Abstract
Chronic persistent inflammation plays a significant role in disease pathology of cancer, cardiovascular disease, and metabolic syndrome (MetS). MetS is a constellation of diseases that include obesity, diabetes, hypertension, dyslipidemia, hypertriglyceridemia, and hypercholesterolemia. Nonalcoholic fatty liver disease (NAFLD) is associated with many of the MetS diseases. These metabolic derangements trigger a persistent inflammatory cascade, which includes production of lipid autacoids (eicosanoids) that recruit immune cells to the site of injury and subsequent expression of cytokines and chemokines that amplify the inflammatory response. In acute inflammation, the transcellular synthesis of antiinflammatory eicosanoids resolve inflammation, while persistent activation of the autacoid-cytokine-chemokine cascade in metabolic disease leads to chronic inflammation and accompanying tissue pathology. Many drugs targeting the eicosanoid pathways have been shown to be effective in the treatment of MetS, suggesting a common linkage between inflammation, MetS and drug metabolism.The cross-talk between inflammation and MetS seems apparent because of the growing evidence linking immune cell activation and metabolic disorders such as insulin resistance, dyslipidemia, and hypertriglyceridemia. Thus modulation of lipid metabolism through either dietary adjustment or selective drugs may become a new paradigm in the treatment of metabolic disorders. This review focuses on the mechanisms linking eicosanoid metabolism to persistent inflammation and altered lipid and carbohydrate metabolism in MetS.
1. Introduction
Eicosanoids represent a diverse group of bioactive lipids synthesized from polyunsaturated fatty acids (PUFA) to either proinflammatory omega-6 arachidonic acid (AA) or anti-inflammatory omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Fig 5.1). These eicosanoids are synthesized from two essential fatty acids (FAs), ω-6 linoleic acid (C18:2n6) and ω-3 linolenic acid (LA) (C18:3n3), by a series of desaturase and elongase enzymes. Both eicosanoids and FAs are partitioned to different organelles by fatty acid transport proteins (FATPs), which transport fatty acid-coenzyme A (CoA) (FA-CoA) or fatty acid binding protein (FABP) that transports free fatty acid (FFA). The FFA is esterified by a group of organelles and FA chain-length-selective acyl-CoA synthetase (ACS) and then incoproation of saturated FA into the sn-1 position or unsaturated FA into sn-2 position of triacylglycerol (TAG) or phospholipids (PLs) by acyltransferase. These bioactive FAs are stored in membranes as PLs [e.g. phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylglycerol] or in the endoplasmic reticulum (ER) or lipid droplets (LDs) as TAG. PLs are polar ionic lipids composed of 1,2-diacylglycerol and sn-3 phosphodiester bridge that links the glycerol backbone to usually a nitrogenous base, choline, serine, ethanolamine inositol or glycerol, while TAG has FAs located at all positions of the glycerol backbone. The release of both saturated and unsaturated FAs from PL or TAG are performed by a group of phospholipases. Phospholipidase A1 (PLA1) releases saturated palmitic acid (C16:0) from the sn-1 producing 2-acyl lysophospholipid. Phospholipase A2 releases unsaturated fatty acid (uSFA) either oleic acid (C18:1n9) or AA from the sn-2 position forming 1-acylphospholipid. Phospholipase C (PLC) hydrolyzes inositol PLs to yield inositol phosphates and diacylglycerol (DAG) as secondary messengers, while phospholipase D produces phosphatidic acid (PA), which is acted upon by PA phosphohydrolase to produce DAG. Lipid peroxidation of membrane PL uSFAs at the sn-2 is removed by PLA2 producing sn-2-lysoPL that is reacylated by either arachidonyl-CoA transacylase or by an exchange reaction catalyzed by lysolecithin:lecithin acyltransferase, which is a major mechanism in membrane remodeling.
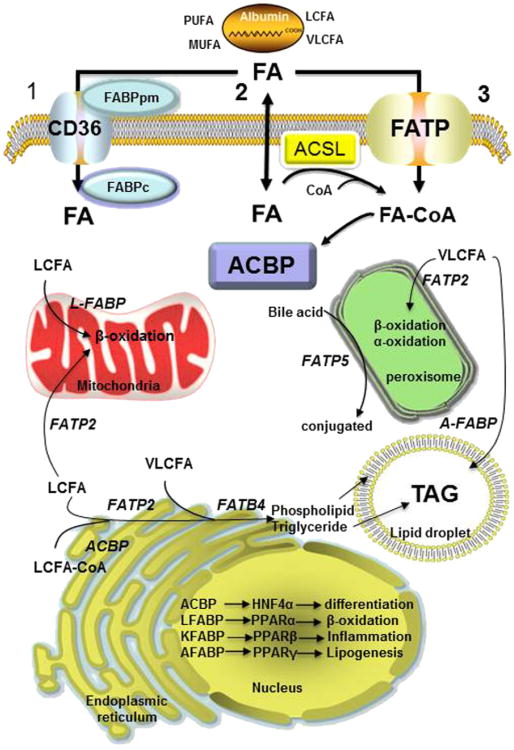
Figure 5.1. Function and subcellular location of fatty acid transport proteins, FABP, FATP/ACSVL, and ACBP in fatty acid transport and channeling.
The extracelluar concentration of fatty acids (FA) varies from 0.3 to up to 2 mM and they are largely bound to albumin (300–600 uM) at a ratio of 5–10 FA molecules per molecule of albumin. Cellular uptake of long-chain fatty acid (LCFA), very-long-chain fatty acid (VLCFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA) occurs through three putative mechanisms: (1) FABPpm (FABPAST) localizes FA to the plasma membrane and CD36 fatty acid translocator facilitates transport across the phospholipid bilayer and is bound by FABPc (l-FABP), (2) FA can cross the membrane by simple passive diffusion or use a flip-fop mechanism resulting in donation of proton to the cytosol. The FA can be bound to 10 different FABPs or converted to FA-CoA by acyl-CoA synthetase (ACSL) to form an acyl-CoA ester, and finally (3) VLCFA are preferentially transported by one of five FATPs that because of their synthetase activity converts VLCFAs to VLC-acyl-CoA esters. In the cytosol, FA and FA-CoA esters are channeled to different organelles and metabolic pathways by FABP and ACBP. Different FABP, FATP, and ACBP show differential selectivity for FAs of different chain length and degree of unsaturation as well as vectorial channeling to different organelles for oxidation or synthesis of complex lipids. The plasma membrane FABPpm is identical to the mitochondrial membrane aspartate aminotransferase (AST) and ths is often identifed as FABPAST. Thus FABPpm has different functions depending on cellular location. Similarly, the cytosolic FABPc is also known as L-FABP and has two FA-binding sites, while other FABPs have a single FA binding site. (For color version of this figure, the reader is referred to the online version of this book).
The release of AA, EPA or DHA by PLA2 is the initial rate-limiting step in the synthesis of bioactive eicosanoids, prostaglandins (PGs), leukotrienes (LTs), and cytochrome P450 metabolites. Although the cyclooxygenase and lipoxygenase (LOX) pathways that produce prostanoids and LTs, respectively, have profound roles in inflammation and regulation of metabolism, the cytochrome P450 epoxygenase and FA omega hydroxylase P450 produce unique eicosanoids that also play a significant role in inflammation and recently, in the regulation of metabolism. The interrelationship between eicosanoid metabolic enzymes and drug-metabolizing enzymes is evident from: 1) many of the same transports for drug metabolites are also used in the trans-cellular synthesis of bioactive eicosanoids, 2) conjugation of drugs with glutathione for transport and synthesis of LTs conjugate to glutathione are performed by glutathione-S-transferase as well as glutathione being necessary for the synthesis of eicosanoids and epoxide hydrolase function in both pathways, and 3) both thromboxane synthase (TXAS) (CYP5), prostacyclin synthase (CYP8), epoxygenase CYP2 and FA omega hydrolase (CYP4) cytochrome P450 enzymes participate in drug metabolism and eicosanoid pathways.
The functional role of eicosanoids in the inflammatory etiology of diseases of metabolic syndrome (MetS) has been extensively studied in relation to immune cell recruitment and cytokine, chemokine production and their activation of inflammatory pathways in cancer, diabetes, and cardiovascular disease (CVD). However, the role of eicosanoids in the regulation of metabolic pathways of lipid and carbohydrate metabolism in obesity, hyperlipidemia, hypertriglyceridemia, hypertension, and insulin resistance has only recently been studied with the use of eicosanoid metabolic enzyme transgenic and global knockout mouse models. These studies in PLA2, 5-lipooxygenase, and 12/15-LOX pathways and knockout mice of fatty acid desaturase (FADS) and elongase (Elovl) in the formation of AA from α-linoleic acid (ALA) have strongly supported eicosanoids as key regulatory molecules in MetS and the progression of hepatic steatosis to steatohepatitis in nonalcoholic fatty liver disease (NAFLD). Furthermore, it is uncertain whether these knockout mice will show alterations in drug-metabolizing enzyme function and regulation. This will be of particular interest with respect to drugs that target inflammation through inhibition of eicosanoid metabolism. These same drugs also target key enzymes in intermediary metabolism and are metabolized by drug-metabolizing cytochrome P450s. The future challenges will include construction of floxed tissue-specific knockout animals to study the role of eicosanoid metabolism in regulation of adipose tissue lipogenesis and lipolysis, in the regulation of pancreas hyperinsulinemia and β-cell destruction, and in progression of steatosis to steatohepatitis and fibrosis in NAFLD.
Finally, we can learn much from patients with sepsis, glucocorticoid disorders and MetS in understanding how eicosanoids link inflammation, drug metabolism and diseases of MetS. The cardinal signs of acute inflammation of dolar (heat), calor (pain), rubor (redness), and tumor (edema) are initiated by a cascade of eicosanoid lipid autacoids, cytokines, and chemokines. Normally, the resolution of inflammation begins with the transcellular synthesis of antiinflammatory lipoxins (LX) and resolvins between different cell types. However, in chronic inflammation, the persistent cellular damage by foreign agents amplifies the inflammatory cascade, which initiates a poorly calibrated immune response that progresses from a local to a systemic response involving multiple organs, leading to immune system repression of drug metabolism and deregulation of basic metabolism. This anomaly is observed in sepsis and septicemia with multiple organ failure, which is the leading cause of surgical deaths, with a death rate equal to that of myocardial infarction. Sepsis is characterized by multiple and systemic changes in several organs that lead to insulin resistance, dyslipidemia, cholestasis, hyperbilirubinemia and vasodilation, vascular leakage, hypovolemia and coagulopathy. These symptoms are also observed in patients with Cushing syndrome and individuals with MetS. Recent studies have revealed alterations in eicosanoid metabolism in septic patients and a downregulation of the major drug-metabolizing cytochrome P450s such as CYP1, CYP2, and CYP3 families that metabolize more than 90% of known drugs, thereby making sepsis a challenge to manage from a therapeutic perspective. It is of interest that CYPs involved in the metabolism of endogenous lipids and eicosanoids have not been characterized with respect to sepsis and metabolic alterations.
The purpose of this review is not to recapitulate the several excellent reviews on eicosanoid metabolism and inflammation but to attempt to link the eicosanoids as pivotal lipid mediators in the control of inflammation and intermediary and drug metabolism in diseases such as MetS, dyslipidemia, hypertriglyceridemia, hypertension, insulin resistance and obesity. We hope that this review will provide insight into the function of eicosanoid metabolites in the regulatory control of lipid and carbohydrate metabolism in adipose tissue, pancreas, liver, and cardiovascular system under MetS.
2. Lipid Metabolism in Control of Eicosanoid Synthesis
2.1. Fatty Acid Transporters
The uptake of essential FFAs, ALA, LA occurs through several transport mechanisms that include caveolins of lipid rafts, FATPs, FABPs, acyl-CoA binding proteins (ACBPs), solute ligand carriers (SLCs), and fatty acid translocases (FATs/CD36) (Table 5.1). The FATPs consist of several integral plasma membrane proteins that show both chain-length and saturation-dependent transport of FFAs (Table 5.1). FATPs have ACS activity and therefore trap FAs inside the cell. FAT/CD36 is expressed in numerous tissues and facilitates FA uptake from serum albumin and insertion into membrane with assistance of FABP. Both FABP and ACBP bind an array of FAs and eicosanoids and function in the intracellular transport of both FAs and FA-CoAs to different organelles including the nucleus (Makowski & Hotamisligil, 2004). The SLC proteins are involved in the uptake of particular PG and other eicosanoids by SLC transporters that have a significant role in the transcellular synthesis of antiinflammatory eicosanoids during the resolution of inflammation (Fig. 5.1).
Table 5.2. Mechanism of regulation of G-protein coupled receptors (GPCRs) and Nuclear hormone receptors (NHRs) by bioactive lipids).
| Gene id | Nomenclature | Tissue | Regulation | Substrate ligand | Protein interaction | Function | ||
|---|---|---|---|---|---|---|---|---|
| FFAR1 | GPR40 | Pancreas | — | C10-C18, TZD | Gq | GSIS secretion | ||
| FFAR2 | GPR43 | Adipose | PPARγ | C2-C5 | Gq,Gi | Inhibit lipolysis | ||
| FFAR3 | GPR41 | Intestine, adipose | — | C2-C4 | Gi | GLP, Leptin secretion | ||
| GPR84 | MT | — | C9-C12 | Gi | IL12-p40 | |||
| GPR119 | Pancreas, intestine | — | LysoPC, 5-HEPE | Gs | GLP-2,PYY secretion | |||
| GPR120 | Colon, adipose | — | C10-C18, PUFA | Gq | GLP-1 secretion | |||
| EP1 | — | |||||||
| EP2 | PGE2 receptor | MT, CNS, T-B | — | PGE2 | Gq | Ca2+↑, CVS, immune | ||
| EP3 | PGE2 receptor | MT, CNS, DC, E, F | — | PGE2 | Gs | cAMP↑, immune | ||
| EP4 | PGE2 receptor | MT, CNS,K, IM | — | PGE2 | GI,G12/13 | cAMP↓, fever | ||
| DP1 | PGE2 receptor | MT, DC, T,CAN | — | PGE2 | Gs,Gi | cAMP↑, inflammation | ||
| DP2 | PGD2 receptor | VSMC, BSMC, P | — | PGD2, PGJ2 | Gs | cAMP↑, intestine | ||
| IP | PGD2 receptor | Th2, MT, IM | — | PGD2, PGJ2 | Gi | cAMP↓,chemotaxis | ||
| FP | PGI2 receptor | VSC, MT, IM | — | PGI2 | Gs,Gq | cAMP↑ | ||
| TP | PGF2 receptor | MT, K, L, IM | — | PGF2α | Gq | Ca2+T | ||
| BLT1 | Thromboxane | MT, IM P, CNS | Sp1,AML1 | TXA2, isoprostanes | Gq | Ca2+T | ||
| BLT2 | LTB4 receptor | Myeloid, spleen | Sp1 | LTB4, 20-LTB4,12-HETE | Gi | Chemotaxis, proliferation | ||
| cysLT1 | LTB4 receptor | Spleen, Liver, | AP-1,GATA | LTB4,12-HETE, 20-LTB4 | Gi | Chemotaxis, proliferation | ||
| LTD4 receptor | myeloid | STAT6 | LTD4 > LTC4 > LTE4 | Gq | SRS-A, inflammation | |||
| cysLT2 | LTC4 receptor | Leukocytes, S,L,I | STAT1,Jak | LTC4 = LTC4 > LTE4 | Gq | SRS-A, inflammation | ||
| ALX/FPR2 | LXA4 receptor | Leukocytes, S,B, heart | — | LXA4, 15epiLXA4,peptides | Gs,i,q | Antiinflammatory | ||
| LPA1-5 | LPA receptor | MT, myeloid MT | — | Lysophosphatidic acid | Gi,Gq,G12/13 | CVS, proliferation | ||
| NR1C1 | PPARα | Liver, kidney | FXRα | LTB4, 8-HETE, EET, EPA, | B-oxidation | |||
| NR1C2 | PPARβ | Muscle | — | DHA, CLA, PL, fibrates | Antiinflammatory | |||
| NR1C2 | PPARγ | C/EBP | 15-keto PGE2, | β-oxidation, antiin-flammatory | ||||
| NR2A1 | HNF4α | Muscle, adipocyte | — | PGI2, 15-HETE,EPA, RA | Adipogenesis | |||
| NR1H3 | LXRα | macrophage | — | 15dPGJ2, 15-HETE, EPA | Antiinflammatory | |||
| NR1H4 | FXRα | Liver, MT | — | 13-HODE, PGF2α,TZD | β-oxidation | |||
| NR5A2 | LRH-1 | Liver | — | Aminosalicylic acid | Antiinflammation | |||
| NR2B1 | RXRα | Liver | — | Linoleic, saturate FA | Cholesterol sensor | |||
| NR1B1 | RARα | Liver | LXRα | Oxysterol | Bile acid sensor | |||
| Gene id | Nomenclature | Tissue | Regulation | Substrate ligand | Protein interaction | Function | ||
| NRA4 | NAR4 | MT | GPCR, | Bile acids | Antiinflammatory | |||
| NROB2 | SHP | MT | TLRs FXRα | Phospholipids | Glucocorticoids | |||
| MT | Cis-retinoic acid, | HNR partner | ||||||
| DHA, | ||||||||
| MT | All-trans retinoic acid Unknown Retinoids? | Differentiation β-adrenergic signal β-oxidation Energy glucose homeostasis | ||||||
AML1, acute myeloid leukemia1; B, basophils; CVS, cardiovascular system; Th2-CRTH2, chemoattractant receptor homologous receptor; CNS, central nervous system; BSM, bronchial smooth muscle; CLA, conjugated linoleic; DC, dendritic cell; E, endothelia; F, fibroblast; GSIS, glucose-stimulated insulin secretion; GLP, glucagon like peptide 1; K, kidney; L, lung; I, intestine; IM, immune cells; M T, multiple tissues; PYY, pancreatic YY peptide; SRSA, slow-reacting substance of anaphylaxis; S, spleen; STAT, signal transducer activator transcription; TZD, thiazolidinedione; TLR, Toll-like receptors.
This table includes receptor's primary tissue of expression, regulation, ligand or substrate activation and primary function in target tissue.
2.1.1. Fatp
The FATP protein family is composed of six members with different preferences for saturated, branched chain and unsaturated FAs. FATP1 (SLC27A1-ACSVL4) is expressed in several tissues including adipocytes and is involved in insulin-induced membrane translocation. Overexpression of this protein increases diacylglyerol transferase activity, indicating that this transporter channels FAs to TAG synthesis (Watkins, 2008). FATP2 (SLC27A2-ACSVL1) is localized in the peroxisome and ER. This protein is believed to have a significant role in the synthesis of TAG and PL by trafficking PUFAs into both phosphatidylcholine (PC) and PI.
FATP3 (SLC27A3-ACSVL3) transports FAs to the microsomes where the FAs associate with cytosolic LDs involved in neutral lipid storage, an early event in hepatic steatosis (Poppelreuther et al., 2012). FATP3 accounts for 30% of activated intracellular FAs, and knockdown of this protein by specific siRNA significantly reduced FA uptake and synthesis of neutral lipids and LD formation. FATP4 (SLC27A4-ACSVL5) knockdown reduces the levels of PLS, cholesterol esters and ceramide in the skin leading to keratinocyte hyperproliferation and hyperkeratosis due to reduced incorporation of very-long-chain ω-hydroxylated FA into ceramide. FATP4 is associated with several organelles and is upregulated in obesity and insulin resistance in humans (Gertow et al., 2004). FATP5 (SLC27A5-ACSVL6) is the major liver transporter of bile acids, while FATP6 (SLC27A6-ACSVL2) is expressed in the placenta and heart. Three FATPs, FATP3, 4, and 6, have a preference in the transport of AA and their association with the ER indicates that they channel FAs for PL synthesis.
The uptake of FAs plays a central role in metabolic homeostasis that is controlled between organs to balance storage with metabolic needs during the fed and fast states. FATPs/SLC27A1-6 not only mediate the organ-specific uptake of FAs but also functions in the intracellular partitioning of selective FAs to different subcellular location. The interplay of organ-specific uptake of FAs is apparent from knockdown of FATP5 and FATP2 in the liver and white adipose tissue (WAT). Liver-specific knockdown of FATP5 and FATP2 not only decreases long-chain fatty acid (LCFA) uptake but also prevents diet-induced hepatic steatosis through lowering hepatic TAG and LD formation, resulting in improved liver morphology, insulin sensitivity, and glucose homeostasis (Kazantzis & Stahl, 2012). Similarly, mice with adipocyte-specific knockdown of FATP1 are resistant to diet-induced diabetes and insulin resistance because of redistribution of LCFA to liver for β-oxidation. The importance of FATP in human MetS is apparent from polymorphisma in the FATP5 promoter that are strongly associated with liver disease susceptibility (Bu & Mashek, 2010) and an intron polymorphism of FATP1 that is associated with increased plasma triglycerides, chylomicrons, and low-density lipoprotein (LDL) particle size. Whether these polymorphisms influence expression of hepatic FATP5 or adipose FATP1 will have to be determined.
2.1.2. Fabp
In contrast to FATP acyl-CoA synthetase activity, the 10 FABP proteins and three ACBP proteins function to distribute acyl-CoA FA between different organelles. There are two groups of FABPs, the plasma membrane FABPS that associate with CD36 and the intracellular FABPs. FABPs are named according to the tissue from which they have been isolated and function as chaperones of FA-CoA to specific organelles. FABPs are involved in the conversion of FAs to eicosanoids, saturation and transport of LTA4 in transcellular metabolism during resolution of inflammation, as well the transport of the PUFAs, DHAs and lysoPLs.
Liver FABPs (L-FABPs) constitute 5% of hepatocyte cytosolic proteins and unlike other FABPs, L-FABP has two distinct binding sites with different affinities for FAs. The notion of how L-FABP discriminates between transporting LCFAs for lipoprotein synthesis and fatty oxidation implies interaction with other FATPs. L-FABP-null mice have diminished FA β-oxidation, indicating a major role in LCFA transport to mitochondria (Atshaves et al., 2010). The intestinal FABP (I-FABP) is expressed in the epithelium of the small intestine with L-FABP and ileal bile acid binding protein (IL-FABP), with each FABP showing regional distribution to different segments of the intestine (i.e. L-FABP, proximal; IL-FABP, distal; and I-FABP, throughout). I-FABP-null mice have an enlarged liver and have weight gain, which suggests that L-FABP and G-FABPG may compensate for loss of I-FABP. L-FABP has a unique role in the intestinal synthesis of chylomicrons, which cannot be replaced by I-FABP. L-FABP seems to function in partitioning LCFA to PL biosynthesis, while I-FABP functions to partition LCFA to TAG synthesis. I-FABP has been linked to MetS through a mutation that increases postprandial serum lipids in humans (Furuhashi & Hotamisligil, 2008).
The heart and skeletal FABPs (H-FABP) function to direct acyl-CoA to mitochondria for FA β-oxidation. H-FABP-null mice show a switch from FA to glucose oxidation similar to the metabolic changes in the heart in ischemia, indicating that H-FABP is required for LCFA transport to maintain mitochondrial FA β-oxidation. H-FABP-null mice also display alteration in TG and PL with an increase in PA and decrease in AA (C20:4n6) incorporation in TG and PL. Both H-FABP and L-FABP regulate LCFA oxidation by activating peroxisome proliferator activated receptor (PPAR)-α. The adipocyte FABP (A-FABP, FAB4-aP2) transcription is controlled by FA, PPARγ, and insulin. A-FABP binds and activates hormone-sensitive lipase (HSL). A-FABP-null mice are protected from atherosclerosis, suggesting a role in MetS. It has been suggested that A-FABP has a central role in foam cell formation in macrophage through suppression of PPARγ-liver-X-receptor (LXR)-α activation of ATP-binding cassette (ABCA1)-mediated cholesterol efflux, induction of inflammatory cytokines, iNOS, and cyclooxygenase 2 (COX 2). The induction of A-FABP in intestinal epithelial cells by Th2 cytokines, interleukin (IL)-4 and IL13, mediated through GPR84 increase T and B cell IL4 secretion and links FA transport and immune response. It will be of interest to determine if the FA receptor (GRP84) and A-FABP are coordinately regulated during MetS.
Epidermal FABP (E-FABP) has a similar FA affinity and reactivity as adipocyte A-FABP. Adipocytes E-FABP-null mice have increased insulin-dependent glucose transport in adipocytes and may function with GPR40 in amplification of glucose-stimulated insulin secretion (GSIS). E-FABP binds retinoic acid and delivers to PPARβ for nuclear hormone receptor (NHR) activation (Schug et al., 2007). The skin type keratinocyte K-FABP functions in brain development where it provides a continuous supply of AA and DHA for neuron growth and axon development in membrane biogenesis. Brain B-FABP is expressed in glia of the white matter and strongly binds ω3-PUFA, and B-FABP-null mice have altered emotional responses typical of schizophrenia. Neuronal FABP (N-FABP) is expressed in the peripheral nervous system where it maintains the lipid composition of myelin.
All FABP-knockout mice exhibit an increase in serum FFAs that are associated with obesity, diabetes and insulin, suggesting that the distribution of FAs to different cells rather than serum levels initiates MetS. In the A-FABP and E-FABP double knockout mice, serum FA composition shifts to short-chain FA (SCFA) with an increase insulin-stimulated glucose uptake, FA oxidation and AMP protein kinase (AMPK) activity. It is likely that serum saturated fatty acids (SFAs) activate adipocyte GPR41 and stimulate leptin release. Two other transporters of FAs are the plasma membrane (FABPpm) and FA translocase FAT/CD36. FABPpm has a unique amino acid sequence, which is identical to that of mitochondrial aspartate aminotransferase, a serum enzyme used to assess organ damage. Muscle contraction and AMPK activity increase the translocation of FABPpm to the plasma membrane in adipocytes and muscle cells. FAT/CD36 is a scavenger receptor protein with multiple functions in metabolic diseases, inflammation, and lipid metabolism. Expression of FAT/CD36 in muscle, adipocytes, and heart is critical for FA oxidation and esterification as revealed in FAT/CD36 knockout mice where muscle contraction was lost. It has been proposed that FAT/CD36 interacts with FATP and FABPpm to mediate FA transport across the lipid bilayer where FABP may facilitate absorption from inner plasma membrane leaflet. Insulin-Insig signaling pathway increases FAT/CD36 translocation to plasma membrane and association with FATP. Upon FA binding, FATP translocates to mitochondria and associates with carnitine palmitoyltransferase (CPT)-I, leading to oxidation of LCFA.
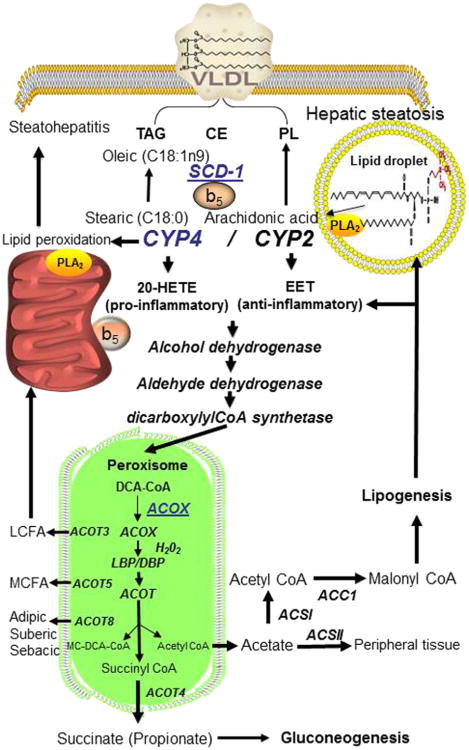
Unlike the close relationship between FATP and long-chain acyl-CoA synthetase (ACSL) where FATP facilitates FA uptake, while ACSL medicates activation, trapping, and vectorial acylation, FABP imports and transports FFAs to different organelles. The 10 organ-specific FABPs show difference in ligand selectively, binding affinity, and the mechanism of ligand binding. Unlike FATPs that solely transport FA-CoAs, FABPs show a broad range of ligands ranging from LCFA, lysoPLs, heme, and cholesterol. Organ-specific FABPs are largely overexpressed in both NAFLD and nonalcoholic steatohepatitis (NASH). Adipocyte FABPa is overexpressed in the liver and found at elevated levels in the serum of patients with NAFLD (Higuchi et al., 2011; Hoo et al., 2012; Kim, et al., 2011). Knockdown of A-FABP in Kupffer cells of mice fed a high-fat (HF) diet and administered LPS leads to resistance to steatohepatitis and hepatic production of proinflammatory cytokines (Higuchi et al., 2011; Hoo et al., 2012; Kim, Cho, et al., 2011). In human patients with NASH, visceral adipose A-FABP levels are significantly higher than in subcutaneous adipose tissue, suggesting that either A-FABP from infiltrating macrophages or visceral adipocytes predisposes patients to progress from NAFLD to NASH. In patients with NAFLD, the level of L-FABP strongly correlates with the extent of obesity and levels of hepatic lipid accumulation (Yoon et al, 2012). The prevention of liver damage in mice fed an HF diet and administered LPS to induce acute liver injury by treatment with an A-FABP inhibitor suggests that targeting the adipocyte-monocyte A-FABP may be a novel therapy to prevent NAFLD progression to NASH (Higuchi et al., 2011).
2.1.3. ACBP-acyl-CoA Binding Protein
ACBPs are multifunctional housekeeping proteins that show tissue-specific distribution similar to FATPs and FABPs and are responsive to glucose and insulin signaling. There are four members of ACBP protein, which all bind C14–C22 acyl-CoA esters with high affinity (Kd = 1 nm) and specificity. ACBPs inhibit acyl-CoA ester hydrolysis and provide acyl-CoA esters to PL, glycerolipid, cholesterol ester, and ceramide synthesis. ACBPs prevent acyl-CoA inhibition of several metabolic enzymes, including acetyl-CoA carboxylase (ACC), ACSL, CPT1, adenine nucleotide translocator and acyl-CoA: cholesterol acyltransferase. Proteolytic fragments of ACBP have been shown to inhibit benzodiazepine receptors that are found at high levels in lymphocytes, macrophages, platelets and granulocytes. Furthermore, ACBPs function in FA metabolism, steroid synthesis and in the regulation of insulin secretion, cholecystokinin secretion, inflammation, and apoptosis. There are four ACBPs in humans that have been extensively characterized: those in liver, testis, brain and recently identified adipose ACBPe (Ludewig et al., 2011). Recently, additional 10 variant human ACBPs have been identified and shown to be transcriptionally controlled by sterol regulatory element binding protein 2 (SREBP2), hepatocyte nuclear factor 4α (HNF4α) and NF-κβ that function as central regulators of cholesterol, glucose metabolism and inflammation (Nitz, Kruse, Klapper, & Doring, 2011). Because ACBPs have very high affinities for plasma membrane, it has been proposed that ACBPs are responsible for PL turnover. Depletion of ACBP in cells increases short-chain unsaturated FA in the membrane PI and PC and reduces SFAs. ACBP has recently been shown to function in maturation of SREBP through activation of SREBP cleavage- activating protein in ER and insulin-induced gene (Insig) pathway. It is not known whether membrane structure or steroid levels prevent maturation of precursor SREBP. Additional studies need to be performed to determine if ACBPs are able to transport PUFAs and possibly eicosanoids (Ludewig et al., 2011).
The role of ACBPs in MetS and NAFLD unlike other FATPs has not been as extensively studied, even though they distribute FA-acyl-CoA to different organelles and have intrinsic responsiveness to glucose and insulin. Furthermore, ACBPs are regulated by transcriptional factors that control fat and glucose metabolism (HNF4α, SREBP) and inflammation (NF-κβ) and regulate the partitioning of FAs between esterification and β-oxidation pathways (Nitz et al., 2011). Transgenic mice overexpressing ACBP show increased accumulation of different lipid classes and increase in liver TAG, suggesting that they have an important role in hepatic steatosis (Huang et al., 2005). The importance of ACBP in hepatic steatosis is further revealed in ACBP- knockout mice that show a delayed induction of lipogenic and cholesterogenic gene pathways due to a decrease in proteolytic processing of SREBP1 and SREBP2 (Neess et al., 2011). Because ACBP and acyl-CoA levels are similar in cells, it is not known whether increased acyl-CoA levels would occur in ACBP knockout mice and lead to increase in peroxisomal, microsomal, and mitochondrial FA oxidation. However, increased acyl-CoA content correlates with muscle insulin resistance but not liver insulin resistance, indicating that excess acyl-CoA has tissue-specific effects in the regulation of metabolism and the prevention of acyl-CoA lipotoxicity Increased acyl-CoA levels have a dramatic effect on metabolism by covalent and allosteric enzyme modification as well as transcriptional mechanism dependent on FA or acyl-CoA chain length and degree of unsaturation. An imbalance in the FA and acyl-CoA pools would also influence β-oxidation and the synthesis of complex lipids, PLs, cholesterol esters, ceramide, and triglycerides.
2.1.4. Cellular Uptake of FFAs
Macrophages express both A-FABP and E-FABP that have distinct functions in lipid metabolism (Storch & Thumser, 2010). Macrophage-specific A-FABP–/–ApoE–/– congenic mice are protected from diet-induced obesity (DIO) and atherosclerosis (Erbay et al., 2009), possibly through A-FABP-mediated cytokines, which activate c-Jun-N-terminal kinase (JNK1). JNK1 has been shown to increase expression of A-FABP, which binds PPARβ and delivers FA ligand to PPARs. FATP and FABP are closely linked to metabolic inflammatory process through their ability to supply FA to critical lipid metabolic pathways for TG, PL synthesis, FA oxidation, and recently as transporter of key LCFA to the nuclear receptor (NR) to modulate both metabolic and inflammatory transcription pathways. It is rather unfortunate that there are no studies on the role of FATPs or FABP in eicosanoid transport, although a number of studies have been performed on the transport of SFAs and uSFAs of different lengths. The expression of similar FABPs in adipocytes and macrophages indicates a link between the energy needs for inflammation and the maintenance of metabolic homeostasis where disruption of this balance initiates an unintended immune response leading to MetS. The development of targeted inhibitors of A-FABP for LCFA transport, similar to knockout mice, displayed enhanced insulin sensitivity, reduced hepatic steatosis and atherosclerosis as well as a reduction in obesity-associated inflammatory cytokines through attenuation of JNK1 activity (Furuhashi et al., 2007). In the human population, a gene variant of A-FABP gene with reduced function protects against MetS (Tuncman et al., 2006). Similarly, reduced activity of FATP4 has a beneficial effect in MetS (Gertow et al., 2004).
2.1.5. ACBP and FABP Fatty Acid Transport to the Nucleus
Several FABPs have the helix-turn-helix domain critical amino acid residues for protein–protein and protein–membrane interaction as well as a nuclear localization region that allows FABPs to transport FAs to nuclear transcription factors. ACBP exerts direct effects on gene regulation through FA acylation of histone 3, which is dependent on CoA ester and reactivity of cysteine (Wilson et al., 2011). Many in vitro studies have shown that FABP and ACBP can bind NHR, PPARα and HNF4α and elicit a transcriptional response by providing an endogenous ligand, either LCFA or LCFA-CoA. Fatty acid NHR ligands in the nucleus have high affinity binding (Kds in nanomolars) for NHRs and the ability to induce conformational changes in NHRs, resulting in coregulator recruitment to NHRs (Atshaves et al., 2010; Schroeder et al., 2008). Support for the role of LCFA-CoA as NHR ligand is seen in peroxisome acyl-CoA oxidase (ACOXI)-null mice with the hyper-activation of PPARα that leads to very-long-chain fatty acid (VLCFA) and VLCFA-CoA accumulation and increased FA β-oxidation. In adrenoleukodystrophy patients, VLCFA accumulates in the cytosol and there is no formation of VLCFA-CoA or hyperactivation of PPARα (Sanders et al., 2006). PPARα has a high affinity for polyunsaturated LCFAs, LCFA-CoAs and VLCFA-CoAs, but not saturated LCFAs or VLCFAs (Schroeder et al., 2008).
One controversial issue concerning eicosanoids as in vivo activators of PPAR is whether micromolar concentrations of PGs, LTs, and cytochrome P450 eicosanoid metabolites for receptor activation can be reached in the nucleus. The transport of eicosanoids by FABP and ACBP may be one mechanism to reach these local HNR-activating concentrations. In contrast to PPARα, HNF4α have high affinity for saturated LCFA and VLCFA acyl-CoA but not polyunsaturated acyl-CoA, suggesting that FA-CoA chain length and degree of unsaturation determines whether HNF4α or PPARα will be activated (Hostetler et al., 2006). FABP binds PUFAs with greater affinity than saturated LCFA and activates PPARα, while ACBP preferentially binds saturated LCFA and activates HNF4α (Schroeder et al., 2008). Recently, HNF4α has been shown to bind linoleic acid (C18:2n6); however, receptor activation was not observed (Yuan, Ta, et al., 2009), suggesting that unsaturated LCFA may inhibit HNF4α activity. These results also suggest that ACBP selectivity cooperates with HNF4α, while L-FABP selectively cooperates with PPARα to mediate downstream coactivator or corepressor association with HNR. Thus saturated LCFA binding to HNF4α would increase activity and inhibit PPARα transactivation, while polyunsaturated LCFA-CoA would decrease HNF4α activity and increase FABP-PPARα transactivation. Because PPARα and HNF4α regulate transcription through a similar direct repeat 1 sequence and compete for the same coactivators and corepressors, receptor activation would be determined by saturated or polyunsaturated LCFA ligands, while crosstalk between these receptors would be determined by FABP/ACBP-mediated coregulator recruitment and cognate receptor repression (http://www.CISREG.ca/tfe).
Future studies need to address the in vivo role of FABP and ACBP protein in the transport of eicosanoids to the nucleus by immunohistochemical colocalization of labeled LCFA ligands, transport protein (FABP, ACBP) and NR (PPAR, HNF4) with receptor-mediated gene activation. Indeed, transgenic mice overexpressing ACBP fed an HF diet have ACBP-induced tissue-specific regulated expression of PPARs and SREBP (Oikari et al., 2008). In contrast, L-FABP gene ablation inhibits PPARα transcription of genes coding for LCFA FA oxidation (Atshaves et al., 2010).
The large number of FA transporters, FATP, FABP, ACBP, and ACSL, in vectorial acylation begs the question why there are so many transporters. Fatty acids have multiple roles in the synthesis of triglycerides, PLs, and eicosanoids, as well as catabolism for energy production, therefore the selective uptake and channeling to different organelles is essential for cell survival. The variety of FA cellular transporters function to control uptake of FA when extracellular FA concentrations are low and to prevent lipid toxicity when extracellular concentrations are high, thus serving as a thermostat in regulating the metabolic needs of the cell in a changing environment. A prevalent view is that passive diffusion and protein-mediated transport contribute to FA uptake. The current concept of FA transport suggests that carboxylated FAs bind to basic residues of caveolin-1 and partitions into the plasma membrane and then internally diffuse to lipid rafts before reabsorption. Membrane proteins function to absorb FA from extracellular media, modulate transport across membrane, trap them intracellulary, and channel FAs to organelles dependent on FA chain length, degree of unsaturation, and metabolic needs.
In recent years, a number of proteins that facilitate FA transport in mammalian cells have been identified. These proteins include CD36/FAT, FABP, FATPS, and ACBP. Although these proteins have different tissue expression patterns, subcellular localization, and FA chain length and unsaturation specificity, each transporter acts independently to modulate FA transport to cellular needs and prevents marked elevation of free FA concentration that can lead to lipid toxicity and cell death. It is apparent that the level of cellular CoA and FABP binding of FFAs is necessary to prevent lipid toxicity.
2.2. Acyl-CoA Synthetase Channeling of FAs
The first step for FAs to be incorporated into cellular PLs is the thioesterification with CoA. The ACSVL/FATP family of ACSL consists of five members, ACSL 1, 2, 4, 5 and 6 with ability to channel FA to different organelles (Watkins et al., 2007). ACSL members show selectivity in the esterification of LCFA depending on degree of unsaturation, with ACSL 3, 4 and 6 showing selectivity toward the thioesterification of arachidonic acid. Gain- and loss-of-function studies have suggested that individual ACSLs channel FA-CoA to different organelles. ACSL1 channels oleic acid toward DAG and PL synthesis and away from cholesterol esterification. ACSL 3 knockdown decreases oleic (C18:1n9) PL incorporation for very low density lipoprotein (VLDL) synthesis (Yao & Ye, 2008). Knockdown of ACSL3 in rat hepatocytes significantly decreased the activation of several lipogenic transcriptional factors, PPARγ, carbohydrate-responsive element binding protein (ChREBP), SREBPIc and LXRα and their target genes suggesting a role in the control of hepatic lipogenesis. ACSL3 associates with the membrane structure of LDs, providing activated FAs for the PC by lysophosphatidylcholine acyltransferase (LPCAT), which enables growth of PL monolayer to keep up with the expanding core when TAG synthesis is high. ACSL3 has a marked preference for AA and EPA over other uSFAs. ACSL4 is predominately expressed in steroidogenic tissues and is localized in peroxisomes and mitochondria and CoA activates preferentially AA and EPA. ACSL5 is located in the outer mitochondrial membrane in intestine and liver and uses a wide range of both SFAs and uSFAs. ACSL5 overexpression in cell increases the incorporation of LCFA into LD for TAG synthesis (Bu & Mashek, 2010). In addition to different FA preference, tissue distribution, and organelle location, ACSLs are differentially regulated by pharmacological inhibitors such thiazolidinedione (TZD).
2.3. Synthesis of Triacylglycerol
Fatty acid transport and channeling have a vital role in the TAG and PL synthesis with regard to lipid metabolism and eicosanoid synthesis, respectively. The synthesis of TAG is initiated by the acylation of glycerol-3-phosphate by several glycerol phosphate acyl transferase (GPAT) isoforms. An extensive review on TAG synthesis has been recently published and the reader is referred to this article for further information (Coleman & Mashek, 2011). We will briefly summarize the steps of TAG and PL synthesis to give the reader a contextual framework to understand their importance in release of eicosanoid precursors by phospholipase A2 enzymes. There are four GPATs with GPAT1 being the major GPAT in the liver and has a preference for C16:0. In GPAT(–/–)–/– mice there is a reduction of palmitic acid at the sn-1 position with an increase in sn-2 AA in PC, PE and PI, suggesting that the sn-1 FA determines the FA incorporated into sn-2 position. GPAT1 location in the outer mitochondrial membrane competes with CPT1 for FA since over-expression of GPAT decreases FA β-oxidation while increasing DAG hepatic content. GPAT also appears to function in the inflammatory response since GPAT1-null mice have an increased abundance of proinflammatory cytokines due to an increase in PGE2 and LTB4 resulting from an increase in hepatic and lymphocyte AA pools (Collison et al., 2008; Karlsson et al., 2009). This is reminiscent of a chronic inflammatory response seen in many diseases of MetS. GPAT2 is a second mitochondrial isoform but unlike GPAT1 has no FA preference. GPAT3 is the first ER GPAT and shows a high preference for C12:0–CoA and is prominently expressed in adipocytes (Kim et al., 2010). GPAT4 is expressed in liver and adipose tissue. GPAT4-null mice show a 50% decrease in hepatic TAG synthesis and increased energy expenditure and are resistant to fatty liver (Nagle et al., 2008).
GPAT-generated lysophosphatidic acid (LPA) is converted to PA by different sn-2-acylglycerol-3-phosphate acyltransferase (AGPAT) located in ER and mitochondria. AGPAT1 incorporates AA and/or stearic-CoAs into the sn-2 when palmitic acid is in the sn-1 position. AGPAT2 is expressed in liver, heart and adipocytes and has a role in adipocyte differentiation since deficiency causes human lipodystrophy. AGPAT3 has high activity toward acylation of lysophosphoinositol and its overexpression increases PL species containing PUFAs. Although all AGPAT isoforms incorporate uSFAs in the sn-2 position, their activities for different lysoPL and type of uSFA remains unclear.
The final step in the synthesis of TAG is the hydrolysis of PA by sn-3-phosphatide phosphohydrolase (PAP/LP) of the lipin family located in the plasma membrane. Other lipin members translocate from ER to cytosol to hydrolyze PA formed by AGPAT to produce DAG that can either be converted to TAG or used for synthesis of PLs by the Kennedy pathway. There are three members of PAP lipin family that contain a nuclear localization sequence that interacts with transcription coactivators. Lipin1 interacts with PPARα, HNF4α, and peroxisome proliferator activated receptor coactivator (PGC)-1α to promote transcription of FABP4 and cytokine expression (Kim et al., 2010). Lipin1 has the highest PAP hydrolase activity of all lipins, yet all lipins have similar coactivator activities (Csaki & Reue, 2010).
PAP hydrolase produces 1, 2-diacylglycerol (DAG) that is converted to TAG by diacylglycerol acyltransferase 1 or 2 (DGAT1) located in ER, mitochondria and LDs. Neither DGAT1 nor DGAT2 has a preference for acyl chain length or degree of FA unsaturation. It is unclear whether these DGATs synthesize different TAGs in LDs or in VLDL synthesis. However, overexpression of either DGAT increases LD formation and steatosis, while DGAT1-null mice are resistant to obesity or steatosis when fed an HF diet (Smith et al., 2000).
The synthesis of DAG from PA by lipin is followed by TAG or PL synthesis. How a cell determines whether to synthesize TAG or PL seems to depend on the ATP levels, with high ATP favoring PL synthesis, while low ATP levels favoring increased TAG synthesis (Wu & Carman, 1994). For PL synthesis, different bases are added to the sn-3 position to produce PC, PE, PS, and PI. The free AA cell pool is controlled by PLA2-mediated cleavage of sn-2 position of PL to produce a free FA and lysoPL and a CoA-dependent acyltransferase acylation reaction reincorporates a different uSFA-CoA to reform the PL. In stimulated cells, PLA2-mediated deacylation is the dominant reaction, while in resting cells, the reacylation reaction dominates. In stimulated cells, the released AA is used for eicosanoid synthesis, while the lysoPL is reacylated by lysophospholipid: acyltransferase (LPLAT) a member of the membrane-bound O- acyltransferase family that uses FA-CoA for incorporation. The LPLATs that show preference for reacylation of lysoPL include lysoPC:acyl-CoA acyltransferase 2 and 3 as well as lysoPI:acyl-CoA acyltransferase and lysophosphatidic acid: acyl-CoA acyltransferase 3. Of particular interest is that the LD lipase CGI-58 has lysophosphatidic acid:acyltransferase activity has a high preference for AA (Moessinger et al., 2011; Shindou et al., 2009) PLs are in constant state of remodeling through the Land's cycle by the action of PLA2 and lysophospholipid:acyltransfera se (LPLAT) that use CoA-independent FFAs as substrate. The cellular AA pool is in constant flux and is determined by PL deacylation by PLA2 and reacylation by lysophospholipid acyltransferase (LPLAT). Thus there are two biosynthetic pathways for the incorporation of AA-CoA into PL. The Land's cycle transacylates lysoPL to ensure the proper distribution of FAs to produce numerous cellular PLs, and the Kennedy pathway for synthesis of lysoPC from PA incorporates AA-CoA in the sn-2 position by lysoPL-CoA-dependent acyltransferase (LPLAT).
The products of TAG and PL metabolic pathways affect several cellular signaling pathways involved in MetS. Increased TAG content in the liver, pancreas and muscle is strongly correlated with insulin resistance. There are four currently known lipid metabolites that may account for insulin resistance, FA-CoA, ceramide, DAG and oxidized lipids. Lipid overload as the cause of insulin resistance is evident from the overexpression of lipoprotein lipase that increases tissue TAG, DAG, acyl-CoA and ceramide, while inhibition of adipocyte lipolysis in diabetic patients decreases muscle acyl-CoA and insulin resistance (Bajaj et al., 2005). The paradox of insulin signaling in insulin resistance is how the phosphorylation of insulin receptor (IR) substrate-1(IRS-1) increases lipogenesis, but phosphorylation of IRS-2, which normally inhibits gluconeogenesis by FOXO1 phosphorylation and exclusion from the nucleus, does not inhibit liver gluconeogenesis (Brown & Goldstein, 2008). It is believed that a lipid metabolite inhibits IRS-2 phosphorylation of FOXO1 and thus prevents inhibition of gluconeogenesis. Strong support for lipid metabolites causing insulin resistance comes from studies of knockout and overexpression of T AG biosynthetic enzymes. Overexpression of GPAT1 in mice causes an increase in DAG activation of protein kinase C (PKC)-ε, leading to insulin resistance with no sign of inflammation, indicating a dissociation of insulin resistance from inflammation (Nagle et al., 2009). In contrast, GPAT1-null mice have a lower hepatic content of DAG and PKCε activation with a twofold increase in acyl-CoA and show reduced steatosis and insulin resistance (Li et al., 2010). Therefore, if DGAT1 activity is associated with insulin resistance through DAG activation of PKCε and phosphorylation and inhibition of IRS-2, then overexpression of DGAT should increase insulin resistance. However, overexpression of DGAT1 or DGAT2, which increased hepatic content of TAG, DAG, ceramide and acyl-CoA, did not show insulin resistance or inflammation. Thus, it is possible that overexpression of GPAT causes insulin resistance, while DGAT overexpression protects against insulin resistance, suggesting that possibly LPA promotes, while PA prevents insulin resistance. Adipose triglyceride lipase (ATGL)-null mice have increased muscle DAG levels, yet are glucose tolerant and insulin sensitive, suggesting that PA or DAG protects against insulin resistance.
The tenet that DAG activation of PKCε-mediated serine phosphorylation of IRS-2 causes insulin resistance is questioned by studies using lipin (phoshatidic acid hydroylase)-null mice that inhibit the formation of DAG from PA. Mutation of Lipin1 gene in fatty liver dystrophy (fld) mice leads to lipodystrophy and insulin resistance, while Lipin1 overexpression leads to obesity. In obese human patients, reduced lipin1 levels were found in insulin-resistant patients. This correlation between high lipin and glucose tolerance and/or low lipin and insulin resistance was observed in other human studies. LIPIN1 polymorphisms are associated with insulin levels, body mass index (BMI) and risk of MetS, suggesting that lipid metabolites between GPAT and Lipin may be responsible for MetS insulin resistance. The numerous PL species produced by the Kennedy pathway and the Land's cycle PL remodeling pathway may have important roles in diabetes, obesity and MetS. Unfortunately, the generation of lysophospholipid: CoA acyltransferase (LPCAT) knockout mice have been lacking except for the recent report of LPCAT3 (lysoPC:acyl-CoA acyltransferase) liver-null mice that showed increased levels of lysoPC that promote VLDL by enhancing microsome transfer protein (MTP) expression and thus hepatic TAG accumulation. However, whether these mice have insulin resistance was not determined.
2.4. Desaturation of Unsaturated Fatty Acid in Eicosanoid Synthesis
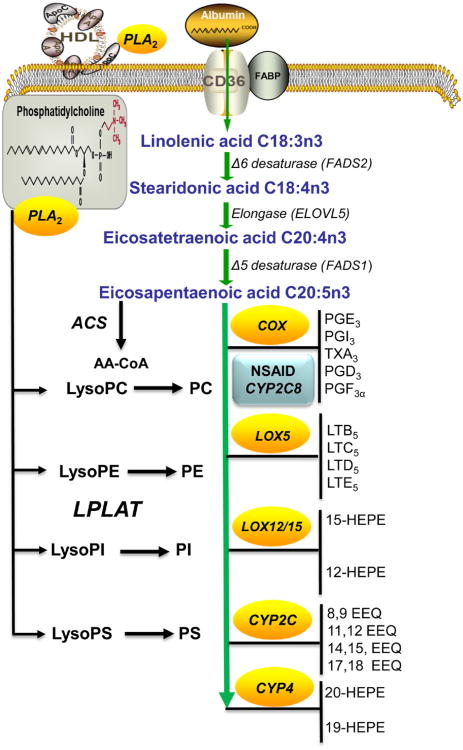
The daily uptake of AA from western diets is calculated to be 0.3–2.0 g/day, while the intake of linoleic acid C18:2n6 is from 10 to 20 g/day and ALA C18:3n3 intake is between 2 and 5 g/day, indicating that the intracellular AA pool is largely determined by our diet. This pool of pro-inflammatory n-6 AA can be modified by reducing consumption of ω-6 linoleic acid and increasing consumption of n-3 linoleic acid. Both, linoleic and ALA are sequentially desaturated and elongated to produce AA and EPA, respectively (Fig. 5.2). The human FA desaturases are encoded by three genes, FADS1, FADS2 and FADS3. FADS1 and FADS2 produce longer chain PUFAs by introduction of double bonds between specific carbons and elongation by Elongase (Elovl) enzymes to produce 20-carbon AA and EPA. Further metabolism of AA and EPA produces eicosanoids by the PG, LT and cytochrome P450 pathways.
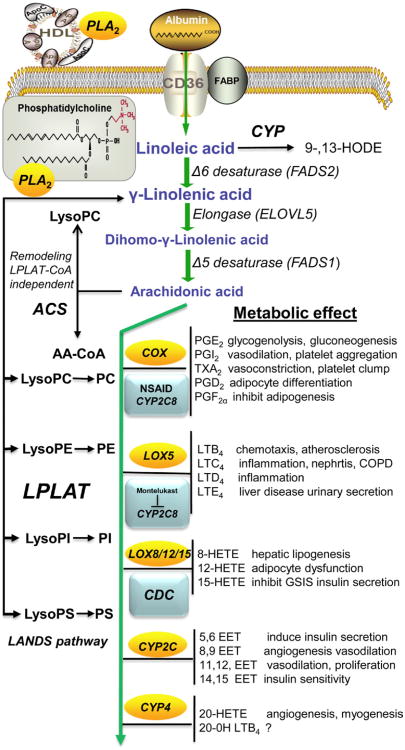
Figure 5.2. Metabolism of linoleic acid to proinflammatory eicosanoids and there function in control of metabolism.
Arachidonic acid (AA) is obtained either directly from diet or synthesis from linoleic acid. This existence of PLA2 in serum lipoproteins provides local release of arachidonic acid from membrane phospholipids and a source of eicosanoids to control inflammation, immune cell function and tissue-specific control of metabolism. Linoleic acid is converted to arachidonic acid by a series of fatty acid desaturase (FADS) and elongase (ELVOL) to produce AA. In humans, there is approximately 100 g of AA distributed between tissue and membrane compartment that have varying turnover rate depending on the metabolic needs of the tissue. Phospholipid AA incorporation occurs through the de novo Kennedy pathway or the Land's membrane remodeling pathway that requires AA-CoA. The remodeling of membrane PL can occur by the Lands pathway that uses lysophospholipid:acyl-CoA acyltransferase (LPLAT) or a CoA-independent transacylase pathway. AA released from membrane phospholipids can be used either to remodel phospholipid membranes or to synthesize eicosanoids, by the prostaglandin, leukotriene, cytochrome P4502 epoxygenase (CYP2), or the cytochrome P450 ω P4504 (CYP4) pathways. The biologically potent autocoid lipids of the series-2 prostanoids and series-4 leukotrienes initiate their biological effects by generally activating prostanoid and leukotriene receptors to initiate a proinflammatory response and activation of nuclear hormone receptor (NHR) and anti-inflammatory response. The CYP2 epoxygenase, epoxyeicosatrienoic acids (EET) also initiate their biological responses through presently unidentified membrane receptors and activation of peroxisome proliferator activated receptors (PPARs). In contrast, the CYP4 ω-hydroxylase metabolite's mechanism of action has not been identified. It is presently believed that 20-hydroxyeicosatetraenoic acid (20-HETE) directly interacts with membrane protein channels to elicit their potent vasculature constrictive and proinflammatory responses. Unlike our extensive understanding of eicosanoids' role in immune cell regulation during inflammation and their function in the cardiovascular system, our understanding of their role in the control of intermediary metabolism is lacking as revealed by our spare knowledge of their metabolic effects. By the use of drugs that inhibit eicosanoid synthesis, including the nonsteroidal antiinflammatory drugs (NSAID), we are able to modulate the cardinal signs of inflammation, pain, heat, redness, edema, and loss of function through possible metabolism by CYP2 epoxygenase resulting in reduced synthesis of EETs and channeling of AA to different eicosanoid metabolic pathways. CYP2C8 metabolizes many of the currently prescribed NSAIDs, and Montelukast inhibits CYP2C8, while CDC, a metabolite of ciprofazacin, inhibits 12/15-lipoxygenase. (For color version of this figure, the reader is referred to the online version of this book).
FADS2 encodes a Δ6-desaturase, which is the first and rate-limiting step in the synthesis of ω-3 or ω-6 EPA and AA, respectively. Deletion of FADS2 abolishes the synthesis of PUFA and eicosanoids. FADS1 Δ5 desaturase activity is 10-fold less than that of FADS2 and produces C20:3 and C20:4 PUFAs that are metabolized to less-active eicosanoids (Fan et al., 2012). FADS-null mice display reduced intestinal crypt proliferation, immune cell homeostasis, and heightened sensitivity to inflammation. The inability of FADS1-null mice to tolerate an intestinal inflammatory challenge is similar to that of the PGE-synthase-null and COX2-null mice (Nakanishi et al., 2011) where PGE2 has a protective role in the intestines. FADS3 is a third gene identified in the FADS cluster that displays a unique expression in other organs different from liver expression of FADS1 and FADS2. Presently, the substrates for FADS3 have not been identified, although its upregulation during oxidative stress suggests a role in the prevention of lipotoxicity. FADS1 and FADS2 expression is increased by insulin, while FADS3 expression is mediated by PPARγ (Arbo et al., 2011; Reardon et al., 2012). There is a strong association of FADS2 gene with plasma liver enzyme levels, MetS, and type II diabetes mellitus (T2DM) (Chambers et al., 2011; Sergeant et al., 2012) and a negative association with FADS1. FADS1 and 2, Δ desaturases, activities require cytochrome b5 and cytochrome b5 reductase that are also used by stearoyl-CoA desaturase (SCD-1) that converts steric and palmitic acids to oleic and palmitoleic acid, respectively. Cytochrome b5 also has an important role in the function and activity of cytochrome P450-mediated drug metabolism since Cyb5-null mice have a 84% decrease in γ-linolenic (GLA) and 200% increase in alpha-linolenic acid (ALA). Cytochrome b5 and cytochrome b5 reductase (Cyb5A, Cyb5R3) or oxidoreductase supplies electrons for desaturase reactions. Cyb5-null mice have impaired desaturation of palmitic and stearic acids and display lipotrophy with increased cytotoxic effects of SFAs. Both Cyb5A and Cyb5R3 reductase genes have been linked to obesity and diabetes susceptibility.
2.5. Elongation of PUFA in Eicosanoid Synthesis
The desaturation of linoleic acid (LA) and ALA by FADS2 produces GLA and stearidonic acids, respectively, which are elongated by elongase 5 (Elovl5). The Elovl family consists of six members in mouse and humans and carries out substrate-specific elongation of FAs of different lengths. The unsaturated VLCFA is elongated by ElovI, 3, 6, and elongation of PUFA is performed by ElovI 2, 4, 5. Elovl1 is involved in membrane modeling and Elovl2 elongates the PUFA, AA, EPA, DHA and docosapentaenoic acid (DPA) and has overlapping function with Elovl5. Elovl3 is found in brown adipose tissue (BAT) and Elovl3-null mice have impaired skin barrier function, complete depletion of fat droplets, and cold intolerance. Elov5 is involved in the elongation C18-C20 unsaturated FAs but not PUFA longer than C22 to produce docosapentanoic acid (C22:5n-6). Elovl3 is found in peroxisomes and produces C24:4n6 and C24:5n3 FAs that are shortened to DPA and DHA. Elovl5-null mice have decreased elongation of 16:1ω7, and Elovl6-null mice show decreased elongation of both C16:1n7 and (Green & Olson, 2011). Both AA and DHA suppress SREBP and its target genes in FA triglyceride synthesis and development of hepatic steatosis (Moon et al., 2009). Elovl4 is expressed in retina, brain and testes and is involved in elongation steps required for DHA synthesis (Yu et al., 2012). Elovl5 induces changes in hepatic FA content and influences multiple pathways in lipid and carbohydrate metabolism and attenuates hyperglycemia in DIO mice and restores insulin sensitivity. Elov6 is involved in the elongation of C12-18 saturated FAs and is found in major metabolic tissues, BAT, WAT, liver and brain. Different single nucleotide polymorphism (SNP) alleles in the human population that have been correlated with insulin sensitivity in human (Morcillo et al., 2011). Recently, a seventh Elovl7 has been identified that has a high activity toward C18:3n-3 (Naganuma et al., 2011). The proper elongation and desaturation of FAs are essential to maintaining lipid homeostasis since disruption of these processes can lead to MetS diseases.
2.6. Phospholipase A2 Role in Formation of Bioactive Lipids in MetS
The incorporation of AA into membrane can occur by three mechanisms:(1) lysophosphatidic acid acyltransferase (LPAAT) synthesis of DAG for PL synthesis through the Kennedy pathway, (2) the CoA-dependent acylation of DAG-CDP choline by lysoPL acyltransferase (LPLAT) or the (3) CoA-independent transacylation by head-group-specific lysophospholipid acyltransferase (LPLAT) of Land's cycle. Membrane lipid PI represents an important cellular signaling molecule. The release of inositol phosphate by PLC is an important regulator of metabolism. Phospholipase-A2-mediated release of AA and lysophosphatidylinositol (lysoPI) also have important roles in both eicosanoid synthesis and metabolic control. LysoPI can bind LysoPI receptors, while AA can be used for reacylation of membrane lipids or used for the production of eicosanoids.
There are a number of recent excellent resources on structure, function and therapeutic modulation of PLA2 (Dennis, Cao, Hsu, Magrioti, & Kokotos, 2011). The multiple functions of PLA2 in blood stream, gastrointestinal system and intracellular location strongly not only implicate that these proteins serve as guardians against foreign insults by activation of inflammation but also suggest that they control basic physiological and metabolic processes.
Therefore the intent of this section is to discuss the role of those PLA2 enzymes in MetS. There are 30 phospholipase A2 and related enzymes that include the 11 secreted Ca2+-requiring extracellular PLA2 (sPLA2), the six Ca2+- dependent cytosolic PLA2 (cPLA2) also known as patatin-like phospholipase domain containing lipase (PNPLA) that function as either phospholipase or lipase, the nine Ca2+-independent PLA2 (iPLA2), the two lysosomal PLA2, the four platelet activating factor acetylhydrolases (PAF-AH) that have specificity for platelet activating factor (PAF) or oxidized PLs, and the adipose-specific PLA2. Phospholipase A2 can be largely divided into two groups: (1) those that function in immune response and inflammation such as, cPLA2, PAF-AH, and sPLA2 and (2) those that function in metabolic disease such as sPLA2, iPLA2, lysosomal, and adipose PLA2.
The secreted family of sPLA2 has 11 members that have antibacterial and antiviral function as well as a role in diseases with an inflammatory etiology and are often referred to as the “inflammatory sPLA2”. These secreted sPLA2 function through two independent pathways, the heparin sulfate proteoglycan (HSPG)-dependent and HSPG-independent pathways, to release AA for PL. Secreted sPLA2 can bind to cell surface HSPG that internalizes the enzyme through caveolin-dependent endocytic pathway where it releases AA from internal anionic PL substrates. In the HSPG-independent pathway, PLA2 binds to bacterial apoptotic cells and exosomes anionic PLs, PLE, PLG, PS, and PA to release AA. PLs entering the digestive tract through food intake or bile acid recycling as PL coated TAG vesicles hydrolyzed to FAs and lysoPLs by PLA2, subsequently allowing digestion bo TAG by pancreatic lipase and carboxyl ester lipase for proper intestinal absorption. The sPLA2IB is a pancreatic enzyme that digests dietary PLs. PLA2IB-null mice are resistant to obesity and show reduced plasma levels of insulin, leptin and glucose on a diabetogenic diet due to reduced intestinal absorption of PL and increased FA β-oxidation by PPARα. Thus inhibition of PLA2IB may be an ideal drug target in the treatment of obesity and diet-induced diabetes.
The relationship between secreted sPLA2 and atherosclerosis is a major area of research since PLA2 is able to modify plasma lipoproteins where upon PC hydrolysis it produces FFA and lysoPC that can trigger vasoactive, chemoactive and proinflammatory conditions (Rosenson & Gelb, 2009). sPLA2 mediates hydrolysis of LDL to small dense proatherogenic LDL particles, while hydrolysis of high-density lipoprotein (HDL) reduces cholesterol efflux from lipid-rich foam cells. Atherosclerotic lesions containing more lysoPC alter apo-B-100 conformation and promote particle aggregation (Rosenson & Gelb, 2009). Transgenic sPLA2IIA mice have an increased incidence of atherosclerotic lesions, while macro-phage sPLA2 exerts a local proatherogenic effect independent of systemic lipoprotein metabolism (Ghesquiere et al., 2005). A hyperlipidemic HF diet increases sPLA2V expression in aorta and induces atherosclerosis by assisting in macrophage uptake of LDL particles. Macrophage sPLA2X is also able to hydrolyze LDL HDL and attenuates cholesterol efflux from macrophage. PLA2X-null mice show an attenuated accumulation of neutrophils in ischemic areas of the heart due to reduced production of LTB4, indicating that sPLA2X has a direct role in neutrophil myocardial injury. It is apparent that sPLA2IIA, sPLA2V and sPLA2X have a significant role in atherosclerosis and CVD; however, their roles in MetS are not fully understood.
Increased release of FFAs and LPC from circulating lipoproteins in diabetic individuals by PLA2V not only increases an individual's risk of CVD but also contributes to MetS. Stress-induced increases in serum glucocorticoids are evident in MetS patients. In sPLA2X-null mice, there is an 80% increase in plasma corticosterone levels, resulting in oxysterol activation of LXRα and macrophage ABCA1 cholesterol efflux transporter. Excessive glucocorticoids can lead to Cushing-like syndrome and associated lipodystrophy observed in obese individuals with MetS. Unfortunately, these lipid derangements associated with MetS have not been investigated in PLA2X-null mice.
The intracellular iPLA2 consists of six calcium-dependent cPLA2 and nine Ca2+-independent iPLA2 that are expressed in many tissues. The most widely studied are these PLA2s because they produce a diverse array of functional lipid products in response to extracellular stimuli. The cytosolic cPLA2 has both phospholipase A2 and lysophospholipase activity. It is the only PLA2 that shows specificity for PLs containing AA exclusively. Thus the cytosolic cPLA2 has a pivotal intracellular role in the production of eicosanoids and a functional role in normal physiological process and the disease pathology. Cytosolic cPLA2IVA is activated by Ca2+-dependent translocation from the cytosol to the perinuclear membrane and functionally couples with COX in PGE2 biosynthesis. The preferred substrate for cPLA2IVA is phosphatidylinositol-4, 5-bisphosphate, which also fully activates cPLA2IVA. Cytosolic cPLA2IVA-null mice display a number of phenotypes due to reduced synthesis of eicosanoids in a broad range of tissues. The broad and extensive pathophysiological role of cPLA2IVA revealed by cPLA2IVA-null mice has been extensively documented (Murakami et al., 2011).
Cytosolic cPLA2IVA-null mice are protected from MetS and associated atherosclerosis, obesity and hepatic steatosis, however its mechanistic role in these diseases has not been studied extensively. Cytosolic cPLA2IVA is localized on LDs, which are present in all cell types and consist of a hydrophobic core of TAG and cholesterol esters surrounded by a monolayer of PLs and cholesterol. Cyotsolic cPLA2IVA phosphorylation by JNK1 and ceramide kinase increases LD formation, while cPLA2IVA knockdown inhibits LD formation (Gubern et al., 2008). Cytosolic cPLA2IVA-null mice are protected from accumulation of LD in adipose tissue and liver (hepatic steatosis) under normal and HF diets. Thus these mice are refractory to atherosclerosis. Because cPLA2IVA-null mice fed an HF diet have no difference in serum leptin, resistin, FFA, VLDL, glucose or insulin compared to the corresponding wild-type mice, it is suggested that cPL2IVA might be an amenable drug target for NAFLD and other obesity-related diseases. The regulation and function of other cytosolic cPLA2 isoforms are less well known with respect to substrate preference and physiological phenotype in disease processes. Cytosolic cPLA2IVB displays a 1000-fold greater lysophospholipase activity compared with other PLA2 (Ghomashchi et al., 2010) and associates with mitochondrial membrane phosphoinositide rich in cardiolipin (CL). Cytosolic cPLA2IVC binds to heart mitochondrial membranes and ER where it is believed to function in membrane PL remodeling because of its high lysophospholipase and transacylase activity.
The human Ca2+-independent iPLA2 consists of nine members that are also known as PNPLA1-9. Patatin is a lipid hydrolase and therefore mammalian PNPLAs have specificity for diverse substrates, including TAG, PL and retinol esters. More than half of the enzymes in this family function as lipases rather than phospholipase. Several of the PNPLA members have important physiological roles in lipid metabolism and energy homeostasis, and thus MetS.
The classical independent iPLA2VIA also known as PNPLA9 exhibits sn-1 lysophospholipase and transacylase activity and thus functions in membrane remodeling through the Land's cycle. It has a fundamental role in cell signaling leading to cell activation, proliferation and migration. Independent iPLA2VIA translocation from cytosol to membrane is mediated by PKC. In pancreatic β-cells, iPLA2VIA overexpression enhances glucose-induced AA release and insulin secretion (Ma et al., 2001). Independent iPLA2IVA functions in the regulation of capacitive Ca+2 entries with calmodulin, which inhibits iPLA2IVA. Upon Ca+2 depletion, calmodulin dissociates from iPLA2IVA, which generates lysoPL that opens Ca+2 entry (SOCE) channels with increased Ca2+, which activates cPLA2IVA-mediated release of AA. Thus cPLA2IVA has an important functional role in Ca2+ homeostasis and eicosanoid synthesis in vascular contraction and relaxation (Xie et al., 2010). Disruption of iPLA2IVA in the pancreas impairs insulin secretory response to glucose, and iPLA2IV-null mice on an HF diet have severe glucose intolerance and coronary-artery-induced occlusion. Intracellular iPLA2IVA, which hydrolyzes membrane PLs, induces lethal malignant ventricular tachyarrhythmia and myocyte apoptosis during acute cardiac ischemia. During apoptosis, cells release FAs and LPC, which are mediated by caspase 3 cleavage of iPLA2IVA to a more active enzyme. The translocation of iPLA2IVA during stress conditions to the mitochondria results in loss of mitochondrial PL and release of cytochrome c and opening of the mitochondrial permeability transition pore.
Independent iPLA2IVA also plays an important role in MetS as revealed not only by impaired glucose-stimulated insulin secretion in iPLA2IVA-null mice but also by the fact that these mice show age-related loss of bone mass with increase in bone marrow fat-laden adipocytes due to alteration in mesenchymal progenitor cells toward adipocytes and osteocyte lineages (Ramanadham et al., 2008). Overexpression of iPLA2IVA increases TAG synthesis and LD formation, suggesting that iPLA2IVA provides FAs for TAG synthesis from the PL pool and also increases LD formation, thus recycling structural PLs for energy-generating substrates. Independent iPLA2IVA has multiple roles at different stages of inflammation by production of lysoPC that attracts neutrophils and monocytes through lysoPC GPCRs, activation of NADPH oxidase in neutrophils during respiratory burst, and neutrophil phosphatidylserine (PS) externalization leading to induction of intrinsic apoptotic pathway (Lei et al., 2010).
Independent iPLA2VIB (PNPLA8) is localized to mitochondria and peroxisome and cleaves PLs at the sn-1 and sn-2 positions depending on the substrate. Mice null for iPLA2IVB are lean and resistant to adiposity, fatty liver, hyperlipidemia, and HF-diet-induced insulin resistance and glucose intolerance (Song et al., 2010); however, these mice display abdominal lipodystrophy and impaired insulin secretion on an HF diet. In skeletal muscle, iPLA2VIB-null mice have impaired mitochondrial β-oxidation as reflected by increased accumulation of long-chain acylcarnitine. In the heart, these knockout mice generate signaling lipid metabolites that modulate energy storage and utilization in different metabolic states by remodeling of CL and thus tailoring mitochondrial lipid composition and metabolism. Knockdown of iPLA2IVB by siRNA reduces cytokine and chemokine overexpression, while overexpression leads to increased PGE2 production through COX1 activation (Murakami et al., 2011). It is uncertain how reduction in selective eicosanoid metabolites in iPLA2IVA-null mice relates to the lean phenotype and resistance to MetS. Data strongly support that iPLA2IVA plays a role in integrating lipid and energy metabolism and possibly through inefficient coupling of electron transport to energy production promotes development of MetS.
Both PNPLA2 (ATGL) and PNPLA3 (adiponutrin) enzymes have attracted much interest in the past few years because of their roles in obesity and MetS. PNPLA2, also known as ATGL, possesses transacylase with weak PLA2 activity and is recruited to LD during lipogenesis (Soni et al., 2009). A number of cofactors regulate PNPLA2/ATGL, which was discussed in an excellent review on ATGL's role in adipose lipolysis (Lass et al., 2011). PNPLA2-null mice display severe defects in TG hydrolysis leading to lipid accumulation in WAT and BAT, while overex-pression promotes lipolysis and inhibits DIO. Several SNPs in PNPLA2 in type II diabetic patients are correlated with reduced plasma FA and TAG. PNPLA2-null macrophages fail to hydrolyze cellular TAGs, thus decreasing cellular levels of FAs, but with an accumulation of LDs. This results in decreased cellular ATP production and impairment of phagocytosis, suggesting that FA must go through a cycle of esterification and hydrolysis before it can be used for energy production (Ahmadian et al., 2009). This implies that FATP, FABP or selective ACSL must channel FA to TG or membrane PL prior to ATGL release and use as an energy substrate. A second TG hydrolyase is PNPLA3 also named adiponutrin, which is found in adipocytes and induced by insulin and in steatotic liver of ob/ob mice where it is induced 100-fold by through LXRα agonist activation of SREBP1 (Huang et al., 2010). PNPLA3 has TAG lipase and transacylase activities with weak PLA2 activity. PNLA3 gene variants are associated with hepatic steatosis and liver function in NAFLD (Tian et al., 2010). A point mutation in PNPLA3 I148M disrupts TAG hydrolytic activity leading to hepatic steatosis (He et al., 2010). Both PNPLA2 and PNPLA3 are TAG hydrolases that modulate TAG content in adipocytes and LD formation in liver and are genetically linked to obesity in humans.
The PAF-AH family members are unique acyl hydrolases that catalyze the release of acetate from the sn-2 position of platelet activity factor (1-0-alkyl-PC). There are four enzymes in this family that are associated with eicosanoid and possibly MetS. The plasma-type PAF-AH (PLA2V11A) has attracted much attention recently in regard to a therapeutic target for atherosclerosis. PAF-AH hydrolyzes acetate or an acyl group up to nine carbons in length from the sn-2 position of PC or PE producing lysoPAF. PAF-AH is associated with apo-B-100 of LDL and HDL where it removes oxidized PC from LDL particles. PAF-AH expression is dramatically increased after LPS administration in several tissues, including circulating leukocytes where it might inactivate PAF and oxidized PL to minimize the pathology of these lipids in sepsis. PAF-AH has a proatherogenic role due to its ability to generate PLC and oxidize FA that recruit and activate leukocytes and induce apoptosis. Thus, pharmacological inhibition of PAF-AH may be of importance in the prevention of atherosclerosis. The intracellular PAF-AH II (PLA2V11B) hydrolyzes sn-2 acyl-chains of up to five carbons and facilitates transfer of acetyl group of PAF to lysoPAF and ceramide in a CoA-independent manner through its transacylase activity. PAF-AHII is highly expressed in liver and kidney where it plays a pivotal role in defense against oxidative stress by degradation of oxidized PLs in membrane. PAF-AHII-null mice are extremely sensitive to chemicals that induce oxidative stress and show elevated levels of esterified 8-iso-PGF2α in the liver. Furthermore, transgenic mice overexpressing PAF-AHII are protected from ischemic injury (Umemura et al., 2007).
There are two distinct members of the lysosome PLA2 family, aiPLA2 (peroxiredoxin6) and macrophage lysosomal PLA2 (LPLA2), which are highly homologous to lecithin:cholesterol acyltransferase. Lysosome aiPLA2-null mice are sensitive to oxidative stress, most likely due to the absence of the enzyme's glutathione peroxidase activity. LPLA2-null mice show a marked accumulation of PL in macrophages with features of foam cells and lamellar inclusion, which is a hallmark of phospholipidosis. Recently, a new adipose-specific PLA2 (AdPLA2) expressed in WAT has been identified. This enzyme exhibits both sn-1 and sn-2 phospholipase activities and releases FA from WAT TG stores (Jaworski et al., 2009). Adipose specific AdPLA2-null mice have reduced WAT and TAG contents but normal adipogenesis with increased FA β-oxidation within adipocytes (Jaworski et al., 2009). AdPLA2-null mice have a higher rate of lipolysis due to a decrease in adipose PGE2 level, which activates the EP3-coupled Gαi receptor, which counteracts cAMP-stimulated lipolysis. Thus AdPLA2 plays an important role in supplying AA for PGE2 synthesis in WAT. Congenic AdPL2-null/ob/ob mice are hyperphagic, yet lean, with increased energy expenditure, but have ectopic TAG storage and insulin resistance reminiscent of human type II diabetes. It is believed that AdPLA2 has a dual role in WAT adipogenesis through both supplying AA for PGE2 synthesis and recruitment of M1 macrophages that induce the cytokine-chemokine cascade during inflammation.
It is very apparent that the once-thought only role of PLA2 enzymes solely in the initiation of inflammation needs to be modified because the results of recent studies that implicate the 30 PLA2 family members as important enzymes in supplying bioactive lipids that control cellular lipid metabolism. However, the cytosolic cPLA2 is the key regulator of AA-mediated eicosanoid metabolism; both the independent iPLA2 and secreted sPLA2 also function in the inflammatory process. Many of the PLA2 enzymes exhibit transacylase activity in addition to phospholipase and lysophospho-lipase activities. Their broad roles in eicosanoid and intermediary metabolism are evident from their diverse roles in FA metabolism. Phospholipase A2 enzymes has an important role in membrane PL remodeling, selective regulation of FA transport proteins (e.g. FABP, ACBP, FATP and ASCL) channeling to meet the metabolic needs of cells, and the systemic energy needs of the organism. Alteration of these processes can lead to diseases associated with MetS. The understanding of the functional role of PLA2 in metabolism in the liver is in its infancy. To further identify PLA2-generated lipid metabolites and especially eicosanoids' role in metabolic disease will require a comprehensive proteomic, lipidomic and genomic approach to provide a metabolomic picture of eicosanoids and their pathways in control of cellular metabolism. These methods will be of an immense value in the future in our dissection of eicosanoids' role in metabolism and their permutation in MetS (Sabido et al., 2012).
3. Metabolism of Eicosanoids in Mets
MetS is a cluster of metabolic and physiological abnormalities that increases an individual's risk for CVD, type II diabetes, obesity, and NAFLD, which includes symptoms of hyperglycemia, insulin resistance, hypertension, hypertriglyceridemia, hyperlipidemia, and hypercholesterolemia. The causes of these abnormalities are currently believed to be dysfunction in lipid metabolism and persistent subacute inflammation caused by alterations in lipid signaling networks that link the immune system and metabolism in metabolic diseases. Although cytokines and chemokines play significant role in the abnormalities of MetS, bioactive lipids may be the early link between inflammation and MetS since drugs that target the synthesis of eicosanoids in inflammation have efficacy in the treatment of MetS. Of equal importance is the observation that dietary ingestion of ω3-PUFAs that produce less-potent eicosanoids and beneficial resolvins reduces the severity of inflammation and many symptoms of MetS. Thus, eicosanoids may provide a common link between inflammation and MetS and targeting selective eicosanoid pathways may provide unexplored opportunities in the current treatment of not only CVD but also other diseases of MetS and NAFLD.
Release of AA from PLs (PI, PC, PE, PS, or CL) by the action of specific and selective PLA2 isoforms can be metabolized by cyclooxygenase, LOX, and cytochrome P450 pathways to produce potent eicosanoids and lipid autacoids. These pathways produce a variety of bioactive eicosanoids including PGs, thromboxanes (TXs), LTs, LX, epoxyeicosatetraenoic acid (EET), hydroxyeicosatetraenoic acid (HETE) and isoprostanes (Fig. 5.2). These eicosanoids elicit their paracrine, autocrine, or intracrine effects through either specific GPCRs, activation of NHRs transcription factors, or alteration of specific eicosanoid enzymes directly.
3.1. Synthesis of Prostaglandins in Intermediary Metabolism
PG and TXs are synthesized from two different PG endoperoxide synthase, prostaglandin endoperoxide H synthase (PGHS)-1 and PGHS2 that catalyze two distinct reactions, a cyclooxygenase and a peroxidase reactions. COX bisoxygenates arachidonic acid, leading to two molecules of oxygen being inserted into AA to yield protaglandin peroxidase. The peroxidase activities of PGHS is performed by a glutathione peroxidase producing PGHS that is rapidly converted by specific synthase to produce PGD2, PGE2, PGF2α, prostacyclin (PGI2), or TXA2. There are two different PGHS (PGHS1 and PGHS2) with different cyclooxygenase activities (COX1, COX2) and different substrate specificities and patterns of regulation. High concentration of palmitic acid in obesity and T2DM stimulates the inflammatory PGHS2 and inhibits the constitutive PGHS1. Cyclooxygenase activity is by two COX monomers, one an allosteric activator site that binds heme and the other a catalytic site. COX activity is regulated by different FAs that elicit a stimulatory or inhibitory effect on AA oxygenation, depending on the FA and PGHS isoforms. Many COX inhibitors that include nonsteroidal anti-inflammatory drugs (NSAID) inhibit both COX1 and COX2, while Coxibs drugs show selectivity in inhibition of COX2. Many of the COX inhibitors are metabolized by cytochrome P450s CYP2C subfamily members. It is also of interest that antiinflammatory ω3-PUFAs are poor substrates for COX isoforms with EPA not being metabolized by COX1 and only weakly metabolized by COX2 (Smith et al., 2011). Thus the differential regulation of COX1 and COX2 activities by saturated SFAs and uSFAs can lead to production of pro- or antiinflammatory eicosanoids. Biologically active 2-series prostanoids are produced in the presence of palmitic acid by COX2, while antiinflammatory eicosanoids would be produced by COX2 or diverted to LOX pathway to produce less-potent LTs. Thus the high consumption of ω-6 linoleic acid seen in a Western diet would produce pro-inflammatory prostanoids and endocannabinoids such as anandamide and 2-acylglycerol.
It is rather surprising that the metabolic phenotypes of COX1 and COX2-null mice have not been completely characterized thus leaving a void in our understanding of prostanoids in the control of intermediary metabolism in MetS. This is especially surprising considering that PGE2 has a pivotal role in adipocyte differentiation and adipogenesis. Of equal importance is the different roles of COX1 and COX2 in the production of different prostanoids, with COX1 coupled with synthesis of TXA2, PGF2α and PGE2 production, and COX2 preferentially channeling to PGI2 and PGE2 synthesis (Smith et al., 2011). This selective channeling is evident in inflammation since COX1- and COX2-null mice have different responses in inflammation with COX1-null showing an attenuated response to AA-induced ear edema, while COX2-null mice having a similar response as wild-type mice. COX2 has a major role in acute and chronic inflammation as well as in the resolution phase of inflammation where antiinflammatory PGD2 and 15-deoxyPGJ2 levels increase, while proinflammatory PGE2 levels drop. The role of COX2 in atherosclerosis is uncertain since COX1-null mice have a marked reduction of lesion development in the apoE lipoprotein (APOE)-null mice (McClelland et al., 2009), while COX2 PGI2 in PGI2 receptor knockout mice show early development of atherosclerosis in hyperlipidemic mice.
The different profile of prostanoids produced by COX1 and COX2 is largely determined by which COX isozyme is expressed in the cells and under what condition. This is clearly evident in normal macrophages that produce equal amounts TXA2 and PGE2, but upon activation, the level of PGE2 dramatically increases. PGE2 is the most abundant prostanoid synthsized from PGH2 by cytosolic PGE-1 synthase (PGES1) and microsomal PGES1 (mPEGS-1) or PGES2 that requires reduced glutathione (GSH) for its activity Like COX2, microsomal PEGS is induced by cytokines and growth factors and is inhibited by glucocorticoids. PGE2 is involved in three of five cardinal signs of inflammation: rubor (redness), tumor (swelling), dolar (pain), calor (heat), and function laesa (loss of function). In inflammatory model of angiogenesis, mPGES-1-null mice show reduced vascular endothelial growth factor (VEGF), suggesting that PGE2 and VEGF cooperate in angiogenesis. PGE2 is actively transported from cell by multidrug resistance protein 4 (MRP4), and binds locally to one of four cognate receptors (EP1-EP4) (Jania et al., 2009). Both EP3 and EP4 are widely expressed in most tissues, therefore the interpretation of EP4-null mice phenotype are difficult to reconcile without the generation of tissue-specific knockout mice.
PGI2 is the most important cardiovascular prostanoid produced by vascular endothelial and vascular smooth muscle (VSM) cells. Prostacyclin synthase is a member of the cytochrome P450 family (CYP8A1), which colocalizes with COX1, yet COX2 is the predominate source of PGI2. Endothelial PGI2 relaxes VSMC and inhibits platelet aggregation, before it is inactivated to 6-keto PGF2α. PGI2 possibly mediates some of its effects through activation of PPARβ, similar to PPARγ activation by 15-deoxyPGD2. PGI2 receptor IP-null mice show accelerated atherogenesis with increased platelet activation and enhanced leukocyte attachment to the vessel in ApoE-null mice (Kobayashi et al., 2004). T cells in adipose tissue are believed to have a significant role in obesity-induced inflammation by modifying adipose tissue macrophage (ATM) numbers and macrophage phenotype to M1 macrophages that secrete pro-inflammatory tumor necrosis factor (TNF)-α and IL1. CD4+ Th1 cells produce inflammatory cytokines, the Th2 cells produce anti-inflammatory cytokines, and regulatory T-cells secrete anti-inflammatory signals that inhibit macrophage migration and induce M2-like macrophage differentiation. Thus PGE2 promotes macrophage differentiation of monocytes to the M1 macrophages and Th1 T cell phenotype, while PGI2 promotes production of M2 antiinflammatory macrophages and Th2 cells.
PGD2 is the major prostanoid synthesized in peripheral tissues by the cytosolic lipocalin-type PGD synthase (L-PGDS) found in mast cells, leukocytes and Th2 cells. PGD2 is further metabolized to PGF2α and a series of cyclopentanone PGs, PGJ2Δ12, and 15-PGJ2. PGD2 is the predominate prostanoid in activated mast cells and appears to mediate its proinflammatory effects through DP1 and DP2 GPCRs. PGD synthase is a member of the commonly known drug metabolizing enzyme glutathione-S-transferase family (α, μ, and π classes) that have the ability to convert PGH2 to a mixture PGD2, PGE2, and PGF2α in the presence of GSH. The PG synthase enzyme glutathione-S-transferase activity most likely complements the glutathione per-oxidase activity of COX enzymes, suggesting a close relationship between drug and eicosanoid metabolism. This suggests a potential source of adverse drug toxicity when glutathione levels are depleted in oxidative stress, which can lead to reduced export of toxic drugs and inhibition of prostanoid synthesis.
PGF synthase produces PGF2α that activates PGF2α FP receptor GPCR coupled to Gα(q/11), leading to elevation of intracellular calcium mobilization. PGF2α can be metabolized to the major plasma metabolite 15-keto dihydroPGF2α by members of the aldol-reductase family. The aldo-keto reductase (AKR) families are widely distributed, consisting of 15 families that metabolize aldehydes, steroids, monosaccharides, aromatic hydrocarbons and prostanoids in the presence of NADPH. A recent study identified that AKR1B7 has a significant role in the detoxification of lipid peroxidation malondialdehyde (MDA), which functions as a chemotactic agent in attracting macrophages and neutrophils to sites of injury (Ge et al., 2011). Surprisingly, adenovirus overexpression of AKR1B7 in liver of diabetic db/db mice lowered blood glucose, hepatic gluconeogenesis, hepatic TAG, and cholesterol. Thus AKRs have dual roles in prostanoid metabolism and intermediary lipid and carbohydrate metabolism. In addition, functional coupling of COX2 and AKR1B7 to produce PGF2α has been demonstrated in HEK 293 cells. Thromoboxane B2 (TXB2) is synthesized in platelets by COX1 as TXA2, which is nonenzymatically degraded to TXB2 with the parallel production of MDA and 12-hydroxyeicosatrienoic acid (12-HHT). TXA2 signal through the thromboxane TP receptor coupled to Gq, G12/13, and other G proteins to influence Rho GEF, adenylate cyclase and PLC that mediate platelet aggregation, adhesion, VSMC contraction, and proliferation during inflammation. Several other eicosanoids including isoprostanes, produced by oxidation of prostanoids and HETEs are potent agonists of T P, while EETs produced by cytochrome P450 epoxygenase are potent antagonists (Behm et al., 2009). TXs are synthesized by TX synthase, a member of the cytochrome P450 family identified as CYP5.
It is apparent that prostanoids can either promote or attenuate acute inflammation through channeling of PGH2 from either COX1 or COX2 to different synthase. Therefore, it is unknown how and why some drugs, that target the prostanoid pathway, are so effective in the treatment of inflammation, CVD, colorectal cancer, asthma, arthritis and thrombosis, but ineffective against the progression of these diseases. Inhibition of one key downstream pathway would have two benefits with regard to drug development in the treatment of inflammatory and metabolic disease. Blockage of the synthesis of proinflammatory prostanoids would inhibit the amplification of the lipid autacoid-cytokine-chemokine cascade and divert the prostanoid substrates through other pathways and thus reduce the probability of toxicity. This tenet is seen in aspirin inhibition of platelet COX1 -derived TXA2 that amplifies platelet aggregation, while aspirin inhibits amplification and persistent activation of platelet aggregation. Targeting the eicosanoid pathway in the treatment of metabolic disease may offer similar benefits of reduced toxicity and attenuation of sustained activation and amplification of disease pathways. Presently, we do not completely understand the role of prostanoids synthesis in the progression of obesity, fatty liver, and diabetes, unlike our detailed understanding of eicosanoid metabolism, in the inflammatory etiology of CVD disease. With regard to prostanoid metabolism, few studies have explored the role of these pathways in obesity and fatty liver disease, which is rather perplexing given the rapid induction of COX2 by peroxides, oxidant stress, NF-κβ cytokines (TNFα, transforming growth factor (TGF)-β1) and chemokines (IL1β, IL6) through activation of lipid autacoid-cytokine-chemokine cascade. COX2 inhibitors Celecoxib and NA-398 inhibited the progression of NAFLD. (Yu et al., 2006). Furthermore, a recent study has provided evidence that drugs targetting eicosanoid metabolism may be effective in the treatment of MetS. Aspirin that targets COX enzymes also has the ability to activate AMPK, the pivotal enzyme in the integration of carbohydrate and lipid metabolism (Hawley et al., 2012). Activation of AMPK inhibits ACC, the rate-limiting enzyme in lipogenesis, and activates FA oxidation. Similarly, niacin, a precursor to NADH and NADPH used in the treatment of MetS dyslipidemia induces COX1 -dependent PGD2 and PGE2 and COX2-dependent PGE2 production, which causes vasodilation. Thus there is a grave need to explore the prostanoid biosynthetic pathway in obesity, fatty liver, and diabetes with regard to alterations in intermediary metabolism in MetS.
3.1.1. Leukotrienes Synthesis in Intermediary Metabolism
LTs, LX, hydroperoxyeicosatetraenoic acid (HPETE), and eoxins are synthesized by 5-, 12-, and 15-LOX that lipoxygenate AA by adding molecular oxygen to form 5-, 12-, or 15-HPETEs, respectively. 5-HPETE is further metabolized by 5-LOX to LTA4, which can be metabolized to LTB4 or the glutathione containing slow-reacting substances of anaphylaxis (LTC4, LTD4, LTE4). 12-LOX produces 12-HETE and 15-LOX produces 15-HETE. LXA4 and LXB4 are produced through transcellular metabolism and have antiinflammatory properties during the resolution of inflammation.
5-LOX is primarily expressed in hematopoietic cells, such as leukocytes, mast cells, dendritic cells, and lymphocytes and its expression is induced by growth factors and cytokines (Haeggstrom & Funk, 2011). 5-LOX activity requires Ca2+ and PC and its activity is induced by FA hydroperoxides that are controlled by glutathione peroxide, thus linking LT synthesis and prostanoid synthesis. 5-LOX activity is regulated by serine phosphorylation by MAPK2, ERK2, and protein kinase A (PKA). Both MAPK2 and ERK2 phosphorylation of 5-LOX induces its translocation to the nucleus, while PKA-mediated phosphorylation inhibits its translocation. For optimal activity, 5-LOX binds 5-lipoxygenase activating protein (FLAP), which stimulates AA utilization and activity to produce LTA4. LTA4 hydrolase is widely expressed in most tissues unlike 5-LOX, which is restricted to hematopoietic cells. LTA4 hydrolase is a bifunctional enzyme having epoxide hydrolase activity and aminopeptidase activity. LTA4 inhibits LTA4 hydrolase activity, but not its aminopeptidase activity. During tissue damage, collagen of the extracellular matrix is broken down to produce the highly chemotactic tripeptide (proline-glycine-proline), which is inactivated by the aminopeptidase activity of LTA4 hydrolase. (Haeggstrom & Funk, 2011). Thus LTA4 inhibits the synthesis of pro-inflammatory LTB4 by blocking LTA4 hydrolase activity without inhibiting the aminopeptidase activity of LTA4 hydrolase.
Presently, we do not know the importance of eicosanoid metabolism and the role of prostanoids, LTs and cytochrome P450 (CYP) AA metabolites in the initiation and progression of MetS and their importance in NAFLD, obesity and insulin resistance. However, several recent studies pointed toward the role of LOX pathways in fatty liver disease based on: (1) the aberrant expression of Alox5 gene in liver during the progression of chronic liver disease (Titos et al., 2010), (2) the protection against hepatic steatosis by administration of 5-LOX inhibitors (Lopez-Parra et al., 2008), and (3) protection against liver inflammation and fibrosis by coadministration of 5-LOX and COX2 inhibitors (Horrillo et al., 2007). In addition, several recent studies have used LOX knockout mice to explore the role of LT metabolites in the control of lipid metabolism and inflammation in liver disease.
The Alox5 gene is highly expressed in several models of liver diseases (El-Swefy & Hassanen, 2009; Lopez-Parra et al., 2008; Titos et al., 2010). Alox5-null mice show a lower degree of hepatic steatosis that a differential regulation of lipid metabolism genes, including the lipogenic factors, Lipin1, c/EBP, Fasn, Acly, and Elovl6 (Titos et al., 2010). The mechanisms by which 5-LOX metabolites initiate liver dysfunction and damage have not been completely identified. It is known that 5-LOX metabolites induce NF-κβ, which increases the expression of proinflammatory cytokines and chemokines (MCP-1, IL1, IL6, and IL8) and cell survival (TNFα). The prevention of steatosis in Alox5-null mice is due to downregulation of FA synthase and ATP-citrate lyase as well as other lipogenic genes and inhibition of microsomal triglyceride transfer protein (MTP), VLDL-TG, and apolipoprotein B secretion (Horrillo et al., 2010). Thus in hepatic steatosis and steatohepatitis, 5-LOX increases lipogenesis and inhibits TAG transport leading to accumulation of TAGs in the liver (Martinez-Clemente et al., 2010). Although 5-LOX-generated LTB4 has a pivotal role in inflammatory chemotaxis and the progression of steatosis to steatohepatitis, it is unknown whether LTB4 has a role in the initiation of hepatic steatosis. LTB4 receptor1 (BLT1)-null mice are resistant to HF-diet-induced obesity and insulin resistance. This is due to reduced accumulation of M1 ATMs and increase in adipose M2 ATMs that prevent lipolysis but promote hepatic triglyceride accumulation and liver insulin resistance (Spite et al., 2011). It is unknown whether BLT2 highly expressed in the liver also has a role in hepatic steatosis.
Although 5-LOX LTB4 appears to be the most relevant metabolite in hepatic inflammation, the cysteine LTs have a significant role in inflammation and hepatocyte survival (LTC4, LTE4) and stellate cell activation (LTD4) (Martinez-Clemente, Ferre, Gonzalez-Periz, et al., 2010; Titos et al., 2010). LTC4 synthase is the committing step in cys-LT synthesis through the conjugation of LTA4 with glutathione. LTC4 synthase is a member of the microsomal GSH transferase (MGST) family of proteins that have LTC4 synthase activity and peroxidase activity toward hydroperoxides (Haeggstrom & Funk, 2011). LTC4 is sequentially metabolized by γ-glutamyl transpeptidase to produce LTD4 (cys–gly), and peptidase to produce LTE4. The cysteinyl LTs increase vascular permeability and plasma leakage of vessels leading to edema. There are no studies on the role cysLT has in either MetS or NAFLD. Even though LTA4 hydrolase-null mice have been generated, but the role of LTA4 deficiency in fatty liver, obesity, or metabolic disease has not been reported.
There are two forms of 12-LOX, platelet and leukocyte forms in mice. In humans, the 12-LOX and human reticulocyte 15-LOX isoform produce similar products and are often referred to as 12/15-LOX. Because of species difference in expression, such as mice expressing the 12-LOX but not 15-LOX, it is difficult to interpret the results of animal studies to humans. The human 12/15-LOX produces a series of important lipid mediators, including 12-HPETE and 15-HPETE, that are further metabolized to 12-HETE and 15-HETE, respectively (Dobrian et al., 2010). The 12/15-LOX also oxygenates linoleic acid (C18:2n6) to produce 9-hydroxyocta-decadienoic acid (HODE) and 13-HODE, as well as hydroxylating FAs esterified in PLs. Also, 9-HODE and 13-HODE are able to activate the tolllike receptor (TLR)-4 in macrophages, and 12/15-LOX metabolites induce production of pro-inflammatory cytokines and chemokines, such as MCP-1, IL6, IL8 and TNFα. The 12/15-LOX-generated hydroperoxides can also serve as precursors in the formation of secondary lipid mediators called LX, hepoxilins (HX), and trioxlins, and antiinflammatory lipid mediators from ω3-PUFA known as resolvins and protectins (Spite et al., 2011) (Fig. 5.3).
Figure 5.3. Metabolism of linolenic acid to anti-inflammatory eicosanoids.
The synthesis of anti-infammatory eicosanoids from ω-3 polyunsaturated fatty acids (PUFA) begins with the desaturation and elongation of the ω-2 linolenic acid to produce eicosapentaenoic acid (EPA). Less potent series-3 prostanoids and series-5 leukotrienes are produced from EPA. However, the CYP2 epoxygenase produces a series of epoxyeicosatetraenoic acids (EEQ) at a 10-fold higher rate than the metabolism of AA, while CYP4 ω-hydroxylase produces hydroxyeicosapentaenoic acid (20-HEPE) at an approximate twofold higher rate than AA and increased rate of ω1 over ω-hydroxylation of EPA compared with AA. In vivo, the EEQ surpasses the role of EETs as endothelium-derived hyperpolarizing factors; however, the role of CYP4-derived 19-HEPE over the 20-HEPE in the vasoconstriction and proinflammatory role of AA are presently uncertain. It will be of particular interest to determine the beneficial effects of EPA metabolites in metabolic disease considering that PUFAs suppress lipogenesis and EPA alleviates obesity-induced insulin resistance by upregulation of glucose transporters GLUT2, GLUT4, insulin receptor substrates IRS-1 and IRS-2 and reduction in cholesterol ester transfer protein (CETP). (For color version of this figure, the reader is referred to the online version of this book).
HX are formed from 12-LOX, 12-HPETE through an intramolecular rearrangement of –OOH group to form a hydroxyl group at C8 (HXA3), C10 (HXB3) by a putative isomerase or an epoxide at the 11,12 position by epoxide hydrolase to form three-hydroxyl-group-inactive metabolites called trioxilins. HX are early signals of inflammation while the 15-LOX-pathway-produced LX (LXA4 and LXB4) have both anti-inflammatory resolving abilities (Spite et al., 2011). The formation of LX begins with 15-HETE that is converted to LXA4 by 5-LOX through transcellular biosynthesis. It is significant that aspirin acetylation of COX2 inhibits COX2 from producing PGH2, but COX2 retains oxygenase activity to produce 15(S) HETE that can be converted to antiinflammatory LX. Over expression of 12/15-LOX in the endothelium increases atherosclerotic plaques, while overexpression in macrophages protects against atherosclerosis in mice. Further perpetuating atherogenesis is angiotensin II that upregulates 12/15-LOX expression in macrophages and endothelium leading to vasoconstriction. It has been reported that polymorphism in the Alox12 gene is associated with subclinical atherosclerosis and serves as a biomarker of disease in families with type II diabetes mellitus (T2DM) (Haeggstrom & Funk, 2011).
Normal blood glucose levels are maintained largely by β-cell insulin and α-cell glucagon secreted from the pancreas. Insulin promotes glucose uptake by adipose, liver and muscle tissue and promotes glycolysis, glycogen synthesis and inhibition of hepatic gluconeogenesis. Both type I and type II diabetes are associated with a significant loss of β-cell function and thus insulin production. Inflammation has a pivotal role in β-cell dysfunction, and upregulation of 12/15-LOX by hyperglycermia and inflammatory cytokines have a central role in diabetes and obesity. Furthermore, both 5-LOX and 12/15-LOX have been implicated in MetS and NAFLD in humans and a recent plasma lipidomic signature of NASH revealed a step-wise increase in 5-LOX 5-HETE, 8-HETE, and 12/15-LOX 15-HETE in the progression of NAFD to NASH (Puri et al., 2009), further supporting the role of LOX in insulin resistance, obesity and fatty liver disease.
3.1.2. Eicosanoids of the P450 Epoxygenase and Omega Hydroxylase Pathways
The third pathway for the metabolism of eicosanoid is mediated by cytochrome P450s of the epoxygenase pathway (CYP2C) and FA omega hydroxylase (CYP4). These drug-metabolizing pathways were once thought to have little function in the control of metabolism; however, recent studies have shown their importance in the disease pathology of MetS. In addition, many of the commonly prescribed antiinflammatory drugs that target PG and LT eicosanoid pathways are metabolized by members of the drug-metabolizing cytochrome P450 family (Figs 5.2 and 5.4). It is also apparent that there are stark similarities in the drug-metabolizing enzymes of phase I oxidation, phase II conjugation, and phase II transport with enzymes of eicosanoid metabolism, which establishes a link between eicosanoid and drug metabolism in metabolic diseases.
Figure 5.4. CYP4A and CYP2C P450s function in liver eicosanoid and fatty acid metabolism.
Fatty acids are delivered to hepatocytes for catabolism by the mitochondria or peroxisome β-oxidation systems or are used for the synthesis and export of triglycerides (TG), phospholipid (PL), cholesterol esters (CE), as very low density lipoprotein (VLDL) particles. Medium- and short-chain-length fatty acids are transported as acylcarnitine derivatives into mitochondria for complete β-oxidation to CO2. Long- and very-long-chain fatty acid CoA esters as well as eicosanoids are transported into the peroxisome where they undergo chain-shortening reactions and then transported as acylcarnitine derivatives to the mitochondria for complete oxidation or as free fatty acids by thioesterase 3, or 5 (ACOT3, 5). Under normal conditions, (5–10%) fatty acids are converted to dicarboxylic acids CoA (DCA-CoA) by CYP4A or CYP4F ω-hydroxylation. Starvation induces the ω-oxidation of fatty acids by 40% through PPARα induction of CYP4A genes. The dicarboxylic acids, adipyl-CoA (C6), sebacyl-CoA (C8) or suberyl-CoA (C10) are exported from peroxisome by the action of acyl-CoA thioesterase 8 (ACOT8), or when ACOT8 is inhibited by free CoASH, the FF-CoA is converted to succinyl-CoA, which is exported as succinate after removal of CoA by ACOT4. Succinate can function as an anaplerotic intermediate in the mitochondria for gluconeogenesis during starvation and excess acetate produced from acetyl-CoA can be used by peripheral tissues after conversion to acetyl-CoA by acetate-CoA synthetase (ACSII). In the fed state, long-chain fatty acids (LCFA) and very-long-chain fatty acids (VLCFA) are metabolized by peroxisome |3-oxidation to shorter chain products, which can be incorporated into phospholipids, cholesterol esters or triglycerides for export as VLDS and stored in peripheral tissues. Excessive acetate in the cytosol is converted to acetyl-CoA by hepatocyte acetate-CoA synthetase I (ACSI) and then converted to malonyl-CoA for fatty acid and cholesterol synthesis by ACC1. CYP4F gene induction by insulin is mediated by SREBP and saturated fatty acid-CoA activation of hepatocyte nuclear factor 4α (HNF4α). In the presence of excessive fatty acids, insulin will activate SREBP1c increasing the synthesis of stearoyl-CoA desaturase 1 (SCD-1) and converting palmitic (C16:0) and stearic (C18:0) acids to palmitoleate (C16:1) and oleic (C18:1), respectively, which are stored as triglycerides. Both CYP4 P450 and SCD-1 use cytochrome b5 and cytochrome b5 reductase in their catalytic cycle. The induction of CYP4A genes by high-fat-diet and an increase in SCD-1 with suppression of CYP4F genes may prevent the liver from lipotoxicity at the expense of steatosis and development of steatohepatitis. ACOX, acyl-CoA oxidase; LBP, L-bifunctional protein; DBP, D-bifunctional protein; TG, triglycerides; PL, phosphor\lipid; CE, cholesterol ester; WE, wax ester. (For color version of this figure, the reader is referred to the online version of this book).
Unlike COX and LOX that insert molecular oxygen into AA and therefore function as dioxygenase, CYP enzymes are monoxygenase inserting one oxygen atom into the substrate and the other in the formation of water. CYPs are able to efficiently use AA and ω3-PUFAs as substrates similar to LOX; however, because COX enzyme metabolizes ω3-PUFAs less efficiently, it is likely that LA-derived EPA (C20:5n3) is preferentially metabolized by CYP or LOX pathways. In addition, neither linoleic acid nor LA is used by either the LOX or COX pathways, but are efficiently metabolized by the CYP pathway. CYP enzymes catalyze the hydroxylation, epoxidation and allylic oxidation of FAs (Oliw et al., 1996). CYP2 members metabolize AA to four regioisomeric cis-epoxyeicostetraenoic acids (5, 6-; 8, 9-; 11, 12-; and 14, 15-EET), with each of these regioisomers forming R, S or S, R enantiomers. This regioselectivity and stereoselectivity of EETs is CYP isoform specific. Human CYP2C8 exclusively metabolizes AA to 14,15- and 11,12-EETs in a ratio of 1.3:1, while CYP2C9 shows less region- and stereoselectivity. CYP2J epoxygenase is highly expressed in the heart and produces all four regioisomers as a mixture of racemers. The human CYP2S1 P450 produces 12-HHET, MDA, 13-HODE, 5-oxo-EET, and 12-oxo-EET (Bui et al., 2011). Allylic oxidation of AA by other P450 isoforms produces hydroxylated metabolites containing cis- and trans-conjugated dienol (5, 8-; 9-; 11-; 12- and 15-HETE). The 12- and 15-HETE are similar to the 12/15-LOX AA metabolites and therefore are believed to have similar function in the liver, adipose tissue and pancreas. These allylic oxidation products are produced by several CYP isoforms including CYP1A2, CYP3A4, CYP2C8 and CYP2C9.
The human epoxygenase CYP2C8, CYP2C9 and CYP2J2 as well as soluble epoxide hydrolase (sEH) that converts EET to dihydroxyeicosatrienoic acid (DHET) are highly polymorphic and several variants have been associated with individual risk for stroke, hypertension, atherosclerosis, myocardial infarction, and cancer (Zordoky & El-Kadi, 2010). EETs are also known as endothelial hyperpolarizing factor and their synthesis is initiated in the vascular bed in response to bradykinin and acetylcholine activation of BK channels in VSMC leading to hyperpolarizations and VSMC relaxation. EETs activate endothelial nitric oxide synthase (eNOS) to cause vasodilation. In the heart, EETs regulate L-type Ca2+ ATP-sensitive potassium (KATP) and Na+ channels thus improving functional recovery from ischemia/reper-fusion (I/R) injury as shown by heart-specific overexpression of CYP2J2.
The ω-3 PUFAs, EPA and DHA are converted by epoxidation and hydroxylation to both 5-regioisomeric epoxyeicosatetraenoic acid (EEQ) and ω-HEPE (Fig. 5.3) (Westphal et al., 2011). The beneficial effects of dietary consumption of ω3-PUFAs are through downregulation of the inflammatory response, and well-established attenuation of lipogenesis. This is the reason we explore how their metabolisms influence metabolic processes of MetS. Both EPA and DHA are efficiently metabolized by CYP-dependent epoxidation and hydroxylation to both Omega and epoxide metabolites of EPA and DHA (Konkel & Schunck, 2011). Several members of the CYP2 family (CYP2C8, C9, C18, C19 and CYP2J2) metabolize EPA (C20:5n3) to 5-regioisomeric EEQ and DHA (C22:6n3) to 6-regioiso-meric epoxydocosapentaenoic acid with equal or higher catalytic activities, but different regioselectivity for EPA versus AA (Fer et al., 2008). CYP2C8 metabolizes AA to 11, 12- and 14, 15-EET, while EPA is metabolized to 17, 18-EEQ and DHA to 19,20-EDP. The heart-specific CYP2J2 metabolizes EPA and DHA at rates 10- and 2-fold greater, respectively, than its rate of AA epoxidation. Presently, the biological activities of EPA and DHA epoxides are similar but have more powerful effects than AA-formed EETs since BK channel activation by 17,18-EEQ greatly exceeds that of 11,12-EET, the strongest AA metabolite activator of BK channels (Westphal et al., 2011). Also, DHA-derived epoxides are 1000-fold more potent than EET in activating BK channels in rat coronary arterioles.
The other CYP eicosanoid metabolizing P450s are members of the FA ω-hydroxylase family (CYP4), which consists of multiple human subfamilies (CYP4A, CYP4B, CYP4F, CYP4Z, CYP4X, and CYP4V), but only members of CYP4A and CYP4F have been studied in detail in regard to eicosanoid metabolism. The CYP4 isoforms ω-hydroxylate the subterminal carbon of AA to produce 20-HETE and ω-hydroxylated LCFAs and eicosanoids that are metabolized by peroxisomal β-oxidation (Fig. 5.4). Hydroxylation of AA is performed by other CYP enzymes forming a series of subterminal regioisomeric HETE, 16-,17-, 18-, and 19-HETE is catalyzed by the ethanol-inducible CYP2E1 (18-, 19-HETE), CYP1A1 and CYP1A2 (16-,17-, 18-HETE); CYP2J9 that produces exclusively 19-HETE; and CYP4F22 (18-HETE) (Nilsson, Ivanov, & Oliw, 2010). A recent study on the activity of the 19-hydroxy-PGH2 CYP4F8 and CYP4F22 P450 in the metabolism of AA and ω3 PUFA showed that CYP4F22 produces 18-HETE, and CYP4F8 metabolizes ω3-PUFA to 8,9- and 11,12- epoxyalcohols (HEETS-hydroxyepoxyeicosatrienoic acid) (Nilsson et al., 2010).
The human CYP4A11 ω-hydroxylase produces 20- and 19-HETEs in a ratio of 90:10, while members of the human CYP4F subfamily CYP4F2, CYP4F3A, CYP4F3B show strict regioselectivity in the ω-hydroxylation of AA (Konkel & Schunck, 2011). The functional role of other CYP4 subfamily members (CYP4F11, CYP4F12, CYP4V2, CYP4Z1, and CYP4F22) in the metabolism of AA and ω-PUFA has not been extensively studied. ω-hydroxylate AA (20-HETE) has been shown to have a vital role in hypertension through its ability to cause vasoconstriction by inhibiting the K+ BK channel, stimulation of Rho kinase, and activation of L-type calcium channels. Vascular production of 20-HETE induces endothelial dysfunction and hypertension through reduced endothelial nitric acid oxidase (eNOS) activity and activation of NF-κβ (Wu, Cheng, et al., 2011). Endothelial dysfunction has been shown to be correlated with the level of urinary 20-HETE. Genetic polymorphisms in CYP4A11 and CYP4F genes are associated with hypertension, stroke and coronary endothelial dysfunction (Fava et al., 2012; Stec et al., 2007; Zordoky & El-Kadi, 2010). CYP4A11, CYP4F2, CYP4F3a, and CYP4F3b efficiently metabolize EPA and DHA (Fer et al., 2008) with CYP4A11 showing a dramatic shift in ω/ω-1 hydroxylase activity ratio of 4:1 for AA to 1:3 with EPA and 1:2 with DHA. This is of particular interest since 19-HETE inhibits 20-HETE-mediated vasoconstriction and endothelial dysfunction, and thus ω3-PUFA may shift CYP4A production of proinflammatory 20-HETE to beneficial effect of 19-hydroxylated EPA or DHA.
CYP4F2 not only produces 20-HETE from AA but also hydroxylates DHA at twofold higher rate than either AA or EPA, while CYP4F3A and CYP4F3b ω-hydroxylate AA and DHA at a similar rate with less activity toward EPA (Fer et al., 2008). Both CYP4F8 and CYP4F12 metabolize EPA and DHA by epoxidation of the ω-double bond to produce 17,18-EEQ and 19,20-EDP, respectively (Stark, Dostalek, & Guengerich, 2008). The brain-and thymus-specific CYP2U1 ω-hydroxylase efficiently ω-hydroxylates ALA, AA, EPA, and DHA (Konkel & Schunck, 2011). Unlike the epoxidation of EPA and DHA that seem to have similar activities as AA-derived EET, the function of ω-hydroxylated metabolites of EPA and DHA are not known, therefore it will be of importance to determine if ω-hydroxylated EPA and DHA have reduced vasoconstrictive and proliferation abilities.
Besides the use of several common enzymes of drug metabolism in eicosanoid metabolism as exemplified by CYP2C epoxygenase and CYP4 ω-hydroxylase, the channeling and partitioning of AA through these three pathways can have a significant role in inflammation, drug metabolism, and intermediary metabolism. This is evident by the partitioning and channeling of EPA that is not efficiently metabolized by COX, but efficiently metabolized by LOX, CYP2 epoxygenase, and CYP4 ω-hydroxylase. Of equal importance is that drugs used to control eicosanoid metabolism (naproxen, ibuprofen, indomethacin, rofecoxib and diclofenac) are metabolized by CYP2 members of the epoxygenase pathways (Fig. 5.2), suggesting that these drugs can have unexpected adverse or beneficial effects in disease management. This is apparent by rofecoxib-mediated cardiovascular events where inhibition of COX2 results in a 120-fold increase in the blood level of 20-HETE and a dramatic increase in coagulation due to 20-HETE platelet aggregation and vessel vasoconstriction (Liu et al., 2010). It is of interest that polymorphism in the CYP4F2 gene has been associated with the idiosyncratic difficulties in warfarin therapy through CYP4F2's ability to metabolize and inactivate vitamin K2 necessary for activation of blood factors (Bejarano-Achache et al., 2012; Pavani et al, 2012). These data indicate that channeling of eicosanoids to different pathways and genetic polymorphisms of CYP genes have a significant role in the effectiveness of drug targeted to eicosanoid metabolism in the treatment of inflammation and metabolic diseases.
3.2. Catabolism of Prostaglandins and Leukotrienes
The inactivation and catabolism of bioactive eicosanoids are important in the inhibition of the inflammatory response and diseases associated with MetS. The design of effective therapies in the treatment of metabolic diseases of eicosanoid metabolism can lead to unanticipated consequences, which are evident in the development of sEH inhibitors used to prevent the inactivation of vasodilatory EET to diHETEs (Panigrahy et al., 2012). EETs are autocrine and paracrine mediators of vasorelaxation in the cardiovascular and renal systems through activated EET receptor-mediated stimulation of Gas and subsequent activation of adenylate cyclase. The production of cAMP activates protein kinase, leading to activation of potassium BKCa++ and KATP K+ efflux and hyperpolarization causing vasodilation, with activation of eNOS and inhibition of NF-κβ. Therefore, sEH inhibitors are in clinical trials as antihypertensive agents and are being evaluated for use in the treatment of diabetes, stroke, dyslipidemia, immunological disorders, vascular remodeling, chronic obstructive pulmonary disease, and atherosclerosis (Shen, 2010). However, using both genetic and pharmacological methods to control the endogenous EET levels in vivo, it was recently demonstrated that EETs are critical for both primary tumor growth and metastasis in several mouse models of cancer (Panigrahy et al., 2012). Increased EETs elevate VEGF receptor 2 and serum VEGF levels, and decreased endogenous levels of angiogenesis inhibitor, thrombospondin. This study raises serious concerns about the chronic use of sEH inhibitors in CVD, which may have adverse effects in cancer patients.
Both EETs and hepoxilin of the 12-LOX pathway are metabolized and inactivated by sEH. EETs have antiinflammatory properties, while hepoxilin are proinflammatory, which suggests that sEH has a central role in modulating the inflammatory response. Although sEH is the major pathway for catabolism for 14,15-EET and is less important in catabolism of 11,12-, 8,9-, or 5,6-EET (Imig, 2012), when sEH is low or inhibited, elongation and peroxisome β-oxidation produce inactive 16-carbon epoxy FA (Spector et al., 2004). Equally, EETs can be ω-hydroxylated by CYP4A and CYP4F and directed to peroxisome for β-oxidation (Fig. 5.4). Many human cancers have increased expression of CYP epoxy-genase that promotes angiogenesis and cancer metastasis. Therefore inhibition of CYP2C and CYP2J2 gene expression or activation of sEH may be an effective treatment for cancer. PPARα ligands downregulate CYP epoxygenase gene expression and thus limit tumor metastasis (Bozza et al., 2011). In addition, CYP4X1 that is highly expressed in tumors and human breast cancer has been shown to metabolize AA to 8, 9-, and 14,15-EET (Stark et al., 2008). Both angiogenesis and inflammation are independent of stromal processes that exert substantial influence on tumor growth and metastasis. EET stimulation of angiogenesis and suppression of inflammation most likely signals through independent pathways either by activation of the unidentified EET receptors (Chen, Wang, et al., 2011) or through activation of PPARα or PPARγ receptors. CYP4A and CYP4F-mediated 20-HETE is a proinflammatory eicosanoid that stimulates production of PGE2; the chemokines IL8, IL13, IL14; and TNFα cytokine (Ishizuka et al., 2008). 20-HETE activates NF-κβ, promoting cell survival, and activates the MAPK/ERK pathways that stimulate endothelial angiogenesis, proliferation and migration (Guo et al., 2007).
The major enzyme in the catabolism of PGs is 15-hydroxy prostaglandin dehydrogenase (15-PGDH). 15-PGDH also oxidizes and inactivates LXA4, 15-HETE, and 12-HHT of the TX synthase pathway (Tai, 2011). The 15-keto metabolites are further metabolized by NAD(P)H-dependent 15-keto prostaglandinΔ13reductase (13-PGR) to produce the inactive 13,14 dihydro-15-keto metabolites. 13-PGR is also known as LTB4-12-hydroxy dehydrogenase and catalyzes the oxidation of 12(R) hydroxyl group of LTB4 to inactive 12-keto-LTB4. Thus both 15-PGDH and 13-PGR are involved in the catabolic inactivation of PGs and LTs. Activation of these enzymes ameliorates inflammation by inactivation of LTB4 and induction of COX2 production of PGE2. In cancer cells, the upregulation of COX2 by IL1β, and TNFα decreases the expression of 15-PGH. In contrast, adenovirus overexpression of 15-PGH induces COX2 upregulation in a dose-dependent manner that is not dependent on the catalytic activity of 15-PGH. It is of interest that the ω3-PUFA increases the expression of 15-PGDH and suppresses COX2 in hepatocellular carcinoma. However, the molecular mechanism responsible for this reciprocal regulation of COX2 and anti-inflammatory 15-PGDH remains to be determined (Tai, 2011). Several studies have shown that NSAIDs induce the expression of antiinflammatory 15-PGDH, while this induction was inhibited by proinflammatory cytokine induction of COX2. The induction of 15-PGDH by PPARγ agonists suggests the possibility that NSAID inhibition of COX2 may channel AA to other eicosanoid pathways producing activators of PPARγ and 15-PGDH expression (Hazra et al., 2007). The parallel regulation of 15-PGDH with the prostaglandin transporter (PGT) in inactivating PGE2 signaling represents an important target in the treatment of metabolic disease with an inflammatory etiology.
The major pathway for the inactivation and catabolism of hydroxyl eicosanoids is through peroxisome β-oxidation and conjugation with glucuronic acid. CYP4 ω-hydroxylase P450 begin the process of catabolism by ω-hydroxylation of eicosanoids to alcohols that are further metabolized to the corresponding aldehydes by alcohol dehydrogenase (ADH4) followed by FA aldehyde dehydrogenase (ALDH32a/FALD) to produce dicarboxylic acids that are solely metabolized by peroxisome β-oxidation (Fig. 5.4). In human neutrophils, CYP4F3a metabolizes and inactivates the proinflammatory chemotactic eicosanoid LTB4. The eicosanoid dicarboxylic acids are chain-shortened by peroxisome β-oxidation and these short-chain products are fully oxidized to CO2 by mitochondrial β-oxidation (Wanders, Ferdinandusse, Brites, & Kemp, 2010). A major difference between PG and LT catabolism by peroxisome β-oxidation is that PGs are chain-shortened from the C1 carboxyl group after CoA activation, while LTs and HETEs are metabolized from the ω-terminal carboxyl end. The peroxisome β-oxidation of eicosanoids and excessive FAs seen in MetS diseases employ the same pathway of CYP4 ω-hydroxylation, ADH4, and FALD to produce dicarboxylic acids. The metabolic catabolism of eicosanoid and excess FFA by the ω-hydroxylase cascade is critical for the termination of bioactive eicosanoids and the prevention of lipotoxicity observed in both MetS and NAFLD.
3.3. Transport and Transcellular Metabolism of Eicosanoids
In order eicosanoids to elicit their paracrine and autocrine effects, eicosanoids must be exported from the cell by a series of efflux transporters that have overlapping substrate specificities with drug metabolites and endogenous toxic biochemicals. Of equal importance is the uptake or influx of eicosanoids for transcellular synthesis by the recipient cells to bioactive eicosanoids that either activate HNRs or are metabolized and inactivated by eicosanoid catabolic enzymes. In general, the efflux transporters are members of the ABC transport superfamily and the influx transporters are members of the SLC family. The ABC efflux and SLC influx transporters function to maintain optimal cell concentrations of nutrients, antioxidants, and signaling molecules. The inactivation of PGE2 occurs through a two-step process where the MRP4-ABCC4 mediates the efflux of PGE2, PGD2 and PGF2α, while importation into the recipient cell for inactivation occurs by the PGT, which is a member of the organic anion SLC carrier organic anion transporter (OAT)/SLC22. Once inside the recipient cell, the PG is inactivated by sequential metabolism by 15-PGDH and 13-PGR.
There are 49 human ABC transporters that function in the efflux of cholesterol, PLs, drugs, nucleosides, peptides, organic anions, and eicosanoids. Several diseases have been associated with defective ABC transporter, including familial intrahepatic cholestasis (ABCB4 and ABCB11), cystic fibrosis (ABCC7), type II diabetes (ABCC8), hyperbilirubinemia (ABCC2), adrenoleukodystrophy (ABCD1), and dyslipidemia syndrome (ABCA1). However, presently, no disease has been associated with eicosanoid efflux transporters. The importance of these transporters in eicosanoid metabolism in inflammatory disease is apparent from their induction by cytokines and regulation by NSAIDs and the inability of defective inflammatory response in MRP1-null mice to efflux LTC4 (Leier, Jedlitschky, Buchholz, & Keppler, 1994). There are eight MRP/ABC transporters that efflux eicosanoids: MRP1/ABCC1 (LTC4, LTD4, LTE4), MRP2/ABCC2 (LTC4, PGA2), MRP3/ABCC3 (LTC4), MRP4/ABCC4 (PGE2, PGF2α, PGD2, TXB2, LTB4, LTC4), MRP5/ABCC5 (cyclic nucleotides), MRP6/ABCC6 (LTC4), MRP7 (LTC4) and MRP8 (LTC4) (van de Ven et al., 2009).
MRP1/ABCC1 is a high-affinity transporter of reduced glutathione (GSH)-conjugated eicosanoids and is also active in transport of drugs and toxic agents, which can lead to GSH depletion and oxidative stress (Henkin et al., 2012). MRP2/ABCC2 functions in the transport of bilirubin, LTC4, and glucuronide-conjugated acetaminophen, and defects in this transporter are responsible for hyperbilirubinemia in Dubin-Johnson patients. MRP is inhibited by MK-571 and Montelukast, both LT receptor antagonists. This transporter also has a significant role in PC transport into the enterohe-patic circulation. MPR3/ABCC3 is upregulated in cholestastic disease and functions to secrete glucuronidated biochemicals into the hepatic sinusoids. MRP4/ABCC4 is the major eicosanoid efflux pump for prostanoids and GSH-conjugated LTB4 and LTC4 that are inhibited by NSAIDs. MRP4 mediates the efflux of several endogenous metabolites that have a critical role in signaling pathways involved in differentiation, pain, and inflammation. Because NSAIDs are strong inhibitors of MRP4 mediated efflux of PGs and LT, this may be another mechanism responsible for the antiinflammatory effects of NSAIDs. In MRP4-null mice, the decreased plasma PG levels were correlated with an increase in the toleration of inflammatory pain (Lin et al., 2008). MRP5/ABCC5 is expressed in most tissues and similar to MRP4 transports cyclic nucleotides and thus functions as an OAT. It is rather surprising that the efflux transporter for LTA4 has not been identified considering its important role in transcellular synthesis of LTs. It is likely that multiple transports function in LTA4 transport similar to those of LTC4. The wide range of immune cells expressing efflux transporters of eicosanoids clearly reveal their role in the orchestration of an effective immune response and significant undetermined role of immune cell involvement in metabolic diseases.
In contrast to ABC efflux transporter of eicosanoids, the SLC protein family members are responsible for the uptake and influx of eicosanoids in recipient cells (Wu et al., 2011). The first identified eicosanoid influx transporter was the PG transporter PGT/SLCO2A1, which is an OAT. Additional members of the OAT family (OATP/SLCO/SLC22) have been identified in the cellular uptake of eicosanoids and display similarities with FA transporter SLC27 members (Emami Riedmaier et al., 2012). The cellular uptake of eicosanoids by SLC22 transporters may interact with the SLC27 FA transport to connect energy needs with an inflammatory response (Niemi et al., 2011). During inflammation, increased circulating levels of PGE2 trigger the PG transporter to normalize PGE2 levels through 15-PGDH and 13-PGR PGE2 catabolism. This homeostatic mechanism is also achieved by the newly identified OAT-PGE2, PGF2α, and PGD2 transporter of the SLC22 family in the kidney proximal tubules where PGE2 activation of PG receptor EP4 increases PGE2 catabolism and activation of the OAT-PG transporter. To date, three influx transporters of eicosanoids have been identified, OATP2A/PGT/SCLC21A2, OATP2B/SLC21A9, and OATP4A/SLC21A2, suggesting that members of the FA transporter family SLC21 may be important in the selective influx, catabolism or trans-cellular metabolism of eicosanoids. The transcellular synthesis of eicosanoids involves a donor cell generating an eicosanoid intermediate that is released from donor cells by MRP and chaperoned to recipient cells by albumin, liposomes or FABP to prevent hydrolytic attack by water, and taken up by SLC transporters.
A number of unanswered questions remain regarding eicosanoid efflux and influx transporters as to their role in modulating the inflammatory response in insulin resistance, diabetes, obesity, and NAFLD. However, their importance in metabolic disease is evident from reduced expression of the efflux transporter in models of NAFLD (Lickteig et al., 2007) and the recent report of a FA efflux transporter in adipocytes (Henkin et al., 2012). Central to understanding the role of eicosanoid transporters in metabolic disease is the characterization of transporter involved in transcellular eicosanoid metabolism and regulatory roles of eicosanoids for the activity of the FA transporter in liver, pancreas, adipose and muscle tissue (Folco & Murphy, 2006).
3.4. Molecular Mechanism of Eicosanoid Regulation through GPCRs
Eicosanoids elicit their effect through paracrine, autocrine, and intracrine mechanisms by either the activation of extracellular G-protein-coupled eicosanoid receptors (GPCR), transcellular synthesis, or eicosanoid activation of transcription factors of the HNR family (Table 5.2). Even though these mechanisms have been well-studied with regard to inflammation and immune response, the role in the control of tissue response to eicosanoids in the regulation of metabolic pathways in MetS and NAFLD has only recently been studied. Excessive lipid droplets (LDs) accumulation of lipids in cells (steatosis) leads to the formation of LDs consisting of neutral lip-ids as TAG surrounded by a PL monolayer with unique FA composition that includes eicosanoids and a distinct set of proteins comprising many of the eicosanoid metabolic enzymes. In leukocytes, a portion of AA is stored in LD triglycerides where it is believed that mobilization of TAG AA by adipose triglyceride lipase (ATGL) replenishes lipid body arachidonyl-phospholipid through activation of LD-associated cPLA2 providing AA for local eicosanoid synthesis (Bozza et al., 2011). Many of the eicosanoid biosynthetic enzymes are associated with LD and actively produce PGE2, LTB4, and LTC4 on inflammatory stimulus. How eicosanoids exit the LD seems to be resolved with the identification of MRP14 transporter that is involved in AA transport and shuttling of uSFA to LD membrane (Vogl et al., 2007). Several studies have shown a correlation between eicosanoid synthesis by COX and LOX and an increase in the LD biogenesis (Bozza & Viola, 2010). In addition, PGD2 activation of DP1 and DP2 receptors on eosinophils increases cytoplasmic LD biogenesis and synthesis of LTC4. Eicosanoid synthesis and LD biogenesis are regulated by distinct signaling pathways through activation of DP1 and DP2 PGD2 receptors. Activation of DP2 does not increase LD biogenesis or eicosanoid synthesis, while DP1 activation increases LD biogenesis, and both are required for eicosanoid synthesis, indicating that these receptors coordinate the increase in LD biogenesis and LTC4 synthesis (Mesquita-Santos et al., 2011).
Table 5.1. Nomenclature and properties of fatty acid transport proteins.
| Gene id | Nomenclature | Tissue | Regulation | Substrate, ligand or binding protein | Subcellular location | Function | ||
|---|---|---|---|---|---|---|---|---|
| SLC27A1 | FATP1-ACSVL4 | Heart, adipose, muscle, brain | PPARγ | C16:0, C18:1, C24:0 | M, PM, ER | β-oxidation TAG synthesis | ||
| SLC27A2 | FATP2-ACSVL1 | Liver, kidney | PPARα, PPARγ | C16:0, C24:0 Phytanic acid, pristanic acid | ER, P | TAG synthesis β-oxidation | ||
| SLC27A3 | FATP3-ACSVL3 | Kidney, ovary, lung, brain, adrenal, testis | C16:0, C18:1, C24:0 | Cytosolic vesicles | Unknown | |||
| SLC27A4 | FATP4-ACSVL5 | Liver, kidney, heart, adipose, skin, muscle, small intestine | PPARγ, SREBP1 | C16:0, C24:0 | ER, P | TAG synthesis β-oxidation | ||
| SCL27A5 | FATP5-ACSVL6 | Liver | Cholate, THCA Chenodeoxycholate Lithocholate, C24:0 Deoxycholate | ER, P | Bile acid conjugation Bile acid synthesis | |||
| SCL27A6 | FATP6-ACSVL2 | Heart, placenta | C18:1, C20:4, C24:0 | PM | Unknown | |||
| FABP1 | L-FABP | Liver, intestine | PPARα, HNF4α | Acyl-CoA, PPARα,γ | Cytosol, N | |||
| FABP2 | I-FABP | Intestine | Acyl-CoA | Cytosol | TAG synthesis | |||
| FABP3 | H-FABP | Heart, kidney muscle, thymus | c/EBPα, SREBP1 | Acyl-CoA, PPARα | Cytosol | β-oxidation | ||
| AP-1 | ||||||||
| FABP4 | A-FABP | Heart, adipose, | cJun, PPARγ | Acyl-CoA, PPARγ | Cytosol | Chylomicron | ||
| epidermis, nerve | ||||||||
| FABP5 | E-FABP | Eye, adipose | PPARδ | Acyl-CoA, PPARβ | Cytosol | Lipogenesis | ||
| FABP6 | Il-FABP | Ileum | Acyl-CoA, FXRα | Cytosol | ||||
| FABP7 | B-FABP | Liver, brain | POU | Acyl-CoA | ||||
| FABP8 | N-FABP | Myelin | Acyl-CoA | Cytosol | Vesicle assembly | |||
| FABP9 | T-FABP | Testis | — | Acyl-CoA | Cytosol | |||
| FABP12 | R-FABP | Retina, testis | — | Acyl-CoA | Cytosol | |||
| ACBP | L-ACBP | Liver, multiple tissues | PPARα, c/EBPα SREBP1c, Sp1 PPARγ | C14:0-C22:0 CoA esters, HNF4α | Cytosol | Glycerolipid, cholesterol synthesis | ||
| ACBP | T-ACBP | Testis, adrenal | — | C14-C22 CoA esters | Cytosol, ER | |||
| ACBP | B-ACBP | Brain | — | C14-C22 CoA | Cytosol | |||
| ABCP | aACBP | Adipocytes | PPARγ | — | — | FA transport | ||
| FAT/ | Multiple tissues | — | Numerous | PM, M | FA transport | |||
| CD36 | ||||||||
| ACSL1 | ACS1 | Adipocytes | PPARγ, PPARγ | C16-C24, C18:1-3,AA | PM, N, | B-oxidation | ||
| ACSL3 | ACS3 | Adipocytes | LXRα, PPARβ | C14-C22.C18:1-3 | M, LD | TAG synthesis | ||
| ACSL4 | ACS4 | Liver | PPARα, SREBP1 | AA, C14-C18 | LD, lipid raft | |||
| ACSL5 | ACS5 | Liver | SREBP1 | C16-C24,C18:1- | ER, P, LD | TAG synthesis | ||
| ACSL6 | ACS2 | Red blood cell | — | C14-C24,C18:1-3 | M, PM, ER, LD Lipid raft | PL synthesis | ||
| FABPpm | AST | Multiple tissue | — | Numerous | PM, M | FA transport | ||
PM, plasma membrane; ER, endoplasmic reticulum; M, mitochondria; LD, lipid droplets; N, nucleus; P, peroxisome; AA arachidonic acid. Characteristics of fatty acid transport protein (FATP-ACSVL), fatty acid binding protein (FABP) and acyl-CoA binding protein (ACBP). This table summarizes tissue-specific expression, regulation by transcription factors, substrate, ligand binding and interaction with nuclear receptors and putative function in the metabolism of fatty binding proteins.
Because LD formation in pancreas and liver increases insulin resistance, hepatic steatosis, and obesity, it is believed that LD may be the primary source of metabolic persistent inflammation in the pathology of MetS. There are few studies to address the issue of LD formation and inflammation in different organs and cell types and whether LD biogenesis and increased eicosanoid synthesis initiate and amplify the lipid actacoid-cytokine-chemokine cascade. LD formation has been strongly associated with muscle insulin resistance in T2DM (Bosma et al., 2012). Muscle insulin resistance is due to SNARE protein that controls the fusion of LDs, which is necessary for the progression of microvesicular steatosis to macrovesicular steatois in the liver. In muscle, SNARE protein associates with the plasma membrane and assists in insulin-mediated translocation of Glut4 from the cytosol. Excessive muscle TAG shifts the SNARE protein from the plasma membrane to LD formation resulting in insulin resistance (Bostrom et al., 2007). Thus, increased LD biogenesis in MetS with elevated tissue synthesis of inflammatory eicosanoids not only promotes recruitment of immune cells but also activates plasma membrane and NHRs in the control of lipid and carbohydrate metabolism through autocrine, paracrine, and intracrine mechanisms.
A broad range of lipid mediators, including FA discussed previously, PGs, TXs, HETE, oxo-ETT, LTs, and lysoPLs act on GPCRs. The plethora of GPCRs, about 376, provide the cell a unique mechanism to both maintain metabolic homeostasis and respond to a changing environment by specific and selective agonists and antagonists. About 50% of the currently prescribed drugs target GPCRs. Although lipid mediators are considered a small group of GPCR activators, they have been proved to be an important viable target in the treatment of inflammation and MetS.
3.4.1. Fatty Acid Receptors in Regulation of Metabolism
The extracellular effects of FFA signal through a group of GCPR (Ichimura et al., 2009). These receptors include FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), GPR84, and GPR120 that recognize FAs with different chain length and degree of unsaturation to initiate both antiinflammatory and anti-obesity effects. These SCFA, medium-chain FA and LCFA receptors function in diabetes and inflammation. Glucagon-like peptide-1(GPL-1) secretion in the intestine and pancreatic insulin secretion are mediated by activation of the GPR120 and GPR40 receptors by ω3-PUFA and C18-20 uSFAs, respectively while GPR40 is also activated by TZD. The divergent response of pancreas to FFAs, where acute exposure stimulates insulin secretion, while chronic exposure impairs insulin secretion, is revealed in GPR40-null mice that display insulinemia and, insulin resistance, but protection from steatosis, hyperglycemia and hypertriglyceridemia (Steneberg et al., 2005). GPR40 is activated by LCFAs (C12-C16) that induce gastrointestinal cells to secrete GLP-1. GRP119 is activated by LCFAs and lysoPLs in the intestine where activation stimulates intestinal K-cells to secrete glucose insulin-trophic peptide and L-cells to discharge GPL-1 incretins. In the pancreas, release of GLP-1 is glucose independent, but GPR40, and FA dependent. GPR43 and GPR41 are activated by SCFAs (propionate, butyrate, pentanoate) that regulate FA and glucose homeostasis in adipose tissue and intestine. SCFA mediated stimulation of GPR43 reduces serum FFAs by inhibiting adipose lipolytic activity and thus has an important role in the lipid profile of MetS patients. GPR43 is prominently expressed in leukocytes and may have a role in leukocyte activation of inflammation in hyperlipidemia. GPR43-null mice fed an HF diet display a lean phenotype with increased energy expenditure and improved glucose tolerance, and the absence of this receptor in adipocytes increases energy expenditure, while absence in macrophages prevents inflammation. GPR41 is also expressed in adipose tissue where it is believed to regulate leptin production since receptor activation increases serum leptin levels. GPR84 is a medium-chain FFA receptor that is highly induced in leukocytes in inflammation. Thus, both GPR84 and GPR43 in leukocytes link FA metabolism to inflammation and immune cell activation. GPR120, usually activated by ω3-PUFAs stimulates intestinal secretion of GLP-1 and increases Glut4 transporter expression in adipose tissue. Importantly, GPR120 attenuates inflammation by inhibiting TLR2, TLR4, and TNFα receptor-mediated inflammation in macrophages (Oh & Olefsky, 2012; Talukdar et al., 2011). Overall, GPRCs regulated by FFAs are direct sensors of nutrients in the extracellular environment that mediate secretion and possibly production of peptide hormones. These GPRs function as important modulators of inflammation and immune system function thus further linking lipid metabolism to inflammation (Oh da & Olefsky, 2012).
3.4.2. Eicosanoid G-protein-Coupled Receptors
Prostanoid receptors consist of five types that bind a diverse array of prostanoids including PGD2 (DP1 and DP2), PGE2 (EP1-4), PGF2α (FP), PGD2 (CRTH2), PGI2 (IP), and TXA2 (TP). Although numerous studies have revealed the role of these receptors in inflammation and immune regulation, only few studies have suggest these receptors in the control of metabolism like the FFA receptors (Hirata & Narumiya, 2011). The prostanoid receptors can be classified by their cellular response as relaxant receptors that increase cAMP through Gαs (DP1,EP2, EP4, and IP), or activation of Ca2+ mobilization and contractile response by Gαq (EP1,FP,TP) while inhibitory receptors connected to Gαi (EP3). The DP1 PGD2 receptor mediates smooth muscle cell vasodilation, inhibition of platelet aggregation and activation of mast cell in allergic inflammation. The DP2 receptor and chemoattractant receptor homolog (CRTH2) in Th2 cells promote immune cell migration in inflammation, and induce basophil and esosinophils to secrete IL4, IL5, and IL13 cytokines. EP1 receptors (PGE2) signal through Gαq to promote tissue edema and pain. EP1-null mice show a significant reduction in systolic blood pressure and thus antagonist can be used to treat hypertension of MetS. An intriguing role of EP1 receptor in the central nervous system is its activation during stress-induced release of glucocorticoids similar to fever generation by hypothalamic-pituitary-adrenal axis stimulation through activation by both EP1 and EP3 receptors (Furuhashi & Hotamisligil, 2008). PGE2 antiinflammatory effects are mediated through EP1/EP4 suppression of TNFα production, enhanced IL10 synthesis, and inhibition of T-cell mitogenesis with enhancement of Th1 cell differentiation and Th17 cell expansion. Blocking both the EP1/EP4 receptors decreases Th1 and Th17 cell accumulation in lymph nodes and suppresses progression of autoimmune encephalomyelitis (Esaki et al., 2010). The EP3 receptor mediates fever generation since EP3-null mice show no fever in response to inflammatory stimuli. The EP4 receptor in the vasculature acts as a potent vasodilator and cooperates with EP2 in inflammation. EP4-null mice develop severe colitis, which can be mimicked by EP4 antagonist in wild-type mice (Kabashima et al., 2002). The IP (prostacyclin) is localized in endothelial cells and its activation has potent antithrombotic and vasodilator effects that oppose the effect of TXA2 since IP-null mice show accelerated atherogenesis in ApoE-deficient mice (Kobayashi et al., 2004). PGI2 has potent antiinflammatory and immunosuppressive effects on Th2-mediated inflammation through IP suppression of dendritic cell activation and maturation of T cells (Zhou et al., 2007) while promoting Th1 cell differentiation. TP (TXA2) induces platelet aggregation and smooth muscle contraction that opposes the prostacyclin vascular effects. TXA2 produced in dendritic cells activates T-cell TP receptors that inhibit dendritic cell immune response while activating inflammation. FP (PGF2α) is coupled to Gαi that inhibits cAMP and Gαq, which mobilizes Ca2+ and has a functional role in lung fibrosis by stimulating fibroblast proliferation and collagen production independent of TGFβ (Oga et al, 2009). It will be of importance to determine if activation of FP in hepatic stellate cells initiates the pathology of steatohepatitis to hepatic fibrosis in NAFLD.
The LT receptors consist of two classes, the LTB4 receptors that are expressed in myeloid cells, endothelial and smooth muscle cells (BLT1), and liver (BLT2) and the cysteine LT receptors (cysLT1 and cysLT2). The BLT receptors are activated by LTB4 and ω-hydroxylase CYP4 LTB4 metabolites, 20-OH LTB4 and 20-COOH LTB4, and TX synthase-produced 12-HHT. BLT1 activation is coupled to Gαq-mediated Ca2+ mobilization and Gαi inhibition of cAMP production. BLT2 has a 20-fold weaker binding of LTB4 (Kd = 1–23 nM) compared to BLT1 (Kd = 0.15 nM) but can be activated by 12(S) HETE, 15(S) HETE and 12-HHT. BLT2 is expressed in liver, ovary, leukocytes, macrophages and mast cells where it mediates chemotaxis similar to BLT1. Both BLT1 and BLT2 activate several kinases, including MAPK, involved in macrophage proliferation, and phosphatidyl kinase that induces Ca2+ mobilization and IL6 gene expression and NF-κβ DNA binding. LTB4 is critical for the induction of the neutrophil respiratory burst in the release of myeloperoxidase, matrix metalloproteinase, elastase and α-defensins. Activation of BLT receptors in macrophages induces IL1β, IL6, and MCP-1 production, and chemotaxis. The presence of both BLT receptors in macrophages allows chemotaxis to occur over a wider range of LTB4 concentrations. In T cells, BLT1 is the dominant chemotactic receptor that induces production of IL1, IL2, IL5 and interferon-γ, which promotes Th17 cell differentiation (Chen et al., 2009). In VSM cells, BLT1 activation initiates SMC migration and proliferation through activation of integrin signaling (Moraes, Assreuy Canetti, & Barja-Fidalgo, 2010) as shown by congenic BLT1-null ApoE-null mice having a dramatic reduction in the number of atherosclerotic lesions (Heller et al., 2005). Endothelial BLT1 is believed to be responsible for release of vasoactive factors, while BLT2 activation is required for angiogenesis, suggesting a correlation between LTB4 levels and function of endothelium. BLT1- and BLT2-null mice show reduced atherosclerosis and an attenuated response to inflammatory arthritis, while only BLT2-null mice have colitis due to disruption of the intestinal barrier (Nancey et al., 2011) that can be mimicked by 12-HHT antagonists. There has been only one study investigating the function of BLT receptors in MetS (Spite et al., 2011). In BLT1-null mice, there is a reduction of adipose tissue M1 macrophages (ATM) while antiinflammatory M2 ATM numbers increase, resulting in decreased expression of proinflammatory chemokines and cytokines. Also, BLT1-null mice fed an HF diet are protected from systemic glucose intolerance and have decreased hepatic steatosis with reduced adipocyte and liver inflammation. It will be of interest to determine the phenotype of BLT2-null mice on an HF diet or on ApoE-null background.
There are two cysteine LT receptors cysLT1 and cysLT2 that are activated by the cysteine leukotrienes (LTC4, LTD4, and LTE4); however, these receptors show difference in regulation by agonists and antagonists. CysLT receptors are important in asthma because of their prominent expression in eosinophils; however, these receptors are also expressed in monocytes, granulocytes, B cells, fibroblasts, myocytes, and plasma where their activation increases release of monocyte chemotactic protein (MCP-1) and its trans-location to the nucleus during inflammation. Both receptors are believed to have different roles in inflammation with cysLT1 mediating the effects of acute inflammation, while cysLT2 functions in chronic inflammation and fibrosis. Activation of cysLTs in the vasculature causes vessel constriction, increase in vascular permeability and cardiac output. Thus blockage of receptor activation inhibits atherosclerotic lesion size and intimal hyperplasia. CysLTs have been implicated in liver disease, which includes hepatic inflammation, cholestasis, portal hypertension and hepatorenal syndrome. Inhibition of receptor activation has been found to be effective in the prevention of liver and intestine injury by reducing apoptosis and oxidative stress (Daglar et al., 2009) and improving hepatic fibrosis in cholestasis (El-Swefy & Hassanen, 2009).
The balance between activation of proinflammatory receptors (LT) and antiinflammatory (LX) is critical in maintaining tissue homeostasis. The antiinflammatory LT LXA4 and LXB4 synthesized by transcellular metabolism from LTA4 have potent antiinflammatory and resolution abilities that signal through LXA4 receptors. LXA4 receptors (LX) are coupled to Gαi/o, reduce chemotaxis and induce phagocytosis of apoptotic neutrophils. Although the pathophysiological role of LXA4 and LX receptor is suggested, their role in human disease has at best been a causal association (Serhan et al., 2007) and thus their role in MetS and NAFLD has not been investigated.
The deacylation and reacylation of PL by the Land's cycle produces lysoPLs that are precursors to LPA, PAF and endocannabinoids. LysoPLC can be converted back to PL by the action of lysophospholipid:acyltransfer ase (LPLAT). LPA is a water-soluble PL that activates six currently known GPCRs for LPA that couple to Gαi, Gαq, and Gα12/13 (Lin et al., 2010). The strong binding of LPA to LPA receptors in the nanomolar range strongly implicates their role in physiological functions. LPA in CVD appears to have a protective role in preventing hypoxia-induced ischemia by activation of PI3/AKT and ERK pathways, while inducing Src-mediated contraction. However, LPA receptors induce intimal hyperplasia, VSMC migration and proliferation, endothelium dysfunction, LDL uptake and monocyte recruitment and adhesion. Overall, LPA has both beneficial effects on I/R injury and adverse effects on the development of atherosclerosis. In the liver, LPA activates hepatic stellate cells, leading to collagen deposition in the extracellular matrix as well as induction of hepatocyte proliferation, which contribute to hepatic cirrhosis (Watanabe et al., 2007). Similar to cysLT receptors the LPA receptors function during the chronic stages of inflammation; however, their role in the progression of NAFLD has not been studied. Furthermore, although the elusive cytochrome epoxygenase EET receptor has not been identified, a recent report has provided conclusive evidence that this receptor mediates many of the physiological effects of EETs (Chen, Falck, Manthati, Jat, & Campbell, 2011).
To date, 30 GPCRs for lipid mediators have been identified and studied (Nakamura & Shimizu, 2011) with particular emphasis on FFA receptors in the control of lipid and carbohydrate metabolism and eicosanoid metabolism in inflammation and immune cell activation. Hopefully, future studies will identify the cross-talk between these FFAs and eicosanoid receptors in inflammation and intermediary metabolism in diabetes, obesity, hyperlipidemia, hypertriglyceridemia, and NAFLD.
3.4.3. Eicosanoid Regulation of Nuclear Hormone Receptors
The original purpose of defining MetS as a constellation of metabolic alterations of insulin resistance, dyslipidemia, hypertension, hypertriglyceridemia and obesity was to define the risk factors that contribute to CVD. The recent pandemic of obesity in the Western population has led to the identification of NAFLD as a disease with many symptoms of MetS. The prevalence of NAFLD in the Western population ranges from 1 to 36% with 90% of obese patients and 70% of type II diabetic patients strongly indicating that NAFLD is a strong predictor of MetS. With the realization that increased influx of free FA was due to alterations in lipid and carbohydrate metabolism controlled by NHRs, it became imperative to identify NHR agonists and antagonists that control FA and carbohydrate oxidation and decrease lipogenesis. The identification of NHR drug agonists that ameliorated some symptoms of insulin resistance and obesity (e.g. PPARγ-TZD agonists) led to the realization that endogenous ligands may be altered thereby leading to MetS associated diseases (Table 5.2). Thus a hunt for endogenous ligands that regulate these orphan NHRs started and continues to of utmost importance in understanding and treatment of NAFLD and MetS. This quest has led to the search for lipid molecules that regulate NHR since lipidemia is a constant in both diseases. Whether eicosanoids are true physiologically important NHR ligands for many orphan NHRs has yet to be resolved through rigorous analytical methods, including in vivo co-localization of eicosanoids with NHR on target genes and testing in eicosanoid enzyme knockout mice. One dilemma in using eicosanoids as physiologic ligands is that even though NHR activation occurs in the eicosanoid nanomolar range, in vitro micromolar or greater levels must be used to observe a response, in vivo. However, the association of several eicosanoid metabolic enzymes with the nuclear envelope and FABP or ACBP transportation into the nucleus can increase eicosanoids levels high enough to activate NHR activation.
The NHR family is the largest group of transcriptional regulators in humans consisting of 48 members that include steroid homodimer receptors: androgen, estrogen, glucocorticoid (GR), mineralocorticoid, and progesterone; nuclear receptor heterodimers with retinoic-X-receptor (RXR); retinoic acid (RAR), thyroid, and vitamin D the partially unknown ligand orphan receptors: farnesoid-X-receptor (FXR), liver-X-receptor, pregnane-X-receptor (PXR), PPAR, estrogen related receptor, HNF receptors, liver-related homolog (LRH), and RAR-related orphan receptor (ROR); and lastly true orphan receptors Reverb, short heterodimer partner (SHP), and chicken upstream promoter transcription factor.
The NHRs most prominently involved in the control of metabolism and collectively known as metabolic sensors are PPARα, PPARβ, PPARγ, LXRα, FXRα, PXRα, constitutive androstane receptor (CAR), RXRα, and HNF4α. The PPARs are the most studied NHRs in regard to lipid activation by FFAs and eicosanoids. PPARα (NR1C1) is expressed in tissues with a high rate of FA oxidation, liver, kidney, intestine, and BATs. PPARβ (NR1C2) is expressed in adipose, skeletal muscle and several other tissues and has a fundamental role in cellular processes. PPARγ (NR1C3) is abundantly expressed in WAT, BAT, and liver where it promotes glucose uptake, lipid storage, adipocyte differentiation and maintenance. The PPARγ2 variant is most prominently expressed in adipose and muscle tissues, while PPARγ1 is broadly expressed in many tissues. A variety of eicosanoids and PLs have been identified as PPAR ligands, connecting inflammation with the control of carbohydrate and lipid metabolism in in the development NAFLD and MetS (Harmon et al., 2011; Wahli & Michalik, 2012).
PPARα (NR1C1) is activated by uSFAs, LTB4, 8(S)-HETE, 8,9-EET, 11,12-EET, 15-HETE, PLs and the synthetic fibrate drugs that reduce serum triglycerides. Activation of PPARα increases the transcription of genes involved in β-oxidation and has antiinflammatory effects. It is apparent that proinflammatory eicosanoids mediate their effects through activation of plasma membrane GPCRs, while these same proinflammatory eicosanoids induce an antiinflammatory response by activation of PPARα, PPARβ, and PPARγ. Activation of PPARα has several antiinflammatory properties that include induction of peroxisome β-oxidation and inactivation of eicosanoids (Narala et al., 2010), induction of I-κβ that blocks NF-κβ transcription of proinflammatory genes, increased expression of soluble interleukin-1 receptor antagonist (Stienstra et al., 2007), as well as transrepression of proinflammatory transcription NF-κβ, activator protein, and nuclear factor of T cell (Poulsen et al., 2012). PPARα also interacts with the GR that mediates an increase in cortisol levels, which that is believed to be responsible for hyperinsulinemia observed in sepsis (Ahmed et al., 2012). PPARα has a central role in metabolism and inflammation which is based on the observation of upregulation of TLR2 and TLR4 in the adipose tissue of PPARα-null mice that exhibit hepatic steatosis after fasting (Wahli & Michalik, 2012). PPARα expression in macrophages modulates cholesterol trafficking and inhibits local vascular inflammation by channeling excess FA to β-oxidation or TAG storage rather than the production of ceramides and DAG that are believed to be instrumental culprits in muscle insulin resistance.
PPARβ (NRIC2) is also activated by uSFA, VLDL constituents, and products of oxidized 4-hydroxy-2-nonenal and 4-hydroxydodeca-2E, 6Z-dienal, and is weakly activated by FAs. PPARβ antiinflammatory effects are evident by its inhibition of NF-κβ activation and transcriptional induction of anti-inflammatory corepressor B-cell lymphoma G, induction of angiopoietin-related protein 4, and increased expression of TGF-β. In adipocytes, PPARβ has a central role in signaling between ATM and adipocytes where it promotes M2 macrophage phenotype over the M1 ATM that is proinflammatory. Activation of PPARβ reduces atherosclerosis in LDLR-null mice by decreasing expression of MCP-1, vascular adhesion molecules, and TNFα. In muscle, PPARβ channels palmitic acid to TAG accumulation and mitochondrial β-oxidation rather than production of lipids that initiate inflammation and insulin resistance. In cardiomyoctes, PPARβ inhibits palmitate and LPS-induced inflammation by transrepression of NF-κβ (Alvarez-Guardia et al., 2011).
PPARγ (NR1C3) is activated by uSFAs, oxidized FA, 9-HODE, 13-HODE, 15-HETE, 13-oxo-ODE, 15-deoxyΔ12,14 prostaglandinJ2, oxo-LDL, LPA, farnesyl phosphate, 15-keto-PGE2, and decanoic acid, while both PGF2α and cyclic PA are antagonists. PPARγ is activated by the synthetic antidiabetic TZDs that reduce serum hyperglycemia and promote adipocyte differentiation. PPARγ inhibits inflammation by repressing NF-κβ, and macrophage and T-cell expression of pro-inflammatory cytokines of the innate immune response (Huang & Glass, 2010). Macrophages from PPARγ-null mice exacerbate metabolic disease by increasing hepatic, adipose, and muscle insulin resistance. Although PPARγ activation increases adipocyte differentiation that has a beneficial effect on increasing systemic insulin sensitivity and reducing hyperglycemia, its induction in the liver promotes hepatic steatosis, while deletion protects against HF-diet-induced hepatic steatosis (Moran-Salvador et al., 2011). The decrease in PPARα and increase in PPARγ expression in hepatic steatosis may be a protective mechanism to prevent liver fibrosis. PPARγ-null mice display muscle and macrophage hypercholesterolemia, leading to atherosclerosis, disruption of endothelial cell dysfunction, and endothelial cell proliferation (Qu et al., 2012).
All three PPARs are therapeutic targets for the treatment of metabolic diseases and are important drug targets to treat the chronically persistent inflammation associated with these diseases. Although fibrates that target PPARα have been very successful in the treatment of dyslipidemia, the PPARγ TZD drugs despite improving insulin sensitivity have a number of undesirable side effects including weight gain, edema, heart failure, and bone fractures. Because PPARγ isoforms are expressed in several tissues associated with MetS, such as adipose, liver, VSMC, endothelial cells and pancreas, it is a desirable target to treat MetS if we can dissociate the undesirable site effects from its efficacy in treating hyperglycemia and hyperlipidemia. Recently, 5-amino salicylic acid, an anti-inflammatory drug that inhibits PGH2 synthase, NF-κβ, 15-LOX, and PLA2, while it activates PPARγ, shows less side effects than TZDs and has greater antiinflammatory properties (Wahli & Michalik, 2012). Also, decanoic acid (C10) a medium-chain FA, activates PPARγ without inducing adipogenesis, yet improves insulin sensitivity and is also a weak agonist for PPARβ and PPARα (Malapaka et al., 2012). Other HNRs are also activated by lipids, but unlike the PPARs, it is not known whether they are also targets for eicosanoid metabolites in the treatment of MetS.
The liver-related homolog-1 (LRH-1/NR5A2), which is a competence factor for SHP, FXR, PPARα, and LXRα. LRH-1 is activated by distinct PLs and has profound antiinflammatory action by induction of glucocorticoid synthesis and reduction in acute-phase protein synthesis of serum amyloid A, haptoglobin, fibrinogen, and inhibition of IL6 and IL1β (Venteclef et al, 2011). LXRα/NRIH3 is a cholesterol sensor that is induced by oxysterol, activating genes in reverse cholesterol transport and repressing proinflammatory cytokine gene expression, thus contributing to reduced atherosclerotic lesions. Unfortunately, LXR activation also increases lipogenesis and VLDL serum levels and decreases ApoA1 necessary for synthesis of HDL particles. LXR coordinates the regulation of neutrophil homeostasis the clearance of senescent neutrophils by antigen-presenting cells in peripheral tissues (Hong et al., 2012). In addition, LXRα inhibits expression of the acute-phase protein, C-reactive protein, through transrepression of proinflammatory transcription factors (Venteclef et al., 2011).
FXRα/nuclear family 4 subgroup A receptors (NR4H4) is the main bile acid sensor that when activated inhibits NF-κβ and profibrogenic collagen 1α. FXR inhibits TAG synthesis and VLDL export through the FXR-SHP cascade as well as inhibits HDL metabolism while inducing VLDL catabolism. PXR/NR1I2 and the CAR/NR1I3 are xenobiotic-activated receptors that promote hepatic lipid storage by decreasing FA β-oxidation. PXR promotes free FA uptake through CD36 induction and thus induces lipogenesis, while CAR regulates serum TAG levels and CAR-null mice are protected from hepatic steatosis. Orphan receptor SHP/NR0B2 is a downstream target of FXR, has no DNA-binding domain. HNF4α/NR4A1 is a master regulator of lipid metabolism and mutations in this HNR lead to maturity onset of diabetes. This orphan receptor ligand is believed to be linoleic acid; however, this FA does not influence HNF4α transcriptional activity (Yuan, et al., 2009).
The orphan NR4A subgroup of receptors include Nur77 (NR4A1), Nurr1 (NR4A2), and Nor-1 (NR4A3); all are associated with lipid and carbohydrate metabolism in muscle, liver, and both WAT and BAT through NR4A activation of β-adrenergic signaling (Pearen & Muscat, 2010). This establishes a link between FA metabolism and eicosanoid pathways. Although NR4A receptors have no known natural ligands, these receptors are key molecular switches linking inflammation to metabolism. Their bulky ligand-binding domain and regulation by PGE2 make them a target for eicosanoids being their possible endogenous agonist (Mohan et al., 2012). NR4A2/NURRI act as monomers to transactivate target genes. NR4A2 is activated by COX2-produced PGE2 that activates the EP1 receptor, leading to induction of FA β-oxidation (Holla et al., 2011). It is especially interesting that NR4A members regulate the expression of FXRα, RXRα, SREBP-1 and PPAR coactivators, PCG1α and PCGβ.
Nuclear hormone receptors have a central role in macrophages' and dendritic cells' ability to sense their lipid environment through eicosanoid and lipid agonists that regulate NHRs, resulting in expression of proinflammatory M1 or antiinflammatory M2 phenotype (Nagy et al., 2012). To deorphanize these NHRs, we need to identify their true endogenous ligands by characterization of receptor–ligand affinities, determination of cellular concentrations of ligands by liquid chromatography tandem mass spectrometry, and also in vivo colocalization of ligand and receptor with a defined physiological response.
4. Eicosanoids in Sepsis and Drug Metabolism
4.1. Links between Sepsis and MetS
Sepsis or septicemia is defined as systemic inflammatory response syndrome (SIRS) that affects over 750,000 patients annually in the United states with a mortality rate of over 30% (Angus et al., 2001). SIRS is a constellation of both metabolic and inflammatory derangements that ultimately lead to multiple organ failure by increased circulating levels of proinflammatory cytokines, cortisol, acute-phase proteins, and apoptotic immune cells. SIRS patients display many of the symptoms of MetS largely due to excessive serum cortisol and adrenocorticotropic hormone (ACTH), which are similar to those of Cushing syndrome patients who have insulin resistance with hyper-insulinemia, hyperlipidemia, hypertriglyceridemia, and hypertension (Macfarlane et al., 2008). The progression of local sepsis to systemic sepsis is initiated by overac-tivation of the lipid autocoid-cytokine-chemokine cascade, leading to overstimulation of immune system and dramatic suppression of drug- and eicosanoid-metabolizing P450s. This situation makes the treatment of sepsis a balance between effective management and the possibility of inadvertent drug toxicity (Seeley et al., 2012). The importance of eicosanoid in the initiation and progression of sepsis is apparent considering clinical trials of sepsis patients with ω3-PUFAs had a 20% decrease in mortality and reduction in serum enzymes, cortisol and ACTH that correlated with a massive increase in EPA-derived LTs (Grimm et al., 2006). Although parenteral and enteral ω3-PUFAs appear to preserve immune function and reduce inflammation, the role of eicosanoids in the progression and management of sepsis has not been extensively studied. Of equal importance is the question how eicosanoids regulate cortisol levels and what effects they have on adrenal gland.
The characterization of a patient's serum profile may have a potential utility in staging of sepsis, which is very difficult due to the heterogeneity of septic patients with respect to sites of infection, type and virulence of pathogens, and comorbidities including liver disease, cancer, age, and environmental variables (Seeley et al., 2012). In septic patients, PGE2 and 11-HETE have recently been identified as differentiating eicosanoid metabolites between healthy subjects and sepsis patients (Bruegel et al., 2012). In septic patients, the levels of PGE2 and 11-HETE are reduced by 80% with a decreased expression of inducible COX2 but not mPGES-1, suggesting that antiinflammatory suppression of the Th-1-mediated immune response is not functional in septic patients. Thus the infusion of PGE2, which mediates suppression of Th-1 and activation of adaptive Th-2 immune response, may have a promise with ω3-PUFAs in the management of sepsis (Nicolete et al., 2008).
It is known that activation of the immune system by inflammation during sepsis leads to a dramatic alteration and repression of the drug-metabolizing enzymes with reduced expression of selective CYP genes. Adverse drug reaction (ADR) is a serious human health problem caused by idiosyncratic effects of drugs during their therapeutic use in the treatment of diseases (Deng et al., 2009). Idiosyncratic adverse drug reactions (IADR) are caused by accumulation of toxic drugs and endogenous biochemical metabolites during inflammation. It may not be a mere coincidence that IADRs are the most common cause of liver failure in sepsis. The COX inhibitor, diclofenac, inhibits COX1 and COX2 enzymes in inflammation as well as being a substrate and inhibitor of CYP2C8 and CYP2C9 that produce antiinflammatory EETs. A nontoxic dose of LPS given to rats rendered a nontoxic dose of diclofenac injurious to the liver (Deng et al., 2006), suggesting that inflammation is a pivotal factor in diclofenac-induced IADR. It is likely that the competition of COX isoforms and CYP2C8 or CYP2C9 for the synthesis of prostanoids and EETs, respectively, is altered by diclofenac in inflammation, where its normal metabolism by CYP2C8 is reduced or completely inhibited leading to diversion of AA from prostanoid and EET synthesis to proinflammatory 5-LOX and 12-LOX pathways (Fig. 5.2). This would explain the beneficial antiinflammatory effects of PGE2 infusion in the treatment of sepsis. The decreased CYP2C19 enzymatic activity in critically ill patients would result in decreased NSAID metabolism and inhibition of COX-mediated production of PGE2, being replaced by AA metabolism to pro-inflammatory LTs. Thus, overexpression of CYP epoxygenase (CYP2C8, CYP2J2) attenuates NF-κB-dependent vascular inflammatory response in vivo and may inhibit chronic inflammation in sepsis patients (Deng et al., 2011).
The cause of IADR in sepsis and inflammation may be suppression of cytochrome P450 activity by unknown mechanisms. It has been suggested that the increased synthesis of acute phase response competes with CYP synthesis in the liver or that inhibition of cytokine mediates inhibition of NHR that regulates the CYPs gene expression. Although numerous studies have shown that selective CYPs are downregulated during inflammation and sepsis, there are few studies on how eicosanoids regulate CYP expression. Kupffer cells, hepatic macrophages, have been shown to mediate the decreased expression and activity of CYP1A1, CYP1A2, and CYP2E1 in the inflammatory response (Kim et al., 2011), and decreased expression of the major liver drug-metabolizing CYP3A4 and CYP2C isoform's mRNA and enzymatic activities has been reported in mice exposed to LPS (Moriya et al., 2012). Furthermore, suppression of hepatic CYP2C and CYP2J mRNA after LPS induction of inflammation decreased levels of EETs and also decreased CYP4A12 and CYP3F13 production of 20-HETE (Theken et al., 2011). The repression of EET and 20-HETE formation by CYP2 and CYP4 P450s, respectively, was also evident in lung, and kidney, but no difference in EET + DHET or 20-HETE was observed in the heart. Although this study needs confirmation, it demonstrates that activation of the innate immune response by inflammation alters the expression of eicosanoid-metabolizing CYPs, leading to reduced synthesis of bioactive EETs and 20-HETE with reduced metabolism of therapeutic drugs that can lead to IADRs.
5. Eicosanoids and Mets Diseases
The epidemics of obesity, T2DM, and atherosclerosis in MetS are increasing yearly worldwide. The constellation of diseases associated with MetS, insulin resistance, hypertriglyceridemia, hyperlipidemia, hypertension, and obesity are largely attributed to derangements in lipid and carbohydrate metabolism. The rate of NAFLD is increasing in the United States with 34% of the population displaying many of the symptoms of MetS, thus making NAFLD an additional characteristic of MetS (Anderson & Borlak, 2008). However, the mechanism in the pathophysiology of fat accumulation in the liver (hepatic steatosis) and how fatty liver communicates with other tissues in the diseases of MetS are not fully understood. Here we will briefly discuss the role of eicosanoids in the control of carbohydrate and lipid metabolism in tissues having a functional role in MetS and NAFLD and how inflammatory effects on CYP drug and eicosanoid metabolism lead to subclinical organ dysfunction in NAFLD, obesity, insulin resistance, dyslipidemia, and hypertension. Our intent in this section is to provide suggestive evidence for eicosanoids functioning as an important link between inflammation and intermediary metabolism and therefore an unrealized therapeutic target to treat MetS.
5.1. Eicosanoids in NAFLD and Obesity
Hepatic steatosis refers to the intracellular accumulation of lipids and the formation of LD in the cytoplasm of hepatocytes that can progress from simple microvesicular steatosis to macrovesicular steatosis and eventually fatty liver inflammation (steatohepatitis), which is also known as NASH. The excessive accumulation of lipids leads to increase in lipid peroxidation by reactive oxygen species (ROS), ultimately leading to immune cell recruitment and infiltration into damaged tissue. The persistent subclinical inflammation is caused by activation of the lipid autocoid-cytokine-chemokine cascade with the eventual activation of hepatic stellate cells with secretion of collagen for increased hepatic fibrosis that can progress to hepatic cirrhosis. The role of eicosanoids in NAFLD until recently has been largely explored with respect to Kupffer cell-mediated cytokine- chemokine-related disease progression.
The importance of eicosanoids in NAFLD is evident from metabolic analysis of eicosanoid-metabolizing enzyme knockout mice and the analysis of the lipidome of NAFLD patients. In patients with NAFLD or NASH, analysis of serum plasma lipidome revealed an increase in monounsaturated fatty acid (MUFA), C16:1n7 and C18:1n9, with an increase in the saturation index (uSFA to SFA) (Puri et al., 2009). In addition, linoleic acid (C18:2n6) decreased with an increase in dihomo-γ-linolenic acid (C20:3n6) and a decrease of antiinflammatory DHA and EPA in PE and PC PLs. Furthermore, there is a stepwise increase in LOX metabolites (5-HETE, 8-HETE, 15-HETE and the nonenzymatic oxidation product HHT) with the progression of NAFLD to NASH. Analysis of oxidized lipids in the plasma of patients with NASH showed increased levels of HETE, HODE, and oxo-octadecadienoic acid (oxoODE) that strongly correlated with free-radical-mediated oxidation of linoleic acid to 9- and 13-HODE and 9,13-oxoODE products that increase with the progression from steatosis, to steatohepatic and fibrosis in patients with NAFLD (Feldstein et al., 2010). Presently, we do not know the source of ROS that initiates progression of NAFLD to NASH (Fig. 5.2). Recent data with CYP2e1-null mice and the corresponding wild-type mice suggest that CYP2E1 is likely to provide ROS in high-fat induced NASH development (Abdelmegeed et al., 2012).
Thus perturbation in hepatocyte lipid metabolism and the accumulation of intrahepatic lipids and LD is the first hit leading to steatosis, while intrahepatic ROS from either altered metabolism of excessive FFA or through recruitment of immune cells constitute the second hit in the progression of steatosis to steatohepatitis (Day & James, 1998). With the excessive lipids in LDs that serve as a source of eicosanoid biosynthesis (Bozza et al., 2011), it is possible that selective eicosanoid metabolites from LD may serve as not only initiators of the lipid-cytokine-chemokine cascade but also regulators of lipid metabolism in hepatic steatosis. Both obesity and insulin resistance are strongly associated with NAFLD with increased insulin resistance in adipocytes, leading to an increase in adipose triglyc-eride lipase (ATGL) and HSL activity, resulting in hydrolysis of adipose TAG and elevation in serum FFAs. Free FA uptake by liver is mediated by FATPs that channel FAs to β-oxidation, production of VLDL for transport to peripheral tissues, or the formation for LDs when excessive lipids are taken up by the liver. Excessive FAs stimulate ACSL3 translocation to nascent LDs (Poppelreuther et al., 2012). Proteomic analysis of LD proteins revealed the association of ADSL1, ACSL3, and AA-CoA-activating ACSL4 with hepatic LDs (Hodges & Wu, 2010). The attenuation of ACSL3 expression correlates with PL class switching, while in ACSL3-null mice, there was a reduced incorporation of FFAs in LDs of human Huh7 hepa-toma cells (Yao & Ye, 2008). The association of lysoPC-acyltransferase with LDs suggests that these organelles are in dynamic equilibrium (Moessinger et al., 2011). Recently, a direct role of LD in ACSL4 esterification of AA-CoA and COX2 activation has been reported in the metastatic potential of breast cancer and LT production. LD ACSL4 may serve a similar function in hepatic steatosis by providing AA for the synthesis of inflammatory 5-LOX metabolites, since ob/ob mice treated with 5-LOX inhibitors show restored microsomal transfer protein (MTP) and VLDL secretion and were protected from hepatic steatosis.
In hepatic steatosis, ACSL5 expression is elevated and channels FAs to LDs thus competing with FA β-oxidation and VLDL transport pathways. Excessive FAs are highly toxic to hepatocytes. Thus, to prevent lipotoxicity excess, FAs are stored as TAG in LD or metabolized by induction of peroxi-somal FA β-oxidation. Peroxisomal FA β-oxidation produces chain-shorted FAs that are completely oxidized by mitochondrial β-oxidation. However, FA mitochondria transport by CPT-1 in hepatic steatosis is inhibited by lipogenic production of the CPT-1 inhibitor malonyl-CoA. Thus LD size expansion through storage of excess FAs as TAG is an adaptive mechanism to prevent lipotoxicity. Central to production of TAG is the synthesis of monounsaturated palmitic and stearic acids by SCD-1. SCD1 and ACC1 are transcriptionally induced by SREBP1 during lipogenesis where SCD-1 regulates the partitioning of monounsaturated and SFAs in steatosis. The critical role of SCD-1 in FA partitioning in steatosis and steatohepati-tis is evident in SCD-1-null mice fed a methionine-choline (MCD) diet, which reduces PC and PE levels. These mice have decreased body weight and hepatic steatosis but markedly increased hepatocellular apoptosis, liver injury, and fibrosis (Li et al., 2009) compared to wild-type SCD +/+ mice fed a MUFA diet that prevented MCD-induced injury. This study indicates the critical role of SCD1 in partitioning of excess FFA into MUFA for either transport as VLDL or storage at TAGs in LDs in hepatic steato-sis. Thus SCD1-null mice accumulate pro-inflammatory saturated FFAs, which leads to hepatic steatohepatitis. Critical for SCD1 enzymatic activity is the role of cytochrome b5 reductase and cytochrome b5 not only in the desaturation of SFA but also in the desaturation and elongase-mediated production of AA. Both cytochrome b5 and reductase also have a critical role in the coupling of electron transport from NADPH cytochrome P450 oxidioreductase (OR) to cytochrome P450 in the metabolism of drugs and production of EETs and 20-HETE by CYP2 and CYP4 P450 isoforms. Both cytochrome b5 and reductase are human obesity susceptibility genes (Rankinen et al., 2006).
Increased intrahepatic SFAs are known to cause lipotoxicity and insulin resistance in the liver, and SCD1 and ACC1 control the ability of the liver to provide nutrition to peripheral tissues through gluconeogenesis, keto-genesis, and VLDL secretion. SFAs differentially affect these key regulatory enzymes causing liver insulin resistance by an unresolved paradox of selective insulin resistance where insulin is able to activate lipogenesis through IRS-1, but not IRS-2-mediated suppression of gluconeogenesis thus causing hyperglycemia (Brown & Goldstein, 2008). Normally, activation of the IR results in phosphorylation and activation of IRS-1 toward SREBP1c increase in lipogenesis, while IRS-2 activation mediates phosphorylation of FOXO-1 and exclusion from the nucleus where it activates the transcription of genes involved in gluconeogenesis. It has been reported that SFAs activate JNK1, which somehow preferentially phosphorylates and inactivates IRS-2 and thus prevents inhibition of gluconeogenesis. However, this idea has been challenged by the fact that hepatocytes from JNK1-null mice show glucose intolerance, insulin resistance and steatosis (Sabio et al., 2009). The mechanism of increased lipogenesis in hepatic insulin resistance may be mediated by ChREBP activation of SREBP-1 transcription in concert with PPARγ2 induction in liver by HF diet and the transcriptional coacti-vator PGC-1β. Recent evidence indicates that SFAs can induce c-Src clustering with plasma membrane subdomains leading to JNK activation, while MUFA blocks this partitioning and activation of JNK (Holzer et al., 2011). It will be of importance to determine if s-Src is associated with LDs and whether LD membranes have higher concentrations of palmitic and AA in their lipid bilayers.
Besides the channeling of excess FA to LD for TAG synthesis, the peroxisome β-oxidation system has a critically significant role in the prevention of FA-induced lipotoxicity. The peroxisome l-bifunctional enzyme enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase (EHHADH) is critical in the metabolism of long-chain dicarboxylic acids and has recently been identified as a candidate gene in NAFLD (Banasik et al., 2011) and a novel regulatory gene of the P450 system (Yang et al., 2010) (Fig. 5.4). At least three genes CYP4A190, CYP4A14, and aldehyde dehydrogenase, Aldh3a2, are involved in the EHHADH peroxisome β-oxidation regulatory network (Houten et al., 2012). In steatosis-resistant A/J mice fed an HF diet all these genes are induced with increased metabolism of long-chain dicarboxylic acids (Hall et al., 2010). A bioinformatics approach identified EHHADH as a candidate gene associated with T2DM, obesity, and glucose intolerance. These data strongly implicate peroxisome β-oxidation as a protective regulatory system to prevent hepatic steatosis and also suggest that the FA ω-hydroxylase CYP4A and CYP4F P450s have a central role in producing dicarboxylic acids from excessive FAs, and an undefined role in the ω-hydroxylation of eicosanoids in NAFLD. Recently, a functional variant of human CYP4F2 V433M was associated with all the features of MetS except glucose (Fava et al., 2012). This variant has previously been associated with hypertension and stroke due to the reduced ability to metabolize particular substrates, including vitamin K in warfarin therapy. However, this variant is as efficient in the ω-hydroxylation of LTB4 as the CYP4F2 wild-type gene indicating that other eicosanoids or LCFA substrates may be inefficient substrates (Stec et al., 2007).
We had previously reported that ob/ob mice fed an HF diet induces the expression of CYP4A genes and protein, while CYP4F expression was reduced (Hardwick, 2008; Zhang & Hardwick, 2000). Moreover, we have observed that insulin induces the expression of the CYP4F2 in human hepatocytes. The CYP4F2 gene is regulated by SREBP-1 (Hsu et al., 2007) and thus may function to metabolize excess FAs in the peroxisome for further metabolism as dicarboxylic acids. The increased expression of CYP4A in hepatic steatosis and steatohepatitis implies that they have a functionally unidentified role in enhancing lipotoxicity as revealed by their dramatic induction in CYP2E1-null mice with an increase in ROS and lipid peroxidation upon exposure to the MCD diet (Leclercq et al., 2000). Increased production of dicarboxylic acid during steatosis by CYP4A isoforms can impair mitochondria function by dissipation of mitochondrial proton gradient and uncoupling of oxidative phosphorylation. In addition, the uncoupling of the P450 catalytic cycle is a major source of microsome ROS (Narasimhulu, 2007) that led to the identification of CYP2E1 as a source of ROS-induced lipid peroxidation in HF-induced steatohepatitis (Abdelmegeed et al., 2012). However, CYP2E1 is not induced in human liver during steatosis or steatohepatitis and in fact decreases with disease progression, while CYP4A11 levels increase suggesting that CYP4A iso-forms may be the major source of ROS in human hepatic steatosis. The recent identification of mitochondrial targeting of CYP2E1 may not only account for CYP2E1 decrease in microsomes but also may account for increase in ROS formation in mitochondria in NAFLD (Knockaert et al., 2011). CYP2E1 can metabolize FAs at the ω-1 position and CYP4A is able to ω-hydroxylate medium-chain FAs. However, as chain length increases, CYP4A11 loses specificity and begins to ω-1 hydroxylate longer chain FAs (Hardwick et al., 2009). It is not known whether this change in FA hydroxylation with different chain length FAs increases uncoupling of the P4504A catalytic cycle and increases ROS production. In addition, we do not know if cytochrome b5 increases coupling of the P450 catalytic cycle and reduces ROS formation. Since cytochrome b5 and reductase are increased in obesity and both enzymes are used for both P450 catalytic cycle and desaturase reactions, sequestering cytochrome b5 by SCD-1 may lead to increased uncoupling of the P450 catalytic cycle in the presence of FA excess. Normally, the induction of CYP4A genes during fasting provides both gluconeogenic precursors and acetate to meet the energy needs of peripheral tissues (Fig. 5.4); however, their induction during NAFLD may increase hyperglycemia, shuttle acetate for lipogenesis and increase ROS by FA uncoupling of the P450 catalytic cycle.
It is not known whether CYP4F2 gene is differentially regulated by SFAs or MUFAs in patients with NAFLD; however, we have evidence that both CYP4F2 and CYP2E1 are repressed in patients with NAFLD, while the neutrophil CYP4F3 expression increases with progression of disease. The CYP4F2 gene expression is downregulated by peroxisome prolifera-tors and fibrate drugs (Zhang et al., 2000), induced by retinoic acid, and increased by lovastatin through SREBP-2 (Hsu et al., 2007). The induction of CYP4F2 and SCD-1 genes by insulin in primary hepatocytes suggests that their differential regulation in insulin resistance may be a determining factor in hepatic steatosis. Thus the induction of CYP4F2 gene expression by retinoic acid, which has been shown to ameliorate steatosis (Ashla et al., 2010), may decrease the formation and storage of TAG in the liver, and prevent recruitment of immune cells by increased metabolism of LTB4 and thus the progression of steatosis to steatohepatitis. Although there are numerous reports of FA induction of cytokine and chemokine production in hepatocytes, we need to further study the function of LTs in the activation of the lipid autocoid-cytokine-chemokine cascade in recruitment of immune cells to the liver in NAFLD. The association of the liver CYP4F2 and neutrophil CYP4F3 gene in inflammatory celiac disease establishes a connection between neutrophil recruitment to the established Th1 innate immune response in disease patients (Curley et al., 2006). In human HepG2 cells treated with saturated FAs (C16:0-C18:0) or unsaturated FAs (C18:1-C18:2) at subphysiological levels from 50 to 200uM, there was a greater induction of CYP4A11 mRNA with SFAs and a marked repression of CYP4F2 by all FAs with no change in CYP4F3b expression (Madec et al., 2011). In addition, in ApoE-null mice fed an HF diet there was a repression of epoxygenase activity and induction of CYP4 ω-hydroxylase activity, leading to a significant increase in the 20-HETE/EET+DHET ratio in kidney compared to no change in the liver (Theken et al., 2012). Furthermore, kidney podocytes exposed to diabetic high glucose concentrations showed increased ROS formation associated with sequential upregulation of CYP4A, 20-HETE and NADPH oxidase activity (Eid et al., 2009), leading to podocyte apoptosis as seen in diabetic patients. Furthermore, the effects of high glucose on induction of NOX mRNA, protein, and activity were blocked by CYP4A inhibitors and mimicked by 20-HETE, indicating that CYP4A induction initiates podocyte apoptosis and diabetic protein-uria.
The importance of drug metabolism in hepatic steatosis is apparent in NADPH cytochrome P450 reductase (OR)-null mice that develop hepatomegaly and fatty liver due to induction of C36 FA transporter, SCD-1, and CYPs involved in bile acid synthesis (CYP7A1), steroid (CYP51), and reti-noic acid metabolism (CYP26a1) with a repression of FA oxidation genes CPT1a, EHHADH and CYP4A10 (Weng, et al., 2005). There is increasing evidence that drug-metabolizing enzymes, consisting of phase I CYP, phase II conjugation, and phase III transporters, are differentially expressed in hepatic steatosis; however, their role in the initiation and progression of NAFLD requires further study especially in the context of eicosanoid metabolism, conjugation and transport in the immunological response to excess FAs (Christensen & Hermann, 2012). In NAFLD, the mRNA and protein for major drug-metabolizing CYP isoforms such as CYP1A2, 2D6, 2E1, 2C19 and 3A4 are decreased with NAFLD progression, while CYP2A6, 2B6 and epoxygenase CYP2C9 increase (Fisher et al., 2009). The decreased expression of CYP correlated with increased expression of pro-inflammatory expression of TNFα, and IL-1β possibly by lipid autocoids. In human hepatocytes treated with 1 mM FFA mixture (2:1 ratio of ole-ate and palmitate), there was a repressed expression of CYP1A2, 2A6, 2B6, 2E1, 3A4 and the epoxygenase CYP2C9 (Donato et al., 2007). These data indicate that FFAs but not cytokines or chemokines control the expression of these CYPs, and that induction of CYP2C9 in vivo, but repression by FFA in vitro, indicates a differential regulation of this epoxygenase in the progression of NAFLD to NASH.
Numerous clinical studies have reported significant changes in drug pharmacokinetics in patients with inflammation, cancer, and sepsis, which is largely due to the differential response of drug-metabolizing enzymes to cytokine- and chemokine-mediated repression. This variable depression of selective CYP can increase patient drug exposure up to 400%, resulting in IADRs, therefore it will be important to determine how NAFLD and NASH influence the pharmacokinetics of drugs, especially drugs targeting eicosanoid metabolism in inflammation, especially in regard to alterations in phase I, II, and III enzyme expression and activity. The role of eicosanoids in the progression of NAFLD, and their role in inflammation have been recently studied in NASH. However, recent studies have also identified a key role of eicosanoids in the regulation of intermediary metabolism in the progression of NAFLD by the use of global knockout mice of eicosanoid-metabolizing enzymes, eicosanoid metabolism inhibitors, and congenic strains developed on mouse models of MetS.
Early studies on the cause of NAFLD focused on the immunological aspects of inflammation in NASH where IL-6, PGE2 and TNFα produced in Kupffer cells directly influence lipid metabolism in hepatocytes (Enomoto et al., 2000). In alcoholic steatohepatitis, increases in TAG correlated with PGE2 levels, indicating that Kupffer cell activation by ethanol enhances PGE2-mediated effects on hepatic lipogenesis. In addition, COX2-derived PGE2 was shown to inhibit TGFβ1-mediated induction of collagen synthesis by activated stellate cells, thus inhibiting fibrosis (Hui et al., 2004). It is apparent that PGE2 has divergent effects by inducing steatosis by inhibiting steatohepatitis possibly by a recent identified mechanism of nonsubstrate FAs binding the allosteric site of COX1, inhibiting catalytic activity, but stimulating COX2 activity and PGE2 production (Zou et al., 2012). These results indicate that COX2 is not only induced by cytokines in inflammation but also by FAs in hepatic steatosis. The progression of steatosis to steatohepatitis is largely mediated by eicosanoids of the LOX pathway. During acute inflammation, 5-LOX and 12/15-LOX pathways are activated and resolution of inflammation is mediated by the transcellular metabolism of hepatocyte 15-HETE by Kupffer cell 5-LOX to produce antiinflam-matory LXA4 and LXB4, which inhibit chemotaxis, selectin and integrin endothelial immune cell adhesion as well as transmigration of neutrophils across the endothelium. It is likely that autocoid lipids mediate the coordinate regulation of metabolic activities in Kupffer cells and hepatocytes through unknown mechanisms that need to be delineated in greater detail in relation to the role of prostanoid and LT pathways in NAFLD.
Disruption of the eicosanoid-metabolizing enzymes has provided insight into the role of eicosanoids in MetS and NAFLD with respect to alterations in carbohydrate and lipid metabolism. In PLA2IVA-null mice fed an HF diet hepatic steatosis, was reduced with smaller adipocytes caused by reduced serum PGE2 that has lipogenic effect on adipocytes. In global knockout of 5-LOX, hepatic necroinflammation, hepatic immune cell infiltration, hepa-tocyte ballooning and serum alanine aminotransferase (ALT) levels were significantly reduced with a marked reduction in hepatic steatosis (Titos et al., 2010). These symptoms were due to a marked reduction of lipogenic gene expression determined by ingenuity pathway analyses that are affected by loss of 5-LOX activity. Congenic mice with double knockout of both ApoE and 5-LOX showed reduced hepatic inflammation and serum ALT due to reduction in serum levels of inflammatory cytokines and chemokines (Martinez-Clemente, Ferre, Gonzalez-Periz, et al., 2010) thus supporting a role of eicosanoids in the initiation of the lipid autocoid-cytokine-che-mokine cascade. Metabolically, ApoE/5-LOX double knockout mice are remarkably insulin sensitive because of upregulation of PPARγ, IRS-1 and serum adiponectin levels, while JNK1 activity is reduced. Furthermore, the 12/15-LOX Alox15 gene is upregulated in ApoE-null mice that spontaneously develop hyperlipidemia (Martinez-Clemente et al., 2010). However, in ApoE/12/15-LOX congenic mice, there is a reduction in serum ALT, hepatic steatosis, inflammation and macrophage infiltration into the liver. In Alox15-null mice fed an HF diet, increased insulin resistance was attenuated and there was an upregulation of IRS-2 and AMPK and inhibition of JNK1 kinase activity, resulting in attenuated hepatocellular injury (Czaja, 2010). Of equal importance, when COX2 knockout mice, which develop atherosclerosis due to the reduced synthesis of PGI2, are crossed with ApoE-null mice, the congenic strain develop accelerated atherogenesis with lesions exhibiting excessive leukocyte infiltration and upregulation of vascular adhesion molecules. These data indicate that diversion of AA to LT pathway exacerbates atherogenesis and thus suggests that chronic administration of NSAIDs may increase cardiovascular risk. It is apparent that global knockout of COX and/or LOX gene activity influences both carbohydrate and lipid metabolism in NAFLD. It is imperative that floxed mice for eicosanoid enzymes be developed to identify the tissue-specific role of eicosanoids in the regulation of metabolism in NAFLD.
5.2. Visceral and Subcutaneous WAT
Adipose tissue consists of several depots located in two body compartments: under the skin (subcutaneous depot) and in the body trunk (visceral depot). The main cell of adipose organ is the adipocyte, which can be white, located in WAT, or brown adipocytes located in BAT. Adipose tissue functions in fuel metabolism, lactation, thermogenesis and immune response and thus represents a dynamic organ in the maintenance of whole-body homeostasis.
Brown adipoctyes function primarily in energy utilization by thermogenesis and thus contain large mitochondria with extensive cristae and elevated levels of uncoupling protein 1 that functions to dissipate the mitochondrial proton motive gradient in the generation of heat. In contrast, WAT stores energy in small adipocytes in the visceral adipose tissue (VAT) or in large adipocytes in the subcutaneous adipose tissue (SAT). WAT express leptin adiponectin, and S100B, and associated with WAT is a lymphocyte population that expresses the leptin receptor, suggesting a relationship that may have importance in the energy requirement of an immunological response (Moro et al., 2010).
The adipose tissue stores display remarkable organ plasticity where WAT can be stimulated to transdifferentiate into BAT after adrenergic stimulus or prolonged exposure to cold. Recently, the adipokine, Irisin, released from exercising muscle has been shown to increase WAT conversion to BAT (Bostrom et al., 2012). The transdifferentiation of WAT to BAT occurs through a number of mechanisms and thus has important implications in MetS since animals with more BAT are resistant to obesity and Type II diabetes, while animals without BAT are prone to obesity and T2DM (Cinti, 2012).Thus exercise or treatment with beta-3-adrenoreceptor agonist, irisin or adiponectin may be important new avenues for the treatment of obesity and T2DM.
Not only the amount of BAT and WAT but also the amount of SAT and VAT have an important role in obesity and T2D. Excessive consumption of fat and carbohydrates increase WAT by adipocyte hypertrophy, differentiation of preadipocyte, and transdifferentiation of BAT by TGFβ. Gender, age, and environmental and genetic factors influence bodily distribution of fat. Since VAT is highly correlated with the development of obesity, T2D, and recently NAFLD, lean patients with abdominal obesity and increased VAT have an increased incidence of NASH (Filik, 2011). It is proposed that fat redistribution to VAT when SAT become full or restricted initiates the development of MetS. Support for this theory comes from studies in which patients treated with PPARγ agonist that promote preadipocyte expansion of SAT, attenuates NAFLD and markedly improves symptoms of MetS. Insight into the mechanism of VAT and SAT ectopic fat redistribution may be stress induced (Mittendorfer, 2011). In patients with NAFLD, expression of 11β-hydroxylsteroid dehydrogenase type I, which converts inactive cortisol to active corticosterone, is elevated in VAT, but not in SAT (Candia et al., 2012). Furthermore, rats fed an HF diet and implanted with corticosterone pellets develop severe insulin resistance, hyperinsulinemia, hyperglycemia, and hypertriglyceridemia, characteristics of MetS that are not evident in either HF diet or corticosterone-treated rats (D'Souza et al., 2012).
As VAT expands, the adipocytes become hypertrophic and produce a signal that attracts macrophage of MI proinflammatory phenotype. These macrophages encompass the dying VAT adipocyte forming crownlike structures (CLS). These CLS are more prevalent in VAT over SAT in obese individuals and these macrophages are often called Mac2 macrophages since they are immune reactive for galactose-specific lectin 3. The Mac2 mac-rophage phagocytoses the dying adipocyte and produces proinflammatory IL-6, TNFα, and IL-1α, which interferes with IR signaling. Obese mice and humans with VAT CLS have increased insulin resistance of peripheral tissues, while hypertrophic VAT that do not display CLS are insulin sensitive (Virtue & Vidal-Puig, 2010). Furthermore, VAT release of FFA is twofold higher than in SAT in humans with NAFLD suggesting a pathological role of VAT, but not SAT adipocyte lipolytic, function in MetS (Thorne et al., 2010). Many clinical and epidemiological studies indicate that VAT is directly associated with abdominal and liver fat content independent of total adipose mass, BMI and SAT (Hall et al., 2012; Hamdy et al., 2006). Many studies have found that VAT and liver fat are associated with MetS independently, such that VAT was more important in lower levels of obesity, while liver fat was associated with severe obesity and the development of MetS (Kim, Nalls, et al., 2011). These findings support the portal vein hypothesis in which FFAs, proinflammatory cytokines, and adipokines from VAT contribute to increased hepatic lipid stores and inflammatory cell recruitment with hepatic and peripheral tissue insulin resistance (Virtue & Vidal-Puig, 2010).
It is generally accepted that metabolic dysfunction arises from lipotoxicity caused by lipid intake that exceeds what an individual adipose tissue can store. Thus the adipose tissue expandability hypothesis suggests that each individual has a threshold for storage of lipids in either the SAT or VAT depots (Virtue & Vidal-Puig, 2010). However, it is uncertain how adipo-cyte dysfunction is initiated and how lipotoxicity in VAT leads to metabolic dysfunction, causing NAFLD and eventually MetS. Recent insight into the metabolic abnormalities associated with obesity and increased VAT depots have come from studies with lean and obese monozygotic twins and large cohort studies where elevated levels of β-hydroxybutyrate ketone bodies in serum were strongly associated with T2D (Gall et al., 2010). Furthermore, obese monozygotic twins have elevated levels of membrane PLs containing PUFA with lower carbon number and increased double bonds than lean nonobese twins (Pietilainen et al., 2011), which is due to increased ELOV6 expression in VAT that is believed to function in membrane remodeling during adipocyte hypertrophy. Therefore, the increase in n-6 PUFAs may be an early metabolic event in VAT that initiates the production of proin-flammatory eicosanoid lipid mediators that modulate events in both lipid storage and lipotoxicity (Du et al., 2012; Murphy, 2001).
The type of PUFA in adipose tissue has a dramatic effect on adipocyte metabolism, adipose dynamics, and inflammatory environment of adipose tissue. The ratio of n-6 and n-3 PUFA in VAT and its metabolism to either pro- or antiinflammatory eicosanoids can have a significant role in adipocyte differentiation, function, and the production of inflammatory chemokines and cytokines. PG of the J2 series derived from AA has a dual effect in adipocytes by increasing adipogenesis through activation of PPARγ and activation of monocyte chemoattractant protein (MCP-1), during the maturation phase of adipogenesis (Hossain et al., 2012). The production of PGJ2 from PGD2 can be prevented by both selective and nonselective COX2 inhibitors, thereby inhibiting the maturation phase of adipogenesis and significantly suppressing the accumulation of adipose fat (Ghoshal et al., 2011). Furthermore, the dynamics of adipocyte remodeling are highly dependent on the ratio of n-6 and n-3 PUFA in adipocyte PLs. The n-6 AA/n3 EPA ratio is elevated in VAT over SAT of severely obese women with metabolic dysfunction, which was strongly negatively correlated with adiponectin levels (Caspar-Bauguil et al., 2012). In contrast, high EPA and DHA levels increased adiponectin mRNA and secreted protein in 3T3-Li adipocytes (Tishinsky, Ma, & Robinson, 2011). EPA inhibits TNFα-induced lipolysis by downregulating HSL and decreases ATGL protein in primary rat adipocytes, resulting in the inhibition of HF-diet-induced hyperglycemia and hyperinsulinemia (Kalupahana et al., 2011; Lorente-Cebrian et al., 2012).
Hypertrophy of V AT adipocyte is an initial step in adipocyte cell death and recruitment of Mac2 macrophage that leads to the formation of CLS. Hypoxia is a key regulatory of adipose tissue dysfunction and heme oxy-genase-2 (HO-2) is an important regulator of physiological levels of ROS. HO-2 knockout mice show depletion of mesenchymal stem cell (MSC) adipocytes that result in increased adipogenesis and production of pro-inflammatory cytokines and decreased HO-1 activity and production of EETs (Burgess et al., 2012). In contrast, upregulation of HO-1 and CYP2J5 production of epoxyeicosatrienoic acid (EET) decreased MSC adipocyte differentiation, increased adiponectin secretion and decreased proinflammatory cytokines, suggesting that HO-1 and EETs protect against adipocyte hypertrophy and ensuing MetS (Burgess, Vanella, Bellner, Gotlinger, et al, 2012; Burgess, Vanella, Bellner, Schwartzman, et al, 2012). Of the 263 secreted proteins from human adipocytes, of which 44 were identified as novel adipokines, HO-1 circulating levels and VAT tissue expression is significantly increased in obese subjects compared to lean controls (Lehr et al., 2012). HO-1 is involved in the reduction of oxidative stress and inflammation and is released by mature SAT in obese individuals. It is of interest that increased TNFα secretion by VAT downregulates HO-1 secretion, while HO-1 induction reduces WAT secretion of TNFα. These data suggest an important link between VAT and SAT in modulating ROS and inflammation. Soluble epoxide hydratase (sEH) levels increase during adipocyte cell differentiation and are markedly elevated in obese mice (De Taeye et al., 2010). Inhibition of sEH leads to an increase in EETs in VAT of mice fed a HF high-fructose diet. Increased EET levels result in a reduction in serum leptin, decrease in VAT, decreased calorie intake, increase in metabolic rate and significant weight loss (do Carmo et al., 2012).
Chronic low-grade persistent inflammation occurring in adipose tissue of obese individuals is linked to the pathogenesis of insulin resistance. Although the exact trigger for this inflammatory process is unknown, adipose tissue hypoxia, ER stress, and SFA activation of innate immune processes have been identified as important processes in these disorders. In hypertrophic VAT, the induction of 12- and 5-LOX enzymes results in the increase in 12-HETE that has been linked to insulin resistance in adipocytes, and 5-HETE and production of chemotactic LTB4 that may be an initiating factor in hypertrophic adipocytes to attract Mac2 macrophages and formation CLS structures (Chakrabarti et al., 2011). The increase in proinflammatory eicosanoids, 12-HETE and 5-HETE, results in induction of NF-κβ and secretion of pro-inflammatory insulin-resistant adipokines, macrophage inflammatory protein MIP-Iγ, TNFα, and IL-6 (Martinez-Clemente et al., 2011). The increased production of proinflammatory adipokines that cause insulin resistance can be reversed by the novel antiinflammatory drug lisofylline, which reduced p-STAT4 in VAT of obese Zucker rats and inhibited the inflammatory response, induced by LO products. These obese Zucker rats show a reduction in fasting plasma glucose and increase in insulin sensitivity (Chakrabarti et al., 2011). How inflammation-driven lipolysis in adipose tissue contributes to insulin resistance remains to be clearly established. TNFα clearly increases lipolysis in adipocytes by both suppression of ATGL inhibitor GOS2 and PKA phosphorylation of ATGL activator, CGI-58/Abhd5 (Glass & Olefsky 2012) as well as activation of HSL. The ability of salicylates to block the lipolytic response of TNFα (Zu et al, 2008) and the recent observation that LTB4 receptor (BLT1)-deficient mice show reduction in Mac2 macrophage in VAT and reduction of proinflammatory cytokines secretion (Spite et al., 2011) suggest that activation of eicosanoid pathways are pivotal in adipose tissue inflammatory response and thus blockage of selective pathway might have insulin-sensitizing effects in obesity and MetS.
5.3. Eicosansoids in Adipocyte Metabolism and Obesity
Prostanoids have a significant role in adipose tissue mass remodeling where PGE2 accelerates adipocyte accumulation of TAG through suppression of cAMP induction of HSL lipolysis. The prostanoid 15-deoxyPGI2, a ligand for PPARγ activation, induces lipogenesis and ameliorates hyperglycemia in T2DM patients (Mazid et al., 2006). Prostacyclin and 15-deoxyPGI2 also induce differentiation of preadipocytes, while PGF2α inhibits preadi-pocyte differentiation. In the IVA PLA2 knockout mice serum levels of PGE2 are reduced, leading to reduced adipose mass and smaller adipocytes. Also, COX2-null mice have a significant reduction in body weight and percentage body fat due to increased metabolic rate. In the adipose tissue of COX2-null mice, the levels of 15-deoxyPGJ2 and markers of adipocyte differentiation were significantly reduced, while preadipocyte marker expression increased along with a reduction in ATMs. Furthermore, the serum lipid lowering effects of niacin are due to promotion of adipogenesis through reduction of antiadipogenic PGF2α and C/EBP activation of COX2, leading to possible synthesis of PGE2 (Song et al., 2012). Adipocytes secrete a variety of adipokines that function to control whole-body metabolism: leptin, adioponectin, resistin, lipocalin2, and retinol binding protein 4 (RBP4) (Ouchi et al., 2011). The function of these adipokines is diverse in controlling the storage or use of lipid stores with leptin controlling nervous system appetite, resistin promoting insulin resistance through cytokine expression with lipocalin2 and RBP4, and adiponection having antiinflammatory and insulin sensitization properties. Adiponectin protects against hepatic steatosis by activation of AMPK and FA β-oxidation. Adiponectin production in adipocytes is inhibited by PGD2, PGJ2, and 15-deoxy PGI2. Leptin expression is also inhibited by PGD2, PGJ2, and 15-deoxy PGI2, but stimulated by PGE2. Increased serum levels of resistin are related to the severity of NAFLD in humans, but no change in resistin levels were noted in IVA PLA2-null mice, indicating a minimal role of eicosanoids in controlling resistin production in adipocytes. These studies strongly indicate that PGs differentially regulate adipokine levels in MetS.
The key factors involved in HF-diet-induced adipose tissue inflammation and macrophage infiltration are not well understood. A recent study demonstrated that the 12/15-LOX pathway is upregulated in the adipose tissue of mice fed an HF diet and that 12/15-LOX metabolites induced adipose tissue inflammation and insulin resistance. This same observation of increased cytokine production and insulin resistance was observed in 3T3-L1 adipocytes incubated with FAs. In 12/15-LOX-null mice fed an HF diet, insulin resistance, proinflammatory M1 macrophage infiltration, and cytokine production by adipose tissue is dramatically reduced. In the adipose tissue of 15-LOX-null mice, there was a reduced expression of proinflammatory adipokines, MCP-1, TNFα and reduced resistin production with an increased expression and activity of the glucose transporter 4 (Glut4) (Martinez-Clemente, Ferre, Titos, et al., 2010). Remodeling of subcutaneous and omental adipose tissue occurs in obesity, MetS, and Cushing syndrome. In obese individuals, 15b-LOX and 12-LOX are expressed in both subcutaneous and omental fat, but only 15a-LOX is expressed in CD34+ cells of subcutaneous fat where increased expression in associated with Homeostatis Model assessment-insulin resistance (HOMA-IR) (Dobrian et al., 2010). In obesity, FAs released from adipose stores are an important source of FAs that are found as TAG within hepatic LD. The regulation of lipid hydrolysis and synthesis of LDs is strongly associated with the differential regulation of LD proteins, perilipin, Cide/FSP27, and Serpin (Greenberg et al., 2011). Unfortunately, there are a limited number of studies that have investigated the role of LD proteins in NAFLD and whether eicosanoids regulate LD protein gene expression or protein function. In addition, the role of CYP2 epoxygenase and CYP4 ω-hydroxylase have not been studied in adipocytes and may be of importance considering the recent identification of FA ω-1-ethanol-inducible CYP2E1 expression in adipose tissue (Sebastian et al., 2011).
5.4. Eicosanoids in Diabetes and Insulin Resistance in Pancreas
The interplay of different organ systems, pancreas, adipose tissue, liver, skeletal muscle and nervous systems determine systemic insulin resistance in T2DM by signaling through different pathways that determine local organ-specific insulin resistance. FFA levels elevated in obese patients are an independent predictor of T2DM and coronary artery disease. Saturated FAs are directly linked to pancreatic β-cell dysfunction through activation of TLR4/MyD88 pathway and induction of cytokines that recruit M1 monocytes/macrophages to pancreatic islets (Eguchi et al., 2012). This data firmly establishes a link between FA-induced islet inflammation and β-cell dysfunction in T2DM that is similar to the role of inflammation in adipose tissue. However, the functions of eicosanoids in different organ systems can have completely divergent effects in the regulation of metabolism. PGE2 appears to be a significant factor in β-cell dysfunction and destruction in complications and pathogenesis of diabetes, while in adipose tissue, PGE2 promotes adipogenesis, reduces serum FAs and glucose levels and thus protects against systemic insulin resistance of T2DM. In pancreatic islets, COX2-produced PGE2 inhibits insulin secretion through autocrine activation of EP3 receptor, leading to decreased cAMP levels and response to GSIS. In contrast, PGI2 increases cAMP through activation of IP receptor and potentiates GSIS. In human islets, COX2 is induced by high glucose (25 mM) and glycation products through advanced glycation end products, while it induces NF-κβ and IL-6 expression and COX promoter binding to induce COX2 gene expression. The inhibition of NF-κβ by sodium salicylates protects islet cells through reduced synthesis of COX2 and EP3 thereby preventing β-cell lost in diabetes. In addition, in liver, the protective effects of NSAID improve insulin resistance through PGE2 involvement in the stimulation of glycogenolysis and gluconeogenesis (Luo & Wang, 2011).
AA is a potent stimulator of insulin secretion that is inhibited by 12/15-LOX activity and 12/15-LOX metabolites mediate cytokine damage to β-cells. In the nonobese diabetic (NOD) mouse model, mice develop autoimmune diabetes, but congenic NOD/12/15-LOX mice are protected from autoimmune diabetes (McDuffie et al., 2008). In 12/15-LOX-null mice fed an HF diet, islet damage was not observed. The functions of LOX metabolites in β-cell function are divergent with 15-HETE, LTB4, and LTC4 inhibiting insulin release and 12-HPETE potentiating insulin secretion (Dobrian et al., 2010). In diabetic Zucker rats, inhibition of 12-LOX activity suppressed AA-induced insulin secretion, while in 12-LOX-null mice, cytokines stimulated GSIS, indicating that 12-LOX products are negative regulators of insulin secretion (Zafiriou et al., 2011). There are three types of 12-LOX: platelet, leukocyte, and epidermal, with leukocyte 12-LOX being the major isoform expressed in pancreatic islets (Chen, Yang, Smith, Carter, & Nadler, 2005). 12-LOX expression is increased in islets of Zucker rats and the pancreatectomy model of diabetes, while 12-LOX expression in human islets by cytokines increases production of 12(S)-HETE that causes β-cell apoptosis. LOX inhibitors protect islet cells from β-cell destruction in diabetic mice.
5, 6-EET produced by CYP epoxygenase stimulates insulin secretion in islets and is regulated by expression of CYP2CJ or inhibition of soluble epoxide hydratase (sEH) that metabolizes EETs to DHET. Inhibition of sEH promotes insulin secretion and GSIS in islets, and EETs have an antiapoptotic effect in β-cells. In sEH-null mice treated with streptozo-cin (STZ) to induce T1DM islet, cell apoptosis was significantly reduced compared to STZ-exposed wild-type mice, supporting the role EETs have in protecting β-cells from apoptosis. In both diabetes and obesity, the pancreatic expression of CYP2C isoforms is reduced (Zhao et al., 2005) with increased expression of CYP4A and sEH (Chen, Wang, et al., 2011). Of importance was the observation that insulin inhibits increased expression of CYP4A in diabetic rats. There are a number of studies associating sEH polymorphisms with insulin resistance in T2DM; however, there seems to be no association of variant alleles of CYP2C9, CYP2C8 or CYP2CJ2 in humans with either T1DM or T2DM (Surendiran et al., 2011). Further studies are necessary to determine the role of CYP2C and CYP4A AA metabolites in the function of β-cells and exocrine islet function in GSIS in hyperglycemia. Understanding how prostanoids regulate the function, proliferation and survival from cytokine- or high-FFA-mediated β-cell dysfunction will increase our understanding of the divergent effects of COX inhibitors on GSIS. Insight into how eicosanoid metabolism is regulated in the early development of T2DM characterized by hyperinsulin secretion to maintain normal glycemia followed by β-cell apoptosis may provide new avenues to protect β-cells from hyperglycemia.
5.5. Eicosanoids in Vascular and Cardiometabolic Diseases
Metabolic conditions such as obesity and insulin resistance lead to cardiovascular abnormalities caused by perturbation in lipid and carbohydrate metabolism. The functional role of eicosanoid pathways of PG, LT, and cytochrome P450 eicosanoids in vascular and cardiometabolic diseases has largely been studied in myocytes, endothelial and immune cells in ischemia, hypoxia, myocardial infarction, stroke, and hypertension. Unfortunately, the role of eicosanoids in the control of carbohydrate and lipid metabolism in these CVDs has not been extensively studied. The effective management of these diseases by NSAID provides therapeutic opportunities to selectively target eicosanoid pathways and prevent the underlying metabolic alteration of these diseases and ameliorate disease symptoms.
The risk of cardiovascular events during treatment with NSAIDs has been one of the most studied ADRs, especially with respect to disruption of vascular TXA2-PGI2 balance by COX2 inhibitors. Similar to Coxib drugs, patients on diclofenac show a higher cardiovascular risk most likely due to metabolism and inactivation of diclofenac by CYP2C8, resulting in reduced synthesis of vasoprotective EETs in combination with the synthesis of pro-inflammatory eicosanoids (Fig. 5.2). It is imperative that we understand not only the mechanism of COX1 and COX2 channeling of PGH1 and PGH2 to selective synthase (isomerase) in the production of prostanoids but also the underlying metabolic changes in the target tissues. The differential expression and regulation of COX1 and COX2 by saturated and PUFAs make these enzyme amenable targets for not only drugs but also dietary manipulation by selective lipids. This is exemplified by palmitic acid stimulation of COX2 and inhibition of COX1 (Yuan, Sidhu, et al., 2009), and the preferential metabolism of AA over the antiinflammatory PUFA, EPA, by COX2. These results suggest that SFAs induce COX2 channeling of PGH2 to different synthase that produce proinflammatory prostanoids. In contrast, EPA is a poor substrate for COX1 and at similar concentrations inhibits AA oxygenation.
The reader is referred to previous section on the role of phospholipase A2 enzymes in CVD. The role of different prostanglandin synthases in vascular disease is revealed by their different roles in either the protection or aggravation of CVD in combination with their cognate GPCRs. Serum lipocalin PGD synthase is a marker of both coronary artery disease and atherosclerosis. Whether the effects of increased L-PGDS on coronary artery disease are due to increased synthesis of PGD2 or the function of L-PGDS in transport of lipophilic lipids have not been determined. It is likely that detrimental effects of PGDS are not due to increased PGD2 production since PGD2 is metabolized by the AKR PGF synthase to antiinflammatory PGJ2 that activates PPARγ (Smith et al., 2011). The inducible mPGES-1 is a member of the membrane-associated proteins in eicosanoid and glutathione metabolism that have glutathione-transferase-mediated drug conjugation capabilities. mPGES-1 is associated with atherosclerosis since mPGES-1-null mice are protected from atherosclerosis but not hypertension through increased production of PGD2. L-PGDS-null mice have atherosclerosis and obesity but no hypertension (Tanaka et al., 2009). PGF synthase of the AKR family metabolizes a number of substrates that include monosac-charides, steroids, xenobiotics, and prostanoids in the presence of NADPH. In vivo PGDS activity is attributed to AKR1B1 that is functionally coupled to COX2 in PGF2α synthesis in the vascular and arterial smooth muscle. Prostacyclin synthase (PGID-CYP8A1) produces PGI2 that has a protective role in CVD and also functions as a PPARβ agonist. Like PGIS, TXAS is a cytochrome P450 (CYP5) that not only produces vasoconstrictive TXA2 but also produces proinflammatory MDS and 12-HETE. TXAS is linked to COX2 in the PGH2 as a TXAS substrate.
A large number of studies clearly show LT involvement in several stages and types of CVD. Increased production of LTB4 in human plaques and increased urinary LTE4 production in patients with myocardial ischemia reveal that increase in 5-LOX activity correlated with the clinical stage of CVD. Both 5-LOX and BLT1 antagonists have protective effects on both ApoE-null and LDLR-null mice against atherosclerosis, myocardial infarction, and stroke. Increased 5-LOX in human artherosclerotic coronary arteries promote macrophage recruitment and production of vasoconstrictive LTC4. In addition, BLT1-null mice show a variable artheroprotective effect by diminishing early but not late stages of lesion formation during intermittent hypoxia-induced atherogenesis (Li et al., 2011). Human Alox5 flap and LTA4 hydrolase variants, which lead to increased LTB4 synthesis, are associated with increased risk of myocardial infarction due to activation of endothelial and VSMC BLT1 (Helgadottir et al., 2006). In aortic angiotensin-II-induced aneurysm in ApoE-null mice, the intraluminal thrombus has increased expression of 5-LOX, FLAP and LTC4 synthase that increases expression of MMP-2 in matrix degradation. The role of 12/15-LOX in atherosclerosis is controversial with overexpression of 15-LOX in macrophages protecting against atherosclerosis, while congenic 12/15-LOX/APOE-null mice are protected against atherosclerosis by production of proresolving lipids (Mesquita-Santos et al., 2011). Expression of both 5-LOX and 15-LOX2 was detected in human atherosclerotic plaques, but no 15-LOX1 enzyme can be detected, suggesting that 15-LOX promotes LDL oxidation and macrophage conversion to foam cells (Nakamura & Shimizu, 2011).
The eicosanoids of the ω-hydroxylase CYP4 pathway production of 20-HETE and epoxygenase CYP2 EET have largely opposing roles in cardiovascular inflammation with 20-HETE being proinflammatory and EETs being antiinflammatory; however, both eicosanoids are potent initiators of cell proliferation in the vascular system (Imig, 2012; Imig et al., 2001). CYP2J2 is highly expressed in the heart and produces EETs that attenuate I/R injury. The CYP cardioprotective effects of EETs are associated with activation of sarcolemmal and mitochondrial K+ channels, MAPK, and PI3K-AKT protective signaling pathways in endothelial and cardiac myocytes. It has also been shown that besides the generation of EETs by CYP2J2 and CYPC8, these P450 are uncoupled during EET formation and produce ROS that can compromise mitochondrial function, suggesting that cardiac CYP enzymes involved in NSAID metabolism can lead to drug efficacy or cardiotoxicity
Within the past few years, the effects of lipid intermediary metabolism have been studied in regard to the risk of CVD in MetS. A relationship has been found between FADS activity with cardiometabolic risk factors of obesity, atherogenic lipoprotein phenotype and inflammation (Do et al, 2011). FADS activity is estimated indirectly by the product-precursor FA ratio of serum PL and cholesterol esters that are affected by both dietary fat intake and endogenous FA metabolism. It was found that a strong correlation exists between Δ6 FADS2 activity, waist circumference, and serum C-reactive protein in the risk for CVD. The desaturases affect lipid metabolism by both dietary intake and endogenous synthesis through Δ5FASD1 and Δ6FASD2 desaturation of dietary LA and linolenic acid. In contrast, Δ9 SCD1 is for the synthesis of palmitoelic and oleic acids for VLDL production. SCD1 in cardiomyocytes has a protective effect through inhibition of apoptosis, ceramide, DAG production, and generation of ROS (Matsui et al., 2012). In contrast, FASD1 Δ6-desaturase activity was positively associated with serum ICAM-1 and proinflammatory cytokines and negatively associated with adiponectin, suggesting that Δ6-desaturase synthesis of eicosanoids or PLs have a functionally undetermined role in cardiometabolic syndrome.
Eicosanoids recently have been shown to have a direct role in mitochondrial function in cardiometabolic diseases through dietary-induced increase in PL AA content in membrane PL. Replacement of mitochondrial PL with ω3-FAs prevented Ca2+-induced mitochondrial depolarization and cell death through opening of the mitochondrial permeability transition pore (Moon et al., 2012). Furthermore, activation of mitochondrial phos-pholipase A2γ (PLA2γ-PNPLA8) increased synthesis of AA eicosanoids and inhibited long-chain acyl-CoA that modulates generation of eicosanoids that regulate mitochondrial bioenergetics and signaling functions. The roles of AA metabolites have a function in cell survival where LOX and COX eicosanoids induce NADPH oxidase and ROS formation (Cho et al., 2011). It has been shown that CYP4 20-HETE induces cardiomyocyte apoptosis through stimulation of caspase 3 activities and Bax expression (Bao et al., 2011). The relationship between acyl-CoA and eicosanoid function in the mitochondria was revealed in aggressive breast cancer where ACSL4 increases intramitochondrial AA levels by ACSL4 induction of COX2 and generation of both LOX and COX eicosanoids that promote cell survival. It is not known how eicosanoids and FATP regulate mitochondrial function of cardiac myocytes in cardiometabolic diseases. There is a need to develop tissue-specific knockout of COX, LOX and cytochrome P450 eicosanoid enzymes in endothelial, VSMC, macrophages, and cardiomyocytes to identify the role of eicosanoids in cardiometabolic diseases and design effective eicosanoid-pathway-targeted treatments to prevent CVD.
6. T Herapies in the Treatment of Nafld
NAFLD is one of the most common causes of chronic liver disease in adults and children worldwide. Even though simple steatosis is initially a benign condition, up to 5% of individuals with NAFLD can progress to chronic diseases of steatohepatitis (NASH), liver fibrosis, liver cirrhosis, and finally either end-stage liver disease or hepatocellular carcinoma. Therefore, early intervention is the key to limit disease progression and realize better outcomes. The cornerstone in the management of NAFLD should be exercise and efforts at weight reduction through dietary changes and increased physical activity. Unfortunately, the constellation of MetS diseases associated with NAFLD of insulin resistance, hypertriglyceridemia, hyperlipidemia, and hypertension often require the clinician to intervene and prescribe therapeutic modalities to control or reduce the progression of symptoms and diseases associated with MetS.
A number of clinical trials (http:www.ClinicalTrials.gov) for NAFLD and NASH have focused on the associated condition of MetS and to date no particular treatment has emerged as safe and effective. Pharmaceutical therapies have had mixed results and presently none have been accepted as a standard therapy. Most of the 78 clinical trials have focused on treating insulin resistance, hypertriglyceridemia, and hyperlipidemia. However, newer studies have begun to focus on the underlying metabolic alteration, oxidative stress, inflammation, and liver injury in the progression of NAFLD to NASH and ensuing chronic liver diseases. The majority of clinical trials for insulin resistance focus on incretin mimics (exenatide and Vildagliptin) to stimulate insulin levels or reduce serum glucose (acarbose-α-glucosidase inhibitor) or the AMPK activator, metformin. To treat hyperlipidemia, the standard has been to use 3-hydroxy-3-methylglutaryl-CoA statin inhibitors (atovastatin) and TZDs (pioglitazone) PPARγ agonist that decrease serum glucose, lipid, inhibits inflammation and delays liver fibrosis. It is rather surprising that there are no trials for a combination study of statin and TZDs in the treatment of NAFLD or trials that use fibrates to increase FA β-oxidation. A rather new study uses arachidylamidocholanoic acid (Aram-chol) that inhibits SCD-1 and upregulates ABCD1 in cholesterol transport. It is uncertain whether inhibition of SCD-1, which decreases steatosis, will increase liver injury as shown in SCD-1 knockout mice. Several studies are focused on treating the metabolic dysfunction by the use of lipoic acid, carnitine, and coenzyme Q targeted to the mitochondria (mitQ). In addition, a number of trials target the underlying causes of MetS, oxidative stress and inflammation by the use of vitamin E (Lovaza) (ω-3 fatty acids) and cysteamine to increase cysteine levels and therefore glutathione. In one randomized control trial, pentoxifylline, which suppresses TNFα synthesis and savages hydroxyl and peroxyl radicals, improves the histological features of NASH. Also, several trials have focused on the prevention of hepatic injury by the use of silymarin and the FXRα agonist 6-ethyl chenodoxycholic acid (obeticholic), which also has antiinflammatory properties.
It is rather disappointing that of the 38 NASH and 40 NAFLD clinical trials none have proposed direct the eicosanoid pathway by the use of salicylates and EPA/DHA for the treatment of NAFLD since salicylates activate AMPK and inhibit TNFα-induced adipose lipolysis, while combination with ω-3 FA produce potent antiinflammatory resolvins, protectins, and LX.
7. Conclusion
Although, COX, LOX, and P450 eicosanoid mediators elicit their own tissue- and cell-selective biological response, the cross-talk between synergistic and antagonistic lipid mediators within these pathways needs to be better understood in the disease process of MetS to design effective treatment with minimal ADRs. The goal will necessitate understanding the functional role of eicosanoids in the control of tissue-specific intermediary metabolism and in particular lipid and carbohydrate metabolism in the fundamental process of cell survival, apoptosis, and proliferation in NAFLD and MetS. Understanding how eicosanoids regulate metabolism is of particular importance considering the link between fatty transporters, channeling, and the differential regulation of eicosanoid pathways.
This challenge of defining tissue-specific eicosanoid pathway cross-talk in inflammation and metabolic disease can only be met by a bioinformatics analysis of the transcriptome, proteome, lipidome, and metabolome in tissues affected by metabolic disease and inflammation in concert with analysis of tissue-specific eicosanoid flox knockout mice to design effective treatments. This fully integrated genomic, proteomic, and metabolomics analysis of eicosanoid pathways has recently been used to quantify eicosanoid metabolite production in macrophages in response to TLR4 signaling (Sabido et al, 2012) and thus adds invaluable insights into the integrated omics analysis of eicosanoid systems biology (Buczynski et al., 2009) http://www.lipidmaps.org. Extending this approach to drugs targeted to selective eicosanoid pathways with analysis of drug metabolism pathways (Guengerich & Cheng, 2011) provides a novel approach to design effective tissue-specific targeted therapies with the reduced ADRs and greater efficacy in the treatment of inflammatory and diseases of MetS and NAFLD (http://bioinformatics.charite.de/supertarget/).
Acknowledgments
This work was supported by National Institute of Health Grants HL32788, RO183366, and RCIHL100828 (to W.M.C), RO1DK093774 (to Yoon Kwang Lee), and grants DK44442 and DK58379 (to J.Y.L.C). We also wish to apologize for not to including recognition of excellent studies on MetS and NAFLD by numerous investigator because of limits on references.
Abbreviations
- AA
Arachidonic acid
- ABC
ATP-binding cassette transporter
- ACBP
Acyl-CoA binding protein
- ACS
Acyl-CoA synthetase
- ACSL
Long-chain acyl-CoA synthetase
- ACSVL
Very-long-chain acyl-CoA synthetase
- ACOX
Acyl-CoA oxidase
- AGPAT
Acyl-CoA:1-acylglycerol-3-phosphate acyltransferase (LPAAT)
- ALA
alpha-linoleic acid
- AMPK
AMP protein kinase
- AP1
Activator protein
- APOE
apoE lipoprotein
- ATGL
Adipose triglyceride lipase
- ATM
Adipose tissue macrophage
- BLT
LTB4 receptor
- CD36/FAT
Fatty acid translocase
- COX
Cylooxygenase
- CL
Cardiolipin
- cysLT
LTC4,LTD4,LTE4 receptor
- CYP
Cytochrome P450
- CVD
Cardiovascular disease
- DAG
Diacylglycerol
- DAGT
Diacylglycerol transferase
- DHA
Docosahexaenoic acid
- DIO
Diet-induced obesity
- DP
Receptor for PGD
- EDP
epoxydocosapentaenoic acid
- EED
DHA CYP2-produced epoxydocosapentaenoic acid
- EEQ
EPA CYP2-produced epoxyeicosatetraenoic acid
- EET
Epoxyeicosatrienoic acid
- EHHADH
Enoyl-CoA hydratase 3-hydroxyacyl-CoA dehydrogenase
- ELOVL
Elongase enzyme
- EP
Receptor for PGE
- EPA
Eicosapentaenoic acid
- ERKS
extracellular receptor kinase 2
- Exoins
Proinflammatory 15-LOX eicosanoids
- FABP
Fatty-acid-binding protein
- FADS
Fatty acid desaturase
- FATP
Fatty acid transport proteins
- FFA
Free fatty acid
- FLAP
5-lipoxygenase activity protein
- FP
Receptor for PGF
- FXR
Farnesoid-X-receptor
- G α
(q/11) G α subunit q that activates phospholipase c
- GEF
guanine exchange factor
- GPAT
Glycerol-3-phosphate acyltransferase
- GSH
Reduced glutathione, GST
- GST
Glutathione-S-transferase
- HDL
High-density lipoprotein
- HEET
hydroxyepoxyeicosatrienoic acid
- HETE
Hydroxeicosatetraenoic acid
- HNF4
Hepatocyte nuclear factor 4
- HODE
Hydroxyoctadecadienoic acid
- HSL
Hormone-sensitive lipase
- HSPG
Heparin sulfate proteoglycan
- IL
Interleukins
- IP
Receptor for PGI
- IRS
Insulin receptor substrate
- LD
lipid droplets
- LDLR
Low-density lipoprotein receptor
- Lipin
Phosphatidic acid phosphatase
- Lipoxin
Antiinflammatory LTA4
- LOX
Lipoxygenase
- LTB4
Leukotriene B4
- LPA
Lysophosphatidic acid
- LPAAT
lysophosphatic acid acyltransferase
- LPCAT
Lysophosphatidylcholine acyl-CoA transferase
- LPLAT
lysophospholipid acid acyltransferase
- LysoPC
Lysophosphatidylcholine
- LXR
Liver-X-receptor
- MAPK2
mitogen activated protein kinase 2
- MBOAT
Membrane-bound O-acyltransferase
- MCP-1
monocyte chemotactic protein
- MGAT
Monoglycerol acyltransferase
- MGST
microsomal GSH transferase
- MetS
Metabolic syndrome
- MRP
Multidrug resistant protein
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NF-κβ
Nuclear factor kappa β
- NSAID
Nonsteroidal antiinflammatory drug
- Null
Gene knockout
- OAT
Organic anion transporter
- Omega-3 PUFA
ω3-PUFA
- PAP
Phosphatidic acid phosphatase
- PNPLA
Patatin-like phospholipase domain containing lipases
- PGC
Peroxisome proliferator activated receptor coactivator
- PLA2
Phospholipase A2
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PG
Prostaglandin
- PGD2
prostaglandin D2
- PGDS
PGD synthase
- PGE2
prostaglandin E2
- PGF2α
prostaglandin E2
- PGES
PGE synthase
- PGFS
PGF synthase
- PGHS
Prostaglandin endoperoxide H synthase
- PGG2
Protaglandin peroxidase
- PGH2
prostaglandin endoperoxide
- PGI
Prostacyclin
- PGIS
Prostacyclin synthase
- PGJ2
prostaglandin J2
- PKA
Protein kinase A
- PKC
Protein kinase C
- PL
Phospholipid
- PLIN
perilipin
- PS
Phosphatidylserine
- PPAR
Peroxisome proliferator activated receptor
- PUFA
Polyunsaturated fatty acid
- PXR
Pregnane-X-receptor
- RAR
Retinoic acid receptor
- RXR
Retinoid-X-receptor
- SCD-1
Stearoyl-CoA desaturase
- sEH
Soluble epoxide hydrolase
- SLC
Solute ligand carrier
- SFA
Saturated fatty acid
- SRSA
Slow-reacting substance of anaphylaxis
- SREBP
Sterol regulatory-element-binding protein
- TAG
Triacylglycerol
- T1DM
Type I diabetes mellitus
- T2DM
Type II diabetes mellitus
- TX
Thromboxane
- TXAS
Thromboxane synthase
- TXA2
thromboxane A2
- TZD
Thiazolidinedione
- usFA
Unsaturated fatty acid
- VLDL
Very-low-density lipoprotein
- 20-HETE
AA CYP4-produced hydroxyeicosatetraenoic acid
- 19-HEPE
EPA CYP4-produced hydroxyeicosapentaenoic acid
- 22-HDoHE-DHA CYP4
Hydroxdocosahexaenoic acid
Footnotes
Conflict of Interest Statement: The authors have no conflict of interests to declare.
References
- Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. Journal of Hepatology. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58(4):855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Rabbitt E, Brady T, Brown C, Guest P, Bujalska IJ, et al. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS One. 2012;7(2):e29531. doi: 10.1371/journal.pone.0029531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Guardia D, Palomer X, Coll T, Serrano L, Rodriguez-Calvo R, Davidson MM, et al. PPARbeta/delta activation blocks lipid-induced inflammatory pathways in mouse heart and human cardiac cells. Biochimica et Biophysica Acta. 2011;1811(2):59–67. doi: 10.1016/j.bbalip.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacological Reviews. 2008;60(3):311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Arbo I, Halle C, Malik D, Brattbakk HR, Johansen B. Insulin induces fatty acid desaturase expression in human monocytes. Scandinavian Journal of Clinical and Laboratory Investigation. 2011;71(4):330–339. doi: 10.3109/00365513.2011.566350. [DOI] [PubMed] [Google Scholar]
- Ashla AA, Hoshikawa Y, Tsuchiya H, Hashiguchi K, Enjoji M, Nakamuta M, et al. Genetic analysis of expression profile involved in retinoid metabolism in nonalcoholic fatty liver disease. Hepatology Research. 2010;40(6):594–604. doi: 10.1111/j.1872-034X.2010.00646.x. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. Journal of Nutritional Biochemistry. 2010;21(11):1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54(11):3148–3153. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- Banasik K, Justesen JM, Hornbak M, Krarup NT, Gjesing AP, Sandholt CH, et al. Bioinformatics-driven identification and examination of candidate genes for non-alcoholic fatty liver disease. PLoS One. 2011;6(1):e16542. doi: 10.1371/journal.pone.0016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Wang X, Li W, Huo D, Shen X, Han Y, et al. 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. Journal of Cardiovascular Pharmacology. 2011;57(3):294–301. doi: 10.1097/FJC.0b013e3182073c78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. Journal of Pharmacology and Experimental Therapeutics. 2009;328(1):231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- Bejarano-Achache I, Levy L, Mlynarsky L, Bialer M, Muszkat M, Caraco Y. Effects of CYP4F2 polymorphism on response to warfarin during induction phase: a prospective, open-label, observational cohort study. Clinical Therapeutics. 2012;34(4):811–823. doi: 10.1016/j.clinthera.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Bosma M, Kersten S, Hesselink MK, Schrauwen P. Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Progress in Lipid Research. 2012;51(1):36–49. doi: 10.1016/j.plipres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nature Cell Biology. 2007;9(11):1286–1293. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;82(4–6):243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, Bandeira-Melo C. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukotrienes and Essential Fatty Acids. 2011;85(5):205–213. doi: 10.1016/j.plefa.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metabolism. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. Comment Review. [DOI] [PubMed] [Google Scholar]
- Bruegel M, Ludwig U, Kleinhempel A, Petros S, Kortz L, Ceglarek U, et al. Sepsis-associated changes of the arachidonic acid metabolism and their diagnostic potential in septic patients. Critical Care Medicine. 2012;40(5):1478–1486. doi: 10.1097/CCM.0b013e3182416f05. [DOI] [PubMed] [Google Scholar]
- Bu SY, Mashek DG. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. Journal of Lipid Research. 2010;51(11):3270–3280. doi: 10.1194/jlr.M009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS, Dennis EA. Thematic review series: proteomics. an integrated omics analysis of eicosanoid biology. Journal of Lipid Research. 2009;50(6):1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui P, Imaizumi S, Beedanagari SR, Reddy ST, Hankinson O. Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids. Drug Metabolism and Disposition. 2011;39(2):180–190. doi: 10.1124/dmd.110.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, et al. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cellular Physiology and Biochemistry. 2012;29(1–2):99–110. doi: 10.1159/000337591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Vanella L, Bellner L, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins and Other Lipid Mediators. 2012;97(1–2):1–16. doi: 10.1016/j.prostaglandins.2011.10.002. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia R, Riquelme A, Baudrand R, Carvajal CA, Morales M, Solis N, et al. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue and portal hypercortisolism in non-alcoholic fatty liver disease. Liver International. 2012;32(3):392–399. doi: 10.1111/j.1478-3231.2011.02685.x. [DOI] [PubMed] [Google Scholar]
- Caspar-Bauguil S, Fioroni A, Galinier A, Allenbach S, Pujol MC, Salvayre R, et al. Pro-inflammatory phospholipid arachidonic acid/eicosapentaenoic acid ratio of dysmetabolic severely obese women. Obesity Surgery. 2012;22(6):935–944. doi: 10.1007/s11695-012-0633-0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. American Journal of Physiology: Endocrinology and Metabolism. 2011;300(1):E175–E187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nature Genetics. 2011;43(11):1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48(3):486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- Chen H, Qin J, Wei P, Zhang J, Li Q, Fu L, et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(4):195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang P, Zhao G, Xu G, Gruzdev A, Zeldin DC, et al. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins and Other Lipid Mediators. 2011;96(1–4):63–71. doi: 10.1016/j.prostaglandins.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry. 2011;50(18):3840–3848. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Molecules and Cells. 2011;32(1):1–5. doi: 10.1007/s10059-011-1021-7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Hermann M. Immunological response as a source to variability in drug metabolism and transport. Frontiers in Pharmacology. 2012;3:8. doi: 10.3389/fphar.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ at a glance. Disease Models and Mechanisms. 2012;5(5):588–594. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chemical Reviews. 2011;111(10):6359–6386. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Murphy EJ, Jolly CA. Glycerol-3-phosphate acyltransferase-1 regulates murine T-lymphocyte proliferation and cytokine production. American Journal of Physiology. 2008;295(6):C1543–C1549. doi: 10.1152/ajpcell.00371.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annual Review of Nutrition. 2010;30:257–272. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley CR, Monsuur AJ, Wapenaar MC, Rioux JD, Wijmenga C. A functional candidate screen for coeliac disease genes. European Journal of Human Genetics. 2006;14(11):1215–1222. doi: 10.1038/sj.ejhg.5201687. [DOI] [PubMed] [Google Scholar]
- Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends in Endocrinology and Metabolism. 2010;21(12):707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglar G, Karaca T, Yuksek YN, Gozalan U, Akbiyik F, Sokmensuer C, et al. Effect of montelukast and MK-886 on hepatic ischemia-reperfusion injury in rats. Journal of Surgical Research. 2009;153(1):31–38. doi: 10.1016/j.jss.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. Editorial. [DOI] [PubMed] [Google Scholar]
- De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, et al. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring) 2010;18(3):489–498. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Stachlewitz RF, Liguori MJ, Blomme EA, Waring JF, Luyendyk JP, et al. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. Journal of Pharmacology and Experimental Therapeutics. 2006;319(3):1191–1199. doi: 10.1124/jpet.106.110247. [DOI] [PubMed] [Google Scholar]
- Deng X, Luyendyk JP, Ganey PE, Roth RA. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacological Reviews. 2009;61(3):262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, et al. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB Journal. 2011;25(2):703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical Reviews. 2011;111(10):6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Morgan J, Jim Wang YX, Munusamy S, Hall JE. Inhibition of soluble epoxide hydrolase reduces food intake and increases metabolic rate in obese mice. Nutrition, Metabolism, and Cardiovascular Diseases. 2012;22(7):598–604. doi: 10.1016/j.numecd.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do HJ, Chung HK, Moon J, Shin MJ. Relationship between the estimates of desaturase activities and cardiometabolic phenotypes in Koreans. Journal of Clinical Biochemistry and Nutrition. 2011;49(2):131–135. doi: 10.3164/jcbn.10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Ma Q, Lindsay JW, Cole BK, Ma K, et al. Differential expression and localization of 12/15 lipoxygenases in adipose tissue in human obese subjects. Biochemical and Biophysical Research. 2010;403(3–4):485–490. doi: 10.1016/j.bbrc.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato MT, Jimenez N, Serralta A, Mir J, Castell JV, Gomez-Lechon MJ. Effects of steatosis on drug-metabolizing capability of primary human hepatocytes. Toxicology In Vitro. 2007;21(2):271–276. doi: 10.1016/j.tiv.2006.07.008. [DOI] [PubMed] [Google Scholar]
- D'Souza AM, Beaudry JL, Szigiato AA, Trumble SJ, Snook LA, et al. Consumption of a high-fat diet rapidly exacerbates the development of fatty liver disease that occurs with chronically elevated glucocorticoids. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2012;302(8):G850–G863. doi: 10.1152/ajpgi.00378.2011. [DOI] [PubMed] [Google Scholar]
- Du ZY, Ma T, Liaset B, Keenan AH, Araujo P, Lock EJ, et al. Dietary eicosapentaenoic acid supplementation accentuates hepatic triglyceride accumulation in mice with impaired fatty acid oxidation capacity. Biochimica et Biophysica Acta. 2012 doi: 10.1016/j.bbalip.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metabolism. 2012;15(4):518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58(5):1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Swefy S, Hassanen SI. Improvement of hepatic fibrosis by leukotriene inhibition in cholestatic rats. Annals of Hepatology. 2009;8(1):41–49. [PubMed] [Google Scholar]
- Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacological Reviews. 2012;64(3):421–449. doi: 10.1124/pr.111.004614. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, et al. Kupffer cell-derived prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2000;279(1):G100–G106. doi: 10.1152/ajpgi.2000.279.1.G100. [DOI] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Natural Medicines. 2009;15(12):1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki Y, Li Y, Sakata D, Yao C, Segi-Nishida E, Matsuoka T, et al. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(27):12233–12238. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Monk JM, Hou TY, Callway E, Vincent L, Weeks B, et al. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. Journal of Lipid Research. 2012;53(7):1287–1295. doi: 10.1194/jlr.M024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Danese E, Sjogren M, Almgren P, Guidi GC, et al. The functional variant V433M of the CYP4F2 and the metabolic syndrome in Swedes. Prostaglandins and Other Lipid Mediators. 2012;98(1–2):31–36. doi: 10.1016/j.prostaglandins.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of Lipid Research. 2010;51(10):3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, et al. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Archives of Biochemistry and Biophysics. 2008;471(2):116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Filik L. Visceral and subcutaneous fat accumulation and nonalcoholic fatty liver disease. Journal of Gastroenterology. 2011;46(21222004):419–420. doi: 10.1007/s00535-010-0362-x. [DOI] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metabolism and Disposition. 2009;37(10):2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell–cell interactions to in vivo tissue responses. Pharmacological Reviews. 2006;58(3):375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature Reviews: Drug discovery. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Yin L, Ma H, Li T, Chiang JY, Zhang Y. Aldo-keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. Journal of Lipid Research. 2011;52(8):1561–1568. doi: 10.1194/jlr.M015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertow K, Pietilainen KH, Yki-Jarvinen H, Kaprio J, Rissanen A, Eriksson P, et al. Expression of fatty-acid-handling proteins in human adipose tissue in relation to obesity and insulin resistance. Diabetologia. 2004;47(6):1118–1125. doi: 10.1007/s00125-004-1417-4. [DOI] [PubMed] [Google Scholar]
- Ghesquiere SA, Hofker MH, de Winther MP. The role of phospholipases in lipid modification and atherosclerosis. Cardiovascular Toxicology. 2005;5(2):161–182. doi: 10.1385/ct:5:2:161. [DOI] [PubMed] [Google Scholar]
- Ghomashchi F, Naika GS, Bollinger JG, Aloulou A, Lehr M, Leslie CC, et al. Interfacial kinetic and binding properties of mammalian group IVB phospholipase A2 (cPLA2beta) and comparison with the other cPLA2 isoforms. Journal of Biological Chemistry. 2010;285(46):36100–36111. doi: 10.1074/jbc.M110.165647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal S, Trivedi DB, Graf GA, Loftin CD. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. Journal of Biological Chemistry. 2011;286(1):889–898. doi: 10.1074/jbc.M110.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metabolism. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CD, Olson LK. Modulation of palmitate-induced endoplasmic reticulum stress and apoptosis in pancreatic beta-cells by stearoyl-CoA desaturase and Elovl6. American Journal of Physiology: Endocrinology and Metabolism. 2011;300(4):E640–E649. doi: 10.1152/ajpendo.00544.2010. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, et al. The role of lipid droplets in metabolic disease in rodents and humans. Journal of Clinical Investigation. 2011;121(6):2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. European Journal of Nutrition. 2006;45(1):55–60. doi: 10.1007/s00394-005-0573-8. [DOI] [PubMed] [Google Scholar]
- Gubern A, Casas J, Barcelo-Torns M, Barneda D, de la Rosa X, Masgrau R, et al. Group IVA phospholipase A2 is necessary for the biogenesis of lipid droplets. Journal of Biological Chemistry. 2008;283(41):27369–27382. doi: 10.1074/jbc.M800696200. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Cheng Q. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharmacological Reviews. 2011;63(3):684–699. doi: 10.1124/pr.110.003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, et al. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. Journal of Pharmacology and Experimental Therapeutics. 2007;321(1):18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chemical Reviews. 2011;111(10):5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Hall D, Poussin C, Velagapudi VR, Empsen C, Joffraud M, Beckmann JS, et al. Peroxisomal and microsomal lipid pathways associated with resistance to hepatic steatosis and reduced pro-inflammatory state. Journal of Biological Chemistry. 2010;285(40):31011–31023. doi: 10.1074/jbc.M110.127159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Kou K, Chen Z, Pietka TA, Kumar M, Korenblat KM, et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. Journal of Lipid Research. 2012;53(5):990–999. doi: 10.1194/jlr.P025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Current Diabetes Reviews. 2006;2(4):367–373. doi: 10.2174/1573399810602040367. Review. [DOI] [PubMed] [Google Scholar]
- Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ. PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-hydroxylase (CYP4) isozymes: implications for prevention of lipotoxicity in fatty liver disease. PPAR Research. 2009;2009:952734. doi: 10.1155/2009/952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochemical Pharmacology. 2008;75(12):2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Harmon GS, Lam MT, Glass CK. PPARs and lipid ligands in inflammation and metabolism. Chemical Reviews. 2011;111(10):6321–6340. doi: 10.1021/cr2001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra S, Batra RK, Tai HH, Sharma S, Cui X, Dubinett SM. Pioglitazone and rosiglitazone decrease prostaglandin E2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Molecular Pharmacology. 2007;71(6):1715–1720. doi: 10.1124/mol.106.033357. [DOI] [PubMed] [Google Scholar]
- He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. Journal of Biological Chemistry. 2010;285(9):6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, Manolescu A, Helgason A, Thorleifsson G, Thorsteinsdottir U, Gudbjartsson DF, et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nature Genetics. 2006;38(1):68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, et al. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation. 2005;112(4):578–586. doi: 10.1161/CIRCULATIONAHA.105.545616. [DOI] [PubMed] [Google Scholar]
- Henkin AH, Ortegon AM, Cho S, Shen WJ, Falcon A, Kraemer FB, et al. Evidence for protein-mediated fatty acid efflux by adipocytes. Acta Physiologica (Oxford, England) 2012;204(4):562–570. doi: 10.1111/j.1748-1716.2011.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi N, Kato M, Tanaka M, Miyazaki M, Takao S, Kohjima M, et al. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP and L-FABP in non-alcoholic fatty liver disease. Experimental and Therapeutic Medicine. 2011;2(22977624):1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Narumiya S. Prostanoid receptors. Chemical Reviews. 2011;111(10):6209–6230. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. Journal of Lipid Research. 2010;51(2):262–273. doi: 10.1194/jlr.R003582. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla VR, Wu H, Shi Q, Menter DG, DuBois RN. Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. Journal of Biological Chemistry. 2011;286(34):30003–30009. doi: 10.1074/jbc.M110.184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147(1):173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Kidani YN, Gonzalez A, Phung T, Ito A, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. Journal of Clinical Investigation. 2012;122(1):337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo RL, Lee IP, Zhou M, Wong JY, Hui X, Xu A, et al. Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non-alcoholic steatohepatitis in mice. Journal of Hepatology. 2012 doi: 10.1016/j.jhep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Horrillo R, Planaguma A, Gonzalez-Periz A, Ferre N, Titos E, Miquel R, et al. Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor. Journal of Pharmacology and Experimental Therapeutics. 2007;323(3):778–786. doi: 10.1124/jpet.107.128264. [DOI] [PubMed] [Google Scholar]
- Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, Lopez-Parra M, Ferre N, Titos E, et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. Journal of Immunology. 2010;184(7):3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- Hossain MS, Nishimura K, Jisaka M, Nagaya T, Yokota K. Prostaglandin J2 series induces the gene expression of monocyte chemoattractant protein-1 during the maturation phase of cultured adipocytes. Gene. 2012;502(2):138–141. doi: 10.1016/j.gene.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Hostetler HA, Kier AB, Schroeder F. Very-long-chain and branched-chain fatty acyl-CoAs are high affinity ligands for the peroxisome proliferator-activated receptor alpha (PPARalpha) Biochemistry. 2006;45(24):7669–7681. doi: 10.1021/bi060198l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Denis S, Argmann CA, Jia Y, Ferdinandusse S, Reddy JK, et al. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. Journal of Lipid Research. 2012;53(7):1296–1303. doi: 10.1194/jlr.M024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. Regulation of human cyto-chrome P450 4F2 expression by sterol regulatory element-binding protein and lovastatin. Journal of Biological Chemistry. 2007;282(8):5225–5236. doi: 10.1074/jbc.M608176200. [DOI] [PubMed] [Google Scholar]
- Huang W, Glass CK. Nuclear receptors and inflammation control: molecular mechanisms and pathophysiological relevance. Arteriosclerosis Thrombosis and Vascular Biology. 2010;30(8):1542–1549. doi: 10.1161/ATVBAHA.109.191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Atshaves BP, Frolov A, Kier AB, Schroeder F. Acyl-coenzyme A binding protein expression alters liver fatty acylcoenzyme A metabolism. Biochemistry. 2005;44(16042405):10282–10297. doi: 10.1021/bi0477891. [DOI] [PubMed] [Google Scholar]
- Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(17):7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AY, Dannenberg AJ, Sung JJ, Subbaramaiah K, Du B, Olinga P, et al. Prostaglandin E2 inhibits transforming growth factor beta 1-mediated induction of collagen alpha 1(I) in hepatic stellate cells. Journal of Hepatology. 2004;41(2):251–258. doi: 10.1016/j.jhep.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins and Other Lipid Mediators. 2009;89(3–4):82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. Journal of Vascular Research. 2001;38(3):247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiological Reviews. 2012;92(1):101–130. doi: 10.1152/physrev.00021.2011. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, et al. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. Journal of Pharmacology and Experimental Therapeutics. 2008;324(1):103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, et al. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E2 biosynthesis. Prostaglandins and Other Lipid Mediators. 2009;88(3–4):73–81. doi: 10.1016/j.prostaglandins.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Natural Medicines. 2009;15(2):159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. Journal of Clinical Investigation. 2002;109(7):883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalupahana NS, Voy BH, Saxton AM, Moustaid-Moussa N. Energy-restricted high-fat diets only partially improve markers of systemic and adipose tissue inflammation. Obesity (Silver Spring) 2011;19(2):245–254. doi: 10.1038/oby.2010.196. [DOI] [PubMed] [Google Scholar]
- Karlsson EA, Wang S, Shi Q, Coleman RA, Beck MA. Glycerol-3-phosphate acyltransferase 1 is essential for the immune response to infection with coxsackievirus B3 in mice. Journal of Nutritional Science. 2009;139(4):779–783. doi: 10.3945/jn.108.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochimica et Biophysica Acta. 2012;1821(21979150):852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Kumar A, Wang L, Liu GH, Keller SR, Lawrence JC, Jr, et al. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Molecular and Cellular Biology. 2010;30(12):3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LJ, Nalls MA, Eiriksdottir G, Sigurdsson S, Launer LJ, Koster A, et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring) 2011;19(6):1265–1271. doi: 10.1038/oby.2010.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Lee SH, Lee SM. Role of Kupffer cells in pathogenesis of sepsis-induced drug metabolizing dysfunction. FEBS Journal. 2011;278(13):2307–2317. doi: 10.1111/j.1742-4658.2011.08148.x. [DOI] [PubMed] [Google Scholar]
- Kim YC, Cho YK, Lee WY, Kim HJ, Park JH, Park DI, et al. Serum adipocyte-specific fatty acid-binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. Journal of Nutritional Biochemistry. 2011;22(20579864):289–292. doi: 10.1016/j.jnutbio.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Knockaert L, Fromenty B, Robin MA. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity. FEBS Journal. 2011;278(22):4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. Review. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. Journal of Clinical Investigation. 2004;114(6):784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochimica et Biophysica Acta. 2011;1814(1):210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in Lipid Research. 2011;50(1):14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. Journal of Clinical Investigation. 2000;105(8):1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr S, Hartwig S, Lamers D, Famulla S, Muller S, Hanisch FG, et al. Identification and validation of novel adipokines released from primary human adipocytes. Molecular and Cellular Proteomics. 2012;11(1):M111.010504. doi: 10.1074/mcp.M111.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang S, Barbour SE, Bohrer A, Ford EL, Koizumi A, et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. Journal of Biological Chemistry. 2010;285(9):6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier I, Jedlitschky G, Buchholz U, Keppler D. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells. European Journal of Biochemistry. 1994;220(2):599–606. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. Journal of Biological Chemistry. 2009;284(9):5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA. Early hepatic insulin resistance in mice: a metabolomics analysis. Molecular Endocrinology. 2010;24(3):657–666. doi: 10.1210/me.2009-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. American Journal of Respiratory and Critical Care Medicine. 2011;184(1):124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, et al. Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metabolism and Disposition. 2007;35(10):1970–1978. doi: 10.1124/dmd.107.015107. [DOI] [PubMed] [Google Scholar]
- Lin ZP, Zhu YL, Johnson DR, Rice KP, Nottoli T, Hains BC, et al. Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Molecular Pharmacology. 2008;73(1):243–251. doi: 10.1124/mol.107.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lee SJ, Shim H, Chun J, Yun CC. The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2010;299(5):G1128–G1138. doi: 10.1152/ajpgi.00321.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Li N, Yang J, Qiu H, Ai D, Chiamvimonvat N, et al. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Parra M, Titos E, Horrillo R, Ferre N, Gonzalez-Periz A, Martinez-Clemente M, et al. Regulatory effects of arachidonate 5-lipoxygenase on hepatic microsomal TG transfer protein activity and VLDL-triglyceride and apoB secretion in obese mice. Journal of Lipid Research. 2008;49(12):2513–2523. doi: 10.1194/jlr.M800101-JLR200. [DOI] [PubMed] [Google Scholar]
- Lorente-Cebrian S, Bustos M, Marti A, Fernandez-Galilea M, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid inhibits tumour necrosis factor-alpha-induced lipolysis in murine cultured adipocytes. Journal of Nutritional Biochemistry. 2012;23(3):218–227. doi: 10.1016/j.jnutbio.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Ludewig AH, Nitz I, Klapper M, Doring F. Identification of a novel human Acyl-CoA binding protein isoform with a unique C-terminal domain. IUBMB Life. 2011;63(7):547–552. doi: 10.1002/iub.471. [DOI] [PubMed] [Google Scholar]
- Luo P, Wang MH. Eicosanoids, beta-cell function, and diabetes. Prostaglandins and Other Lipid Mediators. 2011;95(1–4):1–10. doi: 10.1016/j.prostaglandins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2beta) indicate a signaling rather than a housekeeping role for iPLA2beta. Journal of Biological Chemistry. 2001;276(16):13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. Journal of Endocrinology. 2008;197(2):189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- Madec S, Cerec V, Plee-Gautier E, Antoun J, Glaise D, Salaun JP, et al. CYP4F3B expression is associated with differentiation of HepaRG human hepatocytes and unaffected by fatty acid overload. Drug Metabolism and Disposition. 2011;39(10):1987–1996. doi: 10.1124/dmd.110.036848. [DOI] [PubMed] [Google Scholar]
- Makowski L, Hotamisligil GS. Fatty acid binding proteins—the evolutionary crossroads of inflammatory and metabolic responses. Journal of Nutrition. 2004;134(9):2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapaka RR, Khoo S, Zhang J, Choi JH, Zhou XE, Xu Y, et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. Journal of Biological Chemistry. 2012;287(1):183–195. doi: 10.1074/jbc.M111.294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente M, Ferre N, Gonzalez-Periz A, Lopez-Parra M, Horrillo R, Titos E, et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology. 2010;51(3):817–827. doi: 10.1002/hep.23463. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente M, Ferre N, Titos E, Horrillo R, Gonzalez-Periz A, Moran-Sal-vador E, et al. Disruption of the 12/15-lipoxygenase gene (Alox15) protects hyperlipidemic mice from nonalcoholic fatty liver disease. Hepatology. 2010;52(6):1980–1991. doi: 10.1002/hep.23928. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente M, Clària J, Titos E. The 5-lipoxygenase/leukotriene pathway in obesity, insulin resistance, and fatty liver disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(21587068):347–353. doi: 10.1097/MCO.0b013e32834777fa. [DOI] [PubMed] [Google Scholar]
- Matsui H, Yokoyama T, Sekiguchi K, Iijima D, Sunaga H, Maniwa M, et al. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS One. 2012;7(3):e33283. doi: 10.1371/journal.pone.0033283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazid MA, Chowdhury AA, Nagao K, Nishimura K, Jisaka M, Nagaya T, et al. Endogenous 15-deoxy-Delta(12,14)-prostaglandin J(2) synthesized by adipocytes during maturation phase contributes to upregulation of fat storage. FEBS Letters. 2006;580(30):6885–6890. doi: 10.1016/j.febslet.2006.11.049. [DOI] [PubMed] [Google Scholar]
- McClelland S, Gawaz M, Kennerknecht E, Konrad CS, Sauer S, Schuerzinger K, et al. Contribution of cyclooxygenase-1 to thromboxane formation, platelet–vessel wall interactions and atherosclerosis in the ApoE null mouse. Atherosclerosis. 2009;202(1):84–91. doi: 10.1016/j.atherosclerosis.2008.04.016. [DOI] [PubMed] [Google Scholar]
- McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57(1):199–208. doi: 10.2337/db07-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita-Santos FP, Bakker-Abreu I, Luna-Gomes T, Bozza PT, Diaz BL, Bandeira-Melo C. Co-operative signalling through DP(1) and DP(2) prostanoid receptors is required to enhance leukotriene C(4) synthesis induced by prostaglandin D(2) in eosinophils. British Journal of Pharmacology. 2011;162(8):1674–1685. doi: 10.1111/j.1476-5381.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(6):535–541. doi: 10.1097/MCO.0b013e32834ad8b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. Journal of Biological Chemistry. 2011;286(24):21330–21339. doi: 10.1074/jbc.M110.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, Murphy EP. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clinical Cancer Research. 2012;18(12):3223–3228. doi: 10.1158/1078-0432.CCR-11-2953. [DOI] [PubMed] [Google Scholar]
- Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. Journal of Lipid Research. 2009;50(3):412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SH, Jenkins CM, Liu X, Guan S, Mancuso DJ, Gross RW. Activation of mitochondrial calcium-independent phospholipase A2gamma (iPLA2gamma) by divalent cations mediating arachidonate release and production of downstream eicosanoids. Journal of Biological Chemistry. 2012;287(18):14880–14895. doi: 10.1074/jbc.M111.336776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes J, Assreuy J, Canetti C, Barja-Fidalgo C. Leukotriene B4 mediates vascular smooth muscle cell migration through alphavbeta3 integrin transactivation. Atherosclerosis. 2010;212(2):406–413. doi: 10.1016/j.atherosclerosis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, et al. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB Journal. 2011;25(8):2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- Morcillo S, Martin-Nunez GM, Rojo-Martinez G, Almaraz MC, Garcia-Escobar E, Mansego ML, et al. ELOVL6 genetic variation is related to insulin sensitivity: a new candidate gene in energy metabolism. PLoS One. 2011;6(6):e21198. doi: 10.1371/journal.pone.0021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya N, Kataoka H, Fujino H, Nishikawa J, Kugawa F. Effect of lipopolysac-charide on the xenobiotic-induced expression and activity of hepatic cytochrome P450 in mice. Biological and Pharmaceutical Bulletin. 2012;35(4):473–480. doi: 10.1248/bpb.35.473. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A(2) research: from cells to animals to humans. Progress in Lipid Research. 2011;50(2):152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Murphy RC. Free-radical-induced oxidation of arachidonoyl plasmalogen phospholipids: antioxidant mechanism and precursor pathway for bioactive eicosanoids. Chemical Research in Toxicology. 2001;14(5):463–472. doi: 10.1021/tx000250t. [DOI] [PubMed] [Google Scholar]
- Naganuma T, Sato Y, Sassa T, Ohno Y, Kihara A. Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Letters. 2011;585(20):3337–3341. doi: 10.1016/j.febslet.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Nagle CA, Vergnes L, Dejong H, Wang S, Lewin TM, Reue K, et al. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6-/- mice. Journal of Lipid Research. 2008;49(4):823–831. doi: 10.1194/jlr.M700592-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. Journal of Lipid Research. 2009;50(Suppl):S74–S79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Szanto A, Szatmari I, Szeles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiological Reviews. 2012;92(2):739–789. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Shimizu T. Leukotriene receptors. Chemical Reviews. 2011;111(10):6231–6298. doi: 10.1021/cr100392s. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT, et al. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prevention Research (Philadelphia) 2011;4(8):1198–1208. doi: 10.1158/1940-6207.CAPR-11-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancey S, Boschetti G, Hacini F, Sardi F, Durand PY, Le Borgne M, et al. Blockade of LTB(4)/BLT(1) pathway improves CD8(+) T-cell-mediated colitis. Inflammatory Bowel Diseases. 2011;17(1):279–288. doi: 10.1002/ibd.21404. [DOI] [PubMed] [Google Scholar]
- Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, Reddy RC. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-alpha agonist. Journal of Biological Chemistry. 2010;285(29):22067–22074. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhulu S. Differential behavior of the sub-sites of cytochrome 450 active site in binding of substrates, and products (implications for coupling/uncoupling) Biochimica et Biophysica Acta. 2007;1770(3):360–375. doi: 10.1016/j.bbagen.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Neess D, Bloksgaard M, Bek S, Marcher AB, Elle IC, Helledie T, et al. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. Journal of Biological Chemistry. 2011;286(5):3460–3472. doi: 10.1074/jbc.M110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolete R, Lima Kde M, Junior JM, Jose PJ, Sanz MJ, Faccioli LH. Prostaglandin E(2)-loaded microspheres as strategy to inhibit phagocytosis and modulate inflammatory mediators release. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70(3):784–790. doi: 10.1016/j.ejpb.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacological Reviews. 2011;63(1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Ivanov IV, Oliw EH. LC-MS/MS analysis of epoxyalcohols and epoxides of arachidonic acid and their oxygenation by recombinant CYP4F8 and CYP4F22. Archives of Biochemistry and Biophysics. 2010;494(1):64–71. doi: 10.1016/j.abb.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Nitz I, Kruse ML, Klapper M, Doring F. Specific regulation of low-abundance transcript variants encoding human Acyl-CoA binding protein (ACBP) isoforms. Journal of Cellular and Molecular Medicine. 2011;15(4):909–927. doi: 10.1111/j.1582-4934.2010.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oga T, Matsuoka T, Yao C, Nonomura K, Kitaoka S, Sakata D, et al. Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Natural Medicines. 2009;15(12):1426–1430. doi: 10.1038/nm.2066. [DOI] [PubMed] [Google Scholar]
- Oh da Y, Olefsky JM. Omega 3 fatty acids and GPR120. Cell Metabolism. 2012;15(5):564–565. doi: 10.1016/j.cmet.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikari S, Ahtialansaari T, Huotari A, Kiehne K, Folsch UR, Wolffram S, et al. Effect of medium- and long-chain fatty acid diets on PPAR and SREBP-1 expression and glucose homeostasis in ACBP-overexpressing transgenic rats. Acta Physiologica (Oxford, England) 2008;194(1):57–65. doi: 10.1111/j.1748-1716.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- Oliw EH, Bylund J, Herman C. Bisallylic hydroxylation and epoxidation of polyunsaturated fatty acids by cytochrome P450. Lipids. 1996;31(10):1003–1021. doi: 10.1007/BF02522457. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews: Immunology. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. Journal of Clinical Investigation. 2012;122(1):178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavani A, Naushad SM, Rupasree Y, Kumar TR, Malempati AR, Pinjala RK, et al. Optimization of warfarin dose by population-specific pharmacogenomic algorithm. Pharmacogenomics Journal. 2012;12(4):306–311. doi: 10.1038/tpj.2011.4. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Muscat GE. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Molecular Endocrinology. 2010;24(10):1891–1903. doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietilainen KH, Rog T, Seppanen-Laakso T, Virtue S, Gopalacharyulu P, Tang J, et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biology. 2011;9(6):e1000623. doi: 10.1371/journal.pbio.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppelreuther M, Rudolph B, Du C, Grossmann R, Becker M, Thiele C, et al. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. Journal of Lipid Research. 2012;53(5):888–900. doi: 10.1194/jlr.M024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen LL, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Seminars in Cell and Developmental Biology. 2012 doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50(6):1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A, Shah YM, Manna SK, Gonzalez FJ. Disruption of endothelial peroxi-some proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32(1):65–73. doi: 10.1161/ATVBAHA.111.239137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Novack DV, Christiansen B, et al. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2beta)-null mice. American Journal of Pathology. 2008;172(4):868–881. doi: 10.2353/ajpath.2008.070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Reardon HT, Hsieh AT, Jung Park W, Kothapalli KS, Anthony JC, Nathanielsz PW, et al. Dietary long-chain polyunsaturated fatty acids upregulate expression of FADS3 transcripts. Prostaglandins Leukotrienes and Essential Fatty Acids. 2012 doi: 10.1016/j.plefa.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Gelb MH. Secretory phospholipase A2: a multifaceted family of proatherogenic enzymes. Current Cardiology Reports. 2009;11(6):445–451. doi: 10.1007/s11886-009-0064-2. Review. [DOI] [PubMed] [Google Scholar]
- Sabido E, Quehenberger O, Shen Q, Chang CY, Shah I, Armando AM, et al. Targeted proteomics of the eicosanoid biosynthetic pathway completes an integrated genomics–proteomics–metabolomics picture of cellular metabolism. Molecular and Cellular Proteomics. 2012;11(7):M111.014746. doi: 10.1074/mcp.M111.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, et al. Prevention of steatosis by hepatic JNK1. Cell Metabolism. 2009;10(6):491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RJ, Ofman R, Duran M, Kemp S, Wanders RJ. Omega-oxidation of very long-chain fatty acids in human liver microsomes. Implications for X-linked adrenoleukodystrophy. Journal of Biological Chemistry. 2006;281(19):13180–13187. doi: 10.1074/jbc.M513481200. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43(1):1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian BM, Roychowdhury S, Tang H, Hillian AD, Feldstein AE, Stahl GL, et al. Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. Journal of Biological Chemistry. 2011;286(41):35989–35997. doi: 10.1074/jbc.M111.254201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2012 doi: 10.1152/ajplung.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, Ainsworth HC, et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. British Journal of Nutrition. 2012;107(4):547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB Journal. 2007;21(2):325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC. Soluble epoxide hydrolase inhibitors: a patent review. Expert Opinion on Therapeutic Patents. 2010;20(7):941–956. doi: 10.1517/13543776.2010.484804. Review. [DOI] [PubMed] [Google Scholar]
- Shindou H, Hishikawa D, Harayama T, Yuki K, Shimizu T. Recent progress on acyl CoA: lysophospholipid acyltransferase research. Journal of Lipid Research. 2009;50(Suppl):S46–S51. doi: 10.1194/jlr.R800035-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nature Genetics. 2000;25(1):87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chemical Reviews. 2011;111(10):5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Wohltmann M, Bao S, Ladenson JH, Semenkovich CF, Turk J. Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet. American Journal of Physiology: Endocrinology and Metabolism. 2010;298(6):E1097–E1114. doi: 10.1152/ajpendo.00780.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WL, Stubbe J, Ricciotti E, Alamuddin N, Ibrahim S, Crichton I, et al. Niacin and biosynthesis of PGD(2)by platelet COX-1 in mice and humans. Journal of Clinical Investigation. 2012;122(4):1459–1468. doi: 10.1172/JCI59262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. Journal of Cell Science. 2009;122(Pt 11):1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Progress in Lipid Research. 2004;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. Journal of Immunology. 2011;187(4):1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Dostalek M, Guengerich FP. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS Journal. 2008;275(14):3706–3717. doi: 10.1111/j.1742-4658.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiological Genomics. 2007;30(1):74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metabolism. 2005;1(4):245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Mandard S, Tan NS, Wahli W, Trautwein C, Richardson TA, et al. The interleukin-1 receptor antagonist is a direct target gene of PPARalpha in liver. Journal of Hepatology. 2007;46(5):869–877. doi: 10.1016/j.jhep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. Journal of Biological Chemistry. 2010;285(43):32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendiran A, Pradhan SC, Agrawal A, Subrahmanyam DK, Rajan S, Anichavezhi D, et al. Influence of CYP2C9 gene polymorphisms on response to glibenclamide in type 2 diabetes mellitus patients. European Journal of Clinical Pharmacology. 2011;67(8):797–801. doi: 10.1007/s00228-011-1013-8. [DOI] [PubMed] [Google Scholar]
- Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer and Metastasis Reviews. 2011;30(3–4):409–417. doi: 10.1007/s10555-011-9314-z. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends in Pharmacological Sciences. 2011;32(9):543–550. doi: 10.1016/j.tips.2011.04.004. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Miwa Y, Mou K, Tomikawa M, Eguchi N, Urade Y, et al. Knockout of the l-pgds gene aggravates obesity and atherosclerosis in mice. Biochemical and Biophysical Research. 2009;378(4):851–856. doi: 10.1016/j.bbrc.2008.11.152. [DOI] [PubMed] [Google Scholar]
- Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metabolism and Disposition. 2011;39(1):22–29. doi: 10.1124/dmd.110.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theken KN, Deng Y, Schuck RN, Oni-Orisan A, Miller TM, Kannon MA, et al. Enalapril reverses high-fat diet-induced alterations in cytochrome P450-mediated eicosanoid metabolism. American Journal of Physiology: Endocrinology and Metabolism. 2012;302(5):E500–E509. doi: 10.1152/ajpendo.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne A, Lofgren P, Hoffstedt J. Increased visceral adipocyte lipolysis–a pathogenic role in nonalcoholic fatty liver disease? Journal of Clinical Endocrinology and Metabolism. 2010;95(10):E209–E213. doi: 10.1210/jc.2010-0520. [DOI] [PubMed] [Google Scholar]
- Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nature Genetics. 2010;42(1):21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- Tishinsky JM, Ma DW, Robinson LE. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARgamma-dependent manner in human adipocytes. Obesity (Silver Spring) 2011;19(2):262–268. doi: 10.1038/oby.2010.186. [DOI] [PubMed] [Google Scholar]
- Titos E, Ferre N, Lozano JJ, Horrillo R, Lopez-Parra M, Arroyo V, et al. Protection from hepatic lipid accumulation and inflammation by genetic ablation of 5-lipoxygenase. Prostaglandins and Other Lipid Mediators. 2010;92(1–4):54–61. doi: 10.1016/j.prostaglandins.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hyper-triglyceridemia, type 2 diabetes, and cardiovascular disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura K, Kato I, Hirashima Y, Ishii Y, Inoue T, Aoki J, et al. Neuroprotective role of transgenic PAF-acetylhydrolase II in mouse models of focal cerebral ischemia. Stroke. 2007;38(3):1063–1068. doi: 10.1161/01.STR.0000257981.09329.d2. [DOI] [PubMed] [Google Scholar]
- van de Ven R, Oerlemans R, van der Heijden JW, Scheffer GL, de Gruijl TD, et al. ABC drug transporters and immunity: novel therapeutic targets in autoimmunity and cancer. Journal of Leukocyte Biology. 2009;86(5):1075–1087. doi: 10.1189/jlb.0309147. Review. [DOI] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Steffensen KR, Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends in Endocrinology and Metabolism. 2011;22(8):333–343. doi: 10.1016/j.tem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochimica et Biophysica Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Natural Medicines. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends in Endocrinology and Metabolism. 2012;23(7):351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Ferdinandusse S, Brites P, Kemp S. Peroxisomes, lipid metabolism and lipotoxicity. Biochimica et Biophysica Acta. 2010;1801(3):272–280. doi: 10.1016/j.bbalip.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. Journal of Clinical Gastroenterology. 2007;41(6):616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. Journal of Lipid Research. 2007;48(12):2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- Watkins PA. Very-long-chain acyl-CoA synthetases. Journal of Biological Chemistry. 2008;283(4):1773–1777. doi: 10.1074/jbc.R700037200. Review. [DOI] [PubMed] [Google Scholar]
- Weng Y, DiRusso CC, Reilly AA, Black PN, Ding X. Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. Journal of Biological Chemistry. 2005;280(36):31686–31698. doi: 10.1074/jbc.M504447200. [DOI] [PubMed] [Google Scholar]
- Westphal C, Konkel A, Schunck WH. CYP-eicosanoids—a new link between omega-3 fatty acids and cardiac disease? Prostaglandins and Other Lipid Mediators. 2011;96(1–4):99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Wilson JP, Raghavan AS, Yang YY, Charron G, Hang HC. Proteomic analysis of fatty-acylated proteins in mammalian cells with chemical reporters reveals S-acylation of histone H3 variants. Molecular and Cellular Proteomics. 2011;10(3):M1100.01198. doi: 10.1074/mcp.M110.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WI, Carman GM. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by nucleotides. Journal of Biological Chemistry. 1994;269(47):29495–29501. [PubMed] [Google Scholar]
- Wu CC, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, et al. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension. 2011;57(4):788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Dnyanmote AV, Nigam SK. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Molecular Pharmacology. 2011;79(5):795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z. Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hyper-contractility in diabetic animals. Journal of Biological Chemistry. 2010;285(12):8628–8638. doi: 10.1074/jbc.M109.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang B, Molony C, Chudin E, Hao K, Zhu J, et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Research. 2010;20(8):1020–1036. doi: 10.1101/gr.103341.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Ye J. Long chain acyl-CoA synthetase 3-mediated phosphatidylcholine synthesis is required for assembly of very low density lipoproteins in human hepatoma Huh7 cells. Journal of Biological Chemistry. 2008;283(2):849–854. doi: 10.1074/jbc.M706160200. [DOI] [PubMed] [Google Scholar]
- Yoon YM, Sung MJ, Song SC, et al. Enhanced A-FABP expression in visceral fat: potential contributor to the progression of NASH. Clinical and Molecular Hepatology. 2012;18(23091808):279–286. doi: 10.3350/cmh.2012.18.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ip E, Dela Pena A, Hou JY, Sesha J, Pera N, et al. COX-2 induction in mice with experimental nutritional steatohepatitis: role as pro-inflammatory mediator. Hepatology. 2006;43(4):826–836. doi: 10.1002/hep.21108. [DOI] [PubMed] [Google Scholar]
- Yu M, Benham A, Logan S, Brush RS, Mandal MN, Anderson RE, et al. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. Journal of Lipid Research. 2012;53(3):494–504. doi: 10.1194/jlr.M021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Sidhu RS, Kuklev DV, Kado Y, Wada M, Song I, et al. Cyclooxygenase allosterism, fatty acid-mediated cross-talk between monomers of cyclooxygenase homodimers. Journal of Biological Chemistry. 2009;284(15):10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Ta TC, Lin M, Evans JR, Dong Y, Bolotin E, et al. Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One. 2009;4(5):e5609. doi: 10.1371/journal.pone.0005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiriou MP, Zelarayan LC, Noack C, Renger A, Nigam S, Siafaka-Kapadai A. Hepoxilin A(3) protects beta-cells from apoptosis in contrast to its precursor, 12-hydroperoxyeicosatetraenoic acid. Biochimica et Biophysica Acta. 2011;1811(6):361–369. doi: 10.1016/j.bbalip.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hardwick JP. Regulation of CYP4F2 leukotriene B4 omega-hydroxylase by retinoic acids in HepG2 cells. Biochemical and Biophysical Research. 2000;279(3):864–871. doi: 10.1006/bbrc.2000.4020. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen L, Hardwick JP. Promoter activity and regulation of the CYP4F2 leukotriene B(4) omega-hydroxylase gene by peroxisomal proliferators and retinoic acid in HepG2 cells. Archives of Biochemistry and Biophysics. 2000;378(2):364–376. doi: 10.1006/abbi.2000.1836. [DOI] [PubMed] [Google Scholar]
- Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, et al. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2005;288(1):R188–R196. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hashimoto K, Goleniewska K, O'Neal JF, Ji S, Blackwell TS, et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. Journal of Immunology. 2007;178(2):702–710. doi: 10.4049/jimmunol.178.2.702. [DOI] [PubMed] [Google Scholar]
- Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacology and Therapeutics. 2010;125(3):446–463. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Zou H, Yuan C, Dong L, Sidhu RS, Hong YH, Kuklev DV, et al. Human cyclooxygenase-1 activity and its responses to COX inhibitors are allosterically regulated by nonsubstrate fatty acids. Journal of Lipid Research. 2012;53(7):1336–1347. doi: 10.1194/jlr.M026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu L, Jiang H, He J, Xu C, Pu S, Liu M, et al. Salicylate blocks lipolytic actions of tumor necrosis factor-alpha in primary rat adipocytes. Molecular Pharmacology. 2008;73(1):215–223. doi: 10.1124/mol.107.039479. [DOI] [PubMed] [Google Scholar]