Abstract
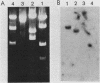
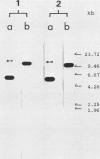
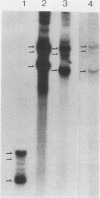
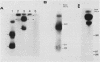
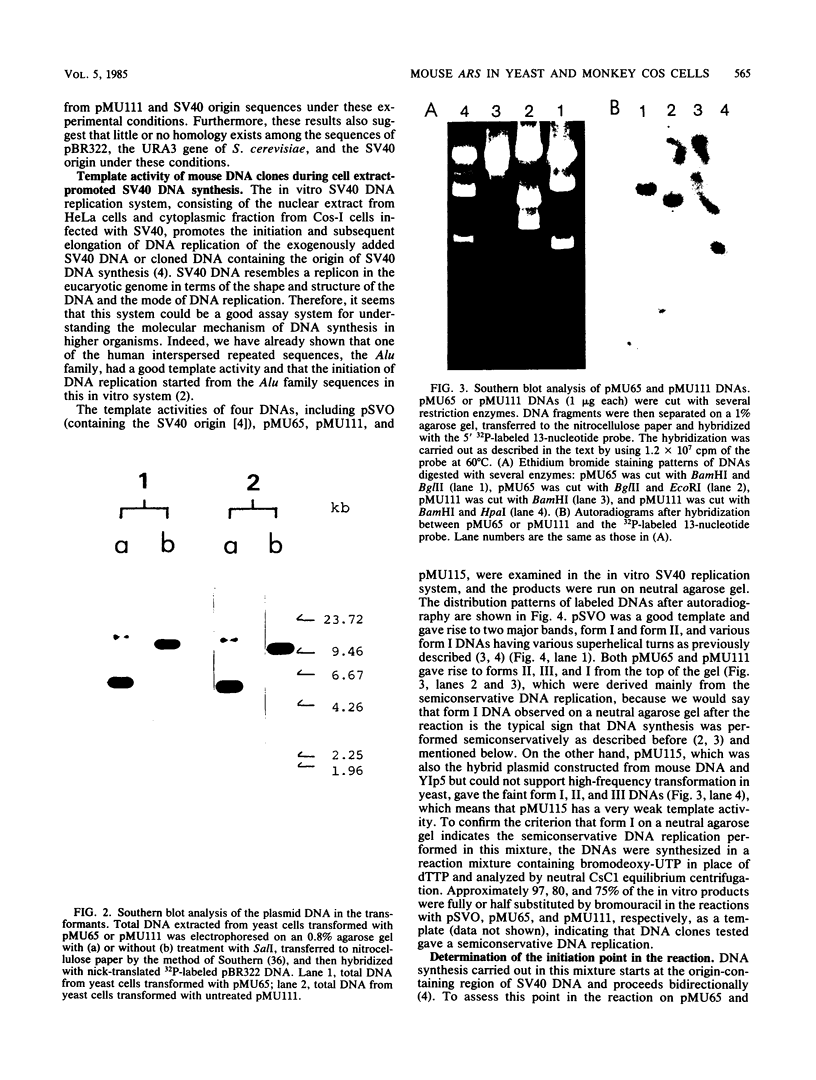
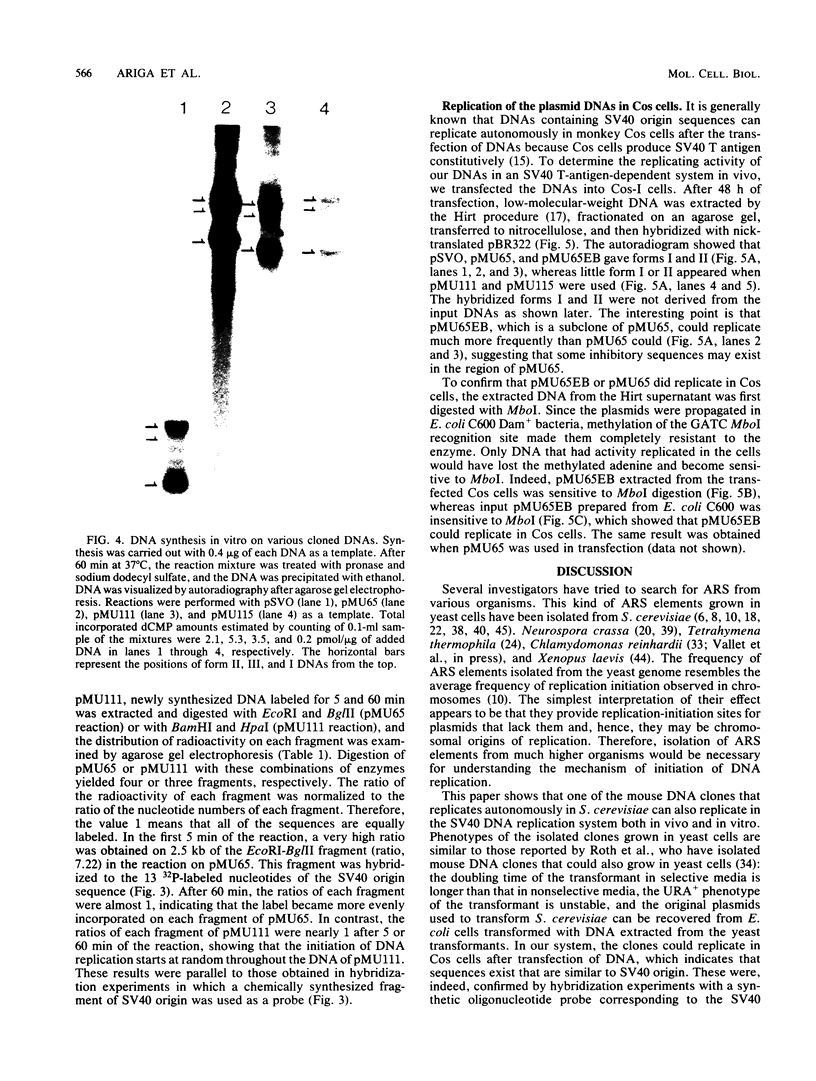
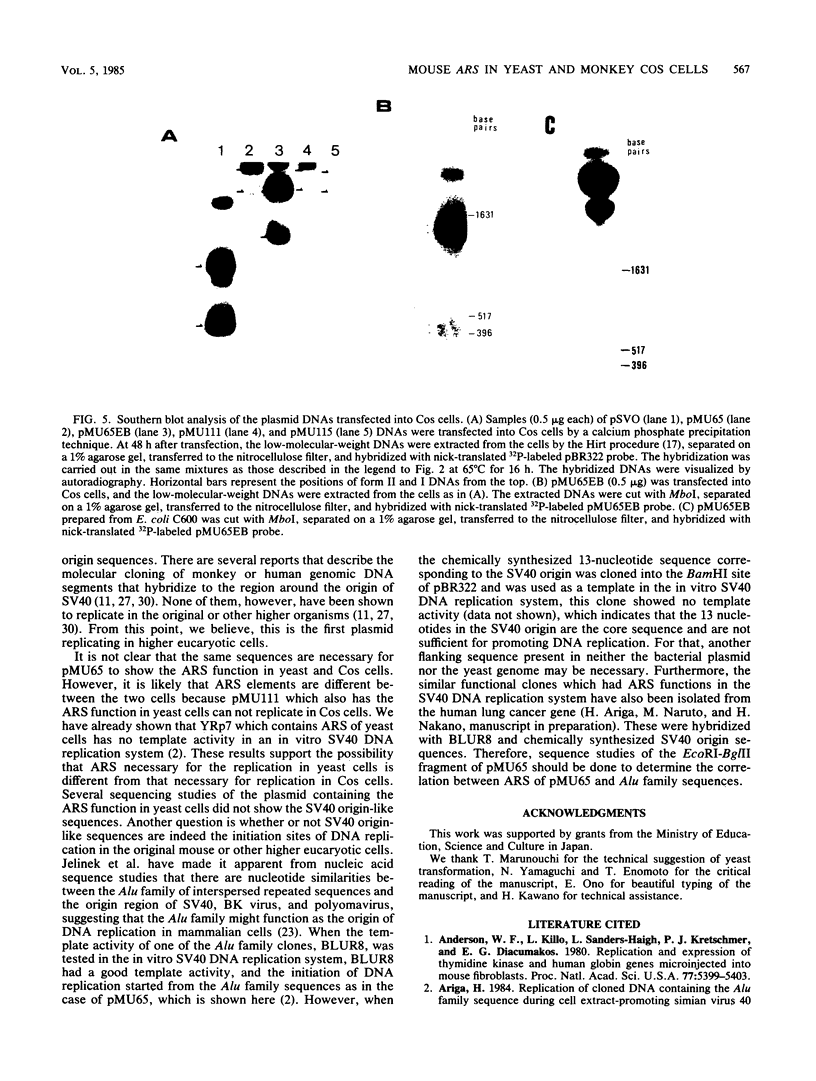
Mouse liver DNA was cut out with BamHI and cloned into YIp5, which contained the URA3 gene of Saccharomyces cerevisiae in pBR322. Of the several plasmids isolated, two plasmids, pMU65 and pMU111, could transform S. cerevisiae from the URA- to the URA+ phenotype and could replicate autonomously within the transformant, indicating that mouse DNA fragments present in pMU65 or pMU111 contain autonomously replicating sequences (ARS) for replication in S. cerevisiae. Furthermore, to determine the correlation between ARS function in yeast cells and that in much higher organisms, we tried to challenge these plasmids with the simian virus 40 (SV40) DNA replication system. Of the two plasmids tested, the EcoRI-BglII region of pMU65 could be hybridized with a chemically synthesized 13-nucleotide fragment corresponding to the origin region of SV40 DNA. Both pMU65 (the EcoRI-BglII region cloned in pBR322) and its subclone pMU65EB could replicate semiconservatively, and initiation of DNA replication started from the EcoRI-BglII region when the replicating activity of these plasmids was tested in the in vitro SV40 DNA replication system we have established before. Furthermore, pMU65 and pMU65EB could replicate autonomously within monkey Cos cells which produce SV40 T antigen constitutively. These results show that a 2.5-kilobase fragment of the EcoRI-BglII region in pMU65 contains the ARS needed for replication in the SV40 DNA replication system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Killos L., Sanders-Haigh L., Kretschmer P. J., Diacumakos E. G. Replication and expression of thymidine kinase and human globin genes microinjected into mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5399–5403. doi: 10.1073/pnas.77.9.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H. Identification of the replicative intermediates in SV40 DNA replication in vitro. Nucleic Acids Res. 1984 Aug 10;12(15):6053–6062. doi: 10.1093/nar/12.15.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H., Sugano S. Initiation of simian virus 40 DNA replication in vitro. J Virol. 1983 Nov;48(2):481–491. doi: 10.1128/jvi.48.2.481-491.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Electron microscopic analysis of replicating DNA of sea urchin embryos. Cell. 1978 Nov;15(3):1095–1107. doi: 10.1016/0092-8674(78)90293-3. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Hicks J. B. Replication and recombination functions associated with the yeast plasmid, 2 mu circle. Cell. 1980 Sep;21(2):501–508. doi: 10.1016/0092-8674(80)90487-0. [DOI] [PubMed] [Google Scholar]
- Cairns J. Autoradiography of HeLa cell DNA. J Mol Biol. 1966 Jan;15(1):372–373. doi: 10.1016/s0022-2836(66)80233-4. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6329–6333. doi: 10.1073/pnas.77.11.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Botchan M. R. Isolation and characterization of human DNA fragments with nucleotide sequence homologies with the simian virus 40 regulatory region. Mol Cell Biol. 1982 Aug;2(8):949–965. doi: 10.1128/mcb.2.8.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Hughes K., Case M. E., Geever R., Vapnek D., Giles N. H. Chimeric plasmid that replicates autonomously in both Escherichia coli and Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1053–1057. doi: 10.1073/pnas.80.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner K. M., Scangos G. A., Ruddle F. H. DNA-mediated gene transfer of a circular plasmid into murine cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5820–5824. doi: 10.1073/pnas.76.11.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. C., Cramer J. H., Rownd R. H. Properties of a Saccharomyces cerevisiae mtDNA segment conferring high-frequency yeast transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1578–1582. doi: 10.1073/pnas.79.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G. B., Amin A. A., Pearlman R. E. Two separate regions of the extrachromosomal ribosomal deoxyribonucleic acid of Tetrahymena thermophila enable autonomous replication of plasmids in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):535–543. doi: 10.1128/mcb.1.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Harland R. M. Replication origins in the eucaryotic chromosome. Cell. 1981 May;24(2):283–284. doi: 10.1016/0092-8674(81)90316-0. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Singer M. F. DNA sequences similar to those around the simian virus 40 origin of replication are present in the monkey genome. Proc Natl Acad Sci U S A. 1981 Jan;78(1):95–99. doi: 10.1073/pnas.78.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Lord S. T., McCutchan T. F., Singer M. F. Three segments from the monkey genome that hybridize to simian virus 40 have common structural elements. Mol Cell Biol. 1981 Dec;1(12):1061–1068. doi: 10.1128/mcb.1.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J., Rahire M. Construction and characterization of autonomously replicating plasmids in the green unicellular alga Chlamydomonas reinhardii. Cell. 1984 Apr;36(4):925–931. doi: 10.1016/0092-8674(84)90042-4. [DOI] [PubMed] [Google Scholar]
- Roth G. E., Blanton H. M., Hager L. J., Zakian V. A. Isolation and characterization of sequences from mouse chromosomal DNA with ARS function in yeasts. Mol Cell Biol. 1983 Nov;3(11):1898–1908. doi: 10.1128/mcb.3.11.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl U., Tudzynski P., Kück U., Esser K. Replication and expression of a bacterial--mitochondrial hybrid plasmid in the fungus Podospora anserina. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3641–3645. doi: 10.1073/pnas.79.11.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Taylor J. H. Cloning of an origin of DNA replication of Xenopus laevis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5292–5296. doi: 10.1073/pnas.77.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Electron microscopic analysis of DNA replication in main band and satellite DNAs of Drosophila virilis. J Mol Biol. 1976 Dec;108(2):305–331. doi: 10.1016/s0022-2836(76)80123-4. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Origin of replication from Xenopus laevis mitochondrial DNA promotes high-frequency transformation of yeast. Proc Natl Acad Sci U S A. 1981 May;78(5):3128–3132. doi: 10.1073/pnas.78.5.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A., Scott J. F. Construction, replication, and chromatin structure of TRP1 RI circle, a multiple-copy synthetic plasmid derived from Saccharomyces cerevisiae chromosomal DNA. Mol Cell Biol. 1982 Mar;2(3):221–232. doi: 10.1128/mcb.2.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]