Abstract
Transferrin is a promising drug carrier that has the potential to deliver metals, small organic molecules and therapeutic proteins to cancer cells and/or across physiological barriers (such as the blood-brain barrier). Despite this promise, very few transferrin-based therapeutics have been developed and reached clinical trials. This modest success record can be explained by the complexity and heterogeneity of protein conjugation products, which also pose great challenges to their analytical characterization. In this work, we use lysozyme conjugated to transferrin as a model therapeutic that targets the central nervous system (where its bacteriostatic properties may be exploited to control infection) and develop analytical protocols based on electrospray ionization mass spectrometry to characterize its structure and interactions with therapeutic targets and physiological partners critical for its successful delivery. Mass spectrometry has already become an indispensable tool facilitating all stages of the protein drug development process, and this work demonstrates the enormous potential of this technique in facilitating the development of a range of therapeutically effective protein-drug conjugates.
Keywords: Biopharmaceuticals, drug delivery, protein-drug conjugate, protein cross-linking, ion exchange chromatography, electrospray ionization, mass spectrometry, protein-receptor binding, enzymesubstrate binding
INTRODUCTION
Transferrin (Tf) is a promising drug carrier that has the potential to deliver metals, small molecule medicines and therapeutic proteins to cancer cells1 and/or across physiological barriers (such as the blood-brain barrier, BBB2). Despite this promise, very few Tf-based therapeutics have been developed and reached clinical trials. This very modest success record can be explained by the complexity and heterogeneity of protein conjugation products, which also pose great challenges to their analytical characterization. Possible interaction of the therapeutic payload with the carrier protein may have a negative impact on the conformational stability of the latter. Even in the absence of such interactions, the mere presence of the conjugate on the protein surface may sterically interfere with the ability of Tf to associate with its receptor at the cell surface, a critical first step in the drug delivery scenarios. Another complication arises due to the need to either have an effective mechanism of liberating the payload from Tf inside the endosome (in order to allow its routing to the intracellular target as opposed to recycling it back to the cell surface), or ensuring that it still exerts its therapeutic action while being attached to Tf if the latter is intended to ferry the payload across a physiological barrier, such as the BBB.
While most of the earlier explorations of Tf as a potential delivery vehicle focused on intracellular drug delivery,3–8 its ability to cross physiological barriers may also be exploited to target difficult-to-reach intracellular targets.9–13 For example, neuroanatomical obstacles frequently limit the effectiveness of antimicrobial therapy in the central nervous system (CNS) by preventing a large number of effective antimicrobials from reaching sufficient concentration levels at the infection site.14 Furthermore, the ever increasing number of bacterial pathogens resistant to common antibiotics has brought to the fore the question of whether the repertoire of antimicrobials should be expanded beyond classical small molecule drugs (be they natural or synthetic products) by considering larger bio-inspired host defense systems, such as amphiphilic peptides15 and other bacteriostatic macromolecules. One particularly attractive class of such bacteriostatic agents is a group of enzymes that compromise the integrity of bacterial cell walls.16 Lysozyme (Lz) is an antibacterial enzyme present in a variety of organisms, which exerts its bacteriostatic function by hydrolyzing the β-1,4-glycosidic bond between the N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) residues of peptidoglycans, resulting in lysis of bacterial cell walls. Although Lz primarily attacks Gram-positive bacteria, where the peptidoglycan layer is not protected by the outer membrane (as it is in Gram-negative bacteria), certain structural modifications can make it effective against Gram-negative bacteria as well.17
While Lz is widely distributed throughout the human body, it is not present in the cerebrospinal fluid (CSF) of healthy subjects18 (detectable levels of Lz in CSF is usually associated with various CNS pathologies19, 20 and are likely to reflect increased permeability of the BBB20). Therefore, the ability to deliver Lz “on demand” across the BBB might lead to development of novel effective therapeutic strategies aimed at the eradication of Gram-positive infections in the CNS, whose carriers gain access to the brain via a variety of routes.14
In this work we explore the properties of Lz conjugated to Tf as a model therapeutic that targets the CNS and develop analytical protocols to characterize its structure and interactions with therapeutic targets and physiological partners critical for its successful delivery. We demonstrate that electrospray ionization (ESI) mass spectrometry (MS) provides a convenient and effective way to probe both the structure of the conjugation products and their ability to interact with physiologically and therapeutically relevant partners, thereby providing important and valuable feedback that that can be used to refine and optimize the conjugation protocols and greatly facilitate the early stages of the drug development process.
EXPERIMENTAL
Preparation of Lz-Tf conjugate
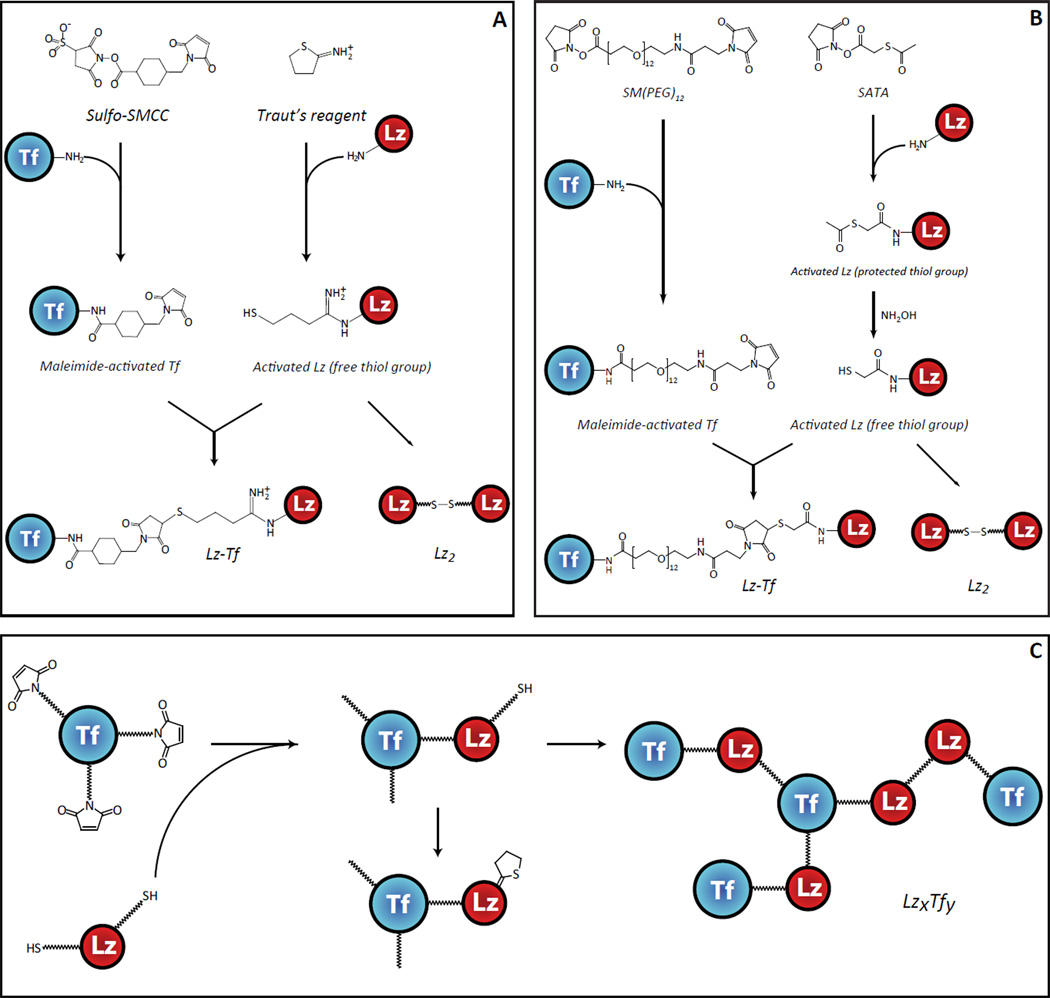
For each reaction the optimal final conditions are given in the text while various reaction parameters tested during optimization are listed in parenthesis. Lz from chicken egg white (Sigma-Aldrich, St. Louis, MO) was activated (decorated with free thiol groups) using either Traut’s reagent (2-iminothiolane hydrochloride; Sigma-Aldrich, St. Louis, MO) or N-succinimidyl-S-acetylthioacetate (SATA; Pierce Biotechnology, Rockford, IL), which target primary amine groups.21 The reaction was carried out by incubating 12 h (0.5, 0.75, 1, 2, 12, 24 h) at 0 °C (0, 4, 25, 37 °C) in 50 mM phosphate buffer with 100 mM NaCl pH 8.0 (7.0, 7.5, 8.0, 8.5, 9.0) and consisted of 500 µM (50, 100, 250, 500 µM) Lz with a 1:1 ratio (2:1, 1:1, 1:2, 1:4) of primary amines relative to the thiolating reagent (freshly prepared in H2O). Incorporation of thiol-reactive maleimide groups into human Tf (provided by Prof. Anne B. Mason, University of Vermont College of Medicine) was carried out by reacting with either sulfosuccinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate (sulfo-SMCC: Pierce Biotechnology, Rockford, IL) or succinimidyl-([N-maleimidopropionamido]-dodecaethyleneglycol) ester (SM(PEG)12; Pierce Biotechnology, Rockford, IL) for 90 min (30, 60, 90, 120 min) at 25 °C (4, 25, 37 °C) in 50 mM phosphate buffer with 100 mM NaCl pH 7.0 (6.0, 7.0, 8.0, 9.0) and consisted of 250 µM (50, 100, 250 µM) Tf with a 1:2 (1:2, 1:4, 1:20) ratio of Tf relative to the activating reagent (freshly prepared in DMSO). Excess activation reagents were removed by centrifugal filtration through a 10 kDa Vivaspin molecular weight filter (Sartorius Stedim Biotech SA, Bohemia, NY) and the non-cleavable thio-ether linkage between activated Lz and Tf was formed by incubating 50 µM (25, 50, 100 µM) each of the two modified proteins together for 12h (1, 2, 4h, 12, 24h) at 4°C (4, 25, 37 °C) in 50 mM phosphate buffer with 100 mM NaCl, pH 7.0 (7.0, 8.0, 9.0) at 1:1 molar ratio. The 1:1 Lz-Tf conjugate was isolated by cation exchange chromatography on a 4.6×100 mm PolyCATA™ column (5 µM, 1000 Å, PolyLC Inc., Columbia, MD) using an Agilent 1100 (Agilent Technologies, Palo Alto, CA) HPLC system. All relevant reaction diagrams are shown in Figure 1.
Figure 1.
Schematic diagrams illustrating conjugation of Tf to Lz using the Traut’s reagent and SMCC (A) and SATA and SM(PEG)12 (B), and possible side reactions due to excessive activation of the two proteins with the Traut’s reagent and SMCC (C).
Mass spectrometry
All ESI MS measurements were carried out with a QStar-XL (ABI/SCIEX, Toronto, Canada) hybrid quadrupole/time-of-flight MS equipped with a nanospray source. Mass profiling of activated Tf, activated Lz, the crude reaction product mixture, and the 1:1 conjugate isolated by cation exchange LC was carried out following extensive buffer-exchange of proteins and placing them in water/methanol/acetic acid (49:49:2) at a concentration of ca. 10 µM. Native ESI MS analyses of the conjugate, and its mixtures with TfR and NAG3 were performed using 20 mM ammonium acetate as a solvent. To ensure the integrity of non-covalent complexes in the gas phase, the declustering potential in the ESI MS interface (DP1) was minimized, unless noted otherwise in the text. Ectodomain of transferrin receptor (TfR)22 used in binding assays was provided by Prof. Anne B. Mason (Univ. of Vermont College of Medicine, Burlington, VT), and N-acetylglucosamine trimer (NAG3) was purchased from Sigma-Aldrich (St. Louis, MO).
Antimicrobial activity assay
Antimicrobial activity of intact Lz, Lz dimers and Lz-Tf conjugate was measured using re-suspended dried cells of Micrococcus lysodeikticus (Worthington Biochemical Corp., Lakewood, NJ) as the substrate. The rate of cell wall lysis in 0.1 M sodium phosphate buffer pH 7.0 at 25°C was monitored by recording the transmission at 450 nm.23 The measurements were carried out in a 1 mL cuvette with a NanoDrop 2000c (Thermo Fisher Scientific, Rockford, IL) UV-Vis spectrophotometer.
RESULTS
Production, purification and characterization of the Lz-Tf conjugate
The classical scheme of conjugating Tf to a protein payload involves derivatizing the Lys side chains and amino terminus of Tf with sulfo-SMCC and activating the protein payload at similar sites using Traut’s reagent, followed by reacting them with each other24. This produces the same thio-ether linkage that was used in the production of TransMID,25 the only Tf-based biopharmaceutical product that ever reached Phase III clinical trials. It is expected that a 1:1 stoichiometry for the conjugate would minimally disturb the functionality of either molecule and was the desired product of our synthesis. Placing a single maleimide group on Tf and a single free thiol group on Lz should lead to the formation of a 1:1 conjugate, with Lz dimers being the only by-product that can form via external disulfide bond formation (Figure 1). While the extent of Tf functionalization with sulfo-SMCC and Lz with Traut’s reagent can be varied over a wide range it is virtually impossible to limit the extent of activation of the two proteins to a single reactive group on each polypeptide chain (Figure 2). Moreover, with 58 Lys residues in Tf and 6 in Lz, an additional level of heterogeneity is introduced by the number of linker positions that can form a 1:1 conjugate. Our initial development embraced this possible heterogeneity, benefitting from a quicker development time. Identifying conjugation sites would be suited for final optimization of the protein drug conjugate.
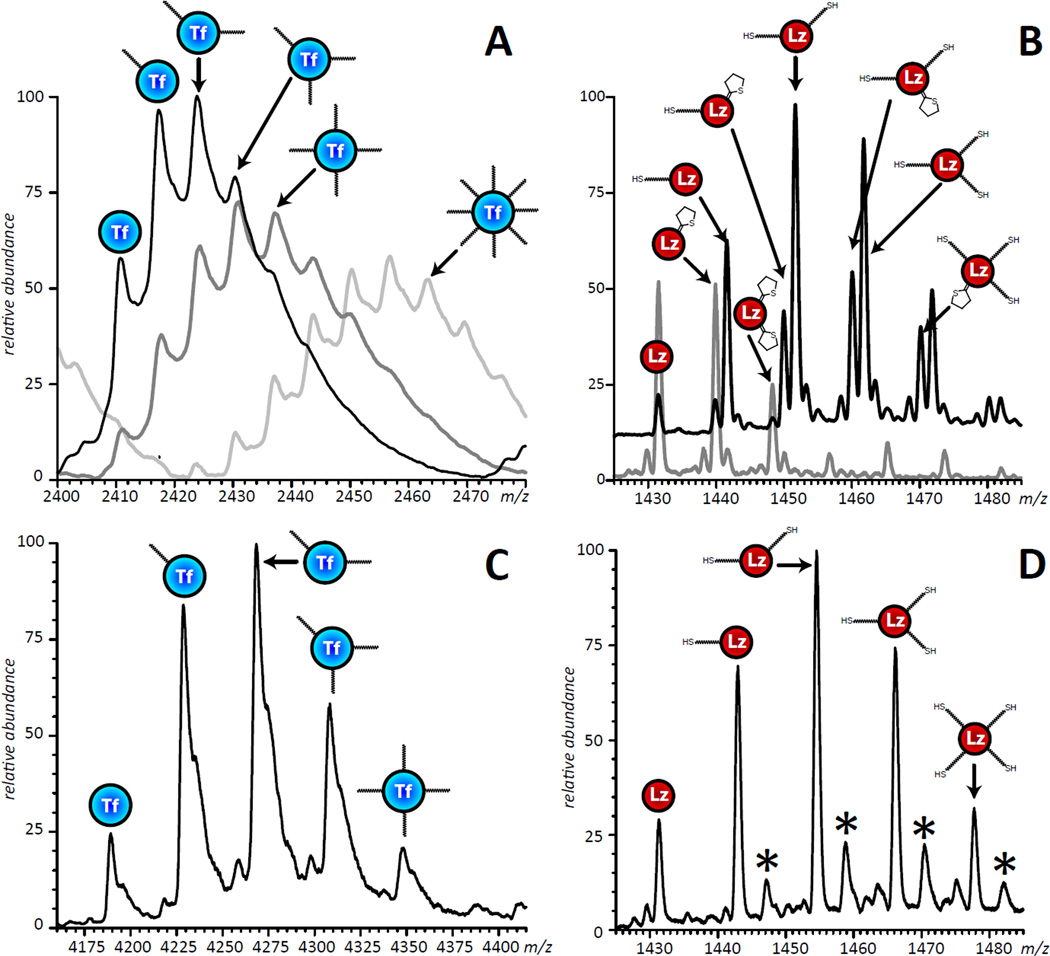
Figure 2.
ESI mass spectra of activated Tf, charge state +32 (A) and Lz, charge state +10 (B) showing a range of reactive groups attached to the surface of each protein. The three traces shown in panel A correspond to a 1:2, 1:4 and 1:20 concentration ratio of Tf to sulfo-SMCC in the reaction. The two traces shown in panel B correspond to Lz modified with Traut’s reagent for 12 hours on ice (the gray trace corresponds to the protein-to-reagent molar ratio 1:2, the product kept at room temperature for 24 hours prior to MS; and the black trace corresponds to the protein-to-reagent molar ratio 1:7, MS analysis of the product was carried out immediately upon reaction completion). The multiple peaks shown in panel B are due to the presence of both chemically active (thiols) and de-activated (rings) groups on the surface of Lz. All mass spectra were acquired under denaturing condition (10µM total protein concentration in water/methanol/acetic acid, 49:49:2 by volume). Panels (C) and (D) show Tf and Lz activated with SM(PEG)12 and SATA, respectively. The series of peaks indicated with an asterisk represent salt adducts.
We found that adequate yields of the conjugation reaction can be achieved only if multiple activation groups are placed on each protein. Placing multiple free thiol groups on Lz is likely to increase the incidence and extent of this protein’s polymerization via formation of external disulfide linkages. While homo-polymerization of the functionalized Tf was not expected to be as significant (at neutral pH maleimide groups are ca. 1000 less reactive towards free amines compared to free sulfhydryls), this process nonetheless was found to occur. Homo-polymerization was particularly apparent when concentrations of Tf in the reaction mixture were elevated compared to that of the activated payload (Lz), most likely due to the presence of a large number of free amine groups (Lys side chains) on the surface of Tf. Above and beyond the formation of Lzn (and, to a lesser extent, Tfn) homo-polymers, polyvalent functionalization of Tf and Lz was also expected to contribute to the extent of heterogeneity of the conjugation products (Figure 1C). Balancing the extent of modification of each protein in order to optimize production of the 1:1 Lz-Tf conjugate was achieved in this work by controlling the following primary variables: reagent concentrations, temperature, incubation time and reaction pH. Mass spectrometry enabled us to determine the effect of altering these variables on the product, allowing us to screen these parameters in an iterative fashion that sought to optimize the yield of conjugation while minimizing undesirable side reactions.
Of the two modification reactions, thiolation by Traut's reagent was the most problematic. Two significant issues that needed to be overcome were the reduction of intramolecular disulfide bonds and formation of N-substituted 2-iminothilane (NSI) products. The abundance of intramolecular disulfide bonds within Tf presented an unexpected obstacle when this protein was initially chosen for activation by Traut’s reagent. While the highest yield of thiolated Tf could be obtained at elevated pH and a high concentration of reagents, the native disulfide bonds of Tf were largely reduced, leading to a drastic and unacceptable change in its higher order structure (see Supporting Information Figure S1 for more detail). Disulfide reduction was still observed (albeit to a much lesser extent) in the thiolation of Lz by Traut’s reagent, but was practically eliminated by reducing reagent concentrations and performing the reaction on ice. Further investigation of this reaction led us to an interesting finding; In addition to thiolated proteins, other chemical modifications were observed with masses consistent with non-thiol by-products reported by Singh.26 These dead-end (non-reactive) products form when the unstable thiol adduct breaks down into a non-reactive 5-membered ring and reduce the number of free thiol groups. Such instability in one of the activating groups can significantly hinder downstream conjugation even when all six Lys residues of Lz have been functionalized (Figure 2B). Formation of NSI by-products was also observed during and after the conjugation reaction, but fortunately could be minimized by lowering the reaction pH and temperature. We investigated the use of N-succinimidyl S-acetylthioacetate (SATA) as an alternative reagent to introduce the desired thiol group. SATA introduces a “protected sulfhydryl” which requires activation by a mild reducing reagent to expose the sulfhydryl prior to the conjugation reaction. Compared to Traut’s reagent, Lz functionalized with SATA had increased stability (did not form NSIs) and reduced heterogeneity (Figure 2B,D).
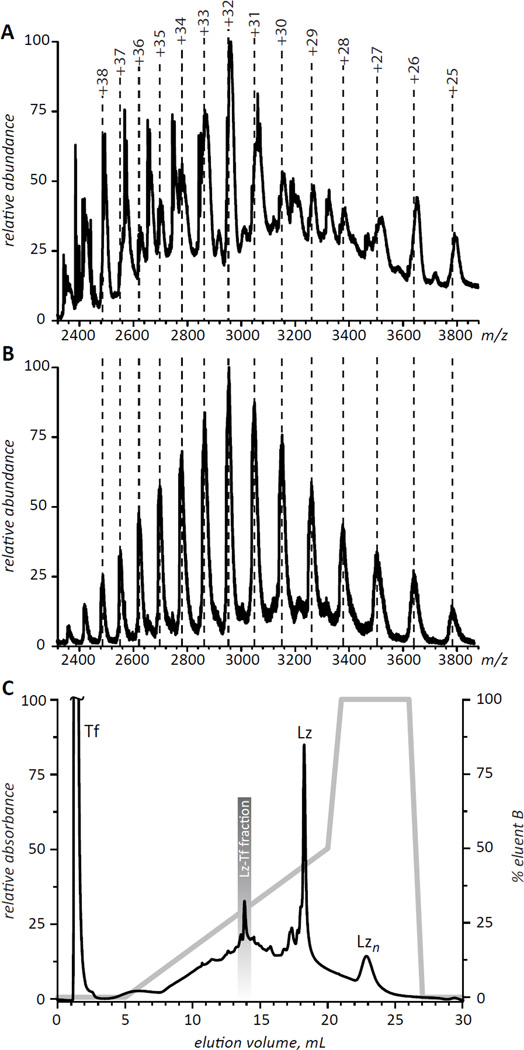
Optimizing the activating steps for each protein focused initially on minimizing deleterious or nonproductive by-products such as the reduction of intramolecular disulfide bonds or the formation of NSIs as monitored by ESI MS. After screening pH values from 7.0 to 9.0, reaction temperatures from 0 to 37°C, and reaction times from 0.5 to 24 hours, the optimized thiolation reaction was performed at pH 8, 0°C, for 12 h. Notably, in the examined range of protein concentrations (50 µM to 500 µM), elevated protein concentration was found to result in a higher yield of modified Lz. Different ratios of protein primary amine to reagent from 2:1 to 1:4 were used to generate a series of differentially modified Lz. The optimal extent of Lz activation was determined in conjunction with the optimal extent of Tf activation selecting for values that produced the highest yield of the 1:1 Lz-Tf conjugate. The final conditions for Lz activation utilized an equimolar ratio of primary amine groups relative to activating reagent and introduced 2 to 2.5 thiol groups per Lz. Similar reaction parameters were screened to optimize the activation of Tf with its thiol reactive group. Tf was optimally activated at pH 7.0, 25 °C using 50 µM of Tf and a ratio of Tf to activating reagent of 1:2 for 90 min. Under these conditions, the average number of functional groups incorporated into Tf was 1.5. In the final round of optimization, formation of the Lz-Tf conjugate was monitored as a function of pH, time, temperature as well as the extent of activation of each protein. The highest yield of a 1:1 Lz-Tf conjugate was obtained at pH 7 allowing optimally activated Tf and Lz (50 µM each) to react for 12 h at 4 °C Nevertheless, even though ESI MS analysis of the optimized conjugation reaction (Figure 3A) clearly shows the presence of the 1:1 Lz-Tf conjugate (charge states assigned based on the calculated mass of 94.4 kDa are shown in Figure 3A with dotted lines), a large number of other species are also present in the mixture. Therefore, evaluation of various properties of Lz-Tf is impossible without its separation from other products and/or reagents. Since the incremental mass increase of Lz-Tf over intact Tf makes use of SEC impractical for purification of the conjugation products, alternative methods of separating the 1:1 Lz-Tf conjugate from other components of the reaction mixture were examined.
Figure 3.
ESI mass spectra of crude (A) and IXC-purified (B) conjugation products of Lz and Tf. Panel C shows IXC chromatogram of the crude mixture (the fraction whose mass spectrum is shown in panel B is highlighted in the chromatogram).
The significant difference in pI values for Tf (5.5–6.3) and Lz (11.0) made ion exchange chromatography (IXC) particularly attractive as a means of purifying the reaction products. Using a weak cation exchange stationary phase, a mobile phase buffered to pH 6.5, and a shallow salt gradient we have been able to achieve separation between the Tf and Lz peaks exceeding 15 minutes (Figure 3C), with Lz homopolymers having even longer elution times. The products of the Lz/Tf conjugation reaction elute within a wide (9–17 min) time period and are mostly unresolved, although a distinct peak is observed at 14 min elution time. Collection of a corresponding IXC fraction (13.5–14.5 min) followed by quick desalting and off-line ESI MS analysis yields a mass spectrum consistent with the 1:1 Lz-Tf conjugate as the major component of this fraction (Figure 3B).
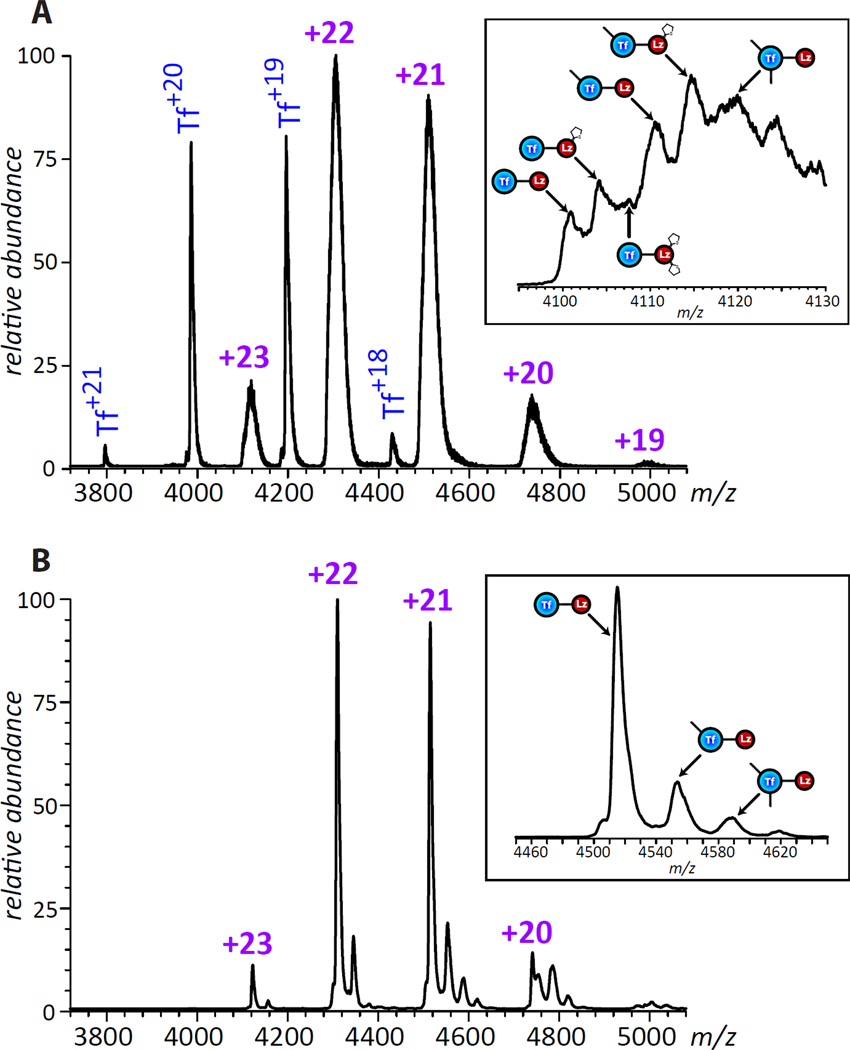
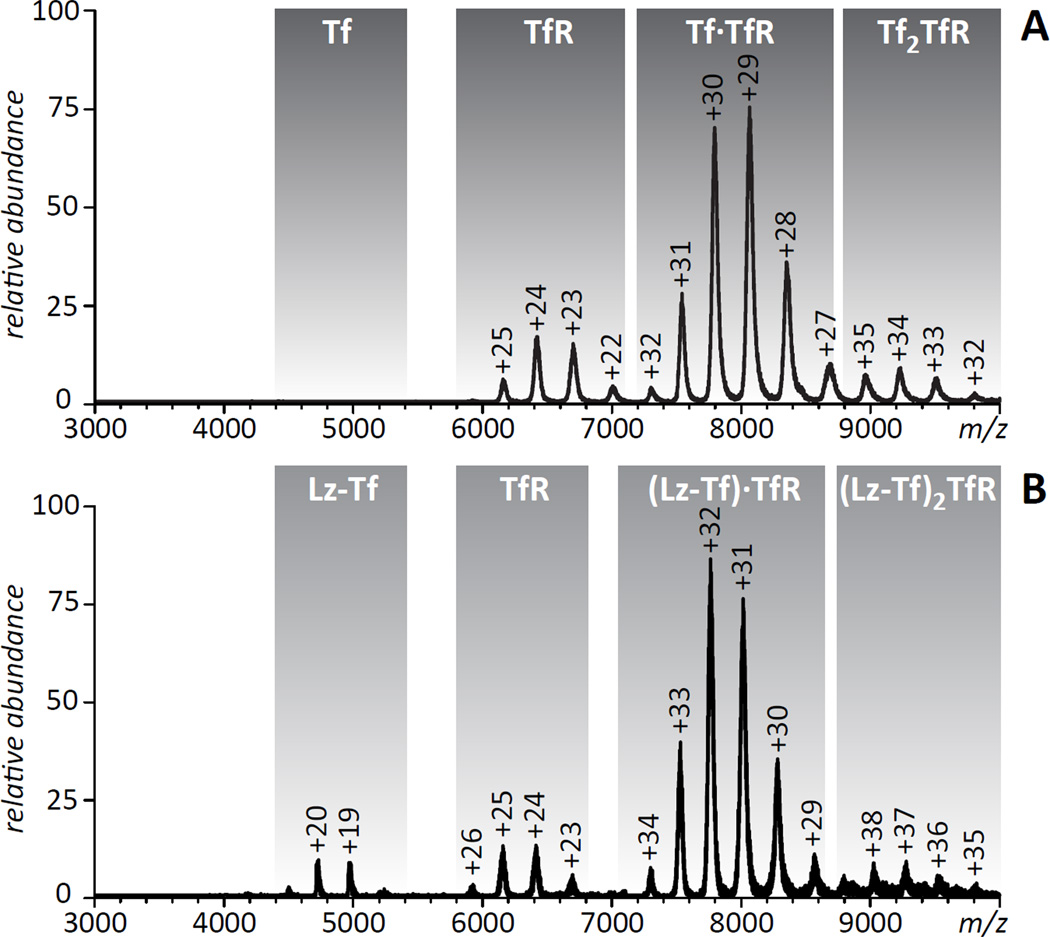
Even though the collected IXC fraction represents a 1:1 Lz-Tf conjugate, there still might be a significant degree of micro-heterogeneity due to the presence of modified Lys side chains on the surfaces of both Tf and Lz. Indeed, native ESI MS analysis of this fraction spiked with intact Tf (Figure 4) clearly shows significantly broader peak shapes for multiply charged Lz-Tf ions compared to intact Tf ions. A more detailed analysis of the mass spectrum reveals very convoluted peak shapes for Lz-Tf ions (insets in Figure 4), where the broad mass distribution of ionic species is due to the presence of either unreacted maleimide groups on the surface of Tf and/or dead-end NSI groups on the surface of Lz. Despite these extensive modifications, no large scale conformational changes are apparent as a result of the conjugation reaction, as the charge state distribution of Lz-Tf ions is consistent with both components of the conjugate maintaining compact structures in solution (no ions were detected in the low m/z region, whose presence in ESI MS usually signals either partial or complete protein unfolding in solution27).
Figure 4.
ESI mass spectra of the purified 1:1 Lz-Tf conjugate (short linker) spiked with intact Tf (A) and a 1:1 conjugate produced with a longer linker (B) acquired under near-native conditions (3 µM of each protein in 20 mM ammonium acetate, pH 7.1). Insets zoom in on a selected charge state for each of the conjugates.
Influence of conjugation and chemical modifications on interaction with transferrin receptor (TfR)
Although examination of the Lz-Tf conjugate with native ESI MS suggests that neither protein undergoes unfolding as a result of the conjugation, the ability of both Tf and Lz to interact with their physiological partners and/or therapeutic targets may nonetheless be compromised as a result of unfavorable location of the cross-link, as well as multiple modification of Lys residues on the surface of either protein beyond the cross-link sites. For example, Lz cross-linked to Tf may interfere with the ability of the latter to bind to TfR, thereby rendering Lz-Tf incapable of crossing the BBB.
Native ESI MS provides an easy way to evaluate protein binding to a variety of ligands, including both small molecules and biopolymers,28, 29 and in some instances allows the binding affinity to be estimated.30, 31 This approach has been used in the past to monitor TfR recognition by wild type Tf and its mutants under a variety of conditions,32, 33 and recently we were successful in using this approach to monitor interactions of a Tf-based fusion protein with TfR.34 However, native ESI MS has never been used to evaluate interaction of protein-protein conjugates with their physiological partners. An ESI mass spectrum of the Lz-Tf/TfR mixture acquired in this work under near native conditions (neutral pH, ionic strength 20 mM) clearly indicates that the receptor does recognize the conjugate, although the binding affinity is diminished compared to intact Tf (Figure 5). Indeed, no ionic signal of unbound Fe2Tf is detected in the mass spectrum of the Fe2Tf/TfR mixture, consistent with the receptor-binding affinity of Fe2Tf being in the sub-µM range (concentration of both proteins in the Fe2Tf/TfR mixture was in the low- µM range, 3 µM). At the same time, the presence of a weak, but detectable ionic signal of unbound Lz-Tf in the mass spectrum of the Lz-Tf/TfR mixture acquired under identical conditions suggests that the TfR binding affinity of the conjugate is in the low-µM range. This affinity range is close to that of intact apo- Tf,33 even though the conjugate was saturated with iron following its isolation from the reaction mixture and its measured mass is in agreement with the diferric form. Nevertheless, even this lower receptor affinity should be sufficient for transient binding to TfR at the cell surface (endogenous Tf is only 30% saturated with iron), and may actually prove beneficial for dissociation from TfR upon crossing the BBB.
Figure 5.
ESI mass spectra of Tf/TfR (A) and Lz-Tf/TfR (B) mixtures acquired under near-native conditions (3 µM of each protein in 20mM ammonium acetate, pH 7.1).
Influence of conjugation and chemical modification on enzymatic activity
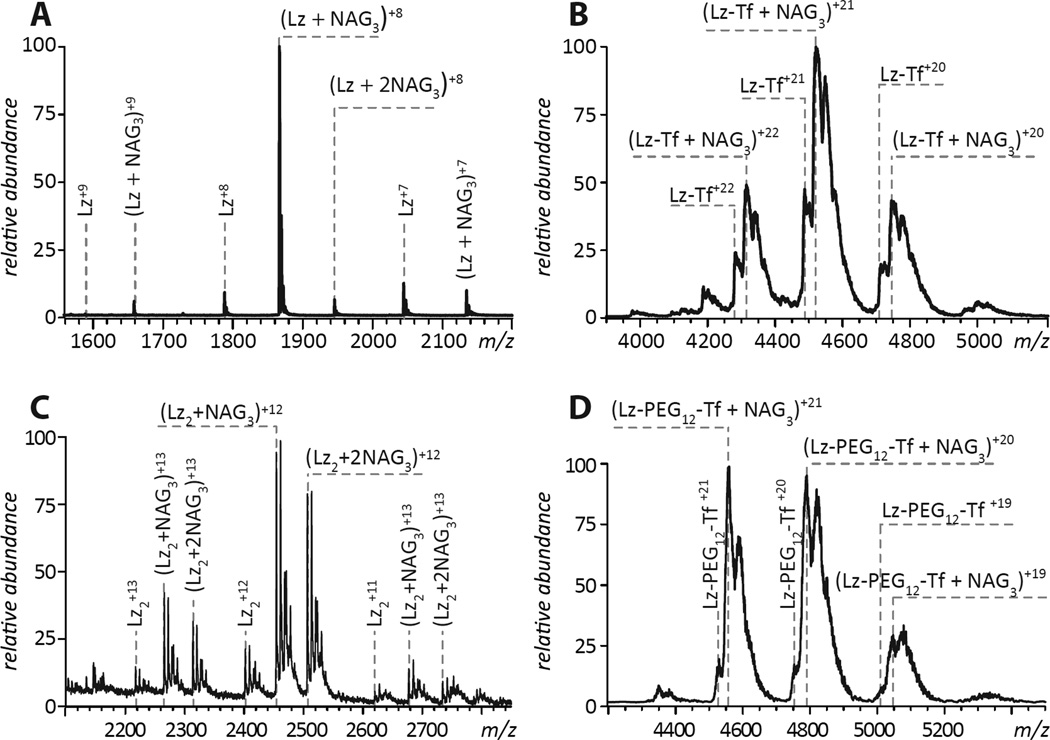
In order to exhibit bacteriostatic properties, enzymatic activity of Lz must be preserved within the conjugate. Enzymatic activity of Lz and its variants is frequently probed using the short tri-saccharide NAG3 as a surrogate substrate to demonstrate the substrate-binding competence of the protein35 (Figure 6A). Despite the proximity of the six Lys residues (targets of modification by Traut’s reagent) to the catalytic site of the enzyme, Lz-Tf retains the ability to bind NAG3, indicated by the presence of the protein-NAG3 complexes in the mass spectrum of a Lz-Tf/NAG3 mixture (Figure 6B) acquired under near-native conditions (neutral pH and 20 mM ionic strength), a behavior very similar to that exhibited by intact Lz.
Figure 6.
ESI mass spectra of NAG3/Lz (A), NAG3/Lz-Tf (B), NAG3/Lz2 (C) and NAG3/Lz-Tf longer linker (D) mixtures acquired under near-native conditions (5µM of proteins and 10µM NAG3 in 20mM ammonium acetate, pH 7.1).
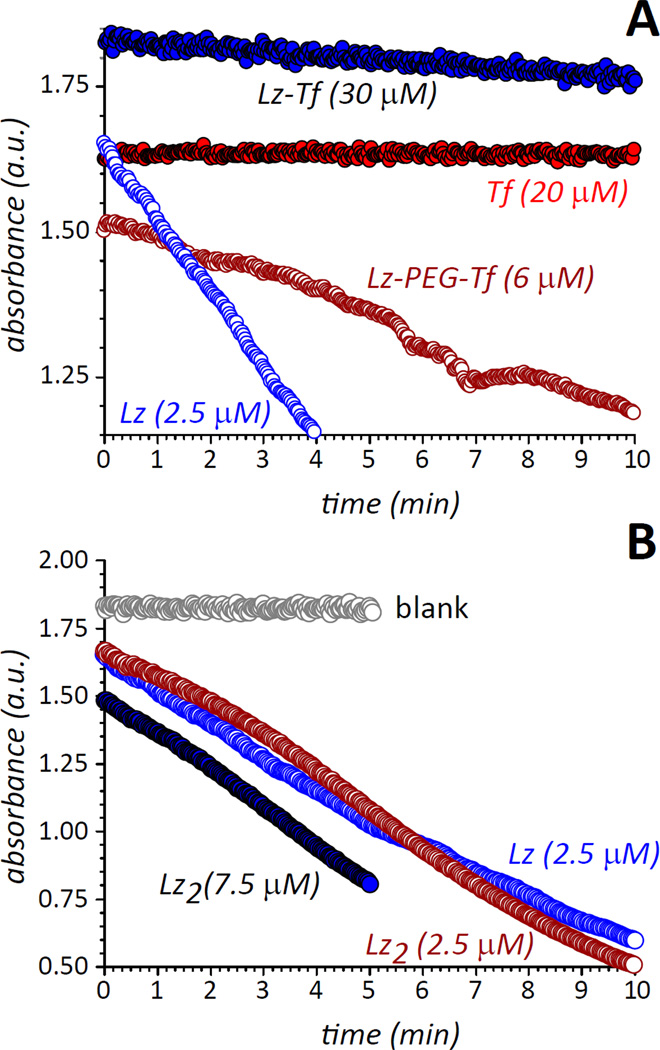
Despite the promising substrate binding results obtained using NAG3, a photometric-based activity assay that measures the lysis of Gram-positive bacteria36 indicated a very significant loss of bacteriolytic activity by Lz-Tf (Figure 7). As can be seen in Table 1, the bacteriolytic activity of Lz-Tf is reduced by two orders of magnitude compared to the control (intact) Lz, which may be attributed to two factors. First, chemical modification of Lys residues on the Lz surface changes the electrostatic properties of this protein (as reflected by its behavior in IXC, vide supra), which may introduce conformational changes in the vicinity of the catalytic site, thereby adversely affecting the ability of this enzyme to recognize large substrates. Second, the presence of a large protein (Tf) anchored to a Lys chain located in the vicinity of the catalytic site of Lz may introduce steric effects, which would make it more difficult for this protein to attack the bacterial cell wall even in the absence of any conformational changes.
Figure 7.
Antibacterial activity data for Lz-Tf conjugates compared to that of intact Lz and Tf (A) and Lz dimers (B).
Table 1.
Bacteriolytic activity of Lz-Tf and related proteins
| Samples | Rate (mAU/min) | Specific activity (%) |
|---|---|---|
| Blank (negative control) | 0.0 | 0.0 |
| Tf (20 µM) | 0.0 | 0.0 |
| Lz control (2.5 µM) | 124 | 100 |
| Lz-Tf conjugate (6.7 µM) | 1.5 | 0.45 |
| Lz-Tf conjugate (30.6 µM) | 6.4 | 0.42 |
| Lz-Tf longer linker (6µM) | 35 | 11.8 |
| Lz dimer 2IT(7.5 µM) | 138 | 18.6 |
| Lz dimer SATA (2.5 µM) | 125 | 50.6 |
Importantly, bacteriolytic activity of the Lz dimer is reduced four-fold, the actual activity of the dimer is 37.2 % compared to that of intact Lz, and is reported as 18.6 % in Table 1 after adjusting it by a factor of 2 due to the presence of two catalytic sites in a single Lz2 molecule. The Lz dimer byproduct conveniently serves as an important control demonstrating that while chemical modification of primary amines on the enzyme surface by Traut’s reagent contributes to the reduction in catalytic activity, it is not the primary reason for Lz inactivation (this is also consistent with the observed ability of Lz-Tf to bind short substrate surrogates, vide supra). Therefore, the impaired ability of Lz-Tf to catalyze the hydrolysis of large glycans is largely due to the significant steric restraints introduced by a bulky “anchor” (Tf). Metaphorically, Tf can be viewed as an “elephant on a leash,” which allows the payload to be delivered to a desired location by overcoming physiological barriers, but also restricts its freedom, thereby making it less effective in exerting the desired therapeutic action.
One possibility to mitigate this negative effect is offered by longer linkers, which should increase the freedom of movement of Lz cross-linked to Tf, allowing it to attack the bacterial cell walls more effectively. A possibility to create protein/protein conjugate with a longer linker is offered by amine-reactive SM(PEG)12, which also introduces a thiol-reactive maleimide group on the protein surface (Figure 1C). Activation of Tf with this reagent and introduction of free thiol groups to Lz using, SATA (to avoid formation of dead-ended by-products introduced by the Traut’s reagent, see Figure 2 B and D) leads to formation of a conjugate that is recognized by TfR (see Supporting Information Figure S2) and has anti-bacterial activity over an order of magnitude higher than that of the conjugate with a short linker (Figure 7). Although a fraction of that increase could be attributed to using SATA as the enzyme-modifying reagent (note that the covalent dimer of SATA-modified Lz retains half the activity of the intact enzyme, see Table 1), the most significant gain is a result of using the longer (and more flexible) linker.
DISCUSSION
The incidence of CNS infections (brain infections) is rising at an alarming rate, while the treatment options remain very limited.14 Only a very small faction of existing small-molecule medicines can penetrate the BBB, and none of the currently approved protein therapeutics is capable of doing so. Coupled with the ever increasing resistance of pathogens to common antibiotics and dire side effects of the immune system’s inflammation response to infection (frequently leading to brain abscess), this presents the clinicians with a grave challenge. Clearly, there exists a significant and unmet need for novel (bio)pharmaceuticals that can control CNS infections in the most efficient way without eliciting an immune response from the host.
Chemical conjugation of a therapeutic payload (a small molecule medicine or a protein drug) to a transport protein, such as Tf, offers a convenient and inexpensive way to produce effective medicines that can be delivered to target tissues and cells. However, only one Tf-based therapeutic has ever reached Phase III clinical trials and was subsequently withdrawn.37, 38 This rather modest record of success can be explained by the tremendous complexity and heterogeneity of conjugate species, a feature that not only complicates the underlying biology, but also frequently prevents effective utilization of state-of-the-art analytical technologies at various stages of the drug development process. MS has been a critical component in the analytical armamentarium supporting protein drug development efforts,39, 40 but its applications in characterization of protein-protein conjugates, such as TransMID, have been very limited so far. Lower-end analytical techniques, such as size-exclusion chromatography (SEC) and circular dichroism (CD) spectroscopy, which have been traditionally applied to characterize protein-protein conjugates,41 do not provide sufficient resolution and may in fact be misleading when relied upon as a sole source of information to describe the conjugate’s molecular weight distribution or its conformation.
In this work we demonstrated that ESI MS can provide characterization of both the products and intermediates of protein-protein conjugation reactions at great detail. Characterization of the activated proteins with ESI MS provides an important feedback for optimization of the conjugation protocol, while MS analysis of the reaction products highlights the enormous degree of structural heterogeneity and underscores the need for chromatographic separation as a means of controlling the extent of heterogeneity. MS characterization of the IXC-purified 1:1 conjugation product demonstrates that its heterogeneity is substantially reduced, but not completely eliminated, as the mass distribution reveals a significant number of additional chemical modifications to both protein components of the conjugate. Another level of structural heterogeneity is due to the large number of combinations that can be used to describe the spatial distribution of both the cross-link and unreacted modification sites on the surface of each protein component. Although structural heterogeneity at this level is not apparent when considering MS data alone, it can be easily visualized when methods of tandem mass spectrometry (MS/MS) are applied either in conjunction with enzymatic degradation of the conjugated protein, or alone (the so-called top-down MS/MS), an approach that has been used successfully in the recent past to characterize the distribution of conjugation sites in PEGylated proteins42 and protein-small molecule drug conjugates.43 Since it is likely that the location of the linker influences the activity of the conjugate, identifying specific conjugation sites utilizing mass spectrometry will be the focus of future work. Ideally, one would be able to correlate linker position with conjugate activity allowing for yet an additional level of optimization in the design of the protein conjugate.
Structural complexity and heterogeneity of the Lz-Tf conjugate highlighted by ESI MS brings to the fore the question of how conjugation and chemical modification may affect the ability of Tf to be recognized by its receptor, a crucial first step in receptor-mediated transcytosis, without which no delivery of the payload to CNS would be possible. ESI MS has been used in the past to monitor protein-receptor interactions involving protein drugs, such as association of interferon β1a (Avonex™) with its cognate receptors, and to evaluate the modulation of its receptor-binding competence by a specific (welldefined) chemical modification.44 Heterogeneity of the 1:1 conjugation product of Lz and Tf does not allow a precise correlation between structural changes and receptor-binding properties to be established. Instead, TfR-binding competence is evaluated in this work for the entire ensemble of Lz-Tf species. Although native ESI MS analysis provides evidence for some loss of the receptor affinity, the conjugate is nonetheless clearly recognized by the receptor. The ability of Lz-Tf to associate with TfR suggests that transfer of this species from the bloodstream to the CNS via receptor-mediated transcytosis is possible (and likely), although the efficiency of this process can be estimated only by in vivo studies capable of measuring protein levels in various biological fluids (a direction actively pursued in our laboratory45).
ESI MS also provides an exciting opportunity to evaluate the retention of enzymatic activity of Lz following its conjugation to Tf. Since the therapeutic targets of Lz (peptidoglycans from the cell walls of Gram-positive bacteria) are too large and heterogeneous for direct ESI MS analysis, most studies use a trisaccharide molecule NAG3 as a surrogate substrate. Interestingly, native ESI MS measurements indicate robust binding of NAG3 to Lz-Tf, while the actual biological activity test indicates a significant (by over two orders of magnitude) loss of its bacteriolytic activity compared to intact Lz. Auto-conjugation of Lz does not result in such a dramatic loss of bacteriolytic activity of this protein, suggesting that it is the presence of a large anchor (Tf), rather than the chemical modification of the enzyme surface, that prevents Lz-Tf from effective execution of its desired therapeutic function.
An obvious solution to a steric hindrance problem is introduction of a longer and/or more flexible linker between the payload and the transport protein. Switching from SMCC (spacer arm length 8.3 Å) to SM(PEG)12 (53.4 Å) results in a dramatic increase of autonomy for each protein component within the conjugate product, leading to a nearly 30-fold increase of its bacteriostatic activity. Such a dramatic recovery of the antibacterial activity of the conjugate is well beyond the much more modest enhancement provided by switching from the Traut’s reagent to SATA. In retrospect, utilization of longer and more flexible linkers may seem an obvious measure to enhance the potency of any conjugate by minimizing interaction of its two protein components; however, this consideration is not always brought to the fore when a specific type of conjugation chemistry is selected. In fact, it was the parallel analysis of ESI MS and biological activity data that illuminated this problem in the Lz-Tf conjugate and allowed for rational optimization of the conjugate product, improving its therapeutic potential.
CONCLUSIONS
A suite of ESI MS-based methods has been applied to characterize the structural and conformational integrity of a model bacteriostatic agent (Lz) conjugated to a transport protein (Tf), as well as its interaction with a physiological partner (TfR) critically important for delivery of this product to the CNS. Interaction of Lz-Tf with therapeutic targets was evaluated initially using ESI MS to monitor binding to a small surrogate substrate (NAG3) followed by measuring its bacteriolytic activity, and comparing its level to that of the intact Lz and Lz dimer. Analysis of these data led to the conclusion that steric hindrance imposed by a large protein anchored closely to the Lz surface reduced its biological activity. Increasing the autonomy of Lz by lengthening the linker lead to a dramatic increase in the bacteriolytic activity of the conjugate. ESI MS has already become an indispensable tool facilitating all stages of the protein drug development process,39 and this work demonstrates the enormous potential of this technique as a means to facilitate development of a range of therapeutically effective protein-drug conjugates. While mass spectrometry is beginning to enjoy wider acceptance in the biopharmaceutical community beyond the trivial tasks of primary structure elucidation,44, 46 its applications for the analysis of protein-drug conjugates have been limited primarily to measuring stoichiometry of the conjugation.47–49 This field has experienced an explosive growth in the past several years due to extensive efforts invested in developing antibody-drug conjugates (ADC),50, 51 and ESI MS clearly has a tremendous potential in this arena by providing invaluable information beyond mass measurement that can be used to optimize protein drug conjugate structures during early stages of development, and further catalyzing the drug design efforts.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health R01 GM061666. The authors are grateful to Prof. Anne B. Mason (Univ. of Vermont College of Medicine, Burlington, VT) for providing TfR sample and to Dr. Stephen J. Eyles (UMass-Amherst) for useful discussions.
Footnotes
SUPPORTING INFORMATION
ESI mass spectra of intact Tf and Tf modified with the Traut’s reagent acquired under denaturing conditions, and an ESI mass spectrum of the mixture of Lz-Tf conjugate (produced with SM(PEG)12 as a linker) and TfR acquired under near-native conditions. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitambar CR, Boon P, Wereley JP. Evaluation of transferrin and gallium-pyridoxal isonicotinoyl hydrazone as potential therapeutic agents to overcome lymphoid leukemic cell resistance to gallium nitrate. Clin. Cancer Res. 1996;2:1009–1015. [PubMed] [Google Scholar]
- 4.Yoon DJ, Chu DSH, Ng CW, Pham EA, Mason AB, Hudson DM, Smith VC, MacGillivray RTA, Kamei DT. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J. Control. Release. 2009;133:178–184. doi: 10.1016/j.jconrel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Sun H, McArdle HJ, Gambling L, Sadler PJ. Ti(IV) uptake and release by human serum transferrin and recognition of Ti(IV)-transferrin by cancer cells: understanding the mechanism of action of the anticancer drug titanocene dichloride. Biochemistry. 2000;39:10023–10033. doi: 10.1021/bi000798z. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Sun H, Qian ZM. The role of the transferrin-transferrin-receptor system in drug delivery and targeting. Trends Pharmacol. Sci. 2002;23:206–209. doi: 10.1016/s0165-6147(02)01989-2. [DOI] [PubMed] [Google Scholar]
- 7.Hartinger CG, Hann S, Koellensperger G, Sulyok M, Groessl M, Timerbaev AR, Rudnev AV, Stingeder G, Keppler BK. Interactions of a novel ruthenium-based anticancer drug (KP1019 or FFC14a) with serum proteins-significance for the patient. Int. J.Clin. Pharmacol. Ther. 2005;43:583–585. doi: 10.5414/cpp43583. [DOI] [PubMed] [Google Scholar]
- 8.Cubo L, Groessl M, Dyson PJ, Quiroga AG, Navarro-Ranninger C, Casini A. Proteins as Possible Targets for Cytotoxic trans-Platinum(II) Complexes with Aliphatic Amine Ligands: Further Exceptions to the DNA Paradigm. Chem Med Chem. 2010;5:1335–1343. doi: 10.1002/cmdc.201000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin SU, Friden P, Moran M, Olson T, Kang YS, Pardridge WM, Morrison SL. Transferrin-antibody fusion proteins are effective in brain targeting. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2820–2824. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah D, Shen WC. Transcellular delivery of an insulin-transferrin conjugate in enterocyte-like Caco-2 cells. Journal of pharmaceutical sciences. 1996;85:1306–1311. doi: 10.1021/js9601400. [DOI] [PubMed] [Google Scholar]
- 11.Widera A, Kim K-JJ, Crandall ED, Shen W-C. Transcytosis of GCSF-transferrin across rat alveolar epithelial cell monolayers. Pharmaceutical research. 2003;20:1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- 12.Widera A, Bai Y, Shen W-C. The transepithelial transport of a G-CSF-transferrin conjugate in Caco-2 cells and its myelopoietic effect in BDF1 mice. Pharmaceutical research. 2004;21:278–284. doi: 10.1023/b:pham.0000016240.81059.ec. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Ann DK, Shen W-C. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peppard WJ, Johnston CJ, Urmanski AM. Pharmacologic options for CNS infections caused by resistant Gram-positive organisms. Expert. Rev. Anti Infect. Ther. 2008;6:83–99. doi: 10.1586/14787210.6.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotech. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 16.Salazar O, Asenjo JA. Enzymatic lysis of microbial cells. Biotechnol. Lett. 2007;29:985–994. doi: 10.1007/s10529-007-9345-2. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim HR, Aoki T, Pellegrini A. Strategies for new antimicrobial proteins and peptides: Lysozyme and aprotinin as model molecules. Curr. Pharm. Des. 2002;8:671. doi: 10.2174/1381612023395349. [DOI] [PubMed] [Google Scholar]
- 18.Stoop MP, Coulier L, Rosenling T, Shi S, Smolinska AM, Buydens L, Ampt K, Stingl C, Dane A, Muilwijk B, Luitwieler RL, Smitt P, Hintzen RQ, Bischoff R, Wijmenga SS, Hankemeier T, van Gool AJ, Luider TM. Quantitative proteomics and metabolomics analysis of normal human cerebrospinal fluid samples. Mol. Cell. Proteomics. 2010;9:2063–2075. doi: 10.1074/mcp.M110.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra OP, Batra P, Ali Z, Anupurba S, Das BK. Cerebrospinal fluid lysozyme level for the diagnosis of tuberculous meningitis in children. J. Trop. Pediatr. 2003;49:13–16. doi: 10.1093/tropej/49.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Rosenling T, Stoop MP, Attali A, Aken Hv, Suidgeest E, Christin C, Stingl C, Suits F, Horvatovich P, Hintzen RQ, Tuinstra T, Bischoff R, Luider TM. Profiling and identification of cerebrospinal fluid proteins in a rat EAE model of multiple sclerosis. J. Proteome Res. 2012;11:2048–2060. doi: 10.1021/pr201244t. [DOI] [PubMed] [Google Scholar]
- 21.Traut RR, Bollen A, Sun TT, Hershey JW, Sundberg J, Pierce LR. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973;12:3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- 22.Byrne SL, Leverence R, Klein JS, Giannetti AM, Smith VC, MacGillivray RTA, Kaltashov IA, Mason AB. Effect of glycosylation on the function of a soluble, recombinant form of the transferrin receptor. Biochemistry. 2006;45:6663–6673. doi: 10.1021/bi0600695. [DOI] [PubMed] [Google Scholar]
- 23.Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochimica et biophysica acta. 1952;8:302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- 24.Lao BJ, Tsai W-LP, Mashayekhi F, Pham EA, Mason AB, Kamei DT. Inhibition of transferrin iron release increases in vitro drug carrier efficacy. Journal of Controlled Release. 2007;117:403–412. doi: 10.1016/j.jconrel.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson VG, Wrobel C, Wilson D, Zovickian J, Greenfield L, Oldfield EH, Youle R. Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. Journal of Neurosurgery. 1989;70:240–248. doi: 10.3171/jns.1989.70.2.0240. [DOI] [PubMed] [Google Scholar]
- 26.Singh R, Kats L, Blä tler WA, Lambert JM. Formation of N-Substituted 2-Iminothiolanes When Amino Groups in Proteins and Peptides Are Modified by 2-Iminothiolane. Analytical Biochemistry. 1996;236:114–125. doi: 10.1006/abio.1996.0139. [DOI] [PubMed] [Google Scholar]
- 27.Kaltashov IA, Abzalimov RR. Do ionic charges in ESI MS provide useful information on macromolecular structure? J. Am.Soc. Mass Spectrom. 2008;19:1239–1246. doi: 10.1016/j.jasms.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Sharon M, Robinson CV. The role of mass spectrometry in structure elucidation of dynamic protein complexes. Annu. Rev. Biochem. 2007;76:167–193. doi: 10.1146/annurev.biochem.76.061005.090816. [DOI] [PubMed] [Google Scholar]
- 29.Heck AJR. Native mass spectrometry: a bridge between interactomics and structural biology. Nat. Meth. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 30.Gabelica V, Galic N, Rosu F, Houssier C, De Pauw E. Influence of response factors on determining equilibrium association constants of non-covalent complexes by electrospray ionization mass spectrometry. J. Mass Spectrom. 2003;38:491–501. doi: 10.1002/jms.459. [DOI] [PubMed] [Google Scholar]
- 31.Boeri Erba E, Barylyuk K, Yang Y, Zenobi R. Quantifying protein-protein interactions within noncovalent complexes using electrospray ionization mass spectrometry. Anal. Chem. 2011;83:9251–9259. doi: 10.1021/ac201576e. [DOI] [PubMed] [Google Scholar]
- 32.Kaltashov IA, Bobst CE, Zhang M, Leverence R, Gumerov DR. Transferrin as a model system for method development to study structure, dynamics and interactions of metalloproteins using mass spectrometry. Biochim. Biophys. Acta. 2012;1820:417–426. doi: 10.1016/j.bbagen.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leverence R, Mason AB, Kaltashov IA. Noncanonical interactions between serum transferrin and transferrin receptor evaluated with electrospray ionization mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8123–8128. doi: 10.1073/pnas.0914898107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobst CE, Wang S, Shen W-C, Kaltashov IA. Mass spectrometry study of a transferrin-based protein drug reveals the key role of protein aggregation for successful oral delivery. Proc. Natl. Acad. Sci. U.S.A. 2012;109:13544–13548. doi: 10.1073/pnas.1206924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennhart N, Letzel T. Mass spectrometric real-time monitoring of enzymatic glycosidic hydrolysis, enzymatic inhibition and enzyme complexes. Anal. Bioanal. Chem. 2006;386:689–698. doi: 10.1007/s00216-006-0604-1. [DOI] [PubMed] [Google Scholar]
- 36.Klass HJ, Hopkins J, Neale G, Peters TJ. The estimation of serum lysozyme: A comparison of four assay methods. Biochemical Medicine. 1977;18:52–57. doi: 10.1016/0006-2944(77)90048-5. [DOI] [PubMed] [Google Scholar]
- 37.Rainov NG, Soling A. Clinical studies with targeted toxins in malignant glioma. Rev. Recent Clin. Trials. 2006;1:119–131. doi: 10.2174/157488706776876454. [DOI] [PubMed] [Google Scholar]
- 38.Rainov NG, Soling A. Technology evaluation: TransMID, KS Biomedix/Nycomed/Sosei/PharmaEngine. Curr. Opin. Mol. Ther. 2005;7:483–492. [PubMed] [Google Scholar]
- 39.Kaltashov IA, Bobst CE, Abzalimov RR, Wang G, Baykal B, Wang S. Advances and challenges in analytical characterization of biotechnology products: Mass spectrometry-based approaches to study properties and behavior of protein therapeutics. Biotechnol. Adv. 2012;30:210–222. doi: 10.1016/j.biotechadv.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobst CE, Kaltashov IA. Advanced Mass Spectrometry-Based Methods for the Analysis of Conformational Integrity of Biopharmaceutical Products. Curr. Pharm. Biotechnol. 2011;12:1517–1529. doi: 10.2174/138920111798357311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundblad RL. Approaches to the conformational analysis of biopharmaceuticals. Vol. Boca Raton: CRC Press/Taylor & Francis; 2010. [Google Scholar]
- 42.Abzalimov RR, Frimpong A, Kaltashov IA. Structural characterization of protein-polymer conjugates. I. Assessing heterogeneity of a small PEGylated protein and mapping conjugation sites using ion exchange chromatography and top-down tandem mass spectrometry. Int. J.Mass Spectrom. 2012;312:144–154. [Google Scholar]
- 43.Hartinger CG, Tsybin YO, Fuchser J, Dyson PJ. Characterization of platinum anticancer drug protein-binding sites using a top-down mass spectrometric approach. Inorganic chemistry. 2008;47:17–19. doi: 10.1021/ic702236m. [DOI] [PubMed] [Google Scholar]
- 44.Kaltashov IA, Bobst CE, Abzalimov RR, Berkowitz SA, Houde D. Conformation and dynamics of biopharmaceuticals: transition of mass spectrometry-based tools from academe to industry. J. Am.Soc. Mass Spectrom. 2010;21:323–337. doi: 10.1016/j.jasms.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SH, Kaltashov IA. A New Strategy of Using O-18-Labeled Iodoacetic Acid for Mass Spectrometry-Based Protein Quantitation. J. Am.Soc. Mass. Spectrom. 2012;23:1293–1297. doi: 10.1007/s13361-012-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkowitz SA, Engen JR, Mazzeo JR, Jones GB. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 2012;11:527–540. doi: 10.1038/nrd3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazar AC, Wang L, Blätler WA, Amphlett G, Lambert JM, Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:1806–1814. doi: 10.1002/rcm.1987. [DOI] [PubMed] [Google Scholar]
- 48.Valliere-Douglass JF, McFee WA, Salas-Solano O. Native intact mass determination of antibodies conjugated with monomethyl auristatin E and F at interchain cysteine residues. Anal. Chem. 2012;84:2843–2849. doi: 10.1021/ac203346c. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Yin S, Wu Y, Ouyang J. Development of a novel native nano-electrospray mass spectrometry method for determination of drug-to-antibody ratio of antibody-drug conjugates. Anal. Chem. 2013;85:1699–1704. doi: 10.1021/ac302959p. [DOI] [PubMed] [Google Scholar]
- 50.Teicher BA, Doroshow JH. The promise of antibody-drug conjugates. N. Engl. J.Med. 2012;367:1847–1848. doi: 10.1056/NEJMe1211736. [DOI] [PubMed] [Google Scholar]
- 51.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Ann. Rev. Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.