Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by progressive loss of dopaminergic (DAergic) neuronal cell bodies in the substantia nigra pars compacta and gliosis. The cause and mechanisms underlying the demise of nigrostriatal DAergic neurons are ill-defined, but interactions between genes and environmental factors are recognized to play a critical role in modulating the vulnerability to PD. Current evidence points to reactive glia as a pivotal factor in PD pathophysiology, playing both protective and destructive roles. Here, the contribution of reactive astrocytes and their ability to modulate DAergic neurodegeneration, neuroprotection and neurorepair in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) rodent model of PD will be discussed in the light of novel emerging evidence implicating wingless-type mouse mammary tumor virus integration site (Wnt)/β-catenin signaling as a strong candidate in MPTP-induced nigrostriatal DAergic plasticity. In this work, we highlight an intrinsic Wnt1/frizzled-1/β-catenin tone that critically contributes to the survival and protection of adult midbrain DAergic neurons, with potential implications for drug design or drug action in PD. The dynamic interplay between astrocyte-derived factors and neurogenic signals in MPTP-induced nigrostriatal DAergic neurotoxicity and repair will be summarized, together with recent findings showing a critical role of glia–neural stem/progenitor cell (NPC) interactions aimed at overcoming neurodegeneration and inducing neurorestoration. Understanding the intrinsic plasticity of nigrostriatal DAergic neurons and deciphering the signals facilitating the crosstalk between astrocytes, microglia, DAergic neurons and NPCs may have major implications for the role of stem cell technology in PD, and for identifying potential therapeutic targets to induce endogenous neurorepair.
Keywords: dopaminergic neurons, neurodegeneration, neurogenesis, neuroprotection Parkinson’s disease, reactive astrocytes
Introduction
Dopamine-synthesizing [dopaminergic (DAergic)] neurons in the ventral midbrain (VM) constitute a pivotal neuronal population controlling motor behaviors, and cognitive and affective brain functions. In Parkinson’s disease (PD), DAergic cell bodies within the substantia nigra pars compacta (SNpc) progressively degenerate, from causes and mechanisms that are poorly understood. The main hallmark of PD, the second most frequent neurodegenerative disorder after Alzheimer’s disease, is the selective loss of DAergic neurons in the SNpc and their projections into the caudate nucleus, leading to substantial decreases in dopamine (DA) levels, which manifest as resting tremor, bradykinesia, rigidity, and gait dysfunction, accompanied by progressive impairment of autonomic, cognitive and mood functions (Di Monte & Langston, 1995; Olanow et al., 2003; Langston, 2006).
Although most cases of PD are observed later in life, there is evidence that the disease has progressed at the point at which it is diagnosed (Olanow et al., 2003). In fact, the clinical symptoms appear only following a > 70–80% loss of midbrain DAergic neurons in the SNpc, suggesting that compensatory mechanisms are established while the neurodegeneration progresses (Hornykiewicz, 1993; Bezard & Gross, 1998).
Current DAergic treatments improve the motor symptoms and quality of life for patients during the early stages of PD, but do not prevent the progression of the disease associated with disabling side-effects (Olanow et al., 2003).
So far, several scenarios regarding the mechanisms by which DAergic neurons degenerate have been suggested, including oxidative stress, deficits in mitochondrial function, excitotoxicity, accumulation of aberrant or misfolded proteins, and impairment of anti-oxidant and neuroprotective mechanisms (Olanow et al., 2003; Abou-Sleiman et al., 2006). In particular, current evidence from epidemiological and post-mortem studies, as well as from experimentally induced PD rodent models, including the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), the rotenone and the 6-hydroxydopamine (6-OHDA) models of basal ganglia injury, point to reactive glia as a pivotal factor in PD, albeit a dual, detrimental/neuroprotective, influence is presently recognized (McGeer et al., 1988; Langston et al., 1999; Olanow et al., 2003; Chen et al., 2005; Marchetti & Abbracchio, 2005; Whitton, 2007; Gao & Hong, 2008; McGeer & McGeer, 2008; Hirsch & Hunot, 2009; Przedborski, 2010; L’Episcopo et al., 2010a).
Astrocytes and microglia normally play neuroprotective roles, but, when chronically activated (i.e. under inflammatory/neurotoxic exposure, or upon brain injury), glial cells produce a wide range of cytotoxic mediators, including reactive oxygen species and reactive nitrogen species, and a panel of proinflammatory cytokines and chemokines that may perpetuate/exacerbate glial activation, thereby increasing neuronal vulnerability, and/or promoting DAergic cell death (Morale et al., 2004; Hu et al., 2008; Hoang et al., 2009; Streit, 2010; Marchetti et al., 2011).
Notably, several genes that cause certain forms of inherited PD (< 10% of cases) have been identified; however, the majority of cases (> 90%) appear to be sporadic, and probably represent an interplay between genetic and environmental influences (Warner & Schapira, 2003), whereby the hormonal background appears to be a critical risk factor (Morale et al., 2004, 2006, 2008; Marchetti et al., 2005a,b,c). Hence, glia may represent a common pathway mediating both genetic and environmental influences on DAergic neurodegeneration/neuroprotection (Marchetti et al., 2005a,b,c; Morale et al., 2006; Marchetti, 2007; Frank-Cannon et al., 2008; Gao & Hong, 2008; Hirsch & Hunot, 2009; L’Episcopo et al., 2010a; Marchetti et al., 2011; Kim et al., 2012; Gillardon et al., 2012).
Recently, the wingless-type mouse mammary tumor virus integration site (Wnt) 1 pathway has emerged as an essential signaling cascade that regulates multiple processes in developing and adult tissues, including differentiation, neuronal survival, axonal extension, synapse formation and plasticity, neurotrophin transcription, neurogenesis, and neuroprotection (Patapoutian & Reichardt, 2000; Ciani & Salinas, 2005; Lie et al., 2005; Maiese et al., 2008; Inestrosa & Arenas, 2010; Zhang et al., 2011; Harrison-Uy & Pleasure, 2012). The interactions of Wnts (Wnt1–Wnt19) with their sevenpass transmembrane receptors of the frizzled (Fzd) family (Fzd1–Fzd10) trigger several signaling pathways, such as the so-called ‘canonical’ Wnt/β-catenin pathway, and the ‘non-canonical’ Wnt/planar cell polarity (PCP) and Wnt/Ca2+ pathways (Clevers, 2006; Clevers & Nusse, 2012; Salinas, 2012).
Compelling evidence clearly indicates that the Wnt/β-catenin signaling pathway plays a central role in midbrain DAergic neurodevelopment (Castelo-Branco et al., 2003, 2004, 2006, 2010; Prakash & Wurst, 2006; Rawal et al., 2006; Joksimovic et al., 2009; Tang et al., 2009, 2010; Inestrosa & Arenas, 2010; Kele et al., 2012). Importantly, mounting evidence points to dysregulation of Wnt/Fzd signaling in major neurodegenerative disorders (Toledo et al., 2008; Inestrosa & Arenas, 2010; Kim et al., 2011; Shruster et al., 2011; Purro et al., 2012), but only recently has the expression of Wnt/β-catenin ligands and Wnt signaling components been characterized in the adult intact and MPTP-injured midbrain. Hence, dysfunctional Wnt/β-catenin signaling was shown to play a causal role in the pathophysiology of nigrostriatal DAergic neurons and PD experimental models (L’Episcopo et al., 2011a,b). In particular, Wnt1/β-catenin signaling and MPTP-reactive astrocytes were uncovered as candidate components of neurorescue pathways involved in nigrostriatal DAergic plasticity (L’Episcopo et al., 2011a,b). Of special interest, emerging evidence indicates that proteins encoded by PARK genes can modify Wnt signaling (Berwick & Harvey, 2012a,b).
Wnt/β-catenin signaling critically contributes to the regulation of adult neurogenesis (Lie et al., 2005; Adachi et al., 2007; Kalani et al., 2008; Wexler et al., 2008; Kuwabara et al., 2009; Shruster et al., 2011; Zhang et al., 2011 for extensive review). In particular, activated astrocytes promote neurogenesis from adult neural stem/progenitor cells (NPCs) (Lim & Alvarez-Buylla, 1999; Jiao & Chen, 2008), also via activation of the Wnt/β-catenin pathway (Lie et al., 2005; Kazanis, 2009; Kuwabara et al., 2009; Zhang et al., 2011). Additionally, reactive VM astrocytes express Wnt1 and promote neurogenesis and DAergic neurogenesis as a function of a specific ‘inflammatory milieu’ in vitro (L’Episcopo et al., 2011a), and efficiently protect adult NPCs of the subventricular zone (SVZ) against MPTP-induced neurogenic impairment, again via Wnt/β-catenin activation (L’Episcopo et al., 2012a,b).
In this review, after a brief summary of neuron–glia interactions in PD, the Wnt signaling cascade and astrocyte–DAergic neuron interactions for neuroprotection and neurorepair will be highlighted. In particular, the evidence gathered on the Wnt/β-catenin signaling pathway as a novel actor at both the neuron–glia and glia–neuroprogenitor interfaces in mice affected by MPTP-induced nigrostriatal degeneration will be discussed in the light of emerging evidence implicating the Wnt signaling cascade in major inflammatory and neurodegenerative diseases, with potential therapeutic implications for PD.
Astrocytes and microglia are key mediators of neuroinflammatory responses
The MPTP-lesioned mouse model of basal ganglia injury recapitulates many of the pathogenetic processes operative in PD (Jackson-Lewis & Przedborski, 2007). The neurotoxin MPTP, converted into its active metabolite, 1-methyl-4-phenylpyridinium ion (MPP+), in astrocytes, is selectively transported into striatal DAergic terminals via the DA transporter (DAT), where it induces oxidative stress, the opening of mitochondrial permeability transition pores, the release of cytochrome c, and the activation of caspases (Vila et al., 2001). In synergy with these early events, which account for ~10% of DAergic neuronal death (Wu et al., 2002), glial inflammatory mechanisms are thought to contribute to nigrostriatal DAergic degeneration (Gao et al., 2003; Hu et al., 2008; Hirsch & Hunot, 2009; Hoang et al., 2009; L’Episcopo et al., 2010a,b; Przedborski, 2010).
Reactive astrocytes, microglial cells and infiltrating monocyte-derived macrophages are the key actors playing both detrimental and neuroprotective roles via an array of growth/neurotrophic factors, proinflammatory/anti-inflammatory cytokines, chemokines, and neurogenic transcription factors (Miller & Streit, 2007; Whitton, 2007; L’Episcopo et al., 2010a,b; Marchetti et al., 2011; Depboylu et al., 2012; Njie et al., 2012) (Fig. 1). The neuroinflammatory reaction also modulates the regenerative capacity of the adult brain, in both positive and negative ways (Butovsky et al., 2006; Pluchino et al. 2008; Ekdahl et al. 2009; Martino et al., 2011; Ekdahl, 2012).
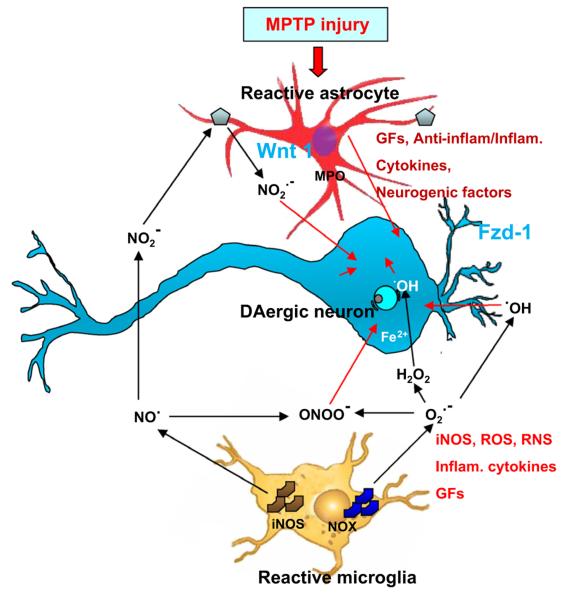
Fig. 1.
A schematic representation of glia-mediated detrimental and beneficial inflammatory pathways in PD. Injury to nigrostriatal DAergic neurons, as a result of specific chemical insults (e.g. MPTP), and/or a combination of genetic and environmental factors, leads to astrocyte and microglia activation. MPTP is metabolized by astrocytes to MPP+, which is concentrated in DAergic neurons. Microglia-derived cytotoxic mediators further exacerbate inflammation and oxidative stress. Astrocytes and microglia can protect neurons by scavenging radicals and glutamate, by harboring receptors for endogenous anti-inflammatory molecules, by providing energy support, trophic factors, and ‘protective’ cytokines, and by stimulating repair/neurogenesis by expressing neurogenic factors, including Wnt1. Under conditions of chronic inflammatory stress, activated astrocytes and microglia may become dysfunctional and over-express a variety of cytoyoxic mediators, eventually resulting in DAergic neuron demise via a synergistic action of reactive oxygen species (ROS) and reactive nitrogen species (RNS), with generation of the potent cytotoxic peroxynitrite (ONOO−). GF, Growth factor; MPO, myeloperoxidase-producing reactive species; NOX, nitric oxide-producing reactive species. Modified from L’Episcopo et al. (2010a).
Activated glia may benefit the host partly by producing cytotoxic molecules that kill pathogens, virally infected cells, or tumor cells, but they may also be detrimental by killing host cells, particularly neurons. Once activated, microglia display conspicuous functional plasticity, and ultimately transform into a macrophage-like phenotype that involves morphological changes, proliferation, increased expression of cell surface receptors, and the production of neurotrophic and neurotoxic factors. Astrocytes respond to injury with hyperplasia and hypertrophy of cell bodies and cell processes, and increased expression of the major astrocytic cytoskeletal protein glial fibrillary acidic protein (GFAP). Importantly, in response to brain injury, astrocytes and microglia play roles that are very dynamic and cell type-dependent, in that they may have ‘harmful’ effects, or they can turn into highly protective cells, and exert anti-inflammatory, neuroprotective and pro-regenerative (‘beneficial’) functions, thereby facilitating neuronal recovery and repair, posing the ‘to be or not to be inflamed’ dilemma (Marchetti & Abbracchio, 2005) (Fig. 1).
Importantly, peripheral immune responses also can trigger inflammation and exacerbation of central nervous system (CNS) degeneration in several neurodegenerative diseases, including PD (Cunningham et al., 2005; Hu et al., 2008; Pott Godoy et al., 2008; L’Episcopo et al., 2011c). Indeed, increasing inflammation and breakdown of the blood–brain barrier upregulates communication between the CNS and peripheral immune systems, with potential harmful consequences for neuronal survival.
Among the cytotoxic molecules produced by activated microglia, nitric oxide, produced by inducible nitric oxide synthase (iNOS), and superoxide, produced by the plasma membrane NADPH oxidase, are two key harmful mediators. iNOS is not normally expressed, but is induced as a part of the activation state in microglia, by cytokines [particularly interferon (IFN)-γ), tumor necrosis factor-α, or interleukin (IL)-1β], bacterial cell wall components [particularly lipopolysaccharide (LPS)], and oxidative stress. Of particular interest is the fact that, if iNOS and NADPH oxidase are active at the same time, then microglia might produce peroxynitrite (ONOO−), a potent toxin, which may promote the nitration of tyrosine, producing hydroxyl radicals (Fig. 1). Hence, the generation of the free radical nitric oxide followed by production of peroxynitrite may be implicated in neuronal cell death (Gao et al., 2003; Gao & Hong, 2008; Gao et al. 2008; Hirsch & Hunot, 2009) .
Reactive astrocytes are characterized by the upregulation of several molecules, including GFAP and S100, and express receptors involved in innate immunity (e.g. Toll-like receptors), participating in the regulation of astrocyte response to injury. In addition, reactive astrocytes express receptors for growth factors, chemokines, and hormones, and produce a wide array of chemokines and cytokines that act as immune mediators in cooperation with those produced by microglia (Gennuso et al., 2004; L’Episcopo et al., 2010a).
Astrocytic reaction represents a pivotal feature of the neuroinflammation accompanying PD. Prolonged activation of astrocytes stimulated by cytokines released from microglia and damaged neurons is implicated in chronic neurodegenerative diseases such as PD, but the crosstalk between damaged neurons and reactive astrocytes still remains poorly understood. Given the cardinal role of astrocytes in the maintenance of brain homeostasis, energy metabolism, and, in particular, the defense against oxidative stress, impairment of astrocyte–neuron crosstalk may contribute to disease progression and impair the recovery process (see McNaught & Jenner, 1999). Dysfunction and/or degeneration of astrocytes may critically reduce their neuroprotective functions, and impair neurogenesis, causing a further delay in the recovery from neurodegeneration. Thus, disturbed and/or insufficient astrocytic function in the face of a highly activated microglial phenotype might represent a critical vulnerability factor compromising the self-repair ability of DAergic neurons.
Wnt signaling cascades, receptors, coreceptors, and endogenous regulators
In the CNS, the Wnt glycoprotein ligands control cell fate during embryonic development, self-renewal in adult neurogenic niches, and neuronal integrity and homeostasis in adult brain (Harrison-Uy & Pleasure, 2012; Salinas, 2012; Willert & Nusse, 2012 and references therein). The Wnt genes encode secretory glycoproteins that activate the Wnt signaling pathway. Wnt signals are context-dependently transduced to the canonical and non-canonical pathways, depending on the expression profiles of Wnt ligands, Wnt antagonists, the Fzd family receptors, coreceptors, and the activity of cytoplasmic Wnt signaling regulators. Wnt proteins share molecular and structural characteristics involving the sequence identity of 39–46-kDa lipid-modified secreted glycoproteins containing 350–400 amino acids with a highly conserved pattern of 23–24 cysteines and several asparagine-linked glycosylation sites (Willert & Nusse, 2012).
Wnt proteins (Wnt homepage: http://www.stanford.edu/~rnusse/wntwindow.html) are generally classified into functional groups according to their ability to induce a secondary body axis in Xenopus embryos and to activate specific signaling cascades, essentially described as the Wnt1 (including Wnt2, Wnt3, Wnt3a, Wnt8, and Wnt8a) and the Wnt5a (including Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11) classes, involving intracellular signaling pathways specifying Wnt signal transduction. Generally, members of the Wnt1 class signal into the cell via the ‘canonical’ Wnt/β-catenin pathway, whereas members of the Wnt5a class signal via the ‘non-canonical’ Wnt/PCP and Wnt/Ca2+ pathways. Common to all three pathways is binding of the Wnt ligand to the seven-pass transmembrane receptors of the Fzd family, recently reviewed by Janda et al. (2012). The hallmark of the Wnt/β-catenin pathway is the stabilization of cytosolic β-catenin. In the absence of Wnt, β-catenin is constantly phosphorylated by a destruction complex consisting of, besides other components, glycogen synthase kinase-3β (GSK-3β), thereby targeting it for ubiquitination and degradation by the proteasome (Aberle et al., 1997; Gordon & Nusse, 2006; Jope et al., 2007).
Wnt signaling inhibits GSK-3β activity, thus increasing the amount of β-catenin, which enters the nucleus, and associates with T-cell factor/lymphoid enhancer-binding factor transcription factors, leading to the transcription of Wnt target genes involved in cell survival, proliferation, and differentiation (Gordon & Nusse, 2006).
The Wnt/β-catenin pathway is involved in several aspects of neural development (Harrison-Uy & Pleasure, 2012), and has been reported to play an important role in midbrain DAergic neuron development, as recently reviewed by Inestrosa & Arenas (2010). Although this article will concentrate on Wnt signaling in the adult midbrain and adult NPCs, it seems crucial to recall that Wnt1, the prototypical ligand of the Wnt/β-catenin pathway, regulates midbrain development, neurogenesis, proliferation of DA progenitors, and differentiation (see Introduction). Additionally, the low-density lipoprotein receptor-related protein (Lrp)6 receptor is important for the onset of midbrain DA differentiation and morphogenesis (Castelo-Branco et al., 2010), and β-catenin is necessary for the integrity of the VM neurogenic niche and the progression from progenitors to DA neurons (Tang et al., 2009, 2010).
Wnt pathways linked to intracellular Ca2+ release are defined as non-canonical or Wnt/Ca2+ pathways (Angers & Moon, 2009). The PCP pathway controls the remodeling of the cytoskeleton via a β-catenin-independent mechanism and controls tissue polarity, coordinated cell migration, and axon guidance. The Wnt5a class binds to Fzd receptors on the cell surface, resulting in several cellular processes that involve stimulation of heterotrimeric G proteins, increased intracellular Ca2+ release, decreased cGMP levels, and activation of the two kinases Ca2+/calmodulin-dependent protein kinase II and calcineurin, and protein kinase C (recently reviewed by van Amerongen, 2012).
A large number of antagonists can modulate the Wnt/β-catenin signaling pathway. These include the Dkkopf (Dkk) family, Wnt inhibitory factor, Fzd-related proteins, and the cerberus and sclerostin families. The Dkk family, composed of Dkk-1, Dkk-2, Dkk-3, Dkk-4, and soggy, is a group of secreted glycoproteins, with one of the best characterized members being Dkk1. Fzd receptors can also bind to proteins from other protein families, such as R-spondin and norrin. Additionally, Wnt–Fzd binding and cooperation with particular coreceptors, such as Lrp5/6, receptor tyrosine kinase-like orphan receptor 1/2, or receptor-like tyrosine kinase, can define downstream signal specificity (van Amerongen et al., 2008; Glinka et al., 2011; Clevers & Nusse, 2012).
The multiplicity of potential interactions between Wnts, their receptors and downstream effectors has exponentially increased the complexity of the signal transduction network. Signaling through each of the Wnt pathways, and crosstalk between them, together play critical roles in all facets of nervous system development, and probably also contribute to adult CNS homeostasis (Ciani & Salinas, 2005; Lie et al., 2005; Prakash & Wurst, 2006; Inestrosa & Arenas, 2010; Marchetti & Pluchino, 2013). Not surprisingly, interruption of Wnt signaling leading to either hypo-functioning or aberrant functioning may promote diverse pathogenic outcomes. Hence, dysregulation of the Wnt/Fzd system is associated with a variety of human hereditary diseases, and modulation of Wnt signaling is actively targeted for cancer, regenerative medicine, stem cell therapy, bone growth, and wound healing (Clevers & Nusse, 2012).
Of specific importance, novel evidence indicates that the leucine-rich repeat kinase 2 (LRRK2), encoded by PARK8 (whose mutations have been linked to PD), has a functional role in canonical Wnt signaling pathway (Berwick & Harvey, 2012a). Hence, LRRK2 interacts with key Wnt signaling proteins of the β-catenin destruction complex and Dvl proteins, in vivo (Berwick & Harvey, 2012a). Following Wnt stimulation, LRRK2 is recruited to membranes, where it binds to the Wnt co-receptor Lrp6 (Berwick & Harvey, 2012a). Pathogenic LRRK2 mutations were shown to reduce Wnt signaling strength and LRRK2-Lrp6 interactions, suggesting that dysregulated Wnt signaling might represent a new pathomechanism leading to PARK8-PD (Berwick & Harvey, 2012a,b).
During the last decade, within the adult CNS, a ‘pro-survival role’ for the Wnt/β-catenin signaling pathway has emerged, and dysregulation of the Wnt/Fzd cascade has been defined as a critical determinant in major neurodegenerative disorders (De Ferrari et al., 2003; Li et al., 2007; Inestrosa & Toledo, 2008; Maiese et al., 2008; Inestrosa & Arenas, 2010; Maiese et al., 2012; Purro et al., 2012; Shruster et al., 2012; and references therein). In this article, we focus on adult midbrain DAergic neurons, and herein trace a candidate role for the Wnt/β-catenin signaling system in healthy DAergic neurons and upon MPTP-induced DAergic plasticity brought about by astrocyte–neuron crosstalk.
Wnt/β-catenin signaling and astrocyte–neuron crosstalk in the MPTP-injured midbrain: an intrinsic self-defense mechanism for DAergic neurorepair
A body of evidence suggests that astrocytes play a vital role in the response of SNpc DAergic neurons to injury or inflammation, by scavenging excess neurotoxic factors, removing dying cells and cellular debris, and stimulating repair processes, whereas impairment of astrocyte function, as a result of aging or exacerbated inflammation, may critically influence neurodegeneration and neurorepair (Morale et al., 2004, 2006; Marchetti & Abbracchio, 2005; Hu et al., 2008; McGeer & McGeer, 2008; Sandhu et al., 2009; L’Episcopo et al., 2010a, 2011a,b,c). Indeed, after injury, many adaptive changes occurring within the astroglial cell compartment may serve to increase the defense against oxidative stress, to reduce inflammation, to improve mitochondrial performance, to increase neurotrophic support, and/or to activate adult neurogenesis (Horner & Palmer, 2003; Barkho et al., 2006; Chen et al., 2009; Sandhu et al., 2009; Voskuhl et al., 2009; Sofroniew & Vinters, 2010).
Hence, astrocytes are known to secrete both inflammatory and anti-inflammatory and neurotrophic and survival factors, and may play a role in modulating microglial activity. Importantly, GFAP-expressing astrocytes can contribute to cell genesis, both as stem cells and as important cellular elements of the neurogenic microenvironment, with implications for self-recovery/neurorepair (Wagner et al., 1999; Alvarez-Builla et al., 2001; Song et al., 2002).
Of special importance is that, in response to injury, the nigrostriatal DAergic system shows compensatory mechanisms, but the degree of plasticity becomes reduced with age (Ricaurte et al., 1987; Hornykiewicz, 1993; Bezard & Gross, 1998; Ho & Blum, 1998). Interestingly, different conditions, including an enriched environment and exercise, by the activation of signaling cascades by neurotrophic factors such as glial cell line-derived neurotrophic factor, can induce neurorestoration in rodent PD models (see Zigmond et al., 2009, 2012).
The idea that astrocytes might harbor Wnt1/β-catenin signaling mechanisms to induce neurorepair arose from gene expression experiments conducted in the MPTP mouse model of basal ganglia injury (L’Episcopo et al., 2011a). Hence, using wide gene expression analysis of 92 mRNA species involved in inflammation, immunity, stemness, self-renewal, DAergic development, and DA metabolism, we discovered major upregulation of certain proinflammatory chemokines and Wnt1 (but not Wnt3a or Wnt5a) during MPTP-induced DAergic degeneration and self-recovery, suggesting a potential involvement of Wnt signaling as an intrinsic response to midbrain injury.
Indeed, the temporal correlation of Wnt1 mRNA expression with Wnt1 protein appeared to be of interest in the light of recent evidence indicating that the Wnt signaling system may be reinduced in the adult CNS after injury (Osakada et al., 2007) and the studies indicating that Wnt/β-catenin activation reduces neurodegeneration in mouse models of Alzheimer’s disease (Chacón et al., 2008; Toledo et al., 2008). The finding that Wnt components are expressed in adult astrocytes (Lie et al., 2005; Cahoy et al., 2008; Kuwabara et al., 2009) prompted us to investigate a possible astrocyte source of Wnt1. Accordingly, in situ hybridization histochemistry revealed MPTP-reactive astrocytes as candidate sources of Wnt1 within the injured VM. In addition, increased transcription of Wnt1 mRNA was observed in astrocytes derived ex vivo from the MPTP-injured VM, raising the possibility that astrocyte-derived Wnt signals might directly and/or indirectly participate in the DAergic neuroplasticity observed upon MPTP exposure (L’Episcopo et al., 2011a).
Earlier studies implicated astrocyte-derived factors in the survival and gowth of several types of neuron, including DAergic neurons (Engele & Bohn, 1991; Takeshima et al., 1994; Gallo et al. 1995; Gallo et al., 2000; Castelo-Branco et al., 2006; Blackburn et al., 2009). Additionally, in vitro astrocyte–neuron co-culture paradigms have underlined the ability of astrocyte-secreted glial cell-derived neurotrophic factor and the glutathione antioxidant system to protect DAergic neurons against 6-OHDA cytotoxicity (Sandhu et al., 2009). Our studies further demonstrated the contribution of the Wnt/β-catenin pathway to VM astrocyte-induced DAergic neuroprotection against MPP+, 6-OHDA, and growth factor deprivation-induced toxicity. Accordingly, inhibition of DAergic neuron survival was obtained by directly blocking Wnt/Fzd-1/β-catenin signaling with the prototypical antagonist of the Wnt canonical pathway, Dkk1 (L’Episcopo et al., 2011a,b), raising the possibility that endogenously secreted Wnt agonists/inhibitors might play a role in directing astrocyte-promoted DAergic cell survival/death (L’Episcopo et al., 2011a,b; Marchetti et al., 2012). Interestingly, exogenous activation of Wnt signaling in MPP+-treated astroglia–neuron co-cultures with a specific GSK-3β antagonist sharply magnified astrocyte-induced DAergic neuroprotection. Moreover, addition of glial inserts or direct application of Wnt1 to purified DAergic neurons just prior to MPP+ insult afforded a significant degree of neuroprotection, an effect counteracted by the addition of Wnt1 blocking antibody, or the Wnt antagonist Fzd-1-cysteine-rich domain, thus supporting the critical role of Wnt1 in DAergic neuron survival (L’Episcopo et al., 2011a).
Cytotoxic insults trigger beneficial astrocyte–DAergic neuron crosstalk: astroglial-born Wnt1 as a critical paracrine protective actor in neuroprotection
That Wnt1 might be the critical Wnt molecule was next corroborated by different lines of evidence. Besides the demonstration of the lack of effect upon tyrosine hydroxylase (TH)-positive neuroprotection induced by VM astrocytes in the presence of a specific Wnt1 antibody (L’Episcopo et al., 2011a), depleting Wnt1 in VM astrocytes by introducing a small interfering RNA targeting Wnt1 resulted in a significant decrease in TH-positive neuron survival upon serum deprivation (SD), 6-OHDA or MPP+ treatment, as compared with neurons co-cultured with AstroCt (L’Episcopo et al., 2011b). Inhibition of TH-positive neuron survival was associated with a marked reduction in β-catenin protein levels, and activation of caspase-3, a recognized apoptotic cell death marker.
Within this context, and of particular interest, co-culture with VM astrocytes markedly increased the Fzd-1 immunofluorescent signal within the rescued TH-positive neurons, at the neurites and growth cones, as opposed to the dramatic downregulation of Fzd-1 receptor observed in purified neurons, either in vitro or in vivo, after the neurotoxic insult. Coupled with the finding that Wnt1 induced upregulation of Fzd-1 receptors in purified DA neurons upon cytotoxic challenge (L’Episcopo et al., 2011b), these findings further suggested that Wnt1 signaling is required to maintain the expression of Wnt signaling components in DA neurons, corroborating the presence of a paracrine astrocyte–neuron autoregulatory loop. It seems important to recall the potential involvement of Fzd receptors localized at growth cones in regenerating neurites, together with the recently characterized role of Fzd-1 receptor in the presynaptic differentiation and function of hippocampal neurons (see Chacón et al., 2008; Toledo et al., 2008, and references herein), and further studies are in progress to clarify the role of Fzd-1 ligands and Fzd-1 receptors localized at the growth cones in TH-positive neurons in neurite outgrowth, maintenance and regeneration in conjunction with astroglia-derived factors.
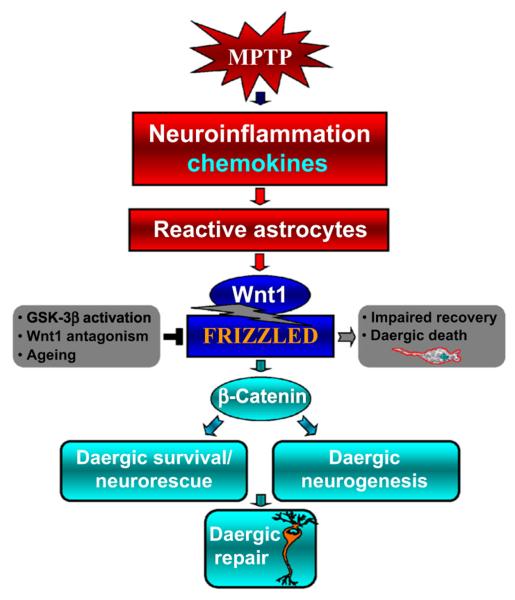
Overall, besides a wide panel of astroglia-derived growth and neurotrophic factors, astrocyte-born Wnt1, via Fzd-1/β-catenin signaling activation, was identified as a chief component of the self-protective machinery of DA neurons, thus prompting us to propose a candidate regulatory autoprotective circuit controlling midbrain DA neuron–astrocyte crosstalk via astroglial Wnt1 (Fig. 2).
Fig. 2.
Schematic illustration of Wnt1/β-catenin signaling as a key player in neuroprotection via DAergic neuron–astrocyte crosstalk and promoting neurogenesis via neuroprogenitor–astrocyte crosstalk. This is a simplified scheme linking reactive astrocytes and Wnt/β-catenin signaling to nigrostriatal injury and repair in the MPTP mouse model of PD. Astrocyte-derived Wnts, particularly Wnt1, control the integrity of DA neurons via blockade of GSK-3β-induced phosphorylation and proteosomal degradation of the neuronal pool of β-catenin. Activation of Wnt/β-catenin signaling can also promote neurogenesis from adult midbrain progenitors. Neurotoxic injury or increased oxidative load as a result of aging may antagonize Wnt/β-catenin signaling in DAergic neurons by upregulating active GSK-3β, leading to β-catenin degradation and increased DAergic neuron vulnerability. Neuronal injury also triggers reactive astrocyte expression of a panel of growth and neurotrophic factors, anti-oxidant and neuroprotective mechanisms, among which astrocyte Wnt1 may function as a vital component of the self-protective machinery of DAergic neurons, shifting the balance towards the programming of cell survival/neurorescue on the on hand, and promoting neurogenesis on the other. From L’Episcopo et al. (2011a) with permission.
Wnt/β-catenin signaling and DAergic neuron integrity in the adult midbrain: building-up a ‘Wnt/β-catenin-linked hypothesis’
In vitro findings
Canonical Wnt signaling has been associated with the control of apoptosis during injury in various cell systems. Although little is known about the receptors that mediate Wnt effects in the adult midbrain, in the hippocampus Fzd-1 represents a potential target, as it is expressed at high levels and mediates the neuroprotective effect of Wnt3a against Aβ toxicity (Chacón et al., 2008; Inestrosa & Arenas, 2010). In addition, the synaptic localization of Fzd-1 receptor in mammalian neurons was recently shown, and it was suggested to mediate the synaptic effects of the Wnt signaling pathway (Inestrosa & Arenas, 2010). We thus verified the expression of Fzd1–10 in developing DAergic neurons in vitro, and, in analogy to studies carried out in primary hippocampal neurons (Chacón et al., 2008), we found that Fzd-1 receptors are expressed in mesencephalic DAT-expressing neurons in primary culture (L’Episcopo et al., 2011b).
Midbrain DAergic neurons are exquisitely sensitive to oxidative stress and growth factor withdrawal, and significant changes indicative of mitochondrial dysfunction, oxidative stress and inflammation, proteasomal deficits and apoptosis have been identified in the human parkinsonian brain (Abou-Sleiman et al., 2006). The ability of exogenous Wnt1 to afford TH neuroprotection in vitro, via β-catenin as a downstream effector, was of specific interest in the light of previous studies reporting the protective abilities of exogenous Wnts against a variety of cytotoxic insults, including SD-induced, Aβ-induced or tumor necrosis factor-α-induced apoptosis (see Chong and Maiese, 2007; Maiese et al., 2008). Both β-catenin-dependent and β-catenin-independent mechanisms have been recognized to play a role, whereas the presence of Wnt antagonists has been largely linked to the occurrence of apoptosis (Maiese et al., 2008). In our studies, by using specific antagonists for the canonical Wnt pathway, or silencing experiments with small interfering RNA to deplete β-catenin, as well as antisense oligonucleotides to knock down Fzd-1, we showed the failure of Wnt1 ligand to efficiently protect TH-positive neurons against SD-induced or 6-OHDA-induced cytotoxicity, identifying for the first time a canonical Wnt1/Fzd-1/β-catenin signaling pathway as a novel potential neuroprotective pathway (L’Episcopo et al., 2011b). In different systems, the anti-apoptotic effects of Wnt signaling were shown to involve β-catenin/Tcf transcription-mediated pathways, the prevention of cytochrome c release from mitochondria, and the subsequent inhibition of caspase-9 activation, by activating nuclear factor-κB, increasing the level of insulin-like growth factor, and inhibiting GSK-3β (see Maiese et al., 2008).
Interestingly, several previous studies defined active GSK-3β as a critical mediator of neuronal apoptosis induced by oxidative stress caused by specific PD mimetics, including 6-OHDA, rotenone, and MPTP/MPP+ (Chen et al., 2004; Wang et al., 2007; Duka et al., 2009; Petit-Paitel et al., 2009). In fact, in the absence of Wnt activity, increased active GSK-3β is known to phosphorylate β-catenin at serine or threonine residues of the N-terminal region, encouraging degradation of β-catenin through ubiquination (Aberle et al., 1997). It should be mentioned that GSK-3β is activated by phophorylation of Tyr216 located in the kinase domain, and inactivated by phosphorylation of the N-terminal Ser9. A potential role of active GSK3β during cytotoxic injury was then verified in mesencephalic neurons in vitro, where we showed the ability of Wnt1 pretreatment to efficiently reverse SD-induced, 6-OHDA-induced and MPP+-induced GSK-3β activation (i.e. increase in the amount of phopshorylated Ser216). Moreover, by the use of specific GSK-3β inhibitors, it was possible to reverse the neurotoxin-induced TH-positive neuron demise, further showing the participation of the Wnt1/Fzd/β-catenin signaling cascade in DAergic neuron death/survival (L’Episcopo et al., 2011b). These findings supported the reports showing the ability of 6-OHDA, rotenone and MPTP/MPP+ to induce neuronal apoptosis in a GSK-3β-dependent manner in SH-SY5Y cells, PC12 cells, cerebellar granule neurons, and primary mesencephalic neurons (Chen et al., 2004; Wang et al., 2007; Duka et al., 2009; Petit-Paitel et al., 2009), and further implicated β-catenin as a chief downstream component.
It is of note that two functional single-nucleotide polymorphisms have been reported in PD brains (see Kwok et al., 2005; Duka et al., 2009). In addition, post-mortem striata from patients diagnosed with PD revealed increased GSK-3β activity as compared with age-matched controls (Duka et al., 2009). It seems also important to recall that the Wnt pathway also uses protein kinase B (Akt) to promote cell survival (see Maiese et al., 2008). Of particular interest is the fact that Akt inhibits the activity of GSK-3β through phosphorylation of this protein to promote cell survival (Chong & Maiese, 2007; Nair & Olanow, 2008), thus anticipating the potential participation of Akt in Wnt1-induced DAergic neuroprotection.
All together, these results indicated the ability of Wnt1 to activate β-catenin via Fzd-1, and also via the inactivation of GSK-3β, thereby blocking the phosphorylation of β-catenin and its proteosomal degradation. This, in turn, results in the stabilization of β-catenin in the cytoplasm, the consequent translocation of β-catenin in the nucleus, followed by β-catenin-mediated transcription of Wnt target genes involved in cell survival/protection. Together, these informations pointed to Wnt1/Fzd-/β-catenin transcriptional activation as a critical downstream pro-survival effector for mesencephalic DAergic neurons (Fig. 3). It is of note that stabilizing neuronal β-catenin was recently shown to render neurons ‘anti-apoptotic’ in cell cultures and transgenic mouse models (Li et al., 2007), and recent in vivo studies have empasized that downregulation of Wnt/β-catenin signaling results in hippocampal neurodegeneration (Kim et al., 2011).
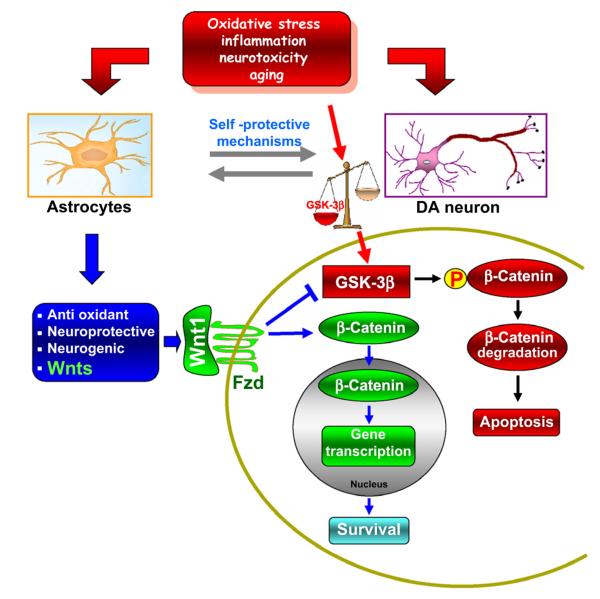
Fig. 3.
Schematic illustration of Wnt1/Fzd-1/β-catenin signaling as a candidate regulatory circuit controlling mesencephalic DAergic neuron–astrocyte crosstalk. In the intact unlesioned midbrain, crosstalk between astrocytes and DA neurons represents a cardinal neuroprotective mechanism against inflammation, oxidative stress, and growth factor deprivation. This function appears to be of paramount importance for DAergic neurons, which are known to be particularly vulnerable to increased inflammation and oxidative stress. Astrocyte-derived Wnts, particularly Wnt1, via activation of Fzd-1 receptors, may contribute to maintain the integrity of DAergic neurons via blockade of GSK-3β-induced phosphorylation (P) and proteosomal degradation of the neuronal pool of β-catenin. Stabilized β-catenin can translocate into the nucleus, associate with a family of transcription factors, and regulate the expression of Wnt target genes involved in DAergic neuron survival. β-Catenin may also function as a pivotal defense molecule against oxidative stress, and can act as a co-activator for several nuclear receptors involved in the maintenance/protection of DA neurons. Crosstalk with upstream survival pathways converging to β-catenin stabilization can also be envisaged. Neurotoxic injury or increased oxidative load as a result of aging may antagonize Wnt/β-catenin signaling in DA ergich neurons by upregulating active GSK-3β, leading to β-catenin degradation and increased DAergic neuron vulnerability. Neuronal injury also triggers reactive astrocyte expression of a panel of growth and neurotrophic factors, anti-oxidant and neuroprotective mechanisms, among which astrocyte Wnt1 may function, via Fzd-1 receptors, as a vital component of the self-protective machinery of DAergic neurons, shifting the balance towards the programming of cell survival/neurorescue. From L’Episcopo et al. (2011b), with permission.
In vivo findings
Mis-regulation of Wnt/β-catenin signaling has been implicated in the pathology of Azheimer’s disease (De Ferrari et al., 2003; Inestrosa & Toledo, 2008; Toledo et al., 2008 and Refs therein). Wnt cascades have recently been linked to early-stage PD. Hence, downregulation of β-catenin in DAergic neurons of the substantia nigra (SN) (Cantuti-Castelvetri et al., 2007) and upregulation of active GSK-3β in the striatum (Duka et al., 2009) have been reported in PD. Consistently, genetic screens have revealed GSK-3β polymorphisms with altered transcription and splicing in PD (Kwok et al., 2005). Other studies have revealed mutations in the Wnt/β-catenin signaling-activated transcription factor Nurr1 (Sleiman et al., 2009), the orphan nuclear receptor involved in DA neurodevelopment and neuroprotection (Kitagua et al., 2007). Gene expression profiling in progressively MPTP-lesioned macaques indicated downregulation of β-catenin and dysregulation of key components of Wnt signaling (Ohnuki et al., 2010). In particular, mutations in PARK8, encoding LRRK2, which constitute a major cause of PD (Healy et al., 2008), were linked to Wnt signaling (Berwick & Harvey, 2012a,b). On the other hand, parkin, an E3 ubiquitin ligase linked to familial PD, regulates β-catenin protein levels in vivo (Rawal et al., 2009). Additionally, α-synuclein, a presynaptic protein that is causal in PD, contributes to GSK-3β-catalyzed phosphorylation of tau (a protein linked to tauopathies, such as Azheimer’s disease) in rodent models of PD, and the importance of this kinase in the genesis and maintenance of neurodegenerative changes associated with PD was recently recognized (Duka et al., 2009).
In our studies, we addressed the physiological relevance of the Fzd-1/β-catenin pathway in the maintenance of adult midbrain DAergic neurons by investigating Fzd receptor and β-catenin expression, and the effect of blocking Fzd/β-catenin signaling in vivo. Previous studies at the mRNA level, using in situ hybridization, indicated that Fzd-1 is expressed in the cortex and in the hilus of the dentate gyrus (see Chacón et al., 2008). In addition, Fzd-1 protein in the adult rodent brain was recently shown to be localized in the cortex and hippocampus (Chacón et al., 2008). Our previous studies documented Fzd-1 receptor expression in the adult VM by real-time polymerase chain reaction and western blot analysis; however, it was not clear which cell type (neurons or glia) might harbor Fzd-1 receptor (L’Episcopo et al., 2011a). We next showed that Fzd-1 receptors and β-catenin colocalize with TH-positive and DAT-positive cells, but not in GFAP-positive cells. Intracerebral infusion of Dkk1, or of inactivating lentiviral vectors expressing Wnt inhibitor/stimulator, was used as a tool to investigate the potential role of the canonical Wnt pathway in physiopathological conditions, including the regulation of hippocampal neurogenesis in intact mice (Lie et al., 2005), or ischemia-induced striatal neurogenesis (Lei et al., 2008), and estrogen-induced neuroprotection in global cerebral ischemia (Zhang et al., 2009). In our hands, unilateral infusion of Dkk1 caused a time-dependent decrease in TH-positive neuron numbers in the ipsilateral-infused, but not in the contralateral-uninfused, SN, whereas unilateral infusion of saline within the SN did not change TH-positive neuron numbers in either the ipsilateral or the contralateral SNpc. That the Dkk1-induced loss of TH-positive neurons was caused by Wnt/β-catenin antagonism, and not by a non-specific effect, was further demonstrated by at least two other lines of evidence: first, early and sharp downregulation of Fzd-1 receptor and β-catenin proteins was revealed in the ipsilateral as opposed to the contralateral SN; second, such β-catenin downregulation preceded and accompanied the tempo of TH-positive neuron degeneration, as revealed by FluoroJade-C staining, in the face of marked upregulation of active GSK-β, which showed, in vivo, a critical role for a paracrine canonical Wnt/β-catenin tone as an endogenous pathway linked to the survival/maintenance of adult midbrain DAergic neurons. This acute decrease in cell number showed an initial return by 7 days post-Dkk1, suggesting the possible activation of repair mechanisms within the SN microenvironment. Interestingly, a marked increase in the number of reactive astrocytes within the ipsilateral Dkk1-infused SN was observed, with hypertrophic GFAP-positive cells abundantly covering the ipsilateral SN, as compared with the saline-infused SN, and longer time-course studies, which are in progress, will verify both astrocyte and TH-positive neuron responses with time. The fact that the preventive activation of β-catenin signaling by pharmacological inhibition of active GSK-3β efficiently promoted TH-positive neuron protection in either the Dkk1-lesioned or the MPTP-lesioned SN thus implied a causative link between the interruption of Wnt signaling and the acute degeneration of SNpc TH-positive neurons. Together with the dysregulation of Wnt signaling in the VM of aging mice being associated with downregulation of β-catenin upon MPTP insult and lack of TH-positive cell recovery (L’Episcopo et al., 2011a; Marchetti et al., 2012), the present findings further provides a new mechanistic insight into the regulation of the pro-survival/anti-apoptotic processes regulating the life and death of adult midbrain DA neurons (Fig. 3).
Activated astrocytes of the VM and Wnt/β-catenin signaling are key players in promoting adult midbrain neurogenesis in vitro: role of a specific inflammatory milieu
Reactive astrocytes express receptors for growth factors, cytokines, chemokines, and hormones, and produce a wide array of neurotrophic and neuroprotective mediators together with those produced by microglia (L’Episcopo et al., 2010a; Sofroniew & Vinters, 2010). In addition, astrocytes are known to release various region-specific signaling molecules, such as Shh and Wnts, which may interact with each other to dictate neurogenic behavior in the adult CNS (Alvarez-Builla et al., 2001; Song et al., 2002; Lie et al., 2005; Barkho et al., 2006; Jiao & Chen, 2008). Indeed, canonical β-catenin-regulatory mechanisms are known to be required for activation of adult neurogenesis both in vitro and in vivo (Lie et al., 2005; Yu et al., 2006; Adachi et al., 2007). Astrocytes have pivotal roles in glia–neuron interactions and in defining the stem cell niche. Hence, embryonic day 13.5 VM astrocytes, but not cortex astrocytes, express Wnt1 and Wnt5a and different DA-specific transcription factors, such as Pax-2, En-1, and Otx-2, and increase the differentiation of VM embryonic precursors into TH-positive neurons in vitro, suggesting that VM astrocytes constitute a part of the neurogenic niche that plays a key role in VM DA neurogenesis (Wagner et al., 1999; Castelo-Branco et al., 2006). In our ex vivo and in vitro studies, we found that MPTP injury and certain proinflammatory chemokines induce the expression of Wnt1 in astrocytes of the VM, indicating not only region specificity but also a defined inflammatory milieu in the modulation of Wnt1 induction in astrocytes (L’Episcopo et al., 2011a).
By culturing NPCs derived from the adult midbrain/hindbrain (MB) (Hermann et al., 2006), using the neurosphere culturing technique, similar to that used for propagation of NPCs derived from the SVZ (Pluchino et al., 2003, 2005), we found that the phenotype of these MB NPCs was similar to that of those derived from the SVZ (Papanikolaou et al., 2008). Accordingly, nestin, a marker for precursor cells in the adult brain, and bromodeoxyuridine, a marker for cell proliferation, were expressed during in vitro clonal expansion of NPCs from the adult MB (L’Episcopo et al., 2011a). In NPC cultures from either the MB or the the SVZ grown in N2 medium, and in the absence of exogenous factors, only rare Tuj1-positive neurons could be detected after only 3 days in vitro, as opposed to direct co-culture of NPCs with astrocytes, which produced a significant increase in the number of Tuj1-positive neuroblasts, in accordance with earlier studies carried out with adult SVZ precursors cultured on type 1 astrocyte monolayers (Lim & Alvarez-Buylla, 1999). Our studies further indicated the ability of VM astrocytes to promote neurogenesis from NPCs derived from the adult midbrain. In addition, chemokine-activated astrocytes significantly increased the proportion of new neurons as compared with co-culture with untreated astrocytes, indicating the ability of a specific inflammatory milieu to sharply increase the neurogenic potential of NPCs. By contrast, blocking Wnt/Fzd signaling with the Wnt antagonist Dkk-1, applied to NPCs just prior to starting co-culture with astrocytes, resulted in significant reductions in both proliferative and neuron differentiation potential, thus suggesting that Wnt/β-catenin signaling was required for activated astrocyte-induced neurogenesis from multipotent NPCs of the MB. Finally, when NPCs isolated from the MB were co-cultured with activated VM astrocytes in differentiating conditions for 7 days in vitro, we found that a certain, albeit small, proportion co-expressed Tuj1 and TH, and extended long and branched TH-positive processes with numerous DAergic varicosities. In sharp contrast, Dkk1 treatment induced a significant reduction in the proportion of TH-positive cells among the total number of Tuj1-positive cells (see L’Episcopo et al., 2011a).
These finding are in line with previous studies suggesting that IL-1β and IL-6 contribute to astroglial modulation of adult neurogenesis (Barkho et al., 2006). Additionally, certain specifically activated microglial cells can induce neural cell renewal in the adult CNS (Butovsky et al., 2006). Hence, microglia pretreated with IL-4 or IFN-γ induced neurogenesis and oligodendrogenesis in NPCs derived from the SVZ, whereas LPS-pretreated microglial cells blocked both processes in adult NPCs (Butovsky et al., 2006), in line with reports that inflammation associated with LPS blocks adult neurogenesis (Butovsky et al., 2006; Ekdahl et al., 2009; Schwartz et al., 2009). Therefore, according to a certain inflammatory microenvironment, factors derived from astrocytes and chemically activated astrocytes, including Wnt/β-catenin signaling, probably contribute to the neuronal and dopaminergic differentiation of adult MB progenitors in vitro, suggesting a critical role for a specific inflammatory milieu in dictating the promotion or inhibition of adult neurogenesis, probably via Wnt (L’Episcopo et al., 2011a,b,c, 2012a,b, 2013; Marchetti & Pluchino, 2013).
Our results, together with data in the literature, suggest a model in which adult VM astrocytes may, under specific controlled conditions, re-express region-specific factors, including Wnt1, contributing to the regulation of diverse aspects of DAergic neuron homeostasis in the injured VM, including amelioration of the impaired nigral milieu, reducing the amount of DAergic neuron death, and/or enhancing the survival, expansion and differentiation of DA progenitors. Although the presence of DA neurogenesis within the SN, either under physiological conditions or in PD animal models, is currently debated (Borta & Holinger, 2007; Hermann & Storch, 2008; Winner et al., 2011), the presence of multipotent clonogenic neural stem cells in the adult mouse MB with functional neurogenic and DAergic potential in vitro was recently reported (see Hermann et al., 2006, 2009; Hermann & Storch, 2008; for review), and further studies are clearly needed to determine whether: (i) NPCs might reside in the MB in vivo, during MPTP-induced nigrostriatal injury; (ii) they can be activated by endogenous astrocyte-derived factors and Wnt/β-catenin signaling activators; and (iii) by manipulating the local microenvironment, it might be possible to boost such endogenous mechanisms to induce the promotion of a DAergic neuronal phenotype in the adult MB (Ourednik et al., 2009; L’Episcopo et al., 2012a,b, 2013).
The inflammatory milieu governs the plasticity of NPCs of the adult SVZ in response to MPTP: involvement of Wnt/β-catenin signaling
The generation of new neurons in the brain SVZ continues throughout adult life, and may contribute to endogenous repair mechanisms after brain damage and/or disease (Curtis et al., 2007; Winner et al., 2011; Curtis et al., 2012). In PD, neurogenesis is impaired. The decreased proliferation of NPCs in the SVZ of human PD brains and in non-human primate and rodent MPTP and 6-OHDA models has been attributed to the loss of the neurotransmitter DA (Baker et al., 2004; Hoglinger et al., 2004; Freundlieb et al., 2006; Borta & Holinger, 2007; O’Keeffe et al., 2009; Winner et al., 2011; Hoglinger et al., 2012). Other studies have proposed DA-independent effects of MPTP, but the cellular contributors and signaling pathways through which MPTP may exert its direct action on SVZ cells have not yet been identified (Hu et al., 2006; Shibui et al., 2009).
In our studies, we decided to focus on glia, and dissected the influence of MPTP, astrocytes and microglia on the SVZ response to PD: first, because NPCs are in intimate contact with the surrounding glia, forming the so-called SVZ ‘stem cell niche’, which can influence NPC proliferation and differentiation (Lim & Alvarez-Buylla, 1999; Alvarez-Builla et al., 2001; Song et al., 2002; Kazanis, 2009); second, because, in the striatum bordering the SVZ of PD experimental models, astrocytes and microglia exhibit remarkable morphological and functional changes, as reported in the previous sections; and third, because although the inflammatory modulatory role on adult neurogenesis was previously reported (see Ekdahl et al., 2009), whether inflammatory glial cells together with their signaling pathways might regulate neurogenesis within the SVZ niche of MPTP-injured mice was ill-defined.
Hence, using in vivo, ex vivo and in vitro experiments, different glia–NPC co-culture paradigms, and pharmacological antagonism/RNA silencing experiments coupled with functional studies, we provided evidence supporting an active and concerted role of reactive astrocytes and microglia in the remodeling of the SVZ niche upon MPTP/MPP+ injury, regulated, at least in part, by crosstalk between inflammation and Wnt/β-catenin signaling cascades, with potential consequences for DAergic neuroprotection and self-repair (L’Episcopo et al., 2012a). We thus showed that, in addition to DA, the PD neurotoxin MPTP/MPP+, directly and/or in conjunction with astrocyte-derived and microglial-derived mediators, may contribute to regulate SVZ plasticity in this PD mouse model. We found that the unfavorable conditions of the SVZ niche during the early degenerative phase resulting from decreased DAergic innervation and MPTP/MPP+-dependent striatal oxidative and nitrosative status probably inhibits the survival and/or the expansion/differentiation and/or migration of endogenous NPCs, at least in part via disruption of β-catenin-mediated Wnt signaling in the SVZ, characterized by increased active GSK-3β associated with β-catenin depletion, and impaired survival/proliferation and neuroblast formation in NPCs of the SVZ (L’Episcopo et al., 2012a,b), in line with studies of Sirerol-Piquer et al. (2011), showing that GSK-3β overexpression induces neuronal death and depletion of the neurogenic niches in the dentate gyrus.
Interestingly, with time, a shift towards a less reactive microglial ‘harmful’ phenotype probably permitted the mitigation of the niche microenvironment, the return of astrocyte ‘beneficial’ expression of growth factors and neurogenic signals, including Wnts, and progressive striatal re-innervation, probably contributing to NPC recovery (L’Episcopo et al., 2012a). In keeping with these findings, exogenous activation of β-catenin signaling or pharmacological mitigation of microglia over-activation upregulated β-catenin in the SVZ and successfully rescued NPC proliferation and neuroblast formation (L’Episcopo et al., 2012a) (Fig. 4).
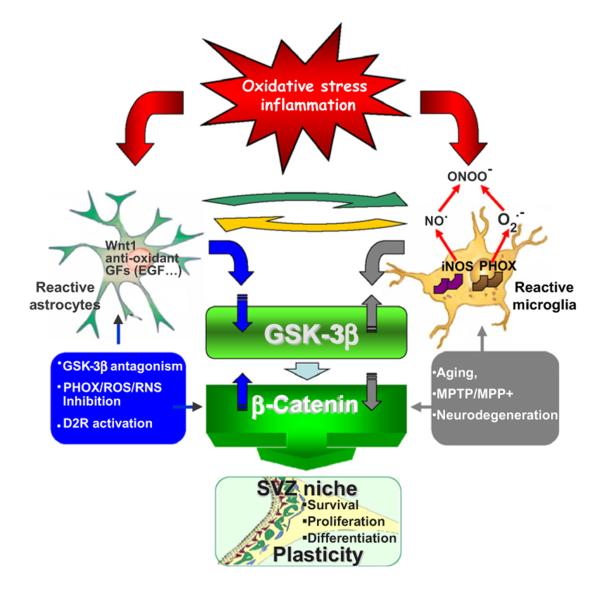
Fig. 4.
Crosstalk between inflammatory and Wnt/β-catenin signaling pathways in MPTP-induced SVZ plasticity. This is a simplified scheme summarizing MPTP-induced neuroinflammation and SVZ plasticity via modulation of Wnt/β-catenin signaling. During the early degeneration phase of MPTP toxicity, hyper-activated microglia contributes to the impairment of SVZ neurogenesis at different levels. By increasing oxidative and nitrosative stress and in synergy with MPTP/MPP+ direct toxicity, microglia-derived mediators [plasma membrane NADPH oxidase (PHOX)-derived reactive oxygen species (ROS) and iNOS-derived nitic oxide (NO) and peroxynitrite] may act as a molecular switch for cell signaling pathways that are critically involved in the physiological control of NPC homeostasis, with harmful consequences for astrocyte and NPC physiology, at least in part through GSK-3β activation, followed by phosphorylation and consequent degradation of β-catenin. By contrast, pharmacological mitigation of inflammation and oxidative stress with Apo, L-Nil or HCT1026 upregulate β-catenin and successfully rescue NPC proliferation and neuroblast formation, a process associated with striatal DAergic neuroprotection, with further positive modulation of SVZ proliferation via DA subtype 2 receptor (D2R)-activated mechanisms. The role of astrocyte–microglia interactions in the plasticity of the SVZ response to MPTP is exemplified by the astrocyte’s ability to overcome microglial inhibitory effects, also via crosstalk with Wnt/β-catenin signaling. EGF, epidermal growth factor; GF, growth factor; RNS, reactive nitrogen species. From L’Episcopo et al. with permission (2012a,b).
Concluding remarks and future directions
We have herein summarized different studies and presented several lines of evidence suggesting that activation of the Wnt/Fzd-1/β-catenin pathway might play a determinant role in the maintenance of a normal complement of TH-positive neurons in the adult midbrain. Fascinatingly, Wnt1-induced neuroprotection is closely integrated with the astroglial response to oxidative stress and inflammation upon injury, and requires stabilization of Fzd-1 receptor and β-catenin, whose expression probably underlies the observed neuroprotection, to convey pro-survival signals to the nucleus. Then, microglia–astrocyte crosstalk may influence the expression/release of Wnt modulators in vivo, thereby modulating Wnt signaling components. Although the Wnt pathway itself is protective in different tissues, receptor context and a particular inflammatory setting may influence the regulation of canonical Wnt signaling by agonist or antagonists, suggesting that a specific receptor component coupled to a specific ligand in the absence or the presence of endogenous inhibitors/activators finally mediates the tissue-specific/cell-specific response. Accordingly, manipulating Wnt expression in normal and injured VM glia might provide an insight into the role of Wnts in midbrain TH-positive neurorecovery (Marchetti & Pluchino, 2013).
It should also be underlined that upstream and downstream modulation of the astroglial Wnt1/Fzd-1/β-catenin pathway may tip the balance between apoptosis and the programming of cell survival/neurorescue in these models (Fig. 3), and an in-depth understanding of the molecular pathways and their crosstalk underlying midbrain neuroprotection will be crucial to identify new avenues for pharmacological and cell replacement therapies for PD (Marchetti & Pluchino, 2013).
Given that Wnt/β-catenin signaling controls the expression of a variety of target genes, mis-regulation of this signaling cascade may be involved in various diseases, particularly neurodegenerative disorders associated with impaired neurogenesis (He & Shen, 2009; Inestrosa & Arenas, 2010; L’Episcopo et al., 2011a,b,c; Marchetti & Pluchino, 2013). Consistently, the signaling mechanisms involved in neuronal and NPC impairment observed in PD appear to target the Wnt/β-catenin signaling pathway (L’Episcopo et al., 2011a,b, 2012a,b).
As far as midbrain DAergic neurogenesis is concerned, further studies are actually in progress: (i) to verify whether the observed in vitro events may also occur in vivo in the MPTP-injured MB; (ii) to ascertain the presence of quiescent and/or activated DAergic NPCs; and (iii) to define the conditions that limit their progression towards the DAergic phenotype as opposed to those promoting acquisition of the mature TH-positive phenotype. Because restoration of tissue integrity and homeostasis is the ultimate goal of next-generation therapies, the reviewed evidence suggests new directions for therapeutic strategies, among which targeted pharmacological interventions by changing environmental signals in situ may have important implications for the design of novel treatments for neurodegenerative diseases (Marchetti & Pluchino, 2013). An understanding of the intracellular signaling networks that dictate the intrinsic beneficial Wnt response is critical for the identification of new potential therapeutic targets and the development of pharmacological and cellular approaches for neurodegenerative diseases, including PD (Cusimano et al., 2012; Hoglinger et al., 2012; L’Episcopo et al., 2012b; Ribeiro et al., 2012; Rueger et al., 2012; Sakata et al., 2012; Shruster et al., 2012; Wallenquist et al., 2012; Marchetti & Pluchino, 2013).
Acknowledgements
The authors wish to thank the Italian Ministry of Health (Con. no. 82; Ps-CAR-DIO ex 56 and PS-NEURO ex 56 to B. Marchetti; Young Investigator Award 2009 to S. Pluchino), the Italian Ministry of Research (Current Research Program 2009-2012 to B. Marchetti), the Italian Multiple Sclerosis Foundation (FISM; grant 2004/R/15 to S. Pluchino), the Italian Ministry of Research and University (MIUR, to B. Marchetti), the European Research Council (Starting Independent Researcher Grant to S. Pluchino), Wings for Life (SE-013/09 to S. Pluchino), the Banca Agricola Popolare di Ragusa (BAPR; unrestricted grant to S. Pluchino), and the OASI (IRCCS) Institution for Research and Care on Mental Retardation and Brain Aging Troina (EN) Italy.
Abbreviations
- CNS
central nervous system
- DA
dopamine
- DAergic
dopaminergic
- DAT
DA transporter
- Dkk
Dkkopf
- Fzd
frizzled
- GFAP
glial fibrillary acid protein
- GSK-3β
glycogen synthase kinase-3β
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- Lrp
low-density lipoprotein receptor-related protein
- LRRK2
leucine-rich repeat kinase 2
- MB
midbrain/hindbrain
- MPP+
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NPC
stem/progenitor cell
- 6-OHDA
6-hydroxydopamine
- PCP
planar cell polarity
- PD
Parkinson’s disease
- SD
serum deprivation
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
- SVZ
subventricular zone
- TH
tyrosine hydroxylase
- VM
ventral midbrain
- Wnt
wingless-type mouse mammary tumor virus integration site
Footnotes
Conflict of interest The authors have no conflict of interest to declare.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Alvarez-Builla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:2287–2293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- van Amerongen R. Alternative wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012;4:pii: a007914. doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor cell proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K. LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6. Hum. Mol. Genet. 2012a;21:4966–4979. doi: 10.1093/hmg/dds342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K. The importance of Wnt signalling for neurodegeneration in Parkinson’s disease. Biochem. Soc. T. 2012b;40:1123–1128. doi: 10.1042/BST20120122. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog. Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function in motor neuron disease: a future therapeutic target? Glia. 2009;57:1251–1264. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]
- Borta A, Holinger GU. Dopamine and adult neurogenesis. J. Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Gennady L, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Keller-Mccgandy C, Bouziou B, Asteris G, Clark TW, Frosh MP, Standaert DG. Effects of gender on nigral gene expression and parkinson disease. Neurobiol. Dis. 2007;26:606–614. doi: 10.1016/j.nbd.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt1, Wnt3a, and Wnt5a. Proc. Natl. Acad. Sci. USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Rawal N, Arenas E. GSK-3β inhibition/β-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J. Cell Sci. 2004;117:5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol. Cell. Neurosci. 2006;31:251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Andersson ER, Minina E, Sousa KM, Ribeiro D, Kokubu C, Imai K, Prakash N, Wurst W, Arenas E. Delayed dopaminergic neuron differentiation in Lrp6 mutant mice. Dev. Dynam. 2010;239:211–221. doi: 10.1002/dvdy.22094. [DOI] [PubMed] [Google Scholar]
- Chacón MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J. Cell. Physiol. 2008;217:215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Non steroidal anti-inflammatory drugs and the risk of Parkinson’s disease. Ann. Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc. Natl. Acad. Sci. USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signalling pathways. Cell. Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Faull RLM, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat. Rev. Neurosci. 2007;8:712–723. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Low FL, Faull RLM. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev. Neurobiol. 2012;72:990–1005. doi: 10.1002/dneu.22028. [DOI] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, Donegà M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GF, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol. Psychiatr. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Stricker S, Ghobril JP, Oertel WH, Priller J, Höglinger GU. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 2012;238:183–191. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, Langston JW. Idiopathic and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University Press; NY: 1995. pp. 997–1019. [Google Scholar]
- Duka T, Duka V, Joyce JN, Sidhu A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT. Microglial activation: tuning and pruning adult neurogenesis. Front. Pharmacol. 2012;3:41. doi: 10.3389/fphar.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Engele J, Bohn MC. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J. Neurosci. 1991;11:3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb N, François C, Tandé D, Ortel WH, Hirsh EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo F, Morale MC, Avola R, Marchetti B. Cross-talk between luteinizing hormone-releasing hormone (LHRH) neurons and astroglial cells: developing glia release factors that accelerate neuronal differentiation and stimulate LHRH release from GT(1-1) neuronal cell line and LHRH neurons induce astroglia proliferation. Endocrine. 1995;3:863–874. doi: 10.1007/BF02738891. [DOI] [PubMed] [Google Scholar]
- Gallo F, Morale MC, Spina-Purrello V, Tirolo C, Testa N, Farinella Z, Avola R, Beaudet A, Marchetti B. Basic fibroblast growth factor (bFGF) acts on both neurons and glia to mediate the neurotrophic effects of astrocytes on LHRH neurons in culture. Synapse. 2000;36:233–253. doi: 10.1002/(SICI)1098-2396(20000615)36:4<233::AID-SYN1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglia NADPH-oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1966. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Kunihiro U, Leight S, Trojanowsski JQ, Lee VM-Y. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J. Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso F, Fernetti C, Tirolo C, Testa N, L’Episcopo F, Caniglia S, Morale MC, Ostrow JD, Pascolo L, Tiribelli C, Marchetti B. Bilirubin protects astrocytes from its own toxicity inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp 1) Proc. Natl. Acad. Sci. USA. 2004;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Harrison-Uy SJ, Pleasure SJ. Wnt signaling and forebrain development. Cold Spring Harb. Perspect. Biol. 2012;4:a008094. doi: 10.1101/cshperspect.a008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of β-catenin signaling reduces neurogenesis in Alzheimer’s disease. J. Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Nakayama H, Dong M, Yamauchi H, Ueno M, Uetsuka K, Doi K. Evidence of apoptosis in the subventricular zone and rostral migratory stream in the MPTP mouse model of Parkinson disease. J. Neuropath. Exp. Neur. 2006;65:873–882. doi: 10.1097/01.jnen.0000235115.29440.ce. [DOI] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Storch A. Endogenous regeneration in Parkinson’s disease: do we need orthotopic dopaminergic neurogenesis? Stem Cells. 2008;26:2749–2752. doi: 10.1634/stemcells.2008-0567. [DOI] [PubMed] [Google Scholar]
- Hermann A, Maisel M, Wegner F, Liebau S, Kim DW, Gerlach M. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949–964. doi: 10.1634/stemcells.2005-0192. [DOI] [PubMed] [Google Scholar]
- Hermann A, Suess C, Fauser M, Kanzler S, Witt M, Fabel K, Schwarz J, Höglinger GU, Storch A. Rostro-caudal loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells. 2009;27:928–941. doi: 10.1002/stem.21. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Ho A, Blum M. Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J. Neurosci. 1998;18:5614–5629. doi: 10.1523/JNEUROSCI.18-15-05614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Choi DK, Nagai M, Wu DC, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, Dawson TM, Chesselet MF, Przedborski S. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radical Bio. Med. 2009;47:1049–1056. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch E. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Barker RA, Hagg T, Arias-Carrion O, Caldwell MA, Hirsch EC. Quantitative evaluation of the human subventricular zone. Brain. 2012;e222:1–6. doi: 10.1093/brain/aws087. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD. New roles for astrocytes: the night life of an ‘astrocyte’. La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson’s disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Adv. Neurol. 1993;60:140–147. [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, Ali SF, Zhang J, Block ML, Hong JS. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J. Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging role of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Toledo EM. The role of Wnt signalling in neuronal dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2008;3:9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP model of Parkinson’s disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 2009;12:125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani MYS, Cheshir SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc. Natl. Acad. Sci. USA. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I. The subependymal zone neurogenic niche: a beating heart in the centre of the brain: how plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed. Brain. 2009;132:2909–2921. doi: 10.1093/brain/awp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kele J, Andersson ER, Villaescusa JC, Cajanek L, Parish CL, Bonilla S, Toledo EM, Bryja V, Rubin JS, Shimono A, Arenas E. SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells. 2012;30:865–875. doi: 10.1002/stem.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Yang MS, Choi D, Kim JH, Kim HS, Seol W, Choi S, Jou I, Kim EY, Joe EH. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS ONE. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Won S, Hwang DY, Lee JS, Kim M, Kim R, Kim W, Cha B, Kim T, Kim D, Costantini F, Jho EH. Downregulation of Wnt/β-catenin signalling causes degeneration of hippocampal neurons in vivo. Neurobiol. Aging. 2011;32:2316.e1–2316.e15. doi: 10.1016/j.neurobiolaging.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Kitagua H, Ray WJ, Glantsching H, Nantermet PV, Yu Y, Leu CT, Harada S, Kato S, Freedman LP. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signalling. Mol. Cell. Biol. 2007;27:7486–7496. doi: 10.1128/MCB.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashira M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JB, Hallupp M, Loy CT, Chan DK, Woo J, Mellick GD, Buchanan DD, Silburn PA, Halliday GM, Schofield PR. GSK3β polymorphism alter transcription and splicing in Parkinson’s disease. Ann. Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reevers AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lei ZNL, Zhang LM, Sun FY. β-Catenin siRNA inhibits ischemia-induced striatal neurogenesis in adult rat brain following a transient middle cerebral artery occlusion. Neurosci. Lett. 2008;435:108–112. doi: 10.1016/j.neulet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Glia as a turning point in the therapeutic strategy of Parkinson’s disease. CNS Neurol. Disord-Dr. 2010a;9:349–372. doi: 10.2174/187152710791292639. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Caniglia S, Testa N, Serra PA, Impagnatiello F, Morale MC, Marchetti B. Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neuroinflamm. 2010b;7:83. doi: 10.1186/1742-2094-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D’Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol. Dis. 2011a;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Serapide MF, Tirolo C, Testa N, Caniglia S, Morale MC, Pluchino S, Marchetti B. A Wnt1 regulated Frizzled-1/β-catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron–astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol. Neurodegener. 2011b;13:6–49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Marchetti B. Switching the microglial harmful phenotype promotes lifelong restoration of substantia nigra dopaminergic neurons from inflammatory neurodegeneration in aged mice. Rejuv. Res. 2011c;14:411–424. doi: 10.1089/rej.2010.1134. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Serapide MF, Deleidi M, Pluchino S, Marchetti B. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves crosstalk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J. Neurosci. 2012a;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Rejuvenated astrocytes and canonical Wnt signaling rescue midbrain stem/neuroprogenitors in ageing mouse model of Parkinson’s disease. Annual Meeting ‘Neuroscience 2012’; New Orleans. 13–17 October; 2012b. Abstract 2012-S-2668-SfN. [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone (SVZ) drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J. Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Wang LL, Liu SJ, Deng YQ, Zhang YJ, Tian YJ, Wang XC, Chen XQ, Yang Y, Zhang JY, Wang Q, Xu H, Liao FF, Wang JZ. Phosphorylation of tau antagonizes apoptosis by stabilizing β-catenin, a mechanism involved in Alzheimer’s degeneration. Proc. Natl. Acad. Sci. USA. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HG, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Diarie AR, Gage FH. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;473:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Faqi L, Chong ZZ, Chen SY. The Wnt signalling pathway: aging gracefully as a protectionist? Pharmacol. Therapeut. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin. Ther. Tar. 2012;16:1203–1214. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: key role of neuron-glia crosstalk. In: Giovanni GD, Esposito E, editors. The Basal Ganglia Pathology: Recent Advances. Transworld Research Network; Kerala, India: 2007. pp. 225–252. [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflamed): is that the question in anti-inflammatory drug therapy of neurodegenerative diseases? Trends Pharmacol. Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes it Wnt! Trends Mol. Med. 2013 doi: 10.1016/j.molmed.2012.12.001. doi: 10.1016/j.molmed.2012.12.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Kettenmann H, Streit WJ. Glia–neuron crosstalk in neuroinflammation, neurodegeneration and neuroprotection. Brain Res. Rev. 2005a;48:129–132. doi: 10.1016/j.brainresrev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Cioni S, Gennuso F, Rocchitta G, Desole MS, Mazzarino MC, Miele E, Morale MC. Hormones are key actors in gene × environment interactions programming the vulnerability to Parkinson’s disease: glia as a common final pathway. Ann. NY Acad. Sci. 2005b;1057:296–318. doi: 10.1196/annals.1356.023. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, Tirolo C, L’Episcopo F, Caniglia S, Gennuso F, Testa N, Miele E, Desole S, Barden N, Morale MC. Glucocorticoid receptor–nitric oxide crosstalk and vulnerability to experimental Parkinsonism: pivotal role for glia–neuron interactions. Brain Res. Rev. 2005c;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Marchetti B, L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC. Vulnerability to Parkinson’s disease: towards a unifying theory of disease etiology. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier Science; London: 2011. pp. 690–704. [Google Scholar]
- Marchetti B, Serapide MF, L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC. Wnt/β-catenin signaling controls the vulnerability of midbrain dopaminergic neurons and spontaneous degeneration in aged mice: critical role of astrocyte–neuron crosstalk. Annual Meeting ‘Neuroscience 2012’; New Orleans, USA. 13–17 October; 2012. Abstract 2012-S-4124-SFN. [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Movement Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Jenner P. Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J. Neurochem. 1999;73:2469–2476. doi: 10.1046/j.1471-4159.1999.0732469.x. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effect of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra P, Delogu MR, Migheli R, Rocchitta G, Tirolo C, Caniglia S, Testa N, L’Episcopo F, Gennuso F, Scoto GM, Barden N, Miele E, Desole MS, Marchetti B. Glucocorticoid receptor deficiency increases vulnerability of the nigrostriatal dopaminergic system: critical role of glial nitric oxide. FASEB J. 2004;18:164–166. doi: 10.1096/fj.03-0501fje. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS, Miele E, Marchetti B. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Morale MC, L’Episcopo F, Tirolo C, Giaquinta G, Caniglia S, Testa N, Arcieri P, Serra PA, Lupo G, Alberghina M, Harada N, Honda S, Panzica GC, Marchetti B. Loss of aromatase cytochrome P450 function as a risk factor for Parkinson’s disease? Brain Res. Rev. 2008;57:431–443. doi: 10.1016/j.brainresrev.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signalling responses. J. Biol. Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and agent rodent brain reveal age-related changes in microglial function. Neurobiol. Aging. 2012;33:195e1–195e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T, Nakamura A, Okuyama S, Nakamura S. Gene expression profiling reveals molecular pathways associated with sporadic Parkinson’s disease. Brain Res. 2010;1346:26–42. doi: 10.1016/j.brainres.2010.05.066. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GO, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc. Natl. Acad. Sci. USA. 2009;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Shapira AHV, Agid Y. Neurodegeneration and prospects for neuroprotection and rescue in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl 3) doi: 10.1002/ana.10566. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signalling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourednik VC, Ourednik J, Xu Y, Zhang Y, Lynch WP, Snyder EY, Schachner M. Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: evidence from an adhesion molecule. Stem Cells. 2009;27:2846–2856. doi: 10.1002/stem.227. [DOI] [PubMed] [Google Scholar]
- Papanikolaou T, Lennington JB, Betz A, Figueiredo C, Salomone JD, Conover JC. In vitro generation of dopaminergic neurons from adult subventricular zone neural progenitor cells. Stem Cells Dev. 2008;17:157–172. doi: 10.1089/scd.2007.0090. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr. Opin. Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvement of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/Mpp+-treated neurons. PLoS ONE. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Canotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, Garcia-Verdugo JM, Comi G, Khoury SJ, Martino G. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J. Physiol. 2006;575:403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Movement Disord. 2010;25:S55–S57. doi: 10.1002/mds.22638. [DOI] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. J. Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal N, Castelo-Branco G, Souse KM, Kele J, Kobayashy K, Okano H, Arenas E. Dynamic temporal and cell type-specific expression of Wnt signaling components in the developing midbrain. Exp. Cell Res. 2006;312:1626–1636. doi: 10.1016/j.yexcr.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Rawal N, Corti O, Sacchetti P, Ardilla-Osorio H, Sehat B, Brice A, Arenas E. Parkin protects dopaminergic neurons from excessive Wnt/β-catenin signalling. Biochem. Bioph. Res. Co. 2009;388:473–478. doi: 10.1016/j.bbrc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Laguna Goya R, Ravindran G, Vuono R, Parish CL, Foldi C, Piroth T, Yang S, Parmar M, Nikkhah G, Hjerling-Leffler J, Lindvall O, Barker RA, Arenas E. Efficient expansion and dopaminergic differentiation of human fetal ventral midbrain neural stem cells by midbrain morphogens. Neurobiol. Dis. 2012;49C:118–127. doi: 10.1016/j.nbd.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Irwin I, Forno LS, DeLanney LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403:43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Rueger MA, Muesken S, Walberer M, Jantzen SU, Schnakenburg K, Backes H, Graf R, Neumaier B, Hoehn M, Fink GR, Schroeter M. Effects of minocycline on endogenous neural stem cells after experimental stroke. Neuroscience. 2012;215:174–183. doi: 10.1016/j.neuroscience.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb. Perspect. Biol. 2012;4:pii: a008003. doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Schwartz M, London A, Shecter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–1142. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Shibui Y, He XJ, Uchida K, Nakayama H. MPTP-induced neuroblast apoptosis in the subventricular zone is not regulated by dopamine or other monoamine transporters. Neurotoxicology. 2009;30:1036–1044. doi: 10.1016/j.neuro.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Shruster A, Eldar-Finkelman H, Melamed E, Offen D. Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid β-peptide. J. Neurochem. 2011;116:522–529. doi: 10.1111/j.1471-4159.2010.07131.x. [DOI] [PubMed] [Google Scholar]
- Shruster A, Ben-Zur T, Melamed E, Offen D. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS ONE. 2012;7:e40843. doi: 10.1371/journal.pone.0040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirerol-Piquer M, Gomez-Ramos P, Hernández F, Perez M, Morán MA, Fuster-Matanzo A, Lucas JJ, Avila J, García-Verdugo JM. GSK3β overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus. 2011;21:910–922. doi: 10.1002/hipo.20805. [DOI] [PubMed] [Google Scholar]
- Sleiman PM, Healy DG, Mugit MM, Yang YX, Van Der Brug M, Holton JL, Revesz T, Quinn NP, Bhatia K, Diss JK, Less AJ, Cookson MR, Latchman DS, Wood NW. Characterization of a novel NR4A2 mutation in Parkinson’s disease. Neurosci. Lett. 2009;457:75–79. doi: 10.1016/j.neulet.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M, Vinters HB. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front. Aging Neurosci. 2010;3:2–22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima T, Johnston JM, Commissiong JW. Mesencephalic type 1 astrocytes rescue dopaminergic neurons from death induced by serum deprivation. J. Neurosci. 1994;14:4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Miyamoto Y, Huang EJ. Multiple roles of β-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development. 2009;136:2027–2038. doi: 10.1242/dev.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Villaescusa JC, Luo SX, Guitarte C, Lei S, Miyamoto Y, Takedo MM, Arenas E, Huang EJ. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J. Neurosci. 2010;7:9280–9291. doi: 10.1523/JNEUROSCI.0860-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Clombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 2008;88:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu D, Teismann P, Choi DK, Tieu K, Przedborski S. The role of glial cells in Parkinson’s disease. Curr. Opin. Neurol. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson R, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Akerud DS, Castro PC, Holm JM, Canals EY, Snyder T, Perlmann T, Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat. Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- Wallenquist U, Holmqvist K, Hånell A, Marklund N, Hillered L, Forsberg-Nilsson K. Ibuprofen attenuates the inflammatory response and allows formation of migratory neuroblasts from grafted stem cells after traumatic brain injury. Restor. Neurol. Neuros. 2012;30:9–19. doi: 10.3233/RNN-2011-0606. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Warner TT, Schapira AHV. Genetic and environmental factors in the cause of Parkinson’s disease. Ann. Neurol. 2003;53:S16–S25. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol. Psychiatr. 2008;13:285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Brit. J. Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JM, Kim JH, Song GS, Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang X, Yang S, Zhang J. The Wnt/β-catenin signaling pathway in adult neurogenesis. Eur. J. Neurosci. 2011;33:1–8. doi: 10.1111/j.1460-9568.2010.7483.x. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Khan M, Maesh V, Brann DW. Role of Dikkopf-1 (Dkk1), an antagonist of the Wnt–β-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of Tau phosphorylation. J. Neurosci. 2009;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak K, Smejne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. D. 2009;15(S3):S42–S45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat. D. 2012;18(Suppl 1):S147–S150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]