Background: The role of caspase 1 in non-immune cells that do not express IL1β/IL18, like hepatocytes, is largely unknown.
Results: Caspase 1 activation limits excessive mitochondrial reactive oxygen species during oxidative stress by up-regulating beclin1 and mitochondrial clearance in hepatocytes.
Conclusion: Caspase 1 is hepatoprotective after oxidative stress.
Significance: Our findings suggest a novel role for caspase 1 activation in promoting adaptive responses to oxidative stress.
Keywords: Autophagy, Cell Death, Liver Injury, Mitochondria, Reactive Oxygen Species (ROS), Hemorrhagic Shock, Mitochondrial Autophagy
Abstract
Caspase 1 activation can be induced by oxidative stress, which leads to the release of the proinflammatory cytokines IL1β and IL18 in myeloid cells and a potentially damaging inflammatory response. However, little is known about the role of caspase 1 in non-immune cells, such as hepatocytes, that express and activate the inflammasome but do not produce a significant amount of IL1β/IL18. Here we demonstrate that caspase 1 activation protects against cell death after redox stress induced by hypoxia/reoxygenation in hepatocytes. Mechanistically, we show that caspase 1 reduces mitochondrial respiration and reactive oxygen species by increasing mitochondrial autophagy and subsequent clearance of mitochondria in hepatocytes after hypoxia/reoxygenation. Caspase 1 increases autophagic flux through up-regulating autophagy initiator beclin 1 during redox stress and is an important cell survival factor in hepatocytes. We find that during hemorrhagic shock with resuscitation, an in vivo mouse model associated with severe hepatic redox stress, caspase 1 activation is also protective against liver injury and excessive oxidative stress through the up-regulation of beclin 1. Our findings suggest an alternative role for caspase 1 activation in promoting adaptive responses to oxidative stress and, more specifically, in limiting reactive oxygen species production and damage in cells and tissues where IL1β/IL18 are not highly expressed.
Introduction
Emerging evidence suggests that caspase 1 activation can be induced by oxidative stress or the release of damage-associated molecular patterns after increased redox stress (1, 2). Caspase 1 mediates the maturation of the proinflammatory cytokines IL1β/IL18 in immune cells and endothelial cells after oxidative stress, which is known to contribute to renal and myocardial ischemia/reperfusion injury (3, 4). However, our previous work demonstrated a protective effect of caspase 1 against liver injury during hemorrhagic shock with bilateral femur fracture independently of IL-1β and IL-18 (5). Moreover, recent research also implicates caspase 1 as a regulator of cellular responses to stress through the regulation of tissue repair and cytoprotective responses (6, 7). This aspect of caspase 1 function is poorly characterized but may be especially important in non-immune cells such as hepatocytes, which express and can activate inflammasome components (8) but are not known to produce a significant amount of IL1β/IL18.
On a subcellular level, mitochondria are organelles central to the regulation of cell death and metabolic adaptation. Mitochondrial respiration is suppressed during hypoxia because of low oxygen tension. The reestablishment of mitochondrial aerobic respiration after reoxygenation results in excessive ROS2 production, which can trigger the release of proapoptotic proteins such as cytochrome c and can lead to cell death (9, 10). Autophagy, be it general or mitochondrial, is responsible for mitochondrial turnover and quality control (11) and has been shown to eliminate dysfunctional mitochondria to reduce mitochondrial ROS production and, therefore, to down-regulate inflammasome and caspase 1 activation (1, 2). However, little is known about the effect of caspase 1 on autophagy or mitochondrial function.
Here we investigate the role of caspase 1 in preventing hepatocyte cell death in the setting of hypoxia/reoxygenation, an in vitro model of HS/R. Mechanistically, we show that caspase 1-mediated protection in mouse hepatocytes after hypoxia/reoxygenation is associated with mitochondrial clearance and reduced mitochondrial ROS production. We also demonstrate a novel role for caspase 1 in the up-regulation of beclin 1 and the initiation of autophagy during hypoxia/reoxygenation, which results in decreased mitochondrial ROS production and improved cytoprotection in mouse hepatocytes. Finally, overexpression of beclin 1 in mouse liver prevents the enhanced liver injury seen in caspase 1−/− mice in the setting of HS/R. Collectively, our study provides an important advance in our understanding of how caspase 1 activation is linked with mitochondrial function and stress-induced autophagy as an adaptive response.
EXPERIMENTAL PROCEDURES
Reagents
Caspase 1 inhibitor (Ac-YVAD-CMK) was from Millipore. Antibodies for Western blot analysis were as follows: rabbit anti-caspase 1 was from Millipore; mouse anti-GAPDH was from Abcam; rabbit anti-beclin 1 was from Abcam and Cell Signaling Technology, Inc.; rabbit anti-cleaved caspase 3, cleaved poly(ADP-ribose) polymerase (PARP), caspase 3, PARP, Atg3, Atg12-Atg5 conjugate, and Atg7 were from Cell Signaling Technology, Inc.; and mouse anti-cytochrome c was from BD Biosciences. For Western blot analysis, radioimmune precipitation assay buffer (Sigma) was used for tissue lysis, and cell lysis buffer (Cell Signaling Technology, Inc.) was used for whole cell lysis together with protease inhibitors. Western gel images were quantified by densitometry using Image J software (National Institutes of Health). Manganese(III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP) was from Enzo Life Sciences. Caspase 1 activity was determined using a caspase 1 activity colorimetric kit (R&D Systems). Caspase 3 activity was determined using a caspase 3 activity fluorometric kit (R&D Systems).
Hepatocyte Isolation and Cell Culture
Hepatocytes were isolated from mice by an in situ collagenase (type VI, Worthington) perfusion technique, modified as described previously (12). Hepatocyte purity exceeded 99% by flow cytometric assay, and viability was typically over 95% by trypan blue exclusion. Hepatocytes (4 × 105 cells/ml for 6-well plates) were plated on gelatin-coated culture plates in Williams medium E with 10% calf serum, 15 mm HEPES, 10−6 m insulin, 2 mm l-glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin. Hepatocytes were allowed to attach to plates for at least 2 h before treatment. Hypoxia/reoxygenation treatment was performed as described previously (13).
Analysis of Cell Death
Hepatocytes (4 × 105 cells/ml for 6-well plates) were cultured under hypoxia (1% oxygen) and then reoxygenation for 1 h or kept for the same duration under normoxic condition with or without caspase 1 inhibitor pretreatment for 1 h (15 μm, Calbiochem). Cell death was measured using the annexin V-FITC apoptosis detection kit (BD Biosciences) according to the instructions of the manufacturer. Briefly, after treatment, cells were collected, washed with PBS, and stained with annexin V-FITC and propidium iodide for 15 min in 1× binding buffer (10 mm HEPES (pH 7.4), 140 mm NaCl, 2.5 mm CaCl2) and analyzed by flow cytometry using a Guava EasyCyte 8HT flow cytometer (Millipore).
DCF Fluorescence
Intracellular ROS generation was assessed with CM-H2DCFDA (10 μm). Microscopy or flow cytometry was performed. Images were acquired with a Zeiss 510 inverted confocal microscope. DCF fluorescence was measured in 15–20 randomly selected fields per group, fluorescence intensity was measured using Metamorph, and values are expressed with respect to the WT control cultured under normoxia. To normalize cell number, Hoechst (Invitrogen) was used as a fluorescent marker for the nucleus, and propidium iodide was used to exclude the dead cells. The experiments were repeated at least three times per treatment. Flow cytometry was performed using a Guava EasyCyte 8HT flow cytometer.
Measurement of Mitochondrial and Cytosolic ROS Production in Hepatocytes
To assess mitochondria- and cytosol-specific ROS production at the single cell level, hepatocytes were transfected with mitochondrially targeted HyPer-Mito or cytoplasm-targeted HyPer-Cyto (Evrogen), which is a genetically encoded fluorescent sensor capable of highly specific detection of mitochondrial or cytosolic H2O2 in live cells. At 36 h after transfection, hepatocytes were subjected to hypoxia (1% O2) for 6 h and reoxygenation or kept under normoxia for the same duration. The green fluorescent signal was observed by fluorescent microscopy in 30 random cells/treatment using an EVOS fluorescence microscope (AMG). Fluorescence intensity was assessed by Image J software and expressed as a fold increase to the WT control. The experiments were repeated at least three times/treatment.
Mitochondrial Volume and Mitochondrial DNA Copy Number
Mitochondrial volume was determined by MitoTracker Red staining (Invitrogen) and divided by cell volume marked by calcein (BD Biosciences), as described previously, with modifications (14). Hepatocytes were loaded with 1 μm of calcein and 100 μm of MitoTracker Red for 30 min before being imaged in a Zeiss LSM 510 laser-scanning confocal microscope using a ×63 oil lens. Z-stacks of individual hepatocyte were acquired at 5-μm intervals. The mitochondrial volume of a random portion of cytoplasm was determined as a fraction of cytoplasm using the three-dimensional Object Counter macro of ImageJ (Fabrice Cordelires and Jonathan Jackson).
The mitochondrial DNA copy number was measured by quantitative PCR as described previously (15). Primers (Integrated DNA Technologies) were as follows: 5′-CCC AGC TAC TAC CAT CAT TCA AGT-3′ (forward) and 5′-GAT GGT TTG GGA GAT TGG TTG ATG-3′ (reverse). The primers used are against part of the mitochondrially encoded NADH dehydrogenase subunit 6 (mt-Nd6), and the results shown are normalized to nuclear DNA copy number.
Measurement of Autophagic Flux
Autophagic flux was assessed by increase in GFP-LC3 puncta or Western blot analysis of LC3 in hepatocytes after treatment with bafilomycin (50 nm, Sigma) for 1 h or transfecting hepatocytes with the GFP-RFP-LC3 plasmid. 24 h after transfection, cells were imaged with a Zeiss LSM 510 laser-scanning confocal microscope using a ×63 oil lens. The numbers of GFP and RFP double-positive (early autophagic vacuoles) and RFP-only (late autophagic vacuoles) puncta were counted for each cell.
Citrate Synthase Activity Assay
Citrate synthase activity was measured in the liver using a citrate synthase assay kit (Sigma). Briefly, livers were collected from control mice or mice that underwent HS/R and homogenized immediately in radioimmune precipitation assay buffer (Sigma) with 50 μg of liver protein loaded per well of a 96-well plate and citrate synthase activity measured according to the instructions of the manufacturer.
Animals and Hemorrhagic Shock
Male C57BL/6 (WT) mice were purchased from The Jackson Laboratory. Caspase 1−/− mice were a gift from Dr. Richard Flavell (Howard Hughes Medical Institute, Yale University) and were bred in our facility. Mice aged 8–12 weeks and weighing 21–30 g were used in experiments. All experimental protocols were approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh. Experimental procedures were carried out in accordance with all regulations regarding the care and use of experimental animals, as published by the National Institutes of Health. Hemorrhagic shock surgery was performed as described previously (16). Briefly, hemorrhage was induced to a mean arterial pressure of 25 mmHg for 1.5 h, followed by fluid resuscitation with Ringer's solution (3× the volume of shed blood) through the catheter. Mice were sacrificed 1.5, 4.5, or 24 h after resuscitation. Control mice were sacrificed without any procedures performed to obtain physiological base line levels.
Immunofluorescence and Confocal Microscopy
Liver tissue from mice was removed after perfusion with cold PBS and 2% paraformaldehyde. Tissue was fixed in 2% paraformaldehyde for 2 h followed by cryopreservation. The level of lipid peroxidation was determined by HNE staining using HNE antibody (1:200, Calbiochem). Liver sections (6 μm) were permeabilized with 0.3% Triton X-100 for 20 min and stained as described previously (17). Images were taken from six random fields/section with a Fluoview 500 confocal microscope (Olympus) at the Center for Biologic Imaging. Imaging conditions were maintained at identical settings with original gating performed using the negative control (no primary antibody). The relative HNE adducts/cell were quantitated using a Metamorph image acquisition system (Universal Imaging) and normalized to the fluorescence intensity of β-actin.
Measurement of Cytochrome c Release
Cytosolic fractions of liver tissue from WT and caspase 1−/− mice were prepared as described previously (18). Briefly, livers from control mice or mice subjected to HS/R were excised, homogenized, and centrifuged to pellet the mitochondria. The supernatant was collected, and protein concentration was determined by BCA assay (Thermo Scientific).
Mouse Caspase 1 Plasmid
To generate the mouse caspase 1 plasmid, mouse caspase 1 was cloned by PCR from cDNA prepared by reverse transcription of normal mouse hepatocyte mRNA. The primers (Integrated DNA Technologies) used for PCR were as follows: 5′-CAT GGC TGA CAA GAT CCT GAG GGC-3′ (forward) and 5′-GTT TAA TGT CCC GGG AAG AGG TAG-3′ (reverse). The amplimer was subcloned into pAdlox.
Mouse Beclin 1 Adenoviral Vectors
To generate adenoviral vectors expressing mouse beclin 1, the open reading frames of murine beclin 1 were inserted into a shuttle plasmid, pAdlox, at the HindIII and BamHI sites, and the sequences were confirmed. The E1/E3-deleted adenoviral vector was then constructed using the Cre-lox recombination system in the adenovirus-packaging cell line CRE8. The recombinant adenoviruses were propagated in 293 cells and purified by cesium chloride density gradient centrifugation and dialysis. Adenovirus particle concentration was determined by spectrophotometric analysis. Ad-beclin 1 or ad-GFP was injected through the tail vein at 4 × 1010 viral particles for each mouse. Two days after injection, mice were subjected to hemorrhagic shock and 4.5 h of resuscitation.
Statistical Analysis
Results are displayed as mean ± S.D. or mean ± S.E. from at least three independent experiments. Two-tailed Student's t test was used to calculate the statistical significance of two experimental groups. p values of less than 0.05 were considered significant.
RESULTS
Caspase 1 Activation Is Protective in Hepatocytes after Hypoxia/Reoxygenation
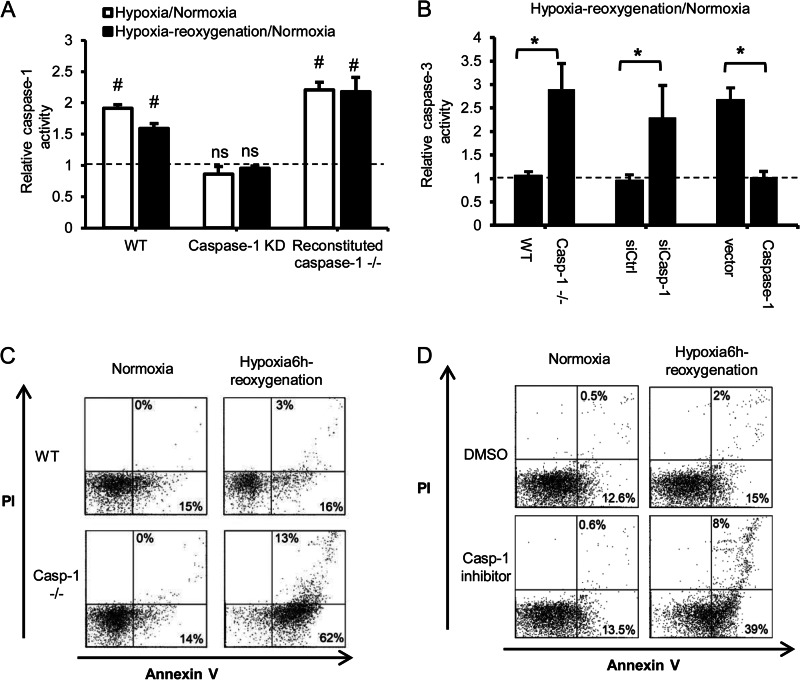
We have shown previously that caspase 1−/− mice exhibit a marked increase in liver damage in a model of HS/R and peripheral tissue injury independent of IL1β and IL18 (5). Hepatocytes are the main cell type in the liver, and recent evidence suggests that hepatocytes express and can activate an inflammasome and caspase 1 (8). Therefore, we investigated the role of caspase 1 in regulating hepatocyte cell death. Because HS/R is characterized by severe redox stress associated with hypoxia/reoxygenation (19), we assessed the response of hepatocytes isolated from WT and caspase 1−/− mice to hypoxia/reoxygenation in vitro as described previously (13). Hypoxia as well as hypoxia/reoxygenation induced the activation of caspase 1 in WT hepatocytes (Fig. 1A). To further confirm the role of caspase 1 in hepatocytes after oxidative stress, caspase 1 was knocked down by ∼51% in WT hepatocytes treated with caspase 1 siRNA as assessed by Western blotting at 24 h (supplemental Fig. S1A). Acute knockdown of caspase 1 significantly decreased caspase 1 activity after hypoxia/reoxygenation as expected (Fig. 1A). Moreover, reversal studies confirm that transfection with mouse caspase 1 is able to significantly restore caspase 1 activity in caspase 1−/− hepatocytes (supplemental Fig. 1, A and B). Consistent with our previous in vivo results showing increased liver cell death after HS/R and peripheral tissue injury, caspase 1 deficiency and acute knockdown resulted in higher levels of hepatocyte apoptosis and necrosis, as determined by increased caspase 3 activity (Fig. 1B) and annexin V/PI staining, after hypoxia/reoxygenation (C). The central effect of caspase 1 activation on hepatocyte cell death during oxidative stress was confirmed by increased cell death in WT hepatocytes pretreated with caspase 1 inhibitor (Ac-YVAD-CMK, 15 μm) shown by annexin V/PI staining as well as around a 2.5-fold increase in cleaved caspase3 and PARP after caspase 1 knockdown (Fig. 1D and supplemental Fig. S1A). The increased cell death in caspase 1−/− cells after hypoxia/reoxygenation was reversed by transfection with a plasmid for mouse caspase 1 (Fig. 1B and supplemental Fig. S1B).
FIGURE 1.
Caspase 1 is protective in hepatocytes after hypoxia/reoxygenation. A, relative caspase 1 activity in WT hepatocytes, WT hepatocytes treated with caspase 1 siRNA, and caspase 1−/− cells transfected with mouse caspase 1 plasmid after normoxia, 6 h of hypoxia, or 6 h of hypoxia/1 h of reoxygenation. Data are shown as fold changes of normoxic levels. Data are mean ± S.D, n = 3. #, p < 0.05, normoxia versus hypoxia-reoxygenation; ns, not significant. B, relative caspase 3 activity in WT and caspase 1−/− hepatocytes, WT hepatocytes treated with caspase 1 siRNA, and caspase 1−/− cells expressing mouse caspase 1 after 6 h of hypoxia/1 h of reoxygenation. Data are shown as fold changes of normoxic levels. Data are mean ± S.D., n = 3. *, p < 0.05. C, representative annexin V/PI flow cytometry dot plots for hepatocytes cultured under normoxia or after 6 h of hypoxia/1 h of reoxygenation. D, representative annexin V/PI analysis of WT hepatocytes pretreated with dimethyl sulfoxide (DMSO) or caspase 1 inhibitor (15 μm) and then 6 h of hypoxia/1 h of reoxygenation. Data shown are representative of three independent experiments.
Caspase 1 Deficiency Increases Mitochondrial ROS Production during Oxidative Stress
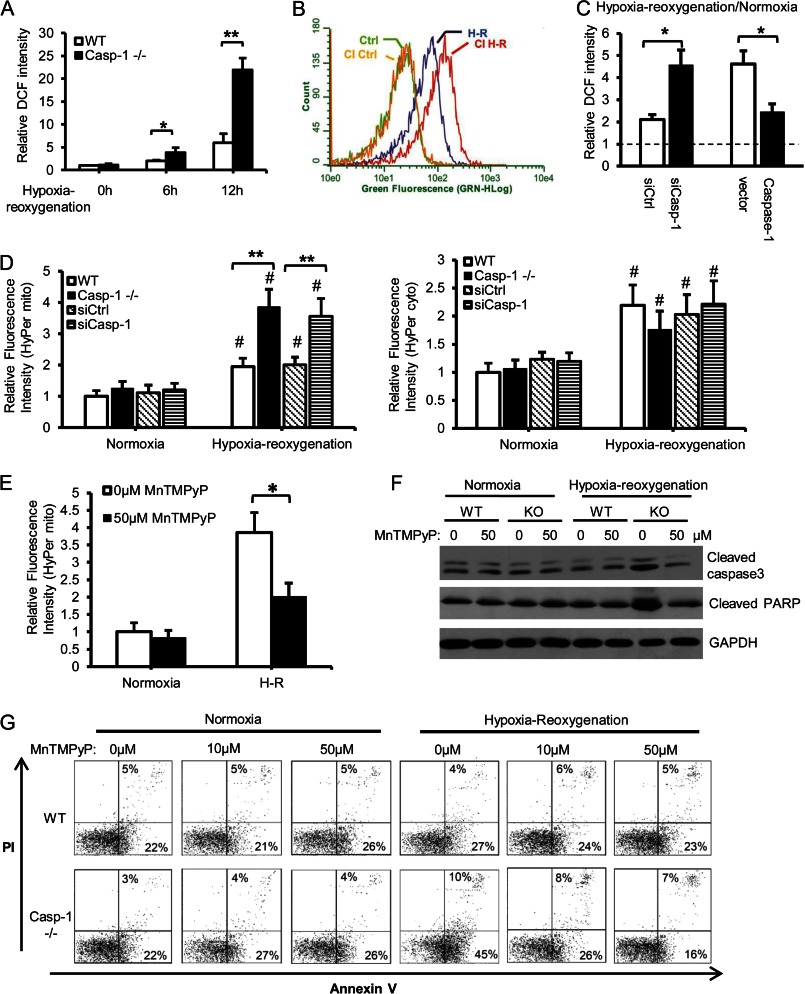
Excessive oxidative stress is one cause of hepatocellular cell death after hypoxia/reoxygenation and HS/R (9, 20, 21). We hypothesized that caspase 1 may be regulating oxidative stress after hypoxia/reoxygenation and, therefore, we assessed intracellular ROS production by DCF staining. As expected, hypoxia/reoxygenation leads to oxidative stress as measured by increased intracellular ROS production (Fig. 2A). We found that ROS production was greater in hepatocytes isolated from caspase 1−/− mice or WT hepatocytes treated with caspase 1 inhibitor than WT hepatocytes alone following hypoxia/reoxygenation (Fig. 2, A and B). The central role of caspase 1 in regulating intracellular ROS production was further confirmed by the acute knockdown and reconstitution studies (Fig. 2C).
FIGURE 2.
Caspase 1 deficiency increases mitochondrial ROS production during hypoxia/reoxygenation. A, intracellular ROS estimated by DCF staining in hepatocytes after normoxia or hypoxia for 6 or 12 h and 1 h of reoxygenation. Fluorescence intensity was quantified and normalized to normoxic WT levels. Data are mean ± S.E. *, p < 0.05; **, p < 0.01. B, DCF fluorescence measured by flow cytometry in WT hepatocytes pretreated with vehicle (dimethyl sulfoxide) or caspase 1 inhibitor (CI) and then 6 h of hypoxia/1 h of reoxygenation (H-R). Data shown are representative histograms from two independent experiments. C, relative intracellular ROS estimated by DCF staining in WT hepatocytes treated with caspase 1 siRNA and caspase 1−/− cells expressing mouse caspase 1 after normoxia or 6 h of hypoxia/1 h of reoxygenation. Data are shown as fold changes of normoxic levels (mean ± S.D., n = 3). *, p < 0.05. D, mitochondrial (left panel) and cytoplasmic H2O2 (right panel) in hepatocytes treated with 6 h of hypoxia/1 h of reoxygenation. Data are mean ± S.E. #, p < 0.05, hypoxia-reoxygenation versus normoxia; **, p < 0.01, WT versus caspase 1−/− or siCtrl versus siCasp-1. E, mitochondrial H2O2 in caspase 1−/− hepatocytes pretreated with 50 μm MnTMPyP for 1 h before they were subjected to 6 h of hypoxia/1 h of reoxygenation. Data are mean ± S.E., n = 3. *, p < 0.05. F, representative Western blot analysis of cleaved caspase 3 and PARP in hepatocytes pretreated with 50 μm MnTMPyP for 1 h before they were subjected to 6 h of hypoxia/1 h of reoxygenation. G, annexin V/PI staining in WT and caspase 1−/− hepatocytes pretreated with 0, 10, or 50 μm MnTMPyP for 1 h and subjected to 6 h of hypoxia/1 h of reoxygenation or normoxia. Data are representative of two independent experiments.
Because ROS can originate from mitochondria or from cytosolic NADPH oxidase/xanthine oxidase, we used fluorescent sensors capable of specific detection of mitochondrial or cytosolic hydrogen peroxide (H2O2) (22) to determine the source of excessive ROS in caspase 1−/− cells. Hypoxia/reoxygenation significantly increased both mitochondrial and cytosolic H2O2, as expected (Fig. 2D). Levels of mitochondrially derived ROS were significantly higher in caspase 1−/− hepatocytes compared with the WT, with no difference in the levels of cytosolic ROS, suggesting that caspase 1 regulates mitochondrial ROS production after hypoxia/reoxygenation. The results were further confirmed by caspase 1 knockdown with siRNA (Fig. 2D). To confirm that excessive mitochondrial ROS contributed to increased cell death in caspase 1−/− hepatocytes, we treated cells with MnTMPyP, a mitochondria-specific ROS scavenger (23), prior to hypoxia/reoxygenation treatment. MnTMPyP pretreatment significantly reduced mitochondrial ROS production in caspase 1−/− cells after hypoxia/reoxygenation, as expected (Fig. 2E). Moreover, MnTMPyP treatment decreased cell death of caspase 1−/− hepatocytes to levels similar to those seen in WT hepatocytes after hypoxia/reoxygenation (Fig. 2, F and G). Altogether, these findings show a role for caspase 1 in mediating protection against mitochondrially derived oxidative stress in hepatocytes.
Caspase 1 Deficiency Results in Impaired Mitochondrial Clearance in Hepatocytes after Hypoxia/Reoxygenation
One adaptive mechanism employed to protect cells from harmful ROS is through regulation of mitochondrial oxidative phosphorylation that generates ROS as a byproduct (24). We analyzed mitochondrial oxygen consumption rate (OCR) using the Seahorse extracellular flux analyzer and confirmed that the OCR in WT cells was reduced after hypoxia/reoxygenation, as expected (Fig. 3A). In contrast, the basal OCR in caspase 1−/− hepatocytes was significantly lower than in WT hepatocytes and remained unchanged after hypoxia/reoxygenation (Fig. 3A), suggesting an important role for caspase 1 in regulating mitochondrial respiration in both basal and hypoxic states.
FIGURE 3.
Defective mitochondrial clearance in caspase 1-deficient cells during oxidative stress. A, OCR in hepatocytes cultured under normoxia or treated with 6 h of hypoxia/1 h of reoxygenation. The OCR was normalized to protein content and is shown as fold changes of normoxic control. Data are mean ± S.D., n = 3. #, p < 0.05, hypoxia-reoxygenation versus normoxia; ***, p < 0.001, WT versus caspase 1−/−. FCCP: Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone 2-DG: 2-deoxyglucose. B, mitochondrial volume in hepatocytes cultured under normoxia or treated with 6 h of hypoxia/1 h of reoxygenation. Data are shown as fold changes of normoxic levels (mean ± S.E.). #, p < 0.05, normoxia versus hypoxia-reoxygenation; **, p < 0.01, WT versus caspase 1−/− or siCtrl versus siCasp-1; *, p < 0.05, vector versus caspase 1. C, mitochondrial content in WT hepatocytes pretreated with dimethyl sulfoxide (DMSO) or caspase 1 inhibitor before normoxic culture or treated with hypoxia/reoxygenation. Data are shown as fold changes of normoxic levels (mean ± S.E.). #, p < 0.05, normoxia versus hypoxia-reoxygenation; **, p < 0.01, WT versus caspase 1 inhibitor. D, the mitochondrial DNA copy number is shown as fold changes of normoxic levels. H-R: hypoxia-reoxygenation. Data are mean ± S.E., n = 3. #, p < 0.05, normoxia versus hypoxia or hypoxia-reoxygenation; *, p < 0.05, WT versus caspase 1−/−. E, the expression (left panel) and quantification (right panel) of Tom20 was assessed in WT hepatocytes transfected with control siRNA (siCtrl) or caspase 1 siRNA (siC1) 24 h before they were treated with hypoxia. Data are mean ± S.E., n = 3). *, p < 0.05. F, the expression (left panel) and quantification (right panel) of Tom20 in WT, caspase 1−/− hepatocytes, and caspase 1−/− hepatocytes reconstituted with mouse caspase 1. Data are mean ± S.E., n = 3. *, p < 0.05; **, p < 0.01.
One explanation for the reduced mitochondrial respiration and ROS production during hypoxia/reoxygenation could be initiation of a rapid decrease in mitochondrial content (25, 26). We found a significantly decreased mitochondrial volume in WT hepatocytes after hypoxia/reoxygenation, measured by quantitation of three-dimensional confocal microscopy images. In contrast, the mitochondrial volume in caspase 1−/− and caspase 1 knockdown cells remained unchanged (Fig. 3B) and was consistent with observed mitochondrial respiration. Our results showing a role for caspase 1 in the reduction in mitochondrial volume after hypoxia were further confirmed in caspase 1−/− cells reconstituted with caspase 1 and WT hepatocytes pretreated with caspase 1 inhibitor (Fig. 3, B and C). To ensure that changes in mitochondrial volume were not secondary to mitochondrial swelling, mitochondrial content was also determined by measuring mitochondrial DNA copy number. As before, mitochondrial DNA significantly decreased in WT hepatocytes after hypoxia or hypoxia/reoxygenation compared with normoxia, whereas caspase 1−/− cells had significantly increased mitochondrial DNA content in comparison with the normoxic level (Fig. 3D), further suggesting an important role of caspase 1 in regulating mitochondrial mass. The effect of caspase 1 on regulating mitochondrial content was further confirmed by an 1.8-fold increase in the levels of translocase of outer membrane 20 (Tom 20) in caspase 1 knockdown cells (Fig. 3E). The defective mitochondrial clearance in caspase 1−/− cells after hypoxia was restored by reconstitution of caspase 1 (Fig. 3F). Caspase 1, therefore, appears to play a vital role in reducing mitochondrial content during hypoxia/reoxygenation, which may form part of the protective mechanism of caspase 1.
Deficiency in Caspase 1 Decreases Autophagic Flux in Hepatocytes after Hypoxia/Reoxygenation
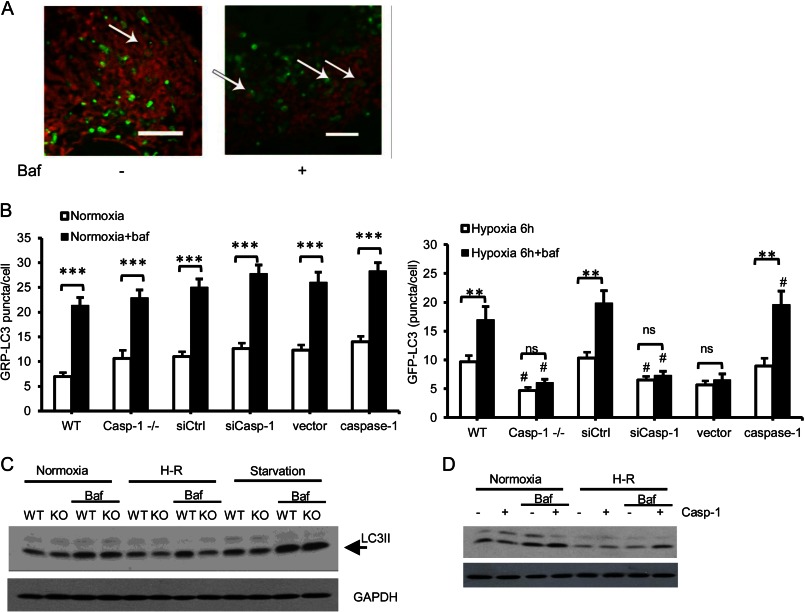
Mitochondrial autophagy is responsible for mitochondrial turnover and clearance of dysfunctional or damaged mitochondria (11, 27). We observed mitochondria-containing autophagosomes in WT hepatocytes after hypoxia/reoxygenation (Fig. 4A, left panel), and this number was increased by blocking degradation of autolysosomes with bafilomycin A1 (right panel). Given our findings above, we hypothesized that caspase 1 would regulate autophagic flux during hypoxia/reoxygenation. Autophagic flux is controlled by both induction and maturation/degradation of autophagosomes. To quantify autophagy, we inhibited lysosomal degradation (with bafilomycin) and analyzed the accumulation of GFP-LC3 puncta (autophagic vacuoles) by microscopy or LC3II by Western blot analysis. This, together with the assessment of steady-state autophagy, allowed us to monitor changes in autophagic flux (28). WT and caspase 1−/− hepatocytes showed similar levels of autophagic flux under normoxic conditions as well as after starvation (Fig. 4, B and C). However, after hypoxia/reoxygenation, caspase 1−/− and caspase 1 knockdown hepatocytes had fewer autophagosomes compared with control groups, and this number did not increase with bafilomycin treatment, suggesting that caspase 1 is important for autophagy induction (supplemental Fig. S2A and Fig. 4B). Reversal studies confirm that transfection with mouse caspase 1 is able to significantly restore autophagic flux in caspase 1−/− hepatocytes (Fig. 4B). Similarly, bafilomycin treatment did not increase the levels of LC3II in caspase 1−/− hepatocytes as in WT cells after hypoxia/reoxygenation, indicating defective autophagic flux in caspase 1−/− cells (Fig. 4C). The central role of caspase 1 in regulating autophagic flux was further confirmed by increased levels of LC3II after bafilomycin in caspase 1 reconstituted hepatocytes (Fig. 4D). The maturation of autophagosomes after caspase 1 activation was analyzed further using a tandem RFP-GFP-LC3 construct (29). GFP fluorescence is quenched in acidic lysosomes, whereas RFP is relatively more resistant to acidic conditions. Therefore, colocalization of green and red puncta indicates early autophagic vacuoles, whereas red puncta alone indicate late autophagic vacuoles. Caspase 1−/− hepatocytes had a significantly reduced number of early autophagic vacuoles after hypoxia/reoxygenation compared with WT hepatocytes (supplemental Fig. S2B), further confirming a role for caspase 1 in promoting autophagy induction.
FIGURE 4.
Caspase 1 regulates autophagic flux in hepatocytes after oxidative stress. A, mitochondria (MitoTracker Red) contained autophagosomes (GFP-LC3) in WT hepatocytes after 6 h of hypoxia/1 h of reoxygenation ± bafilomycin (Baf) (50 nm). Images are representative of at least three separate experiments. Scale bar = 10 μm. B, quantification of autophagosomes (GFP-LC3 puncta) in hepatocytes after 6 h of hypoxia/1 h of reoxygenation ± bafilomycin (50 nm). Data are mean ± S.E. **, p < 0.01; ***, p < 0.001, steady state versus bafilomycin treatment; #, p < 0.05, WT versus caspase 1−/−, siCtrl versus siCasp-1 or vector versus caspase 1; ns, not significant. C, the expression of LC3 in WT and caspase 1−/− hepatocytes left untreated, treated with 6 h of hypoxia/1 of h reoxygenation (H-R), or starvation ± bafilomycin (50 nm). D, the expression of LC3 in caspase 1−/− hepatocytes and caspase 1 reconstituted cells cultured under normoxia or treated with 6 h of hypoxia/1 h of reoxygenation ± bafilomycin (50 nm).
Deficiency in Caspase 1 Impairs Beclin 1 Up-regulation after Hypoxia/Reoxygenation
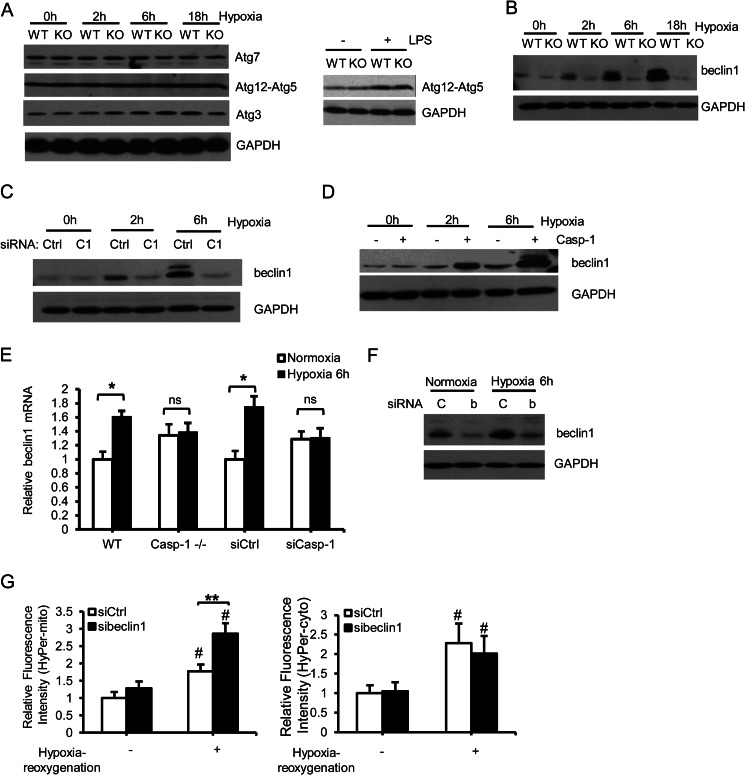
Next we determined the mechanism by which caspase 1 regulates autophagy induction. There was no difference in the levels of Atg3, Atg12-Atg5 conjugate, or Atg7, which are key proteins involved in regulating the initiation of autophagy induction, between WT and caspase 1−/− hepatocytes during oxidative stress (Fig. 5A, left panel) and after lipopolysaccharides (LPS) treatment (right panel). However, expression of beclin 1, a key autophagy initiator, was induced in WT but significantly lower in caspase 1−/− hepatocytes after hypoxia (Fig. 5B) suggesting a previously unidentified role for caspase 1 in regulating beclin 1 expression. Similarly, beclin 1 expression was significantly lower in caspase 1 knockdown hepatocytes, and reconstitution of mouse caspase 1 in caspase 1−/− cells restored beclin 1 expression after hypoxia/reoxygenation (Fig. 5, C and D). Moreover, WT hepatocytes showed increased levels of beclin 1 mRNA after hypoxia, whereas the levels remained unchanged in caspase 1−/− and caspase 1 knockdown cells (Fig. 5E). Beclin 1 has been shown previously to be cleaved by caspase 8 (30). To determine whether caspase 1 induces beclin 1 levels through regulating caspase 8, we assessed the activity of caspase 8 by Western blot analysis. Both WT and caspase 1−/− hepatocytes showed increased caspase 8 after hypoxia, but no difference in caspase 8 cleavage was observed between WT and caspase 1−/− cells (supplemental Fig. S3). To confirm a role for beclin 1-mediated autophagy in reducing mitochondrial content and subsequent ROS production during hypoxia/reoxygenation in hepatocytes, we knocked down beclin 1 in WT hepatocytes (Fig. 5F). Mitochondrial ROS, but not cytosolic ROS production, was significantly higher in sibeclin 1-treated cells compared with controls (Fig. 5G), similar to the results observed in caspase 1−/− cells.
FIGURE 5.
Caspase 1 is required for beclin 1 up-regulation after HS/R and hypoxia/reoxygenation. A, Atg7, Atg12-Atg5 conjugate, and Atg3 expression in WT and caspase 1−/− hepatocytes cultured under normoxia or hypoxia for 2, 6, and 18 h was assessed by Western blot analysis (left panel). The expression of Atg12-Atg5 in WT and caspase 1−/− hepatocytes treated with LPS for 6 h was shown in the right as a positive control. Images are representative of at least three independent experiments. B, representative Western blot analysis of beclin 1 in WT and caspase 1−/− hepatocytes cultured under normoxia or hypoxia for 2, 6, and 18 h. C, representative Western blot analysis of beclin 1 in WT hepatocytes treated with control siRNA or caspase 1 siRNA after normoxia or hypoxia for 2 and 6 h. D, representative Western blot analysis of beclin 1 in caspase 1−/− hepatocytes transfected with vector or mouse caspase 1 plasmid after normoxia or hypoxia for 2 and 6 h. E, beclin1 mRNA levels in hepatocytes after normoxia or 6 h of hypoxia. Data are mean ± S.E., n = 3. *, p < 0.05; ns, not significant. F, beclin 1 expression in WT hepatocytes transfected with siControl (C) or sibeclin1 (b) for 24 h before they were subjected to 6 h of hypoxia. G, mitochondrial (left panel) and cytosolic H2O2 (right panel) production in WT hepatocytes transfected with control siRNA (siCtrl) or siRNA targeting beclin 1 (sibeclin1) and 6 h of hypoxia/1 h of reoxygenation. Data are mean ± S.E. #, p < 0.05, hypoxia-reoxygenation versus normoxia; **, p < 0.01, siCtrl versus sibeclin1.
Caspase 1 Activation Is Protective in the Liver after HS/R through Up-regulating Beclin 1 and Subsequent Clearance of Mitochondria
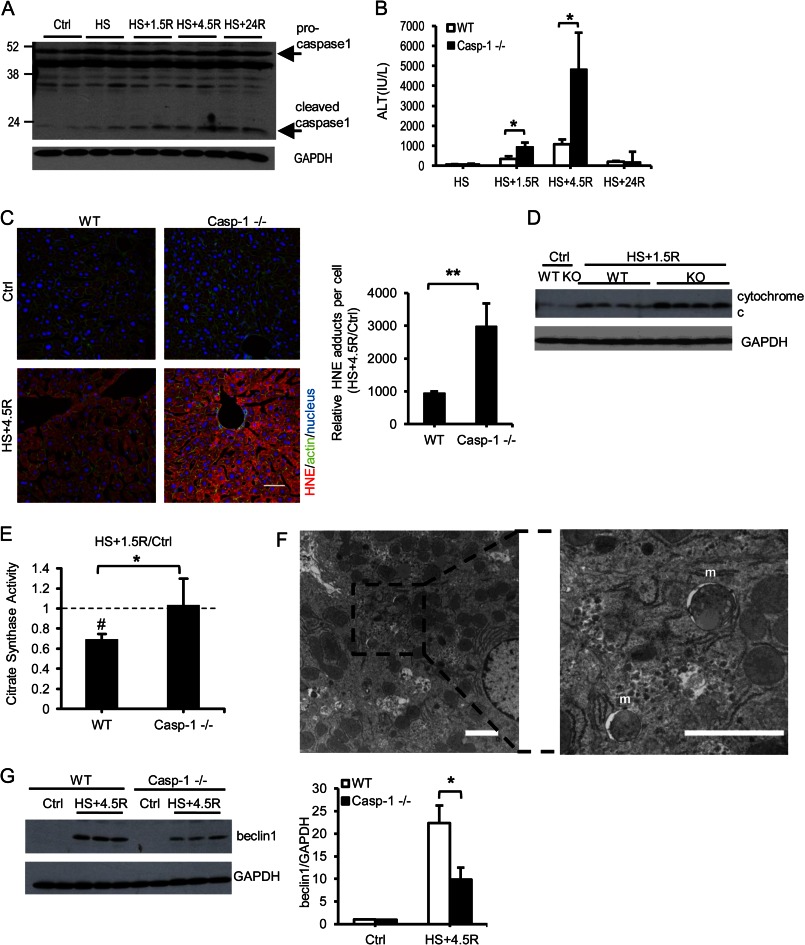
To determine whether similar observations took place in vivo, we carried out experiments in a model of HS/R. Similar to what we have shown before, caspase 1 was activated after 1.5 h of hemorrhagic shock followed by resuscitation in WT mice as shown by increased plasma IL18 levels (supplemental Fig. S4) and increased cleaved caspase 1 in the liver (Fig. 6A). Caspase 1−/− mice exhibited a significant increase in liver injury early after HS/R compared with WT mice (Fig. 6B). This was associated with greater lipid peroxidation in livers of caspase 1−/− mice, measured by a major aldehyde product of lipid oxidation, HNE Michael adducts (Fig. 6C), suggesting increased oxidative stress in the liver of these mice. We also found higher cytosolic cytochrome c levels in caspase 1-deficient liver after HS/R compared with the WT (Fig. 6D). The results are consistent with increased mitochondrial ROS in caspase 1−/− hepatocytes and suggest increased mitochondria-triggered cell death because of caspase 1 deficiency. To assess mitochondrial content in vivo, we measured the activity of citrate synthase, a mitochondrial enzyme relatively resistant to oxidative modification that, therefore, can be used as an indicator of mitochondrial content (31). We found that the mitochondrial content was decreased significantly in WT livers but remained unchanged in caspase 1−/− livers following HS/R (Fig. 6E). Mitochondrial autophagy in WT liver after HS/R was confirmed by electron microscopy analysis (Fig. 6F). Moreover, beclin 1 levels increased in WT and caspase 1−/− livers following HS/R. However, consistent with our findings in vitro, beclin 1 expression was significantly lower in caspase 1−/− livers compared with the WT (Fig. 6G).
FIGURE 6.
Caspase 1 activation reduced liver injury and oxidative stress after HS/R. A, representative Western blot of caspase 1 in liver of WT mice after HS/R. B, plasma alanine aminotransferase (ALT) in mice after HS/R (n = 5 in control groups, n = 7–8 in HS/R groups). Data are mean ± S.E. *, p < 0.05. C, representative staining. Scale bar = 100 μm and quantification of 4-hydroxy-2-nonenal adducts in liver of control mice (Ctrl) and mice subjected to hemorrhagic shock and 4.5 h of resuscitation (HS+4.5R). Data are mean ± S.E., n = 3 for control and n = 5 for HS+4.5R. **, p < 0.01. Quantification is shown as fold increase to control groups. D, cytochrome c release was determined by Western blot analysis of liver cytosolic fraction. E, mitochondrial content in liver was determined by citrate synthase activity and normalized to controls. Data are mean ± S.E., n = 4. #, p < 0.05, HS+1.5R versus control; *, p < 0.05, WT versus caspase 1−/−. F, electron microscopy images of WT liver treated with hemorrhagic shock and 4.5 h of resuscitation. m, mitochondria-containing autophagosomes. Scale bar = 2 μm. G, Western blot analysis and quantification of beclin 1 levels in livers of control mice or mice subjected to HS+4.5R. Data are mean ± S.D., n = 3 for control groups and n = 6 for HS+4.5R groups. *, p < 0.05.
Taken together, our results suggest that caspase 1 deficiency in vivo leads to increased oxidative stress and suppressed mitochondrial clearance in the liver after HS/R. To determine whether this can be rescued by beclin 1 overexpression, we constructed an adenovirus encoding full-length mouse beclin 1 (ad-beclin1) and injected it, or ad-GFP as a control, intravenously into mice 48 h prior to HS/R. Ad-GFP expression in liver was confirmed in control mice (Fig. 7A, left panel), and beclin 1 overexpression in liver was confirmed by immunoblotting (right panel). Beclin 1 overexpression in caspase 1−/− livers led to significantly increased autophagy, indicated by the levels of LC3II (supplemental Fig. S5). Moreover, overexpression of beclin 1was hepatoprotective in caspase 1−/− mice but did not confer a further protection in WT mice, where beclin 1 can be up-regulated normally (Fig. 7B). We also confirmed that beclin 1 overexpression reduced oxidative stress in caspase 1−/− liver, as assessed by HNE adducts (Fig. 7C). Decreased cytosolic cytochrome c levels (Fig. 7D) in caspase 1−/− liver with beclin 1 overexpression further suggests prevention of mitochondria-triggered cell death by beclin 1. Taken together, our results indicate a previously unrecognized role for caspase 1 in the up-regulation of beclin 1 to initiate mitochondrial autophagy in hepatocytes and to reduce excessive mitochondrial ROS production. This is consistent with a known protective role of beclin 1 during hypoxia/reoxygenation (32).
FIGURE 7.
Beclin 1 overexpression rescues caspase 1−/− mice from excessive liver injury. A, effectiveness of adenoviral gene expression in liver after tail vein injection with adenovirus encoding GFP as control (ad-Ctrl) (left panel, scale bar = 50 μm) or mouse beclin 1 (ad-beclin1) (right panel). B, plasma alanine aminotransferase (ALT) (n = 6). Data are mean ± S.E. *, p < 0.05. C, liver HNE adducts. Data are mean ± S.E., n = 5. *, p < 0.05. D, liver cytoplasmic cytochrome c (n = 5). Data are mean ± S.D. #, p < 0.05, WT versus caspase 1−/−. *, p < 0.05, ad-Ctrl versus ad-beclin 1 in mice given ad-GFP or ad-beclin1 (ad-b1) 48 h prior to hemorrhagic shock and 4.5 h of resuscitation (HS+4.5R).
DISCUSSION
This study was undertaken to determine the protective mechanisms of caspase 1 in the setting of hypoxia/reoxygenation in hepatocytes. We show here that caspase 1 deficiency or inhibition leads to enhanced mitochondrial ROS production and a failure to reduce mitochondrial content in hepatocytes subjected to hypoxia/reoxygenation. This was associated with a failure to up-regulate beclin 1 expression and subsequent mitochondrial autophagy. Using a model of hemorrhagic shock with resuscitation, we show that similar events occur in vivo. These results suggest that caspase 1 has a critical role in the regulation of mitochondrial autophagy in hepatocytes.
Our findings in this study establish a mechanism to explain the paradoxical protective role of caspase 1 during HS/R. Our previous results, and the results published here, are in clear contrast to the detrimental effects of caspase 1 in more severe ischemia/reperfusion injury models in heart and kidney, which leave many cells anoxic rather than hypoxic and severely limit the initiation of adaptive survival responses. In these cases caspase 1-mediated inflammation is detrimental (33, 34). Our findings, although initially surprising, are consistent with our understanding of caspase 1-mediated regulation of adaptive responses in non-myeloid cell types. Caspase 1 has been shown previously to regulate adaptive responses to stress and is known to be important in preservation of epithelial integrity by increasing the proliferation of mucosal epithelial cells (6), mediating protein secretion in keratinocytes (35), and promoting membrane repair (7). It now seems likely that caspase 1 activation promotes different effects in different cell types under varying stresses, including a protective role in the liver under conditions of oxidative stress. Indeed, recent studies provide evidence that caspase 1 can reduce the progression of hepatic steatosis (36), in which redox stress is one of the central mediators of the disease process (37). Our data could provide one of the mechanisms behind these effects.
The inhibition of mitochondrial respiration during hypoxia/hemorrhagic shock and the removal of mitochondria to reduce ROS production upon reoxygenation may confer protection against mitochondrial dysfunction and subsequent cell death (10, 24) and is a major cellular adaptive response to hypoxia. Although low levels of ROS may be critical to signaling in cell stress and preconditioning responses (38), our study suggests that high levels of mitochondrial ROS contribute directly to hepatocyte cell death during hypoxia/reoxygenation. Importantly, our results suggest that caspase 1 induces mitochondrial autophagy in response to oxidative stress to promote mitochondrial turnover and maintain a healthy population of mitochondria, which, in turn, regulates ROS production and cell death. This autophagy-mediated turnover of mitochondria may be particularly beneficial in liver, where the half-life of mitochondria is only 1.83 days, much shorter than in organs such as heart and brain (39). Given that lysosomal degradation is the major pathway for mitochondrial turnover (40), the shorter half-life of liver mitochondria suggests that mitochondrial autophagy plays a significant role in mitochondrial quality control in the liver.
Our findings establish a previously unrecognized role for caspase 1 in the regulation of this cell survival response through the regulation of beclin 1 expression. Our results suggest that caspase 1 regulates mRNA levels of beclin 1 as one mechanism for increasing its expression. Previous studies suggest that beclin 1 can be degraded after its ubiquitination and that the process is regulated by ubiquitin-specific peptidases (41). We will further explore whether caspase 1 activation affects beclin 1 stability. Beclin 1 has been shown to confer protection during hypoxia/reoxygenation (32), but here we demonstrate for the first time how beclin 1 expression was regulated during hypoxia and in a clinically relevant model of HS/R. It was shown previously that beclin 1 can be degraded by calpain during anoxia/reoxygenation and liver ischemia/reperfusion (42) in contrast to being up-regulated in our model of hypoxia/reoxygenation and HS/R. This discrepancy may relate to differences in the model and further suggests that adaptive responses during HS/R are essential to protect the liver from further damage, whereas these adaptive responses may be absent during liver ischemia/reperfusion. Beclin 1 was shown previously to be cleaved and inactivated by caspase 8 (30), but our study shows that during oxidative stress, beclin 1 levels are up-regulated by caspase 1, an inflammatory caspase, independently of caspase 8.
Previous studies showed that in cells where autophagy was impaired, enhanced mitochondrial ROS promoted the activation of caspase 1 (1, 2). Our study provides an alternative mechanism of cellular protection in cells that produce little or no IL1β/IL18 cytokines, such as the hepatocytes. In this scenario, caspase 1 is a central driver of mitochondrial autophagy, and the initiating factors leading to caspase 1 activation likely include damage-associated molecular patterns and ROS. Further studies are required to determine the triggers of caspase 1 activation during hypoxia/reoxygenation and to identify the mechanism of how caspase 1 activation up-regulates beclin 1.
Supplementary Material
Acknowledgments
We thank Hong Liao, Meihua Bo, Danielle Reiser, and Carol Meiers for technical assistance and Alicia Frank and Lauryn Kohut for hemorrhagic shock surgery.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HL-079669 (to J. F.) and P50-GM053789 (to T. R. B.). This work was also supported by the Surgical Infection Society (to M. S.).

This article contains supplemental Figs. S1–S5.
- ROS
- reactive oxygen species
- HS/R
- hemorrhagic shock with resuscitation
- MnTMPyP
- manganese(III) tetrakis (1-methyl-4-pyridyl) porphyrin
- DCF
- dichlorofluorescein
- HNE
- 4-hydroxyl-2-nonenal
- PI
- propidium iodide
- OCR
- oxygen consumption rate
- RFP
- red fluorescent protein.
REFERENCES
- 1. Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 2. Nakahira K., Haspel J. A., Rathinam V. A., Lee S. J., Dolinay T., Lam H. C., Englert J. A., Rabinovitch M., Cernadas M., Kim H. P., Fitzgerald K. A., Ryter S. W., Choi A. M. (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pomerantz B. J., Reznikov L. L., Harken A. H., Dinarello C. A. (2001) Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1β. Proc. Natl. Acad. Sci. U.S.A. 98, 2871–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melnikov V. Y., Ecder T., Fantuzzi G., Siegmund B., Lucia M. S., Dinarello C. A., Schrier R. W., Edelstein C. L. (2001) Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J. Clin. Invest. 107, 1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menzel C. L., Sun Q., Loughran P. A., Pape H. C., Billiar T. R., Scott M. J. (2011) Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol. Med. 17, 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaki M. H., Boyd K. L., Vogel P., Kastan M. B., Lamkanfi M., Kanneganti T. D. (2010) The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurcel L., Abrami L., Girardin S., Tschopp J., van der Goot F. G. (2006) Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 [DOI] [PubMed] [Google Scholar]
- 8. Csak T., Ganz M., Pespisa J., Kodys K., Dolganiuc A., Szabo G. (2011) Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malhi H., Gores G. J., Lemasters J. J. (2006) Apoptosis and necrosis in the liver. A tale of two deaths? Hepatology 43, S31–44 [DOI] [PubMed] [Google Scholar]
- 10. Burwell L. S., Nadtochiy S. M., Brookes P. S. (2009) Cardioprotection by metabolic shut-down and gradual wake-up. J. Mol. Cell Cardiol. 46, 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green D. R., Galluzzi L., Kroemer G. (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333, 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seglen P. O. (1976) Preparation of isolated rat liver cells. Methods Cell Biol. 13, 29–83 [DOI] [PubMed] [Google Scholar]
- 13. Kim J. S., Ohshima S., Pediaditakis P., Lemasters J. J. (2004) Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology 39, 1533–1543 [DOI] [PubMed] [Google Scholar]
- 14. Choi S. W., Gerencser A. A., Lee D. W., Rajagopalan S., Nicholls D. G., Andersen J. K., Brand M. D. (2011) Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J. Neurosci. 31, 4524–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos J. H., Meyer J. N., Mandavilli B. S., Van Houten B. (2006) Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 314, 183–199 [DOI] [PubMed] [Google Scholar]
- 16. Prince J. M., Ming M. J., Levy R. M., Liu S., Pinsky D. J., Vodovotz Y., Billiar T. R. (2007) Early growth response 1 mediates the systemic and hepatic inflammatory response initiated by hemorrhagic shock. Shock 27, 157–164 [DOI] [PubMed] [Google Scholar]
- 17. Lagoa C. E., Vodovotz Y., Stolz D. B., Lhuillier F., McCloskey C., Gallo D., Yang R., Ustinova E., Fink M. P., Billiar T. R., Mars W. M. (2005) The role of hepatic type 1 plasminogen activator inhibitor (PAI-1) during murine hemorrhagic shock. Hepatology 42, 390–399 [DOI] [PubMed] [Google Scholar]
- 18. Gusdon A. M., Chen J., Votyakova T. V., Mathews C. E. (2009) Chapter 24 Quantification, localization, and tissue specificities of mouse mitochondrial reactive oxygen species production. Methods Enzymol. 456, 439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCloskey C. A., Kameneva M. V., Uryash A., Gallo D. J., Billiar T. R. (2004) Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock 22, 380–386 [DOI] [PubMed] [Google Scholar]
- 20. Jarrar D., Wang P., Cioffi W. G., Bland K. I., Chaudry I. H. (2000) Critical role of oxygen radicals in the initiation of hepatic depression after trauma hemorrhage. J. Trauma 49, 879–885 [DOI] [PubMed] [Google Scholar]
- 21. Li C., Jackson R. M. (2002) Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 282, C227–241 [DOI] [PubMed] [Google Scholar]
- 22. Belousov V. V., Fradkov A. F., Lukyanov K. A., Staroverov D. B., Shakhbazov K. S., Terskikh A. V., Lukyanov S. (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 [DOI] [PubMed] [Google Scholar]
- 23. Faulkner K. M., Liochev S. I., Fridovich I. (1994) Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 269, 23471–23476 [PubMed] [Google Scholar]
- 24. Yoshioka J., Chutkow W. A., Lee S., Kim J. B., Yan J., Tian R., Lindsey M. L., Feener E. P., Seidman C. E., Seidman J. G., Lee R. T. (2012) Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J. Clin. Invest. 122, 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoppeler H., Vogt M., Weibel E. R., Flück M. (2003) Response of skeletal muscle mitochondria to hypoxia. Exp. Physiol. 88, 109–119 [DOI] [PubMed] [Google Scholar]
- 26. Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., Huang L., Xue P., Li B., Wang X., Jin H., Wang J., Yang F., Liu P., Zhu Y., Sui S., Chen Q. (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 [DOI] [PubMed] [Google Scholar]
- 27. Pua H. H., Guo J., Komatsu M., He Y. W. (2009) Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 182, 4046–4055 [DOI] [PubMed] [Google Scholar]
- 28. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., Blum J. S., Bredesen D. E., Brodsky J. L., Brumell J. H., Brunk U. T., Bursch W., Camougrand N., Cebollero E., Cecconi F., Chen Y., Chin L. S., Choi A., Chu C. T., Chung J., Clarke P. G., Clark R. S., Clarke S. G., Clavé C., Cleveland J. L., Codogno P., Colombo M. I., Coto-Montes A., Cregg J. M., Cuervo A. M., Debnath J., Demarchi F., Dennis P. B., Dennis P. A., Deretic V., Devenish R. J., Di Sano F., Dice J. F., Difiglia M., Dinesh-Kumar S., Distelhorst C. W., Djavaheri-Mergny M., Dorsey F. C., Droge W., Dron M., Dunn W. A., Duszenko M., Eissa N. T., Elazar Z., Esclatine A., Eskelinen E. L., Fesues L., Finley K. D., Fuentes J. M., Fueyo J., Fujisaki K., Galliot B., Gao F. B., Gewirtz D. A., Gibson S. B., Gohla A., Goldberg A. L., Gonzalez R., Gonzalez-Estevez C., Gorski S., Gottlieb R. A., Haussinger D., He Y. W., Heidenreich K., Hill J. A., Hoyer-Hansen M., Hu X., Huang W. P., Iwasaki A., Jaattela M., Jackson W. T., Jiang X., Jin S. K., Johansen T., Jung J. U., Kadowaki M., Kang C., Kelekar A., Kessel D. H., Kiel J. A. K. W., Kim H. P., Kimchi A., Kinsella T. J., Kiselyov K., Kitamoto K., Knecht E., Komatsu M., Kominami E., Konclo S., Kovacs A. L., Kroemer G., Kuan C. Y., Kumar R., Kundu M., Landry J., Laporte M., Le W. D., Lei H. Y., Lenardo M. J., Levine B., Lieberman A., Lim K. L., Lin F. C., Liou W., Liu L. F., Lopez-Berestein G., Lopez-Otin C., Lu B., Macleod K. F., Malorni W., Martinet W., Matsuoka K., Mautner J., Meijer A. J., Melendez A., Michels P., Miotto G., Mistiaen W. P., Mizushima N., Mograbi B., Monastyrska I., Moore M. N., Moreira P. I., Moriyasu Y., Motyl T., Munz C., Murphy L. O., Naqvi N. I., Neufeld T. P., Nishino I., Nixon R. A., Noda T., Nurnberg B., Ogawa M., Oleinick N. L., Olsen L. J., Ozpolat B., Paglin S., Palmer G. E., Papassideri I., Parkes M., Perlmutter D. H., Perry G., Piacentini M., Pinkas-Kramarski R., Prescott M., Proikas-Cezanne T., Raben N., Rami A., Reggiori F., Rohrer B., Rubinsztein D. C., Ryan K. M., Sadoshima J., Sakagami H., Sakai Y., Sandri M., Sasakawa C., Sass M., Schneider C., Seglen P. O., Seleverstov O., Settleman J., Shacka J. J., Shapiro I. M., Sibirny A., Silva-Zacarin E. C. M., Simon H. U., Simone C., Simonsen A., Smith M. A., Spanel-Borowski K., Srinivas V., Steeves M., Stenmark H., Stromhaug P. E., Subauste C. S., Sugimoto S., Sulzer D., Suzuki T., Swanson M. S., Takeshita F., Talbot N. J., Talloczy Z., Tanaka K., Tanaka K., Tanida I., Taylor G. S., Taylor J. P., Terman A., Tettamanti G., Thompson C. B., Thumm M., Tolkovsky A. M., Tooze S. A., Truant R., Tumanovska L. V., Uchiyama Y., Ueno T., Uzcategui N. L., van der Klei I., Vaquero E. C., Vellai T., Vogel M. W., Wang H. G., Webster P., Wiley J. W., Xi Z. J., Xiao G., Yahalom J., Yang J. M., Yap G., Yin X. M., Yoshimori T., Yu L., Yue Z. Y., Yuzaki M., Zabirnyk O., Zheng X. X., Zhu X., Deter R. L., Tabas I. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimura S., Noda T., Yoshimori T. (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 [DOI] [PubMed] [Google Scholar]
- 30. Li H., Wang P., Sun Q., Ding W. X., Yin X. M., Sobol R. W., Stolz D. B., Yu J., Zhang L. (2011) Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of beclin 1. Cancer Res. 71, 3625–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Venkatraman A., Shiva S., Davis A. J., Bailey S. M., Brookes P. S., Darley-Usmar V. M. (2003) Chronic alcohol consumption increases the sensitivity of rat liver mitochondrial respiration to inhibition by nitric oxide. Hepatology 38, 141–147 [DOI] [PubMed] [Google Scholar]
- 32. Hamacher-Brady A., Brady N. R., Gottlieb R. A. (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J. Biol. Chem. 281, 29776–29787 [DOI] [PubMed] [Google Scholar]
- 33. Zhang W. H., Wang X., Narayanan M., Zhang Y., Huo C., Reed J. C., Friedlander R. M. (2003) Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc. Natl. Acad. Sci. U.S.A. 100, 16012–16017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mezzaroma E., Toldo S., Farkas D., Seropian I. M., Van Tassell B. W., Salloum F. N., Kannan H. R., Menna A. C., Voelkel N. F., Abbate A. (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl. Acad. Sci. U.S.A. 108, 19725–19730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keller M., Rüegg A., Werner S., Beer H. D. (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 [DOI] [PubMed] [Google Scholar]
- 36. Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., Camporez J. P., Shulman G. I., Gordon J. I., Hoffman H. M., Flavell R. A. (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pessayre D., Mansouri A., Fromenty B. (2002) Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G193–199 [DOI] [PubMed] [Google Scholar]
- 38. Pagliaro P., Moro F., Tullio F., Perrelli M. G., Penna C. (2011) Cardioprotective pathways during reperfusion. Focus on redox signaling and other modalities of cell signaling. Antioxid. Redox Signal. 14, 833–850 [DOI] [PubMed] [Google Scholar]
- 39. Miwa S., Lawless C., von Zglinicki T. (2008) Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction. Application of a simple dynamic model. Aging Cell 7, 920–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diaz F., Moraes C. T. (2008) Mitochondrial biogenesis and turnover. Cell Calcium 44, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu J., Xia H., Kim M., Xu L., Li Y., Zhang L., Cai Y., Norberg H. V., Zhang T., Furuya T., Jin M., Zhu Z., Wang H., Yu J., Hao Y., Choi A., Ke H., Ma D., Yuan J. (2011) Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147, 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim J. S., Nitta T., Mohuczy D., O'Malley K. A., Moldawer L. L., Dunn W. A., Jr., Behrns K. E. (2008) Impaired autophagy. A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology 47, 1725–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.