Abstract
Transcripts of the CAD gene in Syrian hamster cells are as abundant in the nucleus as in the cytoplasm. This was shown by in situ hybridization of whole cells and by solution and blot hybridization of subcellular fractions. Similar results were obtained both for wild-type cells and for a mutant containing amplified CAD genes in which the level of CAD RNA is 150-fold greater. CAD nuclear RNA is indistinguishable from mature mRNA by gel electrophoresis and blot hybridization. Discrete higher-molecular-weight precursors are undetectable, although the persistence of a short length of intervening sequence in the otherwise fully processed RNA is not excluded. CAD RNA is released from nuclei by sonication in physiological conditions in a ribonucleoprotein form that sediments as a broad peak at about 200S in a sucrose gradient. CAD sequences extracted from nuclei by treatment with EDTA and RNase are found in the 30S particles previously described.
Full text
PDF

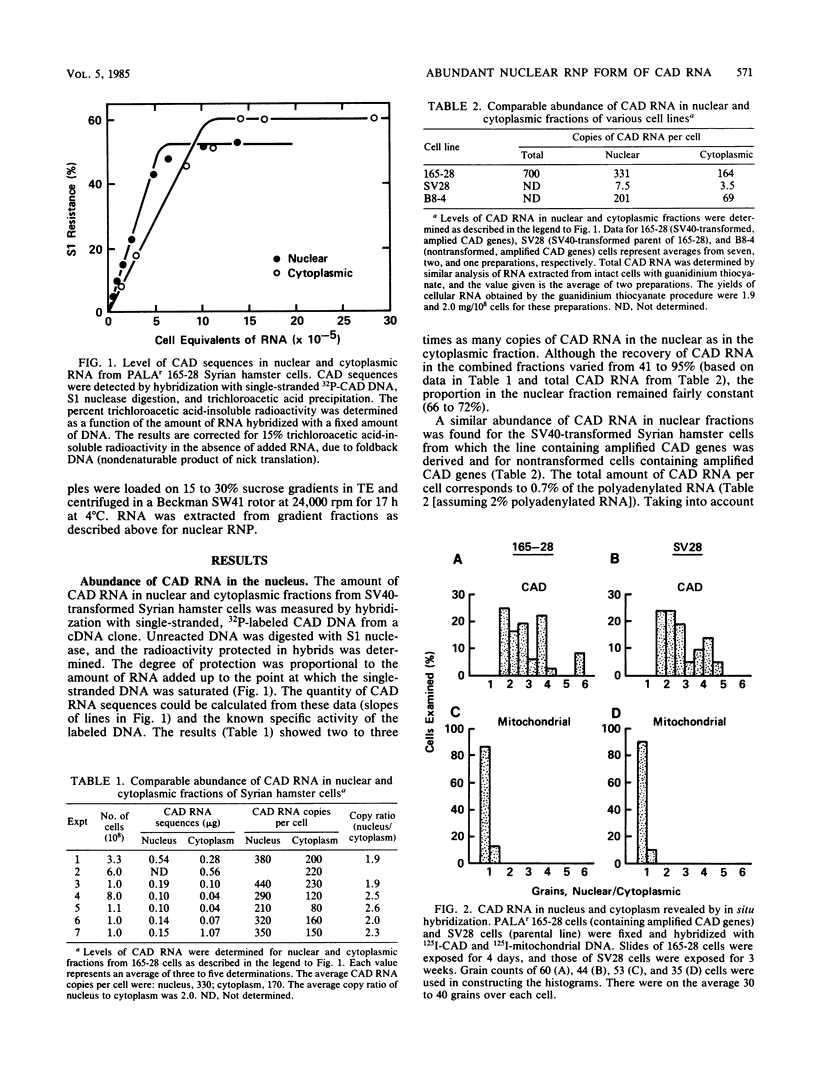

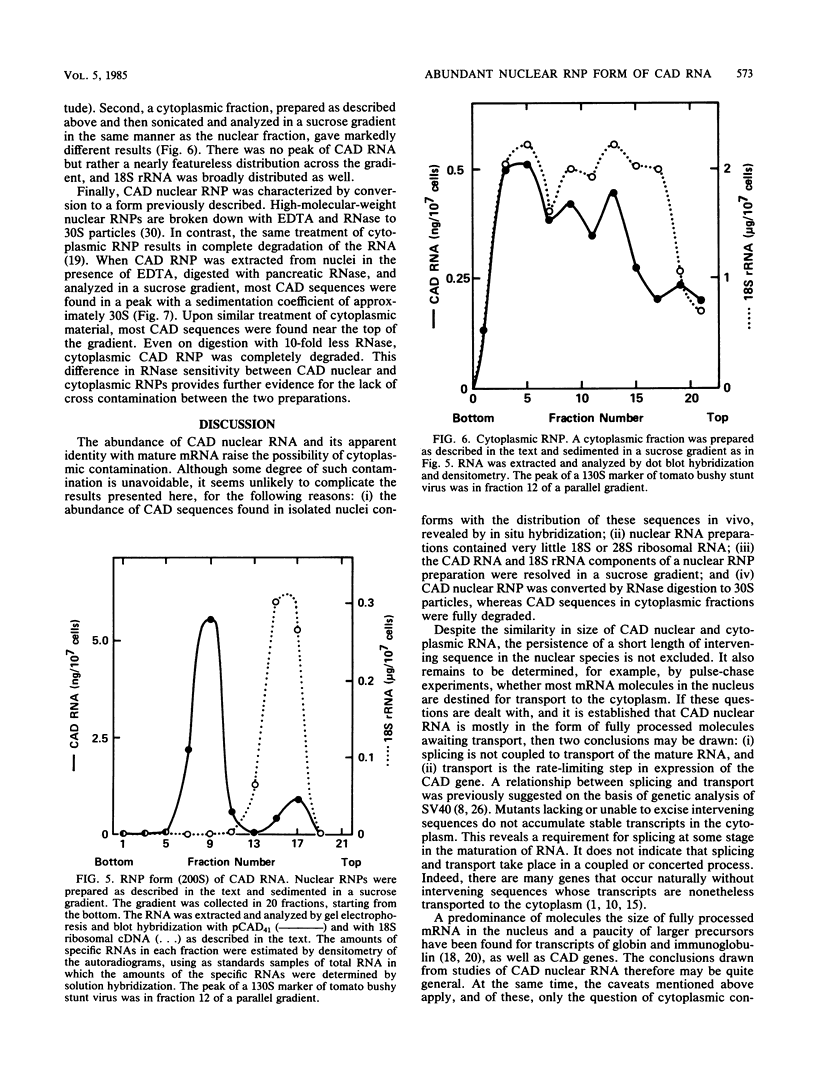
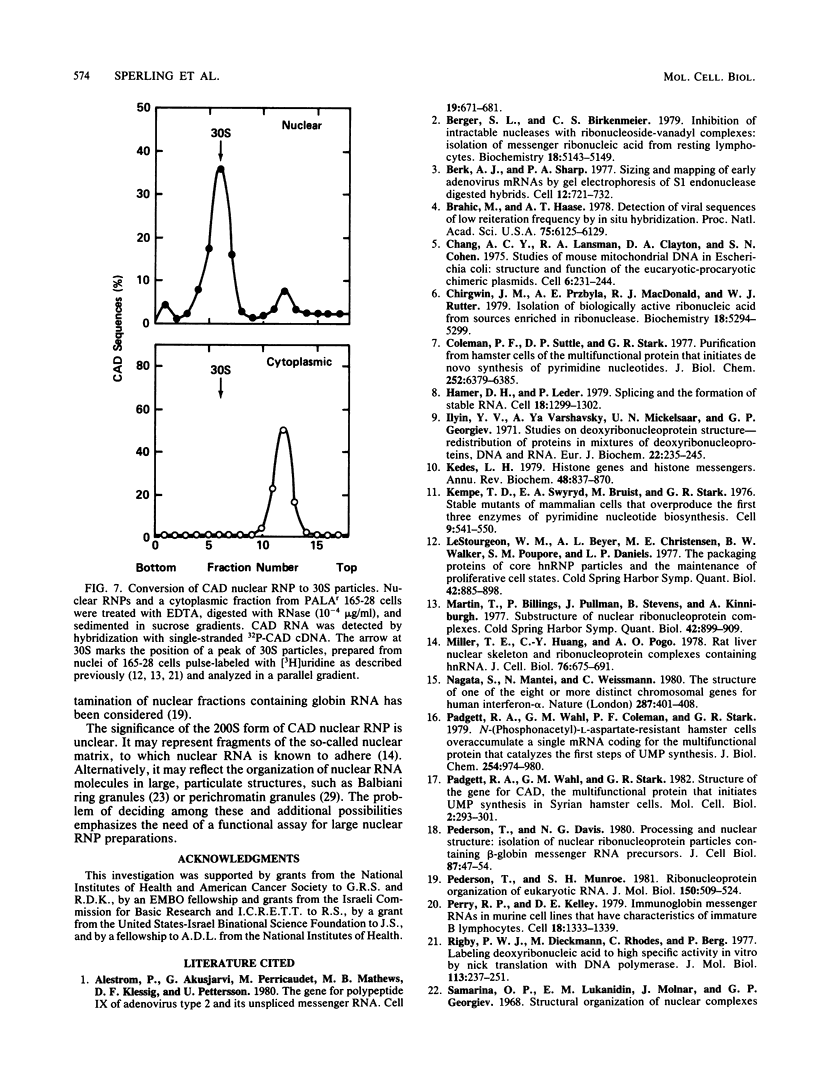

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Lansman R. A., Clayton D. A., Cohen S. N. Studies of mouse mitochondrial DNA in Escherichia coli: structure and function of the eucaryotic-procaryotic chimeric plasmids. Cell. 1975 Oct;6(2):231–244. doi: 10.1016/0092-8674(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Varshavsky A. Y., Mickelsaar U. N., Georgiev G. P. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971 Sep 24;22(2):235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Kedes L. H. Histone genes and histone messengers. Annu Rev Biochem. 1979;48:837–870. doi: 10.1146/annurev.bi.48.070179.004201. [DOI] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- LeStourgeon W. M., Beyer A. L., Christensen M. E., Walker B. W., Poupore S. M., Daniels L. P. The packaging proteins of core hnRNP particles and the maintenance of proliferative cell states. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):885–898. doi: 10.1101/sqb.1978.042.01.090. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Pullman J., Stevens B., Kinniburgh A. Substructure of nuclear ribonucleoprotein complexes. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):899–909. doi: 10.1101/sqb.1978.042.01.091. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Structure of the gene for CAD, the multifunctional protein that initiates UMP synthesis in Syrian hamster cells. Mol Cell Biol. 1982 Mar;2(3):293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Davis N. G. Messenger RNA processing and nuclear structure: isolation of nuclear ribonucleoprotein particles containing beta-globin messenger RNA precursors. J Cell Biol. 1980 Oct;87(1):47–54. doi: 10.1083/jcb.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Munroe S. H. Ribonucleoprotein organization of eukaryotic RNA. XV. Different nucleoprotein structures of globin messenger RNA sequences in nuclear and polyribosomal ribonucleoprotein particles. J Mol Biol. 1981 Aug 25;150(4):509–524. doi: 10.1016/0022-2836(81)90377-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Skoglund U., Andersson K., Björkroth B., Lamb M. M., Daneholt B. Visualization of the formation and transport of a specific hnRNP particle. Cell. 1983 Oct;34(3):847–855. doi: 10.1016/0092-8674(83)90542-1. [DOI] [PubMed] [Google Scholar]
- Swyryd E. A., Seaver S. S., Stark G. R. N-(phosphonacetyl)-L-aspartate, a potent transition state analog inhibitor of aspartate transcarbamylase, blocks proliferation of mammalian cells in culture. J Biol Chem. 1974 Nov 10;249(21):6945–6950. [PubMed] [Google Scholar]
- Thomas P. S., Farquhar M. N. Specific measurement of DNA in nuclei and nucleic acids using diaminobenzoic acid. Anal Biochem. 1978 Aug 15;89(1):35–44. doi: 10.1016/0003-2697(78)90724-8. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Feunteun J., Crawford L. V., Berg P., Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979 Jun;30(3):674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. Observations on a granule associated with chromatin in the nuclei of cells of rat and mouse. J Cell Biol. 1962 Apr;13:162–167. doi: 10.1083/jcb.13.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]