicated concentrations. After treatment, cells were lysed and the resulting TCLs were subjected to Western blot analysis using the indicated antibodies. The signal intensity of the resulting immunoblots was quantified; ratios representing normalized band intensities are shown under the immunoblot. Neutralizing vitreal VEGF-A using α-VEGF at a concentration of 10 μg/mL or higher prevented PVR vitreous-driven repression of p53.
The effect of TRAP alone on vitreous-driven signaling events. This figure is an expansion of Figure 1B, showing that TRAP alone did not affect the ability of RV-PVR to drive PVR-associated signaling events. The p-Akt immunoblot signal (phos) was normalized to total Akt (PAN) and is presented as fold induction over the non-stimulated control.
A single injection with ranibizumab effectively protected rabbits from developing PVR. This figure is an expansion of Figure 2A showing the PVR score from every time point examined during the course of the experiment. The experiment is described in the legend of Figure 2A. Briefly, rabbits received separate, consecutive injections of RCFs, PRP, and either 0.05 mg of ranibizumab (RBZ; white pentagons) or the equivalent amount of isotype control IgG (black triangles). Horizontal bars represent the mean of each group. Statistically significant differences at each time point were determined by Mann-Whitney analysis (P values are indicated above each time point). Significant differences between the two groups were observed as early as day 5, indicating that neutralizing VEGF-A effectively prevented induction of PVR at an early stage.
PDGF-mediated dimerization attenuated indirect activation of PDGFRα. Part of the experiment shown in Figure 3C, which was performed at 4°C, was conducted at 37°C. The results reveal the expected result of a stronger response at the higher temperature.
Heat treatment inactivated VEGF-A in PVR vitreous. PAE-KDR cells were serum starved overnight and then treated with DMEM alone (—) or 200 μL of either human patient or rabbit PVR vitreous (HV-PVR or RV-PVR, respectively) that was unheated or had been heat treated at 90°C for 5 minutes and then rapidly cooled on ice (asterisks). After treatment for 5 minutes at 37°C, cells were lysed and subjected to Western blot analysis using anti–phospho-VEGFR2 and anti-VEGFR2. The phospho-VEGFR2 immunoblot signal was normalized to total VEGFR2. The p-VEGFR2 immunoblot signal (phos) was normalized to total VEGFR2 (PAN) and is presented as fold induction over the non-stimulated control. HV-PVR was pooled from patients with severe (traumatic/hemorrhagic) PVR. The reduced ability of heat-treated vitreous to activate VEGFR2 compared with non–heat-treated controls suggests that VEGF-A is heat labile in both rabbit and human PVR. This idea is supported by the observation that purified VEGF is heat labile.37
References
- 1.Han D: Proliferative Vitreoretinopathy. In: Albert D, JW M, DT A, BA B editor. Philadelphia, PA: Elsevier Saunders; 2008;p. 2315–2324.
- 2.Michels R.G., Wilkinson C.P., Rice T.A. Mosby; St. Louis: 1990. Proliferative Retinopathy. pp 669–706. [Google Scholar]
- 3.Pastor J.C. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43:3–18. doi: 10.1016/s0039-6257(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 4.Patel N.N., Bunce C., Asaria R.H., Charteris D.G. Resources involved in managing retinal detachment complicated by proliferative vitreoretinopathy. Retina. 2004;24:883–887. doi: 10.1097/00006982-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Asaria R.H., Charteris D.G. Proliferative vitreoretinopathy: developments in pathogenesis and treatment. Compr Ophthalmol Update. 2006;7:179–185. [PubMed] [Google Scholar]
- 6.Asaria R.H., Kon C.H., Bunce C., Sethi C.S., Limb G.A., Khaw P.T., Aylward G.W., Charteris D.G. Silicone oil concentrates fibrogenic growth factors in the retro-oil fluid. Br J Ophthalmol. 2004;88:1439–1442. doi: 10.1136/bjo.2003.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campochiaro P.A. Mechanisms in ophthalmic disease: pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 8.Glaser B.M., Cardin A., Biscoe B. Proliferative vitreoretinopathy: the mechanism of development of vitreoretinal traction. Ophthalmology. 1987;94:327–332. doi: 10.1016/s0161-6420(87)33443-8. [DOI] [PubMed] [Google Scholar]
- 9.Abrams G.W., Azen S.P., McCuen B.W., 2nd, Flynn H.W., Jr., Lai M.Y., Ryan S.J. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up: Silicone Study report 11. Arch Ophthalmol. 1997;115:335–344. doi: 10.1001/archopht.1997.01100150337005. [DOI] [PubMed] [Google Scholar]
- 10.Bruhn M.A., Pearson R.B., Hannan R.D., Sheppard K.E. Second AKT: the rise of SGK in cancer signalling. Growth Factors. 2010;28:394–408. doi: 10.3109/08977194.2010.518616. [DOI] [PubMed] [Google Scholar]
- 11.Kim E.K., Choi E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Koenen R.R., Weber C. Chemokines: established and novel targets in atherosclerosis. EMBO Mol Med. 2011;3:713–725. doi: 10.1002/emmm.201100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raman D., Sobolik-Delmaire T., Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–589. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saggini A., Anogeianaki A., Maccauro G., Tete S., Salini V., Caraffa A., Conti F., Fulcheri M., Galzio R., Shaik-Dasthagirisaheb Y.B. Cholesterol, cytokines and diseases. Int J Immunopathol Pharmacol. 2011;24:567–581. doi: 10.1177/039463201102400303. [DOI] [PubMed] [Google Scholar]
- 15.Andrews A., Balciunaite E., Leong F.L., Tallquist M., Soriano P., Refojo M., Kazlauskas A. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:2683–2689. [PubMed] [Google Scholar]
- 16.Banerjee S., Savant V., Scott R.A., Curnow S.J., Wallace G.R., Murray P.I. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007;48:2203–2207. doi: 10.1167/iovs.06-1358. [DOI] [PubMed] [Google Scholar]
- 17.Baudouin C., Fredj-Reygrobellet D., Brignole F., Negre F., Lapalus P., Gastaud P. Growth factors in vitreous and subretinal fluid cells from patients with proliferative vitreoretinopathy. Ophthalmic Res. 1993;25:52–59. doi: 10.1159/000267221. [DOI] [PubMed] [Google Scholar]
- 18.Canataroglu H., Varinli I., Ozcan A.A., Canataroglu A., Doran F., Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–381. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 19.Charteris D.G. Growth factors in proliferative vitreoretinopathy. Br J Ophthalmol. 1998;82:106. doi: 10.1136/bjo.82.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citirik M., Kabatas E.U., Batman C., Akin K.O., Kabatas N. Vitreous vascular endothelial growth factor concentrations in proliferative diabetic retinopathy versus proliferative vitreoretinopathy. Ophthalmic Res. 2012;47:7–12. doi: 10.1159/000324200. [DOI] [PubMed] [Google Scholar]
- 21.Cui J.Z., Chiu A., Maberley D., Ma P., Samad A., Matsubara J.A. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye (Lond) 2007;21:200–208. doi: 10.1038/sj.eye.6702169. [DOI] [PubMed] [Google Scholar]
- 22.Elner S.G., Elner V.M., Jaffe G.J., Stuart A., Kunkel S.L., Strieter R.M. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 23.Harada C., Mitamura Y., Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog Retin Eye Res. 2006;25:149–164. doi: 10.1016/j.preteyeres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Hinton D.R., He S., Jin M.L., Barron E., Ryan S.J. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye (Lond) 2002;16:422–428. doi: 10.1038/sj.eye.6700190. [DOI] [PubMed] [Google Scholar]
- 25.Kim I.K., Arroyo J.G. Mechanisms in proliferative vitreoretinopathy. Ophthalmol Clin North Am. 2002;15:81–86. doi: 10.1016/s0896-1549(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 26.Kon C.H., Occleston N.L., Aylward G.W., Khaw P.T. Expression of vitreous cytokines in proliferative vitreoretinopathy: a prospective study. Invest Ophthalmol Vis Sci. 1999;40:705–712. [PubMed] [Google Scholar]
- 27.La Heij E.C., van de Waarenburg M.P., Blaauwgeers H.G., Kessels A.G., Liem A.T., Theunissen C., Steinbusch H., Hendrikse F. Basic fibroblast growth factor, glutamine synthetase, and interleukin-6 in vitreous fluid from eyes with retinal detachment complicated by proliferative vitreoretinopathy. Am J Ophthalmol. 2002;134:367–375. doi: 10.1016/s0002-9394(02)01536-2. [DOI] [PubMed] [Google Scholar]
- 28.Lei H., Rheaume M.A., Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2010;90:376–381. doi: 10.1016/j.exer.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennock S., Rheaume M.A., Mukai S., Kazlauskas A. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol. 2011;179:2931–2940. doi: 10.1016/j.ajpath.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal R.N., He S., Spee C., Cui J.Z., Ryan S.J., Hinton D.R. In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007;2:67–77. doi: 10.1038/nprot.2007.4. [DOI] [PubMed] [Google Scholar]
- 31.Cui J., Lei H., Samad A., Basavanthappa S., Maberley D., Matsubara J., Kazlauskas A. PDGF receptors are activated in human epiretinal membranes. Exp Eye Res. 2009;88:438–444. doi: 10.1016/j.exer.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikuno Y., Kazlauskas A. An in vivo gene therapy approach for experimental proliferative vitreoretinopathy using the truncated platelet-derived growth factor alpha receptor. Invest Ophthalmol Vis Sci. 2002;43:2406–2411. [PubMed] [Google Scholar]
- 33.Ikuno Y., Leong F.L., Kazlauskas A. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest Ophthalmol Vis Sci. 2000;41:3107–3116. [PubMed] [Google Scholar]
- 34.Zheng Y., Ikuno Y., Ohj M., Kusaka S., Jiang R., Cekic O., Sawa M., Tano Y. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn J Ophthalmol. 2003;47:158–165. doi: 10.1016/s0021-5155(02)00698-6. [DOI] [PubMed] [Google Scholar]
- 35.Lei H., Kazlauskas A. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J Biol Chem. 2009;284:6329–6336. doi: 10.1074/jbc.M808426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei H., Velez G., Hovland P., Hirose T., Gilbertson D., Kazlauskas A. Growth factors outside the PDGF family drive experimental PVR. Invest Ophthalmol Vis Sci. 2009;50:3394–3403. doi: 10.1167/iovs.08-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pennock S., Kazlauskas A. Vascular endothelial growth factor A competitively inhibits platelet-derived growth factor (PDGF)-dependent activation of PDGF receptor and subsequent signaling events and cellular responses. Mol Cell Biol. 2012;32:1955–1966. doi: 10.1128/MCB.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei H., Rheaume M.A., Cui J., Mukai S., Maberley D., Samad A., Matsubara J., Kazlauskas A. A novel function of p53: a gatekeeper of retinal detachment. Am J Pathol. 2012;181:866–874. doi: 10.1016/j.ajpath.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei H., Velez G., Kazlauskas A. Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol Cell Biol. 2011;31:1788–1799. doi: 10.1128/MCB.01321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dieudonne S.C., La Heij E.C., Diederen R.M., Kessels A.G., Liem A.T., Kijlstra A., Hendrikse F. Balance of vascular endothelial growth factor and pigment epithelial growth factor prior to development of proliferative vitreoretinopathy. Ophthalmic Res. 2007;39:148–154. doi: 10.1159/000103234. [DOI] [PubMed] [Google Scholar]
- 41.Lei H., Hovland P., Velez G., Haran A., Gilbertson D., Hirose T., Kazlauskas A. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007;48:2335–2342. doi: 10.1167/iovs.06-0965. [DOI] [PubMed] [Google Scholar]
- 42.Robbins S.G., Mixon R.N., Wilson D.J., Hart C.E., Robertson J.E., Westra I., Planck S.R., Rosenbaum J.T. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci. 1994;35:3649–3663. [PubMed] [Google Scholar]
- 43.Lei H., Velez G., Cui J., Samad A., Maberley D., Matsubara J., Kazlauskas A. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010;177:132–140. doi: 10.2353/ajpath.2010.090604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klinghoffer R.A., Duckworth B., Valius M., Cantley L., Kazlauskas A. Platelet-derived growth factor-dependent activation of phosphatidylinositol 3-kinase is regulated by receptor binding of SH2-domain-containing proteins which influence Ras activity. Mol Cell Biol. 1996;16:5905–5914. doi: 10.1128/mcb.16.10.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakagawa M., Refojo M.F., Marin J.F., Doi M., Tolentino F.I. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1995;36:2388–2395. [PubMed] [Google Scholar]
- 46.Rosenkranz S., DeMali K.A., Gelderloos J.A., Bazenet C., Kazlauskas A. Identification of the receptor-associated signaling enzymes that are required for platelet-derived growth factor-AA-dependent chemotaxis and DNA synthesis. J Biol Chem. 1999;274:28335–28343. doi: 10.1074/jbc.274.40.28335. [DOI] [PubMed] [Google Scholar]
- 47.Wong C.A., Potter M.J., Cui J.Z., Chang T.S., Ma P., Maberley A.L., Ross W.H., White V.A., Samad A., Jia W., Hornan D., Matsubara J.A. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Can J Ophthalmol. 2002;37:211–220. doi: 10.1016/s0008-4182(02)80112-0. [DOI] [PubMed] [Google Scholar]
- 48.Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C.H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 49.Grinnell F., Ho C.H., Lin Y.C., Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- 50.Fastenberg D.M., Diddie K.R., Sorgente N., Ryan S.J. A comparison of different cellular inocula in an experimental model of massive periretinal proliferation. Am J Ophthalmol. 1982;93:559–564. doi: 10.1016/s0002-9394(14)77369-6. [DOI] [PubMed] [Google Scholar]
- 51.Maberley D., Cui J.Z., Matsubara J.A. Vitreous leptin levels in retinal disease. Eye (Lond) 2006;20:801–804. doi: 10.1038/sj.eye.6702011. [DOI] [PubMed] [Google Scholar]
- 52.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 53.Rosenfeld P.J., Heier J.S., Hantsbarger G., Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:623.e1. doi: 10.1016/j.ophtha.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 54.Heldin C.H., Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1:555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross R., Bowen-Pope D.F., Raines E.W. Platelet-derived growth factor and its role in health and disease. Philos Trans R Soc Lond B Biol Sci. 1990;327:155–169. doi: 10.1098/rstb.1990.0051. [DOI] [PubMed] [Google Scholar]
- 56.Raines E.W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- 57.El-Ghrably I.A., Dua H.S., Orr G.M., Fischer D., Tighe P.J. Intravitreal invading cells contribute to vitreal cytokine milieu in proliferative vitreoretinopathy. Br J Ophthalmol. 2001;85:461–470. doi: 10.1136/bjo.85.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azzolini CPI, Pirrone C, Moriondo A, Donati S, Chiaravalli AM, Borroni D, Pasquali F, Porta G: Otx2 and VegfA Expression and P53-Otx1 Differentiation Pathway in Proliferative Vitreoretinopathy. Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, 2012 May 6–12, Fort Lauderdale, FL
- 59.Pastor-Idoate S, Rojas J, Rodriguez-Hernández I, Gonzalez-Sarmiento R, Pastor J: Distribution of p53 Codon 72 Polymorphism in European Patients with Proliferative Vitreoretinopathy (PVR). Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, 2012 May 6–12, Fort Lauderdale, FL
- 60.Charbel Issa P., Finger R.P., Holz F.G., Scholl H.P. Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol. 2008;92:941–945. doi: 10.1136/bjo.2007.129627. [DOI] [PubMed] [Google Scholar]
- 61.Avery R.L., Pieramici D.J., Rabena M.D., Castellarin A.A., Nasir M.A., Giust M.J. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372.e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Yeo J.H., Sadeghi J., Campochiaro P.A., Green W.R., Glaser B.M. Intravitreous fibronectin and platelet-derived growth factor: new model for traction retinal detachment. Arch Ophthalmol. 1986;104:417–421. doi: 10.1001/archopht.1986.01050150119041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of various doses of anti-VEGF on vitreal VEGF-A–driven p53 repression. Primary RCFs were serum starved overnight and either lysed immediately without treatment (—) or continuously treated for 48 hours with 400 μL RV-PVR supplemented with 10 μg/mL nonimmune IgG or the neutralizing anti-VEGF antibody, ranibizumab (α-VEGF), at the indicated concentrations. After treatment, cells were lysed and the resulting TCLs were subjected to Western blot analysis using the indicated antibodies. The signal intensity of the resulting immunoblots was quantified; ratios representing normalized band intensities are shown under the immunoblot. Neutralizing vitreal VEGF-A using α-VEGF at a concentration of 10 μg/mL or higher prevented PVR vitreous-driven repression of p53.
The effect of TRAP alone on vitreous-driven signaling events. This figure is an expansion of Figure 1B, showing that TRAP alone did not affect the ability of RV-PVR to drive PVR-associated signaling events. The p-Akt immunoblot signal (phos) was normalized to total Akt (PAN) and is presented as fold induction over the non-stimulated control.
A single injection with ranibizumab effectively protected rabbits from developing PVR. This figure is an expansion of Figure 2A showing the PVR score from every time point examined during the course of the experiment. The experiment is described in the legend of Figure 2A. Briefly, rabbits received separate, consecutive injections of RCFs, PRP, and either 0.05 mg of ranibizumab (RBZ; white pentagons) or the equivalent amount of isotype control IgG (black triangles). Horizontal bars represent the mean of each group. Statistically significant differences at each time point were determined by Mann-Whitney analysis (P values are indicated above each time point). Significant differences between the two groups were observed as early as day 5, indicating that neutralizing VEGF-A effectively prevented induction of PVR at an early stage.
PDGF-mediated dimerization attenuated indirect activation of PDGFRα. Part of the experiment shown in Figure 3C, which was performed at 4°C, was conducted at 37°C. The results reveal the expected result of a stronger response at the higher temperature.
Heat treatment inactivated VEGF-A in PVR vitreous. PAE-KDR cells were serum starved overnight and then treated with DMEM alone (—) or 200 μL of either human patient or rabbit PVR vitreous (HV-PVR or RV-PVR, respectively) that was unheated or had been heat treated at 90°C for 5 minutes and then rapidly cooled on ice (asterisks). After treatment for 5 minutes at 37°C, cells were lysed and subjected to Western blot analysis using anti–phospho-VEGFR2 and anti-VEGFR2. The phospho-VEGFR2 immunoblot signal was normalized to total VEGFR2. The p-VEGFR2 immunoblot signal (phos) was normalized to total VEGFR2 (PAN) and is presented as fold induction over the non-stimulated control. HV-PVR was pooled from patients with severe (traumatic/hemorrhagic) PVR. The reduced ability of heat-treated vitreous to activate VEGFR2 compared with non–heat-treated controls suggests that VEGF-A is heat labile in both rabbit and human PVR. This idea is supported by the observation that purified VEGF is heat labile.37