Abstract
Evaluation of various solvent systems for lipid extraction of wheat Triticum aestivum L. cv. Rideau seeds showed that boiling 2-propanol followed by the Bligh-Dyer procedure was the most efficient method, with respect to lipid yield and ability to inactivate lipolytic enzymes. Ten phospholipids were identified in dry seeds; the major components being phosphatidylcholine, lysophosphatidylcholine, N-acyl lysophosphatidyl-ethanolamine, N-acylphosphatidylethanolamine, and phosphatidylethanolamine. After growth for 1 week (2 C) or 31 hours (24 C), the proportions of phosphatidylethanolamine + lysophosphatidic acid and phosphatidic acid increased, lysophosphatidylcholine decreased, and the remaining phospholipids showed little change. At 5 weeks (2 C) or 72 hours (24 C), the seedlings showed 5-fold increases in the proportion of phosphatidic acid largely at the expense of phosphatidylcholine, small decreases in N-acyl lysophosphatidylethanolamine and N-acylphosphatidylethanolamine, and significant increases in lysophosphatidylcholine. The changes in phosphatidic acid and phosphatidylcholine are interpreted as being partially due to increasing phospholipase D activity during germination. In general, the phospholipid composition was similar in morphologically equivalent seedlings grown at 2 C or 24 C. The increased membrane content in seedlings grown at 2 C does not reflect any preferential synthesis of individual phospholipids.
Full text
PDF


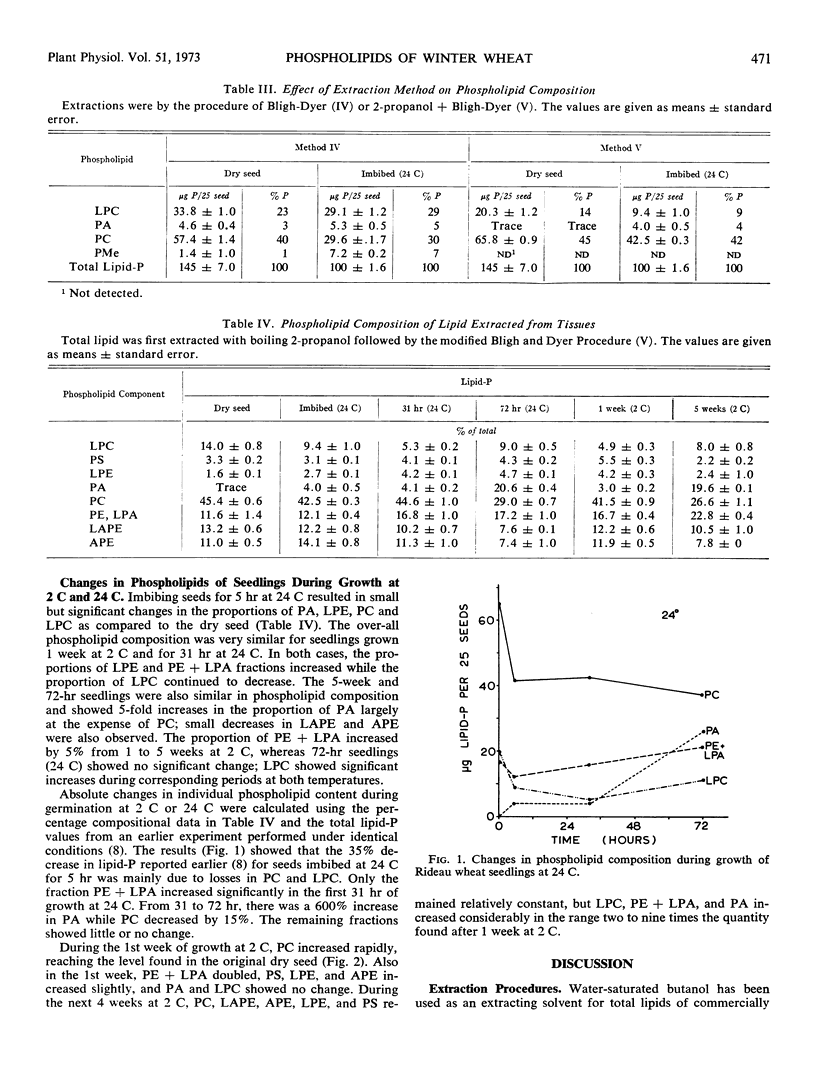
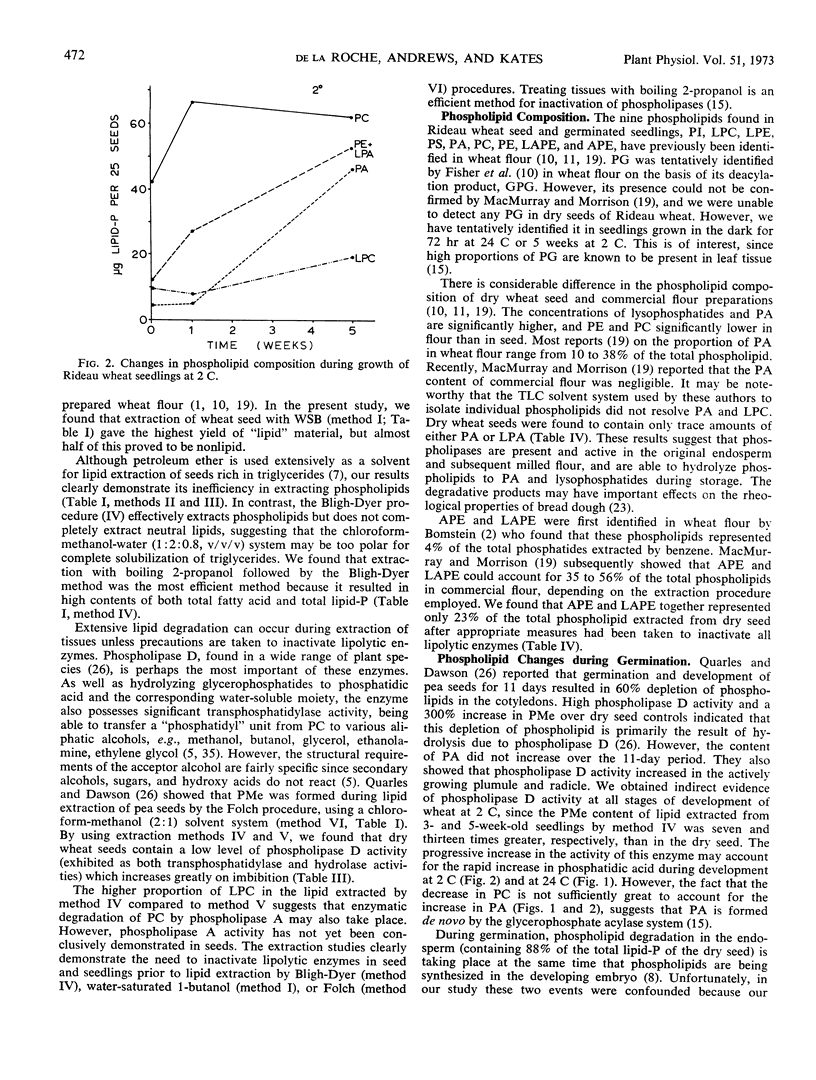

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomstein R. A. A new class of phosphatides isolated from soft wheat flour. Biochem Biophys Res Commun. 1965 Oct 8;21(1):49–54. doi: 10.1016/0006-291x(65)90424-9. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Dawson R. M., Clarke N., Quarles R. H. N-acylphosphatidylethanolamine, a phospholipid that is rapidly metabolized during the arly germnation of pea seeds. Biochem J. 1969 Sep;114(2):265–267. doi: 10.1042/bj1140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967 Jan;102(1):205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland A. Combination of thin layer chromatography and gas chromatography in the analysis on a microgram scale of lipids from wheat flour and wheat flour doughs. J Am Oil Chem Soc. 1968 Dec;45(12):834–840. doi: 10.1007/BF02540164. [DOI] [PubMed] [Google Scholar]
- Harris P., James A. T. The effect of low temperatures on fatty acid biosynthesis in plants. Biochem J. 1969 Apr;112(3):325–330. doi: 10.1042/bj1120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C., Odavić R., Sargent E. J. The chemical nature of the products obtained by the action of cabbage-leaf phospholipase D on lysolecithin: the structure of lysolecithin. Biochem J. 1967 Jan;102(1):221–229. doi: 10.1042/bj1020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of phosphatidylglycerol by cell-free preparations from spinach leaves. Biochim Biophys Acta. 1972 Apr 18;260(4):558–570. doi: 10.1016/0005-2760(72)90005-7. [DOI] [PubMed] [Google Scholar]
- Neskovic N. M., Kostic D. M. Quantitative analysis of rat liver phospholipids by a two-step thin-layer chromatographic procedure. J Chromatogr. 1968 Jun 4;35(2):297–300. doi: 10.1016/s0021-9673(01)82389-x. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Clarke N., Dawson R. M. Isolation of n-acyl phosphatidylethanolamine from pea seeds. Biochem Biophys Res Commun. 1968 Dec 30;33(6):964–968. doi: 10.1016/0006-291x(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON T., TAPPEL A. L. Swelling of fish mitochondria. J Cell Biol. 1962 Apr;13:43–53. doi: 10.1083/jcb.13.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redshaw E. S., Zalik S. Changes in lipids of cereal seedlings during vernalization. Can J Biochem. 1968 Sep;46(9):1093–1097. doi: 10.1139/o68-163. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


