Abstract
Plasma membrane hemichannels composed of connexin (Cx) proteins are essential components of gap junction channels but accumulating evidence suggests functions of hemichannels beyond the communication provided by junctional channels. Hemichannels not incorporated into gap junctions, called unapposed hemichannels, can open in response to a variety of signals, electrical and chemical, thereby forming a conduit between the cell’s interior and the extracellular milieu. Open hemichannels allow the bidirectional passage of ions and small metabolic or signaling molecules of below 1–2 kDa molecular weight. In addition to connexins, hemichannels can also be formed by pannexin (Panx) proteins and current evidence suggests that Cx26, Cx32, Cx36, Cx43 and Panx1, form hemichannels that allow the diffusive release of paracrine messengers. In particular, the case is strong for ATP but substantial evidence is also available for other messengers like glutamate and prostaglandins or metabolic substances like NAD+ or glutathione. While this field is clearly in expansion, evidence is still lacking at essential points of the paracrine signaling cascade that includes not only messenger release, but also downstream receptor signaling and consequent functional effects. The data available at this moment largely derives from in vitro experiments and still suffers from the difficulty of separating the functions of connexin-based hemichannels from gap junctions and from pannexin hemichannels. However, messengers like ATP or glutamate have universal roles in the body and further defining the contribution of hemichannels as a possible release pathway is expected to open novel avenues for better understanding their contribution to a variety of physiological and pathological processes. This article is part of a Special Issue entitled: The Communicating junctions, roles and dysfunctions.
Keywords: Connexin hemichannel, Pannexin hemichannel, Paracrine signaling
1. Introduction
In multicellular systems, proper organ and tissue function is sustained by cell-to-cell signal transduction that coordinates cell function by intercellular communication. This cross-talk between cells can be achieved in a direct manner, by the transfer of signaling molecules through gap junction channels that are composed of connexin proteins [1]. The latter consists of four membrane-spanning domains, two extracellular loops, an intracellular loop and flanking N- and C-termini also located on the intracellular side. At present, 20 connexin genes have been identified in the mouse and 21 in the human genome [2]. Newly synthesized connexins are oligomerized in the Golgi apparatus and the trans-Golgi network to hexameric channels called hemichannels [3]. Following insertion into the plasma membrane, connexin hemichannels interact with their counterparts on neighboring cells in a head-to-head arrangement, forming gap junction channels that coalesce into junctional plaques. Gap junctions allow the direct exchange between cells of metabolic and signaling molecules with molecular weight (MW) below 1–2 kDa [4]. Interestingly, connexin hemichannels may also remain unapposed in non-junctional plasma membrane. When open, unapposed hemichannels form a transmembrane conduit, allowing the passage of ions and molecules with a similar size cut-off limit as for gap junction channels. Table 1 gives an overview of the substances for which there is evidence that they may be released via connexin hemichannels. Cytosolic signaling molecules including adenosine triphosphate (ATP), nicotinamide adenine dinucleotide (NAD+), glutamate, glutathione and prostaglandins thus can diffuse through the channel to reach the extracellular milieu, mediating the spread of the signal followed by binding to receptors on surrounding cells and activating downstream cellular responses. Here, we review the available evidence for connexin hemichannels as a release pathway for paracrine intercellular communication and their role in paracrine signaling under normal and pathological conditions. First, we summarize the factors and conditions leading to connexin hemichannel opening and the methods used to study their function. We then give an overview of paracrine messengers released via hemichannels and discuss their possible contribution to tissue/organ function. In addition, we discuss pannexin (Panx) hemichannels as a release pathway for paracrine messengers (see also Table 1). Panx proteins are orthologues of invertebrate innexins and currently consist of 3 members: Panx1, Panx2 and Panx3 [5]. They do not share a sequence homology with connexins but have a similar structure and membrane topology [6]. Pannexins are considered to predominantly form unapposed hemichannels and not gap junctions [7,8], and for this reason it has been proposed to call pannexin hemichannels just ‘channels’ [7]. We prefer to call pannexin-based channels ‘hemichannels’ until it is mechanistically clear why these proteins cannot form gap junction channels. This is also beneficial in terms of clarity, as the two hemichannel types – composed of pannexins or connexins – form a special class of release channels for paracrine messengers.
Table 1.
Overview of substances for which there is evidence that they may be released via hemichannels.
| Paracrine signaling molecules | Molecular structure | Molecular weight | Net charge at physiological pH | Connexin types involved |
|---|---|---|---|---|
| ATP |

|
505 | −3 or −4 | Cx26 [196] Cx26/Cx30 [38,117,171,174,183] Cx30 [180] Cx36 [186] Cx43 [33,37,42,43,45,58,127,157,159,161,164,190,219] Panx1 [72,100,178,179,200] |
| NAD+ |

|
663 | +1 | Cx43 [206–208] |
| Glutamate |

|
146 | −1 | Cx32 [217] Cx43 [214–216] |
| Glutathione |

|
307 | −1 | Cx43 [224–226] |
| PGE2 |

|
352 | 0 | Cx43 [44,49,138,228] |
| EET |
|
321 | −1 | Panx1 [203] |
PGE2: prostaglandin E2. EET: epoxyeicosatrienoic acids.
2. Triggers for hemichannel opening
2.1. Transmembrane voltage
Connexin hemichannels are present as unapposed channels in the plasma membrane, on their way to being incorporated into gap junctions. Unapposed hemichannels are typically closed under normal conditions and, based on the large unitary conductance of hemichannels composed of certain connexins like Cx26, Cx43 and Cx46 (200–350 pS), they have long been assumed to remain closed until forming gap junctions, to prevent the loss of essential small molecules and the dissipation of ion gradients for Ca2+, Na+ and K+. In the early 90s the first reports appeared suggesting that unapposed hemichannels may open under certain conditions. The opening of unapposed connexin hemichannels in the plasma membrane was initially reported in Xenopus oocytes exogenously expressing lens fiber Cx46 [9,10]. Although the finding was originally thought to be restricted to the oocyte expression system, later evidence brought up the possibility that Cx46 can also form unapposed hemichannels in the lens [11,12]. Up to date, a wide range of connexin types have been reported to form unapposed plasma membrane hemichannels that can be opened by certain stimuli. Electrophysiological evidence documenting hemichannel opening is now available for Cx26, Cx30, Cx31.9, Cx30.2, Cx32, Cx35, Cx36, Cx37, Cx38, Cx43, Cx45, Cx45.6, Cx46, Cx50, Cx55.5 and Cx56 [13–23]. Similar to voltage-sensitive ion channels, connexin hemichannels (except Cx36 and Cx30.2) are sensitive to transmembrane voltage (Vm). Membrane polarization to positive potentials tends to open hemichannels composed of Cx26, Cx30, Cx32, Cx35, Cx37, Cx43, Cx45 and Cx46 (reviewed in [24]). By contrast, Cx50 hemichannels remain open over a wide voltage range and only close when Vm is below −50 mV or above +20 mV [13]. In line with the voltage-dependent profile of hemichannel opening, changes of Vm brought about by oxidative stress have been reported to activate connexin hemichannels [25].
2.2. Extracellular and intracellular ions
Opening of unapposed hemichannels is potentiated by lowering the extracellular calcium concentration ([Ca2+]o; normal level 1–2 mM) [26], a condition that often is associated with organ ischemia [27]. Atomic force microscopy (AFM) of reconstituted Cx43 and Cx40 hemichannels have detailed real-time transitions between closed and open conformations as a function of [Ca2+]o[28,29]. Similar changes of channel structureby external Ca2+ including the confined extracellular vestibule of the lumen was also reported in Cx26 hemichannels dissected from gap junctions making use of the AFM stylus [30]. The initial explanation was that Ca2+ ions attracted by the negative resting Vm enter the channel pore and bind to a channel blocking site at the cytoplasmic end [18]. A later study on Cx46 hemichannels however argued that [Ca2+]o may modulate intrinsic voltage-dependent gating by binding to an extracellular side of the hemichannel, locking the channel to a long-lived closed state [31]. In Cx32 hemichannels, a ring of 12 Asp residues residing at the external vestibule of the channel pore has been identified as a Ca2+-binding site that accounts for the channel block [23]. Interestingly, replacing external Na+ with other monovalent cations such as K+ or Cs+ can reduce Cx50 hemichannel sensitivity to [Ca2+]o[32]. Removal of extracellular Cl− has been reported to potentiate the opening of Cx43 hemichannels as concluded from ATP release measurements [33]. While low [Ca2+]o increases the open probability of hemichannels, changes in intracellular Ca2+ concentration ([Ca2+]i) also affect the function of hemichannels. Hemichannels formed of Cx32 or Cx43 exhibit enhanced ATP release and dye uptake in response to a moderate elevation of [Ca2+]i to ~500 nM [34,35]. Hemichannel opening triggered by [Ca2+]i changes has been reported in embryonic retinal pigment epithelium, in neural precursor cells and in supporting cells of the organ of Corti in the inner ear [36–38]. So far reported data show that hemichannel opening requires an intramolecular binding of C-terminal domain (CT) to cytoplasmic loop (CL) [39], possibly acting via an intermediate signaling pathway including Ca2+/Calmodulin (CaM), Ca2+/CaM-dependent protein kinase II and p38 mitogen-activated protein kinase (MAPK) [35,40]. Cx43 hemichannels are also equipped with inactivation mechanisms and channel closure has been reported to occur when [Ca2+]i rises above 500 nM [34,35]. Recent work has confirmed this inhibition by high [Ca2+]i at the unitary current level (Wang et al., under review).
2.3. Mechanical strain
Mechanical forces acting on the plasma membrane or on the connexin protein also mediate hemichannel opening. This was first demonstrated in Cx46-expressing Xenopus oocytes exposed to a negative pressure or hypotonic solution, which caused hemichannel opening at negative holding potential [41]. Further studies pointed out that many other connexins exhibit a similar mechanosensitivity. In tissues normally exposed to mechanical stress, such as osteocytes, periodontal ligaments and corneal endothelial cells, Cx43 hemichannels tend to open in response to mechanical stimulation and mediate the release of ATP or prostaglandin E2[42–44]. These extracellular signaling molecules, as will be discussed later, are proposed to play a role in controlling tissue formation and regeneration. Activation of hemichannels by mechanical forces has also been reported in astrocytes expressing Cx43, cochlear cells expressing Cx26 as well as hepatocytes expressing Cx26 and Cx32 [45–47].It is still not clear which molecular mechanisms underlie mechanical stress-related activation of connexin hemichannels. Cx43 hemichannels do not appear to respond directly to mechanical strain; in osteocytes, mechanical signals are transduced via the glycocalyx network, leading to rearrangement of actin cytoskeleton and Cx43 hemichannel opening [48]. The fact that stress-induced ATP release disappears by buffering [Ca2+]i changes suggests that mechanical stimuli may also act via [Ca2+]i elevation [43]. In primary osteocytes, shear stress increased the fraction of plasma membrane-associated Cx43 protein relative to the total connexin expression, suggesting that augmented activities of hemichannels are related to their enhanced presence in the plasma membrane [49].
2.4. Post-translational modifications
Many connexins (except Cx26) are phosphoproteins and collected data show that alterations in their phosphorylation status clearly affect functions of connexin hemichannels. In Xenopus oocytes exogenously expressing Cx46, protein kinase C (PKC) directly phosphorylates Cx46, resulting in reduced Cx46 hemichannel-mediated currents [50,51]. Similarly, reconstituted Cx43 hemichannels in liposomes exhibited reduced permeability in response to PKC-mediated phosphorylation of serine 368 [52]. Channels containing the serine 368 substitution to alanine are resistant to PKC modulation and stay preferentially open, suggesting that dephosphorylation of serine 368 favors Cx43 hemichannels opening [52,53]. A MAPK phosphorylation site has also been identified in Cx43, which causes reduced hemichannel function upon phosphorylation. Hyperosmolarity-induced activation of Cx43 hemichannels may be attributed to dephosphorylation at PKC or MAPK consensus site [54]. Intracellular ATP depletion in renal-tubule cells causes dephosphorylation at serine 368 which enhances Cx43 hemichannel activities [55]. The additional post-translational modification of S-nitrosylation of Cx43 CT domains also influenced hemichannel activity and the net effect of such modification appears to depend on the phosphorylation state of the connexin protein [24,56,57].
2.5. Triggers and modulation of pannexin-based hemichannels
Among the pannexins, Panx1 has been best documented in terms of its hemichannel function as a passageway for ions, metabolites and signaling molecules with a MW below 1 kDa, for example ATP [58]. Extensive electrophysiological data demonstrate Panx1 hemichannel opening upon membrane depolarization (see next section). Thus far, there is no evidence that Panx2 forms functional (homomeric) hemichannels, but Panx2 may be involved, together with Panx1, in heteromeric hemichannels [59,60]. Little evidence is yet available concerning Panx3 hemichannels; recent work in osteoblasts anticipates the presence of Panx3 hemichannels both in endoplasmic reticulum and cell surface [61]. Mechanical plasma membrane stress activates Panx1 hemichannels exogenously expressed in Xenopus oocytes [62,63]. An increased extracellular K+ concentration caused by excessive neuronal activity has been reported to stimulate astrocytic Panx1 hemichannels also in a membrane potential-independent manner [64], by an intrinsic yet undefined effect of extracellular K+. Panx1 hemichannels are also activated by purinergic stimulation: when expressed in Xenopus oocytes, they open in response to activation of P2Y receptors [62]. In addition, there is strong evidence that Panx1 hemichannels are the large conductance pore recruited in response to prolonged activation of P2X7 receptors [65]. Thus, P2X7 receptors may act in concert with Panx1 hemichannel as a mechanism of ATP-induced ATP release. Panx1 hemichannels do not respond to changes of [Ca2+]o[66]. However, in cells co-expressing P2X7 receptors and Panx1, removal of extracellular Ca2+ may open the channel via indirect mechanisms including i) a promoted complex formation between P2X7 receptors and Panx1 hemichannels, and ii) an enhanced affinity of P2X7 receptors to such an extent that baseline ATP release (regardless of the mechanisms involved) is sufficient for Panx1 channel activation [67] (Fig. 1).
Fig. 1.
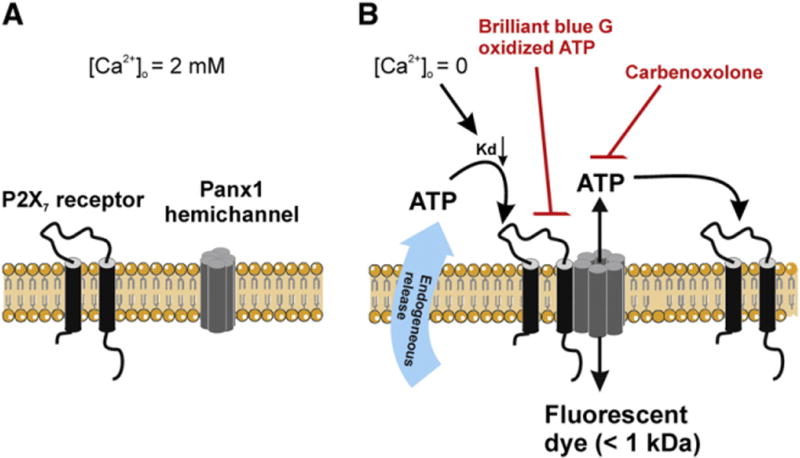
P2X7 receptors are essential to confer sensitivity to extracellular Ca2+ of Panx1 hemichannels. A. At normal extracellular Ca2+ concentration ([Ca2+]o), P2X7 receptors and Panx1 hemichannels both favor the closed states and no interaction takes place between these two channels. B. Removal of extracellular Ca2+ activates P2X7 receptor by increasing the binding affinity (Kd lowering) of the channel for endogenously released ATP. Activation of the P2X7 receptor initiates complex formation with Panx1 hemichannels which in turn serve as an associated portal, allowing the passage of ATP or reporter dyes with a MW below ~1 kDa. Lowering [Ca2+]o may strengthen the interaction between P2X7 receptor and Panx1 hemichannel further by an undefined signaling mechanism, yet independent on purinergic stimulation. Both P2X7 receptor inhibitors such as Brilliant blue G and oxidized ATP and Panx1 hemichannel blockers carbenoxolone abolish the low [Ca2+]o-triggered ATP release.
Like hemichannels composed of Cx32 and Cx43, Panx1 hemichannels are also equipped with an inactivation mechanism, with channel closure occurring as a consequence of ATP-induced inhibitory feedback [68]. Although Panx1 and Panx3 both possess several putative phosphorylation sites at the intracellular domains, treatment of alkaline phosphoatase failed to alter any shifts in electrophoretic mobility of the proteins [8]. Instead, Panx1 and Panx3 are glycosylated at the second and first extracellular loop regions, respectively [8,69]. The impact of glycosylation on pannexin hemichannel functions remains unclear, but this post-translational modification distinct from connexins is crucial for intracellular trafficking and is thought to explain the unlikelihood of docking between two pannexin hemichannels [69–71]. Finally, Panx1 is a substrate of caspase [72]: during apoptosis, caspase-mediated cleavage at the CT domain promotes hemichannel activity.
3. Methodological approaches for studying hemichannels
Before discussing the role of hemichannels as a release pathway for paracrine messengers, it is important to summarize the methods and tools used to study function of hemichannels. Most importantly, not all approaches when used individually lead to equally strong evidence and a multi-method approach is often required for correct interpretation of experimental data [73].
3.1. Biochemical identification of unapposed plasma membrane hemichannels
In the early 1990s Musil and Goodenough introduced a biotinylation technique for the isolation of unapposed hemichannels in the plasma membrane [74]. The technique is based on the labeling of free surface membrane protein containing lysine residues in intact cells, making use of the membrane-impermeable and thiol-cleavable amine-reactive reagent NHS-SS-Biotin. In gap junction channels, the reactive lysine residues at the extracellular side of the connexin protein are sealed into the intercellular space and therefore inaccessible for the biotin assay [75]. Thus, only unapposed hemichannels at non-junctional membranes are labeled by biotin. Further processing by pull down of avidin–biotin–connexin complexes and subsequent separation of connexin from this complex via elution column allow enrichment of the connexin pool associated with unapposed hemichannels. Further work has validated this method for evaluating the role of Cx43 and Cx32 hemichannels in non-junctional membranes [40,49,56,57,76–78].
Biochemical isolation of Triton X-100 (a non-ionic detergent) insoluble fraction [74] is a widely pursued alternative for isolating connexins assembled into gap junction channels. However, the detergent-resistant fraction may be contaminated by hemichannels. Connexin hemichannels are located in ‘lipid rafts’, which are membrane microdomains enriched in sphingolipids and cholesterol [79]. Close lipid packing at these regions limits the accessibility of connexin hemichannels to the detergent which thus should be considered as detergent-resistant. Gap junction plaques do not colocalize with lipid raft structures [79,80] but may also be associated with high cholesterol membrane zones.
Microscopic imaging provides the most direct approach towards identifying unapposed plasma membrane hemichannels. Among the membrane fractions isolated from rat heart, atomic force microscopy characterized a population of native connexin channels that shares similar hexameric arrangement as gap junctions, but has half the thickness of a gap junction channel. These structures described by Lal et al. as ‘hemiplaques’, should be considered as the earliest evidence addressing structural aspects of unapposed hemichannels at the cell surface [81]. In intact cells or tissue, differentiating hemichannels at the non-junctional side of the membrane from whole junctions is based on antibodies directed against conserved sequences of the first or second extracellular loop of the connexin protein; such antibodies are available for Cx26, Cx32 and Cx43 [82,83]. These site-directed antibodies only recognize freely-exposed extracellular loops of the connexins in the plasma membrane, thus specifically reporting the surface distribution of unapposed hemichannels.
3.2. Electrophysiology of unapposed hemichannels
The most direct approach to study functions of connexin hemichannels is by means of electrophysiological recording of unitary currents. The single channel conductance is the signature of connexin isoform being recorded from. The voltage–conductance profile furthermore helps in identifying the hemichannel type [84]. In whole-cell recordings on single non-connected HeLa cells overexpressing Cx43, unitary events associated with Cx43 hemichannel openings can be unambiguously recognized by the single channel conductance (γo) of ~220 pS when stepping the Vm to positive potentials (> +40 mV) [15] (Fig. 2). In astrocytes exposed to pro-inflammatory cytokines, these typical ~220 pS single-channel events appeared at negative Vm, indicating that increased channel opening can also be induced at normal resting potentials [78]. Cx43 hemichannel current reverses near 0 mV, indicating a non-selective nature of the channel. For Cx26 and Cx46 hemichannels characterized in Xenopus oocytes, a moderate depolarization from −80 to −50 mV appears to be sufficient to trigger single channel openings with a γo of ~317 pS and ~300 pS, respectively [16,85]. Hemichannels composed of Panx1 exhibit substantially larger unitary conductance of ~475 pS as reported in Xenopus oocytes and ~520 pS in pyramidal neurons [63,86]. Macroscopic current recordings reported a low voltage threshold for Panx1 hemichannel activation (~−20 mV) [66].
Fig. 2.

Unitary current activities of Cx43 hemichannels. A. A schematic overview of whole cell recording in HeLa cells expressing Cx43 tagged with enhanced green fluorescent protein (EGFP) at the C-terminal. Currents mediated by Cx43 hemichannels are recorded under conditions of blockade of K+ channels using tetra-ammonium chloride (TEACl), BaCl2 and CsCl. B. The fluorescent image of Cx43-EGFP cells, illustrates abundant presence of Cx43 at the plasma membrane. C. Representative whole-cell currents recorded in the Hela Cx43-EGFP cell indicated by an asterisk in panel B. Stepping Vm to +50 mV induces clearly discernible unitary current activities. The expanded trace shows tail currents with step-wise closure of 3 channels upon Vm repolarization to −25 mV. D. All-point histogram of the conductance evaluated from the trace shown in C at +50 mV. The peaks of the event distribution are separated by ~220 pS which equals to the γo of Cx43 hemichannels in the plasma membrane. E. Gently pulling the whole-cell recording electrode away from the cell establishes an outside-out recording configuration (extracellular face directed to the bath solution). F. Outside-out recordings often exhibit only a few active hemichannels as illustrated in the example current trace. G. All-point histogram of the conductance evaluated from the currents shown in E indicates a γo of ~220 pS.
It remains uncertain why different connexins exhibit distinct voltage-sensitivities and a broad range of unitary conductances. Multiple domains including N-terminus, the first transmembrane domain and the first extracellular loop have all been implicated in pore-lining of connexin hemichannels [87–90]. Mutagenesis at these sites is associated with modified charge selectivity and unitary conductance. Diverse unitary conductances are not unexpected given the fact that pore-lining segments may differ across connexins. Moreover, there is no definitive answer yet to where the voltage sensor of a connexin hemichannel is located. It seems that voltage-sensing domain reflects another connexin-specific property [91–93]. Therefore, further studies are needed to ascribe connexin type-dependent differences to intrinsic properties of the hemichannel building blocks. Noteworthy, the biophysical properties of hemichannels also depend on species-specific differences of the same connexin isoform and on expression system in which the hemichannels are examined. For example, Cx43 hemichannels do not open in response to depolarization when expressed in Xenopus oocytes [94] but they do open in mammalian cells endogenously or exogenously expressing Cx43. Xenopus oocytes overexpressing Panx1 exhibit a prominent increase of hemichannel currents by elevating [Ca2+]i[62] but these results cannot be reproduced in mammalian cells stably transfected with Panx1 [95] or in hippocampal neurons [96]. In terms of species-specific differences, it is known that human/sheep Cx26 but not rodent Cx26 are capable of forming functional hemichannels as a consequence of difference in one amino acid [16].
3.3. Uptake or release of reporter molecules
Single channel recordings or biotinylation studies are not always possible in intact tissues. In such cases, hemichannels can be studied making use of the uptake into the cells of fluorescent reporter dyes or the release of endogenous or exogenously loaded molecules with a MW below 1 kDa [9,97]. In connexin hemichannel studies, dye uptake or release studies are performed using various low MW, membraneimpermeable fluorescent probes including Lucifer yellow (LY; MW = 444, net charge = −1), 6-carboxyfluorescein (CF; MW = 376, net charge = −2), calcein (MW = 623, net charge = −4), ethidium bromide (EtBr; MW = 394, net charge = +1), DAPI (MW = 279, net charge = +2) and propidium iodide (PI; MW = 668, net charge = +2) [98,99]. YO-PRO-1 (MW 629, net charge = +2), similar as EtBr or PI, with a dramatically increased fluorescence intensity upon binding to DNA and RNA are extensively used as an indicator for Panx1 hemichannel openings [65,72,100]. In both cultured and primary cells, quantifying uptake or release of the intracellular fluorescence of reporter dye has been extensively pursued as an indirect measure of connexin hemichannel activities [34,97,101,102,102,102,103]. Such experiments allow a dynamic assessment of hemichannel response in real time using time-lapse imaging [104]. An advantage of PI and EtBr as reporter dyes is that the fluorescence of these probes is strongly enhanced upon binding to DNA, increasing the contrast and allowing more easy identification of the cells displaying hemichannel opening. Dye uptake assays are also suitable for hemichannel characterization in intact in vitro tissues. EtBr uptake studies have been applied to mouse hippocampal slices to study hemichannel opening in astrocytes in responseto exogenous glucose deprivation [105]. In mice with bacterial infection-induced brain abscess, a similar set of experiments was conducted to study the effect of inflammation on astrocytic hemichannel openings [106]. In these studies, astrocytes taking up the dye were identified by their GFP expression under control of the glial fibrillary acidic protein (GFAP) promoter in transgenic animals. A major drawback of these assays is that synthetic reporter dyes listed above are biologically irrelevant [99]. This limitation may be overcome by using fluorescently labeled metabolic derivatives such as the fluorescent glucose derivatives 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG) and the nonhydrolyzable 6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-6-deoxyglucose (6-NBDG) [78].
Open connexins and pannexin-based hemichannels also permit the efflux of several bioactive molecules such as ATP, glutamate and prostaglandin E2, which can be detected in the extracellular medium by appropriate measurement techniques. A bioluminescence assay based on luciferin–luciferase has been extensively used to detect extracellular ATP, mainly because of its high sensitivity and linear response over a very wide range of ATP concentrations. Because it has been used so frequently, it suggests the false impression that hemichannels mainly release ATP. Moreover, many other ATP release pathways exist such as via the cystic fibrosis transmembrane regulator (CFTR) protein, volume- and voltage-dependent anion channels, voltage-gated Cl− channels and P2X receptor pores. Work of the Nedergaard lab has brought up an interesting approach that combined ATP release measurements with single hemichannel recordings, to document in an unequivocal manner that hemichannels are indeed permeable to ATP [107]. In this study, an inside-out patch excised from a glioma cell overexpressing Cx43 was subjected to single channel recording of Cx43 hemichannels with ATP as main charge carrier. A second electrode placed adjacent to the excised patch was used to expel small quantities of luciferin/luciferase, allowing the detection of ATP present in the extracellular milieu from the bioluminescent signal. A close correlation between ATP release and unitary current activities of Cx43 hemichannels provided firm evidence that connexin hemichannels are involved in transmembrane flux of ATP.
3.4. Interfering with hemichannel function
Suppression of specific connexin isoforms making use of small interfering RNA technology or knock out (KO) animals are crucial to help in elucidating the nature of the molecular uptake/release pathway [99]. For example, an up to 60% Cx43 knockdown prevented LY uptake in neuroblastoma (N2A) cell cultures and pointed to a potential role of Cx43 hemichannels in oxidative-stress induced cell death [25]. This can be complemented by connexin KO animal approaches, either as global KO, conditional KO in specific cell types or inducible KO by tamoxifen injection (Cx43Cre-ER(T)/fl, one Cx43 coding region replaced by tamoxifen inducible Cre recombinase and the other flanked by two separate loxP sites) [108,109]. A critical limitation of these genetic tools is that such approaches deplete both gap junction channel and hemichannel populations, challenging the validity of the conclusions drawn from such experiments. In addition, in most cases multiple connexin isoforms are coordinately expressed in native cells and interfering with the expression of one connexin can affect the expression of non-targeted connexin isoforms [110–114]. Further work with more refined models making use of inducible knock in technology to switch on the expression of mutant connexins that lack the extracellular loop cysteins and therefore are unable to pair up as gap junctions may be an interesting approach [101]. The use of highly selective hemichannel blockers is another approach. However, gap junctions are composed of the very same molecular building blocks and most gap junction blockers including long-chain alcohols, glycyrrhetinic acid derivatives, and flufenamic acid also abrogate functions of unapposed hemichannels [115]. Some of these agents influence the membrane fluidity and this non-specific effect may have implications on other ion channels present in the plasma membrane. A recent study found that the widely used hemichannel blocker, carbenoxolone, can effectively block volume-regulated anion channels [116]. Conversely, other ion channel blockers like 5-nitro-2-(3-phenylpropyl-amino)-benzoic acid and tamoxifen also inhibit hemichannel openings. Lanthanum (La3+, 100 μM) is a heavy metal ion that blocks depolarization-induced Cx43 hemichannel currents at the single channel [15] and macroscopic levels as well as calcein efflux in HeLa cells overexpressing Cx30-GFP [117], but not Cx43 or Cx30 gap junctions [117,118]. It is hypothesized that La3+ interacts with a site on the extracellular loops of unapposed connexin hemichannels which is freely accessible in the extracellular space, but becomes tightly sealed into the cell–cell interface after docking [99].
Connexin mimetic peptides, like Gap26 and Gap27, that are identical to highly conserved sequences located respectively on the first and second extracellular loop regions of for example Cx32, Cx37 and Cx43 are known as gap junction blockers. They were introduced in the 90s based on the hypothesis that such peptides could prevent docking of two connexin hemichannels, thereby preventing the formation of gap junctions [119,120]. 43Gap26 (VCYDKSFPISHVR) and 43Gap27 (SRPTEKTIFII) do block gap junctions [119,121] but the effect is time and concentration dependent. In most cases, a time window can be defined where hemichannels are inhibited without concurrent effects on gap junctions. The duration of this time window may depend on the connexin turnover rate: in most cases 1 h exposure times have no effect on gap junctions but even 6 h exposures have been reported not to influence gap junctions [122]. Currently, our working hypothesis is that 43Gap26 or 43Gap27 interact with unapposed hemichannels thereby inhibiting these channels, followed by impaired docking and failure to form new gap junction channels [123]. The effectiveness of gap junction blocking also appears to depend on the peptide concentration. Peptides like 43Gap26 and 43Gap27 are often used at concentrations in the range of 100 to 300 μM. ‘Peptide 5’, which mimics a Cx43 sequence of a few amino acids in N-terminal direction as compared to 43Gap27 and also contains the SRPTEK sequence present in 43Gap27, blocks Cx43 hemichannels but does not influence gap junctions when applied at a concentration of 5 μM even with exposure times as long as 24 h [124]. Recent work in ischemia applied to the brain of fetal sheep or to adult rat retina has brought up in vivo evidence that Peptide 5 confers cellular protection by targeting Cx43 hemichannels [125,126].
Our initial studies reporting hemichannel block by connexin mimetic peptides were based on ATP release/dye uptake measurements in response to [Ca2+]i elevation [35,42,123,127]. These findings were complemented by electrophysiology studies indicating that these peptides also inhibited depolarization-induced macroscopic currents in Cx43 expressing taste cells [128]. More recently, we have found that 43Gap26/27 indeed inhibits Cx43 hemichannel-mediated unitary current activities within minutes in cell lines stably transfected with Cx43 and in native cells endogenously expressing Cx43 at concentrations in the 100–200 μM range (Wang et al., under review). In most cases, inhibition of gap junctions is much slower, often in the range of several hours as mentioned before [122,129].
A potential tool for more selective inhibition of hemichannels without associated inhibition of gap junctions consists of a synthetic peptide that corresponds to the L2 sequence on the CL of Cx43 (amino acids 119–144, further called L2 peptide) [39]. Evidence that the L2 domain is an important molecular determinant of Cx43 channel function was obtained in the context of studies performed to understand acidification-induced closure of gap junctions. Divergent techniques including resonance mirror spectrometry, translation diffusion analysis and enzyme-linked sorbent assay all pointed to an increased interaction between the L2 domain and the CT of Cx43 at low pH (~6.5) [130,131]. Over the past decade, a ‘ball-and-chain’ model has been proposed to explain the Cx43 gap junction closure during acidification: driven by the decline of pH, the CT may move as a flexible gating particle and bind to the L2 site at the intracellular vestibule, resulting in a confined channel pore [132–135]. Interestingly, work with L2 peptide has further demonstrated that the ball-and-chain hypothesis may also apply to the voltage-dependent gating of Cx43 gap junction channels. In this case, intramolecular CT–CL interaction is hypothesized to underlie the fast gating transition from the fully open state to the sub-conductance state [136]. L2 peptide, when delivered into the cell via a whole-cell recording pipette, prevented the Cx43 gap junction channel residing from the open to the sub-conductance state at high transjunctional voltage (Vj = ± 60 mV) as well as slightly increased the open time. However, questions as to how one unique molecular basis accounts for two distinct gates, i.e., pH-dependent chemical gate versus voltage-dependent fast gate is yet not resolved. More recently, collaborative work with the Bultynck and Retamal groups has demonstrated that L2 peptide linked to the TAT membrane-translocation sequence (TAT-L2) inhibits Cx43 hemichannel-related ATP release, dye uptake and macroscopic membrane currents [39]. Importantly, this work indicated that CT–CL interaction is actually necessary for hemichannel activation. Thus, preventing CT–CL interaction with L2 peptide inhibits unapposed hemichannels while slightly stimulates gap junctional coupling. A follow-up study in which TAT-L2 was locally injected into the rat basolateral amygdala demonstrated inhibition of fear memory consolidation, indicating a role of astroglial Cx43 hemichannels in memory function [137].
Antibodies targeting the second extracellular loop of Cx43 (aa 185–206; Cx43(E2)) have also been used to block hemichannels [44,138]. Interestingly, Cx43(E2) antibodies inhibit Cx43 hemichannel-associated prostaglandin release in osteocytes, but leave dye coupling and P2X7 receptor unaffected. Further electrophysiological characterization is necessary to define whether these antibodies block hemichannels directly, i.e. via pore block or through modulation of channel gating.
Careful interpretation is warranted when functional roles of connexin hemichannels are investigated with pharmacological tools. In most cases, not only gap junctions are influenced but also Panx1 hemichannels, be it at sometimes different concentrations [6], and several other ion channels and transporter proteins. Panx1 hemichannel currents are rapidly reduced by a low concentration of carbenoxolone (~5 μM) that has no effect on Cx46 connexin hemichannels [66] and show moderate sensitivity to flufenamic acid at concentrations that inhibit connexin hemichannels [99]. La3+ inhibits connexin hemichannels and exerts no effect on gap junctions or Panx1 hemichannels [65]; however, this trivalent ion also inhibits Ca2+ channels [139,140]. Probenecid blocks Panx1 hemichannel currents at concentrations that also inhibit organic anion transporters (150–350 μM) [141]; however, it does not inhibit currents mediated by Cx46 hemichannels or chimeric Cx32E143 hemichannels (the first extracellular loop of Cx32 was replaced with that of Cx43) [141]. The Panx1 mimetic peptide 10Panx1, containing a sequence on the first extracellular loop (WRQAAFVDSY), inhibits Panx1 hemichannels [65] but apparently also inhibits Cx46 hemichannel currents in the same concentration range (~200 μM) [142]. Additionally, Panx1 may form a complex with P2 receptors and therefore can be affected by P2 receptor antagonists [62,65,143,144] including brilliant blue G, pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), oxidized ATP and suramin [67].
Most recently, we characterized a peptide, Gap19, that corresponds to a sequence located in the L2 domain of Cx43. This peptide inhibits Cx43 hemichannels without inhibiting Panx1 hemichannels or gap junctions. In exogenous Cx43 expression systems and in freshly isolated ventricular cardiomyocytes that display strong endogenous expression of Cx43, we demonstrated that Gap19 peptide inhibits hemichannels by shifting the activation voltage for hemichannel opening towards more positive potentials, without affecting the single channel conductance (Wang et al., submitted).
4. Signaling molecules released through hemichannels
4.1. ATP
ATP efflux through open hemichannels is driven by a large gradient between intracellular (several millimolar) and extracellular concentration of ATP (~10,000 fold, which is comparable in magnitude to the Ca2+ concentration gradient but is in the inverse direction). Quantification of ATP release by bioluminescent luciferin–luciferase based assays, demonstrated that the cellular ATP loss is limited (≤ 0.1% of intracellular ATP content). Once released in the extracellular space, ATP and its metabolites ADP, AMP and adenosine diffuse away and can bind to purinergic receptors of the P1 (adenosine) and P2 subtypes on neighboring cells to produce metabotropic (P1 or P2Y)or ionotropic (P2X) downstream responses [145,146].
4.1.1. ATP release in the context of intercellular calcium waves
Significant evidence is available demonstrating connexin hemichannel involvement in ATP release as a paracrine pathway for the propagation of intercellular Ca2+ waves. Intercellular Ca2+ waves are cell-to-cell propagating [Ca2+]i changes that were initially reported to be mediated by the diffusion of the Ca2+ mobilizing messenger inositol 1,4,5-trisphosphate (IP3) via gap junctions [147–149]. The contribution of paracrine signaling was suggested later by the finding that focally triggered intercellular Ca2+ waves could spread to cells separated from the trigger zone by a cell-free region [150]. Medium collected from cell cultures displaying intercellular Ca2+ waves contains ATP and is capable of initiating intercellular Ca2+ waves when applied to non-stimulated cultures [151]. Blockage of purinergic receptors or degradation of ATP suppresses the spread of intercellular Ca2+ waves, supporting a contribution of ATP-mediated purinergic signaling in this process. Extracellular ATP-mediated propagation of intercellular Ca2+ waves have been established in various cell types ranging from glial cells to keratinocytes (reviewed in [152]). The linkage of ATP release to connexin hemichannels was based on the observation of a close correlation of the size of intercellular Ca2+ waves with the level of connexin expression [153], which was caused by increased connexin-related ATP release rather than augmented gap junction coupling [33]. However, connexin hemichannels are not the only pathway of ATP release involved in intercellular Ca2+ waves and there is evidence for vesicular release as well [154]. The trigger for hemichannel opening appears to be IP3[127] and subsequent [Ca2+]i changes [34,35]. If connexin hemichannels are involved, the waves are often larger under conditions of low extracellular Ca2+ or in the presence of quinine which both promote connexin hemichannel opening [45,155]. Interestingly, not only the size but also the frequency of spontaneously occurring intercellular Ca2+ waves is increased by lowering extracellular Ca2+[45,156,157], an effect that may result from increased connexin hemichannel opening and ATP release [158]. Bioluminescent ATP imaging has indicated that under low extracellular Ca2+ conditions, intercellular Ca2+ waves are associated with ATP release from a single cell. A burst of ATP from a single-cell has been concluded from the fact that only one cell of all the cells contributing to the wave displayed uptake of the fluorescent hemichannel-permeable dye propidium iodide [158]. These observations have been confirmed in another study [159].
An interesting approach toward influencing connexin channels involved in intercellular Ca2+ waves consists in the use of connexin targeting peptides. Short incubations with connexin mimetic peptides (Gap26 or Gap27) partly inhibit IP3-triggered intercellular Ca2+ waves, by inhibiting hemichannel ATP [127] while longer applications (> 1 h pre-incubation) more strongly inhibit the waves because of combined inhibition of hemichannels and gap junctions. Application of TAT-L2 peptide also partly inhibits intercellular Ca2+ wave propagation because of more selective hemichannel suppression without influencing gap junction channels [39].
Evidence for a contribution of hemichannel ATP release is also available from brain slices, retina and the organ of Corti in the inner ear. ATP release in embryonic slices from neocortex was assessed using GL261 glioma cells as biosensors that display [Ca2+]i changes in response to ATP [37]. Spontaneous Ca2+ waves and oscillations in neural precursor cells were associated with ATP release and both responses were inhibited by carbenoxolone (100 μM) and Cx43 silencing. A similar biosensor ‘sniffer cell’ approach has been used in neonatal brain slices to document ATP release associated with intercellular Ca2+ waves triggered by electrical stimulation [160]; however, connexin hemichannels did not appear to be involved in this study. By contrast, recent work in neonatal hippocampal slices has brought up clear evidence for hemichannel opening and subsequent ATP release [161]. A local decrease of [Ca2+]o, brought about by photoactivation of the Ca2+ buffer diazo-2, was used to trigger hemichannel opening and this triggered a slowly propagating (4 μm/s) intercellular Ca2+ wave in astrocytes. Imaging of extracellular ATP making use of luciferin–luciferase in combination with a low noise/highly sensitive CCD camera demonstrated a ‘cloud’ of ATP that also expanded in a wave-like manner as a function of time. The ATP release and Ca2+ wave were strongly reduced in Cx30/Cx43 deficient mice. This paper furthermore added evidence that increased neuronal activity is associated with a decrease in [Ca2+]o, which is sufficient to trigger hemichannel opening and ATP release from astrocytes. Importantly, the authors demonstrated that the released ATP activated inhibitory interneurons thereby providing negative feedback to the initially active neuron [161].
In the retina, stimulated intercellular Ca2+ waves propagate through glial cells that consist of both astrocytes and Müller cells [162]. In Müller cells, the waves strongly rely on paracrine purinergic communication and the retinal Ca2+ wave is associated with a wave of extracellular ATP increase [162]. Intercellular Ca2+ waves in retinal glial cells also occur spontaneously, both in vitro and in vivo, and the Ca2+ waves are associated with an extracellular ATP wave [163]. The evidence pointing to connexin hemichannels as the ATP release pathway in Müller cells is based on their abundant Cx43 expression, inhibition of ATP release by 100 μM carbenoxolone (but not 5 μM that only blocks Panx1 hemichannels) and insensitivity to inhibitors of CFTR or P-glycoprotein transporters [164]. In embryonic pigment epithelial cells, Cx43 hemichannels activated by spontaneous intracellular Ca2+ transients release ATP, as evidenced from work with short application of 43Gap26 (30 min) [159].
In organotypic cultures of the organ of Corti, intercellular Ca2+ waves triggered by various stimuli and propagating between supporting cells, were associated with ATP release as measured with biosensor cells [117]. The waves were dependent on Cx26 and Cx30 expression but not on the expression of Panx1 or P2X7, and were inhibited by La3+; collectively, these data indicate a contribution of connexin hemichannels to ATP release in these cells [38,117].
It is important to realize that ATP release via connexin hemichannels is not an isolated process. First of all, a mechanism of ATP-induced ATP release may act to amplify the ATP release [165]. This process involved activation of low affinity P2 receptors, which may well be P2X7 receptors that are coupled to Panx1 hemichannels opening [65,166]. Second, because ATP can be released from cells by diverse pathways, it is possible that an initial hemichannel-related ATP release event is adjoined by a combination of alternative ATP release pathways.
What is the function of hemichannel ATP release associated with intercellular Ca2+ waves? Some suggested functions will be discussed next but it is important to start with some remarks that may help to put this information in the correct context. First, ATP release and paracrine purinergic communication is just one aspect of intercellular Ca2+ waves. Several of the effects may well be the consequence of the associated [Ca2+]i changes or of a combination of [Ca2+]i dynamics and ATP release. Second, and this also holds true for proposed functions of other paracrine messenger released via hemichannels, there is up to now no evidence available that supports a complete paracrine signaling cascade that starts with the hemichannel release process, continues with actions on specific membrane receptors and culminates in clear, fully documented functional responses (preferably recorded in vivo). Obviously, the major problem here is the fact that the currently available tools and technology (KO and transgenic approaches) in most cases influence both gap junctions and hemichannels. Limited tools are becoming available however, as discussed above, and it is anticipated that this will bring up novel insights.
Extracellular ATP-mediated intercellular Ca2+ waves coordinate [Ca2+]i signaling within a group of neighboring cells, synchronizing different phases of cell cycles including resumed proliferation of resting cells, DNA synthesis and mitosis [167,168].
In the brain, during neocortical development, radial glial cells display intercellular Ca2+ waves that appear to be initiated by connexin hemichannel ATP release and that are involved in radial glia cell proliferation in the ventricular zone [157]. Here, as in other organs and tissues, ATP is proposed to act as a mitogenic signal that acts to synchronize cell cycles of a population of cells thereby coordinating proliferation in the ventricular zone [37]. As already referred to above, evidence has recently become available demonstrating astrocyte hemichannel opening and ATP release in response to neuronal activity. The ATP then activates interneurons thereby providing negative feedback [161]. Presumably, this neuron–astrocyte–neuron loop acts in parallel to the well documented direct activation of interneurons by an axon collateral.
In the retina, ATP release from pigment epithelial cells via hemichannels may act as a mitogenic signal to neural progenitor cells in the ventricular zone thereby controlling their proliferation and differentiation [36,159]. It has furthermore been suggested that this Ca2+ wave-associated ATP release maintains Rho-GTPase activity in neural progenitors which is a critical modulator of cytoskeleton-directed nuclear mobility during mitotic division [37].
In the cochlea, a structure of the inner ear, the evidence for hemichannels in ATP release and possible functional consequences is more extensive and for this reason, this subject will be discussed in a separate section that follows.
4.1.2. ATP release in the cochlea
Cx26 and Cx30 are most predominantly expressed in the cochlea supporting and epithelial cells, but are absent in the auditory sensory hair cells [169]. Animals with defective expression of Cx26/Cx30 in inner ear exhibit significant hearing loss. A great number of mutations in Cx26 and Cx30 have been identified in syndromic and nonsyndromic deafness. Functional assessments of Cx26/Cx30 in the cochlea point out that these two connexins can regulate auditory development and transduction in a paracrine manner through formation of connexin hemichannels.
Before the cochlea reaches maturity to convert sound into electrical signals in the auditory nerve fibers [170], sound-independent excitation of auditory nerves is required to fine tune the tonotopic map for optimal sound perception. In the developing cochlea, supporting cells in Köllicker’s organ, a transient structure in the organ of Corti located adjacent to the sensory hair cells, spontaneously release ATP that acts on purinergic receptors on inner hair cells [171]. As already discussed before (Section 4.1.1), there are several arguments pointing to connexin hemichannels as a likely release conduit. The released ATP has been demonstrated to trigger Ca2+ spikes in inner hair cells, triggering bursts of action potentials in the spiral ganglion neurons [171]. In this way, spontaneous activation of spiral ganglion neurons maintains survival of neurons in the cochlea nucleus and optimizes the spatial organization of auditory pathways during development.
In the mature cochlea, evidence from two different labs has demonstrated a unique distribution pattern that Cx26 is colocalized with Cx30 in the cochlear supporting and epithelial cells [172,173], indicating that these two proteins favor co-assembly into heteromeric hemichannels. Various physiological factors including sound-induced mechanical stimulation in the form of basilar membrane vibration, typically low [Ca2+]o (~20 μM) and high extracellular K+ levels ([K+]o ≈ 150 mM) in the endolymph are known as potent modulators of connexin hemichannels. Most of these stimuli (mechanical stimulation and low [Ca2+]o) have been found to trigger ATP release in vitro, and these responses are inhibited by connexin channel blockers but not by inhibiting vesicular ATP release or ATP-binding cassette transporters [174]. Taken together, it is thus plausible that ATP is released through heteromeric Cx26/30 hemichannels. The released ATP may act on outer hair cells, by binding to P2X receptors, which results in a decreased electromotility of these cells. Outer hair cells function as an active cochlear amplifier that promotes the vibrations of the basilar membrane. Thus, because ATP reduces the electromotility of outer hair cells, this will result in a decreased amplifier gain and hearing sensitivity.
4.1.3. ATP release in taste transduction
In taste buds, the signal transduction from taste cells to afferent nerve fibers appears to be more complex than initially thought. Serotonin (5-hydroxytryptamine (5-HT)) has long been considered as the major neurotransmitter between taste cells and gustatory nerve fibers. It was later discovered in a 5-HT3A KO animal model that preventing 5-HT signaling did not alter taste perception, suggesting an additional afferent neurotransmitter active in the taste transduction [175]. ATP became a plausible candidate because of the presence of P2X2/P2X3 receptors in the afferent nerve ending and this was soon confirmed by work in P2X2/P2X3 double KO mice [175]. Interestingly, the ATP releasing cells in the taste bud have been identified as Type II receptor cells which sense bitter, sweet, and umami compounds through activation of G-protein coupled taste receptors [176]. Of equal interest is the fact that these cells lack the vesicular machinery for the classic exocytotic release of neurotransmitters [177]. Further attempts to characterize the molecular basis of the release pathway revealed that ATP release in the receptor cells was driven by repetitive depolarization pulses, as would occur by generator potentials in activated taste cells, and was not dependent on [Ca2+]i changes by Ca2+ influx [176]. The connexin mimetic peptide 43Gap26 (5–15 min) abolished the ATP release in receptor cells while 32Gap27 exerted no effect. Electrophysiological recordings defined a voltage-dependent, 43Gap26-sensitive current whose magnitude was correlated with the intensity of the ATP release [128]. Low concentrations of carbenoxolone (5 μM, 30 min) had little effect suggesting no contribution of Panx1 hemichannels. However, others have reported that low carbenoxolone concentrations do inhibit ATP release and signal transduction pointing to Panx1 hemichannels [178]. ATP released from receptor cells in response to TRPM5 channel-mediated depolarization with concurrent [Ca2+]i elevation [179] may directly activate receptors on the afferent nerve ending, but may also stimulate adjacent presynaptic cells to secrete 5-HT [178]. Taken together, these observations suggest that both Cx43 and Panx1 hemichannels may play a role in taste cell signaling. Fig. 3 summarizes the currently available evidence supporting a role of hemichannels in paracrine signaling in taste cells; this example is perhaps one of the few covering a more complete paracrine signaling cascade with both hemichannel source and functional consequences being characterized. However, further work is still needed to delineate the distinct contribution of Cx43 and Panx1 hemichannels. The involvement of pannexins in ATP release is further considered in more detail in Section 4.1.6.
Fig. 3.
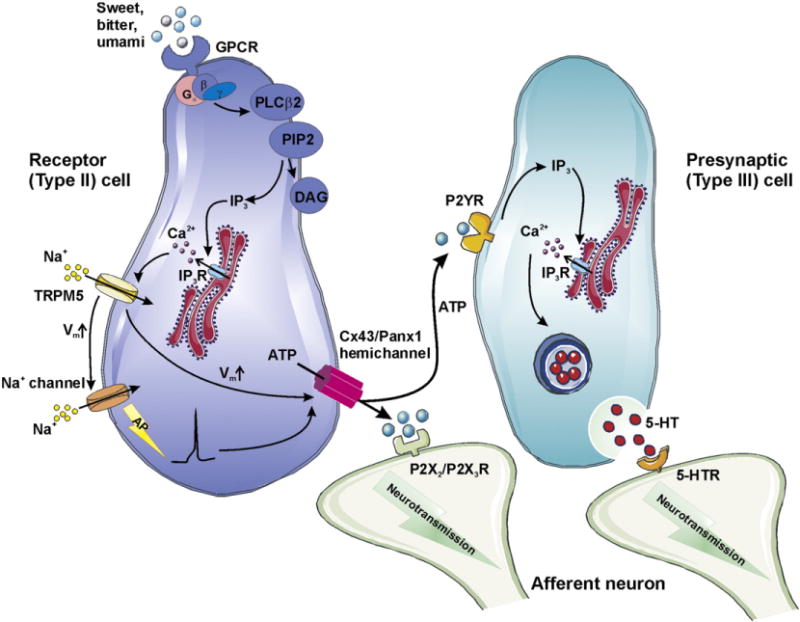
Role of Cx43 and Panx1 hemichannels in paracrine signaling in taste transduction. In taste buds, G-protein coupled receptors (GPCRs) for bitter, sweet and umami tastents are located at the apical membrane of type II receptor cells. Activation of the GPCR signaling pathway leads to the production of IP3 and diacyl glycerol (DAG) through phospholipase Cβ (PLCβ)-mediated cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2). An increase of [Ca2+]i by IP3-induced Ca2+ release opens transient receptor potential cation channel subfamily M member 5 (TRPM5). Na+ influx through TRPM5 channels depolarizes the cell membrane and stimulates voltage-gated Na+ channels, promoting the generation of an action potential (AP). Available evidence indicates that in response to tastent-evoked membrane depolarization, Cx43/Panx1 hemichannels open, facilitating ATP release. Extracellular ATP is thought to function as a neurotransmitter, targeting P2X2/P2X3R at postsynaptic afferent nerves. Alternatively, ATP may diffuse and bind to P2YR at adjacent presynaptic type III receptor cells. Following elevation of [Ca2+]i by IP3, a second taste transmitter 5-HT is secreted from the presynaptic cells through vesicular release.
4.1.4. ATP release influencing cell death/cell survival in disease
Abnormal connexin hemichannel-mediated paracrine signaling has been implicated in a genetic disorder termed hidrotic ectodermal dysplasia [180]. Disease-associated Cx30 mutants (G11R and A88V) form normal gap junctions but defective hemichannels. Uncontrolled ATP efflux through these leaky channels may enhance the proliferation and differentiation of keratinocytes through purinergic signaling [181], giving rise to a severe skin phenotype. Similarly, leaky hemichannels composed of mutated Cx32 (F235C) have been proposed to contribute as a mechanism of cellular toxicity in the X-linked Charcot–Marie–Tooth disease, a demyelinating disorder [182]. It is not known here whether hemichannel ATP release contributes to the presumed toxicity.
Mechanical damage or ototoxic drugs may facilitate hemichannel ATP release from supporting cells in the organ of Corti and may act as a cell death signal via activation of P2 receptors [183]. This triggers an intercellular Ca2+ wave that activates ERK1/2 in supporting cells, which is associated with increased hair cell death.
Connexin hemichannel-dependent ATP release appears to be involved in cell protection in the context of ischemic preconditioning, a process that activates protective signaling cascades in response to repeated episodes of subletal ischemia. Hypoxic preconditioning induced by low concentration of H2O2 or tamoxifen in cultured astrocytes increases the density of membrane surface Cx43 hemichannels as a consequence of impaired Cx43 degradation [184]. ATP release mediated via the expanded pool of Cx43 hemichannels leads to an enhanced accumulation of its metabolite adenosine. Adenosine is a general inhibitor of neurotransmission with neuroprotective effect on the neural tissue [185]. The ischemic preconditioning effect disappears with Cx43 silencing or connexin channel blockers [184]. Additionally, conditional Cx43 KO in astrocytes removes the protective preconditioning effect in mice subjected to occlusion of the middle cerebral artery. Along the same line, ATP release through Cx36 hemichannels has been implicated to increase the tolerance against ischemia provided by cortical spreading depression [186]. Cortical spreading depression denotes the waves of depolarization that spread over the brain cortex in response to focal injury or application of a high K+ solution on the brain surface. Application of high K+ solution to primary cultures of cortical neurons triggers membrane depolarization and release of ATP, which acts, in a paracrine manner, on P2Y receptors and protects neurons against in vitro applied ischemic conditions via PLC and PKA-dependent signaling. The evidence pointing to hemichannels as the ATP release pathway were based on work with connexin channel blockers and Cx36 silencing.
Connexin hemichannel ATP release has also been implicated in ischemic preconditioning in the heart. It is known that mitochondrial Cx43 plays a role in the protective effect of preconditioning [187,188]. Recent work has indicated that opening of mitochondrial Cx43 hemichannels may be involved in the complex cascade leading to protection [189]. It is hypothesized that preconditioning leads to opening of Cx43 hemichannels in the inner mitochondrial membrane, with subsequent loss of ATP from the mitochondrial matrix. This activates ATP-dependent K+ channels that appear to be central in the protective effect.
4.1.5. ATP and immune cell responses
During hypoxia or acute inflammation, increased connexin hemichannel activity in polymorphonuclear leukocytes (PMNs) directly correlates with elevated vascular nucleotides including ATP, ADP and AMP [190]. PMNs activated by hypoxia/inflammation display ATP release that is inhibited by 43Gap27 (but not 40Gap27 targeting Cx40) and not affected by inhibiting the secretion of granular vesicles, nucleoside transporters or transporters from the ATP binding cassette family. PMNs isolated from mice with induced Cx43 deletion had significantly decreased ATP release, pointing to a contribution of Cx43 hemichannels. It is conceivable that Cx43 hemichannel opening is mediated by dephosophorylation at S368 in a phosphatase 2A-dependent signaling pathway [190,191]. It is hypothesized that the ATP released by PMNs is metabolized to AMP by CD39 present at the PMN surface [190] and subsequently converted to adenosine by endothelially located CD73 [192]. Adenosine then activates endothelial A2B receptors [193], leading to downstream cAMP/PKA pathway that inactivates the endothelial cell contraction and enhances the cell-matrix adhesion complex [194], thus self-limiting the disrupted vascular barrier due to PMN migration. Hypoxia upregulates the expression of CD39, CD73 and A2B receptor, further amplifying the interplay of PMNs with endothelial cells. It was later suggested that Cx43 hemichannels in vascular endothelia can additionally contribute to accumulation of extracellular ATP [195]. However hypoxia reduces the ATP release from endothelial cells, making such contribution less likely.
Evidence is also available for bacillar dysentery caused by Shigella flexneri: invasion of the colonic mucosa with this bacteria triggers inflammation and subsequent destruction of the epithelium. During the process of invasion, the dispersion of bacteria from the infection zone to neighboring regions is modulated by ATP release through connexin hemichannels. Evidence for this came from the finding that HeLa cells exogenously expressing human Cx26 (hCx26) showed a larger degree of Shigella invasion and spreading than wild-type cells [196]. hCx26 hemichannel opening in infected Hela cells were indicated by uptake of Lucifer yellow and explained by Shigella-triggered cytoskeleton-reorganization and PLC activation. Released ATP activates purinergic receptors on neighboring cells, triggering [Ca2+]i changes which promote Shigella entry and further bacterial spreading.
4.1.6. ATP and immune effects of pannexin hemichannels
KO of major connexins like Cx26 or Cx43 results in embryonic lethality [197,198] or perinatal death [199]. By contrast, KO of Panx1 gives an apparently normal phenotype [5]. However, there is a significant amount of data available that suggest roles of Panx1 hemichannels. Hemichannel related ATP release was first reported in Panx1 expressing oocytes [62] and supported by follow-up studies performed in erythrocytes that lack connexin expression [200]. Erythrocytes display single-channel events with unitary conductance of ~450 pS that are activated by depolarization and mechanostimulation. The mechanosensitivity of these channels [63] makes it likely that shear stress, caused by the blood flow, stimulates Panx1 hemichannel opening. Released ATP binds to P2Y receptors on neighboring endothelial cells, generating intercellular Ca2+ waves that lead to the generation of nitric oxide that acts as a vasodilator on smooth muscle cells [201,202]. Released ATP may also activate P2X7 receptors in an autocrine and paracrine manner [203], resulting in the release of cis- and trans-epoxyeicosatrienoic acids from erythrocytes, that act as vasotropic agents controlling the local blood flow.
The P2X7–Panx1 hemichannel complex has been implicated in the release of interleukin-1β from macrophages [65]. However, this finding has been challenged by the fact that bone marrow-derived macrophages isolated from Panx1-deficient animals are still capable of secreting pro-inflammatory cytokines upon activation [100]. Similarly, interleukin-1β release in glial cells is explained by a Panx1 hemichannel-independent mechanism [204]. P2X7 stimulation in astrocytes activates acid sphingomyelinase through a p38 MAPK signaling cascade, leading to interleukin-1β release as microparticles. Recent evidence points to an important contribution of ATP/UTP release via Panx1 hemichannels that acts as a ‘find-me’ signal that recruits phagocytes to clear cells undergoing apoptosis [72,100]. As specified in Section 2.5, Panx1 hemichannels are activated during apoptosis by caspase cleavage of the CT tail.
4.2. NAD+
The metabolic substance NAD+ has long been known to be converted to cyclic ADP-ribose (cADPR) by CD38, a transmembrane enzyme with the catalytic domain facing the extracellular side (ectoenzyme). cADPR imported into the cell via CD38 or a family of nucleoside transporters activates type 2 and type 3 ryanodine receptors at the endoplasmic/sarcoplasmic reticulum, resulting in the release of Ca2+[205]. It was however less clear how NAD+ could reach the catalytic site of CD38. Work with unilamellar liposomes with reconstituted purified Cx43 proteins has brought up the possibility that Cx43 hemichannels may act as a diffusive conduit for NAD+[206]. This concept was further validated by the demonstration that Cx43 silencing completely prevented transmembrane efflux of NAD+ in 3T3 fibroblasts [206]. Using the same cells, it was furthermore shown that NAD+, released from cells transfected with CD38 (CD38+), were able to induce [Ca2+]i elevation in surrounding cells lacking CD38 (CD38−) via cADPR action [207], while monocultures of CD38− cells were not responsive. The [Ca2+]i stimulation of CD38− by CD38+ cells disappeared in the presence of a connexin channel inhibitor and by silencing Cx43 in the CD38+ cells. This Cx43 hemichannel related NAD+-mediated paracrine Ca2+ signaling promoted 3T3 fibroblast proliferation with shortened S phase of the cell cycle [207]. A similar effect was found in human hemopoietic progenitors when co-cultured with murine stroma cells overexpressing CD38+[208]. NAD+ release via Cx43 hemichannels may also be involved in mucosal cells; its conversion to cADPR appears to potentiate the contractility of tracheal smooth muscle cells [209].
4.3. Glutamate
Glutamate is a major excitatory neurotransmitter, not only between neurons but also involved in the neuron-glial signaling network [210]. Astrocytes clear extracellular glutamate via glutamate uptake through GLAST and GLT-1 transporters [211]. However, under pathological conditions such as ischemia or inflammation, astrocytes may actually contribute to glutamate release [212], leading to overstimulation of postsynaptic glutamate receptors and neuronal death [213]. In addition to well-defined glutamate release pathways such as glutamate transporters acting in reversed mode and volume-regulated anion channels, connexin hemichannels in astrocytes have been suggested as another favorable candidate. In cultured astrocytes, the presence of unapposed hemichannels was indicated by a unique distribution of Cx43 in the non-junctional membrane and dye uptake triggered by depletion of extracellular Ca2+[214]. Extracellular Ca2+ depletion also triggered glutamate release, which was independent of [Ca2+]i changes, abrogated by several connexin channel blockers, and insensitive to agents inhibiting glutamate transporters or anion channels [214]. Further evidence has implicated Cx43 hemichannels in hyperosmolarity-induced glutamate release from cultured astrocytes [215,216]. The evidence is based on rapid inhibition by 43Gap26 and absence of effects of interfering with glutamate transporters, anion channels, Panx1 hemichannels and P2X7 receptors.
In addition to astrocytes, microglial cells may also display connexin hemichannel-mediated glutamate release [217]. Microglia activated by pro-inflammatory agents produces tumor necrosis factor-α, which upregulates intracellular glutamate synthesis via glutaminase-involved autocrine mechanism and promotes glutamate release. Elevated glutamate level in the extracellular matrix leads to neuronal cell death by activating NMDA receptors, inhibiting mitochondrial respiration and depleting intracellular ATP. Glutamate transport as well as the subsequent neurotoxicity can be suppressed by 32Gap27 but not by 43Gap27, indicating a Cx32 hemichannel-governed release mechanism. Inflammation-stimulated macrophages seem to cause neurotoxicity in a similar manner [218]. The possible contribution of connexin hemichannels in glutamate release has inspired further work towards the molecular mechanism underlying neurodegeneration in Alzheimer’s disease. Amyloid-β activates Cx43 hemichannel openings in both microglial cells and astrocytes [219]. Cytokines secreted by activated microglia may further enhance Cx43 hemichannel activities in astrocytes [78], which promotes the release of glutamate and leads to neuronal cell death.
4.4. Glutathione
During oxidative stress, glutathione can function as an electron donor for the clearance of peroxide [220]. Astrocytes may contribute to neuroprotective effects via glutathione-mediated metabolic interactions with neurons [221]. Astrocytes can release glutathione, which is converted into cysteinylglycine by astrocytic γ-glutamyl transpeptidase [222]. Subsequently, cysteinylglycine is catalyzed by γ-glutamyl transpeptidase resulting in cysteine and glycine [223]. Increased extracellular cysteine potentiates its neuronal uptake and intracellular production of glutathione. Astrocytic glutathione release is stimulated by depletion of extracellular Ca2+ level, inhibited by connexin channel blockers and not affected by P2X7 receptor antagonist, thereby sharing a similar pharmacological profile with connexin hemichannels [224]. The involvement of hemichannels is also suggested by a study investigating the effect of curcumin on glutathione efflux from astrocytes [225]. Curcumin, a compound found in popular Indian spice, upregulates the synthesis of glutathione in astrocytes by stimulating transcriptional genes responsible for astrocytic antioxidant defense. The enhanced glutathione production results in increased efflux that is reduced by 43Gap26 and not influenced by P2X7 receptor inhibition or 10Panx1 peptide. In hippocampal slices, the pharmacological properties of the glutathione efflux triggered by removal of extracellular Ca2+ are identical to those reported in the cell culture systems [226].
4.5. Prostaglandin E2
Mechanical forces regulate a continuous process of bone remodeling. Osteocytes embedded in the bone tissue are endowed with a mechanosensory function, transducing mechanical signals to neighboring osteocytes and cells on the bone cell surface including osteoblasts, osteoclasts and bone-lining cells through both gap junction coupling and paracrine intercellular communication [44]. Prostaglandin E2, identified as one of the paracrine messengers released by osteocytes, stimulates bone formation via its actions on osteoblasts. At high concentrations and long exposure times, prostaglandin E2 induces bone resorption by enhancing osteoclast formation and activation. Bone remodeling is modulated by a well balanced process of bone-resorption and formation. The marked expression of Cx43 in osteocytes together with the fact that Cx43 hemichannels can be activated by mechanical stimuli has led to the hypothesis that these cells release prostaglandin E2via the hemichannel pathway. Fluid flow shear stress resembling the natural mechanical forces-induced interstitial fluid flow [227], has been demonstrated to trigger prostaglandin E2 release from osteocyte-like MLO-Y4 cells in low-density cultures devoid of gap junctions [49,228]. The presence of Cx43 hemichannels at the cell surface was confirmed by biotinylation and cellular dye uptake studies [49]. Shear stress-induced increase of extracellular prostaglandin E2 is inhibited by Cx43 silencing and carbenoxolone, but not by inhibitors of P2X7 receptors or prostaglandin transporters. It appears that mechanostimulation of Cx43 hemichannels are endowed with an inactivation mechanism that limits prostaglandin release: short exposure of osteocytes to fluid flow shear stress (30 min) increases Cx43 hemichannels openings, but leaves the density of the hemichannels at cell surface unaffected. Longer stimulation (2 h) results in increased prostaglandin release as a result of increased density of Cx43 hemichannels in the plasma membrane. Sustained periods of mechanical stimulation (4 h) closes the hemichannels and if the exposure to mechanical stress is further increased (24 h), the plasma membrane density Cx43 hemichannels is downregulated. This adaptive regulation of Cx43 hemichannels prevent overstimulation of osteoclasts by uncontrolled elevation of extracellular prostaglandin E2 and therefore avoid bone loss.
5. Conclusion
Numerous reported data demonstrate that hemichannels may function as a release pathway for paracrine messengers. This includes data showing hemichannel-mediated release of ATP, prostaglandins and other compounds (Table 1) in cells expressing Cx26, Cx32, Cx36, Cx43 and Panx1. However, paracrine signaling involves more than just release of a messenger. At present, there is not enough data to draw a complete signaling cascade that starts with hemichannel-mediated messenger release and its specific triggers, and ends with the target receptors and consequent functional alterations. The reason for this relates to the difficulty of separating the functions of connexin hemichannels from gap junctions composed of the same connexins and, along the same line, distinguishing connexin from pannexin hemichannels. Thus, new tools are urgently needed to overcome this fundamental gap in the connexin/pannexin channel field. The tools needed include novel concepts that can be used for the design of new pharmacological agents and for the design of transgenic approaches to disentangle the connexin and pannexin signaling network. A second reason relates to the master paracrine signaling molecule ATP: this substance has numerous release mechanisms including the diffusive release via plasma membrane channels (hemichannels, maxi-anion channels), carrier-facilitated release and vesicular release, making it very difficult to define the exact contribution of hemichannels in ATP release. Hemichannels may have the advantage that they provide a high-capacity and fast release pathway [229], although the process of opening may be slower. However, strong claims that only hemichannels contribute to the observed responses without contribution of other release mechanisms are currently not possible. At the receptor side, things are also complicated: the purinergic receptor family is extensive and lacks inhibitors with enough specificity to allow clear conclusions on downstream functional responses. However, the good news also comes from this side: ATP, as a paracrine messenger, has such a wide variety of targets and proposed functions that, if hemichannels prove to be an important pathway for its release, the consequences will be significant. This is related to the fact that modulation (inhibition/stimulation) at the level of the source is expected to have stronger functional impact (good as well as bad) than at the target. A third reason why the case for hemichannels in paracrine signaling is still at the weak side relates to the paucity of in vivo data. In this respect, blood cells offer interesting opportunities because of their accessibility and the absence of gap junctions when cells are separated. In conclusion, further efforts toward better defining the role of hemichannels in paracrine signaling should be based on advantaged methodological approaches, new and more selective tools to manipulate hemichannel function and focusing more on in vivo studies.
Acknowledgments
This work was supported by the Fund for Scientific Research, Flanders, Belgium [G.0140.08, 3G.0134.09, G.0298.11N and G.0571.12] and the Interuniversity Attraction Poles Program, Belgian Science Policy [P6/31 and P7] and by NIH grants, R01NS072238 and RO1HL084464.
Footnotes
This article is part of a Special Issue entitled: The Communicating junctions, roles and dysfunctions.
References
- 1.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 2.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 3.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 5.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’hondt C, Ponsaerts R, De SH, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 7.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 9.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert R, Donaldson P, Goldie K, Kistler J. A distinct membrane current in rat lens fiber cells isolated under calcium-free conditions. Invest Ophthalmol Vis Sci. 1998;39:1280–1285. [PubMed] [Google Scholar]
- 12.Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest Ophthalmol Vis Sci. 2011;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc Natl Acad Sci USA. 2006;103:9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez D, Gomez-Hernandez JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. 2006;20:2329–2338. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- 17.Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puljung MC, Berthoud VM, Beyer EC, Hanck DA. Polyvalent cations constitute the voltage gating particle in human connexin37 hemichannels. J Gen Physiol. 2004;124:587–603. doi: 10.1085/jgp.200409023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J. 2006;91:2142–2154. doi: 10.1529/biophysj.106.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valiunas V, Mui R, McLachlan E, Valdimarsson G, Brink PR, White TW. Biophysical characterization of zebrafish connexin35 hemichannels. Am J Physiol Cell Physiol. 2004;287:C1596–C1604. doi: 10.1152/ajpcell.00225.2004. [DOI] [PubMed] [Google Scholar]
- 22.Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci USA. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Retamal MA, Schalper KA, Shoji KF, Orellana JA, Bennett MV, Saez JC. Possible involvement of different connexin43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J Membr Biol. 2007;218:49–63. doi: 10.1007/s00232-007-9043-y. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran S, Xie LH, John SA, Subramaniam S, Lal R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS One. 2007;2:e712. doi: 10.1371/journal.pone.0000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RJ, Symon L. Extracellular pH, potassium, and calcium activities in progressive ischaemia of rat cortex. J Cereb Blood Flow Metab. 1984;4:178–186. doi: 10.1038/jcbfm.1984.26. [DOI] [PubMed] [Google Scholar]
- 28.Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 29.Allen MJ, Gemel J, Beyer EC, Lal R. Atomic force microscopy of Connexin40 gap junction hemichannels reveals calcium-dependent three-dimensional molecular topography and open-closed conformations of both the extracellular and cytoplasmic faces. J Biol Chem. 2011;286:22139–22146. doi: 10.1074/jbc.M111.240002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–327. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J Gen Physiol. 2006;127:67–75. doi: 10.1085/jgp.200509397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Hashimoto-Torii K, Torii M, Ding C, Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci. 2010;30:4197–4209. doi: 10.1523/JNEUROSCI.4187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. ATP-mediated cell–cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponsaerts R, De VE, Retamal M, D’hondt C, Vermeire D, Wang N, De SH, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 40.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martinez AD, Saez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell. 2008;19:3501–3513. doi: 10.1091/mbc.E07-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 42.Gomes P, Srinivas SP, Van DW, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 43.Luckprom P, Kanjanamekanant K, Pavasant P. Role of connexin43 hemichannels in mechanical stress-induced ATP release in human periodontal ligament cells. J Periodontal Res. 2011;46:607–615. doi: 10.1111/j.1600-0765.2011.01379.x. [DOI] [PubMed] [Google Scholar]
- 44.Burra S, Jiang JX. Connexin 43 hemichannel opening associated with Prostaglandin E(2) release is adaptively regulated by mechanical stimulation. Commun Integr Biol. 2009;2:239–240. doi: 10.4161/cib.2.3.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 46.Gossman DG, Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes. 2008;15:305–315. doi: 10.1080/15419060802357217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci USA. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ngezahayo A, Zeilinger C, Todt I, Marten I, Kolb HA. Inactivation of expressed and conducting rCx46 hemichannels by phosphorylation. Pflugers Arch. 1998;436:627–629. doi: 10.1007/s004240050681. [DOI] [PubMed] [Google Scholar]
- 51.Jedamzik B, Marten I, Ngezahayo A, Ernst A, Kolb HA. Regulation of lens rCx46-formed hemichannels by activation of protein kinase C, external Ca2+ and protons. J Membr Biol. 2000;173:39–46. doi: 10.1007/s002320001005. [DOI] [PubMed] [Google Scholar]
- 52.Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 53.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 54.John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 55.Vergara L, Bao X, Bello-Reuss E, Reuss L. Do connexin 43 gap-junctional hemichannels activate and cause cell damage during ATP depletion of renal-tubule cells? Acta Physiol Scand. 2003;179:33–38. doi: 10.1046/j.1365-201X.2003.01198.x. [DOI] [PubMed] [Google Scholar]
- 56.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Retamal MA, Schalper KA, Shoji KF, Bennett MV, Saez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc Natl Acad Sci USA. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’hondt C, Ponsaerts R, De SH, Vinken M, De VE, De BM, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G. Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal. 2011;23:305–316. doi: 10.1016/j.cellsig.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 67.Poornima V, Madhupriya M, Kootar S, Sujatha G, Kumar A, Bera AK. P2X7 receptor-pannexin 1 hemichannel association: effect of extracellular calcium on membrane permeabilization. J Mol Neurosci. 2012;46:585–594. doi: 10.1007/s12031-011-9646-8. [DOI] [PubMed] [Google Scholar]
- 68.Dubyak GR. Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on “a permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP”. Am J Physiol Cell Physiol. 2009;296:C235–C241. doi: 10.1152/ajpcell.00639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 70.Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes. 2008;15:133–142. doi: 10.1080/15419060802014115. [DOI] [PubMed] [Google Scholar]
- 71.Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes. 2008;15:119–132. doi: 10.1080/15419060802013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 74.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schalper KA, Palacios-Prado N, Orellana JA, Saez JC. Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes. 2008;15:207–218. doi: 10.1080/15419060802014198. [DOI] [PubMed] [Google Scholar]
- 76.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez HA, Orellana JA, Verselis VK, Saez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol. 2009;297:C665–C678. doi: 10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Locke D, Liu J, Harris AL. Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry. 2005;44:13027–13042. doi: 10.1021/bi050495a. [DOI] [PubMed] [Google Scholar]
- 80.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 81.Lal R, John SA, Laird DW, Arnsdorf MF. Heart gap junction preparations reveal hemiplaques by atomic force microscopy. Am J Physiol. 1995;268:C968–C977. doi: 10.1152/ajpcell.1995.268.4.C968. [DOI] [PubMed] [Google Scholar]
- 82.Clair C, Combettes L, Pierre F, Sansonetti P, Tran Van Nhieu G. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res. 2008;314:1250–1265. doi: 10.1016/j.yexcr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 83.Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 84.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 87.Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009;65:758–766. doi: 10.1107/S0907444909014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol. 2009;133:555–570. doi: 10.1085/jgp.200910207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem. 2009;284:4484–4493. doi: 10.1074/jbc.M807430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J Gen Physiol. 2003;122:389–405. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res. 2004;95:e22–e28. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- 93.Harris AL. Voltage-sensing and substate rectification: moving parts of connexin channels. J Gen Physiol. 2002;119:165–169. doi: 10.1085/jgp.119.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoang QV, Qian H, Ripps H. Functional analysis of hemichannels and gap-junctional channels formed by connexins 43 and 46. Mol Vis. 2010;16:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 95.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 97.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 99.Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev. 2010;63:160–176. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 101.Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- 102.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 103.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orellana JA, Diaz E, Schalper KA, Vargas AA, Bennett MV, Saez JC. Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun. 2011;409:603–609. doi: 10.1016/j.bbrc.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 106.Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–110. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 109.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 110.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 111.Ortolano S, Di PG, Crispino G, Anselmi F, Mammano F, Chiorini JA. Coordinated control of connexin 26 and connexin 30 at the regulatory and functional level in the inner ear. Proc Natl Acad Sci USA. 2008;105:18776–18781. doi: 10.1073/pnas.0800831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci USA. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 114.Kruger O, Beny JL, Chabaud F, Traub O, Theis M, Brix K, Kirchhoff S, Willecke K. Altered dye diffusion and upregulation of connexin37 in mouse aortic endothelium deficient in connexin40. J Vasc Res. 2002;39:160–172. doi: 10.1159/000057764. [DOI] [PubMed] [Google Scholar]
- 115.D’hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophthalmol Vis Sci. 2007;48:120–133. doi: 10.1167/iovs.06-0770. [DOI] [PubMed] [Google Scholar]
- 116.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia. 2009;57:258–269. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 120.Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol. 1995;488(Pt 3):721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berthoud VM, Beyer EC, Seul KH. Peptide inhibitors of intercellular communication. Am J Physiol Lung Cell Mol Physiol. 2000;279:L619–L622. doi: 10.1152/ajplung.2000.279.4.L619. [DOI] [PubMed] [Google Scholar]
- 122.Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van LL, De Bock M, D’Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009;16:151–163. doi: 10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 123.Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes. 2007;14:265–273. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- 124.O’Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes. 2008;15:27–42. doi: 10.1080/15419060802014164. [DOI] [PubMed] [Google Scholar]
- 125.Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O’Carroll SJ, Nicholson LF, Johnson CS, Green CR. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506–520. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- 126.Davidson JO, Green CR, Nicholson LF, O’Carroll SJ, Fraser M, Bennet L, Gunn AJ. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–132. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- 127.Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 128.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, Dauwe I, Vinken M, Simon AM, Rogiers V, De Ley G, Evans WH, Bultynck G, Dupont G, Cecchelli R, Leybaert L. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J Cereb Blood Flow Metab. 2011;31:1942–1957. doi: 10.1038/jcbfm.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of Connexin43 channels. Cardiovasc Res. 2004;62:268–275. doi: 10.1016/j.cardiores.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 131.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem. 2007;282:5801–5813. doi: 10.1074/jbc.M605233200. [DOI] [PubMed] [Google Scholar]
- 132.Lewandowski R, Procida K, Vaidyanathan R, Coombs W, Jalife J, Nielsen MS, Taffet SM, Delmar M. RXP-E: a connexin43-binding peptide that prevents action potential propagation block. Circ Res. 2008;103:519–526. doi: 10.1161/CIRCRESAHA.108.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shibayama J, Lewandowski R, Kieken F, Coombs W, Shah S, Sorgen PL, Taffet SM, Delmar M. Identification of a novel peptide that interferes with the chemical regulation of connexin43. Circ Res. 2006;98:1365–1372. doi: 10.1161/01.RES.0000225911.24228.9c. [DOI] [PubMed] [Google Scholar]
- 134.Verma V, Larsen BD, Coombs W, Lin X, Spagnol G, Sorgen PL, Taffet SM, Delmar M. Novel pharmacophores of connexin43 based on the “RXP” series of Cx43-binding peptides. Circ Res. 2009;105:176–184. doi: 10.1161/CIRCRESAHA.109.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Verma V, Larsen BD, Coombs W, Lin X, Sarrou E, Taffet SM, Delmar M. Design and characterization of the first peptidomimetic molecule that prevents acidification-induced closure of cardiac gap junctions. Heart Rhythm. 2010;7:1491–1498. doi: 10.1016/j.hrthm.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res. 2004;95:e22–e28. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- 137.Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverría C, Orellana JA, Bultynck G, Ponsaerts R, Leybaert L, Simon F, Sáez JC, Retamal MA. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012 doi: 10.1096/fj.11-198416. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- 138.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Young RC, Schumann R, Zhang P. The signaling mechanisms of long distance intercellular calcium waves (far waves) in cultured human uterine myocytes. J Muscle Res Cell Motil. 2002;23:279–284. doi: 10.1023/a:1022052910585. [DOI] [PubMed] [Google Scholar]
- 140.Mlinar B, Enyeart JJ. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol. 1993;469:639–652. doi: 10.1113/jphysiol.1993.sp019835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 143.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 146.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 147.Leybaert L, Paemeleire K, Strahonja A, Sanderson MJ. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia. 1998;24:398–407. [PubMed] [Google Scholar]
- 148.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 149.Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leybaert L, Sanderson MJ. Intercellular Ca2+ waves: mechanisms and function. Physiol Rev. 2012;92:1359–1392. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stout C, Charles A. Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia. 2003;43:265–273. doi: 10.1002/glia.10257. [DOI] [PubMed] [Google Scholar]
- 156.Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J Neurochem. 1997;69:594–602. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]
- 157.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 158.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 160.Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex. 2006;16:237–246. doi: 10.1093/cercor/bhi101. [DOI] [PubMed] [Google Scholar]
- 161.Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5 doi: 10.1126/scisignal.2002160. (p.ra8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kurth-Nelson ZL, Mishra A, Newman EA. Spontaneous glial calcium waves in the retina develop over early adulthood. J Neurosci. 2009;29:11339–11346. doi: 10.1523/JNEUROSCI.2493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brückner E, Grosche A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Mechanisms of VEGF- and glutamate-induced inhibition of osmotic swelling of murine retinal glial (Müller) cells: indications for the involvement of vesicular glutamate release and connexin-mediated ATP release. Neurochem Res. 2012;37:268–278. doi: 10.1007/s11064-011-0606-z. [DOI] [PubMed] [Google Scholar]
- 165.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 166.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 168.Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 169.Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- 170.Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–242. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- 171.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 172.Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. doi: 10.1016/s0006-291x(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 173.Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessment of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 174.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 176.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–2350. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mol Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 181.Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- 182.Liang GS, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- 183.Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci. 2008;28:4918–4928. doi: 10.1523/JNEUROSCI.4914-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 186.Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 187.Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia-Dorado D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia–reperfusion injury. Cardiovasc Res. 2008;77:325–333. doi: 10.1093/cvr/cvm062. [DOI] [PubMed] [Google Scholar]
- 188.Rodriguez-Sinovas A, Sanchez JA, Gonzalez-Loyola A, Barba I, Morente M, Aguilar R, Agullo E, Miro-Casas E, Esquerda N, Ruiz-Meana M, Garcia-Dorado D. Effects of substitution of Cx43 by Cx32 on myocardial energy metabolism, tolerance to ischaemia and preconditioning protection. J Physiol. 2010;588:1139–1151. doi: 10.1113/jphysiol.2009.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rottlaender D, Boengler K, Wolny M, Schwaiger A, Motloch LJ, Ovize M, Schulz R, Heusch G, Hoppe UC. Glycogen synthase kinase 3beta transfers cytoprotective signaling through connexin 43 onto mitochondrial ATP-sensitive K+ channels. Proc Natl Acad Sci USA. 2012;109:E242–E251. doi: 10.1073/pnas.1107479109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 190.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 191.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 192.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 195.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 197.Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 199.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 200.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- 202.Moerenhout M, Himpens B, Vereecke J. Intercellular communication upon mechanical stimulation of CPAE-endothelial cells is mediated by nucleotides. Cell Calcium. 2001;29:125–136. doi: 10.1054/ceca.2000.0165. [DOI] [PubMed] [Google Scholar]
- 203.Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- 206.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 207.Franco L, Zocchi E, Usai C, Guida L, Bruzzone S, Costa A, De Flora A. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J Biol Chem. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- 208.Zocchi E, Podesta M, Pitto A, Usai C, Bruzzone S, Franco L, Guida L, Bacigalupo A, De FA. Paracrinally stimulated expansion of early human hemopoietic progenitors by stroma-generated cyclic ADP-ribose. FASEB J. 2001;15:1610–1612. doi: 10.1096/fj.00-0803fje. [DOI] [PubMed] [Google Scholar]
- 209.Franco L, Bruzzone S, Song P, Guida L, Zocchi E, Walseth TF, Crimi E, Usai C, De FA, Brusasco V. Extracellular cyclic ADP-ribose potentiates ACh-induced contraction in bovine tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;280:L98–L106. doi: 10.1152/ajplung.2001.280.1.L98. [DOI] [PubMed] [Google Scholar]
- 210.Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11:387–394. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 211.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 212.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 213.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 214.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang S, Yuan H, Duan L, Cao R, Gao B, Xiong YF, Rao ZR. Glutamate release through connexin 43 by cultured astrocytes in a stimulated hypertonicity model. Brain Res. 2011;1392:8–15. doi: 10.1016/j.brainres.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 216.Cao R, Jiang S, Duan L, Xiong YF, Gao B, Rao ZR. Hypertonic stimulation induces synthesis and release of glutamate in cultured rat hypothalamic astrocytes and C6 cells. Neurosci Bull. 2008;24:359–366. doi: 10.1007/s12264-008-0709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 218.Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, Suzumura A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci. 2008;82:1111–1116. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 219.Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011;31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 221.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 222.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li X, Wallin C, Weber SG, Sandberg M. Net efflux of cysteine, glutathione and related metabolites from rat hippocampal slices during oxygen/glucose deprivation: dependence on gamma-glutamyl transpeptidase. Brain Res. 1999;815:81–88. doi: 10.1016/s0006-8993(98)01097-x. [DOI] [PubMed] [Google Scholar]
- 224.Rana S, Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett. 2007;415:45–48. doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 225.Stridh MH, Correa F, Nodin C, Weber SG, Blomstrand F, Nilsson M, Sandberg M. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem Res. 2010;35:1231–1238. doi: 10.1007/s11064-010-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stridh MH, Tranberg M, Weber SG, Blomstrand F, Sandberg M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283:10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278:43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 228.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E(2) by osteocytes in response to mechanical strain. Cell Commun Adhes. 2003;10:259–264. doi: 10.1080/cac.10.4-6.259.264. [DOI] [PubMed] [Google Scholar]
- 229.Dale N. Dynamic ATP signalling and neural development. J Physiol. 2008;586:2429–2436. doi: 10.1113/jphysiol.2008.152207. [DOI] [PMC free article] [PubMed] [Google Scholar]


