Abstract
Fiber intake is critical for optimal health. This review covers the anti-inflammatory roles of fibers using results from human epidemiological observations, clinical trials, and animal studies. Fiber has body weight–related anti-inflammatory activity. With its lower energy density, a diet high in fiber has been linked to lower body weight, alleviating obesity-induced chronic inflammation evidenced by reduced amounts of inflammatory markers in human and animal studies. Body weight–unrelated anti-inflammatory activity of fiber has also been extensively studied in animal models in which the type and amount of fiber intake can be closely monitored. Fermentable fructose-, glucose-, and galactose-based fibers as well as mixed fibers have shown systemic and local intestinal anti-inflammatory activities when plasma inflammatory markers and tissue inflammation were examined. Similar anti-inflammatory activities have also been demonstrated in some human studies that controlled total fiber intake. The anti-inflammatory activities of synbiotics (probiotics plus fiber) were reviewed as well, but there was no convincing evidence indicating higher efficacy of synbiotics compared with that of fiber alone. Adverse effects have not been observed with the amount of fiber intake or supplementation used in studies, although patients with Crohn’s disease may be more sensitive to inulin intake. Several possible mechanisms that may mediate the body weight–unrelated anti-inflammatory activity of fibers are discussed based on the in vitro and in vivo evidence. Fermentable fibers are known to affect the intestinal microbiome. The immunomodulatory role of the intestinal microbiome and/or microbial metabolites could contribute to the systemic and local anti-inflammatory activities of fibers.
Introduction
The nutritional importance of fiber has been long recognized. Fiber has been included in the Nutrition Facts label following the 1990 Nutrition Labeling and Education Act, which sets the daily value (DV)3 for fiber at 12 g/1000 kcal. The 1997 revision of the Dietary Reference Intakes (DRI) established the adequate intake of fiber at ∼14 g/1000 kcal (1). At ∼13 g/1000 kcal (50 g/kg diet), the calculated nutrient density of fiber in purified rodent diets, such as AIN-76 and AIN-93, is similar to the human recommendation (2, 3).
Unfortunately, the presence of these recommendations does not signify a complete understanding of fiber nutrition. The amount of fiber intake that meets the needs for 97–98% of the U.S. population, the recommended dietary allowance, is yet to be determined (1). An even greater deficiency in these recommendations is the lack of consideration given to different types of fibers: both DV and DRI are for total fiber and both AIN-76 and AIN-93 rodent diets use cellulose as the sole source of fiber. Unlike some other fibers, cellulose has little if any growth-promoting activity for intestinal microbes. The primary obstacles in defining the requirement of fiber are a lack of a uniform definition of fiber (4, 5), which makes the estimation and comparison of fiber intake difficult and as a result affects the power of epidemiological analyses, and an incomplete understanding of fiber’s biological effects (6–8), which makes achieving that uniform definition and setting dietary recommendations challenging.
The focus of this review is on the multifaceted role of fiber in modulating tissue inflammation, locally in the intestine and systemically. Although a responsive immune system is necessary for life, proper modulation of inflammation is important for the ultimate restoration of health (9, 10). Experimental evidence as presented in this review supports the presence of both body weight–related and –unrelated anti-inflammatory effects of fiber. Obesity is known to induce a state of chronic inflammation. Fiber intake, through reducing BMI, could limit the obesity-induced inflammation. The body weight–unrelated effect of fiber is likely mediated through the intestinal microbiome. One possible mechanism is that by shaping the intestinal microbiome, fiber intake indirectly affects the immune system.
Current status of knowledge
Classification of fiber
Fiber represents a group of carbohydrates or carbohydrate-containing compounds that are neither digested nor absorbed in the small intestine. The most commonly adopted classification of fiber is based on the source (11). Further structural classifications have also been developed based on either the water solubility or the susceptibility to large intestinal bacterial degradation (i.e., fermentation potential) (12). This last classification carries the most relevance for this review. Although carbohydrates in general are an important source of energy for intestinal microflora (12, 13), various fibers show different fermentation potentials (14–17). Overall, cellulose (β-glucan) in the standard rodent diets showed poor fermentability (17, 18), whereas similarly glucose-based resistant starch is readily fermentable (17). Fructose fibers of varying polymer size are fermented at different rates (16, 19). Human milk–based galactooligosaccharide (GOS) (20) and other carbohydrate heteropolymers (21) are also substrates of commensal microflora. The normal human diet would contain various fibers and an interaction, among fibers with different fermentabilities probably exists. Less fermentable psyllium fiber was shown to shift the fermentation of high-amylase–resistant starch toward the distal colon in rats (22).
Fiber and intestinal microbiome
The intestinal microbiome has been the highlight of recent issues of Science (volume 336, issue 6086, 2012) and Nature (volume 486, number 7402, 2012 and volume 489, number 7415, 2012). Because the fermentation potential of each fiber type is bacterial species and strains dependent (19), dietary patterns with different varieties and amounts of fibers likely will differentially modulate the evolution of the intestinal microbiome. This hypothesis has been supported by the results of fecal analyses performed on humans with different dietary patterns (23–26). Fiber supplementation studies have also examined the hypothesis. Considerable differences in the intestinal microbiome were observed between mice consuming a high-fat, carbohydrate-free diet and those fed the same diet supplemented with glucose oligosaccharide (27). A high-carbohydrate diet was found to promote the growth of Bifidobacterium in the human intestine, i.e., bifidogenic (28), whereas intake of saturated fat has been shown to increase the Firmicutes-to-Bacteroidetes ratio (29).
The bifidogenic activity of individual fiber type has also been confirmed. Plant-based fructooligosaccharide (FOS) is one type of extensively studied bifidogenic fiber (30). Commercially prepared inulin is rich in FOS but also contains longer chain fructose polymers. For the purpose of this review, unless long-chain inulin is specified, the abbreviation FOS is used to represent FOS and FOS-enriched inulin. Fecal sample analyses revealed bifidogenic activity in human feeding trials using FOS (20 g/d) (31) or very long–chain inulin (10 g/d) (32) and in a rat FOS supplementation study (8 g/kg body weight) (33). In addition to the standard fecal analysis, analyses of colon biopsy specimens similarly revealed an increase in Bifidobacterium after FOS supplementation (15 g/d) (34). The increase in the fecal abundance of Bifidobacterium was also observed after supplementation trials with glucose-based soluble fiber from corn (21 g/d) (35), glucose-based resistant starch (∼30 g/d) (36), and GOS (10–21 g/d) (37, 38). Lactose-derived GOS has higher ileal digestibility and thus less bifidogenic effect in rats compared with its less digestible isomer, lactulose (39).
Recent studies have used PCR amplification or 16S ribosomal RNA–based pyrosequencing to evaluate the global change in the intestinal microbiome after fiber supplementation. The results were variable. For example, no changes (37) or a decrease in the Bacteroidetes (37) were reported in GOS supplementation trials. An overall decrease in Firmicutes and an increase in Bacteroidetes and Actinobacteria were reported after the supplementation with resistant starch (36), but similarly glucose-based soluble corn fiber supplementation did not affect the abundance of Bacteroidetes (38). These inconsistencies, although puzzling, were not particular surprising as these supplementation studies often have differences in the duration and other dietary factors, for example, the type and quantity of dietary fat (28). Also, based on the potential mechanisms that mediate the body weight-unrelated anti-inflammatory activity of fiber (see section on Potential mechanisms), it is not clear whether a global change in the intestinal microbiome is required to exert the biological effect on the host.
The fermentability of fiber does not necessarily imply a prebiotic property. The definition of prebiotics is “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora, that confers benefits upon host well-being and health” (40). In the rest of the review, the ability of fibers to confer a health benefit to the host is considered. An anti-inflammatory effect of fiber is of special interest because higher fiber intake has been linked to a decreased overall mortality in older adults including mortality due to infectious, inflammatory, and respiratory diseases (41, 42).
Body weight–related anti-inflammatory effect of fiber
Obesity-related metabolic disorders are associated with inflammation (43, 44); thus, dietary practice that can limit obesity could have a body weight–related anti-inflammatory effect. The potential roles of fiber intake in reducing the risk of obesity are summarized in Figure 1. A long-term dietary pattern high in fiber intake was associated with a lower BMI, and the lower BMI was likely a consequence of the lower energy density in the fiber-rich diet (45, 46) (Fig. 1).
Figure 1.
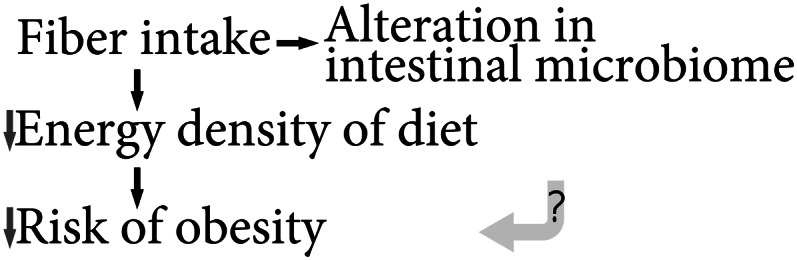
Summary of the current knowledge of the causal relationships among fiber, the intestinal microbiome, and obesity.
Although fiber intake is known to affect the intestinal microbiome, it is not clear whether fiber intake can also affect the risk of obesity through the modification of the microbiome (Fig. 1). By comparing the fecal samples, it was concluded that obesity is associated with an increase in Firmicutes and a decrease in Bacteriodetes (47). However, a direct long-term causal relationship between the intestinal microbiome and obesity is not evident. Microbiome transplantation from ob/ob mice to germ-free mice led to higher body fat after 2 wk compared with a similar transplantation from wild-type mice (48), but long-term weight information was not available. In a clinical trial, energy harvesting, defined as the difference between energy intake and energy loss found in the feces and urine, was greater over a 3-d period of overfeeding, which was coinciding with an increase in Firmicutes and a decrease in Bacteriodetes (49). However, the authors also concluded that changes in the energy harvesting could be a result of the increase in fat intake during the overfeeding condition, which led to higher energy input.
The possible routes by which fiber intake can influence inflammatory response are summarized in Figure 2. Epidemiological studies supported that while reducing the risk of obesity, fiber intake leads to less inflammation. Diets high in fiber intake were linked to a lower BMI and a decreased risk of inflammation-associated metabolic abnormalities (50, 51); and a lower plasma proinflammatory biomarker C-reactive protein (52).
Figure 2.

Summary of the current knowledge of the causal relationships among fiber, the intestinal microbiome, and inflammatory response.
Results of intervention studies also support that fiber can, through its known long-term effect on the BMI, modulate the inflammatory response. In a 1-y diabetes prevention clinical trial, the amount of total fiber intake inversely affected the BMI and the circulating amount of IL-6 and C-reactive protein (53). Another 3-mo fiber intake study of subjects at high risk of cardiovascular disease also reached the same conclusion (54). Using genetically obese Zucker rats or mice with high-fat diet–induced obesity, mixed fibers or FOS supplementation was found to reduce body weight and the obesity-associated increase in inflammatory cytokines (55–57), subcutaneous Toll-like receptor 4, and extracellular antigen F4/80 (58). Long-chain inulin (5% of diet) or FOS (10% diet) supplementation in rats fed a high-fat diet also led to less weight gain, less liver triglyceride accumulation, and lower susceptibility to drug-induced liver damage and inflammation (59, 60). The negative correlation between fiber intake and inflammation indicators found in various epidemiological studies is summarized in Table 1(41, 42, 52, 61–65).
Table 1.
Epidemiological evidence of a considerable negative association between fiber intake and inflammation indicators1
| Population | Study design | Fiber | Indicator | Sample size | Ref. |
| Adults (age ≥20 y) | NHANES, 1999–2000 | Total | C-reactive protein | 3920 | (52) |
| Diabetic women (age ∼60 y) | Nurses’ Health Study | Cereal | C-reactive protein | 902 | (61) |
| TNF-α receptor 2 | |||||
| Adults (age 20–70 y) | SEASONS | Soluble | C-reactive protein | 524 | (63) |
| Insoluble | C-reactive protein | ||||
| Postmenopausal women | Women‘s Health Initiative | Soluble | IL-6; TNF-α receptor 2 | 1958 | (64) |
| Insoluble | IL-6; TNF-α receptor 2 | ||||
| Adults (age 50–71 y) | NIH-AARP study | Total | Infectious disease death | 567,169 | (41) |
| Total | Respiratory disease death | ||||
| Breast cancer (female, age 18–64 y) | HEAL study | Total | C-reactive protein | 1183 | (62) |
| Cancer-free adults | EPIC cohort | Cereal | IL-1β IL-4; IL-5; IL-6; TNF-α | 88 | (65) |
| Adults (age 25–70 y) | EPIC cohort | Total | Inflammatory disease death | 452,717 | (42) |
EPIC, European Prospective Investigation into Cancer and Nutrition; HEAL, Health, Eating, Activity, and Lifestyle Study; SEASONS, Seasonal Variation of Blood Cholesterol Levels Study.
Body weight–unrelated anti-inflammatory effect of fiber
In some larger epidemiological studies, the negative association between fiber intake and plasma amount of inflammatory markers persisted even after adjustment for the BMI. These analyses suggest that fiber intake likely has an impact on inflammation through body weight–unrelated mechanisms as well (Fig. 2). The body weight–unrelated anti-inflammatory effect of fiber is the focus of the rest of the review, and the results of both human trials and animal studies are considered.
Fiber supplementation clinical trials.
In parallel to the epidemiological studies and long-term intervention described above that examined the total intake of mixed fibers, short-term feeding studies were also conducted on healthy subjects and populations at risk of inflammation-associated diseases, as summarized in Table 2. The outcomes of the trials were somewhat inconsistent despite confirmed changes in the intestinal microbiome with fiber supplementation in some studies. Psyllium fiber supplementation (7–14 g/d for 3 mo) to obese individuals led to no changes in the inflammatory markers (66). The blood concentration of proinflammatory cytokines was also not affected by 4-wk supplementation of oat β-glucan (4.8 g/d) in hypercholesterolemic subjects (67). A similar lack of effect was observed in a resistant starch supplementation (12.5 g/d for 4 wk) study on healthy individuals (68). One consistent deficiency of these supplementation trials has been a lack of consideration on other fiber intake through diet before and during the trial. Without the knowledge of total fiber intake, the negative results of these supplementation trials have only limited implication on fiber nutrition. Using a more comprehensive approach, a high fiber diet (27 g/d of fiber) and a diet supplemented with psyllium to a final total fiber concentration of ∼27 g/d were found to similarly decrease the amount of C-reactive protein. The effect was greater in lean normotensive subjects than in obese hypertensive subjects (69).
Table 2.
Clinical trials on the anti-inflammatory property of fiber with positive and negative results1
| Population | Duration | Fiber intake | Measurement | Significance | Ref. |
| Preterm infants | 3–30 d | 1.5 g OS/(kg · d) | Infectious morbidity | NS | (77) |
| Term infants | 6 mo | 8 g FOS+GOS/L | ↓Infectious episodes | P = 0.01 | (74, 75) |
| Adults (age 18–49 y) | 3 wk | Total ∼27 g/d2 | ↓C-reactive protein | P < 0.05 | (69) |
| Obese adults | 3 mo | 7–14 g psyllium/d | C-reactive protein | NS | (66) |
| IL-6; fibrinogen | NS | ||||
| Diabetes | 1 y | 14.4 g/1000 kcal2 | ↓C-reactive protein | P = 0.015 | (53) |
| IL-6 | NS | ||||
| Adults, hypercholesterolemic | 4 wk | 4.8 g oat β-glucan/d | IL-6; IL-8; TNFα | NS | (67) |
| C-reactive protein | NS | ||||
| Elderly (age >70 y) | 12 wk | 5.2 g OS/L | ↓IL-6, TNFα | P ≤ 0.05 | (73) |
| Elderly high cardiovascular disease risk | 3 mo | Total 22.2 g/d2 | ↓C-reactive protein | P = 0.04 | (54) |
| IL-6 | NS | ||||
| Ulcerative colitis patients | 4 wk | 20–30 g GBF/d | ↓Disease activity | P < 0.05 | (71) |
| Ulcerative colitis patients | 2 mo | 30 g GBF/d | ↓IL-6; IL-8 | P < 0.05 | (72) |
| Crohn‘s disease patients | 4 wk | 15 g FOS/d | ↓IL-6; ↑IL-10 in DC | P < 0.05 | (70) |
| Clinical response | NS |
DC, dendritic cell; FOS, fructooligosaccharide; GBF, germinated barley foodstuff; GOS, galactooligosaccharide; NS, not significant; OS, oligosaccharide.
Studies that increased the total fiber intake to the indicated amount. All other studies were trials of supplementation at the amount indicated.
The anti-inflammatory effect of fiber supplementation in the intestine has been examined in clinical trials on patients with inflammatory bowel disease (IBD). Although FOS supplementation (15 g/d) did not provide clinical benefit, it reduced proinflammatory IL-6 and increased anti-inflammatory IL-10 in dendritic cells of patients with active Crohn’s disease compared with the placebo treatment (70). Different from healthy adults (31), FOS supplementation did not lead to a detectable change in the fecal concentration of Bifidobacterium. In addition, using FOS supplementation at the dose for Crohn’s disease patients may not be desirable as the fiber group showed higher dropout rate partly due to a worsening gastrointestinal symptoms (70). When ulcerative colitis patients were given germinated barley foodstuff (GBF), a bifidogenic preparation, at 20–30 g/d along with anti-inflammatory medication, their disease was better controlled compared with the group receiving anti-inflammatory medication alone (71), and a slight decrease in systemic inflammatory cytokines was also observed (72).
Fiber supplementation trials have been conducted in the elderly and infants. Twelve-week oligosaccharide supplementation (1.3 g/d) reduced the low-grade systemic inflammation observed in an undernourished or at-risk older population (73) despite no substantial changes in the intestinal microbiome based on the fecal analysis. GOS/FOS supplementation (8 g/L formula) for the first 6 mo of life in infants with a family history of atopy was shown to reduce episodes of infection during (74) and after intervention (75). There were also fewer cumulative allergic manifestations during the first 2 y of life (75). The same GOS/FOS supplement, when given to mother (18 g/d) during pregnancy, did not affect immune indicators and cytokine amounts of the neonates (76). Including GOS/FOS in the formula also did not protect preterm infants (77) or infants with no family history of allergy (78).
In summary, clinical trials have not uniformly supported an anti-inflammatory role of fiber, although specific benefits were observed in particular populations. Future supplementation studies need to also take into consideration concurrent fiber intake through the diet. There is no evidence to support the use of fiber as an alternative therapy for active IBD, although complementary use of fiber with the anti-inflammatory medication may provide clinical benefits in some patients (71). Other than a trial of patients of Crohn’s disease in which worsening gastrointestinal symptoms was observed in some cases with FOS supplementation (70), no adverse effects were reported in other fiber supplementation trials.
Fiber supplementation animal studies.
Studies using animal models allow for better control of fiber intake and other conditions. Because purified animal diets contain only poorly fermented cellulose (2, 3), these diets are especially useful tools for understanding the benefits of fermentable fibers. The anti-inflammatory effects of fermentable fibers have been examined using mainly two types of rodent IBD models: chemical-induced colitis and genetically susceptible strains. Although both types of models have limitations in mimicking human IBD (79), they nevertheless present a characterized inflammation in which the anti-inflammatory activity of fiber can be assessed.
Effect of fiber in chemical-induced colitis in animal models.
Colitis can be induced by a single intracolonic injection of trinitrobenzene sulfonic acid (TNBS) in rats or by continuous feeding of dextran sulfate sodium (DSS) in mice. FOS supplementation at ∼5% of the food intake or as 5% wt/vol in the fluid changed intestinal microbiome and has been examined extensively for anti-inflammatory activity. FOS supplementation through gastric intubation (80) or as part of the diet (81) reduced weight loss and colonic damage associated with TNBS treatment. In contrast, FOS included in drinking water failed to provide protection in this model (82). FOS showed a different pattern of activity when tested using the DSS model. FOS supplementation by gavaging reduced DSS-induced weight loss and colonic macroscopic damage in mice fed a cereal-based fiber–rich diet (83, 84). In contrast, FOS included in the purified diet (containing cellulose as the only fiber) failed to prevent mucosal damage (84, 85). Clearly, TNBS and DSS models induce different courses of intestinal inflammation (79), and the effect of FOS could be model dependent. Nonetheless, because the amount of food and liquid intake was not always reported in these studies, we cannot rule out the possibility that the variations in the outcome were the results of variable fiber intake.
Several other fermentable fiber preparations have also been shown to alter the intestinal microbiome and were tested for their protective effects on chemical-induced colitis. Resistant starch supplementation (11.5%) reduced cecal and colonic damage in the DSS model (85). Nondigestible lactose-derived synthetic and natural milk saccharides (86), lactulose (0.3–1 mg/g body weight) (87) and goat milk oligosaccharide (2%) (88), both reduced macroscopic damage in the DSS model. Lactulose in water (2.5% wt/vol) also reduced the amount of inflammatory markers in TNBS-induced colitis (89). Including partially hydrolyzed plant polysaccharide guar gum (5%) (90) or enzyme-treated rice fiber (4%) (91) in the diet reduced intestinal inflammation of DSS-treated mice. Two fermentable protein-carbohydrate mixtures, GBF (72, 92) and enzymatic hydrolysate of corn gluten (93), were also tested using rodent models of chemical-induced colitis and were shown to reduce mucosal damage and the amount of inflammatory markers. Further comparison of fiber-enriched GBF and fiber-poor GBF suggested that the fiber component of GBF is likely the active ingredient for the anti-inflammatory activity (94).
Using an animal study, an adverse effect of FOS supplementation was also observed but was limited to a particular small intestine inflammation model. FOS increased the 5-fluorouracil–induced small intestine mucosal damage when given by gavage to rats fed a casein-based fiber-free diet (95). Because most of the inflammation was observed in the jejunum in this model, the relevance of this observation of FOS fermentation in the large intestine is likely limited.
Effect of fiber in genetic models of IBD.
Chemical insult is a rare cause of chronic intestinal inflammation, and thus several complementary genetic models for intestinal inflammation were also used to further test the anti-inflammatory activity of fibers. Rats expressing the HLA-34B gene develop spontaneous gastrointestinal inflammation in the presence of intestinal bacteria (96). Including short-chain or long-chain FOS in the control nonpurified diet was found to alter the intestinal microbiome and reduce the spontaneous inflammation (33, 97). Similar anti-inflammatory activity was observed in this model when fiber-rich Plantago ovata seeds were included at 5% in a diet containing cellulose (98). Supplementation with FOS or glucose-based resistant starch (4–5% in a cellulose-containing diet) also reduced the colon damage in IL10−/− mice (99), another model of spontaneous intestinal inflammation (100). In the same study, soluble corn fiber and some other fibers did not show anti-inflammatory activity at similar doses.
Transferring CD4+CD45RBhigh T cells to SCID mice can lead to the development of colitis resembling human IBD (101). In this model, similar to the observations made in the chemical insult model, supplementation with GBF (10%) or enzyme-treated rice fiber (4%) lessened weight loss and histological damage and reduced the amount of inflammatory markers in the colon compared with mice given the cellulose-containing AIN93G diet (91, 102).
Effect of fiber in modulating the systemic allergic response.
Although fiber per se is not absorbed into the systemic blood circulation, the ability of fermentable fiber to affect the intestinal microbiome may influence the systemic immune response, as proposed in a recent review (103). Thus, not surprisingly, systemic body weight–unrelated anti-inflammatory effects have also been observed after FOS feeding. FOS supplementation (2.5% in diet) in mice suppressed allergic airway inflammation induced by mite allergen (104). Similar FOS supplementation also reduced the ovalbumin-induced allergic peritonitis (105) and allergic asthma (106) in mice. Mice receiving FOS (5% in diet) showed less 2,4-dinitrofluorobenzene-induced contact sensitivity and less increase in plasma inflammatory markers (107, 108). The same amount of FOS supplementation also reduced spontaneous skin lesions and inflammation-related cytokines in NC/Nga mice (109). In this model of spontaneous skin lesions, maternal FOS supplementation provided anti-inflammatory protection for offspring (109).
Implication of fiber supplementation studies in human nutrition.
Overall, it is reasonable to conclude that fermentable fibers have both local and systemic anti-inflammatory activities and should be considered in the dietary recommendation. Despite some variations in experimental outcomes, anti-inflammatory effects were consistently observed in human epidemiological studies and clinical trials as well as in animal studies using various models. Besides the well-studied FOS, other fermentable fiber preparations including glucose and galactose polymers as well as psyllium and the heteropolymer hemicellulose-rich GBF are also promising. Positive effects of supplementations with mixed fiber types are especially encouraging as these supplements better mimic the heterogeneous fiber input of normal human diet. Although most animal studies have used 5% or 10% fiber supplementation, 1% and 2.5% supplementation was also used in a limited numbers of studies that found anti-inflammatory effects. Ranges of doses are needed in future human trials and animal studies to aid in the assessment of fermentable fiber requirement.
FOS supplementation at 15 g/d led to worsening gastrointestinal symptoms in some patients with Crohn’s disease (70). This amount of FOS intake was not found to cause gastrointestinal discomfort in a healthy population, although an increase in bowel movements and flatulence was reported (110). It is possible that the adverse effect of FOS observed in the patients with Crohn’s disease was related to disease-related changes in their basal dietary patterns (111) and intestinal microbiome (112, 113). Based on the literature, it is not clear whether supplementation with other fibers in large amounts poses any risk for IBD patients.
Probiotic and synbiotic supplementation studies
Probiotics and synbiotics are also considered in the review because of their impact on the intestinal microbiome. The definition of probiotics is “live microorganisms that when administered in adequate amounts confer a health benefit on the host” (114). Most commercially available probiotics are strains of Bifidobacterium and Lactobacillus found in normal human gastrointestinal tract (114). Synbiotics represent mixtures of probiotics and prebiotics that beneficially affect the host (115). Because the environmental and dietary exposures to probiotic strains are difficult to quantify, epidemiological data on probiotics and synbiotics are not available. Although probiotic supplementation studies have been published extensively, data interpretation is complicated by the fact that fiber intake, in either humans or in animals, was mostly not monitored or controlled (116–118). Also, although the anti-inflammatory activity of probiotics has been observed in the chemical insult animal model (118), the activity was not found in the genetic model of intestinal inflammation (119). As a result, the benefit of probiotic supplementation has yet to be addressed clearly.
The anti-inflammatory effect of synbiotic supplementation has been examined in clinical trials and animal studies. In human trials of synbiotics, similar to the trials examining prebiotic and probiotic separately, fiber intake from diet was not controlled and information not available. However, reviewing results of synbiotic supplementation studies allowed a limited comparison with the results of fiber supplementation studies. This will help to determine whether additional nutritional benefits exist in synbiotic supplementation compared with fiber supplementation alone.
Synbiotic supplementation trials in patients with local and systemic inflammation.
Synbiotic supplementation has been applied in acute conditions. In acute gastroenteritis in which the intestinal microbiome was likely affected, studies using various different synbiotic preparations found only limited clinical efficacy (120, 121). For critically ill patients, synbiotic supplementation was found to affect the intestinal microbiome (122, 123) and promote weight gain in children (123) but again produced limited clinical benefits if any (122, 123).
Similar to fermentable fiber, synbiotics have been given to IBD patients. In an ulcerative colitis study, 1 mo of treatment with FOS (12 g/d) and a strain of Bifidobacterium (isolated from healthy rectal samples) led to an improvement in the clinical scores and reductions in tissue inflammation and the amount of inflammatory markers (124). Because the probiotics included in the synbiotic preparation originated from healthy human intestines, it is not surprising that the anti-inflammatory effect of this synbiotic preparation is similar to that of the prebiotic GBF alone (72).
Similar to fiber prebiotics, synbiotics have also been used in trials for children with atopic dermatitis. Supplementation with synbiotics, Bifidobacterium plus GOS and FOS, did not help infants (younger than 7 mo) and young children with atopic dermatitis (125, 126). Supplementation of a different synbiotic preparation, Lactobacillus plus FOS, provided some clinical improvement in children with moderate to severe atopic dermatitis. It appeared to be more effective than FOS supplementation alone (127).
Chronic upregulation of inflammatory markers has been observed in immortalized cells (128) and in the tumor microenvironment (129). Synbiotic supplementation trials were thus conducted to examine the potential anti-inflammatory effect of synbiotics on patients with colon cancer and to determine the chemopreventive potential. The outcomes were overall disappointing. FOS (12 g/d) plus Lactobacillus and Bifidobacterium supplementation was carried out on patients who had undergone polypectomy and colon cancer patients for 3 mo. Cancer-related biomarkers in the biopsy specimens were not affected by the supplementation, and only a slight change was observed the in vitro interferon-γ production by their mitogen-activated peripheral blood mononuclear cells (130, 131). The potential chemoprevention effect of a different synbiotic preparation, resistance starch (12.5 g/d) and Bifidobacterium, was also addressed in healthy subjects using a crossover study design. Although changes in the intestinal microbiome were observed, the serum or rectal markers of inflammation were not affected by the supplementation (68).
Overall, limited anti-inflammatory effects of synbiotic supplementation were observed in some cases such as children with atopic dermatitis and patients with ulcerative colitis. Compared with the prebiotic trials, one interesting observation is that although FOS supplementation at 15 g/d may have led to adverse effects in patients with Crohn’s disease (70), FOS supplementation at 12 g/d (6 g per dose, twice daily) as part of the synbiotics has shown anti-inflammatory effects in ulcerative colitis patients (124). There is no strong evidence supporting greater efficacy of synbiotic supplementation compared with prebiotic supplementation.
Animal studies with synbiotic supplementation.
Synbiotic supplementation has been performed with the same animal models that were used to examine the anti-inflammatory effects of fibers. The experiments for these two types of supplementation often showed similar results. Synbiotic supplementation of Lactobacillus, Bifidobacterium, and 10% FOS in rats fed a high-fat, low-fiber diet suppressed intestinal and systemic inflammation similar to FOS supplementation, but probiotic supplementation alone was ineffective (132). Treatment of inflammation-prone HLA-B27 rats with similar synbiotics led to fewer histological changes due to inflammation (133). Again, supplementation with only FOS was just as effective in reducing tissue inflammation in this rat model (33, 97). Subcutaneous injection of azoxymethane induces colon cancer in rats. Including Lactobacillus, Bifidobacterium, and 10% FOS or 10% FOS alone in the diet both reduced inflammation and the colon cancer incidence, but supplementation with probiotics alone had no protective effect (134). In this model, supplementation studies with resistant starch as the source of prebiotics have also been performed. Resistant starch (10%) alone and synbiotic supplementation with resistant starch both reduced the colon cancer incidence (135). In the DSS rat model, a synbiotic dietary supplement containing fiber-rich blueberry husks, oat bran, and a mixture of Bifidobacterium and Lactobacillus was found to reduce the severity of colitis (136, 137) as well as the long-term incidence of colon cancer and liver damage (137). Not surprisingly, blueberry husks alone or blueberry husks plus oat bran offered similar protection against inflammatory damage and cancer (137).
Although at 10% of the diet, fiber supplementation alone has been shown to be as effective as the synbiotic supplementation, there may be an additive effect with lower doses of prebiotics. In a pathogen-mediated intestinal inflammation mouse model, supplementation with FOS (1%) plus Lactobacillus through drinking water led to more anti-inflammatory effect compared with the pre- or probiotic supplementation alone (138). However, the control diet in this study was a fiber-rich, plant-based rodent chow, and the information on fiber in the chow was not available. Future studies using a purified cellulose-only diet as the control is needed to repeat the test of the additive effect.
Implication of synbiotic supplementation studies in human nutrition.
Small human synbiotic supplementation trials were conducted with subjects with a range of health issues. Limited anti-inflammatory effect and clinical benefits were found. It is important to point out that all trials were conducted in conjunction with necessary medical treatments, and thus all benefits were complementary to that of the medication.
Although most animal studies found anti-inflammmatory effects after synbiotic supplementation, a comparison of the outcomes of pre- and synbiotic supplementation studies led to the conclusion that these two approaches were probably similarly effective. This observation is not surprising as most of the probiotic strains were indeed originally obtained from healthy intestine, and fibers serve as the energy source of the intestinal microbiome.
In studies in which a fiber-free diet or fermentable fiber-poor diet was used as the control, probiotic supplementation alone did not promote fermentation and exhibited no anti-inflammatory activity. This is expected based on the contribution of fermentable fiber in shaping the intestinal microbiome. The finding suggests that in cases in which the fiber intake is below the requirement, such as in most of the U.S. population, probiotic supplementation may not compensate for low fiber intake in providing the anti-inflammatory effect.
Potential mechanisms that mediate the body weight–unrelated anti-inflammatory activity of fiber
As shown in Figure 2, three possible mechanisms have been examined for possible contribution in the body weight–unrelated anti-inflammatory activity of fiber.
Fiber represents a group of structurally diverse chemicals with different fermentability. The first possible mechanism suggested that the anti-inflammatory activity is inherent in the chemical structure of fibers. Human colon adenocarcinoma–based cell lines have been used to demonstrate a direct effect of fiber, but the experimental conditions complicated data interpretation. In one study, the effect of fiber treatment on cellular gene expression was performed in the serum-free medium in the absence of any stimulators for inflammation (139). Thus, the experiment did not measure a modulation of inflammatory response. In two other studies, various fibers at 16 g/L of culture medium was shown to block the short-term attachment of Escherichia coli (140) and Cronobacter (141) to epithelial cells grown on the cover glass. It is not clear whether these results can be extrapolated to the in vivo environment with the presence of abundant commensal bacteria on the epithelial surface. To demonstrate an effect of fiber by itself in vivo, animal models should have a microbe-free intestinal environment with no fermentation. However, possible experimental approaches to eliminate the presence of commensal bacteria: antibiotic treatment and the use of germ-free animals are both problematic. In short, these approaches have been shown to lead to compromised intestinal health (142) and overall animal development (143). With the technical limit in the in vivo experiment, it is not possible to conclude that there is any fermentation-independent anti-inflammatory effect of fermentable fibers. Even if a direct action of fiber exists, this mechanism cannot explain the anti-inflammatory effect of fiber outside the gastrointestinal tract.
The second possible mechanism suggests a direct competition between commensal and pathogenic bacteria as has been observed in in vitro studies (144). By producing short-chain fatty acids and other metabolites, the intestinal microbiome may create a nonpermissive environment for the colonization of pathogenic microorganisms (145). Because fiber promotes the growth of commensal bacteria, its anti-inflammatory activity maybe relate to an increased resistance to the colonization of pathogenic bacteria (Fig. 2). In animal models of IBD, the involvement of pathogenic bacteria has been documented (146, 147), and fermentable fiber supplementation has been shown to be effective in controlling the inflammation as described previously. Interestingly, in an epidemiological study, the use of antibiotics, which affects some pathogenic bacteria as well as gram-positive commensal bacteria, was found to increase the risk of IBD (148). The weakness of this mechanism is that it also cannot explain the anti-inflammatory effect of fiber outside the gastrointestinal tract.
In addition to promoting the competitiveness of commensal bacteria toward pathogenic bacteria, it is possible that fiber, by promoting the growth of commensal bacteria, could influence the immune system and thus exert anti-inflammatory activity (Fig. 2). An interaction between the intestinal microbiome and immune system has been studied and reviewed (149–151). Colonization of gnotobiotic mice with commensal bacteria has been shown to promote the differentiation of anti-inflammatory immune cells and increase the expression of anti-inflammatory cytokines (151). The strength of this mechanism is that it can explain both the local and systemic anti-inflammatory effects of fibers. There is indeed evidence that the intestinal microbiome may be involved in the immune response outside the gut. An altered intestinal microbiome has been observed in patients with asthma (152). Based on clinical observations, animal models have been developed, and disruption of the intestinal microbiome was found to promote a pulmonary allergic response (153, 154).
A fourth mechanism that can mediate the anti-inflammatory effect of fiber is through metabolites of the intestinal microbiome (Fig. 2). This mechanism can also explain both the local and systemic anti-inflammatory effects of fibers. Metabolites of the intestinal microbiome were recently reviewed (155). In addition to their appearance in intestinal content, these metabolites appear in host plasma (156) and urine (157) after intestinal absorption. Fiber, by promoting the intestinal microbiome, can change plasma metabolite profiles and thus exert the anti-inflammatory effect locally and systemically (Fig. 2). Although the mechanism is logical, the identification of the crucial metabolites involved is challenging because of the extensive microbiome-induced changes in the plasma profile (156). It is also possible that a cluster of microbial metabolites collectively affects the inflammatory response. Some in vitro evidence suggests an anti-inflammatory function of short-chain fatty acids with a special focus on butyrate.
To ascertain the physiological importance of butyrate or other microbial metabolites, in vivo concentrations of metabolites also need to be taken into consideration. The reported amounts of butyrate in various biological samples are summarized in Table 3. The reported amount of butyrate and other short-chain fatty acids in biological samples showed large intra- and interlaboratory variations. The cecal content of butyrate varied between 0.1 and 30 mmol/L (assuming 1 g = 1 mL) in rodent studies (48, 80, 84, 134, 135, 158). The amount of butyrate probably decreases gradually in the proximal and distal colon (135), but one study reported a concentration of 44 mmol/L in the rat colon (82). The reported fecal butyrate concentration was ∼13 mmol/L (assuming 1 g wet weight = 1 mL) for humans (68), and a range of 14–168 mmol/L was reported for rats (98, 159). The large intestinal and fecal concentrations of other short-chain fatty acids, acetate and propionate, were usually much higher than that of butyrate and can be as high as 1.36 mol/L (98). The plasma concentrations of butyrate and other fatty acids, on the other hand, are in the micromolar range. For example, the plasma concentration of butyrate in human subjects increased from 2.0 to 2.6 and 2.8 μmol/L overnight after a high-fiber dinner (160). In the same study, plasma acetate and propionate concentrations were found to be higher at ∼150 μmol/L and 8 μmol/L, respectively, but their amounts were not affected by fiber ingestion (160).
Table 3.
Butyrate concentrations in biological samples from humans and experimental animals that were or were not treated with dietary fiber
| Species | Samples | Control | Fiber treatment | Ref. |
| mmol/L | ||||
| Human | Feces | 11.7 | 15 | (68) |
| Rat | Feces | 14.3 | (159) | |
| Rat | Feces | 48–98 | 55–168 | (98) |
| Mouse | Cecum | 0.4 | 7.4, 17.3 | (84) |
| Rat | Cecum | 16 | 32 | (80) |
| Rat | Cecum | ∼3 | ∼17 | (134) |
| Rat | Cecum | 8.3 | 11.7 | (135) |
| Rat | Proximal colon | 6 | 9.3 | (135) |
| Rat | Distal colon | 4.1 | 9.8 | (135) |
| Rat | Colon | 44 | (82) | |
| Human | Plasma | 0.0020 | ∼0.0028 | (160) |
Cell lines originating from colon cancer and leukemia have been used as in vitro models to test the biological activity of butyrate. Unfortunately, there has not been a good match between the reported physiological concentrations of butyrate and the concentrations that biological activities of butyrate were identified in vitro. Overall, in vitro anti-inflammatory activities of butyrate were identified at ∼2–3 mmol/L, but the cytotoxicity of butyrate was found at 4–5 mmol/L (161, 162). This narrow dose-response range does not match with the concentrations described above in various types of biological samples (summarized in Table 3). Part of the inconsistency may result from the difficulties in measuring the in vivo concentration of butyrate accurately and in conducting in vitro experiments with volatile butyrate. There could also be cell type– and condition-dependent effects of butyrate, which cannot be captured by using the in vitro cancer cell models. Nevertheless, we cannot exclude the possibility that butyrate plays only a minor role, if any, in mediating the anti-inflammatory effect of fibers. This speculation is consistent with results from a rat study. Intracolonic infusion of 30 mmol/L butyrate plus 10 mmol/L lactate failed to alleviate the TNBS-induced colitis, whereas co-infusion with 109.5 CFU of a Lactobacillus and Bifidobacterium mixture led to a considerable anti-inflammatory effect (80). More multidisciplinary efforts are needed to clarify the involvement of butyrate and/or other microbial metabolites in the biological activity of fibers.
Conclusions
Available literature supports the presence of body weight–related and body weight–unrelated anti-inflammatory activity of fibers. The consistency between observations made using human subjects and animal models helps to strengthen the conclusion. The low energy density of a fiber-rich diet likely contributes to the body weight–related anti-inflammatory activity by curtailing the obesity-induced chronic inflammation. The fermentability of fiber and the consequent changes in the intestinal microbiome and/or their metabolites likely lead to the body weight–unrelated anti-inflammatory activity locally in the intestine and systemically. Although there have been considerable interest and efforts to understand the potential underlying mechanisms, more studies are needed.
From the nutrition point of view, the review provides some supporting evidence of a need to consider the requirement of fermentable and nonfermentable fiber separately. Although the DRI (adequate intake) and DV for total fiber have been established, it is not clear what the optimal ratio between fermentable and nonfermentable fiber should be. In rodent studies that used a purified diet and demonstrated anti-inflammatory effects of fermentable fibers, the ratio of fermentable fiber to cellulose was mostly 1:1 or 2:1, with equal intake of two types of fiber or twice as much fermentable fibers. Although fermentability and solubility are two different approaches used to classify fibers, fermentable fibers are usually soluble, whereas insoluble fibers such as cellulose usually have poor fermentability (18). In the United States across a wide range of total fiber intake, the intake ratio of soluble to insoluble fiber was reported to be ∼1:2–1:3 (62, 63), falling short of the intake of fermentable fiber used in the animal studies. Some large epidemiological studies have taken into consideration the different sources of fibers, cereal, vegetables, fruits, and beans (41, 42). Perhaps the form of fiber consumed, soluble versus insoluble or fermentable versus poorly fermentable, should also be examined in future epidemiological studies to facilitate the establishment of a more precise fiber requirement.
Acknowledgments
The author greatly appreciates the editorial assistance of Shin-Rong Julia Wu. The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: DRI, dietary reference intake; DSS, dextran sulfate sodium; DV, daily value; FOS, fructooligosaccharide; GBF, germinated barley foodstuff; GOS, galactooligosaccharide; IBD, inflammatory bowel disease; TNBS, trinitrobenzene sulfonic acid.
Literature Cited
- 1.Otten JJ, Hellwig JP, Meyers LD. DRI, dietary reference intakes [electronic resource]: the essential guide to nutrient requirements. Washington, DC: National Academies Press; 2006 [Google Scholar]
- 2.Bieri JG, Stoewsand GS, Briggs GM, Phillips RW, Woodard JC, Knapka JJ. Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8 [DOI] [PubMed] [Google Scholar]
- 3.Reeves PG, Nielsen FH, Fahey GCJ. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 4.Spiller GA, Amen R. Letter: Plant fibers in nutrition: need for better nomenclature. Am J Clin Nutr. 1975;28:675–6 [DOI] [PubMed] [Google Scholar]
- 5.Spiller GA, Fassett-Cornelius G, Briggs G. Letter: a new term for plant fibers in nutrition. Am J Clin Nutr. 1976;29:934–5 [DOI] [PubMed] [Google Scholar]
- 6.Lattimer JM, Haub M. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens R, Kranz S, Mobley A, Nicklas T, Raimondi M, Rodriguez J, Slavin J, Warshaw H. Filling America's fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J Nutr. 2012;142:1390S–401S [DOI] [PubMed] [Google Scholar]
- 8.Kranz S, Brauchla M, Slavin J, Miller K. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr. 2012;3:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valledor AF, Comalada M, Santamaría-Babi L, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol. 2010;108:1–20 [DOI] [PubMed] [Google Scholar]
- 10.Mountziaris PM, Spicer P, Kasper F, Mikos A. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17:393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownawell AM, Caers W, Gibson G, Kendall C, Lewis K, Ringel Y, Slavin J. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012;142:962–74 [DOI] [PubMed] [Google Scholar]
- 12.Kay RM. Dietary fiber. J Lipid Res. 1982;23:221–42 [PubMed] [Google Scholar]
- 13.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–29 [DOI] [PubMed] [Google Scholar]
- 14.Fleming SE, Rodriguez M. Influence of dietary fiber on fecal excretion of volatile fatty acids by human adults. J Nutr. 1983;113:1613–25 [DOI] [PubMed] [Google Scholar]
- 15.Bourquin LD, Titgemeyer E, Fahey GJ, Garleb K. Fermentation of dietary fibre by human colonic bacteria: disappearance of, short-chain fatty acid production from, and potential water-holding capacity of, various substrates. Scand J Gastroenterol. 1993;28:249–55 [DOI] [PubMed] [Google Scholar]
- 16.van de Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol. 2007;102:452–60 [DOI] [PubMed] [Google Scholar]
- 17.Kaur A, Rose D, Rumpagaporn P, Patterson J, Hamaker B. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J Food Sci. 2011;76:H137–42 [DOI] [PubMed] [Google Scholar]
- 18.Swanson KS, Grieshop C, Clapper G, Shields RJ, Belay T, Merchen N, Fahey GJ. Fruit and vegetable fiber fermentation by gut microflora from canines. J Anim Sci. 2001;79:919–26 [DOI] [PubMed] [Google Scholar]
- 19.Vernazza CL, Gibson G, Rastall R. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J Appl Microbiol. 2006;100:846–53 [DOI] [PubMed] [Google Scholar]
- 20.Marcobal A, Sonnenburg J. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect. 2012;18: Suppl 4:12–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolam DN, Sonnenburg J. Mechanistic insight into polysaccharide use within the intestinal microbiota. Gut Microbes. 2011;2:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita T, Kasaoka S, Hase K, Kiriyama S. Psyllium shifts the fermentation site of high-amylose cornstarch toward the distal colon and increases fecal butyrate concentration in rats. J Nutr. 1999;129:2081–7 [DOI] [PubMed] [Google Scholar]
- 23.Benson AK, Kelly S, Legge R, Ma F, Low S, Kim J, Zhang M, Oh P, Nehrenberg D, Hua K, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yatsunenko T, Rey F, Manary M, Trehan I, Dominguez-Bello M, Contreras M, Magris M, Hidalgo G, Baldassano R, Anokhin A, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell JH, Foster C, Vishnivetskaya T, Campbell A, Yang Z, Wymore A, Palumbo A, Chesler E, Podar M. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 2012;6:2033–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claesson MJ, Jeffery I, Conde S, Power S, O’Connor E, Cusack S, Harris H, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84 [DOI] [PubMed] [Google Scholar]
- 27.Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fava F, Gitau R, Griffin B, Gibson G, Tuohy K, Lovegrove J. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome 'at-risk’ population. Int J Obes (Lond). Epub 2012 Mar 13. [DOI] [PubMed] [Google Scholar]
- 29.de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Müller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589–99 [DOI] [PubMed] [Google Scholar]
- 30.Kolida S, Gibson G. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137:2503S–6S [DOI] [PubMed] [Google Scholar]
- 31.Joossens M, Huys G, Van Steen K, Cnockaert M, Vermeire S, Rutgeerts P, Verbeke K, Vandamme P, De Preter V. High-throughput method for comparative analysis of denaturing gradient gel electrophoresis profiles from human fecal samples reveals significant increases in two bifidobacterial species after inulin-type prebiotic intake. FEMS Microbiol Ecol. 2011;75:343–9 [DOI] [PubMed] [Google Scholar]
- 32.Costabile A, Kolida S, Klinder A, Gietl E, Bäuerlein M, Frohberg C, Landschütze V, Gibson G. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr. 2010;104:1007–17 [DOI] [PubMed] [Google Scholar]
- 33.Koleva PT, Valcheva R, Sun X, Gänzle M, Dieleman L. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. 2012;16:1–11 [DOI] [PubMed] [Google Scholar]
- 34.Langlands SJ, Hopkins M, Coleman N, Cummings J. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boler BM, Serao M, Bauer L, Staeger M, Boileau T, Swanson K, Fahey GJ. Digestive physiological outcomes related to polydextrose and soluble maize fibre consumption by healthy adult men. Br J Nutr. 2011;106:1864–71 [DOI] [PubMed] [Google Scholar]
- 36.Martínez I, Kim J, Duffy P, Schlegel V, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis LM, Martínez I, Walter J, Goin C, Hutkins R. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE. 2011;6:e25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooda S, Boler B, Serao M, Brulc J, Staeger M, Boileau T, Dowd S, Fahey GJ, Swanson K. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr. 2012;142:1259–65 [DOI] [PubMed] [Google Scholar]
- 39.Hernández-Hernández O, Marín-Manzano M, Rubio L, Moreno F, Sanz M, Clemente A. Monomer and linkage type of galacto-oligosaccharides affect their resistance to ileal digestion and prebiotic properties in rats. J Nutr. 2012;142:1232–9 [DOI] [PubMed] [Google Scholar]
- 40.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137 Suppl 2:830S–7S [DOI] [PubMed] [Google Scholar]
- 41.Park Y, Subar A, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP Diet and Health Study. Arch Intern Med. 2011;171:1061–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang SC, Norat T, Murphy N, Olsen A, Tjønneland A, Overvad K, Boutron-Ruault M, Perquier F, Dartois L, Kaaks R, et al. Fiber intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2012;96:164–74 [DOI] [PubMed] [Google Scholar]
- 43.Stienstra R, Tack C, Kanneganti T, Joosten L, Netea M. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–8 [DOI] [PubMed] [Google Scholar]
- 44.Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. 2011;117:151–64 [DOI] [PubMed] [Google Scholar]
- 45.Farmer B, Larson BT, Fulgoni VL, 3rd, Rainville AJ, Liepa GU. A vegetarian dietary pattern as a nutrient-dense approach to weight management: an analysis of the national health and nutrition examination survey 1999–2004. J Am Diet Assoc. 2011;111:819–27 [DOI] [PubMed] [Google Scholar]
- 46.Shay CM, Van Horn L, Stamler J, Dyer A, Brown I, Chan Q, Miura K, Zhao L, Okuda N, Daviglus M, et al. Food and nutrient intakes and their associations with lower BMI in middle-aged US adults: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am J Clin Nutr. 2012;96:483–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ley RE, Turnbaugh P, Klein S, Gordon J. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3 [DOI] [PubMed] [Google Scholar]
- 48.Turnbaugh PJ, Ley R, Mahowald M, Magrini V, Mardis E, Gordon J. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31 [DOI] [PubMed] [Google Scholar]
- 49.Jumpertz R, Le D, Turnbaugh P, Trinidad C, Bogardus C, Gordon J, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amini M, Esmaillzadeh A, Shafaeizadeh S, Behrooz J, Zare M. Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition. 2010;26:986–92 [DOI] [PubMed] [Google Scholar]
- 51.Rizzo NS, Sabaté J, Jaceldo-Siegl K, Fraser G. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care. 2011;34:1225–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ajani UA, Ford E, Mokdad A. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–5 [DOI] [PubMed] [Google Scholar]
- 53.Herder C, Peltonen M, Koenig W, Sütfels K, Lindström J, Martin S, Ilanne-Parikka P, Eriksson J, Aunola S, Keinänen-Kiukaanniemi S, et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia. 2009;52:433–42 [DOI] [PubMed] [Google Scholar]
- 54.Estruch R, Martínez-González M, Corella D, Basora-Gallisá J, Ruiz-Gutiérrez V, Covas M, Fiol M, Gómez-Gracia E, López-Sabater M, Escoda R, et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J Epidemiol Community Health. 2009;63:582–8 [DOI] [PubMed] [Google Scholar]
- 55.Galisteo M, Sánchez M, Vera R, González M, Anguera A, Duarte J, Zarzuelo A. A diet supplemented with husks of Plantago ovata reduces the development of endothelial dysfunction, hypertension, and obesity by affecting adiponectin and TNF-alpha in obese Zucker rats. J Nutr. 2005;135:2399–404 [DOI] [PubMed] [Google Scholar]
- 56.Sánchez D, Quiñones M, Moulay L, Muguerza B, Miguel M, Aleixandre A. Soluble fiber-enriched diets improve inflammation and oxidative stress biomarkers in Zucker fatty rats. Pharmacol Res. 2011;64:31–5 [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Neyrinck A, Fava F, Knauf C, Burcelin R, Tuohy K, Gibson G, Delzenne N. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83 [DOI] [PubMed] [Google Scholar]
- 58.Dewulf EM, Cani P, Neyrinck A, Possemiers S, Van Holle A, Muccioli G, Deldicque L, Bindels L, Pachikian B, Sohet F, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22:712–22 [DOI] [PubMed] [Google Scholar]
- 59.Sugatani J, Wada T, Osabe M, Yamakawa K, Yoshinari K, Miwa M. Dietary inulin alleviates hepatic steatosis and xenobiotics-induced liver injury in rats fed a high-fat and high-sucrose diet: association with the suppression of hepatic cytochrome P450 and hepatocyte nuclear factor 4alpha expression. Drug Metab Dispos. 2006;34:1677–87 [DOI] [PubMed] [Google Scholar]
- 60.Neyrinck AM, Alexiou H, Delzenne N. Kupffer cell activity is involved in the hepatoprotective effect of dietary oligofructose in rats with endotoxic shock. J Nutr. 2004;134:1124–9 [DOI] [PubMed] [Google Scholar]
- 61.Qi L, van Dam R, Liu S, Franz M, Mantzoros C, Hu F. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11 [DOI] [PubMed] [Google Scholar]
- 62.Villaseñor A, Ambs A, Ballard-Barbash R, Baumgartner K, McTiernan A, Ulrich C, Neuhouser M. Dietary fiber is associated with circulating concentrations of C-reactive protein in breast cancer survivors: the HEAL study. Breast Cancer Res Treat. 2011;129:485–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, 3rd, Li W, Pagoto SL, Hafner AR, Ockene IS. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma Y, Hébert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, Tinker L, Rosal MC, Ockene IS, Ockene JK, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008;24:941–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chuang SC, Vermeulen R, Sharabiani M, Sacerdote C, Fatemeh S, Berrino F, Krogh V, Palli D, Panico S, Tumino R, et al. The intake of grain fibers modulates cytokine levels in blood. Biomarkers. 2011;16:504–10 [DOI] [PubMed] [Google Scholar]
- 66.King DE, Mainous AG, 3rd, Egan BM, Woolson RF, Geesey ME. Effect of psyllium fiber supplementation on C-reactive protein: the trial to reduce inflammatory markers (TRIM). Ann Fam Med. 2008;6:100–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theuwissen E, Plat J, Mensink R. Consumption of oat beta-glucan with or without plant stanols did not influence inflammatory markers in hypercholesterolemic subjects. Mol Nutr Food Res. 2009;53:370–6 [DOI] [PubMed] [Google Scholar]
- 68.Worthley DL, Le Leu R, Whitehall V, Conlon M, Christophersen C, Belobrajdic D, Mallitt K, Hu Y, Irahara N, Ogino S, et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am J Clin Nutr. 2009;90:578–86 [DOI] [PubMed] [Google Scholar]
- 69.King D, Egan B, Woolson R, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. . Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–6 [DOI] [PubMed] [Google Scholar]
- 70.Benjamin JL, Hedin C, Koutsoumpas A, Ng S, McCarthy N, Hart A, Kamm M, Sanderson J, Knight S, Forbes A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60:923–9 [DOI] [PubMed] [Google Scholar]
- 71.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, Naganuma M, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–4 [PubMed] [Google Scholar]
- 72.Faghfoori Z, Navai L, Shakerhosseini R, Somi M, Nikniaz Z, Norouzi M. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48:233–7 [DOI] [PubMed] [Google Scholar]
- 73.Schiffrin EJ, Thomas D, Kumar V, Brown C, Hager C, Van't Hof M, Morley J, Guigoz Y. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging. 2007;11:475–9 [PubMed] [Google Scholar]
- 74.Arslanoglu S, Moro G, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–4 [DOI] [PubMed] [Google Scholar]
- 75.Arslanoglu S, Moro G, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–5 [DOI] [PubMed] [Google Scholar]
- 76.Shadid R, Haarman M, Knol J, Theis W, Beermann C, Rjosk-Dendorfer D, Schendel D, Koletzko B, Krauss-Etschmann S. Effects of galactooligosaccharide and long-chain fructooligosaccharide supplementation during pregnancy on maternal and neonatal microbiota and immunity–a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2007;86:1426–37 [DOI] [PubMed] [Google Scholar]
- 77.Westerbeek EA, van den Berg J, Lafeber H, Fetter W, Boehm G, Twisk J, van Elburg R. Neutral and acidic oligosaccharides in preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:679–86 [DOI] [PubMed] [Google Scholar]
- 78.van Stuijvenberg M, Eisses A, Grüber C, Mosca F, Arslanoglu S, Chirico G, Braegger C, Riedler J, Boehm G, Sauer P. Do prebiotics reduce the number of fever episodes in healthy children in their first year of life: a randomised controlled trial. Br J Nutr. 2011;106:1740–8 [DOI] [PubMed] [Google Scholar]
- 79.Moreau NM, Toquet C, Laboisse C, Nguyen P, Siliart B, Champ M, Dumon H, Martin L. Predominance of caecal injury in a new dextran sulphate sodium treatment in rats: histopathological and fermentative characteristics. Eur J Gastroenterol Hepatol. 2002;14:535–42 [DOI] [PubMed] [Google Scholar]
- 80.Cherbut C, Michel C, Lecannu G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr. 2003;133:21–7 [DOI] [PubMed] [Google Scholar]
- 81.Lara-Villoslada F, de Haro O, Camuesco D, Comalada M, Velasco J, Zarzuelo A, Xaus J, Galvez J. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur J Nutr. 2006;45:418–25 [DOI] [PubMed] [Google Scholar]
- 82.Rodríguez-Cabezas ME, Camuesco D, Arribas B, Garrido-Mesa N, Comalada M, Bailón E, Cueto-Sola M, Utrilla P, Guerra-Hernández E, Pérez-Roca C, et al. The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin Nutr. 2010;29:832–9 [DOI] [PubMed] [Google Scholar]
- 83.Winkler J, Butler R, Symonds E. Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis. Dig Dis Sci. 2007;52:52–8 [DOI] [PubMed] [Google Scholar]
- 84.Goto H, Takemura N, Ogasawara T, Sasajima N, Watanabe J, Ito H, Morita T, Sonoyama K. Effects of fructo-oligosaccharide on DSS-induced colitis differ in mice fed nonpurified and purified diets. J Nutr. 2010;140:2121–7 [DOI] [PubMed] [Google Scholar]
- 85.Moreau NM, Martin L, Toquet C, Laboisse C, Nguyen P, Siliart B, Dumon H, Champ M. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr. 2003;90:75–85 [DOI] [PubMed] [Google Scholar]
- 86.Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137:847S–9S [DOI] [PubMed] [Google Scholar]
- 87.Rumi G, Tsubouchi R, Okayama M, Kato S, Mózsik G, Takeuchi K. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci. 2004;49:1466–72 [DOI] [PubMed] [Google Scholar]
- 88.Lara-Villoslada F, Debras E, Nieto A, Concha A, Gálvez J, López-Huertas E, Boza J, Obled C, Xaus J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin Nutr. 2006;25:477–88 [DOI] [PubMed] [Google Scholar]
- 89.Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi L, Rodriguez-Cabezas M, Concha A, Zarzuelo A, Galvez J. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–71 [DOI] [PubMed] [Google Scholar]
- 90.Naito Y, Takagi T, Katada K, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, Watabe J, Yoshida N, Okanoue T, et al. Partially hydrolyzed guar gum down-regulates colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. J Nutr Biochem. 2006;17:402–9 [DOI] [PubMed] [Google Scholar]
- 91.Komiyama Y, Andoh A, Fujiwara D, Ohmae H, Araki Y, Fujiyama Y, Mitsuyama K, Kanauchi O. New prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of intestinal homeostasis and the mucosal immune system. Scand J Gastroenterol. 2011;46:40–52 [DOI] [PubMed] [Google Scholar]
- 92.Fukuda M, Kanauchi O, Araki Y, Andoh A, Mitsuyama K, Takagi K, Toyonaga A, Sata M, Fujiyama Y, Fukuoka M, et al. Prebiotic treatment of experimental colitis with germinated barley foodstuff: a comparison with probiotic or antibiotic treatment. Int J Mol Med. 2002;9:65–70 [PubMed] [Google Scholar]
- 93.Mochizuki M, Shigemura H, Hasegawa N. Anti-inflammatory effect of enzymatic hydrolysate of corn gluten in an experimental model of colitis. J Pharm Pharmacol. 2010;62:389–92 [DOI] [PubMed] [Google Scholar]
- 94.Kanauchi O, Iwanaga T, Andoh A, Araki Y, Nakamura T, Mitsuyama K, Suzuki A, Hibi T, Bamba T. Dietary fiber fraction of germinated barley foodstuff attenuated mucosal damage and diarrhea, and accelerated the repair of the colonic mucosa in an experimental colitis. J Gastroenterol Hepatol. 2001;16:160–8 [DOI] [PubMed] [Google Scholar]
- 95.Smith CL, Geier M, Yazbeck R, Torres D, Butler R, Howarth G. Lactobacillus fermentum BR11 and fructo-oligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr Cancer. 2008;60:757–67 [DOI] [PubMed] [Google Scholar]
- 96.Taurog JD, Maika S, Satumtira N, Dorris M, McLean I, Yanagisawa H, Sayad A, Stagg A, Fox G, Lê O'Brien A, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–23 [DOI] [PubMed] [Google Scholar]
- 97.Hoentjen F, Welling G, Harmsen H, Zhang X, Snart J, Tannock G, Lien K, Churchill T, Lupicki M, Dieleman L. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–85 [DOI] [PubMed] [Google Scholar]
- 98.Rodríguez-Cabezas ME, Gálvez JC, Calmuesco D, Lorente MD, Concha A, Martinez-Augustin O, Redondo L, Zarzuelo A. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clin Nutr. 2003;22:463–71 [DOI] [PubMed] [Google Scholar]
- 99.Bassaganya-Riera J, DiGuardo M, Viladomiu M, de Horna A, Sanchez S, Einerhand A, Sanders L, Hontecillas R. Soluble fibers and resistant starch ameliorate disease activity in interleukin-10-deficient mice with inflammatory bowel disease. J Nutr. 2011;141:1318–25 [DOI] [PubMed] [Google Scholar]
- 100.Kühn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74 [DOI] [PubMed] [Google Scholar]
- 101.Hirano D, Kudo S. Usefulness of CD4+CD45RBhigh CD25- cell-transferred SCID mice for preclinical evaluation of drugs for inflammatory bowel disease. J Pharmacol Sci. 2009;110:169–81 [DOI] [PubMed] [Google Scholar]
- 102.Kanauchi O, Oshima T, Andoh A, Shioya M, Mitusuyama K. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand J Gastroenterol. 2008;43:1346–52 [DOI] [PubMed] [Google Scholar]
- 103.Kau AL, Ahern P, Griffin N, Goodman A, Gordon J. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yasuda A, Inoue K, Sanbongi C, Yanagisawa R, Ichinose T, Yoshikawa T, Takano H. Dietary supplementation with fructooligosaccharides attenuates airway inflammation related to house dust mite allergen in mice. Int J Immunopathol Pharmacol. 2010;23:727–35 [DOI] [PubMed] [Google Scholar]
- 105.Yasuda A, Inoue K, Sanbongi C, Yanagisawa R, Ichinose T, Tanaka M, Yoshikawa T, Takano H. Dietary supplementation with fructooligosaccharides attenuates allergic peritonitis in mice. Biochem Biophys Res Commun. 2012;422:546–50 [DOI] [PubMed] [Google Scholar]
- 106.Vos AP, van Esch B, Stahl B, M'Rabet L, Folkerts G, Nijkamp F, Garssen J. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol. 2007;7:1582–7 [DOI] [PubMed] [Google Scholar]
- 107.Watanabe J, Sasajima N, Aramaki A, Sonoyama K. Consumption of fructo-oligosaccharide reduces 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Br J Nutr. 2008;100:339–46 [DOI] [PubMed] [Google Scholar]
- 108.Fujiwara R, Sasajima N, Takemura N, Ozawa K, Nagasaka Y, Okubo T, Sahasakul Y, Watanabe J, Sonoyama K. 2,4-Dinitrofluorobenzene-induced contact hypersensitivity response in NC/Nga mice fed fructo-oligosaccharide. J Nutr Sci Vitaminol (Tokyo). 2010;56:260–5 [DOI] [PubMed] [Google Scholar]
- 109.Fujiwara R, Takemura N, Watanabe J, Sonoyama K. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br J Nutr. 2010;103:530–8 [DOI] [PubMed] [Google Scholar]
- 110.Grabitske HA, Slavin J. Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr. 2009;49:327–60 [DOI] [PubMed] [Google Scholar]
- 111.Alastair F, Emma G, Emma P. Nutrition in inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2011;35:571–80 [DOI] [PubMed] [Google Scholar]
- 112.Mahendran V, Riordan S, Grimm M, Tran T, Major J, Kaakoush N, Mitchell H, Zhang L. Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE. 2011;6:e25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q, Wang C, Tang C, Li N, Li J. Molecular-phylogenetic characterization of the microbiota in ulcerated and non-ulcerated regions in the patients with Crohn's disease. PLoS ONE. 2012;7:e34939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boyle RJ, Robins-Browne R, Tang M. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–64 [DOI] [PubMed] [Google Scholar]
- 115.Kolida S, Gibson G. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373–93 [DOI] [PubMed] [Google Scholar]
- 116.Seifert S, Bub A, Franz C, Watzl B. Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J Nutr. 2011;141:978–84 [DOI] [PubMed] [Google Scholar]
- 117.Lam V, Su J, Koprowski S, Hsu A, Tweddell J, Rafiee P, Gross G, Salzman N, Baker J. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu YW, Su Y, Ong W, Cheng T, Tsai Y. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int Immunopharmacol. 2011;11:2159–66 [DOI] [PubMed] [Google Scholar]
- 119.Ganesh BP, Richter J, Blaut M, Loh G. Enterococcus faecium NCIMB 10415 does not protect interleukin-10 knock-out mice from chronic gut inflammation. Benef Microbes. 2012;3:43–50 [DOI] [PubMed] [Google Scholar]
- 120.Passariello A, Terrin G, Cecere G, Micillo M, De Marco G, Di Costanzo M, Cosenza L, Leone L, Nocerino R, Canani R. Randomised clinical trial: efficacy of a new synbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhoea. Aliment Pharmacol Ther. 2012;35:782–8 [DOI] [PubMed] [Google Scholar]
- 121.Vandenplas Y, De Hert S. Randomised clinical trial: the synbiotic food supplement Probiotical vs. placebo for acute gastroenteritis in children. Aliment Pharmacol Ther. 2011;34:862–7 [DOI] [PubMed] [Google Scholar]
- 122.Jain PK, McNaught C, Anderson A, MacFie J, Mitchell C. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr. 2004;23:467–75 [DOI] [PubMed] [Google Scholar]
- 123.Schrezenmeir J, Heller K, McCue M, Llamas C, Lam W, Burow H, Kindling-Rohracker M, Fischer W, Sengespeik H, Comer G, et al. Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections. Clin Pediatr (Phila). 2004;43:239–49 [DOI] [PubMed] [Google Scholar]
- 124.Furrie E, Macfarlane S, Kennedy A, Cummings J, Walsh S, O'Neil D, Macfarlane G. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van der Aa LB, Lutter R, Heymans H, Smids B, Dekker T, van Aalderen W, Sillevis Smitt J, Knippels L, Garssen J, Nauta A, et al. No detectable beneficial systemic immunomodulatory effects of a specific synbiotic mixture in infants with atopic dermatitis. Clin Exp Allergy. 2012;42:531–9 [DOI] [PubMed] [Google Scholar]
- 126.Shafiei A, Moin M, Pourpak Z, Gharagozlou M, Aghamohammadi A, Sajedi V, Soheili H, Sotoodeh S, Movahedi M. Synbiotics could not reduce the scoring of childhood atopic dermatitis (SCORAD): a randomized double blind placebo-controlled trial. Iran J Allergy Asthma Immunol. 2011;10:21–8 [PubMed] [Google Scholar]
- 127.Wu KG, Li T, Peng H. Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol. 2012;166:129–36 [DOI] [PubMed] [Google Scholar]
- 128.Kuo SM, Burl L, Hu Z. Cellular phenotype-dependent and -independent effects of vitamin C on the renewal and gene expression of mouse embryonic fibroblasts. PLoS ONE. 2012;7:e32957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400 [DOI] [PubMed] [Google Scholar]
- 130.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson P, Klinder A, O'Riordan M, O'Sullivan G, Pool-Zobel B, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–96 [DOI] [PubMed] [Google Scholar]
- 131.Roller M, Clune Y, Collins K, Rechkemmer G, Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br J Nutr. 2007;97:676–84 [DOI] [PubMed] [Google Scholar]
- 132.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr. 2004;134:153–6 [DOI] [PubMed] [Google Scholar]
- 133.Schultz M, Munro K, Tannock G, Melchner I, Göttl C, Schwietz H, Schölmerich J, Rath H. Effects of feeding a probiotic preparation (SIM) containing inulin on the severity of colitis and on the composition of the intestinal microflora in HLA-B27 transgenic rats. Clin Diagn Lab Immunol. 2004;11:581–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, Clune Y, Collins K, Paglierani M, Caderni G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–60 [DOI] [PubMed] [Google Scholar]
- 135.Le Leu RK, Hu Y, Brown I, Woodman R, Young G. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010;31:246–51 [DOI] [PubMed] [Google Scholar]
- 136.Håkansson A, Bränning C, Adawi D, Molin G, Nyman M, Jeppsson B, Ahrné S. Blueberry husks, rye bran and multi-strain probiotics affect the severity of colitis induced by dextran sulphate sodium. Scand J Gastroenterol. 2009;44:1213–25 [DOI] [PubMed] [Google Scholar]
- 137.Håkansson A, Bränning C, Molin G, Adawi D, Hagslätt M, Jeppsson B, Nyman M, Ahrné S. Blueberry husks and probiotics attenuate colorectal inflammation and oncogenesis, and liver injuries in rats exposed to cycling DSS-treatment. PLoS ONE. 2012;7:e33510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Foye OT, Huang I, Chiou C, Walker W, Shi H. Early administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunol Med Microbiol. 2012;65:467–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zenhom M, Hyder A, de Vrese M, Heller K, Roeder T, Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J Nutr. 2011;141:971–7 [DOI] [PubMed] [Google Scholar]
- 140.Shoaf K, Mulvey G, Armstrong G, Hutkins R. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 2006;74:6920–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quintero M, Maldonado M, Perez-Munoz M, Jimenez R, Fangman T, Rupnow J, Wittke A, Russell M, Hutkins R. Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Curr Microbiol. 2011;62:1448–54 [DOI] [PubMed] [Google Scholar]
- 142.Aluwihare AP. An ultrastructural study of the effect of neomycin on the colon in the human subject and in the conventional and the germ-free mouse. Gut. 1971;12:341–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bäckhed F, Manchester J, Semenkovich C, Gordon J. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ceuppens S, Van de Wiele T, Rajkovic A, Ferrer-Cabaceran T, Heyndrickx M, Boon N, Uyttendaele M. Impact of intestinal microbiota and gastrointestinal conditions on the in vitro survival and growth of Bacillus cereus. Int J Food Microbiol. 2012;155:241–6 [DOI] [PubMed] [Google Scholar]
- 145.Veiga P, Gallini C, Beal C, Michaud M, Delaney M, DuBois A, Khlebnikov A, van Hylckama Vlieg J, Punit S, Glickman J, et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A. 2010;107:18132–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jurjus AR, Khoury N, Reimund J. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92 [DOI] [PubMed] [Google Scholar]
- 147.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–77 [DOI] [PubMed] [Google Scholar]
- 148.Han DY, Fraser A, Dryland P, Ferguson L. Environmental factors in the development of chronic inflammation: a case-control study on risk factors for Crohn's disease within New Zealand. Mutat Res. 2010;690:116–22 [DOI] [PubMed] [Google Scholar]
- 149.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson A, McCoy K, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maslowski KM, Mackay C. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9 [DOI] [PubMed] [Google Scholar]
- 151.Hooper LV, Littman D, Macpherson A. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Noverr MC, Huffnagle G. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8 [DOI] [PubMed] [Google Scholar]
- 153.Noverr MC, Noggle R, Toews G, Huffnagle G. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Noverr MC, Falkowski N, McDonald R, McKenzie A, Huffnagle G. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7 [DOI] [PubMed] [Google Scholar]
- 156.Wikoff WR, Anfora A, Liu J, Schultz P, Lesley S, Peters E, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Samuel BS, Shaito A, Motoike T, Rey F, Backhed F, Manchester J, Hammer R, Williams S, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Araki Y, Fujiyama Y, Andoh A, Koyama S, Kanauchi O, Bamba T. The dietary combination of germinated barley foodstuff plus Clostridium butyricum suppresses the dextran sulfate sodium-induced experimental colitis in rats. Scand J Gastroenterol. 2000;35:1060–7 [DOI] [PubMed] [Google Scholar]
- 160.Nilsson AC, Östman E, Knudsen K, Holst J, Björck I. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr. 2010;140:1932–6 [DOI] [PubMed] [Google Scholar]
- 161.Zimmerman MA, Singh N, Martin P, Thangaraju M, Ganapathy V, Waller J, Shi H, Robertson K, Munn D, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1405–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gonçalves P, Araújo J, Pinho M, Martel F. In vitro studies on the inhibition of colon cancer by butyrate and polyphenolic compounds. Nutr Cancer. 2011;63:282–94 [DOI] [PubMed] [Google Scholar]


