Abstract
The activities of certain enzymes related to the carbon assimilation pathway in whole leaves, mesophyll cell extracts, and bundle sheath extracts of the C4 plant Panicum miliaceum have been measured and compared on a chlorophyll basis. Enzymes of the C4 dicarboxylic acid pathway—phosphoenolpyruvate carboxylase and NADP-malic dehydrogenase—were localized in mesophyll cells. Carbonic anhydrase was also localized in mesophyll cell extracts. Ribose 5-phosphate isomerase, ribulose 5-phosphate kinase, and ribulose diphosphate carboxylase—enzymes of the reductive pentose phosphate pathway—were predominantly localized in bundle sheath extracts. High activities of aspartate and alanine transaminases and glyceraldehyde-3-P dehydrogenase were found about equally distributed between the photosynthetic cell types. P. miliaceum had low malic enzyme activity in both mesophyll and bundle sheath extracts.
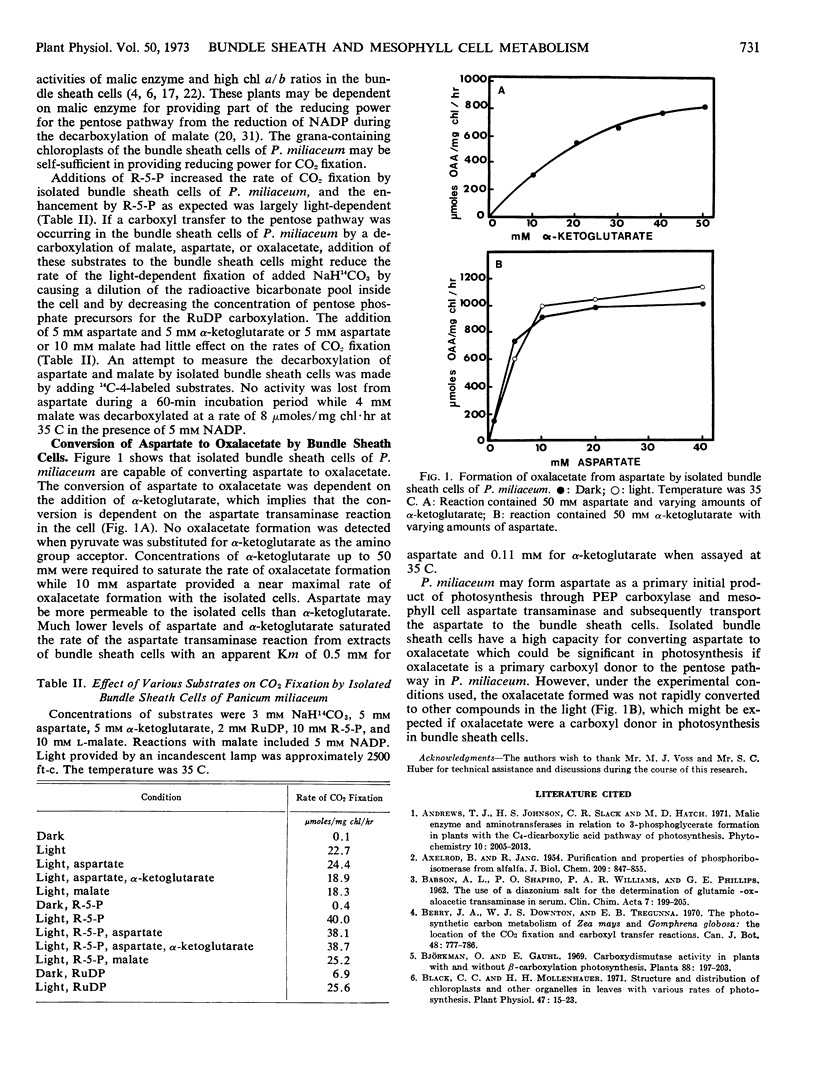
Isolated bundle sheath cells were capable of converting aspartate to oxalacetate at rates approaching the aspartate transaminase activity of bundle sheath extracts. The bundle sheath cells had a light induced CO2 fixation of 23 μmoles of CO2/mg chl·hr in the absence of exogenous substrates.
The photorespiratory enzymes, hydroxypyruvate reductase and glycolic oxidase, were about 3 fold higher in bundle sheath extracts than in mesophyll extracts when compared on a chlorophyll basis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., JANG R. Purification and properties of phosphoribo-isomerase from alfalfa. J Biol Chem. 1954 Aug;209(2):847–855. [PubMed] [Google Scholar]
- BABSON A. L., SHAPIRO P. O., WILLIAMS P. A., PHILLIPS G. E. The use of a diazonium salt for the determination of glutamic-oxalacetic transaminase in serum. Clin Chim Acta. 1962 Mar;7:199–205. doi: 10.1016/0009-8981(62)90010-4. [DOI] [PubMed] [Google Scholar]
- Black C. C., Mollenhauer H. H. Structure and distribution of chloroplasts and other organelles in leaves with various rates of photosynthesis. Plant Physiol. 1971 Jan;47(1):15–23. doi: 10.1104/pp.47.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic CO(2) Fixation Products and Activities of Enzymes Related to Photosynthesis in Bermudagrass and Other Plants. Plant Physiol. 1971 Feb;47(2):199–203. doi: 10.1104/pp.47.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R., Oglen W. L. Oxygen inhibits maize bundle sheath photosynthesis. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2062–2066. doi: 10.1016/0006-291x(72)90759-0. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Wood H. G. The carboxylation of phosphoenolpyruvate and pyruvate. II. The active species of "CO2" utilized by phosphoenlpyruvate carboxylase and pyruvate carboxylase. J Biol Chem. 1971 Sep 10;246(17):5488–5490. [PubMed] [Google Scholar]
- Edwards G. E., Black C. C. Isolation of Mesophyll Cells and Bundle Sheath Cells from Digitaria sanguinalis (L.) Scop. Leaves and a Scanning Microscopy Study of the Internal Leaf Cell Morphology. Plant Physiol. 1971 Jan;47(1):149–156. doi: 10.1104/pp.47.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Kanai R., Black C. C. Phosphoenolpyruvate carboxykinase in leaves of certain plants whick fix CO 2 by the C 4 -dicarboxylic acid cycle of photosynthesis. Biochem Biophys Res Commun. 1971 Oct 15;45(2):278–285. doi: 10.1016/0006-291x(71)90814-x. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicki G. W. Oxaloacetate decarboxylase from cod. A shorter preparation and crystallization. Biochemistry. 1968 Dec;7(12):4299–4302. doi: 10.1021/bi00852a022. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Harris B. Photorespiration during C 4 photosynthesis. Biochim Biophys Acta. 1971 May 11;234(2):270–282. doi: 10.1016/0005-2728(71)90082-x. [DOI] [PubMed] [Google Scholar]
- SCRUTTON M. C., KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. IV. PARTIAL REACTIONS AND THE LOCUS OF ACTIVATION BY ACETYL COENZYME A. J Biol Chem. 1965 Feb;240:574–581. [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. R. Oxalacetate decarboxylase of Aerobacter aerogenes. I. Inhibition by avidin and requirement for sodium ion. Biochemistry. 1967 Nov;6(11):3545–3551. doi: 10.1021/bi00863a028. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. The effects of various vitamin B6 5'-phosphate derivatives on the structure and activities of L-aspartate beta-decarboxylase. Biochemistry. 1969 Mar;8(3):1056–1065. doi: 10.1021/bi00831a037. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Davis J. J., Willard J. M. Phosphoenolpyruvate carboxytransphosphorylase. V. Mechanism of the reaction and the role of metal ions. Biochemistry. 1969 Aug;8(8):3145–3155. doi: 10.1021/bi00836a003. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]


