Abstract
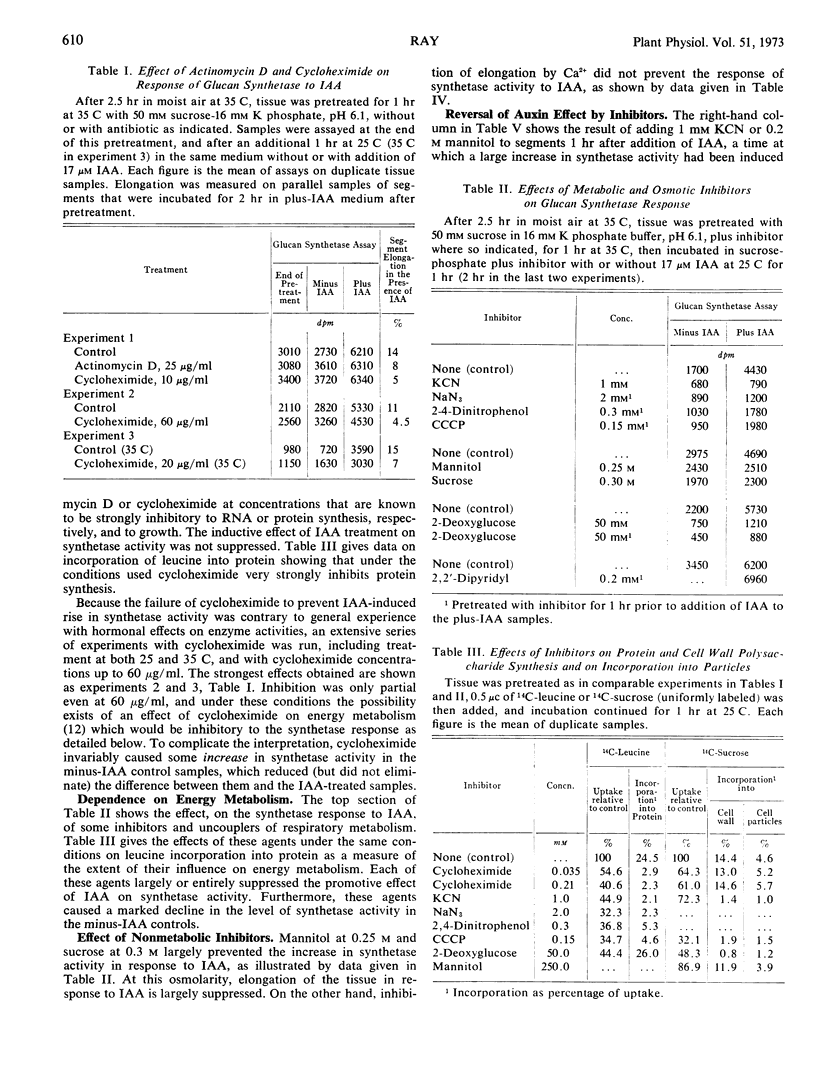
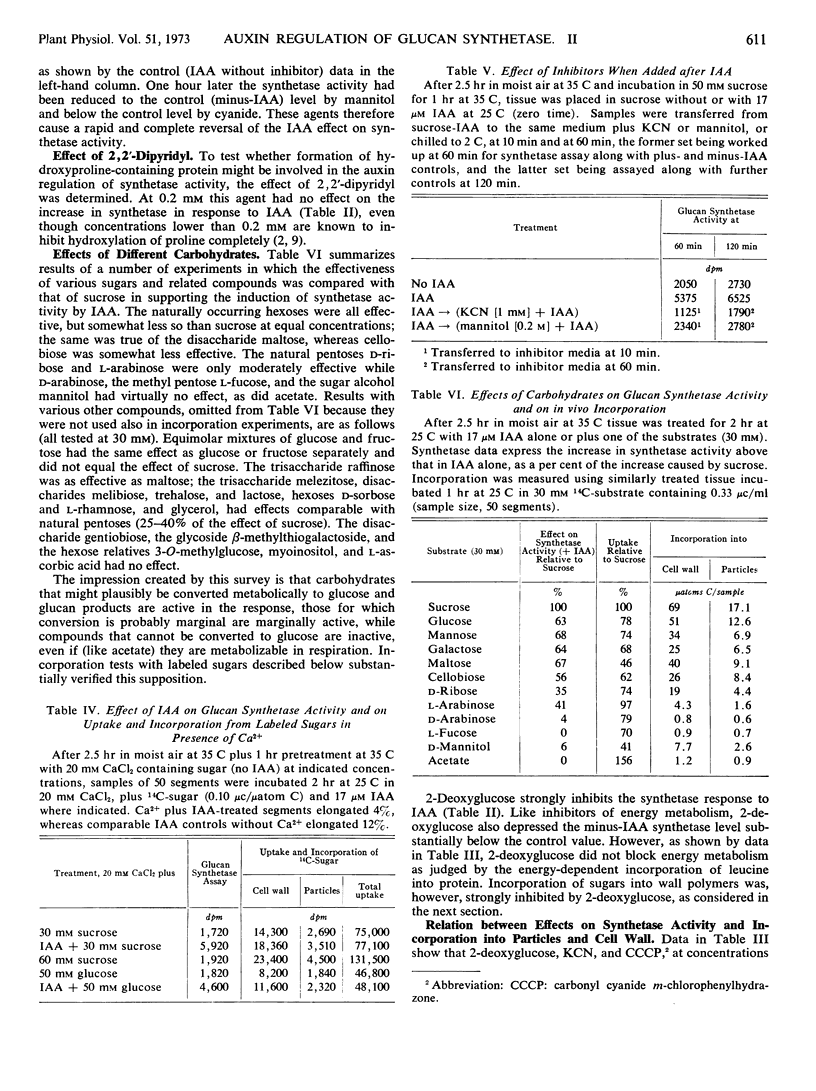
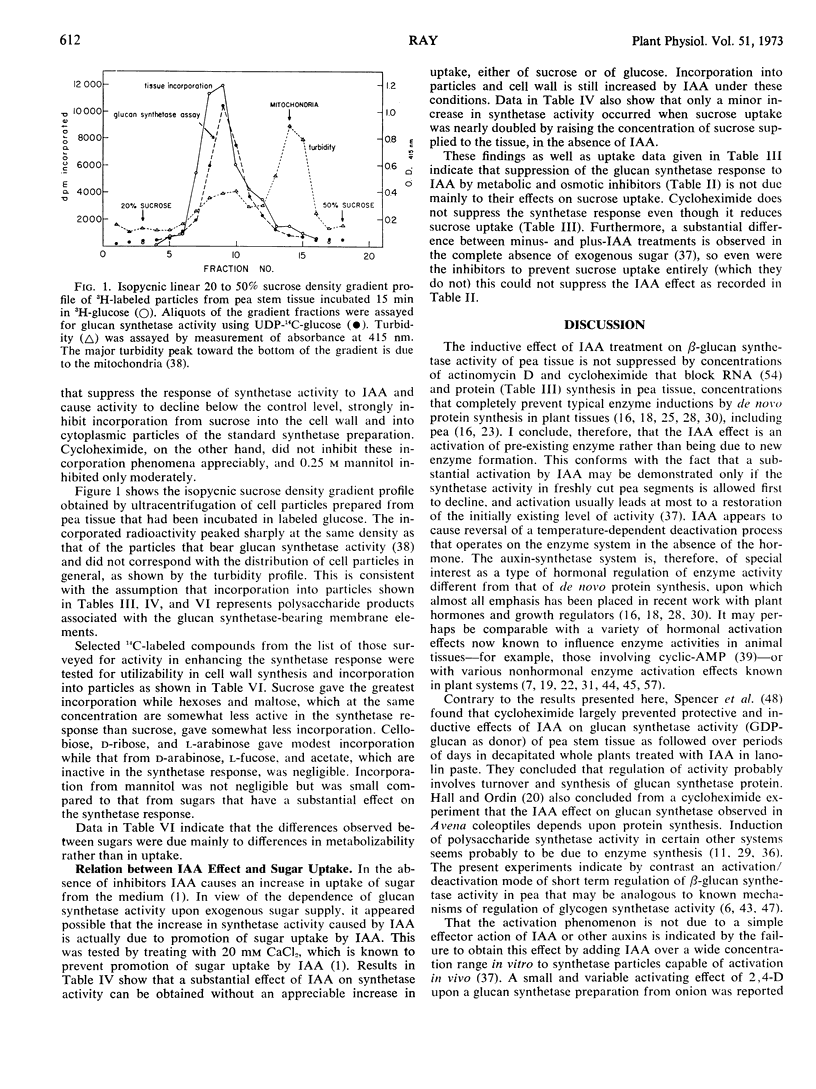
The 2- to 4-fold rise in particle-bound β-glucan synthetase (uridine diphosphate-glucose: β-1, 4-glucan glucosyltransferase) activity that can be induced by indoleacetic acid in pea stem tissue is not prevented by concentrations of actinomycin D or cycloheximide that inhibit growth and macromolecule synthesis. The rise is concluded to be a hormonally induced activation of previously existing, reversibly deactivated enzyme. The activation is not a direct allosteric effect of indoleacetic acid or sugars. It is blocked by inhibitors of energy metabolism, by 2-deoxyglucose, and by high osmolarity, but not by Ca2+ at concentrations that inhibit auxin-induced elongation and prevent promotion of sugar uptake by indoleacetic acid, and not by α, α′-dipyridyl at concentrations that inhibit formation of hydroxyproline. Regulation of the system could be due either to an ATP-dependent activating reaction affecting this enzyme, or to changes in levels of a primer or a lipid cofactor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett N. M. Dipyridyl-induced Cell Elongation and Inhibition of Cell Wall Hydroxyproline Biosynthesis. Plant Physiol. 1970 Feb;45(2):188–191. doi: 10.1104/pp.45.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F., Krisman C. R. The role of dolichol monophosphate in sugar transfer. Arch Biochem Biophys. 1971 Apr;143(2):375–383. doi: 10.1016/0003-9861(71)90224-4. [DOI] [PubMed] [Google Scholar]
- Biely P., Krátký Z., Kovarík J., Bauer S. Effect of 2-deoxyglucose on cell wall formation in Saccharomyces cerevisiae and its relation to cell growth inhibition. J Bacteriol. 1971 Jul;107(1):121–129. doi: 10.1128/jb.107.1.121-129.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt L. M., Kim K. H. Regulation of hepatic glycogen synthetase. Stimulation of glycogen synthetase in an in vitro liver system by insulin bound to sepharose. J Biol Chem. 1971 Aug 25;246(16):4895–4898. [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- Camargo E. P., Meuser R., Sonneborn D. Kinetic analyses of the regulation of glycogen synthetase activity in zoospores and growing cells of the water mold, Blastocladiella emersonii. J Biol Chem. 1969 Nov 10;244(21):5910–5919. [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and Secretion of Hydroxyproline-containing Macromolecules in Carrots: II. In vivo Conversion of Peptidyl Proline to Peptidyl Hydroxyproline. Plant Physiol. 1970 Feb;45(2):223–227. doi: 10.1104/pp.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler T. C. Effects of deoxyglucose on platelet metabolism. Biochim Biophys Acta. 1971 Aug 19;244(2):303–310. doi: 10.1016/0304-4165(71)90230-3. [DOI] [PubMed] [Google Scholar]
- Ellingson J. S., Telser A., Sussman M. Regulation of functionally related enzymes during alternative developmental programs. Biochim Biophys Acta. 1971 Aug 19;244(2):388–395. doi: 10.1016/0304-4165(71)90241-8. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. Synthesis of a beta-1, 3-linked glucan by extracts of Phaseolus aureus seedlings. J Biol Chem. 1958 Oct;233(4):783–788. [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Transglycosylic reactions of nucleotides of 2-deoxy-sugars. I. Biosynthesis of 2-deoxysucrose. Biochim Biophys Acta. 1968 Aug 6;165(1):63–67. doi: 10.1016/0304-4165(68)90188-8. [DOI] [PubMed] [Google Scholar]
- Farkas V., Svoboda A., Bauer S. Secretion of cell-wall glycoproteins by yeast protoplasts. Effect of 2-deoxy-D-glucose and cycloheximide. Biochem J. 1970 Aug;118(5):755–758. doi: 10.1042/bj1180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Studies on the mechanism of activation and inactivation of pyruvate, phosphate dikinase. A possible regulatory role for the enzyme in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 May;112(5):549–558. doi: 10.1042/bj1120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H., Yang S. F. Ethylene-enhanced Synthesis of Phenylalanine Ammonia-Lyase in Pea Seedlings. Plant Physiol. 1971 Jun;47(6):765–770. doi: 10.1104/pp.47.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F. Lysis of yeast cell walls induced by 2-deoxyglucose at their sites of glucan synthesis. J Bacteriol. 1968 Mar;95(3):1169–1172. doi: 10.1128/jb.95.3.1169-1172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F. A., Cabib E. Chitin and yeast budding. Properties of chitin synthetase from Saccharomyces carlsbergensis. J Biol Chem. 1971 Jan 10;246(1):160–166. [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- McVerry P., Kim K. H. Diurnal rhythm of rat liver glycogen synthetase. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1242–1246. doi: 10.1016/s0006-291x(72)80108-6. [DOI] [PubMed] [Google Scholar]
- Müller B., Ziegler I., Ziegler H. Lichtinduzierte, reversible Aktivtätssteigerung der NADP-abhängigen Glycerinaldehyd-3-phosphat-Dehydrogenase in Chloroplasten. Zum mechanismus der reaktion. Eur J Biochem. 1969 May 1;9(1):101–106. doi: 10.1111/j.1432-1033.1969.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Porter C. A., Jaworski E. G. The synthesis of chitin by particulate preparations of Allomyces macrogynus. Biochemistry. 1966 Apr;5(4):1149–1154. doi: 10.1021/bi00868a006. [DOI] [PubMed] [Google Scholar]
- Potter J. L., Weisman R. A. Differentiation in Acanthamoeba: beta-glucan synthesis during encystment. Biochim Biophys Acta. 1971 Apr 20;237(1):65–74. doi: 10.1016/0304-4165(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Regulation of beta-Glucan Synthetase Activity by Auxin in Pea Stem Tissue: I. Kinetic Aspects. Plant Physiol. 1973 Apr;51(4):601–608. doi: 10.1104/pp.51.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Glucose 6-phosphate dependent and independent forms of yeast glycogen synthetase. Their properties and interconversions. Biochemistry. 1971 Mar 30;10(7):1236–1242. doi: 10.1021/bi00783a021. [DOI] [PubMed] [Google Scholar]
- Rothman L. B., Cabib E. Allosteric properties of yeast glycogen synthetase. I. General kinetic study. Biochemistry. 1967 Jul;6(7):2098–2112. doi: 10.1021/bi00859a030. [DOI] [PubMed] [Google Scholar]
- Soderling T. R., Hickenbottom J. P., Reimann E. M., Hunkeler F. L., Walsh D. A., Krebs E. G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1970 Dec 10;245(23):6317–6328. [PubMed] [Google Scholar]
- Spencer F. S., Shore G., Ziola B., Maclachlan G. A. Particulate glucan synthetase activity: generation and inactivation after treatments with indoleacetic acid and cycloheximide. Arch Biochem Biophys. 1972 Sep;152(1):311–317. doi: 10.1016/0003-9861(72)90220-2. [DOI] [PubMed] [Google Scholar]
- Spencer F. S., Ziola B., Maclachlan G. A. Particulate glucan synthetase activity: dependence on acceptor, activator, and plant growth hormone. Can J Biochem. 1971 Dec;49(12):1326–1332. doi: 10.1139/o71-192. [DOI] [PubMed] [Google Scholar]
- Tanner W., Jung P., Behrens N. H. Dolicholmonophosphates: Mannosyl acceptors in a particulate in vitro system of S. cerevisiae. FEBS Lett. 1971 Sep 1;16(4):245–248. doi: 10.1016/0014-5793(71)80361-7. [DOI] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]
- Villemez C. L. Characterization of intermediates in plant cell wall biosynthesis. Biochem Biophys Res Commun. 1970 Aug 11;40(3):636–641. doi: 10.1016/0006-291x(70)90951-4. [DOI] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Warner H. L., Leopold A. C. Timing of growth regulator responses in peas. Biochem Biophys Res Commun. 1971 Aug 20;44(4):989–994. doi: 10.1016/0006-291x(71)90809-6. [DOI] [PubMed] [Google Scholar]
- Wildner G. F., Criddle R. S. Ribulose diphosphate carboxylase. I. A factor involved in light activation of the enzyme. Biochem Biophys Res Commun. 1969 Dec 4;37(6):952–960. doi: 10.1016/0006-291x(69)90223-x. [DOI] [PubMed] [Google Scholar]
- Zemek J., Farkas V., Biely P., Bauer S. Transglycosylic reactions of nucleotides of 2-deoxy-sugars. II. 2-Deoxyglucose incorporation into glycogen. Biochim Biophys Acta. 1971 Dec 21;252(3):432–438. doi: 10.1016/0304-4165(71)90145-0. [DOI] [PubMed] [Google Scholar]


