Abstract
Pathogenic Escherichia coli strains carrying the afa-8 gene cluster are frequently associated with extra-intestinal infections in humans and animals. The afa-8 A to E genes determine the formation of an afimbrial adhesive sheath consisting of the AfaD-VIII invasin and the AfaE-VIII adhesin at the bacterial cell surface. This structure is thought to be required for host colonization. We characterized a new gene encoding the small RNA AfaR, which is transcribed in cis from the complementary strand of the 3′ untranslated region of the afaD messenger RNA, within the afaD–afaE intercistronic region. AfaR is a trans-acting Hfq-dependent antisense small RNA that binds the 5′ untranslated region of the afaD messenger RNA, initiating several ribonuclease E-dependent cleavages, thereby downregulating production of the AfaD-VIII invasin. AfaR transcription is dependent on σE, a member of the stress response family of extracytoplasmic alternative sigma factors. We found that the AfaR-dependent regulatory pathway was controlled by temperature, allowing the production of the AfaD-VIII invasin at temperatures above 37°C. Our findings suggest that the entry of afa-8-positive pathogenic E. coli strains into epithelial cells is tightly regulated by the AfaR small RNA.
INTRODUCTION
Escherichia coli has developed a commensal lifestyle in the lower part of the intestine of humans and other vertebrates, but some strains have the potential to cause a wide spectrum of intestinal and extra-intestinal diseases. Extra-intestinal pathogenic E. coli (ExPEC) strains cause bacteraemia, pyelonephritis, cystitis, prostatitis and neonatal meningitis in humans, and various infections in animals. The virulence of ExPEC strains is largely associated with the presence of virulence factors, including adhesins, toxins, siderophores, capsules, invasins and factors contributing to serum resistance (1,2). During the infection process, ExPEC strains must rapidly adapt the expression of their genes in response to both environmental and host signals. A plethora of sensory systems for activating or repressing the expression of virulence genes has thus coevolved with virulence factors.
In regulatory cascades, sensory systems and effectors may be connected by proteins. However, small non-coding RNAs (sRNAs) have recently emerged as a major class of gene regulators of adaptive responses, relaying information from physicochemical stress ‘sensing’ components to response genes at the posttranscriptional level, without the need for translation (3). A significant proportion of the sRNAs characterized to date interact with dedicated mRNA targets through an antisense-based mechanism, affecting their stability and/or translation (4). Each sRNA is thought to regulate the expression of more than one target, depending on its temporal pattern of expression, thus rendering the system even more complex.
The genome-wide identification of new sRNAs in both gram-negative and gram-positive pathogenic bacteria, by biochemical and in silico approaches and, more recently, by high-throughput sequencing analyses, has highlighted the diversity and role of sRNAs (3,5–7). In particular, virulence-associated sRNA genes are abundant in the core genome and pathogenicity islands of the human pathogens Staphylococcus aureus (8–11), Streptococcus agalactiae (6), Streptococcus pyogenes (12), Salmonella typhimurium (13,14), Listeria monocytogenes (15), Clostridrium perfringens (16) and Vibrio cholerae (17). However, despite several investigations reporting the identification of new sRNAs in E. coli, only a few virulence-associated sRNAs from pathogenic strains have been characterized to date. Studies of the role of the Hfq protein, which facilitates gene regulation by sRNAs, showed virulence to be strongly impaired in hfq-deficient E. coli strains. These strains displayed defects in urinary tract colonization, motility and biofilm production [for uropathogenic E. coli UTI89 (18)], together with an impairment of epithelial cell invasion in the case of adherent-invasive E. coli LF82 (19). These data suggest that several sRNAs are involved in the control of virulence factor expression by pathogenic E. coli/Shigella species. However, these molecules have been little studied, other than in quorum sensing (20), icsA-dependent invasion of the intestine (21) and type 1-mediated adhesion (6).
During host colonization, ExPEC strains produce several adhesins, including afimbrial adhesins of the Afa family (1,22). All afa gene clusters are organized in a similar manner, with six genes (afaA to F) (1) determining the formation at the cell surface of an adhesive sheath consisting of the AfaD invasin and the AfaE adhesin. These proteins are encoded by the afaD and afaE genes, and are responsible for bacterial internalization and binding to the host cell, respectively (23–26). Both these structural components are assembled via the chaperone–usher pathway, which is mediated by the proteins encoded by the afaB and afaC genes (27,28). Finally, the expression of afa gene clusters may be controlled by the afaF and afaA genes, which are homologues of the papI/papB transcriptional regulator genes (27,29,30). Various subtypes of the afa gene cluster (afa-1 to afa-8, dra, daa) have been described, on the basis of comparisons of the sequences of the afa gene cluster, focusing on afaE genes in particular (22). The afa-8 gene cluster is common in pathogenic afa-carrying strains isolated from humans and animals (30,31). ExPEC strains carrying afa-8 gene clusters are frequently detected in humans suffering from pyelonephritis and septicaemia, but these clusters are absent from diarrhoea-associated strains (30). A recent screening for ExPEC-specific sRNA genes showed that some such genes were present in adhesin gene clusters, including the type 1 fimbria operon (6). In particular, a chromosome-borne sRNA candidate gene, SQ109, located in the afa-8 gene cluster of the E. coli AL862 strain, has been identified as a putative riboregulator (6).
We show here that SQ109 sRNA downregulates AfaD-VIII invasin production by acting as an Hfq-dependent antisense sRNA, inducing RNase E-dependent afaD mRNA decay. The expression of this sRNA gene is dependent on the sigma factor σE and temperature, suggesting that AfaD-VIII production is controlled by the environment of the bacterial cell. Based on its location within the afa-8 gene cluster, we renamed this sRNA gene AfaR.
MATERIALS AND METHODS
Strains, plasmids and growth conditions
The E. coli strains and plasmids used in this study are listed in Table 1. All strains were grown in Luria Bertani (LB) broth, at 37°C, with continuous shaking (140 rpm), and were harvested at the indicated OD600. We used the following antibiotics for plasmid selection: 100 µg/ml carbenicillin (Euromedex), 50 µg/ml kanamycin (Sigma), 25 µg/ml chloramphenicol (Sigma), 12.5 µg/ml tetracycline (Sigma) and 100 µg/ml apramycin (Sigma).
Table 1.
Strains and plasmids used in this study
| Name | Description/relevant characteristics | Phenotype | Source/reference |
|---|---|---|---|
| E. coli strain | |||
| 239KH89 | Bovine isolate, afa-8+ | 29 | |
| AL10 | Human clinical isolate, afa-8+ | 31 | |
| AL213 | Human clinical isolate, afa-8+ | 31 | |
| 183 | Human commensal isolate, afa-8+ | 32 | |
| AL511 | Human clinical isolate, afa-8+ | 31 | |
| AL511ΔafaR::KmFRT | afaR-deficient AL511 strain | This study | |
| AL862 | Human clinical isolate, afa-8+ | 29 | |
| N3433 | lacZ43(Fs), LAM-, relA1, spoT1, thi-1 | CGSC# 6976 | |
| N3431 | N3433 rne-3071(ts) | CGSC# 6975 | |
| BW25113 | K-12 Δ(araD-araB)567, lacZ4787Δ(::rrnB-3),LAM-, rph-1, Δ(rhaD-rhaB), hsdR514 | 33 | |
| JW4130-1 | BW25113 Δhfq-722::km | 33 | |
| DH5α | F– endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacIZYA-argF)U169 deoR (ϕ80lacZΔM15) (λpyr+) | Lab. collection | |
| TOP10 | mcrA Δ(mrr-hsdRMS-mcrBC) Ф80lacZΔM15 ΔlacX74 deoR recA1 araD139Δ(ara-leu)7697 galU galK rpsL endA1 nupG | SmR | 34 |
| TOP10Δhfq::KmFRT | Hfq-deficient TOP10 strain | KmR | 34 |
| TOP10Δhfq::FRT | Hfq-deficient strain with the FRT-flanked kanamycin cassette removed by flippase-mediated excision with pCP20 | KmS | This study |
| MC1061 | K-12 F- araD139 Δ(ara-leu)7697 Δ(codB-lacI) galK16 galE15 mcrA0 relA1 rpsL150 spoT1 mcrB9999 hsdR2 lacX74 | Lab. collection | |
| CAG22216 | MC1061 [ϕλrpoH P3::lacZ] ΔrpoE::Cm | CmR | 35 |
| Plasmids | |||
| pKD4 | Source of the KmFRT resistance cassette | KmR | 36 |
| pKOBEG-Apra | Derivative of pKOBEG | ApraR, CmR | 37 |
| pCP20 | FLP flippase expression plasmid | CbR | 38 |
| pCP22 | pCP20 plasmid with insertion of the ble gene into XhoI site conferring resistance to zeocin | CbR, ZeoR | This study |
| pBR322 | Cloning plasmid | CbR, TetR | Lab. Collection |
| pILL1320 | afa-8 gene cluster from 239KH89 inserted into HindIII site of pBR322 | CbR, CmS | This study |
| pILL1322 | pILL1320 ΔPr-afaR::FRT | CbR, TetS | This study |
| pILL1323 | pILL1320 ΔafaR::FRT, complete deletion of the region following the rho-independent terminator of afaD gene with partial removal of afaR gene sequence | CbR, TetS | This study |
| pILL1324 | pILL1320 ΔafaD::FRT | CbR, TetS | This study |
| pILL1325 | pILL1322 ΔPr-afaA::FRT | CbR, TetS | This study |
| pZE2R-gfp | gfp gene under control of the Pλ constitutive promoter | KmR | 39 |
| pZE2R-null | plasmid encoding a ∼60 nt nonsense RNA | KmR | 6 |
| pZE2R-afaR | afaR gene under control of the Pλ constitutive promoter | KmR | This study |
| pZE2R-afaR* | pZE2R-afaR derivative plasmid carrying the GT point mutated allele of AfaR | KmR | This study |
| pQF50 | LacZ fusion plasmid | CbR | 40 |
| pQFafaA::lacZ | 5′ UTR of afaA inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaB::lacZ | 5′ UTR of afaB inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaC::lacZ | 5′ UTR of afaC inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaD::lacZ | 5′ UTR of afaD inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaE::lacZ | 5′ UTR of afaF inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaE::lacZ | 5′ UTR of afaE inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pQFafaR::lacZ | 5′ region of afaR inserted into XhoI/XbaI sites of pQF50 | CbR | This study |
| pXG-0 | Translational fusion control plasmid, gfp- | CmR | 34 |
| pXG-30 | Translational fusion plasmid, gfp+ | CmR | 34 |
| pXGafaD::gfp | Insertion of the 5′ UTR part of afaD into NheI/BfrBI sites of pXG30, giving a afaD::gfp translational fusion | CmR | This study |
| pXGlacZ ::afaD | Insertion of the 3′ UTR part of afaD into NheI/XbaI sites of pXG30, giving a M2-tagged lacZ::afaD translational fusion | CmR | This study |
| pXGafaD*::gfp | pXGafaD::gfp derivative plasmid carrying the AC point mutated allele of AfaD | CmR | This study |
| pACYC184 | Cloning plasmid | TetR, CmR | Lab. Collection |
| pTX381 | hfq gene inserted into pACYC184 | TetR, CmR | 41 |
CGSC, Coli Genetic Stock Center.
Phenotypes: Apra, apramycin; Cb, carbenicillin; Cm, chloramphenicol; Km, kanamycin; Sm, streptomycin; Tet, tetracycline; Zeo, zeocin. S, sensitive; R, resistant.
General DNA techniques
Oligonucleotides were designed, and in silico analysis was carried out with the afa-8 sequence of E. coli 239KH89 (accession number AF072900). The sequences of the oligonucleotides (Eurogentec) used in this study are listed in Supplementary Table S1. All polymerase chain reaction (PCR) products were amplified from the chromosomal DNA of E. coli strain 239KH89 (99.7% nucleotide sequence identity to strain AL862). Enzymatic reactions were performed in accordance with the manufacturer’s instructions.
Plasmids
We generated the expression plasmid pILL1320 by amplifying the full afa-8 gene cluster by PCR with the primers clon.afa8.5 and clon.afa8.3 and Taq DNA polymerase (Qbiogen). The PCR product was digested with HindIII (Roche), purified by agarose gel electrophoresis, and ligated into the HindIII site of pBR322.
For expression of the AfaR sRNA, a fragment containing the afaR gene without its promoter was amplified by PCR with the Cl.afaR.EcoRI and Cl.afaR.XbaI oligonucleotides. The PCR product was purified by agarose gel electrophoresis and inserted into pCRII-TOPO (Invitrogen). The EcoRI/XbaI fragment of the pCRII-afaR plasmid containing the afaR gene was purified by agarose gel electrophoresis, ligated between the EcoRI and XbaI sites of pZE2R-gfp with T4 DNA ligase (Fermentas) and transferred into the TOP10 strain by electroporation.
We constructed the pZE2R-afaR* plasmid, encoding the AfaR* mutated sRNA allele, as described for pZE2R-afaR. We first introduced two mutations by amplifying the 5′ and 3′ regions of the afaR gene with the Cl.afaR.EcoRI/mutafaR5 and mutafaR3/Cl.afaR.XbaI oligonucleotides. The two resulting DNA fragments were assembled by PCR with the Cl.afaR.EcoRI and Cl.afaR.XbaI oligonucleotides.
The pXGafaD::gfp fusion plasmid was constructed by amplifying the sequence of the intergenic region including parts of the 3′ end of the afaC gene and the 5′ end of the afaD gene with the afaC.NsiI and afaD.NheI primers. The PCR product was inserted into pXG-30 digested with NsiI and NheI, as previously described (34). The pXGafaD*::gfp fusion plasmid was constructed by introducing mutations into the afaD gene by the same strategy used for afaR*.
The pXGlacZ::afaD fusion plasmid was constructed in a similar manner. Briefly, pXG30 was digested with NsiI (Fermentas) and XbaI (Roche), and the 3′ end of the afaD gene was amplified by PCR with the afaD.NsiI and afaD.XbaI oligonucleotides, treated with exonuclease I (Fermentas) and digested with XbaI (Roche). The digested plasmid and the PCR product were purified by agarose gel electrophoresis, ligated with T4 DNA ligase (Roche), and introduced into the TOP10 strain by electroporation.
All the promoter fusion plasmids constructed were derivatives of pQF50. The 300 nucleotides upstream from the initiation codon of the afaA to E coding sequences (CDSs) and the promoter region of afaR were amplified by PCR with the primers listed in Supplementary Table S1. PCR products and pQF50 were digested with XhoI and XbaI, purified by agarose gel electrophoresis, ligated with T4 DNA ligase (NEB) and used to transform E. coli strain DH5α by electroporation.
All plasmid constructs were sequenced (GATC Biotech) to check that there were no unwanted mutations.
Promoter fusion plasmid assay
We analysed β-galactosidase activity on LB agar plates containing 40 µg/ml X-Gal and 100 µg/ml carbenicilin by scanning bacterial colonies with a PharosFX (Biorad) at an excitation wavelength of 480 nm. The E. coli DH5α strain bearing pQF50 was used as a negative control.
Inactivation of genes by allelic exchange
Mutant strains were constructed by the allelic exchange recombination method, with the thermosensitive plasmid pKOBEG-Apra. Briefly, long synthetic primers (∼60 nts) overlapping the 5′ and 3′ untranslated regions of target genes were used to amplify the kanamycin FRT-flanked cassette (KmFRT) from pKD4 by PCR. Flippase-mediated excision of the kanamycin resistance cassette was achieved with pCP22.
RNA isolation and reverse transcription
Total RNA was extracted by the hot-phenol protocol (6) and treated twice with DNase I (Roche). We checked the quality of total RNA samples on a Bioanalyzer with an RNA 6000 nanochip (Agilent). Genomic DNA contamination was assessed by PCR amplification of the 5S ribosomal gene with the 5S.Fw and 5S.RT primers, and was considered insignificant if no PCR product was observed on agarose gel electrophoresis. Chimeric DNAs were synthesized from total RNA with random primers for mRNA and specific primers for sRNA, as previously described (6).
Quantitative RT-PCR
For quantitative reverse transcription PCR (qRT-PCR) analyses of gene expression, we used Primer3 software to design specific primer pairs. Real-time PCR was performed with the Bio-Rad iQ system and iQ SYBR Green supermix (Bio-Rad), according to the manufacturer’s instructions. The thermocycling protocol was as follows: denaturation at 94°C for 10 min, 40 cycles of amplification and quantification (95°C for 15 s, 60°C for 1 min with a single fluorescence measurement) and a melting curve program (55°C to 95°C, in increments of 0.5°C). Each assay was performed at least in duplicate, with two independently prepared total RNA samples. The relative expression data obtained were analysed with the optimized amplification efficiency protocol derived from the ΔΔCt method, with the 5S gene used as a reference gene (42).
5′ and circular RACE
The 5′ RACE experiments were carried out as previously described, but with the following modifications (43). Briefly, 10 µg of freshly prepared total RNA from the 239KH89 strain was left untreated or treated with the TAP enzyme (Epicentre) and ligated with the R1 RNA adapter (Sigma Proligo). Chimeric DNA was synthesized with Superscript III reverse transcriptase (Invitrogen) and random primers (Invitrogen). It was then amplified by PCR with P10 and reverse primers and analysed by agarose gel electrophoresis. The largest TAP-treated PCR products were extracted from the gel, inserted into pCRII-TOPO (Invitrogen) and sequenced (GATC Biotech).
Circular RACE was carried out on freshly prepared total RNA. The RNA was first denatured by heating at 80°C for 2 min in 1× buffer (10 mM Tris-HCl pH 8.1 at 37°C, 7% deionized formamide, 0.2% SDS) and was then dephosphorylated with CIP enzyme (NEB). The RNA was purified by phenol/chloroform extraction and isopropanol precipitation. The 5′ ends of RNAs were re-phosphorylated with T4 polynucleotide kinase (NEB) in 1× buffer (80 mM Tris-HCl pH 8.1 at 37°C, 8 mM MgCl2, 4 mM dithiothreitol, 1 mM spermidine), 1 mM ATP and 40 units of RNaseOUT (Invitrogen). The RNA sample was self-ligated, with or without T4 RNA ligase (Epicentre). Circular RNA products were reverse transcribed with random primers, and the products were amplified by PCR with rigBC.RT and rigCD.Fw primers and checked by agarose gel electrophoresis. The DNA of bands of interest (detected only after the use of T4 RNA ligase) was extracted from the agarose gel with the Qiagen gel extraction kit, inserted into pCRII-TOPO and sequenced.
Measurement of RNA decay by rifampicin assay
We measured the rate of decay of AfaR sRNA by growing strain carrying the pZE2R-afaR plasmid in LB broth, at 37°C or 42°C, with continuous shaking (140 rpm) until the OD600 reached 0.4. Rifampicin was added to stop the initiation of transcription (final concentration, 500 µg/ml). Aliquots of cells were removed at several time points (0, 1, 2, 4, 8, 16, 30 and 60 min) after the addition of rifampicin and immediately frozen by mixing with an equal volume of cooled absolute ethanol. RNA preparation, cDNA synthesis and quantitative RT-PCR were performed as described above. The half-life was calculated from the slope of a least-squares regression line of a semi-logarithmic plot of the percentage sRNA remaining as a function of time. Half-life was calculated from the time points at which decay rate was exponential.
Western blotting
Crude extracts of bacterial cell were separated by SDS-PAGE in 14% polyacrylamide gels, and the protein bands were electro-transferred onto PVDF membranes (Millipore). The membranes were then hybridized with the α-GFP antibody (Roche), the α-FLAG antibody (Sigma-Aldrich) and α-GroEL antisera (Sigma-Aldrich) as previously described (34). The AfaD-VIII and AfaE-VIII proteins were detected by incubation with rabbit polyclonal anti-rAfaD-VIII or anti-rAfaE-VIII antibodies (25) at a dilution of 1:2000, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit serum (Zymed). The membranes were then developed with the SuperSignal West pico chemiluminescent reagent (Pierce), and the signals were detected with a ChemiDoc XRS system (Biorad).
RESULTS
Transcriptional organization of the afa-8 gene cluster
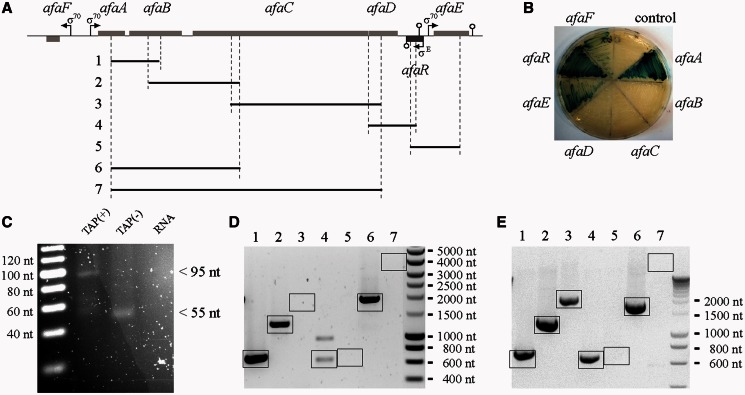
The sequence and genetic organization of the afa-8 gene cluster of E. coli 239KH89 strain were described a few years ago, but we still know little about its transcription (29). We first predicted the transcriptional units of this afa-8 gene cluster in silico by determining the location of the promoters and Rho-independent terminators. Putative σ70 promoters were identified upstream from the afaA, afaE and afaF genes (Figure 1A). These promoters were validated by fusing the 300-bp DNA fragment upstream from the AUG start codon of each afa CDS with the lacZ reporter gene of the pQF50 plasmid. β-galactosidase activity was observed only for strains carrying pQFafaA::lacZ, pQFafaE::lacZ and pQFafaF::lacZ (Figure 1B), confirming our predictions. The identification of a Rho-independent terminator beginning 240 bp downstream from the stop codon of the afaD gene suggests that the afaD mRNA has a long 3′ UTR. Another Rho-independent terminator was found 25 bp downstream from the afaE stop codon. In a previous study, the ∼230-nt SQ109 sRNA gene was identified between the afaD and the afaE genes (6). This sRNA was renamed AfaR (afimbrial adhesin small RNA), as its location suggested a probable role in regulating the expression of the afa-8 gene cluster. BLASTN analysis against the GenBank database (24 January 2012) revealed that the sequence and synteny of the afaR gene were conserved in all sequenced afa-8 gene clusters from E. coli isolates (239KH89, AL511 and AL862 strains) but that this gene was absent from all other afa family gene clusters. We mapped the transcription start site of afaR by 5′ RACE to nucleotide position A5534 of the afa-8 gene cluster of E. coli 239KH89 (Figure 1C). An analysis of the afaR promoter region showed strong sequence identity to the consensus sequence of the σE (also named σ24) promoter. In particular, this region contains the ‘AA’ tract of the -35 element, the invariable C-residue of the -10 box, the 16-bp spacer, the 6-bp discriminator sequence and the conserved -1 T-residue (44). The AfaR promoter was validated by constructing the pQFafaR::lacZ fusion plasmid, which yielded β-galactosidase expression (Figure 1B). The distance between the σE promoter and the Rho-independent terminator confirmed the previous estimate of 230 nt for the size of the AfaR sRNA (6).
Figure 1.
Organization of the transcriptional units of the afa-8 gene cluster from E. coli 239KH89. (A) Genetic organization of the afa-8 gene cluster, with predicted promoters (arrows) and rho-independent terminators (circles). Position of primers (vertical dashed line) for PCR products [horizontal black lines 1 to 7 refer to the RT-PCR shown in (D) and (E)]. (B) β-Galactosidase plate activity assays. Each MC1061 strain carries a pQF50-derived plasmid with or without the region upstream from an afa gene fused to lacZ. (C) 5′ RACE for identification of the AfaR transcription start site. Total RNA from E. coli strain 239KH89 was linked to a 5′ RNA adaptor with [TAP (+) lane] or without [TAP (−) lane] removal of the 5′ triphosphate by TAP treatment. After RT-PCR amplification with specific primers, PCR products were analysed by electrophoresis in 4% agarose gels. A single ∼95 bp band strongly enhanced by TAP treatment was cloned and sequenced to identify the transcription start site of the afaR gene. (D, E) Cotranscription analysis of the afaA to E genes from the afa-8 cluster. (D) Analysis of the BW25113 + pILL1320 strain. (E) Analysis of the BW25113 + pILL1322 strain. The products of RT-PCR amplification were analysed by electrophoresis in a 1% agarose gel, which demonstrated probable linkage between afaA and afaB (lane 1), afaB and afaC (lane 2), afaC and afaD (lane 3), afaD and the 3′ UTR of afaD (lane 4), the 3′ UTR of afaD and afaE (lane 5), afaA and afaC (lane 6), afaA and afaD (lane7). The expected PCR products are indicated by boxes.
Owing to its antibiotic multiresistance phenotype, genetic modification of E. coli strain 239KH89 was not possible. We therefore carried out most of the experiments on the E. coli BW25113 laboratory strain carrying pILL1320, corresponding to the entire afa-8 gene cluster inserted into the pBR322 plasmid. Several RT-PCRs were performed to determine whether the afaABCDE genes were cotranscribed in the BW25113 + pILL1320 strain; for these reactions, we used primers targeting the 5′ and 3′ ends of each gene and the 3′ UTR of afaD (Figure 1A and D). The specificity of the reverse transcription reactions was demonstrated in control experiments by omitting the reverse transcriptase enzyme (data not shown). We checked that the transcription profile of the afaABCDE genes was similar in the E. coli 239KH89 and BW25113 + pILL1320 strains (Supplementary Figure S1). Our findings suggested that afaA and afaB, and afaB and afaC were cotranscribed, generating a single mRNA molecule. However, no linkage was detected between afaC and afaD, afaD and afaE or between afaA and afaD. Amplification of the afaD and afaD 3′ UTR mRNAs but not of the afaD 3′ UTR and afaE mRNAs confirmed the location of the Rho-independent terminator and suggested that afaE was independently transcribed. qRT-PCR analysis of the transcription of the afa-8 gene cluster of the BW25113 + pILL1320 strain revealed that relative transcript levels for the afaAB, afaC and afaD units were significantly different (Supplementary Figure S1). We interpreted these observations as indicating that a putative afaABCD mRNA could be cleaved by one or more endoribonucleases (RNases) at sites surrounding the afaC mRNA, resulting in the release of the afaD mRNA. The cotranscription of the afaABCD mRNA did not rule out the presence of a putative internal promoter. We therefore deleted the entire promoter region between afaF and afaA and the region downstream from the afaD Rho-independent terminator, including the afaR gene promoter from the pill1320 plasmid, giving pILL1325. Analysis, by qRT-PCR, of the transcription of the afa-8 cluster genes from the BW25131 + pILL1325 revealed no significant amplification of afaABCD genes with respect to the 5S gene, indicating that there was not such internal promoter in the gene cluster. In addition to, the afaE gene was amplified, confirming that it was an independent gene (data not shown). Taken together, these results suggest that the afa-8 gene cluster consists of four transcriptional units: afaABCD, afaR, afaE and afaF.
AfaR interacts with the afaD mRNA via an antisense mechanism
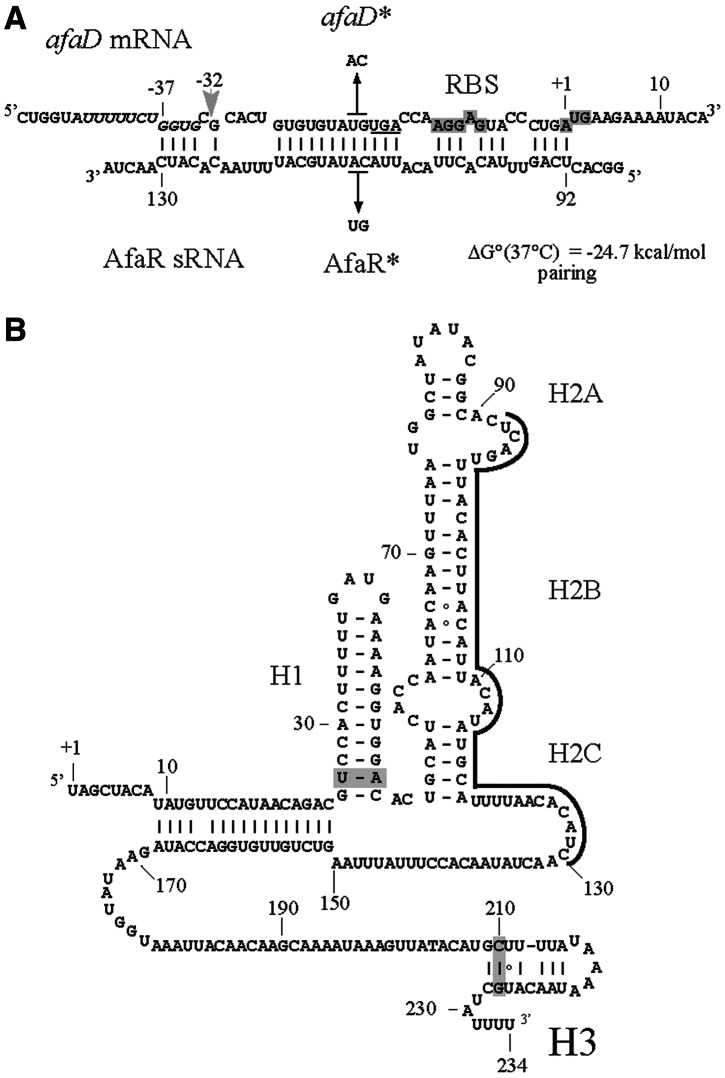
Our working hypothesis is that AfaR is an antisense RNA that regulates AfaD production. We therefore used intaRNA software (45) to analyse in silico the likelihood of AfaR forming double-stranded antisense pairs with other afa-8 gene mRNAs. One significant putative antisense pairing was predicted between nucleotides U92 to C130 of AfaR and nucleotides G−37 to A+1 of the afaD mRNA 5′ UTR, which contains the ribosome-binding site (RBS) (Figure 2A). A second predicted pairing involved the 3′ UTR of the afaD mRNA (residues T490 to G695), which perfectly matched the last 206 nt of the AfaR sequence (data not shown). This putative pairing was predicted on the basis of the presence of afaD and the 3′ end of the afaR gene in the same DNA locus, but in opposite orientations. Both pairing regions were conserved in E. coli strains carrying afa-8 gene clusters, as were AfaR secondary structures (Figure 2B). We thus hypothesized that AfaR bound the 5′ and/or 3′ UTR of the afaD mRNA, thereby modulating its translation and/or stability.
Figure 2.
Predicted RNA secondary structures. (A) Nucleotide pairing of AfaR with the 5′ UTR of the afaD mRNA from E. coli 239KH89. The putative RBS required for afaD translation and the ATG codon are highlighted in grey. Numbers denote the residues in the afaD mRNA with respect to the ATG codon and the mapped +1 site of AfaR. The positions of point mutations introduced into the afaD gene (TG4803 to AC, giving the afaD* allele) and AfaR (AC110 to TG, giving the AfaR* allele) and expected to maintain base-pairing between the afaD*/AfaR* duplex, are indicated by thin black arrows. The light grey arrow indicates the putative RNase E cleavage site at G4791 (-32 relative to the ATG codon). The AU-rich sequence next to a stem–loop helix and a G residue positioned two nucleotides upstream from a cleavage site mimicking the RNase E recognition sequence are displayed in italics. The G residue is shown in italics and underlined. The stop codon of the afaC gene is underlined. (B) Predicted secondary structures of AfaR sRNA. The region of the afaD mRNA to which AfaR binds is underlined. Covariations supporting structure prediction are highlighted in grey.
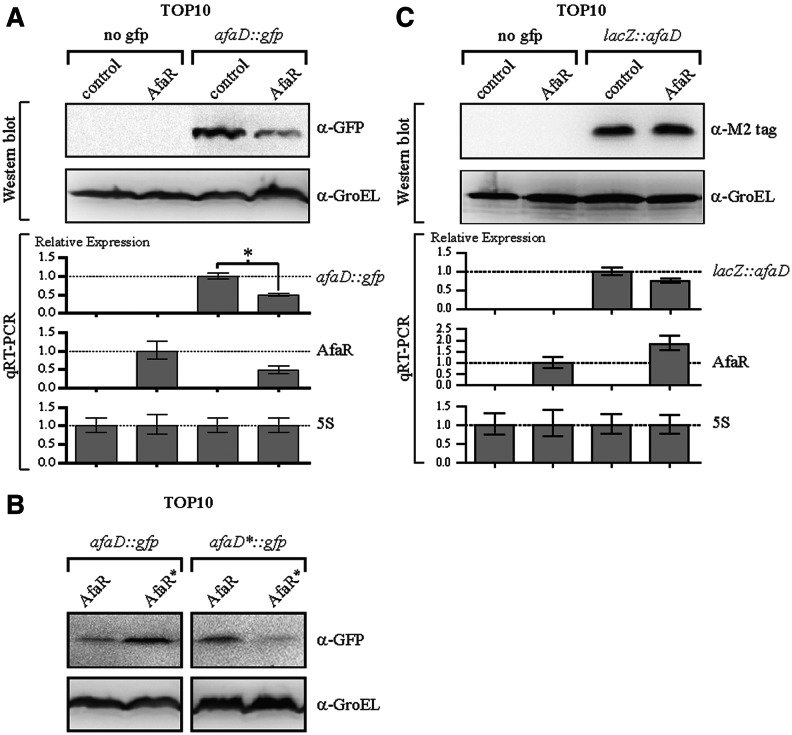
For the validation of these predictions in vivo, we assessed the putative base-pairing interaction between AfaR and these two mRNA targets, using a translational control and target recognition system (34). The afaD 5′ region, spanning the last 66 bp of the afaC gene and the first 60 bp of the afaD gene, was inserted into pXG-30, giving pXGafaD::gfp. The expression of the afaD::gfp translational fusion gene was under the control of the PLtetO-1 promoter. The afaR gene was placed under the control of the strong constitutive Pλ promoter in the pZE2R-afaR plasmid. Four E. coli TOP10 strains harbouring a combination of the pXGafaD::gfp target plasmid or pXG-0 (no target control) and either pZE2R-afaR or pZE2R-null were constructed. We monitored the levels of the sRNA and fusion mRNAs by qRT-PCR and GFP production by Western blotting (Figure 3A). AfaR over-expression was associated with a decrease in the amount of the afaD::gfp fusion mRNA by a factor of 2 to 2.56 (Figure 3A). Interestingly, AfaR levels decreased with those of the afaD mRNA target, suggesting AfaR-guided ribonuclease degradation of the target. Western blot experiments with antibodies directed against GFP showed that AfaR overexpression was associated with a halving of AfaD::GFP protein levels, consistent with the results of the transcriptional analysis (Figure 3A).
Figure 3.
AfaR-induced afaD mRNA decay occurred owing to the binding of AfaR to the 5′ UTR of the afaD mRNA. (A) Analysis of binding to the 5′ UTR of afaD mRNA. Analysis, by qRT-PCR and Western blotting, of afaD::gfp and AfaR gene expression in E. coli strain TOP10 harbouring pZE2R-afaR or pZE2R-null together with pXG-0 (no target control) or pXGafaD::gfp target expression plasmids. The four isolates were cultured in LB medium at 37°C, to an OD600 of 1.0. The expression of the gfp fusion gene was normalized to 1.0 for the TOP10 vector carrying pZE2R-null and pXGafaD::gfp. AfaR expression was normalized to 1.0 for TOP10 carrying pZE2R-afaR and pXG-0. The GroEL protein was used as a loading control. (B) Western-blot analysis with antibodies directed against GFP, for TOP10 harbouring pZE2R-afaR or mutated pZE2R-afaR* together with either wild-type pXGafaD::gfp or mutant pXGafaD*::gfp fusion plasmids. (C) Analysis of binding to the 3′ UTR of afaD mRNA. qRT-PCR and Western blot analysis were performed as described in (A), but with strains carrying pXGlacZ::afaD instead of the pXGafaD::gfp fusion.
The AfaR/afaD RNA interaction was validated by introducing point mutations into the pairing regions of the AfaR gene and the afaD mRNA 5′ UTR (Figure 2A). The TG−19 dinucleotide (UG in RNA) of afaD was changed to AC, yielding the afaD*::gfp allele, and the CA109 dinucleotide in afaR was changed to GT, giving a compensatory afaR* allele. Western blot analyses showed that regulation of the afaD::gfp fusion by AfaR* was strongly impaired. Similarly, the afaD*::gfp fusion was more resistant to regulation by wild-type AfaR allele expression from pZE2R-afaR. The regulation of afaD*::gfp fusion expression was restored by expressing the compensatory AfaR* allele from pZE2R-afaR* (Figure 3B).
The 3′ UTR of the afaD mRNA was the second putative target of AfaR determined in silico. We investigated whether the afaD 3′ UTR played a critical role in regulation by inserting the sequence spanning the last 60 bp of the afaD gene and the following 278 bp sequence, including the afaD rho-independent terminator, into pXG30, to obtain pXGlacZ::afaD. Expression of the lacZ::afaD fusion was monitored by qRT-PCR and Western blotting in E. coli TOP10 harbouring a combination of the pXGlacZ::afaD target plasmid or pXG-0 (control containing no target sequence) and either pZE2R-afaR or pZE2R-null (Figure 3C). A comparison of the relative levels of lacZ::afaD mRNA in strains bearing pZE2R-afaR or pZE2R-null showed that AfaR overexpression had no effect on fusion mRNA levels (Figure 3C). Western blots with antibodies directed against the M2-FLAG tag (LacZ) confirmed this observation (Figure 3C). Thus, AfaR is an antisense sRNA that represses afaD expression in vivo by interacting with the 5′ UTR of the afaD mRNA but not with its 3′ UTR.
AfaR controls afaD mRNA levels posttranscriptionally
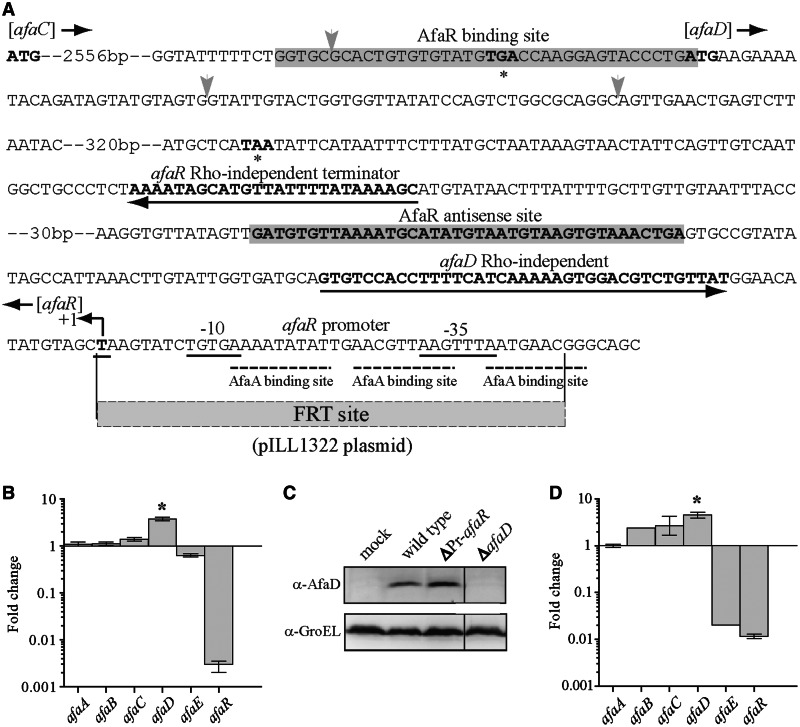
We constructed an afaR mutant strain with a view to confirming the target of AfaR in vivo. E. coli strain 239KH89 is resistant to several antibiotics, AL511 does not express afaE (Supplementary Figure S1) and AL862 carries two afa-8 gene clusters (29). We therefore mutated the sequence inserted into pILL1320. As the 3′ sequences of the afaD and afaR genes overlapped, we had to delete the σE promoter of afaR to abolish the expression of this gene, to generate pILL1322 (Figure 4A). We checked that the afaR sequence in the pILL1322 plasmid was not expressed by qRT-PCR (Figure 4B). A transcriptional linkage analysis of BW25113 + pILL1322 showed that afaC and afaD were cotranscribed. This suggested that AfaR might be involved in the release of afaD mRNA from a primary transcript (Figure 1E), consistent with an interaction between the two RNAs based on an antisense mechanism. We then determined the relative levels of expression of the afaABCDE genes in BW25113 + pILL1320 and BW25113 + pILL1322 by qRT-PCR (Figure 4B). RNA was extracted from cultures at an OD600 of 0.6, in LB medium, in which AfaR levels were maximal (data not shown). No significant difference was observed in the relative levels of expression of afaABC and afaE, indicating that AfaR had little transcriptional or posttranscriptional effect on the expression of these genes.
Figure 4.
Posttranscriptional control of afaD mRNA levels by AfaR, in vivo. (A) Overview of the afaD/afaR locus from the afa-8 gene cluster of E. coli strain 239KH89. The promoter boxes (-10 and -35) and transcription start site (+1) of the afaR gene are underlined. Rho-independent terminators are indicated by an arrow. ‘ATG’ in bold and asterisks indicate the initiation and termination codons, respectively, of the afaC and afaD CDSs. The grey box indicates the binding region of the AfaR sRNA and afaD mRNA. Vertical grey arrows indicate the position of ribonuclease cleavages. The pILL1322 plasmid has the same sequence as pILL1320, except that the σE promoter sequence is replaced by the FRT site, as indicated. (B) Assessment of the relative expression of the afaABCDER genes in strain BW25113 carrying pILL1322 (ΔPr-afaR) versus pILL1320 (wild type), determined by qRT-PCR. Bacteria were grown to an OD600 of 0.6. (C) Western blot analysis (with an anti-rAfaD-VIII antiserum) of AfaD production in a total protein extract obtained at an OD600 of 0.6 from BW25115 carrying pBR322 (mock), pILL1320 (wild type), pILL1322 (ΔPr-afaR) or pILL1324 (ΔafaD). The GroEL protein was used as a loading control. (D) Analysis, by qRT-PCR of the relative expression of the afaABCDER genes from the AL511 ΔafaR::KmFRT and AL511 strains grown to an OD600 of 0.6. Note: the afaE gene was naturally not expressed in the E. coli AL511 wild-type strain.
However, the inhibition of AfaR increased afaD expression by a factor of 3.8 (Figure 4B), and Western blotting indicated an accumulation of the AfaD protein (∼15 kDa) by a factor of 2.6 in the BW25113 + pILL1322 strain (Figure 4C). We validated these results by constructing the AL511 ΔafaR::KmFRT strain and analysing the expression of the afa-8 gene cluster of this mutant by qRT-PCR, comparing the results obtained with those for the wild-type AL511 strain. The transcription levels of the afaA, B and C genes were similar in the two strains. By contrast, the afaD mRNA was overproduced in the afaR-lacking AL511 strain, as previously reported for BW25113 + pILL1322 (Figure 4D). Taken together, these results suggest that AfaD expression is controlled posttranscriptionally by AfaR.
The pairing of the afaR and afaD mRNAs is dependent on Hfq
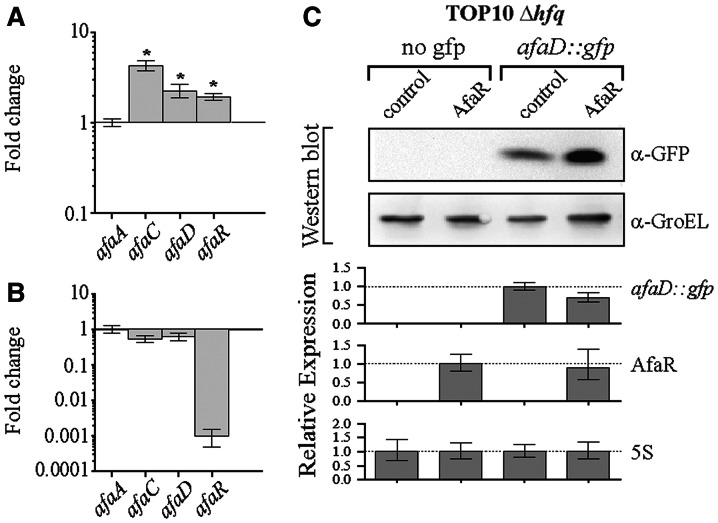
In E. coli, most antisense sRNAs make use of the Hfq protein to capture their mRNA targets (46). We assessed the contribution of Hfq to the AfaR-mediated regulation of afaD mRNA in vivo by comparing the levels of afaD transcripts in the hfq-depleted strain JW4130-1 and the isogenic BW25113 strain, both carrying pILL1320. The relative level of afaA mRNA in the hfq mutant was one tenth that in the wild type, as shown by qRT-PCR, indicating that Hfq affected the transcription of the afa-8 gene cluster (data not shown). When the pTX381 plasmid (carrying the E. coli hfq gene) was introduced into the JW4130-1 + pILL1320 strain, afaA mRNA levels were restored to wild-type values (data not shown). We therefore normalized gene expression with respect to afaA in qRT-PCR analyses, to evaluate the transcription-independent effects of Hfq (Figure 5A). The deletion of hfq had a significant effect on the relative levels of afaC and afaD mRNAs and AfaR (Figure 5A), suggesting possible cooperation between AfaR and Hfq. We tested this hypothesis by carrying out the same experiment with the JW4130-1 + pILL1322 strain, in which expression levels were similar to those for JW4130-1 + pILL1320 (Figure 5B). We characterized this effect in more detail by transforming the TOP10 Δhfq strain harbouring either pZE2R-afaR or pZE2R-null with pXGafaD::gfp and monitoring the expression of the afaD::gfp fusions and AfaR by qRT-PCR. The overtranscription of AfaR did not significantly decrease the amount of fusion mRNA. Furthermore, AfaD::GFP fusion protein levels were slightly higher in the presence of the AfaR sRNA, as shown by Western blots with α-GFP serum. The spot intensities for the fusion proteins in each lane were normalized with respect to the GroEL loading control for the lane concerned. The differences in normalized spot intensities were not statistically significant (t-test) (Figure 5C). We infer from these findings that the Hfq protein is required for the AfaR-mediated regulation of afaD expression.
Figure 5.
Expression of the hfq gene was required for the AfaR-dependent regulation of afaD mRNA decay. (A) Assessment of the relative expression of the genes of the afa-8 cluster in strain JW4130-1 (Δhfq) carrying pILL1320 versus BW25113 carrying pILL1320, determined by qRT-PCR. Asterisks indicate significant differences. (B) Assessment of the relative expression of the genes of the afa-8 cluster in strain JW4130-1 (Δhfq) carrying pILL1322 versus JW4130-1 carrying pILL1320, determined by qRT-PCR. (C) Analysis by Western blotting and qRT-PCR of afaD::gfp and AfaR gene expression in E. coli strain TOP10 Δhfq::FRT harbouring pZE2R-afaR or pZE2R-null plasmids together with pXG-0 (no target control) or pXGafaD::gfp target expression plasmids. The four isolates were cultured in LB medium at 37°C, to an OD600 of 1.0. Expression levels of the gfp fusion gene were normalized to 1.0 for TOP10 Δhfq::FRT carrying pZE2R-null and pXGafaD::gfp. AfaR expression was normalized to 1.0 for TOP10 Δhfq::FRT carrying pZE2R-afaR and pXG-0. GroEL was used as a loading control.
AfaR and RNase E-dependent cleavages of afaD mRNA
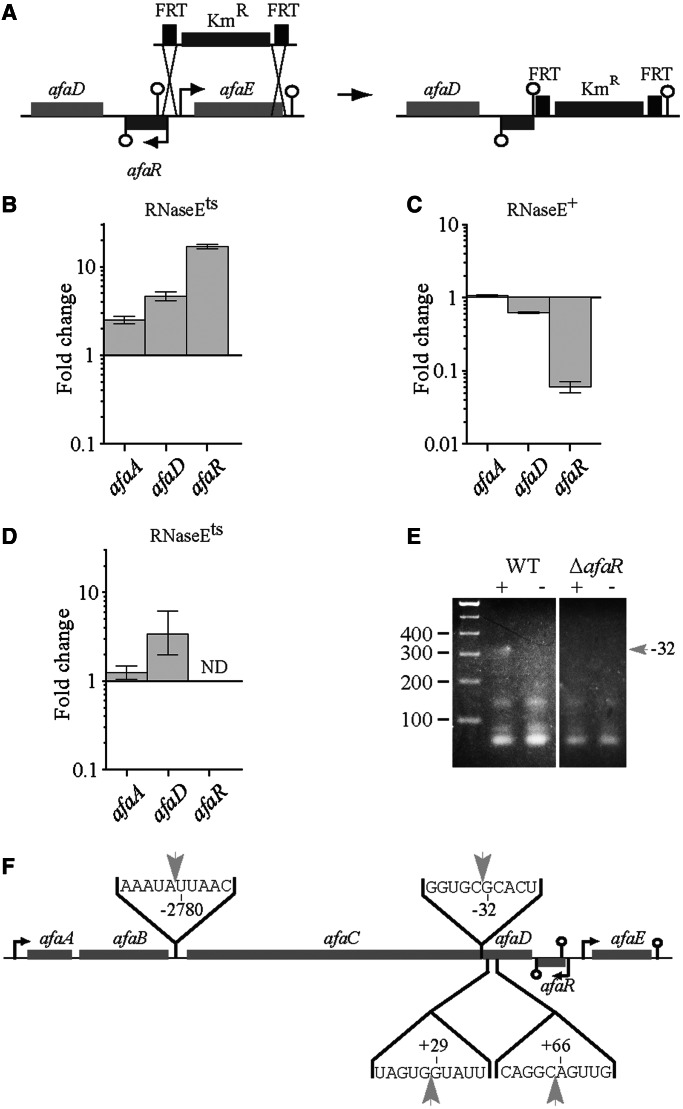
Characterization of the transcriptional units of the afa-8 gene cluster suggested a possible role for RNases in the posttranscriptional regulation of afaABCD mRNA (Figure 1C and D). We tested this hypothesis by analysing the dependence of afa-8 cluster gene expression on RNase E, an RNase frequently associated with sRNA mechanisms. We introduced pILL1320 into the N3431 strain carrying the thermosensitive allele rne-3071 (encoding RNaseEts) and the isogenic strain (N3433) carrying the wild-type rne gene (encoding RNase E). Both strains were grown to an OD600 of 0.6 at 30°C, and half the culture was then shifted to 42°C for 30 min to inactivate rnets gene expression. We analysed the relative levels of afaA and afaD mRNAs and of AfaR in the two strains by qRT-PCR. In the control RNase E+ strain context (Figure 6C), afaA expression levels were unaffecting, indicating that a shift in temperature to 42°C had no visible effect on transcription of the afaABCD operon. By contrast, this shift had a strong effect on AfaR levels. When the rnets gene was inactivated, we observed a small, but marginally significant, increase by a factor of 2.4 in the relative level of the afaA transcript (Figure 6B). By contrast, the levels of the afaD and AfaR RNAs were 4.6 and 16.8 times higher, respectively (Figure 6B), indicating a possible role of RNase E in the posttranscriptional processing of afaD mRNA. A similar analysis was carried out with the RNase Ets strain carrying the pILL1322 plasmid. The levels of the afaA and afaD mRNAs did not change significantly with temperature, indicating that AfaR was required for RNase E-dependent cleavage of the afaABCD mRNA (Figure 6D).
Figure 6.
RNase E dependence of the regulation of afaD expression by AfaR. (A) Schematic representation of the construction of the pILL1323 plasmid by allelic exchange from pILL1320. qRT-PCR analysis confirmed an absence of afaR expression from pILL1323 (See Supplementary Figure S1). (B–D) Relative levels of the afaA and afaD mRNAs and AfaR sRNA, determined by qRT-PCR, in wild-type and RNase Ets thermosensitive strains. The values for each gene are expressed as a ratio of expression at 42°C (low level of RNase E) to that at 30°C (high level of RNase E) for the N3431 + pILL1320 strain (B, [RNase Ets]), the N3433 + pILL1320 strain (C, [RNaseE+]) and the N3431 + pILL1322 strain (D, [RNase Ets]). (E) Circular RACE mapping of the afaC mRNA ends. Total RNA from E. coli BW25113 carrying pILL1320 (wild type) or pILL1323 (ΔafaR) was circularized by end-ligation with or without T4 RNA ligase (lanes + and −, respectively). The ligated 5′ and 3′ ends of the fragment were then amplified by RT-PCR. PCR products were analysed by electrophoresis in a 3% agarose gel. The band of interest was excised, cloned and sequenced. A ∼310 bp DNA fragment (gray arrow) was more abundant after ligation treatment (lane +) in the wild-type strain than in the ΔafaR strain, indicating an AfaR-dependent amplification of the 5′ (cleavage at position -32) and 3′ ends of the afaC mRNA. This suggests that AfaR is involved in the RNase-dependent cleavage of the afaABCD mRNA. (F) Location of RNase cleavage sites in the afaABCD mRNA.
We assessed the possible AfaR-dependent cleavage of the afaABCD mRNA by carrying out differential circular RACE experiments between strains BW25113 carrying pILL1320 or pILL1323, a derivative of pILL1320 containing only the complete afaABCD operon with its natural rho-independent terminator (Figure 6A). The use of this construct overcomes problems due to the influence of downstream sequences on the non-specific expression of AfaR. RNase cleavage occurred around afaC. We therefore grew both isolates to an OD600 of 0.6, extracted total RNA and analysed both AfaR levels and the ligation-dependent circularization of the afaC mRNA (See Methods section, Figure 6E). We sequenced 20 recombinant plasmids containing the ligated 5′ and 3′ ends of the afaC mRNA (Supplementary Figure S2). We filtered out sequences that were not identified at least three times, to distinguish between AfaR-dependent RNase activity and non-specific cleavage. Three cleavage sites were identified at positions U−2780, G−32 and A66, within the 5′ end of the afaC mRNA, the 5′ end of the afaD mRNA that paired with AfaR and within the coding region of the afaD mRNA, respectively (Figure 4A and 6E). Relative levels of afaB and afaC mRNA in BW25113 + pILL1322 and BW25113 + pILL1320 were similar, indicating that cleavage in the 5′ UTR region of the afaC mRNA was independent of AfaR (Figure 4B). For confirmation that afaD mRNA cleavage was dependent on AfaR, we performed a differential 5′ RACE experiment on afaD mRNA in the BW25113 + pILL1320 and BW25113 + pILL1323 strains (data not shown). The AfaR-dependent cleavage site at position A66 within the afaD mRNA was confirmed, and an additional cleavage site was revealed at position G29 of the afaD mRNA (Figure 6F).
RNA secondary structure prediction close to the RBS of afaD (Figure 2A) showed an AU-rich sequence next to a stem–loop helix and a G residue positioned two nucleotides upstream from a cleavage site mimicking the RNase E recognition sequence (47). Thus, cleavage in the 5′ UTR and, probably, within the coding region of the afaD mRNA were dependent on RNase E.
The expression of the afaR gene is controlled by temperature and a σE promoter
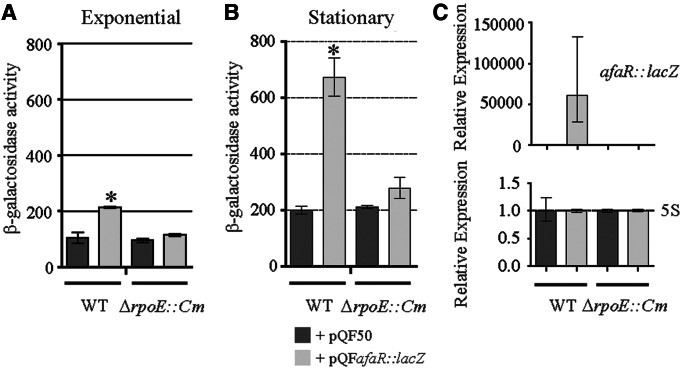
The AfaR gene may be transcribed by the alternative σE, a sigma factor associated with the response to extracytoplasmic stresses, such as increasing temperature. We therefore assessed dependence on the σE promoter by introducing the pQFafaR::lacZ plasmid into E. coli wild-type and isogenic ΔrpoE mutant strains and assessing its activity by measuring β-galactosidase activity (Figure 7A and B). The afaR promoter had no transcriptional activity in the ΔrpoE strain, in either the exponential or the stationary phase. A comparison of afaR::lacZ fusion mRNA levels in the two strains confirmed these findings (Figure 7C). Thus, AfaR transcription is dependent on σE.
Figure 7.
σE is required for induction of the transcription of afaR. (A, B) β-Galactosidase assay on E. coli MC1061 (WT) and CAG22216 (ΔrpoE::Cm) strains carrying the pQF50 or pQFafaR::lacZ plasmid. The promoter of the afaR gene was dependent on σE sigma factor in the (A) exponential and (B) stationary (24 h of culture) phases. The absence of σE totally abolished expression of the afaR::lacZ fusion gene. (C) Expression analysis of the afaR::lacZ fusion mRNA relative to the 5S gene in stationary phase. AfaR expression was abolished in the strain lacking σE. The asterisks indicate significant results.
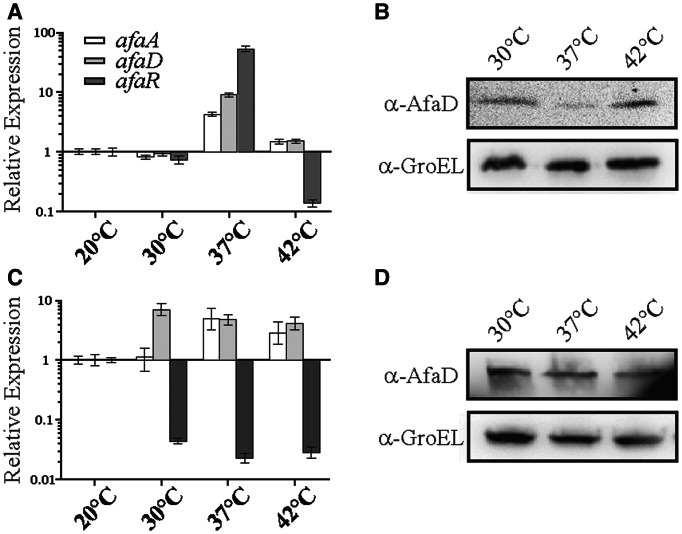
Previous experiments had indicated that expression of the afa-8 gene cluster was temperature dependent and suggested that the σE-dependent AfaR sRNA was responsible for the thermoregulation of AfaD production (C. Le Bouguénec, personal communication). This hypothesis was tested by growing the BW25113 + pILL1320 strain at 20°C, 30°C, 37°C and 42°C, to an OD600 of 0.6. The relative levels of the afaA and afaD mRNAs and AfaR were analysed by qRT-PCR (Figure 8A), and AfaD production was assessed by Western blotting (Figure 8B). AfaR levels were higher at 37°C than at any other temperature. However, although afaD mRNA levels were high at 37°C, the level of AfaD production was lower, indicating that temperature controlled AfaR expression and the fine regulation of AfaD production. We tested this hypothesis by performing the same experiments with the BW25113 + pILL1323 strain. We found that knockout of the afaR gene led to the constitutive production of afaD mRNA and AfaD (Figure 8C and D).
Figure 8.
Control, by temperature, of the production of the AfaD invasin by AfaR regulation. (A) Assessment of the relative levels of expression of afa-8 genes in strain BW25113 carrying pILL1320 grown to an OD600 of 0.6 at 30°C, 37°C and 42°C versus 20°C, as determined by qRT-PCR. The expression levels of all genes at 20°C were normalized to 1. (B) Western blot analysis of AfaD-VIII production by E. coli BW25115 carrying pILL1320 in the same growth conditions as (A). (C, D) as in (A, B), but with the BW25113 strain carrying pILL1323 (ΔafaR::KmFRT). GroEL was used as a loading control.
The abundance of an sRNA at a given time point is a reflection of its rate of synthesis, mode of regulation and decay. We assessed the stability of the AfaR sRNAs by performing qRT-PCR analysis with total RNA isolated from the BW25113 + pZE2R-afaR strain cultured at 37°C and 42°C to an OD600 of 0.4 before treatment with rifampicin to inhibit RNA synthesis. The half-life of AfaR was found to be 6.9 min ± 21 s at 37°C and 5.04 min ± 17.7 s at 42°C. Given that the AfaR sRNA was more abundant at 37°C than 42°C (Figure 8), the apparent slightly lower levels of sRNA at 42°C are not significantly different from those at 37°C (without mRNA target), providing no evidence of lower stability. The stability of individual sRNAs varied widely, from highly stable to highly unstable, as reported for sRNAs in other bacteria (48,49). Taken together, these results indicate that AfaR transcription is controlled by temperature.
DISCUSSION
The invasion of a host by an ExPEC strain requires rapid adaptation to the new growth conditions associated with the transition from a free-living lifestyle to a host-associated state. This involves a reprogramming of the transcriptional activity of the bacterium. sRNAs function as gene regulators, relaying information from environmental sensors to response genes. We have previously identified ExPEC-specific sRNA genes, including four sRNA genes within a pathogenicity island of the E. coli AL862 strain, close to gene clusters encoding known virulence factors (6). Here, we characterized SQ109, a candidate sRNA renamed AfaR, which is encoded by a gene located in the afa-8 gene cluster encoding a virulence-associated adhesive sheath. The σE- and temperature-dependent expression of AfaR is associated with the repression of AfaD-VIII invasin production, suggesting that AfaR is a stress-response regulator involved in the sensing of environmental stimuli relevant to host cell invasion.
The synthesis of outer membrane proteins (OMPs) is controlled by a plethora of sRNAs, acting as antisense RNAs, in gram-negative bacteria (50–52). Some sRNAs are associated with OMPs involved in virulence. The InvR, RnaG and VrrA sRNAs have recently emerged as key regulators of the OmpD, IcsA and OmpA OMPs in pathogenic Shigella flexneri, S. typhimurium and V. cholerae, respectively (21,13,53). We previously showed that the FimR sRNA controls the production of type 1 fimbriae (an adhesion factor of the chaperone/usher family) involved in the colonization and invasion of the urinary tract (54). This sRNA acts by an antisense mechanism, targeting the mRNA encoding the OMP FimD (6). In this study, we characterized AfaR, an sRNA that regulates afaD mRNA decay at the posttranscriptional level, thereby affecting AfaD-VIII invasin production. Recognition of the cellular β1-chain integrin by AfaD-VIII has been implicated in the internalization of beads coated with purified AfaD-VIII in cells derived from the uroepithelium, a target of afa-8-carrying strains, suggesting that such strains are invasive (25). The AfaD-VIII invasin is not strictly an OMP, but our data suggest that it is associated with the bacterial outer membrane. The identification of AfaR thus enlarges the sRNA family of antisense RNAs regulating cell surface proteins involved in pathogenicity.
During the biogenesis of adhesive structures of the chaperone/usher family at the cell surface, structural proteins are required in greater abundance than accessory proteins. Discoordinate gene expression is therefore essential. Regulatory mechanisms involving the RNase-dependent and -independent processing of mRNA from E. coli gene clusters encoding P-, S-fimbriae, CFA/I and F1845 adhesins have been reported (55–58). However, no sRNA has ever before been implicated in these processes. The identification of three sRNA gene candidates (fimR, afaR and prsR) overlapping adhesive structure-encoding gene clusters in a previous publication suggested a possible global involvement of sRNAs in the regulation of such virulence factors (6). In a putative sRNA-independent pathway of regulation, RNase E and RNase III have frequently been implicated in the processing of mRNAs for the biogenesis of adhesive structures (56,57). We therefore assessed the RNase dependence of the afa-8 gene cluster (Supplementary Figure S3A) and found that RNases E, III and LS but not RNases G and P, played a potential role in mRNA processing. Our findings demonstrate that the cleavage activity of RNase E in processing the afaABCD mRNA upstream from afaD is dependent on AfaR. Other RNase-dependent cleavage events remain to be fully characterized and did not seem to involve the AfaR sRNA directly. This suggests that two separate sequential Afa-VIII biosynthesis regulation pathways may occur. The first involves cleavage of the afaABCD mRNA for regulation of the levels of each independent afa mRNA. In the second, the AfaR sRNA then specifically regulates afaD mRNA decay, probably switching AfaD-VIII invasin production on/off at the right time. A putative model of the regulation of the afa-8 gene cluster is presented in Supplementary Figure S3B. Furthermore, the afaD mRNA was found to be subject to several AfaR-dependent RNase E processing events. These cleavages induced rapid afaD mRNA decay, but low levels of translation to generate AfaD protein nevertheless occurred. This suggests that AfaR is a finely tuned regulator that maintains AfaD protein synthesis in some conditions and facilitates invasion in certain circumstances. Thus, FimR and AfaR are the first sRNAs to be implicated in the regulation of OMPs exported by the chaperome/usher pathway.
Pathogenic bacteria are highly adaptive organisms in which a tight control of virulence gene expression is essential, and sRNAs are increasingly considered to be key elements of virulence control in E. coli. Our results indicate that production of the AfaD-VIII invasin is upregulated when AfaR is downregulated (Figure 8A and B), at temperatures above 37°C. The sRNA genes are, in general, regulated by various mechanisms unrelated to RNA stability, although sRNA regulation via transcript stability has not been ruled out in most cases (48,49). Temperature does not seem to affect AfaR stability or AfaR decay in the absence of its mRNA target, indicating that the stability of the AfaR transcript may be crucial for the regulation of afaD mRNA decay at any temperature. First, temperature changes may inform the bacterium that it is within the host, triggering production of the AfaD-VIII invasin. Second, it might indicate that in vivo, variation of the host temperature should not affect the AfaR regulatory pathway. Lastly, the bacteria can produce more AfaD-VIII invasin at higher temperatures, presumably helping then to evade the host response to infection. Several putative temperature sensors might detect and relay information to the AfaR sRNA. We tested the hypothesis that the σE factor plays a crucial role in this process. Indeed, σE is a sigma factor associated with the extracytoplasmic/heat stress responses (59), as observed for expression of the P-fimbria gene cluster, which is controlled by temperature in a σE-dependent manner (60). More generally, the alternative σE factor upregulates the expression of many other sRNA genes, including MicA and RybB in Salmonella species (61) and, probably, FimR in ExPEC strains (6). Our data provide new evidence that the σE regulon may play a more important role in the virulence of gram-negative bacteria than previously suspected.
Expression of the afa-8 gene cluster may also be controlled by the AfaF and AfaA proteins, which are homologues of the transcriptional regulatory proteins of the PapI/PapB family. We identified, in silico, six putative AfaA-binding sites (5′-CTATATTTT-3′ consensus sequence) in the afaA and afaF intergenic region and three other binding sites overlapping the promoter region of the afaR gene (Figure 4A). These findings strongly suggest that afaR transcription may also be dependent on these regulatory proteins. We also found that AfaR expression was strongly downregulated at 42°C, suggesting that AfaR transcription may be dependent on another, as yet undetermined mechanism. Data from several studies have shown the CpxAR two-component systems control the production of curli and P-fimbriae in uropathogenic E. coli, of type IV bundle-forming pili in enteropathogenic E. coli, and downregulate the expression of σE-dependent genes, rendering them of interest for future studies (60).
Comparative genomics of the afaD/afaE intercistronic region of the afa-8 gene cluster showed it to be very specific in size and composition, and no sequence related to the afaR gene was detected in the other gene clusters of the afa family. Thus, AfaR seems to be specific to the afa-8 gene cluster. We also found that the 3′ end of the afaR gene displayed sequence identity to an IS2 insertion sequence, at both the DNA and protein levels. There is therefore evidence to suggest that afa-8 evolved from its ancestor at least partly through IS2-mediated disruption of the afaD/afaE intercistronic region. Pseudogenization of the IS2 transposase gene may then have led to the creation of the afaR gene. This reorganization of the intercistronic sequence also seems to have had consequences for the transcription units specific to the afa-8 gene cluster. Indeed, only in afa-8 was it was possible to identify an afaE promoter and a rho-independent terminator for the afaABCD mRNA. These promoter and terminator sequences may have been acquired from the IS2 sequence, as previously reported following the insertion of IS2 into the ampC promoter (62) or of IS3 insertion into the 5′ end of argE (63). IS elements have been shown to play a role in the dispersal of SprA sRNA genes between strains of the genus Staphylococcus (9). Finally, our study suggests that some of the sRNA genes in E. coli may be derived from IS elements.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1 and 2, Supplementary Figures 1–3, Supplementary Results and Supplementary References [64–66].
FUNDING
Institut Pasteur [PTR165]; Agence National de la Recherche funding for the ERA-NET Pathogenomics project [ANR-06-PATHO-002-03]. Funding for open access charge: Institut Pasteur.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGMENTS
We thank Patrick Trieu-Cuot for his critical comments on the manuscript and Carol Gross, who kindly provided us with E. coli strains.
REFERENCES
- 1.Le Bouguénec C. Adhesins and invasins of pathogenic Escherichia coli. Int. J. Med. Microbiol. 2005;295:471–478. doi: 10.1016/j.ijmm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Khôler CD, Dobrindt U. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011;301:642–647. doi: 10.1016/j.ijmm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Romby P, Vandenesch F, Wagner EGH. The role of RNAs in the regulation of virulence-gene expression. Curr. Opin. Microbiol. 2006;9:229–236. doi: 10.1016/j.mib.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Thomason MK, Storz G. Bacterial antisense RNAs: how many are there, and what are they doing? Annu. Rev. Genet. 2010;44:167–188. doi: 10.1146/annurev-genet-102209-163523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichon C, Felden B. Small RNA gene identifications and mRNA targets predictions in bacteria. Bioinformatics. 2008;24:2807–2813. doi: 10.1093/bioinformatics/btn560. [DOI] [PubMed] [Google Scholar]
- 6.Pichon C, du Merle L, Caliot E, Trieu-Cuot P, Le Bouguénec C. An in silico model for identification of small RNAs in whole bacterial genomes: Characterization of antisense RNAs in pathogenic Escherichia coli and Streptococcus agalactiae strains. Nucleic Acids Res. 2012;40:284–6286. doi: 10.1093/nar/gkr1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storz G, Vogel J, Wassarman K. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 2011;43:915–926. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichon C, Felden B. Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc. Natl Acad. Sci. USA. 2005;102:14249–14254. doi: 10.1073/pnas.0503838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevalier C, Boisset S, Romilly C, Masquida B, Fechter P, Geissmann T, Vandenesch F, Romby P. Staphylococcus aureus RNAIII binds to two distant regions of coa mRNA to arrest translation and promote mRNA degradation. PLoS Pathog. 2010;6:e1000809. doi: 10.1371/journal.ppat.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabelskaya S, Gaillot O, Felden B. A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune-evasion molecule. PLoS Pathog. 2010;6:e1000927. doi: 10.1371/journal.ppat.1000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez N, Trevino J, Liu Z, Ho SCM, Babitzke P, Sumby P. A genome-wide analysis of small regulatory RNAs in the human pathogen group A Streptococcus. PLOS One. 2009;4:e7668. doi: 10.1371/journal.pone.0007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeiffer V, Sittka A, Tomer R, Tedin K, Brinkmann V, Vogel J. A small non-coding RNA of the invasion gene island (SPI-1) represses outer membrane protein synthesis from the Salmonella core genome. Mol. Microbiol. 2007;66:1174–1191. doi: 10.1111/j.1365-2958.2007.05991.x. [DOI] [PubMed] [Google Scholar]
- 14.Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, Margalit H, Altuvia S. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;18:950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol. Microbiol. 2002;43:257–265. doi: 10.1046/j.1365-2958.2002.02743.x. [DOI] [PubMed] [Google Scholar]
- 17.Lenz D, Mok K, Lilley B, Kulkarni R, Wingreen N, Bassler B. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Kulesus R, Diaz-Perez K, Slechta S, Eto DS, Mulvey MA. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect. Immun. 2008;76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen KT, Nielsen G, Bjerrum JV, Kruse T, Kallipolitis BH, Møller-Jensen J. A role for the RNA chaperone Hfq in controlling adherent-invasive Escherichia coli colonization and virulence. PLoS One. 2011;6:e16387. doi: 10.1371/journal.pone.0016387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Attila C, Wang L, Wood T, Valdes J, Bentley W. Quorum sensing in E. coli is signaled by AI-2/LsrR: Effects on sRNA and biofilm. J. Bacteriol. 2007;189:6011–6020. doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giangrossi M, Prosseda G, Tran C, Brandi A, Colonna B, Falconi M. A novel antisense RNA regulates at transcriptional level the virulence gene icsA of Shigella flexneri. Nucleic Acids Res. 2010;38:3362–3375. doi: 10.1093/nar/gkq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bouguénec C, Servin A. Diffusely adherent Escherichia coli strains expressing Afa/Dr adhesins (Afa/DrDAEC): hitherto unrecognized pathogens. FEMS Microbiol. Lett. 2006;256:185–194. doi: 10.1111/j.1574-6968.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 23.Jouve M, Garcia MI, Courcoux P, Labigne A, Gounon P, Le Bouguénec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 1997;65:4081–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia MI, Jouve M, Nataro J, Gounon P, Le Bouguénec C. Characterization of the AfaD-like family of invasins encoded by pathogenic Escherichia coli associated with intestinal and extra-intestinal infections. FEBS Lett. 2000;479:111–117. doi: 10.1016/s0014-5793(00)01898-6. [DOI] [PubMed] [Google Scholar]
- 25.Plançon L, du Merle L, Le Friec S, Gounon P, Jouve M, Guignot J, Servin A, Le Bouguénec C. Recognition of the cellular beta1-chain integrin by the bacterial AfaD invasin is implicated in the internalization of afa-expressing pathogenic Escherichia coli strains. Cell. Microbiol. 2003;5:681–693. doi: 10.1046/j.1462-5822.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 26.Cota E, Jones C, Simpson P, Altroff H, Anderson K, du Merle L, Guignot J, Servin A, Le Bouguénec C, Mardon H, et al. The solution structure of the invasive tip complex from Afa/Dr fibrils. Mol. Microbiol. 2004;62:356–366. doi: 10.1111/j.1365-2958.2006.05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia MI, Labigne A, Le Bouguénec C. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J. Bacteriol. 1994;176:7601–7613. doi: 10.1128/jb.176.24.7601-7613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson K, Billington J, Pettigrew D, Cota E, Simpson P, Roversi P, Chen HA, Urvil P, du Merle L, Barlow P, et al. An atomic-resolution model for assembly, architecture and function of the Dr adhesins. Mol. Cell. 2004;15:647–657. doi: 10.1016/j.molcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Lalioui L, Jouve M, Gounon P, Le Bouguénec C. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 1999;67:5048–5059. doi: 10.1128/iai.67.10.5048-5059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bouguénec C, Lalioui L, du Merle L, Jouve M, Courcoux P, Bouzari S, et al. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do recognize Dr blood group antigens. J. Clin. Microbiol. 2001;39:1738–1745. doi: 10.1128/JCM.39.5.1738-1745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardeau JP, Lalioui L, Said AM, De Champs C, Le Bouguénec C. Extended virulence genotype of pathogenic Escherichia coli isolates carrying the afa-8 operon: evidence of similarities between isolates from humans and animals with extraintestinal infections. J. Clin. Microbiol. 2003;41:218–226. doi: 10.1128/JCM.41.1.218-226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pichon C, Héchard C, du Merle L, Chaudray C, Bonne I, Guadagnini S, Vandewalle A, Le Bouguénec C. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell. Microbiol. 2009;11:616–628. doi: 10.1111/j.1462-5822.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 33.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban JH, Vogel J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007;35:1018–1037. doi: 10.1093/nar/gkl1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouvière PE, De Las Penas A, Mecsas J, Lu CZ, Rudd KE, Gross C. rpoE, the gene encoding the second heat-shock sigma factor, E, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaveroche MK, Ghigo JM, d’Enfert C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000;28:e97. doi: 10.1093/nar/28.22.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassette with the option of Flp-catalyzed excision of the antibiotic resistance determinant. Gene. 1995;158:914. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 39.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, The TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farinha MA, Kropinski AM. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 1990;172:3496–3499. doi: 10.1128/jb.172.6.3496-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziolkowska K, Derreumaux P, Folichon M, Pellegrini O, Régnier P, Boni IV, Hajnsdorf E. Hfq variant with altered RNA binding functions. Nucleic Acids Res. 2006;34:709–720. doi: 10.1093/nar/gkj464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antal M, Bordeau V, Douchin V, Felden B. A small bacterial RNA regulates a putative ABC transporter. J. Biol. Chem. 2005;280:7901–7908. doi: 10.1074/jbc.M413071200. [DOI] [PubMed] [Google Scholar]
- 44.Lane WJ, Darst SA. The structural basis for promoter -35 element recognition by the group IV sigma factors. PLoS Biol. 2006;4:e269. doi: 10.1371/journal.pbio.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busch A, Richter AS, Backofen R. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24:2849–2856. doi: 10.1093/bioinformatics/btn544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel J, Luisi B. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaderdin VR, Walsh AP, Jakobsen T, McDowall KJ, von Gabain A. Enhanced cleavage of RNA mediated by an interaction between substrates and the arginine-rich domain of E. coli ribonuclease E. J. Mol. Biol. 2000;301:257–264. doi: 10.1006/jmbi.2000.3962. [DOI] [PubMed] [Google Scholar]
- 48.Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Lina G, Etienne J, Ehresmann B, Ehresmann C. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005;24:824–835. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichenbach B, Maes A, Kalamorz F, Hajnsdorf E, Gorke B. The small RNA GlmY acts upstream of the sRNA GlmZ in the activation of glmS expression and is subject to regulation by polyadenylation in Escherichia coli. Nucleic Acids Res. 2008;36:2570–2580. doi: 10.1093/nar/gkn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol. 2006;9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Gullier M, Gottesman S, Storz G. Modulating the outer membrane with small RNAs. Genes Dev. 2006;20:2338–2348. doi: 10.1101/gad.1457506. [DOI] [PubMed] [Google Scholar]
- 52.Holmqvist E, Reimegård J, Sterk M, Grantcharova N, Römling U, Wagner EG. Two antisense RNAs target the transcriptional regulator CsgD to inhibit curli synthesis. EMBO J. 2010;29:1840–1850. doi: 10.1038/emboj.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song T, Mika F, Lindmark B, Zhi L, Schiid S, Bishop A, Zhu J, Camilli A, Johansson J, Vogel J, et al. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 2008;70:100–111. doi: 10.1111/j.1365-2958.2008.06392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright K, Seed P, Hultgren S. Development on intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 55.Jordi B, op den Camp I, de Haan L, van der Zeijst B, Gaastra W. Differential decay of RNA of the CFA/I fimbrial operon and control of relative gene expression. J. Bacteriol. 1993;175:7976–7981. doi: 10.1128/jb.175.24.7976-7981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nilsson P, Naureckiene S, Uhlin BE. Mutations affecting mRNA processing and fimbrial biogenesis in the Escherichia coli pap operon. J. Bacteriol. 1996;178:683–690. doi: 10.1128/jb.178.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loomis W, Koo J, Cheung T, Moseley S. A tripeptide sequence within the nascent DaaP protein is required for mRNA processing of a fimbrial operon in Escherichia coli. Mol. Microbiol. 2001;39:693–707. doi: 10.1046/j.1365-2958.2001.02241.x. [DOI] [PubMed] [Google Scholar]
- 58.Balsalobre C, Morschhäuser J, Jass J, Hacker J, Uhlin BE. Transcriptional analysis of the sfa determinant revealing multiple mRNA processing events in the biogenesis of the S fimbriae in pathogenic Escherichia coli. J. Bacteriol. 2003;185:620–629. doi: 10.1128/JB.185.2.620-629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Las Penas A, Connolly L, Gross CA. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol. Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 60.Rowley G, Spector M, Kormanec J, Roberts M. pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nature Rev. 2006;4:383–394. doi: 10.1038/nrmicro1394. [DOI] [PubMed] [Google Scholar]
- 61.Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton J, Vogel J. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaurin B, Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell. 1983;32:809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 63.Charlier D, Piette J, Glansdorff N. IS3 functions as a mobile genetic promoter in E. coli. Nucleic Acids Res. 1982;10:5935–5948. doi: 10.1093/nar/10.19.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wachi M, Umitsuki G, Shimizu M, Takada A, Nagai K. Escherichia coli cafA gene encodes a noval RNase, designated as RNase G, involved in processing of the 5’ end of 16S rRNA. Biochem. Biophys. Res. Commun. 1999;259:482–488. doi: 10.1006/bbrc.1999.0806. [DOI] [PubMed] [Google Scholar]
- 65.Otsuka Y, Yonesaki T. A novel endoribonuclease, RNase LS, in Escherichia coli. Genetics. 2005;169:1320. doi: 10.1534/genetics.104.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kole R, Baer M, Stark B, Altman S. E. coli RNAse P has a required RNA component in vivo. Cell. 1980;19:881–887. doi: 10.1016/0092-8674(80)90079-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.