Abstract
Fluid and HCO3− secretion is a vital function of all epithelia and is required for the survival of the tissue. Aberrant fluid and HCO3− secretion is associated with many epithelial diseases, such as cystic fibrosis, pancreatitis, Sjögren’s syndrome and other epithelial inflammatory and autoimmune diseases. Significant progress has been made over the last 20 years in our understanding of epithelial fluid and HCO3− secretion, in particular by secretory glands. Fluid and HCO3− secretion by secretory glands is a two step process. Acinar cells secrete isotonic fluid in which the major salt is NaCl. Subsequently, the duct modifies the volume and electrolyte composition of the fluid to absorb the Cl− and secrete HCO3−. The relative volume secreted by acinar and duct cells and modification of electrolyte composition of the secreted fluids varies among secretory glands to meet their physiological functions. In the pancreas, acinar cells secrete small amount of NaCl-rich fluid, while the duct absorbs the Cl− and secretes HCO3− and the bulk of the fluid in the pancreatic juice. Fluid secretion appears to be driven by active HCO3− secretion. In the salivary glands, acinar cells secrete the bulk of the fluid in the saliva that contains high concentrations of Na+ and Cl− and fluid secretion is mediated by active Cl− secretion. The salivary glands duct absorbs both the Na+ and Cl− and secretes K+ and HCO3−. In this review, we focus on the molecular mechanism of fluid and HCO3− secretion by the pancreas and salivary glands, to highlight the similarities of the fundamental mechanisms of acinar and duct cell functions, and point the differences to meet glands specific secretions.
I. INTRODUCTION
Bicarbonate (HCO3−) is an indispensible ion in secreted fluids, including the pancreatic juice and saliva. Among other functions, HCO3− is the biological pH buffer that guards against toxic intracellular and extracellular fluctuations in pH (365). As a chaotropic ion, HCO3− facilitates solubilization of macromolecules (like digestive enzymes and mucins) in biological fluids and stimulates mucin secretion (45, 145, 410). HCO3− secreted by the exocrine pancreas neutralizes gastric acid and provides an optimal pH environment for digestive enzymes function in the duodenum (237). HCO3− secretion into the oral cavity protects against enamel erosion by acidic pH (192). Indeed, recent progress in epithelial biology indicates that aberrant HCO3− transport has a fundamental role in human pathophysiology (346, 347). For example, in cystic fibrosis (CF) abnormal HCO3− secretion leads to altered mucin hydration and solubilization (348), resulting in thick mucus that frequently blocks ductal structures of the internal organs. Therefore, altered HCO3− secretion is associated with a wide spectrum of diseases and disorders of epithelial tissues including respiratory, gastrointestinal, and genitourinary systems (61, 284, 346, 347, 432).
At pH 7.4 and 5% CO2, the HCO3− equilibrium concentration is approximately 25 mM. Several bodily fluids have higher HCO3− concentration, and among them the pancreatic juice contains the highest concentration. In humans and several other species, such as dogs, cats, and guinea pigs, HCO3− concentration in the juice secreted by the stimulated pancreas can be higher than 140 mM (86, 237). This remarkable transport feat attracts considerable attention to pancreatic HCO3− secretory mechanism, which is the model of choice to gain insight into the mechanism of epithelial fluid and HCO3− transport. How exocrine glands secrete copious amount of fluid and HCO3− has long been a puzzle. The discovery of acidic pancreatic juice in patients with CF was a milestone in understanding the physiological mechanisms of pancreatic HCO3− secretion (191). In addition, significant progress has been made during the last 20 years with the identification of the molecular nature of many exocrine glands ion channels and transporters, including the cystic fibrosis transmembrane conductance regulator (CFTR) (199), the Na+-HCO3− co-transporter NBCe1-B (also known an pNBC1) (1) and the SLC26 transporters (91, 314). Regulation and coordination of exocrine HCO3− secretion is being defined with understanding the role of regulatory proteins, such as PSD95/discs large/ZO-1 (PDZ)-based adaptor proteins, with-no-lysine (WNK) kinases, the SPAK/OSR1 kinases and of the inositol-1,4,5-triphosphate (IP3) receptor binding protein released with IP3 (IRBIT). However, we have just begun to uncover how the transporting proteins are organized into complexes that function in concert in the luminal (apical) and basolateral membranes and how the high concentration of HCO3− in formed and maintained in the luminal space of exocrine glands.
Another cardinal aspect of exocrine gland function is fluid secretion. While HCO3− secretion is mostly carried out by the gland ducts, the bulk of fluid secretion can be by the duct, as in the exocrine pancreas (237, 404), or by acinar cells, as in the salivary glands (274, 368). While the ionic bases of fluid secretion by the duct are poorly understood, the fundamental mechanism of acinar cell fluid secretion is fairly well characterized. Early mechanistic work defined the basic transport mechanisms at the BLM and LM (331). More recent work relied on gene deletion in mice (50, 369), which confirmed the basic mechanism, but also resulted in unexpected surprises as to the diversity and function of transporter isoforms.
This review is aimed at consolidating the current knowledge of exocrine glands fluid and HCO3− secretion. Since it is understood best, fluid secretion by salivary glands will be emphasized as an example for the mechanism of fluid secretion by acinar cells. Ductal function will be discussed in relation to the molecular mechanism of pancreatic HCO3− secretion with an attempt to explain how the pancreatic juice can accumulate 140 mM HCO3−, a concentration that is more than five times higher than that found in plasma. We will also briefly discuss fluid and HCO3− transport by salivary gland ducts to demonstrate adaptation and alteration of the basic mechanism to meat tissue specific demands.
II. GENERAL CONSIDERATIONS
A. Overview
When Bayliss and Starling described the discovery of the pancreatic secretagogue secretin, they also noticed that the exocrine pancreas secretes alkaline fluids (25). At the time, they assumed that carbonate is responsible for the strong alkalinity of the pancreatic juice. Later, with better understanding of the carbonate/HCO3−/CO2 buffer systems (156), it became clear that the exocrine pancreas secretes fluid in which the dominant anion is HCO3− and HCO3− secretion is coupled to fluid secretion (46, 87, 143). Exocrine glands secrete macromolecules like digestive enzymes and mucins immersed in a HCO3−-rich fluid. Exocrine glands secretion is a two step process (412). The digestive enzymes are synthesized and secreted by the acinar cells. Depending on the gland, acinar cells also secrete a small (pancreas) or a large (salivary) volume of isotonic, plasma-like, NaCl-rich fluid (237, 274). The fluid secreted by acinar cells deliver the macromolecules to the duct. The duct modifies the ionic composition of the fluid along the ductal tree to absorb most of the Cl− and secrete the bulk of the HCO3−. The pancreatic duct also secretes most of the fluid in the pancreatic juice (14, 404) and the salivary duct absorbs the Na+ and secret K+ to form the final saliva (368). Hence, the ductal tree has several functions, providing a structural framework for acinar and endocrine tissues, secreting fluid that acts as a vehicle for the transport of macromolecules out of the glands, and secreting HCO3− to neutralize acid and provide optimal pH environment for the secreted macromolecules at their destination (14, 44).
B. Morphology
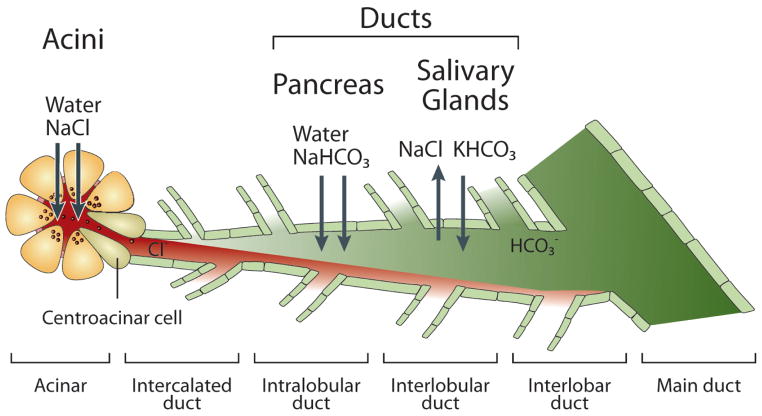
Below, we describe the anatomy of the pancreas as an example of an exocrine gland. However, the anatomy of other exocrine glands (like the salivary, lacrimal and mammary) is similarly organized. The pancreas is a complex endocrine-exocrine organ, with each part developed from the ventral and dorsal surfaces of the primitive foregut, respectively. Similar to other exocrine glands, the exocrine pancreas is composed of two major cell types, the acinar and duct cells. In humans and most other mammals, acinar cells comprise the major mass of the pancreas and duct cells comprise only about 10% of the cells in number and 5% of the total pancreatic gland weight (44). The terminal portion of the ductal tree leading directly from the acinus is called intercalated duct (Fig. 1).
Fig. 1. The acinar and ductal segments of secretory glands and fluid and electrolyte secretory functions.
The figure illustrates the relationships between the acinar and ductal portion of secretory glands. The acini secrete isotonic fluid with NaCl as the major salt. The fluid passes through the centroacinar cells to the duct. The pancreatic duct absorbs the Cl− and secretes HCO3− and most of the water in the pancreatic juice. Active HCO3− secretion drives fluid secretion. The salivary duct absorbs both the Cl− and the Na+ and secretes HCO3− and K+. ENaC is expressed in the salivary glands, but not in the pancreatic duct, and is the main pathway for Na+ absorption by the salivary glands duct.
The acinar cells are the classical model for polarized epithelial cells, having a typical morphology with extensive rough ER at the basal pole and smooth ER at the apical pole. The nucleus is close to the basal pole and is followed by the Golgi apparatus, the secretory granules that are packed at the apical pole, and the luminal membrane (271). The polarity is extended to other intracellular organelles and to single proteins. A good example is the Ca2+ signaling proteins. These proteins are organized as Ca2+ signaling complexes next or at the tight junctions, with polarized expression of Ca2+ pumps (239, 474), Ca2+ channels (240), G protein-coupled receptors (392) and regulatory proteins (163), that are essential for generating the localized Ca2+ signal and the propagated Ca2+ waves (208, 337).
The acinar lobule terminates and the duct starts with the centroacinar cells, which have several ductal characteristics, are regarded as the terminal cells of the ductal tree and connect the acinar and duct cells (237). The duct has several segments based on size and location. Although here we emphasize the secretory (serous) duct cells, it is important to note that the duct has several cell types, including mucosal and ciliated cells. The cellular heterogeneity is found in all segments of the ductal tree. Small intercalated ducts join together and sequentially form the intralobular, interlobular, and interlobar duct segments (Fig. 1). In humans, the interlobar ducts join to form the main pancreatic duct (duct of Wirsung), which shares a duodenal opening with the common bile duct at the Ampulla of Vater. Most individuals have one main pancreatic duct, but some have an additional accessory pancreatic duct, the Duct of Santorini (34). In rodents, a number of interlobular or interlobar ducts open directly into the common pancreaticobiliary duct without forming a main duct (237). Much attention is devoted in recent years to developmental aspects of the duct, since the duct contains the glandular stem cells that are necessary for development and gland repair after injury (195, 219, 248).
The intercalated and small intralobular ducts are the major sites of HCO3− secretion in the human pancreas and salivary glands, whereas in rodents the interlobular duct secretes the bulk of the fluid and HCO3− (259). The HCO3−-secreting portion of these ducts is lined by the principal cells. These cells contain a relatively small amount of cellular organelles required for protein secretion, such as rough endoplasmic reticulum (RER), Golgi complexes and secretory vesicles. Instead, they are rich with mitochondria to satisfy the energy demand of transcellular HCO3− and fluid secretion. The luminal membrane of the principal cells is endowed with extensive microvilli. The lateral membrane is interdigitated and linked by tight and adherent junctions and desmosomes. In the larger ducts the principal cells become columnar and the duct contains goblet cells, which are specialized in mucin secretion (44, 257).
C. Electrolyte Composition of the Secreted Fluids
The human pancreas secretes 1–2 L of pancreatic juice per day. The pancreatic juice is a alkaline, isotonic fluid. The acini secrete isotonic, plasma-like fluid. The pancreatic duct does not absorb the Na+, but absorbs most of the Cl− and secret HCO3−. The human pancreatic duct can secrete a fluid containing 140 mM HCO3− (86). However, the HCO3− concentration in mouse and rat pancreatic juice is 50–70 mM (386). The HCO3− content in the juice increases with increased flow rate. The peak HCO3− content is reached at 30–50% of maximal flow rate. The reciprocal Cl− absorption and HCO3− secretion results in isotonic osmolality at all flow rates (46, 358, 386). The cation composition of the juice is nearly constant, 140 mM Na+ and 10–15 mM K+, regardless of flow rate. Human juice also contains 1–2 mM Ca2+ and a small amount of Mg2+, Zn2+, PO43−, and SO42−.
Humans secrete about 1 L saliva per day. The resting secretion is dominated by the submandibular and sublingual glands, whereas the stimulated secretion is mainly by the parotid gland (369). As is the case in the pancreas, electrolyte composition of the saliva varies with flow rate and among species. Unlike the pancreas, most salivary fluid is secreted by the serous acinar cells, which secrete isotonic, NaCl-rich fluid. The primary fluid in the rat and cat contains 145–160 mM Na+, 5–10 K+, 120–130 Cl− and pH of about 7.5 (382). The salivary gland ducts express the epithelial Na+ channel ENaC (65) and absorbs most of the Na+ and Cl− from the primary saliva while secreting K+ and HCO3− to generate the final saliva. The cholinergic stimulated and Ca2+-dependent saliva in the rat and mouse at steady state contains 2 mM Na+, 135 mM K+, 55 mM HCO−3 and 78 mM Cl− at a transepithelial potential of −11 mV (66, 462). Interestingly, the β-adrenergic stimulated small volume of saliva contains as much as 140 mM HCO3− and as little as 20 mM Cl− (382, 462). This indicates that cholinergic and β-adrenergic stimulation use different pathways to stimulate fluid and electrolyte secretion.
III. HORMONAL CONTROL OF FLUID AND HCO3− SECRETION
A. Overview
Like any physiological system, the function of exocrine glands is controlled by multiple neurohomoral inputs that form intricate regulatory pathways. Here we focus our discussion on the exocrine pancreas, although for the most part similar mechanisms operate in the salivary glands. The fundamental similarities between the two tissues are that fluid secretion by acinar cells is regulated by Ca2+-mobilizing agonist (274, 334, 337) and fluid and HCO3− secretion by duct cells is largely regulated by cAMP generating receptors (237, 265). The most obvious difference that is outside the topic of this review is in enzyme secretion that is Ca2+-activated in the pancreas, but cAMP-activated in the salivary glands. In addition, sympathetic inputs are central in the function of salivary glands, but do not play a role in the function of the exocrine pancreas.
Neurohumoral control of pancreatic fluid and electrolyte secretion was first proposed in the early 20th century (25, 328). Knowledge accumulated over the past 100 years has revealed that regulation of pancreatic secretion is highly complex with multiple stimulatory and inhibitory inputs (398) and varies greatly among species. Basal secretion in rat and mouse is significant, while basal secretion in dog and possibly human is very small, amounting to less than 2% of the HCO3− and 10% of the digestive enzymes secreted during maximal stimulation (73). HCO3− salvage mechanisms in the large pancreatic duct contribute to the low basal HCO3− output (234, 250). Pancreatic enzyme and HCO3− secretion increases in response to a meal. Control of pancreatic secretion is divided into cephalic, gastric, and intestinal phases (44). The intestinal phase is the most important and commences with the passage of chyme into the proximal duodenum. The pancreatic acini and duct express receptors for a battery of hormones and neurotransmitters. The major receptors in acini are for the hormones cholecystokinin (CCK) and bombesin and the neurotransmitter acetylcholine. The hormone secretin, secreted by cells in the upper duodenum, and cholinergic vagal output via an enteropancreatic vagovagal reflex are the two principal receptors controlling ductal fluid and HCO3− secretion. Secretin and cholecystokinin (CCK) are released by distant organs and require high affinity receptors because they reach the pancreas via the bloodstream (237). In addition to these classical hormones, a large number of humoral agents are released by the pancreas to modulate its function. Cells in the islets of Langerhans release insulin, somatostatin, and several other peptide hormones (243), and paracrine agonists released from pancreatic acinar and duct cells, such as purines, prostaglandins and activated trypsin, regulate duct cell function in physiological and pathological states.
B. Endocrine and Paracrine Control
Cholecystokinin (CCK)
Circulating CCK released from the small intestines is a major stimulator of acinar cells enzyme and fluid secretion. This has been amply demonstrated in several rodent species. CCK acting through an increase in cytoplasmic Ca2+ prominently stimulates Ca2+-dependent exocytosis. However, the relevance of CCK-mediated action in human acinar cells has been questioned by several studies, suggested that the effect of CCK is mostly mediated by stimulation of the intestinal vagal afferent fibers (319). This was based on the claim that the human pancreas does not express CCK-A receptors (187, 188, 278). However, this problem was resolved recently with the demonstration of CCK-evoked Ca2+ signals and exocytosis in isolated human acinar cells (286). The endogenously released CCK is heterogeneous and consists of multiple forms including CCK-58, CCK-33, CCK-8 (101). Although the exact role of each isoform is not known and in isolated acini they have similar activity (69), there is evidence for differences in their function in vivo (453).
CCK also acts on the pancreatic duct to affect fluid secretions, but its effect varies among species. In humans, the infusion of CCK alone weakly stimulates fluid secretion, but it greatly potentiates the effects of secretin (460). This suggests prominent synergism between the Ca2+ and cAMP systems in stimulated ductal secretion. This is discussed in more detail in another section of this review.
Secretin
The entry of acidic chyme into the duodenum evokes the release of secretin from neuroendocrine cells in the duodenal mucosa. Intraduodenal pH below 4.5 is a prime stimulus for secretin release (37, 58). Secretin acts mainly on the duct by increasing cAMP to stimulate ductal fluid and HCO3− secretion. That secretin is the principal hormone in postprandial fluid and HCO3− secretion is evident from 1) a rise in plasma secretin after a meal (58, 341), 2) a linear relationship between the rise in plasma secretin and HCO3− output (380) and 3) inhibition of postprandial pancreatic HCO3− secretion by serum anti-secretin (57). Plasma secretin in response to a meal reaches only pM levels, which is sufficient to stimulate modest fluid and HCO3− secretion in all species. CCK and vagal stimulation further potentiates the secretin-stimulated secretion (134, 216, 460).
Purines
Purinergic receptors (P2Rs) are classified into metabotropic P2Y and ionotropic P2X receptors and transduce their signal by increasing cytoplasmic Ca2+. Pancreatic acini store and secret ATP but do not appear to express P2Rs (304). ATP released by acini acts on the duct, which expresses multiple P2YRs and P2XRs both at the apical and basolateral membranes (252). Stimulation of P2Rs in the isolated pancreatic ducts from several species induces fluid secretion and activates membrane transporters that enhance HCO3− secretion (291, 304, 476). However, P2Rs regulation can be quite complicated, for example stimulations of the apical and basolateral P2Rs have opposite effect on guinea pig ductal secretion (176). Possible sources of purinergic ligands include release by nerve terminals at the basolateral space, release of purines stored in zymogene granules of acinar cells, and efflux by ductal ATP transporters (302, 304). Although the purinergic system likely plays a role in the regulation of pancreatic ductal secretion under physiological and pathological states, its specific role in humans yet to be demonstrated.
Other humoral mediators secreted by islets, the gastrointestinal tract and the intrapancreatic nervous system can also modulate the function of the exocrine pancreas, although their exact physiological function remained to be elucidated (see (237) for further details).
C. Neuronal Control
Pancreatic secretion is controlled by the enteric nervous system, which is comprised of a gut-brain axis and an intrapancreatic system. The intrapancreatic nervous system comprises of interconnecting plexus of ganglia and postganglionic fibers lying in the intralobular connective tissues, blood vessels, and occasionally in the neuronal trunk (207, 217). It is supplied by preganglionic parasympathetic (vagal) fibers, postganglionic sympathetic (splanchic) fibers, and possibly other fibers that emanate directly from the gut wall. Nerve fibers travel through the lamina propria of the acini and the duct, with nerve terminals located in close proximity to the basal membrane without forming synapses (257, 418).
The major neurotransmitter acting on acinar and duct cells is acetylcholine secreted by vagal parasympathetic fibers. The acini and duct express M1 and M3 cholinergic receptors, although the M3 is the main receptor type (113) and acts by increasing cytoplasmic Ca2+ ([Ca2+]i) (208). Parasympathetic nerve terminals contain additional neurotransmitters, including vasoactive intestinal peptide (VIP) and ATP (217, 303). The effects of vagal stimulation on pancreatic fluid secretion show species-specific pattern and are quite variable. Vagal stimulation in pigs and guinea pigs causes VIP-stimulated fluid and HCO3− secretion (162, 217). In humans, cholinergic stimulation results in enzyme secretion by acinar cells and enhances the secretin-stimulated ductal secretion, likely by the Ca2+-cAMP synergy. The synergism between the Ca2+ and cAMP system (see below) can explain the strong stimulatory effect of VIP in the pig. VIP (and the β-adrenergic receptors) can stimulate both limbs of the signaling pathways by a Gs/Gi switching mechanism to increase both cellular cAMP and Ca2+ (251).
Other neurotransmitters acting on the exocrine pancreas are Neuropeptide Y (NPY), galanin (93, 390) and histidine isoluecine (161) that have modulatory effect, with NPY mainly controlling blood flow to induce vasoconstriction and inhibit pancreatic secretion. Substance P and the calcitonin gene-related peptide (CGRP) are co-localized in the same neuron and act as inhibitory neurotransmitters (56).
Regulation of salivary glands function is not understood to the same extent as pancreatic function. Salivary gland function is also controlled by multiple inputs, endocrine, paracrine and neuronal (274, 342, 377). The main neuronal regulation is by parasympathetic and sympathetic nerve endings. The parasympathetic neurons release acetylcholine to activate the M1 and M3 receptors in both acinar and duct cells (38, 71, 114, 451) to increase [Ca2+]i and mainly stimulate fluid secretion. The sympathetic nervous system activates β-adrenergic receptors in acinar and duct cells to increase cAMP (23, 48, 75). β-adrenergic stimulation is the main pathway for stimulated enzyme secretion in acinar cell (48). In the duct, β-adrenergic stimulation controls fluid and electrolyte transport (66), including HCO3− secretion (389, 472).
Salivary glands acinar and duct cells express a battery of ionotropic and metabotropic P2 receptors (P2Rs) both in the luminal and basal membranes (415, 465). The P2Y receptors appear most important in gland development (22, 416) and perhaps repair (350). The P2X receptors (287, 307, 465) may have a role in fluid and electrolyte secretion. A recent convincing study showed a prominent stimulation of salivary glands secretion by the P2X7 receptors (287), which are expressed in both acinar and duct cells (242, 354) and act by increasing [Ca2+]i (242, 287, 307). How the function of all inputs is orchestrated to produce the final saliva (and for that matter, the pancreatic juice) is not known at present.
D. Signaling Pathway in Secretory Glands
Acinar and duct cell functions in both the pancreatic and salivary glands is regulated by receptors that change the free cytoplasmic Ca2+ concentration ([Ca2+]i) (208, 337). Changes in [Ca2+]i are also critically involved in pancreatic (205, 330) and salivary glands (205) pathology. Ca2+ signaling entails receptor-mediated activation of the G proteins Gq to generate Gαq·GTP or Gi to release Gβγ, which activate phospholipase C β (PLCβ). PLCβ hydrolyses PIP2 to generate IP3 and diacylglycerol (32). IP3 activates the ER-located Ca2+ release channels IP3 receptors (IP3Rs) (276), with IP3R2 and IP3R3 being the major isoforms in secretory cells (110). Ca2+ release is followed by activation of plasma membrane Ca2+ influx channels, the so called store-operated channels (SOCs). The two SOCs are the Orai (104, 427, 468) and TRPC channels (167, 447, 463). The TRPC and Orai channels are activated by the ER Ca2+ sensor STIM1 (246, 366). In response to Ca2+ stores depletion, STIM1 clusters with TRPC (6, 323, 467) and the Orai channels (83, 246, 249, 419) to activate them. The increase in [Ca2+]i activates the Ca2+ extrusion mechanisms, the sarco/endoplasmic Ca2+ ATPase (SERCA) pumps and the plasma membrane Ca2+ ATPase (PMCA) pumps, to move Ca2+ from the cytosol and restore [Ca2+]i towards the basal level.
The Ca2+ signal in epithelial cells is highly polarized, initiating at the apical pole and propagating to the basal pole (196, 414). Polarization of the Ca2+ signal is achieved by polarized arrangement of all Ca2+ signaling proteins and their assembly into complexes in close proximity of the tight junction. Polarized expression has been demonstrated for all three IP3Rs (240, 293, 446), the SERCA and PMCA pumps (239, 474), GPCRs (357, 392), TRPC channels (203), Orai channels and STIM1 (163, 253). Such an arrangement launches the Ca2+ signal at the cellular domain that initiates the polarized cellular function of the cells, being exocytosis of secretory granules or stimulated fluid and electrolyte secretion.
The Ca2+ signal evoked by physiological agonist concentrations is in the form of Ca2+ oscillations, where the Ca2+ signal is periodically repeated. The frequency and amplitude of the oscillation is determined by the intensity of receptor stimulation (31, 208). The Ca2+ oscillations always start at the apical pole and propagate to the basal pole (196, 414) in the form of Ca2+ waves (197). In pancreatic acinar cells the oscillations can remain confined to the apical pole (333, 337, 414). Interestingly, the spatial and temporal aspects of the Ca2+ oscillations vary among pancreatic and parotid acinar cells (120), likely reflecting adaptation of the Ca2+ signal to the specialized function of the two cell types. The Ca2+ signaling pathway mediates exocytotic enzyme secretion by pancreatic acini (438, 439), fluid secretion by pancreatic and salivary glands acini (274, 331), and modulate ductal fluid and electrolyte secretion (see below).
The second signaling pathway is the cAMP/PKA pathway that is activated by receptors coupled to Gs, as is the case in the ducts. The main stimulator of pancreatic ductal secretion is secretin and of salivary ducts function is norepinephrine acting on the β-adrenergic receptors. Secretin receptors belong to the class B G-protein-coupled receptor (GPCR) and β-adrenergic receptors are class A GPCR. Stimulation of the two receptor types in duct cells evokes an increase in cAMP and activation of PKA (66, 75, 88, 100, 417). Stimulation of the VIP receptors in duct cells (VPAC1), also activates the cAMP/PKA pathway (100, 417). The main transporters activated by cAMP to evoke fluid and HCO3− secretion are the luminal CFTR and the basolateral Na+-HCO3− cotransporter (237, 369) NBCe1-B (458). In the pancreatic duct, anti-secretin antibodies block about 80% of postprandial HCO3− output (57), highlighting the central role of secretin in stimulating HCO3− secretion.
In recent years, a more complicated picture of the regulation of ductal fluid and electrolyte secretion has emerged with the realization that pancreatic and salivary gland cells express a multitude of receptor types. In the pancreatic duct, these include multiple P2 receptors (252, 297) and the protease-activated receptor 2 (PAR2) (9, 290, 292, 299), which in addition to stimulating secretion (287), may mediate critical steps in apoptosis (P2X7 receptors) and the inflammatory response (PAR2) associated with pancreatitis. The P2X7 receptors may have different roles in acinar and duct cells since the receptors show cell specific behavior (242), and pore expansion (241). Functional studies suggest expression of P2Y2R and P2X7R at the luminal membrane and perhaps P2Y1R, P2Y2R and P2X4R at the basolateral membrane of the duct (252). Both the P2Y and P2X receptors signal through changes in [Ca2+]i (302). Subsequently, it was reported that the basolateral P2Rs inhibit HCO3− and fluid secretion in guinea pig duct (176). The P2Y11R that signal through changes in cAMP stimulates a luminal Cl− channel, most likely CFTR (297). The basolateral PAR2 that signals via changes in [Ca2+]i activates the luminal Ca2+-activated Cl− and K+ channels (299) and stimulates ductal HCO3− secretion (9). Again, to what extent these various receptors contribute to fluid and HCO3− secretion in vivo is not clear at present. However, the PAR2 and P2X7 receptors may become particularly active in pathological states when significant trypsin and ATP are released to protect the cells.
E. Synergism
The cAMP and Ca2+ signal pathways show prominent synergism in many physiological functions. A particularly well defined synergism is fluid and HCO3− secretion by the pancreatic duct and will be used here to illustrate the phenomenon. Although secretin is the primary stimulator of pancreatic fluid and HCO3− secretion, exogenous application of secretin that elevates plasma concentration to the level observed in the postprandial state evokes only modest HCO3− and fluid output (87, 136). This suggests that other factors, such as CCK and vagal stimulation, synergize with secretin to stimulate ductal fluid and HCO3− secretion. A decrease in secretin-evoked HCO3− secretion by the cholinergic muscarinic receptors antagonist atropine and by vagal blockade indicates that a vagal cholinergic input is important for postprandial HCO3− secretion (134, 216). In addition, exogenous in vivo application of CCK potentiates the secretin-stimulated fluid and HCO3− secretion (460). Stimulation of both the M1 and M3 and CCK receptors (CCKA) in pancreatic duct cells evoke an increase in [Ca2+]i and potentiate the effect of cAMP agonists such as secretin and VIP (237).
The mechanism of synergism between cAMP and Ca2+ signals in pancreatic duct cells is not yet fully understood. Activation of Ca2+-activated K+ channels in the basolateral membrane, which facilitates anion secretion through CFTR in the apical membrane, seems to be one plausible mechanism that has been demonstrated in several epithelia (60, 226). A second mechanism is Ca2+-dependent activation of CFTR-dependent Cl−/HCO3− exchange shown in CAPAN-1 human pancreatic duct cells (291). This mechanism may involve activation of CFTR by IRBIT. The regulatory role of IRBIT is discussed in detail below and in a recent review (457). For the current discussion, it is necessary to know that IRBIT binds to the IP3Rs to inhibit their function, and it is released from the IP3Rs by IP3 (11). IRBIT also activates both the Na+-HCO3− cotransporter NBCe1-B (394, 458) and CFTR (458). Stimulation of a Ca2+ mobilizing receptors that results in an increase in IP3 may result in dissociation of IRBIT from the IP3Rs and its binding to NBCe1-B and/or CFTR to activate them and increase the cAMP-activated fluid and HCO3− secretion. A reciprocal form of synergism is an augmentation of the [Ca2+]i signal by cAMP. In parotid acinar cells, cAMP/PKA phosphorylates the IP3R2 to increase channel open probability and hence augments the Ca2+ signal (38). Finally, Ca2+ can positively control the cAMP/PKA system. Several adenylyl cyclases (ACs), such as AC1 and AC8, are activated by elevated [Ca2+]i (440).
VI. FLUID AND ELECTROLYTE SECRETION BY ACINAR CELLS
A. Overview
Fluid and electrolyte secretion by secretory glands is a two step process, in which acinar cells secrete isotonic, plasma-like fluid and the duct modifies the electrolyte composition to generate the final fluid. The volume secreted by acinar cells is different in each gland. For example, pancreatic acinar cells secrete a small amount of volume and most fluid in the final pancreatic juice is secreted by the duct (237, 404). On the other hand, in all salivary glands the serous acinar cells specialize in fluid secretion and secrete most of the fluid in the saliva (274). Yet, to the extent that they are known, the fundamental mechanism of fluid and electrolyte secretion by the two acinar cell types is similar, if not identical. The only two known features that may explain the different rate and volume secreted by pancreatic and salivary gland cells are the properties of their receptor-evoked Ca2+ signal, which is much faster in salivary glands acinar cell (120), and perhaps expression of high level of the water channel aquaporin-5 in salivary gland acinar cell (80). Most mechanistical information on pancreatic acinar cells fluid and electrolyte secretion comes from relatively early functional studies and the molecular identity of most transporters is known only to a limited extent (for review see (331)). Most recent work has been done with salivary gland acinar cells and relied on gene deletion in mice, which revealed several unexpected features and role of the transporters (50, 274, 369). Therefore, for the most part, we will discuss fluid and electrolyte secretion by salivary gland cells and will provide information on pancreatic acinar cells when available.
B. Acinar Cells Electrolyte Transporters
The Na+/K+ ATPase pump
Fluid and electrolyte secretion is fueled by the cellular Na+ gradient generated by the basolateral Na+/K+ ATPase pump, which hydrolyses ATP to exchange 3Na+in for 2K+out and one H+in (280) and generate the transcellular Na+ and K+ gradients, and thus also determines the membrane potential (331).
K+ channels
The acinar cell membrane potential is close to the K+ diffusion potential, which is set by K+ channels. The membrane potential provides the driving force for the exit of Cl− at the luminal membrane, which is the key step initiating fluid and electrolyte secretion. Early microelectrodes work recorded K+ conductance in acinar cells that was activated by Ca2+ (332). With the invention of patch-clamp technique (139), it became clear that acinar cells express two type of Ca2+-activated K+ channels (331), a Ca2+- and voltage-activated K+ channel of a large conductance (267) and a time- and voltage-independent K+ channel of intermediate conductance (147, 294). The molecular identity of the channels was subsequently determined as the MaxiK channels coded by the Kcnma1 (294, 360) and the mIK1 channels coded by the Kcnn4 gene (27, 146), respectively. Gene deletion in mice revealed that both channels are required to sustain acinar cells and salivary glands function. Thus, deletion of Kcnn4 had no effect on either resting or stimulated acinar cell volume regulation or glandular fluid and electrolyte secretion, including K+ secretion (27). Moreover, deletion of the Kcnma1 gene had no effect on salivary glands fluid and electrolyte secretion (360), although K+ secretion is impaired in salivary glands lacking the MaxiK channel that is expressed in the luminal membrane of the duct (288). It was necessary to delete both the Kcnn4 and the Kcnma1 genes in mice to reduce receptor-stimulated fluid and K+ secretion by acinar cells and thus by salivary glands (362). Acinar cells appear quite plastic and lack of effect due to deletion of one of the K+ channels may result from adaptation of the acinar cells. Example for acinar cells plasticity and adaptability are also seen in deletion of the Na+/H+ exchange NHE1, which resulted in extensive adaptation to increased Cl−/HCO3− exchange activity, probably by enhanced expression of carbonic anhydrase II, and increased expression of of NKCC1 (121). Another example is the partial deletion of the ER Ca2+ pump SERCA2 that resulted in adaptation of the Ca2+ signaling and granule exocytotic machineries (474).
NKCC1
A key transporter for acinar cells fluid and electrolyte secretion is the Na+/K+/2Cl− co-transporter NKCC1. NKCC1 is ubiquitous and is activated by cell shrinkage to mediate regulatory volume increase to restore cell volume after cell shrinkage (78, 225). NKCC1 is expressed in the basolateral membrane of acinar cells (99) and is inhibited by the diuretics furosemide and bumetanide (137). Manipulation of external ions and the use of NKCC inhibitors showed that NKCC activity mediates about 70% of the Cl− uptake that is secreted across the luminal membrane to drive secretory glands fluid secretion (274, 310, 331). NKCC1 together with the Na+/H+ exchanger NHE1 also provide the cytosol with most of the Na+ necessary to activate the Na+/K+ pump (469, 470). The central role of NKCC1 in salivary glands fluid and electrolyte secretion was further established by deletion of the Nkcc1 gene in mice, which resulted in about 70% inhibition of salivary secretion (99).
NHE1 and AE2
The findings that inhibition (310) and deletion of Nkcc1 in mice (99) inhibited fluid and electrolyte secretion by only 70% indicate that another mechanism can fuel the secretory process. Partial inhibition of glandular secretion by the Na+/H+ exchanger inhibitor amiloride and by the Cl−/HCO3− exchanger inhibitor DIDS (227, 266, 310) suggested that these transporters acting in concert mediate part of the basolateral membrane Cl− uptake necessary for luminal Cl− secretion. The Na+/H+ exchangers family includes five members that are expressed in the plasma membrane NHE1-NHE5 (33, 317) and the Cl−/HCO3− exchangers family includes four members AE1-AE4 (8). Functional (272, 283, 469, 470), immunological (98, 272, 372) and molecular studies (98, 403) identified the acinar cells basolateral membrane Na+/H+ exchanger isoform as NHE1 and the Cl−/HCO3− exchanger isoform as AE2. NHE1 and AE2 are ubiquitous, function as the main regulators of cytoplasmic pH, and are activated by small changes in pHi. NHE1 is activated by acidic pHi (16) to extrude acid generated by cellular metabolism, while AE2 is activated by alkaline pHi to extrude excessive cytosolic base (316). By virtue of regulating pHi in resting and stimulated cells, NHE1 and AE2 are involved in many cellular functions that in secretory gland cells include mediating part of acinar cells fluid and electrolyte secretion. Indeed, deletion of NHE1 (28) and AE2 (115) in mice resulted in severe phenotypes. Although the severe phenotypes precluded using the mice to study the role of NHE1 and AE2 in exocrine glands functions in vivo, the knockout mice were useful in demonstrating the functional role of NHE1 and AE2 in isolated secretory cells.
TMEM16a/Ano1
It has long been recognized that Cl− exits acinar cells by a conductive pathway (186) that was later demonstrated as the apical Ca2+-activated Cl− channel (CaCC) (17, 200, 335). The channel has been extensively characterized biophysically to be a voltage- and Ca2+-activated, time-dependent outwardly rectifying channel (224, 274). However, the molecular identity of the channel eluded extensive searches until recently. Several CLC family (274) and bestrophins, in particular Best2 (186), have been suggested as the molecular identity of CaCC. However, characterization of the various expressed Best channels did not fully recapitulate the features of the native CaCC (224), and even when they closely matched these properties, like Best2, deletion in mice showed that Best2 is not the acinar CaCC (361). Recently, three independent groups have used different approaches to identify TMEM16a/Anoctamin 1 (ANO1) as the CaCC in several cell types (43, 383, 459). Moreover, TMEM16a/ANO1 is expressed at high levels in the luminal membrane of salivary glands (383, 459) and pancreatic acinar cells (166) (but not in the duct), knockdown of TMEM16a/ANO1 by siRNA reduced salivary secretion (459) and knockout in mice eliminated the CaCC activity in acinar cells (361). It was not possible to use mice with global deletion of TMEM16a/ANO1 to study its role in secretory glands function since TMEM16a/ANO1 is required for airway development and most mice die in utero or shortly after birth (359). Targeted knockout of TMEM16a/ANO1 is necessary to overcome this problem and to further examine the role of TMEM16a/ANO1 in acinar cells and glandular function. TMEM16a/ANO1 is expressed in acinar cells, but not the duct. Since the duct also expresses CaCC (128, 465), it is possible that the duct expresses another isoform of the TMEM16 family, which consists of 10 members (224). This is discussed further below.
Aquaporins
Transcellular ion secretion results in the obligatory osmotic water flow. Although water can cross the membrane bilayer, water flow in secretory cells is facilitated by the water channels aquaporins (AQPs). The AQPs family consists of 13 members, all of which can function as water channels, but some can transport other molecules like glycerol, urea, ions and CO2 (425). The AQPs are involved in several cell functions, like cell adhesion, proliferation, migration and cell survival (425). Although our knowledge of the complement of AQPs expressed in secretory glands is not complete and sometime contradictory, the expression and perhaps function of several AQPs is associated with several diseases of secretory cells, like Sjögren’s syndrome (80) and pancreatitis (210, 315). When present in the glands, the AQPs show highly restricted and cell specific expression pattern. For example, AQP1 is expressed in salivary glands endothelial and myoepithelial cells (16), while it is found in the human pancreatic duct (211), in centroacinar cells and in both the apical and basolateral domains of intercalated and intralobular ducts (42). However, deletion of AQP1 in mice has no effect on salivary glands function (272), yet the deletion of AQP1 in mice results in defective dietary fat processing (254) and pancreatic exocrine insufficiency in LXRβ −/− mice is associated with a reduction in AQP1 expression (111). This suggests that perhaps the AQPs have cell specific function in different acinar cells. Another possibility is adaptation of salivary glands to AQP1 deletion.
The established AQP in acinar cells is AQP5, which is expressed in the luminal membrane of acinar cells (80). A critical role of AQP5 in exocrine glands fluid secretion was established by knockout in mice (8, 272) and aberrant trafficking of AQP5 in Sjögren’s syndrome patients (98, 372, 403). AQP5 knockout in mice reduces salivary glands secretion by more than 60% (272) and changes in cell volume in response to osmotic perturbations (317). A key regulatory mechanism of AQP5 activity is trafficking to the plasma membrane in response to cell stimulation (8). In addition, AQP5 appears to regulate the water permeability of the paracellular pathway. Deletion of AQP5 disrupted integrity of the tight junction and reduced the paracellular water permeability (98). Trafficking of AQP5 to the plasma membrane is impaired in salivary glands of patients with Sjögren’s syndrome (98, 372, 403).
The salivary glands duct secretes little fluid (66, 274) and does not express AQP5 (258) or AQP1 (16). However, pointedly, gene transfer of AQP1 to the salivary duct resulted in a marked increase in salivary glands fluid secretion and increased Na+ in the secreted fluid (112). Transduced AQP1 is expressed in the luminal membrane of the rat (283) and mini pigs salivary glands duct (115). This is reminiscent of expression of AQP1 in the pancreatic duct (210–212), which secretes most of the fluid in the pancreatic juice and does not absorb the Na+. The simplest interpretation of these observations is that the pancreatic and salivary gland ducts have different water permeability and luminal membrane water permeability of the ducts dictates their function in secretory glands. In the salivary glands the duct mainly regulates electrolyte composition of the secreted fluid, while the pancreatic duct determines both, electrolyte composition and volume of the secreted fluid. In addition, AQP1 may reduce the function of ENaC of the salivary gland ducts to allow the net Na+ efflux to fuel fluid secretion.
C. Model and Regulation
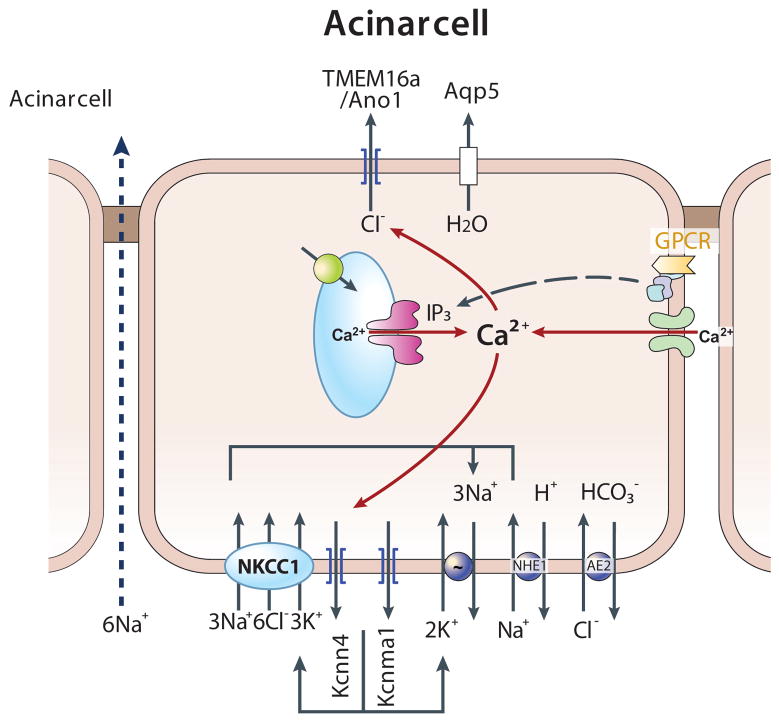
The available information on the localization and function of acinar cells ion transporters leads to the mechanism of acinar cells fluid and electrolyte secretion illustrated in Fig. 2. Acinar cells fluid and electrolyte secretion is fueled by the basolateral Na+/K+ ATPase pump, which set intracellular Na+ at about 20 mM and intracellular K+ at about 140 mM (64, 221, 336, 338). The basolateral NKCC1 and NHE1 are the main routes of Na+ influx that feeds the Na+/K+ ATPase pump (469, 470). The basolateral Ca2+-activated K+ channels set the membrane potential at −50 to −60 mV (186, 331). NKCC1 is the major route of Cl− influx into acinar cells and together with the Cl−/HCO3− exchanger AE2 sets intracellular Cl− at about 60 mM, which is 5 fold above electrochemical equilibrium (186, 472). NHE1 and AE2 also set cytoplasmic pH at about 7.2 (272, 282, 283, 295), which is one order of magnitude below the H+ electrochemical equilibrium, and guard against large fluctuation in pHi. The main transporters at the luminal membrane are the Ca2+-activate Cl− channel TMEM16A/Ano1 (224, 361) and the water channel AQP5 (80, 218, 255). The acinar cells tight junction is permeable to Na+ and is the main route of transcellular Na+ flux (237, 274, 404).
Fig. 2. A model depicting the mechanism of acinar cells fluid and electrolyte secretion.
Shown are the major transporters in the basolateral and luminal membranes of acinar cells and their regulation. The major Cl− loading transporter at the basolateral membrane is NKCC1, with part of the Cl− loading (about 30%) provided by the parallel functioning of NHE1 and AE2. The membrane potential is determined by two Ca2+-activated K+ channels, the MaxiK and mIK1 channels. TMAM16a/Ano1 is the major Ca2+-activated Cl− channel at the luminal membrane that also expresses the water channel AQP5. Fluid and electrolyte secretion by acinar cells is regulated by Ca2+-mobilizing receptors and is a Cl− secretion-driven process. The receptor-evoked [Ca2+]i increase initiates at the apical pole where the Ca2+ signaling complexes are located to activate TMEM16a/Ano1. The Ca2+ signal then propagates to the basal pole to activate the K+ channels. The Ca2+-mediated channels activation results in luminal Cl− efflux and basolateral K+ efflux. Na+ then flows through the tight junction to the luminal space. The secretion of NaCl leads to water efflux through AQP5 and cell shrinkage. Cell shrinkage reduces [Ca2+]i to inhibit the Cl− and K+ channels and at the same time activates the volume-sensitive NKCC1 (and NHE1 and AE2) to restore cell Cl− and K+. The cycle repeats itself during each spike of Ca2+ oscillations.
Acinar cells fluid and electrolyte secretion is a Ca2+-initiated and regulated process that can be augmented by the cAMP/PKA system. The secretory process is initiated by an increase in [Ca2+]i. As outlined above, the physiological receptor-evoked Ca2+ signal is in the form of Ca2+ oscillations that initiate at the apical pole and spread to the basal pole in the form of propagated Ca2+ waves (196, 208, 414). Accordingly, activation of the apical Cl− channel is the key initial step that initiates acinar cells fluid and electrolyte secretion (224, 361, 459). Once the Ca2+ waves arrive at the basolateral membrane, they activate the K+ channels (294, 331, 362). Activation of the Cl− and K+ channels leads to Cl− efflux into the luminal space and K+ efflux to the interstitial space. The Ca2+ increase also activates AQP5 (184, 185). Hence, KCl efflux is followed by the obligatory water efflux through AQP5 to the luminal space and cell shrinkage (8, 17, 335). To compensate for the negative charge due to Cl− secretion, Na+ crosses from the basal to the luminal side mostly through the paracellular pathway, resulting in NaCl secretion.
Cell shrinkage has two consequences, it facilitates reduction in [Ca2+]i back to baseline and it activates ion influx across the basolateral membrane. Thus, increase in cell volume facilitates and a decrease in cell volume decreases the Ca2+ signal (200, 289). Reduction of [Ca2+]i to basal level inhibits the Cl− and K+ channels to temporally stop fluid and electrolyte secretion. Most importantly, cell shrinkage activates the volume-sensitive NKCC1 (137, 159), NHE1 (5) and AE2 (8). The molecular mechanism for activation of NHE1 and AE2 by cell shrinkage is not well understood, although it involves activation of several kinases, like PKC and p38 MAPK, and inhibition of PP1 (329). Cell shrinkage leads to phosphorylation of NKCC1 by the volume sensitive SPAK kinase (76). The role of these regulatory mechanisms in secretory glands function, although likely, is yet to be established. The activated NKCC1 mediates most of the Na+, K+ and Cl− uptake to restore ionic content and volume of acinar cells. The activated AE2 and NHE1 contribute about 30% of the Na+ and Cl−. Restoration of cell volume and ionic content prepares the cell for the next cycle of Ca2+-regulated fluid and electrolyte secretion that occurs during every spike of Ca2+ oscillations.
The cycle of the secretory process is highly synchronized and the Ca2+ oscillations are closely followed by oscillation of intracellular Cl− (and likely K+) that are followed by oscillations in cell volume (106, 107). Moreover, synchronization extends to entire acini that function as a syncytium. The acinar syncytium is coupled by gap junctions made of connexins 26 and 32 (270).
V. DUCTAL FLUID AND HCO3− SECRETION
A. Overview
Exocrine secretion is a two stage process, whereby the acinar cells secret isotonic NaCl-rich fluid and the ducts modify the ionic composition and in some glands, like the pancreas, secrete most of the fluid. For long time studies of ductal function lagged behind studies of acinar cell. Contributing reasons were the fact that acini secret the protein, like digestive enzymes and mucins, or the bulk of the fluid in the case salivary glands, which were considered the central specialized function of secretory glands. In addition, the ducts comprise only between 5% (pancreas and parotid gland) to 20% (submandibular) of the gland cell volume, which limited access to the ducts. Advances in molecular, cellular and physiological techniques over the past 25 years changed this and revealed the molecular identity, function and regulation of ductal ion transporters at the basolateral and luminal membranes (274, 471). Major discoveries are the identification of anion channels in the luminal membrane, such as CFTR and CaCC; identification of the basolateral Na+-HCO3− cotransporter (234, 471), which was shown to be critical for pancreatic HCO3− secretion (180) and cloned as pNBC1 (1) and later re-named NBCe1-B (35). A breakthrough was made with the discovery of the Cl−/HCO3− exchangers SLC26 transporters (160) and the recognition of their essential role in epithelial HCO3− secretion (91, 215). Yet another significant finding was the discovery of HCO3− absorbing mechanisms like NHE3 (234, 250) and NBC3 (NBCn1-A) (250, 327) at the luminal membrane of the ducts, which suggested the ducts absorb and scavenge HCO3− in the resting state. The major ion transporters expressed in the luminal and basolateral membranes of the duct are summarized in Tables 1 and 2, respectively, and illustrated in Fig. 3. Expression and membrane localization of these transporters have been demonstrated in the pancreatic and salivary ducts, including the exception of ENaC, which is expressed only in the luminal membrane of salivary glands duct (65, 66). Accordingly, salivary gland ducts absorb the Na+ from the saliva, whereas the pancreatic duct does not absorb but secrete Na+ into the pancreatic juice.
Table 1.
Transporters in the luminal (apical) membrane of exocrine gland ducts
| Transporter | Protein | Role | References |
|---|---|---|---|
| cAMP-activated Cl− channel | CFTR (ABCC7) | Fluid and HCO3− secretion | (126), (127), (125), (128), (4), (312), (296), (26), (261), (68), (466), (183), (103), (175), (174), (245), (340), (353), (387), (182), (84), (324), (402), (61) |
| Ca2+-activated Cl− channel | TMEM16/Anotcamin family (?) | (?) | (128), (444), (108), (443), (105), (296), (4), (158), (21), (176), (39), (452), (51), (52), (55), (345), (476), (459), (43), (383) |
| Anion exchangers | HCO3− secretion | (47), (220), (19), (407), (306), (471), (235), (153), (20), (97), (291), (238), (213), (386), (401), (183), (179), (178) | |
| SLC26A3 (DRA/CLD) | HCO3− secretion, electrogenic 2Cl−/1HCO3− exchanger | (96), (273), (129) | |
| SLC26A6 (PAT1) | Fluid and HCO3− secretion, electrogenic 1Cl−/2HCO3− exchanger | (96, 247), (429), (209), (190), (434), (449), (213), (235), (215), (122) | |
| Na+/H+ exchangers | NHE3 (SLC9A3) | HCO3− reabsorption (HCO3− salvage) | (118), (263), (471), (264), (234) |
| NHE2 (SLC9A2) | (?) | (118), (263), (471), (264), (234) | |
| Na+-HCO3− cotransporter | NBCn1-A (NBC3, SLC4A7) | HCO3− reabsorption (HCO3− salvage) ? | (327), (59), (250) |
| K+ channels | Maxi- K+ channels (KCNMA1?) | Maintain membrane potential during stimulated secretion | (423) |
| Water channel | Aquaporin 5 (AQP5) | Fluid secretion | (42) |
| Epithelial Na+ channel (ENaC) | SCNN1A (α), SCNN1B (β), SCNN1G (γ), SCNN1D (δ) | Na+ absorption (Functional ENaC channel with αβγ subunits is expressed in salivary, but not in pancreatic duct. ENaCδ is expressed in pancreas; however its role is uncertain.) | (49, 65, 454) |
Table 2.
Transporters in the basolateral membrane of the ducts
| Transporter | Protein | Role | References |
|---|---|---|---|
| Na+/H+ exchangers | NHE1 (SLC9A1) | Na+/H+ exchange, Contributes to basolateral HCO3− influx | (407), (305), (471), (180), (72), (178), (234), (370), |
| NHE4 (SLC9A4) | (?) | (370), (234) | |
| H+-ATPase | V-type H+- ATPase | (?) | (349), (40), (421), (420), (428), (300), (471), (370), (72), (183), (179), (133), (54) |
| Na+-HCO3− cotransporters | NBCe1-B (pNBC1, SLC4A4) | The major Basolateral HCO3− uptake transporter | (221), (471), (428), (180), (72, 178), (396), (182), (1), (413), (260), (379), (371), (131), (131), (132, 179), (173) |
| Anion exchangers | AE2 (SLC4A2) | Housekeeping function Prevent intracellular alkalinization |
(471), (235), (178), (177), (171), (129), (370), (19) |
| Cation-chloride cotransporters | Na+-K+-2Cl− cotransporter (NKCC1, SLC12A2) | Basolateral Cl− uptake in mouse and rat ducts, but not in guinea pig and human) | (103), (54), (397), (67) |
| K+-Cl− cotransporter (KCC1, SLC12A4) | Basolateral K+and Cl− efflux Cell volume regulation? | (119), (373) | |
| K+ channels | Maxi- K+ channels (KCNMA1) | Maintain membrane potential | (305), (157), (308), (320), (168), (152), (124), (296), (15), (19, 123) |
| Small or intermediate conductance K+ channels (mIK1) | Maintain resting membrane potential | (305, 404) | |
| Na+,K+-ATPase | Na+,K+- ATPase (ATP1B1) | Maintain inward Na+ and outward K+ gradients | (305), (256), (41), (399) |
| Water channels | Aquaporin 1 (AQP1) | Water transport. | (109), (212), (42) |
| Aquaporin 5 (AQP5) | Water transport. Role in secretion unknown at present | (42) |
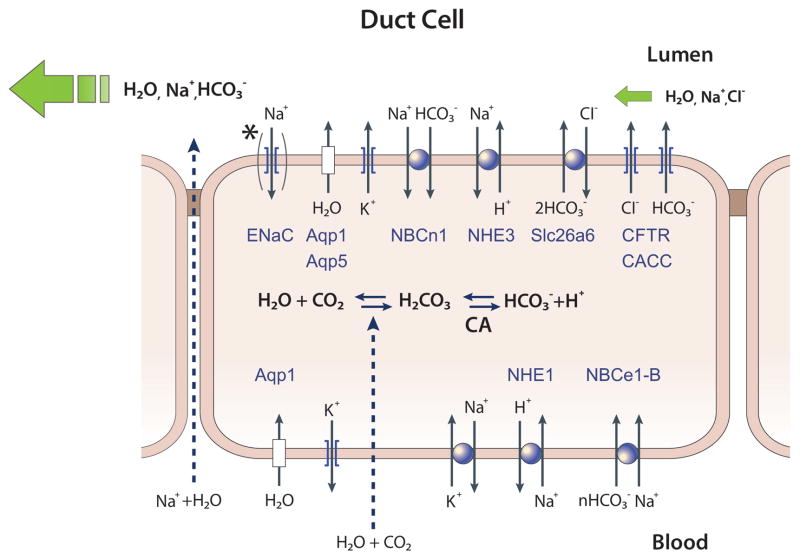
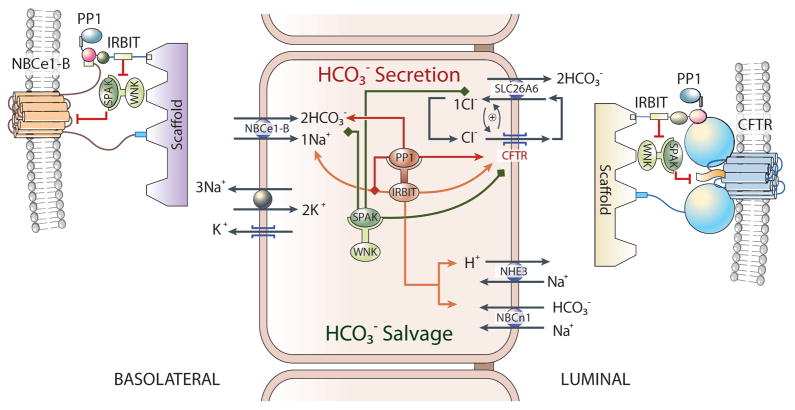
Fig. 3. A model depicting the mechanism of ductal fluid and HCO3− secretion.
Shown are the major transporters in the basolateral and luminal membranes of duct cells. In the pancreas ductal secretion is driven by HCO3− secretion. The major HCO3− loading mechanism is the basolateral Na+-HCO3− cotransporter NBCe1-B. Luminal HCO3− secretion is mediated by the CFTR-Slc26a6 complex. The duct also expresses the HCO3− salvage mechanisms NHE3 and NBCn1-A. The salivary glands, but not the pancreatic, duct also expresses ENaC at the luminal membrane. The functioning of the transporters in ductal fluid and HCO3− secretion is illustrated in Fig. 6.
B. Duct Cells Electrolyte Transporters
Na+/K+ ATPase
The Na+/K+ ATPase pump is abundantly expressed in the basolateral membrane of the ducts (256, 369, 399, 410). The primary Na+/K+ ATPase pump in conjunction with the basolateral K+ channels uses and converts the chemical energy in ATP to osmotic energy in the form of the Na+ and K+ gradients and a negative membrane potential, which fuel fluid and electrolyte secretion by the duct. The Na+ gradient is used for HCO3− accumulation in the cytosol and the membrane potential facilitates HCO3− fluxes by electrogenic transporters at the basolateral and luminal membranes.
K+ channels
Although the activity of several K+ channel is found in duct cells, the molecular identity of the major K+ channel is not fully established. In the pancreatic duct MaxiK channels are the likely candidates maintaining a negative membrane potential during HCO3− secretion (124). These channels are activated by Ca2+, have a large conductance (125–250 pS), and are encoded by the Kcnma1 gene (30). In earlier reports MaxiK+ channels were thought to be localized at the basolateral membrane of pancreatic duct cells (298). However, a recent study revealed that MaxiK channels are expressed at the luminal membrane in guinea pig pancreatic duct cells (423). Such a localization may account for the increased Ca2+ sensitivity of the MaxiK channels by the cAMP/PKA pathway, and contributes to the secretin-induced ductal secretion (124). In addition, activation of luminal MaxiK channels can account for part of the potentiation of ductal fluid and HCO3− secretion by Ca2+ mobilizing receptors.
Luminal localization of the MaxiK channels was also observed in salivary gland ducts (288). Such localization indicates that another K+ channels must be expressed at the basolateral membrane of the duct. Indeed, MaxiK channels do not seem to contribute to duct cells resting membrane potential, possibly because they have very low open probability at the unstimulated state. A potential basolateral K+ channel is Ba2+-sensitive channel of 82 pS conductance, which is the main channel responsible for the resting K+ permeability (305). In this respect, as outlined above, salivary glands express two K+ channels, the MaxiK (294, 360) and the mIK1 channels (27, 146). The salivary glands absorb the Na+ and secrete K+ into the salivary fluid. Deletion of the Kcnma1 gene coding for MaxiK had no effect on salivary glands fluid secretion (360), but impaired K+ secretion (288), establishing the role of MaxiK as the channel that controls K+ efflux and determines the potential of the luminal membrane. Since deletion of the Kcnn4 and the Kcnma1 genes in mice reduced receptor-stimulated fluid and K+ secretion by salivary glands (362), it is likely that mlK1 is the K+ channel at the basolateral membrane of the duct. The use of the available mice with single and combined deletion of the Kcnn4 and Kcnma1 genes should allow addressing this problem directly in the salivary glands and pancreatic ducts.
Na+-HCO3− cotransporters (NBCs)
HCO3− secretion requires HCO3− entry at the basolateral membrane with transport characteristics adequate to maintain HCO3− accumulation in the cytoplasm. A search for such mechanisms identified a Na+/H+ exchange activity with properties of NHE1 and a Na+-HCO3− co-transport (NBC) activity with the properties of what became known as NBCe1-B in the basolateral membrane of the rat pancreatic duct (470). After the initial discovery of NBC activity in the rat pancreatic duct, similar activity was demonstrated in all other species examined, including the guinea pig duct (178, 347). The basolateral NBC isoform was cloned from the pancreas and named pNBC1 (1). After identification of all members of the superfamily of Na+-driven HCO3− transporters it was re-named NBCe1-B (35). NBCe1-B is expressed at the basolateral membrane of most, if not all, epithelia, including salivary gland acinar and duct cells (250).
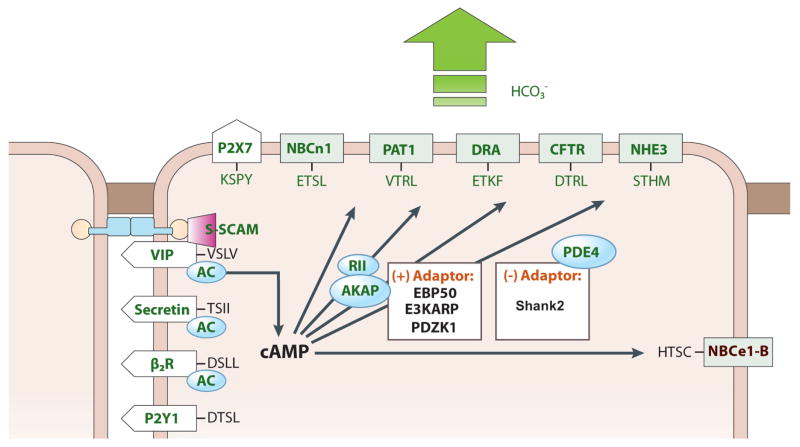
While NHE1 functions as electroneutral exchanger, NBCe1-B is an electrogenic transporter with most likely 1Na+-2HCO3− stoichiometry, although NBCe1-B stoichiometry appears to depend on the cell type in which it is expressed (131), and can be altered by PKA-dependent phosphorylation at Ser1026 (130). The electrogenic NBCe1-B uses the Na+ gradient more efficiently than NHE1 to accumulate cytosolic HCO3− and indeed NBCe1-B mediates the bulk of basolateral HCO3− entry during ductal fluid and HCO3− secretion (180, 183). Significantly, NBCe1-B behaves as a 1Na+-2HCO3− cotransporter when expressed in a pancreatic ductal cell line (131). Although the stoichiometry of the transport was not directly measured in native pancreatic ducts, it is considered to be 1Na+-2HCO3− in the stimulated duct since it mediates HCO3− influx across the basolateral membrane (404), which at a membrane potential of −60 mV is possible only with a 1Na+-2HCO3− stoichiometry. The activity of NBCe1-B is regulated by the protein named IRBIT (394, 457), the mechanism and significance of which is discussed in detail bellow.
The duct also expresses an electroneutral NBC in the luminal membrane (327) that was cloned as NBC3 (343) and later re-named NBCn1-A. The finding of Na+ and HCO3− absorbing mechanisms at the luminal membrane of the duct was unexpected. Similar to the case of NHE3 (see below) (3), NBCn1-A (327) is regulated by CFTR in a cAMP-PKA-dependent manner. Stimulation of CFTR with PKA leads to inhibition of NHE3 and NBCn1-A (3, 327). NHE3 (150) and perhaps NBCn1-A (35) are also activated by IRBIT (see below). Based on their regulation by IRBIT and stimulated CFTR, we proposed that NHE3 and NBCn1-A are part of a HCO3−-regulating complex in the duct, which serves as a HCO3− salvage mechanism at the resting state to maintain acidified pancreatic juice (118, 263) and saliva (250).
Cystic fibrosis transmembrane conductance regulator (CFTR)
CFTR was discovered as the protein mutated in cystic fibrosis (199, 356, 364). Since its discovery, CFTR became one of the most extensively studied protein and serves as a model to understand the function of proteins of similar structure and function. CFTR (ABCC7) belongs to the ATP-binding cassette (ABC) transporters superfamily. Most ABC transporters function as membrane pumps for organic molecules, which transport their substrates against the electrochemical gradient using energy generated from ATP hydrolysis (74). Unlike other ABC transporters, CFTR has anion channel activity that conducts the substrate molecule down the electrochemical gradient. In expression cloning, CFTR functions as a small conductance (5–10 pS) Cl− channel with a linear current-voltage (I-V) relationship that is activated by the cAMP/PKA pathway (411). Channels with similar properties have been identified in the pancreatic and salivary gland duct cells of many species, including humans. The presence of CFTR in the luminal membrane of the pancreatic and salivary glands ducts has now been firmly established in many species by immunohistochemistry (49, 404, 466)
CFTR functions as a Cl− channel with limited permeability to HCO3− at normal intra and extracellular Cl− (245, 340, 387). Based largely on computer modeling, it has been suggested that CFTR may function as a HCO3− channel in the pancreatic duct (181, 312, 353, 387, 437). However, several key findings indicate that CFTR-mediated HCO3− flux has limited and defined role in ductal HCO3− secretion. First, the ducts express luminal, DIDS-sensitive Cl−/HCO3− exchange activity (235, 424, 471) that is mediated by the luminal SLC26 transporters (405, 433). Second, CFTR is regulated by extracellular and intracellular Cl− and at extracellular Cl− of higher than 30 mM it does not transport significant amount of HCO3− (387, 448). Third, Cl− has to be removed from the luminal space for the duct to secrete HCO3− through CFTR (181, 325). Forth, deletion of Slc26a6 impairs ductal fluid and HCO3− secretion (433). Hence, although having a HCO3− selective channel at the apical membrane of duct cells it is theoretically possible to secrete 200 mM HCO3− at a membrane potential of −60 mV, the CFTR PHCO3/PCl is between 0.2–0.5 in pancreatic duct cells when measured with symmetrical Cl− solutions (312, 400). With this permeability ratio, the CFTR anion channel secretes Cl− much faster than HCO3−, thus CFTR would be unable to secrete sufficient HCO3− to account for the bulk of the secreted HCO3−. Finally, fluid secretion requires osmotic HCO3− secretion. Strict exchange of HCO3− for Cl− by CFTR will not result in osmotic solute secretion.
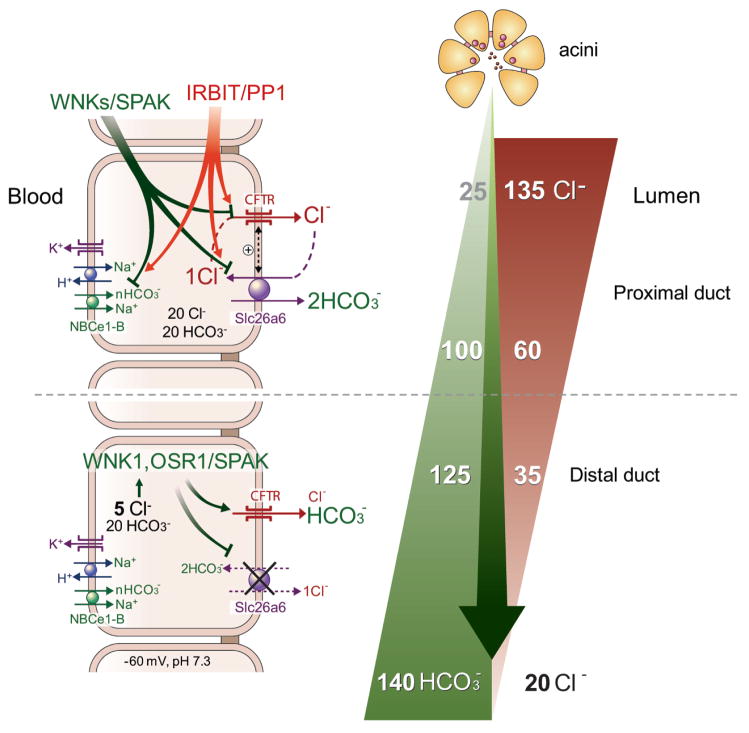
Nevertheless, CFTR-mediated HCO3− flux can become important at the distal portion of the ducts to determine the final HCO3− concentration in the secreted fluids. A unique form of regulation of CFTR activity discovered recently is by the WNK/SPAK pathway. The WNK kinases were discovered in a search for MAPK homologues and the family consists of four members with conserved kinase domain, but diverse N and C termini (reviewed in (164)). Interest in the WNKs increased greatly with the discovery that mutations in WNK1 and WNK4 cause hypertension in humans (442). The WNKs act mostly by regulating surface expression of Na+, K+ and Cl− transporters (194), through regulation of their endocytosis (165). Subsequent work revealed that many functions of the WNK are mediated by the downstream oxidative stress-responsive kinase 1 (OSR1) and STE20/SPS1-related proline/alanine-rich kinase (SPAK) (reviewed in (77)). The WNK/SPAK kinase pathway appears to have dual function in the ducts. At normal physiological [Cl−]i the WNK kinases reduce surface expression of CFTR expression (455, 456). In the pancreatic and salivary glands the WNKs act through SPAK to control the activity of NBCe1-B and CFTR, and knockdown of the WNKs and SPAK increases pancreatic duct fluid and HCO3− secretion (456). However, at low cytoplasmic [Cl−]i, as occurs in the distal duct, the WNK/SPAK pathway appears to have an opposite role. The CFTR HCO3− permeability appears to be dynamically regulated by intracellular Cl− (325). During pancreatic secretion, [Cl−]i can drop to as low as 5 mM. This low [Cl−]i in turn activates WNK1 and the downstream OSR1 and SPAK. Activation of the WNK1/OSR1/SPAK pathway under this [Cl−]i conditions resulted in a dramatic increase in CFTR HCO3− permeability, making CFTR primarily a HCO3− channel (325). Function of CFTR as a HCO3− channel at the distal duct can be essential for the secretion of pancreatic juice containing 140 mM HCO3−. Hence, it appears that under resting conditions and at the proximal duct the WNK/SPAK pathway reduces NBCe1-B and CFTR activity to stabilize the resting state and perhaps minimize HCO3− fluxes by CFTR. On the other hand, at the distal duct an important function WNK/SPAK pathway is to switch CFTR from primarily a Cl− channel to a HCO3− channel to set the final HCO3− concentration in the secreted fluids.
In addition to Cl− and HCO3− channel activity, CFTR functions as a central regulator of ductal fluid and electrolyte secretion by virtue of regulating the function of many transporters at the luminal membrane of the ducts. The central role of CFTR in ductal function is well exemplified by the aberrant fluid and electrolyte transport and pancreatic insufficiency seen in CF patients (94). CFTR exists in a macromolecular complex at the luminal membrane of secretory epithelia, which is assembled with the aid of scaffolding proteins. The three amino acids at the C-terminal end of CFTR form a PDZ ligand that binds to PDZ domains containing scaffolds in epithelia (395, 431). In addition, CFTR interacts with SNARE proteins, AKAPs, kinases and phosphatases (135). In the complexes, CFTR directly or indirectly regulates the activity of several transporters. Functional interactions with CFTR were reported for ENaC, outwardly rectifying Cl− channels, Ca2+-activated Cl− channels, ROMK2 and KvLQT1 K+ channels, the SLC26 transporters, NBCn1-A (NBC3) and perhaps aquaporins (222, 237). Below we discuss the significance of several of these interactions for ductal secretion.
Ca2+-activated Cl− channels (CaCCs)
Ca2+-activated chloride channel activity (CaCCs) is present in the luminal membrane of duct cells (125, 128, 424, 465). The molecular identity of ductal CaCC is still unknown. The discovery of the TMEM16/Anoctamin (ANO) family as the CaCC in acinar cells (43, 383, 459) suggest that the ductal CaCC is likely a member of this family. However, immunostaining shows that TMEM16A/ANO1 is expressed in the luminal membrane of acinar cells and that the duct does not express TMEM16A/ANO1 (383, 459). To date, several members of the TMEM16/ANO family were shown to function as Cl− channels, including TMEM16B/ANO2, TMEM16G/ANO7, TMEM16J/ANO10 and perhaps TMEM16F/ANO6 (406). Human and rodent pancreata and the salivary duct express mRNA for several members of TMEM16/ANO family, including TMEM16B/ANO2, TMEM16F/ANO6 and TMEM16J/ANO10 (MGL and SM, unpublished observation). Although TMEM16F/ANO6 was reported recently to function as a phospholipid scrambler (410), it may also function as Cl− channel. It will be interesting to determine how the two activities of TMEM16F/ANO6 are related. Whether the other TMEM16/ANO isoforms also mediate ductal CaCC awaits further studies.
Interest in the ductal CaCC stems from the possibility that it may replace the Cl− channel function of CFTR in HCO3− secretion. Studies in the human pancreatic duct cell line PANC1 (476), raise questions as to the feasibility of such a role. Moreover, an increase in [Ca2+]i alone by stimulation of the cholinergic and CCK receptors does not evoke significant fluid and HCO3− secretion by the pancreatic duct (460) and cholinergic simulation of salivary glands result in low HCO3− secretion (45). The PHCO3/PCl of heterologously expressed TMEM16A is 0.1–0.5 at [Ca2+]i levels normally induced by receptor stimulation. Interestingly, the PHCO3/PCl permeability of TMEM16A appears to be regulated by [Ca2+]i (MGL, unpublished observation). The physiological role of this finding in epithelial HCO3− secretion is not known at present.
Cl−/HCO3− exchangers: AEs and the SLC26 transporters
The pancreatic duct secretes most of the fluid in the pancreatic juice. The copious fluid secretion must involve large net transcellular salt transport. Since the pancreatic duct absorbs the Cl− and secretes fluid containing Na+ and HCO3−, and since Na+ secretion is largely paracellular, it follows that pancreatic duct HCO3− secretion must be an active process and a 1Cl−/1HCO3− exchange, whether by coupled or uncoupled transport, cannot lead to net electrolyte secretion necessary to drive fluid secretion. This puts a thermodynamic constrain as to the nature of the Cl− absorbing and HCO3− secreting mechanism that must function with a stoichiometry of HCO3−/Cl− >1. We will argue below that SLC26 transporter Slc26a6 fulfill this requirement and is critical for pancreatic duct fluid and electrolyte secretion.
Because the absence of CFTR activity in CF results in acidic pancreatic juice, it was assumed that CFTR directly mediates the tightly coupled Cl− absorption and HCO3− secretion or function in concert with Cl−/HCO3− exchange to mediate HCO3− secretion by the duct (49, 169, 404). An early model for pancreatic HCO3− secretion suggested that electroneutral Cl−/HCO3− exchanger in the apical membrane mediates HCO3− secretion in conjunction with Cl− channel that cycles the Cl− (237, 400, 401, 404). Subsequently, it was revealed that CFTR mediates the apical Cl− channel activity and that the activity of the apical Cl−/HCO3− exchanger is dependent on the expression of CFTR (235, 238). Furthermore, CFTR mutations associated with pancreatic insufficiency exhibited severe defect in CFTR-dependent Cl−/HCO3− exchange activity (61). These findings prompted extensive search to identify the molecular nature and function of the ductal luminal Cl−/HCO3− exchangers.
The first family of Cl−/HCO3− exchangers to be considered are members of the solute-linked carrier 4 (SLC4) family (363). The ducts do express the SLC4 transporter AE2 (SLC4A2), but it is located on the basolateral membrane of pancreatic and salivary gland duct cells (370, 372). AE2 together with NHE1 is essential for regulation of pHi and protects the cells against an alkali load, but does not appear to play a major role in transcellular ductal HCO3− transport. A breakthrough in our understanding of luminal Cl−/HCO3− exchange in epithelia was made with the discovery that the protein previously identified as down regulated in adenoma (DRA), is a Cl− transporter highly expressed at the luminal membrane of the colon, and mutation in which lead to the disease congenital Cl− diarrhea (160, 281). Shortly thereafter it was shown that DRA functions as a Cl−/HCO3− exchanger (273). With the renaming of the family it is designated as SLC26A3 and is expressed in several epithelia.
The SLC26 transporters (SLC26Ts) family consists of 11 genes and 10 members (Slc26A10 is pseudo gene) with several members associated with human diseases (for review see (91)). The family members have diverse functional properties (314), with SLC26A1 and SLC26A2 functioning as SO42− transporters (262), Slc26a3 and Slc26a6 as electrogenic Cl−/HCO3− exchangers (214, 449), Slc26a4 as electroneutral Cl−/HCO3−/I− exchanger (389), SLC26A5 functions as an anion regulated, voltage sensing motor protein (376), and SLC26A7 (204) and SLC26A9 (90) function as bona fide Cl− channels. The function of SLC26A11 is not known at present. Pancreatic and salivary gland ducts express several members of the family, including the ubiquitous SLC26A2 and SLC26A11 and Slc26a6 (215, 314), with salivary glands also expressing Slc26a4 (389). Notably, Slc26a3 functions as an electrogenic Cl−/HCO3− exchanger with a 2Cl−/1HCO3− stoichiometry (215, 388), while Slc26a6 functions as a 2HCO3−/1Cl− exchanger (209, 388). Slc26a3 and Slc26a6 can also mediate uncoupled anion currents, with the mode of transport determined by a glutamate residue that is conserved in all species and in all SLC26Ts (313).
Slc26a6 is the major Cl−/HCO3− exchanger in the salivary and pancreatic duct and is essential for fluid and HCO3− secretion by the ducts (389, 405, 433). SLC26A6 was originally identified in a search for novel SLC26Ts (247), and as the oxalate transporter in the renal proximal tubule (209). Oxalate handling by Slc26a6 mediates oxalate homeostasis by secreting oxalate into the intestinal lumen, and deletion of Slc26a6 results in urolithiasis due to increased renal oxalate load (189). Oxalate transport by Slc26a6 is not likely to be relevant for the pancreatic duct, but may have a role in sialolithiasis (142). An important feature of the SLC26Ts and CFTR is their mutual regulation. Thus, the STAS domain at the C terminus of the SLC26Ts interacts with the R domain of CFTR and the interaction is required for activation of both the SLC26Ts and of CFTR (215). This mode of regulation is discussed further below.
Na+/H+ exchangers (NHEs)
The NHEs are electroneutral 1Na+/1H+ exchangers. Phylogenetic analysis indicates that the mammalian alkali cation/proton gene family (the solute carrier SLC9A or NHE family) is comprised of three general gene clusters: (a) five plasma membrane-type Na+-selective NHEs (NHE1-NHE5); (b) four organellar cation non-selective NHEs (NHE6-NHE9); and (c) two distantly related NHE-like genes, termed Na+/H+ antiporter 1 (NHA1) and NHA2, that have closer homology to the fungal/plant NHA1 and bacterial NhaA (36, 318). The ubiquitous housekeeping NHE1 is essential for pHi homeostasis (28), and is localized at the basolateral membrane in duct cells (151, 234, 250, 326, 370). In addition, NHE1 can provide a portion of HCO3− influx across the basolateral membrane during HCO3− secretion. However, NHE1 contribution to HCO3− influx must be minor, since inhibition of NHE1 by amiloride has minimal effect on secretin-induced fluid and HCO3− secretion by the pancreatic duct in most species (422, 445).
Interestingly, NHEs are also expressed in the luminal membrane of salivary and pancreatic ducts (250, 326), which may salvage HCO3− when HCO3− secretion is not required. Pancreatic juice at resting or low flow rates is acidic and contains high level of CO2, indicating that an active H+ secreting mechanism is functional at this state (118, 263). NHE2 and NHE3 are localized at the luminal membrane of the large-sized mouse pancreatic and salivary glands ducts (33, 234, 250, 264, 326). The role of NHE2 is not clear since deletion of NHE2 in mice had no obvious disease phenotype or altered pH homeostasis and HCO3− metabolism in several organs expressing NHE2 (384), including the pancreatic (234) and salivary glands ducts (98, 250). On the other hand, deletion in mice showed that NHE3 is functional in the luminal membrane of the duct (3). Notably, the luminal NHE3 is associated with CFTR with the aid of PDZ-containing scaffolding proteins and its activity is regulated by CFTR in the HCO3− transporting complex (3). In addition, the activity of NHE3 is regulated by IRBIT (149, 150) (see below).
V-type H+ pump and H+/K+ ATPase pumps
Early studies in pigs suggested that a vesicular V-type H+-ATPase pump can function as a HCO3− loading mechanism at the basolateral membrane of the duct (428). These studies relied mainly on pharmacological tools that may have not reflected the function of the V-type H+ pump. In this respect, no effect of H+ pump inhibition was found on fluid and HCO3− secretion in the guinea pig duct (183). The salivary glands duct was reported to express V-type H+-ATPase pump that shifts from intracellular organelles to the luminal membrane upon chronic acidosis (374). However, no functional evidence is available to show participation of the pump in acid (rather than HCO3−) secretion in salivary ducts.
The topic of ductal H+ pumps was revisited very recently in the rat pancreatic duct to conclude the function of two H+/K+ ATPase pumps in the duct, the gastric and the non-gastric type pumps (309). Immunolocalization suggested that both H+/K+ ATPase pumps are expressed in the lateral and luminal membranes. Based on inhibitor studies, the H+/K+ ATPase pumps were shown to mediate modest H+ clearance from the cytosol of acidified duct cells (309). However, inhibition of the H+/K+ ATPase pumps with omeprazole and SCH-28080 strongly inhibited secretin-stimulated ductal fluid secretion (309). Inhibition of fluid secretion by inhibition of the H+/K+ ATPase pumps appears to be the strongest evidence for their expression, localization and function in the duct. Functioning of H+/K+ ATPase pumps in the basolateral membrane of the duct can be of particular significance since pumps can generate very large gradient using the energy in ATP. The gastric H+/K+ ATPase pump generates the steepest ion gradient in mammalian cells of six orders of magnitude (393). Such a pump in the basolateral membrane can function with NBCe1-B to markedly enhance HCO3− loading of the duct during stimulation of fluid and HCO3− secretion. However, this topic needs significantly additional extensive studies in vivo and in vitro using the available mice with targeted disruption of the H+/K+ ATPase pumps to establish and better understand the function of these pumps in pancreatic and salivary glands ductal function.
Aquaporins
Since the paracellular pathway is permeable to water, early models of epithelial fluid secretion assumed that water follows down its osmotic gradient from the basolateral to the luminal side via the paracellular pathway. In fact, significant portion of the water secreted by salivary glands acini does flow through the paracellular pathway (144, 285), including in salivary glands of AQP5-/- mice (198). However, water flow through the paracellular pathway cannot account for most of the salivary glands water flow. It is now clear that significant portion of water transport is mediated by the water channels aquaporins (AQP) and is a regulated process. There are at least 13 AQP genes (AQP0-AQP12) in mammalian cells (426). Although several members of the AQP family have been reported to be expressed in the exocrine pancreas (170), AQP1 and AQP5 seem to be the major AQPs in the pancreatic duct. Immunolocalization indicates expression of AQP1 at both the basolateral and luminal membranes, and AQP5 at the luminal membrane of pancreatic duct cells (42, 211, 212). Most notably, the salivary glands duct does not express AQP1, but when transduced with AQP1 in the rat (24, 79) and mini-pig (112) it secretes large volume of fluid, highlighting the function and importance of AQP1 in the luminal membrane for fluid secretion by the duct.
The Epithelial Na+ channel ENaC
Like many other epithelia, the salivary duct expresses the epithelial Na+ channel ENaC in the luminal membrane (49, 65). ENaC appears to be the major Na+ absorbing pathway in the salivary glands duct. Deletion of both NHE2 and NHE3 had no effect on ductal Na+ absorption, but inhibition of ENaC by amiloride inhibited both Na+ and Cl− absorption by the duct in stimulated glands (49). However, these findings may indicate that the NHEs’ major role is in HCO3− salvage in the resting state, since they are inhibited in the stimulated state when CFTR is activated (3, 234). ENaC is extensively regulated by multiple physiological pathways, as expected from its central function in salt-sensitive epithelia (228). ENaC activity can be regulated acutely during cell stimulation. In the salivary glands duct, stimulation of luminal and basolateral P2Y2 receptors inhibits ENaC activity by a pertussis toxin-sensitive and pertussis toxin-independent pathways (311). The precise mechanism by which P2Y2 receptors regulate ENaC is yet unknown, although it may involve in part depletion of the lipid PIP2, which activates ENaC (223, 464).
Of particular physiological significance is the regulation of ENaC by receptors that stimulate the glucocorticoid-induced kinase 1 (Sgk1), including mineralocorticoids, such as aldosterone (228). Regulation of ENaC by Sgk1 is biphasic. In the first phase that is completed in about 30 min, activated Sgk1 increases surface expression of the Na+/K+ ATPase pump in the basolateral membrane and of ENaC in the luminal membrane without affecting their transcription and translation. In the second phase that requires 6–8 hrs, Sgk1 increases transcription and translation of ENaC (228). ENaC surface expression is regulated by a well defined endocytotic mechanism that involves the ubiquitin ligase Nedd4-2 (367). Nedd4-2 regulates ENaC by interaction of Nedd4-2 WW domains with the proline-rich (PY) motifs (PPPxYxxL) located in the C-termini of the α, β, and γ subunits of ENaC (141). Nedd4-2 then polyubiquitinates the ENaC subunits to cause their internalization by a clathrin-coated vesicle endocytosis (430). Nedd4-2 appears to interact mostly with mature ENaC and the interaction requires their localization in caveolae and interaction of Nedd4-2 and ENaC with caveolin-1 (229). The key regulation of ENaC expression and activity by Nedd4-2 was recently demonstrated in vivo by showing that deletion of Nedd4-2 in the lung markedly increased ENaC activity and airway Na+ absorption to reproduce the lung phenotype observed in cystic fibrosis (206). However, whether this occurs in cystic fibrosis remains controversial since the lung of the porcine cystic fibrosis model does not show altered ENaC activity and Na+ absorption (53).
Regulation of ENaC by Sgk1 involves the phosphorylation of Nedd4-2 by Sgk1 on specific residues. The phosphorylated Nedd4-2 is then sequestered by the scaffolds 14-3-3 to prevent its interaction with and consequently inhibition of ENaC (228). Phosphorylation of Nedd4-2 by Sgk1 is regulated by the WNK kinases, where the WNKs function as scaffolds to recruit both Nedd4-2 by Sgk1 to allow Nedd4-2 phosphorylation and ENaC surface insertion (155). This system is functional in the salivary glands duct, since modulation of Nedd4-2 and Sgk1 activities have the expected effect on ENaC function (85, 351). Furthermore, treatment of rats with dexamethasone and low Na+ diet resulted in marked upregulation of β and γ ENaC expression (MGL and SM, unpublished observations). In addition, ENaC and CFTR appear to affect the activity of each other, but in a mechanism that appears to be different from that in the lung (29, 49). In the lung, CFTR is proposed to inhibit ENaC channel function and deletion of CFTR may increase ENaC activity, which can be clearly demonstrated in an in vitro models (29). However, in salivary glands deletion of CFTR inhibited Na+ absorption by ENaC and inhibition of ENaC function markedly inhibited CFTR-dependent Cl− absorption (49). Deletion of CFTR resulted in almost elimination of α ENaC expression (49). This likely explains the marked reduction in ENaC activity in CF patients sweat duct (352). It will be of particular interest to determine expression of CFTR and ENaC in different tissues in the various CF models.
C. Diversity of Other Channels and Transporters
Several transporters show organ and species specific expression and in these cases limit the conclusions to the specific organ and species. The pancreatic duct is unique among absorbing and secretory epithelia, including the salivary glands duct, in that it does not express ENaC, and thus it does not absorb Na+. In fact, the pancreatic duct secretes Na+, which passes paracellularly (14, 404). Paracellular Na+ secretion is essential for fluid secretion by the duct not only to maintain electroneutrality, but also as an osmolyte.
NKCC1 (SLC12A2) shows highly species specific expression. It is expressed at the basolateral membrane in a variety of Cl− secreting epithelia and it is expressed in the rat and mouse pancreatic duct (103), but not in the pig and guinea pig pancreatic duct (103, 133) or the salivary glands duct (99, 465). NKCC1 maintains [Cl−]i higher than its equilibrium concentration, and hence facilitates Cl− secretion when apical Cl− channels are opened. This may account for the relatively high Cl− concentration of the secretin-stimulated rat and mouse pancreatic juice and perhaps the lack of pancreatic phenotype in the cystic fibrosis mice models CFTR-/- and ΔF/ΔF. The lack of NKCC activity in pig and guinea pig pancreas (103, 133) (and likely human pancreas), may explain the severe pancreatic phenotype in human with CF (441) and pigs with deleted CFTR (53). As discussed below, a low ductal [Cl−]i is critical for producing pancreatic juice with the final high concentration of HCO3−. The lack of NKCC1 in the pig and human pancreas may serve this purpose to allow the high HCO3− concentration in their pancreatic juice.
VI. REGULATORY PROTEINS OF EPITHELIAL HCO3− SECRETION
A. PDZ-Based Adaptors
HCO3− transport by the duct is mediated by multiple transporters that are assembled into complexes by adaptor proteins containing PDZ domains. PDZ domains were identified as a conserved domain in three proteins, Post-Synaptic-Density-95, Disc-large, and Zonula Occludens-1. The PDZ domain is a protein-protein interaction module consisting of 80–90 amino acids which typically binds to target proteins harboring specific C-terminal sequences called PDZ-binding motifs or ligands (201). PDZ domains have two major functions. 1) They anchor protein, including integral membrane proteins such as receptors, transporters, channels and adhesion proteins, through their PDZ-binding motifs and 2) they bind to the PDZ domains of other PDZ proteins, thus forming scaffolding networks mediated by homo- and heteromultimers (117). Initially, PDZ-based adaptors were known to play an important role in the efficiency and fidelity of synaptic transmission in neurons (201). Subsequent studies revealed that epithelial cells express a battery of adaptors with PDZ domains and these proteins perform critical roles in the transepithelial fluid and electrolyte transport. The role of PDZ-based adaptors in ductal secretion is illustrated in Fig. 4.
Fig. 4. Ductal proteins with PDZ ligands that participate in fluid and HCO3− secretion.
All receptors that stimulate ductal secretion and key transporters that mediate ductal fluid and HCO3− secretion have PDZ ligands, highlighting the key role of PDZ scaffolds in ductal function. See text for details.
The first family of PDZ proteins shown to be involved in epithelial transport is the Na+/H+ exchanger regulatory factor (NHERF) family. NHERF1 (EBP50), NHERF2 (E3KARP), and NHERF3 (CAP70, PDZK1) facilitate the PKA-dependent phosphorylation and membrane trafficking of CFTR and NHE3 in epithelial cells, including duct cells (89, 435). By virtue of expressing multiple PDZ domains, these adaptors assemble a large protein complex in the apical membrane in which CFTR functions as a central regulator of secretory epithelia. The requirement of CFTR and the SLC26 transporters for fluid and HCO3− secretion by duct cells raised the question of how CFTR and the SLC26 transporters communicate to regulate HCO3− secretion. The answer was provided by the discovery of the interaction and reciprocal regulation of CFTR with the ductal HCO3− secretory machinery. Particularly, the interaction and mutual regulation of CFTR with the HCO3−-secreting SLC26 transporters (SLC26Ts) is mediated by the phosphorylated CFTR R domain and the SLC26Ts STAS domain and is enhanced by the interaction of CFTR and the SLC26Ts with PDZ scaffolding proteins (213). In addition, CFTR is involved in the regulation of the luminal HCO3− absorbing transporters NHE3 and NBCn1-A by forming a protein complex via adaptors with multiple PDZ domains such as NHERF1 and NHERF2 (3, 250, 327). As a result, the cAMP/PKA pathway activates CFTR and apical Cl−/HCO3− exchange and inhibits NHE3 and NBCn1-A (89, 435). Assembling a complex with a large number of transporters with the aid of PDZ adaptors greatly enhances signaling efficiency and fidelity of the cAMP/PKA pathway in confined regions of the luminal membrane. Upon stimulation with cAMP, CFTR activates the HCO3−-secreting and at the same time inhibits the HCO3−-absorbing transporters to optimize fluid and HCO3− secretion.
In addition to the NHERFs, duct cells express several other scaffolds with PDZ domains, such as Shank2, S-SCAM, SAP97, and PSD-95 (116, 202). The Shank family of proteins was discovered as molecular scaffolds in neuronal cells, where they serve as coordinators of membrane and cytoplasmic protein complexes in the postsynaptic density (PSD) (244, 391). The Shanks contain multiple protein-protein interacting domains, including ankyrin repeats, an SH3, a PDZ, a long proline-rich region and a sterile α motif (SAM) (391). Currently, there are three known members of the Shank family, Shank1-3. Among them, Shank2 localizes at the apical pole of pancreatic duct cells and modulates the activity of CFTR and NHE3 (140, 202). Expression of Shank2 upregulates the membrane expression of CFTR and NHE3, and this appears to be related to activation of Rho small GTPases by recruiting βPix (PAK-interacting exchange factor), a guanine nucleotide exchange factors (GEF) for the Rho small GTPases (232). By contrast with NHERF proteins that deliver cAMP/PKA signals to CFTR and NHE3, Shank2 mediates an inhibitory effect on the cAMP/PKA pathway. A detailed examination of binding partners of Shank2 revealed that Shank2 associates with phosphodiesterase 4D (PDE4D) that hydrolyzes cAMP, hence lowering local cAMP concentration in the apical microdomains (231). These findings revealed a unique form of regulation, whereby opposite signals can be delivered to the same PDZ-binding motif of a given protein by different adaptors, serving as a homeostatic system to regulate intensity of the stimulated state (231).
Notably, most ductal GPCRs have PDZ ligands at their C-termini (see fig. 4). This recruits the GPCRs to the transporting complex resulting in polarized expression of GPCRs (239, 240, 357, 392) and delivery of the second messengers to the ion transporting microdomain. This should allow confined and better controlled delivery of the stimulus. A good example for such an arrangement is of the VIP receptor VPAC1, which binds through its PDZ ligand to the synaptic scaffolding molecule (S-SCAM), also known as membrane-associated guanylate kinase inverted-2 (MAGI-2) (116). S-SCAM/MAGI-2 associates with E-cadherin, a key protein at the adherens junction, in a β-catenin dependent manner (408) and recruits VPAC1 to the junctional area at the apical pole of epithelial cells. Thus, S-SCAM/MAGI-2 confines VPAC1 to the junctional domain to restrict VIP-induced cAMP signaling to activate CFTR at a microdomain in the vicinity of the apical pole. This, in turn, enables efficient electrolyte and fluid secretion in response to VIP with minimal effects on the cell interior (116). Fig. 4 lists the many ductal HCO3− transporters and GPCRs that have a typical PDZ ligand at their C-termini.
B. With-no-lysine (WNK) and Sterile 20 (STE20)-like Kinases
Recently, two families of protein kinases, the WNK kinases and the sterile 20 (STE20)-like kinases, have emerged as osmotic sensors that modulate the activity of diverse ion transporters (13, 194, 355). The family of the WNKs was discovered by search for a MAPK homologues (450) and were found to lack the conserved lysine in the kinase catalytic site. Crystal structure of the kinase domain showed the lysine is contributed by the β2, rather than the β3 strands (277). The family consists of four large members with only the kinase domain highly conserved (164). The WNKs gained prominence with the seminal discovery that mutations in WNK1 and WNK4 lead to hypertension (442). Subsequent extensive studies of their function showed that WNK1, WNK3 and WNK4 function mainly to reduce the surface expression of various Na+, K+ and Cl− transporters, including NKCC1, NKCC2, NCCT, KCCT, ENaC, ROMK and Cl− channels (164, 165, 194, 355), including CFTR (455, 456) and Slc26a6 (193). The WNKs reduce transporters surface expression by promoting their endocytosis (148, 165). In most cases examined, the WNKs do not act directly on the ion transporters, but act on the downstream kinases, the STE20-like kinase SPAK and the oxidative stress-responsive kinase 1 kinase OSR1. The WNKs can phosphorylate the SPAK/OSR1 on T233, which activates SPAK/OSR1 and the activated SPAK/OSR1 phosphorylates the ion transporters to reduce their surface expression (355). In several cases, the kinase function of the WNKs was not required for regulation of the ion transporters. In these cases the WNKs act as scaffolding proteins that bind SPAK/OSR1 to recruit it to the transporters (13, 148, 155, 164, 456). The scaffolding function of the WNKs resides in their first 119 residues (148, 155, 456). The human mutations in WNK1 and WNK4 result in inhibition of NKCC2, NCCT and ENaC endocytosis to enhance their surface expression in the kidney, leading to excessive Na+ absorption and hypertension (165, 194, 355).
WNK1 (325, 442, 456), WNK3 (456), WNK4 (442, 456), SPAK and OSR1 (325, 456) are abundantly expressed in ductal systems, including the pancreatic (325, 456) and salivary glands ducts (456). Recent studies from our laboratories revealed that the WNK/SPAK pathway appears to have dual roles in regulating ductal function at the resting and stimulated ducts. The WNK/SPAK pathway stabilizes the ductal resting state by prominently reducing the surface expression of all ductal transporters involved in fluid and HCO3− secretion (456). Thus, the WNKs act as scaffolds in which only a short segment of the WNKs N terminal domain that lacks kinase function (155, 456) binds and recruit SPAK to the Na+-HCO3− co-transporter NBCe1-B (pNBC1) and CFTR to reduce their surface expression and dramatically reduce their activity at the plasma membrane (456). The WNK/SPAK pathway also reduces the surface expression (325) and activity (193, 325) of the ductal SLC26 transporters. Moreover, knockdown of the WNKs increases the activity of ductal NBCe1-B, CFTR and stimulated fluid secretion (456). The prominent inhibition of ductal fluid and electrolyte secretion by the WNK/SPAK pathway suggests that the WNK/SPAK pathway stabilize the resting state of the duct.
Regulation of the WNK/SPAK/OSR1 kinase activity is poorly understood and for the most part it is not known how these kinases are activated by stimulation of cell surface receptors or during changes of cellular activity. One well established exception is regulation of the WNK/SPAK/OSR1 kinases by osmotic stress. The WNK kinases, including WNK1, are activated by osmotic stress such as that caused by a decrease in [Cl−]i. The activated WNK1 then phosphorylates and activates the SPAK and OSR1 kinases (355). Indeed, when the human pancreatic duct cell line PANC1 and guinea pig ducts are exposed to low external and intracellular Cl−, the WNK1-SPAK/OSR1 pathway affects the function of two apical HCO3− transporters (325). First, the WNK1-SPAK/OSR1 increases CFTR Cl−/HCO3− selectivity to increase the CFTR HCO3− permeability. Second, the WNK1-SPAK/OSR1 inhibits the apical Cl−/HCO3− exchange by the SLC26 transporters. When occurring in the terminal portion of the stimulated pancreatic duct, the two functions will result in pancreatic juice containing HCO3− at a concentration greater than 140 mM.
C. IRBIT (inositol-1,4,5-triphosphate (IP3) receptors binding protein released with IP3)
IRBIT was discovered several times in different contexts (83, 457), most recently in a search for proteins that interact with the IP3-binding domain of the IP3 receptors (IP3Rs) (11). IRBIT competes with IP3 for interaction with N terminal domain of IP3Rs to play important role in the function of the IP3Rs and Ca2+ signaling (10, 82). Subsequent search for proteins that interact with IRBIT discovered that IRBIT interacts with and regulates the activity of the Na+-HCO3− co-transporter NBCe1-B (394). There are two highly homologous IRBIT isoforms, a short and a long IRBIT, with the long IRBIT having an N terminal extension (12). The domains common to short and long IRBITs are an N terminus PP1 anchoring site that is followed by a PEST domain (81, 83), a coiled-coil domain and a PDZ ligand at the end of the C terminus (83). The PEST domain has multiple phosphorylation sites (10), with phosphorylation of key residues, including S68, S72 and S74, that are required for all known IRBIT functions, including regulation of IP3Rs function (10, 82) and activation of ion transporters (394, 458). Long IRBIT has an N terminal extension rich in prolines and alanines, which differentiates the roles of short and long IRBIT functions (12).
In the duct IRBIT accumulates at the apical pole (458), a site of expression of high level of IP3Rs, but IP3Rs and thus IRBIT are also present in the basal pole (240). At the apical pole IRBIT regulates CFTR (456, 458), and possibly NHE3 (150) and NBCn1-A (35), while at the basal pole IRBIT regulates NBCe1-B (394, 456, 458). The NBCs are members of the superfamily of Na+-coupled HCO3− transporters (NCBT), which includes the electrogenic NBCe1-A~C and the electroneutral NBCn1-A~H (35). The domain structure and function of the NCBTs are discussed in several recent reviews (35, 344). Notably, the first 45–62 residues of N terminus of several NBC, including NBCe1-B and NBCn1-A, forms an inhibitory domain and its deletion results in marked activation of the relevant NCBTs (268). Importantly, IRBIT interacts with the N terminal domain of NBCe1-B to markedly activate NBCe1-B (394). Activation of NBCe1-B by IRBIT is mediated by the PEST domain (394, 458) and required the PDZ ligands to assemble the IRBIT-NBCe1-B complex (458). These findings suggest that IRBIT induce a conformational change in NBCe1-B to dissociate its N terminal inhibitory domain from the transmembrane domain mediating the ion transport. Most notably, IRBIT activates the native NBCe1-B in pancreatic (456, 458) and salivary gland ducts (456).
Activation of NBCe1-B by IRBIT indicates that IRBIT should have a prominent role in the regulation of epithelial HCO3− secretion. Indeed, knockdown of IRBIT markedly inhibited pancreatic duct fluid and HCO3− secretion (458). Another important function of IRBIT is coordination of the ductal secretory process by regulating the activity of CFTR at the luminal membrane of the duct. Hence, IRBIT activates the native ductal CFTR and CFTR expressed in model system by direct interaction with CFTR to increase CFTR open probability (458). As was found with NBCe1-B, activation of CFTR by IRBIT is mediated by the PEST domain and is aided by assembly of IRBIT-CFTR complexes through their PDZ ligands (458). The findings in the native duct and with expressed NBCe1-B and CFTR are summarized in the model in Fig. 5, illustrating how IRBIT coordinates epithelial fluid and HCO3− secretion.
Fig. 5. The IRBIT/PP1 and WNK/SPAK pathways in ductal function.
IRBIT is a key regulator of ductal fluid and HCO3− secretion that regulates both the resting and stimulated states of ductal secretion. PZD scaffolds assemble a basolateral membrane complex composed of NBCe1-B, the WNK/SPAK kinases and IRBIT (which can recruit the phosphatase PP1 to the complex). Similar complex exists in the luminal membrane with CFTR and perhaps Slc26a6 as the major transporters. In the resting state the WNK/SPAK kinases phosphorylate all transporters to reduce their surface expression and thus activity. Part of IRBIT is sequestered by IP3Rs and part is bound to NHE3 and NBCn1-A to activate them and thus affect HCO3− salvage. Upon cell stimulation IRBIT recruits PP1 to the complexes, which overrides the function of the WNK/SPAK pathway and dephosphorylates the HCO3− secreting transporters to increase their surface expression. IRBIT then binds and neutralizes the effect of the HCO3− secreting transporters inhibitory domains. The combined effects stabilize the secretory state of the duct. The mutual stimulation of CFTR and Slc26a6 in the complex further augments ductal secretion.
Activation of NBCe1-B and CFTR by IRBIT sets the ductal stimulated state of fluid and HCO3− secretion. This raised the question of how the duct resting state is set and whether IRBIT communicates with the regulators of the resting state. Inhibition of CFTR (455, 456) and Slc26a6 (193, 325) by the WNK kinases prompted examining the role of the WNK/SPAK kinases in the duct and their communication with IRBIT (456). As discussed above, the first 120 N terminal residues of the WNKs upstream of the kinase domain function as scaffolds (148, 155, 456) that recruit SPAK to NBCe1-B and CFTR. In turn, SPAK phosphorylates NBCe1-B and CFTR to cause their endocytosis and inhibit ductal fluid and HCO3− secretion (456). Significantly, IRBIT overrides the function of the WNK/SPAK pathway by recruiting the phosphatase PP1 to dephosphorylate NBCe1-B and CFTR, insert them into the basolateral and luminal membranes, respectively, and stimulate ductal fluid and HCO3− secretion (456) (Figure 5).
As indicated above, another component of the duct resting state is HCO3− salvage by the luminal NHE3 and NBCn1-A (233, 250). It is of note that other members of the Na+-driven HCO3− transporters have an N terminal domain similar to that of NBCe1-B (35), including the electroneutral NBCn1 and the Na+-dependent Cl−/HCO3− exchangers NDCBE. It is thus possible that NBCn1, including the ductal NBCn1-A, are regulated by IRBIT, as was suggested (35). However, it is not clear which NBCn1 and NDCBE isoforms are activated by IRBIT and whether IRBIT activate them by the same mechanism as NBCe1-B since the N terminal domain of NBCe1-A~C, NBCn1-A~H and NDCBE-A~D differ substantially. NHE3 was also reported to be modestly activated by IRBIT (149), which may be dependent on Ca2+ (150). Interestingly, NHE3 (3) and NBCn1 (327) are inhibited by CFTR at the luminal membrane. Hence, IRBIT appears to participate in the resting and stimulated state. It is possible that in the resting state part of IRBIT is sequestered by IP3Rs and part of IRBIT is associated with NHE3 and NBCn1-A to promote HCO3− salvage. Once the cells are stimulated, IRBIT may dissociate from the HCO3− salvage, interact with the HCO3− secretory transporters and stimulate ductal fluid and HCO3− secretion. The model in Figure 5 illustrates the multiple regulatory roles of IRBIT.
D. Carbonic Anydrases
A class of proteins essential for all HCO3− related functions is the carbonic anhydrases (CAs). The global CA inhibitor acetazolamide significantly inhibits pancreatic (95, 321) and minimally inhibit salivary (461) fluid and HCO3− secretion. Further studies revealed that CAs are present at the HCO3− transporting complex, and supply HCO3− to several transporters. The CAs physically interact with and regulate the activity of several HCO3− transporters, including Na+-HCO3− co-transporters and the AE and SLC26A6 Cl−/HCO3− exchangers (269). Immunolocalization suggested localization of CAII, CAIV, CAIX, and CAXII in pancreatic (301) and multiple isoforms in salivary gland ducts (18, 172). Several of the ductal Ca2+ signal are cytoplasmic and some are integral membrane proteins with the catalytic domain in the cell surface. It is not clear at present which of these CAs is directly coupled to the basolateral NBCe1-B and AE2 in the ducts. Interestingly, the trafficking of CAIV to the luminal membrane is dependent on CFTR, and acetazolamide inhibits HCO3− transport by SLC26A3 (102) and SLC26A6 (269). These findings imply the involvement of the ductal CAs in HCO3− transport by the CFTR-SLC26Ts complex at the luminal membrane.
VII. DISEASES OF DUCTAL HCO3− SECRETION
Altered fluid and HCO3− secretion is associated with several diseases that affect various organs, although the pancreas is the most vulnerable to aberrant fluid and HCO3− secretion. The best known of these diseases is CF (94). It is obvious why a lack of CFTR function virtually eliminates ductal function. However, CF has a spectrum of phenotypes from mild to very severe depending on the mutation and sometime even within the same mutation (475), in particular in lung function (275). Interestingly, the CF genotype-phenotype correlates best with the state of pancreatic function (375, 378). Examining several mutations suggested that this relates to the ability of CFTR to support HCO3−, rather than Cl−, transport (61), probably by stimulating the SLC26 transporters.
Another disease associated with aberrant HCO3− secretion is pancreatitis (70, 211, 409). Pancreatitis is an inflammatory disease that results in destruction of the pancreas by toxins that affect the acinar and duct cells (236). The most common form of pancreatitis is alcoholic pancreatitis, although many other causes of pancreatitis are known (138, 322), including autoimmune pancreatitis (211, 339). Most forms of pancreatitis are considered idiopathic. The central role of CFTR in ductal function led to a search of CFTR mutations associated with pancreatitis (63). The search identified several CFTR mutations that do not result in any of the CF symptoms, but are associated with chronic pancreatitis (62, 63, 230). Interestingly, recent analysis of two such mutations showed that they had no effect on Cl− function of CFTR, but specifically reduced CFTR-mediated HCO3− transport (381, 436), further emphasizing the importance of HCO3− secretion in ductal function.
Of particular interest is a recent study that asked whether aberrant CFTR function is associated with idiopathic forms of chronic pancreatitis (211). Patients with autoimmune pancreatitis have reduced fluid and HCO3− secretion. Examination of CFTR localization in pancreatic biopsies showed mislocalization of CFTR and its accumulation in intracellular compartments (211). Most notably, treating the patients with corticosteroids corrected the mislocalization of CFTR to restored pancreatic HCO3− secretion and ameliorates disease severity (211). Moreover, preliminary results suggest that similar CFTR mislocalization occurs in alcoholic pancreatitis (211). It will be of particular interest to further analyze CFTR expression in all forms of pancreatitis, and if aberrant, whether correction of CFTR localization reduce disease symptoms. In addition, it will be informative to determine the fate of the SLC26 transporters in the disease, as they are tightly associated with CFTR physically and functionally (214, 215).
Another autoimmune inflammatory disease in which HCO3− secretion by salivary glands is aberrant is the dry-mouth, dry-eye disease Sjögren’s syndrome (7). The cause of the aberrant HCO3− secretion is not known. However, one study reported that treating Sjögren’s syndrome patients with the steroid prednisolone reduced damage and increased secretion by salivary glands (279). The results of treating autoimmune pancreatitis with corticosteroids (211) suggest that CFTR and perhaps the SLC26 transporters localization and/or function are altered in Sjögren’s syndrome to reduce HCO3− secretion. Moreover, the reduced ductal fluid and HCO3− secretion in two autoimmune inflammatory diseases raise the possibility that many inflammatory diseases of secretory glands likely lead to some extent of reduced ducal HCO3− secretion.
HCO3− secretion is also reduced in patients undergoing radiation treatment for head and neck malignancies (7, 92). In this case it is likely that radiation resulted in ductal cell damage. Radiation damage is most commonly associated with severe oxidative damage (2, 473). Hence, it is possible that other forms of oxidative damage cause reduced ductal HCO3− secretion, in particular considering that CFTR activity is susceptible to oxidative damage (385). Indeed, in rat and mouse models of bile acid-induced pancreatitis oxidative stress causes marked reduction in ductal function that can be prevented by in vivo stimulation of signaling pathways that reduced oxidative stress (290, 292). Analysis of ductal transporters in these diseases should reveal the cause of the disease, suggest treatment modalities, and further clarify the mechanism and regulation of ductal fluid and HCO3− secretion.
VIII. MECHANISM AND A MODEL FOR DUCTAL HCO3− SECRETION
A. Basic Concepts
A unique feature of the duct in most species, including humans, is the secretion of copious amount of HCO3− with the pancreatic duct secreting fluid containing as much as 140 mM HCO3− (237). Accordingly, the mechanism and regulation of epithelial HCO3− secretion is understood best in the pancreatic duct and thus the model we discuss reflects best the mechanism of fluid and HCO3− secretion by the pancreatic duct. However, although not understood at the same detail, the available data indicates that the mechanism discussed below for the pancreatic duct is applicable to other exocrine gland ducts with variations that meet specific glands specialization, but do not deviate from the basic principles, as we tried to emphasis with the salivary gland ducts example.
The duct secretes most of the fluid in the pancreatic juice and while doing so it absorbs the Cl− and secretes HCO3− to generate pancreatic juice containing about 20 mM Cl− and 140 mM HCO3−. This task imposes several criteria for the mechanism of pancreatic duct fluid and HCO3− secretion that must be met. First, the basolateral HCO3− influx mechanism must be able to concentrate HCO3− in the duct cytosol. Second, HCO3− efflux mechanism at the luminal membrane must be able to concentrate HCO3− at the luminal space and the luminal membrane must have low HCO3− leak and maintain a membrane potential with negative transcellular potential. While absorbing Cl− and secreting HCO3−, the pancreatic duct must mediate large net transcellular salt transport necessary for copious fluid secretion by the duct. Since the only osmolyte actively secreted by the pancreatic duct is HCO3−, the bulk of HCO3− secretion cannot be by osmotically silent HCO3− transport mechanism, such as coupled electroneutral 1Cl−/1HCO3− exchange or uncoupled 1Cl−/1HCO3− exchange by a Cl− and HCO3− permeable channels, such as CFTR, or Cl− and K+ cotransport by luminal Cl− and K+ channels. These set specific constrains on the basolateral HCO3− influx mechanism and the luminal Cl− absorbing and HCO3− secreting mechanism. The capacity of the basolateral HCO3− absorbing mechanism must exceed the capacity of basolateral HCO3− efflux mechanisms, such as AE2, and the luminal HCO3− exit mechanism must function with a stoichiometry of HCO3−/Cl− >1.
The first attempt to explain pancreatic duct fluid and HCO3− secretion was made in the late 1980’s by Argent and Case (14). The basic tenants of their model are the basolateral Na+/K+ pump and K+ channels, which generate the Na+ and K+ gradients and set the membrane potential that are needed to fuel the secretion. A HCO3− entry equivalent is provided by NHE. HCO3− then exits the luminal membrane by way of a Cl−/HCO3− exchange that absorbs the Cl−, with the Cl− re-circulates by exiting through CFTR (14, 404). Although the model does not meet the constraints listed above and thus cannot explain ductal fluid and HCO3− secretion, it was an important model in drawing attention to the problem and stimulating the study of ductal secretion.
Several key discoveries led to significant revision of this model. The major basolateral HCO3− influx mechanism was identified as NBCe1-B, which functions as a 1Na+-2HCO3− cotransporter (1, 180, 471). The main luminal Cl−/HCO3− exchanger in the pancreatic and salivary ducts was identified as Slc26a6 (215, 247), which functions as electrogenic, coupled 1Cl−/2HCO3− exchanger (313, 388) that interacts with CFTR, with Slc26a6 and CFTR activating each other (215). Modeling and studies in guinea pig pancreatic ducts suggested a requirement for HCO3− channel activity to set the final pancreatic juice HCO3− at 140 mM (400, 401, 437). These findings led a two stage model in which initially HCO3− is mediated by electroneutral Cl−/HCO3− exchange. Higher HCO3− concentration in the pancreatic juice is achieved by CFTR acting as a HCO3− channel (for review see (237, 404)). A significant problem with the model is that it does not result in osmotic secretion of HCO3− and thus will not lead to fluid secretion. In addition, the model does not explain how CFTR can conduct HCO3− at the terminal portion of the duct.
B. A Model of Ductal Fluid and HCO3− Secretion
The following findings were used to develop the model in Fig. 6. Loss of pancreatic HCO3− secretion in patients with CF (191) indicates that CFTR plays a critical role in HCO3− secretion. The activity of the duct apical Cl−/HCO3− exchanger is inhibited by DIDS (235, 405) (which does not inhibit CFTR) and is dependent on the expression of CFTR (235, 238). CFTR mutations associated with pancreatic insufficiency exhibit a severe defect in CFTR-dependent Cl−/HCO3− exchange activity (61). Coupled or uncoupled, channel-mediated 1:1 Cl−/HCO3− exchange cannot account for either HCO3− or fluid secretion by the duct (154). HCO3− secretion must be an osmotically active process. Electrogenic HCO3− transporters can generate a fluid with high HCO3− concentrations using the driving force generated by the negative luminal membrane potential. Apical Cl−/HCO3− exchanger by the duct is electrogenic with distinct Cl−:HCO3− stoichiometry (215). The duct expresses Slc26a6 (214, 215) that functions as a 1Cl−/2HCO3− exchanger (313, 388). The WNK/SPAK pathway stabilizes the resting state in the proximal portion of the duct (456), while regulating CFTR HCO3− selectivity and permeability in the distal portion of the duct, switching CFTR into a HCO3−-selective channel (325). IRBIT governs ductal fluid and HCO3− secretion and overrides the inhibitory action of the WNK/SPAK pathway (456). The findings lead to a two stage model: In stage one the proximal duct secretes the bulk of fluid and HCO3− secretion that is mediated by Slc26a6 with CFTR recycling the Cl−, the WNK/SPAK pathway regulating the resting state and IRBIT set the stimulated state. In step two, the WNK/SPAK pathway switches CFTR to largely a HCO3− channel to secret HCO3− and set the final HCO3− secretion of the pancreatic juice (Fig. 6).
Fig. 6. A model of pancreatic duct fluid and HCO3− secretion.
Ductal fluid and HCO3− secretion is a two stage process. In the proximal duct IRBIT antagonizes the effect of the WNK/SPAK pathway to stimulate ductal secretion. HCO3− accumulates in the duct cytosol by NBCe1-B and exits into the lumen mostly by Slc26a6, which mediates 1Cl−/2HCO3− exchange, with CFTR recycling the Cl−. This result with osmotic secretion of HCO3− and together with transcellular Na+ fluxes through the paracellular pathway drives fluid secretion. The water is secreted by AQP1. The proximal duct thus absorbs part of the Cl− and secretes as much as 100 mM HCO3− to secret large fraction of the fluid in the pancreatic juice. As the fluid arrives the more distal portions of the duct, the reduced luminal Cl− and activated CFTR results in intracellular Cl− concentration ([Cl−]i) of less than 10 mM. The low [Cl−]i activates WNK1 that phosphorylates SPAK/OSP1, which, in turn, acts on CFTR to change its Cl−/HCO3− selectivity, converting it primarily a HCO3− channel. At the same time the WNK/SPAK pathway inhibits the function of Slc26a6 to prevent HCO3− re-absorption. HCO3− efflux by CFTR thus determines the final HCO3− concentration in the secreted fluid.
With the steep inward Na+ gradient of about 15 fold, 1Na+-2HCO3− co-transport by NBCe1-B leads to active accumulation of cytosolic HCO3−. Secretion of HCO3− by the 1Cl−/2HCO3− exchanger Slc26a6 leads to osmotic secretion of HCO3−. The obligatory movement of Na+ through the paracellular pathway results in net Na+-HCO3− secretion that drives water efflux by AQP1 and starts the process of accumulation of HCO3− in the duct lumen. In the resting state, the WNK/SPAK pathway keeps large fraction of NBCe1-B and of CFTR in intracellular compartments while IRBIT may be associated with and activate NHE3 and NBCn1-A (not included in the model) to salvage any Na+ and HCO3− leaking to the duct lumen. Initiation of ductal secretion requires antagonism of the WNK/SPAK pathway by IRBIT-mediated recruitment of PP1 to dephosphorylate the transporters NBCe1-B and CFTR and exocytose them to their respective plasma membrane. Once at the plasma membrane the transporters are phosphorylated by PKA and now IRBIT interacts directly with the inhibitory domains in NBCe1-B, CFTR and perhaps Slc26a6 to activate them and set the stimulated state. At a luminal membrane potential of −50 mV or more negative, and the ionic gradients of HCO3− and Cl− set by NBCe1-B and CFTR, 1Cl−: 2HCO3− exchange by Slc26a6 can achieve a luminal HCO3− concentration of about 136 mM (404).
To transport events in the proximal duct outlined above can account for the bulk of fluid and HCO3− secretion by the rodent duct. However, the guinea pig, pig and human pancreatic ducts can generate pancreatic juice containing more than 120–130 mM HCO3−. As luminal Cl− is absorbed, the reduced Cl− gradient across the luminal membrane limits HCO3− secretion by Slc26a6, and thus alternative pathway if HCO3− efflux is required to maintain HCO3− secretion. The decrease in luminal Cl− and the activated CFTR at a membrane potential of −50 mV or more negative reduces [Cl−]i to below 10 mM, which serves as an alternative signal to modulate the activity of WNK/SPAK pathway. Activation of the WNK/SPAK pathway by low [Cl−]i converts CFTR from a Cl− channel to preferably HCO3− channel, allowing further HCO3− efflux. The WNK/SPAK pathway inhibits any luminal Cl−/HCO3− exchange activity to avoid HCO3− absorption due to the steep HCO3− gradient set by CFTR (325). IRBIT activity in this portion of the duct needs to be reduced (not included in the model), to allow the WNK/SPAK pathway to act on CFTR and the SLC26 transporters.
The model in Fig. 6 can explains the available information on the mechanism of fluid and HCO3− secretion by secretory gland ducts, most importantly how the duct can secrete both large fluid volume and high amount of HCO3−. By no means, this intent to be the last word in the mechanism by which secretory gland ducts secrete fluid and electrolytes. As with other models, including our earlier models, it will continue to evolve as knowledge of existing pathway increases and new pathways are discovered. However, we hope that at this stage the model will stimulate further research into this so very important topic of fluid and HCO3− secretion that is aberrant in large number of epithelial diseases.
Acknowledgments
We thank Dong-Su Jang for preparing the illustrations. This work was supported by grants 2010-0001670 and 2010-0017752 from the National Research Foundation of Korea, the Ministry of Education, Science, and Technology, Korea to M.G.L. and by the intramural research funds of the NIH/NIDCR ZIA DE000735-01 to S.M.
References
- 1.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 2.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3- salvage mechanisms in model systems and the mouse pancreatic duct. J Biol Chem. 2001;276:17236–17243. doi: 10.1074/jbc.M011763200. [DOI] [PubMed] [Google Scholar]
- 4.al-Nakkash L, Cotton CU. Bovine pancreatic duct cells express cAMP- and Ca2+-activated apical membrane Cl− conductances. Am J Physiol. 1997;273:G204–216. doi: 10.1152/ajpgi.1997.273.1.G204. [DOI] [PubMed] [Google Scholar]
- 5.Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–167. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 6.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Almstahl A, Wikstrom M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol. 2003;48:337–344. doi: 10.1016/s0003-9969(02)00200-5. [DOI] [PubMed] [Google Scholar]
- 8.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez C, Regan JP, Merianos D, Bass BL. Protease-activated receptor-2 regulates bicarbonate secretion by pancreatic duct cells in vitro. Surgery. 2004;136:669–676. doi: 10.1016/j.surg.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 12.Ando H, Mizutani A, Mikoshiba K. An IRBIT homologue lacks binding activity to inositol 1,4,5-trisphosphate receptor due to the unique N-terminal appendage. J Neurochem. 2009;109:539–550. doi: 10.1111/j.1471-4159.2009.05979.x. [DOI] [PubMed] [Google Scholar]
- 13.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argent B, Case R. Pancreatic ducts. Cellular mechanism and control of bicarbonate secretion. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1994. pp. 1473–1497. [Google Scholar]
- 15.Argent BE, Gray MA. Regulation and Formation of Fluid and Electrolyte Secretions by Pancreatic Ductal Epithelium. GASTROENTEROLOGY AND HEPATOLOGY. 1997;3:349–378. [Google Scholar]
- 16.Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- 17.Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asari M, Kimura H, Ichihara N, Kasuya T, Nishita T. Immunohistochemistry of carbonic anhydrase isozymes (CA-I, II and III) in canine salivary glands: a distributional and comparative assessment. Anat Histol Embryol. 2000;29:9–12. doi: 10.1046/j.1439-0264.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 19.Ashton N, Argent BE, Green R. Characteristics of fluid secretion from isolated rat pancreatic ducts stimulated with secretin and bombesin. J Physiol. 1991;435:533–546. doi: 10.1113/jphysiol.1991.sp018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashton N, Argent BE, Green R. Effect of vasoactive intestinal peptide, bombesin and substance P on fluid secretion by isolated rat pancreatic ducts. J Physiol. 1990;427:471–482. doi: 10.1113/jphysiol.1990.sp018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashton N, Evans RL, Elliott AC, Green R, Argent BE. Regulation of fluid secretion and intracellular messengers in isolated rat pancreatic ducts by acetylcholine. J Physiol. 1993;471:549–562. doi: 10.1113/jphysiol.1993.sp019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker OJ, Camden JM, Ratchford AM, Seye CI, Erb L, Weisman GA. Differential coupling of the P2Y1 receptor to Galpha14 and Galphaq/11 proteins during the development of the rat salivary gland. Arch Oral Biol. 2006;51:359–370. doi: 10.1016/j.archoralbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Baum BJ. Neurotransmitter control of secretion. J Dent Res. 1987;66(Spec No):628–632. doi: 10.1177/00220345870660S104. [DOI] [PubMed] [Google Scholar]
- 24.Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, Chiorini JA, Voutetakis A, Leakan RA, Van Waes C, Mitchell JB, Delporte C, Wang S, Kaminsky SM, Illei GG. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758:1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becq F, Hollande E, Gola M. Phosphorylation-regulated low-conductance Cl− channels in a human pancreatic duct cell line. Pflugers Arch. 1993;425:1–8. doi: 10.1007/BF00374496. [DOI] [PubMed] [Google Scholar]
- 27.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 28.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 29.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst. 2009;5:123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkefeld H, Fakler B, Schulte U. Ca2+-activated k+ channels: from protein complexes to function. Physiol Rev. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 32.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 33.Bobulescu IA, Di Sole F, Moe OW. Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr Opin Nephrol Hypertens. 2005;14:485–494. doi: 10.1097/01.mnh.0000174146.52915.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bockman DE. Anatomy and fine structure. In: Beger H, Buchler M, Kozarek R, Lerch M, Neoptolemos J, Warshaw A, Whitcomb D, Shiratori K, editors. Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery. Oxford, U.K: Blackwell Publishing; 2008. pp. 50–57. [Google Scholar]
- 35.Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 37.Brooks AM, Grossman MI. Postprandial pH and neutralizing capacity of the proximal duodenum in dogs. Gastroenterology. 1970;59:85–89. [PubMed] [Google Scholar]
- 38.Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. doi: 10.1074/jbc.M106609200. [DOI] [PubMed] [Google Scholar]
- 39.Bruce JI, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 40.Buanes T, Grotmol T, Landsverk T, Raeder MG. Ultrastructure of pancreatic duct cells at secretory rest and during secretin-dependent NaHCO3 secretion. Acta Physiol Scand. 1987;131:55–62. doi: 10.1111/j.1748-1716.1987.tb08205.x. [DOI] [PubMed] [Google Scholar]
- 41.Bundgaard M, Moller M, Poulsen JH. Localization of sodium pump sites in cat pancreas. J Physiol. 1981;313:405–414. doi: 10.1113/jphysiol.1981.sp013673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52:1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 44.Case R, Argent B. Pancreatic duct cell secretion: control and mechanisms of transport. In: Go VLW, editor. The Pancreas : biology, pathobiology, and disease. New York: Raven Press; 1993. pp. 301–350. [Google Scholar]
- 45.Case RM, Conigrave AD, Novak I, Young JA. Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol. 1980;300:467–487. doi: 10.1113/jphysiol.1980.sp013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Case RM, Harper AA, Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969;201:335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Case RM, Hotz J, Hutson D, Scratcherd T, Wynne RD. Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol. 1979;286:563–576. doi: 10.1113/jphysiol.1979.sp012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castle D, Castle A. Intracellular transport and secretion of salivary proteins. Crit Rev Oral Biol Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- 49.Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catalan MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest. 2009;56 (Suppl):192–196. doi: 10.2152/jmi.56.192. [DOI] [PubMed] [Google Scholar]
- 51.Chan HC, Cheung WT, Leung PY, Wu LJ, Chew SB, Ko WH, Wong PY. Purinergic regulation of anion secretion by cystic fibrosis pancreatic duct cells. Am J Physiol. 1996;271:C469–477. doi: 10.1152/ajpcell.1996.271.2.C469. [DOI] [PubMed] [Google Scholar]
- 52.Chan HC, Law SH, Leung PS, Fu LX, Wong PY. Angiotensin II receptor type I-regulated anion secretion in cystic fibrosis pancreatic duct cells. J Membr Biol. 1997;156:241–249. doi: 10.1007/s002329900204. [DOI] [PubMed] [Google Scholar]
- 53.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng HS, Leung PY, Cheng Chew SB, Leung PS, Lam SY, Wong WS, Wang ZD, Chan HC. Concurrent and independent HCO3− and Cl− secretion in a human pancreatic duct cell line (CAPAN-1) J Membr Biol. 1998;164:155–167. doi: 10.1007/s002329900401. [DOI] [PubMed] [Google Scholar]
- 55.Cheng HS, Wong WS, Chan KT, Wang XF, Wang ZD, Chan HC. Modulation of Ca2+-dependent anion secretion by protein kinase C in normal and cystic fibrosis pancreatic duct cells. Biochim Biophys Acta. 1999;1418:31–38. doi: 10.1016/s0005-2736(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 56.Chey WY, Chang T. Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology. 2001;1:320–335. doi: 10.1159/000055831. [DOI] [PubMed] [Google Scholar]
- 57.Chey WY, Kim MS, Lee KY, Chang TM. Effect of rabbit antisecretin serum on postprandial pancreatic secretion in dogs. Gastroenterology. 1979;77:1268–1275. [PubMed] [Google Scholar]
- 58.Chey WY, Lee YH, Hendricks JG, Rhodes RA, Tai HH. Plasma secretin concentrations in fasting and postprandial state in man. Am J Dig Dis. 1978;23:981–988. doi: 10.1007/BF01263096. [DOI] [PubMed] [Google Scholar]
- 59.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- 60.Choi JY, Joo NS, Krouse ME, Wu JV, Robbins RC, Ianowski JP, Hanrahan JW, Wine JJ. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest. 2007;117:3118–3127. doi: 10.1172/JCI31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohn JA. Reduced CFTR function and the pathobiology of idiopathic pancreatitis. J Clin Gastroenterol. 2005;39:S70–77. doi: 10.1097/01.mcg.0000155522.89005.bf. [DOI] [PubMed] [Google Scholar]
- 63.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–658. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 64.Compton J, Martinez JR, Martinez AM, Young JA. Fluid and electrolyte secretion from the isolated, perfused submandibular and sublingual glands of the rat. Arch Oral Biol. 1981;26:555–561. doi: 10.1016/0003-9969(81)90017-0. [DOI] [PubMed] [Google Scholar]
- 65.Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA. Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys. 2002;36:105–113. doi: 10.1385/cbb:36:2-3:105. [DOI] [PubMed] [Google Scholar]
- 66.Cook DI, Van Lennep EW, Roberts MLYJ. Secretion by the major salivary glands. In: Johnson L, Christensen J, Jackson M, Jacobson E, Walsh J, editors. Physiology of the Gastrointestinal Tract. 3. Vol. 2. Raven Press; New York: 1994. pp. 1061–1117. [Google Scholar]
- 67.Cotton CU. Ion-transport properties of cultured bovine pancreatic duct epithelial cells. Pancreas. 1998;17:247–255. doi: 10.1097/00006676-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Criddle DN, Booth DM, Mukherjee R, McLaughlin E, Green GM, Sutton R, Petersen OH, Reeve JR., Jr Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1085–1092. doi: 10.1152/ajpgi.00119.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Czako L, Yamamoto M, Otsuki M. Exocrine pancreatic function in rats after acute pancreatitis. Pancreas. 1997;15:83–90. doi: 10.1097/00006676-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Dai YS, Ambudkar IS, Horn VJ, Yeh CK, Kousvelari EE, Wall SJ, Li M, Yasuda RP, Wolfe BB, Baum BJ. Evidence that M3 muscarinic receptors in rat parotid gland couple to two second messenger systems. Am J Physiol. 1991;261:C1063–1073. doi: 10.1152/ajpcell.1991.261.6.C1063. [DOI] [PubMed] [Google Scholar]
- 72.de Ondarza J, Hootman SR. Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. Am J Physiol. 1997;272:G124–134. doi: 10.1152/ajpgi.1997.272.1.G124. [DOI] [PubMed] [Google Scholar]
- 73.Debas HT, Grossman MI. Pure cholecystokinin: pancreatic protein and bicarbonate response. Digestion. 1973;9:469–481. doi: 10.1159/000197476. [DOI] [PubMed] [Google Scholar]
- 74.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 75.Dehaye JP, Valdez IH, Turner RJ. Beta-adrenergic stimulation and cAMP mobilize Ca2+ from an IP3-insensitive pool in rat submandibular granular ducts. Am J Physiol. 1993;265:C1356–1362. doi: 10.1152/ajpcell.1993.265.5.C1356. [DOI] [PubMed] [Google Scholar]
- 76.Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl- cotransport in primary afferent neurons. J Physiol. 2010;588:3365–3373. doi: 10.1113/jphysiol.2010.190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 78.Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu Rev Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 79.Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P, Baum BJ. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758:1061–1070. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 81.Devogelaere B, Beullens M, Sammels E, Derua R, Waelkens E, van Lint J, Parys JB, Missiaen L, Bollen M, De Smedt H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem J. 2007;407:303–311. doi: 10.1042/BJ20070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Devogelaere B, Nadif Kasri N, Derua R, Waelkens E, Callewaert G, Missiaen L, Parys JB, De Smedt H. Binding of IRBIT to the IP3 receptor: determinants and functional effects. Biochem Biophys Res Commun. 2006;343:49–56. doi: 10.1016/j.bbrc.2006.02.119. [DOI] [PubMed] [Google Scholar]
- 83.Devogelaere B, Sammels E, De Smedt H. The IRBIT domain adds new functions to the AHCY family. Bioessays. 2008;30:642–652. doi: 10.1002/bies.20772. [DOI] [PubMed] [Google Scholar]
- 84.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proc Natl Acad Sci U S A. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Domschke S, Domschke W, Rosch W, Konturek SJ, Sprugel W, Mitznegg P, Wunsch E, Demling L. Inhibition by somatostatin of secretin-stimulated pancreatic secretion in man: a study with pure pancreatic juice. Scand J Gastroenterol. 1977;12:59–63. [PubMed] [Google Scholar]
- 87.Domschke S, Domschke W, Rosch W, Konturek SJ, Wunsch E, Demling L. Bicarbonate and cyclic AMP content of pure human pancreatic juice in response to graded doses of synthetic secretin. Gastroenterology. 1976;70:533–536. [PubMed] [Google Scholar]
- 88.Dong M, Miller LJ. Molecular pharmacology of the secretin receptor. Receptors Channels. 2002;8:189–200. [PubMed] [Google Scholar]
- 89.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl(−) channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- 92.Dreizen S, Brown LR, Handler S, Levy BM. Radiation-induced xerostomia in cancer patients. Effect on salivary and serum electrolytes. Cancer. 1976;38:273–278. doi: 10.1002/1097-0142(197607)38:1<273::aid-cncr2820380141>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 93.Dunning BE, Havel PJ, Veith RC, Taborsky GJ., Jr Pancreatic and extrapancreatic galanin release during sympathetic neural activation. Am J Physiol. 1990;258:E436–444. doi: 10.1152/ajpendo.1990.258.3.E436. [DOI] [PubMed] [Google Scholar]
- 94.Durie PR. The pathophysiology of the pancreatic defect in cystic fibrosis. Acta Paediatr Scand Suppl. 1989;363:41–44. doi: 10.1111/apa.1989.78.s363.41. [DOI] [PubMed] [Google Scholar]
- 95.Dyck WP, Hightower NC, Janowitz HD. Effect of acetazolamide on human pancreatic secretion. Gastroenterology. 1972;62:547–552. [PubMed] [Google Scholar]
- 96.Elgavish A, Meezan E. Altered sulfate transport via anion exchange in CFPAC is corrected by retrovirus-mediated CFTR gene transfer. Am J Physiol. 1992;263:C176–186. doi: 10.1152/ajpcell.1992.263.1.C176. [DOI] [PubMed] [Google Scholar]
- 97.Evans RL, Ashton N, Elliott AC, Green R, Argent BE. Interactions between secretin and acetylcholine in the regulation of fluid secretion by isolated rat pancreatic ducts. J Physiol. 1996;496 (Pt 1):265–273. doi: 10.1113/jphysiol.1996.sp021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic agonist-induced up-regulation of Na(+)/H(+) exchange in mouse parotid acinar cells. J Biol Chem. 1999;274:29025–29030. doi: 10.1074/jbc.274.41.29025. [DOI] [PubMed] [Google Scholar]
- 99.Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J Biol Chem. 2000;275:26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- 100.Evans RL, Perrott MN, Lau KR, Case RM. Elevation of intracellular cAMP by noradrenaline and vasoactive intestinal peptide in striated ducts isolated from the rabbit mandibular salivary gland. Arch Oral Biol. 1996;41:689–694. doi: 10.1016/s0003-9969(96)00028-3. [DOI] [PubMed] [Google Scholar]
- 101.Eysselein VE, Eberlein GA, Hesse WH, Schaeffer M, Grandt D, Williams R, Goebell H, Reeve JR., Jr Molecular variants of cholecystokinin after endogenous stimulation in humans: a time study. Am J Physiol. 1990;258:G951–957. doi: 10.1152/ajpgi.1990.258.6.G951. [DOI] [PubMed] [Google Scholar]
- 102.Fanjul M, Salvador C, Alvarez L, Cantet S, Hollande E. Targeting of carbonic anhydrase IV to plasma membranes is altered in cultured human pancreatic duct cells expressing a mutated (ΔF508) CFTR. Eur J Cell Biol. 2002;81:437–447. doi: 10.1078/0171-9335-00264. [DOI] [PubMed] [Google Scholar]
- 103.Fernandez-Salazar MP, Pascua P, Calvo JJ, Lopez MA, Case RM, Steward MC, San Roman JI. Basolateral anion transport mechanisms underlying fluid secretion by mouse, rat and guinea-pig pancreatic ducts. J Physiol. 2004;556:415–428. doi: 10.1113/jphysiol.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 105.Fong P, Argent BE, Guggino WB, Gray MA. Characterization of vectorial chloride transport pathways in the human pancreatic duct adenocarcinoma cell line HPAF. Am J Physiol Cell Physiol. 2003;285:C433–445. doi: 10.1152/ajpcell.00509.2002. [DOI] [PubMed] [Google Scholar]
- 106.Foskett JK. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol. 1990;259:C998–1004. doi: 10.1152/ajpcell.1990.259.6.C998. [DOI] [PubMed] [Google Scholar]
- 107.Foskett JK, Melvin JE. Activation of salivary secretion: coupling of cell volume and [Ca2+]i in single cells. Science. 1989;244:1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- 108.Fuller CM. Calcium-activated chloride channels. Amsterdam, Boston: Academic Press; 2002. [Google Scholar]
- 109.Furuya S, Naruse S, Ko SB, Ishiguro H, Yoshikawa T, Hayakawa T. Distribution of aquaporin 1 in the rat pancreatic duct system examined with light- and electron-microscopic immunohistochemistry. Cell Tissue Res. 2002;308:75–86. doi: 10.1007/s00441-002-0527-x. [DOI] [PubMed] [Google Scholar]
- 110.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 111.Gabbi C, Kim HJ, Hultenby K, Bouton D, Toresson G, Warner M, Gustafsson JA. Pancreatic exocrine insufficiency in LXRbeta−/− mice is associated with a reduction in aquaporin-1 expression. Proc Natl Acad Sci U S A. 2008;105:15052–15057. doi: 10.1073/pnas.0808097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao R, Yan X, Zheng C, Goldsmith CM, Afione S, Hai B, Xu J, Zhou J, Zhang C, Chiorini JA, Baum BJ, Wang S. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011;18:38–42. doi: 10.1038/gt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- 114.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 115.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem. 2004;279:30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 116.Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, Kim KS, Kim H, Baba A, Yang J, Kim E, Kim KH, Lee MG. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology. 2009;137:607–617. e601–604. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 117.Gee HY, Park HW, Kim KH, Lee MG. PDZ-based adaptor proteins in epithelial anion transport and VIP receptor regulation. J Med Invest. 2009;56 (Suppl):302–305. doi: 10.2152/jmi.56.302. [DOI] [PubMed] [Google Scholar]
- 118.Gerolami A, Marteau C, Matteo A, Sahel J, Portugal H, Pauli AM, Pastor J, Sarles H. Calcium carbonate saturation in human pancreatic juice: possible role of ductal H+ secretion. Gastroenterology. 1989;96:881–884. [PubMed] [Google Scholar]
- 119.Gillen CM, Brill S, Payne JA, Forbush B., 3rd Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 120.Giovannucci DR, Bruce JI, Straub SV, Arreola J, Sneyd J, Shuttleworth TJ, Yule DI. Cytosolic Ca(2+) and Ca(2+)-activated Cl(−) current dynamics: insights from two functionally distinct mouse exocrine cells. J Physiol. 2002;540:469–484. doi: 10.1113/jphysiol.2001.013453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzalez-Begne M, Nakamoto T, Nguyen HV, Stewart AK, Alper SL, Melvin JE. Enhanced formation of a HCO3- transport metabolon in exocrine cells of Nhe1−/− mice. J Biol Chem. 2007;282:35125–35132. doi: 10.1074/jbc.M707266200. [DOI] [PubMed] [Google Scholar]
- 122.Gray MA. Bicarbonate secretion: it takes two to tango. Nat Cell Biol. 2004;6:292–294. doi: 10.1038/ncb0404-292. [DOI] [PubMed] [Google Scholar]
- 123.Gray MA, Argent BE. Non-selective cation channel on pancreatic duct cells. Biochim Biophys Acta. 1990;1029:33–42. doi: 10.1016/0005-2736(90)90433-o. [DOI] [PubMed] [Google Scholar]
- 124.Gray MA, Greenwell JR, Garton AJ, Argent BE. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol. 1990;115:203–215. doi: 10.1007/BF01868636. [DOI] [PubMed] [Google Scholar]
- 125.Gray MA, Harris A, Coleman L, Greenwell JR, Argent BE. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989;257:C240–251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- 126.Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993;264:C591–602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- 127.Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990;259:C752–761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- 128.Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol. 1994;266:C213–221. doi: 10.1152/ajpcell.1994.266.1.C213. [DOI] [PubMed] [Google Scholar]
- 129.Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1301–1308. doi: 10.1152/ajpgi.2001.281.5.G1301. [DOI] [PubMed] [Google Scholar]
- 130.Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. J Physiol. 2003;549:673–682. doi: 10.1113/jphysiol.2003.042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol. 2001;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser(982) in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3− : Na+ stoichiometry from 3 : 1 to 2 : 1 in murine proximal tubule cells. J Physiol. 2001;537:659–665. doi: 10.1111/j.1469-7793.2001.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grotmol T, Buanes T, Raeder MG. NN′-dicyclohexylcarbodiimide (DCCD) reduces pancreatic NaHCO3 secretion without changing pancreatic tissue ATP levels. Acta Physiol Scand. 1986;128:547–554. doi: 10.1111/j.1748-1716.1986.tb08011.x. [DOI] [PubMed] [Google Scholar]
- 134.Grundy D, Hutson D, Scratcherd T. The response of the pancreas of the anaesthetized cat to secretin before, during and after reversible vagal blockade. J Physiol. 1983;342:517–526. doi: 10.1113/jphysiol.1983.sp014866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guggino WB. The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc Am Thorac Soc. 2004;1:28–32. doi: 10.1513/pats.2306011. [DOI] [PubMed] [Google Scholar]
- 136.Gyr K, Beglinger C, Fried M, Grotzinger U, Kayasseh L, Stalder GA, Girard J. Plasma secretin and pancreatic response to various stimulants including a meal. Am J Physiol. 1984;246:G535–542. doi: 10.1152/ajpgi.1984.246.5.G535. [DOI] [PubMed] [Google Scholar]
- 137.Haas M, Forbush B., 3rd The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 138.Halangk W, Lerch MM. Early events in acute pancreatitis. Clin Lab Med. 2005;25:1–15. doi: 10.1016/j.cll.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 140.Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG. Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem. 2006;281:1461–1469. doi: 10.1074/jbc.M509786200. [DOI] [PubMed] [Google Scholar]
- 141.Hanwell D, Ishikawa T, Saleki R, Rotin D. Trafficking and cell surface stability of the epithelial Na+ channel expressed in epithelial Madin-Darby canine kidney cells. J Biol Chem. 2002;277:9772–9779. doi: 10.1074/jbc.M110904200. [DOI] [PubMed] [Google Scholar]
- 142.Harrison JD. Causes, natural history, and incidence of salivary stones and obstructions. Otolaryngol Clin North Am. 42:927–947. doi: 10.1016/j.otc.2009.08.012. Table of Contents, 2009. [DOI] [PubMed] [Google Scholar]
- 143.Hart WM, Thomas JE. Bicarbonate and choloride of pancreatic juice secreted in response to various stimuli. 1945;4:409–420. [Google Scholar]
- 144.Hashimoto S, Murakami M. Morphological evidence of paracellular transport in perfused rat submandibular glands. J Med Invest. 2009;56 (Suppl):395–397. doi: 10.2152/jmi.56.395. [DOI] [PubMed] [Google Scholar]
- 145.Hatefi Y, Hanstein WG. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969;62:1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hayashi M, Kunii C, Takahata T, Ishikawa T. ATP-dependent regulation of SK4/IK1-like currents in rat submandibular acinar cells: possible role of cAMP-dependent protein kinase. Am J Physiol Cell Physiol. 2004;286:C635–646. doi: 10.1152/ajpcell.00283.2003. [DOI] [PubMed] [Google Scholar]
- 147.Hayashi T, Young JA, Cook DI. The Ach-evoked Ca2+-activated K+ current in mouse mandibular secretory cells. Single channel studies. J Membr Biol. 1996;151:19–27. doi: 10.1007/s002329900054. [DOI] [PubMed] [Google Scholar]
- 148.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2010;285:27869–27878. doi: 10.1074/jbc.M110.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem. 2008;283:33544–33553. doi: 10.1074/jbc.M805534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- 152.Hede SE, Amstrup J, Christoffersen BC, Novak I. Purinoceptors evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits K+ conductance, and P2X stimulates cation conductance. J Biol Chem. 1999;274:31784–31791. doi: 10.1074/jbc.274.45.31784. [DOI] [PubMed] [Google Scholar]
- 153.Hegyi P, Gray MA, Argent BE. Substance P inhibits bicarbonate secretion from guinea pig pancreatic ducts by modulating an anion exchanger. Am J Physiol Cell Physiol. 2003;285:C268–276. doi: 10.1152/ajpcell.00574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hegyi P, Rakonczay Z, Jr, Farkas K, Venglovecz V, Ozsvari B, Seidler U, Gray MA, Argent BE. Controversies in the role of SLC26 anion exchangers in pancreatic ductal bicarbonate secretion. Pancreas. 2008;37:232–234. doi: 10.1097/MPA.0b013e318164f8ff. [DOI] [PubMed] [Google Scholar]
- 155.Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285:25161–25167. doi: 10.1074/jbc.M110.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Henderson LJ. The Regulation of Neutrality in the Animal Body. Science. 1913;37:389–395. doi: 10.1126/science.37.950.389. [DOI] [PubMed] [Google Scholar]
- 157.Henriksen KL, Novak I. Effect of ATP on intracellular pH in pancreatic ducts involves P2X7 receptors. Cell Physiol Biochem. 2003;13:93–102. doi: 10.1159/000070253. [DOI] [PubMed] [Google Scholar]
- 158.Ho MW, Kaetzel MA, Armstrong DL, Shears SB. Regulation of a human chloride channel. a paradigm for integrating input from calcium, type ii calmodulin-dependent protein kinase, and inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 2001;276:18673–18680. doi: 10.1074/jbc.M101128200. [DOI] [PubMed] [Google Scholar]
- 159.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 160.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 161.Holst JJ, Fahrenkrug J, Knuhtsen S, Jensen SL, Nielsen OV, Lundberg JM, Hokfelt T. VIP and PHI in the pig pancreas: coexistence, corelease, and cooperative effects. Am J Physiol. 1987;252:G182–189. doi: 10.1152/ajpgi.1987.252.2.G182. [DOI] [PubMed] [Google Scholar]
- 162.Holst JJ, Fahrenkrug J, Knuhtsen S, Jensen SL, Poulsen SS, Nielsen OV. Vasoactive intestinal polypeptide (VIP) in the pig pancreas: role of VIPergic nerves in control of fluid and bicarbonate secretion. Regul Pept. 1984;8:245–259. doi: 10.1016/0167-0115(84)90066-1. [DOI] [PubMed] [Google Scholar]
- 163.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang CL, Cha SK, Wang HR, Xie J, Cobb MH. WNKs: protein kinases with a unique kinase domain. Exp Mol Med. 2007;39:565–573. doi: 10.1038/emm.2007.62. [DOI] [PubMed] [Google Scholar]
- 165.Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens. 2008;17:519–525. doi: 10.1097/MNH.0b013e32830dd580. [DOI] [PubMed] [Google Scholar]
- 166.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A. 2009;106:21413–21418. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 168.Hug M, Pahl C, Novak I. Effect of ATP, carbachol and other agonists on intracellular calcium activity and membrane voltage of pancreatic ducts. Pflugers Arch. 1994;426:412–418. doi: 10.1007/BF00388304. [DOI] [PubMed] [Google Scholar]
- 169.Hug MJ, Tamada T, Bridges RJ. CFTR and bicarbonate secretion by [correction of to] epithelial cells. News Physiol Sci. 2003;18:38–42. doi: 10.1152/nips.01412.2002. [DOI] [PubMed] [Google Scholar]
- 170.Hurley PT, Ferguson CJ, Kwon TH, Andersen ML, Norman AG, Steward MC, Nielsen S, Case RM. Expression and immunolocalization of aquaporin water channels in rat exocrine pancreas. Am J Physiol Gastrointest Liver Physiol. 2001;280:G701–709. doi: 10.1152/ajpgi.2001.280.4.G701. [DOI] [PubMed] [Google Scholar]
- 171.Hyde K, Harrison D, Hollingsworth MA, Harris A. Chloride-bicarbonate exchangers in the human fetal pancreas. Biochem Biophys Res Commun. 1999;263:315–321. doi: 10.1006/bbrc.1999.1367. [DOI] [PubMed] [Google Scholar]
- 172.Ichihara N, Tsukamoto A, Kasuya T, Shibata S, Nishita T, Murakami M, Amasaki H, Asari M. Gene expression of secretory carbonic anhydrase isozymes in striated ducts of canine salivary glands using laser microdissection system. Anat Histol Embryol. 2007;36:357–360. doi: 10.1111/j.1439-0264.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 173.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 174.Illek B, Tam AW, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelium. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- 175.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am J Physiol. 1997;272:L752–761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- 176.Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC. Luminal ATP stimulates fluid and HCO3− secretion in guinea-pig pancreatic duct. J Physiol. 1999;519(Pt 2):551–558. doi: 10.1111/j.1469-7793.1999.0551m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ishiguro H, Naruse S, Kitagawa M, Mabuchi T, Kondo T, Hayakawa T, Case RM, Steward MC. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2002;539:175–189. doi: 10.1113/jphysiol.2001.012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 2000;528(Pt 2):305–315. doi: 10.1111/j.1469-7793.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol. 1998;511 (Pt 2):407–422. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3- by Na+-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495 (Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133:315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ishiguro H, Steward MC, Sohma Y, Kubota T, Kitagawa M, Kondo T, Case RM, Hayakawa T, Naruse S. Membrane potential and bicarbonate secretion in isolated interlobular ducts from guinea-pig pancreas. J Gen Physiol. 2002;120:617–628. doi: 10.1085/jgp.20028631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495 (Pt 1):179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 185.Ishikawa Y, Yuan Z, Inoue N, Skowronski MT, Nakae Y, Shono M, Cho G, Yasui M, Agre P, Nielsen S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am J Physiol Cell Physiol. 2005;289:C1303–1311. doi: 10.1152/ajpcell.00211.2005. [DOI] [PubMed] [Google Scholar]
- 186.Iwatsuki N, Petersen OH. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977;269:735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells do not respond to cholecystokinin. Pharmacol Toxicol. 2002;91:327–332. doi: 10.1034/j.1600-0773.2002.910610.x. [DOI] [PubMed] [Google Scholar]
- 188.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- 189.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 190.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 191.Johansen PG, Anderson CM, Hadorn B. Cystic fibrosis of the pancreas. A generalised disturbance of water and electrolyte movement in exocrine tissues. Lancet. 1968;1:455–460. [PubMed] [Google Scholar]
- 192.Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35:889–896. doi: 10.1016/j.jdent.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 193.Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc Natl Acad Sci U S A. 2004;101:2064–2069. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 195.Karbanova J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, Hollerova H, Jaszai J, Ehrmann J, Kolar Z, Liebers C, Arl S, Subrtova D, Freund D, Mokry J, Huttner WB, Corbeil D. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem. 2008;56:977–993. doi: 10.1369/jhc.2008.951897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kasai H, Augustine GJ. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990;348:735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- 197.Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- 198.Kawedia JD, Nieman ML, Boivin GP, Melvin JE, Kikuchi K, Hand AR, Lorenz JN, Menon AG. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci U S A. 2007;104:3621–3626. doi: 10.1073/pnas.0608384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 200.Kidd JF, Thorn P. Intracellular Ca2+ and Cl- channel activation in secretory cells. Annu Rev Physiol. 2000;62:493–513. doi: 10.1146/annurev.physiol.62.1.493. [DOI] [PubMed] [Google Scholar]
- 201.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 202.Kim JY, Han W, Namkung W, Lee JH, Kim KH, Shin H, Kim E, Lee MG. Inhibitory regulation of cystic fibrosis transmembrane conductance regulator anion-transporting activities by Shank2. J Biol Chem. 2004;279:10389–10396. doi: 10.1074/jbc.M312871200. [DOI] [PubMed] [Google Scholar]
- 203.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 204.Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl- channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- 205.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1010334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.King BF, Love JA, Szurszewski JH. Intracellular recordings from pancreatic ganglia of the cat. J Physiol. 1989;419:379–403. doi: 10.1113/jphysiol.1989.sp017877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 209.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ko SB, Mizuno N, Yatabe Y, Yoshikawa T, Ishiguro H, Yamamoto A, Azuma S, Naruse S, Yamao K, Muallem S, Goto H. Aquaporin 1 water channel is overexpressed in the plasma membranes of pancreatic ducts in patients with autoimmune pancreatitis. J Med Invest. 2009;56 (Suppl):318–321. doi: 10.2152/jmi.56.318. [DOI] [PubMed] [Google Scholar]
- 211.Ko SB, Mizuno N, Yatabe Y, Yoshikawa T, Ishiguro H, Yamamoto A, Azuma S, Naruse S, Yamao K, Muallem S, Goto H. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology. 2010;138:1988–1996. doi: 10.1053/j.gastro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ko SB, Naruse S, Kitagawa M, Ishiguro H, Furuya S, Mizuno N, Wang Y, Yoshikawa T, Suzuki A, Shimano S, Hayakawa T. Aquaporins in rat pancreatic interlobular ducts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G324–331. doi: 10.1152/ajpgi.00198.2001. [DOI] [PubMed] [Google Scholar]
- 213.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(−) transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kohler H, Nustede R, Barthel M, Schafmayer A. Exocrine pancreatic function in dogs with denervated pancreas. Pancreas. 1987;2:676–683. doi: 10.1097/00006676-198711000-00009. [DOI] [PubMed] [Google Scholar]
- 217.Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481:1–14. doi: 10.1016/j.ejphar.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 218.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 219.Ku HT. Minireview: pancreatic progenitor cells--recent studies. Endocrinology. 2008;149:4312–4316. doi: 10.1210/en.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kuijpers GA, Van Nooy IG, De Pont JJ, Bonting SL. Anion secretion by the isolated rabbit pancreas. Biochim Biophys Acta. 1984;774:269–276. doi: 10.1016/0005-2736(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 221.Kuijpers GA, Van Nooy IG, De Pont JJ, Bonting SL. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta. 1984;778:324–331. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- 222.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 223.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 224.Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. Bestrophin and TMEM16-Ca(2+) activated Cl(−) channels with different functions. Cell Calcium. 2009;46:233–241. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 225.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 226.Larsson O, Olgart L. The enhancement of carbachol-induced salivary secretion by VIP and CGRP in rat parotid gland is mimicked by forskolin. Acta Physiol Scand. 1989;137:231–236. doi: 10.1111/j.1748-1716.1989.tb08743.x. [DOI] [PubMed] [Google Scholar]
- 227.Lau KR, Howorth AJ, Case RM. The effects of bumetanide, amiloride and Ba2+ on fluid and electrolyte secretion in rabbit salivary gland. J Physiol. 1990;425:407–427. doi: 10.1113/jphysiol.1990.sp018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee IH, Campbell CR, Cook DI, Dinudom A. Regulation of epithelial Na+ channels by aldosterone: role of Sgk1. Clin Exp Pharmacol Physiol. 2008;35:235–241. doi: 10.1111/j.1440-1681.2007.04844.x. [DOI] [PubMed] [Google Scholar]
- 229.Lee IH, Campbell CR, Song SH, Day ML, Kumar S, Cook DI, Dinudom A. The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J Biol Chem. 2009;284:12663–12669. doi: 10.1074/jbc.M809737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee JH, Choi JH, Namkung W, Hanrahan JW, Chang J, Song SY, Park SW, Kim DS, Yoon JH, Suh Y, Jang IJ, Nam JH, Kim SJ, Cho MO, Lee JE, Kim KH, Lee MG. A haplotype-based molecular analysis of CFTR mutations associated with respiratory and pancreatic diseases. Hum Mol Genet. 2003;12:2321–2332. doi: 10.1093/hmg/ddg243. [DOI] [PubMed] [Google Scholar]
- 231.Lee JH, Richter W, Namkung W, Kim KH, Kim E, Conti M, Lee MG. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J Biol Chem. 2007;282:10414–10422. doi: 10.1074/jbc.M610857200. [DOI] [PubMed] [Google Scholar]
- 232.Lee JS, Lee YM, Kim JY, Park HW, Grinstein S, Orlowski J, Kim E, Kim KH, Lee MG. BetaPix up-regulates Na+/H+ exchanger 3 through a Shank2-mediated protein-protein interaction. J Biol Chem. 2010;285:8104–8113. doi: 10.1074/jbc.M109.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na(+)-dependent transporters mediate HCO(3)(−) salvage across the luminal membrane of the main pancreatic duct. J Clin Invest. 2000;105:1651–1658. doi: 10.1172/JCI9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S. Na+-dependent transporters mediate HCO3− salvage across the luminal membrane of the main pancreatic duct. J Clin Invest. 2000;105:1651–1658. doi: 10.1172/JCI9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. J Biol Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 236.Lee MG, Muallem S. Pancreatitis: the neglected duct. Gut. 2008;57:1037–1039. doi: 10.1136/gut.2008.150961. [DOI] [PubMed] [Google Scholar]
- 237.Lee MG, Muallem S. Physiology of duct cell secretion. In: Beger H, Buchler M, Kozarek R, Lerch M, Neoptolemos J, Warshaw A, Whitcomb D, Shiratori K, editors. Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery. Oxford, U.K: Blackwell Publishing; 2008. pp. 78–90. [Google Scholar]
- 238.Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, Muallem S. Regulation of Cl−/HCO3− exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J Biol Chem. 1999;274:3414–3421. doi: 10.1074/jbc.274.6.3414. [DOI] [PubMed] [Google Scholar]
- 239.Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15771–15776. doi: 10.1074/jbc.272.25.15771. [DOI] [PubMed] [Google Scholar]
- 240.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 241.Li Q, Luo X, Muallem S. Regulation of the P2X7 receptor permeability to large molecules by extracellular Cl- and Na+ J Biol Chem. 2005;280:26922–26927. doi: 10.1074/jbc.M504966200. [DOI] [PubMed] [Google Scholar]
- 242.Li Q, Luo X, Zeng W, Muallem S. Cell-specific behavior of P2X7 receptors in mouse parotid acinar and duct cells. J Biol Chem. 2003;278:47554–47561. doi: 10.1074/jbc.M308306200. [DOI] [PubMed] [Google Scholar]
- 243.Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466–473. [PubMed] [Google Scholar]
- 244.Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E. Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem. 1999;274:29510–29518. doi: 10.1074/jbc.274.41.29510. [DOI] [PubMed] [Google Scholar]
- 245.Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 2000;70:102–112. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- 248.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem. 2001;276:9808–9816. doi: 10.1074/jbc.M008548200. [DOI] [PubMed] [Google Scholar]
- 251.Luo X, Zeng W, Xu X, Popov S, Davignon I, Wilkie TM, Mumby SM, Muallem S. Alternate coupling of receptors to Gs and Gi in pancreatic and submandibular gland cells. J Biol Chem. 1999;274:17684–17690. doi: 10.1074/jbc.274.25.17684. [DOI] [PubMed] [Google Scholar]
- 252.Luo X, Zheng W, Yan M, Lee MG, Muallem S. Multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells. Am J Physiol. 1999;277:C205–215. doi: 10.1152/ajpcell.1999.277.2.C205. [DOI] [PubMed] [Google Scholar]
- 253.Lur G, Haynes LP, Prior IA, Gerasimenko OV, Feske S, Petersen OH, Burgoyne RD, Tepikin AV. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ma T, Jayaraman S, Wang KS, Song Y, Yang B, Li J, Bastidas JA, Verkman AS. Defective dietary fat processing in transgenic mice lacking aquaporin-1 water channels. Am J Physiol Cell Physiol. 2001;280:C126–134. doi: 10.1152/ajpcell.2001.280.1.C126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 256.Madden ME, Sarras MP., Jr Distribution of Na+,K+-ATPase in rat exocrine pancreas as monitored by K+-NPPase cytochemistry and [3H]-ouabain binding: a plasma membrane protein found primarily to be ductal cell associated. J Histochem Cytochem. 1987;35:1365–1374. doi: 10.1177/35.12.2824600. [DOI] [PubMed] [Google Scholar]
- 257.Madden ME, Sarras MP., Jr The pancreatic ductal system of the rat: cell diversity, ultrastructure, and innervation. Pancreas. 1989;4:472–485. doi: 10.1097/00006676-198908000-00013. [DOI] [PubMed] [Google Scholar]
- 258.Mahnensmith RL, Aronson PS. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985;56:773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- 259.Mangos JA, McSherry NR. Micropuncture study of excretion of water and electrolytes by the pancreas. Am J Physiol. 1971;221:496–503. doi: 10.1152/ajplegacy.1971.221.2.496. [DOI] [PubMed] [Google Scholar]
- 260.Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol. 1999;277:G487–494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- 261.Marino CR, Matovcik LM, Gorelick FS, Cohn JA. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88:712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol. 2007;69:361–375. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- 263.Marteau C, Blanc G, Devaux MA, Portugal H, Gerolami A. Influence of pancreatic ducts on saturation of juice with calcium carbonate in dogs. Dig Dis Sci. 1993;38:2090–2097. doi: 10.1007/BF01297090. [DOI] [PubMed] [Google Scholar]
- 264.Marteau C, Silviani V, Ducroc R, Crotte C, Gerolami A. Evidence for apical Na+/H+ exchanger in bovine main pancreatic duct. Dig Dis Sci. 1995;40:2336–2340. doi: 10.1007/BF02063234. [DOI] [PubMed] [Google Scholar]
- 265.Martinez JR. Ion transport and water movement. J Dent Res. 1987;66(Spec No):638–647. doi: 10.1177/00220345870660S206. [DOI] [PubMed] [Google Scholar]
- 266.Martinez JR, Cassity N. Effects of 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid and amiloride on salivary secretion by isolated, perfused rat submandibular glands. Arch Oral Biol. 1985;30:797–803. doi: 10.1016/0003-9969(85)90134-7. [DOI] [PubMed] [Google Scholar]
- 267.Maruyama Y, Petersen OH, Flanagan P, Pearson GT. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature. 1983;305:228–232. doi: 10.1038/305228a0. [DOI] [PubMed] [Google Scholar]
- 268.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 270.Meda P. The role of gap junction membrane channels in secretion and hormonal action. J Bioenerg Biomembr. 1996;28:369–377. doi: 10.1007/BF02110113. [DOI] [PubMed] [Google Scholar]
- 271.Meldolesi J. Membranes and membrane surfaces. Dynamics of cytoplasmic membranes in pancreatic acinar cells. Philos Trans R Soc Lond B Biol Sci. 1974;268:39–53. doi: 10.1098/rstb.1974.0014. [DOI] [PubMed] [Google Scholar]
- 272.Melvin JE, Moran A, Turner RJ. The role of HCO3- and Na+/H+ exchange in the response of rat parotid acinar cells to muscarinic stimulation. J Biol Chem. 1988;263:19564–19569. [PubMed] [Google Scholar]
- 273.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- 274.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 275.Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597–607. doi: 10.1016/s0025-7125(05)70243-1. [DOI] [PubMed] [Google Scholar]
- 276.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 277.Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12:1303–1311. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 278.Miyasaka K, Shinozaki H, Jimi A, Funakoshi A. Amylase secretion from dispersed human pancreatic acini: neither cholecystokinin a nor cholecystokinin B receptors mediate amylase secretion in vitro. Pancreas. 2002;25:161–165. doi: 10.1097/00006676-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 279.Miyawaki S, Nishiyama S, Matoba K. Efficacy of low-dose prednisolone maintenance for saliva production and serological abnormalities in patients with primary Sjogren’s syndrome. Intern Med. 1999;38:938–943. doi: 10.2169/internalmedicine.38.938. [DOI] [PubMed] [Google Scholar]
- 280.Morth JP, Pedersen BP, Buch-Pedersen MJ, Andersen JP, Vilsen B, Palmgren MG, Nissen P. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol. 2011;12:60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- 281.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol. 1999;276:G185–192. doi: 10.1152/ajpgi.1999.276.1.G185. [DOI] [PubMed] [Google Scholar]
- 282.Muallem S, Loessberg PA. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. I. Characterization of H+ and HCO3- transporters. J Biol Chem. 1990;265:12806–12812. [PubMed] [Google Scholar]
- 283.Muallem S, Loessberg PA. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. II. Regulation of H+ and HCO3- transporters by Ca2(+)-mobilizing agonists. J Biol Chem. 1990;265:12813–12819. [PubMed] [Google Scholar]
- 284.Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol. 2010;588:2329–2342. doi: 10.1113/jphysiol.2010.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Murakami M, Murdiastuti K, Hosoi K, Hill AE. AQP and the control of fluid transport in a salivary gland. J Membr Biol. 2006;210:91–103. doi: 10.1007/s00232-005-0848-2. [DOI] [PubMed] [Google Scholar]
- 286.Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Green GM, Reeve JR, Jr, Petersen OH, Sutton R. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 287.Nakamoto T, Brown DA, Catalan MA, Gonzalez-Begne M, Romanenko VG, Melvin JE. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem. 2009;284:4815–4822. doi: 10.1074/jbc.M808597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol. 2008;294:C810–819. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, Begenisich T, Melvin JE. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2380–2390. doi: 10.1152/ajpregu.00591.2006. [DOI] [PubMed] [Google Scholar]
- 290.Namkung W, Han W, Luo X, Muallem S, Cho KH, Kim KH, Lee MG. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 2004;126:1844–1859. doi: 10.1053/j.gastro.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 291.Namkung W, Lee JA, Ahn W, Han W, Kwon SW, Ahn DS, Kim KH, Lee MG. Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl−-dependent HCO3− transport in pancreatic duct cells. J Biol Chem. 2003;278:200–207. doi: 10.1074/jbc.M207199200. [DOI] [PubMed] [Google Scholar]
- 292.Namkung W, Yoon JS, Kim KH, Lee MG. PAR2 exerts local protection against acute pancreatitis via modulation of MAP kinase and MAP kinase phosphatase signaling. Am J Physiol Gastrointest Liver Physiol. 2008;295:G886–894. doi: 10.1152/ajpgi.00053.2008. [DOI] [PubMed] [Google Scholar]
- 293.Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J Biol Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- 294.Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol. 2003;284:C535–546. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- 295.Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl(−)/HCO(3)(−) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [Ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G312–320. doi: 10.1152/ajpgi.00158.2003. [DOI] [PubMed] [Google Scholar]
- 296.Nguyen TD, Koh DS, Moody MW, Fox NR, Savard CE, Kuver R, Hille B, Lee SP. Characterization of two distinct chloride channels in cultured dog pancreatic duct epithelial cells. Am J Physiol. 1997;272:G172–180. doi: 10.1152/ajpgi.1997.272.1.G172. [DOI] [PubMed] [Google Scholar]
- 297.Nguyen TD, Meichle S, Kim US, Wong T, Moody MW. P2Y(11), a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G795–804. doi: 10.1152/ajpgi.2001.280.5.G795. [DOI] [PubMed] [Google Scholar]
- 298.Nguyen TD, Moody MW. Calcium-activated potassium conductances on cultured nontransformed dog pancreatic duct epithelial cells. Pancreas. 1998;17:348–358. doi: 10.1097/00006676-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 299.Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nishi T, Forgac M. The vacuolar H+-ATPases--nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 301.Nishimori I, FujikawaAdachi K, Onishi S, Hollingsworth MA. Carbonic anhydrase in human pancreas: hypotheses for the pathophysiological roles of CA isozymes. Ann N Y Acad Sci. 1999;880:5–16. doi: 10.1111/j.1749-6632.1999.tb09505.x. [DOI] [PubMed] [Google Scholar]
- 302.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 303.Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 304.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Novak I, Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflugers Arch. 1988;411:58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- 306.Novak I, Greger R. Properties of the luminal membrane of isolated perfused rat pancreatic ducts. Effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 1988;411:546–553. doi: 10.1007/BF00582376. [DOI] [PubMed] [Google Scholar]
- 307.Novak I, Jans IM, Wohlfahrt L. Effect of P2X(7) receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol. 2010;588:3615–3627. doi: 10.1113/jphysiol.2010.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Novak I, Pahl C. Effect of secretin and inhibitors of HCO3−/H+ transport on the membrane voltage of rat pancreatic duct cells. Pflugers Arch. 1993;425:272–279. doi: 10.1007/BF00374178. [DOI] [PubMed] [Google Scholar]
- 309.Novak I, Wang J, Henriksen KL, Haanes KA, Krabbe S, Nitschke R, Hede SE. Pancreatic bicarbonate secretion involves two proton pumps. J Biol Chem. 2011;286:280–289. doi: 10.1074/jbc.M110.136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Novak I, Young JA. Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch. 1986;407:649–656. doi: 10.1007/BF00582647. [DOI] [PubMed] [Google Scholar]
- 311.O’Mullane LM, Cook DI, Dinudom A. Purinergic regulation of the epithelial Na+ channel. Clin Exp Pharmacol Physiol. 2009;36:1016–1022. doi: 10.1111/j.1440-1681.2009.05256.x. [DOI] [PubMed] [Google Scholar]
- 312.O’Reilly CM, Winpenny JP, Argent BE, Gray MA. Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions. Gastroenterology. 2000;118:1187–1196. doi: 10.1016/s0016-5085(00)70372-6. [DOI] [PubMed] [Google Scholar]
- 313.Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137:239–251. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol. 2009;587:2179–2185. doi: 10.1113/jphysiol.2008.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ohta E, Itoh T, Nemoto T, Kumagai J, Ko SB, Ishibashi K, Ohno M, Uchida K, Ohta A, Sohara E, Uchida S, Sasaki S, Rai T. Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am J Physiol Cell Physiol. 2009;297:C1368–1378. doi: 10.1152/ajpcell.00117.2009. [DOI] [PubMed] [Google Scholar]
- 316.Olsnes S, Tonnessen TI, Sandvig K. pH-regulated anion antiport in nucleated mammalian cells. J Cell Biol. 1986;102:967–971. doi: 10.1083/jcb.102.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 318.Orlowski J, Grinstein S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol. 2007;19:483–492. doi: 10.1016/j.ceb.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 320.Pahl C, Novak I. Effect of vasoactive intestinal peptide, carbachol and other agonists on the membrane voltage of pancreatic duct cells. Pflugers Arch. 1993;424:315–320. doi: 10.1007/BF00384358. [DOI] [PubMed] [Google Scholar]
- 321.Pak BH, Hong SS, Pak HK, Hong SK. Effects of acetazolamide and acid-base changes on biliary and pancreatic secretion. Am J Physiol. 1966;210:624–628. doi: 10.1152/ajplegacy.1966.210.3.624. [DOI] [PubMed] [Google Scholar]
- 322.Pandol SJ, Lugea A, Mareninova OA, Smoot D, Gorelick FS, Gukovskaya AS, Gukovsky I. Investigating the Pathobiology of Alcoholic Pancreatitis. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106:20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Paradiso AM, Coakley RD, Boucher RC. Polarized distribution of HCO3− transport in human normal and cystic fibrosis nasal epithelia. J Physiol. 2003;548:203–218. doi: 10.1113/jphysiol.2002.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 326.Park K, Olschowka JA, Richardson LA, Bookstein C, Chang EB, Melvin JE. Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. Am J Physiol. 1999;276:G470–478. doi: 10.1152/ajpgi.1999.276.2.G470. [DOI] [PubMed] [Google Scholar]
- 327.Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem. 2002;277:50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- 328.Pavlov I. The work of the digestive glands. Philadelphia: Lippincott; 1910. pp. 59–64. [Google Scholar]
- 329.Pederson SF, Varming C, Christensen ST, Hoffmann EK. Mechanisms of activation of NHE by cell shrinkage and by calyculin A in Ehrlich ascites tumor cells. J Membr Biol. 2002;189:67–81. doi: 10.1007/s00232-001-0190-2. [DOI] [PubMed] [Google Scholar]
- 330.Petersen OH. Ca2+ signaling in pancreatic acinar cells: physiology and pathophysiology. Braz J Med Biol Res. 2009;42:9–16. doi: 10.1590/s0100-879x2009000100003. [DOI] [PubMed] [Google Scholar]
- 331.Petersen OH. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986;251:G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]
- 332.Petersen OH. Increase in membrane conductance by adrenaline in parotid acinar cells. Experientia. 1976;32:471–472. doi: 10.1007/BF01920803. [DOI] [PubMed] [Google Scholar]
- 333.Petersen OH. Local and global Ca2+ signals: physiology and pathophysiology. Biol Res. 2004;37:661–664. doi: 10.4067/s0716-97602004000400023. [DOI] [PubMed] [Google Scholar]
- 334.Petersen OH. Stimulus-excitation coupling in plasma membranes of pancreatic acinar cells. Biochim Biophys Acta. 1982;694:163–184. doi: 10.1016/0304-4157(82)90023-5. [DOI] [PubMed] [Google Scholar]
- 335.Petersen OH, Philpott HG. Mouse pancreatic acinar cells: the anion selectivity of the acetylcholine-opened chloride pathway. J Physiol. 1980;306:481–492. doi: 10.1113/jphysiol.1980.sp013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Petersen OH, Poulsen JH. Inhibition of salivary secretion and secretory potentials by g-strophantin, dinitrophenol and cyanide. Acta Physiol Scand. 1967;71:194–202. doi: 10.1111/j.1748-1716.1967.tb03725.x. [DOI] [PubMed] [Google Scholar]
- 337.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 338.Petersen OH, Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol. 1977;264:819–835. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pickartz T, Mayerle J, Lerch MM. Autoimmune pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:314–323. doi: 10.1038/ncpgasthep0837. [DOI] [PubMed] [Google Scholar]
- 340.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Preshaw RM, Cooke AR, Grossman MI. Quantitative aspects of response of canine pancreas to duodenal acidification. Am J Physiol. 1966;210:629–634. doi: 10.1152/ajplegacy.1966.210.3.629. [DOI] [PubMed] [Google Scholar]
- 342.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 343.Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- 344.Pushkin A, Kurtz I. SLC4 base (HCO3 -, CO3 2-) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol. 2006;290:F580–599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- 345.Qu Z, Hartzell HC. Anion permeation in Ca2+-activated Cl− channels. J Gen Physiol. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 347.Quinton PM. The neglected ion: HCO3−. Nat Med. 2001;7:292–293. doi: 10.1038/85429. [DOI] [PubMed] [Google Scholar]
- 348.Quinton PM. Role of epithelial HCO3 transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299:C1222–1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Raeder MG. The origin of and subcellular mechanisms causing pancreatic bicarbonate secretion. Gastroenterology. 1992;103:1674–1684. [PubMed] [Google Scholar]
- 350.Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI, Erb L, Weisman GA. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem. 2010;285:7545–7555. doi: 10.1074/jbc.M109.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Rauh R, Dinudom A, Fotia AB, Paulides M, Kumar S, Korbmacher C, Cook DI. Stimulation of the epithelial sodium channel (ENaC) by the serum- and glucocorticoid-inducible kinase (Sgk) involves the PY motifs of the channel but is independent of sodium feedback inhibition. Pflugers Arch. 2006;452:290–299. doi: 10.1007/s00424-005-0026-5. [DOI] [PubMed] [Google Scholar]
- 352.Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 353.Reddy MM, Quinton PM. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature. 2003;423:756–760. doi: 10.1038/nature01694. [DOI] [PubMed] [Google Scholar]
- 354.Reyes JP, Perez-Cornejo P, Hernandez-Carballo CY, Srivastava A, Romanenko VG, Gonzalez-Begne M, Melvin JE, Arreola J. Na+ modulates anion permeation and block of P2X7 receptors from mouse parotid glands. J Membr Biol. 2008;223:73–85. doi: 10.1007/s00232-008-9115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121:3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 356.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 357.Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111. [PubMed] [Google Scholar]
- 358.RMC . Pancreatic exocrine secretion: mechanisms and control. In: Beger HG, editor. The pancreas. Oxford, UK: Blackwell Publishing; 1998. pp. 63–100. [Google Scholar]
- 359.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 360.Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem. 2006;281:27964–27972. doi: 10.1074/jbc.M603871200. [DOI] [PubMed] [Google Scholar]
- 361.Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–13001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol. 2007;581:801–817. doi: 10.1113/jphysiol.2006.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 364.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 365.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 366.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rotin D, Schild L. ENaC and its regulatory proteins as drug targets for blood pressure control. Curr Drug Targets. 2008;9:709–716. doi: 10.2174/138945008785132367. [DOI] [PubMed] [Google Scholar]
- 368.Roussa E. Channels and transporters in salivary glands. Cell Tissue Res. 2011;343:263–287. doi: 10.1007/s00441-010-1089-y. [DOI] [PubMed] [Google Scholar]
- 369.Roussa E. Channels and transporters in salivary glands. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1089-y. [DOI] [PubMed] [Google Scholar]
- 370.Roussa E, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J Histochem Cytochem. 2001;49:463–474. doi: 10.1177/002215540104900406. [DOI] [PubMed] [Google Scholar]
- 371.Roussa E, Nastainczyk W, Thevenod F. Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun. 2004;314:382–389. doi: 10.1016/j.bbrc.2003.12.099. [DOI] [PubMed] [Google Scholar]
- 372.Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2 and Na(+)-HCO(−)(3) cotransporter in rat parotid and submandibular glands. Am J Physiol. 1999;277:G1288–1296. doi: 10.1152/ajpgi.1999.277.6.G1288. [DOI] [PubMed] [Google Scholar]
- 373.Roussa E, Shmukler BE, Wilhelm S, Casula S, Stuart-Tilley AK, Thevenod F, Alper SL. Immunolocalization of potassium-chloride cotransporter polypeptides in rat exocrine glands. Histochem Cell Biol. 2002;117:335–344. doi: 10.1007/s00418-002-0393-3. [DOI] [PubMed] [Google Scholar]
- 374.Roussa E, Thevenod F, Sabolic I, Herak-Kramberger CM, Nastainczyk W, Bock R, Schulz I. Immunolocalization of vacuolar-type H+-ATPase in rat submandibular gland and adaptive changes induced by acid-base disturbances. J Histochem Cytochem. 1998;46:91–100. doi: 10.1177/002215549804600112. [DOI] [PubMed] [Google Scholar]
- 375.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 376.Rybalchenko V, Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys J. 2008;95:4439–4447. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sabbatini ME. Natriuretic peptides as regulatory mediators of secretory activity in the digestive system. Regul Pept. 2009;154:5–15. doi: 10.1016/j.regpep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 378.Salvatore F, Scudiero O, Castaldo G. Genotype-phenotype correlation in cystic fibrosis: the role of modifier genes. Am J Med Genet. 2002;111:88–95. doi: 10.1002/ajmg.10461. [DOI] [PubMed] [Google Scholar]
- 379.Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso ON, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G. Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- 380.Schaffalitzky de Muckadell OB, Fahrenkrug J, Watt-Boolsen S, Worning H. Pancreatic response and plasma secretin concentration during infusion of low dose secretin in man. Scand J Gastroenterol. 1978;13:305–311. doi: 10.3109/00365527809179825. [DOI] [PubMed] [Google Scholar]
- 381.Schneider A, Larusch J, Sun X, Aloe A, Lamb J, Hawes R, Cotton P, Brand RE, Anderson MA, Money ME, Banks PA, Lewis MD, Baillie J, Sherman S, Disario J, Burton FR, Gardner TB, Amann ST, Gelrud A, George R, Rockacy MJ, Kassabian S, Martinson J, Slivka A, Yadav D, Oruc N, Barmada MM, Frizzell R, Whitcomb DC. Combined bicarbonate conductance-impairing variants in CFTR and SPINK1 variants are associated with chronic pancreatitis in patients without cystic fibrosis. Gastroenterology. 2011;140:162–171. doi: 10.1053/j.gastro.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Schneyer LH, Young JA, Schneyer CA. Salivary secretion of electrolytes. Physiol Rev. 1972;52:720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- 383.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Oxidative stress caused by pyocyanin impairs CFTR Cl(−) transport in human bronchial epithelial cells. Free Radic Biol Med. 2008;45:1653–1662. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Sewell WA, Young JA. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975;252:379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Shcheynikov N, Kim KH, Kim KM, Dorwart MR, Ko SB, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem. 2004;279:21857–21865. doi: 10.1074/jbc.M313323200. [DOI] [PubMed] [Google Scholar]
- 388.Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sheikh SP, Holst JJ, Skak-Nielsen T, Knigge U, Warberg J, Theodorsson-Norheim E, Hokfelt T, Lundberg JM, Schwartz TW. Release of NPY in pig pancreas: dual parasympathetic and sympathetic regulation. Am J Physiol. 1988;255:G46–54. doi: 10.1152/ajpgi.1988.255.1.G46. [DOI] [PubMed] [Google Scholar]
- 391.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113 (Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 392.Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem. 2001;276:44146–44156. doi: 10.1074/jbc.M105203200. [DOI] [PubMed] [Google Scholar]
- 393.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3- cotransporter 1 (pNBC1) Proc Natl Acad Sci U S A. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 396.Shumaker H, Amlal H, Frizzell R, Ulrich CD, 2nd, Soleimani M. CFTR drives Na+-nHCO3− cotransport in pancreatic duct cells: a basis for defective HCO3− secretion in CF. Am J Physiol. 1999;276:C16–25. doi: 10.1152/ajpcell.1999.276.1.C16. [DOI] [PubMed] [Google Scholar]
- 397.Shumaker H, Soleimani M. CFTR upregulates the expression of the basolateral Na+-K+-2Cl− cotransporter in cultured pancreatic duct cells. Am J Physiol. 1999;277:C1100–1110. doi: 10.1152/ajpcell.1999.277.6.C1100. [DOI] [PubMed] [Google Scholar]
- 398.SJP Neurohumoral control of exocrine pancreatic secretion. Curr Opin Gastroenterol. 2003;19:443–446. doi: 10.1097/00001574-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 399.Smith ZD, Caplan MJ, Forbush B, 3rd, Jamieson JD. Monoclonal antibody localization of Na+-K+-ATPase in the exocrine pancreas and parotid of the dog. Am J Physiol. 1987;253:G99–109. doi: 10.1152/ajpgi.1987.253.2.G99. [DOI] [PubMed] [Google Scholar]
- 400.Sohma Y, Gray MA, Imai Y, Argent BE. HCO3− transport in a mathematical model of the pancreatic ductal epithelium. J Membr Biol. 2000;176:77–100. doi: 10.1007/s00232001077. [DOI] [PubMed] [Google Scholar]
- 401.Sohma Y, Gray MA, Imai Y, Argent BE. A mathematical model of the pancreatic ductal epithelium. J Membr Biol. 1996;154:53–67. doi: 10.1007/s002329900132. [DOI] [PubMed] [Google Scholar]
- 402.Spiegel S, Phillipper M, Rossmann H, Riederer B, Gregor M, Seidler U. Independence of apical Cl−/HCO3− exchange and anion conductance in duodenal HCO3− secretion. Am J Physiol Gastrointest Liver Physiol. 2003;285:G887–897. doi: 10.1152/ajpgi.00083.2003. [DOI] [PubMed] [Google Scholar]
- 403.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren’s syndrome patients. Lab Invest. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 404.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 405.Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl-/HCO3- exchange with CFTR in stimulated HCO3- secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1307–1317. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29:6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Stuenkel EL, Machen TE, Williams JA. pH regulatory mechanisms in rat pancreatic ductal cells. Am J Physiol. 1988;254:G925–930. doi: 10.1152/ajpgi.1988.254.6.G925. [DOI] [PubMed] [Google Scholar]
- 408.Subauste MC, Nalbant P, Adamson ED, Hahn KM. Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein β-catenin with the scaffolding protein MAGI-2. J Biol Chem. 2005;280:5676–5681. doi: 10.1074/jbc.M405561200. [DOI] [PubMed] [Google Scholar]
- 409.Sugiyama M, Kobori O, Atomi Y, Wada N, Kuroda A, Muto T. Pancreatic exocrine function during acute exacerbation in WBN/Kob rats with spontaneous chronic pancreatitis. Int J Pancreatol. 1996;20:191–196. doi: 10.1007/BF02803768. [DOI] [PubMed] [Google Scholar]
- 410.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 411.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- 412.Thaysen JH, Thorn NA, Schwartz IL. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 1954;178:155–159. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- 413.Thevenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na+ bicarbonate cotransporter. Biochem Biophys Res Commun. 1999;264:291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- 414.Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- 415.Turner JT, Landon LA, Gibbons SJ, Talamo BR. Salivary gland P2 nucleotide receptors. Crit Rev Oral Biol Med. 1999;10:210–224. doi: 10.1177/10454411990100020701. [DOI] [PubMed] [Google Scholar]
- 416.Turner JT, Park M, Camden JM, Weisman GA. Salivary gland nucleotide receptors. Changes in expression and activity related to development and tissue damage. Ann N Y Acad Sci. 1998;842:70–75. doi: 10.1111/j.1749-6632.1998.tb09633.x. [DOI] [PubMed] [Google Scholar]
- 417.Ulrich CD, 2nd, Holtmann M, Miller LJ. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology. 1998;114:382–397. doi: 10.1016/s0016-5085(98)70491-3. [DOI] [PubMed] [Google Scholar]
- 418.Ushiki T, Watanabe S. Distribution and ultrastructure of the autonomic nerves in the mouse pancreas. Microsc Res Tech. 1997;37:399–406. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<399::AID-JEMT4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 419.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 420.Veel T, Buanes T, Engeland E, Raeder MG. Colchicine inhibits the effects of secretin on pancreatic duct cell tubulovesicles and HCO3− secretion in the pig. Acta Physiol Scand. 1990;138:487–495. doi: 10.1111/j.1748-1716.1990.tb08876.x. [DOI] [PubMed] [Google Scholar]
- 421.Veel T, Buanes T, Grotmol T, Ostensen J, Raeder MG. Secretin dissipates red acridine orange fluorescence from pancreatic duct epithelium. Acta Physiol Scand. 1991;141:221–226. doi: 10.1111/j.1748-1716.1991.tb09070.x. [DOI] [PubMed] [Google Scholar]
- 422.Veel T, Villanger O, Holthe MR, Cragoe EJ, Jr, Raeder MG. Na(+)-H+ exchange is not important for pancreatic HCO3- secretion in the pig. Acta Physiol Scand. 1992;144:239–246. doi: 10.1111/j.1748-1716.1992.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 423.Venglovecz V, Hegyi P, Rakonczay Z, Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA. Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut. 2010 doi: 10.1136/gut.2010.214213. [DOI] [PubMed] [Google Scholar]
- 424.Venglovecz V, Rakonczay Z, Jr, Ozsvari B, Takacs T, Lonovics J, Varro A, Gray MA, Argent BE, Hegyi P. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut. 2008;57:1102–1112. doi: 10.1136/gut.2007.134361. [DOI] [PubMed] [Google Scholar]
- 425.Verkman AS. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:F13–28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 427.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Villanger O, Veel T, Raeder MG. Secretin causes H+/HCO3− secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphosphatase. Gastroenterology. 1995;108:850–859. doi: 10.1016/0016-5085(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 429.Waldegger S, Moschen I, Ramirez A, Smith RJ, Ayadi H, Lang F, Kubisch C. Cloning and characterization of SLC26A6, a novel member of the solute carrier 26 gene family. Genomics. 2001;72:43–50. doi: 10.1006/geno.2000.6445. [DOI] [PubMed] [Google Scholar]
- 430.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281:14129–14135. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 431.Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- 432.Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y, Lam SY, Chan LN, Zhao WC, Chung YW, Chan HC. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- 433.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: relevance to cystic fibrosis. EMBO J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G573–579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 435.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- 436.Weiss FU, Simon P, Bogdanova N, Shcheynikov N, Muallem S, Lerch MM. Functional characterisation of the CFTR mutations M348V and A1087P from patients with pancreatitis suggests functional interaction between CFTR monomers. Gut. 2009;58:733–734. doi: 10.1136/gut.2008.167650. [DOI] [PubMed] [Google Scholar]
- 437.Whitcomb DC, Ermentrout GB. A mathematical model of the pancreatic duct cell generating high bicarbonate concentrations in pancreatic juice. Pancreas. 2004;29:e30–40. doi: 10.1097/00006676-200408000-00016. [DOI] [PubMed] [Google Scholar]
- 438.Williams JA. Receptor-mediated signal transduction pathways and the regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2008;24:573–579. doi: 10.1097/MOG.0b013e32830b110c. [DOI] [PubMed] [Google Scholar]
- 439.Williams JA, Groblewski GE, Ohnishi H, Yule DI. Stimulus-secretion coupling of pancreatic digestive enzyme secretion. Digestion. 1997;58 (Suppl 1):42–45. doi: 10.1159/000201524. [DOI] [PubMed] [Google Scholar]
- 440.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 441.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56:1153–1163. doi: 10.1136/gut.2004.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 443.Winpenny JP, Harris A, Hollingsworth MA, Argent BE, Gray MA. Calcium-activated chloride conductance in a pancreatic adenocarcinoma cell line of ductal origin (HPAF) and in freshly isolated human pancreatic duct cells. Pflugers Arch. 1998;435:796–803. doi: 10.1007/s004240050586. [DOI] [PubMed] [Google Scholar]
- 444.Winpenny JP, Verdon B, McAlroy HL, Colledge WH, Ratcliff R, Evans MJ, Gray MA, Argent BE. Calcium-activated chloride conductance is not increased in pancreatic duct cells of CF mice. Pflugers Arch. 1995;430:26–33. doi: 10.1007/BF00373836. [DOI] [PubMed] [Google Scholar]
- 445.Wizemann V, Schulz I. Influence of amphotericin, amiloride, ionophores, and 2,4-dinitrophenol on the secretion of the isolated cat’s pancreas. Pflugers Arch. 1973;339:317–338. doi: 10.1007/BF00594167. [DOI] [PubMed] [Google Scholar]
- 446.Wojcikiewicz RJ, Ernst SA, Yule DI. Secretagogues cause ubiquitination and down-regulation of inositol 1, 4,5-trisphosphate receptors in rat pancreatic acinar cells. Gastroenterology. 1999;116:1194–1201. doi: 10.1016/s0016-5085(99)70023-5. [DOI] [PubMed] [Google Scholar]
- 447.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wright AM, Gong X, Verdon B, Linsdell P, Mehta A, Riordan JR, Argent BE, Gray MA. Novel regulation of cystic fibrosis transmembrane conductance regulator (CFTR) channel gating by external chloride. J Biol Chem. 2004;279:41658–41663. doi: 10.1074/jbc.M405517200. [DOI] [PubMed] [Google Scholar]
- 449.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- 450.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 451.Xu X, Diaz J, Zhao H, Muallem S. Characterization, localization and axial distribution of Ca2+ signalling receptors in the rat submandibular salivary gland ducts. J Physiol. 1996;491 (Pt 3):647–662. doi: 10.1113/jphysiol.1996.sp021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yamamoto A, Ishiguro H, Ko SB, Suzuki A, Wang Y, Hamada H, Mizuno N, Kitagawa M, Hayakawa T, Naruse S. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol. 2003;551:917–926. doi: 10.1113/jphysiol.2003.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Yamamoto M, Reeve JR, Jr, Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol. 2005;288:G866–879. doi: 10.1152/ajpgi.00389.2003. [DOI] [PubMed] [Google Scholar]
- 454.Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Protons activate the delta-subunit of the epithelial Na+ channel in humans. J Biol Chem. 2004;279:12529–12534. doi: 10.1074/jbc.M400274200. [DOI] [PubMed] [Google Scholar]
- 455.Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Commun. 2007;353:535–540. doi: 10.1016/j.bbrc.2006.11.151. [DOI] [PubMed] [Google Scholar]
- 456.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011 doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Yang D, Shcheynikov N, Muallem S. IRBIT: It Is Everywhere. Neurochem Res. 2010 doi: 10.1007/s11064-010-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 460.You CH, Rominger JM, Chey WY. Potentiation effect of cholecystokinin-octapeptide on pancreatic bicarbonate secretion stimulated by a physiologic dose of secretin in humans. Gastroenterology. 1983;85:40–45. [PubMed] [Google Scholar]
- 461.Young JA, Cook DI, Evans LA, Pirani D. Effects of ion transport inhibition on rat mandibular gland secretion. J Dent Res. 1987;66:531–536. doi: 10.1177/00220345870660022401. [DOI] [PubMed] [Google Scholar]
- 462.Young JA, Martin CJ, Asz M, Weber FD. A microperfusion investigation of bicarbonate secretion by the rat submaxillary gland. The action of a parasumpathomimetic drug on electrolyte transport. Pflugers Arch. 1970;319:185–199. doi: 10.1007/BF00586449. [DOI] [PubMed] [Google Scholar]
- 463.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Yue G, Malik B, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 465.Zeng W, Lee MG, Muallem S. Membrane-specific regulation of Cl- channels by purinergic receptors in rat submandibular gland acinar and duct cells. J Biol Chem. 1997;272:32956–32965. doi: 10.1074/jbc.272.52.32956. [DOI] [PubMed] [Google Scholar]
- 466.Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997;273:C442–455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- 467.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Zhao H, Muallem S. Agonist-specific regulation of [Na+]i in pancreatic acinar cells. J Gen Physiol. 1995;106:1243–1263. doi: 10.1085/jgp.106.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Zhao H, Muallem S. Na+, K+, and Cl− transport in resting pancreatic acinar cells. J Gen Physiol. 1995;106:1225–1242. doi: 10.1085/jgp.106.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Zhao H, Xu X, Diaz J, Muallem S. Na+, K+, and H+/HCO3- transport in submandibular salivary ducts. Membrane localization of transporters. J Biol Chem. 1995;270:19599–19605. [PubMed] [Google Scholar]
- 473.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 474.Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+-dependent exocytosis in SERCA2(+/−) mice. EMBO J. 2001;20:2680–2689. doi: 10.1093/emboj/20.11.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]
- 476.Zsembery A, Strazzabosco M, Graf J. Ca2+-activated Cl− channels can substitute for CFTR in stimulation of pancreatic duct bicarbonate secretion. FASEB J. 2000;14:2345–2356. doi: 10.1096/fj.99-0509com. [DOI] [PubMed] [Google Scholar]